(C) 2012 Rogério Bertani. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three aviculariine genera endemic to Brazil are revised. Typhochlaena C. L. Koch, 1850 is resurrected, including five species; Pachistopelma Pocock, 1901 includes two species; and Iridopelma Pocock, 1901, six species. Nine species are newly described: Typhochlaena amma sp. n., Typhochlaena costae sp. n., Typhochlaena curumim sp. n., Typhochlaena paschoali sp. n., Pachistopelma bromelicola sp. n., Iridopelma katiae sp. n., Iridopelma marcoi sp. n., Iridopelma oliveirai sp. n. and Iridopelma vanini sp. n. Three new synonymies are established: Avicularia pulchra Mello-Leitão, 1933 and Avicularia recifiensis Struchen & Brändle, 1996 are junior synonyms of Pachistopelma rufonigrum Pocock, 1901 syn. n., and Avicularia palmicola Mello-Leitão, 1945 is a junior synonym of Iridopelma hirsutum Pocock, 1901 syn. n. Pachistopelma concolor Caporiacco, 1947 is transferred to Tapinauchenius Ausserer, 1871, making the new combination Tapinauchenius concolor (Caporiacco, 1947) comb. n. Lectotypes are newly designed for Pachistopelma rufonigrum Pocock, 1901 , Iridopelma hirsutum Pocock, 1901 and Pachistopelma concolor Caporiacco, 1947. Cladistic analyses using both equal and implied weights were carried out with a matrix comprising 62 characters and 38 terminal taxa. The chosen cladogram found with X-Pee-Wee and concavity 6 suggests they are monophyletic. All species are keyed and mapped and information on species habitat and area cladograms are presented. Discussion on biogeography and conservation is provided.
Brazilian Atlantic rainforest, bromeliads, campo rupestre, cerrado, endemism, new species, restinga, systematics, tarantula
Theraphosidae is the richest mygalomorph family, comprising ca. 1/3 of the 2, 693 known mygalomorph species (
This work focuses on those aviculariines occurring mainly in Northeastern Brazil from where the type species of aviculariine genera Typhochlaena C. L. Koch, 1850, Pachistopelma Pocock, 1901 and Iridopelma Pocock, 1901 were described. Within all aviculariine genera presently accepted (
Typhochlaena and Iridopelma share a history of synonymies and revalidations, whereas Pachistopelma has lacked important taxonomic change since its description. Typhochlaena was erected by C. L.
Two years later,
Roewer’s (1942) Catalog listed both Iridopelma and Typhochlaena as junior-synonyms of Avicularia.
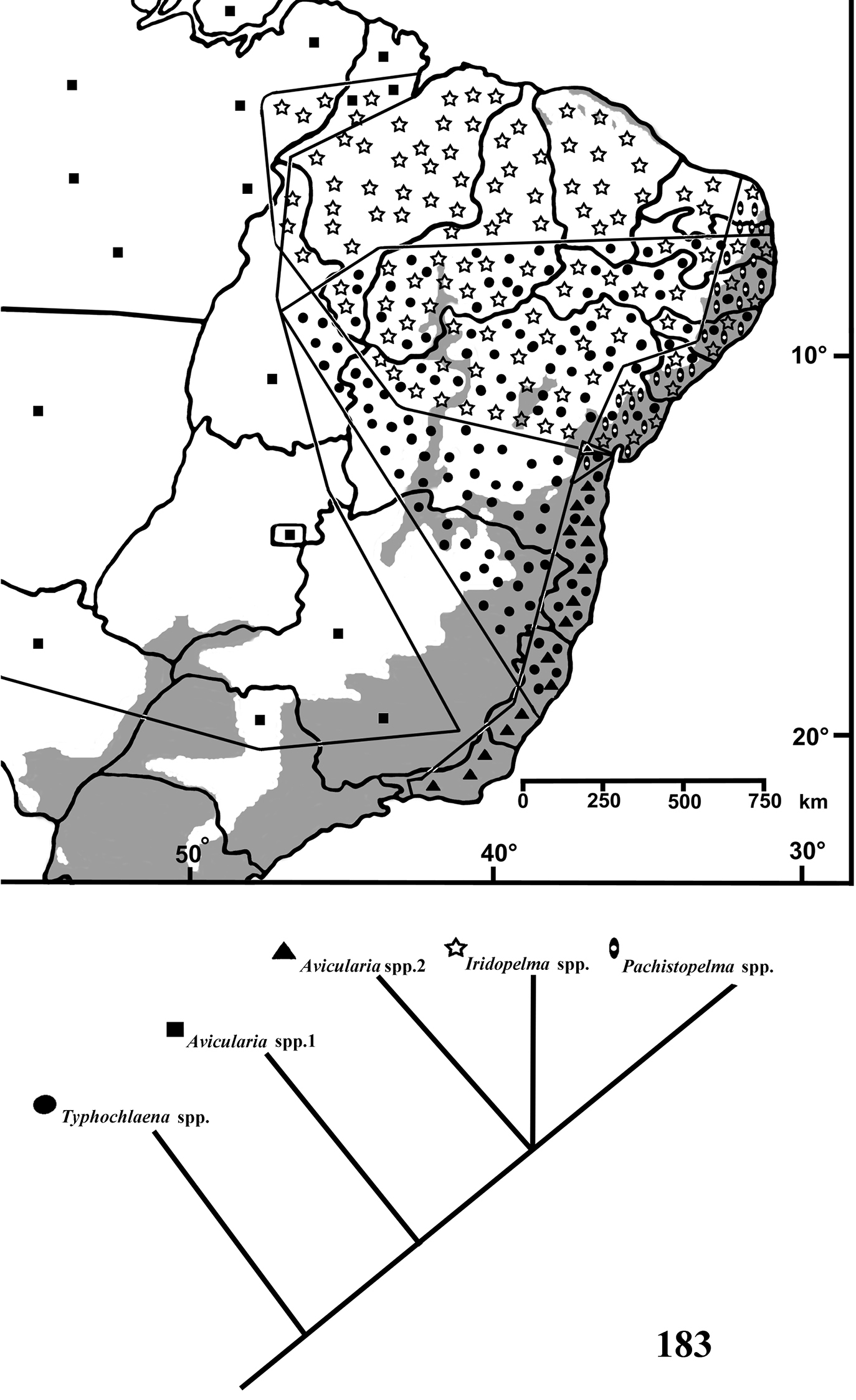
Herein, species of Typhochlaena, Pachistopelma and Iridopelma are revised and relationships are analyzed using cladistic methods. Geographical distribution and notes on habitats of species are given with area cladograms also presented. Thirteen species are herein recognized, five in Typhochlaena, six in Iridopelma and two in Pachistopelma, nine of which are newly described, showing an unexpected high richness of aviculariine species out of their core distribution within and immediately surrounding the Amazon region.
MethodsThe general description format follows
Specimens from the following institutions were examined: DZUB – Departamento de Zoologia da Universidade de Brasília, Brasilia; IBSP – Instituto Butantan, São Paulo; MIZA – Instituto de Zoologia, Universidad Central de Venezuela, Maracay; MNRJ – Museu Nacional do Rio de Janeiro, Rio de Janeiro; MPEG – Museu Paraense Emilio Goeldi, Belém; MZSP – Museu de Zoologia da Universidade de São Paulo, São Paulo; MZUF – Museo Zoologico “La Specola”, Firenze; NHM – The Natural History Museum, London; SMF – Senckenberg Museum, Frankfurt.
Geographical coordinates: primary sources are between round brackets and secondary sources (Google Earth©) are between square brackets (following
Cladistic analyses were based mainly on the matrix of
Matrix data deposited in the Dryad repository: doi: 10.5061/dryad.bv0kr
Examined specimens for cladisticsFor Iridopelma, Pachistopelma and Typhochlaena species, it was used the same specimens used in descriptions.
Paratropididae: Glabropelmatinae. Melloina sp., male (MIZA 520), female (MNRJ 12961), Venezuela, Lara, Cueva El Santuario [9°49'N, 70°03'W], O. Villarreal, 19 April 2000.
Theraphosidae: Aviculariinae: Avicularia sp. male, Brazil, state of Pará, U.H.E. Tucuruí [4°20'S, 49°31'W], Equipe Resgate de Fauna, 1984 (IBSP 7879 IBA 1002), female, same locality and collectors (IBSP 8845 Ref 48186); Avicularia diversipes (C. L. Koch, 1842), female, Brazil, state of Bahia, Ilhéus, CEPLAC [14°46'S, 39°13'W], R. Bertani and G. Puorto, March 1991 (IBSP 11754), male, same data (IBSP 119271); Avicularia gamba Bertani and Fukushima, 2009, holotype male, Brazil, state of Bahia, Elísio Medrado, RPPN Jequitibá (12°52'3.20"S, 39°28'9.09"W), R. Bertani, C.S. Fukushima and R.H. Nagahama, 07 October 2007 (MZSP 31115), paratype female, same data (MZSP 31116); Avicularia juruensis Mello-Leitão, 1923, male, Brazil, state of Rondônia, Porto Velho [8°45'S, 63°54'W] (IBSP 2503), female, Brazil, state of Mato Grosso, between Vale de São Lourenço and Pontes e Lacerda, U.H.E. Guaporé [14°40'S, 56°51'W] (IBSP 10279); Avicularia sooretama Bertani and Fukushima, 2009, holotype male, Brazil, state of Espírito Santo, Reserva Biológica de Sooretama [18°59'S, 40°07'W], AMNRJ, 18 April 2006 (MNRJ 18435), paratype female, Brazil, state of Espírito Santo, Pinheiros, Reserva Biológica Córrego do Veado (18°37'0.16"S, 40°14'1.60"W), AMNRJ, 22 October 2005 (MNRJ 12930); Avicularia taunayi (Mello-Leitão, 1920), female, Brazil, state of Minas Gerais, Barão de Cocais [19°56'S, 43°28'W], J. P. Couto, 5 November 1970 (IBSP 199), male, Brazil, Distrito Federal, Brasília, Lago Sul [15°50'S, 47°49'W], R.A. Brandão and G. Zerbini (DZUB 1675); Ephebopus murinus (Walckenaer, 1837), male (IBSP 9650), female (IBSP 9658), Brazil, state of Pará, Tucuruí, U.H.E. Tucuruí [4°20'S, 49°31'W], Equipe de Resgate de Fauna, 1984; Ephebopus uatuman Lucas, Silva & Bertani, 1992, male holotype (IBSP 4939), female paratype (IBSP 4940), Brazil, state of Amazonas, Presidente Figueiredo, Uatuman River, Balbina Hydroelectric Power Station [2°02'S, 60°01'W], M. Costa, 19.II.1988; Ephebopus cyanognathus West & Marshall, 2000, Ephebopus rufescens West & Marshal, 2000 and Ephebopus foliatus West, Marshall, Fukushima and Bertani, 2008 data were taken from
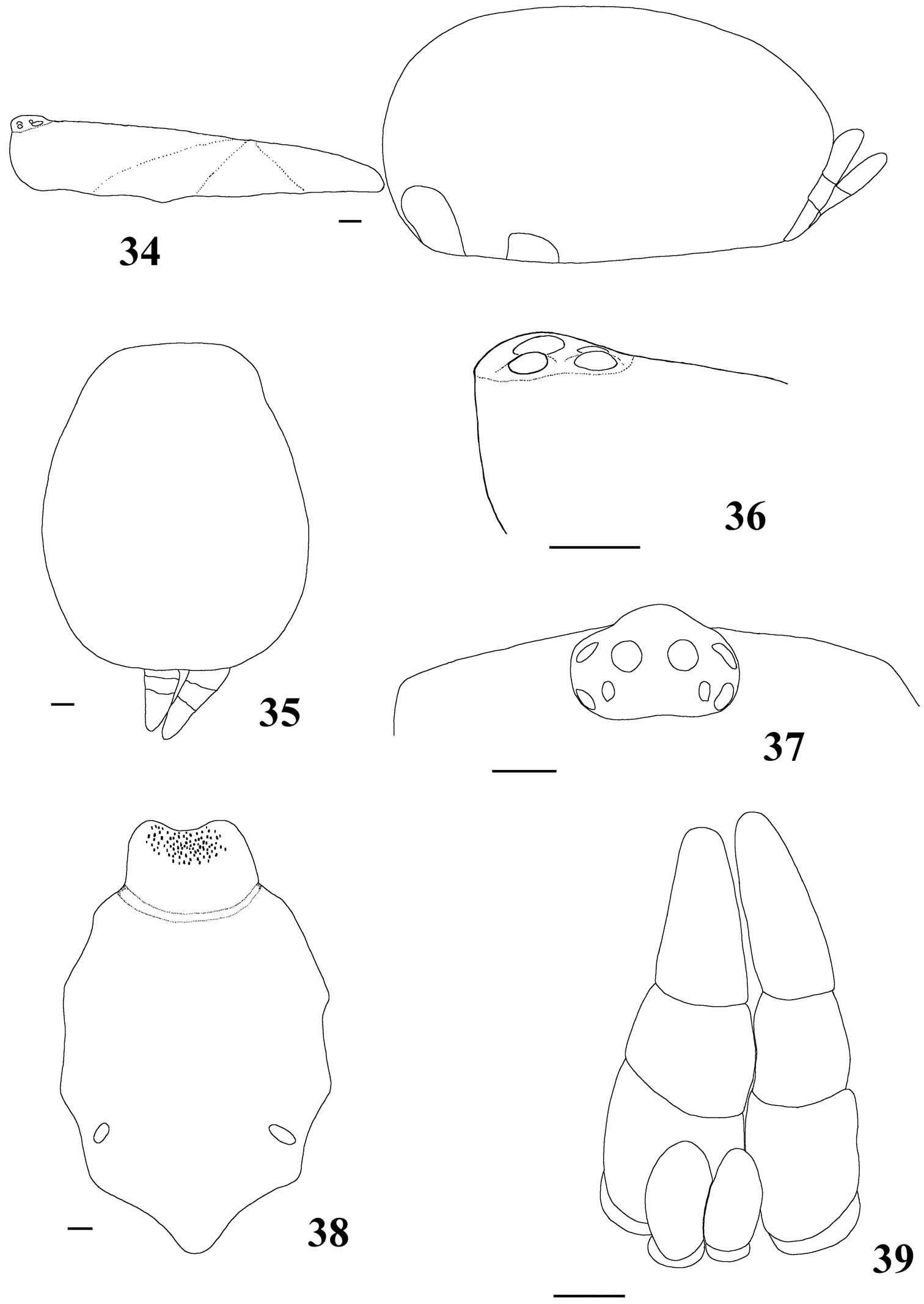
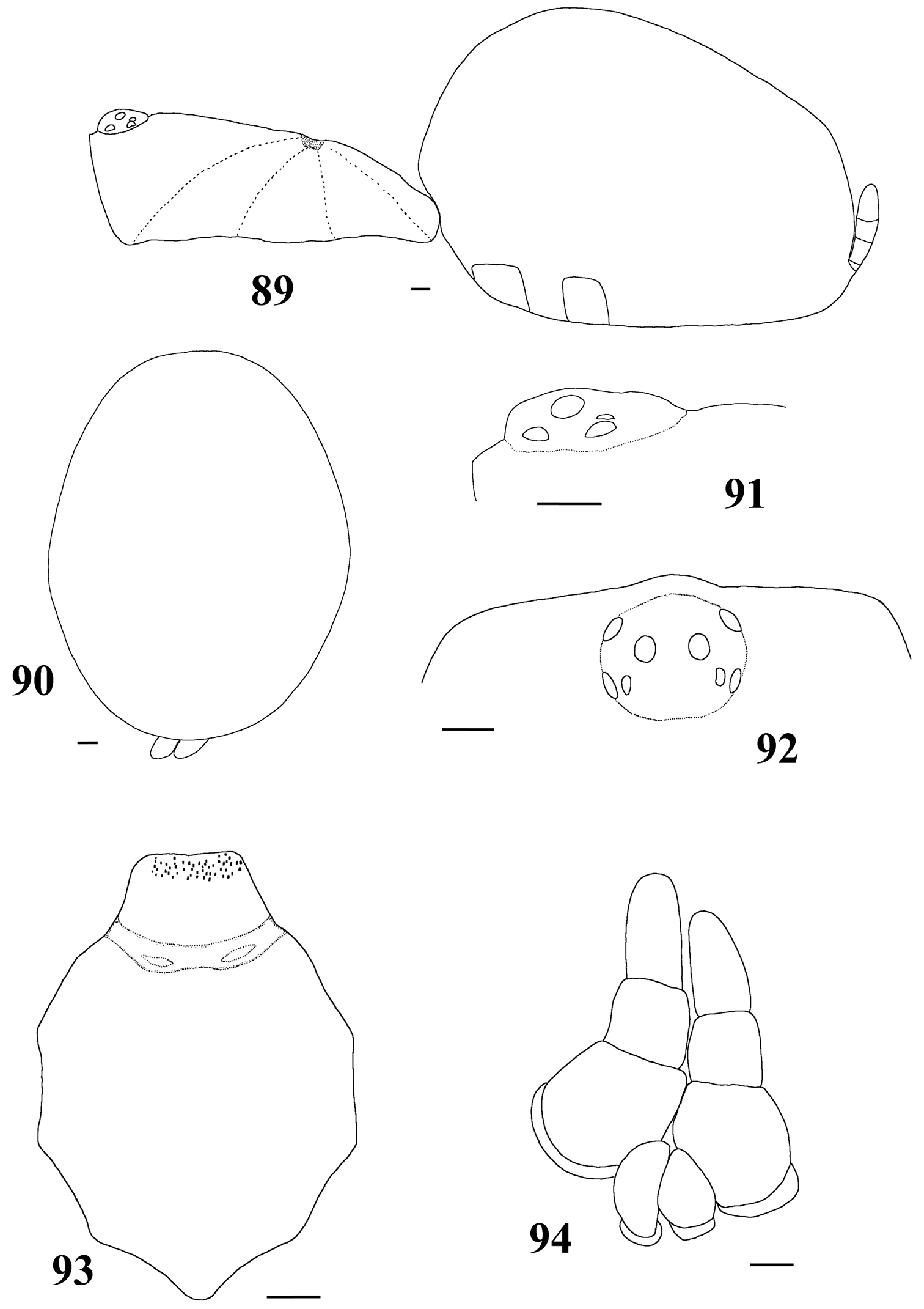
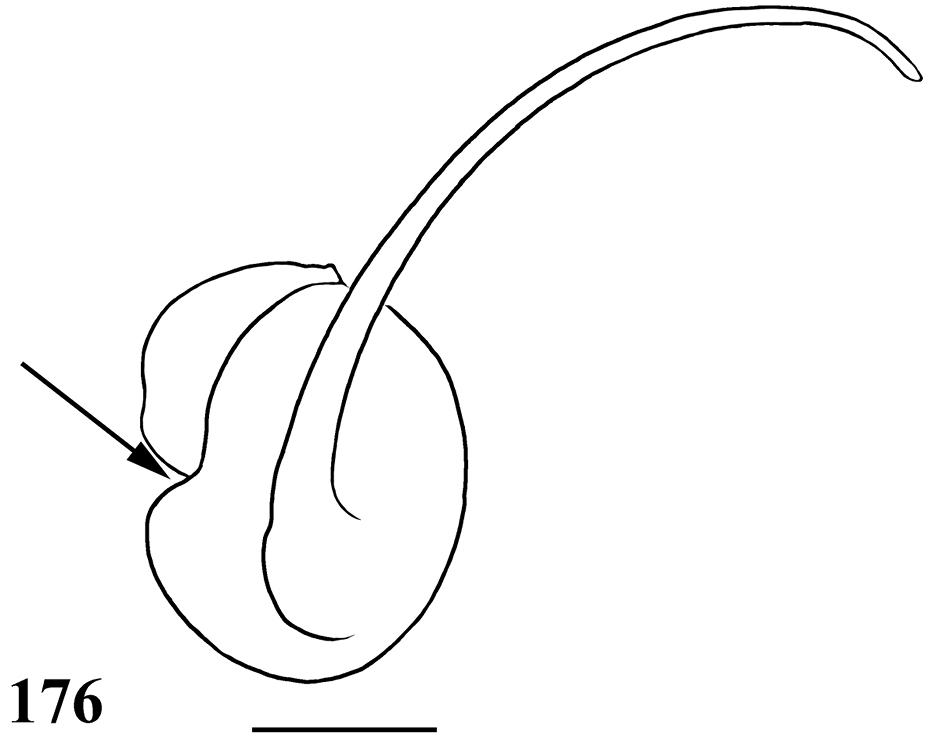
List of Characters: (0) - Eye tubercle in males and females: (0) low (Fig. 36), (1) high (Fig. 91). (1) Anterior row of eyes in males and females, measured from the anterior margins: (0) procurved (Fig. 92), (1) straight (Fig. 37). (2) Clypeus in males and females: (0) not evident (Fig. 37), (1) narrow (Fig. 92), (2) wide. (3) Fovea in males and females, curvature: (0) straight, (1) recurved, (2) procurved. (4) Fovea in males and females, closure: (0) slit-like (closed), (1) pit-like (open). (5) Fovea in males and females, depth: (0) shallow, (1) deep. (6) Labial cuspules in males and females, number: (0) 30–300, (1) 0–20, (2) 350–450. (7) Sternum, shape: (0) longer than wide, not truncated behind (Fig. 93), (1) as long as wide, truncated behind (Fig. 20). Sternum is typically longer than wide in adult theraphosids, with few exceptions. However, in Typhochlaena, it is always as long as wide, or wider than long and truncated behind. Since this condition is present in immatures of other theraphosid species, this state seems to be neotenic in Typhochlaena, in agreement with the small size of adult individuals in all species of this genus. (8) Sigilla, posterior pair in males and females, position: (0) marginal, less than 1.5 times its own diameter from margin (Fig. 20), (1) located closer to the center of the sternum separated from marginby twice its own diameter. (9) Abdomen in females, (0) ovoid, about a half longer than wide (Figs 89–90), (1) dorso-ventrally flattened, about a quarter longer than wide (Figs 34–35). (10) Posterior lateral spinnerets, distal segment, (0) digitiform (Fig. 39), (1) domed (Fig. 21).
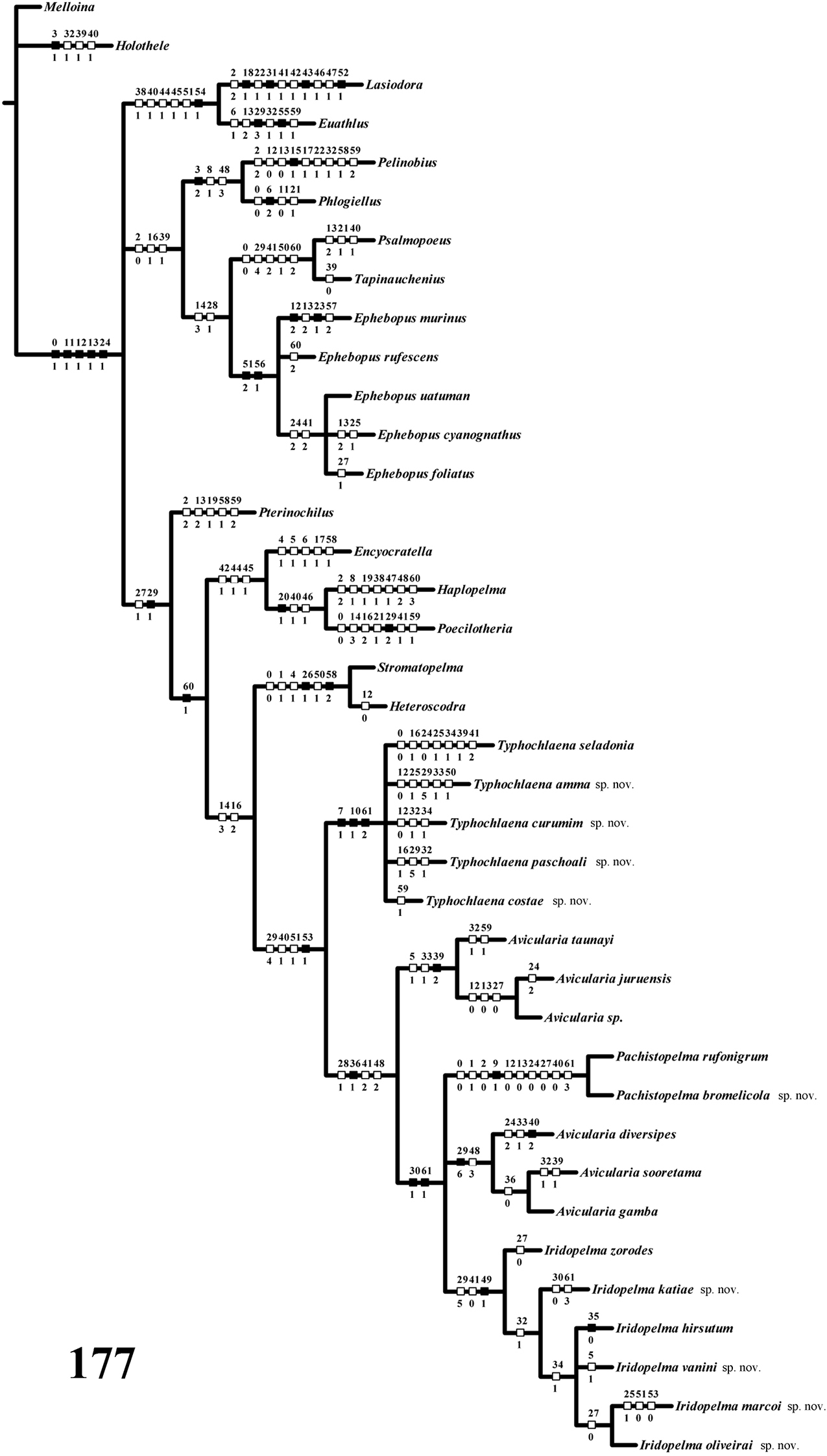
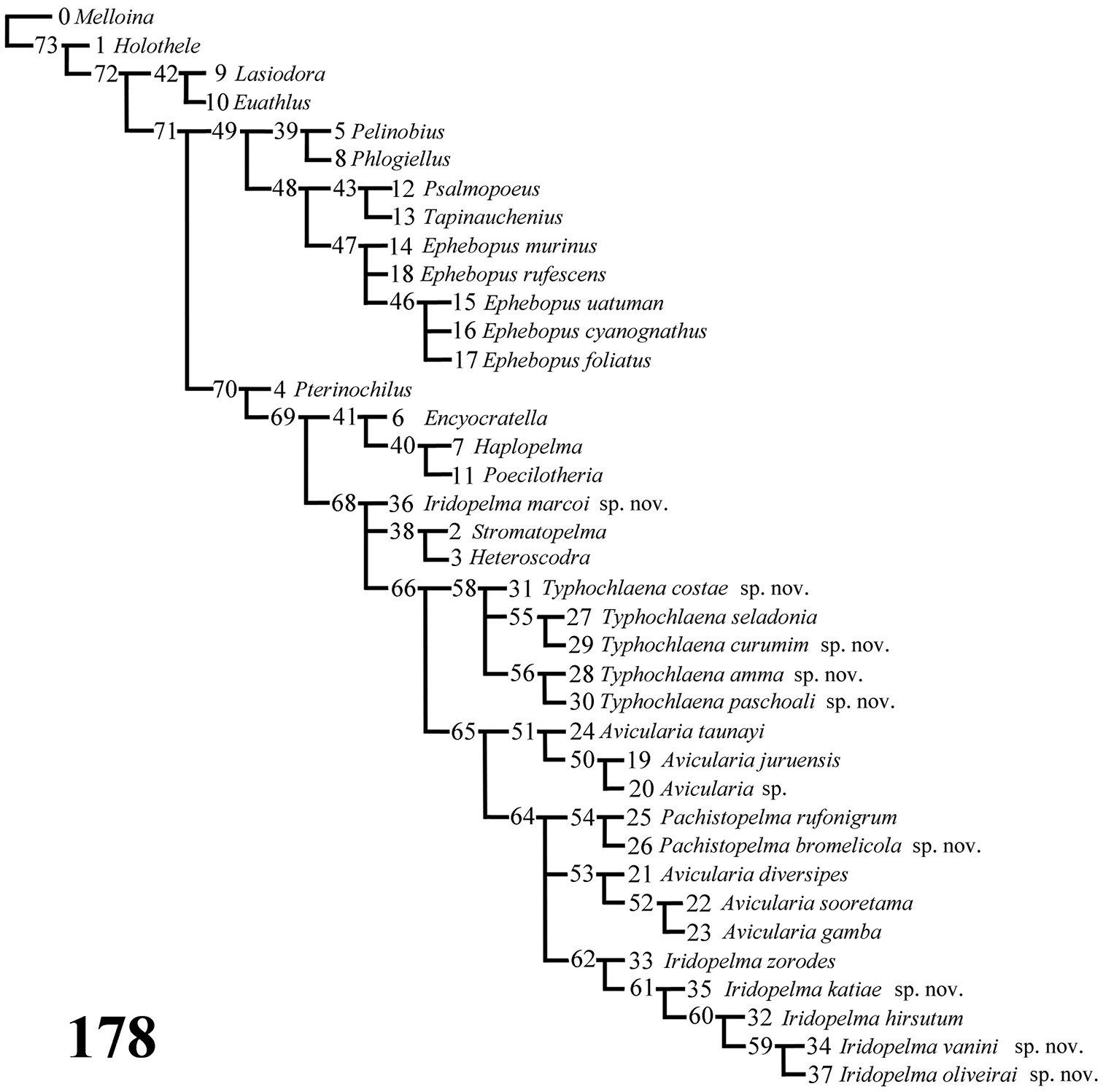
Computer methods: A data matrix (Table 1) with 62 characters and 38 taxa was analyzed with NONA 2.0 for Windows (
Data matrix showing distribution of character states in cladistic analysis. (?=unknown, - = non-applicable, both treated as missing data).
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melloina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | ? | 0 | 0 | - |
| Holothele | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | - |
| Stromatopelma | 0 | 1 | 1 | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 2 | 0 | 1 | 0 |
| Heteroscodra | 0 | 1 | 1 | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 2 | 0 | 1 | 0 |
| Pterinochilus | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | - |
| Pelinobius | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | - |
| Encyocratella | 1 | 0 | 1 | - | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | - | - | - | - | - | 0 | 1 | 0 | ? | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 1 | ? | 1 | 0 |
| Haplopelma | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | - | 0 | - | - | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | - |
| Phlogiellus | 0 | 1 | 0 | 2 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | - |
| Lasiodora | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | - | - | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | - |
| Euathlus | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | ? | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | - | - | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 3 | - |
| Poecilotheria | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | - | 0 | - | - | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 |
| Psalmopoeus | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 2 | - |
| Tapinauchenius | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | 2 | - |
| Ephebopus murinus | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | - |
| Ephebopus uatuman | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | - |
| Ephebopus cyanognathus | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | ? | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | - |
| Ephebopus foliatus | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | ? | ? | ? | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | ? | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | ? | ? | 0 | ? | - |
| Ephebopus rufescens | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | - |
| Avicularia juruensis | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 1 | 0 | - | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 |
| Avicutaria sp. | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 1 | 0 | - | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 1 | 0 |
| Avicularia diversipes | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 6 | 1 | 0 | 0 | 1 | 0 | - | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 1 | 1 |
| Avicularia sooretama | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 | 1 | 0 | 1 | 0 | 0 | - | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | 1 |
| Avicularia gamba | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 | 1 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | 1 |
| Avicularia taunayi | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | 0 | 0 | 1 | 1 | 0 | - | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 1 | 1 | 0 |
| Pachistopelma rufonigrum | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | 1 | 3 |
| Pachistopelma bromelicola sp. n. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | - | 1 | 3 |
| Typhochlaena seladonia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | 2 |
| Typhochlaena amma sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ? | 5 | 0 | 0 | 0 | 1 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | ? |
| Typhochlaena curumim sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ? | 0 | ? | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 1 | 0 | 1 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | 2 |
| Typhochlaena paschoali sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ? | 1 | ? | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 1 | 0 | 0 | - | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | ? | ? |
| Typhochlaena costae sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 1 | ? | ? |
| Iridopelma hirsutum | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | 1 |
| Iridopelma zorodes | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 0 | 1 | 1 |
| Iridopelma vanini sp. n. | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5 | ? | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 1 | 1 | ? |
| Iridopelma katiae sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5 | 0 | 0 | 1 | 0 | 0 | - | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | 0 | 3 | 1 | 3 |
| Iridopelma marcoi sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 1 | ? | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ? | ? | ? | 0 | 1 | 0 | 1 | 1 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 0 | 0 | 0 | 0 | - | ? | 1 | 1 | ? |
| Iridopelma oliveirai sp. n. | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 5 | ? | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | - | ? | 1 | 1 | ? |
http://species-id.net/wiki/Typhochlaena
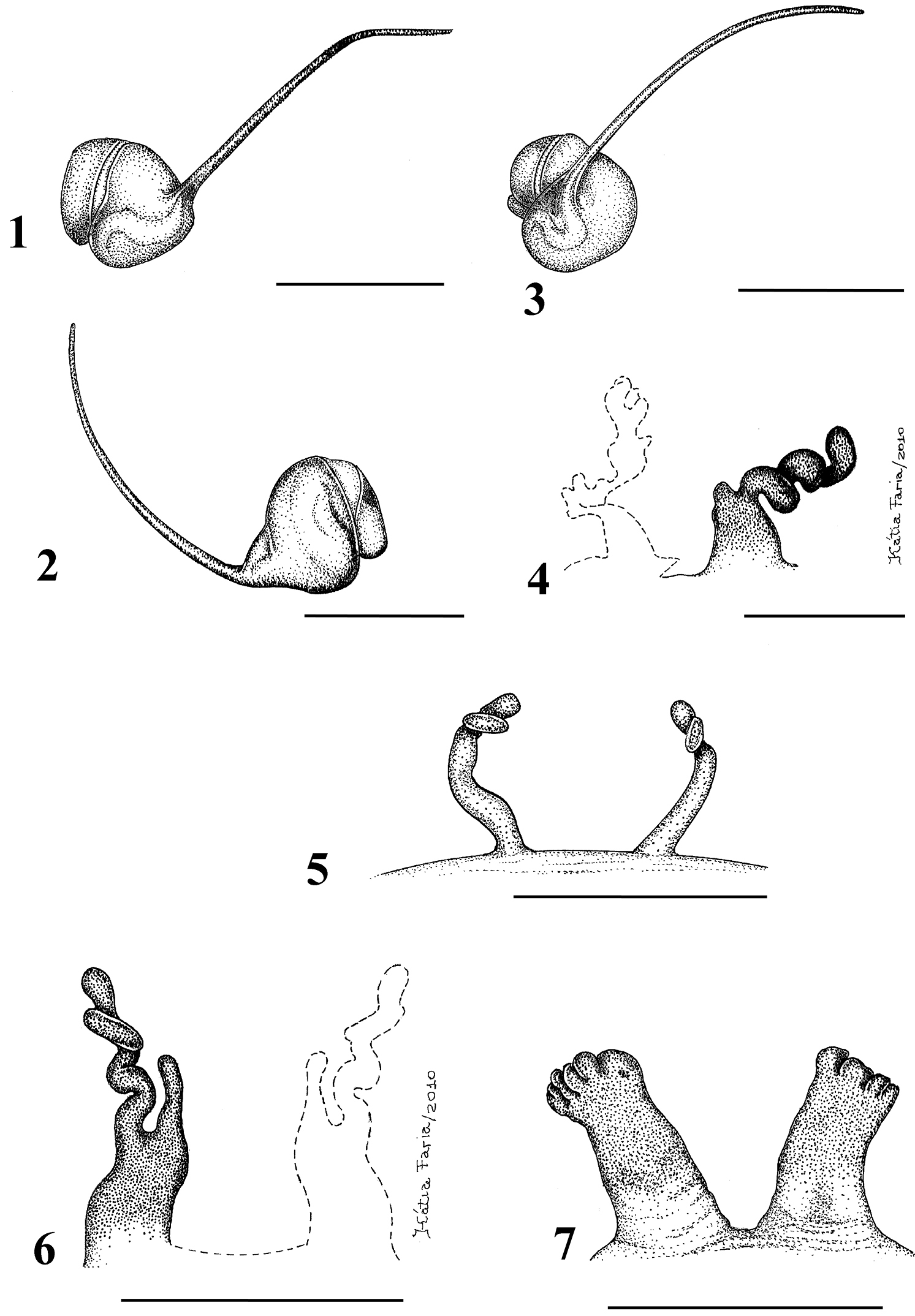
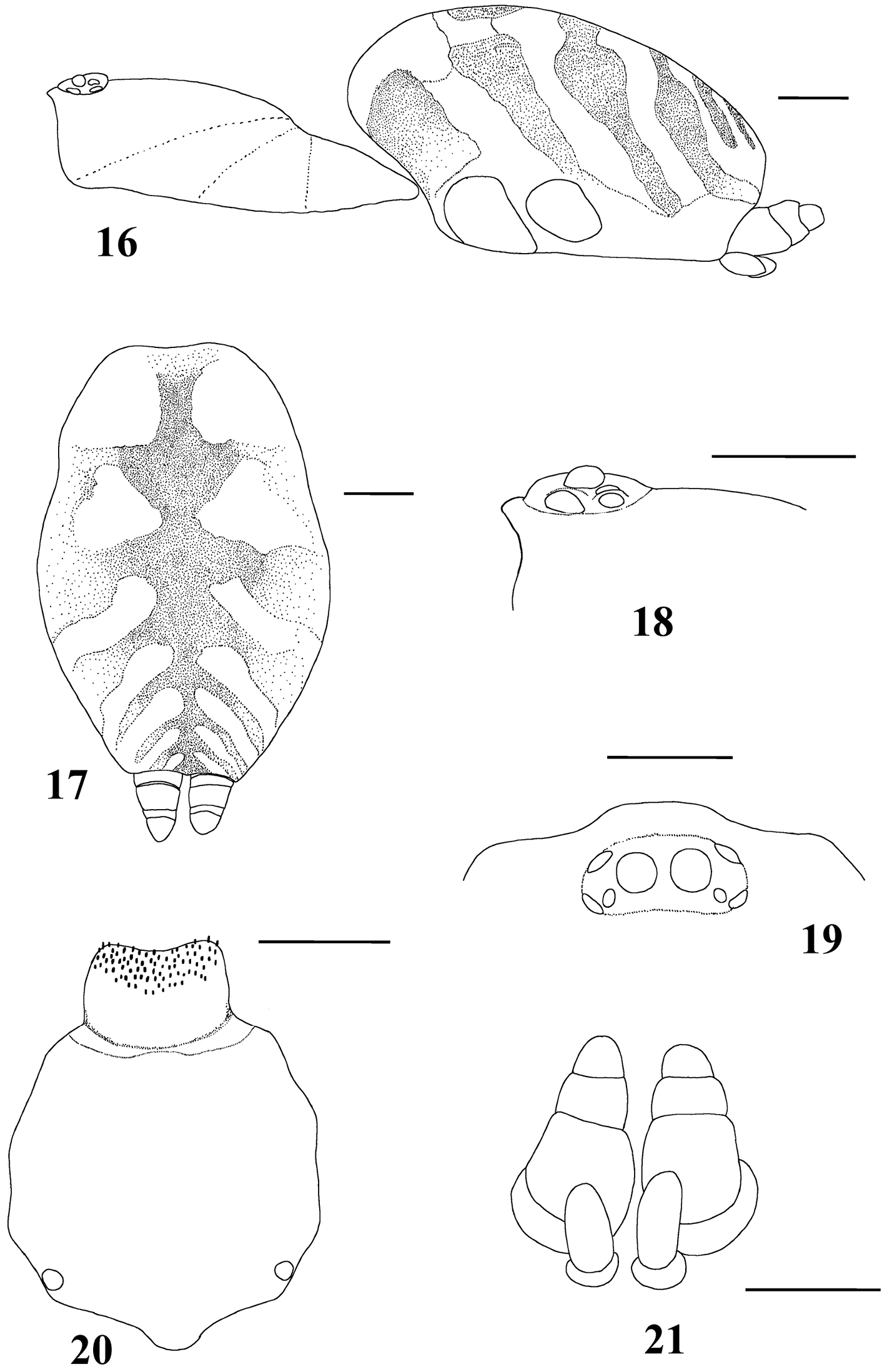
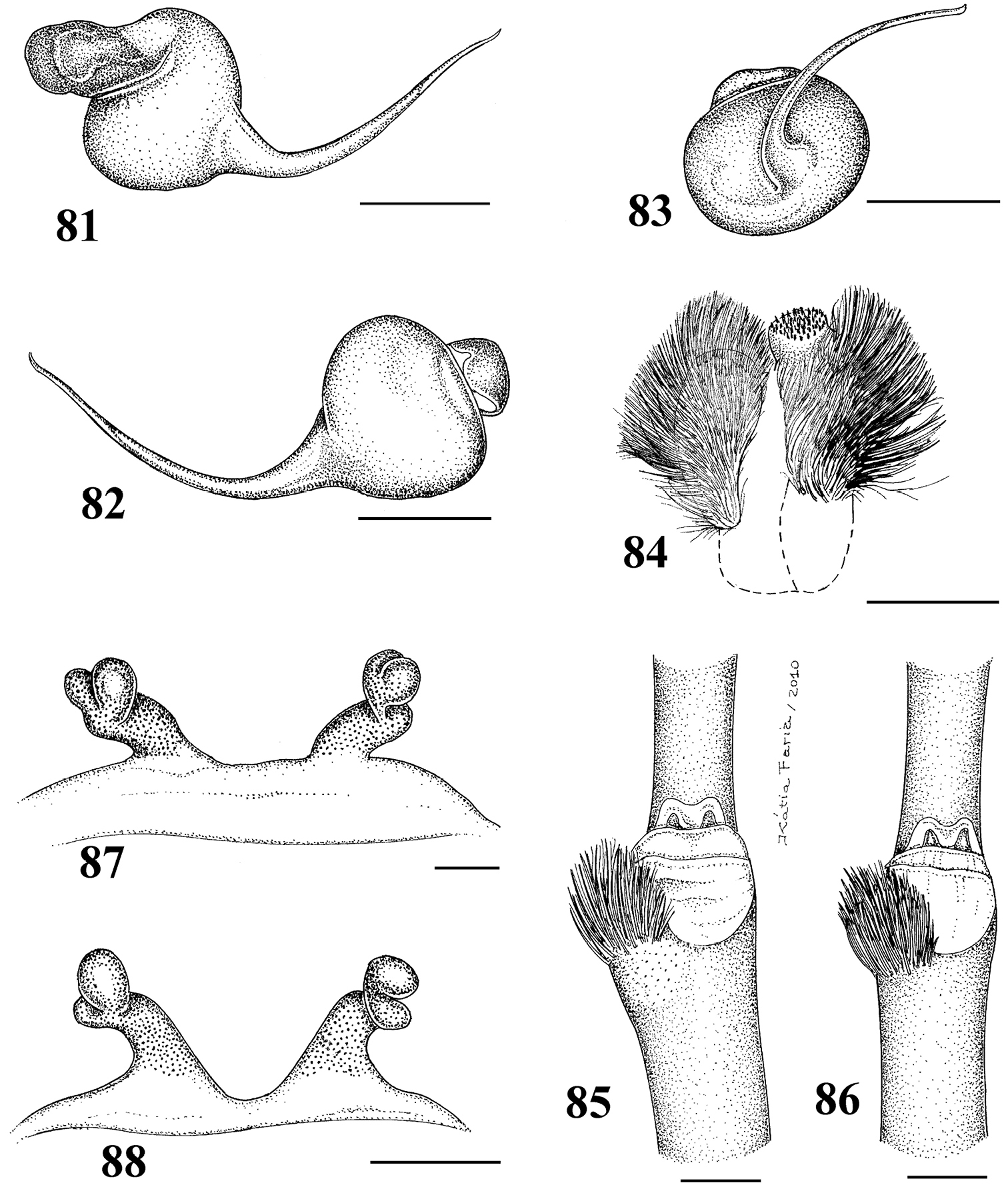
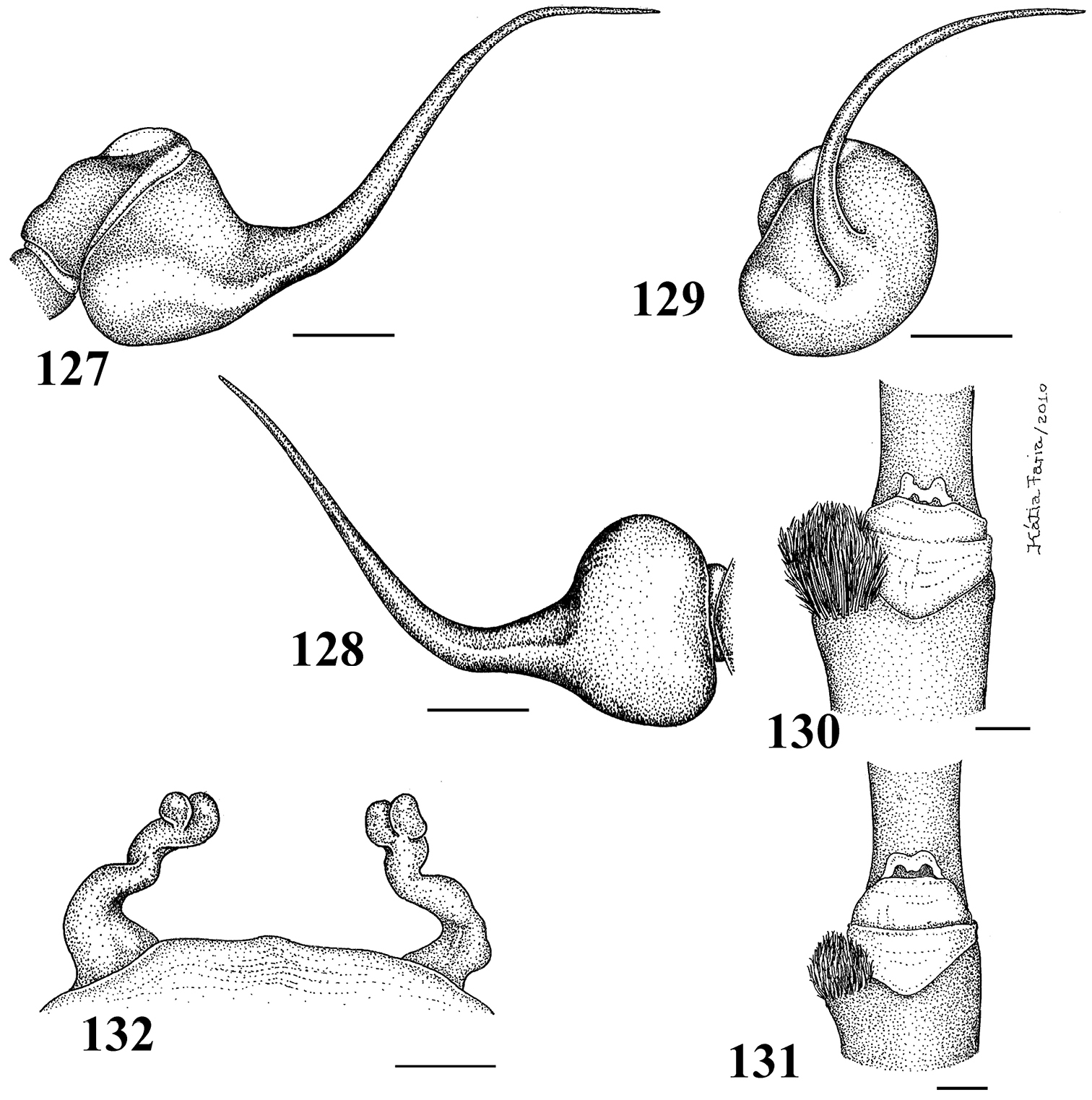
Figs 1–28Mygale seladonia C. L. Koch, 1841 by subsequent designation (
Differs from all other aviculariine genera by the domed, short distal segment of PLS (Fig. 21) and adults with sternum as long as wide, truncated behind (Fig. 20). Additionally, they are very small aviculariines having urticating hair type II on the dorsum of the abdomen and males lack both tibial spurs and spiniform process on the cymbium.
Carapace as long as wide, or slightly longer than wide, cephalic region moderately raised (Fig. 16). Cephalic and thoracic striae shallow. Fovea shallow, straight. Chelicerae without rastellum. Eye tubercle low (Typhochlaena seladonia) (Fig. 18) or raised (other species), wider than long. Clypeus narrow (Figs 18–19). Anterior eye row procurved (Fig. 19). Labium wider than long, with ca. 58–122 cuspules concentrated on anterior half. Maxillary lyra absent. Maxilla subrectangular, anterior lobe distinctly produced into conical process, inner angle bearing (40–69) cuspules. Sternum wider than long or as long as wide, truncated behind (Fig. 20). Posterior angle not separating coxae IV. Three pairs of sigillae, all rounded, less than a quarter diameter from margin, sometimes not evident. Leg formula: IV I II III (except male Typhochlaena amma sp. n.: I IV II III). Clavate trichobothria on the distal 1/2 of tarsi I-IV. STC of males and femalewithout teeth. Tarsi I–III fully scopulated, IV divided by a band of setae. Scopulae of tarsi and metatarsi I–II extended very laterally giving them a spatulate appearance. Femur IV without retrolateral scopula. Legs lacking spines in males, female legs aspinose or with two ventro-apical spines on metatarsi III and/ or IV (Typhochlaena seladonia, Typhochlaena paschoali sp. n.). Posterior lateral spinnerets with distal article short, domed (Fig. 21). Stridulatory setae absent. Cymbium with two subequal lobes, the prolateral one triangular in shape (fig. 25 in
Males of Typhochlaena curumim sp. n. and Typhochlaena paschoali sp. n. are unknown.
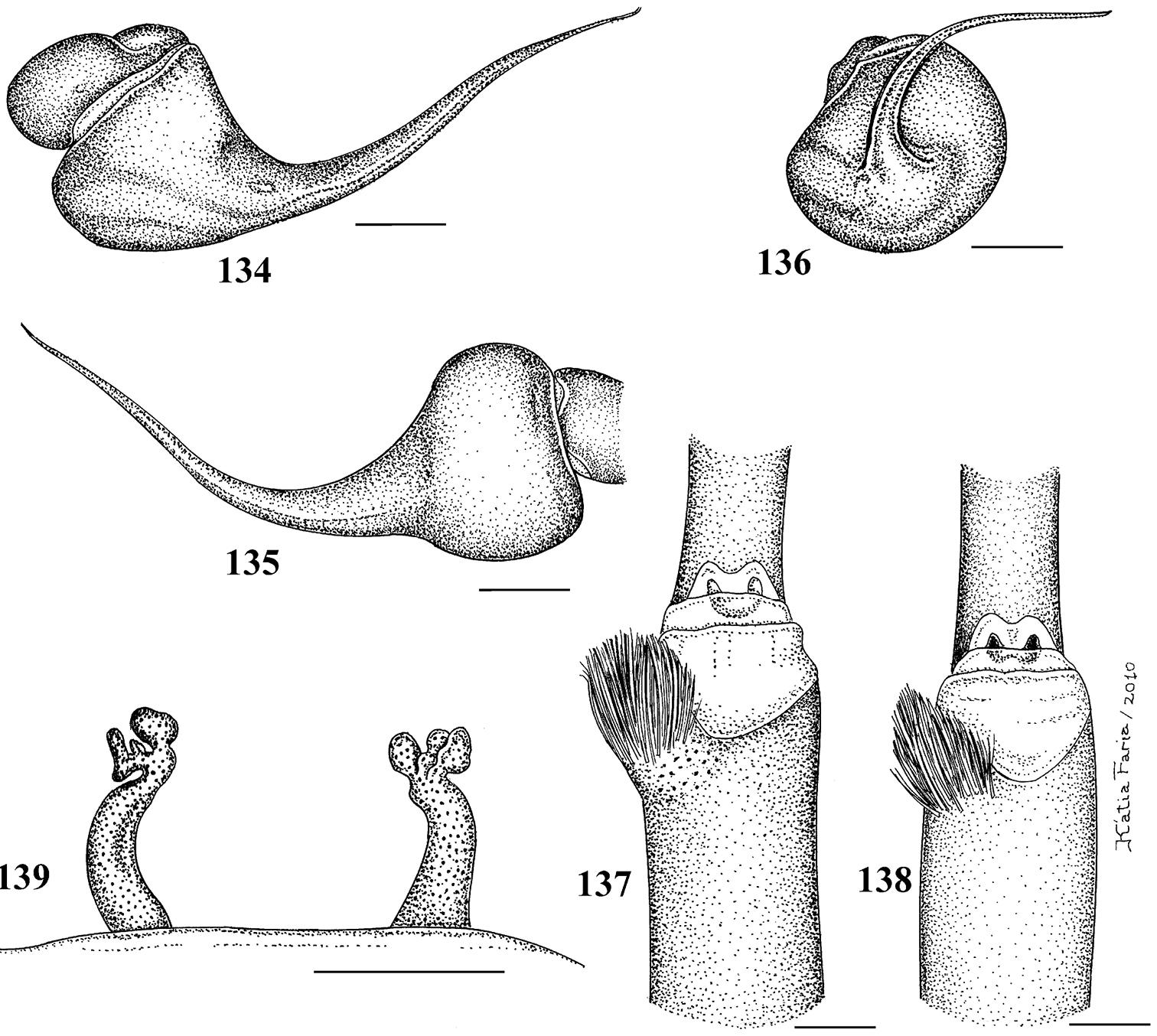
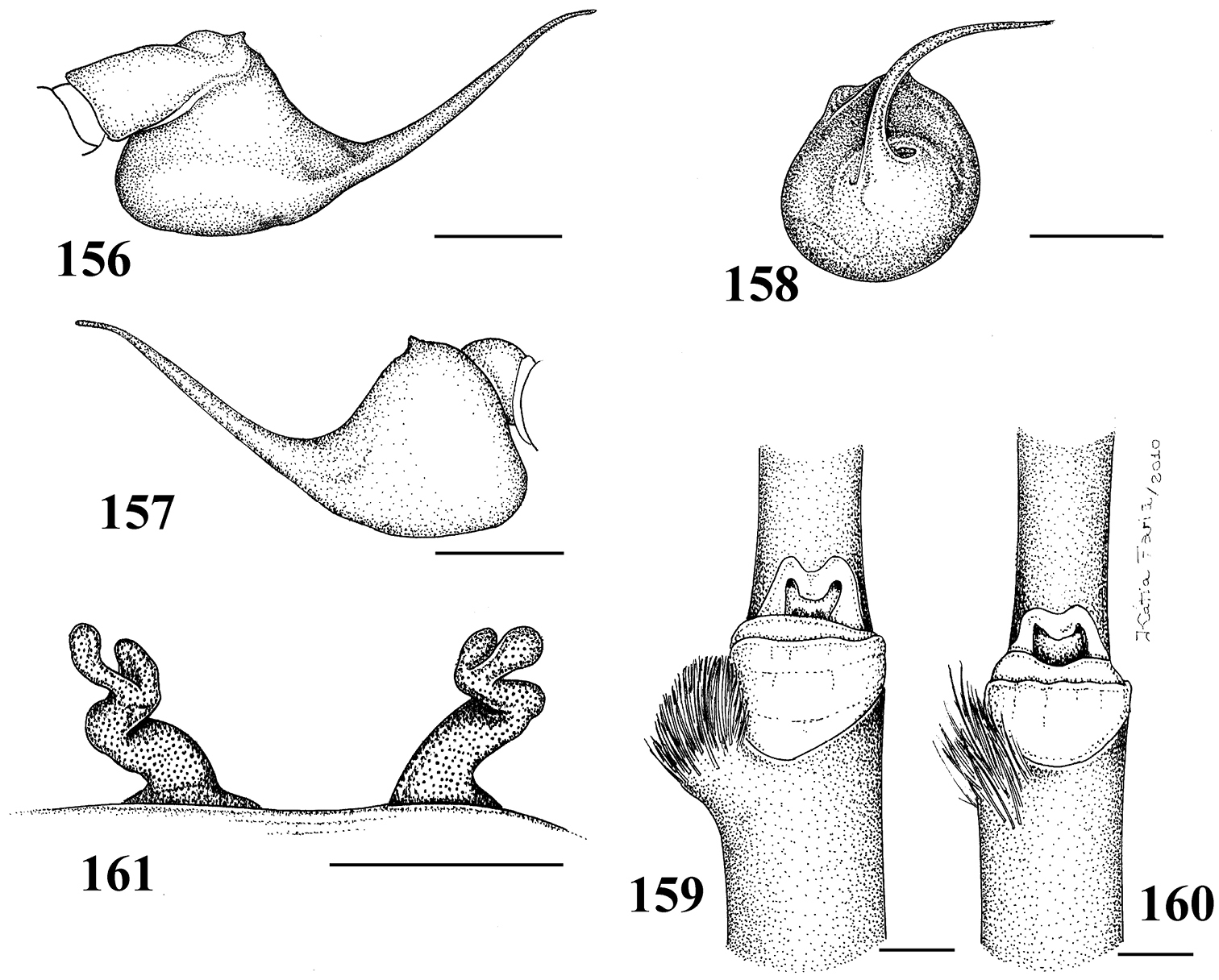
1–5 Typhochlaena seladonia C. L. Koch, 1841 1–3 male (IBSP 4551) left palpal bulb 1 prolateral 2 retrolateral 3 frontal 4–5 females, spermathecae 4 exuvium (IBSP 4551) 5 female (IBSP 109718) 6 Typhochlaena curumin sp. n. holotype female (IBSP 8701) spermathecae 7 Typhochlaena paschoali sp. n., paratype female (MNRJ 12928), spermathecae. Scale bar = 1mm.
8–11 Typhochlaena amma sp. n. 8–10 paratype male (MNRJ 12926), left palpal bulb 8 prolateral 9 retrolateral 10 frontal 11 holotype female (MNRJ 06239), spermathecae 12–15 Typhochlaena costae sp. n. 12–14 paratype male (IBSP unnumbered), left palpal bulb 12 prolateral 13 retrolateral 14 frontal 15 holotype female (IBSP unnumbered), spermathecae. Scale bar = 1mm.
Typhochlaena seladonia C. L. Koch, 1841, female (IBSP 109718) 16 carapace and abdomen, lateral 17 abdomen, dorsal 18 eye tubercle, lateral 19 eye tubercle, dorsal 20 labium and sternum 21 spinnerets, ventral. Scale bar = 1mm.
Species included: Typhochlaena seladonia (Figs 1–5, 16–23), Typhochlaena curumim sp. n. (Figs 6, 26), Typhochlaena paschoali sp. n. (Figs 7, 27), Typhochlaena amma sp. n. (Figs 8–11, 24) and Typhochlaena costae sp. n. (Figs 12–15, 25).
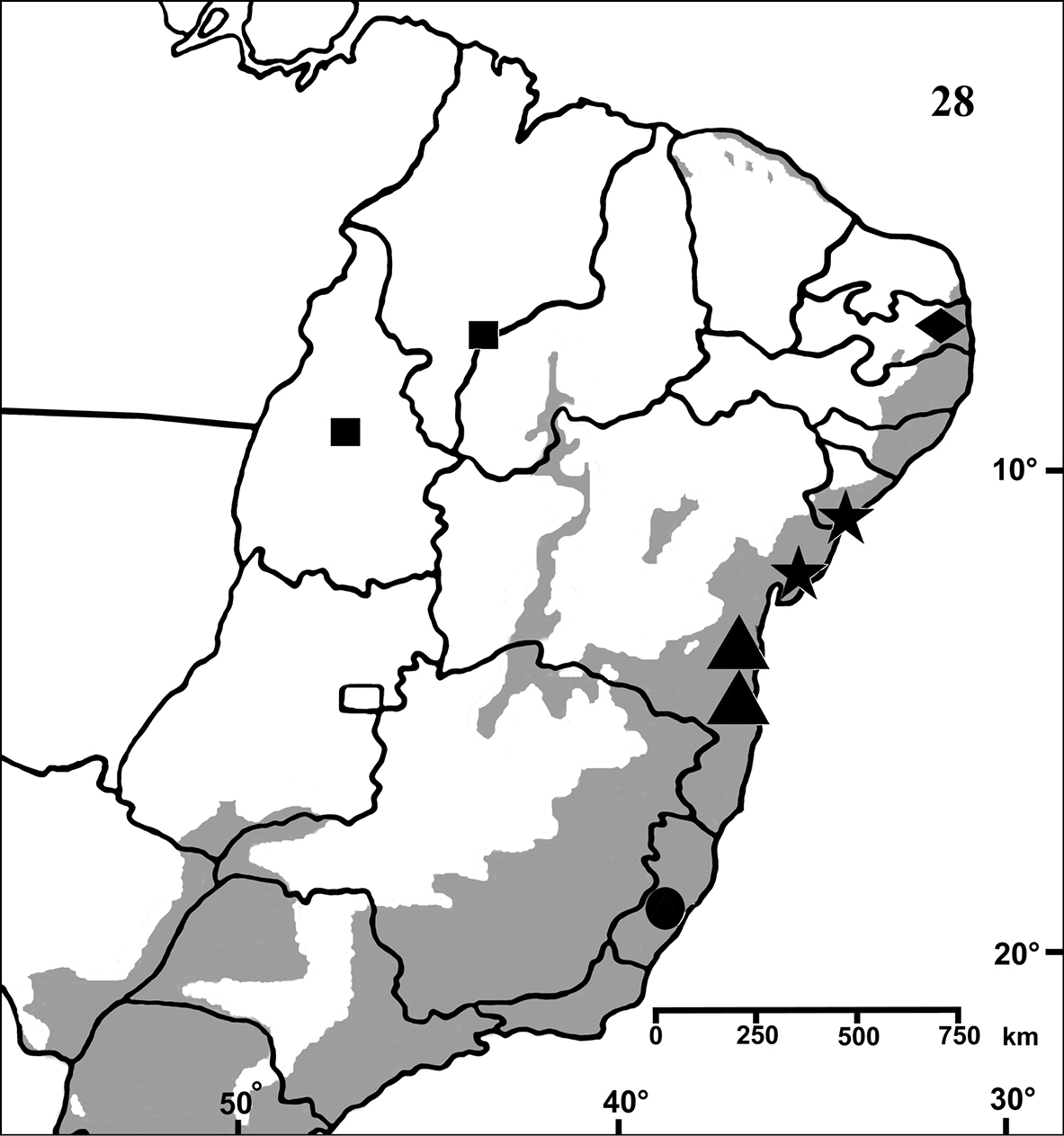
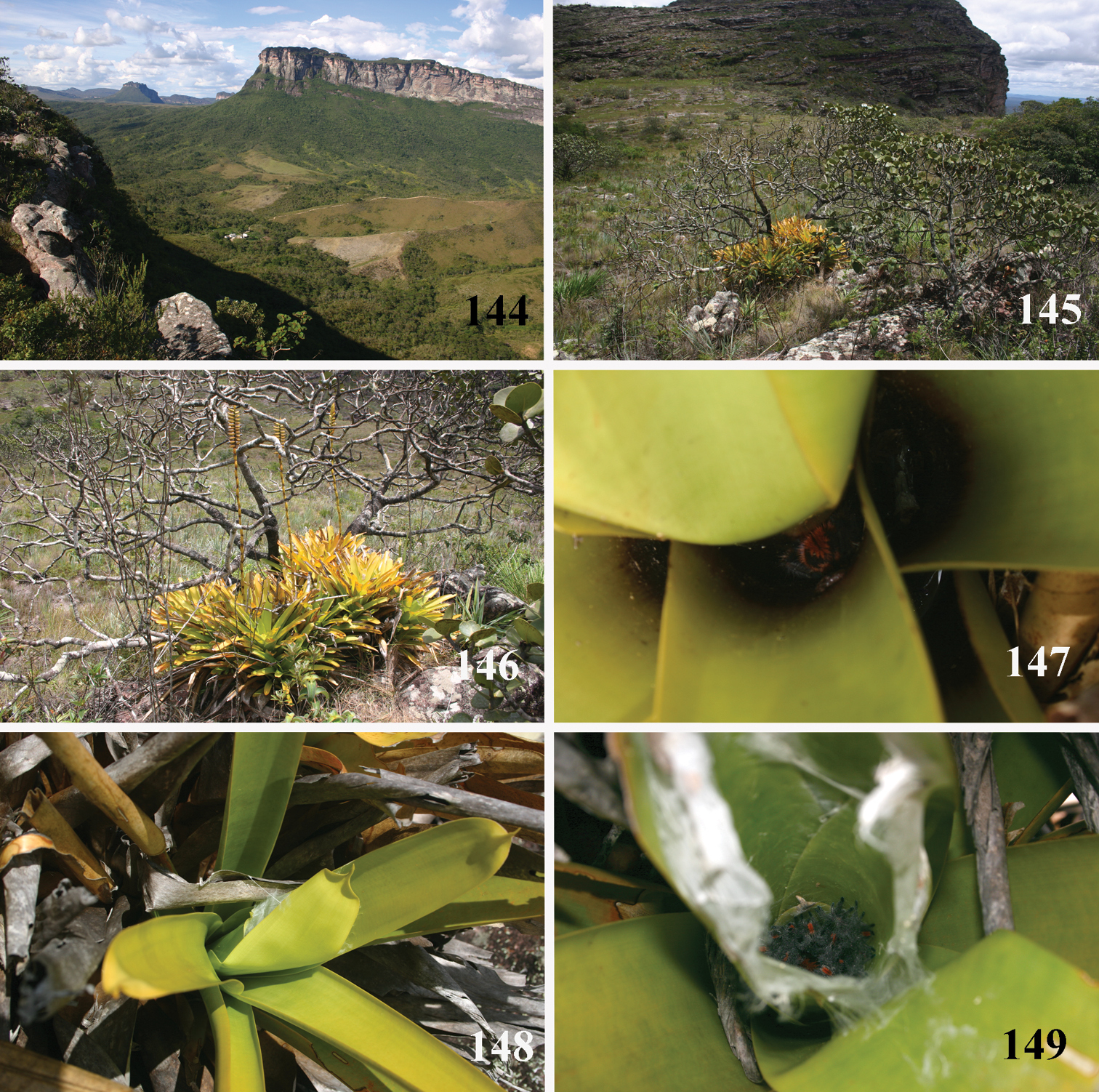
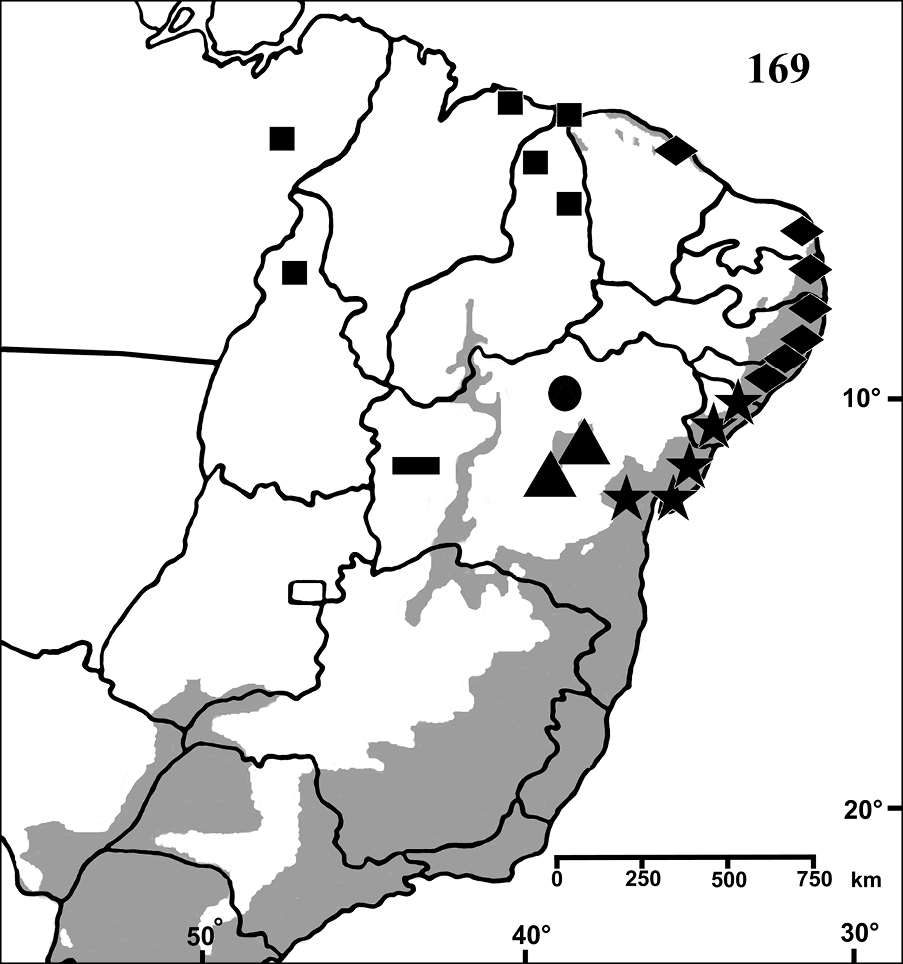
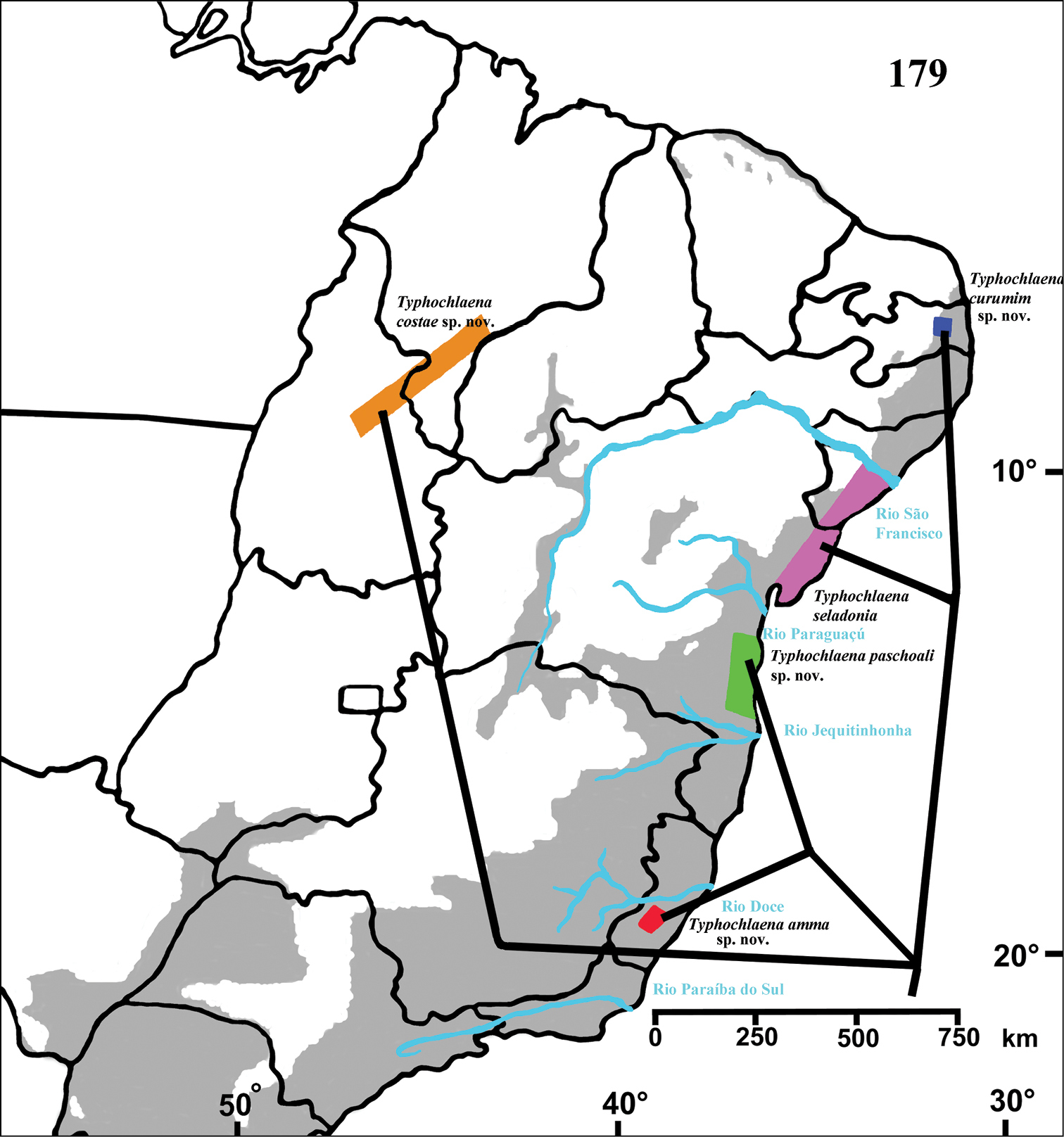
Brazil: Northeastern, part of Central-West (state of Tocantins) and part of Southeastern (state of Espirito Santo) (Fig. 28). Specimens of Typhochlaena spp. are mostly found in Brazilian Atlantic rainforest, but one species seems to occur in drier, open environments (Typhochlaena costae sp. n.). Available data for two species (Typhochlaena seladonia and Typhochlaena curumim sp. n.) indicates they build a silky retreat under loose tree bark (Fig. 23).
22–23 Typhochlaena seladonia C. L. Koch, 1841, habitus 22 female, Santa Luzia do Itanhy, state of Sergipe 23 immature inside its retreat in tree bark, same locality 24 Typhochlaena amma sp. n., female, Santa Teresa, state of Espirito Santo 25 Typhochlaena costae sp. n., female, Palmas, state of Tocantins 26 Typhochlena curumim sp. n., female, Areia, state of Paraiba 27 Typhochlaena paschoali sp. n., preserved female, Camacam, state of Bahia (holotype MNRJ 13723). Photos: R. Bertani.
Map showing records of Typhochlaena species in Northestern, Central western and Southeastern Brazil. Star = Typhochlaena seladonia C. L. Koch, 1841, square = Typhochlaena costae sp. n., diamond = Typhochlaena curumim sp. n., triangle = Typhochlaena paschoali sp. n., circle = Typhochlaena amma sp. n. The gray area represents the approximate original distribution of Brazilian Atlantic rainforest. White area represents open environment (cerrado and caatinga).
Contrary to most aviculariines, ontogenetic changes are not known in species of Typhochlaena.
(Males of Typhochlaena curumim sp. n. and Typhochlaena paschoali sp. n. are unknown)
| 1 | Male | 2 |
| – | Female | 4 |
| 2 | Embolus very long and slender (Figs 1–3); most of carapace and legs metallic green (Fig. 22); abdomen pattern as in Fig. 22 | Typhochlaena seladonia |
| – | Embolus short (Figs 9, 13); coloration brownish or dark with blue sheen | 3 |
| 3 | Embolus too short (Figs 12–14), some long curled yellow setae over carapace and chelicerae (Fig. 25) | Typhochlaena costae sp. n. |
| – | Embolus longer (Figs 8–10), setae over carapace and chelicerae brownish | Typhochlaena amma sp. n. |
| 4 | Spermathecae strongly curved outwards from its base (Fig. 11); legs dark with blue sheen and with long reddish setae (Fig. 24) | Typhochlaena amma sp. n. |
| – | Spermathecae straight or almost so (Figs 5–7, 15); coloration otherwise | 5 |
| 5 | Spermathecae broad, straight, with multiple lobes at their tip (Fig. 7) | Typhochlaena paschoali sp. n. |
| – | Spermathecae slender or tapering from base to tip, without multiple lobes. (Figs 5–6, 15) | 6 |
| 6 | Spermathecae with lobes or folds (Figs 5–6); yellow curled setae over carapace and chelicerae lacking | 7 |
| – | Spermathecae lacking lobes and folds (Fig. 15); some long curled yellow setae over carapace and chelicerae (Fig. 25) | Typhochlaena costae sp. n. |
| 7 | Spermathecae slender throughout their length (Fig. 5); carapace and legs mostly metallic green (Fig. 22) | Typhochlaena seladonia |
| – | Spermathecae broad at base, tapering distally (Fig. 6); carapace and legs dark brown (Fig. 26) | Typhochlaena curumimsp. n. |
http://species-id.net/wiki/Typhochlaena_seladonia
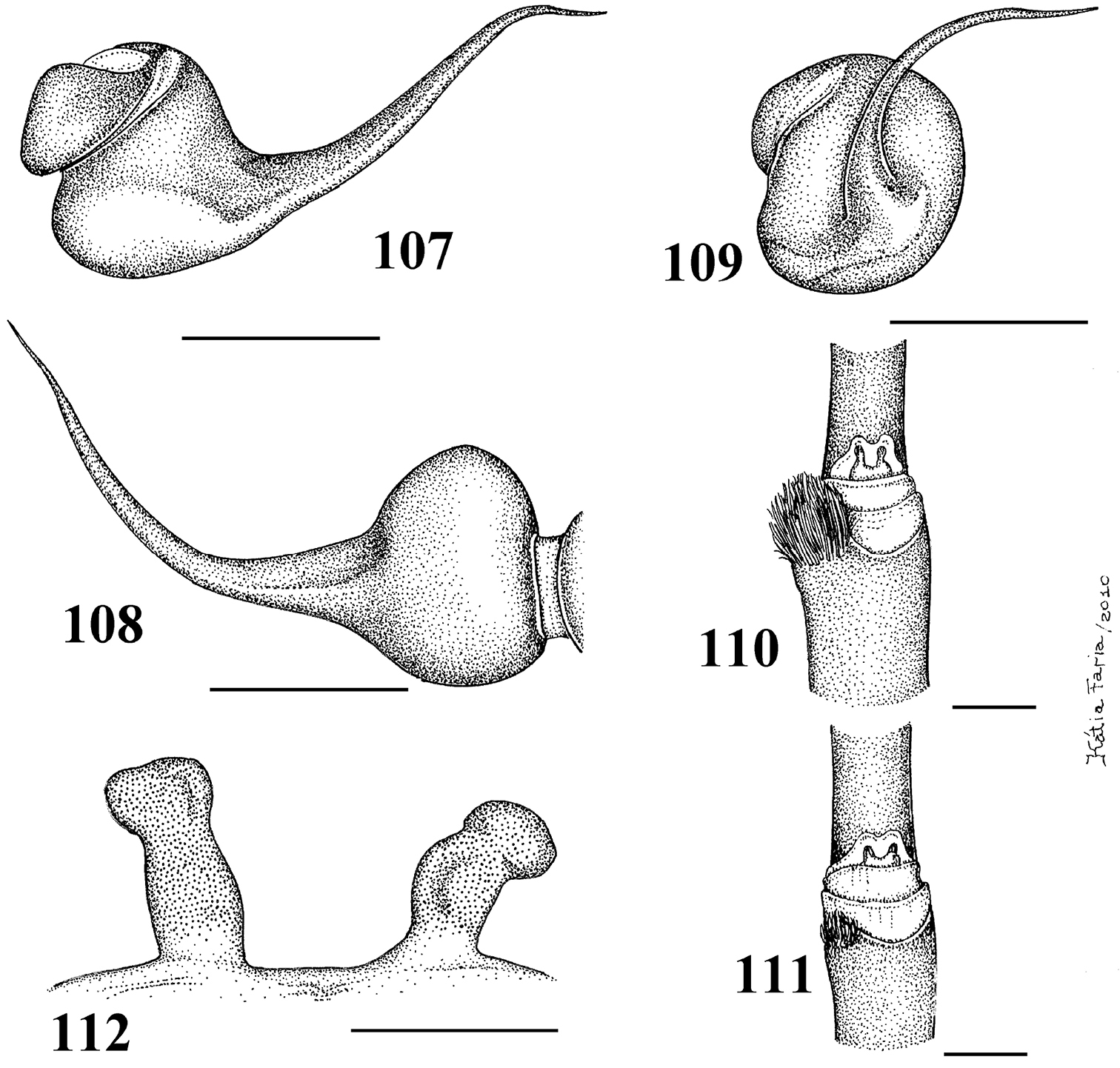
Figs 1–5, 16–23, 28Males differ from those of other species by the very long male palpal bulb embolus (Figs 1–3). The females differ by the long and slender spermathecae, spiraled distally (Figs 4–5). Additionally, males and females have cephalothorax metallic green and abdomen dorsum black with two series of six spots, the most anterior pair reddish, other yellowish (Fig. 22).
Holotype female of Mygale seladonia C. L. Koch, 1841 [dry, pinned] from Brazil, State of Bahia, Gomez, deposited at Museum für Naturkunde, Berlin (ZMB 2033), not examined, too brittle for mail.
Brazil: Sergipe: Santa Luzia do Itanhy, Mata do Crasto (11°23'S, 37°24'W) 1 immature male, A. D. Brescovit, 6 December 1996 (IBSP 115372); Bahia: Camaçari, Jacuipe [12°42'S, 38°07'W], 1 male, 1 female exuvium, T. Brazil ded., August 1980 (IBSP 4551); Salvador, Alphaville [12°56'S, 38°21'W], 1 female, G. G. Montingelli, 11 December 2001 (IBSP 109718).
Male (IBSP 4551). Carapace 4.6 long, 4.9 wide, chelicerae 2.4. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 5.6, 2.7, 4.0, 3.6, 2.0, 17.9. II: 5.5, 2.6, 3.7, 3.3, 1.7, 16.8. III: 4.7, 2.3, 3.4, 3.3, 1.7, 15.4. IV: 5.8, 2.4, 4.6, 4.6, 1.8, 19.2. Palp: 3.7, 2.0, 2.6, –, 0.9, 9.2. Mid-widths (lateral): femora I –IV = 1.1, 1.2, 1.2, 1.0, palp=0.8; patellae I–IV = 1.0, 1.1, 1.0, 1.0, palp = 1.0; tibiae I–IV = 0.9, 0.8, 0.8, 0.8, palp = 1.0; metatarsi I–IV = 0.7, 0.7, 0.6, 0.6; tarsi I–IV = 0.7, 0.7, 0.6, 0.5, palp = 0.9. Abdomen 5.2 long, 3.6 wide. Spinnerets: PMS, 0.6 long, 0.3 wide, 0.1 apart; PLS, 0.9 basal, 0.4 middle, 0.6 domed distal; mid-widths (lateral), 0.6, 0.4, 0.3, respectively. Carapace: length to width 0.94. Fovea 0.8 wide. Eyes: tubercle 0.1 high, length 0.6, width 1.3. Clypeus 0.3. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.3, ALE 0.3, PME 0.1, PLE 0.2, AME–AME 0.2, AME–ALE 0.1, AME–PME 0.05, ALE–ALE 0.9, ALE–PME 0.2, PME–PME 0.7, PME–PLE 0.08, PLE–PLE 1.0, ALE–PLE 0.1, AME–PLE 0.2. Ratio of eye group width to length 2.54. Maxillae: length to width: 2.0. Cuspules: 46 spread over ventral inner heel. Labium: 0.6 long, 0.9 wide, with 58 cuspules spaced by one diameter from each other on the anterior half. Labio-sternal groove shallow, flat, sigilla not evident. Chelicerae: basal segments with seven teeth, the two more distal closely positioned, others spaced by at least one diameter from each other. Sternum: 2.1 long, 2.2 wide (Fig. 20). Legs: leg formula: IV I II III. Scopula: tarsi I–IV fully scopulate, IV divided by a four setae wide row. Metatarsi I–II fully scopulate; III 1/2 distal scopulate; IV 1/4 distal scopulate. IV divided by three wide row of setae. Spines absent on all legs and palps. Urticating hairs: type II (0.25 long, 0.01 wide) on the abdomen dorsum. Palp: embolus 2.4 in length, with a 60° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.1, 0.07 and 0.03, respectively. Tegulum 0.6 long. (Figs 1–3). Cymbium: spiniform process lacking. Color pattern: carapace and chelicerae covered with metallic green setae, and pale yellow setae on both margins. Legs, palps, labium and sternum metallic green. Longitudinal stripes on femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi rings very discrete, whitish. Abdomen dorsum black with two series of six spots. Most anterior pair reddish, other yellowish. Abdomen laterally and venter metallic green (Fig. 22).
Female (IBSP 109718) Carapace 5.4 long, 5.2 wide, chelicerae 2.5. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 4.2, 2.5, 2.5, 2.0, 1.7, 12.9. II: 3.7, 2.2, 2.4, 1.8, 1.7, 11.8. III: 3.5, 2.1, 2.1, 2.1, 1.4, 11.2. IV: 4.4, 2.1, 2.9, 2.7, 1.3, 13.4. Palp: 3.0, 1.9, 2.0, –, 2.3, 9.2. Mid-widths (lateral): femora I –IV = 1.1, 1.2, 1.2, 1.0, palp = 1.0; patellae I–IV = 1.3, 1.3, 1.2, 1.1, palp = 0.9; tibiae I–IV = 1.2, 1.2, 1.1, 1.0, palp = 1.0; metatarsi I–IV = 1.0, 0.9, 0.8, 0.8; tarsi I–IV = 1.0, 1.0, 0.8, 0.8, palp = 1.0. Abdomen 6.0 long, 4.0 wide. Spinnerets: PMS, 0.9 long, 0.3 wide, 0.1 apart; PLS, 0.7 basal, 0.3 middle, 0.3 domed distal; mid-widths (lateral), 0.6, 0.5, 0.4, respectively. Carapace: length to width 1.03. Fovea 0.8 wide. Eyes: tubercle 0.02 high, 0.7 long, 1.4 wide. Clypeus 0.3. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.3, ALE 0.3, PME 0.1, PLE 0.2, AME–AME 0.1, AME–ALE 0.1, AME–PME 0.08, ALE–ALE 0.9, ALE–PME 0.2, PME–PME 0.8, PME–PLE 0.04, PLE–PLE 1.0, ALE–PLE 0.1, AME–PLE 0.2. Ratio of eye group width to length 2.72. Other characters as in male, except: maxillae: length to width: 1.6. Cuspules: ca. 50 spread over ventral inner heel. Labium: 0.8 long, 1.1 wide, with ca. 87 cuspules. Sternum: 2.3 long, 2.5 wide. Scopula: tarsi I–II fully scopulate, III with some sparse setae along its center, IV divided by a four setae row. Metatarsi I–II fully scopulate; III 1/2 distal scopulate; IV 1/5 distal scopulate. IV divided by three wide row of setae. Spines: metatarsi III and IV with two ventro-apical spines. Urticating hairs type II (0.43 to 0.48 long, 0.015 wide) on the abdomen dorsum. Genitalia: paired long, slender, curved, weakly sclerotized spermathecae spiraled distally.
Brazil, state of Sergipe and northeastern Bahia (Fig. 28).
One female was found under loose tree bark in Santa Luzia do Itanhy SE (September 1999). Fig. 23. Two females were found in a similar retreat in nature, in Bahia, and one specimen built a retreat with small pieces of tree bark in captivity (
urn:lsid:zoobank.org:act:818C7745-D8D8-4B32-B5F4-08059D64DDCA
http://species-id.net/wiki/Typhochlaena_curumim
Figs 6, 26, 28The female differs by the spermatheca broad at its base, tapering to a single or bifid slender spiraled distal region (Fig. 6). Additionally, females have cephalothorax and legs dark brown or brown, and the abdomen metallic yellowish-green, dorsally with central longitudinal black stripe and a series of five black stripes on both sides, extending laterally (Fig. 26).Male unknown.
The specific nameis derived fromthe Brazilian indigenous Tupi language, meaning “child”. It refers to the local children that found the type specimens high in a tree in Areia, State of Paraíba, Brazil, during an arachnological expedition.
Types. Holotype female, Brazil, Paraíba, Areia, Reserva Ecológica Estadual Mata do Pau-Ferro [6°58'S, 35°42'W], 500 m a.s.l., under tree bark, A.D. Brescovit, R. Bertani, A.B.Bonaldo, S.C.Dias, September 1999 (IBSP 8701); Paratype female, same data (IBSP 8354).
None.
Holotype female (IBSP 8701). Carapace 5.0 long, 4.8 wide, chelicerae 2.5. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 3.2, 2.4, 2.3, 1.8, 1.3, 11.0. II: 3.1, 2.2, 2.2, 1.9, 1.3, 10.7. III: 2.7, 1.9, 2.1, 2.0, 1.4, 10.1. IV: 3.6, 2.2, 2.6, 2.6, 1.6, 12.6. Palp: 2.5, 1.7, 1.7, –, 2.0, 26.9. Mid-widths (lateral): femora I –IV = 1.2, 1.1, 1.1, 1.0, palp = 0.8; patellae I–IV = 1.0, 1.2, 1.0, 0.9, palp = 0.9; tibiae I–IV = 1.1, 1.1, 1.0, 0.9, palp = 1.0; metatarsi I–IV = 1.0, 1.0, 0.8, 0.7; tarsi I–IV = 1.1, 1.0, 0.8, 0.7, palp = 0.9. Abdomen 5.3 long, 3.3 wide. Spinnerets: PMS, 0.5 long, 0.3 wide, 0.1 apart; PLS, 0.4 basal, 0.3 middle, 0.3 domed distal; mid-widths (lateral), 0.4, 0.4, 0.3, respectively. Carapace: length to width 1.04. Fovea 1.4 wide. Eyes: tubercle 0.2 high, 1.0 long, 1.5 wide. Clypeus 0.2. Anterior eye row procurved, posterior straight. Eye sizes and inter-distances: AME 0.3, ALE 0.3, PME 0.2, PLE 0.2, AME–AME 0.2, AME–ALE 0.1, AME–PME 0.1, ALE–ALE 0.9, ALE–PME 0.3, PME–PME 0.8, PME–PLE 0.1, PLE–PLE 1.0, ALE–PLE 0.3, AME–PLE 0.3. Ratio of eye group width to length 2.1. Maxillae: length to width: 1.45. Cuspules: ca. 51 spread over ventral inner heel. Labium: 0.6 long, 1.0 wide, with 122 cuspules spaced by one diameter from each other on the anterior half. Labio-sternal groove shallow, flat, sigilla not evident. Chelicerae: basal segments with six larger teeth and three very small after the 3°, 5° and 7° teeth. Sternum: 1.9 long, 2.1 wide. Legs: leg formula: IV I II III. Scopula: tarsi I–III fully scopulate, IV divided by row of 6–7 setae. Metatarsi I–II 4/5 scopulate; III 1/2, IV 1/3 distal scopulate. IV divided by a six wide row of setae. Spines absent on all legs and palps. Urticating hairs type II (0.3 mm long, 0.01 wide) on the abdomen dorsally. Genitalia: paired spermathecae with two lobes distally, a long spiraled and a straight short (Fig. 6). Color pattern: carapace and chelicerae dark brown with pale yellow long hairs on the carapace border. Legs and palps dark brown, except for brown femora. Cephalic region, legs, palps and chelicerae covered with long and abundant chestnut setae. Coxae brown. Labium, sternum and maxilla dark brown. Longitudinal stripes on femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi rings whitish. Abdomen metallic yellowish-green, dorsally with central longitudinal black stripe and a series of five black stripes on both sides, extending laterally. Several scattered white and very long guard hairs over abdomen dorsally (Fig. 26).
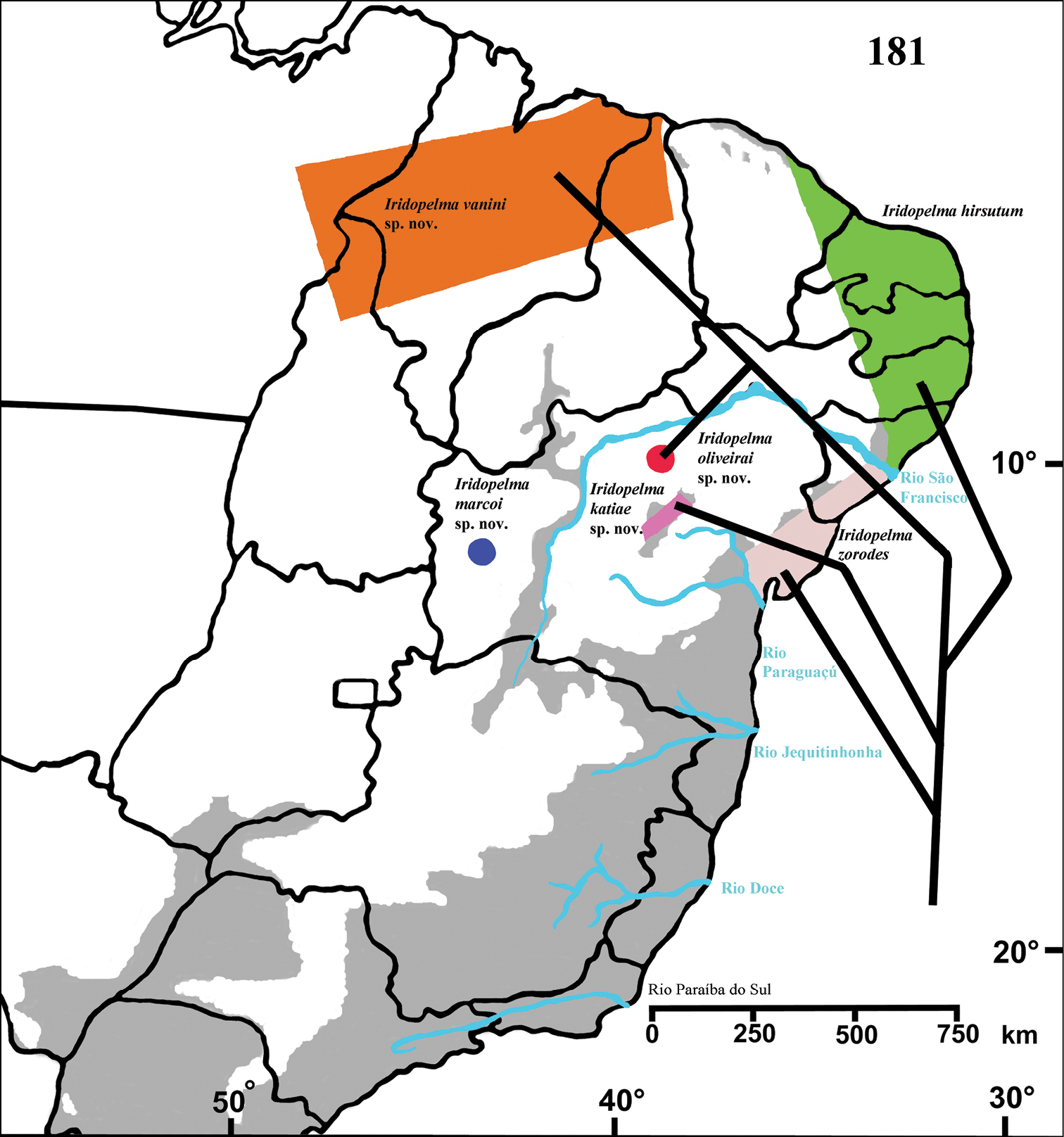
Known only from “Mata do Pau-Ferro”, Areia, in the state of Paraiba, Brazil (Fig. 28).
Three specimens were found high in a tree, under loose bark in “Mata do Pau-Ferro” reserve, September, 1999.
urn:lsid:zoobank.org:act:962A2C3A-127A-4656-9D3E-E9A1A9639CAD
http://species-id.net/wiki/Typhochlaena_paschoali
Figs 7, 27–28The females differ from those of all other Typochlaena spp. by an almost straight, broad and short spermathecae, with a multilobular distal portion (Fig. 7). Male unknown. Additionally, females have cephalothorax and legs brown, abdomen black with dorsum with a central longitudinal white pattern having zigzag border (Fig. 27).
The specific name is a patronym in honour of Elbano Paschoal de Figueiredo Moraes, a Brazilian environmentalist who was died early on April 2011. He was one of the founders of the NGO “GAMBA – Grupo Ambientalista da Bahia”, and was well known for his efforts in preserving Brazilian Atlantic rainforest remnants in the state of Bahia, Brazil.
Holotype female and immature paratype, Brazil, state of Bahia, Camacan [15°24'S, 39°30'W], no further data (MNRJ 13723); Paratypes 1 female, 1 subadult male, 9 immatures, Brazil, state of Bahia, Jussari [15°10'S, 39°29'W], no further data (MNRJ 12928 – R2981).
Brazil, Pernambuco: Tapera [8°23'S, 38°05'W], 1 female, no further data (MNRJ 13615) (probably mislabeled); Bahia: Uruçuca, Faz. S. Teresa [14°35'S, 39°17'W], 2 females, 1 immature, no further data (MNRJ 12919 – R 2955); (CEPLAC), 1 subadult male, N. Tingarine (MNRJ 13761).
Holotype female (MNRJ 13723). Carapace 6.4 long, 6.0 wide, chelicerae 3.0. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 4.6, 3.3, 3.2, 2.4, 2.0, 15.5. II: 4.2, 2.8, 3.3, 2.4, 2.1, 14.8. III: 3.6, 2.6, 2.6, 2.5, 1.6, 12.9. IV: 4.6, 2.9, 3.7, 3.5, 1.8, 16.5. Palp: 3.5, 2.4, 2.1, –, 2.2, 10.2. Mid-widths (lateral): femora I –IV = 1.5, 1.5, 1.4, 1.3, palp = 0.9; patellae I–IV = 1.3, 1.4, 1.3, 1.3, palp = 1.2; tibiae I–IV = 1.3, 1.3, 1.2, 1.1, palp = 1.1; metatarsi I–IV = 1.3, 1.2, 0.9, 0.8; tarsi I–IV = 1.2, 1.1, 0.9, 0.7, palp = 1.2. Abdomen 6.4 long, 4.7 wide. Spinnerets: PMS, 0.5 long, 0.4 wide, 0.1 apart; PLS, 0.9 basal, 0.5 middle, 0.5 domed distal; mid-widths (lateral), 0.7, 0.6, 0.4, respectively. Carapace: length to width 1.0. Fovea 1.5 wide. Eyes: tubercle 0.1 high, 1.1 long, 1.8 wide. Clypeus 0.2. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.3, ALE 0.4, PME 0.2, PLE 0.3, AME–AME 0.4, AME–ALE 0.2, AME–PME 0.2, ALE–ALE 1.3, ALE–PME 0.3, PME–PME 1.1, PME–PLE 0.1, PLE–PLE 1.5, ALE–PLE 0.3, AME–PLE 0.4. Ratio of eye group width to length 2.4. Maxillae: length to width: 1.7. Cuspules: 64 spread over ventral inner heel. Labium: 0.9 long, 1.3 wide, with 76 cuspules spaced by one diameter from each other on the anterior half. Labio-sternal groove shallow, flat, sigilla not evident. Chelicerae: basal segments with 8 teeth having similar size and well spaced from each other. Sternum: 3.1 long, 3.0 wide. Legs: leg formula: IV I II III. Scopula: tarsi I–III fully scopulate, IV divided by row of 6 setae. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/4 distal scopulate. IV divided by six wide row of setae. Spines: two apical spines on ventral metatarsus IV. Urticating hairs type II (0.32 to 0.4 long, 0.01 wide) on the abdomen dorsum. Genitalia: paired broad spermathecae very slightly curved outwards, ending in multilobular apex (Fig. 7). Color pattern (preserved in alcohol): carapace and chelicerae brown. Legs and palps light brown, except for tarsi and metatarsi I–III and tarsi of palp and leg IV, darker. Coxae, maxillae and labium light brown. Sternum dark brown. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi with white rings. Abdomen black, dorsum with a central longitudinal white pattern having zigzag border. Cephalic area and chelicerae with long stiff setae. Abdomen dorsum with several scattered, very long, white guard-hairs (Fig. 27).
Known from southern state of Bahia, Brazil. A single record for Tapera, in the state of Pernambuco, Brazil is probably a label mistake (Fig. 28).
No data available.
urn:lsid:zoobank.org:act:C177BD29-700C-4B94-AFA5-211FA5C37A1A
http://species-id.net/wiki/Typhochlaena_amma
Figs 8–11, 24, 28The male differs from those of Typhochlaena seladonia by shorter and broader embolus (Figs 8–10). Differs from Typhochlaena costae sp. n. by the embolus longer and stouter. The females differ by the strongly curved spermathecae, diverging, in their basal portion (Fig. 11). Additionally, the females have cephalothorax and legs covered with dark setae having a blue metallic sheen under light, abdomen black ventrally and dorsally with a central pinkish stripe having zigzag border (Fig. 24). The males are light brown and the abdomen is black with a dorsally pale stripe similar in shape to that of the female.
The specific name refers to the project AMMA – arachnids and myriapods from Brazilian Atlantic rainforest carried out by the arachnologists from Museu Nacional, Rio de Janeiro, who collected the types.
Holotype female, Brazil, Espírito Santo, Santa Teresa, Estação Ecológica de Santa Lúcia [19°58'S, 40°32'W], 672 m.a.s.l, A. P. L. Giupponi, February 2008 (MNRJ 06239); Paratype female, same locality, A. Pérez Gonzalez, January 2005 (MNRJ 06240); Paratype male, same locality, no further data (MNRJ 12926); Paratypes 2 males, Domingos Martins, Pico do Eldorado [20°22'S, 40°39'W], M. T. Tavares et al., 13 December 2004 (IBSP 13005).
None.
Holotype female (MNRJ 06239). Carapace 10.3 long, 10.3 wide, chelicerae 5.1. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 7.3, 5.4, 5.7, 4.5, 2.8, 25.7. II: 7.1, 4.8, 5.1, 4.5, 2.7, 24.2. III: 6.3, 4.5, 5.0, 4.7, 3.0, 23.5. IV: 7.9, 4.6, 6.8, 6.4, 2.9, 28.6. Palp: 5.1, 3.5, 3.4, –, 3.5, 15.5. Mid-widths (lateral): femora I–IV=2.5, 2.6, 2.6, 2.4, palp = 2.0; patellae I–IV = 2.3, 2.3, 2.2, 2.2, palp = 1.9; tibiae I–IV = 2.3, 2.3, 2.1, 2.0, palp = 2.0; metatarsi I–IV = 1.9, 2.0, 1.6, 1.2; tarsi I–IV = 1.6, 1.8, 1.6, 1.4, palp = 1.9. Abdomen 12.0 long, 8.9 wide. Spinnerets: PMS, 1.6 long, 0.7 wide, 0.2 apart; PLS, 1.6 basal, 0.9 middle, 1.0 domed distal; mid-widths (lateral), 1.2, 1.0, 0.6, respectively. Carapace: length to width 1.0. Fovea 1.8 wide. Eyes: tubercle 0.1 high, 1.1 long, 1.8 wide. Clypeus 0.3. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.5, ALE 0.5, PME 0.3, PLE 0.5, AME–AME 0.6, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.9, ALE–PME 0.5, PME–PME 1.6, PME–PLE 0.1, PLE–PLE 2.1, ALE–PLE 0.5, AME–PLE 0.6. Ratio of eye group width to length 2.3. Maxillae: length to width: 1.8. Cuspules: 51 spread over ventral inner heel. Labium: 1.7 long, 2.0 wide, with 79 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove shallow, flat, sigilla not evident. Chelicerae: basal segments with 7 teeth well spaced from each other. Sternum: 4.7 long, 4.3 wide. Leg formula: IV I II III. Scopula: tarsi I–III fully scopulate, IV divided by six wide row of setae. Metatarsi I–II 4/5 scopulate; III 1/2, IV 1/4 distal scopulate. IV divided by six wide row of setae. Spines absent on all legs and palps. Urticating hairs type II (0.42 to 0.56 long, 0.01 wide) on the abdomen dorsum. Genitalia: paired spermathecae curving abruptly outwards from their base (more than 90°) and with a partial division of their apex into two lobes (Fig. 11). Color pattern: carapace, chelicerae, sternum, maxillae and legs covered with dark setae having a blue metallic sheen under light. Legs, mainly ventrally, with long chestnut-brown setae. Longitudinal stripes on femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi with white rings. Abdomen black ventrally and dorsally with a central pinkish stripe having zigzag border (Fig. 24).
Paratype male (MNRJ 12926). Carapace 7.3 long, 7.7 wide, chelicerae 3.2. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 8.9, 4.4, 7.7, 6.7, 3.0, 30.7. II: 7.8, 4.2, 6.9, 6.0, 2.6, 27.5. III: 6.0, 3.5, 5.1, 4.8, 2.4, 21.8. IV: 8.1, 4.0, 7.8, 7.2, 2.4, 29.5. Palp: 4.6, 2.9, 3.5, –, 1.6, 12.6. Mid-widths (lateral): femora I–IV = 1.1, 1.4, 1.8, 1.1, palp = 0.8; patellae I–IV = 1.5, 1.5, 1.4, 1.6, palp = 1.1; tibiae I–IV = 1.3, 1.1, 1.5, 1.4, palp = 1.3; metatarsi I–IV = 1.1, 1.0, 1.0, 0.9; tarsi I–IV = 1.0, 1.1, 1.0, 0.9, palp = 1.1. Abdomen 6.6 long, 3.8 wide. Spinnerets: PMS, 0.8 long, 0.4 wide, 0.1 apart; PLS, 1.1 basal, 0.6 middle, 0.6 domed distal; mid-widths (lateral), 0.6, 0.5, 0.3, respectively. Carapace: length to width 0.95. Fovea 1.4 wide. Eyes: tubercle 0.6 high, 1.4 long, 2.3 wide. Clypeus 0.1. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.5, ALE 0.5, PME 0.2, PLE 0.3, AME–AME 0.3, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.6, ALE–PME 0.5, PME–PME 1.4, PME–PLE 0.1, PLE–PLE 1.7, ALE–PLE 0.3, AME–PLE 0.5. Ratio of eye group width to length 2.3. Other characters as in female, except: maxillae: length to width: 1.9. Cuspules: 40 spread over ventral inner heel. Labium: 0.9 long, 1.5 wide, with 71 cuspules spaced by one diameter from each other on the anterior half. Chelicerae: basal segments with seven teeth spaced by at least one diameter from each other. Sternum: 3.4 long, 3.2 wide. Legs: leg formula: I IV II III. Scopula: tarsi I–IV fully scopulate, IV divided by six wide row of setae. Metatarsi I–II 4/5 scopulate; III 1/2 distal scopulate; IV 1/4 distal scopulate. IV divided by six wide row of setae. Urticating hairs type II (0.51 to 0.70 long, 0.01 wide) on the abdomen dorsum. Palp: embolus 2.2 long, with a 60° curvature retrolaterally. Embolus basal, middle and distal width of 0.3, 0.2 and 0.05, respectively. Tegulum 0.9 long. (Figs 8–10). Color pattern: (specimen is preserved and faded) carapace, chelicerae, palps, legs, sternum and labium are light brown. Abdomen black with a dorsally pale stripe similar in shape to the female.
Known only from Santa Teresa and Domingos Martins, in the mountain range of state of Espirito Santo, Brazil (Fig. 28).
urn:lsid:zoobank.org:act:1EAFD951-7AE2-46AA-9373-618A78B1A8FB
http://species-id.net/wiki/Typhochlaena_costae
Figs 12–15, 25, 28Males differ from those of other species by the short and slender embolus (Figs 12–14). Females differ by the non-spiraled spermathecae, lacking lobes, diverging on their base and converging on their distal portions (Fig. 15). Additionally, males and females have cephalothorax brown and abdomen dorsally black with two series of four red spots extending laterally. Carapace, dorsum of chelicerae and most anterior dorsal region of abdomen with very long, yellow, stiff setae (Fig. 25).
The specific name is a matronym in honour of Miriam Costa, who collected the holotype and several other new spider species during several years she worked at Instituto Butantan.
Holotype female, Brazil, state of Tocantins, Palmas, U.H.E. Luis Eduardo Magalhães [10°12'S, 48°24'W], 211 m.a.s.l, during faunal rescuing, M. Costa and D. Candido, 12 January 2002 (IBSP unnumbered); Paratype male, Brazil, state of Tocantins, Lajeado, (9°46'4.85"S, 48°21'6.69"W), 226 m.a.s.l, G. Puorto, R. Martins, I. Knysak (pitfall trap), April 2002 (IBSP unnumbered).
Brazil: Maranhão/Piaui border, 1 immature, C. E. V. Toledo, February 2010 (MZSP 36880).
Holotype female (IBSP unnumbered). Carapace 6.2 long, 6.0 wide, chelicerae 2.9. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 4.1, 2.9, 2.8, 2.4, 1.9, 14.1. II: 4.0, 2.8, 2.8, 2.2, 1.7, 13.5. III: 4.1, 2.6, 2.5, 2.2, 1.8, 13.2. IV: 4.9, 2.7, 3.3, 2.9, 1.8, 15.6. Palp: 3.4, 2.2, 1.8, –, 2.3, 9.7. Mid-widths (lateral): femora I –IV = 1.4, 1.5, 1.2, 1.2, palp = 1.0; patellae I–IV = 1.4, 1.4, 1.3, 1.2, palp = 1.1; tibiae I–IV = 1.3, 1.5, 1.2, 1.2, palp = 1.3; metatarsi I–IV = 1.2, 1.3, 0.9, 0.9; tarsi I–IV = 1.2, 1.2, 1.0, 0.9, palp = 1.2. Abdomen 8.3 long, 5.5 wide. Spinnerets: PMS, 0.9 long, 0.5 wide, 0.3 apart; PLS, 1.3 basal, 0.7 middle, 0.5 distal; mid-widths (lateral), 0.9, 0.6, 0.5, respectively. Carapace: length to width 1.03. Fovea 1.4 wide. Eyes: tubercle 0.3 high, 1.2 long, 1.7 wide. Clypeus 0.3. Anterior eye row procurved, posterior slightly recurved. Eyes size and inter-distances: AME 0.4, ALE 0.5, PME 0.2, PLE 0.4, AME–AME 0.3, AME–ALE 0.2, AME–PME 0.2, ALE–ALE 1.1, ALE–PME 0.4, PME–PME 1.0, PME–PLE 0.1, PLE–PLE 1.3, ALE–PLE 1.3, AME–PLE 0.2. Ratio of eye group width to length 2.0. Maxillae: length to width: 1.9. Cuspules: 69 spread over ventral inner heel. Labium: 0.8 long, 1.3 wide, with 80–90 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove shallow, flat, sigilla not evident. Chelicerae: basal segments with six teeth having similar size and well spaced from each other. Sternum: 2.7 long, 2.8 wide. Legs: leg formula: IV I II III. Scopula: tarsi I–III fully scopulate, IV divided by row of 6–7 setae. Metatarsi I–II fully scopulate; III 4/5, IV 1/3 distal scopulate. IV divided by six wide row of setae. Spines absent on all legs and palps. Urticating hairs type II (0.33 long, 0.01 wide) on the abdomen dorsum. Genitalia: paired spermathecae diverging basally and curving abruptly inwards medially (Fig. 15). Color pattern: carapace, chelicerae and dorsum of legs and palps dark brown with light brown hairs. Coxae, labium, maxilla and legs ventrally brown. Sternum darker. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi light brown. Distal femora, patellae, tibiae and metatarsi with white rings. Abdomen dorsally black with two series of four red spots extending laterally. Abdomen ventrally black with three transversal grayish stripes. Carapace covered with very long, yellow, stiff setae in cephalic region and in front of eye tubercle. Same type of yellow setae on dorsum of chelicerae, in internal half area and in most anterior dorsal region of abdomen. Rest of abdomen dorsum has some long, scattered, white setae (Fig. 25).
Paratype male (IBSP unnumbered). Carapace 6.5 long, 6.1 wide, chelicerae 2.8. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 7.0, 3.2, 5.3, 4.4, 2.7, 22.6. II: 6.4, 3.1, 4.8, 4.0, 2.3, 20.6. III: 5.7, 2.8, 4.2, 4.0, 2.4, 19.1. IV: 6.9, 2.9, 5.7, 5.8, 2.4, 23.7. Palp: 3.5, 2.0, 2.6, –, 1.1, 9.2. Mid-widths (lateral): femora I–IV = 1.2, 1.4, 1.3, 1.1, palp = 0.9; patellae I–IV = 1.2, 1.3, 1.2, 1.3, palp = 1.1; tibiae I–IV = 1.2, 1.0, 1.1, 0.9, palp = 1.1; metatarsi I–IV = 0.9, 0.9, 0.9, 0.8; tarsi I–IV = 1.0, 0.9, 1.0, 0.8, palp = 1.0. Abdomen 6.4 long, 4.0 wide. Spinnerets: PMS, 0.7 long, 0.4 wide, 0.1 apart; PLS, 0.8 basal, 0.5 middle, 0.6 distal; mid-widths (lateral), 0.8, 0.7, 0.5, respectively. Carapace: length to width 1.06. Fovea 0.9 wide. Eyes: tubercle 0.4 high, 1.2 long, 1.7 wide. Clypeus 0.1. Anterior eye row procurved, posterior straight. Eye sizes and inter-distances: AME 0.4, ALE 0.4, PME 0.2, PLE 0.3, AME–AME 0.3, AME–ALE 0.2, AME–PME 0.1, ALE–ALE 1.2, ALE–PME 0.2, PME–PME 1.0, PME–PLE 0.1, PLE–PLE 1.4, ALE–PLE 0.1, AME–PLE 0.3. Ratio of eye group width to length 2.5. Other characters as in female, except: maxillae: length to width: 1.4. Cuspules: 45 spread over ventral inner heel. Labium: 0.7 long, 1.2 wide, with 70–80 cuspules. Chelicerae: basal segments with seven teeth. Sternum: 3.0 long, 2.9 wide. Scopula: tarsi I–IV fully scopulate, IV with a few sparse setae. Metatarsi I–II 4/5 scopulate; III 1/2 distal scopulate; IV 1/3 distal scopulate. IV divided by three wide row of setae. Urticating hairs type II (0.42 to 0.68 long, 0.01 to 0.02 wide) on the abdomen dorsum. Palp: embolus 1.2 long, with a 45° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.15, 0.06 and 0.05, respectively. Tegulum 0.8 long. (Figs 12–14).
Brazil, states of Tocantins, Maranhão and Piaui (Fig. 28).
No available data. The male was collected by pitfall trap, the female was taking during a faunal rescue in a flooded area, and the immature in fossil tree samples coming from states of Maranhão and Piauí border.
http://species-id.net/wiki/Pachistopelma
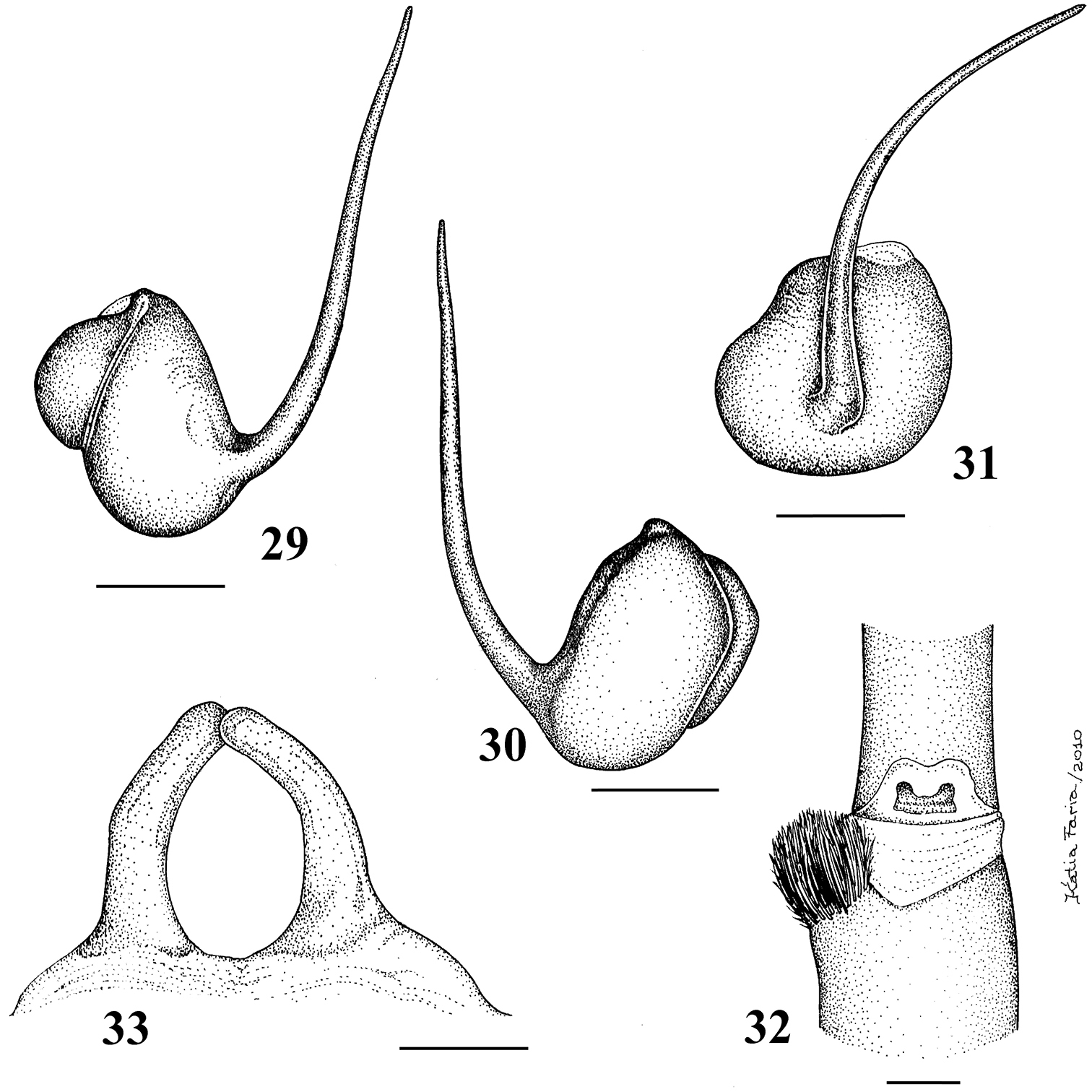
Figs 29–80Pachistopelma rufonigrum Pocock, 1901, by original designation.
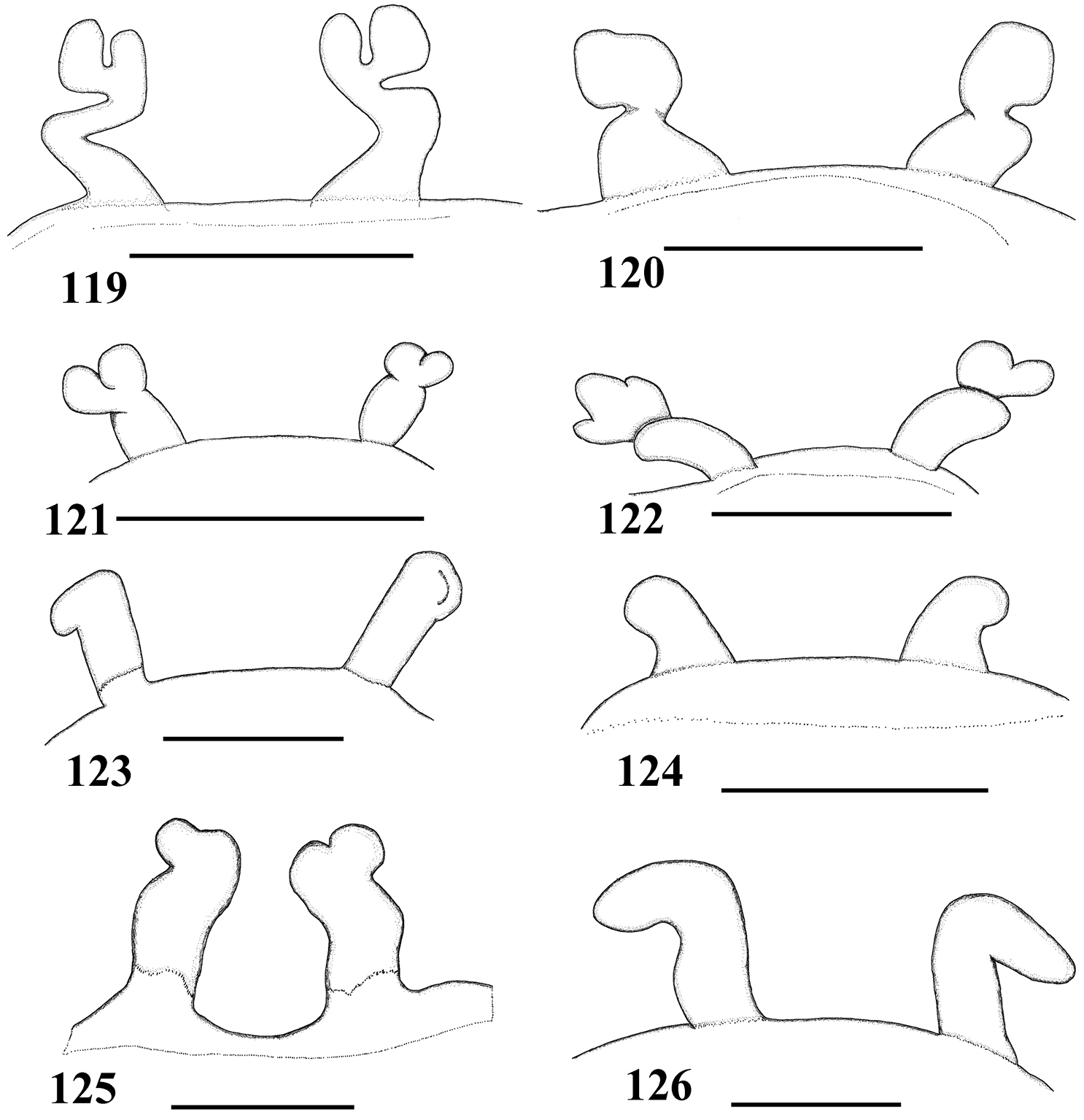
Male and female Pachistopelma differ from most other aviculariines, except Ephebopus, Tapinauchenius and Psalmopoeus by the straight to sligthly procurved first eye row (Fig. 37). Pachistopelma males differ from these genera by having a spinose spur on tibia I and females differ by having a dorso-ventrally flattened abdomen in combination with paired long spermathecae with a slight curvature medially and lacking constrictions or lobes. Additionally, males and females differ from these genera by both the absence of leg spines and the presence of urticating type II hair on the abdomen dorsum (except in mature females, that lack them).
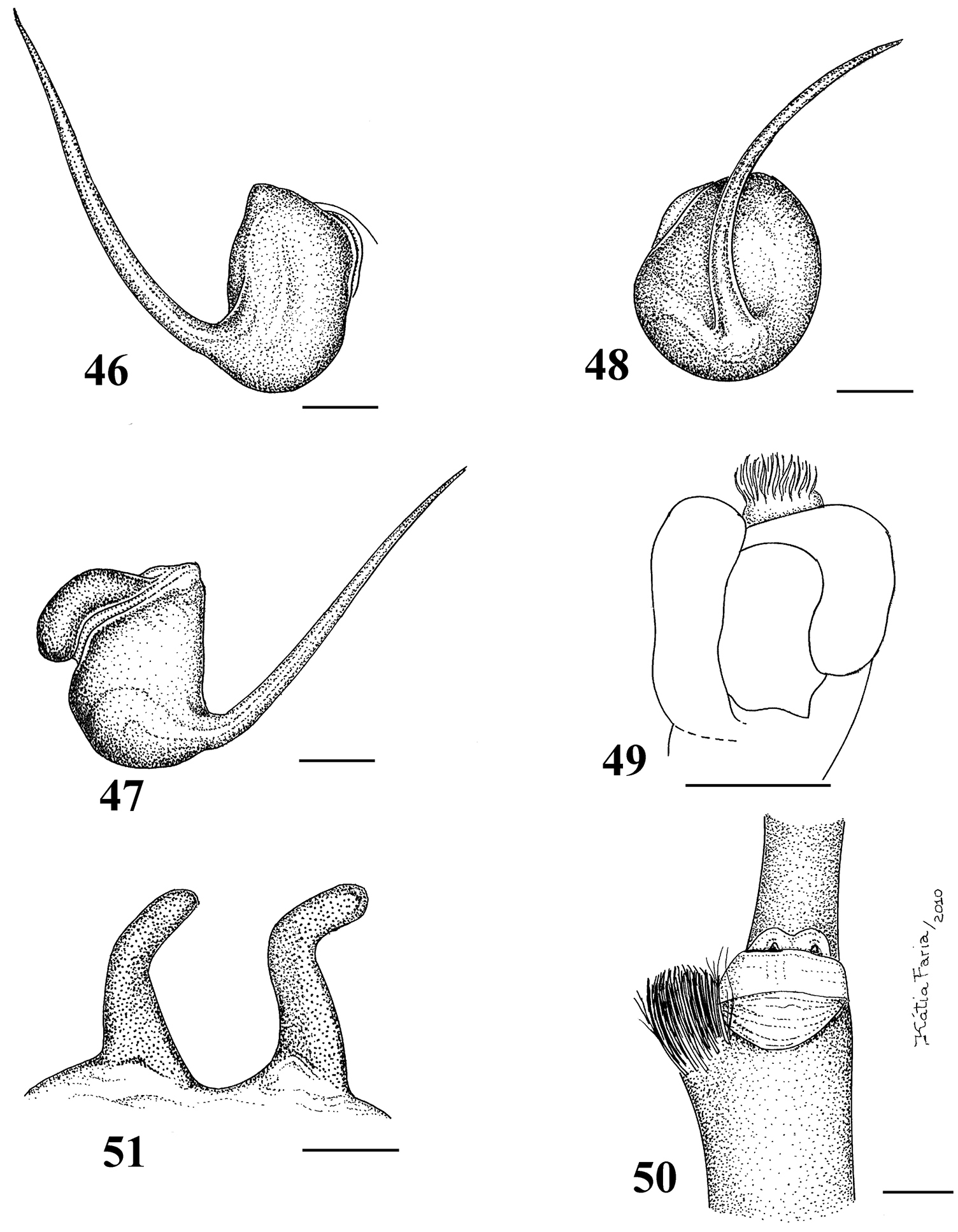
Carapace longer than wide; cephalic region low (mainly in female) (Fig. 34). Cephalic and thoracic striae hardly distinct. Fovea shallow, straight. Chelicerae without rastellum. Eye tubercle low (mainly in female), wider than long (Fig. 36). Clypeus absent (Fig. 37). Anterior eye row straight in female, slightly procurved in male (Fig. 37). Labium wider than long, with 80–150 cuspules concentrated on anterior half. Maxillary lyra absent. Maxilla subrectangular, anterior lobe distinctly produced into conical process, inner angle bearing numerous cuspules (130–200). Sternum longer than wide. Posterior angle not separating coxae IV. Posterior sigilla submarginal, less than one diameter from margin. Leg formula: IV I II III. Clavate trichobothria on distal 2/3 of tarsi I–IV. STC of males and femalewithout teeth. Tarsi I–IV fully scopulated. Scopulae of tarsi and metatarsi I–II extended very laterally giving them a spatulate appearance. Femur IV without retrolateral scopula. Legs lacking spines. Posterior median spinneret with distal article digitiform (Fig. 39). Abdomen dorso-ventrally flattened in female (Figs 34–35). Stridulatory setae absent. Male spur on tibia I, consists of a low elevation with numerous spiniform setae on its tip (Fig. 33). Male palpal bulb globose narrowing abruptly forming long slender embolus, 3 times longer than tegulum length, with a curvature of roughly 45° to retrolateral side, keels absent, tegulum without prolateral depression (Figs 29–31). Two long, uniform, weakly sclerotized spermathecae with slight curvature in their middle (Fig. 32). Cymbium with a short spiniform process (Fig. 49). Type II of urticating hair on abdomen dorsum of immatures and males, absent in mature females.
Pachistopelma rufonigrum Pocock, 1901 29–32 male (MNRJ 06247), left palpal bulb 29 prolateral 30 retrolateral 31 frontal 32 male tibial spur of left leg I 33 female (MNRJ 06246) spermathecae. Scale bar = 1mm.
Pachistopelma rufonigrum Pocock, 1901, female (MNRJ 06246) 34 carapace and abdomen, lateral 35 abdomen, dorsal 36–37 eye tubercle 36 lateral 37 dorsal 38 labium and sternum 39 spinnerets, ventral. Scale bar = 1mm.
Pachistopelma rufonigrum Pocock, 1901, habitus 40–43 immatures in progression 44 female 45 male, all from E.E. Murici, Murici, state of Alagoas. Photos: R. Bertani. Scale bar = 10 mm.
Pachistopelma bromelicola sp. n. 46–50 male, holotype (MNRJ 06241) left palpal bulb 46 retrolateral, 47 prolateral, 48 frontal 49 male cymbium showing protuberance 50 male tibial spur of left leg I 51 female paratype (MNRJ 06242) spermathecae. Scale bar = 1mm.
Pachistopelma rufonigrum Pocock, 1901 (Figs 29–45, 58–61, 67–68, 76–77), Pachistopelma bromelicola sp. n. (Figs 46–57, 62–68, 75, 78–80).
Pachistopelma bromelicola sp. n., habitus 52–55 immatures in progression 56 female 57 male, all from RPPN Sapiranga, Mata de São João, state of Bahia, except male, from Jeremoabo, state of Bahia. Photos: R. Bertani. Scale bar = 10 mm.
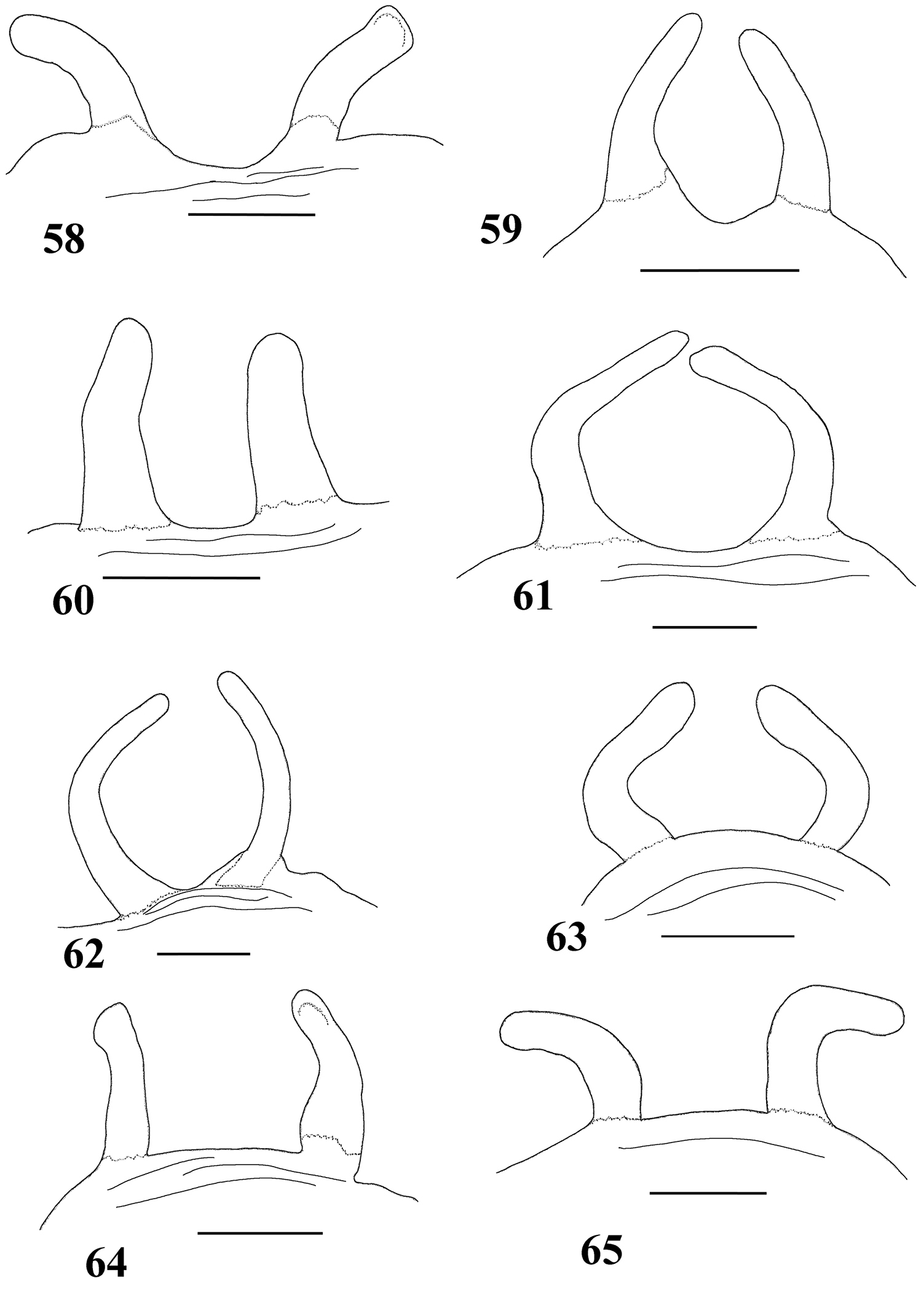
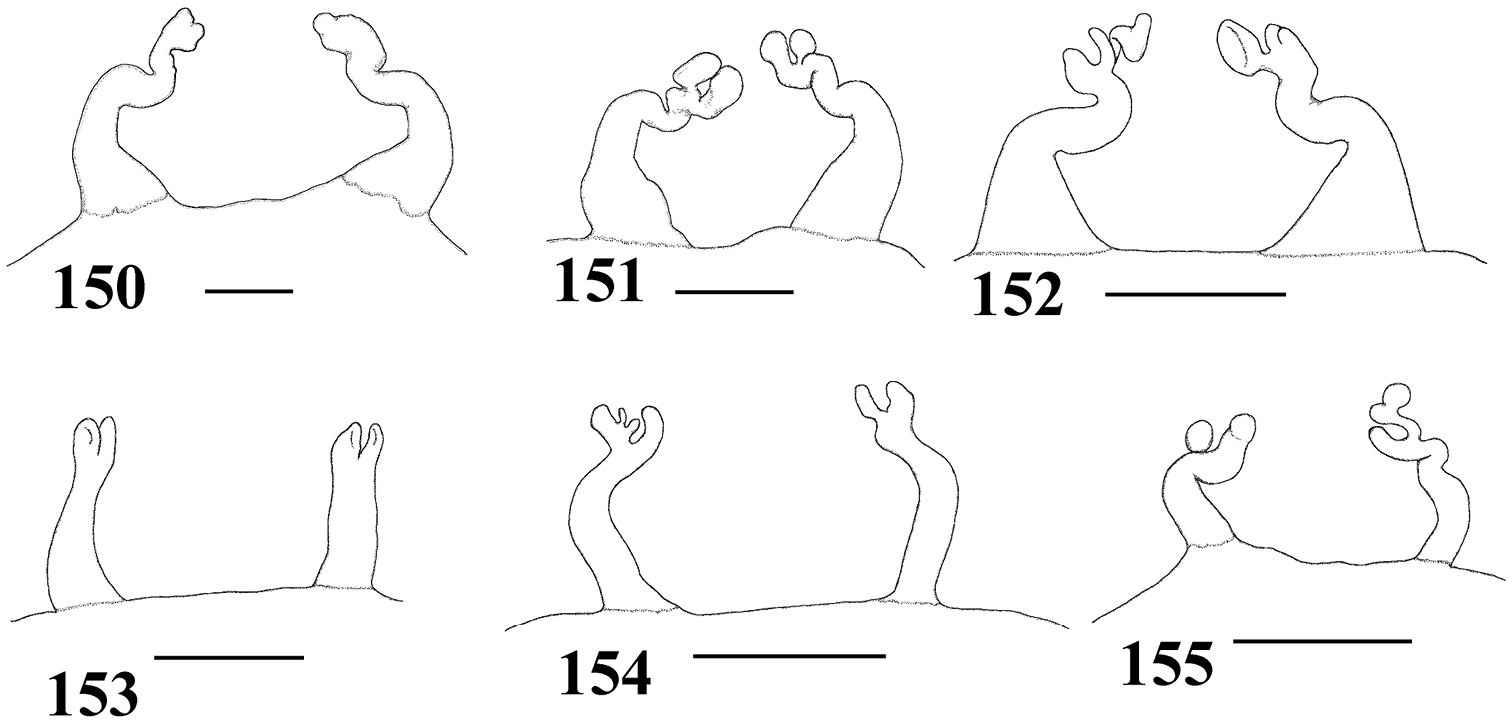
Variation in spermathecae. 58–60 Pachistopelma rufonigrum Pocock, 1901 58 Alhandra, state of Paraiba (MZSP 10839) 59 Mamanguape, state of Paraíba (IBSP 9756) 60 Igarassu, state of Pernambuco (MZSP 10862) 61–65 Pachistopelma bromelicola sp. n. 61 Nossa Senhora da Glória, state of Sergipe (IBSP ref 28482) 62–64 Santo Amaro das Brotas, state of Sergipe (MZSP 10847, 10847, 10846) 65 Maracás, state of Bahia (IBSP 7889). Scale bar = 1mm.
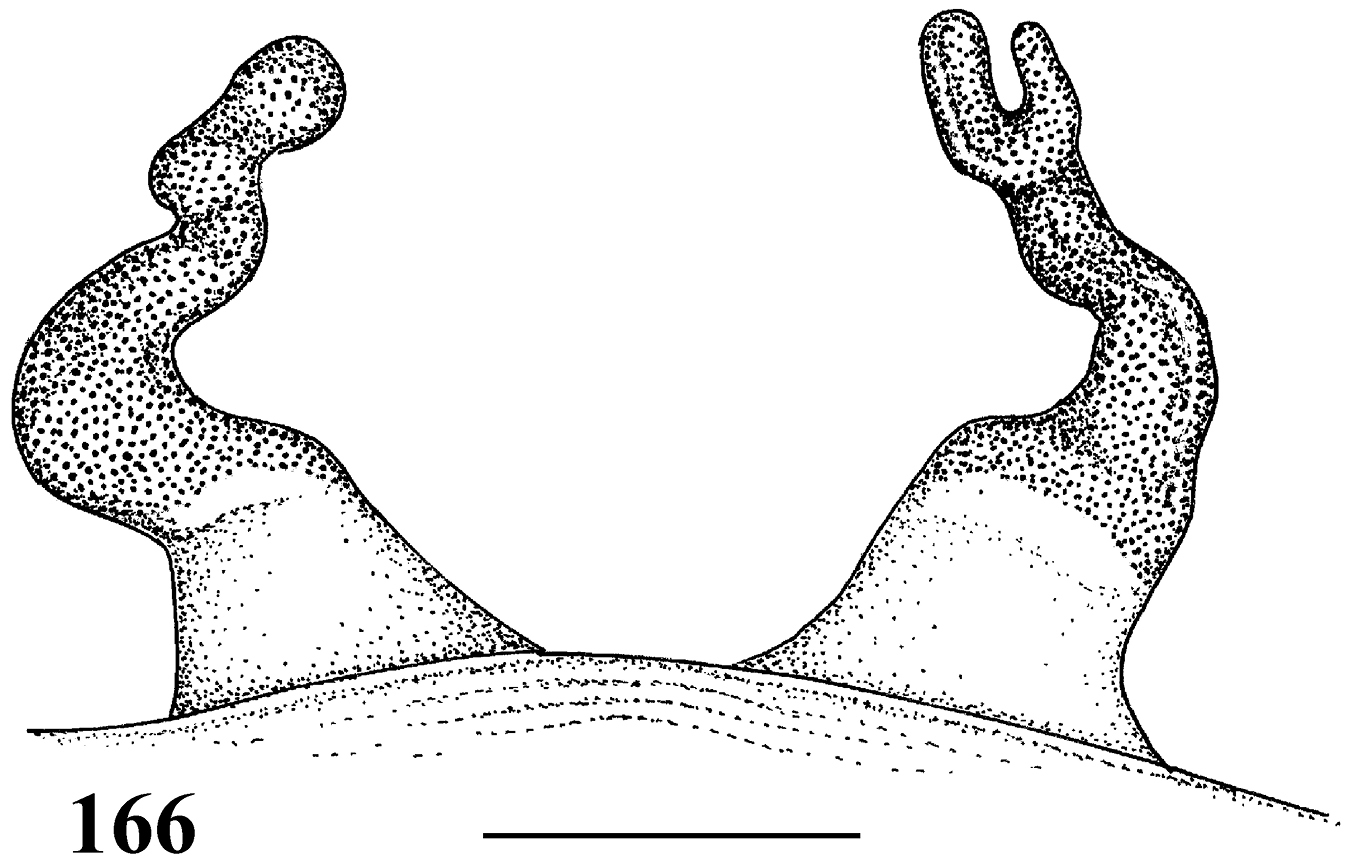
Female leg IV retrolateral view 66 Pachistopelma bromelicola sp. n. 67 Pachistopelma rufonigrum Pocock, 1901.
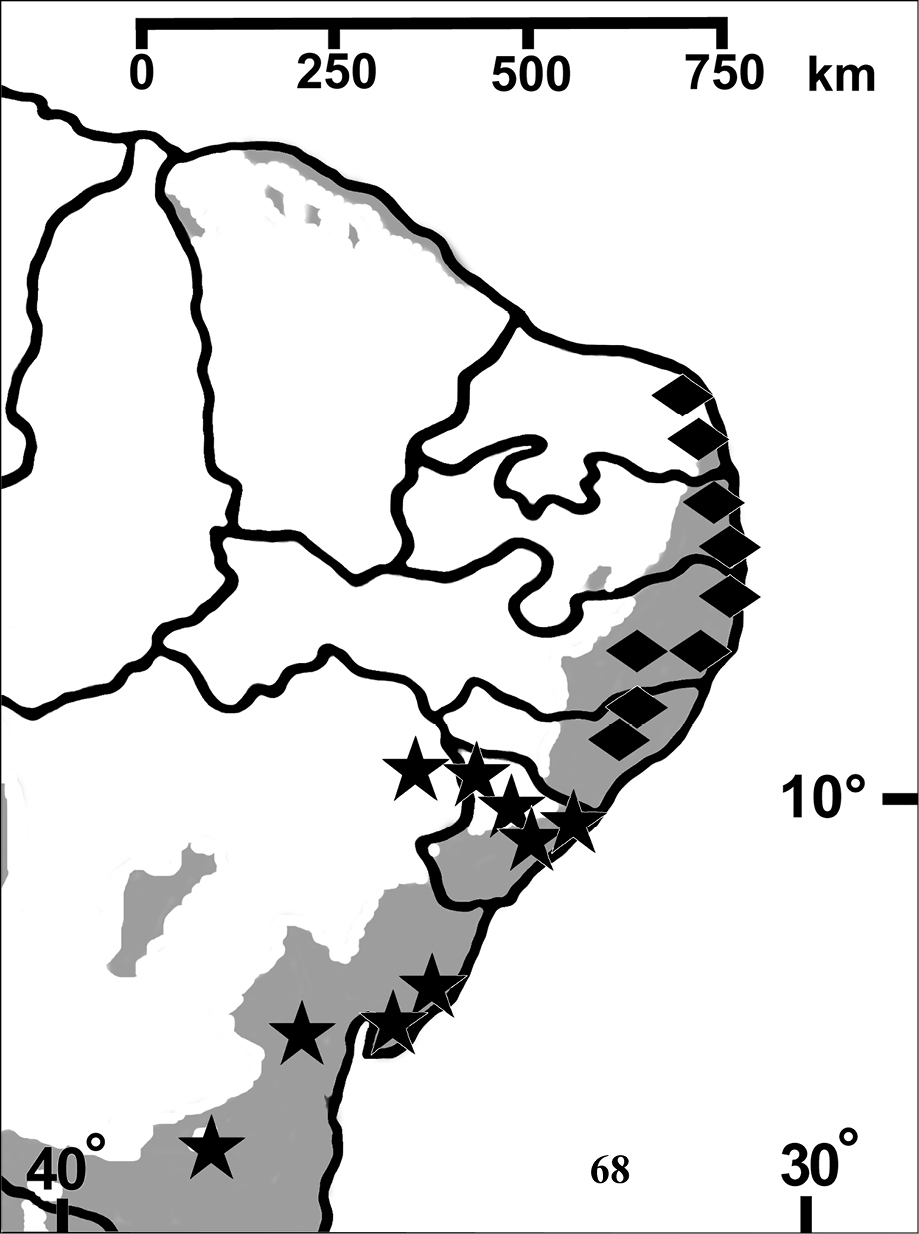
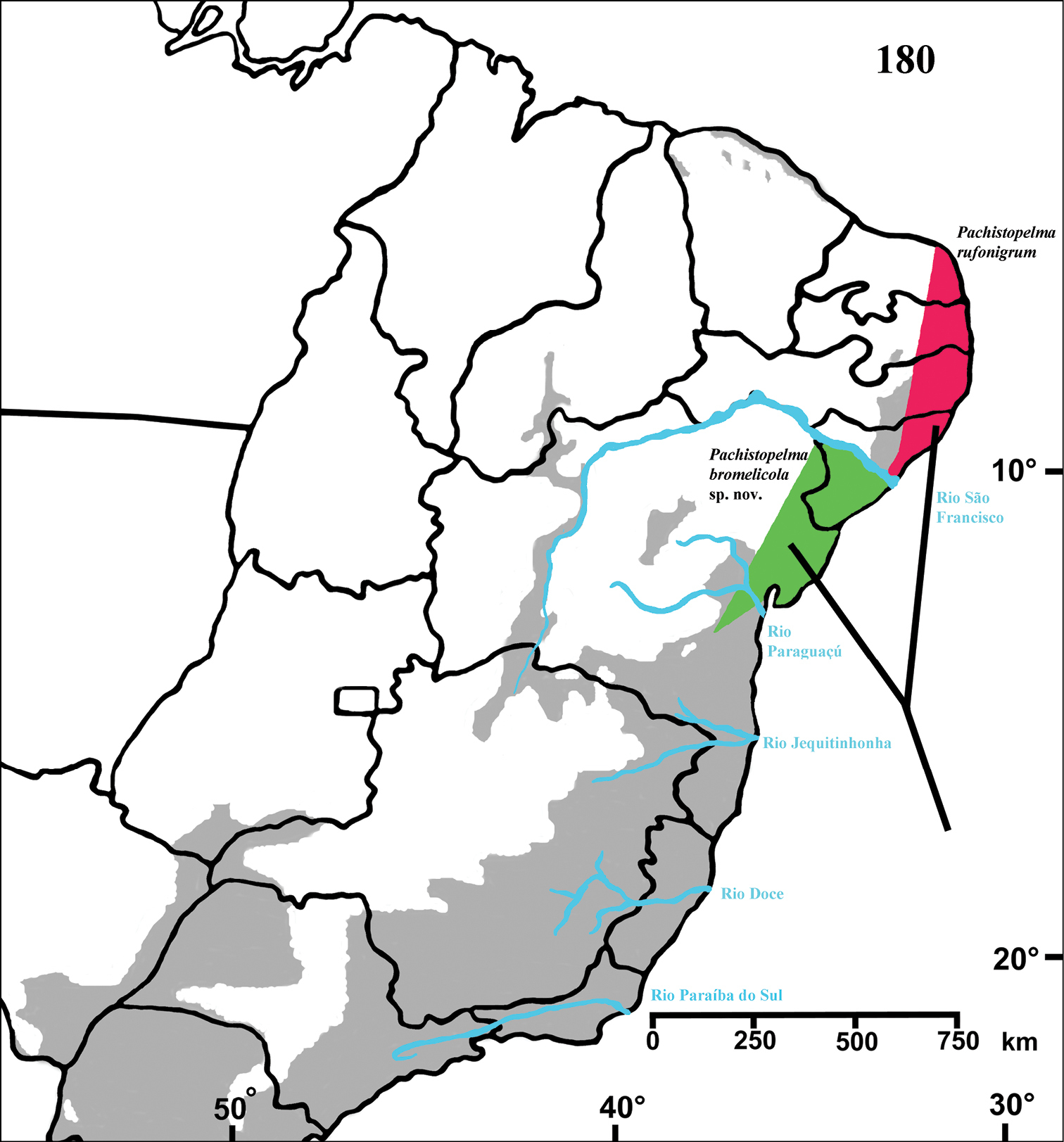
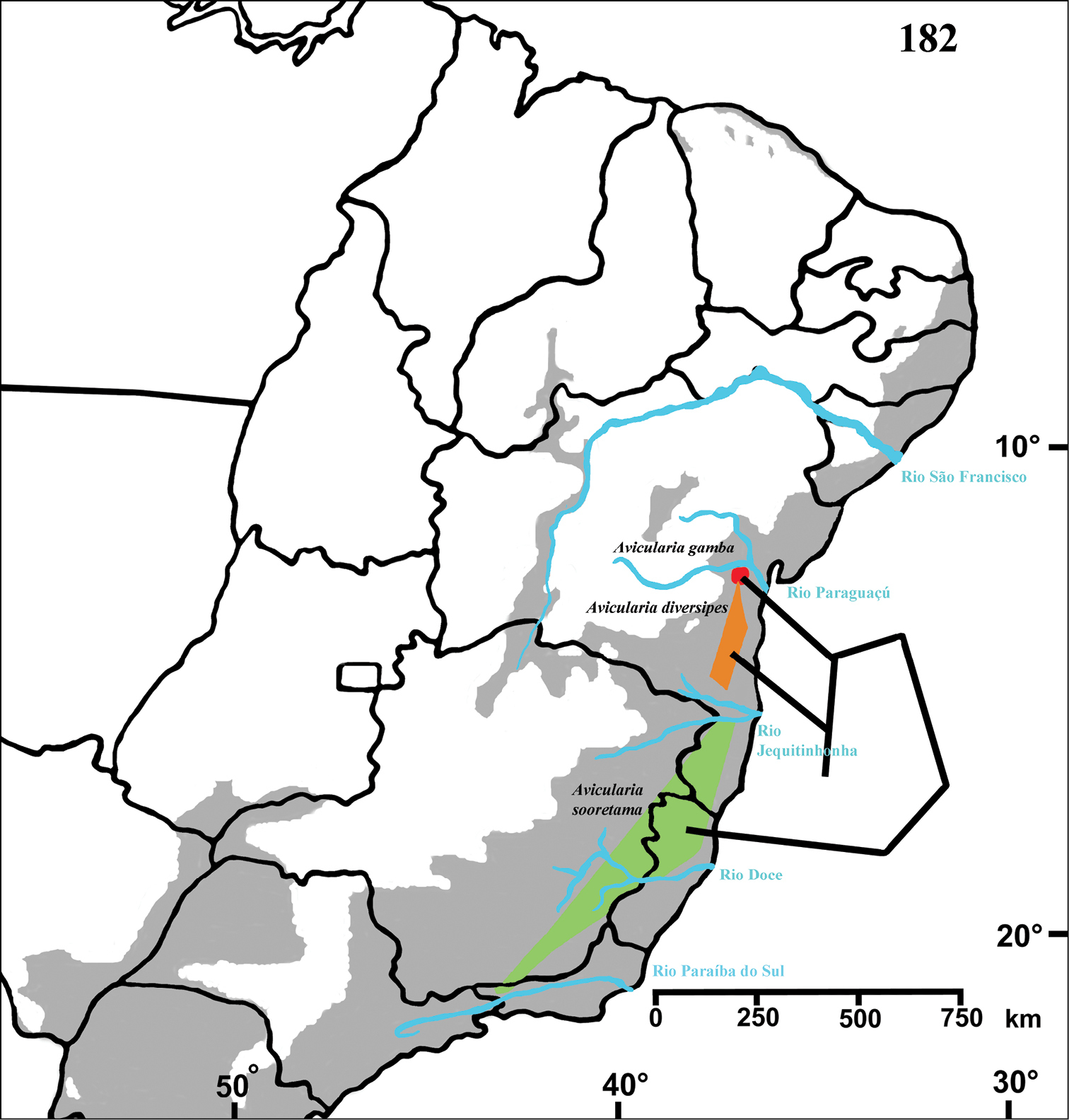
Northeastern Brazil, from the state of Rio Grande do Norte [6°22'S] to state of Bahia [13°25'S], mostly in the coastal region (Fig. 68). Both species of Pachistopelma inhabit tank bromeliads exclusively, e. g. Aechmea aquilega (
Figure 68. Map showing records of Pachistopelma species on Northeastern Brazil. Losangle = Pachistopelma rufonigrum, star = Pachistopelma bromelicola sp. n. The gray area represents the approximate original distribution of Brazilian Atlantic rainforest. White area represents open environments (cerrado and caatinga).
Pachistopelma spp. habitats 69–71 E. E. Murici, Murici, state of Alagoas 69 general view of a hill covered with Brazilian Atlantic rainforest and showing rocky formation on its tip, lower, yellowish area is a secondary grassland 70 same, viewed from its tip and showing bromeliads on the lower right of the photo 71 details of the rocky area with bromeliads intermixed with Brazilian Atlantic rainforest 72–74 Itabaiana National Park, Areia Branca, state of Sergipe, sandy areas resembling “restinga” vegetation with bromeliad “islands”. Photos: R. Bertani.
Pachistopelma spp. microhabitats 75, 79–80 Pachistopelma bromelicola sp. n. 75 Itabaiana National Park, Areia Branca, state of Sergipe 79–80 RPPN Sapiranga, São João da Mata, state of Bahia 79 closed retreat has a female with eggsac 80 same retreat opened 76–78 Pachistopelma rufonigrum Pocock, 1901, E. E. Murici, Murici, state of Alagoas. Photos: R. Bertani.
Pachistopelma juveniles possess a metallic green general pattern, and the dorsum of abdomen having a black longitudinal central stripe and five lateral black stripes that may connect with the central stripe. Adults are brownish to pinkish or blackish, without abdominal pattern (Figs 40–45, 52–57).
Pachistopelma concolor Caporiacco, 1947, lectotype (herein designated), immature male, Guyana, Campo di Marlissa, 31 December (MZUF 507); paralectotype, immature, Guyana, Campo I Demerara (MZUF 505), deposited in Museo Zoologico “La Specola”, Firenze, examined. Specimens have spines on apex of tibiae and metatarsi, lack urticating hair both on abdomen dorsum as well as on prolateral distal femur of palp, first ocular eye row is almost straight, and maxillary lyra is absent. This combination of characters is diagnostic for Tapinauchenius, and, therefore, Pachistopelma concolor Caporiacco, 1947 is transferred to Tapinauchenius concolor (Caporiacco, 1947) comb. n.
| 1 | Metatarsus IV incrassate, with stiff setae (Fig. 66); general coloration dark (Fig. 56), male with longer setae on legs whitish and abdomen dorsum reddish (Fig. 57) | Pachistopelma bromelicola sp. n. |
| – | Metatarsus IV not incrassate, with long and flexible reddish setae (Fig. 67); general coloration dark brown with pinkish setae (Figs 44–45) | Pachistopelma rufonigrum |
http://species-id.net/wiki/Pachistopelma_rufonigrum
Figs 29–45, 58–60, 67–68, 76–77Males and females differ from Pachistopelma bromelicola sp. n. by the slender metatarsi IV without stiff bristles (Fig. 67) and legs pinkish with black tarsi (Figs 43–45).
Lectotype (herein designated) a dissected adult female and 8 paralectotypes (herein designated), comprising 4 females, 2 males and 2 immatures fom Iguarassu, Brazil, G. A. Ramage, BMNH 1888.47, examined.
BRAZIL: Rio Grande do Norte: Baia Formosa [6°22'S, 35°00'W], inside bromeliads, 1 female, 8 immatures, A. L. Castro, 29 January 1964 (MNRJ 12922); Natal, road to Zumbi [5°42'S, 35°16'W], 1 female, 3 immatures, M. Aranha, M. Uvana, M. C. B. Pereira, H. R. Silva, 1 February 1989 (MNRJ 13750); Paraíba: Alhandra [7°26'S, 34°54'W], inside bromeliads, 1 female, 44 immatures, Exp. ABC MZUSP, 8 May 1971 (MZSP 10839); same data, 1 female, 2 immatures (MZSP 28579); Igarassú [7°50'S, 34°54'W], 2 females, 4 immatures, Vanzolini, 7 June 1963 (MZSP 10862); Mamanguape, Reserva Biológica de Mamanguape [6°47'S, 34°59'W], inside bromeliads, 1 male, 2 females, Cascone and Alonso, 31 October 1982 (IBSP 9756); same data, 4 immatures (IBSP 14276); Pernambuco: Agrestina, Serra da Quitéria [8°27'S, 35°56'W], inside bromeliads, 1 female, 2 immatures, Exp. ABC MZUSP, 15 May 1971 (MZSP 28580); Rio Formoso, Estação Ecológica de Saltinho [8°43'S, 35°10'W], 1 immature, 12 May 1969 (IBSP 3986B); Alagoas: Murici, Estação Ecológica de Murici, Triunfo (9°14'9.52"S, 35°48'0.25"W), 245 m.a.s.l., inside bromeliads, 3 immatures, R. Bertani, R. H. Nagahama, D. R. M Ortega, 11 August 2006 (MNRJ 06244); same data, 5 immatures, 2 males, 1 female, 12 August 2006 (MNRJ 06245); same data, 6 immatures, 1 male, 5 females, 13 August 2006 (MNRJ 06246); Jitituba (9°14'9.52"S, 35°48'0.25"W), 295 m.a.s.l., inside bromeliads, 2 males, 3 females, R. Bertani, R. H. Nagahama, D. R. M Ortega, 14 August 2006 (MNRJ 06247); Fazenda Santa Fé (9°26'1.83"S, 35°50'3.93"W), 362 m.a.s.l., inside bromeliads, 1 male, 2 females, R. Bertani, R. H. Nagahama, D. R. M. Ortega, 15 August 2006 (MNRJ 06248); Passo do Camaragibe, Fazenda Santa Justina [9°14'S, 35°29'W], 2 females, 1 immature, H. R. Silva and C. A. Caetano, 13–18 January 1988 (MNRJ 1781).
Female (MNRJ 06246, Al 1110) from Brazil, state of Alagoas, Murici, Estação Ecológica de Murici. Carapace 15.1 long, 13.3 wide, chelicerae 7.3. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 9.7, 6.6, 7.3, 6.8, 4.1, 34.5. II: 9.2, 6.3, 6.8, 6.5, 3.9, 32.7. III: 8.8, 5.5, 6.3, 6.8, 4.0, 31.4. IV: 11.0, 6.1, 9.1, 9.7, 4.3, 40.2. Palp: 7.3, 4.7, 4.8, –, 5.1, 21.9. Mid-widths (lateral): femora I–IV=2.8, 2.8, 3.0, 3.1, palp=2.4; patellae I–IV=2.7, 2.8, 2.8, 2.9, palp=2.4; tibiae I–IV=2.4, 2.5, 2.4, 2.8, palp=2.3; metatarsi I–IV=1.9, 1.9, 1.8, 1.7; tarsi I–IV=1.8, 1.8, 1.7, 1.9, palp=2.0. Abdomen 14.5 long, 12.0 wide. Spinnerets: PMS, 1.8 long, 0.7 wide, 0.3 apart; PLS, 2.7 basal, 2.1 middle, 2.6 distal; mid-widths (lateral), 1.6, 1.3, 0.8, respectively. Carapace: length to width 1.13; Fovea 1.6 wide. Eyes: tubercle 0.3 high, 1.7 long, 2.6 wide. Anterior eye row straight, posterior slightly recurved. Eye sizes and inter-distances: AME 0.6, ALE 0.6, PME 0.3, PLE 0.5, AME–AME 0.4, AME–ALE 0.3, AME–PME 0.3, ALE–ALE 1.7, ALE–PME 0.3, PME–PME 1.3, PME–PLE 0.2, PLE–PLE 1.8, ALE–PLE 0.3, AME–PLE 0.5. Ratio of eye group width to length 2.2. Maxillae: length to width: 1.7. Cuspules: 150–200 spread over ventral inner heel. Labium: 1.8 long, 2.7 wide, with 100–120 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove shallow, flat, sigilla not evident. Chelicerae: basal segments with ten teeth decreasing in size from distal to basal portion. Sternum: 7.4 long, 6.2 wide. Sigilla: three pairs, ellipsoid, less than one diameter from margin. Scopula: tarsi I–IV fully scopulate, IV divided by five wide row of setae. Metatarsi I 3/4 scopulate; II 2/3 scopulate; III 1/2, IV 1/3 distal scopulate. IV divided by three wide row of setae. Urticating hair absent. Genitalia: paired long, uniform, weakly sclerotized spermathecae with a slight curvature medially (Fig. 32). Color pattern: carapace and chelicerae brown, covered with golden hairs intermixed with metallic pinkish hairs. Legs and palps dark brown, covered with dark hairs having metallic green/blue iridescence. Coxae, labium, maxilla and sternum black. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi narrow, whitish. Distal femora, patellae, tibiae and metatarsi rings absent. Abdomen dorsum black with long reddish hairs mainly on lateral and posterior regions. Abdomen ventrally black (Fig. 44).
Male (MNRJ 06245, Al 1100) fromBrazil, state of Alagoas, Murici, Estação Ecológica de Murici. Carapace 12.7 long, 11.4 wide, chelicerae 5.6. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 9.8, 5.5, 7.5, 7.8, 4.8, 35.4. II: 9.3, 5.6, 7.2, 7.2, 4.0, 33.3. III: 8.6, 4.6, 6.5, 7.3, 3.9, 30.9. IV: 10.4, 5.5, 9.1, 10.4, 3.9, 39.3. Palp: 6.8, 4.1, 5.3, –, 1.8, 18.0. Mid-widths (lateral): femora I–IV=2.2, 2.3, 2.6, 2.8, palp=1.8; patellae I–IV=2.3, 2.2, 2.3, 2.3, palp=1.7; tibiae I–IV=1.8, 1.9, 1.9, 2.1, palp=1.6; metatarsi I–IV=1.1, 1.1, 1.3, 1.1; tarsi I–IV=1.2, 1., 1.2, 1.3, palp=1.5. Abdomen 13.9 long, 9.2 wide. Spinnerets: PMS, 1.2 long, 0.5 wide, 0.4 apart; PLS, 2.1 basal, 1.4 middle, 2.0 distal; mid-widths (lateral), 1.0, 0.9, 0.6, respectively. Carapace: length to width 1.11; Fovea: 0.9 wide. Eyes: tubercle 0.4 high, 1.7 long, 2.3 wide. Anterior eye row slightly procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.4, ALE 0.6, PME 0.3, PLE 0.4, AME–AME 0.4, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.6, ALE–PME 0.5, PME–PME 1.1, PME–PLE 0.1, PLE–PLE 1.5, ALE–PLE 0.3, AME–PLE 0.5. Ratio of eye group width to length 1.9. Other characters as in female, except: labium: 1.5 long, 2.0 wide, with ca. 80 cuspules spaced by one diameter from each other on the anterior third center. Chelicerae: basal segments with nine teeth decreasing in size from distal to basal portion. Sternum: 6.0 long, 4.9 wide. Sigilla: three pairs, small, rounded, first and last pairs 1.5 diameter from margin, second less than half diameter from margin. Scopula: metatarsi I–II 4/5 scopulate; III 1/3 distal scopulate; IV 1/4 distal scopulate. IV divided by five wide row of setae. Tibial spur 0.7 high, 1.3 wide; with numerous spiniform setae on the tip (Fig. 33). Metatarsus I straight. Urticating hairs type II (0.51 to 0.58 long, 0.012 wide) on the abdomen dorsum. Palp: embolus 3.4 long, with a 45° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.3, 0.2 and 0.04, respectively. Tegulum 1.0 long, 1.7 wide (Figs 29–31). Cymbium: two subequal lobes, the prolateral one triangular in shape. Spiniform process 0.5 long, 0.6 wide on the apex. Color pattern: carapace, chelicerae, legs and palps dark brown, covered with dense layer of pinkish hairs and longer hairs of the same color. Tarsi black, metatarsi dorsum with black stripe. Coxae and sternum dark brown with pinkish hairs. Labium and maxillae dark brown. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi discrete, light brown. Abdomen dorsum orange with long reddish hairs. Abdomen ventrally orange with a greyish area on its center (Fig. 45).
Females and immatures have a very low cephalic region, when compared to the males, and the abdomen is dorso-ventrally flattened in the former (Fig. 34). The eye tubercle is very low in females (Fig. 36) and immatures, and the first ocular row is straight (Fig. 37). Males possess a more developed eye tubercle and the anterior ocular row is slightly procurved. Immatures and adult males have type II urticating hairs on abdomen dorsum, which becomes lost in adult females.
A typical Pachistopelma rufonigrum spermatheca is weakly sclerotized, long, tapering distally, without constrictions or lobes, and slightly curved inwards (Figs 32, 59). Nevertheless, shorter (Figs 58, 60) spermathecae are not rare, as well as broader ones (Fig. 60). Most are sligthly curved inwards (Figs 32, 59) whereas a fraction are curved outwards (Fig. 58).
Brazil, from state of Rio Grande do Norte southwards to state of Alagoas, mainly in coastal region (Fig. 68).
All specimens with recorded field data indicate Pachistopelma rufonigrum individuals were found inside bromeliads (
Juveniles are almost completely metallic green, except for a pattern on dorsum of abdomen comprising a central longitudinal black stripe connected with five lateral black stripes (Fig. 40). In larger individuals carapace border and dorsum of chelicerae, coxae and trochantera are light brown. Dorsum of abdomen is light brown with a reddish area posteriorly. The black stripes remain (Fig. 41). In a next stage carapace is completely pink, as well as dorsum of coxae, trochantera and most femora. Remaining parts of legs retain the metalic-green. The clear part of abdomen is now of a vivid red, and the black stripes remain (Fig. 42). Subadults have carapace and legs brown with sparse pinkish long hairs. Dorsum of abdomen is still reddish, but the black stripes begin to fade (Fig. 43). Adult female is completely brown with long pinkish setae on legs, carapace and chelicerae. Abdominal pattern is lacking or very inconspicuous (Fig. 44). Adult male carapace, chelicerae and legs are brown and covered by pinkish setae, except for the tarsi and a stripe on metatarsi that are black. Abdomen is a vivid orange/red, there is no vestige of any pattern (Fig. 45).
Avicularia pulchra Mello-Leitão, 1933, holotype from Brazil, Pernambuco, D. Bento Pickel (MNRJ 29180), examined, is an immature specimen with a low eye tubercle, almost straight anterior row of eyes, abdomen flattened dorso-ventrally, and typical abdominal pattern, all characteristics of Pachistopelma. The color pattern and geographical distribution are consistent with Pachistopelma rufonigrum. Therefore, Avicularia pulchra Mello-Leitão, 1933 is transferred to Pachistopelma and considered a junior-synonym of Pachistopelma rufonigrum Pocock, 1901, syn. n.
Avicularia recifiensis Struchen & Brändle, 1996, holotype female (SMF 39873) and paratype male (SMF 39872), from Brazil, Recife, Martin Weber, 1993 according to original description. Specimen labels indicate holotype male and paratype female contrary to paper. The holotype female, examined, has a very low eye tubercle, straight anterior eye row, dorso-ventrally flattened abdomen, and spermathecae, though dissected, was not in the vial. Paratype male has a poorly developed tibial spur typical of the species (contrary to description that states it lacks a tibial spur), a spiniform process between cymbium lobes, and a sligthly procurved anterior eye row. All characters of both male and female agree with those of Pachistopelma, and color pattern and geographical distribution agree with Pachistopelma rufonigrum. Therefore, Avicularia recifiensis Struchen & Brändle, 1996 is transferred to Pachistopelma and considered a junior-synonym of Pachistopelma rufonigrum Pocock, 1901, syn. n.
urn:lsid:zoobank.org:act:0D248413-EA0C-4187-8E15-C71B93A27D9E
http://species-id.net/wiki/Pachistopelma_bromelicola
Figs 46–57, 61–68, 75, 78–80Males and females differ from those of Pachistopelma rufonigrum by the incrassate metatarsus IV with stiff bristles (Fig. 66) and blackish color of the legs (Figs 54–57).
The specific name refers to the lifestyle habits of this species, bromeliad endemism.
Holotype male (MNRJ 06241), Brazil, State of Bahia, Elísio Medrado, RPPN Jequitibá (12°52'3.20"S, 39°28'9.09"W), R. Bertani, C.S. Fukushima and R.H. Nagahama, 07 October 2007, collected at night, found immature inside bromeliads, matured in captivity in May 2010; Paratype female (MNRJ 06242), same data.
Additional material examined. BRAZIL: Sergipe: Areia Branca, Parque Nacional Serra de Itabaiana [10°44'S, 37°22'W], inside bromeliads, 4 males, R. Bertani, A. D. Brescovit, A. B. Bonaldo, September 1999 (IBSP 10010, 9789, 9832, 8716); 5 females, same data (IBSP 10216, 10463, 10399, 10035, 10748); 3 immatures, same data (IBSP 8644, 8599, 8525); 1 female, 1 immature, A. C. M. Fernandes, 12 November 1996 (IBSP 11762); Barra dos Coqueiros [10°54'S, 37°01'W] 1 immature, without collector, 20 February 1994 (IBSP 8087); 2 males, without data (IBSP Ref. 79901); Brejo Grande [10°25'S, 36°28'W], 1 female, no collector data, 26 October 1998 (IBSP 8085); Nossa Senhora da Gloria [10°13'S, 37°25'W], 1 female, S. Lucas, 1980 (IBSP 7892); 1 male, 1 female, same data (IBSP 7893); 1 female, same data (IBSP Ref. 28482); Pirambú [10°40'S, 36°52'W], 1 immature, without collector, 19 February 1998 (IBSP 8088); Poço Redondo [9°47'S, 37°41'W], 1 female, S. Lucas, August 1980 (IBSP 4712); Santo Amaro das Brotas [10°46'S, 37°03'W], inside bromeliad, 1 female, A. V. Alcântara, 09 September 1978 (MZSP 10846); 1 female, 2 immatures, no collector data, 7 October 1978 (MZSP 10842); 1 female, 2 immatures, no collector data, 2 June 1979 (MZSP 10843); 1 female, no collector data, 11 November 1978 (MZSP 10841); 14 females, 6 immatures, no collector data, 23 March 1978 (MZSP 10847); 1 female, no collector data, 02 July 1979 (MZSP 10845). Bahia: Acajutiba [11°39'S, 38°01'W], 2 females, 10 immatures, E. Boaventura, 18 April 1991 (MZSP 32179, col. Bock. 699–710); Camaçari, Praia do Jacuípe [12°42'S, 38°07'W], 1 male, L. Stabile, 7 September 2006 (IBSP 12991); Elísio Medrado, RPPN Jequitibá (12°52'3.20"S, 39°28'9.09"W), inside bromeliads, 2 males, 3 females, R. Bertani, R. H. Nagahama, C. S. Fukushima, 7 October 2007 (MZSP 36881); 1 female, 4 immatures, inside bromeliads, M. A. Freitas, April 2010 (MZSP 36882); Jeremoabo, [10°03'S, 38°20'W], 1 male, A. J. Silva, November 1989 (MZSP 32177, col. Bock. 770); 1 male, A. J. Silva (MZSP 32175, col. Bock. 787); 1 male, A. J. Silva, 15 July 1989 (MZSP 32172, col. Bock. 786); 1 male, A. J. Silva, 28 October 1989 (MZSP 32178, col. Bock. 792); 1 male, 2 females, A. J. Silva, December 1988 (MZSP 32169, col. Bock. 837–839); 3 females, A. J. Silva, 28 October 1999 (MZSP 32166, col. Bock. 827–829); 1 male, 7 females, 2 immatures, A. J. Silva, December 1988 (MZSP 32163, col. Bock. 840–849); 2 males, 6 females, 1 immature, A. J. Silva, 16 January 1989 (MZSP 32170, col. Bock. 861–869); 2 males, 14 females, 1 immature, A. J. Silva, 29 June 1989 (MZSP 32171, col. Bock. 711–713, 716, 748–755, 768–769, 772–774); 2 males, 8 females, 1 immature, A. J. Silva, December 1988 (MZSP 32167, col. Bock. 850–859); 6 females, A. J. Silva, 16 January 1989 (MZSP 32165, col. Bock. 880–884); 2 males, 6 females, A. J. Silva, 16 January 1989 (MZSP 32168, col. Bock. 870, 872–875, 877–879); 5 females, A. J. Silva, 28 October 1989 (MZSP 32164, col. Bock. 830, 832, 834–836); Maracás [13°25'S, 40°26'W], 1 female, Werner, November 1965 (IBSP 7889); Mata de São João, RPPN Sapiranga (12°34'0.58"S, 38°02'3.38"W), inside bromeliads, 2 females, 1 immature, R. Bertani, R. H. Nagahama, C. S. Fukushima, 1 October 2007 (MNRJ 06243); Minuim [9°50'S, 38°05'W], 1 male, without colector, 06 June 1987 (MZSP 32176, col. Bock. 714); Salvador, Itapoã [12°57'S, 38°21'W] 1 male, 1 female, A. Travassos, 1951 (IBSP 2369); inside bromeliads, 2 immatures, Vanzolini and Rebouças, December 1962 (MZSP 10840); 1 immature, same data and colectors (MZSP 10863); 1 male, 1 female, 1 immature, Vanzolini, 3 June 1963 (MZSP 4992); Ondina [13°00'S, 38°30'W], 1 female, T. B. Nunes, December 1982 (IBSP 7905); Santa Brigida [9°43'S, 38°07'W], 1 female, J. P. Carvalho, 23 October 1987 (MZSP 32174, col. Bock. 789).
Holotype male (MNRJ 06241). Carapace 11.6 long, 11.4 wide, chelicerae 5.3. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 9.8, 6.0, 7.6, 8.0, 4.6, 36.0. II: 9.5, 5.4, 7.1, 7.7, 4.1, 33.8. III: 8.5, 4.7, 7.2, 7.6, 4.1, 32.1. IV: 11.0, 5.4, 9.5, 10.7, 4.2, 40.8. Palp: 7.3, 4.2, 5.6, –, 2.3, 19.4. Mid-widths (lateral): femora I–IV = 2.4, 2.4, 2.6, 2.7, palp= 1.7; patellae I–IV = 2.2, 2.1, 2.2, 2.3, palp = 1.7; tibiae I–IV = 2.0, 1.9, 1.9, 2.1, palp = 1.7; metatarsi I–IV = 1.3, 1.2, 1.2, 1.4; tarsi I–IV = 1.2, 1.2, 1.2, 1.2, palp = 1.3. Abdomen 13.9 long, 9.4 wide. Spinnerets: PMS, 1.2 long, 0.4 wide, 0.4 apart; PLS, 2.2 basal, 1.1 middle, 1.5 distal; mid-widths (lateral), 1.0, 0.7, 0.6, respectively. Carapace: length to width 1.01; Fovea 1.1 wide. Eyes: tubercle 0.3 high, 1.2 long, 1.9 wide. Anterior eye row slightly procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.4, ALE 0.4, PME 0.2, PLE 0.4, AME–AME 0.3, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.5, ALE–PME 0.3, PME–PME 1.1, PME–PLE 0.2, PLE–PLE 1.6, ALE–PLE 0.3, AME–PLE 0.5. Ratio of eye group width to length 2.1. Maxillae: length to width: 1.6. Cuspules: 130–150 spread over ventral inner heel. Labium: 1.2 long, 1.9 wide, with ca. 90 cuspules spaced by one diameter from each other on the anterior third center. Labio-sternal groove shallow, flat, with two sigilla. Chelicerae: basal segments with eigth teeth decreasing in size from distal to basal portion. Sternum: 5.6 long, 4.7 wide. Sigilla: three pairs, small, ellipsoid, less than one diameter from margin. Scopula: tarsi I–IV fully scopulate, IV divided by four wide row of setae. Metatarsi I 4/5 scopulate; II 2/3 scopulate; III 1/2 distal scopulate; IV 1/3 distal scopulate. IV divided by five wide row of setae. Tibial spur 0.8 high, 1.4 wide; with numerous spiniform setae on tip (Fig. 51). Metatarsus I straight. Urticating hairs type II (0.63 to 1.0 long, 0.012 to 0.016 wide) on the abdomen dorsum. Palp: embolus 2.9 long, with a 45° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.3, 0.2 and 0.08, respectively. Tegulum 1.0 long, 1.8 wide. (Figs 46–48). Cymbium: two subequal lobes, the prolateral one triangular in shape. Spiniform process 0.3 long, 0.4 wide on the apex (Fig. 49). Color pattern: carapace and chelicerae dark brown, covered with golden hairs. Legs and palps black, longer hairs with distal half light brown. Coxae, labium, maxilla and sternum black. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi without rings. Abdomen dorsum orange with long reddish hairs. Abdomen ventrally grayish (Fig. 57).
Paratype female (MNRJ 06242). Carapace 15.7 long, 14.8 wide, chelicerae 7.3. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 10.1, 6.7, 7.6, 6.7, 4.0, 35.1. II: 9.2, 6.5, 6.6, 6.6, 3.8, 32.7. III: 8.9, 5.6, 6.7, 7.0, 3.9, 32.1. IV: 11.0, 6.5, 9.7, 9.8, 4.1, 41.1. Palp: 7.2, 5.1, 4.8, –, 4.9, 22.0. Mid-widths (lateral): femora I–IV = 2.8, 3.1, 3.4, 3.4, palp = 2.2; patellae I–IV = 2.9, 2.8, 3.0, 3.0, palp=2.3; tibiae I–IV = 2.7, 2.6, 2.8, 3.1, palp = 2.3; metatarsi I–IV = 2.0, 1.9, 2.0, 2.4; tarsi I–IV = 1.9, 1.9, 1.9, 1.9, palp = 2.0. Abdomen 17.4 long, 13.3 wide. Spinnerets: PMS, 1.7 long, 0.8 wide, 0.6 apart; PLS, 2.6 basal, 2.1 middle, 2.3 distal; mid-widths (lateral), 1.4, 1.1, 0.9, respectively. Carapace: length to width 1.06. Fovea 1.6 wide. Eyes: tubercle 0.3 high, 1.8 long, 2.6 wide. Anterior eye row straight, posterior slightly recurved. Eye sizes and inter-distances: AME 0.5, ALE 0.5, PME 0.3, PLE 0.4, AME–AME 0.4, AME–ALE 0.3, AME–PME 0.3, ALE–ALE 1.7, ALE–PME 0.4, PME–PME 1.3, PME–PLE 0.2, PLE–PLE 2.0, ALE–PLE 0.4, AME–PLE 0.5. Ratio of eye group width to length 2.4. Maxillae: length to width: 1.7. Cuspules: 150–200 spread over ventral inner heel. Labium: 2.0 long, 2.7 wide, with ca. 150 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove without evident sigilla. Chelicerae:basal segments with eleven teeth decreasing in size from distal to basal portion.Sternum: 7.4 long, 6.4 wide. Urticating hairs on abdomen dorsum lacking. Genitalia: paired long, uniform, weakly sclerotized spermathecae with a slight curvature in their middle (Fig. 50). Color pattern: as in male, except abdomen dorsum black with sparse long reddish hairs, ventrally black (Fig. 56).
Brazil: States of Sergipe and Northern State of Bahia, mainly in coastal regions (Fig. 68).
The cephalic region of female and immatures is very low in profile when compared with those of male, and abdomen is dorso-ventrally flattened in the former (Fig. 34). The eye tubercle is very low in female (Fig. 36) and immature, and first ocular row is straight (Fig. 37). Males have a more developed eye tubercle, the anterior ocular row is slightly procurved. Immatures and adult males have urticating hair type II on abdomen dorsally, which becomes lost in adult females.
As Pachistopelma rufonigrum, the typical spermatheca is weakly sclerotized, long, tapering apically, without constrictions or lobes, and slightly curved inwards (Figs 61–62). Shorter spermathecae (Figs 63–65) can be found, as well as almost straight ones (Fig. 64) and a few are curved outwards (Fig. 65). In the paratype female (MNRJ 06242), one spermatheca is curved inwards whereas the other is curved outwards (Fig. 50). Spermathecae of Figs 62–64 are from specimens of same population.
As with Pachistopelma rufonigrum, all specimens examined and labeled with field data indicate they were found inside bromeliads, which agrees with field observations (Dias et al 2000;
The color pattern is similar to Pachistopelma rufonigrum, mainly in early instars. However, the lateral black stripes of abdomen dorsum are almost always connecting with the longitudinal central stripe (Figs 52–53) whereas in Pachistopelma rufonigrum they normally do not connect (Figs 41–43). Larger individuals have dark legs and dark-brown carapace, and abdominal pattern is conspicuous (Fig. 54). In subadults, abdomen is very dark, lacks a pattern, or is inconspicuous (Fig. 55). Adult female is almost completely black, except for some brown setae over carapace (Fig. 56). Adult male is also completely black, but with many long whitish setae on legs, carapace and chelicerae. Abdomen is black with long red setae (Fig. 57).
http://species-id.net/wiki/Iridopelma
Figs 81–169Iridopelma hirsutum Pocock, 1901 by original designation.
Males of Iridopelma species differ from those of other aviculariines in the presence of tibial spurs on both leg I (Fig. 85) and II (Fig. 86). Females differ from those of all other aviculariines, except Avicularia and Typhochlaena, in that the anterior eye row is strongly procurved. Iridoplema further differs from Typhochlaena in that the most distal PLS segment is digitiform; from Avicularia in possessing spermatheca lacking an accentuated curvature medially.
Iridopelma hirsutum Pocock, 1901 81–86 male (MZSP 36884) 81–83 left palpal bulb 81 prolateral 82 retrolateral, 83 frontal 84 cymbium showing protuberance 85 tibial spur of left leg I 86 tibial spur of left leg II 87–88 female spermathecae 87 (MNRJ 06251) 88 (MNRJ 06249). Scale bar = 1mm.
Carapace longer than wide, cephalic region moderately raised. Cephalic and thoracic striae conspicuous. Fovea straight, shallow or deep. Chelicerae without rastellum. Eye tubercle raised (Fig. 91), wider than long. Clypeus narrow (Fig. 92). Anterior eye row procurved (Fig. 92). Labium wider than long, with 40–200 cuspules spaced by one diameter from each other on the anterior third center. Maxillary lyra absent. Maxilla subrectangular, anterior lobe distinctly produced in to conical process, inner angle bearing (75–150) cuspules. Sternum wider than long (Fig. 93). Posterior angle not separating coxae IV. Three pairs of sigillae, all rounded, less than one diameter from margin. Leg formula: I=IV II III (all females, males of Iridopelma vanini sp. n., Iridopelma katiae sp. n. andIridopelma zorodes) or I IV II III (males of Iridopelma hirsutum and Iridopelma oliveirai sp. n.). Clavate trichobothria on the distal 2/3 of tarsi I–IV. STC of males and femalewithout teeth. Tarsi I–III fully scopulated, IV divided by a band of setae or scopula integral. Scopulae of tarsi and metatarsi I–II extended very laterally giving them a spatulate appearance. Femur IV without retrolateral scopula. Legs lacking spines. Posterior lateral spinneret distally elongating, digitiform (Fig. 94). Stridulatory setae absent. Cymbium with two subequal lobes, the prolateral one triangular in shape. Male spur on tibia I (Fig. 85) and II (Fig. 86). Male metatarsus I straight. Male palpal bulb globose narrowing abruptly forming a long slender embolus, 1.8 times (Iridopelma oliveirai sp. n.) (Figs 156–158), 2.4–2.7 times (Iridopelma hirsutum, Iridopelma zorodes) (Figs 81–83, 107–109) or 3.3 times (Iridopelma vanini sp. n., Iridopelma katiae sp. n.) (Figs 127–129, 134–136) longer than tegulum length, with curvature of roughly 60° (Iridopelma hirsutum) (Fig. 83), 90° (Iridopelma katiae sp. n.) (Fig. 136), 105° (Iridopelma oliveirai sp. n.) (Fig. 158), 115° (Iridopelma zorodes) (Fig. 109) or 135° (Iridopelma vanini sp. n.) (Fig. 129) to retrolateral side; keels absent, tegulum without prolateral depression. Paired straight spermathecae ending in two or three lobes (Iridopelma hirsutum, Iridopelma oliveirai sp. n., Iridopelma vanini sp. n., Iridopelma marcoi sp. n., Iridopelma katiae sp. n.) (Figs 87–88, 161, 132, 166, 139), or a single lobe (Iridopelma zorodes) (Fig. 112); with a single apical fold (Iridopelma hirsutum, Iridopelma oliveirai sp. n.) (Figs 87–88, 161), double folded (Iridopelma marcoi sp. n., Iridopelma vanini sp. n.) (Figs 166, 132), or without folds (Iridopelma zorodes, Iridopelma katiae sp. n.) (Figs 112, 139). Cymbium with well developed spiniform process (Fig. 84). Type II urticating hair on abdomen dorsum of males and females (most species), or absent in adult females (Iridopelma marcoi sp. n.). Color pattern ontogeny present.
Male Iridopelma marcoi sp. n. are currently unknown.
Iridopelma hirsutum Pocock, 1901, female (MNRJ 06249) 89 carapace and abdomen, lateral 90 abdomen, dorsal 91–92 eye tubercle 91 lateral 92 dorsal 93 labium and sternum 94 spinnerets, ventral. Scale bar = 1mm.
Iridopelma hirsutum Pocock, 1901, habitus 95–98 immatures in progression 99 female 100 male, all from Reserva Biológica de Saltinho, Rio Formoso, state of Pernambuco, except female, from E. E. Murici, Murici, state of Alagoas. Photos: R. Bertani.
Iridopelma hirsutum Pocock, 1901, microhabitats 101 immature in retreat made with palm/Melastomatacea leaves 102, 104 immature in retreat made of two large unidentified leaves 103 immature in a retreat made of a Heliconiacea leaf 105 adult female in a retreat made of two leaves of “Guapeba” (Sapotaceae) 106 female on an abandoned house wall 101–104 Saltinho, state of Pernambuco, 105–106 E. E. Murici, Murici, state of Alagoas. Photos: R. Bertani.
Iridopelma zorodes (Mello-Leitão, 1926) 107–111 male (MNRJ 06254) 107–109 left palpal bulb 107 prolateral 108 retrolateral 109 frontal 110 tibial spur of left leg I 111 tibial spur of left leg II 112 female (IBSP 11760) spermathecae. Scale bar = 1mm.
Iridopelma zorodes (Mello-Leitão, 1926), habitus 113–115 immatures in progression 116 female 117 male 118 female 113–116 immatures and female from Itabaiana National Park, Areia Branca, state of Sergipe 117 male from RPPN Camurujipe, São João da Mata, state of Bahia 118 female from Elísio Medrado, RPPN Jequitiba, state of Bahia (MNRJ 06253). Photos: R. Bertani.
Variation in spermathecae 119–122 Iridopelma hirsutum Pocock, 1901 119 João Pessoa, state of Paraiba (IBSP 8076) 120 Sucupira, state of Pernambuco (IBSP 3986A) 121 Cabedelo, Ilha da Restinga, state of Paraiba (IBSP 8082) 122 Olinda, state of Pernambuco (IBSP 8080) 123–126 Iridopelma zorodes (Mello-Leitão, 1926) 123 Santo Amaro das Brotas, state of Sergipe (MZSP 10847) 124, 126 Acajutiba, state of Bahia (MZSP 32160–698, MZSP 32181–800) 125 Elísio Medrado, RPPN Jequitibá, state of Bahia (MNRJ 06253). Scale bar = 1mm.
Iridopelma vanini sp. n. 127–131 male 127–129 left palpal bulb (IBSP 11328) 127 prolateral 128 retrolateral 129 frontal 130 tibial spur of left leg I 131 tibial spur of left leg II 132 holotype female (IBSP Ref. 74.595) spermathecae. Scale bar = 1mm.
Iridopelma hirsutum Pocock, 1901 (Figs 81–106, 119–122, 169), Iridopelma zorodes (Mello-Leitão, 1926) (Figs 107–118, 123–126, 169), Iridopelma vanini sp. n. (Figs 127–133, 150–152, 169), Iridopelma katiae sp. n. (Figs 134–149, 153–155, 169), Iridopelma oliveirai sp. n. (Figs 156–165, 169), Iridopelma marcoi sp. n. (Figs 166–169).
Brazil: Northeastern, from Reconcave region in state of Bahia northwards. Few records for states of Para and Tocantins (Fig. 169). Specimens of Iridopelma spp. are mostly found in Brazilian Atlantic rainforest (Figs 101–105). Some species occur in drier, open environments such as “cerrado” (Fig. 168), “caatinga” and “campo rupestre” (Figs 145–146). A single record is known from the Amazon.
Iridopelma hirsutum and Iridopelma zorodes build retreats connecting leaves with silk threads (Figs 101–105). Iridopelma katiae sp. n. make retreats inside bromelids (Figs 147–149). Habitat preference of other Iridopelma species is in need of confirmation.
Iridopelma vanini sp. n., holotype female, habitus, from Parnamirim, state of Piaui. Photo: R. Bertani.
Figures 134–139. Iridopelma katiae sp. n. 134–138 paratype male (MZSP 36888) 134–136 left palpal bulb 134 prolateral 135 retrolateral 136 frontal 137 tibial spur of left leg I 138 tibial spur of left leg II 139 holotype female (MZSP 36887) spermathecae. Scale bar = 1mm.
Iridopelma katiae sp. n., habitus 140 small immature 141 large immature 142 female 143 male, all from Chapada Diamantina National Park, Mucuge, state of Bahia. Photos: R. Bertani.
Iridopelma katiae sp. n. habitats 144 general view of Chapada Diamantina showing “Chapada” formation 145 detail of top of “Chapada” formation showing “campo rupestre” area and a bromeliad (Vriesea atra)“island” 146 detail of the bromeliad “island” 147 Iridopelma katiae sp. n. inside Vriesea atra leaves 148 Vriesea atra leaf folded and linked with silk threads making retreat 149 opened retreat, showing young. Photos: R. Bertani.
Variation in spermathecae 150–152 Iridopelma vanini sp. n. 150–151 Barreirinhas, state of Maranhão (IBSP 11343, IBSP 11330) 152 Colinas de Tocantins, state of Tocantins (IBSP 3918) 153–155 Iridopelma katiae sp. n. 153 Rio de Contas, state of Bahia (IBSP 10317) 154 Mucugê, Parque Nacional da Chapada Diamantina, state of Bahia (MZSP 36890) 155 Ibicoara, state of Bahia (MZSP 22762). Scale bar = 1mm.
Iridopelma oliveirai sp. n. 156–160 male (IBSP 10100) 156–158 left palpal bulb 156 prolateral 157 retrolateral 158 frontal 159 tibial spur of left leg I 160 tibial spur of left leg II 161 female (IBSP 8714), spermathecae. Scale bar = 1mm.
Iridopelma oliveirai sp. n., habitus 162–163 immatures in progression 164 paratype female (IBSP 8714) 165 holotype male (IBSP 10100), all from Central, state of Bahia. Photos: R. Bertani.
Iridopelma marcoi sp. n. holotype female (IBSP 36891), spermathecae. Scale bar = 1 mm.
Iridopelma marcoi sp. n. 167 holotype female (IBSP 36891), habitus 168 habitat, São Desidério, state of Bahia. Photos: Marco A. Freitas.
Map showing records of Iridopelma species in Northestern, Central western and Northern Brazil. Diamond = Iridopelma hirsutum Pocock, 1901, star = Iridopelma zorodes (Mello-Leitão, 1926), square = Iridopelma vanini sp. n., retangle = Iridopelma marcoi sp. n., triangle = Iridopelma katiae sp. n., circle = Typhochlaena oliveirai sp. n. The gray area represents the approximate original distribution of Brazilian Atlantic rainforest. White area represents open environments (cerrado and caatinga).
Iridopelma hirsutum, Iridopelma zorodes and Iridopelma katiae sp. n. color pattern changes dramatically from juvenile to adult stages. Data is insufficient for Iridopelma vanini sp. n., Iridopelma oliveirai sp. n. and Iridopelma marcoi sp. n.
(Male Iridopelma marcoi sp. n. is unknown)
| 1 | Male | 2 |
| – | Female | 6 |
| 2 | Leg I longer than leg IV (leg IV/I length 0.84 or less) | 3 |
| – | Leg I and IV roughly same length (leg IV/I length 0.93 or more) | 4 |
| 3 | Leg I much longer than leg IV (leg IV/I length 0.73), pattern lacking on abdomen dorsum (Fig. 165); embolus short (Figs 156–158) | Iridopelma oliveirai sp. n. |
| – | Leg I not so longer than leg IV (leg IV/I length 0.84), pattern on abdomen dorsum present (Fig. 100); embolus long (Figs 81–83) | Iridopelma hirsutum |
| 4 | General coloration black with reddish pattern on abdomen dorsum (Fig. 143) | Iridopelma katiae sp. n. |
| – | General coloration brown or light-brown, lacking pattern on abdomen dorsum | 5 |
| 5 | Large spiders, embolus very long (Figs 127–129) | Iridopelma vanini sp. n. |
| – | Small to medium-sized spiders, embolus shorter (Figs 107–109) | Iridopelma zorodes. |
| 6 | Spermathecae with a single lobe and lacking folds (Figs 112, 123–126) | Iridopelma zorodes |
| – | Spermathecae with two or more lobes | 7 |
| 7 | Spermathecae slender from base to tip, lacking folds (Fig. 139, 153–155); general coloration black with reddish pattern on abdomen dorsum (Fig. 142) | Iridopelma katiae sp. n. |
| – | Spermathecae tapering from base to tip, with at least one fold; general coloration brown or metallic green | 8 |
| 8 | Spermathecae very long, double folded (Figs 132, 150–152) | Iridopelma vanini sp. n. |
| – | Spermathecae not too long, with single fold (Figs 87–88) | 9 |
| 9 | Pattern on abdomen dorsum present, with longitudinal reddish central stripe (Fig. 99) | Iridopelma hirsutum. |
| – | Abdomen dorsally lacking pattern (Fig. 164) | 10 |
| 10 | Carapace metallic green (Fig. 167); urticating hair lacking on abdomen dorsum | Iridopelma marcoi sp. n. |
| – | Carapace brownish to grayish (Fig. 164); urticating hair type II present on abdomen dorsum | Iridopelma oliveirai sp. n. |
http://species-id.net/wiki/Iridopelma_hirsutum
Figs 81–106, 119–122, 169Males resemble those of Iridopelma oliveirai sp. n. by leg I longer than leg IV (leg IV/I length = 0.84, SD = 0.05). Can be further distinguished by narrow embolus ending in a curved tip (Figs 81–83) and by a pattern present on the abdomen dorsally (Fig. 100). Female resembles Iridopelma oliveirai sp. n. by diverging spermathecae twisted in apical portions (Figs 87–88). It differs by brown carapace and legs, and an obvious abdominal pattern (Fig. 99).
Lectotype female (herein designated) a dissected adult female and 4 paralectotypes, 1 male, 3 immatures fom Pernambuco, Brazil, Royal Soc, H. N. Ridley (BMNH 1889-8-9-19) examined. Remarks:
BRAZIL: Ceará: Fortaleza [3°43'S, 38°32'W], 1 male, H. A. Braga, 10 April 1947 (IBSP 3505); 1 male, M. P. Paiva, 16 November 1960 (IBSP 3555); 2 males, 1 female, D. Rocha (IBSP 3362); 1 immature, same collector (IBSP 3361); Rio Grande do Norte: Parnamirim [5°54'S, 35°15W], 1 male, C. A. Almeida, 9 April 1973 (IBSP 2741); 1 male, F. Kawata, November 2007 (IBSP 14685); Paraíba: 1 male, A. R. Roman, October 1980 (IBSP 4658); 1 immature, M. Ihering (MNRJ 42344); Cabedelo, Ilha da Restinga [7°00'S, 34°50'W], 1 female, P. F. L. Duarte, 12 November 1978 (IBSP 8082); João Pessoa [7°08'S, 34°51W], 2 males, P. F. L. Duarte (IBSP 9758); Campus da U.F.P.B., 1 male (IBSP 8077), 1 female, Robson and Paula, 29 September to 3 October (IBSP8079), 1 female, B. M. Grisi, 23 August 1977 (IBSP 8076), 1 male, P. F. L. Duarte, 30 November 1979 (IBSP 8078), 1 female, Carrocinha, 17 September 1976 (MNRJ 13778), 1 male, P. F. L. Duarte, 1976 (MNRJ 13745), 1 immature, P. F. L. Duarte, 5 May 1977 (MNRJ 13549); Pernambuco: 1 male, P. F. L. Duarte (IBSP 9757); Camocim de São Félix [8°21'S, 35°45'W], 1 immatue, Vera Lúcia, 25 November 1969 (IBSP 8081); Jaboatão dos Guararapes, Sucupira [8°06'S, 34°58'W], 1 immature, J. M. Arcanjo, 21 May 1969, on bromeliads (IBSP 3986A); Igarassu [7°50'S, 34°54'W], 1 male, G. A. Ramage (BMNH 1888-47); Olinda [8°00'S, 34°51'W], 2 females, 8 immatures, A. T. Coelho, 1963, on bushes (IBSP 8080); Recife [8°03'S, 34°52'W], 1 female, M. F. Santos, May 1985 (IBSP 2741); Rio Formoso, Reserva Biológica de Saltinho (8°43'5.99"S, 35°10'7.08"W), 1 male, R. Bertani, D. R. M. Ortega and R. H. Nagahama, 9 August 2006, at night, with retreat made with silk and two leaves, 1 m above the ground (MZSP 36884); 1 female, same colectors, 8 August 2006 (MZSP 36885); 1 female, same collectors, 10 August 2006, at night, on a leaf 0.5 m above the ground (MZSP 36886); 1 immature, same collectors, 9 August 2006, at night in palm tree, inside retreat made with silk and two leaves, 1 m above the ground (MZSP 36887), 2 immatures, 12 May 1969 (MNRJ 13721); Alagoas: Coruripe, Barragem da Fazenda Capiatã I, Usina Coruripe [10°08'S, 36°10'W], 1 male, E. C. Santos and R. C. T. Aquino (ex MUFAL 269), October 2004 (IBSP 12583), Murici, Estação Ecológica de Murici, Ufal (9°15'1.86"S, 35°51'0.17"W), 200 m a.s.l., 1 female, R. Bertani, D. R. M. Ortega and R. H. Nagahama, 17 August 2006, at night, inside reatreat made with silk and two leaves, 1 m above the ground (MNRJ 06249); 1 immature, same colectors, 12 August 2006, at night, in a web built between two Heliconia leaves (MNRJ 06250); (9°14'1.73"S, 35°50'1.61"W), 575 m a.s.l., 1 female, same collectors, 16 August 2006, at night in a wall of an abandoned house (MNRJ 06251); 1 female, same data (MNRJ 06252); without locality (Cidade Universitaria), 1 male, M. N. Marques, 11 May 1980 (MNRJ 13782).
Female (MNRJ 06249) from Murici, state of Alagoas, Brazil. Carapace 15.0 long, 14.4 wide, chelicerae 9.0. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 13.4, 7.6, 10.4, 9.6, 5.3, 46.3. II: 12.3, 7.0, 8.8, 9.3, 4.9, 42.3. III: 10.4, 6.0, 8.0, 8.6, 4.6, 37.6. IV: 12.6, 6.2, 10.5, 10.9, 4.7, 44.9. Palp: 9.4, 5.2, 6.2, –, 6.2, 27.0. Mid-widths (lateral): femora I –IV = 2.8, 2.8, 3.1, 2.8, palp = 2.2; patellae I–IV = 2.9, 2.8, 2.7, 2.7, palp = 2.4; tibiae I–IV = 2.7, 2.5, 2.5, 2.6, palp = 2.3; metatarsi I–IV = 2.2, 2.2, 1.8, 1.6; tarsi I–IV = 2.2, 2.2, 2.0, 2.3, palp = 2.5. Abdomen 21.5 long, 16.0 wide. Spinnerets: PMS, 1.8 long, 0.7 wide, 0.4 apart; PLS, 2.3 basal, 1.6 middle, 2.5 distal; mid-widths (lateral), 1.9, 1.4, 0.9, respectively. Carapace: length to width 1.04. Fovea: shallow, 2.3 wide. Eyes: tubercle 0.6 high, 2.4 long, 2.8 wide. Clypeus 0.6. Anterior eye row procurved, posterior straight. Eye sizes and inter-distances: AME 0.6, ALE 0.6, PME 0.4, PLE 0.6, AME–AME 0.6, AME–ALE 0.5, AME–PME 0.3, ALE–ALE 2.0, ALE–PME 0.7, PME–PME 1.6, PME–PLE 0.08, PLE–PLE 2.1, ALE–PLE 0.4, AME–PLE 0.4. Ratio of eye group width to length 2.0. Maxillae: length to width: 2.1. Cuspules: 90–100 spread over ventral inner heel. Labium: 2.1 long, 2.2 wide, with 70–90 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove deep, narrow, sigilla not evident. Chelicerae: basal segments with ten teeth decreasing in size from distal to basal portion. Sternum: 6.9 long, 6.2 wide. Legs: leg formula: I=IV II III. Scopula: tarsi I–III fully scopulate, IV basal half divided by row of 6–7 setae. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/3 distal scopulate. IV divided by three wide row of setae. Urticating hairs type II (0.53 to 0.61 long, 0.015 wide) on abdomen dorsum. Genitalia: paired diverging spermathecae tapering from the basally to the apex, folding on medially and with a strong constriction forming two (more rarely one or three) distal lobes (Figs 87–88). Color pattern: carapace, chelicerae, legs and palps brown with light brown setae, except for femora, patellae and tibiae of leg I-II and patellae and tibiae of leg III, blackish ventrally and prolaterally and tibiae and metatarsi of leg IV, darkened ventrally. Sternum, coxae, labium, maxillae black. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi whitish, more conspicuous on the patellae. Distal femora, patellae, tibiae and metatarsi with white rings. Tarsi with a dorsal “U” shaped orange stripe. Abdomen dorsum brown with long light brown setae; a longitudinal large darker stripe on its center. Ventrally black (Fig. 99).
Male (MZSP 36884) from Rio Formoso, state of Pernambuco, Brazil. Carapace 10.4 long, 10.0 wide, chelicerae 5.7. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 13.2, 5.8, 10.9, 11.0, 5.3, 46.2. II: 11.9, 5.3, 9.7, 9.6, 4.9, 41, 4. III: 9.8, 4.5, 7.6, 8.3, 3.9, 34.1. IV: 11.7, 4.7, 10.2, 11.0, 4.3, 41.9. Palp: 7.1, 3.9, 5.3, –, 2.2, 18.5. Mid-widths (lateral): femora I–IV = 1.8, 1.7, 2.0, 1.8, palp = 1.3; patellae I–IV = 1.8, 1.9, 1.7, 1.9, palp = 1.6; tibiae I–IV = 1.5, 1.4, 1.5, 1.6, palp = 1.5; metatarsi I–IV = 1.1, 1.0, 1.1, 1.1; tarsi I–IV = 1.1, 1.3, 1.2, 1.1, palp = 1.4. Abdomen 11.2 long, 7.6 wide. Spinnerets: PMS, 1.4 long, 0.5 wide, 0.2 apart; PLS, 2.3 basal, 1.1 middle, 1.7 distal; mid-widths (lateral), 0.8, 0.6, 0.5, respectively. Carapace: length to width 1.01. Fovea: shallow, 1.6 wide. Eyes: tubercle 0.4 high, 1.6 long, 2.1 wide. Clypeus 0.5. Anterior eye row procurved, posterior straight. Eye sizes and inter-distances: AME 0.5, ALE 0.5, PME 0.4, PLE 0.5, AME–AME 0.6, AME–ALE 0.3, AME–PME 0.1, ALE–ALE 1.3, ALE–PME 0.5, PME–PME 1.2, PME–PLE 0.08, PLE–PLE 1.3, ALE–PLE 0.3, AME–PLE 0.4. Ratio of eye group width to length 2.0. Other characters as in female, except: maxillae: length to width: 2.1. Cuspules: 75–80 spread over ventral inner heel. Labium: 1.2 long, 1.6 wide, with 50 cuspules spaced by one diameter from each other on the anterior third center. Labio-sternal groove shallow, flat, with two sigilla. Chelicerae: basal segments with eigth teeth decreasing in size from distal to basal portion. Sternum: 5.0 long, 4.7 wide. Legs: leg formula: I IV II III. Scopula: tarsi I–IV fully scopulate, IV divided by three row of setae. Metatarsi I 4/5 scopulate; II 2/3 scopulate; III 1/2 distal scopulate; IV 1/3 distal scopulate. IV divided by three wide row of setae. Tibial spurs. Legs: I, single, 0.6 long, 1.1 wide, with numerous spiniform setae on its tip (Fig. 85); II, single, without elevation, formed by numerous spiniform setae (Fig. 86). Urticating hairs type II (0.88 to 0.95 long, 0.015 wide) on abdomen dorsum. Palp: embolus 2.6 long, with a 60° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.5, 0.1 and 0.05, respectively. Tegulum 1.1 long, 1.5 wide. (Figs 81–83). Cymbium: spiniform process 0.8 long, 0.4 wide on the apex (fig. 84). Color pattern as in female, except for legs dorsally and ventrally brown with light brown setae; coxa I with distal ventro-retrolateral margin, II with distal half and III–IV completely brown with light brown setae; dorsum of abdomen with a longitudinal stripe of reddish urticating hairs (Fig. 100).
Brazil: From state of Ceará southwards to the state of Alagoas (Fig. 169).
Typical spermatheca diverges and tapers from base to apex, folds after its middle and has a strong constriction forming two distal lobes (Figs 87, 119, 121). More rarely, there is one lobe on both (Figs 88, 120) spermathecae or even three lobes (Fig. 122). Relative length and width vary, mainly in smaller specimens (Figs 119–122). However, even small spermathecae are already folded after their middle.
Individuals of Iridopelma hirsutum are known to inhabit remnants of Brazilian Atlantic rainforest. Of eight specimens collected in Reserva Biológica de Saltinho, state of Pernambuco, and Estação Ecológica de Murici, state of Alagoas, six were found inside or close to retreats on leaves and two adult females were walking on the inner walls of an abandoned house (Fig. 106). They normally build a retreat with two leaves connected with silk threads (Figs 101, 102, 104–105) on different plants such as Piperaceae, Melastomataceae, Palmae (Fig. 101), “Guapeba” (Sapotaceae) (Fig. 105) and other not identified (Figs 102, 104). Ocasionally, they can be found with retreats in a single leaf, as in rolled Heliconiaceae leaf (Fig. 103). Retreat was above ground 0.5 to 1.0 m, but this was affected by collector’s inability to search for specimens high in the vegetation. No adult males or females with eggsacs were found in the excursion period (August).
Juveniles have most of the carapace, chelicerae and legs metallic green (Figs 95–96). Coxae, trochantera, basal femora dorsally and parts of the chelicerae are light brown. The abdomen is black with a light central longitudinal large spot having zigzag edges and a longitudinal central black stripe over a more reddish area. The sternum, coxae, labium and abdomen are ventrally black. As the juveniles mature, the metallic green coloration changes to brown (Fig. 97) and the central spot on dorsal abdomen becomes totally reddish (Fig. 98). In the adult females, parts of the abdominal pattern remain, the longitudinal central black stripe is broader, and the reddish area becomes inconspicuous (Fig. 99). Adult males also retain an abdominal pattern, and the reddish area is normally conspicuous (Fig. 100).
Avicularia palmicola Mello-Leitão, 1945, holotype female from Brazil, Paraíba, Mumbaba, Aristóteles Silva, collected in coconut tree (MNRJ 2328), examined. The specimen is an aviculariine female with first eye row procurved and spermathecae having a single fold and bilobed at distal portion. These characters agree with Iridopelma and spermathecae shape is typical of Iridopelma hirsutum. Therefore, Avicularia palmicola Mello-Leitão, 1945 is transferred to Iridopelma and considered a junior-synonym of Iridopelma hirsutum Pocock, 1901 syn. n.
http://species-id.net/wiki/Iridopelma_zorodes
Figs 107–118, 123–126, 169The males resemble those of Iridopelma vanini sp. n.and Iridopelma katiae sp. n. by leg I and IV having similar length (leg IV/I length = 0.93, SD = 0.01). It differs from Iridopelma vanini sp. n. by the shorter embolus (Figs 107–109). It differs from Iridopelma katiae sp. n. by general brown pattern and lack of a reddish pattern on abdomen dorsally (Fig. 117). Females differ from all other species by two straight to slightly curved spermathecae tapering little from base to apex, not folding and with a very slight constriction forming a single distal lobe (Fig. 112).
Holotype male of Typhochlaena zorodes [dry, pinned] from Bahia, O. Torres, MNRJ 1408 [MLPC 900], not located (see
BRAZIL: Sergipe: Areia Branca, Parque Nacional Serra de Itabaiana [10°44'S, 37°22'W], 1 male, A. C. M. Fernandes, 12 November 1996 (IBSP 11761); 1 female, R. Bertani, A. D. Brescovit, A. B. Bonaldo, September 1999 (IBSP 9421); 1 female, same data (IBSP 8545); 1 male, same data (IBSP 8543); 1 immature, same data (IBSP 8722); 1 immature, same data (IBSP 8954); 1 female, I. Matos, 1 November 1992 (IBSP 11760); Lagarto [10°54'S, 37°39'W], 1 female, Maxwell ded. September 1999 (IBSP 8026); Santa Luzia do Itanhy, Mata do Crasto [11°23'S, 37°24'W], 1 female, R. Bertani, A. D. Brescovit, A. B. Bonaldo, September 1999 (IBSP 8782); Santo Amaro das Brotas [10°46'S, 37°03'W], no collector data, 23 March 1978, inside bromeliad (MZSP 10847); São Cristóvão [11°00'S, 37°13'W], 1 female, A. D. Brescovit, 6 December 1996 (IBSP 9664); Bahia: Acajutiba [11°39'S, 38°01'W], 1 male, 1 female, E. Boaventura, 27 March 1991 (MZSP 32159, col. Bock. 696–697); 1 female, same data (MZSP 32160, col. Bock. 698); 5 females, 17 immatures, same collector, 18 April 1991 (MZSP 32181, col. Bock. 716, 800–820); 3 females, 2 immatures, same data (MZSP 32180, col. Bock. 795–799); Elísio Medrado, RPPN Jequitibá (12°52'3.20"S, 39°28'9.09"W) 1 female, R. H. Nagahama, R. Bertani, C. S. Fukushima, 7 October 2007, at night, inside bromeliad (MNRJ 06253); Salvador, Alphaville [12°56'S, 38°21'W], 1 male, G. G. Montingelli, 11–29 November 2001 (IBSP 9717); Ondina [13°00'S, 38°30'W] 1 immature, T. B. Nunes Ded. December 1982 (IBSP 7906); 1 male (REF 42901C); Mata de São João, RPPN Camarujipe [12°31 S, 38°02'W], 1 male, C. S. Fukushima, R. Bertani and R. H. Nagahama, 4 October 2007, at night on a leaf (MNRJ 06254); Paraná: Curitiba [25°25'S, 49°16'W], 1 immature, F. Cominese (MZSP 32161, Bock. 695) (prabably mislabeled).
Female (IBSP 11760) from Areia Branca, Sergipe, Brazil. Carapace 12.8 long, 12.0 wide, chelicerae 6.3. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 10.8, 6.4, 8.1, 8.4, 4.1, 37.8. II: 9.8, 5.6, 6.9, 6.8, 3.6, 32.7. III: 8.4, 4.6, 6.0, 6.7, 3.4, 29.1. IV: 10.2, 5.3, 8.3, 8.8, 3.7, 36.3. Palp: 7.6, 4.4, 5.0, –, 5.3, 22.3. Mid-widths (lateral): femora I–IV = 2.3, 2.3, 2.4, 2.1, palp = 1.8; patellae I–IV = 2.4, 2.4, 2.4, 2.4, palp = 2.1; tibiae I–IV = 2.2, 2.1, 1.9, 2.1, palp = 1.9; metatarsi I–IV = 1.6, 1.5, 1.5, 1.4; tarsi I–IV = 1.8, 1.8, 1.8, 1.7, palp = 1.8. Abdomen 12.8 long, 9.5 wide. Spinnerets: PMS, 1.3 long, 0.7 wide, 0.3 apart; PLS, 1.6 basal, 1.0 middle, 1.9 distal; mid-widths (lateral), 1.1, 0.9, 0.7, respectively. Carapace: length to width 1.07. Fovea: deep, 2.1 wide. Eyes: tubercle 0.9 high, 1.6 long, 2.6 wide. Clypeus 0.4. Anterior eye row procurved, posterior straight. Eye sizes and inter-distances: AME 0.5, ALE 0.6, PME 0.4, PLE 0.5, AME–AME 0.4, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.6, ALE–PME 0.5, PME–PME 1.4, PME–PLE 0.1, PLE–PLE 2.1, ALE–PLE 0.2, AME–PLE 0.4. Ratio of eye group width to length 2.2. Maxillae: length to width: 1.8. Cuspules: 90–100 spread over ventral inner heel. Labium: 1.2 long, 2.1 wide, with 80–100 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove deep, narrow, with two sigilla. Chelicerae: basal segments with nine teeth decreasing in size from distal to basal portion. Sternum: 5.9 long, 4.8 wide. Legs: leg formula: I=IV II III. Scopula: tarsi I–IV fully scopulate, IV with some sparse setae. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/3 distal scopulate. IV divided by three wide row of setae. Urticating hairs type II (0.40 to 0.45 long, 0.01 wide) on the abdomen dorsum. Genitalia: paired straight to slightly curved spermathecae tapering slightly from base to apex, not folding, with very slight constriction forming single distal lobe (Fig. 112). Color pattern: carapace, chelicerae, legs and palps brown with light brown hairs; metatarsi, tibiae and patellae ventrally dark. All tarsi dorsally with a “U” shaped orange stripe. Sternum, labium and maxillae black. Coxae I black, except by brown retrolateral apical third; Coxae II–IV basal half black, distal half brown. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi light. Distal femora, patellae, tibiae and metatarsi with white rings. Abdomen venter black; lateral light brown; dorsal central area dark with reddish urticating hairs (Fig. 116).
Male (MNRJ 06254) from Mata de São João, Camurujipe, state of Bahia, Brazil. Carapace 8.0 long, 7.6 wide, chelicerae 4.5. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 10.2, 4.7, 8.1, 8.3, 4.0, 35.3. II: 9.3, 4.1, 7.3, 7.5, 3.5, 31.7. III: 7.6, 3.6, 5.8, 6.4, 3.2, 26.6. IV: 9.5, 3.7, 7.9, 8.7, 3.4, 33.2. Palp: 5.6, 3.0, 4.1, –, 2.1, 14.8. Mid-widths (lateral): femora I –IV = 1.4, 1.5, 1.5, 1.4, palp = 1.2; patellae I–IV = 1.6, 1.5, 1.5, 1.8, palp = 1.3; tibiae I–IV = 1.4, 1.2, 1.1, 1.3, palp = 1.3; metatarsi I–IV = 0.9, 0.9, 0.9, 0.8; tarsi I–IV = 1.1, 1.1, 1.0, 1.0, palp = 1.2. Abdomen 8.7 long, 4.7 wide. Spinnerets: PMS, 0.9 long, 0.4 wide, 0.1 apart; PLS, 1.2 basal, 0.8 middle, 1.1 distal; mid-widths (lateral), 0.6, 0.6, 0.5, respectively. Carapace: length to width 1.05. Fovea: shallow, 1.3 wide. Eyes: tubercle 0.5 high, 1.4 long, 1.7 wide. Clypeus 0.2. Anterior eye row procurved, posterior straight. Eyes sizes and inter-distances: AME 0.5, ALE 0.3, PME 0.2, PLE 0.3, AME–AME 0.3, AME–ALE 0.1, AME–PME 0.1, ALE–ALE 1.1, ALE–PME 0.3, PME–PME 1.1, PME–PLE 0.08, PLE–PLE 1.2, ALE–PLE 0.2, AME–PLE 0.2. Ratio of eye group width to length 2.1. Other characters as in female, except: maxillae: length to width: 1.8. Cuspules: 90–100 spread over ventral inner heel. Labium: 0.8 long, 1.5 wide, with 70–80 cuspules spaced by one diameter from each other on the anterior third center. Labio-sternal groove shallow, flat, with two sigilla. Chelicerae: basal segments with eleven teeth decreasing in size from distal to basal portion. Sternum: 3.9 long, 3.3 wide. Legs: leg formula: I=IV II III. Scopula: Tarsi I–IV fully scopulate, IV with some sparse setae. Metatarsi I–II 2/3 scopulate; III 2/3 distal scopulate; IV 1/3 distal scopulate. IV divided by four wide row of setae. Tibial spur. Legs: I, single, 0.8 long, 1.0 wide (Fig. 110); II, 0.2 long, 0.7 wide (Fig. 111); both with numerous spiniform setae on tips. Urticating hairs type II (0.75 to 0.85 long, 0.015 to 0.02 wide) on the abdomen dorsum. Palp: embolus 2.2 long, with a 115° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.4, 0.2 and 0.03, respectively. Tegulum 0.8 long, 1.2 wide. (Figs 107–109). Cymbium: spiniform process 0.4 long, 0.4 wide on the apex.
Brazil: states of Sergipe and Northern Bahia (Fig. 169).
Typical spermatheca is straight, or diverges little from its base; has no fold and apical portion is dilated (Figs 112, 123). Some spermathecae are shorter and taper considerably (Fig. 124), whereas others are long and curved distally (Fig. 126). The spermatheca of a specimen from Elísio Medrado, state of Bahia, is partially divided distally (Fig. 125). It is from the southermost distribution of the species and shows a different color pattern as well (Fig. 118).
Individuals of Iridopelma zorodes inhabits remnants of Brazilian Atlantic rainforest. My own field observations in state of Sergipe (Areia Branca, Parque Nacional Serra de Itabaiana) and Santa Luzia do Itanhy (Mata do Crasto), indicate that individuals make retreats similar to those of Iridopelma hirsutum, i. e., leaves connected with silk threads. Eventually, they were found inside bromeliads. An adult male was found on a leaf in Camurujipe, São João da Mata, state of Bahia, in October.
As Iridopelma hirsutum, but adult males and females lack or have an inconspicuous abdominal pattern (Figs 113–118).
Even though the holotype is lost (
urn:lsid:zoobank.org:act:8302CBCC-2045-4E2B-943E-2FE15BFB7F5E
http://species-id.net/wiki/Iridopelma_vanini
Figs 127–133, 150–152, 169Males resemble those of Iridopelma zorodes and Iridopelma katiae sp. n. by leg I and IV having similar length (leg IV/I length = 0.98, SD = 0.02). It differs from Iridopelma zorodes and Iridopelma katiae sp. n. by longer embolus (Figs 127–129). Additionally, it differs from Iridopelma katiae sp. n. by a general brown pattern. Female resembles those of Iridopelma marcoi sp. n. by longer spermathecae folded twice (Fig. 132), and differs by having type II urticating setae on abdomen dorsum.
The specific name is a patronym in honour of Professor Sérgio Antonio Vanin, a Brazilian entomologist. As advisor of some arachnologists, myself included, he contributed to the development of modern Brazilian arachnology.
Holotype female (IBSP Ref. 74.595), Brazil, state of Piauí, Parnaíba [2°53'S, 41°41'W], 5 m a.s.l., R. Bertani, November 1994; Paratype male (IBSP 11328), Brazil, state of Maranhão, Barreirinhas, Parque Nacional dos Lençóis Maranhenses [2°41'S, 42°55'W), 32 m a.s.l., Equipe Biota, 12–18 October 2001.
BRAZIL: Pará: Rio Tocantins, margem direita, Chiqueirão [4°20'S, 49°25'W], B. M. Mascarenhas, 1 April 1984 (MPEG-ARA 005080); Maranhão: Barreirinhas, Parque Nacional dos Lençóis Maranhenses [2°41'S, 42°55'W), 32 m a.s.l., Equipe Biota, 12–18 October 2001, 1 male (IBSP 11346), 1 female (IBSP 11343), 1 female (IBSP 11330), 1 female, 1 immature (IBSP 11345), 1 female, 2 immatures (IBSP 11342); Piauí: Castelo do Piauí, E. C. B. Rochas Ornamentais (5°13'50.8"S, 41°42'01.1"W), F. M. Oliveira-Neto, 27 October 2005, 1 male (MPEG-ARA 002297); José de Freitas, Nazareth Eco Resort [4°46'S, 42°34'W], S. Brasil, 3 males (IBSP 11349); Tocantins: Colinas de Tocantins [8°03'S, 48°28'W], W. R. Soares, 10 December 1968, 1 female (IBSP 3918).
Holotype female (IBSP Ref. 74.595). Carapace 21.3 long, 20.0 wide, chelicerae 11.0. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 17.4, 9.8, 13.1, 11.9, 6.5, 58.7. II: 15.9, 9.1, 11.9, 11.2, 5.9, 54.0. III: 14.0, 8.5, 10.6, 10.7, 5.8, 49.6. IV: 16.5, 9.1, 13.5, 13.9, 5.9, 58.9. Palp: 12.0, 7.0, 8.0, –, 8.1, 35.1. Mid-widths (lateral): femora I–IV = 4.2, 4.2, 4.5, 4.2, palp = 3.3; patellae I–IV = 4.0, 4.3, 3.9, 3.8, palp = 3.4; tibiae I–IV = 3.5, 3.3, 3.1, 3.5, palp=3.0; metatarsi I–IV = 2.6, 2.7, 2.4, 2.4; tarsi I–IV = 2.9, 2.9, 2.9, 2.9, palp = 3.0. Abdomen 22.1 long, 16.9 wide. Spinnerets: PMS, 2.2 long, 1.3 wide, 0.5 apart; PLS, 3.7 basal, 2.5 middle, 3.8 distal; mid-widths (lateral), 2.1, 1.9, 1.4, respectively. Carapace: length to width 1.06. Fovea: deep, 4.4 wide. Eyes: tubercle 1.0 high, 2.5 long, 3.5 wide. Clypeus 1.4. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.6, ALE 0.7, PME 0.4, PLE 0.7, AME–AME 1.0, AME–ALE 0.5, AME–PME 0.4, ALE–ALE 2.4, ALE–PME 0.9, PME–PME 2.1, PME–PLE 0.2, PLE–PLE 2.7, ALE–PLE 0.5, AME–PLE 0.7. Ratio of eye group width to length 2.1. Maxillae: length to width: 2.2. Cuspules: 100–150 spread over ventral inner heel. Labium: 2.5 long, 3.0 wide, with ca. 100 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove deep, narrow, sigilla not evident. Chelicerae: basal segments with ten teeth decreasing in size from distal to basal portion. Sternum: 9.9 long, 7.5 wide. Legs: leg formula: I=IV II III. Scopula: tarsi I–IV fully scopulate. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/3 distal scopulate. IV divided by five wide row of setae. Urticating hairs type II (0.65 to 0.70 long, 0.02 wide) on abdomen dorsum. Genitalia: paired long and converging spermathecae tapering strongly from base to apex, double folded and with strong constriction forming two (more rarely one or three) distal lobes (Fig. 132). Color pattern: carapace, chelicerae and dorsum of legs and palps covered with short setae having green/blue iridescence and with abundant longer light brown setae giving a brownish appearance. Tarsi dorsally with a “U” orange stripe. Femora ventrally of the same color as the dorsum; patellae, tibiae, metatarsi and tarsi ventrally blackish. Coxae, labium, sternum and maxillae black. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi whitish, conspicuous. Distal femora, patellae, tibiae and metatarsi with white rings. Abdomen dorsum and lateral light-brown with long setae of same color. Central dorsal area blackish, forming a pattern, with long setae having basal part blackish, distal light brown. Urticating hairs reddish. Abdomen ventrally black (Fig. 133).
Paratype male (IBSP 11328). Carapace 17.7 long, 17.4 wide, chelicerae 9.6. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 17.5, 9.1, 13.9, 13.6, 7.3, 61.4. II: 16.4, 8.3, 13.0, 12.7, 6.5, 56.9. III: 14.0, 7.0, 11.0, 11.6, 5.8, 49.4. IV: 16.7, 7.7, 14.4, 14.9, 6.3, 60.0. Palp: 9.9, 5.8, 7.7, –, 3.6, 27.0. Mid-widths (lateral): femora I–IV = 3.7, 3.6, 3.7, 3.4, palp = 2.5; patellae I–IV = 3.6, 3.5, 3.5, 3.3, palp = 2.6; tibiae I–IV = 3.0, 2.9, 2.9, 2.7, palp = 2.4; metatarsi I–IV = 2.2, 2.1, 1.9, 1.8; tarsi I–IV = 2.4, 2.5, 2.1, 2.3, palp = 2.4. Abdomen 19.3 long, 13.1 wide. Spinnerets: PMS, 1.7 long, 0.8 wide, 0.3 apart; PLS, 2.9 basal, 2.3 middle, 2.9 distal; mid-widths (lateral), 1.3, 1.1, 0.8, respectively. Carapace: length to width 1.01. Fovea 2.1 wide. Eyes: tubercle 0.9 high, 1.9 long, 3.0 wide. Clypeus 1.0. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.6, ALE 0.7, PME 0.5, PLE 0.7, AME–AME 0.7, AME–ALE 0.5, AME–PME 0.3, ALE–ALE 1.9, ALE–PME 0.6, PME–PME 1.8, PME–PLE 0.3, PLE–PLE 2.3, ALE–PLE 0.2, AME–PLE 0.6. Ratio of eye group width to length 2.0. Other characters as in female, except: maxillae: length to width: 2.3. Cuspules: 150–200 spread over ventral inner heel. Labium: 2.3 long, 2.9 wide, with 150–200 cuspules spaced by one diameter from each other on the anterior third center. Labio-sternal groove shallow, flat, sigilla not evident. Sternum: 8.2 long, 6.8 wide. Legs: leg formula: I=IV II III. Tibial spurs. Legs: I, single, 0.8 long, 2.2 wide (Fig. 130); II, single, 0.5 long, 2.1 wide (Fig. 131); both with numerous spiniform setae on their tips. Urticating hairs type II (1.13 long, 0.02 wide) on the abdomen dorsum. Palp: embolus 4.9 long, with a 135° curvature to the retrolateral side. Embolus basal, middle and distal width of 0.9, 0.3 and 0.04, respectively. Tegulum 1.5 long, 2.3 wide. (Figs 127–129). Cymbium: spiniform process 1.0 long, 0.7 wide on apex. Color pattern and setation mostly as in female. Dorsal abdominal pattern not as conspicuous as in female.
Brazil, states of Piaui, Maranhão and possibly Tocantins and Pará (Fig. 169). There is a single record for both Tocantins (Colinas de Tocantins) and Pará (Tocantins River). These specimens are smaller than those from Maranhão and Piauí, and the specimen from Pará has both male palpal bulb emboli broken, therefore its identification is only tentative.
Typical spermatheca is long, converging, tapers strongly from base to apex, double folded and with a strong constriction forming two distal lobes (Figs 132, 151). In some specimens the apical region ends in a single lobe, that can be partially divided (Fig. 150) or in two lobes with one partially or completely divided, in this case resulting in multilobular spermathecae (Fig. 152).
Little is known of this species. The holotype female was collected with an eggsac in November, under a fallen tree trunk in a moderately anthropized area in Parnaíba, state of Piaui. Other specimens were collected in Barreirinhas, State of Maranhão. Both localities are on coastal region characterized by huge sandy dunes and sparse vegetation. Other localities, as Jose de Freitas (
No data available.
urn:lsid:zoobank.org:act:E4ED25DB-B675-4BC5-9E5D-E5A9C9159DB6
http://species-id.net/wiki/Iridopelma_katiae
Figs 134–149, 153–155, 169Male resemble those of Iridopelma zorodes and Iridopelma vanini sp. n. in similar leg I and IV lengths (leg IV/I length =1.04, SD= 0.02). It differs from Iridopelma zorodes by blackish coloration with a reddish pattern on abdomen dorsum (Fig. 143). It differs from Iridopelma vanini sp. n. by shorter embolus (Figs 134–136). Female differs from those of all species in spermathecal morphology, two straight to slightly curving spermathecae tapering little from base to apex, lacking folds and with constriction forming two to multiple distal lobes (Fig. 139). Additionally, females have a general blackish coloration with a reddish pattern on abdomen dorsum (Fig. 142).
The specific name is a matronym in honour of my wife, Kátia de Mendonça Faria, who illustrated several plates for spider taxonomy papers, this work included.
Holotype female, Brazil, Bahia, Mucugê, Parque Nacional da Chapada Diamantina (12°45'7.00"S, 41°30'2.18"W), 1399 m a.s.l., R. Bertani, C. S. Fukushima and R. H. Nagahama, 18 February 2008 (MZSP 36887); Paratype male (12°45'3.77"S, 41°30'4.01"W), 1377 m a.s.l., 17 February 2008 (MZSP 36888), same data; Paratype male (12°45'4.18"S, 41°30'3.73"W), 1366 m.a.s.l, same data (MZSP 36889); Paraype female (12°45' 2.50"S, 41°30'4.15"W), 1388 m.a.s.l, same data, found with ca. 100 young (MZSP 36890). All specimens found inside bromeliads (Vriesea atra) in a campo rupestre area.
BRAZIL: Bahia: Ibicoara, Serra do Sincorá [13°24'S, 41°15'W], G. Machado, 12 March 2000, 2 females (MZSP 22762); Rio de Contas [13°35'S, 41°48'W], O. A. V. Marques and G. Skuk, 09 October 1991, 1 female, 1 immature (IBSP 10317).
Holotype female (MZSP 36887). Carapace 13.3 long, 12.7 wide, chelicerae 7.7. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 11.3, 6.5, 8.2, 7.6, 4.6, 38.2. II: 10.0, 6.1, 7.2, 7.0, 4.3, 34.6. III: 9.3, 5.3, 6.4, 6.9, 4.1, 32.0. IV: 10.9, 5.2, 8.8, 9.6, 4.7, 39.2. Palp: 7.9, 4.6, 5.3, –, 5.9, 23.7. Mid-widths (lateral): femora I–IV = 2.4, 2.5, 2.8, 2.4, palp = 2.2; patellae I–IV = 2.7, 2.6, 2.5, 2.5, palp = 2.3; tibiae I–IV = 2.1, 2.1, 2.1, 2.0, palp = 2.1; metatarsi I–IV = 1.9, 1.7, 1.6, 1.6; tarsi I–IV = 2.1, 2.1, 2.1, 2.0, palp = 2.1. Abdomen 18.4 long, 13.0 wide. Spinnerets: PMS, 1.7 long, 0.7 wide, 0.3 apart; PLS, 2.1 basal, 1.4 middle, 1.5 distal; mid-widths (lateral), 1.3, 1.1, 0.8, respectively. Carapace: length to width 1.04. Fovea: shallow, 2.3 wide. Eyes: tubercle 0.5 high, 1.8 long, 2.4 wide. Clypeus 0.6. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.4, ALE 0.6, PME 0.5, PLE 0.6, AME–AME 0.6, AME–ALE 0.4, AME–PME 0.2, ALE–ALE 1.5, ALE–PME 0.3, PME–PME 1.4, PME–PLE 0.08, PLE–PLE 1.8, ALE–PLE 0.3, AME–PLE 0.5. Ratio of eye group width to length 2.0. Maxillae: length to width: 1.9. Cuspules: 100–120 spread over ventral inner heel. Maxillary lyra absent. Labium: 1.6 long, 2.0 wide, with ca. 100 cuspules spaced by one diameter from each other on the anterior third. Labio-sternal groove deep, narrow, sigilla not evident. Chelicerae: basal segments with ten teeth decreasing in size from distal to basal portion. Sternum: 6.2 long, 5.3 wide. Legs: leg formula: I=IV II III. Scopula: Tarsi I–III fully scopulate, IV basal half divided by row of 6–7 setae. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/3 distal scopulate. IV divided by three wide row of setae. Urticating hairs type II (0.45 to 0.51 long, 0.012 wide) on abdomen dorsum. Genitalia: paired slightly curving inwards spermathecae tapering slightly from base to apex, not folded, with constriction forming two to multiple distal lobes (Fig. 139). Color pattern: carapace, chelicerae, legs and palps black, covered with hairs having a weak golden iridescence. Coxae, labium, sternum, and maxillae black. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi with discrete whitish rings. Abdomen dorsum black with longitudinal large band of long reddish setae on the central area, forming a pattern. Sparse long red setae over lateral area of abdomen. Abdomen ventrally black (Fig. 142).
Paratype male (MZSP 36888). Carapace 12.1 long, 11.2 wide, chelicerae 6.5. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 11.7, 6.6, 9.1, 10.5, 5.2, 43.1. II: 10.8, 6.0, 8.3, 9.3, 4.8, 39.2. III: 9.5, 4.6, 7.0, 8.2, 4.6, 33.9. IV: 11.4, 5.5, 9.7, 11.0, 4.5, 42.1. Palp: 6.6, 4.4, 4.9, –, 2.6, 18.5. Mid-widths (lateral): femora I–IV = 2.1, 2.0, 2.4, 2.4, palp=1.7; patellae I–IV = 2.1, 2.3, 2.2, 2.2, palp = 2.0; tibiae I–IV = 1.9, 1.8, 1.6, 1.9, palp = 1.7; metatarsi I–IV = 1.3, 1.3, 1.3, 1.2; tarsi I–IV = 1.2, 1.3, 1.5, 1.2, palp = 1.8. Abdomen 12.3 long, 8.1 wide. Spinnerets: PMS, 1.4 long, 0.5 wide, 0.2 apart; PLS, 2.4 basal, 1.2 middle, 1.7 distal; mid-widths (lateral), 0.9, 0.8, 0.6, respectively. Carapace: length to width 1.08. Fovea 1.8 wide. Eyes: tubercle 0.8 high, 1.5 long, 2.1 wide. Clypeus 0.4. Anterior eye row procurved, posterior straight. Eyes size and inter-distances: AME 0.4, ALE 0.4, PME 0.3, PLE 0.4, AME–AME 0.4, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.3, ALE–PME 0.4, PME–PME 1.1, PME–PLE 0.1, PLE–PLE 1.6, ALE–PLE 0.3, AME–PLE 0.5. Ratio of eye group width to length 2.1. Maxillae: length to width: 1.6. Cuspules: 120–150 spread over ventral inner heel. Labium: 1.3 long, 2.1 wide, with ca. 90 cuspules spaced by one diameter from each other on the anterior third center. Labio-sternal groove shallow, flat, with two sigilla. Chelicerae: basal segments with eleven teeth decreasing in size from distal to basal portion. Sternum: 5.1 long, 4.9 wide. Legs: leg formula: I=IV II III. Scopula: Tarsi I–IV fully scopulate, IV with a row of sparse setae. Metatarsi I–II 4/5 scopulate; III 1/2 distal scopulate; IV 1/3 distal scopulate. IV divided by three wide row of setae. Tibial spurs. Legs: I, single, 0.4 long, 1.2 wide (Fig. 137); II, 0.2 long, 1.1 wide (Fig. 138); both with numerous spiniform setae on their tips. Urticating hairs type II (0.72 to 0.75 long, 0.015 wide) on abdomen dorsum. Palp: embolus 3.2 long, with a 90° curvature to retrolateral side. Embolus basal, middle and distal width of 0.8, 0.2 and 0.04, respectively. Tegulum 1.0 long, 1.5 wide. (Figs 134–136). Cymbium: spiniform process 0.7 long, 0.5 wide on the apex. Color pattern and setation as in female (Fig. 143).
Brazil: state of Bahia, endemic to Chapada Diamantina and its immediate surrounding regions, in Espinhaço mountain range (Fig. 169).
Typical spermatheca is slender, slightly curving inwards, tapering little from base to apex, not folded, with a distal constriction forming two to multiple distal lobes (Fig. 139, 153–155). Detected variation concerns shape and number of lobes.
Two specimens were found under rocks in Ibicoara (G. Machado, pers. comm.) and in Rio de Contas, both in state of Bahia. I found four specimens in Mucugê, Parque Nacional da Chapada Diamantina, all inside bromeliads (Vriesea atra) (Fig. 147). One of the specimens folded a bromeliad leaf and connected it with silk threads, making a retreat (Fig. 148). Inside with the mother were ca. 100 small juveniles (Fig. 149). The region where they were found is high (1400 m. a. s. l.) (Fig. 144) and vegetation is sparse, growing over a rocky substratum with shallow soil (campo rupestre) (Figs 145–146). Temperature is high during the day, falling abruptly at night. Water is largely available only during the rainy period. That’s a very distinct environmental condition when compared with the habitat in rainforest of Iridopelma hirsutum and Iridopelma zorodes, and seems inhospitable, mainly for an arboreal species. In this environment, bromeliads and space under rocks are among the few available shelter for spiders.
Cladistic analysis suggests that a retreat made with tree leaves and silk is primitive for Iridopelma species, and bromelicolous habitat in Iridopelma katiae sp. n. is derived. A possibility to explain the habit change could be the existence of a primitive forest in the area which became drier more recently in geological time, resulting in grasslands over a rocky or sandy shallow soil and lack of high vegetation. Bromeliads then become the primary (and relatively scarce) available microhabitat with similar conditions and resources, e. g. leaves as substratum and high humidity, necessities for an aviculariine spider. The environmental conditions in campo rupestre areas in Chapada Diamantina could aid in understanding the specialization to endemic bromelicolous lifestyle in other spider species, such as Pachistopelma spp.
Juveniles have light brown legs, except for black tarsi. Carapace is black and abdomen has a broad central longitudinal red stripe over a black area. Abdomen laterals are light brown (Fig. 140). When the individual grows, it becomes darker and retains the abdominal pattern, now with a longitudinal central black stripe (Fig. 141). Adults males and females are completely black and have a conspicuous abdominal pattern remains (Figs 142–143).
urn:lsid:zoobank.org:act:11BF0C3A-82BB-4337-8441-4446A8E9EB02
http://species-id.net/wiki/Iridopelma_oliveirai
Figs 156–165, 169The male differs from those of all other species by very long leg I (leg IV/I length = 0.73) (Fig. 165). The female resembles Iridopelma hirsutum by diverging spermathecae folded distally (Fig. 161), but differs by having a grayish carapace and legs and lacking an abdominal pattern (Fig. 164).
Specific name is a patronym in honor of Judge João Carlos Sá Moreira de Oliveira, for allowing the author to have access to part of the aviculariine specimens deposited in the Instituto Butantan collections, which were fundamental for the completion of this present work.
Holotype male (IBSP 10100), Brazil, state of Bahia, Central, Toca dos Pilões [11°08'S, 42°06'W], A. D. Brescovit, July 2000, and paratype female (IBSP 8714), at night in “Macambira” bromeliad (Bromelia laciniosa), same data.
Only the types.
Holotype male (IBSP 10100). Carapace 14.7 long, 12.7 wide, chelicerae 7.1. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 16.5, 7.9, 16.7, 17.8, 7.9, 68.8. II: 14.3, 7.3, 13.4, 14.4, 6.1, 55.5. III: 10.8, 5.6, 9.0, 10.2, 4.4, 40.0. IV: 13.1, 6.2, 12.1, 13.4, 4.8, 49.6. Palp: 9.1, 5.1, 6.9, –, 2.4, 23.5. Mid-widths (lateral): femora I –IV = 2.5, 2.4, 2.3, 2.3, palp = 1.8; patellae I–IV = 2.5, 2.4, 2.3, 2.4, palp = 1.9; tibiae I–IV = 1.9, 1.9, 1.9, 2.0, palp = 1.7; metatarsi I–IV = 1.3, 1.2, 1.2, 1.2; tarsi I–IV = 1.1, 1.2, 1.4, 1.5, palp = 1.6. Abdomen 12.8 long, 6.1 wide. Spinnerets: PMS, 1.1 long, 0.5 wide, 0.2 apart; PLS, 1.7 basal, 1.1 middle, 1.6 distal; mid-widths (lateral), 1.1, 0.9, 0.7, respectively. Carapace: length to width 1.16. Fovea: deep, 2.0 wide. Eyes: tubercle 0.9 high, 1.8 long, 2.5 wide. Clypeus 0.8. Anterior eye row procurved, posterior straight. Eye sizes and inter-distances: AME 0.4, ALE 0.5, PME 0.3, PLE 0.5, AME–AME 0.6, AME–ALE 0.3, AME–PME 0.2, ALE–ALE 1.6, ALE–PME 0.5, PME–PME 1.5, PME–PLE 0.1, PLE–PLE 1.9, ALE–PLE 0.5, AME–PLE 0.5. Ratio of eye group width to length 1.9. Maxillae: length to width: 2.0. Cuspules: 90–100 spread over ventral inner heel. Labium: 1.4 long, 2.3 wide, with ca. 40 cuspules. Labio-sternal groove deep, flat, with two sigillae. Chelicerae: basal segments with eleven teeth decreasing in size from distal to basal portion. Sternum: 6.6 long, 5.0 wide. Sigilla: not evident. Legs: leg formula: I II IV III. Scopula: tarsi I–IV fully scopulate. Metatarsi I–II 4/5 fully scopulate; III 3/4 distal scopulate; IV 1/2 distal scopulate. IV divided by three wide row of setae. Tibial spurs. Legs: I, single, 2.0 long, 1.2 wide, with numerous spiniform setae on its tip (Fig. 159); II, single, without elevation, formed by numerous spiniform setae (Fig. 160). Urticating hairs type II (0.73 to 0.77 long, 0.015 wide) on abdomen dorsum. Palp: embolus 2.2 long, with 105° curvature to retrolateral side. Embolus basal, middle and distal width of 0.2, 0.1 and 0.03, respectively. Tegulum 1.2 long, 1.5 wide. (Figs 156–158). Cymbium: Spiniform process 0.6 long, 0.5 wide on apex. Color pattern: carapace, chelicerae, legs and palps brown with light brown setae. Sternum, labium, maxillae and anterior half of coxa I black. Posterior half of coxa I and coxae II–IV brown with light brown setae. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi whitish. Distal femora, patellae, tibiae and metatarsi with white rings. Tarsi with dorsal “U” shaped orange stripe. Abdomen dorsum brown with long light brown setae. Ventrally black (Fig. 165).
Paratype female (IBSP 8714). Carapace 13.2 long, 11.4 wide, chelicerae 6.7. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 11.0, 6.2, 8.6, 8.0, 4.6, 38.4. II: 9.7, 5.8, 7.6, 7.1, 4.0, 34.2. III: 8.3, 4.8, 5.9, 6.3, 3.8, 29.1. IV: 10.2, 5.2, 8.6, 8.8, 4.0, 36.8. Palp: 7.4, 4.2, 5.0, –, 5.1, 21.7. Mid-widths (lateral): femora I –IV = 2.0, 2.0, 2.3, 2.1, palp = 1.8; patellae I–IV = 2.2, 2.1, 2.1, 2.2, palp = 2.0; tibiae I–IV = 2.0, 1.8, 1.8, 2.1, palp = 1.8; metatarsi I–IV = 1.7, 1.5, 1.5, 1.2; tarsi I–IV = 1.8, 1.7, 1.8, 1.5, palp = 1.9. Abdomen 13.2 long, 8.8 wide. Spinnerets: PMS, 1.6 long, 0.9 wide, 0.2 apart; PLS, 1, 8 basal, 1.3 middle, 1.8 distal; mid-widths (lateral), 1.3, 1.1, 0.8, respectively. Carapace: length to width 1.16. Fovea: 2.4 wide. Eyes: tubercle 0.7 high, 1.6 long, 2.3 wide. Clypeus 0.4. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.4, ALE 0.4, PME 0.4, PLE 0.4, AME–AME 0.5, AME–ALE 0.2, AME–PME 0.5, ALE–ALE 1.7, ALE–PME 0.5, PME–PME 1.4, PME–PLE 0.1, PLE–PLE 1.8, ALE–PLE 0.5, AME–PLE 0.5. Ratio of eye group width to length 2.1. Other characters as in male, except: maxillae: length to width: 1.8. Cuspules: 100–150 spread over ventral inner heel. Labium: 1.5 long, 1.9 wide, with ca. 50 cuspules. Labio-sternal groove deep, narrow, with two sigillae. Chelicerae: basal segments with thirteen teeth decreasing in size from distal to basal portion. Sternum: 5.8 long, 5.1 wide. Legs: leg formula: I=IV II III. Scopula: tarsi I–IV fully scopulate. Metatarsi I–II fully scopulate; III 2/3, IV 1/2 distal scopulate, IV divided by three wide row of setae. Urticating hairs type II (0.57 to 0.61 long, 0.015 wide) on abdomen dorsum. Genitalia: paired diverging spermathecae tapering from base to apex, folded medially, with strong distal constriction forming two distal lobes (Fig. 161). Color pattern as in male, except: carapace, chelicerae, legs and palps grayish with pinkish setae; patellae and tibiae of palps and legs I–II and tibiae and metatarsi of leg III ventrally dark. Sternum, labium, maxillae and coxae black. Abdomen dorsum brown with long pinkish setae.
Brazil: known only from Central, State of Bahia (Fig. 169).
Little is known regarding this species. The vegetation in Central, state of Bahia, comprises mainly caatinga (a xerophilous vegetation). One female was found at night in a bromeliad (Bromelia laciniosa).
Data for small juveniles is lacking. Large immatures specimens have a pattern similar to Iridopelma hirsutum (Figs 162–163). Adult male and female lack or have a very inconspicuous abdominal pattern (Figs 164–165).
urn:lsid:zoobank.org:act:4ED0A9EA-7460-4CAE-82B8-EB38B489CBE0
http://species-id.net/wiki/Iridopelma_marcoi
Figs 166–169The female resembles that of Iridopelma vanini sp. n. by long double folded spermathecae (Fig. 166), but differ by lacking type II urticating setae on abdomen dorsum. Male unknown.
The specific name is a patronym in honour of Marco Antonio de Freitas, a Brazilian zoologist and geographer who collected the holotype and some other aviculariine specimens studied in this work.
Holotype female, Brazil, state of Bahia, São Desidério (12°28'52"S, 45°09'10"W), 724 m a.s.l, M. A. Freitas, October 2009, under tree bark, 1 m above the ground, area of carrasco vegetation (MZSP 36891).
Only holotype.
Holotype female (MZSP 36891). Carapace 14.9 long, 14.6 wide, chelicerae 9.3. Legs (femur, patella, tibia, metatarsus, tarsus, total): I: 12.4, 7.8, 8.9, 8.3, 4.5, 41.9. II: 11.4, 6.9, 8.1, 7.6, 4.4, 38.4. III: 10.0, 6.1, 7.3, 6.5, 4.3, 34.2. IV: 12.2, 6.5, 9.7, 10.1, 4.7, 43.2. Palp: 8.7, 5.5, 5.6, –, 5.9, 25.7. Mid-widths (lateral): femora I–IV = 3.0, 3.1, 3.2, 2.5, palp = 2.4; patellae I–IV = 2.8, 2.8, 2.8, 2.8, palp = 2.4; tibiae I–IV = 2.6, 2.6, 2.5, 2.4, palp = 2.3; metatarsi I–IV = 2.1, 2.0, 2.0, 1.6; tarsi I–IV = 2.2, 2.2, 229, 2.2, palp = 2.2. Abdomen 16.7 long, 11.7 wide. Spinnerets: PMS, 1.9 long, 0.9 wide, 0.3 apart; PLS, 2.3 basal, 1.5 middle, 2.2 distal; mid-widths (lateral), 1.3, 1.2, 1.0, respectively. Carapace: length to width 1.02. Fovea: deep, 2.5 wide. Eyes: tubercle 0.8 high, 2.1 long, 2.6 wide. Clypeus 1.0. Anterior eye row procurved, posterior slightly recurved. Eye sizes and inter-distances: AME 0.5, ALE 0.5, PME 0.4, PLE 0.4, AME–AME 0.6, AME–ALE 0.3, AME–PME 0.1, ALE–ALE 1.8, ALE–PME 0.5, PME–PME 1.6, PME–PLE 0.1, PLE–PLE 2.1, ALE–PLE 0.4, AME–PLE 0.4. Ratio of eye group width to length 2.0. Maxillae: length to width: 2.0. Cuspules: 100–150 spread over ventral inner heel. Labium: 1.9 long, 2.6 wide, with ca. 150 cuspules. Labio-sternal groove deep, narrow, sigilla not evident. Chelicerae: basal segments with ten teeth decreasing in size from distal to basal portion. Sternum: 7.5 long, 6.6 wide. Legs: leg formula: I=IV II III. Scopula: tarsi I–IV fully scopulate. Metatarsi I–II 4/5 scopulate; III 2/3, IV 1/2 distal scopulate. IV divided by three wide row of setae. Urticating hairs lacking on abdomen dorsum. Genitalia: paired long and converging spermathecae tapering strongly from base to apex, double folded and with a strong distal constriction forming one or two distal lobes (Fig. 166). Color pattern: carapace, chelicerae, and femora of legs and palps dark brown covered with dense layer of metallic green setae. Other leg and palp segments dorsally dark brown with brown setae. Tibiae and patellae covered with metallic blue setae prolaterally and retrolaterally; ventrally black. Tarsi with a dorsal “U” orange stripe. Sternum, labium, maxillae and coxae black. Abdomen ventrally black; dorsally dark brown with a large lighter central spot. Longitudinal stripes on dorsum of femora, patellae, tibiae and metatarsi inconspicuous. Distal femora, patellae, tibiae and metatarsi with broad white rings (Fig. 167).
Male: unknown.
Brazil: known only from São Desidério, state of Bahia (Fig. 169).
The only known specimen was found in cerrado/carrasco vegetation (Fig. 168), under loose tree bark about one meter above the ground (M. A. Freitas, pers. comm.).
Only the adult female holotype is known.
Searches using NONA resulted in 12 trees and strict consensus is used (Fig. 177). With X-PEE-WEE, it was found from 1 to 6 trees, depending on the concavity used (Table 2). Cladogram obtained with concavity 6 is shown in Figs 178. The main difference of cladograms obtained with the different strategies comprise the position of clade having Ephebopus, Tapinauchenius and Psalmopoeus. In NONA and X-PEE-WEE with concavities 2–6 that clade is paraphyletic in respect to other aviculariine genera (Figs 177–178); with X-PEE-WEE and concavity 1 Aviculariinae is retrieved as monophyletic. Topologies of clade Typhochlaena (Avicularia spp.1 (Pachistopelma, Avicularia spp.2, Iridopelma)) were retrieved in all employed search strategies. Resolution of the terminal clade retrieving Iridopelma as sister group of Avicularia spp.2 was obtained with X-PEE-WEE and concavities 1–5, whereas Iridopelma and Avicularia spp.2 were in a tricotomy with Pachistopelma when using NONA and X-PEE-WEE with concavity 6. Internal relationship of Typhochlaena is totally unresolved with NONA, and partially resolved with X-PEE-WEE, independently of the concavity used. Position of Iridopelma marcoi sp. n. and Iridopelma katiae sp. n. change more markedly. With concavities 1–5 Iridopelma katiae sp. n. is sister-group of Pachistopelma (Iridopelma + Avicularia spp.2). With NONA and X-PEE-WEE with concavity 6 it is part of Iridopelma clade. Iridopelma marcoi sp. n. is part of Iridopelma clade only with NONA. With X-PEE-WEE it is always polyphyletic. Its position will be fully discussed below.
Number of trees obtained, length and fit using different concavities and X-PEE-WEE.
| Trees | Length | FIT | |
|---|---|---|---|
| K = 1 | 2 | 221 | 2945, 4 |
| K = 2 | 6 | 213 | 3549, 1 |
| K = 3 | 6 | 213 | 3919, 8 |
| K = 4 | 6 | 213 | 4172, 5 |
| K = 5 | 1 | 211 | 4361, 2 |
| K = 6 | 1 | 209 | 4507, 4 |
Dorsal abdominal pattern in immatures 170 herringbone (Pterinochilus sp.) 171 with a zigzag central longitudinal dark stripe over a clear spot, which is marginated in black and connects to five narrow transversal black stripes (Poecilotheria sp.) 172 two median dorso-lateral spots (Euathlus vulpinus (Karsch, 1880)) 173 central longitudinal black stripe with 5–6 lateral stripes, connecting or not with the central stripe (Pachistopelma bromelicola sp. n.) 174 black with a light central longitudinal large spot having zigzag edges and a longitudinal central black stripe over a more reddish area (Iridopelma hirsutum Pocock, 1901) 175 two longitudinal dark stripes with five lateral stripes connecting to them (Avicularia diversipes (C. L. Koch, 1842)).
Avicularia taunayi (Mello-Leitão, 1920), left male palpal bulb, frontal view. Arrow indicates accentuated depression on retrolateral tegulum. Scale bar = 1 mm.
The results obtained herein were compared with other published analyses for Ephebopus spp. and Aviculariinae genera relationship of
Strict consensus of 12 trees obtained with Nona and all characters non-additive. Black square = synapomorphy, white square = homoplasy. Length = 211, Ci = 0.44, Ri = 0.68.
Single tree obtained with X-Pee-Wee, all characters non-additive and concavity 6. Fit = 4507.4 length = 209.
In agreement with other studies (
Characters, Fits, Steps and Extra Steps for characters of cladogram of Fig 178.
| Character | Fit | Steps | Extra Steps | Character | Fit | Steps | Extra Steps |
|---|---|---|---|---|---|---|---|
| 0 | 50 | 7 | 6 | 31 | 100 | 2 | 0 |
| 1 | 66.7 | 4 | 3 | 32 | 42.86 | 9 | 8 |
| 2 | 54.5 | 7 | 5 | 33 | 75 | 3 | 2 |
| 3 | 100 | 2 | 0 | 34 | 75 | 3 | 2 |
| 4 | 85.71 | 2 | 1 | 35 | 100 | 2 | 0 |
| 5 | 60 | 5 | 4 | 36 | 85.71 | 2 | 1 |
| 6 | 85.71 | 3 | 1 | 37 | 100 | 1 | 0 |
| 7 | 100 | 1 | 0 | 38 | 85.71 | 2 | 1 |
| 8 | 85.71 | 2 | 1 | 39 | 60 | 6 | 4 |
| 9 | 100 | 1 | 0 | 40 | 54.54 | 7 | 5 |
| 10 | 100 | 1 | 0 | 41 | 54.54 | 7 | 5 |
| 11 | 85.71 | 2 | 1 | 42 | 85.71 | 2 | 1 |
| 12 | 50 | 8 | 6 | 43 | 85.71 | 3 | 1 |
| 13 | 40 | 11 | 9 | 44 | 85.71 | 2 | 1 |
| 14 | 75 | 5 | 2 | 45 | 85.71 | 2 | 1 |
| 15 | - | - | - | 46 | 85.71 | 2 | 1 |
| 16 | 66.67 | 5 | 3 | 47 | 85.71 | 2 | 1 |
| 17 | 85.71 | 2 | 1 | 48 | 66.67 | 6 | 3 |
| 18 | - | - | - | 49 | 100 | 1 | 0 |
| 19 | 85.71 | 2 | 1 | 50 | 75 | 3 | 2 |
| 20 | 100 | 1 | 0 | 51 | 85.71 | 3 | 1 |
| 21 | 75 | 3 | 2 | 52 | - | - | - |
| 22 | 85.71 | 2 | 1 | 53 | 100 | 1 | 0 |
| 23 | - | - | - | 54 | 100 | 1 | 0 |
| 24 | 60 | 6 | 4 | 55 | - | - | - |
| 25 | 66.67 | 4 | 3 | 56 | 100 | 1 | 0 |
| 26 | 100 | 1 | 0 | 57 | 85.71 | 3 | 1 |
| 27 | 50 | 7 | 6 | 58 | 75 | 4 | 2 |
| 28 | 85.71 | 2 | 1 | 59 | 50 | 9 | 6 |
| 29 | 75 | 8 | 2 | 60 | 75 | 5 | 2 |
| 30 | 85.71 | 2 | 1 | 61 | 85.71 | 4 | 1 |
Synapomorphies for cladogram of Fig. 178.
| Taxa or Node | Character | Change | Taxa or Node | Character | Change | Taxa or Node | Character | Change |
|---|---|---|---|---|---|---|---|---|
| Holothele | 3 | 0 → 1 | Ephebopus cyanognathus | 13 | 1 → 2 | 29 | 0 → 4 | |
| 32 | 0 → 1 | 25 | 0 → 1 | 50 | 0 → 1 | |||
| 39 | 0 → 1 | Ephebopus foliatus | 27 | 0 → 1 | 60 | 3 → 2 | ||
| Heteroscodra | 12 | 1 →0 | Ephebopus rufescens | 60 | 3 → 2 | Node 46 | 24 | 1 → 2 |
| Pterinochilus | 2 | 1 → 2 | Avicularia sp.1 | 24 | 1 → 2 | Node 47 | 51 | 0 → 2 |
| 13 | 1 → 2 | Avicularia diversipes | 24 | 1 → 2 | 56 | 0 → 1 | ||
| 19 | 0 → 1 | 33 | 0 → 1 | Node 48 | 14 | 2 → 3 | ||
| 58 | 0 → 1 | 40 | 1 → 2 | 28 | 0 → 1 | |||
| 59 | 0 → 2 | Avicularia sooretama | 32 | 0 → 1 | Node 49 | 2 | 1 → 0 | |
| Pelinobius | 2 | 0 → 2 | 39 | 0 → 1 | 16 | 0 → 1 | ||
| 12 | 1 → 0 | Avicularia taunayi | 32 | 0 → 1 | 39 | 0 → 1 | ||
| 13 | 1 → 0 | 59 | 0 → 1 | Node 50 | 12 | 1 → 0 | ||
| 15 | 0 → 1 | Typhochlaena seladonia | 0 | 1 → 0 | 13 | 1 → 0 | ||
| 17 | 0 → 1 | 16 | 2 → 1 | 27 | 1 → 0 → 1 | |||
| 22 | 0 → 1 | 24 | 1 → 0 | Node 51 | 5 | 0 → 1 | ||
| 32 | 0 → 1 | 25 | 0 → 1 | 33 | 0 → 1 | |||
| 58 | 0 → 1 | Typhochlaena amma | 12 | 1 → 0 | 39 | 0 → 2 | ||
| 59 | 0 → 2 | 25 | 0 → 1 | Node 52 | 36 | 1 → 0 | ||
| Haplopelma | 2 | 1 → 2 | 33 | 0 → 1 | Node 53 | 29 | 4 → 6 | |
| 8 | 0 → 1 | Typhochlaena curumim | 12 | 1 → 0 | Node 54 | 0 | 1 → 0 | |
| 19 | 0 → 1 | 32 | 0 → 1 | 1 | 0 → 1 → 0 | |||
| 38 | 0 → 1 | Typhochlaena paschoali | 16 | 2 → 1 | 2 | 1 → 0 | ||
| 47 | 0 → 1 | 32 | 0 → 1 | 9 | 0 → 1 | |||
| 48 | 3 → 2 | Typhochlaena costae | 59 | 0 → 1 | 12 | 1 → 0 | ||
| 60 | 1 → 3 | Iridopelma hirsutum | 35 | 1 → 0 | 13 | 1 → 0 | ||
| Phlogiellus | 0 | 1 → 0 | Iridopelma vanini | 5 | 0 → 1 | 24 | 1 → 0 | |
| 6 | 0 → 2 | Iridopelma katiae | 30 | 1 → 0 | 40 | 1 → 0 | ||
| 11 | 1 → 0 | 59 | 0 → 3 | 61 | 1 → 3 | |||
| 21 | 0 → 1 | 61 | 1 → 3 | Node 55 | 34 | 0 → 1 | ||
| Lasiodora | 2 | 1 → 2 | Iridopelma marcoi | 25 | 0 → 1 | Node 56 | 29 | 4 → 5 |
| 18 | 0 → 1 | 27 | 1 → 0 | Node 58 | 7 | 0 → 1 | ||
| 22 | 0 → 1 | 32 | 0 → 1 | 10 | 0 → 1 | |||
| 31 | 0 → 1 | 34 | 0 → 1 | Node 59 | 59 | 0 → 1 | ||
| 41 | 0 → 1 | 59 | 0 → 1 | Node 60 | 34 | 0 → 1 | ||
| 42 | 0 → 1 | Iridopelma oliveirai | 27 | 1 → 0 | Node 61 | 32 | 0 → 1 | |
| 43 | 0 → 1 | Encyocratella | 4 | 0 → 1 | Node 62 | 29 | 4 → 5 | |
| 46 | 0 → 1 | 5 | 0 → 1 | 41 | 2 → 0 | |||
| 47 | 0 → 1 | 6 | 0 → 1 | 49 | 0 → 1 | |||
| 52 | 0 → 1 | 17 | 0 → 1 | Node 64 | 30 | 0 → 1 | ||
| Euathlus | 6 | 0 → 1 | 58 | 0 → 1 | 61 | 0 → 1 | ||
| 13 | 1 → 2 | Node 38 | 0 | 1 → 0 | Node 65 | 28 | 0 → 1 | |
| 29 | 0 → 3 | 1 | 0 → 1 | 36 | 0 → 1 | |||
| 32 | 0 → 1 | 4 | 0 → 1 | Node 66 | 29 | 1 → 4 | ||
| 55 | 0 → 1 | 26 | 0 → 1 | 40 | 0 → 1 | |||
| 59 | 0 → 1 | 50 | 0 → 1 | 51 | 0 → 1 | |||
| Poecilotheria | 0 | 1 → 0 | 58 | 0 → 2 | 53 | 0 → 1 | ||
| 14 | 2 → 3 | Node 39 | 3 | 0 → 2 | Node 68 | 14 | 2 → 3 | |
| 16 | 0 → 2 | 8 | 0 → 1 | 16 | 0 → 2 | |||
| 21 | 0 → 1 | Node 40 | 20 | 0 → 1 | Node 69 | 60 | 3 → 1 | |
| 29 | 1 → 2 | 40 | 0 → 1 | Node 70 | 27 | 0 → 1 | ||
| 41 | 0 → 1 | 46 | 0 → 1 | 29 | 0 → 1 | |||
| 59 | 0 → 1 | Node 41 | 42 | 0 → 1 | Node 71 | 37 | 0 → 1 | |
| Psalmopoeus | 13 | 1 → 2 | 44 | 0 → 1 | Node 72 | 0 | 0 → 1 | |
| 21 | 0 → 1 | 45 | 0 → 1 | 11 | 0 → 1 | |||
| 40 | 0 → 1 | Node 42 | 38 | 0 → 1 | 12 | 0 → 1 | ||
| Tapinauchenius | 39 | 1 → 0 | 44 | 0 → 1 | 13 | 0 → 1 | ||
| Ephebopus murinus | 12 | 1 → 2 | 45 | 0 → 1 | 24 | 0 → 1 | ||
| 13 | 1 → 2 | 51 | 0 → 1 | Node 73 | 2 | 0 → 1 | ||
| 23 | 0 → 1 | 54 | 0 → 1 | 60 | 0 → 3 | |||
| 57 | 1 → 2 | Node 43 | 0 | 1 → 0 |
Typhochlaena is monophyletic and supported by characters sternum as long as wide, truncated behind (character 7, Table 4), and posterior lateral spinnerets with distal segment short, domed (character 10). The topology of Typhochlaena clade shows a tricotomy. In that Typhochlaena seladonia and Typhochlaena curumim sp. n. are sister-groups supported by spiraled spermathecae (character 34) with homoplasies in Iridopelma marcoi sp. n. and Iridopelma hirsutum (Iridopelma vanini sp. n. + Iridopelma oliveirai sp. n). Typhochlaena amma sp. n. and Typhochlaena paschoali sp. n. are sister-groups supported by abdomen pattern in immatures (state 5 of character 29) with homoplasies with most Iridopelma species.
Pachistopelma is monophyletic and supported by 9 synapomorphies: low eye tubercle (character 0), straight anterior row of eyes (character 1), clypeus absent (character 2), flattened abdomen a quarter longer than wide in females (character 9), leg IV longer than leg I in females (character 12) and in males (character 13), leg rings on distal tibiae and metatarsi not evident (character 24), embolous straight or very slightly curved (character 40), bromelicolous lifestyle (61). All these synapomorphies are very homoplasious (3 additional steps or more), except for character 9, which is exclusive for the genus, and character 61, homoplastic with Iridopelma katiae sp. n.
Iridopelma is monophyletic only if Iridopelma marcoi sp. n. is excluded. However, some important characters for the analyses are exclusive to males (i. e., tibial spurs, cymbium protuberance) and at least one, exclusive to Iridopelma (tibial spur presence on leg II). The lack of a known male in this species undoubtedly influenced its position in the cladogram topology. Also, the absence of urticating hairs in female, that is an important synapomorphy for a large aviculariine clade, surely contributed for this result as well. On the other hand, the spermathecae shape, as well as some somatic characters indicate it is an Iridopelma species. The discovery of the male of Iridopelma marcoi sp. n. migth confirm its position.
Other Iridopelma species are in a monophyletic clade. Three synapomorphies support Iridopelma monophyly: dorsal abdominal pattern in immatures (Character 29), homoplasious with Typhochlaena amma sp. n.; embolous length that changes from long to median (Character 41), extremally homoplasious, and presence of a tibial spur also on leg II, exclusive for the genus (character 49). Therefore, the main synapomorphy for Iridopelma is still the classical character proposed by
Typhochlaena, Pachistopelma and Iridopelma species are highly endemic, and there is no overlapping in distribution of species belonging to the same genus (Figs 179–181). Most areas of endemism for species of those genera are mostly concordant with river systems, as proposed for Atlantic rainforest in Northeastern and Southeastern Brazil (
Area cladogram for Typhochlaena species in Brazil.
Area cladogram for Pachistopelma species in Brazil.
Area cladogram for Iridopelma species in Brazil.
Area cladogram for Avicularia spp.2 (Avicularia diversipes (C. L. Koch, 1842), Avicularia gamba Bertani and Fukushima, 2009 and Avicularia sooretama Bertani and Fukushima, 2009) in Brazil.
At least two hypotheses were proposed to explain the existence of endemism areas in Atlantic forest of Northeastern and Southeastern Brazil.
Whereas distribution of congeners did not overlap, there is strong ovelap of species of Typhochlaena with Pachistopelma, Iridopelma and Avicularia spp.2 as well as of Iridopelma with Pachistopelma (Fig. 183). Typhochlaena spp. overlap with Avicularia spp. 2 on state of Espírito Santo and Southern state of Bahia, with Iridopelma on Northern state of Bahia, Sergipe, Paraíba, Piauí, Maranhão and possibly Pernambuco, Alagoas and Tocantins. They also overlap with Pachistopelma spp. in Northern Bahia, Sergipe, Alagoas, Pernambuco and Paraíba. Pachistopelma spp. overlap with Iridopelma spp. and Typhochlaena spp. over almost all their distribution (from Rio Grande do Norte to Northern Bahia). Iridopelma possibly overlaps slightly with Avicularia spp.1 in parts of Maranhão, Pará and Tocantins, with Typhochlaena and Pachistopelma on the coast and with Typhochlaena on Northern Bahia, Sergipe, Alagoas, Pernambuco, Paraiba and Southern Ceara, Piauí, Maranhão and parts of Tocantins. Most of the overlap involves Typhochlaena – the most basal genus of the clade, and, if this genus is not considered, only a marginal overlapping of Avicularia spp.1, Avicularia spp.2 and Iridopelma will remain (except Pachistopelma, which will be discussed separately).
Area cladogram for Avicularia spp.1, Avicularia spp.2, Iridopelma spp., Pachistopelma spp. and Typhochlaena spp. in Brazil.
The overlap of Iridopelma spp. with Pachistopelma spp. in most of the distribution of the latter deserves a more detailed discussion. Pachistopelma spp. are strictly associated with bromeliads (
Pachistopelma spp. inhabit tank bromeliads - Aechmea aquilega (
Pachistopelma spp. seem specialized to live inside bromeliads, mainly by the dorso-ventrally flattened body of immatures and female which aids the spider in moving between the narrow inter-leaves spaces (Bertani 1994, Bertani in
At least five other aviculariine species are found in open vegetation regions - cerrado (Typhochlaena costae sp. n., Iridopelma marcoi sp. n., Iridopelma vanini sp. n.), caatinga (Iridopelma oliveirai sp. n.), sandy dunes/restinga (Iridopelma vanini sp. n.), or campo rupestre (Iridopelma katiae sp. n.). For all species with available field data, there are records of them living on trees (Iridopelma marcoi sp. n.) or in bromeliads (Iridopelma oliveirai sp. n., Iridopelma katiae sp. n.). The holotype of Iridopelma vanini sp. n. was found under a fallen tree trunk on a sandy dune region in Parnaíba, state of Piaui. Iridopelma katiae sp. n. habitat is interesting, because it is in a high region (1200–1300 m a.s.l.) in a campo rupestre area. As restinga and caatinga, the climate is severe, with rocky formations, water stress present most times of year, high temperatures during the day and low temperatures at night, and few sparse trees (
Another bromelicolous spider, the salticid Psecas chapoda (Peckham & Peckham, 1894), is specialized to living inside the bromeliad Bromelia balanseae (
This complex history may be responsible for the extraordinary diversity of aviculariines in Northeastern, Southeastern and Central-western Brazil, which rivalizes with the aviculariine forms found in Northern South America and Central America in number of species, morphological variation and habitat use. Specifically, parts of Northeastern Brazil, as Reconcavo Bahiano, has one of the richest aviculariine fauna in the world, with records for Avicularia diversipes, Avicularia gamba, Iridopelma zorodes, Pachistopelma bromelicola sp. n., and possibly at least one Typhochlaena species.
Due to their limited dispersal abilities and high endemism, theraphosids are important taxa for biogeographic and evolutionary studies. Phylogeographical studies with species of aviculariines would aid in understanding important evolutionary processes and biogeographical patterns in South America, and I expect the present paper stimulates this kind of study with these animals.
As with other Brazilian Atlantic forest aviculariines (Avicularia diversipes, Avicularia sooretama and Avicularia gamba –
Iridopelma hirsutum and Iridopelma zorodes are abundant, but they are known only from patches of Atlantic forest, which is limited to 7.91% of its original distribution (
I thank Ibama and ICMBio for collection permits. Directors of the conservation areas of Reserva Biológica de Saltinho (Fábio Cunha), Parque Nacional de Itabaiana, Estação Ecologica de Murici (Jaílton J. F. Fernandes), Parque Nacional da Chapada Diamantina (Cezar Gonçalves) are thanked for allowing the development of this project in the area and giving support for field work. Private Natural Heritage Reserves (RPPNs) of Charles Darwin (Roberto Siqueira), Jequitibá (Elbano Paschoal de F. Moraes in mem., Maria Theresa S. Stradmman), Sapiranga and Camarujipe (Fundação Garcia D’Ávila, Naína) and Reserva Ecológica do Pau Ferro are thanked for allowing and supporting collection of specimens and field work. Several colleagues helped with specimens and field data: Marco Antonio de Freitas, AMMA project (mainly Abel Pérez Gonzalez, Alessandro Giupponi and Adriano Kury), Glauco Machado, Carlos E. V. Toledo, Rafael A. Sanfilippo and Luiz Sanfilippo. Adriano Kury (MNRJ), Alexandre Bonaldo (MPEG), Janet Becalloni (NHM), Jose Clavijo, Osvaldo Villarreal (MIZA), Luca Bartolozzi (MZUF), Paulo Motta (DZUB), Peter Jäger (SMF) and Ricardo Pinto-da-Rocha (MZSP) kindly loaned specimens for study and/or allowed the examination of specimens from collections under their care. Roberto H. Nagahama, Caroline Sayuri Fukushima, Diego Ribeiro Migueis Ortega, Felipe Borba, Missinho (Chapada Diamantina), Francisco Félix da Silva (Murici), helped in field work. Carol Fukushima critically read the manuscript and made suggestions. Katia M. Faria kindly made most of the illustrations. Volker von Wirth, Ray Gabriel and Andrew Smith are thanked for their hospitality when I was in Europe consulting arachnological collections. The referees Robert Raven, Rick West, Ray Gabriel and Richard Gallon improved the manuscript. Support: FAPESP 03/12587-4.