(C) 2013 Laura Montes de Oca. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73–87. doi: 10.3897/zookeys.337.5779
We describe two new species of the nemesiid spider genus Chaco from Rocha Province, Uruguay. These new species are diagnosed based on genital morphology, male tibial apophysis spination, and burrow entrance. We test cospecificity of one species, Chaco costai, via laboratory mating experiments. The new species are diagnosed and illustrated and habitat characteristics, and capture behavior are described. We conduct a cladistic analysis based on a previously published morphological character matrix that now includes the newly described species.
En este trabajo se describen dos nuevas especies de arañas, nemésidas del género Chaco recolectadas en el departamento de Rocha, Uruguay. Las nuevas especies se distinguen de las conocidas por caracteres tomados de la morfología de la genitalia, número de espinas en la apófisis tibial de los machos y la tapa-cortina en la cueva. Se realizó un análisis filogenético de las especies del género Chaco incluyendo las nuevas especies en una matriz previa.
Spiders, Taxonomy, Cladistics, Chaco, Nemesiidae, Natural history
The family Nemesiidae Simon, 1889 comprises 43 genera (
The genus Chaco was originally described on the basis of the type species Chaco obscura Tullgren, 1905 known from a female specimen. The species was characterized as having posterior lateral spinnerets with two apical segments of equal length and the absence of labial cuspules (
The characteristics of some individual specimens discovered along the coast of Uruguay have the diagnostic characteristics described for Chaco but differ from all the known species. In this article we describe, diagnose, and illustrate two new species: Chaco castanea sp. n. and Chaco costai sp. n. We present a cladistic reanalysis of the genus with newly described species and present some natural history data for the new taxa.
Specimens were examined using an Olympus SZH stereomicroscope. The description of color was based on live organisms when possible. Abbreviations: AME anterior median eyes, ALE anterior lateral eyes, PME posterior median eyes, PLE posterior lateral eyes, OQ ocular quadrangle, P prolateral, R retrolateral, D distal, STC superior tarsal claw, ITC inferior tarsal claw, FCE-MY Collection of Facultad de Ciencias, Entomología – Mygalomorphae. All measurements are in mm and were taken with an ocular micrometer. Total body length excludes chelicerae and spinnerets. Lengths were measured along a dorsal longitudinal line and widths were taken at maximum values. The OQ length was measured from the anterior edge of ALE to the posterior edge of PLE; the sternum length from the posterior angle to the labium edge (
Cladistic analysis. We scored the newly described species (Chaco castanea and Chaco costai) for 32 characters (Table 1) from
Data matrix for the genus Chaco and the outgroup.
| 1 | 2 | 3 | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | |
| Lycinus longipes | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ? |
| Diplothelopsis bonariensis | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | ? | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | ? |
| Chilelopsis calderoni | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | ? |
| Chaco obscura | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Chaco tucumana | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Chaco socos | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Chaco tigre | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Chaco patagonica | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | ? | 1 | 0 | 0 | ? | 1 | 1 | ? | ? | 0 | 1 | 1 | 1 | ? | ? | ? | 1 | 0 | 0 | 0 | ? | ? | ? |
| Chaco tecka | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ? | 1 | 0 | 0 | ? | 1 | 1 | ? | ? | 0 | 0 | 1 | 1 | ? | ? | ? | 1 | 0 | 1 | 0 | ? | ? | ? |
| Chaco sanjuanina | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | ? | 0 | 0 | 0 | 0 | 1 |
| Chaco castanea | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | ? | 2 | 0 | 0 | 0 | 1 |
| Chaco costai | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
The data matrix (Table 1) was constructed using Nexus Data Editor ver 0.5.0 software (
Characters scored. (0) Clypeus: 0, wide; 1, narrow (1) PE row: 0, recurved; 1 procurved (2) Eyes: 0, AME and PME subequal size; 1, AME much larger than PME (3) Pubescence: 0, absent; 1, light; 2, dense (4) Sternum: 0, wide; 1, normal; 2, narrow (5) Sternal sigilla: 0, conspicuous; 1, inconspicuous (6) Leg color: 0, uniform; 1, patterned (7) Setae on female posterior legs: 0, normal; 1, dense (8) Maxillary cuspules in females: 0, few (0-10); 1, medium (11-30); 2, many (over 30) (9) Maxillary cuspules in males: 0, few (0-10); 1, medium (11-30); 2, many (more than 30) (10) Rastellum: 0, weak; 1, strong (11) Female tarsi: 0, rigid; 1, flexible (12) Scopula IV: 0, absent/ very light; 1, light; 2, dense (13) Trichobothria on male cymbium: 0, medial third; 1, basal half (14) PMS spigot number: 0, many; 1, few (15) Male metatarsus IV: 0, 1-1-1P SUP; 1, 0-0-1P SUP (16) Dorsal spines in male palpal tibia: 0, absent; 1, present (17) Spines on male patella I-II: 0, 0/1P; 1, 1-1-1P (18) Female patella IV: 0, 0/1P; 1, 1-1-1P (19) Spines on female tarsi IV: 0, absent; 1, present (20) Spines on female tibia/metatarsus I: 0, short; 1, long (21) Male tibial spur: 0, absent; 1, present (22) Male palpal tibia: 0, short; 1, long (23) Male bulb keels: 0, absent; 1, parallel keels or ridges along embolous base; 2, lateral keels or flanges (24) Male bulb duct: 0, basal portion evenly curved; 1, basal portion strongly sinuous (25) Female spermathecae: 0, no basal sphere; 1, with basal sphere (26) Habits: 0, flap door; 1, trap door. (27) Spermathecae fundus 0, subspherical; 1, reniphorm (28) female tibiae 0, normal; 1, short (29) Setae on male cymbium 0, thin hair like setae; 1, thickened setae (30) Two long dorsal setae on palpal tibiae setae 0, absent; 1, present (31) Spines on male tibial apophysis 0, five or less; 1, more than 5.
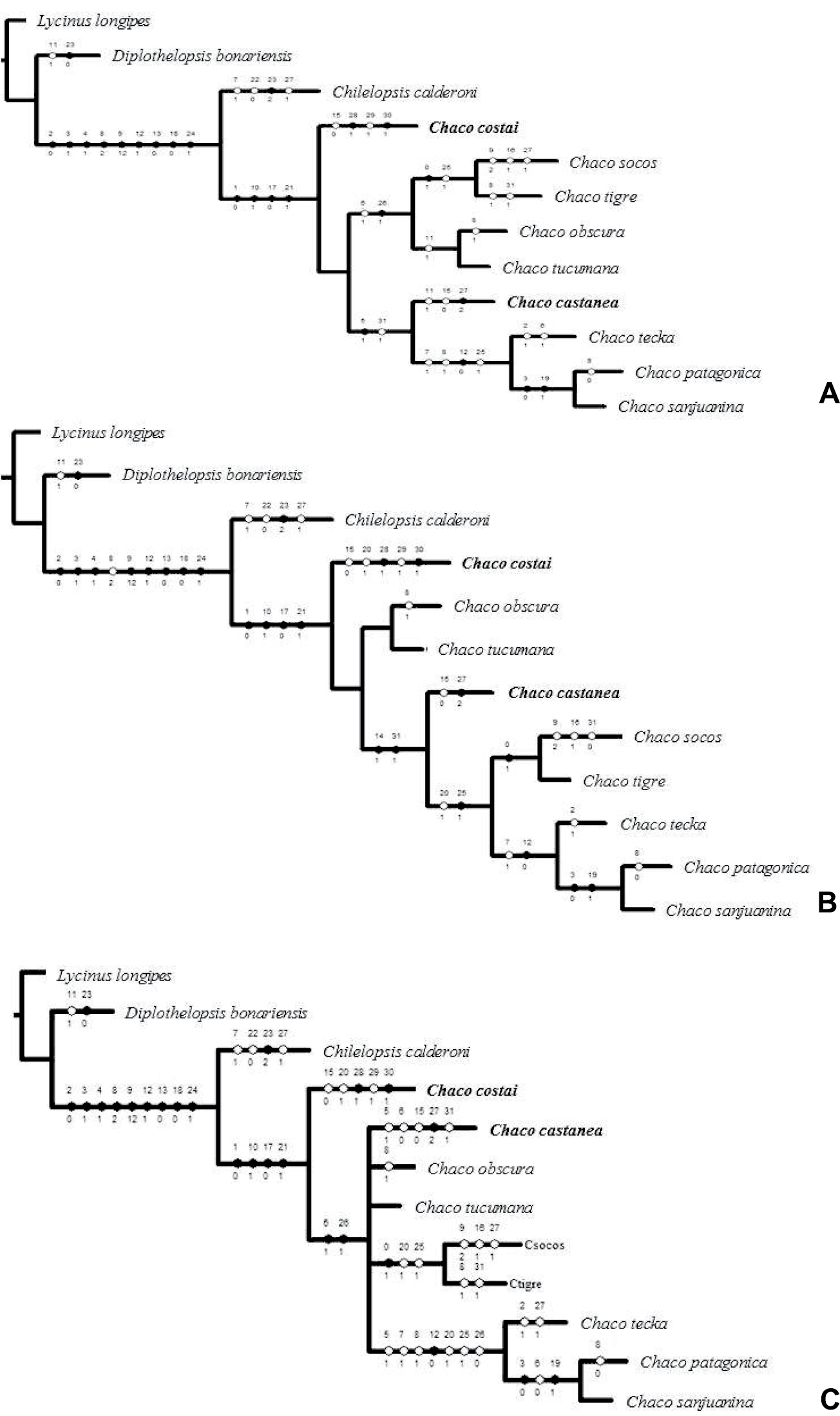
Analysis of the morphological data using implied weighting and implicit enumeration resulted in 2 most parsimonious trees. Topologies were stable across K values 1-6 (62 steps, CI = 0.61, RI = 0.65, K = 1, fit = 8.75, K = 6, fit = 2.79; Fig. 5A-B). The genus Chaco was recovered as monophyletic including the two new species, supported by 4 characters: PE row procurved (1), strong rastellum (10), spination of male patellae I-II (17) and the presence of male palpal tibial spur (21). The main difference between the topology of the two recovered trees was: Chaco socos + Chaco tigre is the sister group of Chaco obscura + Chaco tucumana in one tree, while in the other Chaco socos + Chaco tigre is the sister group of (Chaco teka (Chaco patagonica, Chaco sanjuanina)). The consensus tree (Fig. 5C) recovers a polytomy for Chaco castanea, Chaco obscura, Chaco tucumana, and the clades (Chaco obscura, Chaco tucumana) and (Chaco teka (Chaco patagonica, Chaco sanjuanina)). Based on these data the monophyly of the genus Chaco appears to be well supported with the inclusion of the new species; the addition of new characters in the future will be necessary to improve the resolution of relationships among several species. Regarding the biology of Chaco costai, the flap-like door of the burrow may be explained as an adaptation to sandy soil habitat.
Chaco costai specimens are typically found in sandy soils of oceanic and river coastal areas associated with psammophyte vegetation (Fig. 2F). Individuals were collected from tubular vertical burrows of about 100mm length; the entrance diameter is about 10mm. The spider closes the burrow entrance with sand and silk when disturbed. Chaco patagonica, Chaco costai make a burrow that is covered with a thin, flaplike door (Fig. 2E). The door actually consists of a prolongation of the silk layer lining the interior of the burrow, covered by grains of sand; it is flexible and loosely articulated. According to field and laboratory observations, the spider begins foraging at night by standing at the top of the burrow with legs I-III extended lying in the substrate (Fig. 2E); similar to that reported by
A copulation event observed in the laboratory occurred over an eight minute time period at 18 °C. The male appeared to initiate courtship with body vibrations and pulling silk threads with his chelicerae. Body vibrations were caused by spasmodic contraction of legs I and II. The male approached the female burrow entrance and opened it with his chelicerae; the female then emerged from the burrow. Copulation took place at the burrow entrance; the male clasped his tibial apophysis with female chelicerae. The male performed 23 palpal insertions, alternating right and left palps. The mean duration of the insertions was 21.09±12.73 seconds. After copula the male retreated with legs I extended and female retreated in the burrow but maintained her first legs out towards the entrance. After 17 minutes the female closed the burrow flap-door.
http://zoobank.org/42AA4036-8DA2-4C0C-8AAB-92B5A15649DC
http://species-id.net/wiki/Chaco_castanea
Figs 1A–D, 3A–EMale holotype (deposited in FCE-MY 0767) from, Rocha, Perla de Rocha, 34°25.0'S, 53°51.0'W, i.2001, coll. G. Calixto Female paratype (deposited in FCE-MY 0770) from Rocha, Cabo Polonio, 34°24.0'S, 53°47.0'W, 24.i–18.iii.2003, coll. F. Achaval. Additional material examined. Male from Rocha, Cabo Polonio, 19.xii.2003–18.iii.2004, coll. F. Achaval. 1m (deposited in FCE-MY 0769), Female from Rocha, Perla de Rocha, 34°25.0'S, 53°51.0'W, i.2001, coll. G. Calixto, 1f (FCE-MY 0766), female from Rocha, Cabo Polonio, 34°24.0'S, 53°47.0'W, 18.i–18.iii.2005, coll. F. Achaval, 1f (deposited in FCE-MY 0797).
The specific epithet is a noun taken in apposition (chestnut) and is in reference to the brownish coloration of this species.
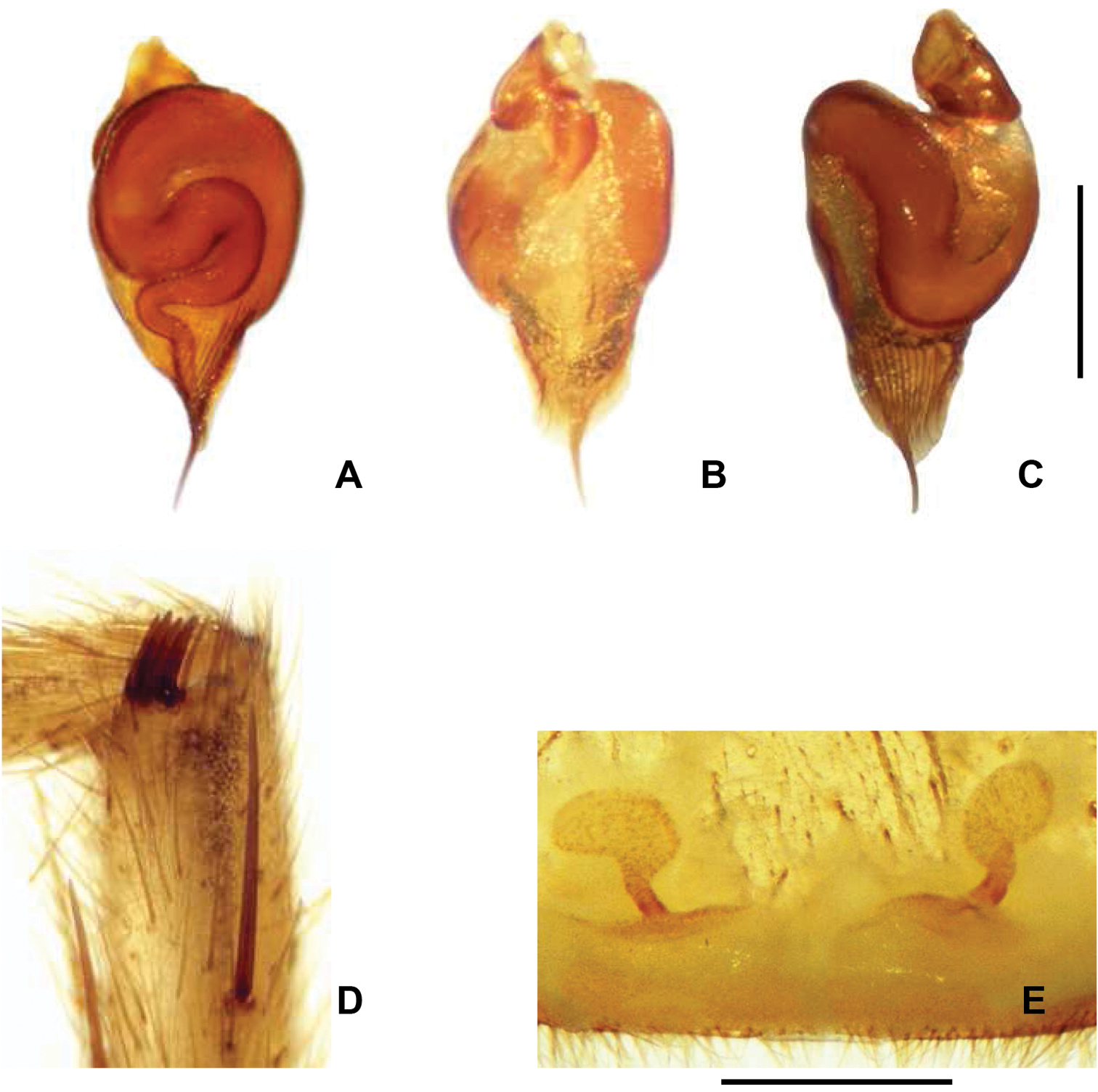
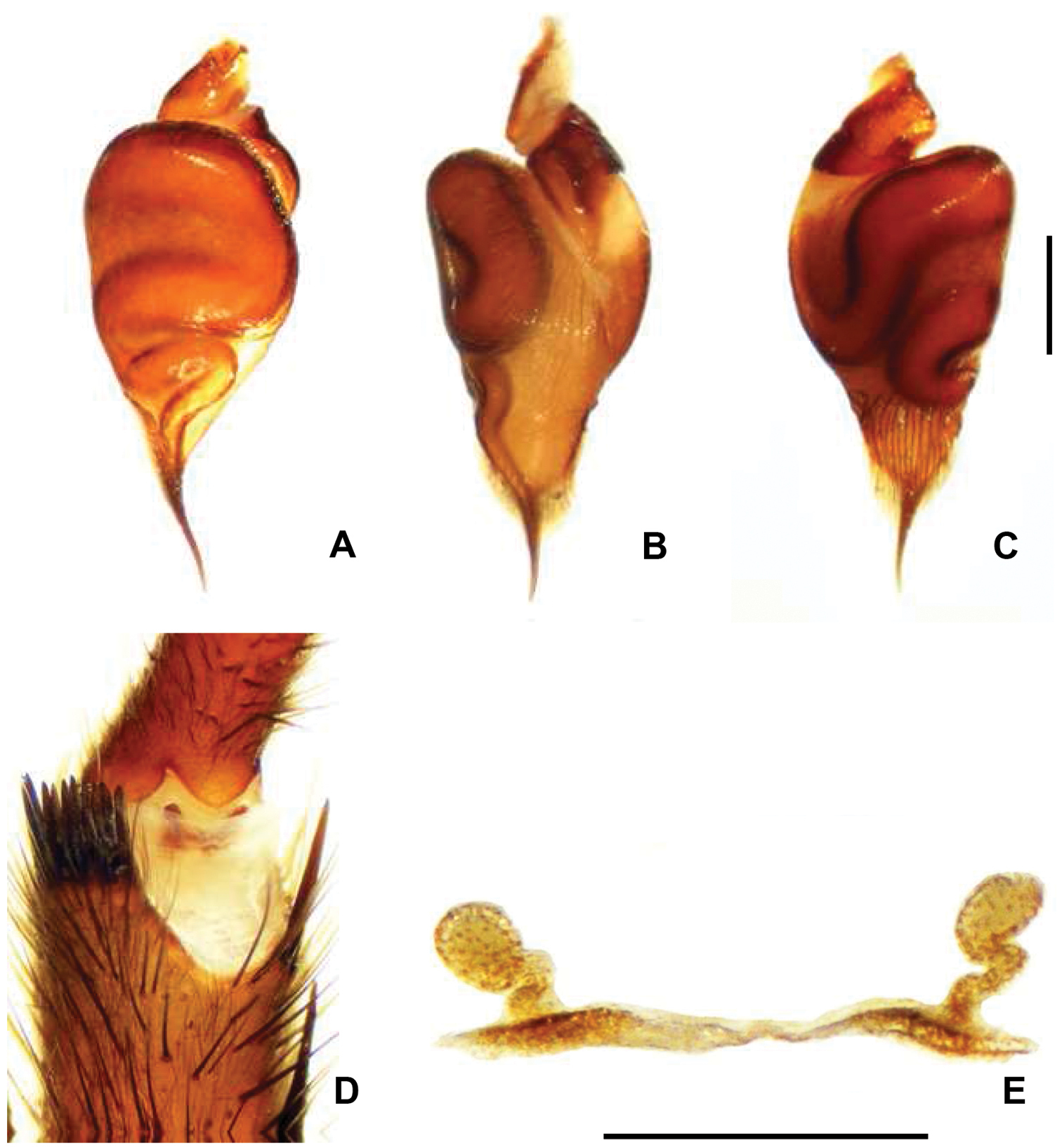
Diagnosis. Males (Fig. 1A) uniquely possess a tibial apophysis with 4 spines (Fig. 3D). Chaco castanea males differ from Chaco tigre and Chaco socos males by having a palpal organ with a sinuous spermophor and parallel longitudinal ridges (Figs 3A–C), and by having a PME and AME that are subequal in diameter. Females of Chaco castanea (Fig. 1C) differ from the other known Chaco species by the presence of a large reniform spermathecal receptacle in combination with a short sinuous duct (Fig. 3E).
Chaco castanea. A Male holotype habitus, dorsal view B Male holotype maxillae and labium showing cuspules C Female paratype habitus, dorsal view D Female paratype maxillae and labium showing cuspules.
Chaco costai. A Male habitus, dorsal view B Male maxillae and labium showing cuspules C Female habitus, dorsal view D Female maxillae and labium showing cuspules E Chaco costai female ambushing in the burrow entrance, see the flap-like door F Habitat of Chaco costai showing psammophyte vegetation.
Chaco castanea. A Palpal organ, dorsal view B Palpal organ, retrolateral view C Palpal organ prolateral view D Male tibial apophysis with 4 spines, prolateral view E Spermathecae, dorsal view. Scale = 5mm.
Male (holotype). Total length 7.75; carapace 4.13 long, 3.27 wide; eight eyes grouped on a tubercle, AME 0.17; PME 0.13; ALE 0.16; PLE 0.16; OQ 0.86 long, 0.44 wide; clypeus 0.06; fovea 0.63; sternum oval, 1.93 long, 1.56 wide. Posterior sigillae sub-circular, sub-marginal. Labium sub-rectangular, 0.2 long, 0.56 wide; labial cuspules absent; 18/20 maxillary cuspules (Fig. 1B); chelicerae with 7 promarginal teeth of similar size; rastellum with 26 short, thick conical setae on promargin narrowing through retromargin. Leg, palpal measurements in Table 2; chaetotaxy in Table 3. Tarsus I-IV scopula entire; metatarsi I-II distal third, III-IV absent. Anterior tibiae without scopula. STC with numerous teeth in two lateral rows. ITC I-IV absent. Claw tufts absent. Tibial apophysis with 4 prolateral apical spines (Fig. 3D). Palpal organ spermophor strongly sinuous (Figs 3A–C). Four spinnerets, PMS short monoarticulated, PLS triarticulated apical article short, domed. Spigots without pumpkin-like socket. Body, legs light brown, abdomen with dark brown spots.
Female (paratype). Total length 18.2; carapace 7.2 long, 5.6 wide; caput raised; eight eyes grouped on a tubercle, AME 0.24; PME 0.25; ALE 0.36; PLE 0.31; OQ 0.75 long, 1.6 wide; clypeus 0.14; fovea 0.9; sternum oval, 2.3 long, 2.2 wide. Posterior sigillae sub-circular, sub-marginal. Labium sub-rectangular, 0.7 long, 1.3 wide; 1 labial cuspule; maxillary cuspules 48/62 (Fig. 1D). Chelicerae with 6 promarginal teeth; first tooth smaller than second, decreasing thereafter; 10 retromarginal denticles; rastellum with 45 short, thick conical setae on promargin. Leg, palpal measurements in Table 4; chaetotaxy in Table 5. Tarsus I-IV scopula entire, metatarsus I-II entire, III–IV absent. Anterior tibiae without scopula. STC with numerous teeth in two lateral rows. ITC I-IV absent. Claw tufts absent. Palpal claw with 4 teeth in prolateral median line. Two spermathecal receptacles, single sinuous neck; reniform fundus (Fig. 3E). Four spinnerets, PMS short monoarticulated, PLS triarticulated apical article short, domed. Spigots without pumpkin-like socket. Coloration as in male.
Length of legs palpal segments of the holotype male of Chaco castanea.
| Fe | Pa | Ti | Mt | Ta | Total | |
|---|---|---|---|---|---|---|
| Palp | 2 | 0.83 | 1.71 | – | 0.83 | 5.37 |
| I | 3.4 | 2 | 2.53 | 2.67 | 2.07 | 12.67 |
| II | 3.27 | 1.67 | 0.87 | 2.67 | 2.2 | 10.68 |
| III | 3 | 1.45 | 2.1 | 3.17 | 2.43 | 12.15 |
| IV | 3.93 | 1.6 | 3.73 | 4.33 | 2.93 | 16.52 |
Spination of legs and palps of holotype male Chaco castanea.
| Fe | Pa | Ti | Mt | Ta | |
|---|---|---|---|---|---|
| Palp | 2-1-1-0 | 0 | 0-3-1-0 | – | 0 |
| I | 4-1-1-0 | 0 | 0-2-0-6 | 0-1-1-3 | 0 |
| II | 5-2-3-0 | 0-1-0-0 | 0-2-0-3 | 0-1-1-3 | 0 |
| III | 0-0-0-6 | 0-3-1-0 | 6-2-2-6 | 3-4-4-6 | 0 |
| IV | 6-6-3-0 | 0-1-1-0 | 3-2-3-6 | 3-3-2-6 | 0 |
Length of legs and palpal segments of the paratype female of Chaco castanea.
| Fe | Pa | Ti | Mt | Ta | Total | |
|---|---|---|---|---|---|---|
| Palp | 3.6 | 2.1 | 2.2 | – | 2.2 | 10.1 |
| I | 4.7 | 2.8 | 3.1 | 2.8 | 2.0 | 15.4 |
| II | 4.2 | 2.7 | 3.0 | 2.8 | 1.9 | 14.6 |
| III | 3.6 | 2.4 | 2.3 | 3.5 | 2.4 | 14.2 |
| IV | 5.0 | 3.2 | 4.2 | 5.1 | 2.9 | 20.4 |
Spination of legs and palps of paratype female Chaco castanea.
| Fe | Pa | Ti | Mt | Ta | |
|---|---|---|---|---|---|
| Palp | 0-1-0-0 | 0-1-0-0 | 0-4-1-6 | – | 0 |
| I | 0-1-0-0 | 0-0-0-0 | 0-0-0-1 | 0-0-0-3 | 0 |
| II | 0 | 0-2-0-0 | 0-2-0-2 | 0-1-0-2 | 0 |
| III | 0-1-0-0 | 0-3-1-0 | 1-2-2-2 | 2-2-3-6 | 0 |
| IV | 1-0-1-0 | 0-0-0-0 | 0-2-3-6 | 0-2-3-8 | 0 |
Uruguay, Rocha, Perla de Rocha and Cabo Polonio.
http://zoobank.org/65418A73-632C-4AEF-8CE9-2740E0F09EC4
http://species-id.net/wiki/Chaco_costai
Figs 2A–F, 4A–EMale holotype (FCE-MY 1007) female paratype (FCE-MY 1006) and from Rocha, Perla de Rocha, 34°25.63'S, 53°52.27'W, 26-28.xii.2011, coll. A. Laborda, C. Castro, L. Montes de Oca.
The specific epithet is a patronym in honor of Fernando G. Costa, a recognized Uruguayan arachnologist who greatly contributed to the knowledge of spiders and has inspired many colleagues and students.
Males of Chaco costai (Fig. 2A) differ from the other species of the genus, except Chaco obscura, by the presence of numerous spines (7–10) on tibial apophysis (Fig. 4D); they can be distinguished from Chaco obscura by having a shorter embolous (Figs 4A–C). Female Chaco costai specimens (Fig. 2C) differ from most species of Chaco by having spermathecae with a sinuous neck (Fig. 4E). The species is distinguished from the geographically proximate species Chaco castanea by having a longer spermathecal neck and from Chaco obscura by the sinuous neck. Chaco costai differ from all other species of the genus (except Chaco patagónica and Chaco tecka) by having a flap door that covers the burrow (Fig. 2E).
Chaco costai. A Palpal organ, dorsal view B Palpal organ retrolateral view C Palpal organ prolateral view D Male apophysis with 10 spines, prolateral view E Spermathecae, dorsal view. Scale = 5mm.
Results from cladistics analyses. A–B Most parsimonious trees obtained by TNT (implied weighting). Length = 62, CI = 0.61, RI = 0.65, K =1 Fit = 10.33, K = 6 Fit = 3.48 C Strict consensus of cladograms A and B.
Male (holotype). Total length 14.9; carapace 7.7 long, 6.2 wide; eight eyes grouped on a tubercle, AME 0.28; PME 0.23; ALE 0.38; PLE 0.36; OQ 0.79 long, 1.04 wide; clypeus 0.22; fovea transverse, slightly procurve 1.5; sternum oval, 3.1 long, 2.8 wide. Posterior sigillae sub-circular, sub-marginal. Labium sub-rectangular, 1.2 long, 3.5 wide; 4 labial cuspules; 42/38 maxillary cuspules (Fig. 2B); chelicerae with 2 row of teeth, 6 promarginal, 5 retromarginal denticles; rastellum with 16 short, thick conical setae on promargin. ITC absent. Claw tufts absent. Leg, palpal measurements in Table 6; chaetotaxy in Table 7. Tarsus I-III scopula entire, IV divided; metatarsi I 4:5D, II 3:4 D, III 1:5 D, IV absent. Anterior tibiae without scopula. STC with numerous teeth in two lateral rows. Tibial apophysis with 7-10 prolateral apical spines (Fig. 4D). Palpal tibia with 2 dorsal long thin setae. Palpal organ spermophor very sinuous (Figs 4A–C). Four spinnerets, PMS short monoarticulated, PLS triarticulated apical article short, domed. Spigots without pumpkin-like socket. Cephalothorax, legs dorsally light brown, and ventrally dark brown; abdomen lighter with dark brown pattern.
Length of legs palpal segments of the holotype male of Chaco costai.
| Fe | Pa | Ti | Mt | Ta | Total | |
|---|---|---|---|---|---|---|
| Palp | 3.9 | 1.9 | 2.4 | – | 1.2 | 9.4 |
| I | 6.7 | 3.8 | 4.7 | 5.5 | 3.8 | 24.5 |
| II | 5.9 | 3.5 | 4.4 | 5.3 | 3.8 | 22.9 |
| III | 5.0 | 3.2 | 3.8 | 5.7 | 4.4 | 22.1 |
| IV | 7.2 | 3.5 | 6.1 | 8.0 | 4.9 | 29.7 |
Spination of legs and palps of male Chaco costai. The formula gives the number of spines in the following order: dorsal–prolateral–retrolateral–ventral.
| Fe | Pa | Ti | Mt | Ta | |
|---|---|---|---|---|---|
| Palp | 2-0-0-0 | 0-2-0-0 | 0-4-1-0 | – | 0 |
| I | 8-0-0-0 | 0-1-0-0 | 0-2-2-5 | 0-2-1-4 | 0 |
| II | 9-1-0-0 | 0-2-0-0 | 0-4-0-6 | 2-3-1-6 | 0 |
| III | 8-1-0-0 | 0-3-1-0 | 4-2-0-7 | 9-4-3-7 | 1-0-0-0 |
| IV | 9-0-0-0 | 0-1-1-0 | 4-1-4-6 | 7-3-3-8 | 1-0-0-0 |
Female (paratype). Total length 19.9; carapace 7.1 long, 5.7 wide; caput raised; eight eyes grouped on a tubercle, AME 0.2; PME 0.17; ALE 0.36; PLE 0.35; OQ 0.63 long, 1.16 wide; clypeus 0.27; fovea slightly procurved 1.10; sternum oval, 3.4 long, 2.9 wide. Posterior sigillae sub-circular, sub-marginal. Labium sub-rectangular, 0.47 long, 0.97 wide; 3 labial cuspules; 43/36 maxillary cuspules (Fig. 2D). Chelicerae with 8 promarginal teeth; 9 retromarginal denticles; rastellum with 18 short, thick conical setae on promargin. Leg, palpal measurements in Table 8; chaetotaxy in Table 9. Tarsus I-II scopula entire, III- IV divided by a wide band of longer conical setae, metatarsus I complete, II 2:3, III–IV absent. Anterior tibiae without scopula. STC with numerous teeth in two lateral rows. ITC I-IV absent. Claw tufts absent. Palpal claw with 4 teeth in median line. Two spermathecal receptacles, single sinuous long neck; sub-espheric fundus (Fig. 4E). Four spinnerets, PMS short monoarticulated, PLS triarticulated apical article short, domed. Spigots without pumpkin-like socket. Cephalothorax, legs brown, abdomen very light brown with darker dots.
Length of legs and palpal segments of the paratype female of Chaco costai.
| Fe | Pa | Ti | Mt | Ta | Total | |
|---|---|---|---|---|---|---|
| Palp | 3.8 | 1.9 | 2.1 | – | 1.8 | 9.6 |
| I | 4.8 | 3.3 | 2.8 | 2.7 | 2.1 | 15.6 |
| II | 3.3 | 2.2 | 1.7 | 2.2 | 1.8 | 11.2 |
| III | 3.7 | 2.6 | 1.4 | 2.9 | 2.5 | 13.1 |
| IV | 3.8 | 2.3 | 2.8 | 3.1 | 2.0 | 14 |
Spination of legs and palps of female Chaco costai.
| Fe | Pa | Ti | Ta | ||
|---|---|---|---|---|---|
| Palp | 0-1-0-0 | 0-4-0-0 | 0-1-0-9 | – | 0 |
| I | 2-0-0-0 | 0-1-0-0 | 0-2-0-4 | 0-0-0-4 | 0 |
| II | 1-0-0-0 | 0-1-0-0 | 2-0-0-3 | 1-1-0-5 | 0 |
| III | 2-0-0-0 | 0-3-1-0 | 1-2-2-5 | 1-4-3-7 | 0 |
| IV | 0-0-0-0 | 0-0-0-0 | 0-0-2-2 | 0-3-3-8 | 0 |
Only known from the type locality.
We thank F. Achaval, G. Calixto for collecting the first specimens and M.J. Albo, C. Castro, A. Laborda, L. Ziegler for their help in the fieldwork. We are also deeply indebted with Dr. J. Bond and Dr. P. Goloboff for their critical comments which help us to improve the manuscript.