(C) 2013 Andrés Arias. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Arias A, Barroso R, Anadón N, Paiva PC (2013) On the occurrence of the fireworm Eurythoe complanata complex (Annelida, Amphinomidae) in the Mediterranean Sea with an updated revision of the alien Mediterranean amphinomids. ZooKeys 337: 19–33. doi: 10.3897/zookeys.337.5811
The presence of two species within the Eurythoe complanata complex in the Mediterranean Sea is reported, as well as their geographical distributions. One species, Eurythoe laevisetis, occurs in the eastern and central Mediterranean, likely constituting the first historical introduction to the Mediterranean Sea and the other, Eurythoe complanata, in both eastern and Levantine basins. Brief notes on their taxonomy are also provided and their potential pathways for introduction to the Mediterranean are discussed. A simplified key to the Mediterranean amphinomid genera and species of Eurythoe and Linopherus is presented plus an updated revision of the alien amphinomid species reported previously from the Mediterranean Sea. A total of five exotic species have been included; information on their location, habitat, date of introduction and other relevant features is also provided.
Alien polychaetes, cryptic species, Gibraltar Strait, Lessepsian migrant
Introductions of alien species are threatening the economic and ecological well-being of marine ecosystems worldwide. The impacts of alien species on their new environments include alterations of established food webs, importation of new diseases or parasites, competition with native species for food and space, and even changing gene pools (
Amphinomidae is a well-known family of polychaetes that is globally distributed, reaching its highest diversity in shallow tropical and subtropical waters (
Shallow water forms play an important ecological role mainly in rocky and coral reef environments, where species such as Hermodice carunculata (Pallas, 1766) are major predators of both soft corals (Alcyonacea) and hard corals (Scleractinia) (
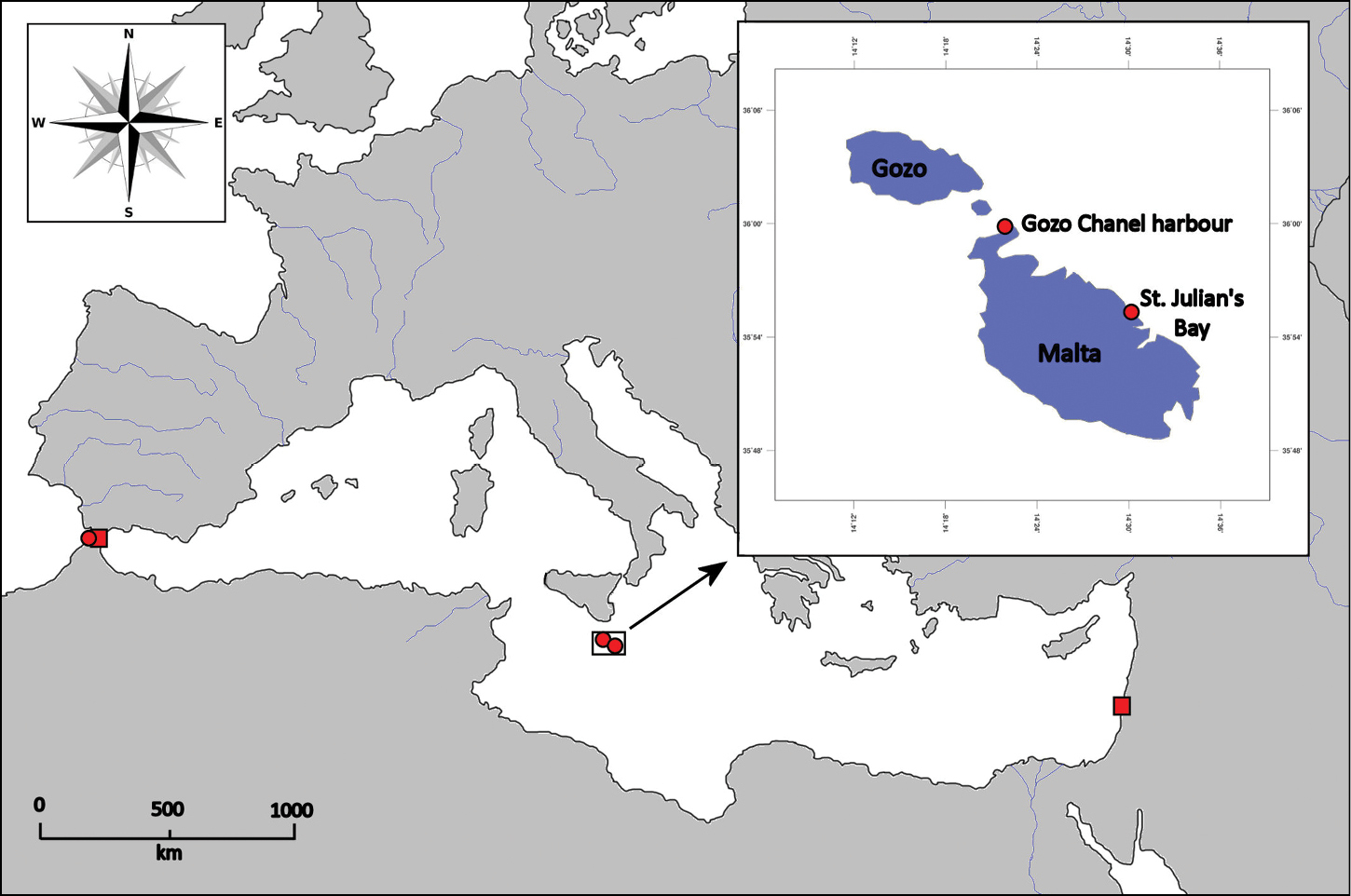
Field collections were made along the Maltese Islands, Central Mediterranean, on hard substrata from the shallow subtidal rocky areas at Ċirkewwa Harbour (35°59'N, 14°19'E) and St. Julian’s Bay (35°55'N, 14°29'E) in March 2011 (Figure 1). Large specimens were randomly removed by a swift hand motion. Small specimens were collected using grabs and screened using a 1 mm mesh sieve. The worms were removed from the residue under a stereomicroscope. Then, all specimens were relaxed in MgCl2 isotonic with seawater, fixed in 10% formaldehyde solution, rinsed in fresh water and finally transferred to 70% ethanol. Photographs were taken using a stereomicroscope Nikon SMZ-1000 equipped with a digital camera; before photography, specimens were stained with lithic carmine solution. Lithic carmine staining increased the contrast of some morphological structures, such as caruncle, branchiae, parapodial lobes and cirri. Glycerol slides of parapodial sections, examined under a compound light microscope Leica DM 2500, were used for the detailed examination of chaetal morphology and distribution.
Current distribution of Eurythoe laevisetis (red circles) and Eurythoe complanata (red squares) along the Mediterranean Sea.
The examined material was deposited at the Invertebrate Collection of the Department of Biology of Organisms and Systems (BOS) of University of Oviedo. Detailed location data is given below in the ‘Material examined’ sections of the respective species. The number of specimens in each sample is given in parentheses after the museum abbreviation and registration number. Furthermore, preserved specimens identified as Eurythoe complanata from the Gibraltar Strait, eastern Mediterranean (deposited in the MNCN), and the coasts of Atlit, Israel (deposited in the BMNH), were re-examined.
Additionally, comparative material was also studied: Eurythoe laevisetis Fauvel, 1914: São Tomé Island: IBUFRJ 0545; Eurythoe cf. laevisetis: Sal Island (Cape Verde): BOS-Amp1; Gran Canary (Canary Islands): BOS-Amp2; Eurythoe complanata: Bocas del Toro, Panamá (Caribbean): IBUFRJ 0542. Red Sea (unknown locality): BMNH 1923.3.20.8.
BMNH The Natural History Museum, London, U.K.
BOS Biology of Organisms and Systems, University of Oviedo, Spain
IBUFRJ Instituto de Biologia, Universidade Federal do Rio de Janeiro, Brazil
MNCN Museo Nacional de Ciencias Naturales, Madrid, Spain
An updated check-list of the alien amphinomid species is provided based on an exhaustive review of the species records in the literature. The species data were mainly extracted from the regional reviews on alien species and compilations of polychaete species. We have also included data on their ecology, distribution and other relevant features.
The revision of the literature along with our results (observations on 28 Mediterranean specimens belonging to Eurythoe complanata complex) revealed that five amphinomid species belonging to three genera were determined to be alien species in the Mediterranean Sea: Eurythoe laevisetis, Eurythoe complanta, Linopherus acarunculatus (Monro, 1937), Linopherus canariensis Langerhans, 1881 and Notopygos crinita Grube, 1855. The diagnostic differences between these species are summarised in the key provided. Furthermore, information about location, habitat, date of introduction and other relevant features are provided in Table 1.
Summary of current knowledge on exotic Mediterranean Amphinomidae.
| Species | Locality | Year | Mediterranean area | Habitat | Others features | Reference |
|---|---|---|---|---|---|---|
| Eurythoe complanata (Pallas, 1766) | Atlit (Israel) | 1937 | Eastern | |||
| Gulf of Eilat (Israel) | 1976 | Eastern | intertidal reefs of Dendropoma spp | Occurring together with another amphinomid Linopherus acarunculatus | ||
| Isabel II Island Gibraltar Strait (Spain) | September 1992 | Western | On rocks, 3–6 m depth | Occurring sympatrically with Eurythoe laevisetis | Current work | |
| Isabel II Island Gibraltar Strait (Spain) | July 1993 | Western | On rocks, 3–6 m depth | |||
| Congreso Island Gibraltar Strait (Spain) | July 1993 | Western | On rocks, 3 m depth | Current work | ||
| Chafarinas Islands Gibraltar Strait (Spain) | 1995 | Western | Rocky substrate | |||
| Eurythoe laevisetis Fauvel, 1914 | Isabel II Island Gibraltar Strait (Spain) | September 1992 | Western | On rocks, 3–6 m depth | Occurring sympatrically with Eurythoe complanata | Current work |
| Isabel II Island Gibraltar Strait (Spain) | July 1993 | Western | On rocks, 3–6 m depth | |||
| Gozo Harbour (Malta) | March 2011 | Central | Rocky bottom 0.5–1 m depth | Associated with the invasive Branchiomma bairdi | Current work | |
| Linopherus acarunculatus (Monro, 1937) | Lebanon | 1966 | Eastern | Shallow waters | Referred to as Pseudeurythoe acarunculata Monro, 1937. |
|
| Gulf of Elat (Israel) | 1976 | Eastern | Intertidal reefs of Dendropoma spp | |||
| Linopherus canariensis Langerhans, 1881 | Kemer (Turkey) | July, 1993 | Eastern | 5 m depth on algae | ||
| Cyprus | May 1997 | Eastern | 35 m depth on sandy substrate | Associated with Brachiomma lanceolatum | ||
| Antalya Bay (Turkey) | 1997 | Eastern | Referred to as Pseudeurythoe acarunculata Monro, 1937 | |||
| Cyprus | 2005 | Eastern | ||||
| Turkey | September-October 2005 | Eastern | On rocks between 0.1–5 m. Mainly in Calendula officinalis substrate | |||
| Italy | 2005 | Central | ||||
| Lake of Faro (Italy) | May 2008 | Central | Artificial modules with a neighboring sandy bottom, 1.2 m depth | Showed an invasive behaviour, reaching densities of 41.86 ind / m2 | ||
| Notopygos crinita Grube, 1855 | Italy | 1983 | Central | Currently this species is considered as not established in the Mediterranean ( |
Type species. Eurythoe capensis Kinberg, 1857, subsequent designation: Eurythoe complanata (Pallas, 1766).
http://species-id.net/wiki/Eurythoe_laevisetis
Fig. 2A–FEurythoe cf. complanata: Gozo Harbour (Malta), 35°50'N, 14°35'E (Mar. 2011): BOS-Amp3 (2 specimens), BOS-Amp4 (9 specimens).
Eurythoe complanata: Isabel II Island (Chafarinas Islands, Spain), 35°11'N, 2°26'W (Sep. 1992): MNCN 16.01/3340 (1 specimen); (Jul. 1993) MNCN 16.01/33394 (1 specimen).
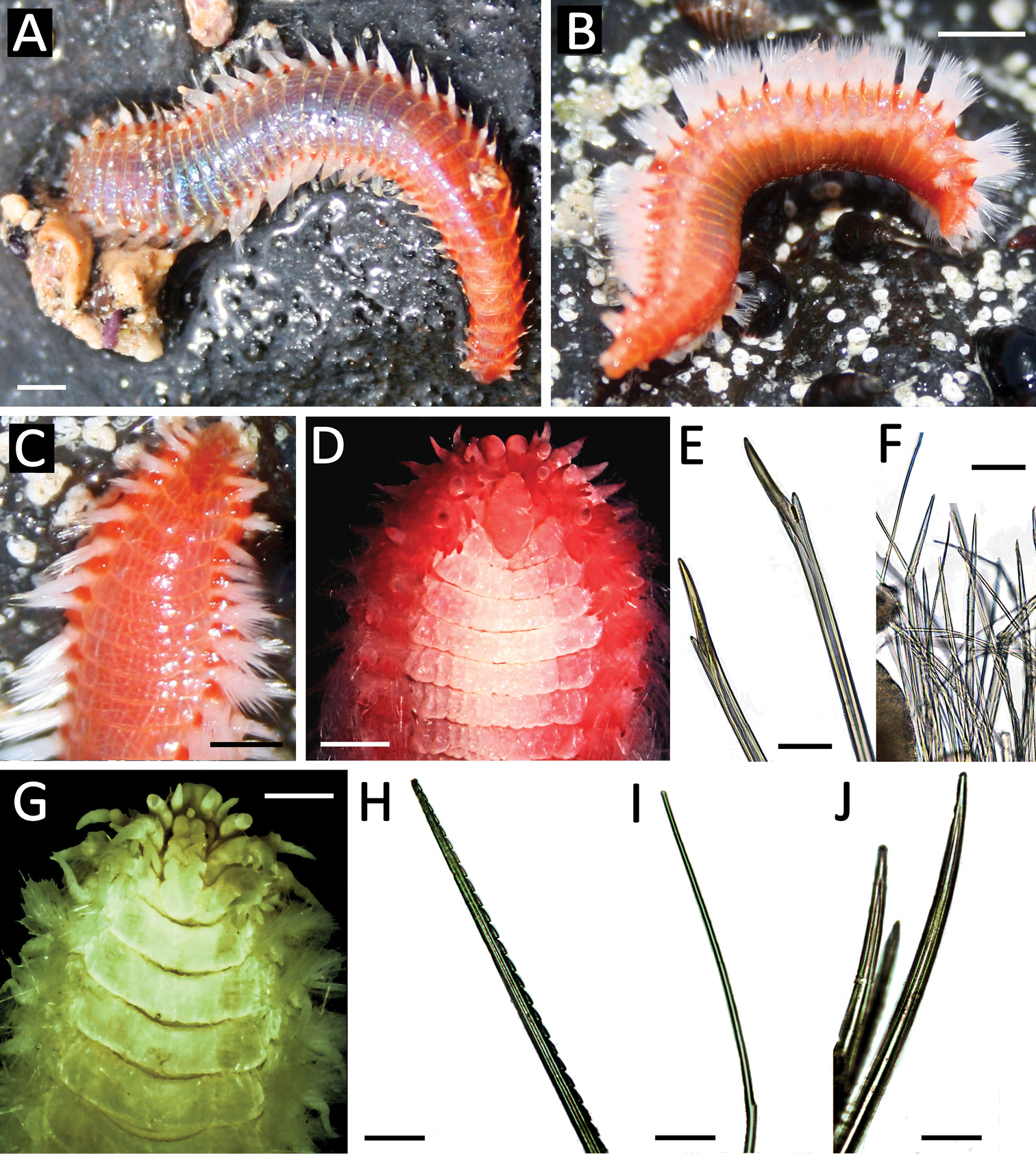
Body depressed elongated, rectangular in cross section. Specimens from Malta ranged in length from 14 to 52 mm with a mean of 39 mm (N=11, SD=12.09). Live specimens have a uniform orange-pinkish colour (Fig. 2A–C), on which the gills and a bright red caruncle stand out, and white chaeta fascicles forming two longitudinal bands along the body (Fig. 2A, B). Prostomium rounded with 2 pairs of inconspicuous eyes arranged in a square and three antennae, two lateral ones in an anterior position and one slightly behind the others. The anterior end has a bilobed prebuccal lobe where are inserted a pair of cirriform palps (Fig. 2D). The caruncle is elongated and extends until the third chaetiger (Fig. 2C, D). Each segment is provided with a pair of arborescent gills that are present from the second chaetiger to the posterior region (Fig. 2C, D). Biramous parapodia with digitiform dorsal and ventral cirri, similar in size. Notochaetae of two types: very fine with a small spur that continues in a capillary-like thorn; and thicker with a marked spur (spurred capillary notochaeta) (Fig. 2F). The neurochaetae are spur-type and thick, slightly denticulate on juveniles (Fig. 2E).
Eurythoe laevisetis from Malta. A live specimen, general view B live specimen, lateral view C live specimen anterior end, dorsal view D detailed view of anterior end, dorsal view E neurochaetae F spurred capillary notochaetae. Eurythoe complanata from Israel G detailed anterior end, dorsal view H harpoon notochaeta I notopodial spurred capillar notochaeta J notoacicular spines.
Several Maltese specimens present evidence of regeneration of the anterior and posterior end. All preserved specimens have whitish colour and lack the characteristic harpoon notochaetae. The two pairs of eyes are extremely inconspicuous, the anteriormost being similar in size to the posterior one. Specimens from Malta and Chafarinas Islands were morphologically identical to the Atlantic Eurythoe laevisetis from the Canary Islands and Cape Verde and Eurythoe laevisetis from São Tomé Island.
http://species-id.net/wiki/Eurythoe_complanata
Fig. 2G–JEurythoe complanata: Isabel II Island (Chafarinas Islands, Spain), 35°11'N, 2°26'W (Sep. 1992): MNCN 16.01/3337 (2 specimens), MNCN 16.01/3338 (2 specimens), MNCN 16.01/3340 (1 specimen); Congreso Island (Chafarinas Islands, Spain), 35°11'N, 2°26'W (Jul. 1993): MNCN 16.01/3336 (1 specimen); Isabel II Island (Chafarinas Islands, Spain), 35°11'N, 2°26'W: MNCN 16.01/33394 (2 specimens). Atlit (Israel), 32°41'N, 34°56'E (1937): BMNH 1937.4.7.1-5 (7 specimens).
Israeli specimens ranged from 20 to 45 mm in length with a mean of 31 mm (N=7, SD=9.77). Prostomium rounded with 2 pairs of eyes arranged in a square, the first being larger (Fig. 2G), and with three antennae, lateral ones in an anterior position and the single one slightly posterior. Anterior end with a bilobed prebuccal lobe, carrying a pair of cirriform palps. The caruncle is elongated and extends until the third chaetiger (Fig. 2G). Each segment is provided with a pair of arborescent branchiae that are present from the second chaetiger to the posterior end. Biramous parapodia with dorsal and ventral cirri digitiform, similar in size. Notochaetae of three types: harpoon-like (Fig. 2H); spurred capillaries with small spurs (Fig. 2I) and thicker smooth notochaetal spines (Fig. 2J). Notoacicula are very small, hastate, limited in number and always form an arc immediately in front of the dorsal cirrus. Neurochaetae are bifurcate, with prongs of different lengths.
One specimen regenerating the posterior end. Pairs of eyes inconspicuous in some specimens, but always with the anterior pair larger than posterior pair. Specimens from Chafarinas Islands had a mean size of 37 mm (N= 8, SD = 7.24). All preserved specimens had a brownish colour.
| 1 | Caruncle absent | Hipponoa |
| – | Caruncle present, variably developed | 2 |
| 2 | Oval body | 3 |
| – | Elongated body; subcylindrical or quadrangular cross section | 4 |
| 3 | Dorsal accessory (branchial) cirri plus dorsal cirri on anteriormost abranchiate chaetigers; in branchiate chaetigers, one dorsal cirri per notopodium; bipinnate branchiae | Chloeia |
| – | Dorsal accessory (branchial) cirri plus dorsal cirri on all chaetigers; palmate branchiae | Notopygos |
| 4 | First chaetiger dorsally continuous, complete | 5 |
| – | First chaetiger dorsally discontinuous, not complete | 7 |
| 5 | Hooks present in the first chaetiger; caruncle round | Paramphinome |
| – | Hooks not present in the first chaetiger | 6 |
| 6 | Branchiae limited to anterior segments | Linopherus 10 |
| – | Branchiae on all segments after the chaetiger 2 or 3 | Amphinome |
| 7 | Caruncle large and conspicuous, extending beyond one chaetiger posteriorly | 8 |
| – | Caruncle small and inconspicuous, not extending beyond one chaetiger posteriorly | Cryptonome |
| 8 | Caruncle without a median lobe, with folds obliquely arranged | Hermodice |
| – | Caruncle with a smooth median lobe | 9 |
| 9 | Caruncle not sinusoidal | Eurythoe 11 |
| – | Caruncle sinusoidal | Pareurythoe |
| 10 | First branchiae present on chaetiger 3 | Linopherus canariensis |
| – | First branchiae present on chaetiger 4 | Linopherus acarunculatus |
| 11 | Three types of notochaetae present: spurred capillary, notoacicular spine and harpoon | Eurythoe complanata |
| – | Two types of notochaetae present: spurred capillary and notochaetal spine; harpoon absen | Eurythoe laevisetis |
Members of the family Amphinomidae have a number of characteristics that gives the group high invasive potential. They show high biological plasticity and reproductive habits that include both sexual and asexual reproduction; possess a great capacity of regeneration and a large dispersal capability due to their long-term rostraria larvae (
The use of the term ‘morphospecies’ for referring to Eurythoe complanata has been proposed as an alternative to overcome the identification difficulties associated with this species complex, which includes two cryptic species along with Eurythoe laevisetis. Here, we have an example of two species that are genetically distinct but morphologically identical under the same ‘morph’, named as Eurythoe complanata. So, the Eurythoe complanata complex erected by
The Eurythoe complanata complex represents one more case of species group that is likely to be introduced in the Mediterranean, but which has been underestimated and misidentified. Re-examination of specimens from Malta, Chafarinas Islands and Israel demonstrates the existence of two morphospecies belonging to the Eurythoe complanata complex in the Mediterranean Sea: Eurythoe laevisetis in the western and central Mediterranean and Eurythoe complanata in the western and Levantine basins. Moreover, the Israeli Eurythoe complanata is not a recently introduced species, but one that had been present since, at least 1937. All examined specimens from Malta and two from Chafarinas Islands belong to the species Eurythoe laevisetis, characterized by the absence of the harpoon notochaetae. According to
On the other hand, all examined specimens from Israel and nine from Chafarinas Islands were morphologically identical to Eurythoe complanata from the Atlantic and Pacific sensu
The origins, plausible pathways and introduction vectors of these related amphinomids into the Mediterranean may be discerned by focusing on populations of the central (Eurythoe laevisetis), western (Eurythoe laevisetis and Eurythoe complanata) and Levantine (Eurythoe complanata) regions. For example, Maltese and Chafarinas populations of Eurythoe laevisetis may have originated from Atlantic islands through the Gibraltar Strait. Such a scenario is wholly consistent with arrivals of other Atlantic species of marine invertebrates into the Mediterranean such as the gastropod Marginella glabella (Linnaeus, 1758), which is presently colonizing the coasts of Málaga (SE Spain, western Mediterranean) from the Canary Islands and West Africa (
We are grateful to C. Glasby, J. Kudenov and one anonymous reviewer for valuable comments and suggestions that greatly improved the manuscript.The first author (AA) is supported by a Severo Ochoa fellowship from the FICYT Foundation (Principado de Asturias, Spain). CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) provided support to RB. CNPq and FAPERJ (Fundação Carlos Chagas Filho de Apoio à Pesquisa do Estado do Rio de Janeiro) provided financial support to PCP.