(C) 2013 Brian J. Stucky. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Stucky BJ (2013) Morphology, bioacoustics, and ecology of Tibicen neomexicensis sp. n., a new species of cicada from the Sacramento Mountains in New Mexico, U.S.A. (Hemiptera, Cicadidae, Tibicen). ZooKeys 337: 49–71. doi: 10.3897/zookeys.337.5950
Tibicen neomexicensis sp. n., a new species of cicada found in the Sacramento Mountains of southcentral New Mexico, is described. Tibicen neomexicensis closely resembles Tibicen chiricahua Davis morphologically, but males of the two species have highly distinct calling songs that differ in phrasal structure, amplitude burst rates, and pulse structure. Unlike Tibicen chiricahua, male Tibicen neomexicensis use conspicuous dorso-ventral abdominal movements to modulate the amplitude and frequency of their calls. Tibicen neomexicensis is also smaller on average than Tibicen chiricahua, and differences in the color patterns of the wing venation identify these two species morphologically. Both species are dependent on pinyon-juniper woodlands and have similar emergence phenologies. These species appear to be allopatric, with Tibicen chiricahua found west of the Rio Grande in New Mexico, Arizona, and Mexico, and Tibicen neomexicensis so far known only from New Mexico, east of the Rio Grande. Tibicen chiricahua and Tibicen neomexicensis males share a common genitalic structure that separates them from all other species of Tibicen, and the possible evolutionary and biogeographic history of these likely sister species is also discussed.
Cicadidae, Tibicen, bioacoustics, cicada, cryptic species
Cicadas, crickets, katydids, and many other insects produce airborne acoustic signals that play an essential role in reproduction (
During fieldwork in New Mexico in 2012, I observed cicadas that fit the morphological description of Tibicen chiricahua Davis (
In this paper, I describe Tibicen neomexicensis and compare its morphology to Tibicen chiricahua, describe and compare the calling songs and calling behaviors of Tibicen neomexicensis and Tibicen chiricahua, and compare the geographic distributions of the two species. Finally, I discuss the general ecology, phenology, and daily activity patterns of Tibicen neomexicensis and consider its possible evolutionary relationship with Tibicen chiricahua.
All field work was conducted during May and June of 2012. Cicadas identified as Tibicen chiricahua were observed and audio recorded in the Magdalena Mountains west of Socorro, New Mexico, and at the type locality for Tibicen chiricahua, Pinery Canyon in the Chiricahua Mountains of southeastern Arizona (
To estimate the geographic ranges of the two species and better understand morphological variation across these ranges, I examined a total of 202 specimens previously identified as Tibicen chiricahua from the collections of the Arthropod Museum at New Mexico State University (NMSU), the C. P. Gillette Museum of Arthropod Diversity at Colorado State University (CSUC), the Frank M. Hasbrouck Insect Collection at Arizona State University (ASUT), the Snow Entomological Museum at the University of Kansas (SEMC), the Texas A&M University Insect Collection (TAMU), the University of Arizona Insect Collection (UAIC), and the University of Colorado Museum of Natural History (UCMC). The SEMC specimens included a male paratype of Tibicen chiricahua from Davis’s original type series. I also examined high-resolution digital photographs of the holotype male and allotype female of Tibicen chiricahua, which are currently housed in the collection of the Academy of Natural Sciences of Drexel University (ANSP).
Morphological terminology follows
Cicada calling songs were recorded in the field using a Sennheiser ME 66 shotgun microphone with an MZW 66 PRO windscreen connected to a Sony PCMM10 digital audio recorder. All recordings were made as uncompressed, 16-bit PCM audio at a sampling rate of 44.1 kHz. For each recording, the microphone was held between 0.5 and 2 meters away from the calling cicada. This was close enough to minimize background noise, but far enough away to avoid any near-field acoustic effects in the frequency range of the calling songs (
Cicada calls were analyzed to determine peak frequencies, amplitude burst rates, and the number of sound pulses per amplitude burst. In this paper, I use the term “pulse” in the sense of
Analyses were conducted using Audacity® (
The calls of both Tibicen chiricahua and Tibicen neomexicensis can be divided into three phrases (see results below), but the boundaries between phrases are often indistinct. To avoid the non-repeatability and potential bias of estimating the phrase durations by simple visual or aural inspection of the call oscillograms, I used objective criteria based directly on the audio data. All audio data were first normalized so that the peak amplitude was at 0 dBFS. For both Tibicen chiricahua and Tibicen neomexicensis, the first phrase began at the start of the call, and the end of the first phrase and beginning of the second phrase was defined by the first amplitude burst that reached -3 dBFS. For Tibicen chiricahua, the end of the second phrase was defined by the last amplitude burst to reach -3 dBFS, while for Tibicen neomexicensis, the end of the second phrase was defined as the end of the modulated portion of the call. For both species, the third phrase consisted of all audio from the end of the second phrase to call termination.
I did not include ambient air temperatures in the acoustic analyses. North American cicadas utilize a variety of behavioral and physiological thermoregulation tactics, so ambient temperature is often a poor indicator of a calling cicada’s body temperature (
The locations of field sites that I personally visited were determined using a Garmin nüvi 260 GPS receiver. Specimen label data lacking latitude and longitude information were georeferenced primarily using data from the Geographic Names Information System of the United States Geological Survey (http://geonames.usgs.gov/), and in some cases using Google Earth (http://earth.google.com/). Landcover data were from the Southwest Regional Gap Analysis Project (
Acoustic and morphometric data were analyzed in R (
USA, New Mexico, Lincoln County, Lincoln National Forest, near the junction of Forest Road 105 and State Highway 37, 33.5287°N, 105.6939°W (datum: WGS84), elevation 2188 m, pinyon-juniper forest.
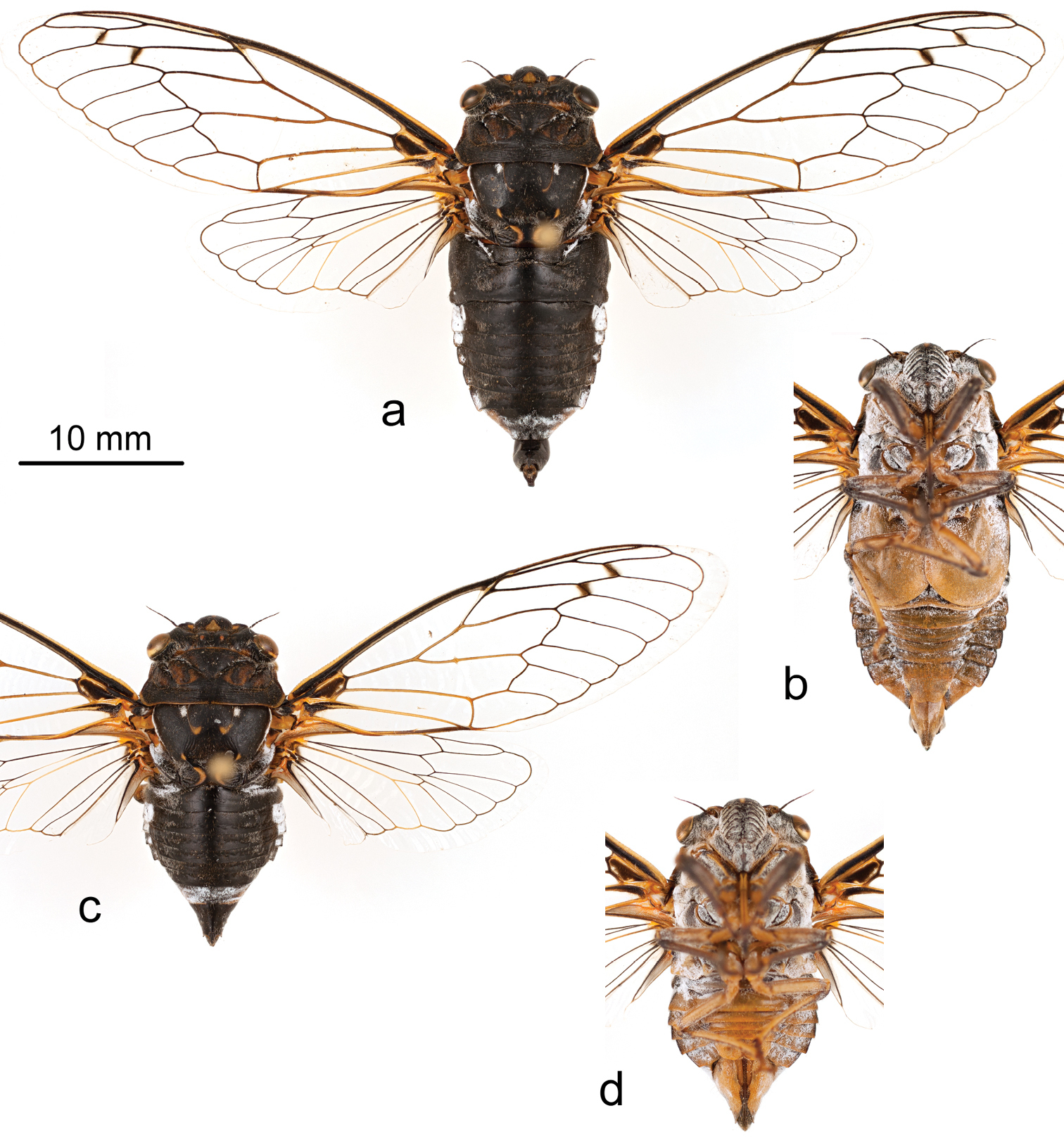
Holotype male. Pinned specimen (Figures 1–3). Original label: “NM: Lincoln Co. | Lincoln NF, FR 105 | 33.5287°N, 105.6939°W | May 31, 2012 7178 feet | Brian and Erin Stucky”. UCMC, specimen identifier UCMC 0046172.
Holotype male of Tibicen neomexicensis sp. n.: a dorsal view b ventral view; and paratype female: c dorsal view d ventral view.
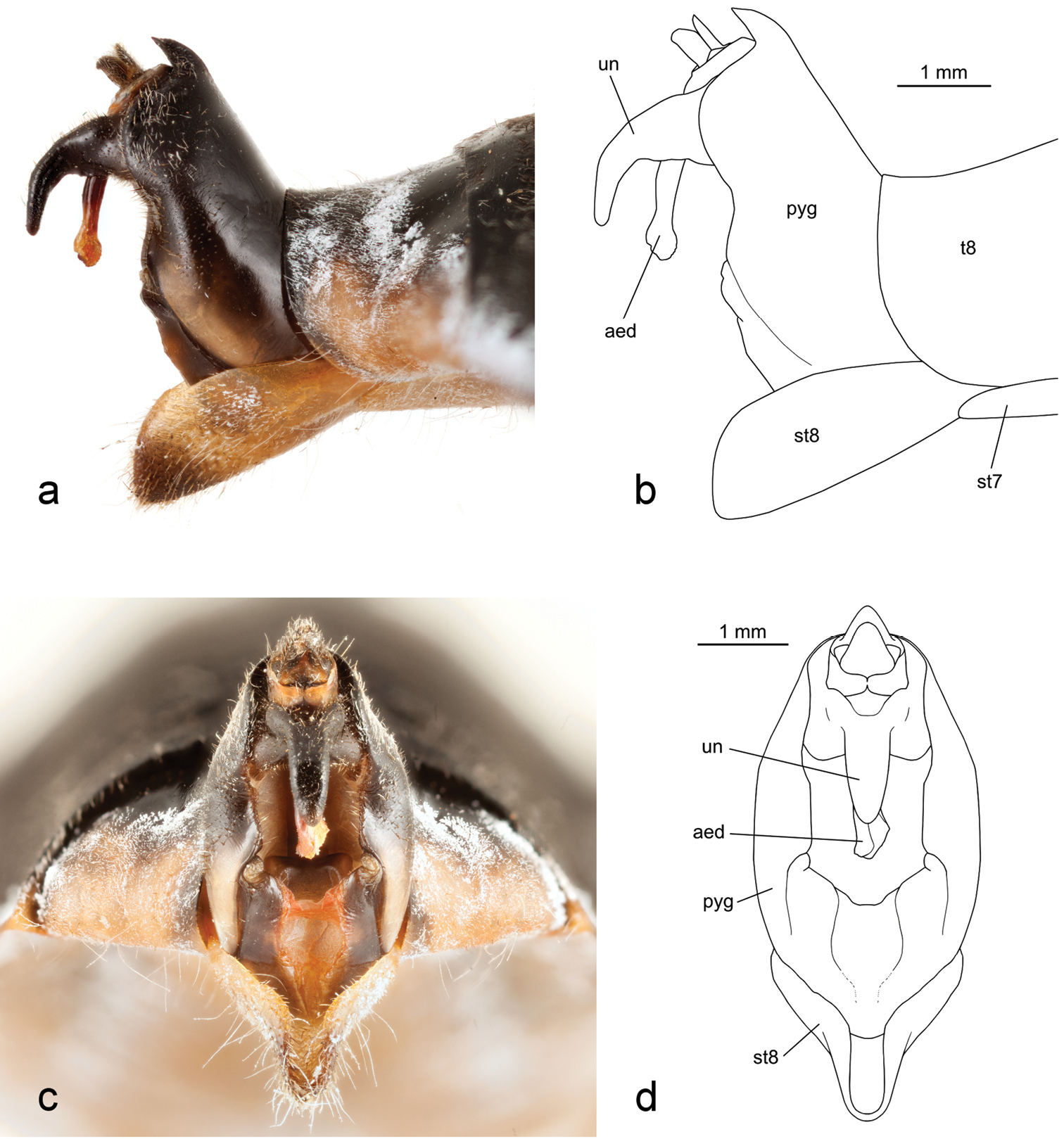
Terminalia of holotype male Tibicen neomexicensis sp. n.: a and b lateral view; and c and d posterior view. Abbreviations: aed–aedeagus, pyg–pygofer, st–sternite, t–tergite, un–uncus.
Key morphological features separating Tibicen neomexicensis sp. n. (a) and Tibicen chiricahua (b): 1) the color of the cubitus anterior vein (CuA) and its second branch (CuA2) in the hind wing, and 2) the color of the anterior margin of the subcostal vein (Sc) of the fore wing.
Paratypes. 8 males and 3 females, same label data as holotype; 2 males and 2 females, same label data as holotype except collected on May 30, 2012. The paratypes are currently housed in the UCMC and the author’s collection. Upon publication, paratypes will also be transferred to the ANSP, the Smithsonian National Museum of Natural History (NMNH), NMSU, and the SEMC.
Head. Slightly wider than anterior margin of pronotum. Vertex and frons black, marked with orange-brown on the posterior margin near the eyes and immediately lateral of the lateral ocelli. Supra-antennal plates black dorsally with an orange-brown mark adjacent to the postclypeus, orange-brown ventrally marked with black immediately above the antennae, and orange-brown along the anterior margin except for immediately adjacent to the postclypeus. Antennae mostly black with distal margin of scape yellowish, proximal half of pedicel dark brown in some specimens. Dorsal surface of head sparsely covered with short golden hairs and with longer, silvery-white hairs behind the eyes. Ventral surface mostly covered with dense, silvery-white hairs. Postclypeus black, marked with orange-brown on the anterior-medial margin and with a triangular orange-brown mark adjacent to the frontoclypeal suture. Transverse grooves of postclypeus lined with pruinosity and silvery-white hairs. Anteclypeus black, yellowish posterolaterally, with a medial brown spot at the junction with the postclypeus. Lora mostly black, marked with yellow along the lateral margins. Genae black anteriorly, yellowish posteriorly where they border the lora. Proximal two thirds of rostrum yellowish, labrum and distal one third of rostrum black, with the apex extending posteriorly to the hind coxae.
Thorax. Pronotum black, marked faintly with dark brown between the paramedian and lateral fissures and between the lateral fissures and pronotal collar, brown markings often more extensive in females. Pronotal collar black, lined with orange along the anterior margin between the eyes and along the lateral margins, extending to the posterior margin and fading to black medially. Some specimens have the entire posterior margin lined with orange. Pronotum sparsely covered with fine golden hairs. Mesonotum black marked with orange as follows: two J-shaped lines following the parapsidal suture, a small spot at the terminal end of each anterior arm of the cruciform elevation, two C-shaped marks starting at the origin of the anterior arms of the cruciform elevation and curving medially then laterally towards the posterior arms, and a large mark near the base of each fore wing. Mesonotum with two small pruinose spots on the anterior margin just lateral of the parapsidal sutures, lateral margin also pruinose. Mesonotum sparsely covered with fine golden hairs, with longer silvery-white hairs in the depressions of the cruciform elevation and along the posterolateral margins. Visible portion of metanotum black, covered with silvery-white hairs laterally. Ventral surface of thorax often heavily pruinose and covered with silvery-white hairs, yellowish except for katepisternum 2, anterior portion of basisternum 2, anepimeron 2, central part of katepimeron 2, meron 2, anterior portions of trochantins 2 and 3, episternum 3, and basisternum 3, all of which are black.
Legs. Fore coxae orange marked with brown apically and with the anterolateral surface dark brown except along the margins. Middle and hind coxae orange marked with dark brown laterally. Coxae covered with silvery-white hairs and often pruinose. Trochanters orange, variably marked with brown. Femora orange, apex mostly yellow, brown ventrally, with longitudinal brown stripes that often merge apically and basally. Silvery-white hairs on femora mostly confined to brown markings. Femoral spines brown basally with dark brown apices. Tibiae orange ventrally, brown dorsally with brown markings expanded at the base, covered with silvery-white hairs. Tibial spurs and comb dark brown. Tarsi variable in color but usually dark brown dorsally and light brown to orange ventrally. Claws brown basally with dark brown apices.
Wings. Fore wings hyaline with 8 apical cells, crossveins r and r-m usually strongly infuscated. Costal margin yellow, C vein black, R+Sc vein black with posterior margin pale along the radial cell. Sc vein black beyond the node, subcostal margin brown to dark yellow. Basal cell mostly black, anterior and posterior borders yellow. M vein yellowish-black from its base to the junction with M1+2, black beyond. M3+4 yellowish-black. M1+2 yellowish-black becoming black apically. CuA vein yellow from its base to the junction with CuA2, yellowish-black beyond. CuA2 yellowish-black. CuP+1A and 2A+3A veins mostly yellow, ambient vein dark yellowish-black, remaining venation black. Hind wings hyaline with 6 apical cells. Sc+RA, RA, CuA between base and CuA2, and CuA2 veins mostly yellow to yellowish-orange. CuA between CuA2 and m-cu, and CuA1 veins yellow to yellowish-black. Ambient vein black marked with yellow along 1st cubital cell and 6th apical cell. Remaining venation mostly black or dark brown. 3rd anal cell gray marked with reddish-orange basally.
Opercula. Male opercula yellowish marked with black on the anterolateral and anteromedial margins, overlapping medially. Posterior margins smoothly rounded, not quite reaching the posterior margin of sternite II. Female opercula yellowish, becoming black anterolaterally. Posterior margin sinuate, reaching the anterior margin of sternite II. Meracanthus black basally with a yellow apex.
Abdomen. Dorsal surface of abdomen almost entirely black, sparsely covered with short golden and silvery hairs. Tergite 8 orange-brown laterally. Tergites 3-7 often marked with orange-brown laterally, markings usually strongest on tergite 3. Timbal covers black, sometimes dark brown centrally, completely concealing timbal. Timbal with 3 long ribs, 4 intercalary ribs, and an incomplete 4th long rib. Dorsal abdomen pruinose at the following locations: along the anteromedial margins of the timbal covers in males; along the anterolateral margins of tergite 2 in females; the lateral margins of tergites 3-7, most prominently on tergite 3; the lateral margins of tergite 8, often extending medially to cover most of the tergite. Sternites orange to yellowish, usually dark brown laterally and anterolaterally. Epipleurites orange to yellowish, indistinctly marked with dark brown or black. Ventroposterior portion of male sternite VIII dark brown.
Male terminalia. Pygofer black, becoming brown or yellowish laterally along the lobes, and with a small brown spot dorsally at the base of the dorsal beak. Dorsal beak not quite as long as anal styles. Anal styles black. Median lobe of uncus slender, black, strongly bent ventrally and terminating in a rounded point. Aedeagus reddish-brown.
Female terminalia. Abdominal segment 9 yellowish-orange ventrally, black dorsally starting at about the lateral mid-line. Dorsal beak about as long as anal styles. Sternite VII yellowish-orange, usually brown laterally, deeply notched at the middle of the posterior margin. Visible portion of gonocoxite IX yellowish-orange, indistinctly marked with brown near the posterior end. Ovipositor sheath black, ventromedial margins partially lined with orange. Ovipositor sheath extends posteriorly about as far as anal styles.
Measurements. All measurements are reported in mm as mean (range, standard deviation). Males (n = 11): head width: 8.3 (8.1–8.5, 0.13); pronotum width: 8.9 (8.5–9.3, 0.25); fore wing length: 28.1 (26.8–29.6, 0.76); fore wing width: 9.8 (9.3–10.5, 0.41); body length: 24.9 (23.1–26.3, 1.05). Females (n = 5): head width: 7.8 (7.7–7.9, 0.07); pronotum width: 8.4 (8.2–8.5, 0.14); fore wing length: 26.7 (26.2–27.4, 0.48); fore wing width: 9.1 (8.9–9.3, 0.20); body length: 20.0 (19.3–20.9, 0.64).
The specific epithet refers to the U.S. state of New Mexico. As far as is currently known, Tibicen neomexicensis is endemic to this state.
Five morphometric measurements were taken for both Tibicen chiricahua and Tibicen neomexicensis: fore wing length, fore wing width, head width, pronotum width, and total body length. The correlation coefficient matrix for these five variables revealed that all five measurements were very strongly correlated with one another. All pairwise correlation coefficients excluding body length were > 0.91, and all pairwise correlation coefficients including body length were > 0.80. Body length in adult cicadas is not constant and instead varies according to a cicada’s abdominal posture, so the lower correlation coefficients for body length were not surprising. Given the high correlation among the five variables, analyzing each separately would have been largely redundant, so comparative analysis focused on fore wing length (Table 1). Fore wing length is invariant in adult cicadas and easily measured for either live or preserved specimens.
Summary statistics for fore wing length and six acoustic variables for Tibicen neomexicensis sp. n. and Tibicen chiricahua (M = male, F = female).
| variable | species | mean | 95% CI | range | std. dev. | n |
|---|---|---|---|---|---|---|
| fore wing length (mm) | Tibicen chiricahua, M | 31.3 | 30.6–32.0 | 29.4–33.1 | 1.06 | 11 |
| Tibicen neomexicanus, M | 28.1 | 27.6–28.6 | 26.8–29.6 | 0.76 | 11 | |
| Tibicen chiricahua, F | 29.1 | 28.0–30.2 | 28.0–30.1 | 0.88 | 5 | |
| Tibicen neomexicanus, F | 26.7 | 26.1–27.3 | 26.2–27.4 | 0.48 | 5 | |
| peak frequency (kHz) | Tibicen chiricahua | 7.12 | 6.56–7.67 | 5.73–8.47 | 0.82 | 11 |
| Tibicen neomexicanus | 7.27 | 7.02–7.52 | 6.07–7.81 | 0.45 | 15 | |
| amp. burst rate (bursts/s) | Tibicen chiricahua | 54 | 52.4–55.6 | 50.5–59.3 | 2.32 | 11 |
| Tibicen neomexicanus | 27.8 | 27.4–28.3 | 26.5–29.5 | 0.86 | 15 | |
| pulses per amplitude burst | Tibicen chiricahua | 5.02 | 4.54–5.51 | 3.92–6.42 | 0.723 | 11 |
| Tibicen neomexicanus | 8.34 | 7.70–8.98 | 7.25–11.33 | 1.066 | 13 | |
| phrase 1 length (s) | Tibicen chiricahua | 1.72 | 1.21–2.23 | 0.49–2.80 | 0.758 | 11 |
| Tibicen neomexicanus | 2.04 | 1.66–2.42 | 1.25–3.24 | 0.6 | 12 | |
| phrase 2 length (s) | Tibicen chiricahua | 7.82 | 6.95–8.68 | 6.02–10.83 | 1.286 | 11 |
| Tibicen neomexicanus | 6.68 | 5.77–7.59 | 4.86–10.96 | 1.575 | 14 | |
| phrase 3 length (s) | Tibicen chiricahua | 3.75 | 3.10–4.39 | 2.37–5.82 | 0.963 | 11 |
| Tibicen neomexicanus | 1.65 | 1.36–1.94 | 0.75–2.33 | 0.479 | 13 |
Analysis of the linear model including both species and sex as predictors of fore wing length revealed that this simple model explained much of the variation in size among the cicadas, and that the effects of both predictors were highly significant (R2 = 0.805, p < 0.000001 for both variables). After adjusting for the size differences between males and females, the fore wings of Tibicen neomexicensis are, on average, about 2.9 mm shorter than those of Tibicen chiricahua (95% CI: 2.3–3.5). An F-test comparing this simple two-factor model to a model that included a (species∙sex) interaction term revealed that the two models were not significantly different (F = 1.202, p = 0.282). Therefore, the data show that for the morphometric measurements used in this study, Tibicen neomexicensis is significantly smaller than Tibicen chiricahua, and that for both species, females are significantly smaller than males. It must be noted, though, that this analysis was limited to localities for which acoustic data were available, and it is possible that these species exhibit greater variation in size across their full ranges.
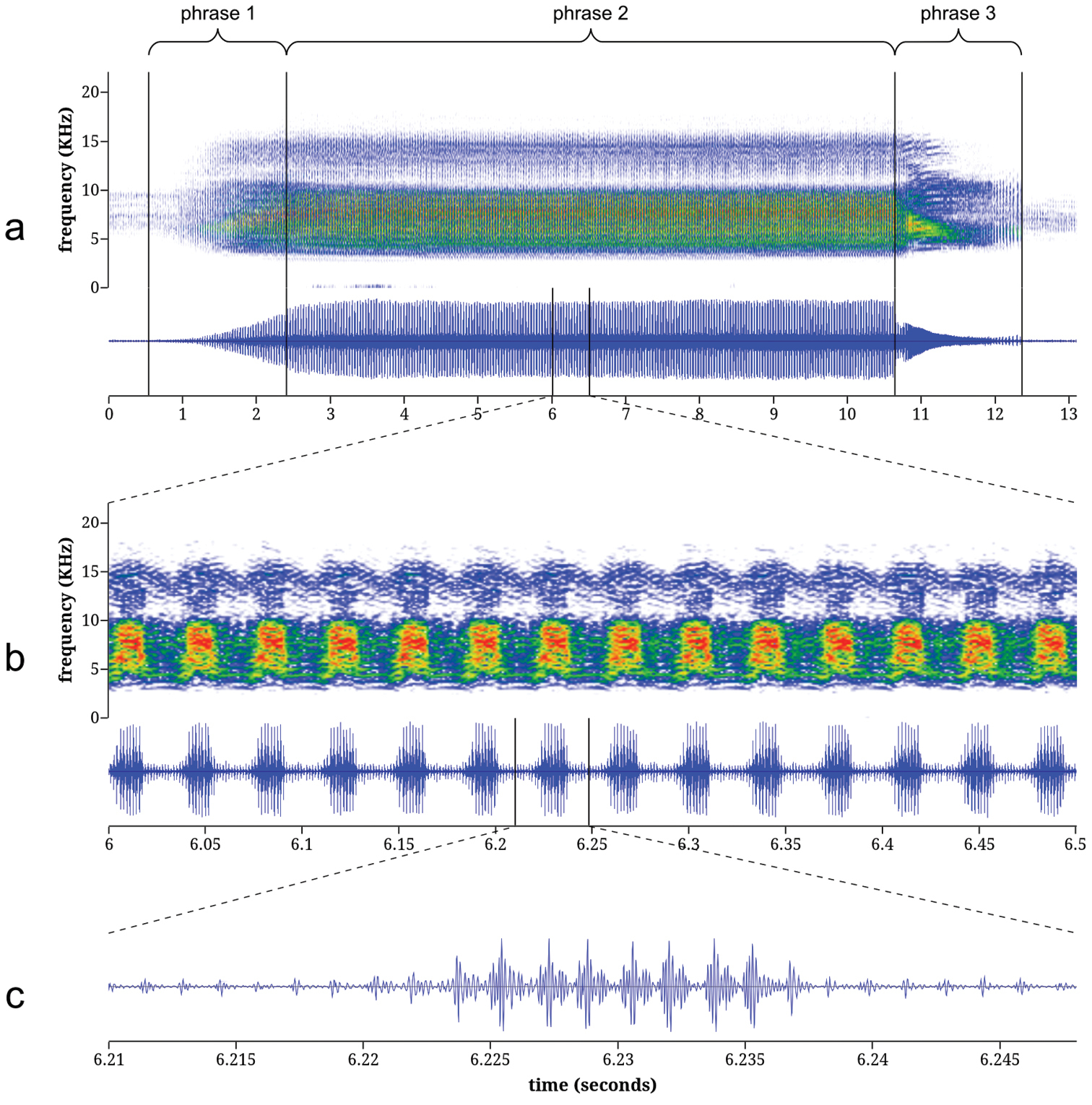
Calling song of Tibicen neomexicensis. The calling song of Tibicen neomexicensis can be divided into three phrases, each of which consists of a continuous train of pulses (Figure 4). The first phrase represents the initial increase in amplitude as the cicada begins calling and lasts an average of 2.04 seconds (95% CI: 1.66–2.42; full descriptive statistics for all acoustic parameters are given in Table 1). The second phrase is the main phrase of the call and is produced at or near maximum amplitude. This phrase lasts an average of 6.68 seconds (95% CI: 5.77–7.59) and has a mean peak frequency of 7.27 kHz (95% CI: 7.02–7.52). The first two phrases are characterized by distinctive amplitude and frequency modulations that group pulses into regular “bursts” of high amplitude. During the main phrase, these amplitude bursts are delivered at a mean rate of 27.8 bursts/s (95% CI: 27.4–28.3) and each amplitude burst consists of 8.34 pulses on average (95% CI: 7.70–8.98). The amplitude and frequency modulations are accompanied by rapid dorso-ventral movements of the cicada’s abdomen. These movements modulate frequency and amplitude by changing the acoustic properties of the sound-producing system (
Spectrograms and oscillograms of the calling song of Tibicen neomexicensis sp. n.: a complete call b 0.5 seconds from the middle of phrase 2, illustrating 14 amplitude bursts c a single amplitude burst from b, illustrating the pulse structure. Spectrograms were generated using a 256-sample Fast Fourier Transform with the Hamming window function.
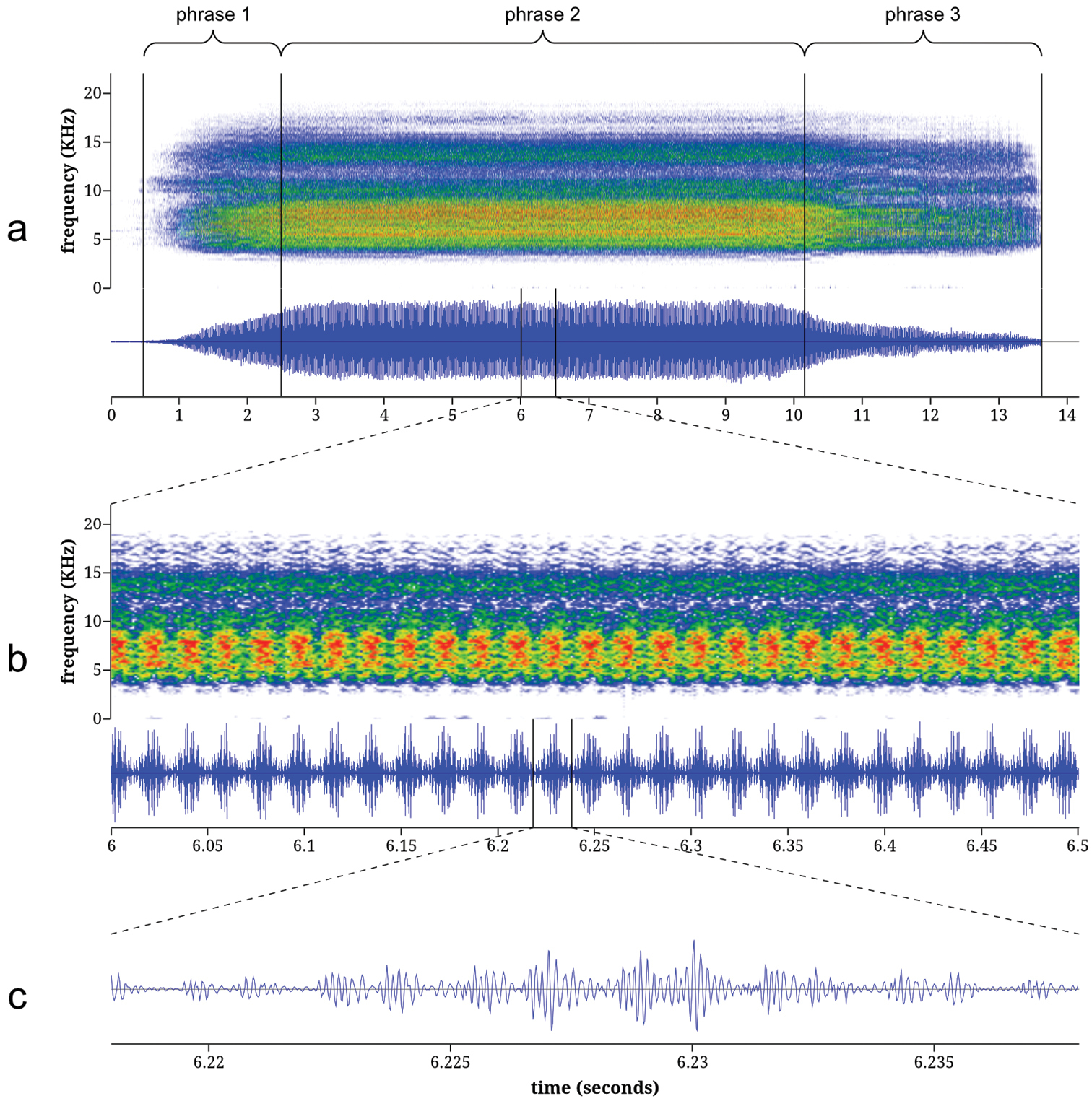
Calling song ofTibicen chiricahua. The calling song of Tibicen chiricahua is also naturally divided into three phrases (Figure 5). The first phrase is the initial crescendo as the call begins and lasts an average of 1.72 seconds (95% CI: 1.21–2.23). The second, main phrase of the call has a mean duration of 7.82 seconds (95% CI: 6.95–8.68) with a peak frequency of 7.12 kHz (95% CI: 6.56–7.67). The third phrase is a gradual decrescendo as the calling song terminates and lasts an average of 3.75 seconds (95% CI: 3.10–4.39). The entire call consists of an amplitude-modulated train of pulses. Pulses are grouped into high-amplitude bursts that, during the main phrase of the call, contain an average of 5.02 pulses per burst (95% CI: 4.54–5.51) and are delivered at a mean rate of 54.0 bursts/s (95% CI: 52.4–55.6).
Spectrograms and oscillograms of the calling song of Tibicen chiricahua: a complete call b 0.5 seconds from the middle of phrase 2, illustrating 27 amplitude bursts c a single amplitude burst from b, illustrating the pulse structure. Spectrograms were generated using a 256-sample Fast Fourier Transform with the Hamming window function.
Comparison of calling songs. Comparison of acoustic parameters, song structure, and physical behavior during call production verified that the calls of these two species are distinct. First, the underlying structures of the amplitude modulations of the calls differ. The mean amplitude burst rate of the call of Tibicen chiricahua is nearly twice that of Tibicen neomexicensis (54.0 and 27.8 bursts/s, respectively, t = 37.4, p < 0.000001), and the amplitude bursts of Tibicen chiricahua contain about 3.3 fewer pulses per burst, on average, than those of Tibicen neomexicensis (5.02 and 8.34 pulses/burst, respectively, t = 18.0, p < 0.000001). There was no overlap in the ranges of observed values for either of these variables. Second, the phrasal structures of the calls also differ. The phrases in the call of Tibicen chiricahua are defined merely by the overall pattern of amplitude changes in the call and have a relatively uniform sound quality throughout, while the third phrase of the call of Tibicen neomexicensis is markedly different in quality from the other two phrases, lacking the characteristic modulations of phrases one and two. Furthermore, the beginning of the third phrase in Tibicen neomexicensis is usually marked by an abrupt drop in amplitude, but the amplitude decreases gradually and smoothly from the second to the third phrases of Tibicen chiricahua. Finally, the amplitude and frequency modulations in the call of Tibicen neomexicensis are a result of rapid dorso-ventral movements of the abdomen during the calling song, but no such movements were apparent in the calling behavior of Tibicen chiricahua.
The observed mean peak frequency of the main phrase of the call of Tibicen neomexicensis was slightly higher than that of Tibicen chiricahua, although the difference was not significant (7.27 and 7.12 kHz, respectively, t = 0.623, p = 0.539). Peak calling song frequency is constrained by body size for most cicadas, with larger cicadas having lower-frequency calls (
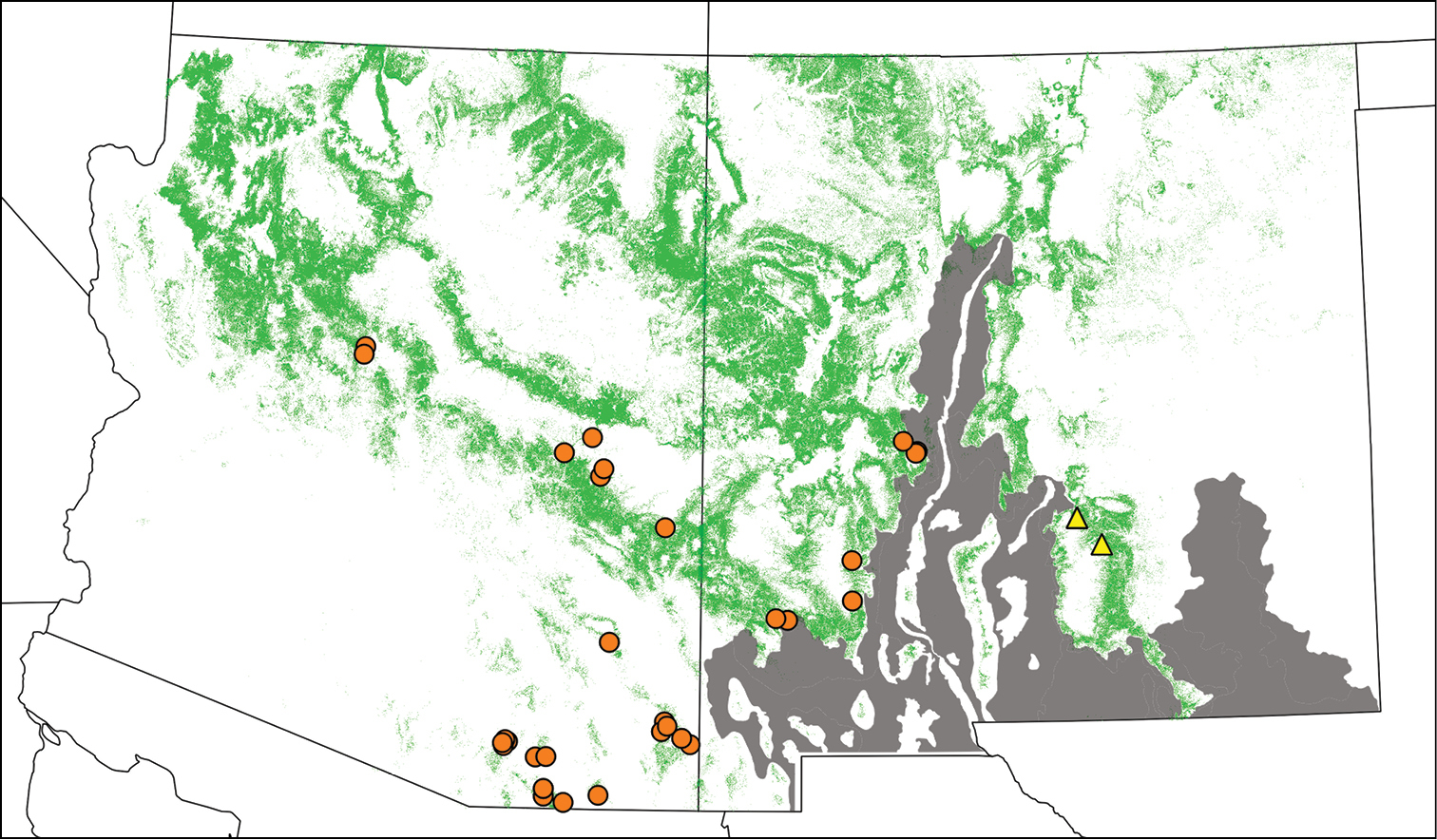
Tibicen chiricahua is more widely distributed than Tibicen neomexicensis, ranging from central and southeastern Arizona to southwestern New Mexico (Figure 6). Although not depicted in Figure 6, Tibicen chiricahua is also known from Chihuahua, Mexico (
Geographic distribution of Tibicen chiricahua (orange circles) and Tibicen neomexicensis sp. n. (yellow triangles), estimated from field observations and museum specimens. Green regions indicate pinyon-juniper habitats. The gray region represents the Albuquerque Basin and Chihuahuan Desert. Tibicen chiricahua is also found in Mexico (
Four museum specimens, representing two unique collecting localities, could not be conclusively identified. One, a female collected June 15, 1937 in “Big Bend Park, ” Brewster Co, TX (TAMU), appeared to be Tibicen neomexicensis. However,
Tibicen is the second most diverse cicada genus in North America north of Mexico (
Tibicen neomexicensis can be separated from all other North American species of Tibicen except for Tibicen chiricahua by the combination of its size, almost entirely black dorsal color pattern (Figure 1), and the male’s genitalia, particularly the shape of the uncus (Figure 2). Within Tibicen, this uncal structure is unique to Tibicen neomexicensis and Tibicen chiricahua (see
In the field, Tibicen neomexicensis and Tibicen chiricahua are most easily distinguished by the unique calling songs of the males. Audio recordings of both species are available as online supplementary data for this paper. To human ears, the first and second phrases of the call of Tibicen neomexicensis sound like a high-pitched, whiny buzz with easily discernible pulsations that correspond to the amplitude and frequency modulations. At the beginning of the third phrase, the abrupt transition to an unmodulated, uniform whine is perhaps the most aurally distinctive feature of the calling song.
In contrast, the call of Tibicen chiricahua sounds like a monotonous, coarse buzz that rapidly increases in amplitude during the first phrase and then slowly fades away during the final phrase. Apart from the amplitude changes in the first and third phrases, there are no obvious changes in sound quality during the course of the call.
Both male and female specimens of Tibicen neomexicensis and Tibicen chiricahua can usually also be separated by the coloration of the wing venation. In Tibicen neomexicensis, the anterior margin of the subcostal vein (Sc) of the fore wing is usually yellowish or at least noticeably lighter in color than the main part of the vein, which is dark black (Figure 3). In Tibicen chiricahua, both the vein and its anterior margin are black (Figure 3). In addition, the cubitus anterior vein (CuA) in the hind wing of Tibicen neomexicensis is yellow from its base to the junction with its second branch (CuA2), and the basal two-thirds or more of CuA2 is usually also yellow (Figure 3). In Tibicen chiricahua, these two veins tend to be mostly or entirely black (Figure 3).
Although none of these morphological characters are 100% reliable, when used in combination, they identify nearly all specimens. The color of the margin of the Sc vein is the most reliable single morphological diagnostic character. Out of nearly 200 specimens examined, only 7 might have been misidentified by the color of the Sc vein alone. The color of CuA and CuA2 is more variable, with some overlap between the two species, and the utility of this character seems to vary among populations of Tibicen chiricahua. Unfortunately, in very old specimens, the colors of the wing veins sometimes fade, making identification difficult. Fore wing length can also be used to help confirm an identification, especially when the wing vein colors are ambiguous.
Both Tibicen neomexicensis and Tibicen chiricahua are associated with pinyon-juniper woodlands, and neither species seems to occur in habitats where both pinyon pines (Pinus edulis, primarily) and junipers (Juniperus sp.) are absent (B. Stucky, pers. observation;
Specimen label data and field observations indicate that adults of Tibicen neomexicensis and Tibicen chiricahua emerge in early summer and are mostly gone by the end of July. The earliest record for Tibicen chiricahua is May 25 (in 1997) (specimen, CSUC). Although the UAIC has a specimen of Tibicen chiricahua from Arizona with the date recorded as “September, ” the next latest collecting date is July 22 (in 1975) (UAIC), so the September date is either very unusual or in error. The majority of collecting events for Tibicen chiricahua were in June, and
Phenological data for Tibicen neomexicensis are much more limited, but consistent with an annual pattern similar to Tibicen chiricahua. The earliest record for Tibicen neomexicensis is for May 30 (in 2012), at the type locality (B. Stucky, pers. observation), but at this time, there were already large numbers of females ovipositing, so the cicadas must have emerged some number of days earlier. The latest record is from June 7 (in 2005) (specimen, NMSU).
The daily activity patterns of these two cicada species are also similar. Once the sun warms them sufficiently, males of both species will sing throughout much of the day, with peak calling activity occurring from about mid-day through early afternoon (B. Stucky, pers. observation;
Although the nymphal host plants of Tibicen chiricahua and Tibicen neomexicensis are not known with certainty, there is anecdotal evidence that females of these species have different oviposition preferences.
Both Tibicen chiricahua and Tibicen neomexicensis are commonly found with Tibicen duryi Davis, another species that is specialized on pinyon-juniper habitats (
Tibicen neomexicensis and Tibicen chiricahua are not only extremely similar morphologically, but the shared structure of the male genitalia separates them from all other species of Tibicen. It therefore seems probable that Tibicen neomexicensis and Tibicen chiricahua are sister species, although a broader phylogenetic analysis of Tibicen is needed to confirm this.
Today, these species are apparently entirely allopatric, separated from one another by the uninhabitable Albuquerque Basin and Chihuahuan Desert. This might be a relatively recent phenomenon, though. At the time of the last glacial maximum, pinyons and junipers were widespread across much of what is today the Chihuahuan Desert (
These habitat changes must have certainly affected the distributions of and interactions among the ancestors of modern Tibicen neomexicensis and Tibicen chiricahua. What impact, if any, this had on population divergence and speciation is unknown. However, theory predicts that secondary sexual traits can diverge rapidly in allopatry (
Acoustic, morphometric, and behavioral data all indicate that the cicadas resembling Tibicen chiricahua from New Mexico’s Sacramento Mountains should be recognized as a distinct species, described here as Tibicen neomexicensis. In particular, analysis of audio recordings confirms that the calls of these two species have significant, consistent structural and temporal differences, which provide the simplest means for identifying these cicadas in the field.
With the discovery of Tibicen neomexicensis, the North American Tibicen are now known to encompass at least three complexes of morphologically cryptic species with distinct male calling songs: the chiricahua group [Tibicen chiricahua and Tibicen neomexicensis], the dorsatus group [Tibicen dorsatus (Say) and Tibicen tremulus Cole], and the pruinosus group [Tibicen linnei (Smith & Grossbeck), Tibicen pruinosus (Say), and Tibicen robinsonianus Davis]. A phylogeographic and divergence-time analysis of the North American Tibicen species based on molecular data could not only help clarify the relationship between Tibicen neomexicensis and Tibicen chiricahua, but also shed light on the broader patterns of diversification for one of the most species-rich cicada genera in North America.
The geographic ranges of these species are still rather poorly documented, especially in Mexico, where Tibicen chiricahua is currently known only from a single specimen (
I am grateful to the following individuals for providing information about specimens, photographs, loans, or access to collections: Sandra Brantley (UNMC), Graeme Davis (NMSU), Boris Kondratieff (CSUC), Sangmi Lee (ASUT), Carl Olson (UAIC), Ed Riley (TAMU), Virginia Scott (UCMC), Jennifer Thomas (SEMC), and Jason Weintraub (ANSP). I thank the Bowers Lab at the University of Colorado, Rob Guralnick, Erin Stucky, and two anonymous reviewers for helpful feedback on the manuscript and figures. Erin Stucky also assisted with the fieldwork in NM and AZ. Publication of this article was generously funded by the University of Colorado Boulder Libraries Open Access Fund.
Calling song of Tibicen neomexicensis sp. n. (doi: 10.3897/zookeys.337.5950.app1) File format: Waveform Audio File Format (wav).
Explanation note: Recorded on May 31, 2012, at the type locality for Tibicen neomexicensis. Details of the recording methods are provided in the main text.
Calling song of Tibicen chiricahua Davis. (doi: 10.3897/zookeys.337.5950.app2) File format: Waveform Audio File Format (wav).
Explanation note: Recorded on June 1, 2012, at the type locality for Tibicen chiricahua. Details of the recording methods are provided in the main text.