(C) 2014 Bert W. Hoeksema. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Hoeksema BW (2014) The “Fungia patella group” (Scleractinia, Fungiidae) revisited with a description of the mini mushroom coral Cycloseris boschmai sp. n. ZooKeys 371: 57–84. doi: 10.3897/zookeys.371.6677
The recent taxonomic history of extant free-living Cycloseris species is briefly reviewed, resulting in the description of Cycloseris boschmai sp. n. (Scleractinia, Fungiidae) and a discussion on the validity of two other recently described species. Some Cycloseris species were previously considered to belong to the Fungia patella group, which also concerned misidentified museum specimens that actually belong to the new species. Other specimens of C. boschmai sp. n. were photographed and collected in the course of 30 years of fieldwork. The new mushroom coral is compared with other free-living Cycloseris species by means of an identification key. With a maximum diameter of 50 mm, it is the smallest free-living mushroom coral discovered so far. It can also be distinguished by its large primary order costae and variable colouration. Its distribution range is limited to the Coral Triangle, where it can be observed as an uncommon species on lower reef slopes.
Coral reef, free-living, fungiid, solitary, budding, collection, fieldwork, Coral Triangle, Indo-Pacific
Mushroom corals (Scleractinia, Fungiidae) form a common element in the fauna of most Indo-Pacific coral reefs. Depending on the species, full-grown specimens are either attached or free-living, which are character states occurring in various evolutionary lineages and therefore do not necessarily reflect phylogenetic relationships among the Fungiidae (
The smallest species among free-living mushroom corals appear to be among the most difficult to identify because they show relatively few distinguishing characters and much ecophenotypic variation (
Previously, species of the Fungia patella group were considered to belong to the genera Cycloseris Milne Edwards & Haime, 1849, consisting of complete corals, and Diaseris Milne Edwards & Haime, 1849, representing radially fragmenting corals of the same species (see
Oddly, both Fungia patella and Fungia patellaris are junior synonyms of Fungia fungites (Linnaeus, 1756), the type species of Fungia (see
The two specimens (ZMA Coel. collection) earlier described by
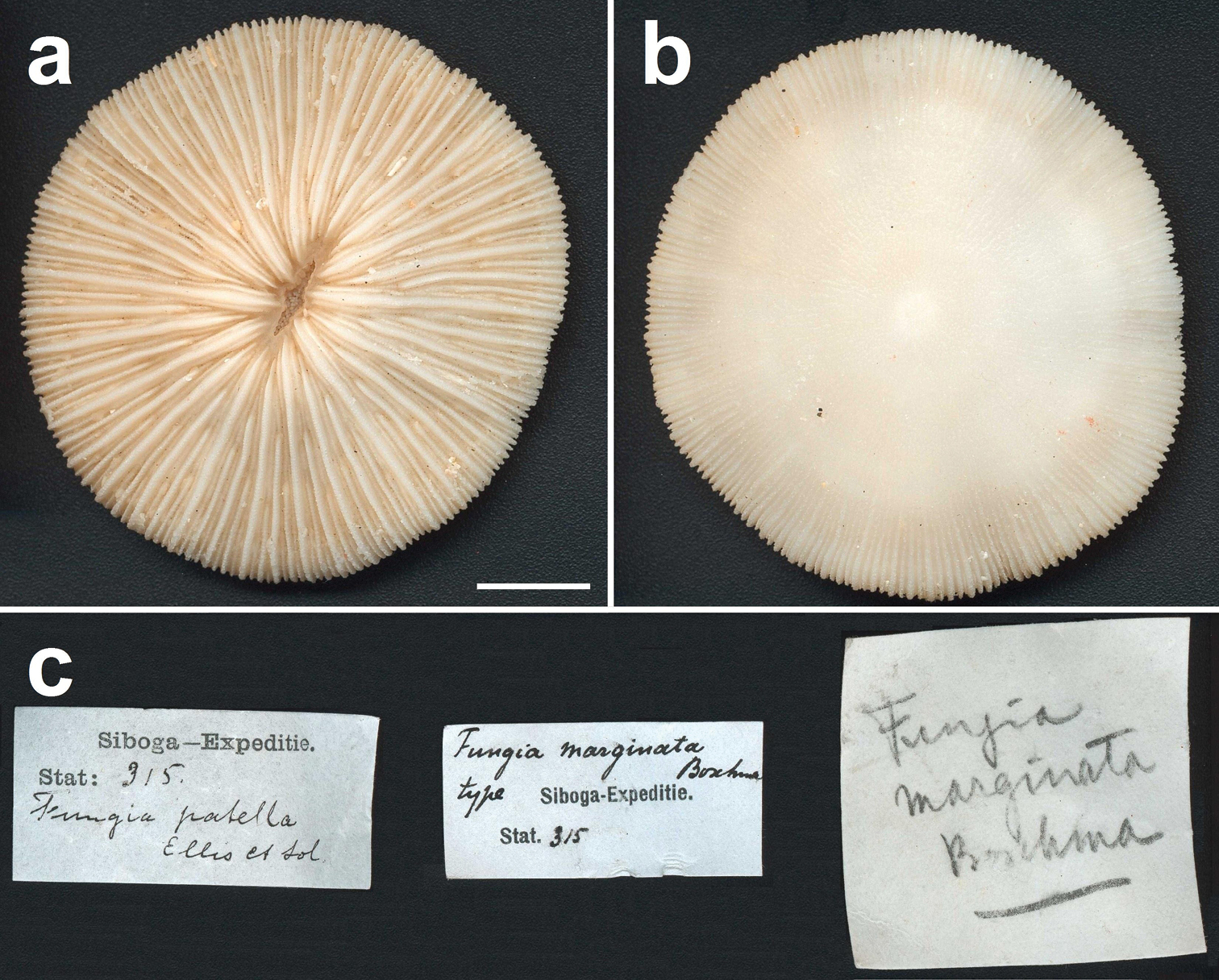
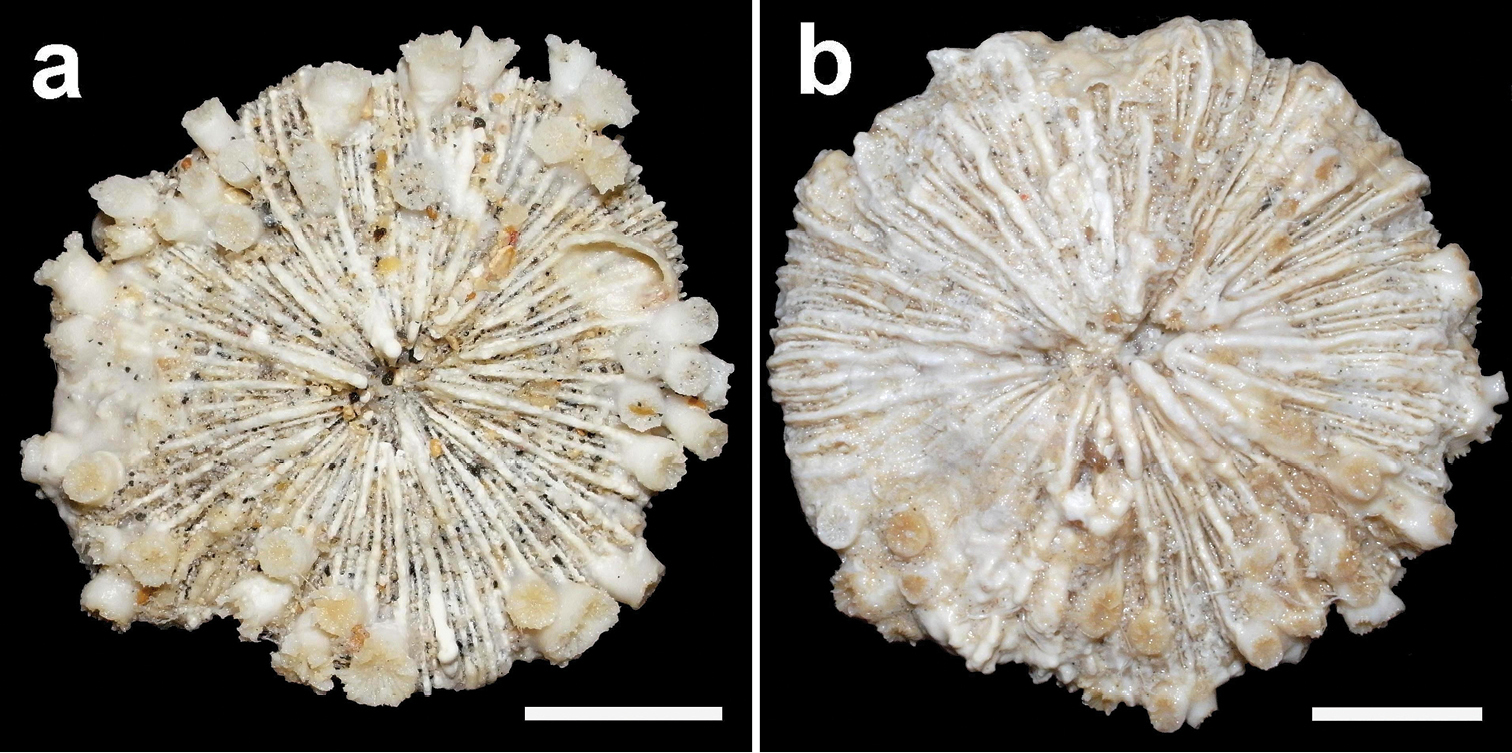
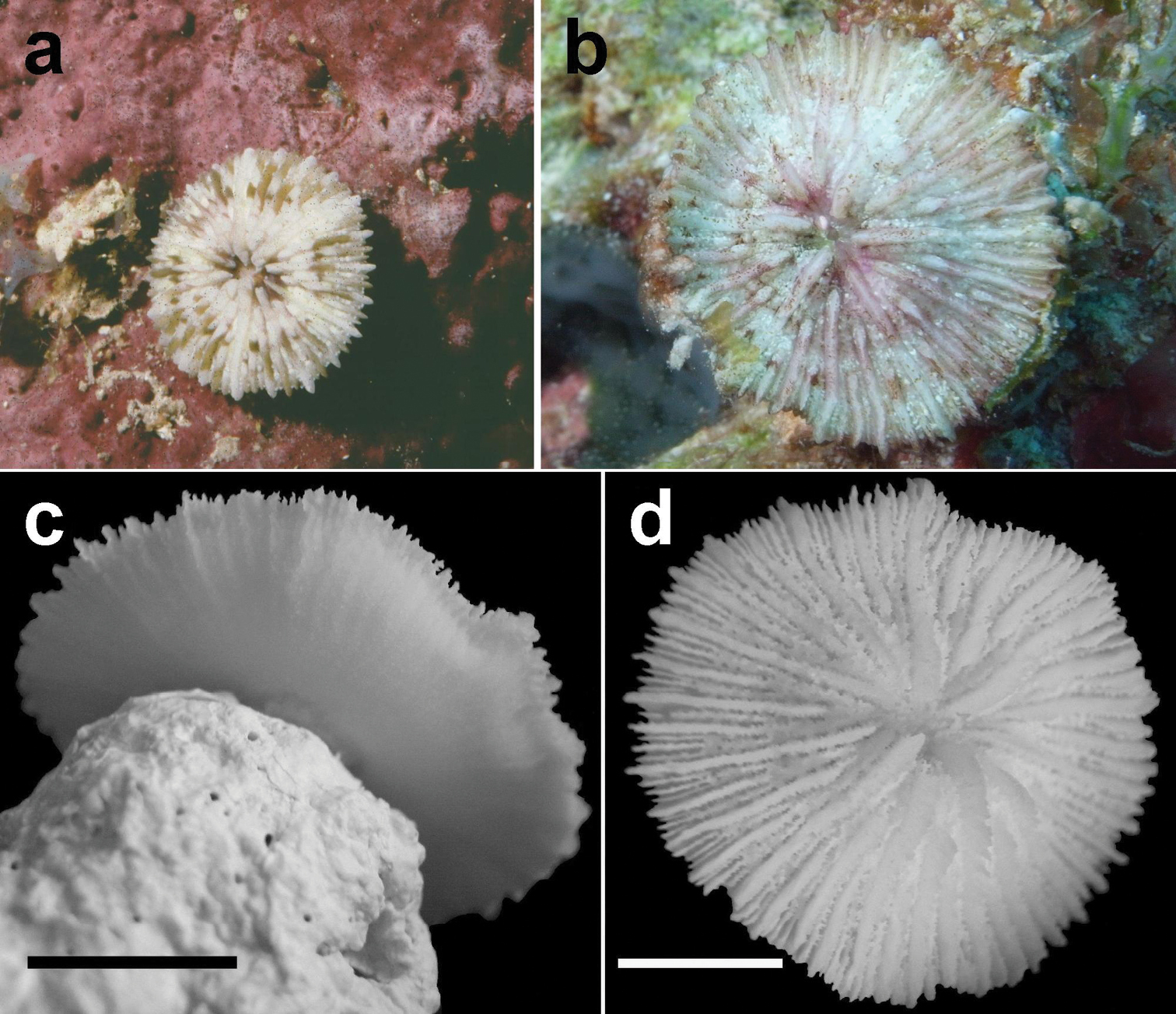
Lectotype of Fungia marginata Boschma, 1923 (ZMA Coel. 604, ethanol), which is a specimen of Cycloseris costulata Ortmann, 1889, collected at Siboga Expedition Sta. 315, Anchorage East of Sailus Besar, in the Paternoster islands, Indonesia. a Upper side b Lower side c Collection labels indicating the first identification by Van der Horst (Fungia patella) and the later one by Boschma (Fungia marginata). Scale bar: 0.5 cm.
Paralectotype of Fungia marginata Boschma, 1923 (ZMA Coel. 723, dry), which may be a juvenile specimen of Cycloseris costulata Ortmann, 1889 or Cycloseris hexagonalis Milne Edwards & Haime, 1849, collected at Siboga Expedition Sta. 315, Anchorage East of Sailus Besar, in the Paternoster islands, Indonesia a Specimen with collection box and labels; scale bar: 1 cm. Collection labels indicating the first identification by Van der Horst (Fungia patella) and the later one by Boschma (Fungia marginata) b Close–up upper side c Close–up lower side.
All species of the former Fungia patella-group belong to Cycloseris, previously considered a subgenus of Fungia (see
BWH = B.W. Hoeksema; Exp. = Expedition; I. = Island; Sta. = Station; MTQ = Museum of Tropical Queensland, Townsville, Australia; RMNH Coel. = Rijksmuseum van Natuurlijke Historie, Coelenterate collection (Naturalis Biodiversity Center), Leiden, the Netherlands; UZMK = Zoological Museum – University of Copenhagen, Denmark; ZMA Coel. = Zoological Museum of Amsterdam Coelenterate collection (Naturalis Biodiversity Center), Leiden, the Netherlands.
Fungia cyclolites
Cycloseris
Diaseris
Fungia (Cycloseris) –
Adult corals either encrusting and polystomatous or free-living and monostomatous (
http://zoobank.org/8FA4CA99-7074-4425-A7ED-D051D6AB3311
http://species-id.net/wiki/Cycloseris_boschmai
Figures 3–13Type specimens of Cycloseris boschmai from Banda, Moluccas, Indonesia (Danish Exp. to the Kei Islands, 1922), previously identified as Fungia marginata by
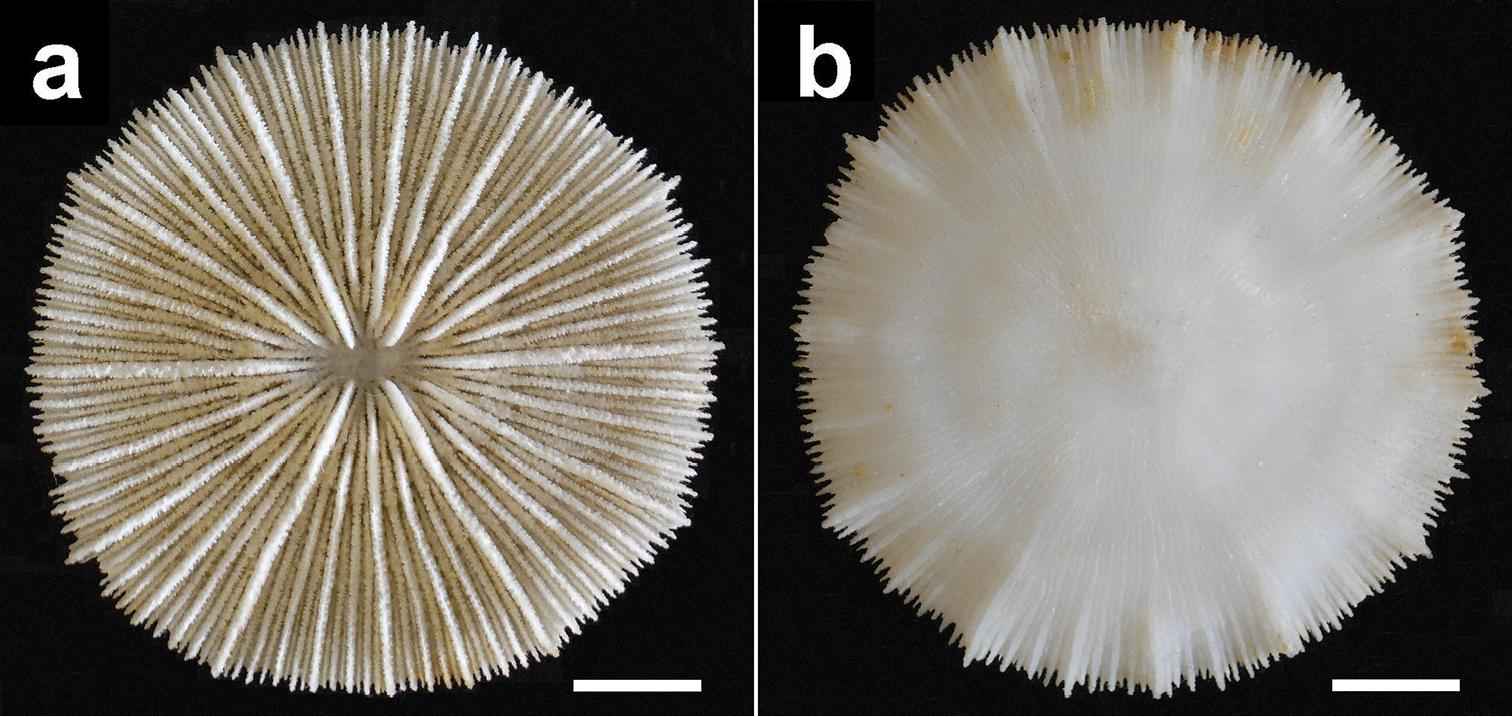
Holotype of Cycloseris boschmai sp. n. (RMNH Coel. 8333). Indonesia, Banda, Danish Exp. to the Kei Islands, 1922. a Upper side b Lower side. Scale bar: 0.5 cm.
Bali: RMNH Coel. 40146 (2 dry specimens: 25, 31 mm), NE Bali, Tulamben, 3-5m, 08°16'36"S, 115°35'37"E, Bali Lombok Strait Exp. Sta. BAL.20, 09.iv.2001, coll. BWH; RMNH Coel. 40147 (1 dry specimen: 18 mm), NE Bali, Tulamben, 5m depth, 08°16'26"S, 115°35'28"E, Bali Lombok Strait Exp. Sta. BAL.21, 11.iv.2001, coll. BWH. Nusa Tenggara Timur (Lesser Sunda Islands): RMNH Coel. 21471 (1 dry specimen: 38 mm), NE Komodo, S Gili Lawa Laut, 08°27'00"S, 119°34'24"E, Snellius-II Exp. Sta. 4.253, 27.x.1984, coll. BWH; RMNH Coel. 40145 (4 dry specimens: 24–32 mm), SE Komodo, N side bay S of Tanjung Lohnamu, 08°38'19"S, 119°28'45’’ E, TNC Komodo Rapid Ecological Assessment Sta. KOM.16, coll. BWH; RMNH Coel. 31190 (1 dry specimens: 32 mm), N Sumbawa, Bay of Sanggar, 08°19'36"S, 118°15'12"E, Snellius-II Exp. Sta. 4.132, 30.x.1984, coll. BWH. South Sulawesi. RMNH Coel. 31188 (1 dry specimen: 31 mm), Spermonde Archipelago, N Bone Tambung Island, 05°01'50"S, 119°16'25"E, 13.vi.1986, coll. BWH; RMNH Coel. 31189 (5 dry specimens: 29–50 mm), Spermonde Archipelago, W Kudingareng Keke I., 9–18 m depth, 05°06'30"S, 119°17'04"E, 6.xii.1984, coll. BWH; RMNH Coel. 31192 (5 dry specimens: 17–31 mm), SW Selayer I., NW Bahuluang I., 06°28'00"S, 120°25'30"E, Snellius-II Exp. Sta. 4.202, 10.x.1984, coll. BWH; RMNH Coel. 31191 (3 dry specimens: 35–37 mm), NE Taka Bone Rate, E Tarupa Besar, 06°28'S, 121°08'E, Snellius-II Exp. Sta. 4.140, 25.ix.1984, coll. BWH. Central Sulawesi, Tomini Bay, Togian Islands: RMNH Coel. 24278 (2 dry specimens: 34, 39 mm), S Talatakoh I., 00°26'34"S, 122°06'07"E, Tethyana Exp. Sta. 10, 21.ix.1999, coll. BWH; RMNH Coel. 24291 (1 dry specimen: 48 mm), S Togian I., 00°20'10"S, 121°59'00"E, Tethyana Exp. Sta. 14, 23/24.ix.1999, coll. BWH; RMNH Coel. 24706 (5 dry specimens: 30-42 mm), S Batudaka I., 00°35'25"S, 121°41'38"E, Tethyana Exp. Sta. 15, 24.ix.1999, coll. BWH; RMNH Coel. 31193 (7 dry specimens: 29-50 mm), S Waleabahi I., 00°26'16"S, 122°15'16"E, Tethyana Exp. Sta. 8, 19.ix.1999, coll. BWH. North Sulawesi: RMNH Coel. 40156 (4 dry specimens; 36-40 mm), Lembeh Strait, Tanjung Mawali, 14 m depth, 01°26'36"N, 125°13'46"E, Lembeh Strait Exp. Sta. LEM.04, 31.i.2012, coll. BWH. SE Sulawesi, Tukang Besi Islands (Wakatobi): RMNH Coel. 40143 (1 dry specimen: 12 mm), NW Tomia, 05°43'59"S, 123°53'35"E, TNC-WWF Wakatobi Rapid Ecological Assessment Sta. WAK.25, 13.v.2003, coll. BWH; RMNH Coel. 40144 (1 dry specimen: 40 mm), SW Karang Kaledupa, lagoon, 05°51'46"S, 123°43'17"E, TNC-WWF Wakatobi Rapid Ecological Assessment Sta. WAK.28, 14.v.2003, coll. BWH. Moluccas: RMNH Coel. 33586 (2 dry specimens: 47, 58 mm), Ambon, N coast near Morela, 03°33'S, 128°12'E, Fauna Malesiana Maluku Exp. Sta. MAL.12, 13.xi.2002, coll. BWH; Northern Moluccas: RMNH Coel. 8286 (10 dry specimens, some with buds, 32-50 mm, previously identified as Fungia marginata), Banda, off Lontor, to 13 m depth, 4°33'S, 129°52'E, Danish Exp. to the Kei Islands, 15.vi.1922; UZMK (5 specimens in ethanol, 13-40 mm, previously identified as Fungia marginata), Gunung Api, 20-25 m depth, Danish Exp. to the Kei Islands, 13.vi.1922; UZMK (9 specimens in ethanol, 26-50 mm, previously identified as Fungia marginata), Lontor, 13 m depth, Danish Exp. to the Kei Islands, 15.vi.1922; RMNH Coel. 40096 (1 dry specimen: 29 mm), Halmahera, East coast Teluk Dodinga, Karang Galiasa, 00°50'46"N, 127°35'07"E, Ternate Exp. Sta. TER.38, 14.xi.2009, coll. BWH; RMNH Coel. 40102 (1 dry specimen: 34 mm), Hiri I., Tanjung Ngafauda, 00°54'38"N, 127°19'03"E, Ternate Exp. Sta. TER.14, 16 m depth, 31.x.2009, coll. BWH; RMNH Coel. 40103 (1 dry specimen: 30 mm), Ternate, Sulamadaha I., 00°52'04"N, 127°19'33"E, Ternate Exp. Sta. TER.22, 18 m depth, 6.xi.2009, coll. BWH; RMNH Coel. 40104 (1 dry specimen: 19 mm), Ternate, Dufadufa, Benteng Toloko, 00°48'49"N, 127°23'22"E, Ternate Exp. Sta. TER.24, 8 m depth, 7.xi.2009, coll. BWH; RMNH Coel. 40105 (1 dry specimen: 31 mm), Halmahera, W Pasir Lamo, 00°53'21"N, 127°27'34"E, Ternate Exp. Sta. TER.26, 8.xi.2009, coll. BWH; RMNH Coel. 40106 (1 dry specimen: 48 mm), Tidore, north Pilongga, 00°42'50"N, 127°28'45"E, Ternate Exp. Sta. TER.34, 12 m depth, 12.xi.2009, coll. BWH; RMNH Coel. 40173 (1 dry specimen: 29 mm), Ternate, Tanjung Tabam, 00°50'05"N, 127°23'10"E, Ternate Exp. Sta. TER.12, 11 m depth, 30.x.2009, coll. BWH; RMNH Coel. 40174 (1 dry specimen: 17 mm), Ternate, outside harbor to the east, 00°46'55"N, 127°30'20"E, Ternate Exp. Sta. TER.25, 8 m depth, 7.xi.2009, coll. BWH. East Kalimantan, Berau Islands: RMNH Coel. 31922 (1 dry specimen: 19 mm), W Derawan I., 7 m depth, 02°16'53"N, 118°13'39"E, East Kalimantan Berau Exp. Sta. BER.02, 4.x.2003, coll. BWH; RMNH Coel. 31923 (1 dry specimen: 15 mm), Berau Delta, Lighthouse-2 Reef, 6 m depth, 02°09'34"N, 118°10'11"E, East Kalimantan Berau Exp. Sta. BER.05, 5.x.2003, coll. BWH; RMNH Coel. 31924 (2 dry specimen: 22, 31 mm), Samama I., 8 m depth, 02°07'32"N, 118°20'10"E, East Kalimantan Berau Exp. Sta. BER.10, 7.x.2003, coll. BWH; RMNH Coel. 31925 (1 dry specimen: 42 mm), NE Kakaban I., 14 m depth, 02°08'53’’ 118°32'32"E, East Kalimantan Berau Exp. Sta. BER.49, 28.x.2003, coll. BWH; RMNH Coel. 40149 (1 dry specimen: 14 mm), S of Samama I., NE Buliulin I., 14 m depth, 02°07'07"N, 118°20'32"E, East Kalimantan Berau Exp. Sta. BER.26, 15.x.2003, coll. BWH; RMNH Coel. 40153 (1 dry specimen: 46 mm), N Maratua I., near entrance lagoon, 9 m depth, 02°14'53"N, 118°37'36"E, East Kalimantan Berau Exp. Sta. BER.29, 17.x.2003, coll. BWH; RMNH Coel. 40154 (1 dry specimen: 21 mm), S Derawan I., 13 m, 02°15'04"N, 118°15'04"E, East Kalimantan Berau Exp. Sta. BER.04a, 18.x.2003, coll. BWH; RMNH Coel. 40155 (1 dry specimen: 50 mm), E Sangalaki I., Lighthouse, 12 m depth, 02°04'54"N, 118°24'30"E, East Kalimantan Berau Exp. Sta. BER.22, 14.x.2003, coll. BWH. West Papua, Raja Ampat Islands: RMNH Coel. 40140 (1 dry specimen: 30 mm), S. Mansuar (Sawandarik village), 00°35'26"S, 130°36'12"E, Raja Ampat Exp. Sta. RAJ.06, 20.xi.2007, coll. BWH; RMNH Coel. 40141 (1 dry specimen: 33 mm), Yeffam I., E Penemu I., 8 m depth, 00°35'20"S, 130°17'06"E, Raja Ampat Exp. Sta. RAJ.66, 13.xii.2007, coll. BWH. Malaysia, Sabah, northern Borneo: RMNH Coel. 33545 (1 dry specimen: 20 mm), W Sabah, Gaya Islands off Kota Kinabalu, W Sapi I., 06°00'26"N, 116°00'13"E, 28.vii.2005, coll. BWH. Layang-Layang: RMNH Coel. 40095 (2 dry specimens: 18 mm attached, 37 mm free-living), Easternmost point, Sta. LAC.14, 15-25 m depth, 07°22'34"N, 113°51'15"E, 28.iii.2013, coll. BWH. Philippines: RMNH Coel. 24908 (2 dry specimens: 20, 32 mm), Cebu Strait, West of Bohol, NW Cabilao I., 09°53'20"N, 123°45'53"E, 2.x.1999, coll. BWH. Palau: RMNH Coel. 40225 (1 dry specimen), SE off Garreru I., S Goraklbad Passage, 07°19'15"N, 134°35'50"E, 29.vii.2002, coll BWH.
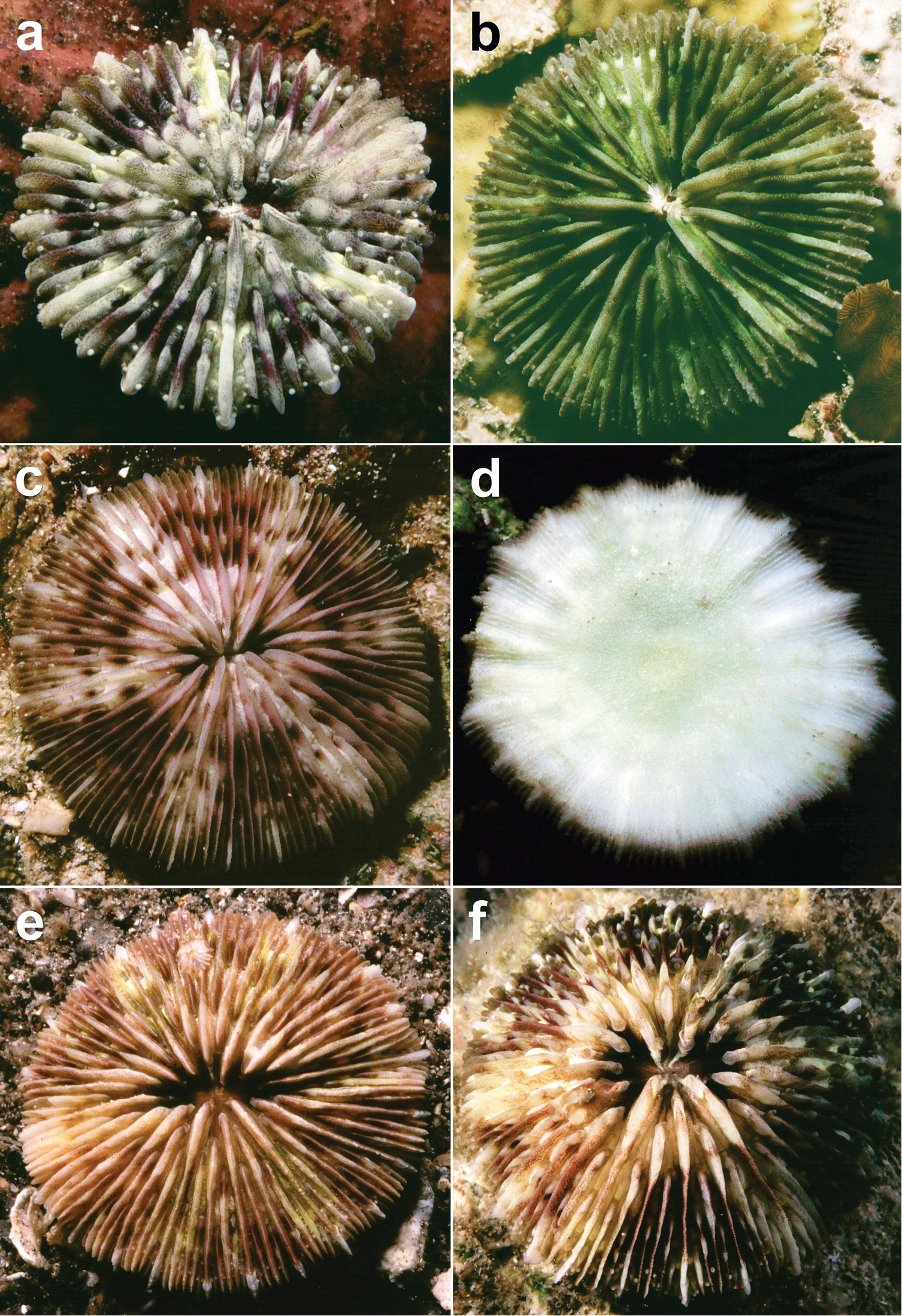
(Figures 3–13) The diameter of the examined specimens ranges between 12 and 50 mm. Corals mostly flat, moderately thick and robust. Adult animals unattached and monostomatous with septa-costae extending outside the circular to slightly oval corallum outline. Juvenile specimens vary from round to slightly hexagonal. Wedge-shaped, regenerating fragments not known. The length of the fossa, measured at its bottom, is 1/9 to 1/6 of the corallum length. The columella is formed by a mingled mass of tightly to loosely packed trabeculae. Septa densely packed and straight, unequal in thickness and height. The relatively thick and high septa of lower orders are solid; they are flanked by perforated septa of higher orders. Tentacular lobes absent. Septal margins are finely ornamented with sharp and granular dentations. Their number varies from 20 to 70 per cm. Septal sides are densely covered by fine granulations, which are irregularly dispersed or arranged in rows perpendicular to the septal margin. Compound synapticulae (fulturae) connecting the septa laterally cannot easily be distinguished because of tight septal arrangement. The solid corallum wall is granulated and may show a detachment scar. The lower side varies from flat to slightly convex. Costae unequal in size, straight and prominent near the corallum margin but less distinct at the centre. Corallum margin may be slightly undulating because of enlarged lower order costae. Costae ornamented with fine granular or acute spines. Their number varies from 15 to 80 per cm. Some individuals have small buds over their surface, especially in the proximity of the corallum margin (Figure 10). Attached juveniles (anthocaulus stage) are rare (Figure 11). The color of the living animal is variable with hues of red or green (Figures 11–13). Tentacles small and transparent with white acrospheres at their tips (Figure 13).
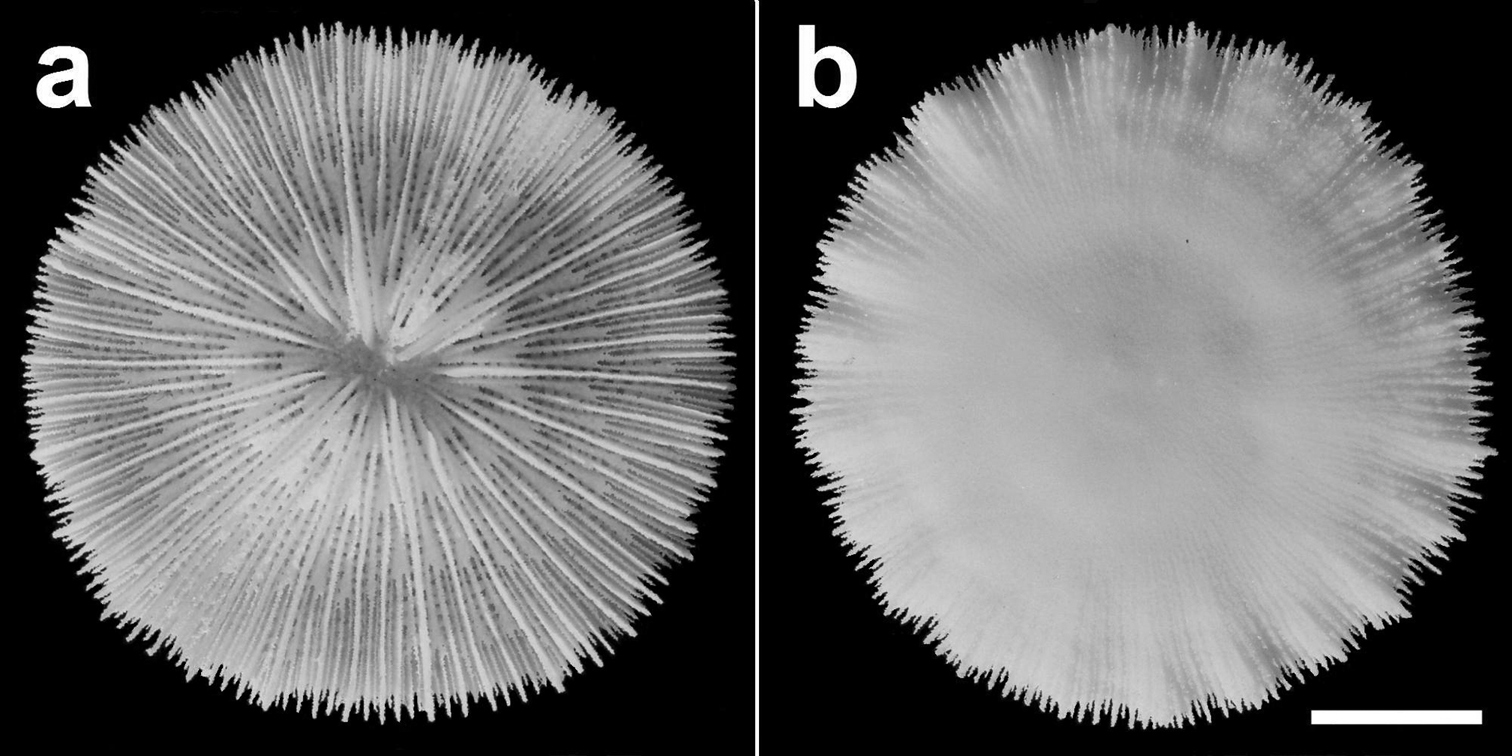
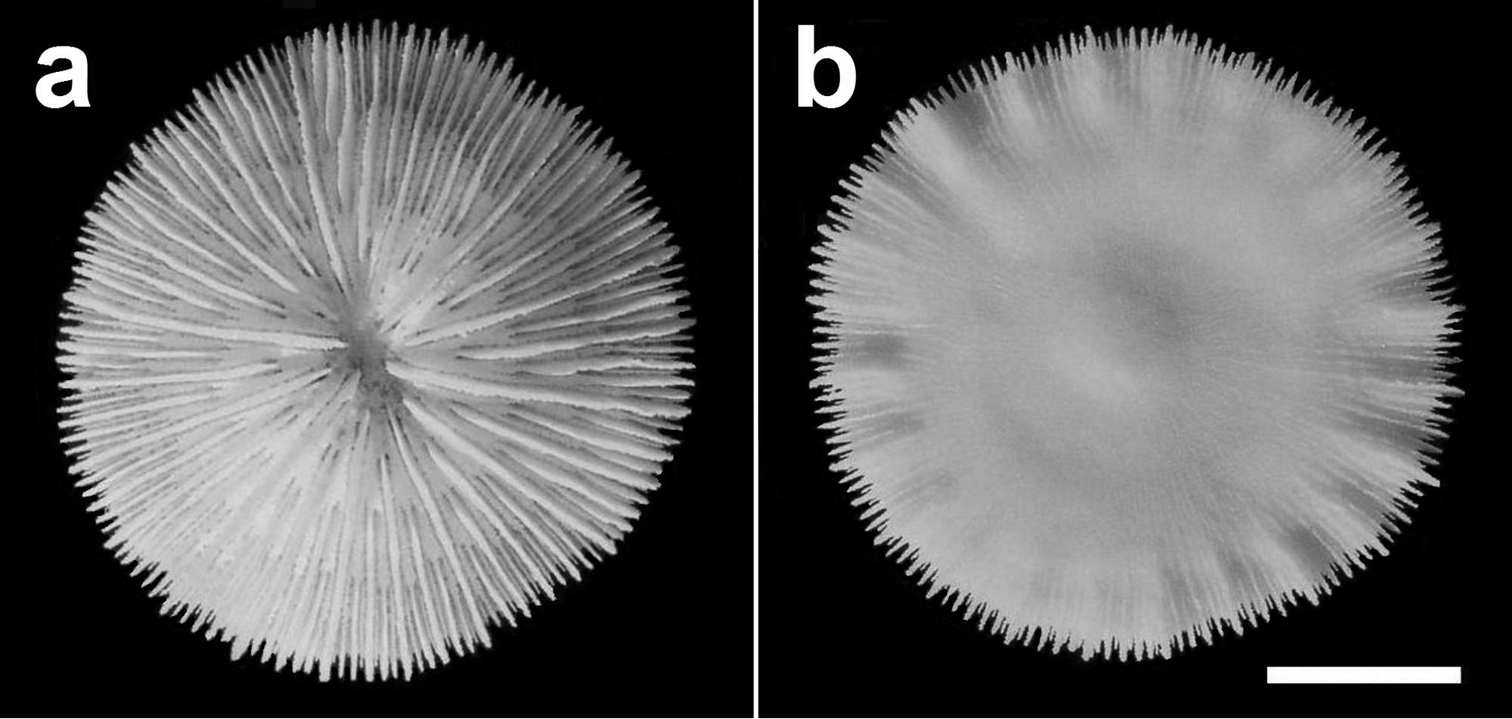
Specimen of Cycloseris boschmai sp. n. (RMNH Coel. 21471). Indonesia, NE Komodo, S Gili Lawa Laut, Snellius-II Exp. Sta. 4.253, 27 October 1984. a Upper side b Lower side. Scale bar: 0.5 cm.
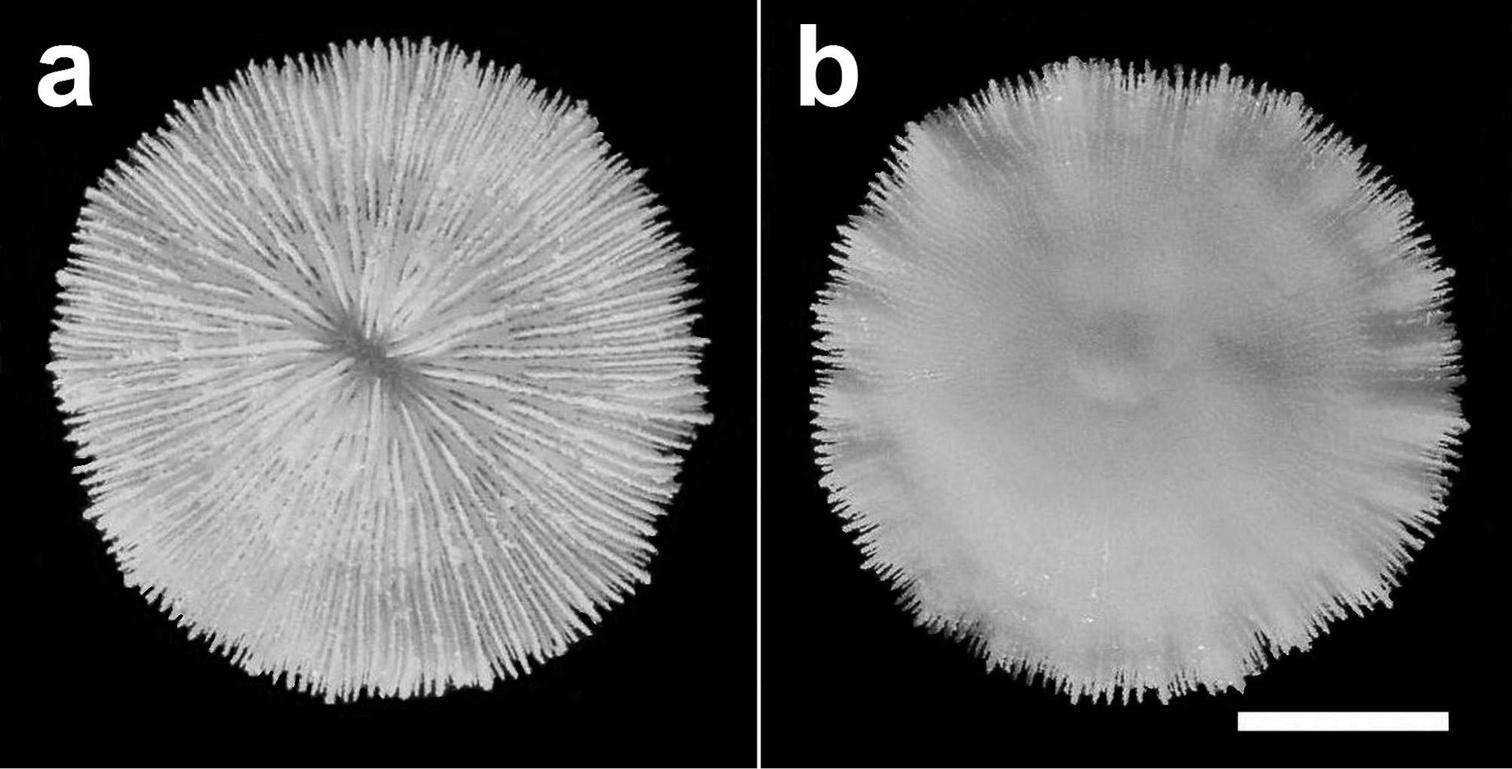
Specimen of Cycloseris boschmai sp. n. (RMNH Coel. 31190). Indonesia, N Sumbawa, Bay of Sanggar, Snellius-II Exp. Sta. 4.132, 30 October 1984 a Upper side b Lower side. Scale bar: 0.5 cm.
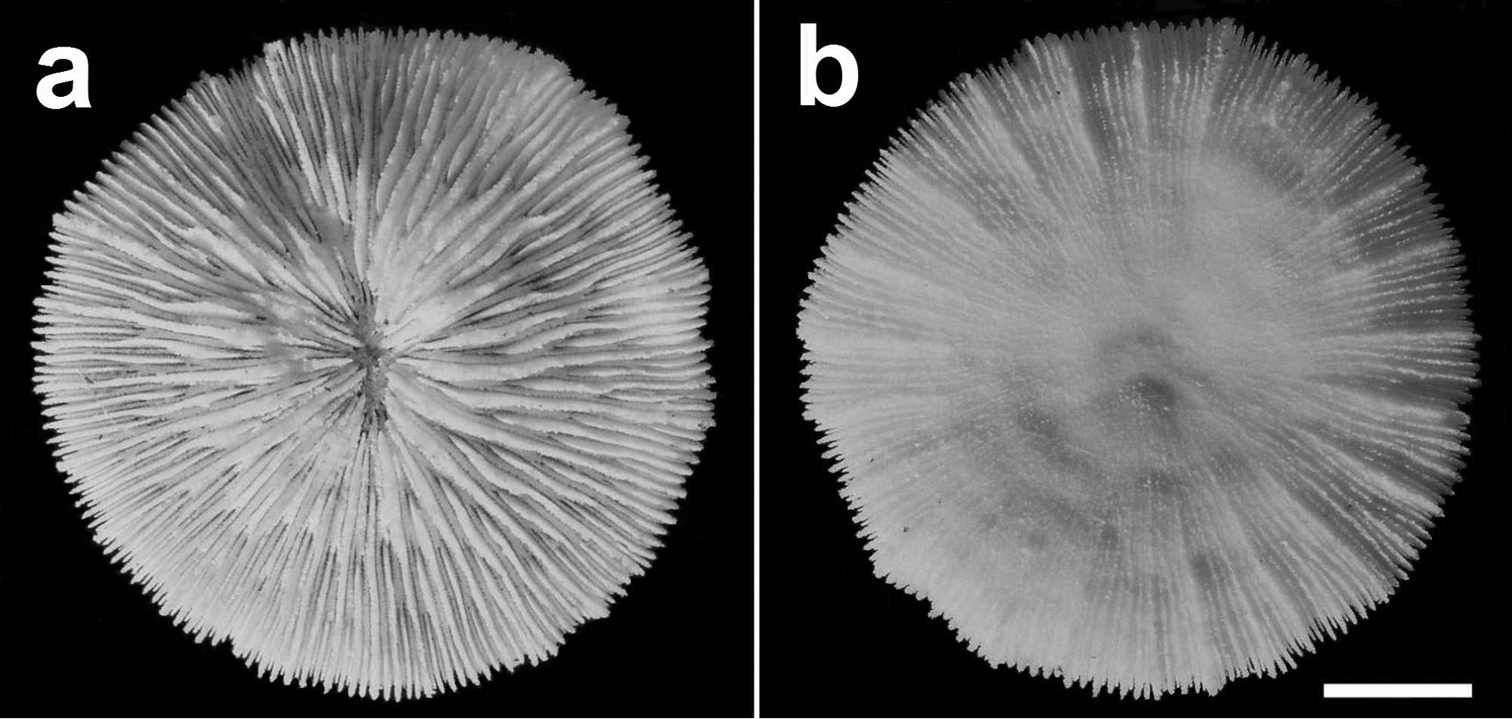
Specimen of Cycloseris boschmai sp. n. (RMNH Coel. 31188). Indonesia, South Sulawesi, Spermonde Archipelago, north side of Bone Tambung Island, 13 June 1986. a Upper side b Lower side. Scale bar: 0.5 cm.
Specimen of Cycloseris boschmai sp. n. (RMNH Coel. 24291). Indonesia, Central Sulawesi, Togian Islands, S Togian I., Tethyana Exp. Sta. 14, 23/24 September 1999 a Upper side b Lower side. Scale bar: 0.5 cm.
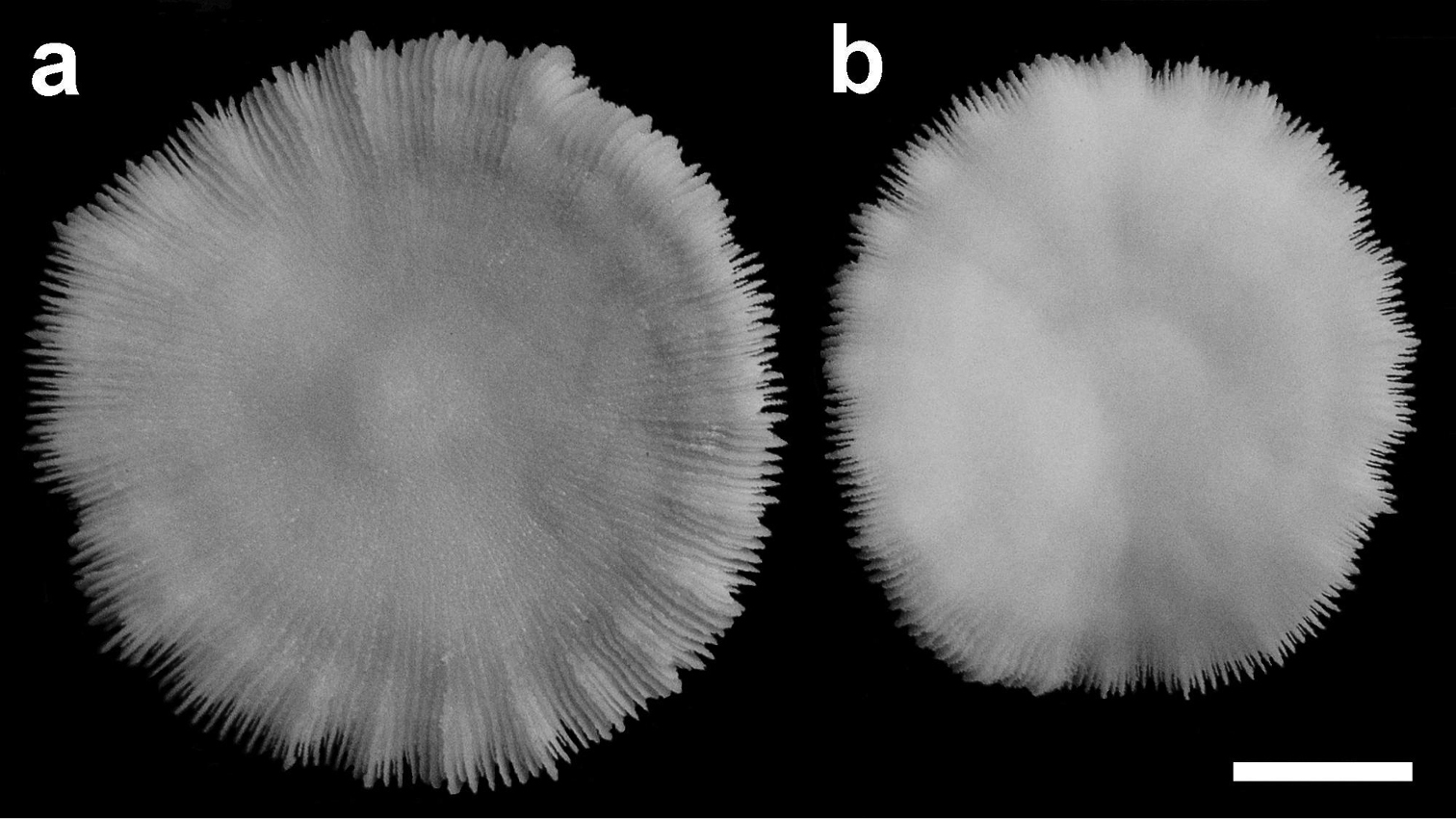
Specimens of Cycloseris boschmai sp. n. (RMNH Coel. 31193). Indonesia, Central Sulawesi, Togian Islands, S Waleabahi I., Tethyana Exp. Sta. 8, 19 September 1999 a Upper side b Lower side. Scale bar: 0.5 cm.
Two specimens of Cycloseris boschmai sp. n. (RMNH Coel. 24278). Indonesia, Central Sulawesi, Togian Islands, S Talatakoh I., Tethyana Exp. Sta. 10, 21 September 1999. Scale bar: 0.5 cm.
Two specimens of Cycloseris boschmai sp. n. (RMNH Coel. 8286) with marginal buds and sand in the mouths. Indonesia, Banda, off Lontor, Danish Exp. to the Kei Islands, 15 June 1922.
Juvenile, attached specimens of Cycloseris boschmai sp. n. a Papua New Guinea, Bismarck Sea, Madang, June 1992 b–d Malaysia, South China Sea, Layang Layang, Easternmost point, (RMNH Coel. 40095), 28 March 2013 b In situ (bleached) c Lower side d Upper side. Scale bars: 0.5 cm.
Cycloseris boschmai sp. n. a Indonesia, Bali, Tulamben, September 1997 b Philippines, Cebu, November 1999 c–e Indonesia, Central Sulawesi, Togian Islands, September 1999 f Indonesia, South Sulawesi, Spermonde Archipelago, Bone Lola reef, August 1997.
Cycloseris boschmai sp. n. specimen showing transparent extended tentacles with white acrospheres at their tips; Indonesia, North Sulawesi, Lembeh Strait, Lobangbatu, February 2012.
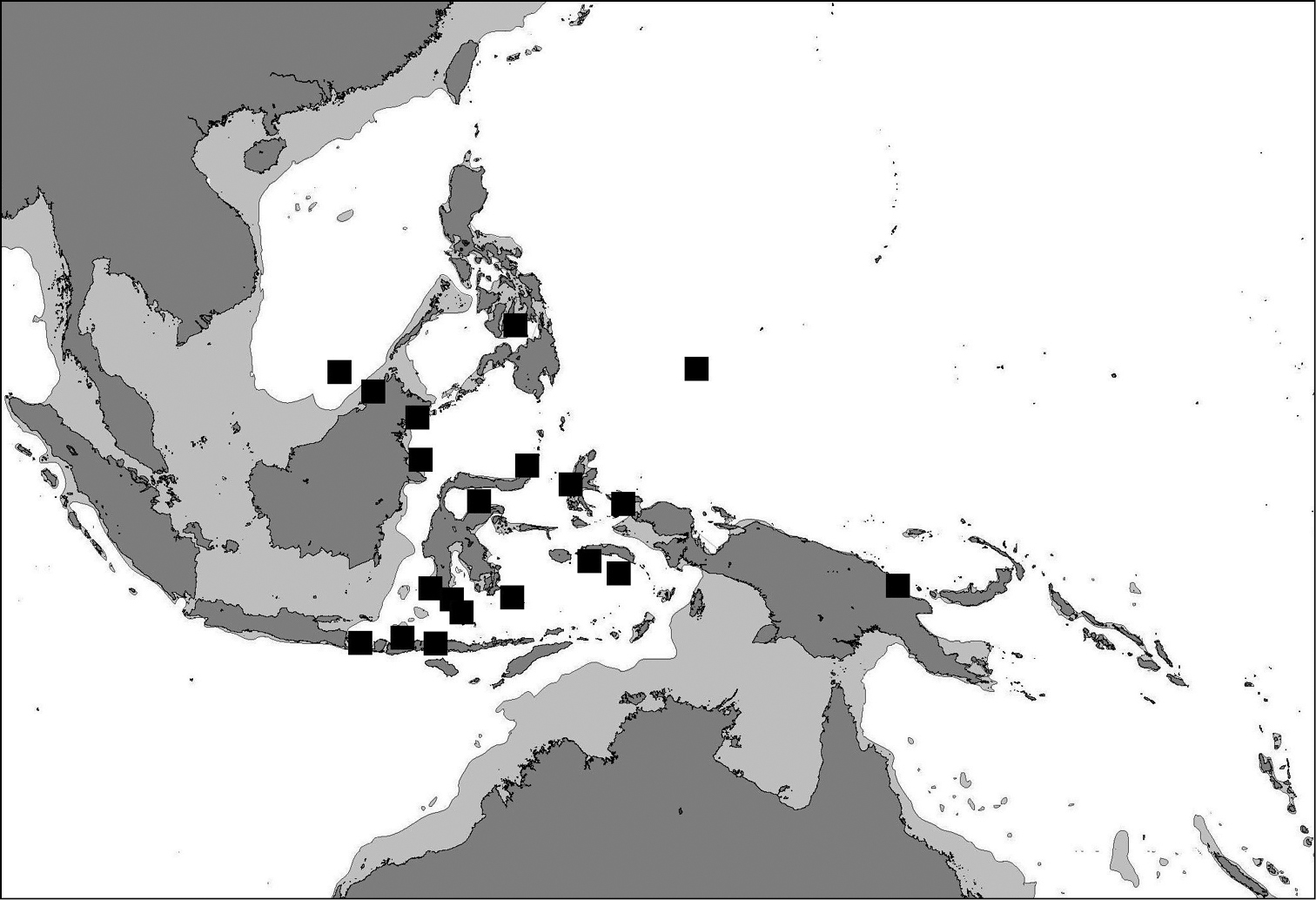
(Figure 14). The distribution range is limited to the Coral Triangle (
Map of the Central Indo-Pacific indicating localities where Cycloseris boschmai sp. n. has been recorded.
The species is named after the late Prof. Hilbrand Boschma, former director of the Rijksmuseum van Natuurlijke Historie (now Naturalis Biodiversity Center), who devoted much of his research time to the study of mushroom corals, including specimens of the new species.
Adult corals small (< 50 mm) with uneven circular corallum margin owing to enlarged costae. Live specimens with variable, patchy colouration.
| 1a | Lower order costae distinctly larger than other ones | 2 |
| 1b | Costae fine, adjacent ones equal to almost equal | 5 |
| 2a | Coralla flat and thin, corallum outline circular | 3 |
| 2b | Coralla thick and slightly arched, corallum outline slightly or much oval | 4 |
| 3a | Lower order costae thicker and longer than higher order costae, ornamentation fine (20–70 / cm), maximum corallum diameter 5 cm, habitat mostly consisting of reef slopes and sandy reef bases | Cycloseris boschmai sp. n. |
| 3b | Lower order costae sharp, ornamentation very fine (40–80 / cm) on lower order costae and indistinct on higher order costae, maximum corallum diameter 8 cm, habitat mostly consisting of deep, sandy reef bases | Cycloseris vaughani |
| 4a | Corallum outline slightly oval, lower order costae irregularly and roughly ornamented (20–70 / cm), maximum corallum diameter 8.5 cm, habitat consisting of upper reef slopes | Cycloseris tenuis |
| 4b | Corallum outline clearly oval, lower order costae sharp, costal ornamentation very fine (40–90 / cm) and nearly absent on higher order costae, maximum corallum diameter 12.5 cm, habitat mostly consisting of deep, sandy reef bases | Cycloseris somervillei |
| 5a | Septa densely packed and (almost) equal in height | 6 |
| 5b | Septa loosely packed, septa of lower orders thicker and more exsert than others | 7 |
| 6a | Central fossa short (< 10% of corallum diameter); all septa perforated, nearly equal in size and tightly packed with little space in between them, maximum corallum diameter 8.5 cm, habitat mostly consisting of deep, sandy reef bases | Cycloseris sinensis |
| 6b | Length of central fossa > 10% of corallum diameter, septa of lower order solid and thicker than adjacent septa with distinct space in between them, maximum corallum diameter 7.5 cm, habitat consisting of deeper reef slopes or reef bases | Cycloseris distorta |
| 7a | Corallum outline oval | 8 |
| 7b | Corallum outline circular or irregularly round with folds or undulations | 9 |
| 8a | Coralla thick; underside flat or arched, costae equal, maximum corallum diameter 9 cm, habitat consisting of lower reef slopes or sandy reef bases | Cycloseris cyclolites |
| 8b | Coralla convex around fossa (humped), costae equal in juveniles, maximum corallum diameter 12.5 cm, habitat mostly consisting of deep, sandy reef bases | Cycloseris somervillei |
| 9a | Coralla with folded, undulating margin | 10 |
| 9b | Coralla with regular, smooth periphery | 11 |
| 10a | Coralla thin, central fossa short (< 10% of corallum diameter), margin undulating (hexagonal in juveniles) maximum corallum diameter 8.5 cm, habitat consisting of sandy reef slopes or sandy reef bases | Cycloseris hexagonalis |
| 10b | Coralla thick and usually strongly arched, margin with folds, maximum corallum diameter 8.5 cm, habitat consisting of lower reef slopes or sandy reef bases | Cycloseris curvata |
| 11a | Coralla and septa thin, adjacent costae slightly alternating in size maximum corallum diameter 15 cm, habitat mostly consisting of sandy reef bases | Cycloseris fragilis |
| 11b | Coralla moderately thick, lower order septa thicker than others, costae nearly similar in size, maximum corallum diameter 12 cm, habitat consisting of lower reef slopes or sandy reef bases | Cycloseris costulata |
Although some material of Cycloseris boschmai sp. n. was already available in museum collections (RMNH, UZMK), the species could only be discovered because of much fieldwork (1983–2013) with proportionate opportunities for observations and sampling to enable separation of the new species from resembling ones.
The Fungia marginata material from Banda is suitable as type material of the new species. Because the Banda specimens were wrongly identified by him, this does not concern a new name for an existing species but an entirely new species (
Cycloseris boschmai sp. n. is the smallest mushroom coral known so far (see key). Superficially, it resembles Cycloseris costulata, which has less prominent costae, a more even corallum margin (not undulating), a larger maximum size and less colourful appearance (see
Cycloseris boschmai sp. n. also resembles Cycloseris tenuis, which is more oval, less corlourful (see
With the inclusion of Cycloseris boschmai sp. n., 11 free-living Cycloseris species are distinguished. Since the taxonomic revision of the Fungiidae by
Cycloseris colini Veron, 2000 is a synonym of Lithophyllon spinifer (
Cycloseris densicolummelus Latypov, 2006 has not been described in an official publication but in an electronic document that was distributed via a CD-ROM. This work should have contained a clear publication date and a statement naming at least five major publicly accessible libraries in which copies of the optical disc were to have been deposited (
Because Cycloseris boschmai is a rare species (considering that most material was gathered during fieldwork in a time span of 30 years) and its geographic distribution range is restricted to the Coral Triangle, not much can be said about its ecology. Specimens are difficult to find, owing to their small body size compared to other mushroom coral species (
Small-sized free-living mushroom corals have been reported to show much mobility (
Although free-living Cycloseris species were previously considered primitive, this is not the case according to their phylogeny reconstruction (
I am grateful to the following people, organizations and institutes who assisted during the fieldwork or other parts of the research: Dr. Alfian Noor and Drs Willem Moka (Universitas Hasanuddin, Makassar), Dr. Carden C. Wallace (MTQ, Tethyana Expedition to the Togian Islands), Dr. Filipina B. Sotto and Dr Thomas Heeger (University of San Carlos, Cebu City), Dr Jacob van der Land (Naturalis, Snellius-II Expedition), Dr. Pat Colin and Ms Lori Colin (Coral Reef Research Foundation, Palau), Dr. Phillipa Mansell (Operation Wallacea, Wakatobi, Indonesia), Dr. Jos Pet (The Nature Conservancy, Bali), Dr Suharsono (Research Centre for Oceanography, Indonesian Institute of Sciences, Jakarta), Ms. Zarinah Waheed (Borneo Marine Research Institute, Kota Kinabalu). The research was partly financed by the Netherlands Foundation for the Advancement of Tropical Research (WOTRO, grant W77-96) and by the Schure-Beijerinck-Popping Fonds. I thank Ms. Majken T. Tøttrup and Dr. M.V. Sørensen for the loan of specimens from the UZMK. I thank Dr Carden C. Wallace and Mrs. Barbara Done for the loan of specimens from the MTQ. I am grateful to Mrs. Elly Beglinger and Mr. Koos van Egmond for their help in accessing ZMA and RMNH specimens. Comments given by two anonymous reviewers helped considerably to improve the text.