(C) 2014 Anna Halász. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Halász A, McFadden CS, Aharonovich D, Toonen R, Benayahu Y (2014) A revision of the octocoral genus Ovabunda Alderslade, 2001 (Anthozoa, Octocorallia, Xeniidae). ZooKeys 373: 1–41. doi: 10.3897/zookeys.373.6511
The family Xeniidae (Octocorallia) constitutes an abundant benthic component on many Indo-West Pacific coral reefs and is ecologically important in the Red Sea. The genus Ovabunda Alderslade, 2001 was recently established to accommodate previous Xenia species with sclerites comprised of a mass of minute corpuscle-shaped microscleres. The aim of the present study was to examine type material of Xenia species in order to verify their generic affiliation. We present here a comprehensive account of the genus Ovabunda, using scanning electron microscopy to depict sclerite microstructure. We assign three Xenia species to the genus: O. ainex comb. n., O. gohari comb. n., and O. crenata comb. n.; and synonymize several other species of Ovabunda. We provide a key to Ovabunda species and conclude that they are mainly confined to the Red Sea, with some occurrence in the West Indian Ocean.
Red Sea, sclerite microstructure, taxonomy, Xenia
Members of the octocoral family Xeniidae form a major component of shallow coral-reef communities in the tropical Indo-West-Pacific region, and in the Red Sea in particular (e.g.,
The xeniids comprise for the most part small and soft colonies, which are often slippery due to the secretion of large amounts of mucus (
Most xeniids feature a high density of sclerites in all parts of the colony, such as members of the genera Asterospicularia Utinomi, 1951; Sansibia Alderslade, 2000 (see
Over the years studies have revealed that a number of taxa have relatively simple sclerites in the form of round platelets, including those of the genera Cespitularia Milne-Edwards & Haime, 1850; Heteroxenia Kölliker, 1874; Funginus Tixier-Durivault, 1970; Sansibia Alderslade, 2000; Sarcothelia Verrill, 1928; Sympodium Ehrenberg, 1834 and Xenia Lamarck, 1816 (see also
Over the last two decades the use of scanning electron microscopy (SEM) has revealed microstructural features of xeniid sclerites, which were not visible under a light microscope.
The discovery of a corpuscular sclerite-type among previously described Xenia species led
In a later study,
Establishment of the genus Ovabunda by Alderslade led us to examine type material that was originally described as Xenia, in order to verify its generic affiliation. Not only were the types of the species assigned to Ovabunda by Alderslade examined, and their sclerites studied using SEM, but the types of a number of other nominal species of Xenia were also studied (see Table 1). Consequently, the current study has assigned three Xenia species to Ovabunda and retained 20 in the former genus; it also synonymizes several other species and designates a neotype for Ovabunda macrospiculata. The findings of the study emphasize the importance of re-examination of type material and the use of SEM to study xeniid sclerite microstructure for taxonomic purposes.
List of Xenia type material examined during the current study along with corresponding museum numbers.
| Species name | Type | Museum and museum number |
|---|---|---|
| Xenia actuosa Verseveldt & Tursch, 1979 | Holotype | RMNH Coel. 12866 |
| Xenia antarctica Kükenthal, 1902 | Syntype | MNHHWU 63 |
| Xenia bauiana May, 1899 | Syntype | ZMB 3673 |
| Xenia blumi Schenk, 1896 | Holotype | SMF 44 |
| Xenia crassa Schenk, 1896 | Holotype | SMF 39 |
| Xenia delicata Roxas, 1933 | Syntype | ZMB 6908 |
| Xenia fusca Schenk, 1896 | Syntype | SMF 40 |
| Xenia garciae Bourne, 1894 | Type | BML 1921.11.18.1 |
| Xenia grasshoffi Verseveldt, 1974 | Holotype | SMF 2616 |
| Xenia kükenthali Roxas, 1933 | Holotype | ZMB 6917 |
| Xenia lepida Verseveldt, 1971 | Holotype | RMNH Coel. 6703 |
| Paratype | RMNH Coel. 6704 | |
| Xenia membranacea Schenk, 1896 | Holotype | SMF 41 |
| Xenia mucosa Verseveldt & Tursch, 1979 | Holotype | RMNH Coel. 12867 |
| Xenia multispiculata Kükenthal, 1909 | Syntype | ZMB 6920 |
| Xenia novaebritanniae Ashworth, 1900 | Type | BML 1962.7.20.148 |
| Syntype | BML 1962.7.20.149 | |
| Xenia plicata Schenk, 1896 | Holotype | SMF 45 |
| Xenia rubens Schenk, 1896 | Holotype | SMF 46 |
| Xenia sansibariana May, 1899 | Syntype | ZMB 3828 |
| Xenia ternatana Schenk, 1896 | Holotype | SMF 43 |
| Xenia viridis Schenk, 1896 | Holotype | SMF 42 |
The study examined ethanol-preserved type specimens obtained on loan from the British Museum of Natural History (BML); National History collections of the Hebrew University of Jerusalem (HUJ); Museum of Natural History, Wroclaw University, Poland (MNHHWU); The Naturhistorisches Museum Wien (NHMW); the Naturalis Biodiversity Centre, formerly Rijksmuseum van Natuurlijke Historie, Leiden (RMNH); Senckenberg Naturmuseum Frankfurt (SMF); Zoologisches Museum Berlin (ZMB); and the Zoological Museum of Tel Aviv University (ZMTAU). Neotype material of Ovabunda macrospiculata was collected by the last author from the northern Red Sea, Gulf of Suez, Shag Rock from ~5 meters, 11 July 1986 and preserved in 70% ethanol.
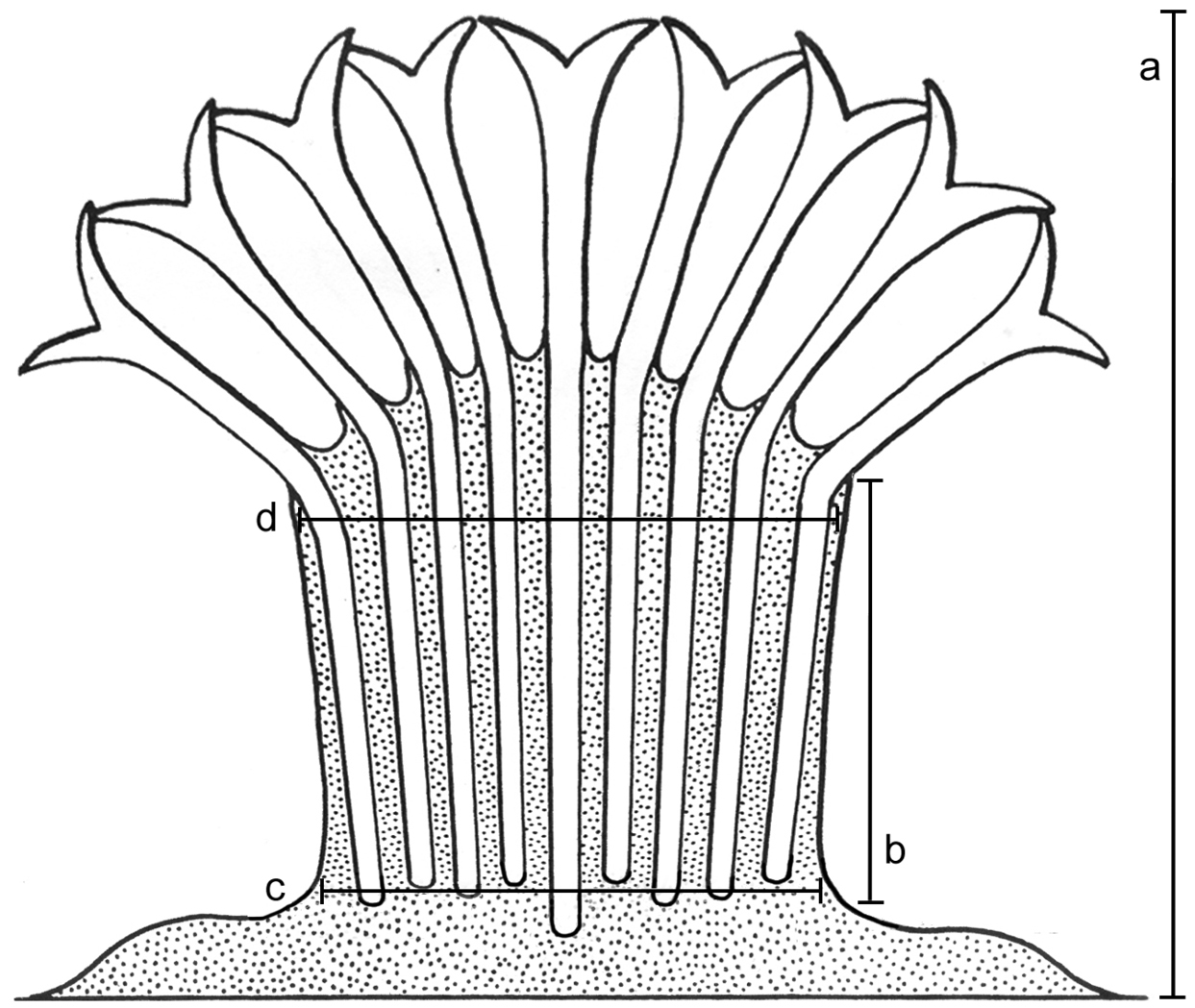
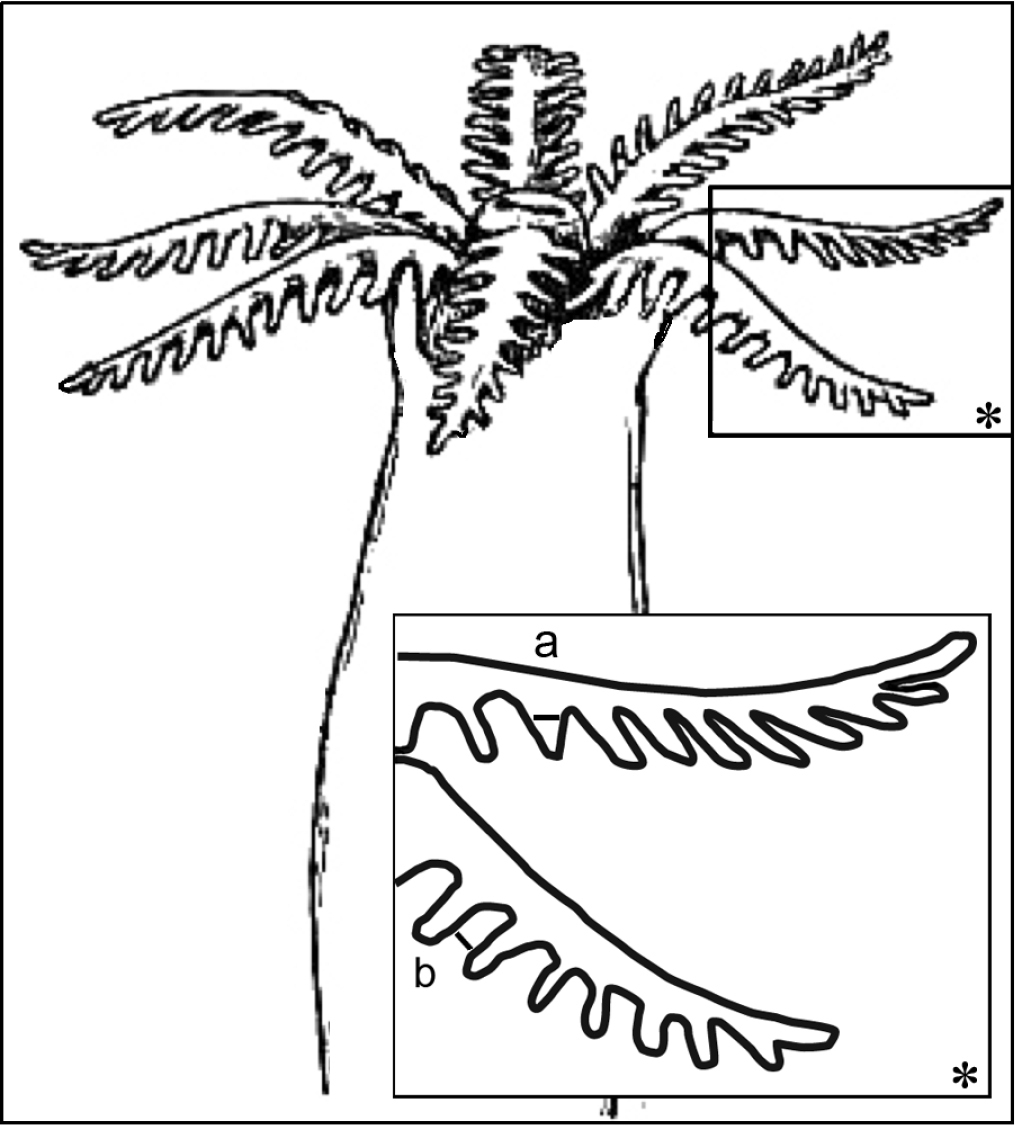
Morphological features of the preserved colonies were recorded: colony height, numbers of branches, stalk length, width at colony-base, and width at the uppermost part below the capitulum where the polyps arise (Fig. 1). The number of rows of pinnules and number of pinnules in the outermost row were counted under a compound microscope from five to ten tentacles, as much as possible from different polyps. The polyps examined were the biggest within the colony and preferably from the mid-part of the capitulum, and not from the outer part which contains the newly grown and smaller polyps. In addition, the length of the anthocodiae – consisting of the polyp body and extended tentacles – the dimensions of the pinnules (length and width at base), and the distance between adjacent pinnules were recorded (Fig. 2).
Illustration of colony dimensions. a Colony height b Stalk length c Stalk width at base d Stalk width at uppermost part. Illustration adopted from
Illustration of polyp dimensions. a Pinnule width at its base b Gap between adjacent pinnules; asterisk indicates magnified area. Illustration adopted from Encyclopedia Britannica, 11th Edition, Volume 3 (http://www.digilibraries.com/html_ebooks/105312/34018/www.digilibraries.com@34018@34018-h@34018-h-7.htm).
To examine the sclerites, the tissue was treated with 10% sodium hypochlorite followed by repeated rinses in distilled water. Wet preparations of the clean sclerites from polyps, colony stalk, the coenenchyme and canal walls within were examined and photographed under an Optishot Nikon light microscope at × 400 magnification. After comparison of these sclerites we concluded that there are no differences in appearance and dimensions between sclerites in different colony parts. Therefore, only polyp sclerites were examined under SEM and presented in this paper. SEM stubs for polyp sclerites were prepared following
The zoogeographical species distribution is based on the examination of types and other material, unless specified otherwise in the text. Table 1 lists Xenia types that were examined by us and maintained their original generic affiliation.
http://species-id.net/wiki/Xeniidae
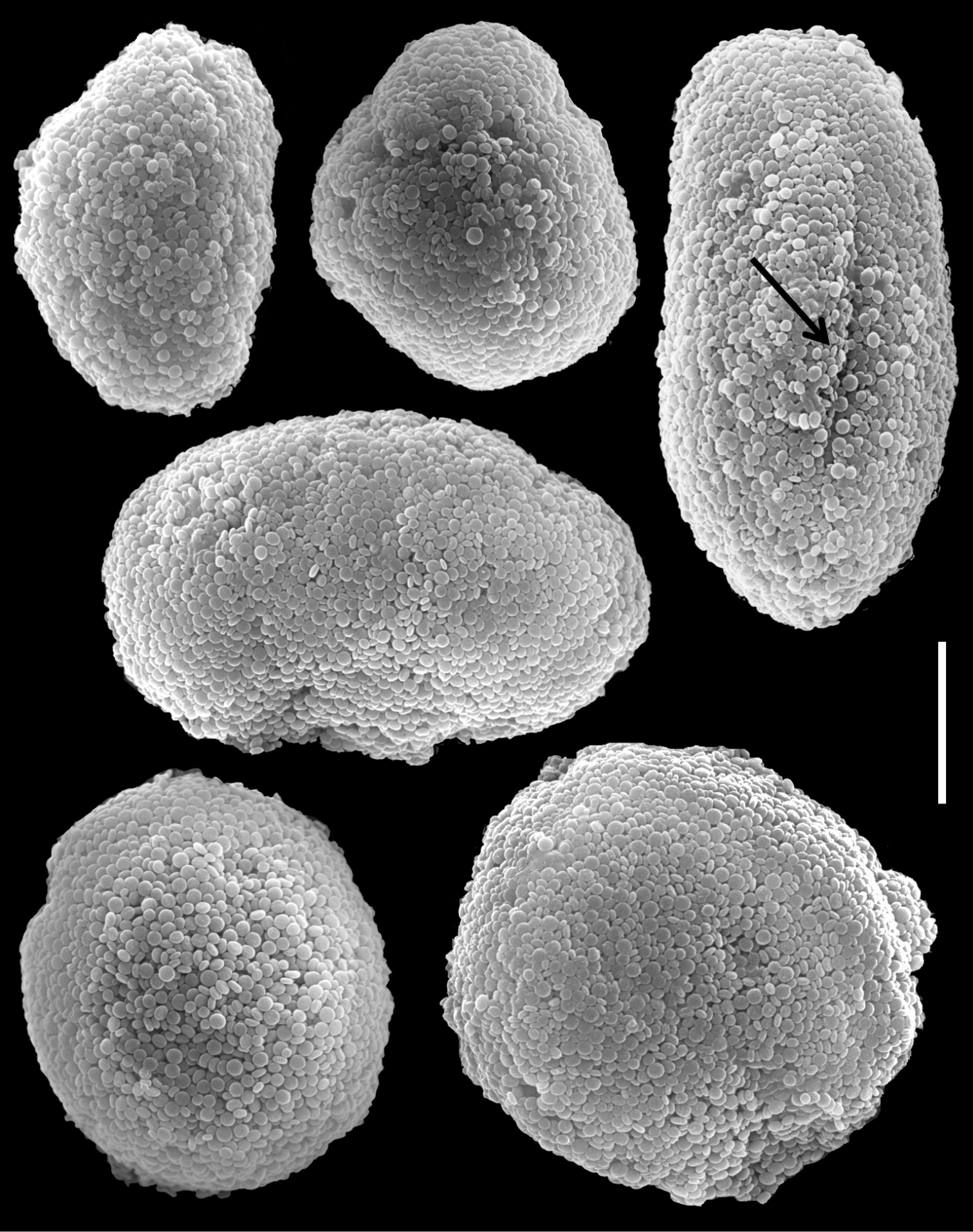
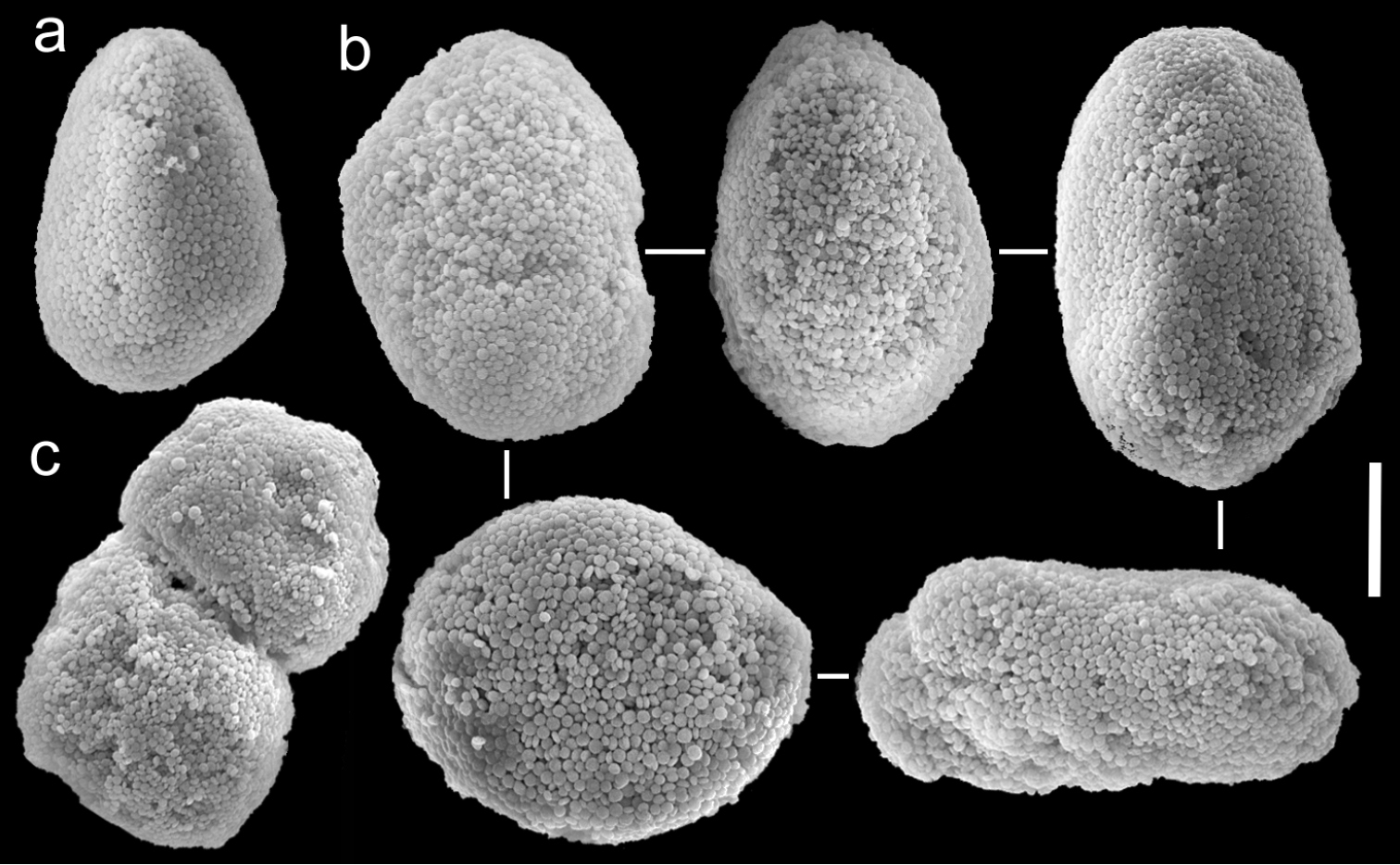
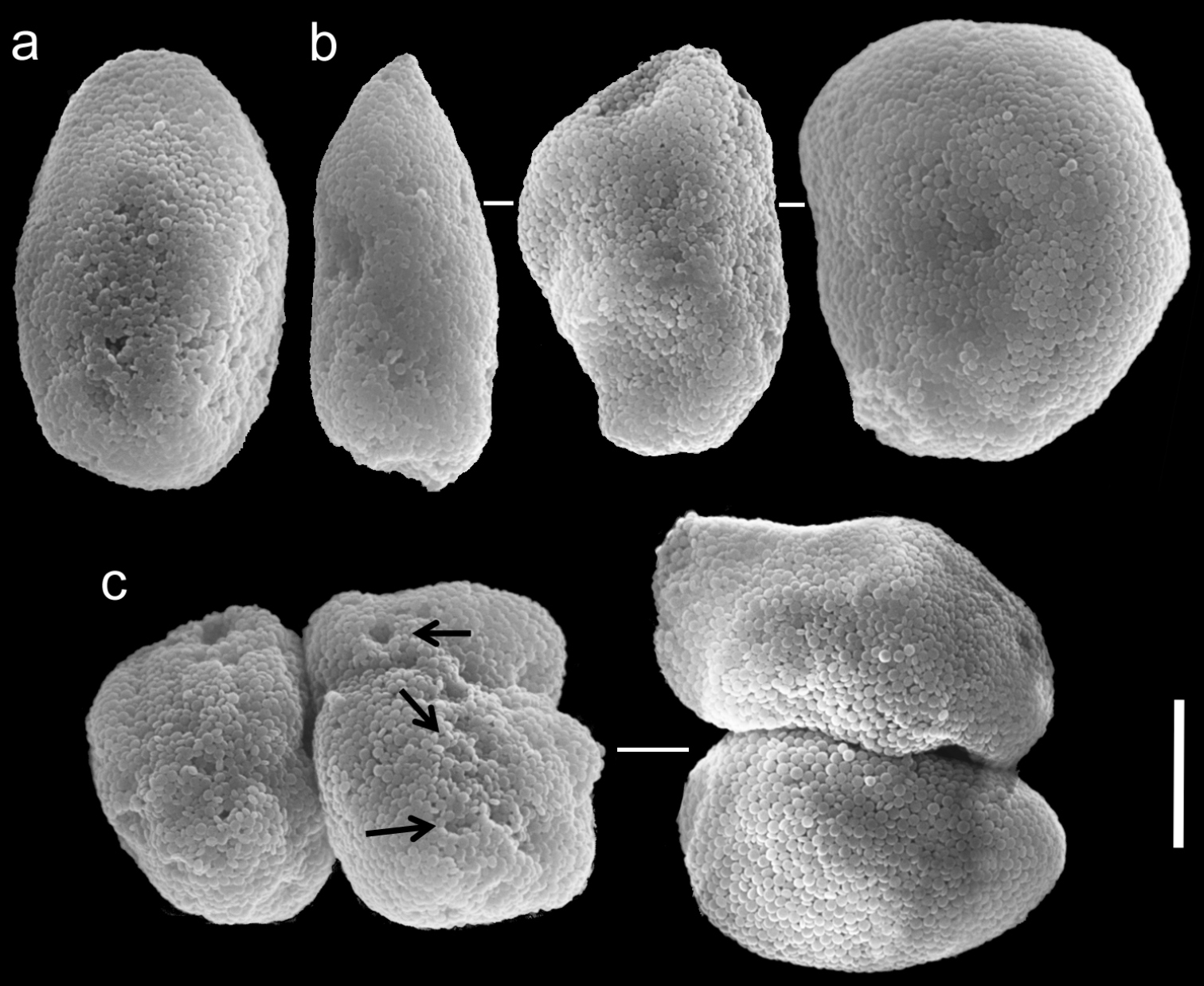
Colonies are small and soft with cylindrical stalks, undivided or branched, terminating in one or more domed polyp-bearing regions. Polyps are not retractile and are always monomorphic. Sclerites are oval spheroids, usually abundant in all parts of the colony, mostly up to 0.035 mm in maximal diameter, and comprised of a mass of minute corpuscle-shaped microscleres.
| 1 | Non-pulsating polyps in live colonies | |
| 1.1 | One row of pinnules on each side of the tentacles | |
| 6–7 pinnules in each row | Ovabunda benayahui | |
| 12–18 pinnules in each row | Ovabunda verseveldti (No data on pulsation present in the literature) | |
| 1.2 | Mostly one row, but sometimes two rows of pinnules on each side of the tentacles | |
| 18–22 pinnules in the outermost row | Ovabunda gohari | |
| 1.3 | Two rows of pinnules on each side of the tentacles | |
| 8–11 pinnules in the outermost row | Ovabunda impulsatilla | |
| 13–16 pinnules in the outermost row | Ovabunda biseriata | |
| 17–24 pinnules in the outermost row | Ovabunda faraunensis | |
| 24–29 pinnules in the outermost row | Ovabunda arabica | |
| 1.4 | Mostly two, but sometimes three rows of pinnules in the outermost row | |
| 12–16 pinnules in the outermost row | Ovabunda crenata | |
| 1.5 | Three rows of pinnules on each side of the tentacles | |
| 15–20 pinnules in the outermost row | Ovabunda ainex | |
| 1.6 | Mostly three but sometimes four rows of pinnules in the outermost row | |
| 17–22 pinnules in the outermost row | Ovabunda hamsina | |
| 2 | Pulsating polyps in live colonies | |
| 2.1 | Three rows of pinnules on each side of the tentacles | |
| 14–18 pinnules in the outermost row | Ovabunda macrospiculata | |
http://species-id.net/wiki/Ovabunda_ainex
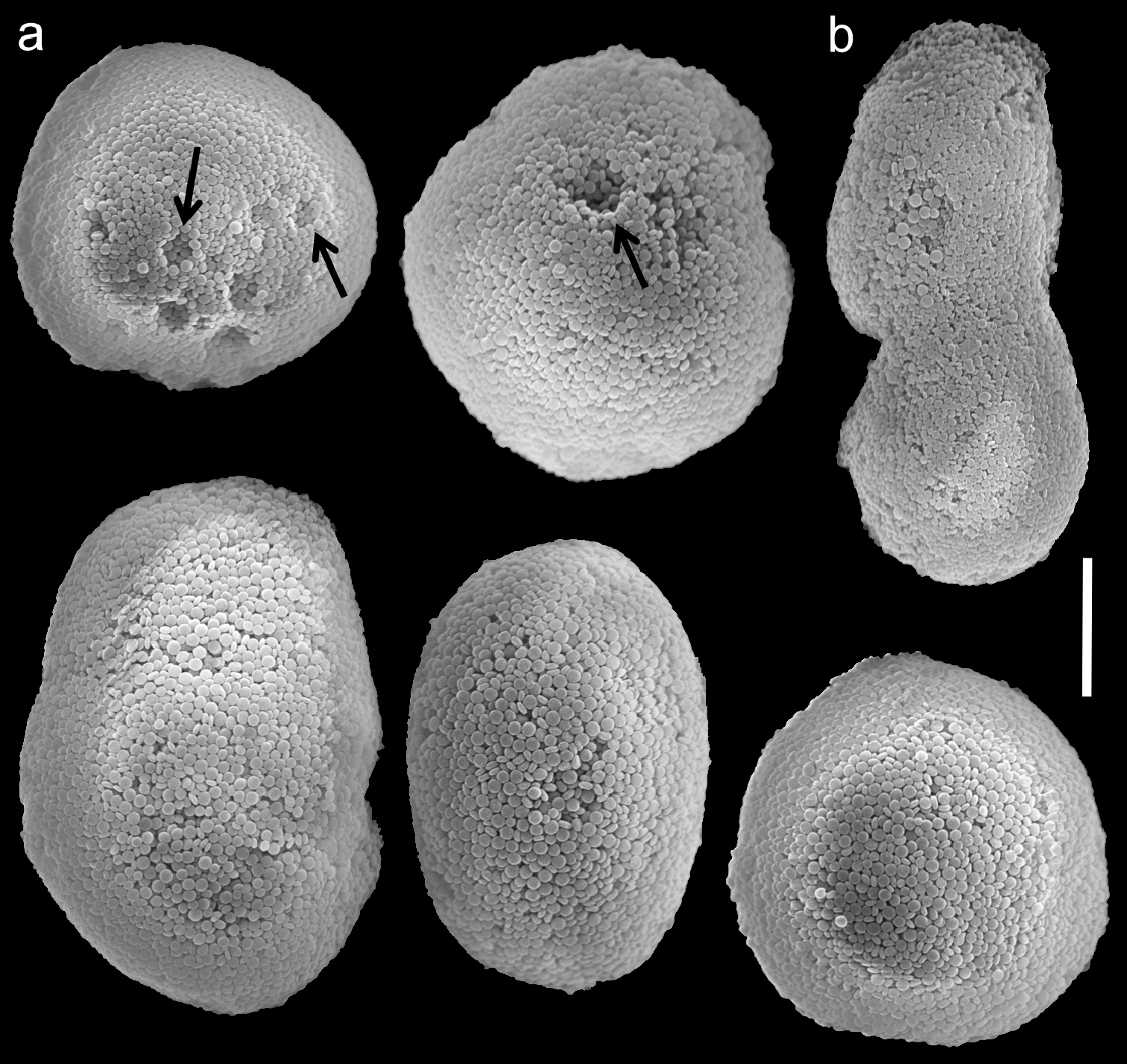
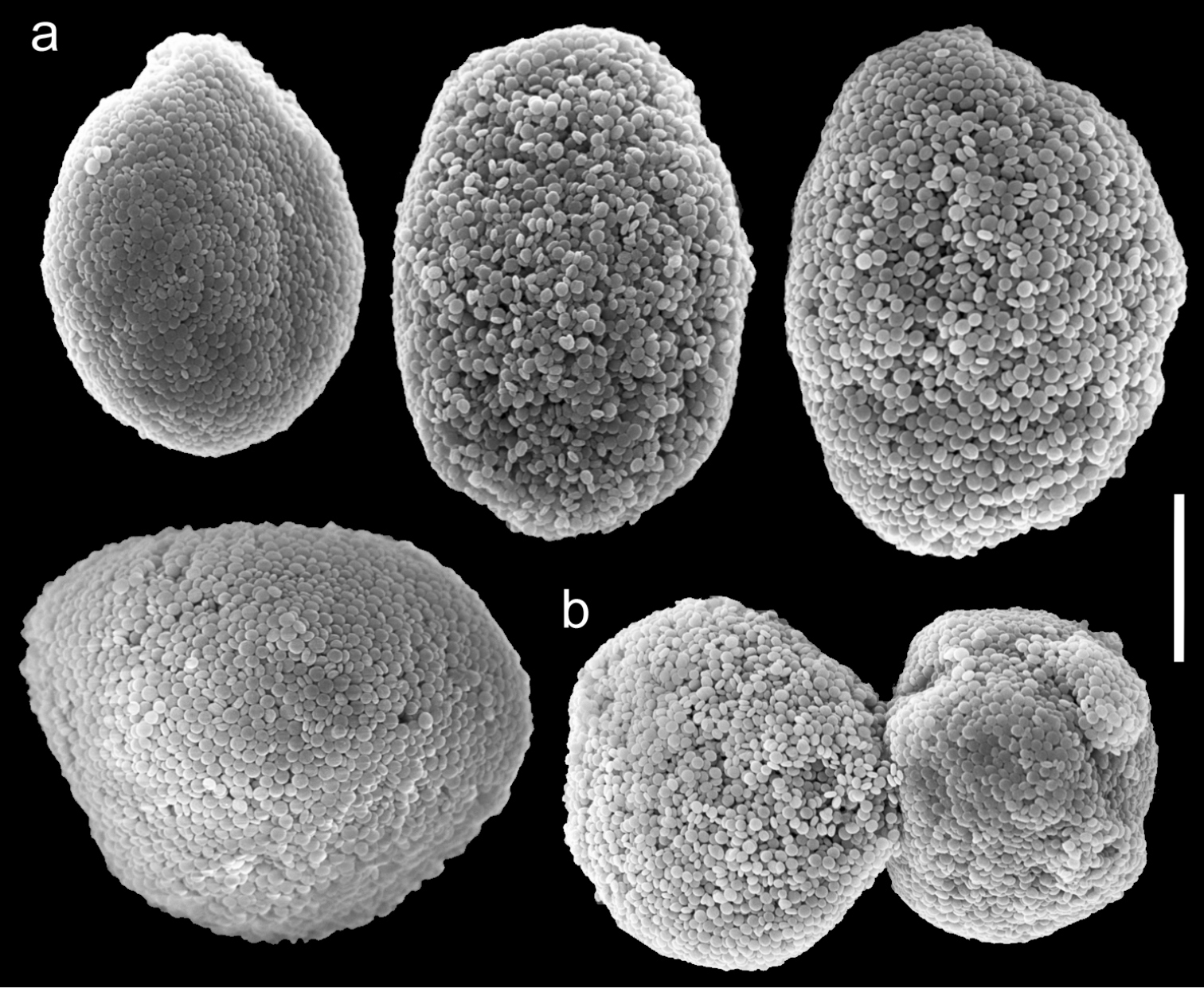
Fig. 3Holotype: RMNH Coel. 23539, Sudanese Red Sea, Sanganeb Atoll, 20 km off Port Sudan, southern-slope near jetty (19°21'33.81"N, 37°19'37.66"E), 6 m, April 1991, coll. G. B. Reinicke; paratype: RMNH Coel. 23540, same location, 5 m S- jetty, October 1992, coll. G. B. Reinicke; additional material: RMNH Coel. 23535, Red Sea, Gulf of Aqaba, Aqaba, Saudi Arabian border Bay (29°21'37.31"N, 34°57'39.48"E), October 1989, coll. G. B. Reinicke; NHMW 2250, Saudi Arabia; holotype of Xenia crassa SMF 39, Indonesia, Ternate island, 1894, coll. Kükenthal; holotype of Xenia ternatana SMF 43, Indonesia, Ternate island, 1894, coll. Kükenthal.
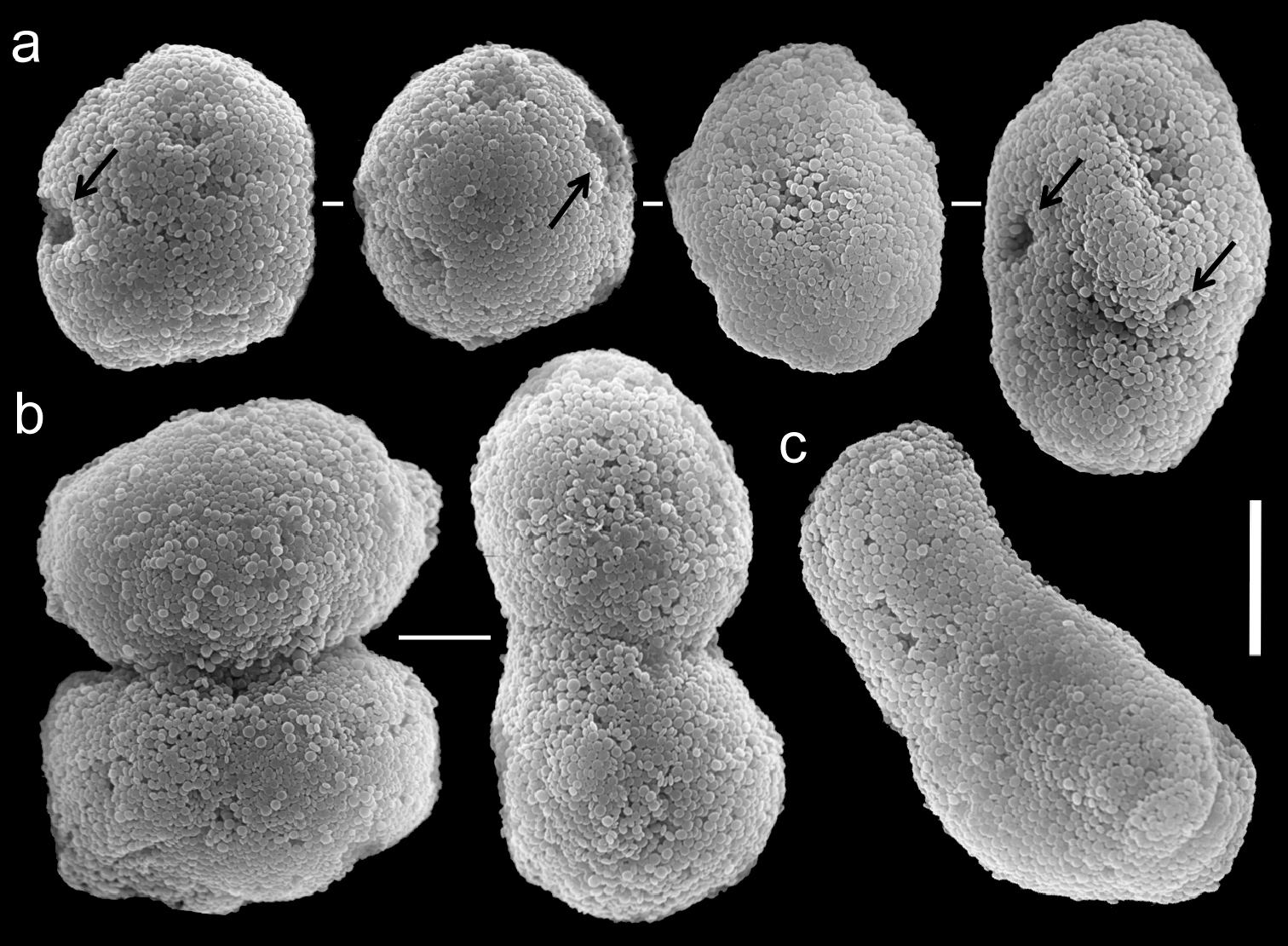
The holotype is 45 mm high; its stalk is 15 mm long and splits at the base into three branches, two of which split again into two, 22 and 18 mm above the colony base; the latter branches are 35 and 30 mm long, 8 and 10 mm wide at their base, 4 and 6 mm wide at their uppermost part, respectively. The third branch splits 20 mm above the base into three branches, and is 40 mm long and 7 mm wide at its base and 5-7 mm wide at the uppermost part. The polyp’s body is up to 10 mm long, and the tentacles are up to 5 mm long, featuring three rows of pinnules on each side. The pinnules are relatively slender, up to 1 mm long and 0.08 mm wide, 17-20 in the outermost row with a space of one to two pinnule widths between adjacent pinnules. The spheroidal sclerites, typical regular Ovabunda sclerites, are scarce in all parts of the colony, measuring 0.011–0.030 × 0.018–0.042 mm in diameter (n = 25), composed of a mass of corpuscle-shaped microscleres. The original description indicated non-pulsating polyps for the live colonies. The ethanol-preserved holotype is light yellowish-beige, almost transparent.
The paratype is 20 mm long; its stalk is 15 mm wide at its base, splitting into two branches, each 5 mm long and 10 and 8 mm wide at the base. The polyp’s body is up to 5 mm long, and the tentacles up to 5 mm long, bearing three rows of pinnules and 15–17 pinnules in the outermost row. The pinnules are 1.6 mm long and 0.16 mm wide, with a gap between adjacent pinnules ranging from one to three pinnule widths. The sclerites are Ovabunda vabunda-type, regular or irregular in shape (Fig. 3a, c), 0.012–0.026 × 0.02–0.04 mm in diameter (n = 21 sclerites). When two sclerites fuse they reach 0.039 mm in maximum diameter (Fig. 3b). The original description indicated non-pulsating polyps for live colonies. The ethanol-preserved colony is light beige.
Scanning electron micrographs of polyp sclerites of Ovabunda ainex (Reinicke, 1997) paratype (RMNH Coel. 23540). a Regular sclerites b Fused sclerites c Irregular sclerite. Arrows indicate surface dents. Scale bar: 10 µm.
RMNH Coel. 23535 features polyps with three rows of pinnules on each side of the tentacles and 16–19 pinnules in the outermost row. The sclerites are Ovabunda-type, measuring 0.016–0.026 × 0.022–0.030 mm in diameter (n =13).
Our findings confirm the original description of
Ovabunda ainex is most similar to Ovabunda macrospiculata. Although they both have three rows of pinnules, the number of pinnules in the outermost row ranges from 15–20 in Ovabunda ainex compared to 14–18 in Ovabunda macrospiculata. The major difference between them, however, is that Ovabunda macrospiculata has pulsating polyps in live colonies and Ovabunda ainex does not.
Red Sea: Gulf of Aqaba, Sudan.
http://species-id.net/wiki/Ovabunda_arabica
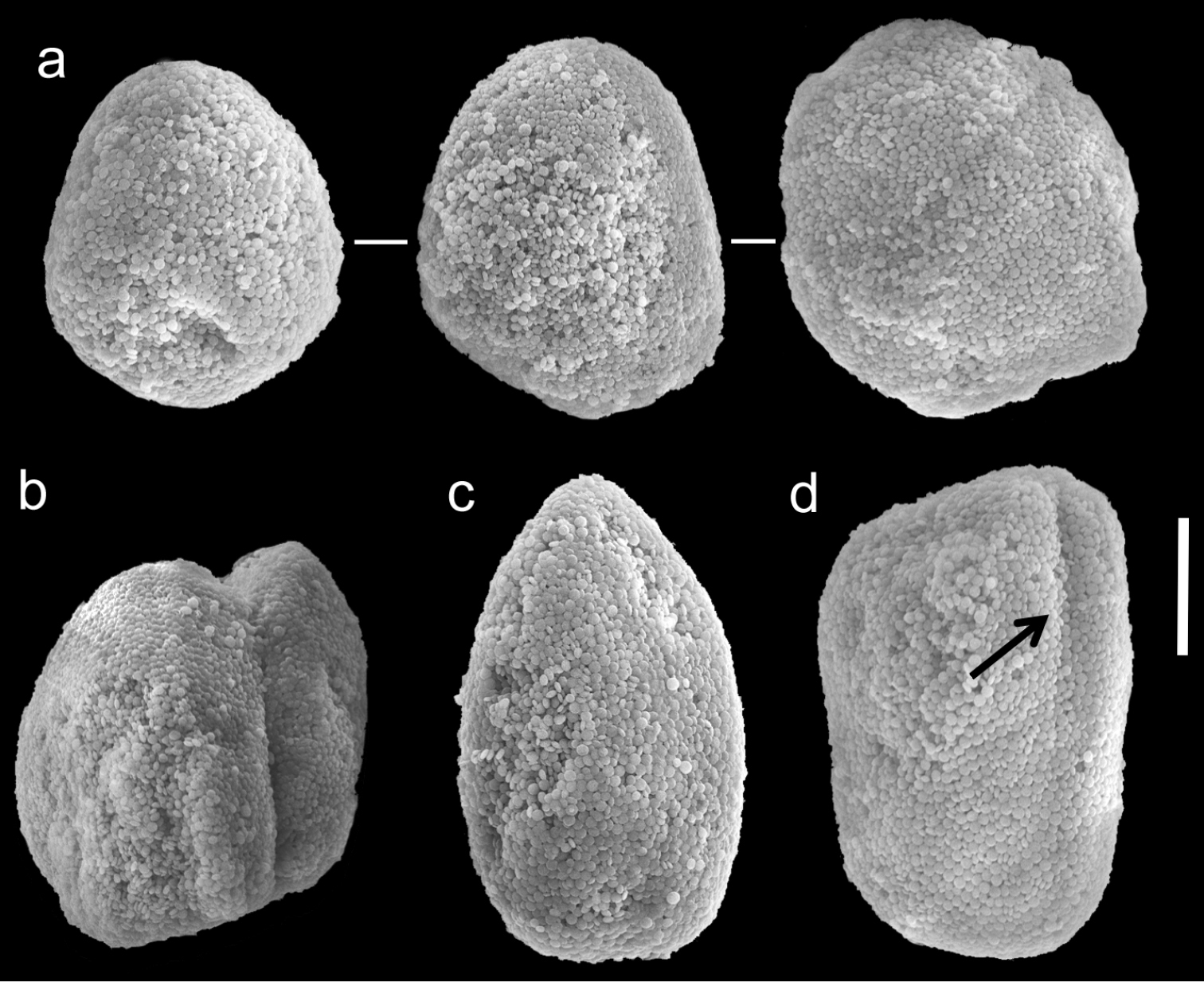
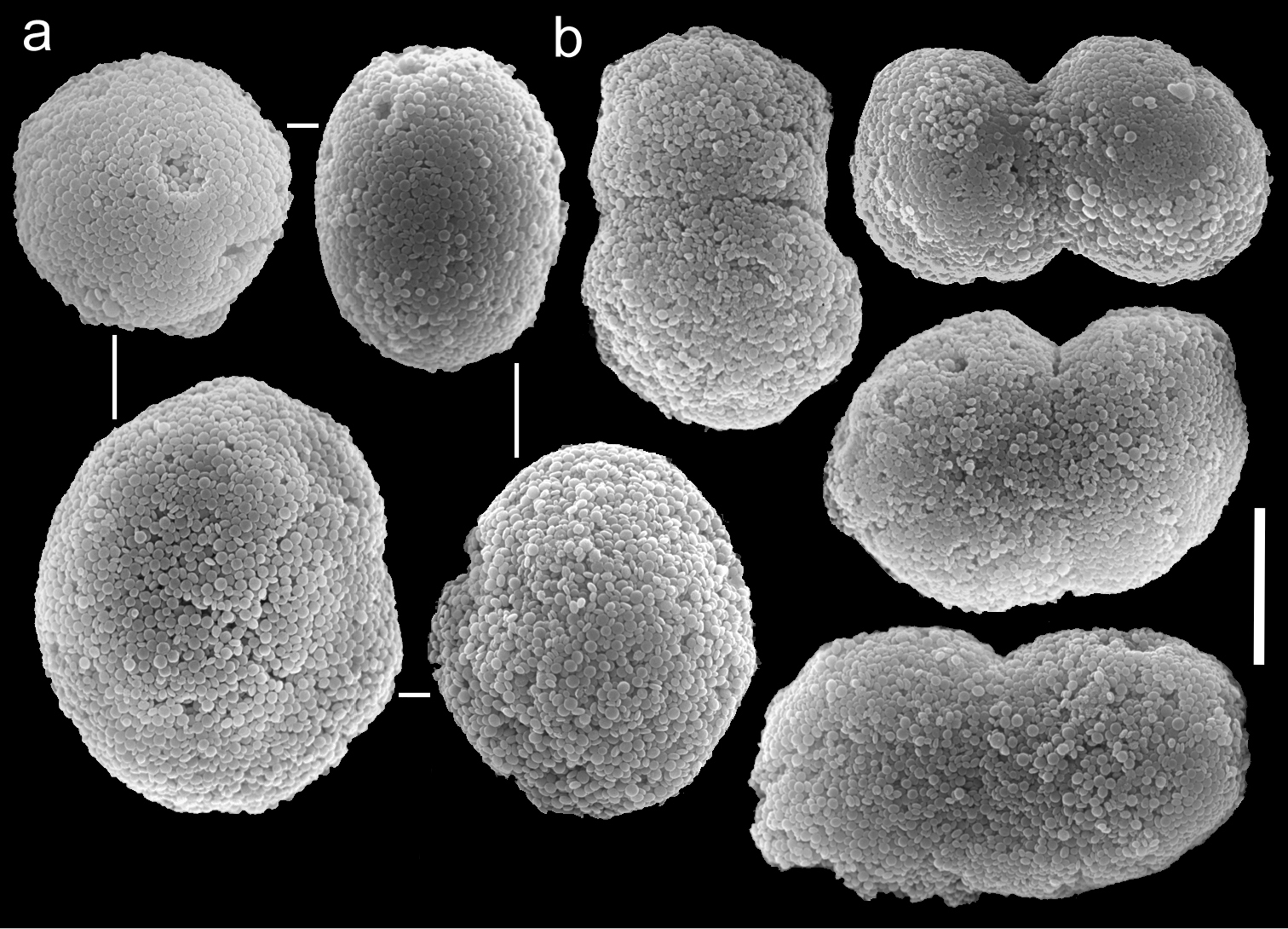
Figs 4, 5Holotype: RMNH Coel. 18673, northern Red Sea, Gulf of Aqaba, Saudi Arabian border bay, 20 km South of Aqaba (29°21'37.31"N, 34°57'39.48"E), 15 m, 12 November 1991, coll. G. B. Reinicke. Paratypes: RMNH Coel. 18675, northern Red Sea, Gulf of Aqaba, Nature Reserve (Aqaba), Marine science station (MSS), 10 km South of Aqaba (29°27'27.33"N, 34°58'24.19"E), 15 m, 4 November 1991, coll. G. B. Reinicke; RMNH Coel. 18676, location as above, 12 m, 5 November 1991, coll. G. B. Reinicke; additional material: holotype of Xenia crista RMNH Coel. 18677, northern Red Sea, Gulf of Aqaba, Nature Reserve (Aqaba), Marine science station (MSS), 10 km South of Aqaba (29°27'27.33"N, 34°58'24.19"E), 12 m, October 1989, coll. G. B. Reinicke; paratype of Xenia crista RMNH Coel. 18678, location as above, 15 m, October 1990, coll. G. B. Reinicke.
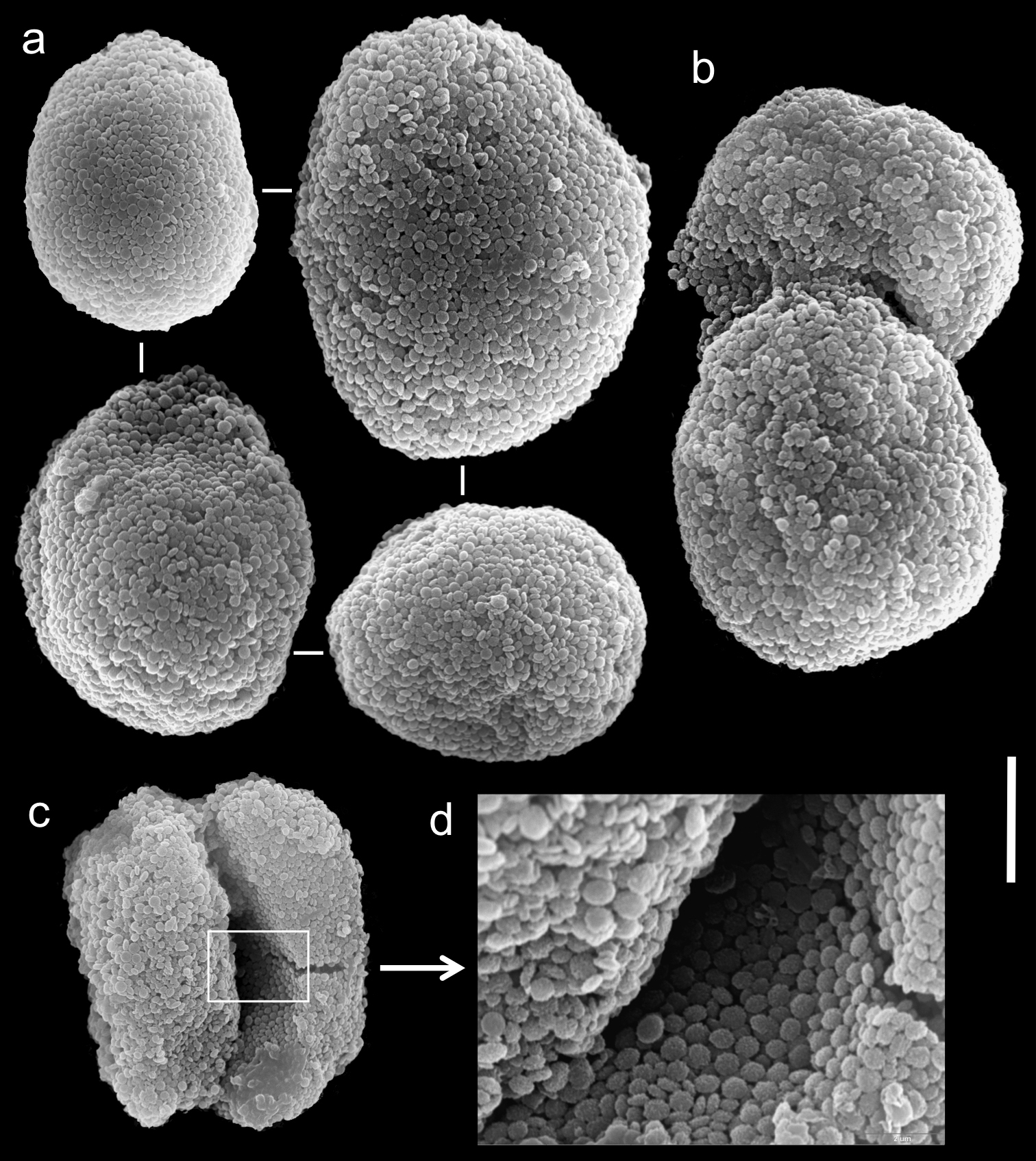
The holotype is 45 mm high; its stalk is 15 mm long, 7 mm wide at its base and 7 mm wide at its upper part. The polyp’s body is 12 mm long, and tentacles up to 11 mm long. The pinnules are 2 mm long and 0.2 mm wide at the base; the gap between adjacent pinnules is one pinnule wide. There are two rows of pinnules on each side and 24-25 pinnules in the outermost row. The sclerites are spheroids of the Ovabunda-type, measuring 0.028–0.036 mm at largest diameter.
One paratype (RMNH Coel. 18675) has a total height of 20 mm; its stalk is 16 mm long, 10 mm wide at the base, and 18 mm wide at its uppermost part. The polyp’s body is 9 mm long; the tentacles are 6 mm long, featuring two rows of pinnules 2.2 mm long and 0.2 mm wide on each side, and 24–29 pinnules in the outermost row. The pinnules mostly feature a one pinnule width gap. Sclerites are Ovabunda-type, regular and pear- shaped (Fig. 4a, c), measuring 0.016–0.030 × 0.022–0.038 mm in diameter (n = 27 sclerites). Occasionally, two sclerites are fused (Fig. 4b), measuring up to 0.048 mm in diameter. The sclerites are more abundant on the aboral side of the tentacles than the oral one. The second paratype (RMNH Coel. 18676) has 2 rows of pinnules on each side of the tentacles, 24–27 in the outermost row. The sclerites are Ovabunda-type, measuring 0.026–0.036 mm at maximal diameter. The original description indicated non-pulsating polyps for live colonies. Colour of the ethanol-preserved holotype is light brown.
Scanning electron micrographs of polyp sclerites of Ovabunda arabica (Reinicke, 1995) paratype (RMNH Coel. 18675). a Regular sclerites b Fused sclerites c Pear-shaped sclerite. Arrow indicates surface irregularity, might represent the fusion area of two individual sclerites. Scale bar 10 µm.
The number of rows of pinnules and pinnules in the outermost row correspond to the original description of the holotype and paratypes of Xenia arabica. Their sclerites are Ovabunda-type (Fig. 4) and, therefore, the species should be reassigned to that genus. Sclerite sizes given for the holotype and paratype in the original description exceed those obtained by us, as follows: holotype 0.043–0.053 mm vs. 0.028–0.036 mm at the larger diameter, and paratypes (RMNH Coel. 18675) 0.039 × 0.043 vs. 0.016–0.030 × 0.022–0.038 mm and (RMNH Coel. 18676) 0.043 × 0.051 vs. 0.026–0.036 mm, respectively. The larger size of 0.053 and 0.051 mm indicated by
The holotype of Xenia crista (RMNH Coel. 18677) is 50 mm high and its stalk is 27 mm long, split into two branches 17 mm above its base. The branches are 10 mm long and 8 mm wide at their base and uppermost part. The polyp’s body is up to 10 mm long. The tentacles are up to 10 mm long; pinnules up to 1.5–2 mm long and 0.2 mm wide, featuring one pinnule-width gap between adjacent pinnules. The tentacles have two rows of pinnules on each side, with 22–30 pinnules in the outermost row. Sclerites are Ovabunda-type (Fig. 5a), measuring 0.014–0.030 × 0.017–0.037 mm in diameter (n = 48 sclerites). Occasionally, two sclerites are fused, reaching a maximal diameter of 0.042 mm (Fig. 5b, c).
Scanning electron micrographs of polyp sclerites of Xenia crista Reinicke, 1997 holotype (RMNH 18677). a Regular sclerites b–c Fused sclerites d White rectangle in c indicates magnified area. Scale bar 10 µm.
The paratype of Xenia crista (RMNH Coel. 18678) features tentacles with two rows of pinnules and 26–29 pinnules in the outermost row. The sclerites are Ovabunda-type, 0.025–0.036 mm in maximal diameter. The original description of the species indicated non-pulsating polyps in live colonies.
Both the original description of Xenia crista and the current examination revealed two rows of pinnules; however, we found 22–30 pinnules in the holotype and 26–29 in the paratypes, compared to 29–33 and 28–32, respectively, in the original description. The taxonomic features of Xenia crista overlap those of Ovabunda arabica and, therefore, they should be considered as synonyms, giving an alphabetical priority to Ovabunda arabica.
Ovabunda arabica is most similar to Ovabunda faraunensis. Although they both have two rows of pinnules and both have non-pulsating polyps in living colonies, the number of pinnules in the outermost row ranges from 24–29 in Ovabunda arabica compared to 17–24 in Ovabunda faraunensis.
Red Sea: Gulf of Aqaba.
http://species-id.net/wiki/Ovabunda_benayahui
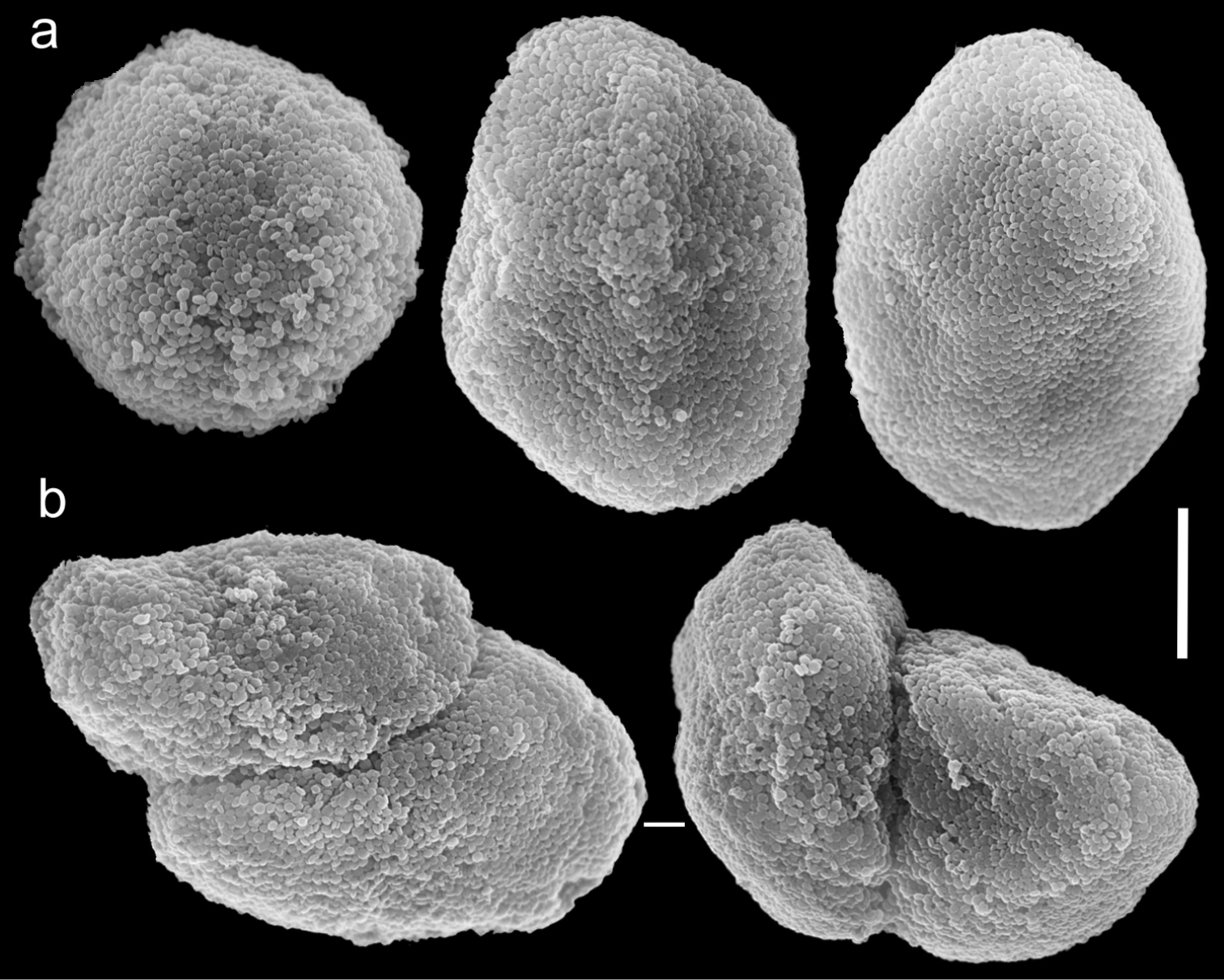
Figs 6, 7Holotype and two paratypes(the holotype is the largest colony): RMNH Coel. 19664, northern Red Sea, Gulf of Aqaba, Saudi Arabian border bay, 20 km south to Aqaba (29°21'37.31"N, 34°57'39.48"E), 21 m, 3 October 1989, coll. G. B. Reinicke; additional material: ZMTAU Co 26043, northern Red Sea, Gulf of Suez, Shag Rock (27°47'13.59"N, 34°0'22.61"E), 14 July 1987, coll. Y. Benayahu; ZMTAU Co 26044, northern Red Sea, Gulf of Suez, Southern tip of Sinai, Shaab el Utaf (27°45'9.00"N, 34°10'18.00"E), 10 m, 8 July 1986, coll. Y. Benayahu.
The holotype is 25 mm high; stalk is 10 mm long, 6 mm wide at colony-base, and 15 mm wide at the uppermost part. The polyp’s body is up to 4 mm long, and the tentacles up to 4 mm long, featuring a single row of 6–7 pinnules along each edge. The pinnules are short, up to 0.7 mm long and 0.4 mm wide with a 0.2–0.3 mm gap between adjacent pinnules. The sclerites are Ovabunda-type spheroids (Fig. 6a) and measure 0.019–0.035 × 0.027–0.041 mm in diameter (n = 22 sclerites). When two sclerites are fused they measure up to 0.043 mm in maximal diameter (Fig. 6b). The original description indicated non-pulsating polyps in live colonies. The ethanol-preserved colony is light brown-beige.
Scanning electron micrographs of polyp sclerites of Ovabunda benayahui (Reinicke, 1995) holotype (RMNH Coel. 19664). a Regular sclerites b Fused sclerite. Arrows indicate surface dents. Scale bar 10 µm.
ZMTAU Co 26043, originally identified by
Scanning electron micrographs of polyp sclerites of Ovabunda benayahui Reinicke, 1995 (ZMTAU Co 26043). a Regular sclerites b Fused sclerites c Egg-shaped sclerite d Rectangular sclerite. Arrow indicates surface crest. Scale bar 10 µm.
The features of the holotype and paratypes of Ovabunda benayahui agree with the original description of the species, and the assignment to Ovabunda by
Ovabunda benayahui is most similar to Ovabunda verseveldti. Although they both have one row of pinnules, the numbers of pinnules in the outermost row ranges from 6–7 in Ovabunda benayahui compared to 12–18 in Ovabunda verseveldti.
Red Sea: Gulf of Aqaba, Southern tip of Sinai Peninsula; Seychelles (see
http://species-id.net/wiki/Ovabunda_biseriata
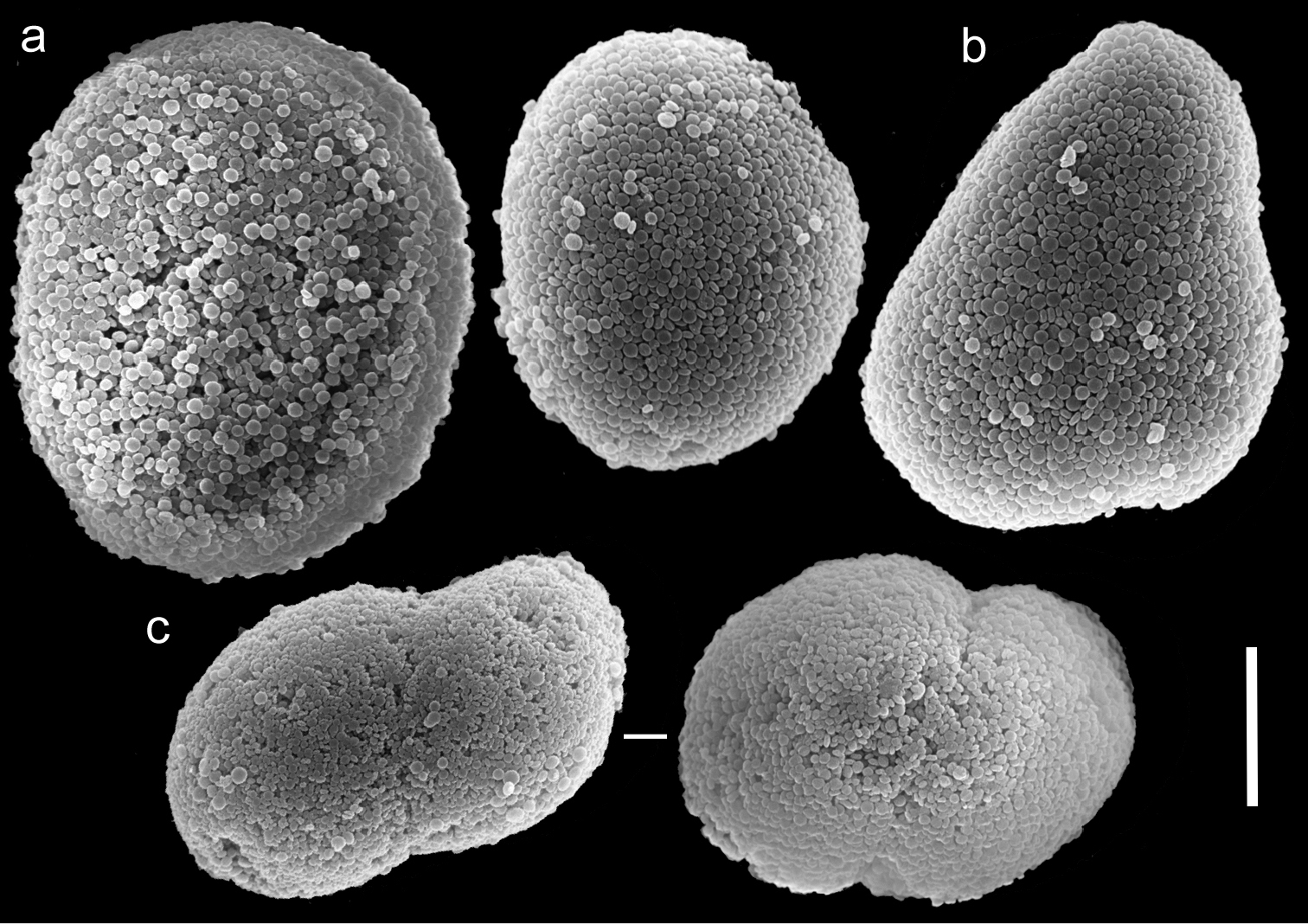
Figs 8, 9Holotype and five paratypes: HUJ I Co. 72, northern Red Sea, Gulf of Aqaba, Marsa Murach (29°25'34.44"N, 34°50'10.46"E), 1–4 m, 15 September 1969, coll. Y. Cohen; holotype of Ovabunda obscuronata HUJ I Co. 120, northern Red Sea, Gulf of Aqaba, Ras el Muqebla (29°24'1.20"N, 34°48'41.99"E), 12 m, 16 August 1971, coll. Y. Cohen; holotype of Xenia ternatana SMF 43, Indonesia, Ternate island, 1894, coll. Kükenthal.
The holotype is 28 mm high, stalk is 15 mm long, 11 mm wide at its base and 10 mm wide at the uppermost part; it is attached to the skeleton of a stony coral. Polyp’s body reaches up to 8–10 mm, and the tentacles 6–7 mm, bearing pinnules up to 1 mm long and 0.24 mm wide, separated by less than a pinnule-width. Two rows of pinnules are aligned along each of the tentacles margins, with 13–15 pinnules in the outermost row. There are numerous sclerites, abundant in all parts of the colony except for the mid-line of the oral side of the tentacles, where they are scarce. The sclerites are Ovabunda-type (Fig. 8), measuring 0.013–0.029 × 0.018–0.039 mm in diameter (n = 100 sclerites). Rarely, two sclerites are fused, reaching a maximum diameter of 0.048 mm and occasionally 0.060 mm. The paratypes are of similar size to the holotype, featuring tentacles with two rows of pinnules and 13–16 pinnules in the outermost row. The stalk of the smallest paratype is divided into two branches; the paratype tentacles bear two rows of pinnules with 10–12 pinnules in the outermost row. The sclerites of all paratype colonies are Ovabunda-type, ranging 0.018–0.025 × 0.023–0.039 mm in diameter. The original description of the species indicated non-pulsating polyps in live colonies. The preserved colonies are yellowish-light beige in colour.
Scanning electron micrographs of polyp sclerites of Ovabunda biseriata (Verseveldt & Cohen, 1971) holotype (HUJ I Co. 72). Arrow indicates surface dents. Scale bar 10 µm.
In general the original description of the species corresponds to the current findings. SEM micrographs of the holotype sclerites (Fig. 8) indicate that they are indeed Ovabunda-type, and therefore further confirm the previous assignment to that genus (
Scanning electron micrographs of polyp sclerites of Ovabunda obscuronata (Verseveldt & Cohen, 1971) holotype (HUJ I Co. 120). Scale bar 10 µm.
Examination of the holotype of Ovabunda biseriata and Ovabunda obscuronata revealed certain similarities, as already noted in the original description (
Ovabunda biseriata is most similar to Ovabunda impulsatilla and Ovabunda faraunensis. Although they all have two rows of pinnules and non-pulsating polyps in living colonies, the number of pinnules in the outermost row ranges from 13–16 in Ovabunda biseriata compared to 8–11 in Ovabunda impulsatilla and 17–24 in Ovabunda faraunensis.
Red Sea: Gulf of Aqaba, Gulf of Suez, Sudan.
http://species-id.net/wiki/Ovabunda_crenata
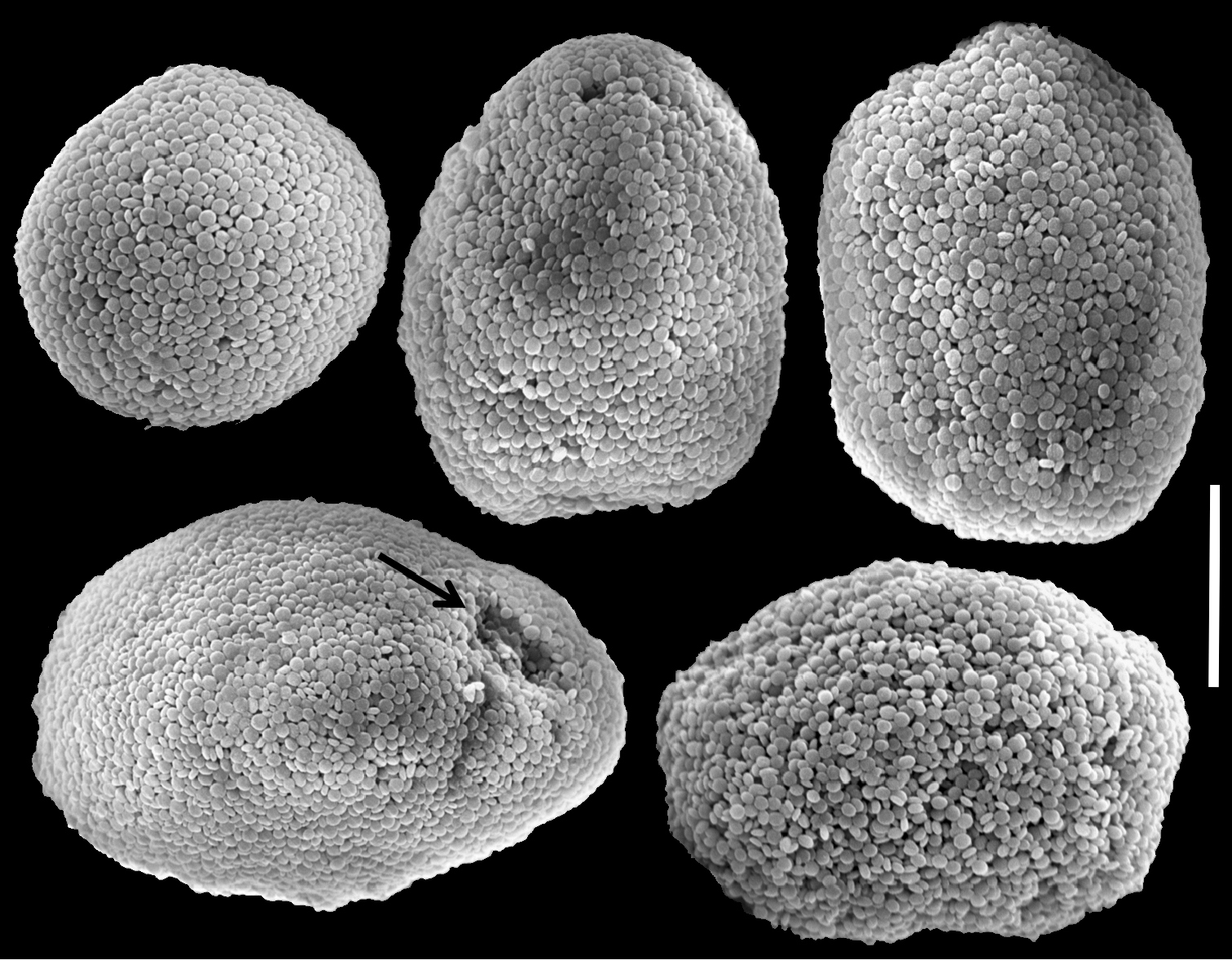
Fig. 10Holotype: RMNH Coel. 23538, Sudanese Red Sea, Sanganeb Atoll, 20 km off Port Sudan, S-slope near jetty (19°21'33.81"N, 37°19'37.66"E), 10 m, April 1991, coll. G.B. Reinicke; additional material: RMNH Coel. 23517, Sudanese Red Sea, Sanganeb Atoll, lagoon slope, TQ II station, 8 m, March 1991, coll. G.B. Reinicke; type of Xenia viridis SMF 42, Indonesia, Ternate island, 1894, coll. Kükenthal; type of Xenia blumi SMF 44, Indonesia, Ternate island, 1894, coll. Kükenthal.
The holotype is 30 mm high and 10 mm wide at its base. The stalk splits into two branches. The first is 15 mm long, 6 mm wide at its base and 10 mm wide at its uppermost part; the second splits further into two branches, 15 and 7 mm long, each 5 mm wide at the base, and 10 and 7 mm wide at the upper part, respectively. Polyp’s body is up to 5 mm long and the tentacles up to 5 mm long. The pinnules are mostly arranged in two rows, with an occasional third row. There are 12–16 pinnules in the outermost row, 1 mm long and 0.14 mm wide, with a 0.2 mm space between adjacent pinnules. Sclerites are very scarce in all parts of the holotype; they are Ovabunda-type spheroids, measuring 0.012–0.028 × 0.018–0.036 mm in diameter (n = 35 sclerites). Occasionally, two sclerites are fused, reaching 0.050 mm in maximal diameter. SEM micrographs of sclerites were obtained only from the additional material due to low density of sclerites in the holotype.
RMNH Coel. 23517 is similar in size to the holotype. Polyp’s body is up to 2 mm long, with 2 mm long tentacles, mostly bearing two rows of 1 mm long and 0.1 mm wide slender pinnules on each of the tentacle sides, and, rarely, a third row. The outermost row features 12–15 pinnules, up to one pinnule-width apart. Sclerites are of the Ovabunda-type, varying in shape from regular (Fig. 10a), egg-shaped (Fig. 10c) and more rectangular (Fig. 10d), ranging 0.016–0.026 × 0.028–0.040 mm (n = 25 sclerites) in diameter. Occasionally, two sclerites are fused, reaching 0.047 mm in maximal diameter (Fig. 10b).
Scanning electron micrographs of polyp sclerites of Ovabunda crenata (Reinicke, 1997) (RMNH Coel. 23517). a Regular sclerites b Fused sclerites c Egg-shaped sclerites d Rectangular sclerite. Arrows indicate surface dents. Scale bar 10 µm.
The original description of the holotype indicated three rows of pinnules, whereas the present examination revealed mostly two rows, with an indication of a third one. There is agreement between our findings and the original description regarding the number of pinnules in the outermost row of the tentacles (12–16 vs. 12–15, respectively). Sclerites correspond to the original description in size but are Ovabunda-type spheroids; therefore, it is concluded that the species should be assigned to Ovabunda.
Ovabunda crenata is most similar to Ovabunda biseriata. Although they both have overlapping number of pinnules in the outermost row, 12–16 and 13–16, respectively, Ovabunda biseriata has two rows of pinnules and Ovabunda crenata occasionally presents a third row. They both have non-pulsating polyps in live colonies.
Red Sea: Gulf of Aqaba, Sudan.
http://species-id.net/wiki/Ovabunda_faraunensis
Fig. 11Holotype: HUJ I. Co. 140, northern Red Sea, Gulf of Aqaba, opposite Gezirat Fara’un (Coral Island) (29°27'46.95"N, 34°51'27.38"E), 18 m, 15 October 1969, coll. Y. Cohen.
The holotype is 16 mm high; its stalk is 6-10 mm long, 4 mm wide at the base and 7 mm wide at its uppermost part. Polyp’s body is up to 2-4 mm long and the tentacles 3-5 mm long, featuring two rows of pinnules on each side with 17-24 pinnules in the outermost row. The sclerites are Ovabunda-type spheroids (Fig. 11a), measuring 0.013–0.028 × 0.019–0.033 mm in diameter (n = 46 sclerites). Occasionally, two sclerites are fused, reaching up to 0.044 mm in maximal diameter (Fig. 11b).
Scanning electron micrographs of polyp sclerites of Ovabunda faraunensis (Verseveldt & Cohen, 1971) holotype (HUJ I. Co. 140). a Regular sclerites b Fused sclerite. Scale bar 10 µm.
At the time of examination the holotype was dry, and therefore precise dimensions of the pinnules could not be obtained. The original description (
The current examination of the holotype (Fig. 11) along with the examination of the holotype by
Ovabunda faraunensis is most similar to Ovabunda arabica. Although they both have two rows of pinnules and both have non-pulsating polyps in living colonies, the number of pinnules in the outermost row ranges from 17–24 in Ovabunda faraunensis compared to 24–29 in Ovabunda arabica.
Red Sea: Gulf of Aqaba, Sudan (see
http://species-id.net/wiki/Ovabunda_gohari
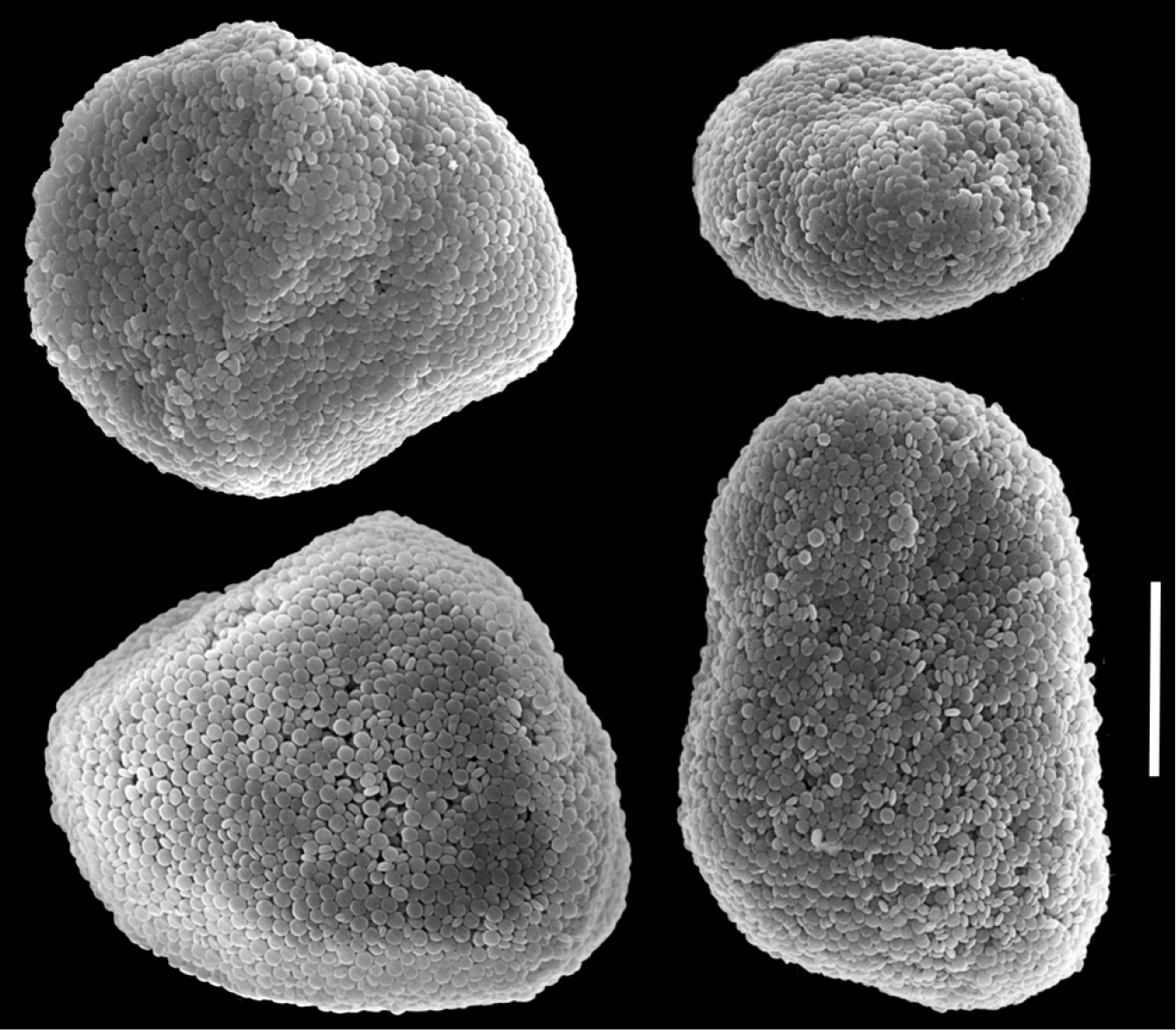
Fig. 12Holotype: RMNH Coel. 23435 and a paratype: RMNH Coel. 23436 northern Red Sea, Gulf of Aqaba, 10 km south to Aqaba, Nature Reserve (Agaba) (29°26'29.03"N, 34°58'0.50"E), 10 m, November 1991 and 3 December 1991, respectively; coll. G. B. Reinicke.
The holotype is 25 mm high; the stalk is 15 mm long, 10 mm wide at its base and 20 mm wide at its uppermost part and is divided into two. Polyp’s body reaches 5 mm and the 11 mm long tentacles mostly feature a single row bearing 19–22, 3 mm long and 0.2 mm wide pinnules. The gap between the pinnules is up to twice their width; occasionally a second row exists and then the outermost row bears 19–22 pinnules. The gap between adjacent pinnules in the outmost row is almost twice their width, as in the case of a single row. The sclerites are Ovabunda-type spheroids, measuring 0.028–0.045 mm in diameter (Fig. 12).
The paratype is 18 mm long. Its stalk is divided into two branches, 8 mm and 4 mm long; each branch is 3 mm wide at its base, and 5 and 4 mm wide at the uppermost part, respectively. Polyp’s body is up to 3 mm long, and tentacles 5–8 mm long, each have one row of 18–21 pinnules on each side; some small polyps have only 11–13 pinnules. The pinnules are up to 3 mm long and 0.24 mm wide at the base and the gap between the pinnules is up to twice their width. The sclerites (Fig. 12a) measure 0.015–0.035 × 0.028–0.055 mm in diameter (n = 30 sclerites). Occasionally, two sclerites are fused, measuring up to 0.060 mm in maximal diameter; the fusion of sclerites can be partial or almost complete (Fig. 12b). The sclerites are more abundant at the base of the pinnules along the tentacle midline compared to their distal part, and similarly at the aboral side of the tentacles compared to their oral side. The original description indicated non-pulsating polyps in live colonies. The ethanol-preserved colony is light yellowish, beige.
Scanning electron micrographs of polyp sclerites of Ovabunda gohari (Reinicke, 1997) paratype (RMNH Coel. 23436). a Regular sclerites b Fused sclerite. Scale bar 10 µm.
The current study confirmed the number of rows of pinnules and number of pinnules of the original description. Sclerite dimensions of the latter are larger than our findings, probably due to measurements that included fused sclerites.
The sclerites are Ovabunda-type spheroids and justify assignment to that genus.
Ovabunda gohari is most similar to Ovabunda faraunensis. Although they both have overlapping number of pinnules in the outermost row, 18–22 and 17–24, respectively, Ovabunda gohari has mostly one row of pinnules and Ovabunda faraunensis presents two rows of pinnules. They both have non-pulsating polyps in live colonies.
Red Sea: Gulf of Aqaba, Sudan (see
http://species-id.net/wiki/Ovabunda_hamsina
Fig. 13Holotype: RMNH Coel. 23904, Sudanese Red Sea, Sanganeb Atoll, off Port Sudan, reef flat (19°21'33.81"N, 37°19'37.66"E), 6 April 1991, coll. G. B. Reinicke; paratypes: RMNH Coel. 23902, same data as above, April 1991; RMNH Coel. 25906, same locality SW corner, 15 m; RMNH Coel. 23553, Sudanese Red Sea, Sanganeb Atoll, lagoon slope near TQ II, 12 m, October 1992, coll. G. B. Reinicke; additional material: RMNH Coel. 23552, same locality, W-slope, TQ IV, 12 m, April 1991, coll. G. B. Reinicke; RMNH Coel. 25903, same locality, SE corner, reef flat; RMNH Coel. 25905 SW corner, 15 m; RMNH Coel. 23907, near southern jetty, 10 m; all April 1991, all coll. G. B. Reinicke; RMNH Coel. 23908, Indian Ocean, Madagascar, 1960, coll. M. Cherbounier, MNHN Oct.A.1993.16; holotype of Xenia grasshoffi SMF 2616, northern Red Sea, Gulf of Aqaba, Elat, 1 January 1968, coll. Grasshoff M.
The holotype is 25 mm high and its stalk is 18 mm long, 15 mm wide at its base and 12 mm wide at its uppermost part. Polyp’s body reaches up to 2 mm long; tentacles 5 mm long featuring three rows of 1 mm long and 0.16 mm wide pinnules, 19-21 in the outermost row. An incomplete fourth row is occasionally present. The pinnules are separated by a two-pinnule width space. The sclerites are Ovabunda-type (Fig. 13a), 0.015–0.025 × 0.016–0.035 mm in diameter (n = 25). Occasionally, two sclerites are partially fused, reaching 0.034 mm in maximal diameter (Fig. 13b). The tentacles of the paratypes (RMNH Coel. 25902, 23533, 25906) bear three rows of pinnules on each side, with 19–22, 19–21 and 17–22 pinnules in the outermost row, respectively, and all feature Ovabunda-type sclerites. The original description indicated non-pulsating polyps in live colonies. The ethanol-preserved colony is light beige.
Scanning electron micrographs of polyp sclerites of Ovabunda hamsina (Reinicke, 1997) holotype (RMNH Coel. 23904). a Regular sclerites b Fused sclerites. Scale bar 10 µm.
The colonies RMNH Coel. 25905, 23907, 23908 feature three rows of pinnules with 17–22 pinnules in the outermost row. The tentacles of RMNH Coel. 23552 have four rows of pinnules and 18–21 pinnules in the outermost row; RMNH Coel. 25903 has three rows of pinnules with an indication of a fourth row, and 19–22 pinnules in the outermost row. All colonies feature Ovabunda-type sclerites.
Similar species. Ovabunda hamsina is most similar to Ovabunda ainex. Although they both have overlapping number of pinnules in the outermost row, 17–22 and 15–20, respectively, Ovabunda ainex has three rows of pinnules and Ovabunda hamsina presents three and sometimes four rows of pinnules. They both have non-pulsating polyps in live colonies.
Distribution. Sudanese Red Sea, Madagascar, Seychelles (see
http://species-id.net/wiki/Ovabunda_impulsatilla
Figs 14, 15, 16Holotype: HUJ I Co. 84 northern Red Sea, Gulf of Aqaba, near Solar Pond (Sinai), (29°25'44.43"N, 34°49'50.31"E), 2 m, 15 August 1969, Coll. Y. Cohen. Eight colonies on a sponge, one of them is the holotype. Additional material: the holotype of Xenia miniata: RMNH Coel. 23514, Sudanese Red Sea, Sanganeb Atoll, 20 km off Port Sudan, W-slope, TQ IV, (19°21'33.81"N, 37°19'37.66"E), 12 m, March 1991; paratypes of Xenia miniata: RMNH Coel 25412, RMNH Coel 25413, details as above, RMNH Coel. 25411, same location, SW-corner slope, TQ I, 12 m, March 1991, coll. G. B. Reinicke; RMNH Coel. 6848, northern Red Sea, gulf of Suez, El Tur (28°14'10.99"N, 33°36'51.06"E), 6 July 1969, coll. L. Fishelson; RMNH Coel. 6847, same details; RMNH Coel. 8938, same details, Abu Durbah (28°28'27.56"N, 33°19'30.13"E); the holotype of Ovabunda aldersladei RMNH Coel. 38681, Indian Ocean, Seychelles, northern coast of Bird Island (03°42'S, 55°12'E), <30 m, 21 December 1992, Tyro expedition; type of Xenia ternatana SMF 43, Indonesia, Ternate island, 1894, coll. Kükenthal; type of Xenia garciae BML 1921.11.18.1, Indian Ocean, Chagos Archipelago, coll. Diego Garcia; RMNH Coel. 8938 Red Sea, Gulf of Suez, Abu Zanima; RMNH Coel. 6847, Red Sea, Gulf of Suez, El Tur, 6 July 1969, coll. L. Fishelson; RMNH Coel. 6848, same location, misidentified as Xenia miniata
The now dry holotype is 18 mm high. The stalk is divided into two branches, 10 and 8 mm long, 6 and 4 mm wide at the base, and 8 and 4 mm wide at the uppermost part, respectively. Polyp’s body is up to 2–2.5 mm long, and tentacles up to 1.5–2 mm, featuring two rows of pinnules on each side, with 8–11 pinnules in the outermost row. The sclerites areOvabunda-type (Fig. 14a) and are almost evenly distributed in all parts of the colony, measuring 0.013–0.028 × 0.019–0.039 mm in diameter (n = 36 sclerites). Occasionally, two sclerites are fused, measuring up to 0.045 mm in maximal diameter (Fig. 14b). The original description stated that polyp pulsation in this species occurs in live colonies. The ethanol-preserved colony is light brown.
Scanning electron micrographs of polyp sclerites of Ovabunda impulsatilla (Verseveldt & Cohen, 1971) holotype (HUJ I Co. 84). a Regular sclerites b Fused sclerites. Scale bar 10 µm.
At the time of examination the type was dry and, therefore, the dimensions of the pinnules are lacking and the current measurements do not reflect the original ones given by
The holotype of Xenia miniata (RMNH Coel. 23514) features two rows of pinnules on each side of the tentacles, with 8–13 pinnules in the outermost row. The sclerites are Ovabunda-type, some regular (Fig. 15a), some pear-shaped (Fig. 15b) measuring 0.017–0.034 × 0.022–0.053 mm in diameter (n = 30 sclerites). Occasionally, two sclerites are fused, reaching 0.059 mm in maximal diameter (Fig. 15c). The paratypes of that species (RMNH 25411, 25412, 25413) feature tentacles with two rows, 11–13 pinnules in the outermost row and Ovabunda-type of sclerites, measuring 0.018–0.030 × 0.024–0.034 mm in diameter (n = 24 sclerites). Although the original description of Xenia miniata indicated three rows of pinnules in the holotype and paratypes, we found only two rows. The original description of the species indicated non-pulsating polyps in live colonies. Colour of the ethanol preserved colony is beige. Consequently, we conclude that Xenia miniata should be synonymised with Ovabunda impulsatilla.
Scanning electron micrographs of polyp sclerites of Xenia miniata Reinicke, 1997 holotype (RMNH Coel. 23514). a Regular sclerites b Pear-shaped sclerite c Fused sclerites. Scale bar 10 µm.
We also examined additional colonies that were identified by
Our measurements of the dimensions of the holotype of Ovabunda aldersladei (RMNH Coel. 38681) agree with those of the original description. It features 3 mm long polyp’s body, 2 mm long tentacles, with two rows of pinnules on each side, and 8–12 pinnules in the outermost row. The densely set pinnules are up to 0.6 mm long and 0.2–0.3 mm wide and with almost no gap between adjacent pinnules. The sclerites are Ovabunda-type spheroids (Fig. 16), measuring 0.012–0.030 × 0.018–0.042 mm in diameter (n = 46 sclerites).
Scanning electron micrographs of polyp sclerites of Ovabunda aldersladei Janes, 2008 holotype (RMNH Coel. 38681). Arrow indicates surface crest. Scale bar 10 µm.
Ovabunda impulsatilla is most similar to Ovabunda biseriata. Although they both have two rows of pinnules and non-pulsating polyps in living colonies, the numbers of pinnules in the outermost row ranges from 8–11 in Ovabunda impulsatilla and 13–16 in Ovabunda biseriata.
Distribution. Red Sea: Egypt, Sudan; Seychelles.
http://species-id.net/wiki/Ovabunda_macrospiculata
Figs 17, 18, 19Neotype: ZMTAU Co 25635, northern Red Sea, Gulf of Suez, Shag Rock (27°47'1.48"N, 33°59'23.17"E), <5m, 11 July 1986, coll. Y. Benayahu. Paratypes: ZMTAU Co 35789 (field number A69) northern Red Sea, Gulf of Aqaba, Underwater Restaurant (29°32'49.43N, 34°57'14.51"E), 5 m, 3 May 2010, coll. A. Halász; ZMTAU Co 35790 (field number A70) northern Red Sea, Gulf of Aqaba, The Interuniversity Institute of Marine Sciences in Eilat (IUI) (29°30'6.54"N, 34°55'4.44"E), 22 m, 4 May 2010, coll. A. Halász; ZMTAU Co 35791 (field number A72), collection details as above, 9 m.
The neotype is 20 mm high. Its stalk is 10 mm wide at its base, splitting into three branches, 7, 5 and 5 mm long; 5, 4 and 4 mm wide at the base and 10, 6 and 7 mm wide at the uppermost part, respectively. Polyp’s body reaches up to 2 mm and tentacles up to 5 mm, bearing three, occasionally two, rows of pinnules. The pinnules are 1 mm long and 0.2 mm wide, with less than a pinnule-width space between them, and there are 15–17 pinnules in the outermost row on each side of the tentacle. The sclerites are Ovabunda-type, both regular and pear-shaped, measuring 0.015–0.025 × 0.023–0.041 mm (n = 34, Fig. 17a, b). Occasionally two sclerites are fused, reaching 0.041 mm in maximal diameter (Fig. 17c).
The other specimens (ZMTAU Co 35789, 35790, 35791) are of similar size to the neotype; all with three rows of pinnules aligned on both sides of the tentacles, and 14–18, 14–17 and 14–17 pinnules in the outermost row, respectively. Their sclerites are of the Ovabunda-type and vary in shapes from regular to irregular, pear-shaped or fused. Their size ranges through 0.016–0.025 × 0.023–0.043 mm; 0.014–0.03 × 0.019–0.043 mm (Fig. 18) and 0.015–0.027 × 0.022–0.046 mm (Fig. 19) in diameter, respectively (n = 21 for each colony).
Scanning electron micrographs of polyp sclerites of Ovabunda macrospiculata (Gohar, 1940) neotype (ZMTAU Co 25635). a Pear-shape sclerite b Regular sclerites c Fused sclerite. Scale bar 10 µm.
Scanning electron micrographs of polyp sclerites of Ovabunda macrospiculata (Gohar, 1940) paratype (ZMTAU Co 35790). a Regular sclerites b Irregular sclerite c Fused sclerites d Pear-shaped sclerite. Scale bar 10 µm.
Scanning electron micrographs of polyp sclerites of Ovabunda macrospiculata (Gohar, 1940) paratype (ZMTAU Co 35791). a Regular sclerite b Irregular sclerites c Fused sclerites. Arrows indicate surface dents. Scale bar 10 µm.
Xenia macrospiculata was originally described by
The neotype was collected in proximity to the collection site of the original specimen collected by Gohar. The neotype is located at ZMTAU, and available upon request for future examination.
Ovabunda macrospiculata was also described by
Ovabunda macrospiculata is most similar to Ovabunda ainex. Although they both have three rows of pinnules, the numbers of pinnules in the outermost row ranges from 14–18 in Ovabunda macrospiculata compared to 15–20 in Ovabunda ainex. The major difference between them is that Ovabunda macrospiculata presents pulsating polyps in live colonies and Ovabunda ainex does not.
Red Sea: Gulf of Aqaba, southern tip of Sinai Peninsula.
http://species-id.net/wiki/Ovabunda_verseveldti
Fig. 20Holotype: ZMTAU Co 26048 and four paratypes: ZMTAU Co 31625 northern Red Sea, Gulf of Aqaba, Dahab (28°30'34.21"N, 34°31'18.26"E), 1 m, 9 November 1979, coll. Y. Benayahu.
The holotype is 18 mm high; its stalk is 9 mm long, 5 mm wide at its base and 13 mm wide at its uppermost part. Polyp’s body is 1–4 mm long, and the tentacles 6 mm long, bearing 1.8 mm long and 0.2 mm wide pinnules separated from each other by a small gap, less than one pinnule width. A single row of 14–18 pinnules is aligned on each side of the tentacles. There are numerous sclerites in all parts of the colony, densely packed in the pinnules and scarce in the mid-line of the tentacles’ oral side. The sclerites are Ovabunda-type, varying in shape from regular spheroids (Fig. 20b) to egg-shaped (Fig. 20a) and more rectangular forms (Fig. 20c), measuring 0.014–0.033 × 0.022–0.046 mm in diameter (n = 45). Rarely, two sclerites are fused, reaching a diameter of up to 0.051 mm (Fig. 20d).
Scanning electron micrographs of polyp sclerites of Ovabunda verseveldti (Benayahu, 1990) holotype (ZMTAU Co 26048). a Egg-shaped sclerite b Regular sclerite c Rectangular sclerites d Fused sclerites e Irregular sclerite. Scale bar 10 µm.
The four paratypes (ZMTAU Co 31625) are smaller than the holotype, 12–17 mm high; the stalk is 10–15 mm long, 4–6 mm wide at the stalk base and 6–8 mm wide at the upper part. Polyp’s body is up to 5 mm long, and tentacles up to 3 mm long, featuring one row of 12–17 pinnules on each side. The pinnules are 1.2 mm long and 0.2 mm wide at their base, densely set in each row, almost touching each other. The sclerites are Ovabunda-type, measuring 0.018–0.030 × 0.022–0.049 mm in diameter (n = 38). Occasionally, two sclerites are fused, reaching a maximum size of 0.049 mm. The original description did not mention polyp pulsation. The ethanol-preserved colonies are light brown.
The features of the holotype and paratypes agree with the original description of the species. The species was assigned by
Ovabunda verseveldti is most similar to Ovabunda benayahui. Although they both have one row of pinnules the numbers of pinnules in the outermost row ranges from 12–18 in Ovabunda verseveldti compared to 6–7 in Ovabunda benayahui.
Red Sea: Gulf of Aqaba, southern tip of Sinai Peninsula.
The current study revises the genus Ovabunda Alderslade, 2001, following examination of relevant type material. Examination of the types has confirmed the previous assignment of the following four species to this genus: Ovabunda benayahui, Ovabunda faraunensis, Ovabunda hamsina, Ovabunda verseveldti (see
The first taxonomic revision of the family Xeniidae was that by Kükenthal in
Here we discuss the reliability of each of the characteristics used to diagnose species, according to the order they appear in the species descriptions for each species. Colony dimensions as presented in the current study might in part be determined by age (
The application of SEM for octocoral taxonomic studies, and Xeniidae in particular, has significantly increased the resolution of sclerite imaging and led to the establishment of new taxa based on their microstructural features (e.g. Ovabunda Alderslade, 2001; Fasciclia Janes, 2008; Yamazatum Benayahu, 2010). In the current study, SEM revealed for Ovabunda species the full shape and size range of the spheroidal sclerites including fused ones. The latter type of sclerite is found in all species, although sometimes rare (as in the case of Ovabunda verseveldti). For each species the dimensions of the individual sclerites and the fused ones are presented, which together are necessary for future species description. The range of the smallest diameter of the single Ovabunda spheroids was found to be similar in all the types, ranging 0.026–0.035 mm (e.g., Ovabunda ainex: Fig. 3, Ovabunda biseriata: Fig. 8). Their maximal diameter is mostly 0.035–0.040 mm (e.g., Ovabunda benayahui: Figs 6–7; Ovabunda macrospiculata: Figs 17–19; Ovabunda gohari Fig. 12); and in some species, such as Ovabunda gohari (Fig. 12) and Ovabunda verseveldti (Fig. 20), they occasionally reach a larger size, up to 0.046–0.055 mm. It is important to emphasize that these larger sclerites are rare in the above-mentioned types, which mainly have sclerites within a range of 0.035–0.040 mm. In order to present the actual range of sclerite sizes we measured at least 20 sclerites from each colony, a standard that we recommend for future studies. The lack of such a detailed account in past studies has led to taxonomic errors, as in the establishment of Ovabunda aldersladei (
Polyp pulsation of living xeniid colonies was first noted by
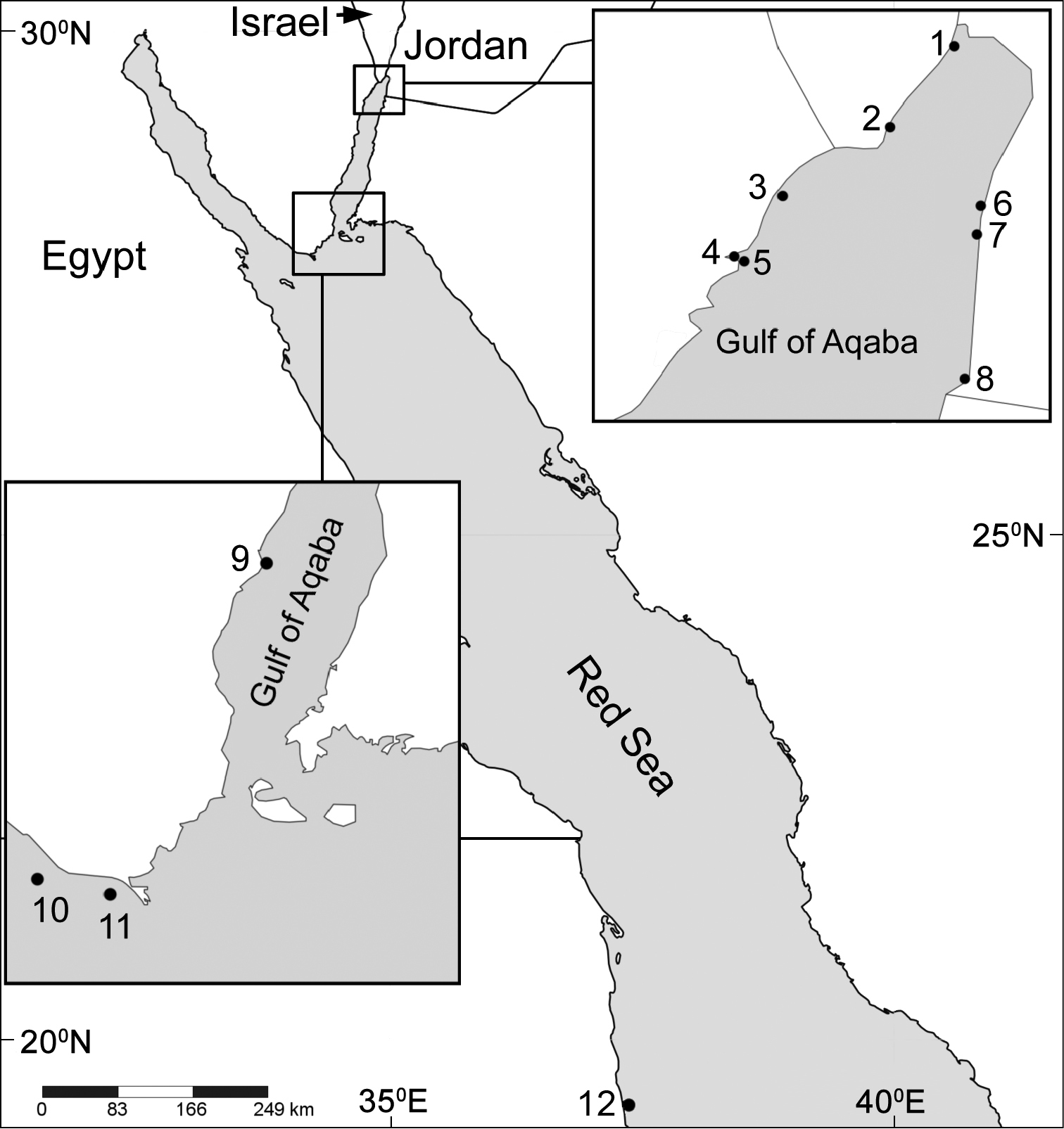
The current study indicates that the Red Sea is the type locality of most Ovabunda species (Fig. 21). Some species, such as Ovabunda hamsina, Ovabunda impulsatilla, and Ovabunda macrospiculata, were also recorded in the West Indian Ocean (e.g., Madagascar and the Seychelles). The possibility that the genus has a wider distributional range is not excluded, and remains to be confirmed by re-examination of already collected material deposited in various collections, or of freshly collected material from throughout the Indo-Pacific basin.
Re-examination and appropriate re-descriptions of octocoral type material, as conducted in the current study, is highly important in an era of molecular phylogeny and increasing phylogeographic studies, despite the difficulty or inability to extract DNA from the types themselves. This kind of comprehensive study based mainly on type material is a critical first step in the process of understanding phylogenetic relationships among species and genera, and their ecology. Due to similar morphologies in the case of Xenia and Ovabunda, further analysis is needed in order to reveal their radiation, especially in regions where they have a sympatric distribution. There is also a need to validate the current Ovabunda species, through an integrated taxonomic effort, combining molecular genetic evidence of species boundaries, ecological, and reproductive differences.
Distribution map of type localities of Ovabunda species. Areas of small rectangles are represented in respective large ones: 1 Underwater Restaurant, Eilat 2 The Interuniversity Institute of Marine Sciences (IUI), Eilat 3 opposite Gezirat Fara’un 4 Solar pond 5 Marsa Murach 6 Marine Science Station, Jordan 7 Nature reserve, Jordan 8 Saudi Arabia border bay 9 Dahab 10 Shag Rock 11 Shaab el Utaf 12 Sanganeb Atoll.
Support for this project came from the U.S.-Israeli Binational Science Foundation grant #2008186 to Y.B., C.S.M. & R.J.T. and from the Israel Taxonomy Initiative (ITI). This research (Applications DE-TAF-662, AT TAF 2064, and GB TAF 3027) received support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP7 “Capacities” Program. It also was in part supported by a Temminck Fellowship, the Naturalis Biodiversity Centre, and The Israel Cohen Chair in Environmental Zoology to Y.B. We thank curators of the following museums for loan of material: A. Cabrinovic, The Natural History Museum London (BML); A.D. Chipman, National History collections of the Hebrew University of Jerusalem (HUJ); J. Jurkowska, Museum of Natural History, Wroclaw University, Poland (MNHHWU); H. Sattmann, The Naturhistorisches Museum Wien (NHMW); L.P. van Ofwegen, the Naturalis Biodiversity Centre, formerly Rijksmuseum van Natuurlijke Historie, Leiden (RMNH); M. Grasshoff, Senckenberg Naturmuseum Frankfurt (SMF) and C. Lüter, Zoologisches Museum Berlin (ZMB). We thank The Interuniversity Institute for Marine Sciences in Eilat (IUI) for assistance and use of facilities. We acknowledge Alex Shlagman for professional curatorial skills, Y. Delaria and V. Holdengreber for SEM work, V. Vexler for digital editing and N. Paz for editorial assistance. We thank Dr. E. Gavish-Regev and Dr. N. Dorchin for taxonomic assistance and M. Weis for technical laboratory assistance. We would like to thank the reviewers of this manuscript: P. Alderslade, M. Janes and G.B. Reinicke for their constructive comments. This work was completed by A.H. as partial fulfilment of the requirements for a PhD at Tel Aviv University.