(C) 2014 Théo Léger. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Léger T, Landry B, Nuss M, Mally R (2014) Systematics of the Neotropical genus Catharylla Zeller (Lepidoptera, Pyralidae s. l., Crambinae). ZooKeys 375: 15–73. doi: 10.3897/zookeys.375.6222
The Neotropical genus Catharylla Zeller, 1863 (type species: Crambus tenellus Zeller, 1839) is redescribed. Catharylla contiguella Zeller, 1872, C. interrupta Zeller, 1866 and Myelois sericina Zeller, 1881, included by Munroe (1995) in Catharylla, are moved to Argyria Hübner. Catharylla paulella Schaus, 1922 and C. tenellus (Zeller, 1839) are redescribed. Six new species are described by Léger and Landry: C. bijuga, C. chelicerata, C. coronata, C. gigantea, C. mayrabonillae and C. serrabonita. The phylogenetic relationships were investigated using morphological as well as molecular data (COI, wingless, EF-1α genes). The median and subterminal transverse lines of the forewing as well as the short anterior and posterior apophyses of the female genitalia are characteristic of the genus. The monophyly of Catharylla was recovered in all phylogenetic analyses of the molecular and the combined datasets, with three morphological apomorphies highlighted. Phylogenetic analyses of the morphology of the two sexes recovered three separate species groups within Catharylla: the chelicerata, the mayrabonillae, and the tenellus species groups. The possible position of Micrelephas Schaus, 1922 as sister to Catharylla, based on both morphological and molecular data, and the status of tribe Argyriini are discussed. The biogeographical data indicate that the chelicerata species group is restricted to the Guyanas and the Amazonian regions whereas the tenellus group is restricted to the Atlantic Forest in the South-Eastern part of Brazil. The mayrabonillae group is widespread from Costa Rica to South Bolivia with an allopatric distribution of the two species. COI barcode sequences indicate relatively strong divergence within C. bijuga, C. mayrabonillae, C. serrabonita and C. tenellus.
Argyria, Argyriini, Atlantic forest, biogeography, Crambini, Micrelephas, morphology, new species, phylogeny, Pyraloidea, taxonomy
Pyraloidea is one of the largest superfamilies of the order Lepidoptera. The monophyly of the group and that of its two main lineages, the Pyraliformes and Crambiformes (or Pyralidae and Crambidae, depending on authors), are well supported by morphological characters (
Catharylla moths are of medium size, with snow white to cream-colored wings with two ochreous to brown transverse lines on the forewings. Species of Catharylla occur in tropical Central and South America. Nothing is known on the immature stages and biology. The original description of
In this work, Catharylla is revised using both morphological and molecular data. Phylogenetic relationships within Catharylla and with putatively related taxa as well as the distribution of each species along with biogeographical hypotheses are analysed.
Catharylla and outgroup taxa investigated were borrowed from museums and private collectors as listed in Table 1, which also gives the acronyms used throughout the text.
Collections from which Catharylla specimens were borrowed.
| Acronym | Museum or Collection | Locality | Number of specimens |
|---|---|---|---|
| AMNH | American Museum of Natural History | USA, New York, New York | 1 |
| Becker Coll. | Collection of V. O. Becker | Brazil, Bahia, Camacan | 57 |
| BMNH | Natural History Museum | Great Britain, London | 34 |
| CMNH | Carnegie Museum of Natural History | USA, Pennsylvania, Pittsburgh | 9 |
| CNC | Canadian National Collection of Insects, Arachnids and Nematodes | Canada, Ontario, Ottawa | 14 |
| ISZP | Institute of Systematic Zoology | Poland, Krakow | 9 |
| INBio | Instituto Nacional de Biodiversidad | Costa Rica, Santo Domingo de Heredia | 5 |
| MHNG | Muséum d’histoire naturelle de Genève | Switzerland, Geneva | 28 |
| NMW | Naturhistorisches Museum Wien | Austria, Vienna | 4 |
| Schouten Coll. | Collection R. T. A. Schouten | Netherlands, The Hague | 6 |
| SMNS | Staatliches Museum für Naturkunde, Stuttgart | Germany, Stuttgart | 1 |
| SMTD | Staatliches Museum für Tierkunde, Dresden | Germany, Dresden | 1 |
| USNM | National Museum of Natural History | USA, D. C., Washington | 33 |
Several of the MHNG specimens were kindly given to this institution by collaborators mentioned in the acknowledgments section. The specimens were usually well preserved, the color being sometimes faded. Many specimens from the BMNH were dissected by S. Bleszynski. Unfortunately, his preparations were usually poorly made and badly mounted, sometimes hampering the investigation of genitalia characters.
The dissection and slide mounting methods follow
Apart from the eight Catharylla species, four additional crambine species were included in the dataset for phylogenetic analyses. The material investigated to build the morphological matrix is reported below in Table 2.
Material used for the morphology-based phylogenetic analysis of Catharylla species and related genera.
| Subfamily | Genus Species | Sex | Collecting locality | Collection | Dissection number |
|---|---|---|---|---|---|
| Crambinae | Argyria lacteella | M | USA, Florida, Lake Placid, Archbold Biological Station | MHNG | BL 064 |
| Crambinae | Argyria lacteella | F | USA, Florida, Lake Placid, Archbold Biological Station | MHNG | BL 067 |
| Crambinae | Argyria lacteella | F | Brazil, Bahia, Camacan, Serra Bonita Reserve | MHNG | TL 15 (wing prep.) |
| Crambinae | Catharylla bijuga | M | French Guiana, Saint-Jean-du-Maroni | BMNH | BL 1719 |
| Crambinae | Catharylla bijuga | F | Suriname, Sipaliwini District, Thibiti area, partly swampy, primary forest on hilly slopes, ca. 2km from river | Schouten Coll. | BL 1732 |
| Crambinae | Catharylla chelicerata | M | Brazil, Reserva Ducke, km. 26 Manaus–Itacoatiara Highway | CNC | BL 1721 |
| Crambinae | Catharylla chelicerata | F | Brazil, Reserva Ducke, km. 26 Manaus–Itacoatiara Highway | CNC | BL 1711 |
| Crambinae | Catharylla chelicerata | M | French Guiana, 36 km SE Roura (Camp Patawa) | MHNG | MHNG 6272 (wing prep.) |
| Crambinae | Catharylla coronata | M | Brazil, Espirito Santo, Linhares, 40m | Becker Coll. | BL 1743 |
| Crambinae | Catharylla coronata | F | Brazil, Rio Negro, 900 m | ISZP | BL 1731 |
| Crambinae | Catharylla gigantea | M | Guyana, Potaro | BMNH | BL 1716 |
| Crambinae | Catharylla gigantea | F | French Guiana, Saint-Jean-du-Maroni | BMNH | Pyralidae Brit. Mus. Slide N° 11342 |
| Crambinae | Catharylla mayrabonillae | M | Peru, Agnaytia, Huallaga, Peru, 400m | CNC | BL 1724 |
| Crambinae | Catharylla mayrabonillae | F | French Guiana, Saint-Jean-du-Maroni | BMNH | BL 1720 |
| Crambinae | Catharylla paulella | M | Bolivia, Provincia del Sara, 450 m | BMNH | Pyralidae Brit. Mus. Slide N° 15890 |
| Crambinae | Catharylla paulella | F | Brazil, Mato Grosso, Urucum, 15 miles South of Columbá, 650 feet | BMNH | BL 1712 |
| Crambinae | Catharylla serrabonita | M | Brazil, Espirito Santo, Linhares, 40m | USNM | BL 1745 |
| Crambinae | Catharylla serrabonita | F | Brazil, Espirito Santo, Linhares, 40m | Becker Coll. | BL 1759 |
| Crambinae | Catharylla serrabonita | M | Brazil, Bahia, Camacan, Serra Bonita Reserve | MHNG | TL 8 (wing prep.) |
| Crambinae | Catharylla tenellus | M | Brazil, Minas Gerais, Caraça, 1300 m | Becker Coll. | BL 1746 |
| Crambinae | Catharylla tenellus | F | Brazil, São Paulo, Bertioga, 5 m | Becker Coll. | BL 1742 |
| Crambinae | Crambus uliginosellus | M | Germany, Oberstdorf, Allgäu | SMTD | MTD prep. N°327 |
| Crambinae | Crambus uliginosellus | F | Switzerland, St. Gallen | SMTD | MTD prep. N°329 |
| Crambinae | Crambus pascuella | M | Germany, Coswig, Dresden | SMTD | MTD prep. N°325 |
| Crambinae | Crambus pascuella | F | Germany, Koetzschenbroda, Dresden | SMTD | MTD prep. N°326 |
| Crambinae | Micrelephas pictellus | M | Brazil, Bahia, Camacan, 400–700m | MNHG | MHNG ENTO N°2831 |
| Crambinae | Micrelephas pictellus | F | Panama, Barro Colorado Island | MNHG | MHNG ENTO N°2829 |
| Crambinae | Micrelephas pictellus | M | Brazil, Bahia, Camacan, Serra Bonita Reserve | MNHG | TL 17 (wing prep.) |
The types of the two species Catharylla contiguella Zeller, 1872, and Catharylla interrupta Zeller, 1866 could not be found. With the help of the descriptions and illustrations, they were excluded from Catharylla because of the forewing pattern, which is like that of Argyria Hübner, 1818, with only one median transverse line. For Catharylla sericina Zeller, 1881, based on the description and a photograph of the type in the BMNH, the species was rejected from Catharylla based on the elongated forewing shape and the silvery white pattern without transverse lines. For Catharylla paulella Schaus, 1922, a photograph of the habitus and the genitalia of the female type from the USNM allowed to find other specimens of the same species; the male and female were then associated based on wing pattern. For Catharylla tenellus Zeller, 1839, a photograph of the habitus and the genitalia of the female type were available and the male of the species was associated based on wing pattern. For the descriptions, we followed the nomenclature and terminology used by
The following measurements were made with the use of an ocular micrometer: length of labial palpus (base of segment I to apex of segment III), diameter of eye (greatest vertical width), length of forewing (from base to apex), length of uncus (from tegumen-uncus junction to apex of uncus), length of tegumen connection (from tegumen arms connection medially to tegumen-uncus junction), length of papillae anales (dorso-ventral length), lengths of anterior and posterior apophyses (from base to apex).
Regarding the holotype data, the information was copied exactly as found on the labels with vertical slashes to express changes of lines. Abbreviations are spelled out in square brackets. We assume that the labels are rectangular and white, and that the text is in black ink, otherwise differences are indicated in brackets. Paratype data are reported by country in alphabetical order and the information is recorded without indication of line change. Collecting localities are reported as written on labels, with a question-mark when the locality could not be recovered. Dates and collectors’ information were standardized and the latter placed in parentheses. The specimen depositories are reported with the use of the corresponding acronyms.
The coordinates of the localities were found using Google Earth (2011). The localities that were not registered in Google Earth were localized more or less precisely with the help of internet search engines or with gazeeters from the GEOnet Names Server (GNS) of the National Geospatial Intelligence Agency (http://earth-info.nga.mil/gns/html/). The localities were reported on a text file (*.txt) and loaded on a map using DIVA-GIS 7.4.0.1 (
The genes investigated are the mitochondrial COI gene (1474 bp) and the two nuclear genes wingless (353 bp) and EF-1α (679 bp). These genes show different rates of substitution through time, with COI >> wingless > EF-1α (
Specimens used for molecular investigations are listed in Table 3, with the sequencing success for the different investigated genes. Specimens were preserved in 95% ethanol under cool conditions until molecular investigations, or were pinned and dried. Sequences for the additional species were obtained from GenBank (see Table 3).
List of the material used in the molecular work with voucher numbers, database of origin, collecting depository, and sequencing results for each gene. LEP references refer to the Lepidoptera DNA database of M. Nuss at the SMTD. BC MTD references refer to the barcode sequence voucher of the Barcoding Of Life Database (BOLD). HG references refer to the European Nucleotide Archive. Amplicon length (in basepairs) is given in brackets. The sequences with an asterisk were those used to build the datasets used in the phylogenetic analyses.
| species | Voucher number | Collecting locality and specimen depository | sequencing results | |||
|---|---|---|---|---|---|---|
| COI | wingless | EF-1α | ||||
| 1st part | 2nd part | |||||
| Catharylla | ||||||
| Catharylla bijuga | - | French Guiana, Roura, road to Crique Gabrielle, 3.6 km East Roura (AMNH) | BC MTD 01839 (1–654) | |||
| Catharylla bijuga | - | Brazil, Amazonas, Parque nacional do Jaú, 1°57'S, 61°49'W (USNM) | BC MTD 01840 (1–654)* | |||
| Catharylla chelicerata | LEP 963 | French Guiana, 36 km SE Roura (Camp Patawa) (MHNG) | BC MTD 01703 (1–654) | |||
| Catharylla chelicerata | - | French Guiana, 36 km SE Roura (Camp Patawa) (MHNG) | BC MTD 01704 (1–654) | |||
| Catharylla chelicerata | LEP 1290 | French Guiana, 600, Parcelles CIRAD de Combi, plantations expérimentales, pk 1.8 5°18''N, 52°55'30''W (MHNG) | HG793015 (1–1474)* | HG793008 (46–353)* | HG793003 (1–452)* | |
| Catharylla coronata | - | Brazil, Espiritu Santo, Linhares, 40m (USNM) | BC MTD 01890 (1–654)* | |||
| Catharylla mayrabonillae | LEP 1126 | Peru, Huánuco, Rio Llullapichis, Panguana, 74, 945°W / 9, 614°S (SMTD) | HG793014 (6–1474)* | HG793009 (1–353)* | HG793004 (1–199)* | |
| Catharylla mayrabonillae | - | Costa Rica, Alajuela, Area de Conservacion Guanacaste, Estacion Caribe (INBio) | 07-SRNP-113921 (1–654) | |||
| Catharylla paulella | LEP 965 | Brazil, Sao Paulo, Sao Luiz do Paraitinga, 900 m, 23°20'S, 45°06'W(V. O. Becker n°132357) (Becker Coll.) | HG793016 (1–978)* | |||
| Catharylla serrabonita | LEP 970 | Brazil, Bahia, Porto Seguro, A. d'Ajuda, 16°27'S, 39°03'W, 20m (V. O. Becker n°144140) (Becker Coll.) | BC MTD 01887 (1–654) | |||
| Catharylla serrabonita | LEP 979 | Brazil, Bahia, Camacan, Reserva Serra Bonita, 800m (MHNG) | HG793017 (35–556)* | HG793018 (751–1333)* | HG793010 (734–1474)* | HG793005 (106–679)* |
| Catharylla serrabonita | - | Brazil, Espiritu Santo, Linhares, 40m (USNM) | BC MTD 01843 (1–654) | |||
| Catharylla tenellus | - | Brazil, Bahia, Porto Seguro, A. d'Ajuda, 16°27'S, 39°03'W, 20m (V. O. Becker n°142784) (Becker Coll.) | BC MTD 01708 (15–654) | |||
| Catharylla tenellus | LEP 973 | Brazil, Bahia, Porto Seguro, A. d'Ajuda, 16°27'S, 39°03'W, 20m (V. O. Becker n°144140) (Becker Coll.) | BC MTD 01709 (1–654) | HG793011 (23–353)* | ||
| Catharylla tenellus | - | Brazil, Bahia, Porto Seguro, A. d'Ajuda, 16°27'S, 39°03'W, 20m (V. O. Becker n°140808) (Becker Coll.) | BC MTD 01710 (1–654) | |||
| Catharylla tenellus | - | Brazil, São Paulo, Ubatuba, Picinguaba, 23°22'S, 44°50'W (Becker Coll.) | BC MTD 01842 (1–654) | |||
| Catharylla tenellus | LEP 972 | Brazil, Bahia, Porto Seguro, A. d'Ajuda, 16°27'S, 39°03'W, 20m (V. O. Becker n°140808) (Becker Coll.) | BC MTD 01888 (1–654)* | HG793020 (792–1473)* | ||
| Other Crambinae | ||||||
| Argyria lacteella | LEP 976 | Brazil, Bahia, Camacan, Reserva Serra Bonita, 800m (MHNG) | HG793013 (6–689; 709–1474)* | HG793006 (57–355)* | HG793001 (1–654)* | |
| Crambus pascuella | - | USA: North Carolina, Swain County, 1720m, 35°35'45''N, 83°27'42''W | GU089400 (1–657)* | |||
| Crambus uliginosellus | - | unknown | GU828691 (1–668)* | GU828487 (716–1474)* | GU829571 (1–353)* | GU829302 (269–679)* |
| Micrelephas pictellus | LEP 977 | Brazil, Bahia, Camacan, Reserva Serra Bonita, 800m (MHNG) | HG793012 (26–1474)* | HG793007 (1–353)* | HG793002 (1–626)* | |
For Catharylla specimens collected no more than twenty years ago, a leg was sent to the Canadian Centre for DNA Barcoding (CCDB) at the Biodiversity Institute of Ontario (BIO) in Guelph. The barcode sequences of Catharylla are reported in each species description. The protocol for DNA extraction is found in the supplementary material of
DNA extractions were performed following the method of
The primers used are recorded in Table 4.
Primers used for DNA sequencing. (F) stands for Forward primers, (R) for Reverse primers. The primers of the Nymphalidae Systematics Group can be found at http://nymphalidae.utu.fi/Nymphalidae/Molecular.htm
| Origine | gene | Primers | References |
|---|---|---|---|
| mitochondrial | COI | HybLCO (F) | |
| Nancy (R) | |||
| HybJerry (F) | |||
| HybPat (R) | |||
| K699 (R) | The Nymphalidae Systematics Group | ||
| Ron (F) | The Nymphalidae Systematics Group | ||
| Mila (R) | The Nymphalidae Systematics Group | ||
| Brian (F) | The Nymphalidae Systematics Group | ||
| nuclear | wingless | HybLepWG1 (F) | |
| HybLepWG2 (R) | |||
| EF-1α | Oscar-6143 (F) | ||
| Bosie-6144 (R) |
PCRs were performed using peqGOLD Taq DNA polymerase (PeqLab). In cases of weak or absent PCR result, a re-examination PCR was done using BIO-X-ACT Short Taq polymerase (Bioline). PCR protocols are given in Appendix II - Tables 1 and 2. Potential contamination was tested along with PCRs by control sample without DNA.
Success of gene amplification was evaluated by an electrophoresis with 1% agarose gel, subsequent gel dying with GelRed and analysis under ultraviolet light. PCR products were purified using ExoSAP-IT (USB Corporation).
Sequence PCR was done with the BigDye Terminator-Kit of Applied Biosystems. The amount of each product is reported in Appendix II - Table 3. The PCR programme is reported in the BigDye Terminator Sequencing Kit protocol. The sequences were obtained from the sample analysis on a 3130 Genetic Analyzer (Applied Biosystems).
Sequence alignment was done with BIOEDIT 7.1.3 (
http://www.ebi.ac.uk/ena/data/view/HG793012-HG793020; http://www.ebi.ac.uk/ena/data/view/HG793012-HG793020
Genetic distances between barcoding sequences of COI are given in Table 5. Distances were calculated using DAMBE. The GTR model was used as the substitution model.
Distance matrix between Catharylla species calculated with GTR correction. Values are given in %.
| Catharylla bijuga BC MTD 1839 | Catharylla bijuga BC MTD 1840 | Catharylla chelicerata LEP 1290 | Catharylla chelicerata BC MTD 1703 | Catharylla chelicerata BC MTD 1704 | Catharylla coronata BC MTD 1890 | Catharylla mayrabonillae LEP 1126 | Catharylla mayrabonillae 07-SRNP-113921 | Catharylla paulella LEP 965 | Catharylla serrabonita LEP 979 | Catharylla serrabonita BC MTD 1887 | Catharylla serrabonita BC MTD 1843 | Catharylla tenellus BC MTD 1842 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catharylla bijuga BC MTD 1840 | 5.05 | ||||||||||||
| Catharylla chelicerata LEP 1290 | 16.61 | 14.63 | |||||||||||
| Catharylla chelicerata BC MTD 1703 | 16.84 | 14.83 | 0.62 | ||||||||||
| Catharylla chelicerata BC MTD 1704 | 16.63 | 14.63 | 0.46 | 0.15 | |||||||||
| Catharylla coronata BC MTD 1890 | 11.55 | 10.61 | 13.34 | 13.35 | 13.14 | ||||||||
| Catharylla mayrabonillae LEP 1126 | 15.55 | 15.8 | 16.6 | 16.83 | 16.61 | 11.22 | |||||||
| Catharylla mayrabonillae 07-SRNP-113921 | 14.82 | 15.07 | 15.63 | 15.63 | 15.42 | 9.79 | 4.34 | ||||||
| Catharylla paulella LEP 965 | 13.66 | 13.32 | 16.41 | 16.63 | 16.42 | 11.13 | 7.57 | 6.29 | |||||
| Catharylla serrabonita LEP 979 | 10.16 | 9.75 | 13.46 | 13.46 | 13.46 | 8.48 | 14.58 | 13.11 | 12.59 | ||||
| Catharylla serrabonita BC MTD 1887 | 11.1 | 10.41 | 15.06 | 14.65 | 14.86 | 7.74 | 14.64 | 13.68 | 13.57 | 3.24 | |||
| Catharylla serrabonita BC MTD 1843 | 10.37 | 9.7 | 14.48 | 14.07 | 14.28 | 7.59 | 14.49 | 13.11 | 13.01 | 2.21 | 0.77 | ||
| Catharylla tenellus BC MTD 1842 | 12.29 | 9.87 | 14.72 | 14.95 | 14.73 | 7.3 | 13.42 | 13.06 | 12.93 | 7.45 | 6.44 | 6.47 | |
| Catharylla tenellus BC MTD 1709 1710 1888 | 11.8 | 10.52 | 15.01 | 15.24 | 15.03 | 7.31 | 13.15 | 12.08 | 12.14 | 6.72 | 7.17 | 6.49 | 3.34 |
The 21 characters are listed in Table 6. Characters 12, 16 and 17 were polarized into two sets of continuous values of ratios, with the limit selected subjectively where the gap between two groups of values appeared to be the largest. Phylogenetic analyses were run under PAUP 4.0b10 (
Character matrix of Catharylla and related taxa. A question mark refers to an unknown state for character 21, or an uncoded state (Crambus pascuella, character 7).
| characters \ taxa | Catharylla bijuga | Catharylla chelicerata | Catharylla coronata | Catharylla gigantea | Catharylla mayrabonillae | Catharylla paulella | Catharylla serrabonita | Catharylla tenellus | Argyria lacteella | Micrelephas pictellus | Crambus pascuella | Crambus uliginosellus |
| 1. Labial palpi ringed with brown at 1/3 and 2/3: absent (0); present (1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2. Length of labial palpi/eye diameter: >2/1 (0); < 2/1 (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 3. Hindwing color: yellowish / creamy white (0); white (1) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 4. Uncus in lateral view: horizontally straight (0); downcurved (1) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 5. Dorsal furrow on uncus: absent (0); present (1) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 6. Uncus dorsally setose (0); bare, or with few setae (1) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| 7. Uncus apex: unilobed, regularly rounded (0); regularly rounded with narrow tip (1); narrowing to a point (2); apically slightly bifid (3) | 2 | 1 | 3 | 1 | 2 | 2 | 3 | 3 | 2 | 0 | ? | 1 |
| 8. Apex of valva: narrowed or rounded (0); quadrangular, truncated (1) | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9. Gnathos: almost straight with apex pointing upward (0); bent of about 90° angle (1); regularly curved (2) | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 10. Transtilla: absent (0); present (1) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 11. Lateroventral projections on juxta: absent (0); present (1) | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 12. Ratio of dorsal roof of tegumen on uncus length: < 1/2 (0); > 1/2 (1) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 13. Latero-basal tuft of hairs on uncus: absent (0); present (1) | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 14. Vesica: with one cornutus (0); with crest of cornuti (1); without cornuti (2) | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 2 | 1 | 2 | 2 | 0 |
| 15. Ventral shape of papillae anales in lateral view: not produced (0); slightly produced (1) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 16. Ratio of anterior apophyses/papillae anales: > 0.1 (0); < 0.1 (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 17. Ratio of posterior apophyses/papillae anales: > 0.5 (0); < 0.5 (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 18. Ventral tongue shaped pronounced sclerotization postero-ventrally on ductus bursae: absent (0); present (1) | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19. Ventral membrane of segment VIII: without tiny setae (0); with tiny setae (1) | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 20. Postvaginal sterigma: present (0); absent (1) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| 21. Forewing R4: free (0); stalked with R2+3 (1) | ? | 1 | ? | ? | 1 | ? | 1 | ? | 0 | 0 | ? | ? |
Because of the age of some of the material used, only the COI barcode sequences were available for Catharylla bijuga and Catharylla coronata, and the whole COI gene for Catharylla paulella. For Catharylla bijuga, we used the sequence BC MTD 1840 to build the datasets as this sequence performed better than BC MTD 1839 in phylogenetic analyses. The COI sequence of LEP 972 was combined with that of wingless for LEP 973 given that the two samples come from the same population and are genetically very similar as attest the barcode sequences. We generated four different datasets from the sequence data: a complete dataset with all three genes sequences available for the 12 taxa (mol_1), a 12-taxa dataset with the 3rd codon position of COI deleted (mol_2), a dataset restricted to the 7 taxa for which the sequence of the COI gene and at least of one nuclear gene were available (nucl_1), and the same data as nucl_1 but with the 3rd codon position of the COI deleted (nucl_2). We used the programme RAxML (
A nexus file was created for the 12 taxa investigated, with the four following partitions: (1) the morpho-matrix (Table 6), (2) COI, (3) wingless, (4) EF-1α, and was analysed using Mr-Bayes 3.2.1 (
http://species-id.net/wiki/Catharylla
Crambus tenellus Zeller, 1839, by subsequent designation by
Catharylla species have snow white to creamy white wings and short labial palpi. They can be separated from other Argyriini by the presence on the forewing of median and subterminal thin transverse lines, slightly curved, convex on costal 1/3. The labial palpi are also shorter in comparison to those of Vaxi. The highly variable male genitalia do not show any synapomorphy or generic diagnostic character. In females, a possible synapomorphy is the strongly reduced anterior and posterior apophyses of abdominal segments VIII and IX, but this is shared with some Crambini and a few other Crambinae (see
Head white, chaetosemata present. Antenna brown, covered with light ochreous to brown scales. Maxillary palpus light ochreous to brown, white tipped. Labial palpus 1.0–1.95 × width of head, curved upward; white basally, light ochreous to ochreous, white tipped, with some brown or dark brown. Thorax white, with ochreous to brown scales at collar. Foreleg coxa white to whitish brown, femur dorsally brown to dark brown, tibia and tarsomeres distally ringed with dark brown. Midleg white to light ochreous with tibia-femur joint ashen brown, with pair of spurs at apex of tibia, tarsomeres II–V dorsally brown to dark brown, with white tips. Hindleg white, with 2 pairs of spurs on tibia, tarsomeres as on midleg. Male frenulum simple, frenulum hook present; female frenulum with 3 or 4 acanthae. Forewing length: 7.5–15 mm in males; 9.5–22 mm in females. Wing venation (of Catharylla chelicerata) (Fig. 9): R1 present and free, not connected to Sc; R2 free; R3 connected with R4 at 3/4; R5 stalked with R3+R4 at 1/4; M1 from upper corner of cell; cell opened between M1 and M2; M2 and M3 not stalked; CuA1 from lower corner of cell; CuA2 at distal 1/3 of cell; 1A+2A strong. Hindwing Sc+R1 connected to Rs at distal 1/3; M1 connected to Sc+R1 by short narrow vein; M3 connecting to M2 at distal 1/3, CuA1 connecting to M2 at half of length and CuA2 connecting at basal 2/5; 1A unforked; 2A unforked, strong; 3A present, unforked. Forewing (Figs 1–8) background snow white; pattern with costal margin ochreous to brown, sometimes faded; median and subterminal transverse lines thin, ochreous to brown, convex toward costa; outer margin ochreous, sometimes with dark brown spots between veins, or spots forming a continuous line; fringes white; verso light ochreous to ochreous; with marginal spots pronounced. Hindwing snow white to cream-colored; with hairs along 2A and root of M2; with small dark brown spots on outer margin, sometimes in continuous line; sometimes with postmedian transverse line; verso white with marginal spots pronounced.
Habitus of Catharylla species: 1 Catharylla bijuga sp. n., holotype, French Guiana, Pied Saut, Oyapock River (CMNH) 2 Catharylla chelicerata sp. n., Parcelles CIRAD de Combi, plantations expérimentales pk 1.8, 5°18'N, 52°55'30"W (MHNG) 3 Catharylla gigantea sp. n., holotype, Brazil, Amazonas, Manaus, Reserva Ducke, AM-010, km 26, 2°55'S, 59°59'W 4 Catharylla tenellus Zeller, Brazil, Saõ Paulo, Bertioga (Becker Coll.) 5 Catharylla coronata sp. n., holotype, Brazil, Espirito Santo, Linhares (USNM) 6 Catharylla serrabonita sp. n., holotype, Brazil, Bahia. Camacan, Reserva Serra Bonita, 15°23'S, 39°33'W, 800m (Becker Coll.) 7 Catharylla mayrabonillae sp. n., holotype, Ecuador, Napo, Misahualli (Becker Coll.) 8 Catharylla paulella Schaus, holotype, Brazil, Sao Paulo state, Sao Paulo (USNM).
Male wing venation of Catharylla species: Catharylla chelicerata sp. n., paratype ♂, French Guiana, Roura, Camp Patawa; slide MHNG 6272 (MHNG).
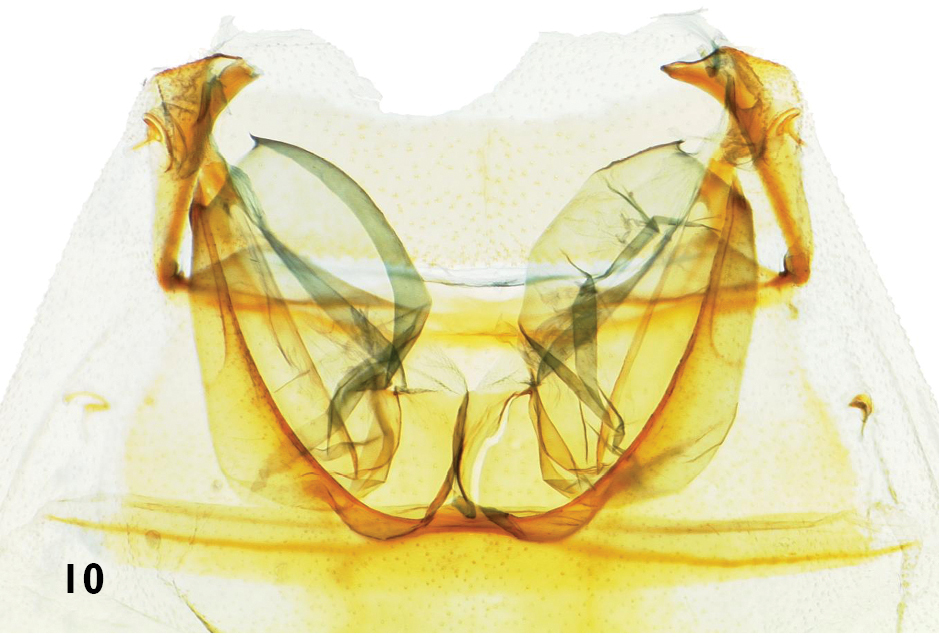
Tympanal organs (Fig. 10): Transverse ridge regularly rounded. Tympanic pockets more or less extending beyond transverse ridge. Tympanic bridge present, straight, lightly sclerotized. Tympanic drum more or less ovoid.
Tympanal organs of Catharylla species: Catharylla paulella Schaus, ♀, Brazil, Distrito Federal, Planaltina, slide BL 1751 (Becker Coll.).
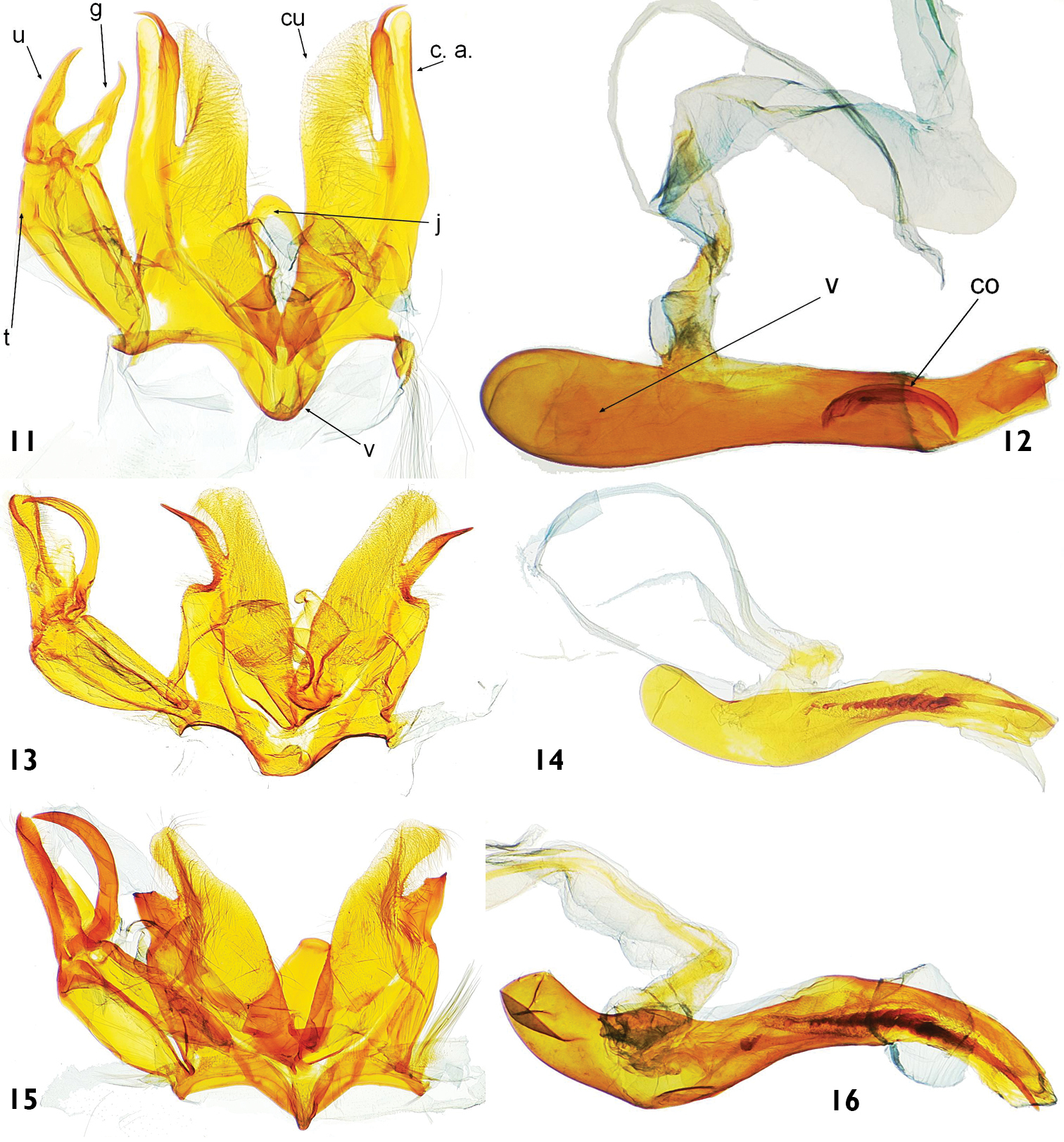
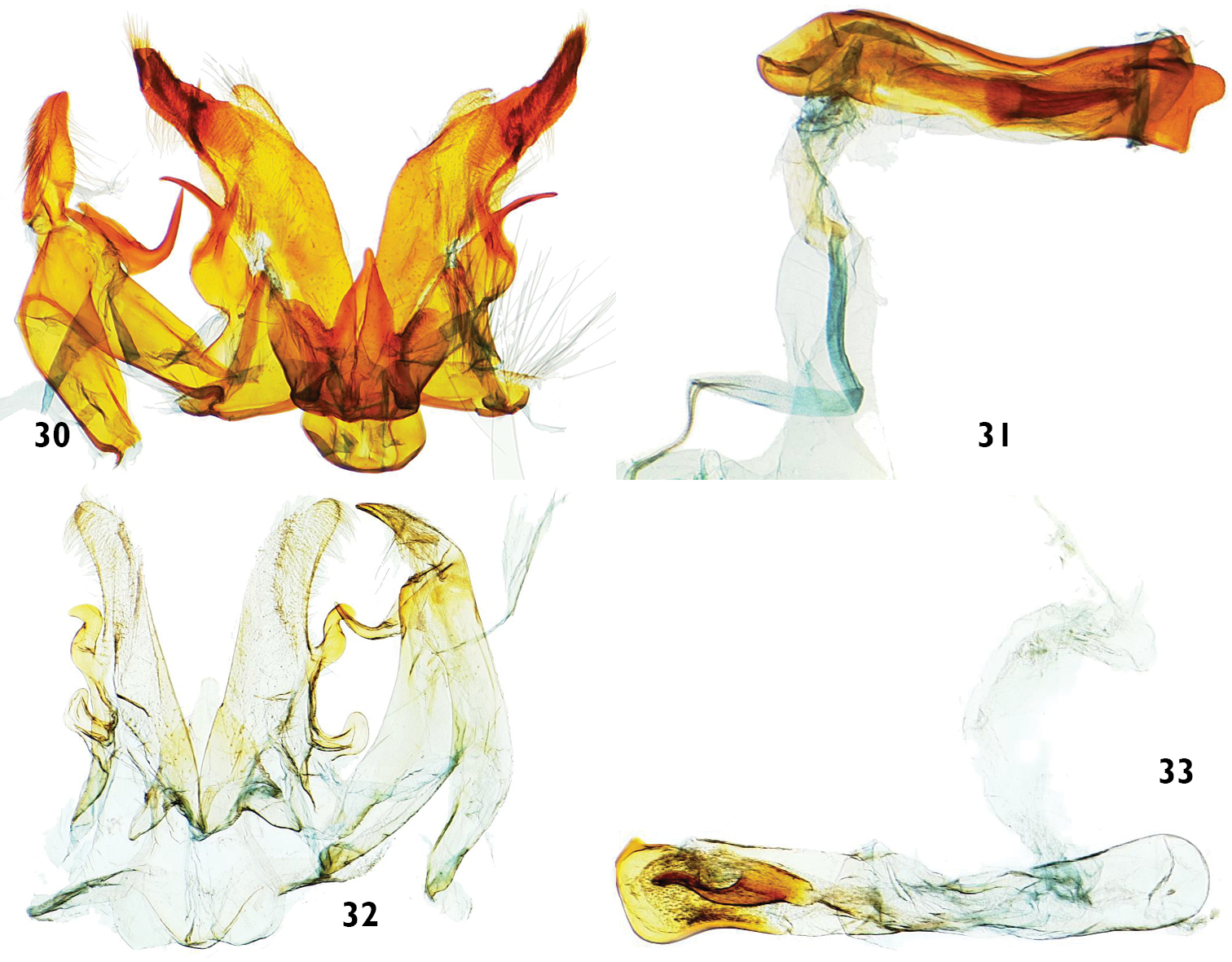
Male genitalia (Figs 11–33): Uncus long, basally wide, straight or downcurved, bare or setose. Gnathos arms connecting at 1/4 to 1/2 from base, thin, slightly curved to hook shaped, with apex pointing upward. Tegumen arms enlarging toward uncus, connecting dorsally. Valva with cucculus long, densely setose on inner side, apically more or less rounded, slightly curved upward; costal arm present, variable in shape. Transtilla present only in Catharylla tenellus group, strongly developed. Juxta medially curved downward, narrowing toward rounded apex, slightly directed downward apically, basally triangular, sometimes with additional lobes at base or ventro-lateral projections. Vinculum of medium width; saccus short, rounded, directed anterad and slightly upward. Phallus straight or curved, usually more strongly sclerotized at apex; vesica without cornuti, with one cornutus, or with crest of cornuti.
Male genitalia features of Catharylla species: 11–12 Catharylla bijuga sp. n., paratype, French Guyana, St-Jean-de-Maroni, slide BL 1719 ♂ (BMNH) 11 Genitalia without phallus. cu: cucculus; c. a.: costal arm; g: gnathos; j: juxta; t: tegumen; u: uncus; v: vinculum 12 Phallus in lateral view. v: vesica; c: cornutus 13–14 Catharylla chelicerata sp. n., paratype, Brazil, Amazonas, Rio Negro, Mirapinima, slide BL 1714 ♂ (MHNG) 13 Genitalia without phallus 14 Phallus in lateral view 15–16 Catharylla gigantea sp. n., holotype, Brazil, Amazonas, Reserva Ducke, slide BL 1747 ♂ (BMNH) 15 Genitalia without phallus. 1 6 Phallus in lateral view.
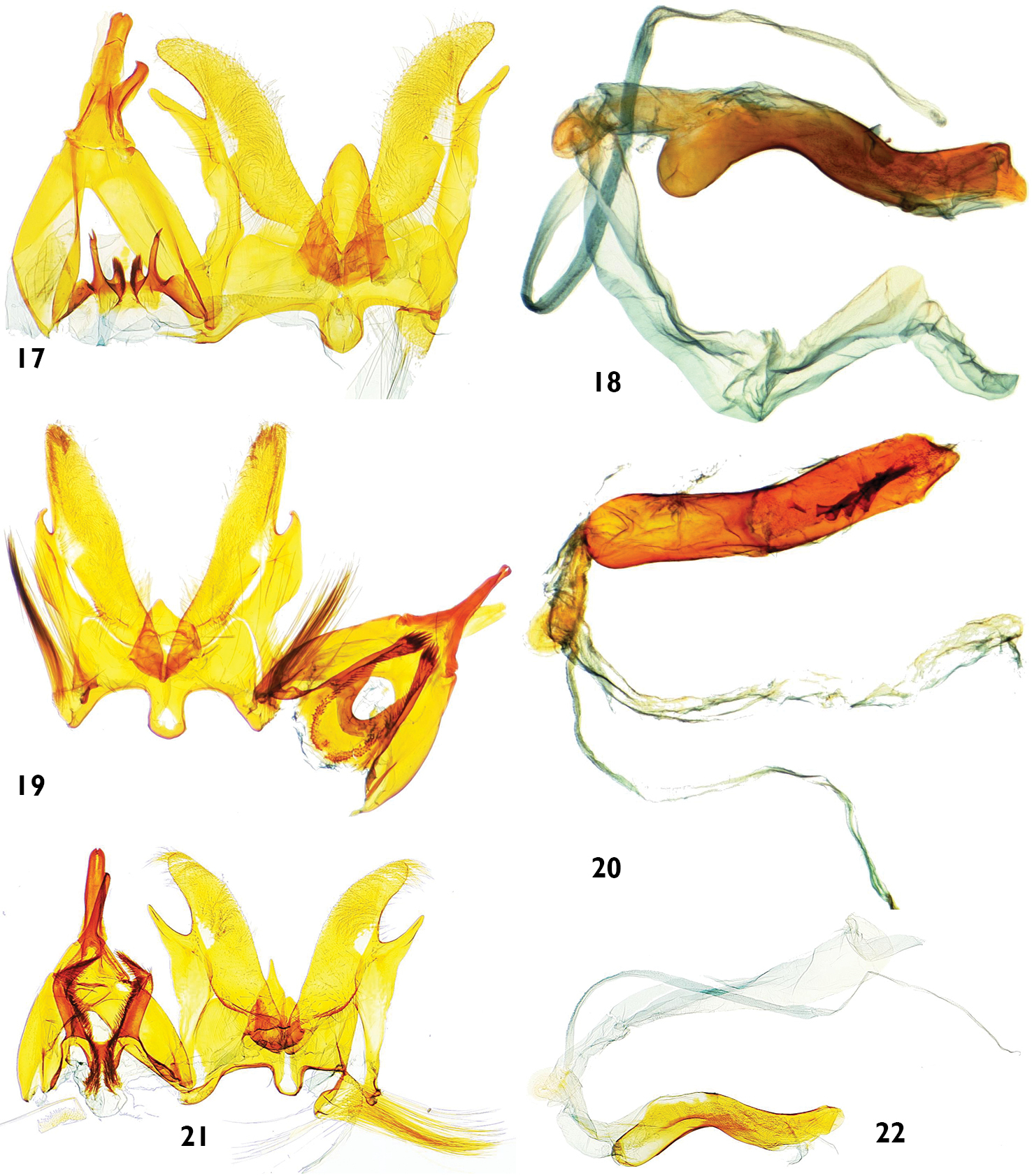
Male genitalia features of Catharylla species: 17–18 Catharylla tenellus Zeller 17 Genitalia without phallus, Brazil, Minas Gerais, Caraça, slide BL 1755 ♂ (Becker Coll.) 18 Phallus in lateral view, Brazil, Saõ Paulo, Bertioga, slide BL 1746 ♂ (USNM) 19–20 Catharylla coronata sp. n. paratype, Brazil, Paranà, Rio Negro, slide BL 1730 ♂ (ISZP) 19 Genitalia without phallus 20 Phallus in ventral view 21–22 Catharylla serrabonita sp. n. paratype, Brazil, Bahia, Camacan, Serra Bonita Reserve, slide BL 1776 ♂ (MHNG) 21 Genitalia without phallus 22 Phallus in lateral view.
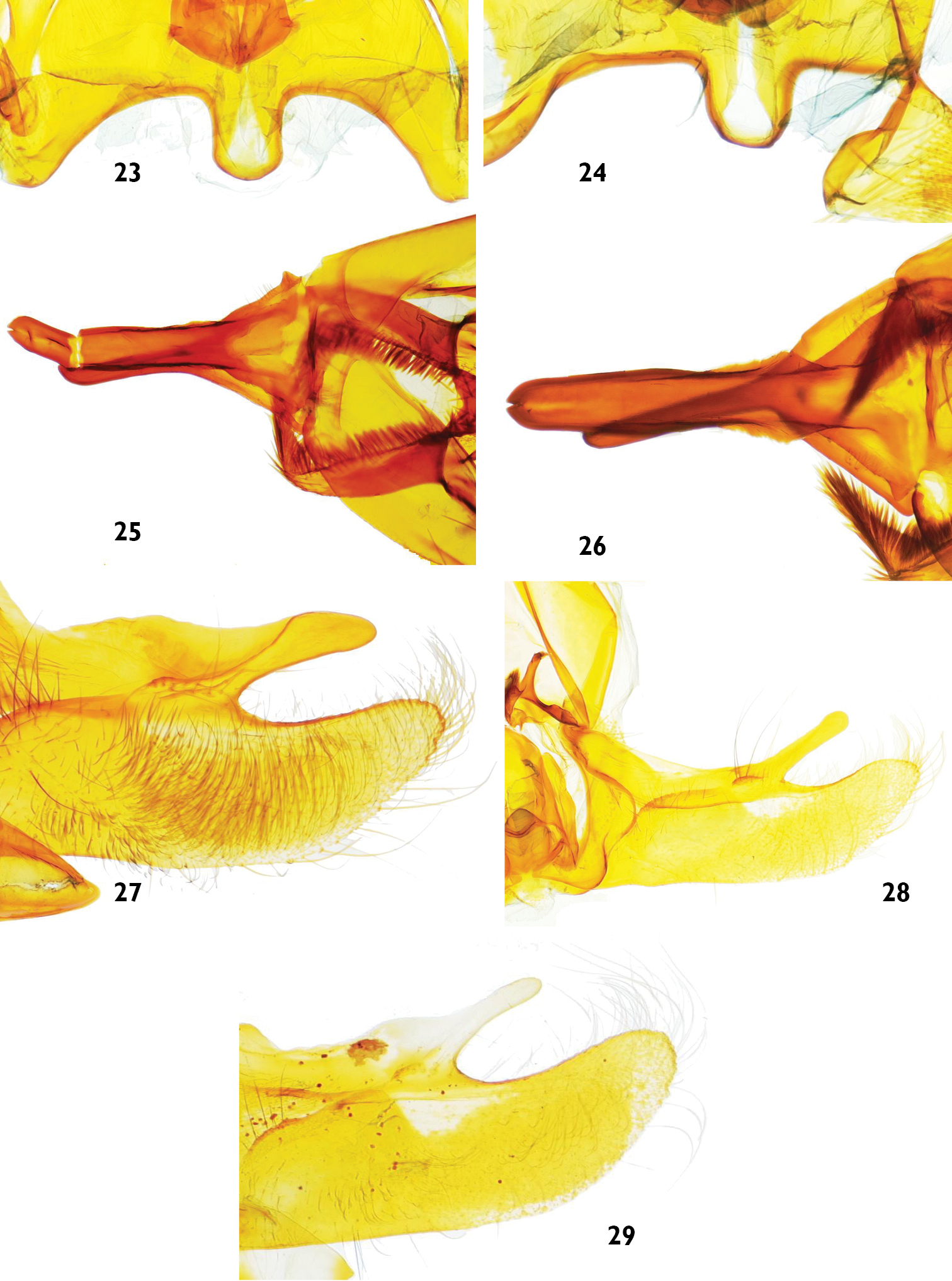
Male genitalia features of Catharylla species: 23–24 Vinculum of Catharylla serrabonita sp. n. 23 Brazil, Espírito Santo, Linhares, slide BL 1745 ♂ (USNM) 24 Brazil, Bahia, Camacan, Serra Bonita Reserve, slide BL 1776 ♂ (MHNG) 25–26 Uncus of Catharylla serrabonita sp. n. 25 Brazil, Espírito Santo, Linhares, slide BL 1745 ♂ (USNM) 26 Brazil, Bahia, Camacan, Serra Bonita Reserve, slide BL 1776 ♂ (MHNG) 27–29 Costal arm of Catharylla tenellus 27 São Paulo, Ubatuba, Picinguaba, slide BL 1757 ♂ 28 Minas Gerais, Caraça, slide BL 1746 ♂ 29 Brazil, Bahia, Porto Seguro, A. d’Ajuda, slide TL 9 ♂.
Male genitalia features of Catharylla species. 30–31 Catharylla mayrabonillae sp. n., paratype, Peru, Agnaytia, Huallaga, 400 m, slide BL 1724 ♂ (CNC) 30 Genitalia without phallus 31 Phallus in lateral view 32–33 Catharylla paulella Schaus, Bolivia, provincia del Sara, slide GS-6682-SB (BMNH) 32 Genitalia without phallus 33 Phallus in lateral view.
Female genitalia (Figs 34–41): Papillae anales strongly setose, connecting dorsally and ventrally, usually slightly produced dorsally, with basal band of sclerotization. Posterior apophyses 0.25–0.45 × length of papillae anales, straight, regularly thin. Tergite VIII narrow, with postero-dorsal line of setae. Anterior apophyses reduced, 0.01–0.1 × length of papillae anales. Sternite VIII about twice length of tergite, not connecting ventrally in tenellus species group. Sterigma present, strongly sclerotized except in tenellus species group, usually forming pockets of variable shape; reduced to sclerotized lamella antevaginalis in Catharylla bijuga. Ductus seminalis connecting posteriorly at base of ductus bursae. Ductus bursae long, at least 2 × length of corpus, wide, basally curved. Corpus bursae usually rounded, egg-shaped, often enlarging progressively from ductus bursae, usually with one signum, sometimes without; corpus and ductus bursae covered with minute spicules.
Female genitalia of Catharylla species. 34 Catharylla bijuga sp. n. in lateral view, paratype, Suriname, Sipaliwini District, Thibiti area, Kabo-Creek, slide BL 1732 ♀ (Schouten Coll.) 35 Catharylla chelicerata sp. n., paratype, Brazil, Reserva Ducke, slide BL 1711 ♀ (CNC); pap: papillae anales; p. a.: posterior apophyses; s: segment VIII; a. a.: anterior apophyses; st: sterigma; d: ductus bursae; c: corpus bursae 36 Catharylla gigantea sp. n. paratype, French Guyana, Saint-Jean-du-Maroni, slide n°6679SB (BMNH) 37 Catharylla tenellus Zeller, Brazil, Rio de Janeiro, slide BL 1733 ♀ (CMNH) 38 Catharylla coronata sp. n. paratype, Brazil, Paranà, Curitiba, slide BL 1753 ♀ (Becker Coll.) 39 Catharylla serrabonita sp. n. paratype, Brazil, Espírito Santo, Linhares, slide BL 1759 ♀ (Becker Coll.) 40 Catharylla mayrabonillae sp. n. paratype, French Guyana, Saint-Jean-du-Maroni, slide BL 1720 ♀ (BMNH) 41 Catharylla paulella Schaus, Brazil, Distrito Federal, Planaltina, slide BL 1751 ♀ (Becker Coll.).
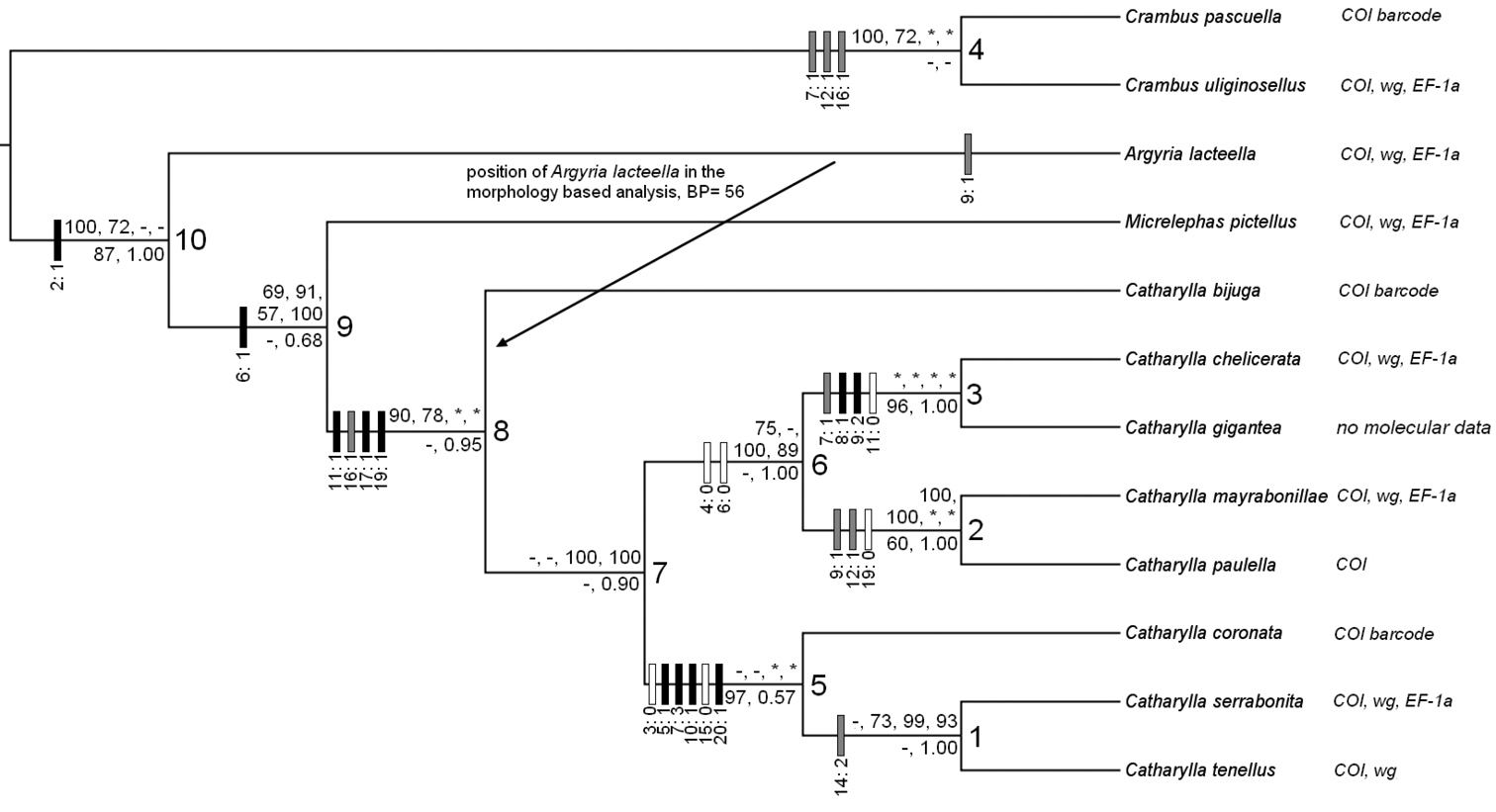
50% majority-rule consensus tree of the combined Bayesian analysis of the morphological and molecular datasets combined with 3'000'000 generations. Values above branches are the respective BS supports of the phylogenetic analyses with Maximum Likelihood algorithm of mol_1 (gene + codon partition, 800 BS replicates), mol_2 (gene partition, 450 BS replicates), nucl_1 (gene partition, 150 BS replicates), and nucl_2 (gene partition, 150 BS replicates). Values below branches are the BS supports of the phylogenetic analysis of the morphological data and the PP of the combined Bayesian analysis. “-” represent values under the majority rule; “*” represent the absence of value because of the absence of one or several taxa in the considered analysis. Boxes upon branches represent character changes: black boxes represent unique transformations to the apomorphic state; grey boxes represent multiple transformations to the apomorphic state; white boxes represent reversals to the plesiomorphic state.
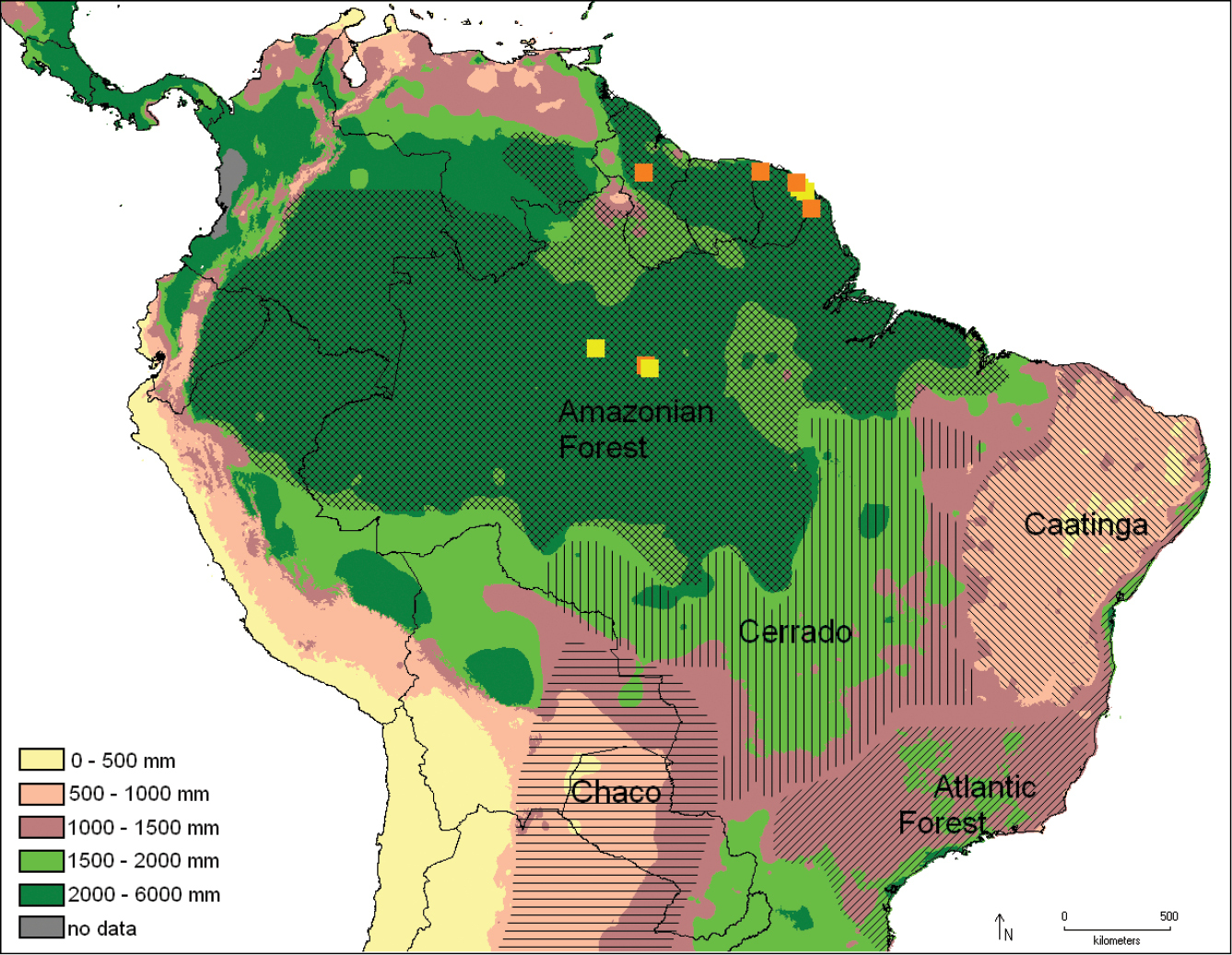
The genus is restricted to the Neotropical Region, from Costa Rica to Santa Catarina, Brazil, from sea level to 1300 m (Figs 43–46).
Distribution of Catharylla chelicerata (yellow) and Catharylla gigantea (orange) with the pluviometry and the biomes reported. Distribution of the biomes taken from
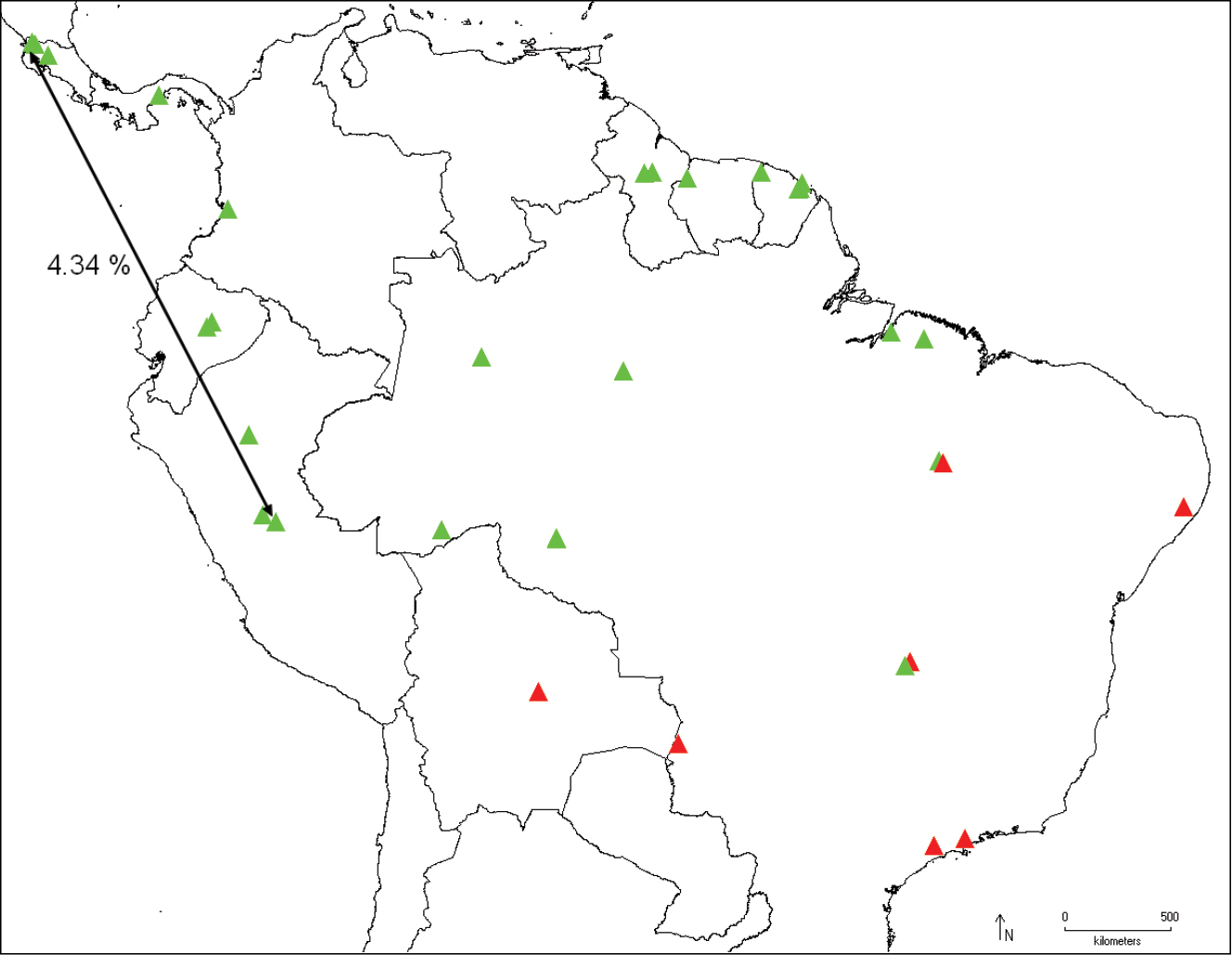
Distribution of Catharylla mayrabonillae (green) and Catharylla paulella (red), and distance between barcode haplotypes 07-SRNP-113921 and LEP 1226 (Catharylla mayrabonillae).
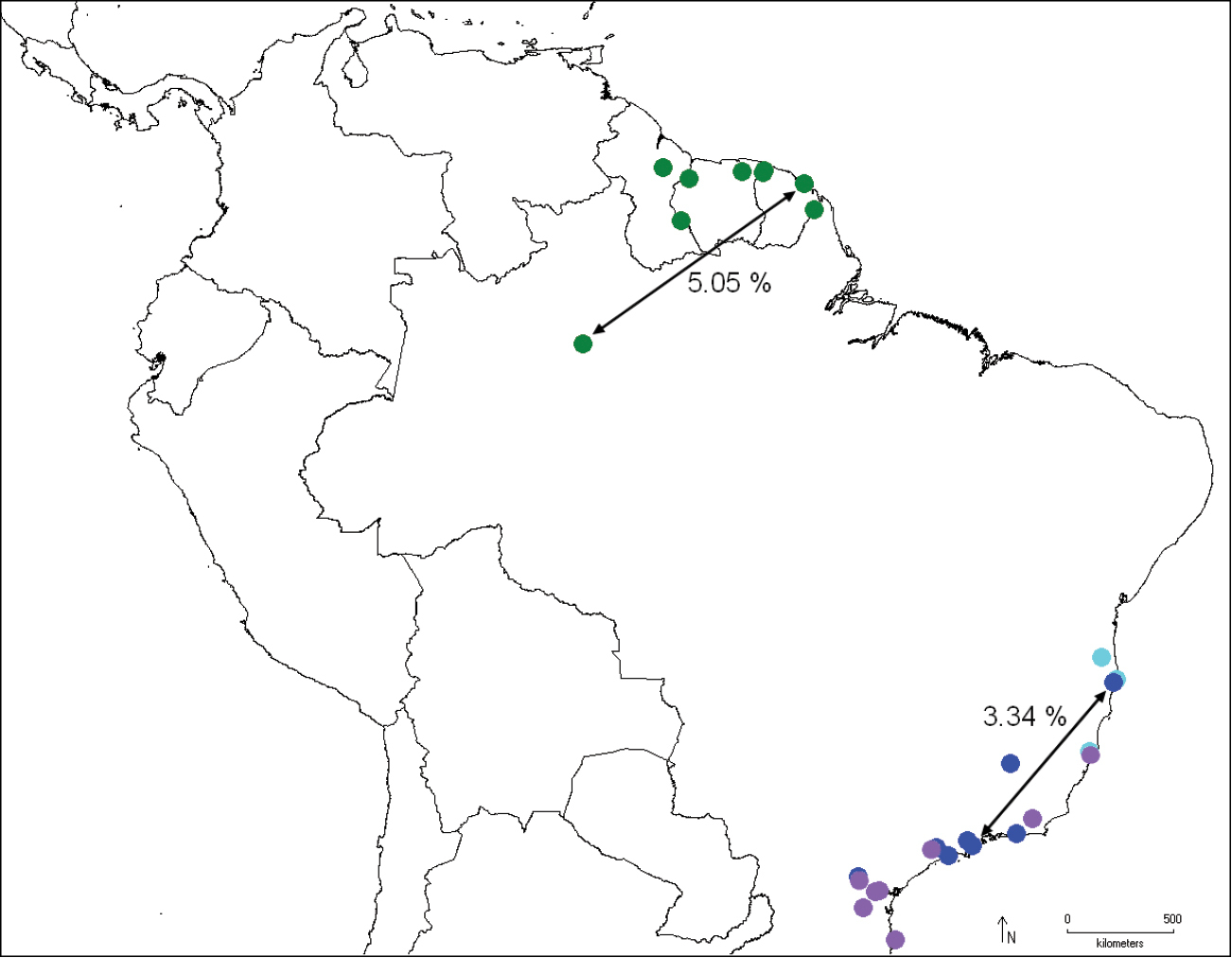
Distribution of Catharylla bijuga (green), Catharylla coronata (light purple), Catharylla serrabonita (sky blue) and Catharylla tenellus (blue), and distances between barcode haplotypes BC MTD 01839 and BC MTD 1840 (Catharylla bijuga), and BC MTD 1709 and BC MTD 1842 (Catharylla tenellus).
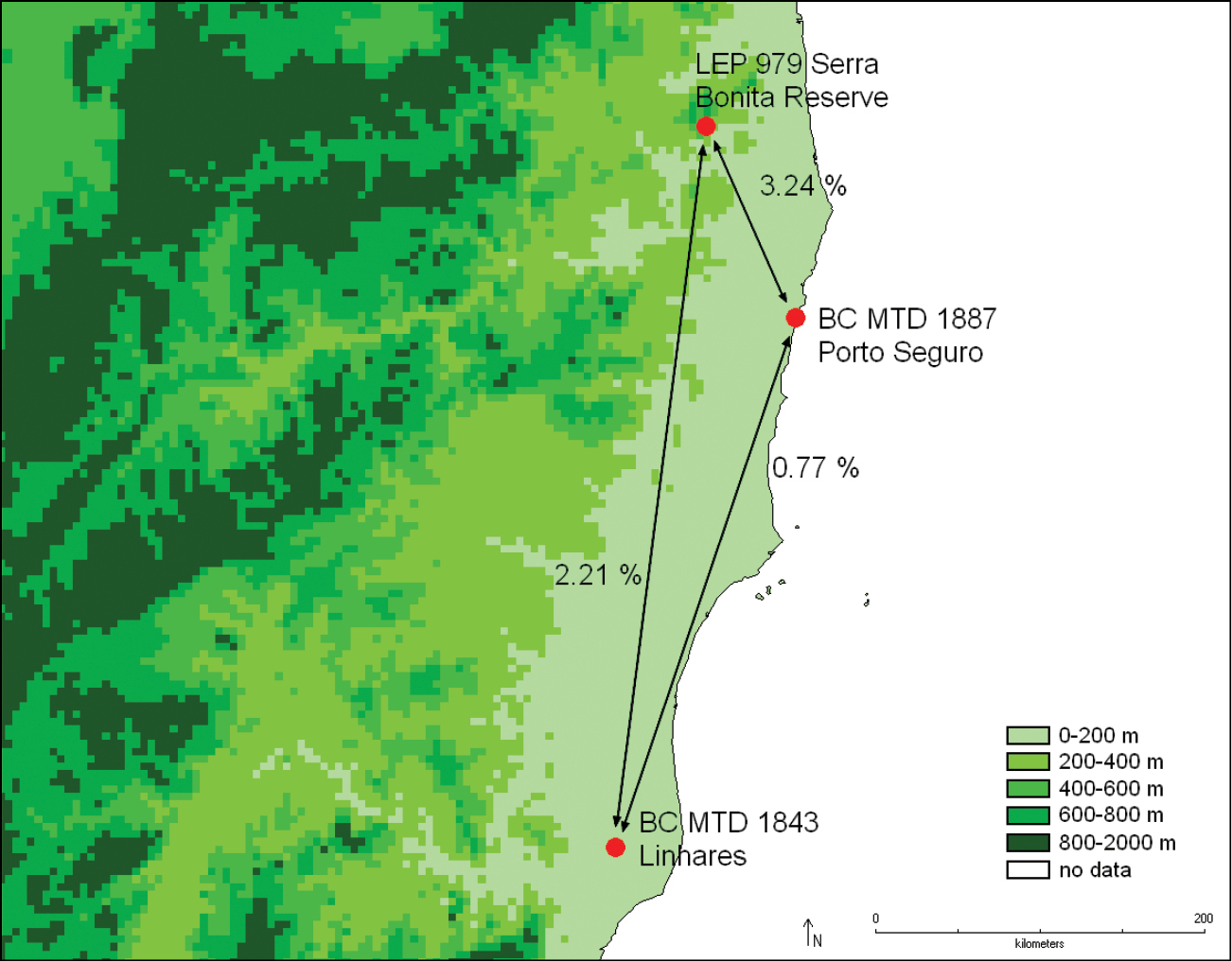
Distribution of Catharylla serrabonita and percentage of nucleotide differences in barcode sequences between different samples.
The biology of the species remains unknown. In the Serra Bonita Reserve in march 2011, we observed Catharylla serrabonita in its environment, i.e. forested hills up to about 950 m in elevation, surrounded by cacao or coffee plantations in the lowlands. The moths were coming to light, usually very late (after 23:00).
Presumably, given the reduced labial palpi and the forewing color and pattern, Catharylla has been placed in the Argyriini (
http://zoobank.org/9FC3D8A6-A172-443C-A52A-BE510976DD0A
http://species-id.net/wiki/Catharylla_bijuga
Figs 1, 11, 12, 34, 45Holotype. ♂, with labels as follows: “Pied Saut, | Oyapok [sic] River, | French Guiana, | S. M. Klages | C. M. Acc. 6111.”; “Dec[ember]. | 1917”; “HOLOTYPE | Catharylla bijuga | T. Léger & B. Landry” [red label]; “Catharylla | ramona sp. n. | det. Bleszynski, 1969”; “MANUSCRIPT | NAME” [white card with red lettering and thin red rectangle submarginally]; “BL 1744 ♂”. Deposited in CMNH.
Paratypes. 16 ♂, 9 ♀. BRAZIL: 1 ♂ (genitalia on slide BL 1748, used for DNA Barcoding BC MTD 01840), Amazonas, P[ar]q.[ue] Nac.[ional] do Jaú, Rio Jaú, bg. Miratucú, 1°57'S, / 61°49'W, 26–27.vii.1995, U[ltra] V[iolet] light sheet (R. W. Hutchings) (USNM). FRENCH GUIANA: 2 ♂ (1 with genitalia on slide Pyralidae Brit. Mus. slide N°15893) with same data as holotype (BMNH); 5 ♂, 1 ♀ (2 ♂ with genitalia on slides Pyralidae Brit. Mus. Slide N°15891, N°15892, ♀ with genitalia on slide BL 1735) with same locality as holotype except i.1918 (1 ♂), ii.1918 (4 ♂, 1 ♀) (S. M. Klages) (BMNH, CMNH); 1 ♂, 2 ♀, Parcelles CIRAD de Combi, plantations expérimentales pk 1.5, 5°18'N, 52°55'30 W, 4.iii.2011, piège lumineux [light trap] (B. Hermier) (♂ with Hermier n° 24340, 2 ♀ with labels Hermier n° 24341 & 24345) (MHNG); 3 ♂, 2 ♀ (2 ♂ with genitalia on slides BL 1719 and Pyralidae Brit. Mus. Slide N° 7815, 2 ♀ with genitalia on slides BL 1739 and BL 1740), Saint-Jean-du-Maroni (Le Moult) (BMNH); 1 ♂, Saint-Laurent-du-Maroni (USNM); 1 ♂ (genitalia on slide BL 1694, (used for DNA barcoding BC MTD 01839) Roura, 3.6km E[ast] Roura at r[oa]d to Crique Gabrielle, 50m, 20.iv.1994, at light (J. S. Miller & C. Snyder) (AMNH); 1 ♀ (genitalia on slide BL 1734), Cayenne, iii.1917 (CMNH). GUYANA: 1 ♂, New River 1938 (C.A. Hudson) (BMNH); 1 ♂, Mallali [sic] (USNM). SURINAME: 1 ♀ (genitalia on slide USNM 52888), Geldersland, Surinam River (USNM); 1 ♀ (genitalia on slide BL 1732), Sipaliwini Distr[ict], Thibiti area, Kabo Creek, partly swampy, primary forest on hilly slopes, ca. 2km from river, 29.v.1989 (J. Beerlink) (Schouten Coll.).
COI barcode sequence of paratype BC MTD 01839 (654 bp): ACATTATATTTTATCTTCGGAATTTGAGCAGGAATAGTTGGAACATCCCTAAGACTTTTAATTCGAGCAGAATTAGGTAATCCAGGTTCTCTTATTGGTGACGACCAAATTTATAATACTATTGTTACTGCTCATGCATTTATTATAATTTTTTTTATAGTTATGCCAATTATAATTGGAGGATTCGGTAATTGATTAGTTCCATTAATATTGGGAGCACCAGATATAGCATTCCCACGAATAAATAATATAAGATTTTGATTACTCCCCCCCTCTTTAATCCTATTAATTTCTAGAAGAGTTGTAGAAAATGGAGCTGGAACAGGATGAACAGTTTACCCCCCACTTTCATCAAATATTGCTCATAGTGGTAGATCTGTAGATTTAGCAATTTTTTCTCTACACTTAGCAGGAATTTCATCAATCTTAGGAGCTATTAATTTTATTACAACAATTCTTAATATACGAATTAATGGTTTATCTTTCGATCAAATACCTTTATTTGTTTGATCTGTAGGAATTACAGCTTTACTTCTTCTCTTATCCTTACCCGTATTAGCTGGTGCTATTACTATACTTTTAACTGATCGAAATTTAAATACATCTTTTTTTGATCCTGCTGGAGGAGGAGATCCTATCCTTTACCAACACTTA
On the forewing (Fig. 1), the seven, thin, marginal dark brown dashes with the most tornal two shaped like spots will separate this species from the others. In male genitalia (Fig. 11), the strongly sclerotized double costal arm of the valva with the ventral arm tubular is a distinctive character. In female genitalia, the best diagnostic character is the sclerotized projection latero-ventrally on sternite VIII (Fig. 34).
Male (n = 17) (Fig. 1): Antenna brown with light ochreous scales; with patch of dark brown scales at base. Maxillary palpus ringed with brown at base and half of length, white tipped. Labial palpus: 1.4–1.6 mm long; ochreous, slightly lighter basally, ringed with dark brown at 2/3, white tipped. Thorax slightly ochreous at collar. Foreleg coxa white; femur ochreous, dark brown dorsally; tibia and tarsomeres ochreous, distally ringed with brown. Midleg and hindleg light ochreous; tibia-femur joint brown on midleg; tarsomeres II–V brown to dark brown on upperside, with white ringed tips. Abdomen dull white to greyish brown. Forewing length: 9.0–10.5 mm; snow white, with yellow ochreous to brown costal margin, partially disrupted when meeting transverse lines; median line yellow ochreous; subterminal line yellow ochreous to brown, forming small triangular spot on costal margin; subapical triangle on costal margin ochreous; outer margin slightly ochreous with five dark brown dashes regularly spaced or sometimes forming faintly continuous line, and one cubital and one anal spots, with cubital spot slightly displaced toward base; fringes brass colored; underside dull white to light ochreous along costal margin, with marginal dashes pronounced. Hindwing snow white, veins slightly ochreous, with shiny aspect; marginal line thin, brown, pronounced up to CuA1, then shiny white; fringes white; underside white, with same margin as on recto.
Tympanal organs (n = 8): Tympanic pockets extending slightly beyond transverse ridge, rounded. Tympanic drum elongate, more or less oval, postero-laterally extended beyond transverse ridge.
Male genitalia (n = 8) (Figs 11, 12): Uncus slightly down-curved, about 3/5 length of tegumen arms, with few setae laterally; tip pointed; uncus arms not separated at base, forming low bump medio-ventrally. Gnathos arms joining at half their length; distal half with short, rounded, dorsal projection at base; directed upward subapically at about 50° angle; slightly shorter than uncus and thinner. Tegumen pedunculi progressively widening toward uncus; dorsal connection of tegumen about 1/3 length of pedunculi; ventral margin straight; dorsal margin slightly convex, bare. Cucculus moderately wide, narrowing in distal 1/4; costal arm of valva double, bare, about as long as cucculus, joined to cucculus until 3/5 of its length; ventral arm thin, tubular, strongly sclerotized, slightly curved inward, apex directed upward, narrowed, pointed; dorsal arm broader, slightly shorter than ventral arm, straight, apically rounded, less thickly sclerotized than ventral arm. Vinculum enlarging latero-dorsally, ventrally narrow; saccus short, rounded. Juxta triangular, apically broadly rounded, slightly curved downward, basally projected into two large lateral lobes. Phallus almost straight, with slightly upturned sclerotized apex; vesica covered with microspicules barely visible, with one large, curved, pointed cornutus.
Female (n = 10): Labial palpi: 1.1–1.4 mm long. Forewing length: 11–15 mm; frenulum triple.
Female genitalia (n = 6) (Fig. 34): Papillae anales slightly projected ventrally and dorsally, dorsally forming prominent sclerotized rounded bulge. Posterior apophyses widened basally, 0.35–0.5 × length of papillae anales. Segment VIII circular in cross section, enlarging progressively toward papillae. Tergite VIII narrow, about 2/5 length of sternite VIII, with short setae along posterior edge. Anterior apophyses wide at base, about 0.1 × length of papillae. Sterigma with thin slightly sclerotized membrane covered with minute spicules dorsad of ostium bursae, with posterior margin slightly indented; with sclerotized projection laterad from sternite VIII antero-ventrally, with tip bifid, longer part directed downward, shorter part lateral, curved posterad. Basal part of ductus bursae ventrally sclerotized, looping and narrow, progressively widening toward corpus bursae. Corpus bursae poorly differenciated from ductus, twice as long as wide, without signum.
The species occurs in lowlands in the three Guianas and Brazil (Fig. 45).
Bijuga comes from the Latin bijugus, a, um which means “yoked together, double”, in reference to the bifid costal arm of the male genitalia.
In some paratypes from French Guiana the collecting data mention a “pk” (=”point kilométrique”). This kilometric marker refers to the distance of the collecting spot on the forest road to the nearest main road. CIRAD (Centre de coopération internationale en recherche agronomique pour le développement) refers to the name of the research institution leading agronomical research on the Combi site. the Combi site. When S. Bleszynski looked into Catharylla, he gave the manuscript name Catharylla ramona to this species, but never published it. The comparison of the tip of the tubular costal arm of the male genitalia and the female lateral projections of sternite VIII shows rather nicely that the male hooks the female genitalia during the mating process. The specimen collected in Parque Nacional do Jaú, Brazil, shows a divergence in COI barcode sequence of 5.05% with that of Roura, French Guiana. In morphology we find no significant difference corroborating this divergence. The relationships of this species to the others remain uncertain in our phylogenetic analyses.
Diagnosis. The synapomorphies of the group are the quadrangular valva with a truncated apex and the hook-shaped gnathos in the male genitalia. The chelicerata species group can be separated from the other Catharylla species based on additional diagnostic characters. Externally, the forewing has a clear, dark brown costal band, and its length is usually over 14 mm. In male genitalia, the apex of the uncus is regularly rounded with a short, narrow projection medially and the vesica shows one large, curved, pointed cornutus, preceded by a string of 13–14 smaller cornuti increasing in size toward apex. In female genitalia, the ductus bursae shows a pronounced, tongue-shaped projection postero-ventrally.
Notes. This group includes two species. The phylogenetic analyses restricted to the nuclear genes and the combined Bayesian analysis place the group as sister-group of the mayrabonillae species group (Fig. 42).
http://zoobank.org/10B2350D-F0E5-4E09-BDE8-CE7D3F58A33F
http://species-id.net/wiki/Catharylla_chelicerata
Figs 2, 9, 13, 14, 35, 43Holotype. ♂, with labels as follows: “/600/ Parcelles CIRAD de Combi, | plantations expérimentales pk 1, 8 | 5°18'N, 52°55'30"W | 3.XII.2010 | B[ernard]. Hermier | [piège lumineux]”; 1 ♂, “Hermier | n° 23939”; “MHNG | ENTO ♂ | 00007213”; “Don de Bernard | Hermier | MHNG 2013”; “HOLOTYPE | Catharylla | chelicerata | T. Léger & B. Landry” [red label]. Deposited in MHNG.
Paratypes. 20 ♂, 4 ♀. BRAZIL: 1 ♂ (genitalia on slide BL 1714), Amazonas, Rio Negro, Mirapinima, 8.iv.1972 (E. G., I. & E. A. Munroe) (CNC); 9 ♂, 1 ♀, Reserva Ducke, km 26 Manaus–Itacoatiara Highway, 15.iv.1972 (1 ♂), 18.iv.1972 (1 ♂, with genitalia on slide BL 1721), 21.iv.1972 (2 ♂, one with genitalia on slide BL 1709), 16.v.1972 (2 ♂), 17.v.1972 (2 ♂, one with genitalia on slide BL 1738), 18.v.1972 (1 ♂, 1 ♀ with genitalia on slide BL 1711) (E.G., I. and E.A. Munroe) (CNC). FRENCH GUIANA: 5 ♂, 1 ♀, 36km SE, Roura (Camp Patawa), 21.xi.2007 (1 ♂, genitalia on slide MHNG ENTO 6239), 29–30.xi.2007 (3 ♂, 1 ♀, one ♂ with wings on slide MHNG ENTO 6272, 2 ♂ used for DNA sequencing and barcoding, one with labels LEP 963, BC MTD 01703, genitalia on slide TL 1, one with labels LEP 964, BC MTD 01704, genitalia on slide TL 2, ♀ with genitalia on slide MHNG ENTO 6240), 30.xi.2007 (1 ♂) (MHNG); 1 ♀ (abdomen used for DNA sequencing LEP 1290, genitalia on slide BL 1750) with same data as holotype; 1 ♂, same data as holotype except 2.ix.2011 (Hermier n° 24755); 1 ♀, same data as holotype except /604/ and 4.iii.2011 (Hermier n° 24344); 2 ♂, Beauséjour, N[ationale] 1 pk 28.5, 4°42'30"N, 52°23'30"W, 3.vi.2011, piège lumineux (Hermier n° 24545 & 24546) (B. Hermier) (MHNG); 1 ♂, Route d’Apatou pk 25.5 spk 2+4.4, 1.x.2011, piège lumineux (B. Hermier) (Hermier n° 24956) (MHNG); 1 ♂ (genitalia on slide BL 1749), R[ou]te forestière de Saut Léodate pk 4.5, 4°55'N, 52°33'W, 31.x.1995 piège lumineux (B. Hermier) (Hermier n° 8457) (MHNG).
Other specimens. 1 ♀ (genitalia on slide GS-5949-SB), Nova Olinda, Rio Purus, v.1922 (S. M. Klages) (CMNH); 1 ♀ (genitalia on slide Pyralidae Brit. Mus. Slide N° 17693), Teffé [sic], vi.1906 (W. Hoffmanns) (BMNH).
COI barcode sequence of paratype BC MTD 01703 (654 bp): ACTTTATATTTTATCTTTGGAATTTGAGCAGGAATAATTGGAACATCCTTAAGACTACTAATTCGAGCAGAATTAGGTAATCCTGGATCTCTTATCGGGGATGACCAAATTTATAACACTATTGTTACTGCTCATGCATTTGTAATAATCTTTTTTATAGTTATACCAATTATAATTGGTGGATTTGGAAACTGATTAGTACCTTTAATGCTAGGGGCACCAGATATAGCATTCCCTCGTATAAATAATATAAGATTTTGACTTCTTCCCCCCTCTTTAACCCTATTAATTTCAAGTAGAATTGTAGAAAATGGGGCAGGAACAGGATGAACCGTTTATCCACCTTTATCATCTAATATTGCCCATGGAGGCAGATCAGTAGATCTGGCAATTTTTTCACTACATTTAGCTGGAATTTCATCAATTTTAGGGGCAATTAATTTTATTACAACAATTATTAATATACGAATTAATAATCTTTCATTTGATCAAATACCCCTATTTGTTTGATCAGTAGGTATTACAGCATTACTATTACTTCTATCTTTACCAGTATTGGCGGGAGCTATTACCATACTTCTAACTGACCGAAATCTCAATACTTCCTTTTTTGATCCAGCAGGGGGGGGAGACCCTATTTTATATCAACACCTA
From Catharylla gigantea, Catharylla chelicerata differs in having the male costal arm hook shaped, longer, and thinner than in Catharylla gigantea, and the juxta is strongly downcurved, apically conical whereas it is long, almost straight, without apical conical projection downward in Catharylla gigantea. In female genitalia the sterigma forms a strongly sclerotized symmetrical structure made of two asymmetrical bell-shaped cavities, opened anterad in Catharylla chelicerata whereas it forms a pair of shallow pockets opened posterad in Catharylla gigantea.
Male (n = 21) (Figs 2, 9): Head with ochreous to brown chaetosemata. Antenna greyish brown with light brown scales, with patch of brown scales at base. Maxillary palpus ochreous to dark brown, lightly ringed with dark brown at 2/3, white tipped. Labial palpus: 1.3–2.0 mm long; ochreous, basally white, tip of segment II light greyish-brown; white tipped. Thorax with dark brown patch at collar. Foreleg coxa white; femur white, ashen brown dorsally, tibia and tarsomeres ochreous, distally ringed with dark brown. Midleg femur white, tibia ashen brown basally, tarsomeres ochreous, brown to ashen brown on upperside, with white ringed tips. Hindleg white, tarsomere I ochreous; II–V brown on upperside, with white ringed tips. Abdomen dull white to light ochreous. Forewing length: 10.5–15.0 mm; costal band wide, brown from base to apex; median and subterminal transverse lines faded brown, sometimes completely faded; dark brown spots on apical margin forming more or less continuous line; fringes brass colored; underside white with costal margin brown; outer margin with somewhat triangular spots. Hindwing snow white, with marginal spots between veins; fringes white; underside silvery white with marginal spots pronounced.
Tympanal organs (n = 9): Transverse ridge almost straight medially. Tympanic pockets conical, extending slightly beyond transverse ridge. Tympanic bridge lightly sclerotized, dorsal base of praecinctorium sclerotized. Tympanic drums elongate, bean shaped, posteriorly reaching transverse ridge or slightly beyond.
Male genitalia (n = 9) (Figs 13, 14): Uncus straight, of about 4/5 length of tegumen arms, dorso-ventrally compressed, with setae dorsally and laterally; apex truncated, slightly rounded, tip with short projection pointing posterad, ventrally convex, sometimes with median bump. Gnathos arms joining at 1/5 of length, regularly hook shaped, forming angle of about 100° with axis of basal arms, about 1/4 longer than uncus. Tegumen arms narrow at base, enlarging progressively toward dorsum to 2 × basal width, projected dorsally with bump at connection, connecting at distal 1/6. Cucculus densely setose, slightly directed upward on distal 1/3, apically truncated; basal 2/3 of costa of valva dorso-ventrally and laterally widened; costal arm hook-shaped, strongly sclerotized, directed upward at about 45° from costal arm base. Juxta triangular, curved downward, tip rounded and sac-like, basal lateral lobes curved ventrally. Saccus short, curved upward medially. Phallus narrow, S-shaped; vesica covered with tiny spicules, with one large, curved, pointed cornutus apically, preceded by string of 13–14 smaller cornuti increasing in size toward apex.
Female (n = 4): Labial palpi: 1.8–2.2 mm long. Forewing: 15–19.5 mm. Frenulum quadruple.
Female genitalia (n = 3) (Fig. 35): Papillae anales dorsally strongly produced posterad, with rounded bulge dorso-apically; ventrally slightly produced. Posterior apophyses 0.3–0.45 × length of papillae. Tergite VIII about half of length of sternite VIII. Anterior apophyses about 0.1 × length of papillae anales, slightly wider and rounded apically. Sternite VIII narrowing ventrally, densely covered with spinules, slightly connected medially at lamella antevaginalis; lamella antevaginalis slightly projected downward. Sterigma forming strongly sclerotized ventro-laterally symmetrical structure made of two asymmetrical bell-shaped cavities in ventral view, opened anterad, with dorsal lobe longer, expanding upward, slightly indented along latero-anterior margin; covered with minute punctuation. Ventro-basal section of ductus bursae tongue shaped, strongly sclerotized; ductus bursae long, ventrally sclerotized, widened and looped in basal half; enlarging progressively into corpus bursae. Corpus bursae egg-shaped with one signum.
The species was found in French Guiana and Brazil (Amazonas) (Fig. 43).
“Chelicerata” refers to the shape of the costal arms of the male valva, which look like mygalomorph chelicerae.
Two females included here have been named Catharylla robustella (genitalia on slide GS-5949-SB, CMNH) and Catharylla tenellina (genitalia on slide Pyralidae Brit. Mus. Slide N°17693, BMNH) by S. Bleszynski, as indicated on labels, but these names were never published. These two specimens are probably Catharylla chelicerata, but the bad genitalia preparations do not allow to see details, and therefore they are not included as paratypes.
http://zoobank.org/06B7837B-A5DE-4347-824E-B74127F028E2
http://species-id.net/wiki/Catharylla_gigantea
Figs 3, 15, 16, 36, 43Holotype. ♂, with labels as follows: “Brazil: Amazonas, Manaus, | Reserva Ducke, AM-010, k[ilo]m[eter]. 26 | 2°55'S, 59°59'W, Dec[ember].13, 1993 | J. Bolling Sullivan & | Roger W. Hutchings | U[ltra]V[iolet] Light (Plateau Hut)”; “HOLOTYPE | Catharylla gigantea | T. Léger & B. Landry” [red label]; “BL 1747 ♂” [light green label]. Deposited in USNM.
Paratypes. 5♂, 2 ♀. BRAZIL: 1 ♂, Amazonas, Reserva Ducke, km. 26, Manaus–Itacoatiara Highway, 15.v.1972 (E. G., I. and E. A. Munroe) (CNC). FRENCH GUIANA: 1 ♂, 1 ♀ (genitalia respectively on Pyralidae Brit. Mus. slides N° 11224 and 11342), Saint-Jean-du-Maroni (E. Le Moult) (BMNH); 1 ♂ (genitalia on slide GS-6694-SB), Oyapok [sic] River, Pied Saut, iii.1918 (S. M. Klages) (CMNH). GUYANA: 2 ♂, 1 ♀ (1 ♂ with genitalia on slide BL 1716, ♀ with genitalia on Pyralidae Brit. Mus. Slide N° 19017), Potaro, ii.1908 (2 ♂), v.1908 (1 ♀) (S. M. Klages) (BMNH).
From Catharylla chelicerata, Catharylla gigantea differs in having the male costal arm shorter, basally wide and tooth shaped while it is long, narrow throughout and hook shaped in Catharylla chelicerata. The juxta is long, tongue shaped, almost straight, and apically rounded, whereas it is downcurved and apically conical in Catharylla chelicerata. In female genitalia, the sterigma forms a pair of shallow pockets opened posterad whereas in Catharylla chelicerata the sterigma forms a strongly sclerotized symmetrical structure made of two asymmetrical bell-shaped cavities opened anterad.
Male (n = 6) (Fig. 3): Head with ochreous chaetosemata. Antenna brown with light brown scales, with patch of dark brown scales at base. Maxillary palpus brown with dark brown spot at half of length, white tipped. Labial palpus: 1.6–2.4 mm long; ochreous to brown ochreous, basally white, with patch of dark brown scales at half of length, white tipped. Thorax with some brown at collar. Foreleg coxa white, femur white, ashen brown dorsally; tibia and tarsomeres brown-ochreous, distally ringed with dark brown. Midleg white with tibia-femur joint and base of tibia ashen; tarsomeres ochreous to brown ochreous with upperside brown to dark brown, white tipped. Hindleg white with tarsomeres II–V ochreous to brown ochreous, upperside brown, with white tips. Forewing length: 13.5–14.5 mm; snow white with wide brown to dark brown costal line from base to apex; median and subterminal transverse lines faded brown; dark brown spots on termen forming more or less continuous line; fringes brass colored; underside white, with costal margin brown ochreous, outer margin with subtriangular spots. Hindwing snow white; marginal spots dark brown between R5, M1, M2, M3, and CuA1; fringe white; underside snow white, with same spots as on upperside.
Tympanal organs (n = 5): Transverse ridge medially convex. Tympanic pockets extending slightly beyond transverse ridge, rounded. Tympanic bridge lightly sclerotized, dorsal base of praecinctorium sclerotized. Tympanic drums elongate, bean shaped.
Male genitalia (n = 5) (Figs 15, 16): Uncus straight, about 3/4 length of tegumen arms, dorso-ventrally flattened, dorsally convex, ventral margin convex in basal half, concave in distal half; basally and laterally setose; apex slightly rounded, medially with short projection pointing postero-ventrally. Gnathos arms joining at 1/5, about 1/4 longer than uncus, regularly curved. Tegumen arms narrow at base, widening regularly to reach 1.5 × basal width dorsally, with connection at distal 1/6. Cucculus densely setose, broad at base, slightly widening and truncate at apex; costal arm of valva basally wide, short, tooth shaped, slightly curved inward. Juxta long, tongue shaped, almost straight, apically rounded, with basal lateral lobes curved ventrally. Saccus short, curved upward. Phallus narrow, S-shaped; vesica covered with tiny spicules, with string of 14 small cornuti increasing in size toward apex, with apical cornutus up to 5 × length of previous one.
Female (n = 2): Labial palpi: 2.5–3.1 mm long. Forewing length: 17.5–22 mm; frenulum triple.
Female genitalia (n = 2) (Fig. 36): Papillae anales ventrally projected. Posterior apophyses about 0.35 × length of papillae anales, narrow. Segment VIII narrowing ventrally, densely covered with spinules; narrow connection at lamella antevaginalis; lamella antevaginalis slightly projected downward. Sterigma forming pair of shallow pockets opened posterad at base of segment VIII. Anterior apophyses about 0.08 × length of papillae anales, of medium width, basally wide. Ductus bursae wide, as long as twice segment VIII, regularly enlarging into corpus bursae. Corpus bursae with one rounded signum.
Catharylla gigantea has been found in French Guiana, Guyana, and Brazil (Amazonas) (Fig. 43).
The name comes from the Latin giganteus, a, um meaning very large.
The name was given to the species on manuscript labels by S. Blezynski, probably in reference to the large size of the female.
Diagnosis. The synapomorphies of the group are the dorsal furrow on the uncus, the uncus apex slightly bifid, the presence of a transtilla in male genitalia, and the absence of a ventral connection of sternite VIII in female genitalia. The tenellus species group can also be separated from the other Catharylla species based on the following additional diagnostic characters: the hindwings are creamy-white, and in female genitalia, the papillae anales are not produced.
Notes. This group includes three species, including two new ones. Catharylla serrabonita and Catharylla tenellus form a monophyletic group within the tenellus species group (Fig. 42). Relationships to other species groups are unknown.
http://species-id.net/wiki/Catharylla_tenellus
Figs 4, 17, 18, 27–29, 37, 45Holotype. ♀, “Type” [red ringed]; “Catharylla | tenella Z[eller]. | Mon[ograph]. p[age]. 50 Am. anftr.” [not clearly readable]; “Zell[er]. Coll[ection]. | 1884”; “♀ | Pyralidae | Brit[ish]. Mus[eum]. | Slide N° | 1094”. Deposited in BMNH.
Other specimens examined. 20 ♂, 7 ♀. BRAZIL: 3 ♂ (1 ♂ with leg used for DNA barcoding BC MTD 01842, 1 ♂ with genitalia on slide BL 1757), São Paulo, Ubatuba, Picinguaba, 23°22'S, 44°50'W, 2–20m, 22–24.ix.2001 (V. O. Becker n°132820) (Becker Coll.); 2 ♂ with same data except 10–12.xi.2001 (V. O. Becker n°133712) (Becker Coll.); 2 ♂, 1 ♀ (1 ♂ with genitalia on slide BL 1741, ♀ genitalia on slide BL 1742), São Paulo, Bertioga, 5 m, 5.xi.1995 (V. O. Becker n°99090) (USNM); 1 ♂ (genitalia on slide BL 1778) with same data (Becker Coll.); 1 ♂ (genitalia on slide BL 1775) with same data except 15–17.v.1996 (V. O. Becker n° 99386) (Becker Coll.); 1 ♂ with same data except 7–9.x.1996 (V. O. Becker Coll. n°99757) (Becker Coll.); 1 ♂ (used for DNA sequencing and barcoding LEP 975, BC MTD 01711, genitalia on slide TL 13), São Paulo, São Luiz do Paraitinga, 23°20'S, 45°06'W, 900 m, 13–20.iii.2001 (V. O. Becker n°132356) (Becker Coll.); 1 ♂ (genitalia on slide Pyralidae Brit. Mus. Slide N° 19065), São Paulo, 700 m (E. D. Jones) (BMNH); 1 ♂ (genitalia on slide BL 1746), Minas Gerais, Caraça, 1300 m, 1–2.iv.1992 (V. O. Becker n°85081) (USNM); 1 ♀ (genitalia on slide BL 1754) with same data (Becker Coll.); 1 ♂ (genitalia on slide BL 1755) with same data except 25.x.1994 (V. O. Becker & K. S. Sattler, n°93291) (Becker Coll.); 1 ♂, 1 ♀ (♂ used for DNA sequencing and barcoding LEP 973, BC MTD 01709, genitalia on slide TL 11, ♀ genitalia on slide BL 1758), Bahia, Porto Seguro, A. d’Ajuda, 16°27'S, 39°03'W, 20 m, 12.vii.2009 (V. O. Becker n°144140) (Becker Coll.); 2 ♂ (used for DNA sequencing and barcoding, one with labels LEP 972, BC MTD 01888, genitalia on slide TL 10, other with labels LEP 974, BC MTD 01710, genitalia on slide TL 12) with same data except 15.viii.2008 (V. O. Becker n°140808) (Becker Coll.); 1 ♂ (used for DNA sequencing and barcoding LEP 971, BC MTD 01708, genitalia on slide TL 9) with same data except 1–3.v.2009 (V. O. Becker n°142784) (Becker Coll.); 1 ♂, Paranà, Castro (USNM); 1 ♀ (genitalia on slide BL 1733), Rio de Janeiro, xi[day and year data missing] (H. H. Smith) (CMNH); 1 ♀ (genitalia on Pyralidae Brit. Mus. Slide N°19069), Rio de Janeiro, Corcovado, 457 m, 26.xii.1958 (E. P. Wiltshire) (BMNH). No locality data: 1 ♂, 1 ♀ (♂ genitalia on slide Nat[ur]. hist[orisches]. Mus[eum]. Wien Gen[italia]. Praep[aration]. MV 9022a, ♀ genitalia on slide Nat. hist. Mus. Wien Gen. Praep. MV 9022b); 1 ♀ (genitalia on slide Nat. hist. Mus. Wien Gen. Praep. MV 9022c), 1869 (NMW).
COI barcode sequence of specimen BC MTD 1710 (654 bp): ACTCTATATTTTATCTTTGGAATTTGATCAGGAATAATTGGAACATCTTTAAGATTATTAATTCGAGCAGAATTAGGGAATCCTGGATCTCTAATTGGAGATGATCAAATTTATAACACTATTGTAACAGCCCATGCATTTATTATAATTTTTTTTATGGTTATACCAATTATAATTGGTGGATTTGGAAATTGATTGGTTCCATTAATATTAGGAGCCCCAGATATAGCTTTCCCCCGAATAAATAACATAAGATTTTGGTTATTACCCCCTTCCTTAACTCTTTTAATTTCTAGAAGAATTGTAGAAAATGGAGCTGGAACAGGATGAACGGTCTACCCCCCCCTTTCATCTAATATTGCCCATAGTGGAAGATCTGTAGATTTAGCAATCTTTTCTCTTCATTTAGCTGGAATTTCATCAATTTTAGGAGCTATTAATTTTATTACAACAATTATTAATATACGAATTAGTAATTTATCTTTTGATCAAATACCTTTATTTGTTTGATCAGTCGGTATTACAGCTTTACTTCTTCTTCTATCTTTACCTGTATTAGCAGGAGCTATTACTATACTTTTAACTGATCGAAATTTAAATACATCTTTTTTTGATCCTGCAGGAGGAGGAGATCCTATCTTATATCAACATTTA
From Catharylla serrabonita and Catharylla coronata, Catharylla tenellus can be separated by the median transverse line, which is faintly convex towards costa, whereas it is more strongly convex in Catharylla coronata and Catharylla serrabonita. The male genitalia provide the best diagnostic characters. The most obvious refers to the transtilla, which forms a pair of short, narrow sclerotized arms with pointed tips, projecting posterad, with, in between, a pair of brushes directed medio-ventrally, whereas it forms a pair of arms pointing posterad with a string of spines ventrally in Catharylla serrabonita and Catharylla coronata. In female genitalia, the anterior angle of sternite VIII is directed downward into a more or less rounded projection covered with short spinules of same length, whereas it is projected anterad in Catharylla serrabonita, and it is not projected in Catharylla coronata.
Male (n = 20) (Fig. 4): Head white with ochreous chaetosemata. Antenna brown with ochreous scales. Maxillary palpi ochreous to brown, white tipped. Labial palpi: 1.1–1.4 mm long; basally white, medially brown ochreous with white tips. Thorax slightly ochreous at collar. Foreleg coxa white; femur ochreous, dorsally dark brown; tibia and tarsomeres ochreous, distally ringed with dark brown. Midleg light ochreous with tibia-femur joint brown; tarsomeres II–V dark brown on upperside, with white ringed tips. Hindleg white; tarsomeres as midleg. Forewing length: 10.5–12 mm; snow white; costal line ochreous, lightly pronounced from base to apex; median and subterminal transverse lines ochreous, median transverse line faintly convex towards costa; outer margin ochreous with 7 dark brown spots often triangular, strongly pronounced; fringes brass colored; underside ochreous with costal margin pronounced in basal half and marginal spots pronounced. Hindwing cream-coloured; outer margin with small ochreous brown spots forming more or less continuous line between Sc+R1, Rs, M1, M2, M3, CuA1 and CuA2; underside light ochreous, with marginal spots pronounced; fringes white.
Tympanal organs (n = 13): Transverse ridge more or less regularly rounded, medially more straight. Tympanic pocket extending slightly beyond transverse ridge. Tympanic drum ovoid, posteriorly not extended beyond transverse ridge. Tympanic bridge faintly sclerotized.
Male genitalia (n = 13) (Figs 17, 18, 27–29): Uncus about half of length of tegumen arms, broadly downcurved; uncus arms connecting at base, with ventro-lateral tuft of setae; dorsal furrow with few short setae on each side, tip rounded, slightly indented medially, slightly convex in apical 1/3. Gnathos short and thick, arms joining at half of length, laterally compressed toward apex, almost straight, slightly downcurved, with apex pointing upward. Tegumen arms slightly enlarging toward apex; connecting at 3/4, slightly projected dorsally at connection. Costa of valva at 2/3 with arm directed postero-dorsally with rounded tip, without basal projection on dorsal edge or narrow with low basal projection, or wide with basal projection; cucculus upcurved in apical 1/4. Juxta ogival, posteriorly directed downward, with pair of thumb-like lobes ventrally reaching about 2/3 of length. Transtilla strongly sclerotized, with pair of narrow arms on each side of middle, pointing posterad, with 3 spines apically, also with pair of shorter brushes of tightly set spines medio-ventrally; in some specimens triangular median projection dorsally with few tiny setae. Phallus S-shaped, subapically with dorsal bump, apically lightly sclerotized, truncated, covered with microspicules barely visible; vesica without cornuti.
Female (n = 7): Labial palpi: 1.6–2 mm; forewing length 12–16 mm; frenulum triple.
Female genitalia (n = 7) (Fig. 37): Papillae anales straight, thick. Posterior apophyses narrow 0.3–0.45 × length of papillae anales, slightly wider basally. Intersegmental membrane between segments VIII and IX covered with microspines. Tergite VIII laterally about 2X longer than dorsally; sternite VIII formed by 2 lobes regularly narrowing downward in more or less triangular shape, not connected ventrally, densely covered with short spinules of same length; ventro-anterior angle of sternite VIII slightly projected downward, rounded, covered with short spinules; anterior margin of segment VIII latero-dorsally strongly sclerotized, thicker; posterior margin with dorsal line of setae. Anterior apophyses 0.02–0.05 × length of papillae anales. Sterigma membranous, covered with spinules. Ductus bursae about 3× length of corpus bursae, narrow. Corpus bursae elongate, sometimes with one signum.
The species is known from Brazil in the Atlantic Forest (Bahia, Minas Gerais, Paraná, Rio de Janeiro, Saõ Paulo) (Fig. 45).
Notes. The species was described from “one female collected in Brazil, near Rio de Janeiro”. Hence, the lectotype designated by a label by S. Bleszynski is not warranted. This designation is presumably based on the fact that
Specimens from Porto Seguro, Brazil show a divergence of 3.34% in COI barcode sequences with the specimen from Ubatuba, Brazil. In morphology, differences in male genitalia are also observed: in the specimens from Bertioga, Caraça, São Paulo and Ubatuba the costal arm of the valva is wide and 1/3 of the length of the cucullus, almost reaching its tip, and the dorsal edge at base is slightly produced (Fig. 27). In the specimens from Porto Seguro, the costal arm is about 1/5 the length of the cucullus, relatively narrow, and the dorsal edge is slightly produced at base (Fig. 29). Another form, from Caraça, Minas Gerais (Fig. 28) was also found. No differences were found in the female genitalia. We feel that specimens and data are currently lacking to conclude that possibly more than one taxon should be recognized under Catharylla tenellus, or that there is indeed a deep divergence in the COI barcode between populations of this species.
http://zoobank.org/7E0EB0BE-44C4-42EC-9F4D-2C923E9299E6
http://species-id.net/wiki/Catharylla_coronata
Figs 5, 19, 20, 38, 45Holotype. ♂, with labels as follows: “Col. BECKER | 81552”; “BRASIL:ES | Linhares, 40m | 20-29.ii.1992 | V.O.Becker Col”; “HOLOTYPE | Catharylla | coronata | Léger & Landry” [red label]. Deposited in Becker Collection.
Paratypes. 21 ♂, 4 ♀. BRAZIL: 5 ♂ with same data as holotype (1 used for DNA barcoding BC MTD 01890, 1 with genitalia on slide BL 1743); 2 ♂ with same data as holotype (1 used for DNA barcoding BC MTD 01891) except 05–09.iv.1992 (V. O. Becker n°82486); 6 ♂, 1 ♀ (1 ♂ with genitalia on slide BL 1730, ♀ with genitalia on slide BL 1731), Paranà, Rio Negro, 900 m, 8.ii.1973 (2 ♂), 10.ii.1973 (1 ♂), 11.ii.1973 (3 ♂), 13.ii.1973 (1 ♀) (A. & J. Razowski) (ISZP); 2 ♂, 2 ♀, Paranà, Curitiba, 920 m, 17.ii.1975 (1 ♂) (V. O. Becker n°10167), 20.ii.1975 (1 ♀, genitalia on slide BL 1756) (V. O. Becker n°10168), 12.iii.1975 (1 ♀, genitalia on slide BL 1753) (V. O. Becker n°10166), 10.x.1975 (1 ♂) (V. O. Becker n°4010) (Becker Coll.); 1 ♂ (genitalia on Pyralidae Brit. Mus. Slide No. 11357), Paranà, Castro, 950 m (E. D. Jones) (BMNH); 1 ♂, Paranà, Quatro Barras, 850 m, 27.ii.1970 (Laroca & Becker) (V. O. Becker n°15442) (Becker Coll.); 1 ♂ (genitalia on Pyralidae Brit. Mus. Slide No. 11337) Rio de Janeiro, Novo Friburgo (BMNH); 1 ♂ (genitalia on Pyralidae Brit. Mus. Slide. No. 19019) Sao Paulo, 700 m (E. D. Jones) (BMNH); 1 ♂, 1 ♀ (♂ with genitalia on slide BL 1774, ♀ with genitalia on slide BL 1736), Santa Catarina, Rio Vermelho, 968 m, 18.ii.1973 (♂), 28. ii. 1973 (♀) (A. & J. Razowski) (ISZP); 1 ♂, no locality data (V. O. Becker) (Becker Coll.).
COI barcode sequence of paratype BC MTD 01890 (654 bp): ACTTTATATTTTATTTTTGGAATTTGAGCAGGAATAGTAGGAACATCATTAAGATTATTAATTCGAGCTGAATTAGGTAATCCTGGATCTCTTATTGGAGATGATCAAATCTATAATACTATTGTAACCGCTCATGCATTTATTATAATTTTTTTTATAGTTATACCAATTATAATTGGTGGATTTGGAAATTGATTAGTTCCCTTAATATTAGGAGCACCAGATATAGCTTTTCCTCGAATAAATAACATAAGATTTTGATTATTACCCCCCTCTTTAACTCTTTTAATTTCAAGAAGAATTGTAGAAAATGGAGCTGGAACAGGATGAACAGTTTACCCCCCACTTTCATCTAATATTGCCCATAGTGGAAGATCCGTAGATTTAGCAATCTTTTCCCTTCATTTAGCTGGAATTTCTTCAATTTTAGGAGCAATTAATTTTATTACAACAATTATTAATATACGAATCAATAATCTTTCATTTGATCAAATACCTCTTTTTGTTTGATCAGTAGGAATTACAGCTTTACTTCTTCTTTTATCATTACCAGTATTAGCTGGAGCTATTACTATACTTTTAACTGATCGAAATTTAAATACATCTTTTTTTGATCCCGCAGGAGGAGGAGATCCTATTTTATATCAACATTTA
From Catharylla serrabonita and Catharylla tenellus, Catharylla coronata can be separated with characters of the male genitalia: the uncus is apically bifid and grooved on distal 1/5 in Catharylla coronata whereas it is only indented medially at apex in Catharylla serrabonita and Catharylla tenellus; the costal arm of the valva is short and the apex is curved inward in Catharylla coronata whereas the costal arm is longer and points postero-dorsally in the other two species; the transtilla forms a pair of sclerotized arms slightly bent inward distally, ventrally with a row of short spines increasing in size from base to apex whereas it forms a pair of short, narrow sclerotized arms with pointed tips, projecting posterad, and with a pair of brushes directed medio-ventrally in Catharylla tenellus and a pair of sclerotized arms strongly bent inward on distal 1/4 and with a string of long spines of same length medially along it in Catharylla serrabonita; the juxta is shorter than in Catharylla tenellus, and regularly narrowing toward apex whereas it is strongly narrowing on distal 1/4 in Catharylla serrabonita; the ventral projections of the juxta form a pair of shallow pockets whereas they are bell-shaped in Catharylla serrabonita and thumb-like in Catharylla tenellus; the vesica has a row of 6–7 cornuti in Catharylla coronata whereas it does not show any cornuti in Catharylla serrabonita and Catharylla tenellus. In the female genitalia of Catharylla coronata, the anterior angle of sternite VIII is not projected whereas it is rounded, projected anterad and covered with short spinules in Catharylla serrabonita, and projected downward in Catharylla tenellus. The anterior apophyses are quadrangular, anvil shaped whereas they are spine like in the other two species.
Male (n = 21) (Fig. 5): Head white with ochreous chaetosemata. Antenna brown, with whitish ochreous scales and patch of brown scales at base. Maxillary palpi light ochreous to ochreous, white tipped. Labial palpi: 1.6–1.85 mm long; light ochreous, white tipped. Thorax white, with ochreous patch at collar. Foreleg coxa white; femur white, dorsally dark brown; tibia and tarsomeres ochreous, distally ringed with brown; midleg and hindleg white to light ochreous, tarsomeres II–V ochreous, upperside brown, with white ringed tips. Forewing length: 10–13 mm; costal margin line thin, light ochreous, apically faded; median transverse line light ochreous, concave on costal half, more or less disrupted; subterminal transverse line ochreous, curving toward base on costal half; R5 vein faintly marked apically with ochreous; outer margin ochreous with 7 pronounced dark brown spots more or less triangular between veins, sometimes connecting; fringes brass colored; underside white ochreous to ochreous, costal margin basally brown; outer margin with pronounced spots. Hindwing white to creamy white, usually with marginal brown spots between Sc+R1, Rs, M1, M2, M3, CuA1 and CuA2, forming more or less continuous line; fringes white; underside light ochreous, with dark brown marginal spots pronounced.
Tympanal organs (n = 7): Transverse ridge more or less regularly rounded. Tympanic pocket extending faintly beyond transverse ridge, rounded. Tympanic drum glomerular, not reaching transverse ridge.
Male genitalia (n = 7) (Figs 19, 20): Uncus about 3/4 length of tegumen arms, downcurved; uncus arms basally with ventro-lateral tuft of setae; dorsal furrow pronounced medially with row of few setae on each side; thin, bifid on distal 1/5, slightly grooved, with apex slightly pointed; with shallow cavity ventro-apically. Gnathos arms connecting at 1/3 of length; shaft slightly downcurved, with apex pointing upward. Tegumen arms enlarging progressively toward uncus; tegumen connection about 1/3 arms length. Costa of valva basally narrow, with quadrangular projection, apically narrowing into arm pointing posterad with short tip curved inward; cucullus curved upward in distal 1/3, with apex rounded. Juxta triangular, regularly narrowing toward apex with shallow pockets projected ventro-laterally; with baso-lateral angles curved upward. Transtilla modified into two arms projecting posterad, slightly curved inward in distal 1/4, with longitudinal string of short spines ventrally at base, medially along arms, and at apex, increasing in size from base to apex in factor of about 1 to 4–5. Phallus almost straight, apex dorsally triangular; vesica basally covered with tiny spicules, microspicules barely visible all along vesica, also with row of 5–6 straight, short spine-like cornuti wider at their base.
Female (n = 4): Labial palpi: 1.6–2.2 mm long. Forewing length 14–16 mm. Frenulum triple.
Female genitalia (n = 4) (Fig. 38): Papillae anales straight, thick. Posterior apophyses 0.3–0.5 × length of papillae anales, wide at base, about half of length of papillae. Intersegmental membrane between segment VIII and IX covered with microspines. Sternite VIII laterally about 1/3 longer than tergite VIII. Sternite VIII formed by 2 lobes regularly narrowing downward into triangle, not connected ventrally, densely covered with spinules, with spinules longer ventrally. Anterior apophyses about 0.05 × length of papillae anales, quadrangular, anvil shaped. Anterior margin of sternite VIII latero-dorsally strongly sclerotized, thicker; posterior margin with dorsal line of setae. Sterigma membranous, covered with spinules. Ductus bursae regularly enlarging into corpus bursae, basally directed downward. Corpus bursae more or less rounded, faintly delimited from ductus bursae, with one oval signum.
The species occurs in Brazil in the following states: Bahia, Espirito Santo, Paranà, Rio de Janeiro, Santa Catarina, Saõ Paulo (Fig. 45).
The name comes from the latin coronatus, a, um: crowned, referring to the longitudinal string of short spines of the transtilla in the male genitalia.
Based on our combined phylogenetic analysis, Catharylla coronata is the sister species of the Catharylla tenellus + Catharylla serrabonita pair (Fig. 42).
http://zoobank.org/8B0F3E46-1CA6-47C3-A5AC-D9A9A01AAA29
http://species-id.net/wiki/Catharylla_serrabonita
Figs 6, 21–26, 39, 45, 46Holotype. ♂, with labels as follows: “BRASIL: BA, Camacan | Res[erva]. Serra Bonita | 15°23'S, - 39°33'W, | 800m, 06.iv.2011 | B. Landry, V. Becker”; “HOLOTYPE | Catharylla serrabonita | T. Léger & B. Landry” [red label]. Deposited in Becker Collection.
Paratypes. 21 ♂, 1 ♀. BRAZIL: 5 ♂ (1 used for DNA barcoding BC MTD 01843, 1 with genitalia on slide BL 1745), Espírito Santo, Linhares, 40m, 25–30.i.1998 (V. O. Becker n°113929) (Becker Coll., USNM); 2 ♂, 1 ♀ (♀ with genitalia on slide BL 1759) with same except 20–29.ii.1992 (V. O. Becker n°81552) (Becker Coll., USNM); 2 ♂ with same data as holotype except 05–09.iv.1992 (V. O. Becker n°82486) (USNM); 10 ♂ (1 in alcohol, thorax used for DNA sequencing LEP 979, genitalia on slide TL 7, wing on slide TL 8) Bahia, Camacan, Serra Bonita Reserve, 15°23'S, 39°33'W, 800 m, B. Landry, V. O. Becker, 1.iv.2011 (1 ♂), 2.iv.2011 (2 ♂), 3.iv.2011 (1 ♂, genitalia on slide BL 1776), 5.iv.2011 (3 ♂), 6.iv.2011 (1 ♂), 7.iv.2011 (1 ♂) (MHNG); 1 ♂ with same data except vii.2010 (V. O. Becker) (Becker Coll.); 1 ♂ (used for DNA sequencing and barcoding LEP 970, BC MTD 01887, genitalia on slide TL 6) Bahia, Porto Seguro, A. d’Ajuda, 16°27'S, 39°03'W, 20 m, 12.vii.2009 (V. O. Becker n°144140) (Becker Coll.).
COI barcode sequence of holotype LEP 979 (516 bp): TAGTTGGAACATCATTAAGACTATTAATTCGAGSAGAGTTAGGGAATCCTGGATCTCTTATTGGAGATGATCAAATTTATAATACTATTGKAACAGCTCATGSATTTATTATAATTTTTTTTATAGTTATACCAATTATAATTGGTGGATTTGGAAACTGACTAGTTCCATTAATATTAGGAGCCCCAGACATAGCTTTCCCCCGAATAAATAATATAAGATTTTGATTACTCCCCCCCTCTTTAACCCTTTTAATTTCCAGAAGAATTGTAGAGAATGGAGCTGGAACAGGATGAACGGTTTACCCCCCCCTTTCATCTAATATTGCTCATAGKGGAAGATCTGTAGATTTAGCAATTTTTTCTCTTCATTTAGSAGGAATTTCATCAATTTTAGGAGCAATTAATTTTATTACAACAATTATTAATATACGAATTAATAATTTATCTTTTGATCAAATACCGTTATTTGTCTGATCAGTTGGTATTACAGCTTTACTCCTTCTTTTATCTTTAC
From Catharylla coronata and Catharylla tenellus, Catharylla serrabonita can be separated by the zigzagging median transverse line with the short triangular dent at CuA2 and the pronounced creamy color of the hindwing. The male genitalia provide the best discriminant characters: in Catharylla serrabonita, the transtilla forms a pair of sclerotized arms bent inward in distal 1/4 and with a string of long spines of same length medially along it, whereas it forms a pair of short, narrow sclerotized arms with pointed tips projecting posterad, and with a pair of brushes directed medio-ventrally in Catharylla tenellus, and two sclerotized arms slightly bent inward distally, with a row of short spines increasing in size from base to apex in Catharylla coronata, and the juxta is apically narrow and pointed whereas it is triangular and regularly narrowed in Catharylla coronata and Catharylla tenellus. In female genitalia, the anterior angle of sternite VIII is projected anterad into a rounded protrusion covered with short spinules in Catharylla serrabonita, whereas it is projected downward in Catharylla tenellus and it is not projected in Catharylla coronata.
Male (n = 21) (Fig. 6): Head white with ochreous chaetosemata. Antenna brown with whitish-ochreous scales and patch of brown scales at base. Maxillary palpus light ochreous, with patches of dark brown scales at 1/3 and 2/3, white tipped. Labial palpus: 1.7–2.5 mm long; light ochreous, white tipped. Thorax white, with ochreous patch at collar. Foreleg coxa white; femur white, dorsally dark brown; tibia and tarsomeres ochreous, distally ringed with brown. Midleg and hindleg white to light ochreous; tarsomeres II–V ochreous, brown on upperside, with white ringed tips. Forewing length: 10–14 mm; costal line ochreous; median transverse line ochreous to brown, zigzagging with short brown pronounced spot at M1 and short triangular dent at CuA2; subterminal transverse line ochreous to brown, regularly curved up to CuA2, then curved again; R5 faintly marked apically with ochreous; outer margin ochreous with 7 more or less triangular and connected dark brown spots between veins; fringes brass colored; underside ochreous, outer margin with pronounced spots. Hindwing cream-coloured, usually with more or less connected marginal brown spots between Sc+R1, Rs, M1, M2, M3, CuA1 and CuA2; fringes white; underside light ochreous, with marginal spots pronounced.
Tympanal organs (n = 4): Transverse ridge more or less rounded, medially slightly flattened. Tympanic pocket extending faintly beyond transverse ridge, rounded. Tympanic drum glomerular, not reaching transverse ridge.
Male genitalia (n = 4) (Figs 21–26): Uncus about as long as tegumen arms, downcurved; uncus arms connecting basally, with ventro-lateral tuft of setae at base; dorsal furrow pronounced medially with row of few hairs on each side; apex rounded, slightly indented medially, slightly convex ventro-apically. Gnathos arms connecting at 1/3; main shaft slightly downcurved with apex pointing upward. Tegumen arms regularly enlarging toward apex, connection at about 4/5 length of arms. Costa with apically rounded arm pointing postero-dorsally; cucullus curved upward in distal 1/3, with apex rounded. Juxta triangular, narrowing in distal 1/4 with bell-shaped ventro-lateral projections, regularly curved with apex horizontally straightened; baso-lateral angles curved upward. Transtilla with two very large sclerotized arms projecting posterad, bent inward in apical 1/4, with longitudinal string of long spines medially. Phallus slightly S-shaped, with apex dorsally sclerotized; vesica covered with microspicules, without cornuti.
Female (n = 1): Labial palpi: 1.9 mm long. Forewing length: 14 mm. Frenulum triple.
Female genitalia (n = 1) (Fig. 39): Papillae anales straight, thick. Posterior apophyses 0.4 × length of papillae anales, narrow, wider at base. Intersegmental membrane between segment VIII and IX covered with microspines. Sternite VIII laterally about 5/3 length of tergite VIII; posterior margin of tergite VIII with line of setae; sternite VIII forming 2 triangular lobes regularly narrowing downward, not connected, densely covered with short spinules of same length; anterior angle of sternite VIII slightly projected anterad, rounded, covered with short spinules of same length. Anterior apophyses 0.03 × length of papillae anales. Sterigma membranous, covered with spinules. Ductus bursae about 3 × length of corpus bursae, narrow, basally directed downward and then bent upward. Corpus bursae elongate, ovoid, with one tiny signum.
The species occurs in Brazil (Bahia, Espirito Santo) (Figs 45 & 46).
The name comes from that of the Serra Bonita Reserve founded by Vitor O. Becker and Clemira de Souza. It is managed by Instituto Uiraçu in the State of Bahia, Brazil.
Serra Bonita Reserve is located in the Atlantic Forest, in a hilly region of cacao plantations and scattered forest. Adults came late to light, usually after 23:00. Our molecular analysis of the COI barcode sequences highlighted that specimens from Serra Bonita respectively show 3.24 and 2.21 % base differences with those of Porto Seguro and Linhares. This divergence is possibly associated with slight morphological differences in male genitalia as shown in Figs 23–26. No females were found at Serra Bonita.
Diagnosis. We have not recovered any obvious synapomorphy for this group. It can be separated from the other Catharylla species based on the shorter forewing length, usually between 7.5 and 9.0 mm (maximum 10.5 mm). In male genitalia, the tegumen connection is more than two times longer than the uncus, the uncus is beak-shaped, with the apex narrowing to a point, the gnathos is bent at an angle of about 90°. In female genitalia, the basal line along the anal papillae is ventrally expanded onto a triangle, and the sterigma forms a pair of sclerotized pockets on each side of the middle, covered with short spines or spicules. The sterigma does not bear tiny setae on the ventral membrane of segment VIII.
Notes. This group includes two species. The phylogenetic analyses restricted to the nuclear genes and the combined Bayesian analysis place the mayrabonillae group as sister to the chelicerata group (Fig. 42).
http://zoobank.org/5078E6D0-DDA4-4B9F-8089-F7FFECC725C6
http://species-id.net/wiki/Catharylla_mayrabonillae
Figs 7, 30, 31, 40, 44Holotype. ♂, with labels as follows: “Col. BECKER | 101668”; “ECUADOR: NAPO | Misahualli | 450m xii.1992 | V.O.Becker Col”; “HOLOTYPE | Catharylla | mayrabonillae | Léger & Landry” [red label]. Deposited in Becker Collection.
Paratypes. 16 ♂, 37 ♀. BRAZIL: 1 ♀ (genitalia on slide BL 1729), [Acre] Rio Branco, 1924 (Dengler) (SMNS); 2 ♀, Amazonas, Manaus, Reserva Ducke, AM-010, km 26, 2°55'S, 59°59'W, 15.xii.1993, U[ltra]V[iolet] Light (J. B. Sullivan & R. W. Hutchings) (USNM); 1 ♂ (genitalia on Pyralidae Brit. Mus. Slide No. 11341), Amazonas, Fonte Boa, ix.1906 (S. M. Klages) (BMNH); 1 ♀ (genitalia on slide BL 1713), Federal District, Estaçao Florestal, Cabeca do Vedao, 1100m, 18.x.1971 (E.G., I. & E.A. Munroe) (CNC); 2 ♂, Maranhão, Feira Nova, Faz[enda]. Retiro, 480m, 07°00'S, 46°26'W, 1–3.xii.2011 (V. O. Becker n°148263) (Becker Coll.); 2 ♀, Pará, Belém, 20m, i.1984 (V. O. Becker n°46981) (Becker Coll.); 1 ♀, Pará, Capitao Poco, 25–31.i.1984 (V. O. Becker n°97880) (Becker Coll.); 1 ♂, Rondonia, Cacaulãndia, 140m, xi.1991 (V. O. Becker n°79592) (Becker Coll.); 1 ♀, Rondonia, 62km S[outh] Ariquemes, Fazenda Rancho Grande, 165m, 10°32'S, 62°48'W, 18–26.iv.1991 (R. Leuschner) (USNM). COLOMBIA: 1 ♂, Valle, J[un]ct[ion]. Old B’[uena]v[en]tura R[oa]d. and Rio Dagua, 50m, 8.ii.1989 (J. B. Sullivan) (USNM). COSTA RICA: 1 ♀ (used for DNA Barcoding by Janzen, 07-SRNP-113921), Alajuela, Area de Conservacion Guanacaste, Estacion Caribe, 12.xi.2007 (S. Rios & H. Cambronero) (INBio); 1 ♀, Alajuela, Area de Conservacion Guanacaste, Rio Negro, 25.i.2009 (H. Cambronero & F. Quesada) (INBio); 2 ♂ (one used for DNA sequencing LEP 966, with genitalia on slide TL 3, other used for DNA sequencing LEP 967, with genitalia on slide TL 4), Alajuela, San Carlos, Arenal National Park, Send[ero] Pilón, Rio Celeste, 700m, light trap, 17–19.x.2001 (G. Rodriguez) (INBio); 1 ♂, Prov[incia] Guanacaste, F[in]ca Pasmompa, Est[acion] Pitilla, 5km SO S[an]ta Cecilia, 400m, xii.1990 (P. Rios & C. Moraga) (INBio). ECUADOR: 4 ♀ with same data and deposition as holotype; 4 ♂, 8 ♀ (1 ♂ used for DNA barcoding BC MTD 01844) with same data (USNM); 1 ♀ (genitalia on slide BL 1726, used for DNA sequencing and barcoding LEP 969, BC MTD 1707), Napo, 6km NW Tena, Lumu Caspi, 0°54'37"S, 77°49'32"W, 590m, 29.ix.2002 (Schouten Coll.); 1 ♀ (genitalia on slide BL 1715, used for DNA sequencing and barcoding LEP 968, BC MTD 1706), Pastaza, 1km N Santa Clara, 1°16'02"S, 77°52'57"W, 630m, 28.ix.2002 (Schouten Coll.). FRENCH GUIANA: 1 ♂, 4 ♀ (♂ genitalia on Pyralidae Brit. Mus. Slide No 7816, ♀ genitalia on slides BL 1720, BL 1725, and Pyralidae Brit. Mus. Slides No. 5953 and 19020), Saint Jean de Maroni (E. Le Moult) (BMNH); 2 ♀, Piste Nancibo, km 6, 4°41'N, ; 52°25'W, in logged rain forest, at 125W[atts] mer[cury]-vapor light and 15W[atts] U[ltra]V[iolet], 11.i.1985 (J.[-]F. Landry) (USNM); 1 ♂, Roura, Montagne des Chevaux, xii.2008 (S. Delmas) (MHNG); 1 ♂ (genitalia on slide Pyralidae Brit. Mus. Slide No 7792), Cayen[ne] (BMNH). GUYANA: 1 ♀ (genitalia on slide BL 1723), Omai, vi.1908 (S. M. Klages) (BMNH); 1 ♀ (genitalia on slide BL 1722), Potaro i.1908 (S. M. Klages) (BMNH). PANAMA: 1 ♀, Rio Trinidad, 12.iii [no year data] (A. Busck) (USNM). PERU: 1 ♂ (genitalia on slide BL 1724), Agnaytia, Huallaga, 400m, ix.1961 (F. H. Walz) (CNC); 1 ♀ (genitalia on slide GS-6908-SB), Yurimaguas, Huallaga 14.iv.[19]20 (CMNH). SURINAME: 2 ♀ (genitalia on slides BL 1727 and BL 1728), Kabo, 5°16'N, 55°44'W, Saramaca, black light, respectively 15–16.iii.1983 and 13–14.i.1983 (K.E.Neerling) (Schouten Coll.); 1 ♀ (genitalia on slide BL 1710), Sipaliwini Distr[ict]., Tibiti area, Kabo Creek, partly swampy primary forest on hilly slopes, ca 2km from river, vi.1989 (J. Beerlink) (Schouten Coll.).
Other specimen examined. 1♀ (used for DNA sequencing Lep 1126), Peru, Huánuco, Rio Llullapichis, Panguana, 74, 945°W / 9, 614°S, 23.9.–10.10.2011 (SMTD).
COI barcode sequence of paratype 07-SRNP-113921 (654 bp): ACATTATATTTTATTTTCGGGATTTGAGCAGGTATAGTAGGAACTTCACTTAGATTATTAATTCGTGCTGAATTAGGTAACCCTGGCTCTCTTATTGGAGATGATCAAATTTATAATACTATTGTAACAGCCCATGCATTTATTATAATTTTTTTTATAGTTATACCTATTATAATCGGTGGATTTGGAAATTGATTAGTTCCTTTAATATTAGGGGCACCAGATATAGCTTTCCCTCGAATAAATAACATAAGATTTTGATTATTACCACCATCATTAACTCTTTTAATTTCTAGAAGAATTGTAGAAAATGGAGCTGGAACAGGATGAACAGTTTATCCACCTTTATCATCTAATATTGCCCATGGGGGTAGATCTGTAGATTTAACAATTTTTTCATTACATTTAGCTGGAATTTCATCAATTTTAGGAGCTATTAATTTTATTACAACAATTATTAATATACGAATTAATAATTTATCATTTGATCAATTATCATTATTTATTTGATCAGTAGGAATTACTGCTTTACTTTTATTATTATCATTACCAGTTTTAGCTGGGGCTATTACTATACTTTTAACTGATCGAAATCTTAATACATCATTTTTTGATCCAGCAGGAGGAGGAGATCCAATTTTATATCAACATTTA
The best discriminant characters externally between the two species of the mayrabonillae group are the shape of the forewing outer margin, which is slightly produced apically in Catharylla mayrabonillae and not produced in Catharylla paulella, and the forewing median transverse line with two strongly pronounced spots at 1/3 and 2/3 in Catharylla paulella, whereas these spots are lacking in Catharylla mayrabonillae. The hindwing of Catharylla mayrabonillae has a faded subterminal transverse line on costal half whereas the hindwing of Catharylla paulella lacks this marking. In male genitalia, the heavily sclerotized sacculus bears a dorso-lateral sclerotized string of short spines on distal 1/4 whereas the two processes of the costa are S-shaped in Catharylla paulella, and the apex of the phallus is trifid, rounded medially, shortly triangular laterally, whereas it is simply rounded in Catharylla paulella. In female genitalia, the sterigma forms double rounded cavities with a mustachio-shape arrangement of short spines in ventral view, and the ductus bursae is wide, progressively widening toward corpus in Catharylla mayrabonillae, whereas the sterigma forms a pair of shallow rounded pockets on each side of middle and the ductus bursae is narrow, with the rounded corpus bursae clearly differentiated from it in Catharylla paulella.
Male (n = 17) (Fig. 7): Head with light ochreous chaetosemata. Antenna brown, with white scales dorsally and patch of dark brown scales at base. Maxillary palpus light ochreous, ringed with dark brown at 2/3; white tipped. Labial palpus: 1–1.4 mm; white, with patch of dark brown scales at 1/3 and 2/3 laterally. Thorax with patch of light ochreous scales at collar. Foreleg coxa whitish brown, femur white, dorsally ashen brown, tibia and tarsomeres ochreous, distally ringed with dark brown. Midleg femur white, tibia light ochreous, basally brown, tarsomeres II–V ochreous with tips ringed white. Hindleg white, except tarsomeres, as in midleg. Abdomen dull white. Forewing length: 7.5–8.5 mm; with apex slightly produced; costal line thin, ochreous or white in basal half, white in apical half; median transverse line ochreous, slightly undulated; subterminal transverse line ochreous; transverse lines enlarging into brown spot on costal margin with ochreous bar on costa following subterminal transverse line; terminal sector with light ochreous between veins, margin with thin, dark brown line from apex to CuA1, with two dark brown spots in cubital sector, with spot between CuA1 and CuA2 slightly displaced toward base; fringes brass colored; underside light ochreous with some brownish scales, with thin brown margin. Hindwing white with thin transverse subterminal line faded ochreous, in continuity with forewing median transverse line; outer margin line pronounced, dark brown; underside dull white with thin faded brown margin; fringes white.
Tympanal organs (n=7): Transverse ridge regularly rounded, medially slightly flattened. Tympanic pockets broadly rounded, extended widely beyond transverse ridge, connected medially at base of praecinctorium. Tympanic drum bean-shaped, elongated, extended beyond tympanic pockets.
Male genitalia (n = 7) (Figs 30, 31): Uncus thick and wide, about 2/5 length of tegumen arms, densely setose, with shortly projecting apex dorsally rounded. Gnathos reaching about 1/4 longer than uncus; arms wide, joining at 2/5 of length; distal 2/5 at angle of about 85°. Tegumen almost regularly narrow, joined in last 1/4. Cucculus narrow, shorter than sacculus, apically rounded; sacculus greatly enlarged, thickly sclerotized, directed upward, then apically straight, slightly narrowing toward apex, laterally with string of short spines and 2–3 longer basal spines pointing downward; costal arm of valva directed upward, located at about 1/3 of costal margin, thin, strongly sclerotized, slightly curved. Vinculum arms narrow; saccus short and wide, tongue shaped, projecting posterad apically. Juxta elongate, distal 1/4 narrowed with rounded tip; wide base with ear-like lobes laterally and baso-lateral angle projected anterad. Phallus slightly bent sideways in distal 1/4, with trifid sclerotized apex rounded medially and shortly triangular laterally; vesica basally covered with tiny spicules, microspicules barely visible all along, with long spine-like, down-curved cornutus of about 2/5 length of phallus.
Female (n = 37): Labial palpi length: 1.1–1.3 mm. Forewing length: 9.5–10.5 mm; frenulum triple.
Female genitalia (n = 16) (Fig. 40): Papillae anales strongly curved in lateral view; sclerotized line along papillae expanding ventrally into triangle. Posterior apophyses 0.35–0.45 × length of papillae anales. Tergite VIII about 1/3 length of sternite VIII; postero-dorsal margin with few setae of moderate length; anterior apophyses 0.03–0.1 × papillae anales; sternite VIII with patches of minute setae antero-ventrally on each side of bare median band. Sterigma forming double rounded cavities with mustachio-shaped arrangement of short spines (in ventral view); remaining cavity wall with tiny spines. Ductus bursae short and wide, enlarged near middle; partly sclerotized on right side of enlargement and posterior section. Corpus bursae circular to elongate, about as long as tergite VII; single signum faintly pronounced.
The species has been found so far in Panama, Costa Rica, Colombia, Venezuela, Guyana, Suriname, French Guiana, Ecuador, Peru and Brazil (Acre, Amazonas, Distritò Federal, Pará, Rondônia) (Fig. 44). It is the most widespread species of Catharylla and the only one so far found in Central America and in Venezuela, Columbia, Ecuador and Peru.
Catharylla mayrabonillae is named in honor of Ms. Mayra Bonilla of San Jose, Costa Rica, in recognition of her artistic portrayal of the biodiversity and ecosystems of Costa Rica and her many years of support for the existence of the rain forest in Area de Conservacion Guanacaste.
The relatively strong COI barcode divergence of 4.34% between samples LEP 1126 from Peru and 07-SRNP-113921 from Costa Rica (Table 5) is notable but it is not associated with morphological variation.
http://species-id.net/wiki/Catharylla_paulella
Figs 8, 10, 32, 33, 41, 44Holotype. ♀, with labels as follows: “Sao Paulo | S.E. Brazil.”; “Collection | W[illia]mSchaus”; “Type No. | 25533 | U.S.N.M.” [orange label]; “SLIDE | SB ♀ | No.4641” [light blue label]; “Catharylla | paulella | type Sch[au]s” [hand written]; “Genitalia Slide | By SB | USNM 111, 535” [green rectangular label with thin black line submarginally]. Deposited in USNM.
Other specimens examined. 2 ♂, 7 ♀. BOLIVIA: 2 ♂ (genitalia on slides GS-6652-SB and Pyralidae Brit. Mus. Slide No. 15890), Prov.[incia] del Sara, 450 m, iv.1910 (J. Steinbach) (CMNH, BMNH). BRAZIL: 1 ♀ (genitalia on slide BL 1752), Federal District, Planaltina, 15°35'S, 47°42'W, 1000 m, 3.xi.1977 (V. O. Becker n°22055) (Becker Coll.), 1 ♀ (genitalia on slide BL 1751) with same locality, 16.x.1990 (V. O. Becker n°96854) (Becker Coll.); 1 ♀, Maranhão, Feira Nova, Faz[enda]. Retiro, 480m, 07°00'S, 46°26'W, 1–3.xii.2011 (V. O. Becker n°148263) (Becker Coll.); 1 ♀ (genitalia on slide BL 1712), Mato Grosso, Urucum, 15 miles S[outh]. of Columbá, 650 f[ee]t, 19. iv. [19]27, at light (C. L. Collenette) (BMNH); 1 ♀, Parà, Belém, 20m, i.1984 (V. O. Becker n°46993) (Becker Coll.); 1 ♀ (genitalia on Pyralidae Brit. Mus. Slide N° 17692), São Paulo (BMNH); 1 ♀ (used for DNA sequencing and barcoding LEP 965, BC MTD 1705, genitalia on slide TL 5), São Paulo, São Luiz do Paraitinga, 23°20'S, 45°06'W, 900 m, 13–20.iii.2001 (V. O. Becker n°132357) (Becker Coll.).
COI barcode sequence of specimen LEP 965 (654 bp): ACATTATATTTTATTTTTGGAATTTGAGCAGGTATACTAGGAACTTCACTTAGATTATTAATTCGTGCTGAATTAGGTAATCCTGGATCTCTTATTGGTGATGATCAAATTTATAATACTATTGTAACAGCTCATGCATTTATTATAATTTTTTTTATAGTTATACCTATTATAATTGGTGGATTTGGAAATTGATTAGTTCCTTTAATATTAGGTGCACCAGATATAGCTTTCCCTCGAATAAATAATATGAGATTTTGATTATTACCCCCATCATTAACTCTTTTATTTT?TAGAAGAATTGTCGAAAATGGAACTGGAACAGGATGAACAGTTTACCCACCCTTATCATCCAATATTGCTCATAGAGGTAGATCAGTAGATCTAGCAATTTTTTCTTTACATTTGGCTGGAATTTCATCAATCTTAGGAGCTATTAATTTTATTACAACAATTATCAATATACGAATTAATAATTTATCTTTTGATCAATTATCATTATTTATTTGATCTGTAGGTATTACAGCTTTACTTTTATTATTATCATTACCAGTTCTAGCTGGAGCTATTACTATACTTTTAACTGATCGAAATCTTAATACATCATTTTTTGATCCTGCAGGAGGAGGTGATCCTATCTTGTATCAACATTTA
This species can be easily separated from the other Catharylla species by the forewing median transverse line with two strongly pronounced spots at 1/3 and 2/3. The forewing is also sparkled with dark brown scales, which is unique in the genus. In male genitalia, the two S-like projections of the costal arm of the valva discriminate this species from the other species of Catharylla. In female genitalia, the sterigma forms a pair of shallow rounded pockets on each side of middle, and the ductus bursae is narrow, with the rounded corpus bursae clearly differentiated from it in Catharylla paulella, whereas it forms double rounded cavities with a mustachio-shape arrangement of short spines in ventral view, and the ductus bursae is wide, progressively widening toward corpus in Catharylla mayrabonillae.
Male (n = 2): Head with ochreous chaetosemata. Antenna ochreous with white scales, with patch of dark brown scales at base. Maxillary palpus light ochreous, white tipped. Labial palpus: 1.4–1.7 mm long, light ochreous, white tipped. Thorax light ochreous at collar. Foreleg coxa whitish ochreous, femur light ochreous, dorsally ochreous; tibia and tarsomeres greyish brown, distally ringed with dark brown. Midleg and hindleg whitish ochreous; midleg tibia basally brown, hindleg tibia white; midleg and hindleg tarsomeres with white tips. Forewing length: 7–8 mm; costal line thin, brown or dirty white; median transverse line ochreous, with two dark brown strongly pronounced spots at 1/3 and 2/3; subterminal transverse line thin, ochreous, with small triangular spot on costal margin; with ochreous bar on costal margin following subterminal transverse line; outer margin ochreous with short dark brown lunules or dashes; fringes brass colored; underside light ochreous with brownish suffusion; with pronounced marginal spots. Hindwing white; outer margin with thin ochreous line in apical half; fringes white; underside dull white with dark brown marginal spots more or less connected on apical half.
Male genitalia (n = 2) (Figs 32, 33): Uncus almost straight, densely setose, about 1/4 length of tegumen arms. Gnathos with arms joining at 3/5, then directed upward at slightly less than 90° angle; apically narrowly rounded. Tegumen arms regularly widening toward uncus, connecting at about half their length. Cucculus of medium width, slightly curved upward in distal 1/4; costal arm of valva divided with short spatula at base and S-shaped projection with rounded apex apically. Juxta triangular with distal third narrower, apically rounded, with baso-lateral narrow, triangular projections pointing anterad. Phallus with apex more thickly sclerotized, with blunt apical margin, with short triangular ventral projection; vesica covered on basal 1/4 with tiny spicules, with barely visible microspicules all along, with one wide and curved cornutus at about 1/4 length of phallus.
Female (n = 7) (Figs 8, 10): Labial palpi: 1.4–1.7 mm long; forewing length: 9–9.5 mm; frenulum triple.
Tympanal organs (n = 5) (Fig. 10): Transverse ridge medially straight. Tympanic pockets not reaching beyond transverse ridge, rounded. Tympanic drum bean shaped, somewhat oval, just reaching transverse ridge.
Female genitalia (n = 5) (Fig. 41): Papillae anales ventrally slightly projected; sclerotized line at base enlarging medially to triangular shape covered by minute punctuation. Posterior apophyses 0.4–0.5 × length of papillae anales, narrow, tubular, with rounded tips. Tergite VIII short, about half of length of greatly enlarged sternite VIII. Anterior apophyses 0.05–0.1 × length of papillae anales. Lamella antevaginalis of sterigma dorsally covered with minute spicules; pair of shallow rounded pockets on each side of middle opened posterad. Base of ductus bursae sclerotized, forming circular membranous and narrow pocket. Corpus bursae regularly rounded, without signum.
The species has been found in Brazil (Federal District, Maranhão, Mato Grosso, Pará, Saõ Paulo) and in Bolivia (Fig. 44).
The original description doesn’t mention the original number or sex of the specimens but it is assumed that there was only one. S. Bleszynski gave the new name of Catharylla hibisca to specimens that appear to be Catharylla paulella. The BMNH São Paulo specimen is associated with slide n° 17692, but the genitalia on this slide seem to be wrongly associated, given the inscription “wrong abdomen?” on the label, as well as the size of the abdomen, which is much bigger than those of Catharylla paulella. Therefore, this specimen cannot be identified with certainty. An error is possible in the association of the sexes of this species as there are no series of both sexes from the same locality or other means of associating them with 100% confidence.
| 1 | Forewing costa with thick, brown to greyish brown stripe (Figs 2, 3); forewing length usually > 14 mm. Hindwing white. In male genitalia, gnathos regularly curved, juxta without latero-ventral projections (Figs 13, 15) | 2 |
| – | Forewing costa with thin, ochreous stripe or none (Figs 1, 4–8); forewing length 7–14 mm. Hindwing white, cream-coloured, or yellowish. In male genitalia, gnathos more or less straight, or bent at about 90° angle; juxta with latero-ventral projections (Figs 11, 17, 19, 21, 30, 32) | 3 |
| 2 | Male genitalia with costal arm long, narrow throughout, hook shaped, slightly curved, posteriorly reaching beyond cucculus (Fig. 13). In female genitalia, sterigma forming strongly sclerotized ventro-laterally symmetrical structure made of two asymmetrical bell-shaped cavities (Fig. 35) | Catharylla chelicerata |
| – | Male genitalia with costal arm short, basally wide, tooth shaped, not reaching cucculus tip (Fig. 15). In female genitalia, sterigma forming a pair of shallow pockets opened posterad, antero-ventrad of segment VIII (Fig. 36) | Catharylla gigantea |
| 3 | Forewing length 7–10 mm, forewing costa slightly ochreous or white on basal 1/2, white on distal 1/2 (Figs 7, 8). In male genitalia, gnathos bent at about 90° angle; uncus less than twice length of tegumen connection, beak shaped (Figs 30, 32) | 4 |
| – | Forewing length 10–16 mm, forewing costa with thin ochreous to brown stripe (Figs 1, 4–6). In male genitalia, gnathos more or less straight; uncus more than twice length of tegumen connection (Figs 11, 17, 19, 21) | 5 |
| 4 | Forewing outer margin slightly produced at apex, median transverse line without spots at 1/3 and 2/3 (Fig. 7). In male genitalia, costal arm of valva simple, narrow; sacullus heavily sclerotized, large, laterally with string of sclerotized spines with 2–3 longer basal spines pointing downward (Fig. 30). In female genitalia, sterigma forming mustachio-shaped double rounded cavities set with short spines (Fig. 40) | Catharylla mayrabonillae |
| – | Forewing outer margin not produced at apex, median transverse line with two clearly pronounced spots at 1/3 and 2/3 (Fig. 8). In male genitalia, costal arm of valva with two well-separated projections; sacullus not strongly sclerotized, densely setose, apically rounded (Fig. 32). In female genitalia, sterigma forming double rounded pockets opened posterad (Fig. 41) | Catharylla paulella |
| 5 | Hindwing white (Fig. 1). In male genitalia, costal arm of valva double, with ventral arm tubular, apically pointing upward, and dorsal arm slightly shorter, flattened, apically rounded; transtilla absent; vesica with one cornutus (Fig. 11). In female genitalia, sternite VIII ventrally connecting, with latero-ventral projections (Fig. 34) | Catharylla bijuga |
| – | Hindwing cream colored or yellowish (Figs 4–6). In male genitalia, costal arm of valva simple; transtilla present; vesica without cornutus, or with crest of cornuti (Figs 17–22). In female genitalia, sternite VIII narrowing ventrally, ventrally not connected, without latero-ventral projection (Figs 37–39) | 6 |
| 6 | Forewing median transverse line more or less straight, shortly curved inward in costal 1/3 (Fig. 4). In male genitalia, transtilla laterally with short, narrow sclerotized arms with pointed tips, projecting posterad, and medially with pair of brushes directed medio-ventrally (Fig. 17). In female genitalia, anterior angle of sternite VIII projected downward into more or less rounded projection covered with short spinules of same length (Fig. 37) | Catharylla tenellus |
| – | Forewing median transverse line not regular, slightly curved outward at M1 and CuA2, curved inward in costal 1/3 (Figs 5–6). In male genitalia, transtilla forming pair of sclerotized arms slightly bent inward with longitudinal row of spines ventrally or medially (Figs 19, 21). In female genitalia, anterior angle of sternite VIII not projected or projected anterad (Figs 38, 39) | 7 |
| 7 | Forewing median transverse line without short marked triangular dent at CuA2 (Fig. 5). In male genitalia, uncus bifid and grooved in distal 1/5; transtilla forming pair of sclerotized arms slightly bent inward distally, ventrally with row of short spines increasing in size from base to apex; juxta regularly narrowing toward apex; vesica with row of 6–7 cornuti (Figs 19, 20). In female genitalia, anterior angle of sternite VIII not projected; anterior apophyses quadrangular, anvil shaped (Fig. 38) | Catharylla coronata |
| – | Forewing median transverse line with short triangular dent at CuA2 (Fig. 6). In male genitalia, uncus apically indented medially; transtilla forming pair of sclerotized arms strongly bent inward in distal 1/4 and with string of long spines of same length medially along it; juxta strongly narrowing in distal 1/4; vesica without cornuti (Figs 21, 22). In female genitalia, anterior angle of sternite VIII projected anterad into rounded projection covered with short spinules; anterior apophyses spine-like (Fig. 39) | Catharylla serrabonita |
The number of bases obtained for each barcode sequence is given in Table 3 and the genetic distances between specimens in Table 5.
As most of the data were restricted to few sequence samples per species, and some of the sequences came from the same populations, we don’t have the definitive picture of the intraspecific variation in the COI barcode sequences of Catharylla species. We observe a relatively high divergence between different barcode haplotypes in Catharylla bijuga (5.05%), Catharylla mayrabonillae (4.34%), Catharylla serrabonita (3.24%) and Catharylla tenellus (3.34%), sometimes possibly associated with differences in morphological characters (see Notes of the descriptions of Catharylla bijuga, Catharylla tenellus) and with geographical distances. The divergence between Catharylla chelicerata samples LEP 1703 and LEP 1704 is of 0.15% (1 base) because they are issued from the same population. Sample LEP 1290 differs from samples LEP 1703 and LEP 1704 respectively by 0.62 and 0.46%. We observe no variation between samples LEP 1708, LEP 1709, LEP 1710 and LEP 1888 because they are all issued from the same population. The inter-specific variation in COI barcode sequences (6.29–16.84%) is always higher than the intraspecific variation (0.15–5.05%).
The monophyly of Catharylla is supported by the analysis of mol_1 and the combined Bayesian analysis (BS support = 90, PP = 0.95) and by the analyses of nucl_1 and nucl_2 (Catharylla chelicerata, Catharylla mayrabonillae, Catharylla serrabonita and Catharylla tenellus represented). Three synapomorphies (11:1, 17:1, 19:1, with one reversal to the ancestral state in the chelicerata group for character 11) and one non-unique apomorphy (16:1, apomorphy observed in the two outgroups as well) support the group. The mayrabonillae group is well supported in all analyses (BS supports = 100 in mol_1 and mol_2 analyses, PP = 1.00 in the combined Bayesian analysis) except in the morphology-based analysis (BS support = 60), because no clear synapomorphy was found for the group. However, two non-unique synapomorphies and one reversal (observed only once in Catharylla) are observed (9:1, 12:1, 19:0). The tenellus group is well supported by the morphology-based analysis (BS support = 97) with four clear synapomorphies (5:1, 7:3, 10:1, 20:1) and two reversals (3:0, 15:0), but show no support in other analyses, probably because only the barcode sequence was available for Catharylla coronata. The closer relationship between Catharylla serrabonita and Catharylla tenellus within the tenellus group is supported by the combined Bayesian analysis and by one non-unique apomorphy (but unique in Catharylla). The tenellus group as represented in the analyses of nucl_1 and nucl_2 (Catharylla serrabonita + Catharylla tenellus) is well supported (BS supports of 99 and 93, respectively). The chelicerata group is well supported by the morphology-based analysis (BS support = 96) and the combined Bayesian analysis (PP = 1.00). The group shows two synapomorphies (8:1, 9:2), one non-unique apomorphy (7:1) and one reversal (11:0). Unfortunately, no sequence was available for Catharylla gigantea, thus we cannot compare the morphology with the molecular-based analyses. The closer relationships between the chelicerata and the mayrabonillae groups is strongly supported by the nucl_1 analysis (BS support = 100) and the combined Bayesian analysis (PP = 1.00). Two reversals (4:0, 6:0) support the group. The position of Catharylla bijuga as sister group of the other Catharylla species is weakly supported by the combined Bayesian analysis (PP = 0.90), and show no support in other analyses. The position of Micrelephas pictellus as sister group of Catharylla is supported by the analysis of mol_2 and nucl_2 (respective BS supports of 91 and 100). This node is supported by one synapomorphy (6:1, with one reversal in the chelicerata + mayrabonillae clade). Node 10 (Argyria lacteella+ Micrelephas pictellus+ Catharylla) is supported by the analysis of mol_1 (BS support = 100) and the combined Bayesian analysis (PP = 1.00). One synapomorphy (2:1) supports the group. The monophyly of the two Crambus species chosen as outgroups is well supported in the mol_1 analysis (BS support of 100). The settings of MrBayes do not allow to choose two outgroups, therefore the monophyly of the two Crambus species is not supported by the combined Bayesian analysis. Three non-unique synapomorphies support the genus based on these two species (7:1, 12:1, 16:1). The synapomorphies of the different groups highlighted by the phylogeny are reported here below:
Chelicerata group (node 3)
8:1. Apex of valva quadriangular, truncated
9:2. Gnathos regularly curved
Tenellus group (node 5)
5:1. Presence of a dorsal furrow on the uncus
7:3. Apex of uncus slightly bifid
10:1. Transtilla present
20:1. Postvaginal sterigma absent
Catharylla (node 8)
11:1. Lateroventral projections on juxta
17:1. Posterior apophyses/papillae anales < 0.5
19:1. Ventral membrane of segment VIII with tiny setae
Catharylla + Micrelephas (node 9)
6:1. Uncus dorsally bare, or with few setae
Catharylla + Micrelephas + Argyria (node 10)
2:1. Length of labial palpi/eye diameter < 2/1
Catharylla occurs northward from Middle America with locality records in Costa Rica (Area de Conservacion Guanacaste) at a latitude of 10°54 N, southward to Rio Vermelho (Brazil, Santa Catarina) at a latitude of 27°30 S, from sea level up to 1300 m (Brazil, Minas Gerais, Serra do Caraça). Within Catharylla, species and species groups show distinct distribution patterns. The chelicerata group is widespread in the Northern Amazonian rainforest of Brazil, and in the three Guyanas (Fig. 43). The tenellus group is restricted to the south-eastern Atlantic coast of Brazil in the Atlantic Forest, which is formed by tropical moist forests (
The analyses of nucl_1 and nucl_2 datasets brought better BS supports than the analyses of mol_1 and mol_2 on nodes 1, 6, 9, and for the monophyly of Catharylla (nodes 7 & 8). The reduced number of taxa (7 taxa in nucl datasets vs 11 in mol datasets, with 4 Catharylla species in nucl_1 and nucl_2 vs 7 in mol_1 and mol_2), as well as the quality of the datasets (complete sets of genes for nucl_1 and nucl_2 vs partly complete sets of genes in mol_1 and mol_2) explain the better results obtained in nucl_1 and nucl_2, because taxa with incomplete data tend to lower the resolution and the bootstrap supports of the tree (
Our molecular-based analyses failed to place with certainty two of the four taxa lacking nuclear sequences (Catharylla bijuga and Catharylla coronata) because of the great divergence of these sequences from those of other Catharylla species. However, the clear support brought by the morphological analysis places Catharylla coronata together with Catharylla serrabonita and Catharylla tenellus. The neighbor-joining (NJ) analysis of barcode sequences of Catharylla species (not represented here) places Catharylla coronata together with Catharylla serrabonita (divergence of 7.59% with the sequence BC MTD 1843) and Catharylla tenellus (divergence of 7.3% with the sequence BC MTD 1842), and thus corroborates the findings of the morphology-based analysis. Moreover, the species is morphologically very similar to Catharylla serrabonita especially regarding the transtilla in the male genitalia. The basal position of Catharylla bijuga in Catharylla, as sister taxon of other Catharylla species, is doubtful (PP = 0.90) and may be an artefact due to the great divergence of the barcode sequence (the lowest divergence with other Catharylla species is of 9.7% with sample BC MTD 1843 of Catharylla serrabonita). The supports brought by the combined Bayesian analysis have to be carefully considered since the results are sensitive to small variations of the taxon and character sampling, and the posterior probabilities tend to overestimate the strongness of the nodes (
The intra-specific divergence in barcode sequences within Catharylla (Table 5; distances mapped on Figs 44, 45 and 46) is higher than the threshold of 2–3% predicted to delimit the species level in Lepidoptera according to
The different species groups of Catharylla highlighted by the phylogenetic analyses show distinct distribution patterns, with species widely spread, such as Catharylla mayrabonillae and Catharylla paulella (Fig. 44) and other species confined to smaller areas, such as Catharylla bijuga (Fig. 45), and the chelicerata and tenellus species groups (Figs 43 & 45). The humid formations of the Amazonian and Atlantic Forests constituted two different refuges separated by the Caatinga and the Cerrado xerothermic formations, and vicariant evolutionary processes in plants (
We thank the Swiss Academy of Sciences for awarding T. Léger a travel grant to Brazil, the University of Geneva for an Erasmus grant also to T. Léger to study in Dresden, Franziska Bauer for her help with lab techniques, Anja Rauh, Anke Müller and Christian Kehlmaier in the DNA laboratory of the SMTD for DNA sequencing, Andreas Schmitz, Juan Montoya and Raphaël Covain for their help with phylogenetic programmes, V. O. Becker and Clemira de Souza for welcoming B. Landry and T. Léger in Serra Bonita, and Lionel Monod and Mickaël Blanc for their helpful comments. For the barcode sequences we thank the Canadian Centre for DNA Barcoding (CCDB). We also thank the online database Genbank for allowing us access to sequences and the people who deposited the sequences we used. For the loan of specimens we thank the following curators: S. Rab-Green (AMNH), J. Rawlins (CMNH), J.-F. Landry (CNC), L. Przybylowicz (ISZP), E. Phillips (InBIO), S. Gaal-Maszler (NHM), K. Tuck (BMNH), A. Zwick (SMNS), M. A. Solis (USNM), as well as private collection owners R. T. A. Schouten and V. O. Becker. We also thank S. Delmas, B. Hermier and L. Przybylowicz for their gifts of specimens to the MHNG. Last but not least, we thank V. O. Becker for having suggested to BL to select Catharylla as a M.Sc. project for TL.
Coordinates of the localities pertaining to the Catharylla specimens studied.
| Country | Locality | Province / State / Subdivision | latitude, longitude | |
|---|---|---|---|---|
| Bolivia | ? | Sara | -16°55, -63°37 | |
| Brazil | Belem | Pará | -1°27, -48°30 | |
| Bertioga | São Paulo | -23°51, -46°8 | ||
| Cacaulândia | Rondônia | -10°20, -62°53 | ||
| Capitão Poço | Pará | -1°44, -47°3 | ||
| Caraça | Minas Gerais | -20°07, -43°28 | ||
| Castro | Paraná | -24°47, -50°0 | ||
| Corcovado | Rio de Janeiro | -22°57, -43°13 | ||
| Curitiba | Paraná | -25°25, -49°16 | ||
| Fazenda Rancho Grande | Rondônia | -10°18, -62°52 | ||
| Feira Nova | Maranhão | -07°00, -46°26 | ||
| Fonte Boa | Amazonas | -2°31, -66°05 | ||
| Ibateguara | Alagoas | -8°58, -35°55 | ||
| Lago Teffé | Amazonas | -3°27, -64°53 | ||
| Linhares | Espírito Santo | -19°23, -40°3 | ||
| Manaus | Amazonas | -3°7, -60°0 | ||
| Mirapinima | Amazonas | -2°10, -61°9 | ||
| Nova Friburgo | Rio de Janeiro | -22°17, -42°32 | ||
| Nova Olinda do Norte | Amazonas | -3°54, -59°05 | ||
| Parque Nacional de Jaú | Amazonas | -1°57, -61°49 | ||
| Planaltina | Federal District | -15°36, -47°40 | ||
| Porto Seguro | Bahia | -16°27, -39°3 | ||
| Quatro Barras | Paraná | -25°22, -49°4 | ||
| Reserva Ducke | Amazonas | -2°55, -59°58 | ||
| Rio Branco | Acre | -9°58, -67°48 | ||
| Rio Negro | Paranà | -26°6, -49°48 | ||
| Rio Vermelho | Santa Catarina | -27°29, -48°24 | ||
| Sao Luiz do Paraitinga | São Paulo | -23°13, -45°18 | ||
| São Paulo | São Paulo | -23°32, -46°38 | ||
| Serra Bonita | Bahia | -15°23, -39°33 | ||
| Ubatuba | São Paulo | -23°26, -45°5 | ||
| Urucum | Mato Grosso do Sul | -19°8, -57°38 | ||
| Colombia | Buenaventura | Valle del Cauca | 3°49, -77°00 | |
| Costa Rica | Estacion Caribe | Alajuela | 10°54'06, -85°16'30 | |
| Pitilla | Guanacaste | 10°59'21, -85°25'32 | ||
| Parque Nacional Arenal | Alajuela | 10°26, -84°44 | ||
| Ecuador | Misahualli | Napo | -1°1, -77°40 | |
| Santa Clara | Pastaza | -1°15, -77°53 | ||
| French Guiana | Beauséjour | 4°42, -52°23 | ||
| Cayenne | 4°55, -52°19 | |||
| Montagne des Chevaux | 4°43, -52°25 | |||
| Nancibo | 4°40, -52°28 | |||
| Oyapock, Pied Saut | 3°48, -51°53 | |||
| Parcelles CIRAD de Combi | 5°18, -52°55 | |||
| Roura, road to Crique Gabrielle | 4°44, -52°19 | |||
| Roura, Camp Patawa | 4°33, -52°9 | |||
| Route d’Apatou pk 25.5 | 5°15, -54°13 | |||
| Route de Saut Léodate | 4°55, -52°33 | |||
| Saint-Jean-du-Maroni | 5°24, -54°4 | |||
| Saint-Laurent-du-Maroni | 5°30, -54°01 | |||
| Guyana | Malali | Upper Demerara–Berbice | 5°37, -58°21 | |
| New River | East Berbice–Corentyne | 3°21, -57°35 | ||
| Omai | Cuyuni–Mazaruni | 5°25, -58°45 | ||
| Potaro | Potaro–Siparuni | 5°22, -59°7 | ||
| Panama | Rio Trinidad | Nuevo Emperador | 8°43, -79°58 | |
| Peru | Aguaytía | Ucayali | -9°20, -75°30 | |
| Panguana | Huánuco | -9°36, -74°56 | ||
| Yurimaguas | Loreto | -5°54, -76°7 | ||
| Suriname | Gelderland | Para | 5°27, -54°59 | |
| Kabo | Sipaliwini | 5°16, -55°44 | ||
| Kabo Kreek | Sipaliwini | 5°8, -57°16 | ||
Molecular material and techniques.
PCR mix for respective genes and primer pairs.
| Product | LCO/Pat, LCO/Nancy, Jerry/ Pat (COI) HybLepWg1 / HybLepWg2 (wingless) | LCO/K699, Ron/Nancy, Jerry/Mila, Brian/Pat (COI), HybLepWg1/ HybLepWg2 (wingless), Oscar-6143 /Bosie-6144 (EF-1α) | HybLepWg1/HybLepWg2 (wingless), Oscar-6143/ Bosie-6144 (EF-1α) |
|---|---|---|---|
| H2O (ultraclean) | 15.9 μl | 12.55 μl | 11.95 μl |
| 10X Taq buffer (15 mM MgCl2) | 2.5 μl | 2.5 μl | 2.5 μl (10X OptiBuffer) |
| MgCl2 (25 mM) | 1 μl | 0.75 μl | 0.75 μl (50 mM) |
| Forward primer (10 pmol/μl) | 0.5 μl | 1.2 μl | 1.5 μl |
| Reverse primer (10 pmol/μl) | 0.5 μl | 1.2 μl | 1.5 μl |
| dNTP (each 10 mM) | 0.5 μl | 2.5 μl | 2.5 μl |
| Taq DNA polymerase (5 units/μl) | 0.1 μl | 0.3 μl | 0.3 μl (BIO-X-ACT Short TaqDNA polymerase; 4 units/μl) |
| DNA | 4 μl | 4 μl | 4 μl |
PCR programmes for COI, wingless and EF-1α.
| Gene | COI, wingless | wingless | EF-1α | |
|---|---|---|---|---|
| Polymerase | Taq polymerase | BIO-X-ACT Short Taq | Taq polymerase | |
| programme | initial: | 95 °C (5 min) | 95 °C (5 min), 48 °C (1 min) | 95 °C (5 min) |
| 40 cycles: | 94 °C (30 sec) | 95 °C (30 sec) | 94 °C (30 sec) | |
| 48 °C (30 sec) | 51 °C (30 sec) | 51 °C (30 sec) | ||
| 72 °C (1 min 30) | 70 °C (1 min) | 72 °C (1 min 30) | ||
| final: | 72 °C (10 min) | 70 °C (10 min) | 72 °C (10 min) | |
| cooling: | 8 °C (∞) | 8 °C (∞) | 8 °C (∞) | |
PCR-mix for sequencing reaction.
| Product | low DNA concentration | high DNA concentration |
|---|---|---|
| cleaned PCR-product | 4 μl | 1 μl |
| H2O (ultraclean) | 2 μl | 5 μl |
| Forward primer (10 pmol/μl) | 1 μl | 1 μl |
| T-mix (2.5×) | 1 μl | 1 μl |
| Buffer (5×) | 2 μl | 2 μl |