(C) 2013 Ferenc Kozár. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The number of scale insect species (Hemiptera: Coccoidea) known from Hungary has increased in the last 10 years by 39 (16.6 %), to a total of 274 species belonging to 112 genera in10 families. The family Pseudococcidae is the most species rich, with 101 species in 34 genera; Diaspididae contains 59 species in 27 genera; Coccidae contains 54 species in 27 genera; and the Eriococcidae contains 33 species in 8 genera. The other 6 coccoid families each contain only a few species: Asterolecaniidae (7 species in 3 genera); Ortheziidae (7 species in 4 genera); Margarodidae sensu lato (5 species in 5 genera); Cryptococcidae (3 species in 2 genera); Kermesidae (4 species in 1genus); and Cerococcidae (1 species). Of the species in the check list, 224 were found in outdoor conditions, while 50 species occurred only in indoor conditions. This paper contains 22 species recorded for the first time in the Hungarian fauna.
Introduced pests, insect invasion, distribution, taxonomy, Palaearctic Region
Scale insects (Hemiptera: Coccoidea) live on a wide variety of plant species and many of them are important agricultural pests. Publication of new knowledge of this insect group is therefore very important from a practical viewpoint. The distribution data of different species may serve also as a reliable biodiversity indicator in different territories, such as nature reserves and agricultural or urban landscapes. The distribution data may also reflect the progress of climatic changes (
The world distribution of insect pests has changed greatly in recent decades, mainly due to increasing international trade in plant material. Scale insects are particularly well adapted to accidental introduction because their habits are often cryptic, so they can escape detection during quarantine inspections (
Early data on the distribution of scale insects in Hungary were summarized by
Scale insect species in Hungary are grouped into three categories. True members of the Hungarian fauna can be found regularly in outdoor habitats and typically overwinter outdoors. The second category of species is generally found in greenhouses or other buildings, mainly on ornamental plants. These introduced species are, in some cases, well-established in Hungary and may occur regularly, but are unable to overwinter outdoors. The third category consists of relatively few, introduced species that occur typically on imported tropical or subtropical fruits for consumption. Some of these species have not been able to establish at all, even in greenhouses, despite repeated introductions over several decades. All the species in the following checklist are assigned to one of these three categories.
The list below is based on the collection data of the authors between 2003 and 2013 and includes earlier records from
The scales were mounted on microscope slides following the method described by
The nomenclature of the scale insects has frequently been changed, even within the last decade. The scientific names used below therefore are annotated to relate them to those that were used in earlier Hungarian publications. We have endeavoured to maintain conformity with our previous works, as well as with the international scale insect database on “ScaleNet” (
For the zoogeographical and zoological subregion of Central Europe, we used the Palaearctic concept of
The number of scale insect species in Hungary has increased by 39 (16.6 %) in the last ten years, and currently totals 274 species in ten families (Tables 1 and 2). The largest families in order of species richness are: Pseudococcidae with 101 species, Diaspididae (59 species), Coccidae (54 species) and Eriococcidae with 33 species. The new species to the Hungarian fauna recorded here belong to the Pseudococcidae, Diaspididae and Eriococcidae. Most of the species in the checklist (224; 81.75 %) are native and live outdoors. The check list contains 50 introduced (generally cosmopolitan) species, mainly occurring indoors in Hungary on ornamental plants in greenhouses and buildings. Of these indoor species, 33 occurred only in greenhouses or buildings (mainly on ornamental plants) and 7 were found exclusively on imported tropical/subtropical fruits for consumption. Four of the species living in greenhouses sometimes also occur outdoors. Four other species, which are typically found on imported fruit, also appear in greenhouses from time to time. Two of the newly recorded species were found on imported nursery plant material. In the present list, 22 species are new to the Hungarian fauna. According to these data, Hungary is the most scale-insect-species-rich country in in Central Europe (Fig. 1).
The number of scale insect species in different categories.
| Number of species | % | |
|---|---|---|
| New to the Hungarian fauna | 22 | 8.03 |
| Only found outdoors | 224 | 81.75 |
| Introduced on propagation plant material (outdoor conditions) | 2 | 0.73 |
| Only found indoors (in greenhouses and buildings) | 33 | 12.40 |
| Only found on imported fruit | 7 | 2.55 |
| Mainly found in greenhouses | 4 | 1.46 |
| Mainly on imported fruits, but occasionally in greenhouses | 4 | 1.46 |
| Total | 274 | - |
Updated checklist of scale insects (Homoptera: Coccoidea) of Hungary (2013), with comments and nomenclatural changes. Information on the original decriptions of species can be found in ScaleNet database (
| Taxon | Comment |
|---|---|
| Asterolecaniidae (3 genera) | |
| Asterodiaspis bella (Russell, 1941) | |
| Asterodiaspis quercicola (Bouché, 1851) | |
| Asterodiaspis roboris (Russell, 1941) | |
| Asterodiaspis variolosa (Ratzeburg, 1870) | |
| Asterodiaspis viennae (Russell, 1941) | |
| Asterolecanium epidendri (Bouché, 1844) | |
| Planchonia arabidis Signoret, 1877 | Previously recorded as Asterolecanium fimbriatum (Leonardi, 1920). |
| Cerococcidae (1 genus) | |
| Cerococcus cycliger Goux, 1932 | |
| Coccidae (27 genera) | |
| Ceroplastes japonicus Green, 1921 | Found in Hungary in 2011 ( |
| Ceroplastes rubens Maskell, 1893 | Found in Hungary in 2011 ( |
| Ceroplastes rusci (Linnaeus, 1758). | According to |
| Chloropulvinaria floccifera (Westwood, 1870) | |
| Coccus hesperidum Linnaeus, 1756 | Found on Maclura sp. outdoors in recent years in Hungary (Velence) ( |
| Eriopeltis festucae (Fonscolombe, 1834) | |
| Eriopeltis lichtensteini Signoret, 1876 | |
| Eriopeltis stammeri Schmutterer, 1952 | |
| Etiennea villiersi Matile-Ferrero, 1984 | It has not been found since its first record ( |
| Eucalymnatus tessellatus (Signoret, 1873) | |
| Eulecanium ciliatum (Douglas, 1891) | |
| Eulecanium franconicum (Lindinger, 1912) | |
| Eulecanium tiliae (Linnaeus, 1758) | Previously recorded as Eulecanium mali (Schrank, 1781). |
| Eupulvinaria hydrangeae (Steinweden, 1946) | |
| Exaeretopus formiceticola Newstead, 1894 | |
| Exaeretopus mahunkai Kozár & Drozdják, 1990 | |
| Gascardia hodgsoni Matile-Ferrero & Le Ruyet, 1985 | It has not been found since its first record ( |
| Lecanopsis formicarum Newstead, 1893 | Previously recorded as Lecanopsis terrestris Borchsenius, 1952. |
| Lecanopsis subterranea (Gomez Menor Ortega, 1948 | Previously recorded as Longicoccus festucae Borchsenius, 1952. |
| Lecanopsis turcica (Bodenheimer, 1951) | Previously recorded as Lecanopsis porifera Borchsenius, 1952 |
| Luzulaspis kosztarabi Koteja & Kozár, 1979 | |
| Luzulaspis nemorosa Koteja, 1966 | Previously recorded as Lecanopsis luzulae (Dufour, 1864). |
| Luzulaspis rajae Kozár, 1981 | |
| Luzulaspis scotica Green, 1926 | Previously recorded as Lecanopsis borchsenii Rehacek 1959. |
| Palaeolecanium bituberculatum (Targioni Tozzetti, 1868) | |
| Parafairmairia bipartita (Signoret, 1872) | |
| Parafairmairia gracilis Green, 1916 | |
| Parthenolecanium corni (Bouché, 1844) | |
| Parthenolecanium fletcheri (Cockerell, 1893) | |
| Parthenolecanium persicae (Fabricius, 1776) | |
| Parthenolecanium pomeranicum (Kawecki, 1954) | |
| Parthenolecanium rufulum (Cockerell, 1903) | |
| Phyllostroma myrtilli (Kaltenbach, 1874). | The occurrence of this species in Hungary was mentioned by |
| Physokermes hemicryphus (Dalman, 1826) | |
| Physokermes inopinatus Danzig & Kozár, 1973 | |
| Physokermes piceae (Schrank, 1801) | |
| Poaspis intermedia (Goux, 1939) | Previously recorded as Luzulaspis. |
| Poaspis jahandiezi (Balachowsky, 1932) | Previously recorded as Luzulaspis. |
| Poaspis lata (Goux, 1939) | |
| Psilococcus ruber Borchsenius, 1952 | |
| Pulvinaria ribesiae Signoret, 1873 | |
| Pulvinaria vitis (Linnaeus, 1758) | Previously recorded as Pulvinaria betulae (Linnaeus, 1758). |
| Pulvinariella mesembryanthemi (Vallot, 1830) | It has not been found since its first record ( |
| Rhizopulvinaria artemisiae (Signoret, 1873) | |
| Rhizopulvinaria gracilis Canard, 1967 | |
| Rhizopulvinaria spinifera Borchsenius, 1952 | |
| Rhodococcus perornatus (Cockerell & Parrott, 1899) | Previously recorded as Rhodococcus bulgariensis (Wünn, 1939) or Rhodococcus rosophilus Borchsenius, 1953. |
| Rhodococcus spireae (Borchsenius, 1949) | |
| Saissetia coffeae (Walker, 1852) | Previously recorded as Saissetia hemisphaerica (Targioni Tozzetti, 1867). |
| Saissetia oleae (Olivier, 1791) | |
| Scythia craniumequinum Kiritchenko, 1938 | |
| Scythia festuceti (Šulc, 1941) | |
| Sphaerolecanium prunastri (Fonscolombe, 1834) | |
| Vittacoccus longicornis (Green, 1916) | |
| Cryptococcidae (2 genera) | |
| Cryptococcus aceris Borchsenius, 1937 | |
| Cryptococcus fagisuga, Lindinger, 1912 | |
| Pseudochermes fraxini (Kaltenbach, 1860) | |
| Diaspididae (27 genera) | |
| Abgrallaspis cyanophylli (Signoret, 1869) | Found in 2013 by K. Fetykó on Globularia punctata outdoors in Hungary (Budapest, Sashegy). Overwintering method unknown. |
| Aonidia lauri (Bouché, 1833) | |
| Acanthomytilus jablonowskii Kozár & Matile-Ferrero, 1983 | |
| Aonidiella aurantii (Maskell, 1879) | |
| Aspidiotus nerii Bouché, 1833 | Previously recorded as Aspidiotus hederae Signoret, 1869. |
| Aspidiotus destructor Signoret, 1869 | New to the Hungarian fauna. Found in 2013 by K. Fetykó on Phoenix roubellini indoors in Hungary (Kecskemét). |
| Aulacaspis rosae (Bouché, 1833) | |
| Aulacaspis yatsumatsui Takagi, 1977 | New to the Hungarian fauna. Found in 2012 by K. Fetykó on Cycas revoluta indoors in Hungary (Kecskemét). |
| Carulaspis carueli (Signoret, 1869) | New to the Hungarian fauna. Found in 2009-2012 by F. Kozár indoors and outdoors in Hungary (Csepel, Nagykovácsi, Solymár, Zalakomár), on nursery plants (Thuja sp., Chamaecyparis sp., Juniperus sp.). |
| Carulaspis juniperi (Bouché, 1851) | In high densities on ornamental plants in recent years. |
| Carulaspis visci (Schrank, 1781) | According to |
| Chionaspis austriaca Lindinger, 1912 | According to |
| Chionaspis lepineyi Balachowsky, 1928 | |
| Chionaspis salicis (Linnaeus, 1958) | |
| Chortinaspis subterraneus (Lindinger, 1912) | |
| Chrysomphalus aonidum (Linnaeus, 1758) | Previously recorded as Chrysomphalus ficus Ashmead, 1880. |
| Chrysomphalus dictyospermi (Morgan, 1889) | |
| Diaspidiotus alni (Marchal, 1909) | |
| Diaspidiotus bavaricus (Lindinger, 1912) | |
| Diaspidiotus gigas (Thiem & Gerneck, 1934) | Previously recorded as Quadraspidiotus. |
| Diaspidiotus labiatarum (Marchal, 1909) | Previously recorded as Quadraspidiotus. |
| Diaspidiotus lenticularis (Lindinger, 1912) | Previously recorded as Quadraspidiotus. |
| Diaspidiotus marani Zahradnik, 1952 | Previously recorded as Quadraspidiotus. |
| Diaspidiotus ostreaeformis (Curtis, 1843) | Previously recorded as Quadraspidiotus. |
| Diaspidiotus perniciosus (Comstock, 1881) | Previously recorded as Quadraspidiotus. |
| Diaspidiotus pyri (Lichtenstein, 1881) | Previously recorded as Quadraspidiotus. |
| Diaspidiotus sulci Balachowsky, 1950 | Previously recorded as Quadraspidiotus. |
| Diaspidiotus wuenni (Lindinger, 1923) | |
| Diaspidiotus zonatus (Frauenfeld, 1868) | Previously recorded as Quadraspidiotus; Diaspidiotus hungaricus Kosztarab, 1956 is a synonym. |
| Diaspis bouisduvali Signoret, 1869 | New to the Hungarian fauna. Found in 2006 by É. Szita on Ananas sp. indoors in Hungary (Budapest). |
| Diaspis bromeliae (Kerner, 1778) | |
| Diaspis echinocacti (Bouché, 1833) | |
| Dynaspidiotus abietis (Schrank, 1776) | Previously recorded as Nuculaspis. |
| Dynaspidiotus britannicus (Newstead, 1896) | |
| Epidiaspis leperii (Signoret, 1869) | |
| Ferreroaspis hungaricus (Vinis, 1981) | Previously recorded as Acanthomytilus |
| Hemiberlesia rapax (Comstock, 1881) | |
| Lepidosaphes beckii (Newman, 1869). | Often in high densities on imported lemon and orange fruit. |
| Lepidosaphes conchiformis (Gmelin, 1789) | Previously recorded as Mytilaspis rubri Thiem, 1931. |
| Lepidosaphes gloverii (Packard, 1869) | |
| Lepidosaphes granati Koroneos, 1934 | Previously recorded as Mytilococcus. |
| Lepidosaphes newsteadi (Šulc, 1895) | |
| Lepidosaphes ulmi (Linnaeus, 1758) | The validity of the synonyms Lepidosaphes tiliae Savescu, 1957 and Lepidosaphes populi Savescu, 1957 and/or their presence in Hungary is questionable. |
| Leucaspis loewi Colvée, 1882 | Previously recorded as Anamaspis. In very high densities in recent years ( |
| Leucaspis pini (Hartig, 1839). | In very high densities in recent years ( |
| Leucaspis pusilla Löw, 1883. | In very high densities in recent years ( |
| Mohelnaspis massiliensis (Goux, 1937) | |
| Mycetaspis personata (Comstock, 1883) | |
| Parlatoria crotonis Douglas, 1887 | |
| Parlatoria pergandii Comstock, 1881 | |
| Parlatoria ziziphi (Lucas, 1853) | |
| Pinnaspis aspidistrae (Signoret, 1869) | |
| Pinnaspis strachani (Cooley, 1899) | |
| Pseudaulacaspis pentagona (Targioni Tozzetti, 1886) | Important outdoor pest of fruit and and ornamental plantss in Hungary; found in 2012 by K. Fetykó on kiwi fruit imported from Greece. |
| Rhizaspidiotus balachowskyi Kozár & Matile-Ferrero, 1983 | |
| Syngenaspis parlatoriae Šulc, 1895 | Placed by some authors in Parlatoria. |
| Targionia vitis (Signoret, 1876) | |
| Unaspis euonymi (Comstock, 1881) | A very important pest of Euonymus in towns in recent years. |
| Unaspis yanonensis (Kuwana, 1923) | New to the Hungarian fauna. Found in 2013 in Hungary (Budapest) indoors. |
| Eriococcidae (8 genera) | |
| Acanthococcus aceris Signoret, 1875 | |
| Acanthococcus melnikensis Hodgson & Trencheva, 2008 | New to the Hungarian fauna. Found in 1969 in Hungary (Vászoly) by F. Kozár on Quercus sp. |
| Acanthococcus roboris (Goux, 1931) | |
| Acanthococcus thymi (Schrank, 1801) | |
| Anophococcus agropyri Borchsenius, 1949 | Previously recorded as Acanthococcus or Rhizococcus. |
| Anophococcus cingulatus (Kiritchenko, 1940) | Previously recorded as Acanthococcus or Rhizococcus. |
| Anophococcus cynodontis (Kiritchenko, 1940) | Previously recorded as Acanthococcus or Rhizococcus. |
| Anophococcus granulatus (Green, 1931) | New to the Hungarian fauna. Found in 2007 in Hungary (Vászoly) by F. Kozár on Poaceae. |
| Anophococcus herbaceus Danzig, 1962 | Previously recorded as Acanthococcus or Rhizococcus. |
| Anophococcus insignis (Newstead, 1891) | Previously recorded as Acanthococcus or Rhizococcus. |
| Anophococcus species nova Kozár & Konczné Benedicty, 2013 | Previously recorded as Rhizococcus cistacearum (Goux, 1936), a misidentification of Anophococcus sp. n.). New to the Hungarian fauna. Found in 2008 in Hungary (Fehérszék) by G. Konz on Festuca sp. |
| Anophococcus pseudinsignis (Green, 1921) | Previously recorded as Acanthococcus or Rhizococcus. |
| Gossyparia spuria (Modeer, 1778) | |
| Greenisca brachypodii Borchsenius & Danzig, 1966 | |
| Greenisca gouxi (Balachowsky, 1954) | |
| Gregoporia erwini Kozár, 1996 | Previously recorded as Greenisca. |
| Kaweckia glyceriae (Green, 1921) | Previously recorded as Greenisca. |
| Kaweckia laeticoris (Tereznikova, 1965) | Previously recorded as Greenisca. |
| Ovaticoccus agavium (Douglas, 1888) | |
| Rhizococcus artiguesi Goux, 1991 | New to the Hungarian fauna. Found in 2011 in Hungary (Budaörs) by F. Kozár on Thymus glabrescens. |
| Rhizococcus baldonensis Rasina, 1966 | |
| Rhizococcus cantium (Williams, 1985) | Previously recorded as Acanthococcus. |
| Rhizococcus echinatus (Goux, 1936) | New to the Hungarian fauna. Found by D-vac method in 1982 in Hungary (Sashegy) by A. Rákóczi, on Festucetum. |
| Rhizococcus desertus Matesova, 1957 | Previously recorded as Acanthococcus. |
| Rhizococcus devoniensis (Green, 1896) | Previously recorded as Acanthococcus. |
| Rhizococcus gnidii Silvestri, 1875 | New to the Hungarian fauna. Found in 1981 in Hungary (Budaörs) by F. Kozár on Thymus glabrescens. |
| Rhizococcus greeni (Newstead, 1898) | |
| Rhizococcus istresianus (Goux, 1989) | New to the Hungarian fauna. Found in 2007 in Hungary (Törek) by F. Kozár on Hieracium sp. |
| Rhizococcus micracanthus Danzig, 1975 | Previously recorded as Acanthococcus. |
| Rhizococcus munroi Boratynski, 1962 | Previously recorded as Acanthococcus. |
| Rhizococcus reynei (Schmutterer, 1952) | Previously recorded as Acanthococcus. |
| Rhizococcus targassoniensis (Goux, 1993) | New to the Hungarian fauna. Found in 2008 in Hungary (Bócsa) by Z. Konczné Benedicty on Artemisia sp. |
| Rhizococcus zernae (Tereznikova, 1977) | New to the Hungarian fauna. |
| Kermesidae (1 genus) | |
| Kermes bacciformis Leonardi, 1908 | |
| Kermes gibbosus Signoret, 1875 | |
| Kermes quercus (Linnaeus, 1758) | |
| Kermes roboris (Fourcroy, 1785) | |
| Margarodidae (5 genera) | |
| Dimargarodes mediterraneus Silvestri, 1906 | |
| Icerya purchasi (Maskell, 1878) | |
| Matsucoccus pini (Green, 1925) | Previously recorded as Matsucoccus matsumurae (Kuwana, 1905). |
| Neomargarodes festucae Archangelskaja, 1935 | |
| Porphyrophora polonica (Linnaeus, 1758) | |
| Ortheziidae (4 genera) | |
| Insignorthezia insignis (Browne, 1887) | Previously recorded as Orthezia. |
| Newsteadia floccosa (De Geer, 1778) | |
| Orthezia arenariae Vayssiere, 1923 | |
| Orthezia urticae (Linnaeus, 1758) | |
| Orthezia yashusi Kuwana, 0923 | |
| Ortheziola britannica Kozár & Miller, 2000 | |
| Ortheziola vejdovskyi Šulc, 1895 | |
| Pseudococcidae (35 genera) | |
| Atrococcus achilleae (Kiritchenko, 1936) | |
| Atrococcus arakelianae (Ter-Grigorjan, 1964) | |
| Atrococcus bejbienkoi Kozár & Danzig, 1976 | |
| Atrococcus cracens Williams, 1962 | |
| Atrococcus paludinus (Green, 1921) | |
| Balanococcus boratynskii Williams, 1962 | |
| Balanococcus singularis Schmutterer, 1952 | Previously recorded as Trionymus. |
| Boreococcus ingricus Danzig, 1960 | |
| Brevennia pulveraria (Newstead, 1892) | |
| Ceroputo pilosellae (Šulc, 1898) | Previously recorded as Puto. |
| Chaetococcus phragmitis (Marchal, 1909) | |
| Chaetococcus sulci (Green, 1934) | |
| Chnaurococcus danzigae Kozár & Kosztarab, 1976 | |
| Chorizococcus rostrellum (Lobdell, 1930) | |
| Chorizococcus senarius McKenzie, 1967 | Previously this species was known only from USA (California) ( |
| Coccidohystrix samui Kozár & Benedicty, 1997) | |
| Coccura comari (Künow, 1880) | |
| Dysmicoccus brevipes (Newstead, 1891) | |
| Dysmicoccus walkeri (Newstead, 1891) | |
| Fonscolombia europeae (Newstead, 1897) | |
| Fonscolombia graminis Lichtenstein, 1877 | |
| Fonscolombia tomlini (Newstead, 1892) | Previously recorded as Phenacoccopsis. |
| Geococcus coffeae Green, 1933 | |
| Heliococcus bohemicus Šulc, 1912 | |
| Heliococcus danzigae Bazarov, 1974 | |
| Heliococcus glacialis (Newstead, 1900) | Previously recorded as Heliococcus cydoniae Borchsenius, 1937. |
| Heliococcus radicicola Goux, 1934 | |
| Heliococcus salviae Borchsenius, 1949 | |
| Heliococcus sulci Goux, 1934 | |
| Heterococcus agropyri Savescu, 1985 | Proposed as a synonym of Heterococcus nudus (Green, 1926). |
| Heterococcus nudus (Green, 1926) | |
| Heterococcus tritici (Kiritchenko, 1932) | |
| Kissrhizoecus hungaricus Kozár & Konczné Benedicty, 2004 | An element from the steppes, origin unknown. |
| Longicoccus ashtarakensis Ter-Grigorjan, 1964 | New to the Hungarian fauna. Found in 2004 in Hungary (Orgovány) by F. Kozár and Z. Konczné Benedicty, on Festuca sp. Probably native. |
| Longicoccus festucae (Koteja, 1971) | |
| Longicoccus psammophilus (Koteja, 1971) | |
| Metadenopus festucae Šulc, 1933 | |
| Mirococcopsis avetianae Ter-Grigorian, 1964 | |
| Mirococcopsis borchsenii (Ter-Grigorian, 1964) | Previously recorded as Eumirococcus. |
| Mirococcopsis elongatus Borchsenius, 1948 | |
| Mirococcopsis nagyi Kozár, 1981 | |
| Mirococcopsis subterraneus (Newstead, 1893) | Previously recorded as Chnaurococcus. |
| Nipaecoccus nipae (Maskell, 1892) | |
| Peliococcus balteatus (Green, 1928) | |
| Peliococcus chersonensis (Kiritchenko, 1935) | |
| Peliococcus marrubii (Kiritchenko, 1935) | Previously recorded as Spinococcus. |
| Peliococcus rosae Danzig, 2001 | Previously recorded as Spinococcus morrisoni Kiritchenko, 1935. |
| Peliococcus turanicus (Kiritchenko, 1931) | |
| Pelizzaricoccus gabrielis Kozár, 1991 | New to theHungarian fauna. Found in 2005 by D-vac in Hungary (Nagykovácsi), by F. Samu and E. Botos. Origin unknown. |
| Phenacoccus abditus Borchsenius, 1949 | |
| Phenacoccus aceris (Signoret, 1875) | Previously recorded by the synonym Phenacoccus mespili (Signoret, 1875). No voucher specimens available. |
| Phenacoccus avenae Borchsenius, 1949 | |
| Phenacoccus bicerarius Borchsenius, 1949 | |
| Phenacoccus evelinae (Tereznikova, 1975) | Previously recorded as Paroudablis graminis Tereznikova, 1968. |
| Phenacoccus ferulae Borchsenius, 1949 | |
| Phenacoccus hordei (Lindeman, 1886) | |
| Phenacoccus interruptus Green, 1923 | Previously recorded as Paroudablis. |
| Phenacoccus persimplex Borchsenius, 1949 | |
| Phenacoccus phenacoccoides (Kiritchenko, 1932) | |
| Phenacoccus piceae Löw, 1883 | Previously recorded as Paroudablis. |
| Phenacoccus pumilus Kiritchenko, 1935 | |
| Planococcus citri (Risso, 1813) | In high densities in greenhouses and buildings; males were caught by pheromone traps in Central Europe. |
| Planococcus vovae (Nassonov, 1908) | Previously recorded as Allococcus. In high densities in recent years on Thuja sp., Juniperus sp., and Chamaecyparis sp. ( |
| Polystomophora ostiaplurima (Kiritchenko, 1940) | |
| Pseudococcus elisae Borchsenius, 1947 | New to the Hungarian fauna. Found in 2007 in Hungary (Gyál) by K. Fürst on Musa sp. fruits. Unknown origin. |
| Pseudococcus longispinus (Targioni Tozzetti, 1868) | Previously recorded as Pseudococcus microadonidum. |
| Pseudococcus microadonidum Beardsley, 1966 | |
| Pseudococcus viburni (Signoret, 1875) | Previously recorded as Pseudococcus affinis (Maskell, 1894), Pseudococcus obscurus Essig, 1909, or Pseudococcus maritimus Ehrhorn, 1900). |
| Puto superbus (Leonardi, 1907) | Previously recorded as Macrocerococcus. |
| Rhizoecus albidus Goux, 1936) | |
| Rhizoecus cacticans (Hambleton, 1946) | |
| Rhizoecus falcifer Künckel d’Herculais, 1878 | |
| Rhizoecus franconiae Schmutterer, 1956 | |
| Rhizoecus kazahstanus Matesova, 1980 | |
| Rhodania porifera Goux, 1935 | |
| Ripersiella caesii Schmutterer, 1956 | New to the Hungarian fauna. Found in 2007 in Hungary (Sárbogárd) by B. Kiss on Festuca sp. Probably native. Previously recorded as Rhizoecus. |
| Ripersiella halophila (Hardy, 1868) | Previously recorded as Rhizoecus. |
| Ripersiella lelloi (Mazzeo, 1995) | |
| Ripersiella periolana (Goux, 1985) | Previously recorded as Ripersiella halophilus. |
| Ripersiella poltavae Laing, 1929 | Previously recorded as Rhizoecus. |
| Ritsemia pupifera Lichtenstein, 1879 | |
| Spilococcus artemisiphilus Tang, 1988 | New to the Hungarian fauna. Found in 2009 in Hungary (Csepel) by F. Kozár and É. Szita on Lotus corniculatus. Probably native. |
| Spilococcus furcatissispinus (Borchsenius, 1937) | New to the Hungarian fauna. Found in 2009 in Hungary (Lajosmizse) by F. Kozár on Festuca sp. Probably native. |
| Spilococcus halli (McKenzie & Williams, 1965) | Previously recorded as Chorizococcus viktorina. |
| Spilococcus mamillariae (Bouché, 1844) | Previously recorded as Spilococcus cactearum. |
| Trionymus aberrans Goux, 1938 | |
| Trionymus dactylis Green, 1925 | |
| Trionymus elymi (Borchsenius, 1949) | |
| Trionymus graminellus Borchsenius, 1949 | New to the Hungarian fauna. Found in 2010 in Hungary (Törökbálint) by F. Kozár on Festuca sp. Probable native. |
| Trionymus hamberdi (Borchsenius, 1949) | |
| Trionymus multivorus (Kiritchenko, 1935) | |
| Trionymus newsteadi (Green, 1917) | |
| Trionymus perrisii (Signoret, 1875) | |
| Trionymus phalaridis Green, 1925 | |
| Trionymus radicum (Newstead, 1895) | |
| Trionymus singularis Schmutterer, 1952 | New to the Hungarian fauna. Found in Hungary (Gyál) by F. Kozár on Agropyron sp. Probably native. |
| Trionymus thulensis Green, 1931 | |
| Trionymus tomlini Green, 1925 | |
| Volvicoccus stipae Borchsenius, 1949 | Previously recorded as Mirococcopsis. |
| Volvicoccus volvifer (Goux, 1945) | New to the Hungarian fauna. Found in Hungary (Sashegy) by D-vac (leg: E. Botos) on Brometum. Probably native. |
| Vryburgia brevicruris (McKenzie, 1960) |
Comments:
i. The record of the presence of Acanthomytilus sacchari (Hall, 1923) in Hungary was given by Danzig and Pellizzari in
ii. The presence of Lepidosaphes shanxiensis Shi, 1990 in Hungary, cited by ScaleNet, is not proven (error or misidentification).
iii. The record of the presence of Parlatoria oleae (Colvée, 1880) in Hungary given by
iv. The record of Kermes ilicis (Linnaeus, 1758) given by
v. The record of the presence of Luzulaspis frontalis Green, 1928, cited by ScaleNet as a scale distribution record for Hungary, is probably a misunderstanding of the text of
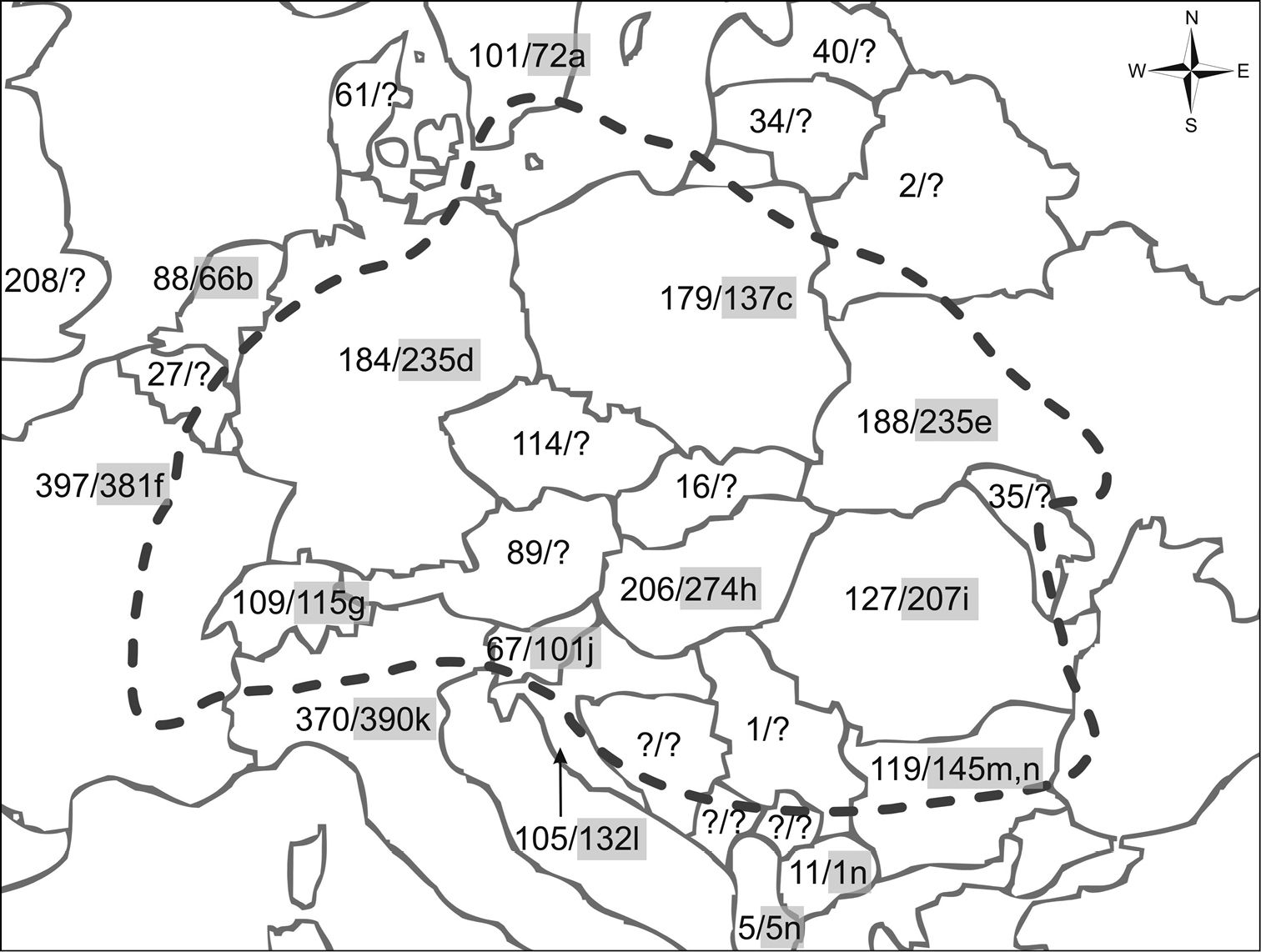
Biogeographic map of Central Europe after
No species should be considered as truly endemic only on the basis of its presence in a checklist, because the lack of a species in the surrounding countries is most likely due to inadequate exploration of those areas (Fig. 1). Out of the above list, 106 (38.69%) species are considered as widely distributed Pan-Palaearctic species, 75 (27.37%) are widely distributed Euro-Siberian species, 91 (33.21%) are cosmopolitan, and only two species are known to originate from the Mediterranean subregion.
Our data from Hungary shows a substantially different picture from that of earlier analyses dealing with scale insect zoogeography (
Number of scale insect species in Hungary, by family
| Family | Number of species | % of the Hungarian fauna | Number of new records |
| Asterolecaniidae | 7 | 2.55 | 0 |
| Cerococcidae | 1 | 0.36 | 0 |
| Coccidae | 54 | 19.71 | 0 |
| Cryptococcidae | 3 | 1.09 | 0 |
| Diaspididae | 59 | 21.53 | 5 |
| Eriococcidae | 33 | 12.04 | 9 |
| Kermesidae | 4 | 1.46 | 0 |
| Margarodidae s.l. | 5 | 11.82 | 0 |
| Ortheziidae | 7 | 2.55 | 0 |
| Pseudococcidae | 101 | 36.86 | 8 |
In Central Europe in the wide bio-geographic sense of
Concerning the category of species found indoors in greenhouses and buildings (Table 4) detailed information on these species are available in
List of scale insect species found indoors in greenhouses and buildings (on ornamental plants) in Hungary
| Abgrallaspis cyanophylli (Signoret, 1869) |
| Aonidia lauri (Bouché, 1833) |
| Aspidiotus nerii Bouché, 1833 |
| Aspidiotus destructor Signoret, 1869. |
| Asteroleanium epidendri (Bouché, 1844) |
| Aulacaspis yatsumatsui Takagi, 1977 |
| Ceroplastes japonicus Green, 1921 |
| Ceroplastes rubens Maskell, 1893 |
| Ceroplastes rusci (Linnaeus, 1758) |
| Chrysomphalus aonidum (Linnaeus, 1758) |
| Chrysomphalus dictyospermi (Morgan, 1889) |
| Coccus hesperidum Linnaeus, 1758 |
| Diaspis bouisduvali Signoret, 1869 |
| Diaspis bromeliae (Kerner, 1778) |
| Diaspis echinocacti (Bouché, 1833) |
| Dynaspidiotus britannicus (Newstead, 1896) |
| Etiennea villiersi Matile-Ferrero, 1984 |
| Eucalymnatus tessellatus (Signoret, 1873) |
| Gascardia hodgsoni Matile-Ferrero & Le Ruyet, 1985 |
| Geococcus coffeae Green, 1933 |
| Hemiberlesia rapax (Comstock, 1881) |
| Icerya purchasi (Maskell, 1878) |
| Mycetaspis personata (Comstock, 1883) |
| Nipaecoccus nipae (Maskell, 1892) |
| Prelongorthezia insignis Browne, 1887 |
| Ovaticoccus agavium (Douglas, 1888) |
| Parlatoria crotonis Douglas, 1887 |
| Pinnaspis aspidistrae (Signoret, 1869) |
| Pinnaspis strachani (Cooley, 1899) |
| Planococcus citri (Risso, 1813) |
| Pseudococcus longispinus (Targioni Tozzetti, 1868) |
| Pseudococcus microadonidum Beardsley, 1966 |
| Pseudococcus viburni (Signoret, 1875) |
| Pulvinariella mesembryanthemi (Vallot, 1830) |
| Rhizoecus cacticans (Hambleton, 1946) |
| Rhizoecus falcifer Künckel d’Herculais, 1878 |
| Saissetia coffeae (Walker, 1852) |
| Saissetia oleae (Olivier, 1791) |
| Spilococcus mamillariae (Bouché, 1844) |
| Unaspis yanonensis (Kuwana, 1923) |
| Vryburgia brevicruris (McKenzie, 1960) |
A detailed study of the scale insects introduced into Hungary on tropical and subtropical fruits was published by
Scale insect species found in Hungary on imported (mainly subtropical and tropical) fruits for consumption.
| Aonidiella aurantii (Maskell, 1879) |
| Aspidiotus nerii Bouché, 1833 |
| Carulaspis caruelii (Signoret, 1869) |
| Chrysomphalus aonidum (Linnaeus, 1758) |
| Chrysomphalus dictyospermi (Morgan, 1889) |
| Dysmicoccus brevipes (Newstead, 1891) |
| Lepidosaphes beckii (Newman, 1869) |
| Lepidosaphes gloverii (Packard, 1869) |
| Parlatoria pergandii Comstock, 1881 |
| Parlatoria ziziphi (Lucas, 1853) |
| Planococcus citri (Risso, 1813) |
| Pseudaulacaspis pentagona (Targioni Tozzetti, 1886) |
| Pseudococcus elisae Borchsenius, 1957 |
| Pseudococcus viburni (Signoret, 1875) |
Our thanks are due to numerous colleagues who collected scale insects for us, particularly Dr. Barnabás Nagy, Dr. Ferenc Szentkirályi, Dr. Klára Reiderné Saly, Dr. Géza Ripka, Dr. György Csóka, and Zoltán Avar. We also thank Dr. Bora Kaydan (Cukorova University, Department of Plant Protection, Adana, Turkey) and Dr Gillian W. Watson (California Department of Food & Agriculture, Sacramento, California, U.S.A.) for helpful suggestions on the manuscript. Our work was financially supported by various grants from the Hungarian National Research Found (OTKA: K75889, K83829, K81971, K91104).