(C) 2013 Marco Bertolino. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Bertolino M, Cerrano C, Bavestrello G, Carella M, Pansini M, Calcinai B (2013) Diversity of Porifera in the Mediterranean coralligenous accretions, with description of a new species. ZooKeys 336: 1–37. doi: 10.3897/zookeys.336.5139
Temperate reefs, built by multilayers of encrusting algae accumulated during hundreds to thousands of years, represent one of the most important habitats of the Mediterranean Sea. These bioconstructions are known as “coralligenous” and their spatial complexity allows the formation of heterogeneous microhabitats offering opportunities for a large number of small cryptic species hardly ever considered.
Although sponges are the dominant animal taxon in the coralligenous rims with both insinuating and perforating species, this group is until now poorly known. Aim of this work is to develop a reference baseline about the taxonomic knowledge of sponges and, considering their high level of phenotypic plasticity, evaluate the importance of coralligenous accretions as a pocket for biodiversity conservation.
Collecting samples in four sites along the coast of the Ligurian Sea, we recorded 133 sponge taxa (115 of them identified at species level and 18 at genus level). One species, Eurypon gracilis is new for science; three species, Paratimea oxeata, Clathria (Microciona) haplotoxa and Eurypon denisae are new records for the Italian sponge fauna, eleven species are new findings for the Ligurian Sea. Moreover, seventeen species have not been recorded before from the coralligenous community. The obtained data, together with an extensive review of the existing literature, increase to 273 the number of sponge species associated with the coralligenous concretions and confirm that this habitat is an extraordinary reservoir of biodiversity still largely unexplored, not only taxonomically, but also as to peculiar adaptations and life histories.
Porifera, cryptic species, bioconstructions, Ligurian Sea
The term “coralligenous” refers to a secondary hard substrate, formed by the concretion of algal thalli and, to a lesser extent, by animal skeletons. Two main types of coralligenous concretions can be distinguished: banks, which are built over more or less horizontal substrata, and rims, which develop in the outer parts of marine caves and on vertical cliffs (
Sponges, with 142 recorded species, are one of the most diverse group of sessile animals of the coralligenous assemblage (
In the present paper, the species diversity of the coralligenous sponge fauna was studied in four sites of the Ligurian Sea, focusing on the relatively poorly known cryptic species boring or insinuating into the calcareous concretions. A new species for science and ten poorly known species, rarely recorded in the Mediterranean Sea, are treated exhaustively.
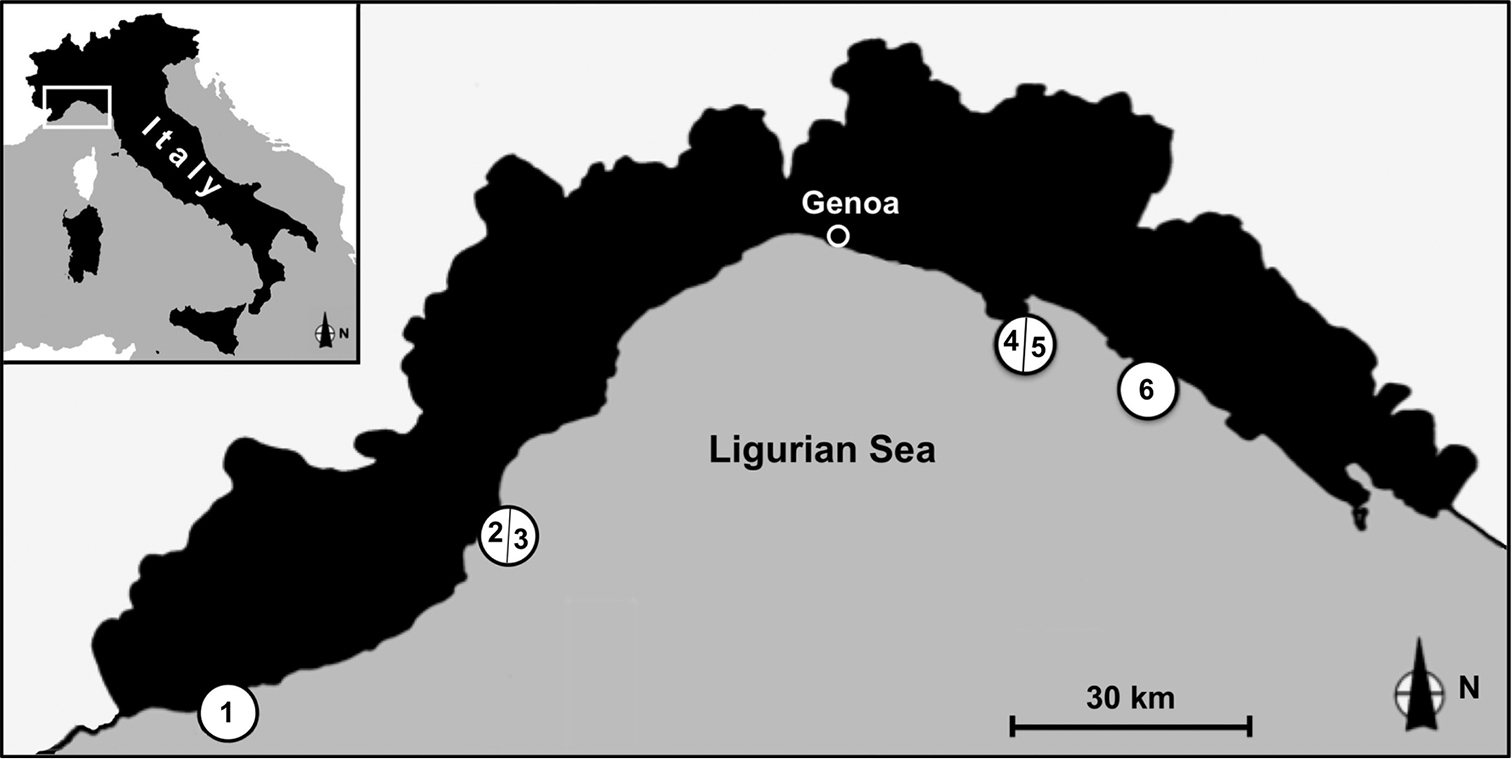
Samples were collected between 30 and 40 m depth by SCUBA diving from 6 stations along the Ligurian coast where coralligenous is more developed (Fig. 1). Stations (from West to East) are: Santo Stefano Shoals, station 1; Gallinara Island, station 2 (Falconara) and station 3 (Sciusciaù); Portofino Promontory, Punta del Faro, station 4 and 5 (northern and southern side of the point); Punta Manara, station 6. Four blocks of coralligenous concretion, with an average volume of 20 l, were collected from each station.
The four studied localities along the Ligurian Coast: Santo Stefano Shoal (station 1), Gallinara Island (station 2–3), Punta del Faro (Portofino Promontory) (station 4–5) and Punta Manara (station 6).
All the sponge species settled on the surface of these blocks were sampled and identified.
Two of the four blocks from each station were cut into slices about 2 cm thick and observed by a stereomicroscope to detect the cryptic, generally small, endolithic sponges.
The spicule complement of each sponge specimen was analysed according to
We followed the classification given by
During this survey we have recorded 133 sponge taxa (115 of them identified at species level and 18 at genus level). One species is new for science, 17 are new findings for the coralligenous conglomerate, 11 of which for the Ligurian Sea and 3 for the Italian sponge fauna (Table 1). In the following taxonomic part we provide the description of the new species and of ten poorly known ones.
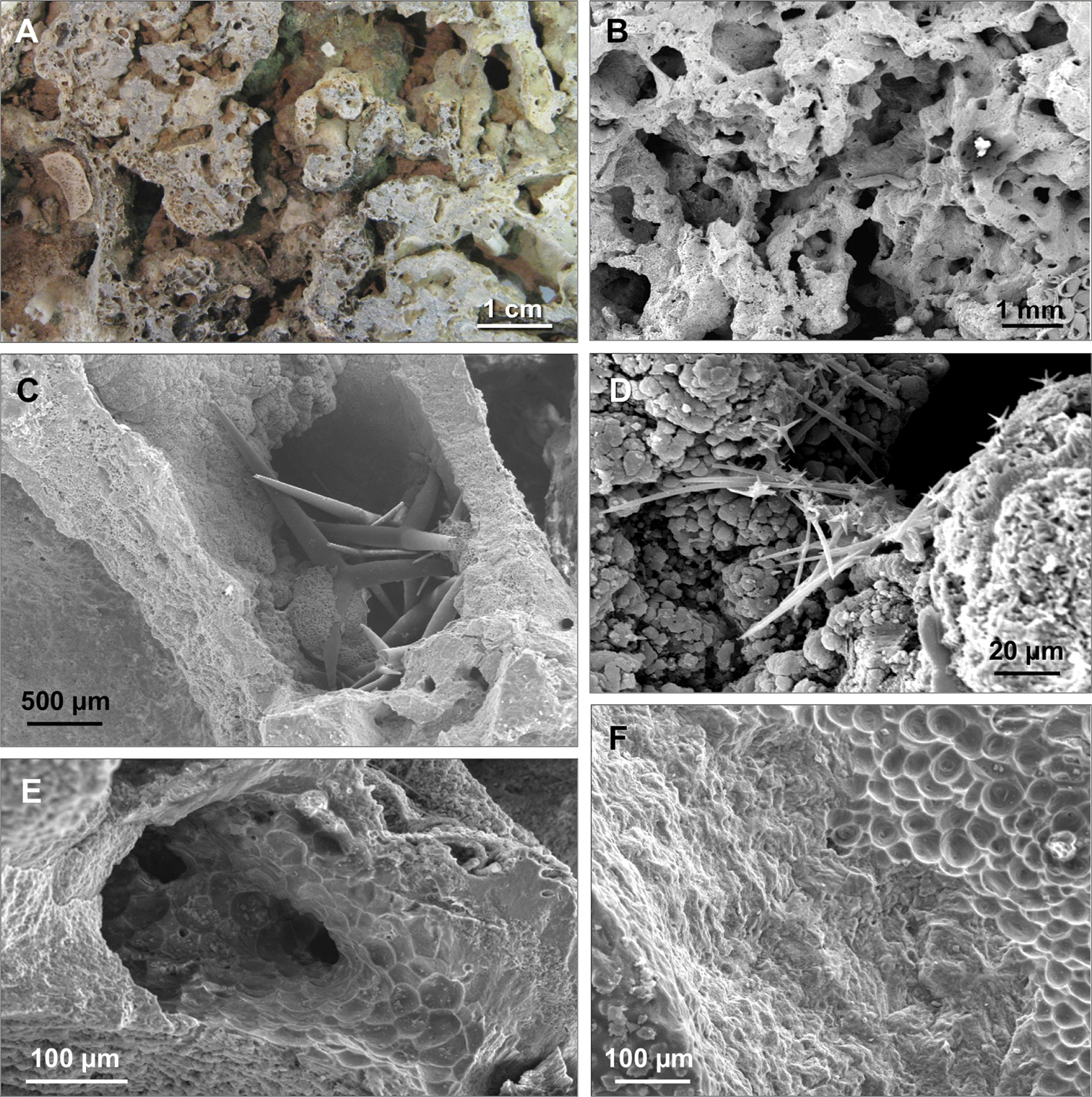
On the surfaces of the blocks 103 massive or encrusting species were recorded; inside the crevices of the conglomerate 63 species were observed and 33 shared both positions. Thirty species are exclusively endolithic demonstrating the abundance of cryptic sponges thriving inside the porous matrix of the coralligenous substrate (Table 1) (Fig. 2).
List of Demospongiae and Homoscleromorpha species living outside and inside the coralligenous blocks (SSS: Santo Stefano Shoals, station 1; GI: Gallinara Island, station 2-3; PF: Punta del Faro, station 4-5; PM: Punta Manara, station 6; * new finding for the coralligenous concretion; ** new finding for the Ligurian Sea; *** new finding for the Italian sponge fauna).
| Species \ Sites | SSS | GI | PF | PM | Epilithic | Endolithic |
|---|---|---|---|---|---|---|
| Oscarella lobularis (Schmidt, 1862) | + | + | + | |||
| Plakina trilopha Schulze, 1880 | + | + | + | |||
| Plakinastrella copiosa Schulze, 1880 | + | + | ||||
| Plakortis simplex Schulze, 1880 | + | + | + | |||
| Samus anonymus Gray, 1867 | + | + | + | |||
| Stelletta grubii Schmidt, 1862 | + | + | ||||
| Stelletta lactea Carter, 1871 * | + | + | ||||
| Stelletta stellata Topsent, 1893 * | + | + | ||||
| Jaspis incrustans Topsent, 1890 ** | + | + | + | + | ||
| Jaspis johnstoni (Schmidt, 1862) | + | + | + | + | + | + |
| Penares euastrum (Schmidt, 1868) | + | + | + | + | + | |
| Dercitus (Stoeba) plicatus (Schmidt, 1868) | + | + | + | + | + | + |
| Pachastrissa sp. | + | + | ||||
| Erylus discophorus (Schmidt, 1862) | + | + | + | + | ||
| Geodia conchilega Schmidt, 1862 | + | + | + | + | + | |
| Geodia cydonium Schmidt, 1862 | + | + | + | + | ||
| Pachastrella monilifera Schmidt, 1868 | + | + | + | |||
| Poecillastra compressa (Bowerbank, 1866) | + | + | + | + | ||
| Triptolemma simplex (Sarà, 1959) | + | + | + | + | + | |
| Cliona burtoni Topsent, 1932 *, ** | + | + | ||||
| Cliona celata Grant, 1826 | + | + | + | + | + | |
| Cliona janitrix Topsent, 1932 | + | + | + | + | + | + |
| Cliona schmidtii (Ridley, 1881) | + | + | + | |||
| Cliona viridis Schmidt, 1862 | + | + | + | + | + | |
| Cliona sp. | + | + | + | |||
| Dotona pulchella mediterranea Rossell & Uriz, 2002 | + | + | ||||
| Spiroxya corallophila (Calcinai et al., 2002) | + | + | ||||
| Spiroxya heteroclita Topsent, 1896 | + | + | + | + | + | |
| Spiroxya sarai Melone, 1965 | + | + | + | |||
| Delectona ciconiae Bavestrello, Calcinai & Sarà, 1996 | + | + | ||||
| Delectona sp. | + | + | + | |||
| Paratimea oxeata Pulitzer-Finali, 1978 *, **, *** | + | + | ||||
| Polymastia sp. | + | + | + | |||
| Diplastrella bistellata (Schmidt, 1862) | + | + | + | + | + | |
| Aaptos aaptos (Schmidt, 1864) | + | + | + | + | ||
| Prosuberites longispinus Topsent, 1893 | + | + | ||||
| Pseudosuberites sulphureus (Bowerbank, 1866) | + | + | + | |||
| Suberites carnosus (Johnston, 1842) | + | + | ||||
| Suberites domuncula (Olivi, 1792) | + | + | ||||
| Suberites sp. | + | + | + | |||
| Terpios gelatinosa (Bowerbank, 1866) | + | + | + | |||
| Timea stellata (Bowerbank, 1866) | + | + | + | + | + | |
| Timea unistellata (Topsent, 1892) | + | + | + | + | ||
| Chondrosia reniformis Nardo, 1847 | + | + | + | + | ||
| Acarnus souriei Levi, 1952 *, ** | + | + | ||||
| Acarnus sp. | + | + | ||||
| Clathria (Microciona) armata (Bowerbank, 1866) *, ** | + | + | ||||
| Clathria (Microciona) atrasanguinea (Bowerbank, 1862) | + | + | + | |||
| Clathria (Microciona) gradalis Topsent, 1925 | + | + | ||||
| Clathria (Microciona) haplotoxa (Topsent, 1928) *, **, *** | + | + | ||||
| Clathria (Microciona) toxistyla (Sarà, 1959) | + | + | ||||
| Clathria (Microciona) toxivaria (Sarà, 1959) | + | + | ||||
| Clathria (Microciona) sp. | + | + | + | |||
| Antho (Antho) involvens (Schmidt, 1864) | + | + | ||||
| Eurypon cf. cinctum Sarà, 1960 | + | + | + | |||
| Eurypon clavatum (Bowerbank, 1866) | + | + | + | + | + | |
| Eurypon coronula (Bowerbank, 1874) ** | + | + | ||||
| Eurypon denisae Vacelet, 1969 *, ** | + | + | ||||
| Eurypon gracilis sp. n. Bertolino, Calcinai & Pansini | + | + | + | |||
| Eurypon major Sarà & Siribelli, 1960 | + | + | + | + | + | |
| Eurypon topsenti Pulitzer-Finali, 1983 | + | + | + | |||
| Eurypon vesciculare Sarà & Siribelli, 1960 | + | + | + | + | + | |
| Eurypon sp. | + | + | + | + | + | |
| Raspaciona aculeata (Johnston, 1842) | + | + | ||||
| Raspaciona sp. | + | + | ||||
| Forcepia (Leptolabis) brunnea (Topsent, 1904) ** | + | + | + | |||
| Lissodendoryx (Lissodendoryx) isodictyalis (Carter, 1882) | + | + | ||||
| Lissodendoryx (Anomodoryx) cavernosa (Topsent, 1892) | + | + | + | + | + | |
| Crambe crambe (Schmidt, 1862) | + | + | + | + | ||
| Crella (Crella) elegans (Schmidt, 1862) | + | + | ||||
| Crella (Crella) mollior Topsent, 1925 | + | + | ||||
| Crella (Grayella) pulvinar (Schmidt, 1868) | + | + | + | + | + | |
| Hemimycale columella (Bowerbank, 1864) | + | + | ||||
| Hymedesmia (Hymedesmia) baculifera Topsent, 1901 * | + | + | + | |||
| Hymedesmia (Hymedesmia) rissoi Topsent, 1936 | + | + | + | + | ||
| Hymedesmia sp. | + | + | + | |||
| Hymedesmia (Stylopus) coriacea (Fristedt, 1866) | + | + | + | + | ||
| Phorbas fictitius Bowerbank, 1866 | + | + | + | + | ||
| Phorbas mercator (Schmidt, 1868) * | + | + | ||||
| Phorbas lieberkuhni (Burton, 1930) | + | + | ||||
| Phorbas tenacior (Topsent, 1925) | + | + | + | + | + | |
| Phorbas sp. | + | + | + | |||
| Plocamionida ambigua (Bowerbank, 1866) * | + | + | + | + | + | |
| Tedania (Tedania) anhelans (Lieberkühn, 1859) | + | + | ||||
| Mycale (Aegogropila) tunicata (Schmidt, 1862) * | + | + | ||||
| Mycale (Paresperella) serrulata Sarà & Siribelli, 1960 **, *** | + | + | ||||
| Merlia normani Kirkpatrick, 1908 * | + | + | ||||
| Axinella damicornis (Esper, 1794) | + | + | + | + | + | |
| Axinella polypoides Schmidt, 1862 | + | + | ||||
| Axinella verrucosa (Esper, 1794) | + | + | + | |||
| Phakellia sp. | + | + | ||||
| Bubaris carcisis Vacelet, 1969 | + | + | + | + | ||
| Bubaris vermiculata (Bowerbank, 1866) | + | + | ||||
| Hymerhabdia oxytrunca Topsent, 1904 | + | + | ||||
| Hymerhabdia typica Topsent, 1892 * | + | + | ||||
| Hymerhabdia sp. | + | + | ||||
| Halicnemia geniculata Sarà, 1958 *, ** | + | + | ||||
| Halicnemia patera Bowerbank, 1864 | + | + | ||||
| Acanthella acuta Schmidt, 1862 | + | + | + | + | + | |
| Dictyonella incisa (Schmidt, 1880) | + | + | + | + | + | |
| Dictyonella marsilii (Topsent, 1893) | + | + | ||||
| Dictyonella pelligera (Schmidt, 1862) | + | + | + | + | ||
| Dictyonella sp. | + | + | ||||
| Halichondria (Halichondria) contorta Sarà, 1961 | + | + | + | |||
| Halichondria (Halichondria) cf. convolvens Sarà, 1960 | + | + | ||||
| Halichondria (Halichondria) genitrix Schmidt, 1862 | + | + | + | |||
| Halichondria (Halichondria) panicea Pallas, 1766 | + | + | + | |||
| Halichondria sp. | + | + | + | |||
| Agelas oroides Schmidt, 1864 | + | + | + | + | ||
| Dendroxea lenis (Topsent, 1892) | + | + | + | + | ||
| Haliclona (Gellius) angulata (Bowerbank, 1866) | + | + | + | + | ||
| Haliclona (Gellius) marismedi (Pulitzer-Finali, 1978) *, ** | + | + | + | + | ||
| Haliclona (Halichoclona) fulva (Topsent, 1893) | + | + | + | + | + | |
| Haliclona (Halichoclona) parietalis (Topsent, 1893) | + | + | + | |||
| Haliclona (Haliclona) sp. | + | + | + | |||
| Haliclona (Reniera) cinerea Grant, 1826 | + | + | ||||
| Haliclona (Reniera) citrina (Topsent, 1892) | + | + | + | |||
| Haliclona (Reniera) sp. | + | + | + | + | ||
| Haliclona (Soestella) arenata Griessinger, 1971 | + | + | ||||
| Haliclona (Soestella) mucosa (Griessinger, 1971) | + | + | ||||
| Haliclona sp. | + | + | ||||
| Siphonodictyon insidiosum (Johnson, 1899) | + | + | + | + | + | + |
| Petrosia (Petrosia) clavata (Esper, 1794) | + | + | + | + | ||
| Petrosia (Petrosia) ficiformis (Poiret, 1798) | + | + | + | + | + | |
| Ircinia variabilis (Schmidt, 1862) | + | + | + | + | + | + |
| Sarcotragus spinosulus Schmidt, 1862 | + | + | + | + | + | + |
| Cacospongia mollior Schmidt, 1862 | + | + | ||||
| Spongia (Spongia) officinalis Linnaeus, 1759 | + | + | ||||
| Spongia (Spongia) virgultosa (Schmidt, 1868) | + | + | + | + | + | + |
| Dysidea avara (Schmidt, 1862) | + | + | + | + | ||
| Dysidea sp. | + | + | ||||
| Pleraplysilla spinifera (Schulze, 1879) | + | + | + | + | ||
| Aplysina cavernicola Vacelet, 1959 | + | + | ||||
| Total number of species | 61 | 70 | 71 | 61 | 103 | 63 |
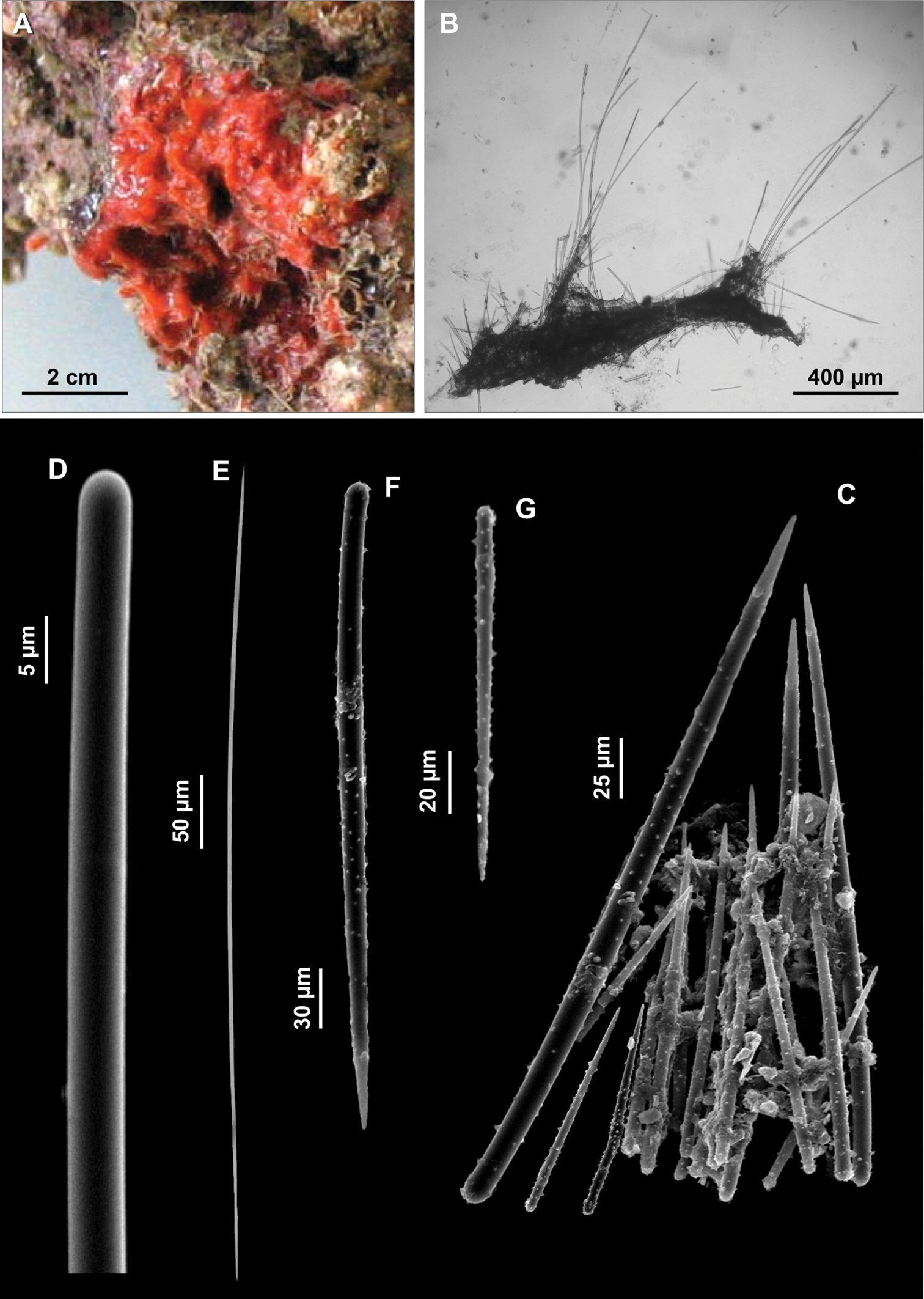
Porosity of the coralligenous concretion. A Holes and cavities of the coralligenous concretion B Magnification of the holes C Magnification of a natural hole occupied by spicules of Pachastrella monilifera D Spicules of Jaspis johnstoni in a natural cavity in the coralligenous concretion E Cavity excavated by a boring sponge with excavation marks (pits) on the wall F Border between the area excavated by a boring sponge (right) and the not excavated area (left).
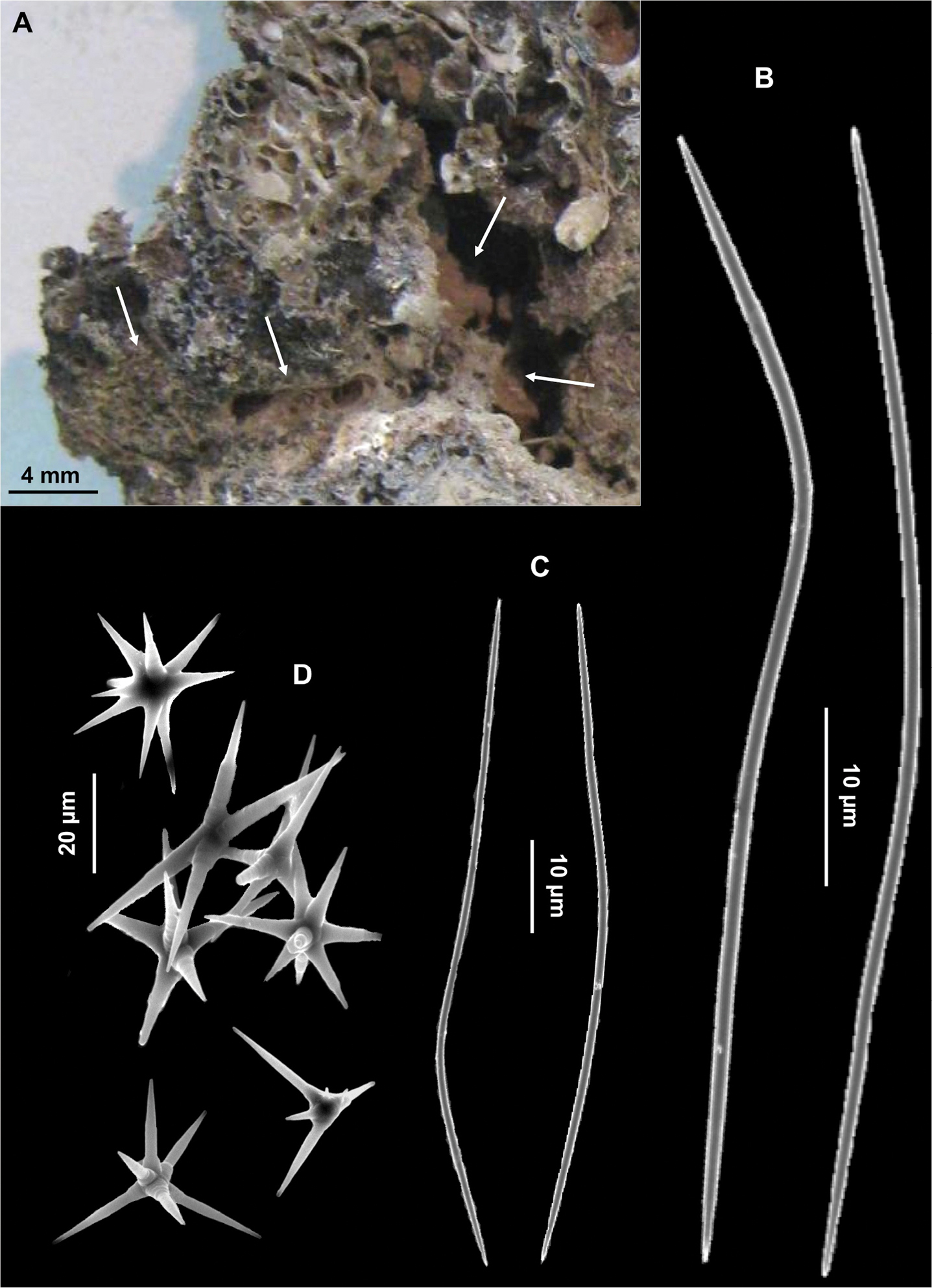
Among the 63 species recorded inside the conglomerate, 53 were insinuating and 10 boring (Table 1). From the first group six species: Geodia cydonium (Jameson, 1811) (Fig. 3A), Poecillastra compressa (Bowerbank, 1866) (Fig. 3D), Stelletta grubii Schmidt, 1862, Paratimea oxeata Pulitzer-Finali, 1978 (Fig. 3E), Hymedesmia (Hymedesmia) baculifera (Topsent, 1901) and Mycale (Paresperella) serrulata (Sarà & Siribelli, 1960) were hitherto recorded encrusting or massive; four species: Erylus discophorus (Schmidt, 1862), Penares euastrum (Schmidt, 1868), Geodia conchilega Schmidt, 1862 (Fig. 3B) and Pachastrella monilifera Schmidt, 1868 (Fig. 3C) were generally recorded as massive but also described as insinuating by Pulitzer-Finali (1970, 1983) and
Insinuating sponges. A Geodia cydonium B Geodia conchilega C Pachastrella monilifer a D Poecillastra compressa E Paratimea oxeata F Spongia virgultosa.
http://species-id.net/wiki/Cliona_burtoni
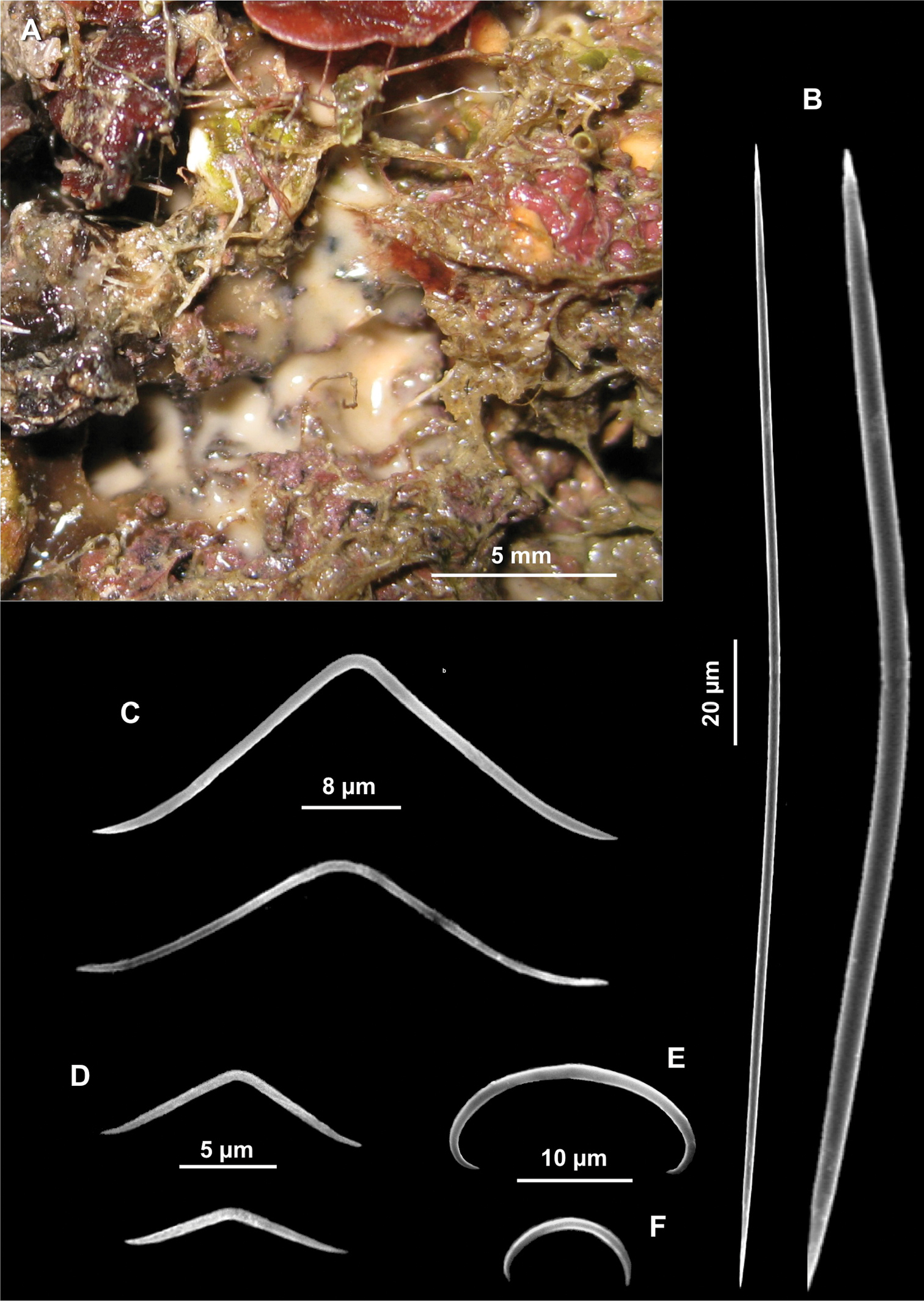
Figs 4A–LSpecimen IG-S-BL1-F5B-spB; dry state, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected 17-06-2009. The specimen was entirely used for spicule preparations.
Boring sponge in alpha growth form, occupying a surface of 1 cm2 in a section of conglomerate. Colour beige in dry state.
Skeleton. Not observed.
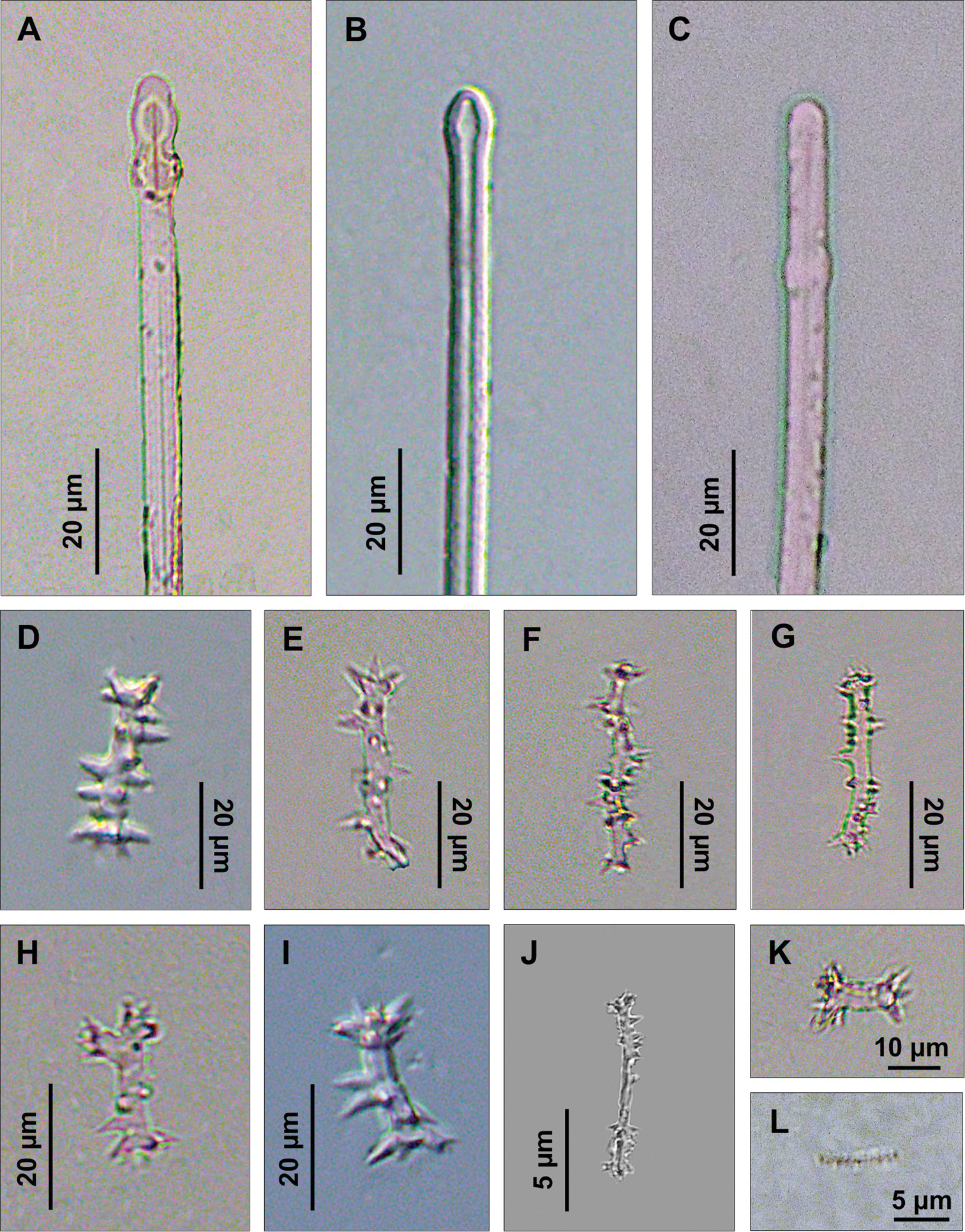
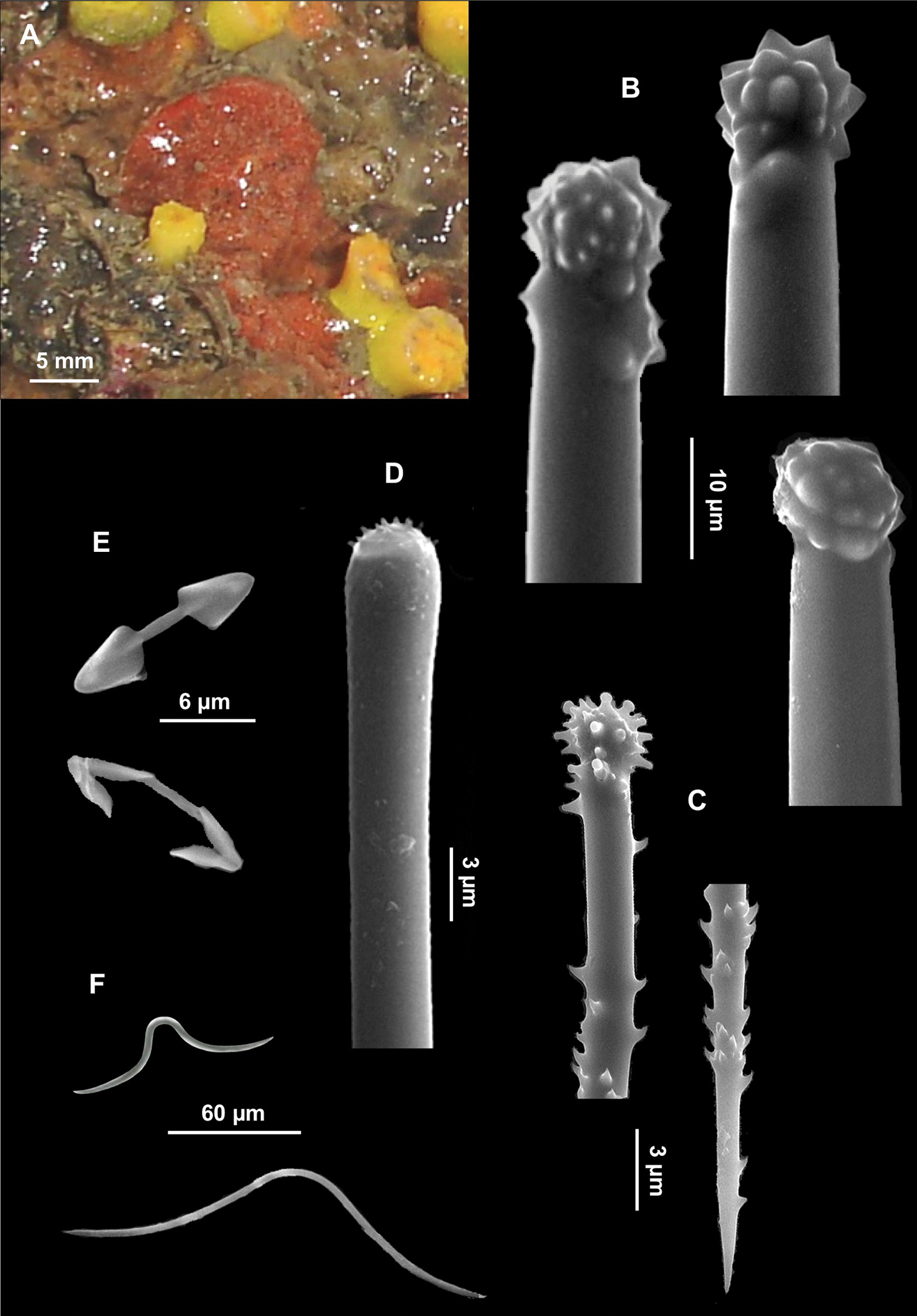
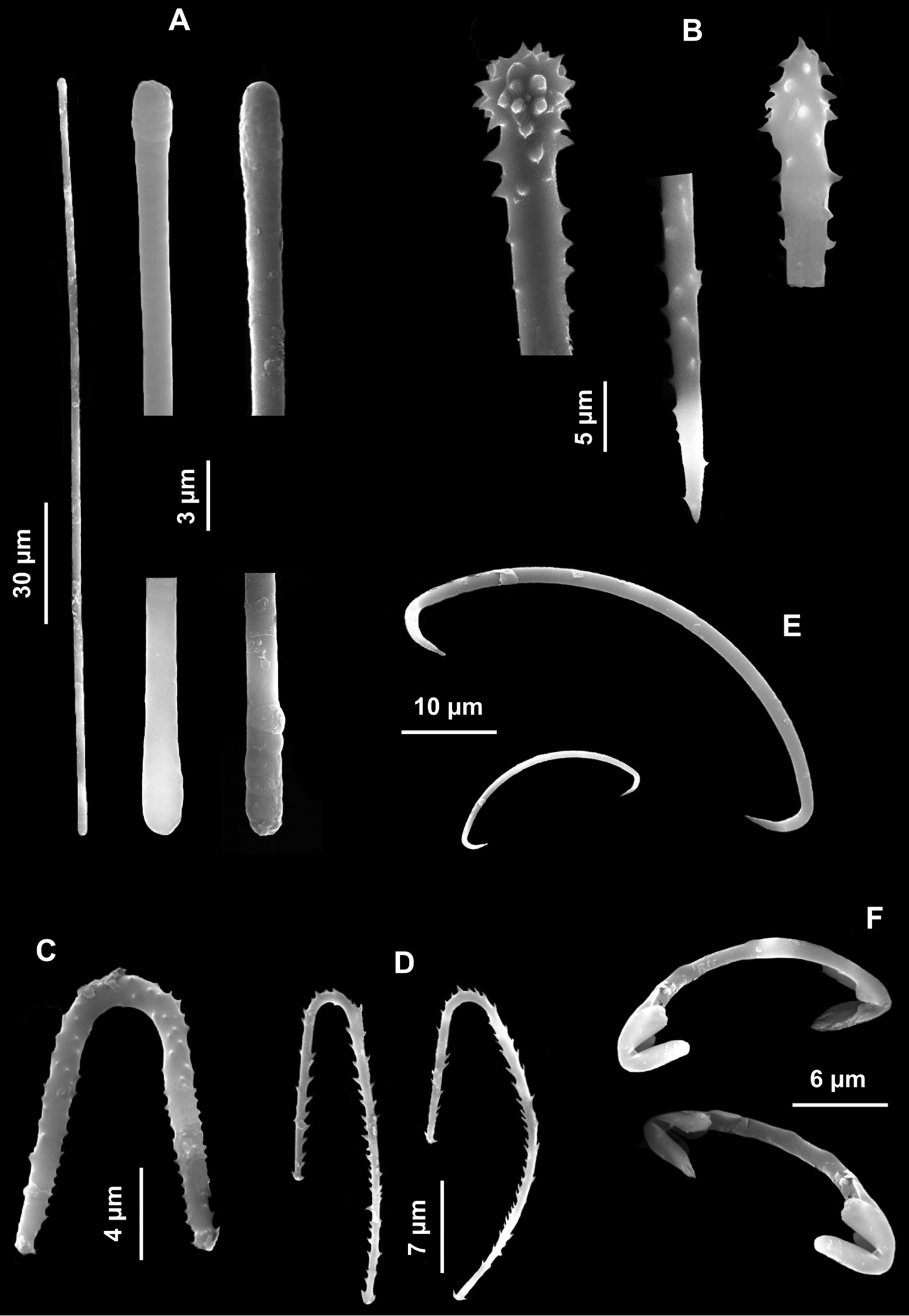
Spicules. Macroscleres: tylostyles to subtylostyles straight or slightly curved, 132 (225) 287 × 5 (6) 7.5 μm. Heads with a rounded or oval tyle, sometimes in terminal position but more often shifted along the shaft (Figs 4A, B, C). Microscleres: spirasters of various shape and thickness, straight or curved, 10 (26.5) 45 × 1.25 (10) 17.5 μm. The most abundant have scattered conical spines (Figs 4D, E, F, G, H, I, J, K) and numerous are amphiaster-like (Figs 4H, I, K). The smaller ones are microspinated (Fig. 4J, L).
Cliona burtoni. A–C Tylostyle heads D–L Spirasters of various shape and thickness.
This is a Mediterranean endemic species (
http://species-id.net/wiki/Paratimea_oxeata
Figs 5A–DSpecimen SSS-BL1-F3A-spH; alcohol and dry state; Santo Stefano Shoals (station 1), 43°49'N, 7°54'E, depth 35 m, collected 14-02-2008. The specimen was entirely used for spicule preparations.
Very small (0.5 cm2) insinuating sponge (Fig. 5A) detected inside a cavity of a slice of a coralligenous block. Grey coloured in dry state.
Skeleton. Not observed.
Spicules. Macroscleres: oxeas in two size categories: I) large oxeas curved, bent or flexuous, with hastate tips (Fig. 5B), 810 (961.25) 1200 × 15 (18) 25 μm; II) small oxeas curved or flexuous (Fig. 5C), 300 (546.6) 700 × 2.5 (4.75) 5 μm. Microscleres: oxyasters with more or less marked centrum with 9-12 conical rays, 25 (41.5) 60 μm in diameter. In some cases the number of rays is reduced (Fig. 5D).
Paratimea oxeata. A Specimen in the coralligenous accretions (arrows) B Large oxeas C Small oxeas D Oxyasters.
The species wasdescribed from Naples (
http://species-id.net/wiki/Clathria_armata
Figs 6A–FSpecimen IG-F-BL4-sp2-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected 31-7-2009.
Description. Thickly encrusting sponge (3-5 mm thick) covering a surface of 1.5 cm2 on a coralligenous block (Fig. 6A). Surface irregular, smooth. Consistency soft. The red-orange colour of the living specimen slightly fades when alcohol preserved.
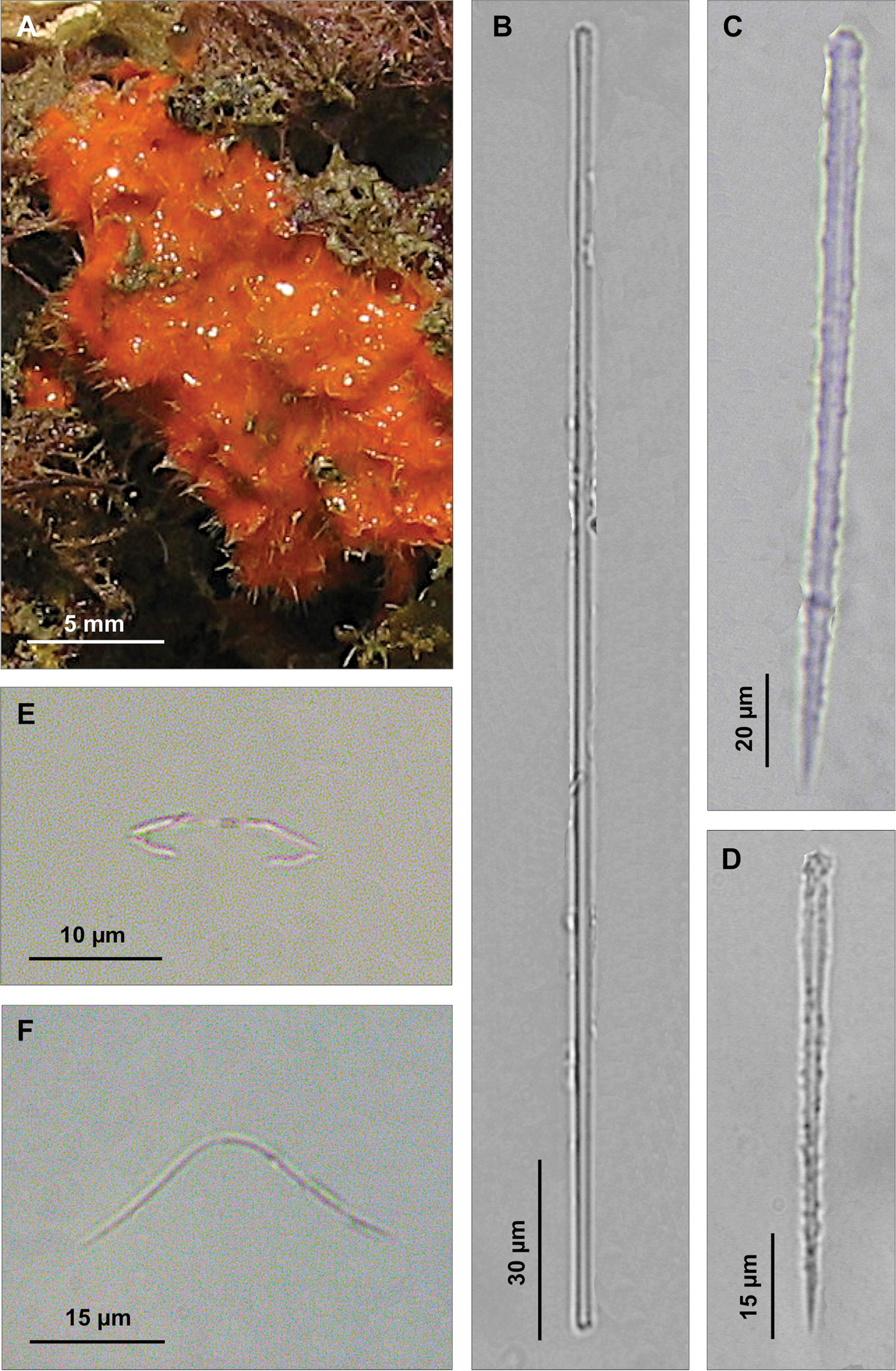
Clathria (Microciona) armata. A Specimen on the surface of the coralligenous block B Large acanthostyle heads C Small acanthostyle D Subtylostyle with spined head E Palmate isochelae F Toxas of variable size, with smooth extremities.
Skeleton. Not observed.
Spicules. Macroscleres: acanthostyles in two size categories: I) large acanthostyles slightly curved, with obtuse spines concentrated on the head (Fig. 6B), 220 (484.5) 830 × 3.75 (8.5) 12 μm; II) small acanthostyles, with scattered spines, but more concentrated on the head (Fig. 6C), 100 (110) 122.5 × 3.75 (5) 6 μm; subtylostyles straight, often with slightly spined head (Fig. 6D), 440 (503.7) 550 × 2.5 (2.9) 3.8 μm. Microscleres: palmate isochelae (Fig. 6E), 10 (12.5) 13.5 μm long. Toxas of variable size, with more or less wide central curvature and slightly reflexed smooth points (Fig. 6F), 80 (114.5) 210 μm long.
This species has been recorded on rocky walls and on mollusc shells from 10 to 180 m depth (
This specimen, like that described by
http://species-id.net/wiki/Clathria_haplotoxa
Figs 7A–FSpecimen IG-F-BL3-sp5-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected 17-06-2009. The specimen was entirely used for spicule preparations.
Encrusting sponge on the surface of a coralligenous block, 2 cm in diameter. Surface hispid. Colour brick red (Fig. 7A).
Skeleton. Not observed.
Spicules. Macroscleres: strongyles straight, smooth, 112.5 (178) 215 × 2.5 μm (Fig. 7B); acanthostyles straight with a characteristic constriction under the head, in two size categories: I) large acanthostyles (Fig. 7C), 150 (175.5) 210 μm and II) small acanthostyles (Fig. 7D), 55 (74.5) 102.5 × 2.5 (3.5) 5 μm. Microscleres: palmate isochelae with straight shaft (Fig. 7E), 12.5 (13.8) 15 μm long; toxas thin, smooth, with wide central curvature and slightly reflexed points, 30 (32.5) 37.5 μm long (Fig. 7F).
Distribution and discussion. Described from Porto Santo Bay (Madeira) the species extends south to the Sahelian Upwelling (
Clathria (Microciona) haplotoxa. A Specimen on the surface of a coralligenous block B Strongyle C Large acanthostyle D Small acanthostyle E Isochela F Toxa.
http://species-id.net/wiki/Eurypon_denisae
Figs 8A–ESpecimen IG-S-BL3 sp10-fot.; alcohol preserved, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected 31-07-2009.
Encrustingsponge covering a surface of 3 cm2 on a coralligenous block. Surface hispid. Colour in life white.
Skeleton. Ectosomal skeleton absent. Choanosomal skeleton consisting of basal acanthostyles with heads embedded in a spongin layer and bundles of very long tylostyles protruding through the sponge surface which appears hispid.
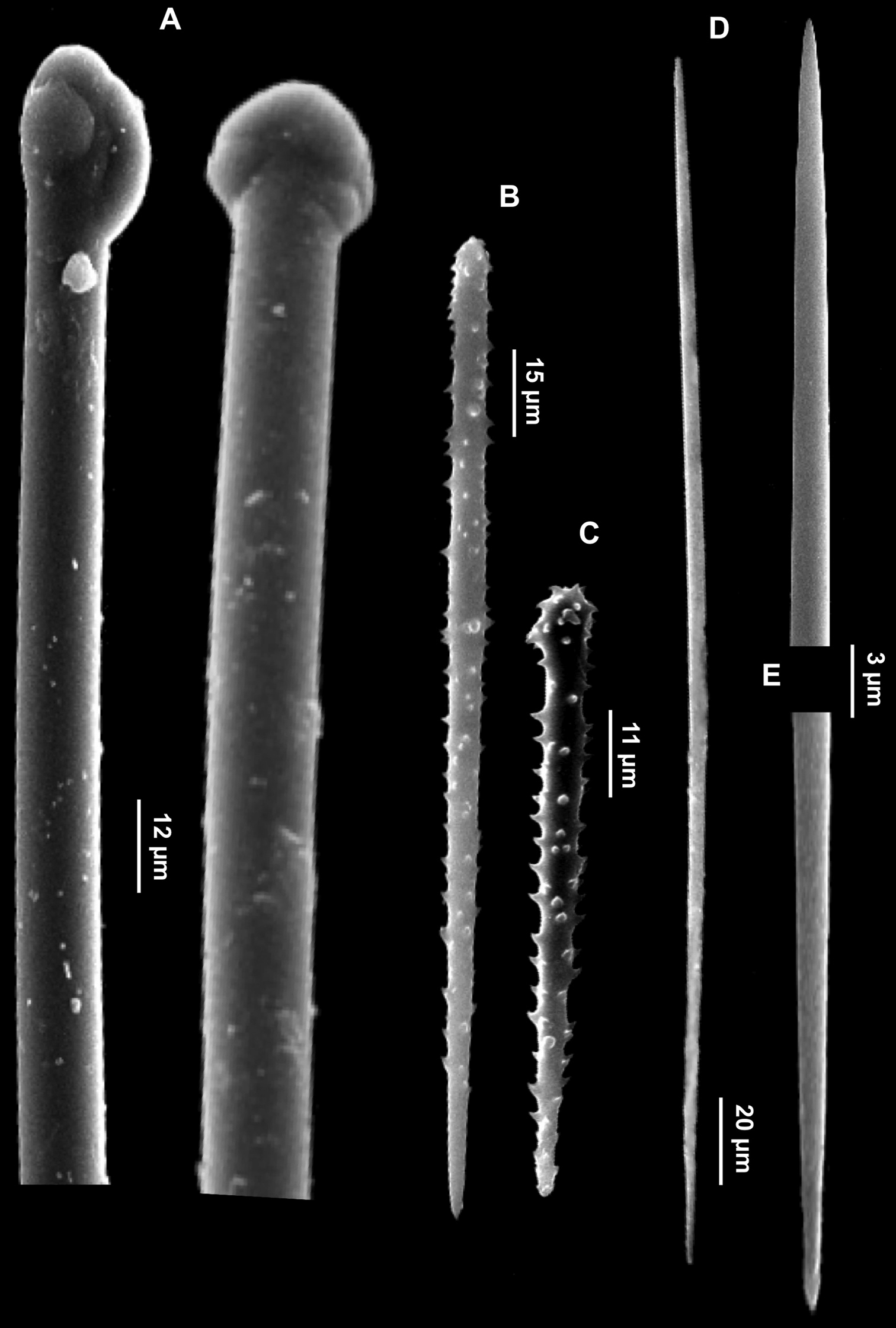
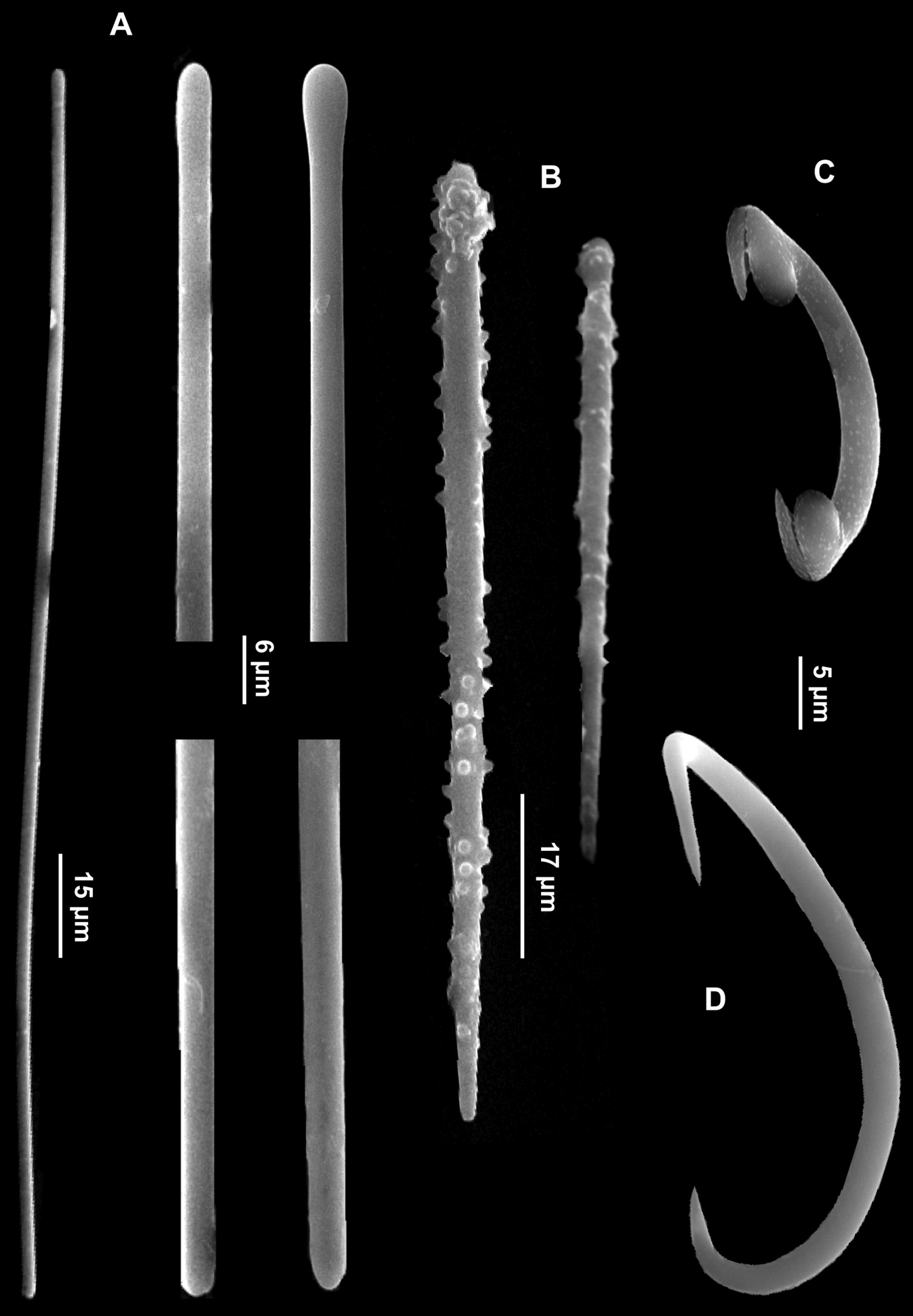
Spicules. Long tylostyles, slightly curved or straight, with rather irregular heads, 1066 (1774) 2236 × 5 (8.5) 12.5 μm (Fig. 8A); anisoxeas straight or faintly curved, 200 (220) 250 × 5 (5.5) 7 μm (Figs 8D-E); acanthostyles in two size categories: I) large, straight acanthostyles, often with inconspicuous heads, uniformly but faintly spined, 107.7 (134.5) 170 × 7.5 (9) 12 μm (Fig. 8B); II) small, straight acanthostyles with stouter and longer spines, 60 (68) 77.5 × 7.5 (8) 10 μm (Fig. 8C).
Eurypon denisae. A Tylostyles with variable head B Large acanthostyles C Small acanthostyles D Anisoxeas E Magnifications of the extremities of an anisoxea.
The species was originally described by
http://zoobank.org/E2792BEE-BEC2-41E5-BB7E-E32969E50A1C
http://species-id.net/wiki/Eurypon_gracilis
Figs 9A–GType specimen: Holotype MSNG 57017. Specimen PdF-S-BL4-sp18-sciaf., on a coralligenous concretion, depth 40 m, Stat. 4, 27-07-2009. leg. M. Bertolino, alcohol preserved.
Italy, Ligurian Sea, Portofino Promontory (Punta del Faro) 44°17'54.20"N, 9°13'06.93"E.
Specimen IG-F-BL1-sp4-fot.; specimen IG-F-BL1-sp15-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected 17-06-2009; specimen IG-S-BL3-sp6-fot.; alcohol preserved, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected 17-06-2009; specimen PM-BL1-sp9-sciaf.; alcohol preserved, Punta Manara (station 6) 44°15'05.61"N, 9°24'09.33"E, depth 35 m, collected 13-06-2009.
All the specimens were encrusting on the surface of coralligenous blocks, covering surfaces up to 2 cm2. The sponge surface is corrugated, hispid. The colour in life is brick red (Fig. 9A).
Eurypon gracilis sp. n. A Holotype B Skeleton C Portion of the skeleton with large and small echinating acanthostyles D Long style E Oxea F Large acanthostyle with scattered small spines G Small acanthostyle.
Skeleton. The skeleton consists of a basal layer of spongin in which the spicules are vertically positioned, perpendicular to the substrate. Both the categories of acanthostyles are close one another (Fig. 9C) with the heads embedded in the basal spongin layer. Styles and oxeas–with the same vertical arrangement–are grouped in bundles which are faintly echinated, in their lower part, by the smaller acanthostyles (Fig. 9B). Oxeas are positioned in the basal part of the bundles. The styles protrude trough the sponge surface making it hispid.
Spicules. Long styles to tylostyles, curved or flexuous (Fig. 9D), 788 (1101) 1280 × 5 (6.8) 10 µm; oxeas thin, almost straight or with a slight curvature (Fig. 9E), 365 (483) 650 × 2.5 µm; acanthostyles without head and uniformly spined, in two sizes categories: I) large acanthostyles, straight or slightly curved with rather small spines (Fig. 9F), 200 (253) 320 × 5 (6) 7.7 µm; II) small acanthostyles straight, with spines more robust than in the previous category (Fig. 9G), 90 (119.5) 160 × 2.5 (3.8) 5 µm.
The species is named after the slenderness of all the spicule types.
So far known only from the Ligurian Sea.
It lives at 30–40 m depth on coralligenous concretion, characterized by the presence of a Paramuricea clavata facies.
This species, characterized by a microcionid skeleton with a basal layer of spongin, extra-axial spicules and echinating achantostyles embedded in spongin fibres, clearly belongs to the genus Eurypon.
Only five, out of the numerous species of the genus Eurypon found in the temperate Western Atlantic have oxeas or tornotes as structural megascleres together with styles or tylostyles. All of them (Eurypon cinctum Sarà, 1960, Eurypon denisae Vacelet, 1969, Eurypon obtusum Vacelet, 1969, Eurypon major Sarà & Siribelli, 1960 and Eurypon lacazei (Topsent, 1891) occur in the Mediterranean Sea. Eurypon cinctum showing a lilac colour, achantostyles with discrete heads and different size in the other megascleres is not close to the new species. Eurypon denisae is also different according to the description given above. Eurypon obtusum is grey in colour and has smaller oxeas and acanthostyles than those of the present species, but the maximum length of its tylostyles is unknown. Eurypon lacazei remarkably differs from the present species for the green colour and spicule shape and size. The closest species to the new one is Eurypon major but its tylostyles are longer and stouter (1445–2210 × 10–17 µm) and differ in the shape of the heads, while the acanthostyles, in a single size category, have well formed heads. Only two other species from the temperate Atlantic: Eurypon lictor (Topsent, 1904) and Eurypon (Acantheurypon) mucronale (Topsent, 1928) present oxeas. However, they are both deep species (recorded deeper than 1500 m from the Azores) and they differ also in the spicule characters from Eurypon gracilis sp. n. There are two other species of Eurypon with oxeas reported in the literature: Eurypon calypsoi Lévi, 1958 from the Red Sea which is blue in colour and Eurypon fulvum Lévi, 1969 from South Africa which is yellow. Both have a single size category of acanthostyles and differ in the spicule morphology. Eurypon gracilis therefore has to be considered as new for science.
http://species-id.net/wiki/Forcepia_brunnea
Figs 10A–FSpecimen PdF-NE-BL2A-sp15-sciaf.; alcohol preserved, Portofino Promontory (Punta del Faro, station 4) 44°17'55.61"N, 9°13'07.95"E, 40 m depth, collected on 27-08-2009; specimen IG-S-BL3-sp13-sciaf.; alcohol preserved, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45" E, depth 30 m, collected on 17-06-2009; specimen PdF-BL8-sp50-sciaf.; alcohol preserved, Portofino Promontory (Punta del Faro, station 4) 44°17'55.61"N, 9°13'07.95"E, 30 m depth, collected on 25-01-2013.
Thin, smallencrusting sponges (up to 0.5 cm2) on the surface of coralligenous blocks. Colour in life yellow-orange.
Skeleton. Basal acanthostyles erect on the substrate in a hymedesmioid arrangement. Other spicule types not detectable from the skeleton.
Spicules. Megascleres: anisotylotes straight or faintly curved, with slightly different extremities and a few malformations along the shaft (Fig. 10A), 127.5 (157.7) 280.5 × 1.25 (2.3) 2.5 μm; acanthostyles straight, conical with discrete but not swollen heads. Spines evenly distributed, slightly stouter on the spicule head (Fig. 10B), 61.2 (92.2) 142.8 × 5.2 (7.5) 10.4 μm. Microscleres: acanthose symmetric forceps with straight legs, ending in small, button-like swellings with toothed margin (Fig. 10C). They measure 12.5 (15.8) 17.5 × 2.5 μm in length, the distance between the legs being 5.2 (7.2) 7.5 μm. Acanthose asymmetric forceps, very thin, have unequal legs (Fig. 10D), the longer of which is straight or curved inward, 20.4 (22.3) 25 × 1.5 μm. Sigmas in two size categories: the larger ones, “C" shaped (Fig. 10E) or more rarely “S" shaped, 40.8 (64.3) 80 × 2.5 μm are very abundant, the smaller, 17.5–25.5 μm are rare. Palmate isochelae (Fig. 10F), 18 (20) 20.8 μm long.
Forcepia (Leptolabis) brunnea. A Anisotylotes B Acanthostyles C Symmetric forceps D Asymmetric forceps E Large and small sigmas F Isochelae.
Leptolabis brunnea was afterwards recorded from the Far-Oer Islands, the Azores, Spain (NW coast, Strait of Gibaltar, Castellón, Girona), France (Marseille, Monaco), Italy (Gulf of Naples), between 4 and 1360 m depth. It lives in caves, detritic bottoms, coralligenous concretions and epibiotic on other organisms (
http://species-id.net/wiki/Hymedesmia_rissoi
Figs 11A–DSpecimen IG-F-BL3-F18b-spA; Specimen IG-F-BL4-sp9-sciaf.; specimen IG-F-BL4 sp11-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected on 17-06-2009; specimenSSS-BL1-sp11-sciaf.; Santo Stefano Shoals, (station 1), 43°49'N, 7°54'E, depth 35 m, collected on 14-02-2008.
Small (0.5 cm2), slimy, coriaceous encrusting sponge, grey in colour after alcohol preservation, recorded both on the surface and inside the coralligenous blocks.
Skeleton. Not observed.
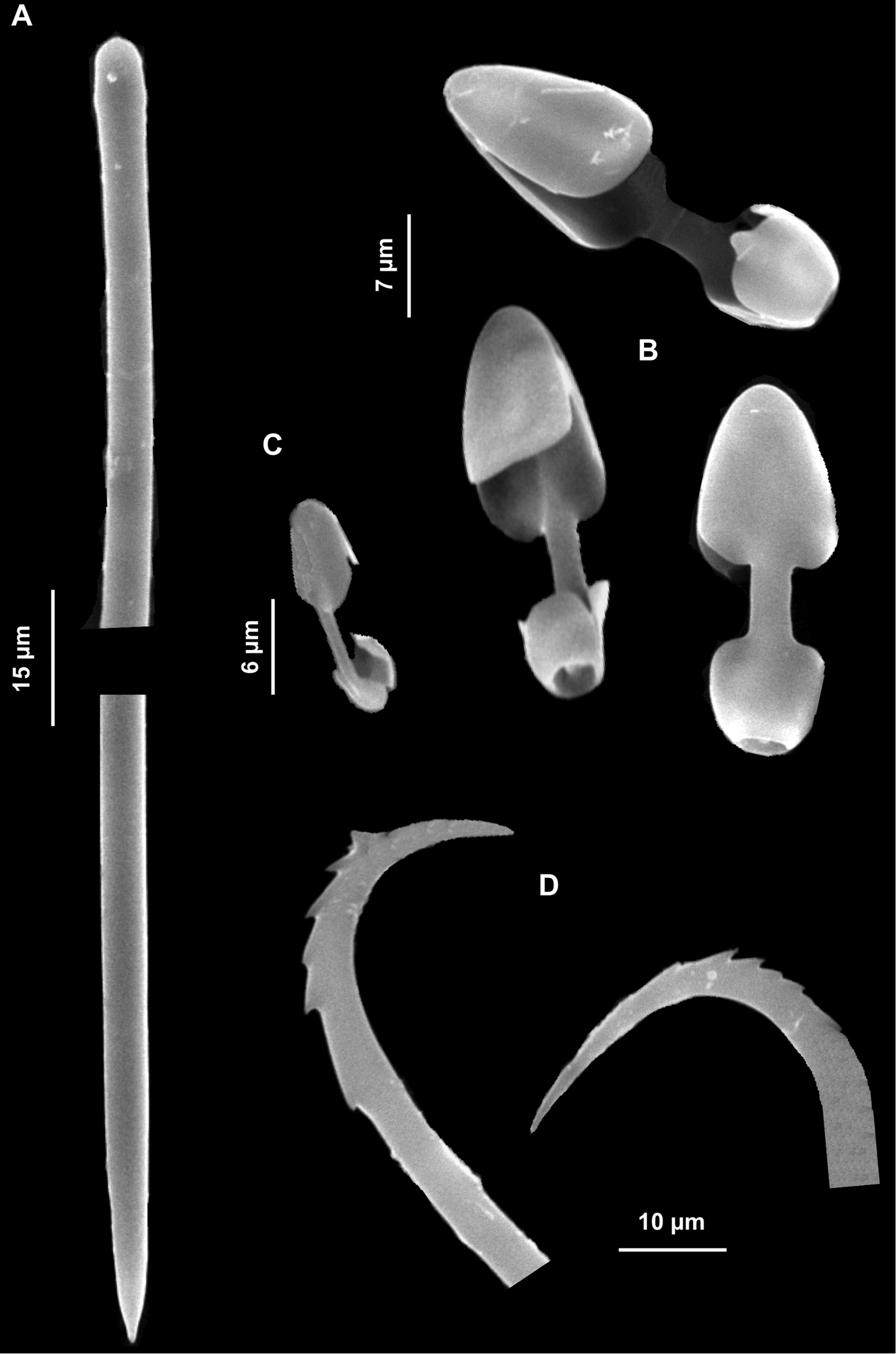
Spicules. Megascleres: straight or slightly sinuous anisotornotes, sometimes modified in anisotylotes or strongyles (Fig. 11A), 140 (175) 177.5 × 2.5 (2.7) 3.75 μm; acanthostyles in a single size category, 67.5 (84) 105 × 2.5 (3.5) 3.75 μm, devoid of conspicuous heads. The extremities may be pointed or blunt (Figs 11B, C). Microscleres: arcuate isochelae (Fig. 11D), 25 (25.6) 27.5 μm long; thin sigmas “C” (Fig. 11E) and “S” shaped, 32.5 (35) 37.5 × 1.25 μm.
Hymedesmia (Hymedesmia) rissoi. A Tornote, sometimes modified into subtylotes and strongyles B Acanthostyles C Arcuate isochelae D Thin sigmas.
In the original description
http://species-id.net/wiki/Mycale_serrulata
Figs 12A–DSpecimen IG-F-BL3-F4B-spA; specimen IG-F-BL3-F17B-spA alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected on 31-07-2009. The specimen was entirely used for spicule preparations.
Description. Small, encrusting and insinuating sponge, beige in the dry state, occupying a small cavity (1 cm3) in a coralligenous block.
Skeleton. Not observed.
Spicules. Megascleres: mycalostyles straight or flexuous, with acerate tip (Fig. 12A), 310 (325) 340 × 3.75 (5) 7.5 µm. Microscleres: anisochelae in two size categories. I) The larger ones, 25 (29.5) 35 µm, have the bigger tooth palmate and the smaller often characterized by a conspicuous point and slightly diverging outwords alae; a hole is detectable at the smaller extremity (Fig. 12B). II) The smaller ones measure, 12.5 (13.7) 15 µm (Fig. 12C). Sigmas “C” shaped, 64 (78) 100 × 2.5 (2.7) 5 µm, with the convex edge serrated (Fig. 12D).
Mycale (Paresperella) serrulata. A–B Mycalostyles B Large anisochelae C Small anisochelae D Magnifications of the serrated edge of a sigma.
Mycale (Paresperella) serrulata Sarà & Siribelli, 1960, was originally described from a detritic bottom of the Gulf of Naples at 30-40 m depth.
http://species-id.net/wiki/Halicnemia_geniculata
Figs 13A–DSpecimen IG-F-BL4-sp1-sciaf.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected on 17-06-2009. The specimen was entirely used for spicule preparations.
Small and thin, yellow-ochre encrustation (1 cm2) on a coralligenous block.
Skeleton. Not observed.
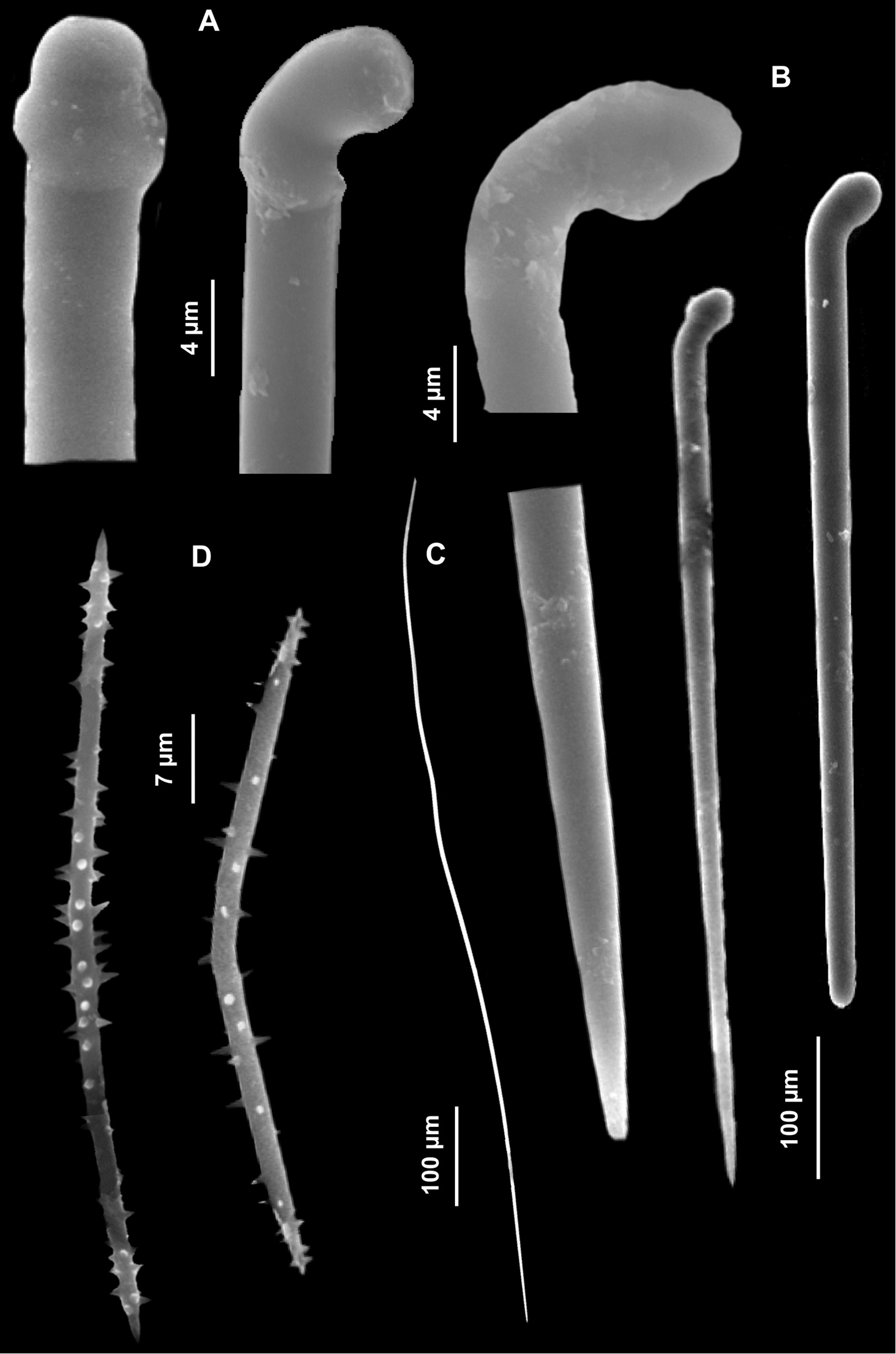
Spicules. Long tylostyles, 405 (1351.7) 1976 × 1.5 (2.7) 4 μm, generally straight, with terminal or subterminal swellings variable in shape; irregular and polytylote forms are to be found (Fig. 13A). Rabdhotylostyles with heads as above, 147(242)705 × 1.5 (2.7) 4 μm (Fig. 13B); oxeas long, sinuous and thin, 460 (757) 1118 × 1.5 (2.5) 5 mm (Fig. 13C); acanthoxeas slightly curved or bent, uniformly spined, 42.5 (51.8) 62.5 × 1.5 (1.8) 2 μm (Fig. 13D).
Halicnemia geniculata. A Magnifications of the tylostyle heads B Rabdhotylostyles C Oxeas, long, sinuous and thin D Acanthoxeas.
This species, originally described from a superficial cave of the Gulf of Naples (
http://species-id.net/wiki/Haliclona_marismedi
Figs 14A–FSpecimen PM-BL1-sp7-sciaf.; specimen PM-BL1-sp8-sciaf.; specimen PM-BL2b-sp6-sciaf.; specimen PM-BL2b-sp6a-sciaf.; Punta Manara (station 6) 44°15'05.61"N, 9°24'09.33"E, depth 35 m, collected 13-07-2009; specimen IG-S-BL1-sp2-sciaf.; Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected on17-06-2009.
Description. Small (1-1.5 cm2) encrusting and insinuating sponge, beige or brown, detected on the surface and inside a coralligenous block. Surface smooth, consistency soft (Fig. 14A).
Haliclona (Gellius) marismedi. A Specimen on the surface of the coralligenous block and insinuating into it B Oxeas C Large toxas D Small toxas E Large sigma F Small sigma.
Skeleton. The choanosome consists of multispicular primary lines connected by unispicular secondary tracts, creating a confused reticulation.
Spicules. Oxeas gently curved with hastate extremities detectable only in the larger spicules (Fig. 14B), 220 (245) 275 × 2.5 (4.5) 6.25 μm; toxas with more or less angulate central curvature and slightly reflexed points in two size categories: I) 27.5 (45.5) 57.5 μm (Fig. 14C) and II) 10 (11.5) 12.5 μm (Fig. 14D); two types of thin sigmas, “C” shaped, I) 22.5 (23.7) 25 μm and II) 10 (13.6) 17.5 μm (Figs 14E, F).
Distribution and discussion.
This is a new finding for the Ligurian Sea and the coralligenous community and the third record after the original description.
According to the latest available revision of coralligenous biodiversity (
List of sponge species (Demospongiae and Homoscleromorpha) hitherto recorded associated to the coralligenous community.
| Oscarellidae | |
| 1. | Oscarella lobularis (Schmidt, 1862) |
| Plakinidae | |
| 2. | Corticium candelabrum Schmidt, 1862 |
| 3. | Placinolopha moncharmonti (Sarà, 1960) |
| 4. | Plakina monolopha Schulze, 1880 |
| 5. | Plakina dilopha Schulze, 1880 |
| 6. | Plakina trilopha Schulze, 1880 |
| 7. | Plakinastrella copiosa Schulze, 1880 |
| 8. | Plakinastrella mixta Maldonado, 1992 |
| 9. | Plakortis simplex Schulze, 1880 |
| Tetillidae | |
| 10. | Craniella cranium (Müller, 1776) |
| Samidae | |
| 11. | Samus anonymus Gray, 1867 |
| Ancorinidae | |
| 12. | Stelletta dorsigera Schmidt, 1862 |
| 13. | Stelletta grubii Schmidt, 1862 |
| 14. | Stelletta lactea Carter, 1871 |
| 15. | Stelletta stellata Topsent, 1893 |
| 16. | Jaspis incrustans (Topsent, 1890) |
| 17. | Jaspis johnstonii (Schmidt, 1862) |
| 18. | Stryphnus mucronatus (Schmidt, 1868) |
| 19. | Stryphnus ponderosus (Bowerbank, 1866) |
| 20. | Penares candidata (Schmidt, 1868) |
| 21. | Penares euastrum (Schmidt, 1868) |
| 22. | Penares helleri (Schmidt, 1864) |
| 23. | Holoxea furtiva Topent, 1892 |
| 24. | Dercitus (Dercitus) bucklandi (Bowerbank, 1858) |
| 25. | Dercitus (Stoeba) plicata (Schmidt, 1868) |
| Calthropellidae | |
| 26. | Calthropella (Calthropella) pathologica (Schmidt, 1868) |
| 27. | Calthropella (Corticellopsis) stelligera (Schmidt, 1868) |
| Geodiidae | |
| 28. | Erylus discophorus (Schmidt, 1862) |
| 29. | Erylus papulifer Pulitzer-Finali, 1983 |
| 30. | Caminus vulcani Schmidt, 1862 |
| 31. | Pachymatisma johnstonia (Bowerbank in Johnston, 1842) |
| 32. | Geodia anceps (Vosmaer, 1894) |
| 33. | Geodia conchilega Schmidt, 1862 |
| 34. | Geodia cydonium Jamenson, 1811 |
| 35. | Caminella intuta (Topsent, 1892) |
| Pachastrellidae | |
| 36. | Pachastrella monilifera Schmidt, 1868 |
| 37. | Poecillastra compressa (Bowerbank, 1866) |
| 38. | Nethea amygdaloides (Carter, 1876) |
| 39. | Thenea muricata (Bowerbank, 1858) |
| 40. | Triptolemma simplex (Sarà, 1959) |
| 41. | Vulcanella (Vulcanella) gracilis (Sollas, 1888) |
| 42. | Annulastrella verrucolosa (Pulitzer-Finali, 1983) |
| Clionaidae | |
| 43. | Cliona burtoni Topsent, 1932 |
| 44. | Cliona carteri (Ridley, 1881) |
| 45. | Cliona celata Grant, 1826 |
| 46. | Cliona lobata Hancock, 1849 |
| 47. | Cliona janitrix Topsent, 1932 |
| 48. | Cliona rhodensis Rützler & Bromley, 1981 |
| 49. | Cliona schmidtii (Ridley, 1881) |
| 50. | Cliona thoosina Topsent, 1888 |
| 51. | Cliona vermifera Hancock, 1867 |
| 52. | Cliona viridis Schmidt, 1862 |
| 53. | Dotona pulchella mediterranea Rosell & Uriz, 2002 |
| 54. | Pione vastifica (Hancock, 1849) |
| 55. | Spiroxya corallophila (Calcinai, Cerrano & Bavestrello, 2002) |
| 56. | Spiroxya heteroclita Topsent, 1896 |
| 57. | Spiroxya levispira (Topsent, 1898) |
| 58. | Spiroxya sarai (Melone, 1965) |
| Thoosidae | |
| 59. | Alectona millari Carter, 1879 |
| 60. | Delectona ciconiae Bavestrello, Calcinai & Sarà, 1996 |
| 61. | Delectona madreporica Bavestrello et al., 1997 |
| 62. | Thoosa armata Topsent, 1888 |
| 63. | Thoosa mollis Volz, 1939 |
| Hemiasterellidae | |
| 64. | Paratimea constellata (Topsent, 1893) |
| 65. | Paratimea oxeata Pulitzer-Finali, 1978 |
| Stelligeridae | |
| 66. | Stelligera rigida (Montagu, 1818) |
| Polymastiidae | |
| 67. | Polymastia inflata Cabioch, 1968 |
| 68. | Polymastia mamillaris (Müller, 1806) |
| 69. | Polymastia polytylota Vacelet, 1969 |
| 70. | Quasillina brevis (Bowerbank, 1861) |
| 71. | Pseudotrachya hystrix (Topsent, 1890) |
| Spirastrellidae | |
| 72. | Diplastrella bistellata (Schmidt, 1862) |
| 73. | Spirastrella cunctatrix Schmidt, 1868 |
| Suberitidae | |
| 74. | Aaptos aaptos (Schmidt, 1864) |
| 75. | Prosuberites longispina Topsent, 1893 |
| 76. | Protosuberites ectyoninus (Topsent, 1900) |
| 77. | Protosuberites epiphytum (Lamarck, 1815) |
| 78. | Protosuberites rugosus (Topsent, 1893) |
| 79. | Pseudosuberites hyalinus (Ridley & Dendy, 1867) |
| 80. | Pseudosuberites sulphureus (Bowerbank, 1866) |
| 81. | Suberites carnosus (Johnston, 1842) |
| 82. | Suberites carnosus incrustans Topsent, 1900 |
| 83. | Suberites domuncula (Olivi, 1792) |
| 84. | Suberites syringella (Schmidt, 1868) |
| 85. | Terpios gelatinosa (Bowerbank, 1866) |
| Tethyidae | |
| 86. | Tethya aurantium (Pallas, 1766) |
| 87. | Tethya citrina Sarà & Melone, 1965 |
| Timeidae | |
| 88. | Timea cumana Pulitzer-Finali, 1978 |
| 89. | Timea fasciata Topsent, 1934 |
| 90. | Timea irregularis Sarà & Siribelli, 1960 |
| 91. | Timea stellata (Bowerbank, 1866) |
| 92. | Timea stellifasciata Sarà & Siribelli, 1960 |
| 93. | Timea unistellata (Topsent, 1892) |
| Trachycladidae | |
| 94. | Trachycladus minax (Topsent, 1888) |
| Chondrillidae | |
| 95. | Chondrosia reniformis Nardo, 1847 |
| 96. | Chondrilla nucula Schmidt, 1862 |
| Desmanthidae | |
| 97. | Desmanthus incrustans (Topsent, 1889) |
| Acarnidae | |
| 98. | Acarnus souriei (Lévi, 1952) |
| 99. | Acarnus tortilis Topsent, 1892 |
| Microcionidae | |
| 100. | Clathria (Clathria) compressa (Schmidt, 1862) |
| 101. | Clathria (Clathria) coralloides (Olivi, 1792) |
| 102. | Clathria (Clathria) depressa Sarà & Melone, 1966 |
| 103. | Clathria (Clathria) toxivaria (Sarà, 1959) |
| 104. | Clathria (Microciona) armata (Bowerbank, 1862) |
| 105. | Clathria (Microciona) assimilis Topsent & Olivier, 1943 |
| 106. | Clathria (Microciona) gradalis Topsent, 1925 |
| 107. | Clathria (Microciona) haplotoxa (Topsent, 1928) |
| 108. | Clathria (Microciona) spinarcus (Carter & Hope, 1889) |
| 109. | Clathria (Microciona) toxistyla (Sarà, 1959) |
| 110. | Antho (Antho) inconstans (Topsent, 1925) |
| 111. | Antho (Antho) involvens (Schmidt, 1864) |
| 112. | Antho (Acarnia) coriacea (Bowerbank, 1874) |
| 113. | Antho (Acarnia) cf. novizelanica (Ridley & Duncan, 1881) |
| Raspailiidae | |
| 114. | Raspailia (Raspailia) viminalis Schmidt, 1862 |
| 115. | Aulospongus spinosus (Topsent, 1927) |
| 116. | Eurypon cinctum Sarà, 1960 |
| 117. | Eurypon clavatum (Bowerbank, 1866) |
| 118. | Eurypon coronula (Bowerbank, 1874) |
| 119. | Eurypon denisae Vacelet, 1969 |
| 120. | Eurypon gracilis Present paper |
| 121. | Eurypon lacazei (Topsent, 1891) |
| 122. | Eurypon major Sarà & Siribelli, 1960 |
| 123. | Eurypon topsenti Pulitzer-Finali, 1983 |
| 124. | Eurypon vesciculare Sarà & Siribelli, 1960 |
| 125. | Eurypon viride (Topsent, 1889) |
| 126. | Raspaciona aculeata (Johnston, 1842) |
| Rhabderemiidae | |
| 127. | Rhabderemia gallica van Soest & Hooper, 1993 |
| 128. | Rhabderemia indica Dendy, 1905 |
| 129. | Rhabderemia minutula (Carter, 1876) |
| 130. | Rhabderemia cf. topsenti van Soest & Hooper, 1993 |
| Chondropsidae | |
| 131. | Batzella inops (Topsent, 1891) |
| Coelosphaeridae | |
| 132. | Chaetodoryx insinuans (Topsent, 1936) |
| 133. | Forcepia (Leptolabis) apuliae (Sarà, 1969) |
| 134. | Forcepia (Leptolabilis) brunnea (Topsent, 1904) |
| 135. | Forcepia (Leptolabis) cf. luciensis (Topsent, 1888) |
| 136. | Forcepia (Leptolabis) megachela (Maldonado, 1992) |
| 137. | Lissodendoryx (Lissodendoryx) isodictyalis (Carter, 1882) |
| 138. | Lissodendoryx (Anomodoryx) cavernosa (Topsent, 1892) |
| Crambeidae | |
| 139. | Crambe crambe (Schmidt, 1862) |
| 140. | Crambe tuberosa Maldonado & Benito, 1991 |
| Crellidae | |
| 141. | Crella (Crella) elegans (Schmidt, 1862) |
| 142. | Crella (Crella) mollior Topsent, 1925 |
| 143. | Crella (Grayella) pulvinar (Schmidt, 1868) |
| 144. | Crella (Pytheas) fusifera Sarà, 1969 |
| 145. | Crella (Pytheas) sigmata Topsent, 1925 |
| 146. | Crella (Yvesia) rosea (Topsent, 1892) |
| Desmacididae | |
| 147. | Desmacidon adriaticum Sarà, 1969 |
| 148. | Desmacidon fruticosum (Montagu, 1818) |
| Hymedesmiidae | |
| 149. | Hemimycale columella (Bowerbank, 1864) |
| 150. | Hymedesmia (Hymedesmia) baculifera (Topsent, 1901) |
| 151. | Hymedesmia (Hymedesmia) paupertas (Bowerbank, 1866) |
| 152. | Hymedesmia (Hymedesmia) peachi Bowerbank, 1882 |
| 153. | Hymedesmia (Hymedesmia) plicata Topsent, 1928 |
| 154. | Hymedesmia (Hymedesmia) rissoi Topsent, 1936 |
| 155. | Hymedesmia (Hymedesmia) versicolor (Topsent, 1893) |
| 156. | Hymedesmia (Stylopus) coriacea (Fristedt, 1885) |
| 157. | Phorbas dives (Topsent, 1891) |
| 158. | Phorbas fibulatus (Topsent, 1893) |
| 159. | Phorbas fictitius Bowerbank, 1866 |
| 160. | Phorbas mercator (Schmidt, 1868) |
| 161. | Phorbas tenacior (Topsent, 1925) |
| 162. | Plocamionida ambigua (Bowerbank, 1866) |
| Myxillidae | |
| 163. | Myxilla (Myxilla) rosacea (Lieberkühn, 1859) |
| Tedaniidae | |
| 164. | Tedania (Tedania) anhelans Lieberkühn, 1849 |
| Desmacellidae | |
| 165. | Biemna parthenopea Pulitzer-Finali, 1978 |
| 166. | Biemna variantia (Bowerbank, 1858) |
| 167. | Desmacella annexa Schmidt, 1870 |
| 168. | Desmacella inornata (Bowerbank, 1866) |
| Esperiopsidae | |
| 169. | Ulosa stuposa (Esper, 1794) |
| Hamacanthidae | |
| 170. | Hamacantha (Vomerula) falcula (Bowerbank, 1874) |
| Mycalidae | |
| 171. | Mycale (Mycale) lingua (Bowerbank, 1866) |
| 172. | Mycale (Mycale) massa (Schmidt, 1862) |
| 173. | Mycale (Aegogropila) contarenii (Lieberkühn, 1859) |
| 174. | Mycale (Aegogropila) tunicata (Schmidt, 1862) |
| 175. | Mycale (Paresperella) serrulata Sarà & Siribelli, 1960 |
| Merliidae | |
| 176. | Merlia normani Kirkpatrick, 1908 |
| Podospongiidae | |
| 177. | Podospongia lovenii Bocage, 1870 |
| Latrunculiidae | |
| 178. | Latrunculia (Biannulata) citharistae Vacelet, 1969 |
| 179. | Sceptrella biannulata (Topsent, 1892) |
| 180. | Sceptrella insignis (Topsent, 1890) |
| Axinellidae | |
| 181. | Axinella cannabina (Esper, 1794) |
| 182. | Axinella damicornis (Esper, 1794) |
| 183. | Axinella rugosa (Bowerbank, 1866) |
| 184. | Axinella polypoides Schmidt, 1862 |
| 185. | Axinella verrucosa (Esper, 1794) |
| 186. | Phakellia robusta Bowerbank, 1866 |
| 187. | Phakellia ventilabrum (Linnaeus, 1767) |
| Bubaridae | |
| 188. | Bubaris carcisis Vacelet, 1969 |
| 189. | Bubaris vermiculata (Bowerbank, 1866) |
| 190. | Cerbaris curvispiculifer (Carter, 1880) |
| 191. | Monocrepidion vermiculatum Topsent, 1898 |
| Hymerhabdiidae | |
| 192. | Hymerhabdia oxytrunca Topsent, 1904 |
| 193. | Hymerhabdia typica Topsent, 1892 |
| Heteroxyidae | |
| 194. | Halicnemia geniculata Sarà, 1958 |
| 195. | Halicnemia patera Bowerbank, 1864 |
| Dictyonellidae | |
| 196. | Acanthella acuta Schmidt, 1862 |
| 197. | Dictyonella incisa (Schmidt, 1880) |
| 198. | Dictyonella marsilii (Topsent, 1893) |
| 199. | Dictyonella obtusa (Schmidt, 1862) |
| 200. | Dictyonella pelligera (Schmidt, 1862) |
| Halichondriidae | |
| 201. | Axinyssa aurantiaca (Schmidt, 1864) |
| 202. | Halichondria (Halichondria) bowerbanki Burton, 1930 |
| 203. | Halichondria (Halichondria) contorta (Sarà, 1961) |
| 204. | Halichondria (Halichondria) convolvens Sarà, 1960 |
| 205. | Halichondria (Halichondria) genitrix (Schmidt, 1870) |
| 206. | Halichondria (Halichondria) panicea (Pallas, 1766) |
| 207. | Halichondria (Halichondria) semitubulosa Lieberkühn, 1859 |
| 208. | Hymeniacidon perlevis (Montagu, 1818) |
| 209. | Hymeniacidon rugosa (Schmidt, 1868) |
| 210. | Laminospongia subtilis Pulitzer-Finali, 1983 |
| 211. | Spongosorites intricatus (Topsent, 1892) |
| 212. | Spongosorites flavens Pulitzer-Finali, 1983 |
| 213. | Topsentia glabra (Topsent, 1898) |
| 214. | Topsentia vaceleti Kefalas & Castritsi–Catharios, 2012 |
| Agelasidae | |
| 215. | Agelas oroides Schmidt, 1864 |
| Callyspongiidae | |
| 216. | Callyspongia subcornea Griessinger, 1971 |
| Chalinidae | |
| 217. | Dendroxea lenis (Topsent, 1892) |
| 218. | Haliclona (Gellius) angulata (Bowerbank, 1866) |
| 219. | Haliclona (Gellius) dubia (Babic, 1922) |
| 220. | Haliclona (Gellius) flagellifer (Ridley & Dendy, 1866) |
| 221. | Haliclona (Gellius) lacazei (Topsent, 1893) |
| 222. | Haliclona (Gellius) marismedi (Pulitzer-Finali, 1978) |
| 223. | Haliclona (Gellius) tenuisigma (Sarà & Siribelli, 1960) |
| 224. | Haliclona (Halichoclona) fulva (Topsent, 1893) |
| 225. | Haliclona (Haliclona) simulans (Johnston, 1842) |
| 226. | Haliclona (Reniera) aquaeductus (Schmidt, 1862) |
| 227. | Haliclona (Reniera) citrina (Topsent, 1892) |
| 228. | Haliclona (Reniera) cratera (Schmidt, 1862) |
| 229. | Haliclona (Reniera) mediterranea Griessinger, 1971 |
| 230. | Haliclona (Rhizoniera) rosea (Bowerbank, 1866) |
| 231. | Haliclona (Rhizoniera) sarai (Pulitzer-Finali, 1969) |
| 232. | Haliclona (Soestella) arenata Griessinger, 1971 |
| 233. | Haliclona (Soestella) implexa (Schmidt, 1868) |
| 234. | Haliclona (Soestella) mamillata (Griessinger, 1971) |
| 235. | Haliclona (Soestella) mucosa (Griessinger, 1971) |
| 236. | Haliclona (Soestella) valliculata (Griessinger, 1971) |
| 237. | Haliclona elegans (Lendenfeld, 1887) |
| Phloeodictyidae | |
| 238. | Siphonodictyon coralliirubri (Calcinai et al., 2007) |
| 239. | Siphonodictyon insidiosum (Johnson, 1899) |
| 240. | Calyx nicaeensis (Risso, 1826) |
| Petrosiidae | |
| 241. | Petrosia (Petrosia) clavata (Esper, 1794) |
| 242. | Petrosia (Petrosia) ficiformis (Poiret, 1798) |
| Irciniidae | |
| 243. | Ircinia dendroides (Schmidt, 1862) |
| 244. | Ircinia oros (Schmidt, 1864) |
| 245. | Ircinia variabilis (Pallas, 1766) |
| 246. | Sarcotragus fasciculatus (Pallas, 1766) |
| 247. | Sarcotragus foetidus Schmidt, 1862 |
| 248. | Sarcotragus pipetta (Schmidt, 1868) |
| 249. | Sarcotragus spinosulus Schmidt, 1862 |
| Thorectidae | |
| 250. | Cacospongia mollior Schmidt, 1862 |
| 251. | Cacospongia scalaris Schmidt, 1862 |
| 252. | Hyrtios collectrix (Schulze, 1880) |
| 253. | Fasciospongia cavernosa (Schmidt, 1862) |
| Spongiidae | |
| 254. | Spongia (Spongia) agaricina Pallas, 1766 |
| 255. | Spongia (Spongia) nitens (Schmidt, 1862) |
| 256. | Spongia (Spongia) officinalis Linnaeus, 1759 |
| 257. | Spongia (Spongia) virgultosa (Schmidt, 1868) |
| 258. | Spongia (Spongia) zimocca Schmidt, 1862 |
| 259. | Hippospongia communis (Lamarck, 1814) |
| Dysideidae | |
| 260. | Dysidea avara (Schmidt, 1862) |
| 261. | Dysidea fragilis (Montagu, 1818) |
| 262. | Dysidea tupha (Martens, 1824) |
| 263. | Pleraplysilla spinifera (Schulze, 1879) |
| Darwinellidae | |
| 264. | Aplysilla rosea (Barrois, 1876) |
| 265. | Aplysilla sulfurea Schulze, 1878 |
| 266. | Chelonaplysilla noevus (Carter, 1876) |
| Dictyodendrillidae | |
| 267. | Spongionella gracilis (Vosmaer, 1883) |
| 268. | Spongionella pulchella (Sowerby, 1804) |
| Halisarcidae | |
| 269. | Halisarca dujardini Johnston, 1842 |
| Aplysinidae | |
| 270. | Aplysina aerophoba Nardo, 1843 |
| 271. | Aplysina cavernicola Vacelet, 1959 |
| Ianthellidae | |
| 272. | Hexadella pruvoti Topsent, 1896 |
| 273. | Hexadella racovitzai Topsent, 1896 |
This increasing is related to the difficulty of studying the organisms inhabiting the coralligenous concretions due to the complexity of the habitat, the high diversity, and the depth where these structures are located (
Among the insinuating species observed in the coralligenous crevices we have found several species previously recorded with a massive habitus in deeper waters. Pachastrella monilifera Schmidt, 1868 and Poecillastra compressa (Bowerbank, 1866) were the species with the highest phenotypic plasticity, since they usually appear with large, fun shaped specimens, in deep habitats (
As to the boring sponges, Cliona janitrix is indicated by