(C) 2012 Jordi Corbera. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new cumacean genus and species, Ithyleucon sorbei gen. et sp. n., was described from material collected in the southern margin of the Cap Ferret Canyon (Bay of Biscay, NE Atlantic). Although the new genus resembles Pseudoleucon Zimmer, 1903, in terms of the general aspect of the carapace and the pseudo-rostrum position, it shows important differences in the uropod structure and in the size of the antenna 1 accessory flagellum. In addition, some comments regarding the morphology of certain rare species (Mesolamprops denticulatus Ledoyer, 1983, Hemilamprops normani Bonnier, 1896 and Schizocuma spino-culatum (Jones, 1984)) are also provided.
Cumacea, new species, deep sea, suprabenthos, Atlantic Ocean
Cumaceans display a wide diversity in deep waters (
Following the study of suprabenthic communities of the Cap Ferret Canyon, this work deals with some rare and undescribed cumacean species that have since been discovered there.
Material and methodsThe present material was collected within the framework of a study on the suprabenthic community structure of the continental margin in the Bay of Biscay (
The type material was deposited in the Biological Reference Collection (CBR) of the Institut de Ciències del Mar, CSIC, Barcelona.
Taxonomy Family Lampropidae Sars, 1878http://species-id.net/wiki/Mesolamprops_denticulatus
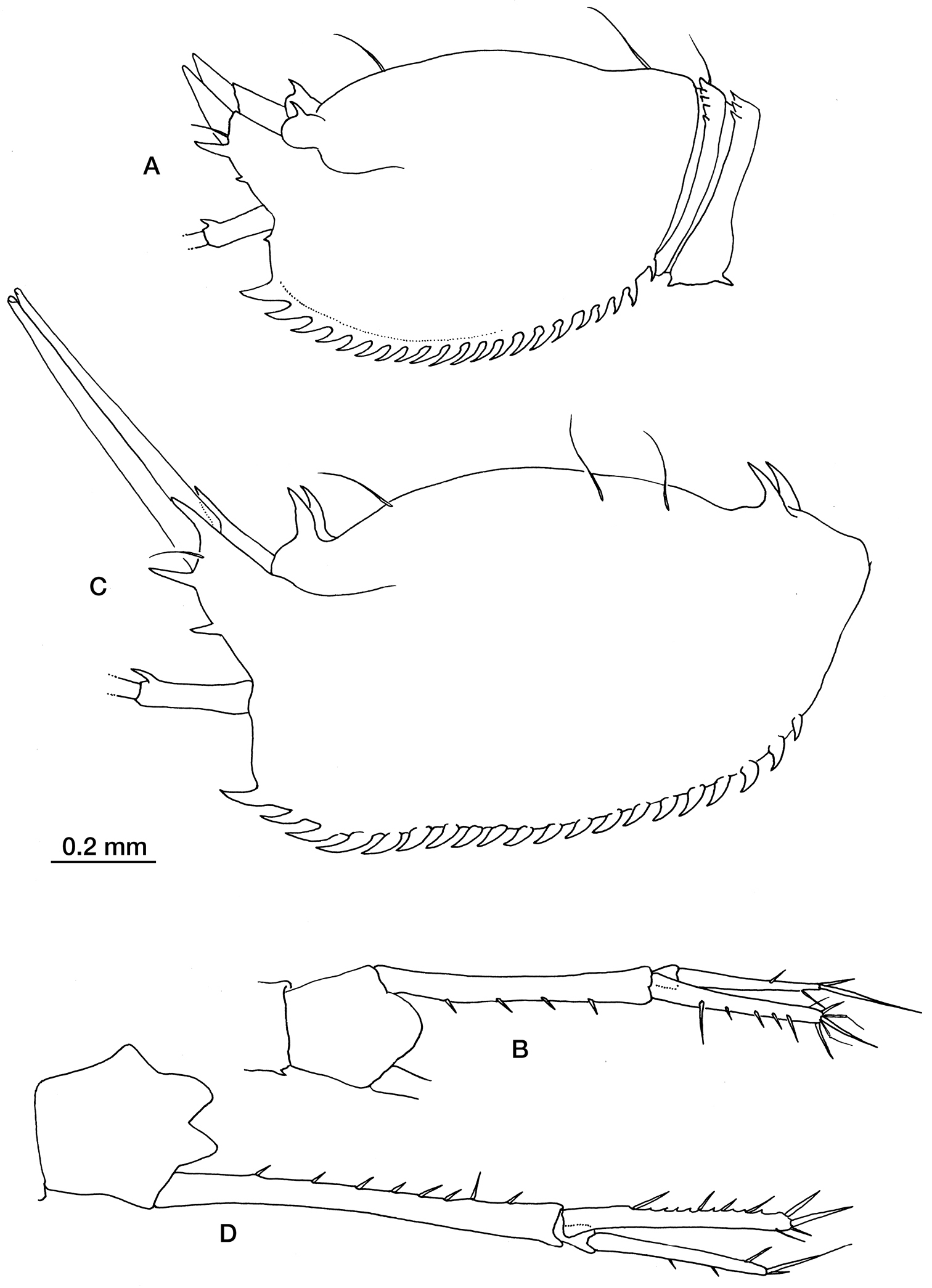
Fig. 1CCap Ferret Canyon, Bay of Biscay, ESSAIS I: stn TS01, 44°33.30'N, 2°08.30'W, 346–347 m, 21/04/89, 2 pread. female. ESSAIS II: stn TS04, 44°34.380'N, 2°10.18'W, 484–485 m, 18/05/89, 1 pread. female. ECOFER I: stn TS05, 44°35.57'N, 2°11.21'W, 522–523 m, 1/07/89, 2 pread. female, 1 pread. male, 1 adult male. J.-C. Sorbe leg.
Mesolamprops denticulatus was described from the Mediterranean Sea by
Mesolamprops denticulatus was for a long time considered an endemic Mediterranean species until
http://species-id.net/wiki/Hemilamprops_normani
Fig. 1A, BCap Ferret Canyon, Bay of Biscay, ESSAIS II: stn TS10, 44°33.10'N, 2°13.13'W, 791–790 m, 18/05/89, 3 mancas, 2 pread. female, 1 ad. male; stn TS11, 44°32.89'N, 2°14.24’W, 923–924 m, 18/05/89, 6 mancas, 2 pread. males; stn TS13, 44°34.19'N, 2°16.18'W, 1097–1099 m, 17/05/89, 4 mancas, 2 imm. males. J.-C. Sorbe leg.
Although
Hemilamprops normani is known to inhabit the waters of the Bay of Biscay (
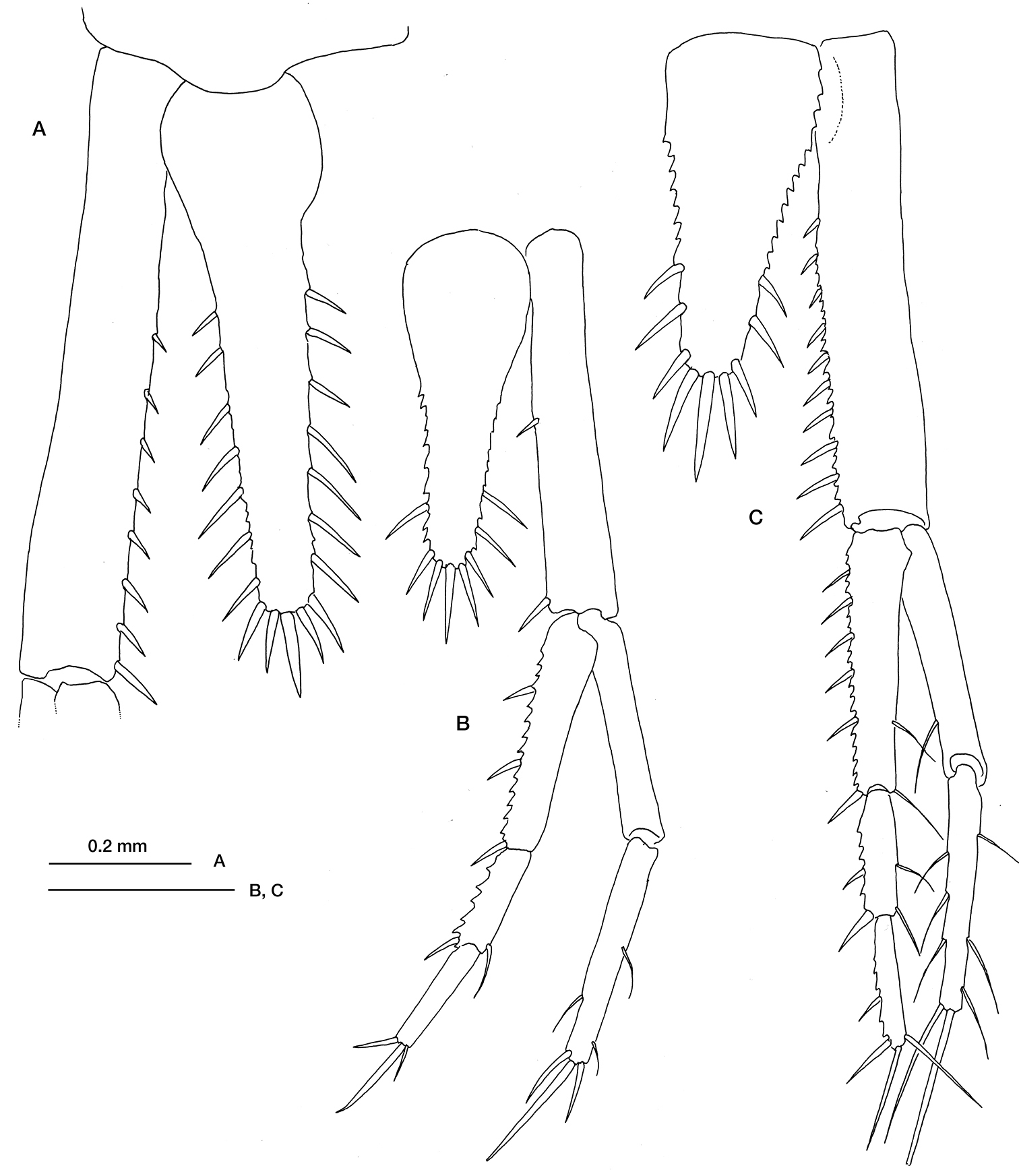
Hemilamprops normani Bonnier, 1896: A preadult female, telson and left uropod peduncle B manca, telson and right uropod. Mesolamprops denticulatus Ledoyer, 1983 C preadult female, telson and right uropod. Scale bar: 0.2 mm.
urn:lsid:zoobank.org:act:D9D1A1D7-C6EA-4E26-9C19-7AE5DE138F31
Pseudorostrum extending anterodorsally and upturned; antenna 1 geniculate between peduncle article 1 and 2; accessory flagellum longer than main flagellum article 1; female with exopods on maxilliped 3 and pereopods 1–3; male with exopods on maxilliped 3 and pereopods 1–4; pereopod 2 ischium very short; uropod endopod 2-articulate; male with two pairs of pleopods.
The shape of the carapace and the position of the pseudorostrum of Ithyleucon gen. n. resemble those of Pseudoleucon Zimmer, 1903. However, Ithyleucon differs from the latter by 1) the size of the uropod endopod, which is longer than the peduncle and of similar length as the exopod (i.e., as long as the peduncle and certainly shorter than the exopod in Pseudoleucon) and by 2) the antenna 1 accessory flagellum, which is longer than the main flagellum article 1 (shorter in Pseudoleucon). Although these two features, as well as the geniculated antenna 1, are in agreement with the diagnosis of Bytholeucon Watling, 1991, the anterolateral corner is strongly angular in this genus and the known males observed up until now have had only one pair of pleopods.
In addition to these morphological differences, the only two known Pseudoleucon species also show divergence in terms of their ecology and biogeography. They inhabit shallow bottoms of the northeastern Pacific and a phylogenetic relationship with the genus described herein seems to be highly unlikely.
From the Greek ithys, meaning upright, referring to the position of the pseudorostrum, and Leucon, the stem genus. Gender masculine.
Ithyleucon sorbei sp. n.
urn:lsid:zoobank.org:act:B29AE99A-81B2-431D-B9DB-221C28671864
http://species-id.net/wiki/Ithyleucon_sorbei
Figs 2–4Holotype: Cap Ferret Canyon, Bay of Biscay, ESSAIS II, stn TS13, 44°34.19'N, 2°16.18'W, 1097–1099 m, 17/05/89, preadult female (ICMU12101901), Jean-Claude Sorbe leg.
Paratypes: Same data as the holotype, 1 preadult female (ICMU12101903), 1 preadult female dissected in two slides (ICMU12101902), 2 preadult males (ICMU12101904 and ICMU12101905); ESSAIS I, stn TS12, 44°32.30'N, 2°15.10'W, 1024–1043 m, 22/04/89 1 immature male (ICMU12101906), Jean-Claude Sorbe leg.
Carapace without ridges, frontal lobe with two teeth and others located posteriorly. Pseudorostral lobes extending anterodorsally, upturned, anterior margin serrate. Antenna 1 geniculate between peduncle articles 1 and 2, accessory flagellum extending beyond the mid-length of main flagellum. Female with exopods on pereopods 1–3; male with exopods on pereopods 1–4. Uropod peduncle shorter than rami; endopod bi-articulate, slightly shorter than exopod. Male with 2 pairs of pleopods.
Preadult female 3.125 mm total length. Carapace (Fig. 2) slightly longer than a fourth of the total length; frontal lobe with two teeth and others (3–4) positioned posteriorly on the middorsal line. Pseudorostral lobes extending anterodorsally, upturned by an angle of about 90°, anterior margin serrate; antennal notch small, anterolateral angle acute with 0–3 serrations on the lower margin
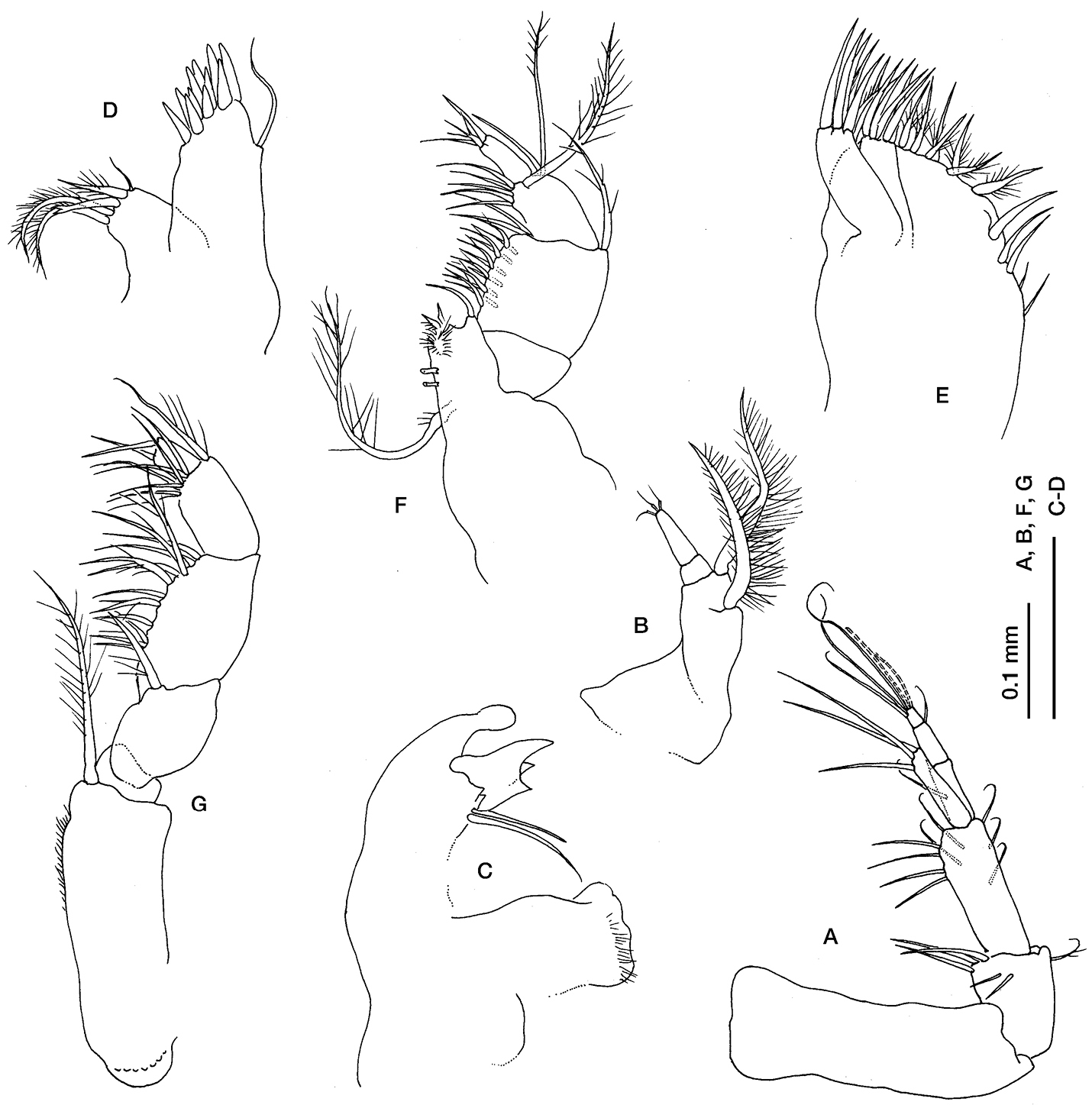
Antennula (Fig. 3A), peduncle 3-articulate, geniculate between articles 1 and 2; article 1 longer than the combined lengths of articles 2 and 3; article 2 shorter than article 3; main flagellum 3-articulate, shorter than the last peduncle article, with two aesthetascs and three long simple setae terminally; accessory flagellum longer than the main flagellum of article 1, with three long simple setae positioned terminally.
Antenna 2 (Fig. 3B) 3-articulate, with two pappose setae on article 1.
Mandible (Fig. 3C) base truncate, lacina mobilis with three teeth, two simple setae between lacina mobilis and pars molaris.
Maxillula (Fig. 3D) inner endite with five setae, one simple, three pappose and one bifid; outer endite with cuspidate setae.
Maxilla (Fig. 3E) with 3 endites; broad endite with 5 simple and several pappose setae terminally; narrow endites not extending beyond the distal margin of broad endite; inner narrow endite with 5 simple setae terminally; outer narrow endite with 4 simple setae terminally.
Maxilliped 1 (Fig. 3F) reduced with only three articles, dactylus minute.
Maxilliped 2 (Fig. 3G) basis shorter than rest of appendage, with a pappose seta on distal inner corner; merus with a long seta; carpus longer than merus with several simple setae on inner margin; propodus shorter than carpus, with a pappose seta on distal outer corner and several setae on inner margin; dactylus with two simple setae terminally.
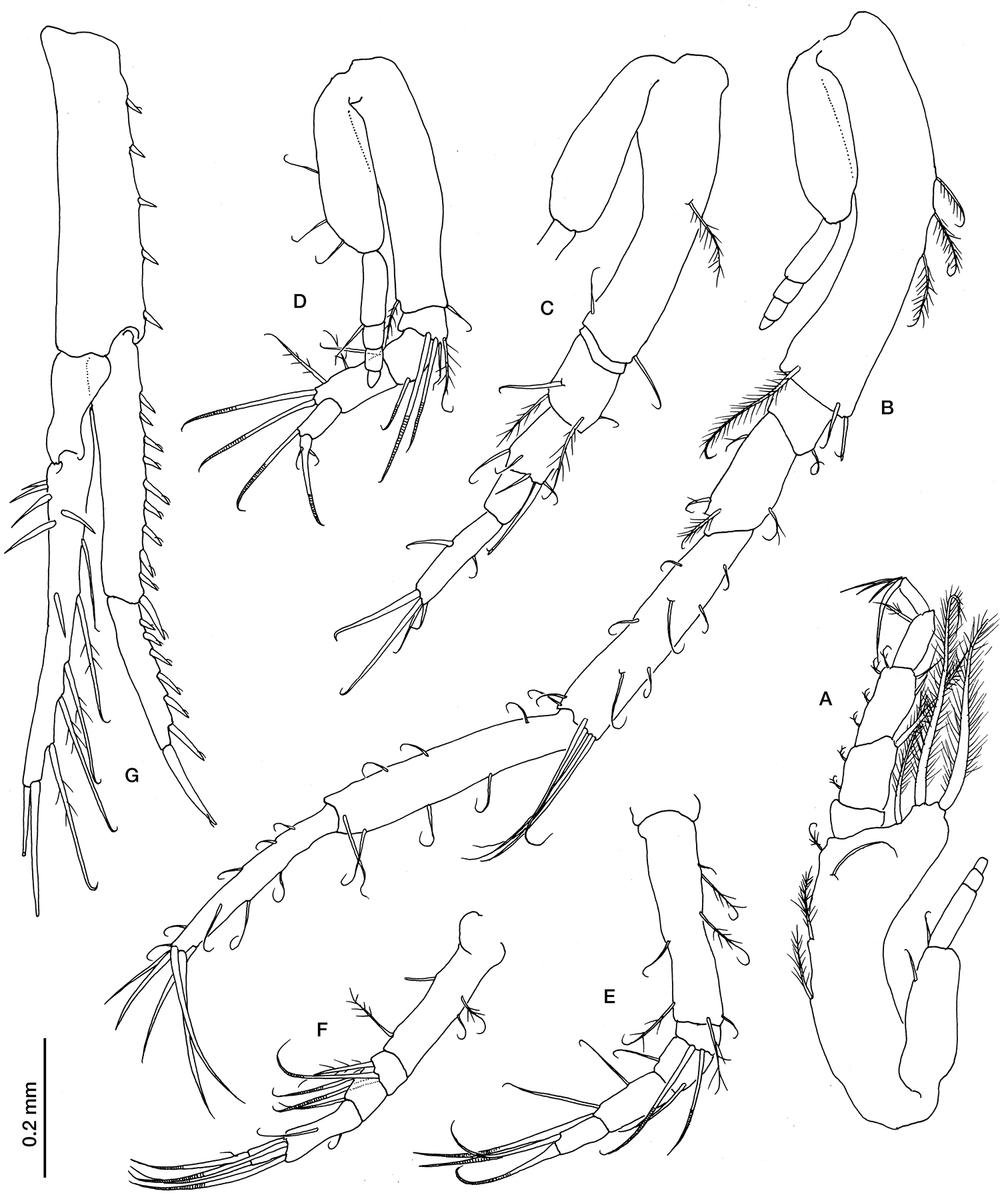
Maxilliped 3 (Fig. 4A) with well developed exopod, basis longer than rest of appendage, produced distally, with three long pappose setae on distal outer corner and three pappose setae on inner margin; merus with small pappose sete on inner margin and a long pappose seta on distal outer corner; carpus as long as merus, with pappose seta on inner margin and two simple setae on distal outer corner; propodus shorter than carpus with a pappose seta on inner margin; dactylus shorter than propodus.
Pereopod 1 (Fig. 4B) with well developed exopod, basis shorter than the following three articles combined, with three pappose setae on its inner margin and a longer one on distal corner; ischium with a small simple seta on inner margin; merus half the length of carpus, with small pappose setae; carpus as long as propodus, with short simple setae on both margins and four long simple setae distally; propodus with simple setae on both margins; dactylus shorter than propodus, with five long simple setae terminally and some smaller ones along the margins.
Pereopod 2 (Fig. 4C) with well-developed exopod, basis as long as rest of appendage, with three pappose setae on inner margin and a long one on distal outer corner; ischium very short; as long as carpus; carpus with simple setae on distal margin; propodus half length of dactylus; dactylus with a simple setae on each margin and four terminally (the longest longer than the article).
Pereopod 3 (Fig. 4D) with well-developed exopod, basis longer than the rest of appendage, with a simple seta on distal anterior corner; ischium with three simple and a pappose setae on distal corner; merus twice as long as ischium, with a simple seta on distal corner; carpus twice as long as merus, with two long simple setae (distally annulated) on distal corner; propodus longer than half length of carpus with a long simple seta (distally annulated) on distal corner.
Pereopod 4 (Fig. 4E) basis as long as the rest of appendage, with simple and pappose setae on both margins; ischium with two long simple setae; merus with a simple seta on distal corner; carpus 1.5 times as long as merus, with two simple seta on the margin and two (distally annulated) on distal corner; propodus as long as merus, with a long simple seta (distally annulated) on distal corner.
Pereopod 5 (Fig. 4F), basis as long as the three following article combined length; carpus twice as long as merus, with two simple setae (distally annulated) on distal corner; propodus as long as merus, with a long simple seta (distally annulated) on distal corner.
Uropod peduncle (Fig. 4G) slightly longer than the last pleonite and 0.66 times as long as exopod, with five small cuspidate setae on inner margin. Endopod 2-articulate; article 1, 1.6 times as long as article 2, with 10 cuspidate setae on inner margin; article 2 with six cuspidate setae on inner margin and one terminally. Exopod 2-articulate, slightly longer than endopod; article 2 with simple setae on the outer margin and upper face, five pappose setae on inner margin, and two long simple setae terminally.
Preadult male 3.63 mm total length (Fig. 2B). Similar in most characteristics—apart from the sexual ones—to the female but with a shorter pseudorostrum, a lower number of teeth on the middorsal line and without antennal notch. However, the pseudorostrum of the immature male (pleopods reduced to a single bud with few terminal simple setae) is long as it is in females (Fig. 2C).
The new species is named in honour of Jean-Claude Sorbe (Arcachon, France) in recognition of his extensive work studying suprabenthic communities.
Bay of Biscay, N Atlantic between 1024 and 1099 m depth.
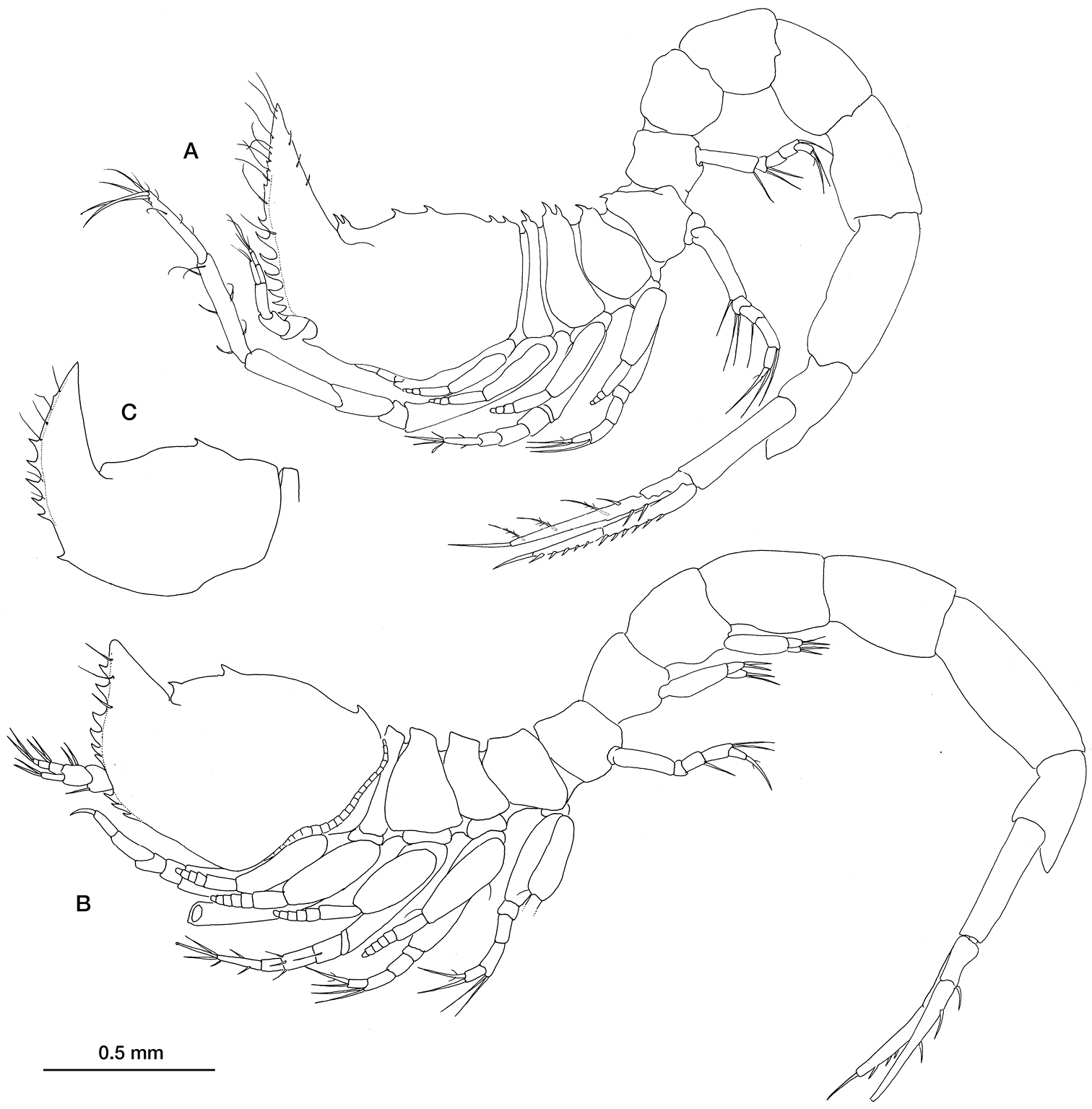
Ithyleucon sorbei gen. et sp. n. A preadult female holotype (ICMU12101901), whole animal in lateral view B preadult male paratype (ICMU12101904) C carapace of immature male paratype (ICMU12101906).
Ithyleucon sorbei gen. et sp. n. preadult female paratype (ICMU12101902): A antenna 1 B antenna 2 C left mandible D maxilla 1 E maxilla 2 F maxilliped 1 G maxilliped 2.
Ithyleucon sorbei gen. et sp. n. preadult female paratype (ICMU12101902): A maxilliped 3 B pereopod 1 C pereopod 2 D pereopod 3 E pereopod 4 F pereopod 5 G uropod.
http://species-id.net/wiki/Schizocuma_spinoculatum
Fig. 5Schizocuma spinoculatum: ESSAIS II, stn TS13, 44°34.19'N, 2°16.18'W, 1097–1099 m, 17/05/89, 7 pread. females, 2 imm. males, 1 ad. male.
Schizocuma molosa (Zimmer, 1907): BENTART 06; stn 30, 69°58'24"S, 87°26'54"W, 1798–1799 m, 27/01/2006, 1 ad. male, 1 imm; stn 31, 69°57'46"S, 87°22'08"W, 1395 m, 29/01/2006, 2 imm. females, 1 ad. male; stn 38, 69°15'11"S, 80°12'11"W, 1339–1343 m, 5/02/2006, 1 imm. female.
When
Schizocuma spinoculatum (Jones, 1984): A carapace in lateral view B uropod. Schizocuma molosa (Zimmer, 1907) from Bellingshausen Sea, Antarctica: C carapace in lateral view D uropod.
The author wishes to express his sincere gratitude to Jean-Claude Sorbe (Arcachon, France) for a long and friendly collaboration and for the loan of this interesting cumacean collection. Joseph Moore was also thanked for his careful revision of the English language. Comparative Antarctic material of Schizocuma molosa was collected during the BENTART 06 cruise carried out under the auspices of the Spanish Ministry of Science and Technology (MCYT) Antarctic Programme (CGL2004-01856).