(C) 2013 Terue C. Kihara. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Kihara CT, Rocha CEF (2013) First record of Clausidium (Copepoda, Clausidiidae) from Brazil: a new species associated with ghost shrimps Neocallichirus grandimana (Gibbes, 1850) (Decapoda, Callianassidae). ZooKeys 335: 47–67. doi: 10.3897/zookeys.335.5490
A new clausidiid copepod was found living in galleries of ghost shrimps Neocallichirus grandimana (Gibbes, 1850) in Natal, Brazil. The new species resembles to Clausidium senegalense Humes, 1957 and Clausidium vancouverense (Haddon, 1912) in the armature of P2–P5 of the female, and shares with Clausidium senegalense similar segmentation and armature of the antenna and maxilla of the female. Nevertheless, it can be easily distinguished from its congeners by the unique characteristics observed in the antenna, maxilliped and first leg of males, as well as by the anal somite, maxillule and maxilliped of the females. This new species extends the group distribution to the Southwest Atlantic and represents the first record of the genus in Brazil. A key for the identification of the species based on females of Clausidium is provided.
Biodiversity, CLSM, Crustacea, Poecilostomatoida, taxonomy, identification key
Clausidiids characterized by the presence of sucking discs on endopods of legs 1 to 4, the genus Clausidium Kossmann, 1874 was established to accommodate Clausidium apodiforme (Philippi, 1839). During the revision by
The representatives of Clausidium are typically external associates of marine decapods. They can be found inhabiting the burrows of ghost or mud shrimps of the families Callianassidae Dana, 1852 and Upogebiidae Borradaile, 1903 (Table 1).
A list of species of Clausidium including known distributional records, hosts and references.
| Species | Distribution | Hosts | References |
|---|---|---|---|
| Clausidium apodiforme (Phillippi, 1839) | North Atlantic Ocean | Calianassa subterranea (Montagu, 1808) | |
| Syn: Hersilia apodiformis Phillipi, 1839 | (Adriatic and | Calianassa sp. | |
| Clausidium testudo Kossman, 1874 | Mediterranean sea) | Pestarella candida (Olivi, 1792) | |
| Clausidium caudatum (Say, 1818) | North Atlantic Ocean | Callichirus major (Say, 1818) | |
| Clausidium chelatum Pillai, 1959 | Indian Ocean | Calianassa sp. | |
| Clausidium dissimile Wilson C.B., 1921 | North Atlantic Ocean | Calianassa sp. | |
| Gilvossius setimanus (De Kay, 1844) | |||
| Lepidophthalmus louisianensis (Schmitt, 1935) | |||
| Sergio trilobata (Biffar, 1970) | |||
| Clausidium saldanhae Kensley, 1974 | South Atlantic Ocean | Pestarella rotundicaudata (Stebbing, 1902) | |
| Clausidium searsi Wilson C. B., 1937 | South Pacific Ocean | Calianassa sp. | |
| Clausidium senegalense Humes, 1957 | South Atlantic Ocean | Calianassa sp. | |
| Clausidium tenax Humes, 1949 | North Atlantic Ocean | Callichirus islagrande (Schmitt, 1935) | |
| Clausidium travancorense Pillai, 1959 | Indian Ocean | Neocallichirus maxima (A. Milne-Edwards, 1870) | |
| Clausidium vancouverense (Haddon, 1912) | North Pacific Ocean | Callichirus seilacheri (Bott, 1955) | |
| Syn: Hersilia (Clausidium) vancouverensis | South Pacific Ocean | Neotrypaea californiensis (Dana, 1854) | |
| Haddon, 1912 | Neotrypaea gigas (Dana, 1852) | ||
| Clausidium californiense Wilson C. B., 1935 | Upogebia pugettensis (Dana, 1852) | ||
| Clausidium sp. | South Pacific Ocean | Callichirus seilacheri (Bott, 1955) | |
| Clausidium rodriguesi sp. n. | South Atlantic Ocean | Neocallichirus grandimana (Gibbes, 1850) |
Although Clausidium is reported in population studies of the ghost shrimp Callichirus seilacheri (Bott, 1955) from Chilean coast (
A new clausidiid copepod, which can not be reconciled to any of the 10 species of Clausidium that have been described so far, was found living in galleries of ghost shrimps Neocallichirus grandimana (Gibbes, 1850) in the intertidal zone of a beach in Natal, state of Rio Grande do Norte (N.E. of Brazil). This is the first record of genus Clausidium in Brazil.
The copepods were recovered from water drawn from the burrows and collected from pleopods of the ghost shrimp Neocallichirus grandimana in the intertidal zone of a beach in Natal, state of Rio Grande do Norte, Brazil (5°45'S, 35°11'W).
Whole specimens were examined in temporary lactic acid mounts. Chips of cover slip were used to support the cover glass of the preparation. After examination, material was returned to and preserved in 70% ethanol. Dissections were made in glycerine and the dissected parts were placed on slides and sealed with Glyceel.
A Leitz Laborlux D® phase-contrast microscope and a Zeiss Axioskop 2 Plus® compound microscope equipped with differential interference contrast, digital camera Nikon Coolpix 995® and camera lucida were used to examine and prepare illustrations of the specimens.
Two females and two males were prepared for scanning electron microscopy (SEM). Specimens were dehydrated through a series of graded acetone; critical-point dried, mounted on stubs, sputter-coated with palladium and observed using a Philips XL 30 Field Emission Scanning Electron microscope (Philips, Eindhoven, Netherlands).
For confocal laser scanning microscopy (CLSM), a female was stained with Congo Red and mounted on slide following the procedure described by
Series of stacks were obtained, collecting overlapping optical sections throughout the whole preparation; the optimal number of scans and the imaging settings according to the software, are given in Table 2. Final images were obtained by maximum projection, and CLSM illustrations were composed and adjusted for contrast and brightness using the software Adobe Photoshop CS4 (Adobe Systems, San José, U.S.A.).
Total body length was measured from the anterior margin of the rostrum to the posterior margin of the caudal rami. The descriptive terminology follows
The type material is deposited in the collection of the Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil.
Microscope lens and confocal laser scanning microscopy (CLSM) settings used for the observation of the specimens; Ch1 and Ch2 = detection channels 1 and 2.
| Lens | HC PL APO CS (High-grade colour-corrected Plan Apochromat lens for confocal) |
|---|---|
| Objective | 20× |
| Numerical aperture | 0.7 |
| Immersion | Oil |
| Excitation wavelength | 488 and 633 nm |
| Laser intensity | 50% and 33%, respectively |
| Excitation beam splitter | TD 488/561/633 |
| Detected emission wavelength | Ch1: 493 – 600 nm |
| Ch2: 650 – 750 nm | |
| Detector gain | 833.8 and 791.6 V |
| Amplitude offset | -0.9 and -1.0 % |
| Electronic zoom | 3X |
| Pinhole aperture | 54.6 μm |
| Image format | 2048 × 2048 dpi |
http://zoobank.org/96A49C80-55DA-484C-AC7A-08A84A5AE21F
http://species-id.net/wiki/Clausidium_rodriguesi
Figs 1 – 11Holotype female (reg. no. MZUSP 16464) in ethanol, dissected paratypes consist of 2 females and 2 males (reg. no. MZUSP 19628–19631) undissected paratype consist of 1 female (reg. no. MZUSP 19632) deposited in the collection of the Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil. All material collected in 02/1984 from the type locality by Prof. Dr. G. Shimizu.
Rio Grande do Norte, Natal, margin of Potengi river (5°45'S, 35°11'W). All specimens from water drawn from the burrows and pleopods of the ghost shrimp Neocallichirus grandimana.
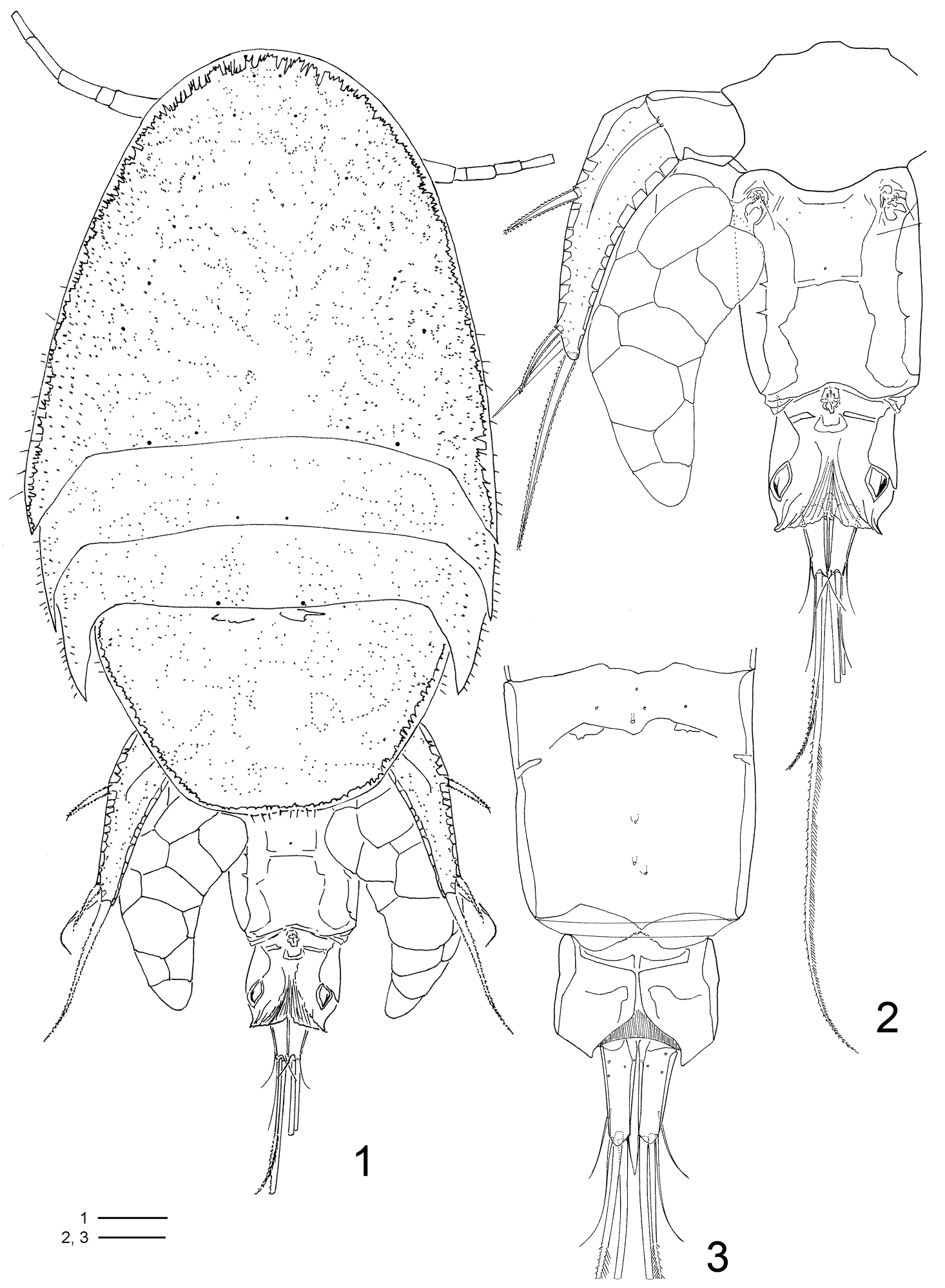
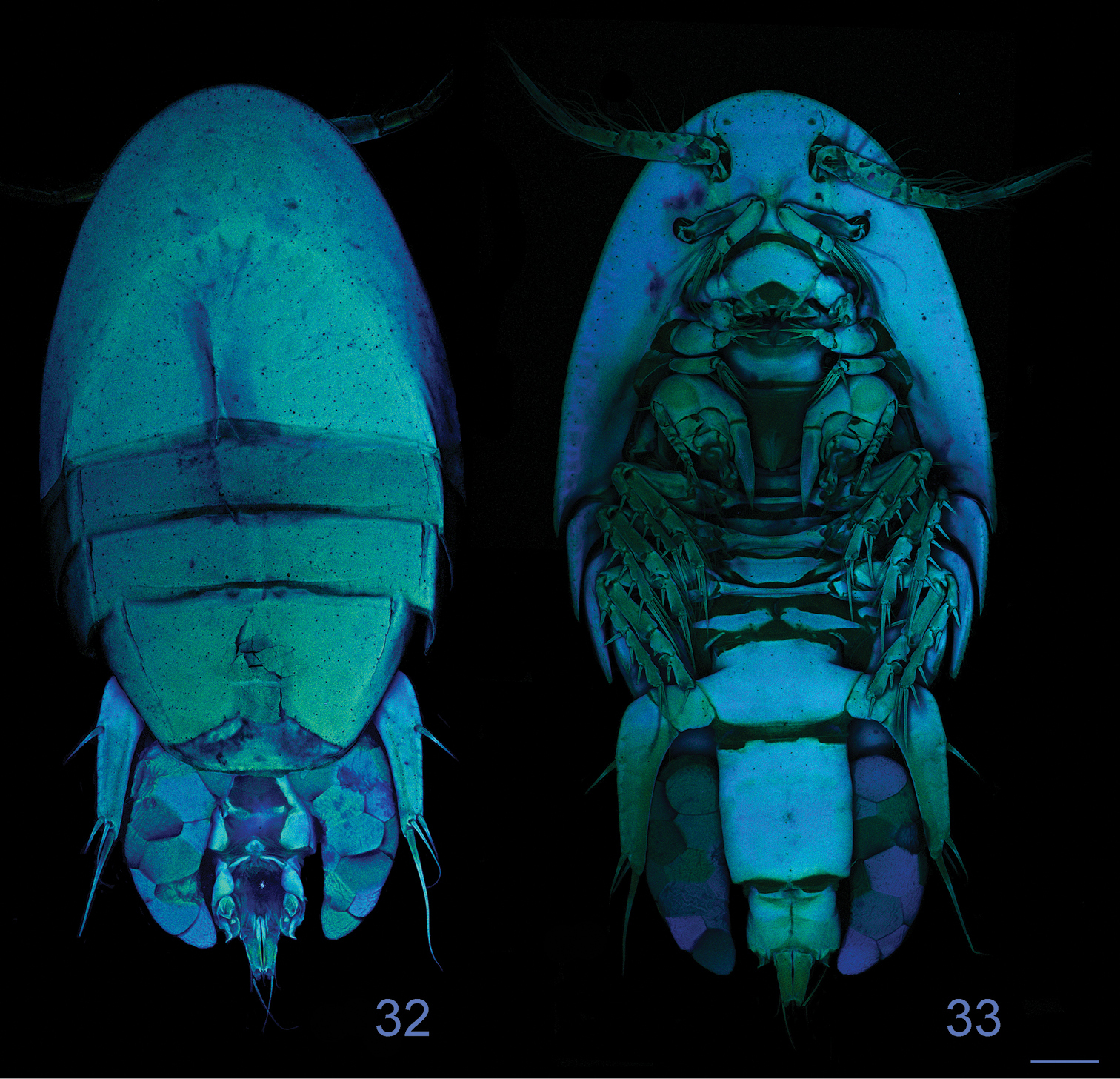
FEMALE (Figs 1–19, 32–38, 42, 45, 47, 48): Total length, excluding setae on caudal rami, 1.36–1.40mm (N=6). Body cyclopiform (Figs 1, 32–33), maximum width measured at posterior margin of cephalic shield. Prosome twice longer than urosome. First pedigerous somite fused with cephalosome. Body prosomites with minute integumental pits, sensilla and numerous pores distributed as illustrated in Fig. 1. Somites bearing P2–P3 subequal, both with latero-posterior margin sharply drawn out and posterior margin smooth. Somite bearing P4 trapezoid in form, longer than the two anterior somites combined, posterior margin with row of sensilla. Urosome (Figs 1–3, 32–35) 3-segmented, distinctly narrower than prosome. Urosome comprising fifth pedigerous somite, genital double-somite, and anal somite. Somite bearing P5 (Figs 1–2, 32–33) 1.4 times broader than long in dorsal view and with P5 arising ventrolaterally. Genital double-somite (Figs 1–3, 32–33) 1.3 times longer than broad, dorsal and ventro-lateral cuticular ridges marking plane of fusion between genital and first abdominal somite. Genital apertures (Fig. 2) located dorsolaterally on each side, near posterior margin of fifth pedigerous somite. Presence of pairs of pores near genital apertures and medial pore on dorsal view. Ventral surface with pores along medial region (Fig. 3). Egg sacs dorsolaterally located on each side, reaching posterior edge of anal somite and containing 13–15 eggs each. Anal somite (Figs 1–3, 32–33) well developed, formed by second to fourth abdominal somites fused in single somite; dorsal surface with well sclerotized leaf-like areas laterally displaced and intricate folders as illustrated in Figures 2 and 34, clearly incised medially, posterior borders with pointed curved extensions on outer corners; almost quadrate in ventral view, with pointed posterior inner corners and fringed with membrane medially.
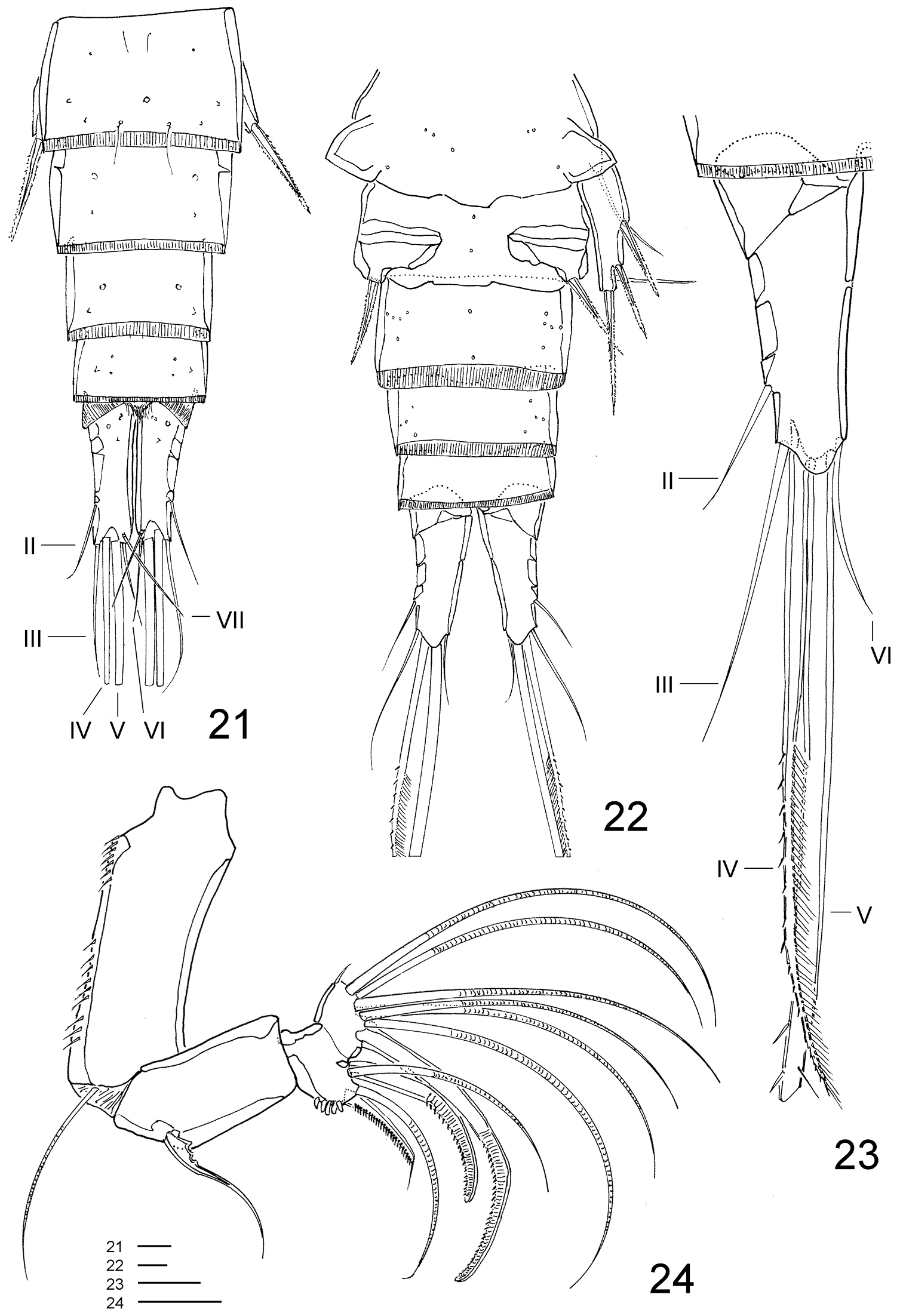
Clausidium rodriguesi sp. n. Female: 1 habitus, dorsal 2 urosome, dorsal 3 urosome lacking somite bearing P5, ventral. Scale bars: 1 = 100 μm; 2, 3 = 50 μm.
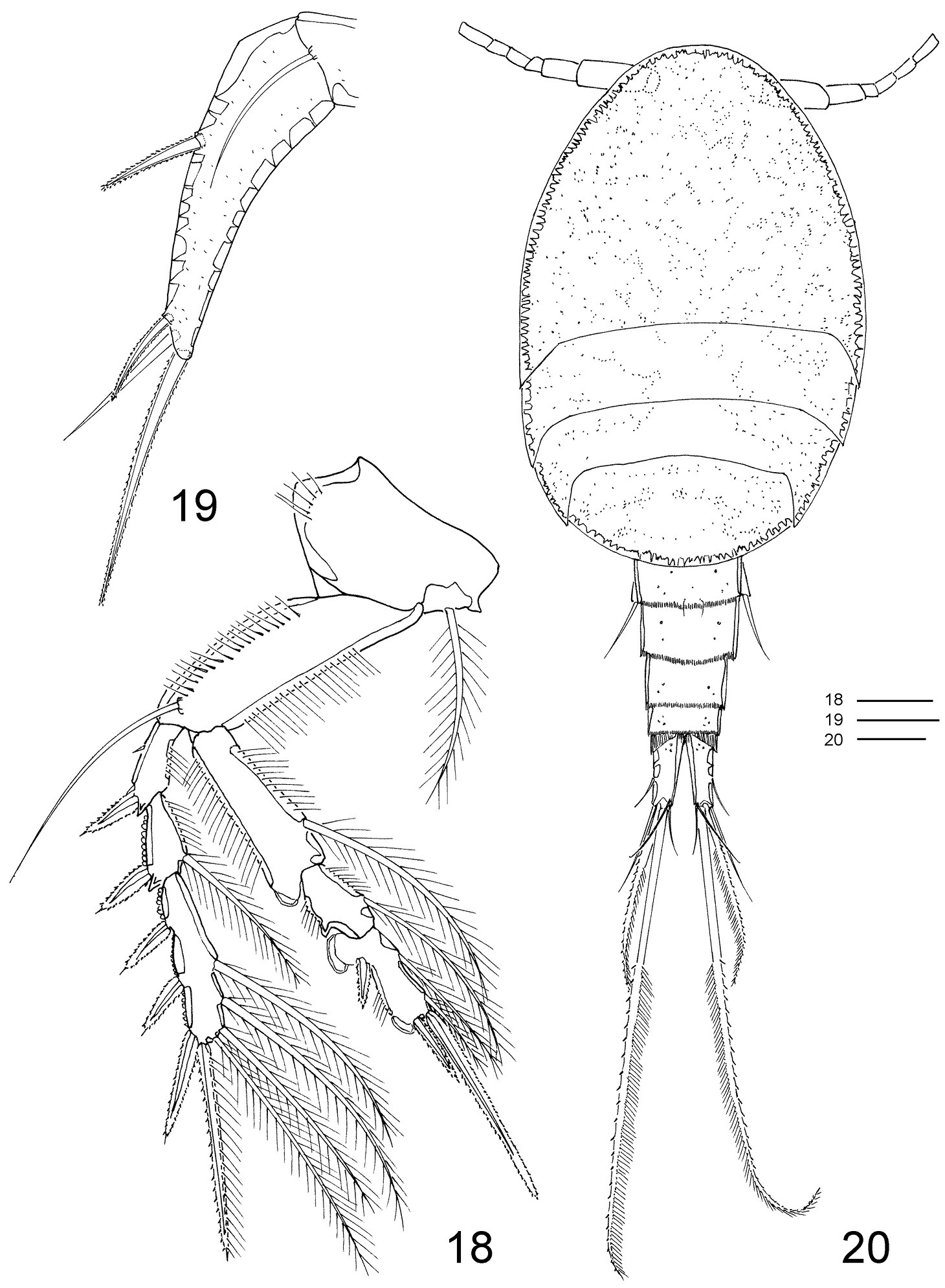
Clausidium rodriguesi sp. n. Female: 4 caudal rami, dorsal 5 caudal rami, ventral 6 antennule (arrow head indicating bipinnate seta). Scale bar = 50 μm.
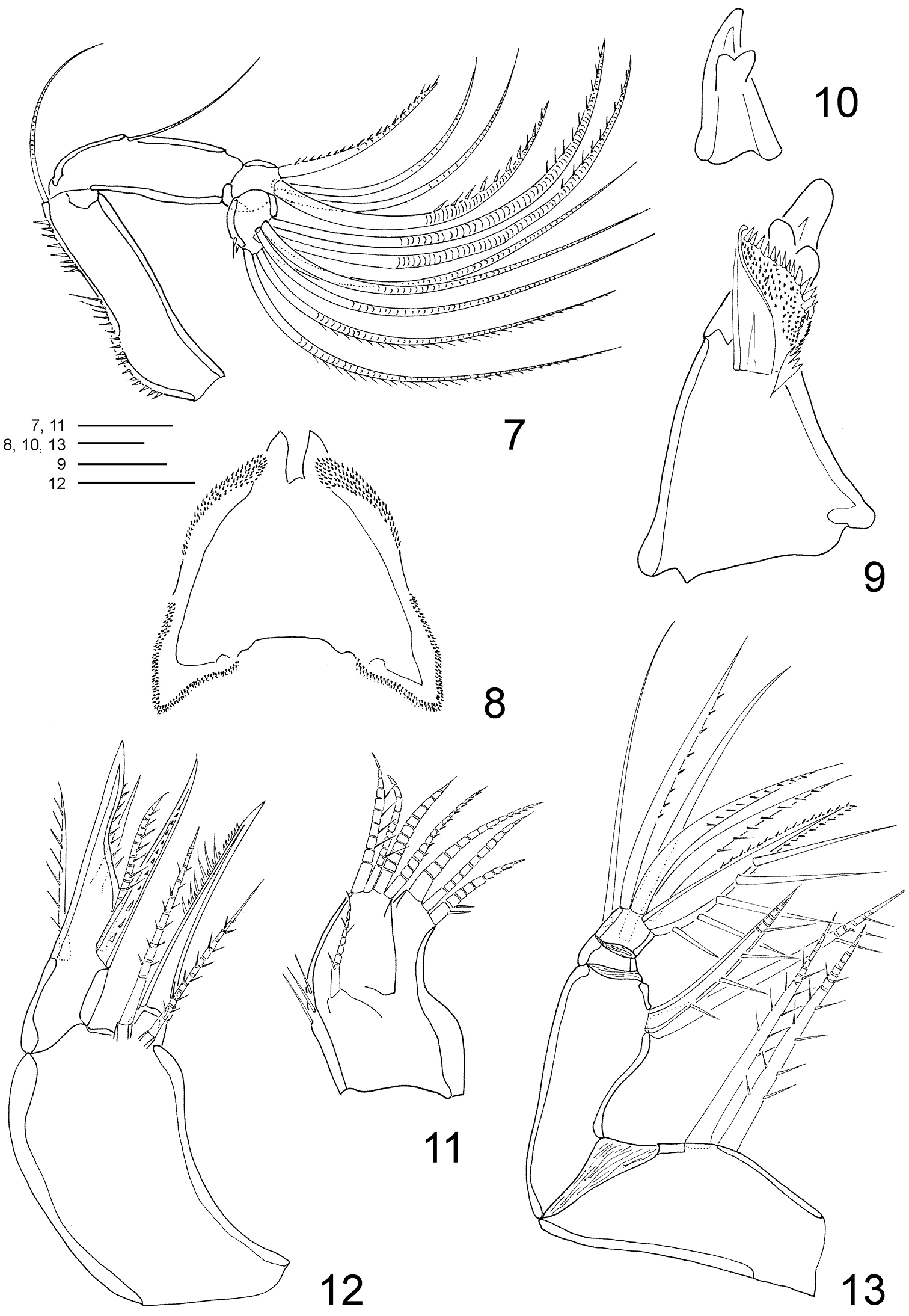
Clausidium rodriguesi sp. n. Female: 7 antenna 8 labrum 9 mandible 10 Detail of mandible tooth 11 maxillule 12 maxilla 13 maxilliped. Scale bars: 7 = 50 μm; 8 = 10 μm; 9, 10 = 25 μm; 11–13 = 20 μm.
Clausidium rodriguesi sp. n. Female: 14 P1, anterior (arrows indicating adhesive fringe) 15 detail of distal area of P1 endopod, posterior 16 P2, anterior 17 P3, anterior. Scale bars: 14 = 20 μm; 15 = 10 μm; 16, 17 = 50 μm.
Clausidium rodriguesi sp. n. Female: 18 P4, anterior 19 P5, anterior. Male: 20 habitus, dorsal. Scale bars: 18 = 20 μm; 19 = 50 μm; 20 = 100 μm.
Caudal ramus (Figs 2–5) about 3.5 times longer than wide, and armed with 6 setae. Seta I absent, setae II and III slender and naked; setae IV and V strongly developed and bipinnate, plumose on inner edge and spinulose on outer edge (seta V, 2.5 times longer than seta IV); seta VI the shortest; seta VII triarticulate and located at inner posterior corner, both naked. Caudal ramus with rounded lappet on posterior margin of ventral surface covering basal portion of setae III–V.
Rostrum (Fig. 33) incorporated into cephalothorax, demarcated by sclerotized areas laterally; with pair of sensilla ventrally and pattern of pores as illustrated.
Antennule (Fig. 6) 7-segmented. Segment 2 longest, with well-developed pinnate seta inserted on inner distal corner and extending over tip of antennule (arrowed in Fig. 6). Aestethasc inconspicuous, very similar to other setae. Segment 6 with aesthetasc fused basally to seta. Armature formula: I-[5], II-[14 + 1 bipinnate], III-[6], IV-[3], V-[4 + ae], VI-[2 + ae], VII-[7 + ae].
Antenna (Figs 7, 36) 4-segmented. Coxobasis elongated, with row of spinules along inner margin, with single seta on inner distal corner. Endopod 3-segmented; segment 1 with seta along inner margin; segment 2 with 4 setae (2 pinnate and 2 naked); segment 3 with row of spinules along distal margin, 7 apical setae, 2 of them with setules and 2 with spinules.
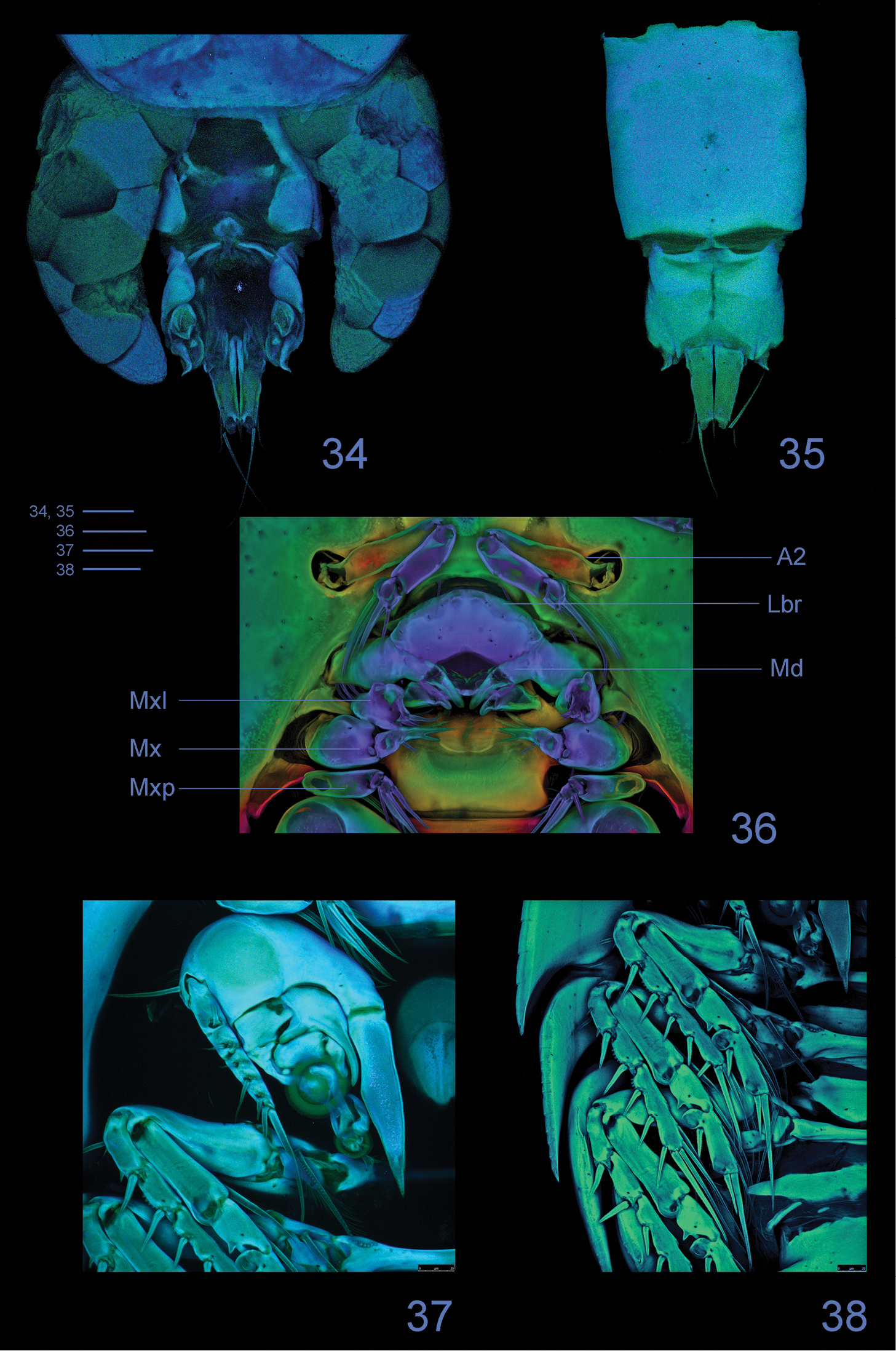
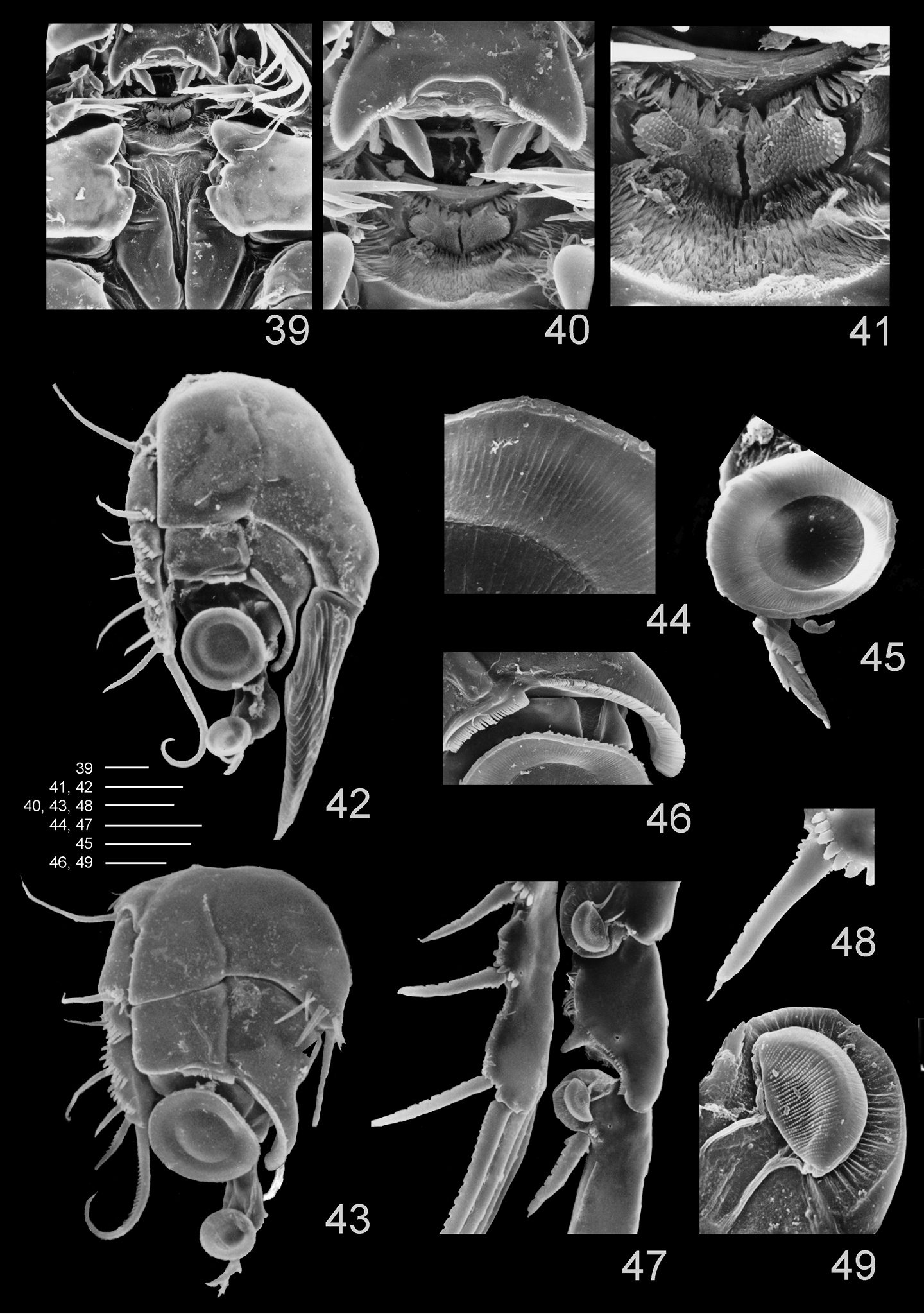
Labrum (Figs 8, 36, 39–40) twice wider than long; lateral projections with row of denticles. Metastomal area ornamented as in figures 40 and 41.
Mandible (Figs 9–10, 36) well developed. Armed with 3 elements, 1 toothed projection, 1 small seta, and 1 conical structure covered with minute spinules covering inner surface and spines along distal margin.
Maxillule (Figs 11, 36) bilobed, with 1 lateral seta pinnate. Outer lobe with row of spinules along outer margin and 4 setae (2 pinnate and 2 naked). Inner lobe with 3 setae (2 pinnate and 1 naked).
Maxilla (Figs 12, 36) 2-segmented. Syncoxa with 2 bipinnate setae and one stout spine with spinules on distal edge. Basis with large spinous process with spinules along concave margin, bearing 3 setae (2 pinnate and 1 naked) and 1 pinnate spine.
Maxilliped (Figs 13, 36) 4-segmented. Syncoxa with 2 bipinnate setae along inner margin. Basis with 1 pinnate seta and 1 spine with long spinules. Endopod 2-segmented; first segment unarmed; second segment bearing 2 naked lateral setae, 3 pinnate distal setae and stout distal spine with long and slender spinules along inner margin; minute spinules on opposite margin.
P1 (Figs 14–15, 33, 37, 42) biramous, both rami 3-segmented, and highly modified for prehension. Coxa and basis fused forming well-developed segment with row of spines along proximal margin, plumose seta on outer proximal corner; large blade-like seta with acute apex and concentric lines on inner distal corner. Exp-1 and -2 with 1 outer seta each; exp-2 with row of denticles along outer margin. Exp-3 with row of denticles along outer margin, 3 outer setae (2 naked and 1 pinnate), 2 apical setae (outer one pinnate, inner one longest and bipinnate) and 2 inner bipinnate setae. Enp-1 with 1 stout curved process with an adhesive fringe (arrowed in Fig. 14). Enp-2 with pinnate seta. Enp-3 elongated, irregular segment ending in a lobe with serrate margin and armed with 1 seta and 2 sucking discs (Fig. 15); proximal sucking disc 1.6 larger than distal one.
P2–P4 (Figs 16–18, 33, 38, 47) biramous, with both rami 3-segmented. Coxae with inner plumose seta, row of sparse setules along outer margin and row of spinules along distal margin (P3). Basis of P2–4 longitudinally elongate, with naked seta on outer distal corner, row of spinules along outer margin and row of setules along inner margin. Exp-1 and Exp-2 with row of setules along inner margin and row of spinules (exp-1) or denticles (exp-2) along outer margin, but exp-3 with denticles along outer margin; exopod outer spines serrate and with terminal flagellum (Fig. 48), apical spine with serrate outer margin and spinulose inner margin. Enp-1 and Enp-2 with row of setules along both margins; endopod outer andapical spines serrate and with terminal flagellum, inner apical spines with serrate outer margin and spinulose inner margin (P2–P3) (Figs 16, 17) or both margins serrate (P4) (Fig. 18); sucking discs (Fig. 47, detail in Fig. 49) on distal inner edge of enp-1 and proximal and subterminal inner edges of enp-3.
Armature formula of P2–P4 (Figs 13–16) as follows (Roman numerals representing spines, Arabic numerals representing setae):
P5 (Figs 2, 19, 33) uniramous, 2-segmented and located laterally on somite. Protopod with 1 outer seta; free exopodal segment elongated with 2 serrate spines, 1 naked seta along outer margin and serrate spine apically; dorsal punctuations as in figures 19 and 33.
P6 (Fig. 2) consisting of 3 small setae.
MALE (Figs 20–31, 39–41, 43, 44, 46): Total length, excluding setae on caudal rami, 0.75–0.79mm (N=4). Body cyclopiform (Fig. 20). Prosome longer than urosome (1.5:1). First pedigerous somite fused with cephalosome. Body prosomites with minute integumental pits, sensilla and numerous pores distributed as illustrated in Figure 20. Cephalosome and 3 free prosomites with posterior borders smooth; somites bearing P2–P3 subequal; somite bearing P4 with distal margin rounder than in female. Urosome (Figs 21–22) 6-segmented, distinctly narrower than prosome. Somite bearing P5 (Fig. 22) 1.5 times broader than long in ventral view and with P5 arising ventrolaterally. Pores and sensilla as illustrated in Figures 21–22. Hyaline frills of first to third abdominal somites finely striated. Anal somite (Figs 21–22) extremely reduced and deeply incised medially, with hyaline frill on dorsal posterior margin.
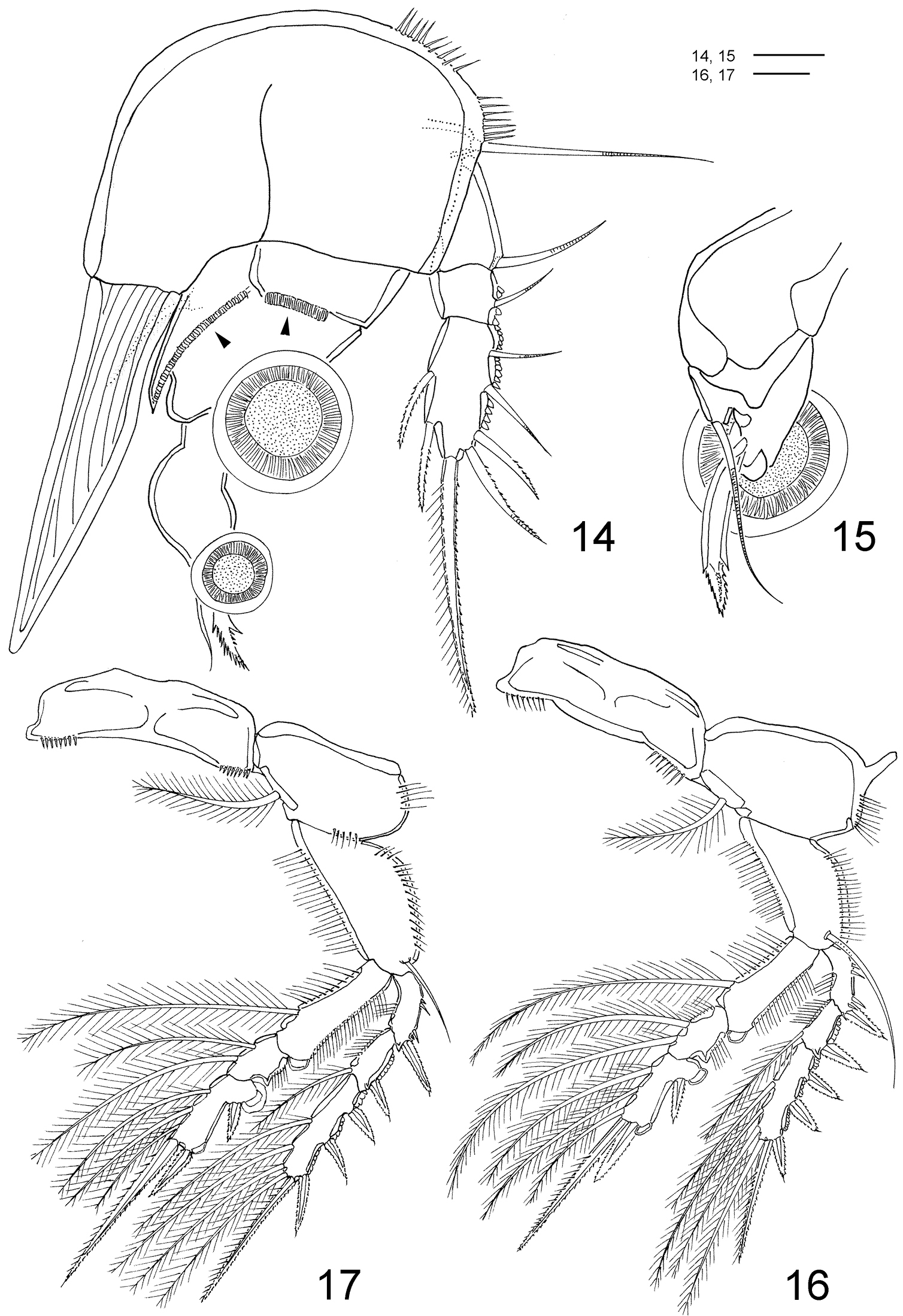
Clausidium rodriguesi sp. n. Male: 21 urosome lacking somite bearing P5, dorsal 22 urosome, ventral 23 caudal ramus, dorsal 24 antenna. Scale bars: 21–23 = 20 μm; 24 = 25 μm.
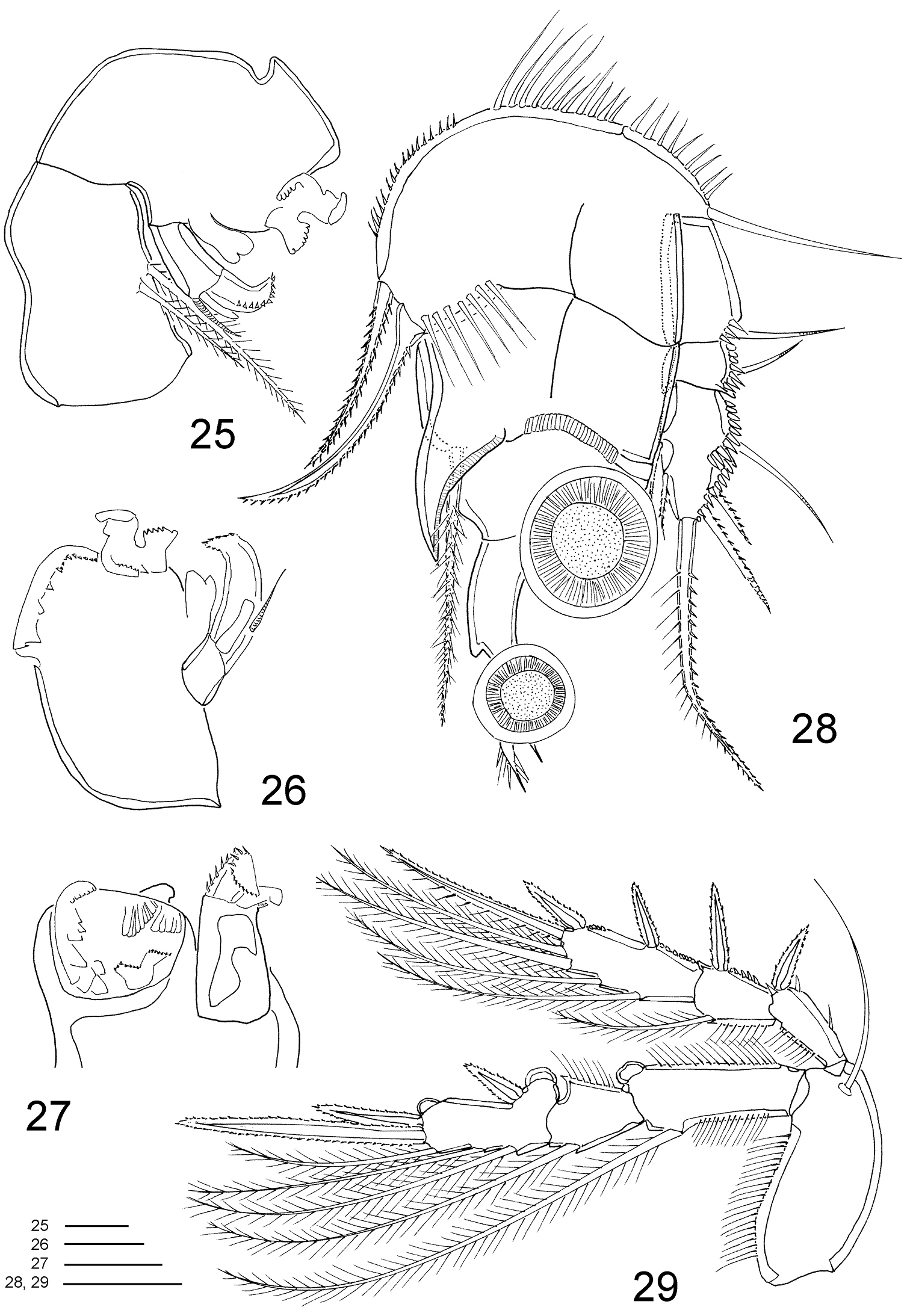
Clausidium rodriguesi sp. n. Male: 25 mandible 26 mandible, detail, ventral 27 mandible, detail, dorsal 28 P1, anterior 29 P2, anterior. Scale bars: 25–27 = 10 μm; 28, 29 = 20 μm.
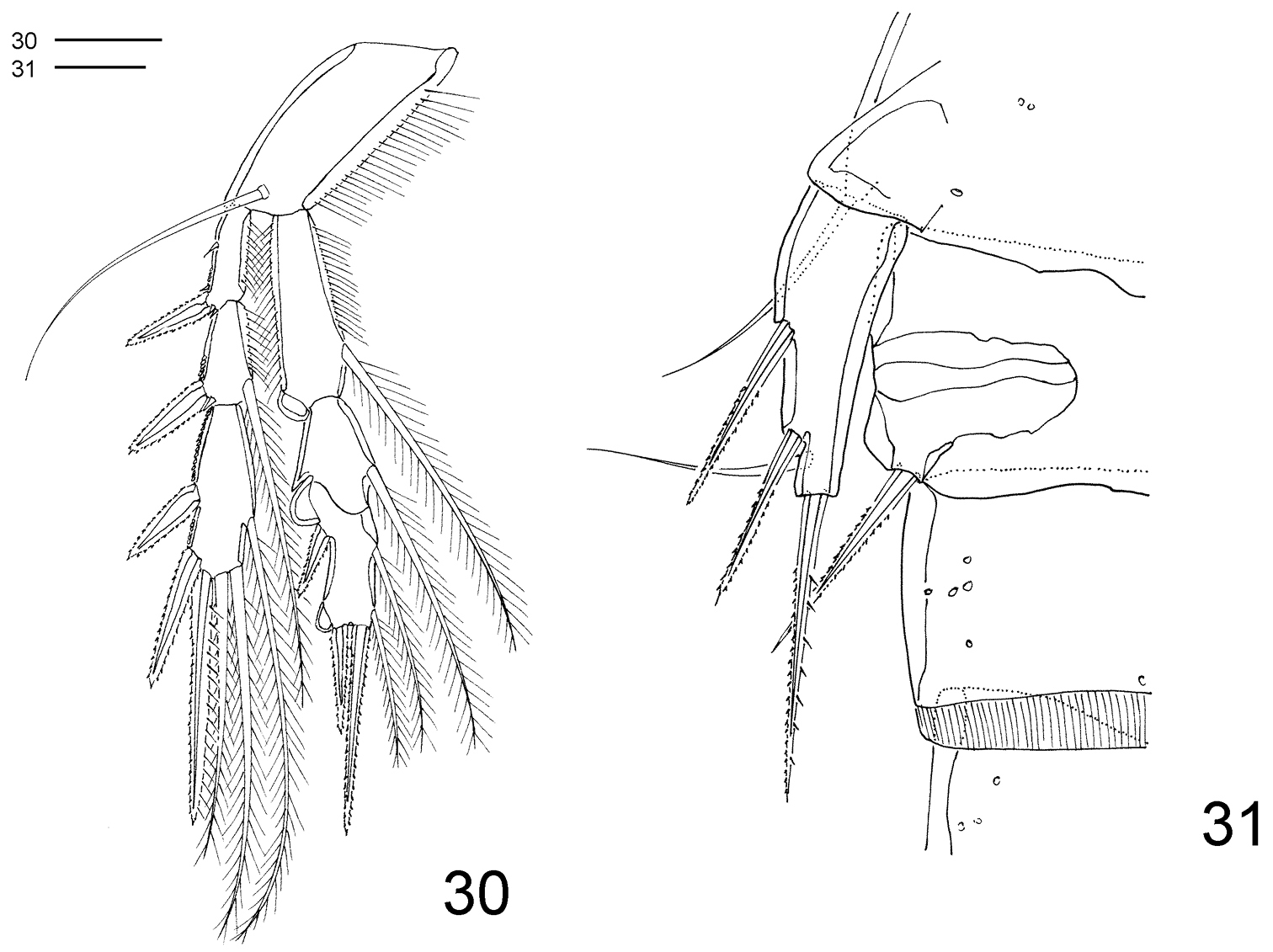
Clausidium rodriguesi sp. n. Male: 30 P4, anterior 31 P5 and P6. Scale bar: 20 μm.
Clausidium rodriguesi sp. n. Female: Confocal laser scanning microscopy maximum projections 32 habitus, dorsal 33 habitus, ventral. Scale bars: 100 μm.
Clausidium rodriguesi sp. n. Female: Confocal laser scanning microscopy maximum projections 34 urosome, dorsal 35 urosome lacking somite bearing P5, ventral 36 antenna and oral region 37 P1, anterior 38 P2-P4, anterior. Scale bars: 50 μm.
Clausidium rodriguesi sp. n.: Scanning electron microscopy photos 39 metastomal area, male 40 detail of metastomal area, male 41 detail of metastomal area, male 42 P1, anterior, female 43 P1, anterior, male 44 detail of sucking disc of P1, male 45 detail of lobe with serrate margin and distal sucking disc of enp-3 of P1, female 46 detail of P1 enp-1 adhesive fringe, male 47 sucking discs of P2, female 48 detail of serrate spine with apical flagellum of P2, female 49 detail of sucking disc of P2, female. Scale bars: 39, 40, 47 = 25 μm; 41, 44–46 = 10 μm; 42 = 35 μm; 43 = 20 μm; 48 = 12.5 μm; 49 = 4 μm.
Caudal ramus (Figs 21–23), antennule, mandible, maxillule and maxilla resembling those of female.
Antenna (Fig. 24) 4-segmented. Coxobasis elongated, with row of spinules along inner margin, with single seta on inner distal corner. Endopod 3-segmented; seta on segment 1 with proximal third enlarged and irregular, inserted along inner margin; segment 2 with row of denticles, 2 naked setae, spinulose spine with terminal flagellum and curved spine with serration along distal inner margin; segment 3 with row of spinules along distal margin, 6 naked setae and 1 curved spine with serration along distal inner margin.
Maxilliped (Figs 25–27, 39) well developed, strongly modified. Syncoxa with 2 pinnate setae. Basis with unequal denticulate projections and distal half of border curved and with irregular margin. Endopod 1-segmented; with strong serrate claw implanted near curved projection, and 1 small seta.
P1 (Figs 28, 43) similar to female. Coxa and basis fused, with rows of stout spinules along proximal margin, row of long spinules near inner distal corner, long naked seta on outer edge and 2 pinnate setae on inner distal corner. Exp-1 and Exp-2 with 1 outer seta each, and row of spinules along outer margin. Exp-3 with row of denticles along outer margin, 3 outer setae (2 pinnate and 1 naked), 1 apical bipinnate seta and 2 inner bipinnate setae. Enp-1 with adhesive fringe along distal margin, stout curved process with adhesive fringe (Fig. 46) and long pinnate seta on inner distal corner. Enp-2 and Enp-3 as in female.
P2–P4 (Figs 29–30) lacking outer spine on Exp-3. P4 (Fig. 30) without inner seta on Exp-3. Armature formula of P2–P4 as follows (Roman numerals representing spines, Arabic numerals representing setae):
P5 (Fig. 31) smaller than in female.
P6 (Fig. 31) represented by membranous flaps with bipinnate seta.
One female paratype showed left P3 endopod modified - enp-2 with only 1 seta and enp-3 with 8 elements in total (I, II, I+4).
The new species is named in honor of Prof. Dr. Sérgio de A. Rodrigues (Universidade de São Paulo) in recognition of his significant contributions to the taxonomy of Callianassidae and who kindly made available the studied material.
Although the new species Clausidium rodriguesi resembles Clausidium senegalense and Clausidium vancouverense in the armature of P2–P5 of the female, and shares with Clausidium senegalense similar segmentation and armature of the female antenna and maxilla, it can be easily distinguished from its congeners by the unique characteristics observed in the males, - i. e., antenna with modified elements (enlarged seta on endopod-1 and spines on endopod-2 and endopod-3); maxilliped with distinct denticulate projections; and P1 coxobasis with 1 outer and 2 inner setae.
Other differential features refer to the morphology of the anal somite with sclerotized leaf-like areas and intricated folders dorso-laterally, posterior borders with pointed curved extensions on outer corners and clearly incised medially; maxillule with apical outer lobe bearing 2 pinnate and 2 naked setae, as well as maxillular inner lobe bearing 2 pinnate and 1 naked setae; and female maxilliped with 1 pinnate seta and 1 pinnate spine on basis, and endopod-2 bearing 2 naked lateral setae and 4 pinnate apical elements (3 setae and 1 spine).
This new species, the first record of Clausidium in Brazil, not only extends the group distribution to the Southwest Atlantic but also enlarges the host list for the genus by adding Neocallichirus grandimana (Gibbes, 1850).
A dichotomous key to the 11 valid species of Clausidium based exclusively on females is given below. Males differ from females by shape and size of the body, as well as by small differences in the segmentation and armature of antenna, setal formulae of legs, maxilliped strongly modified - adapted to grasp the female. In addition, many records of males are not well detailed. Thus, any identification of males must be verified against the best available description of the species.
| 1 | Antenna 3-segmented | 2 |
| – | Antenna 4-segmented | 3 |
| 2 | Antennary distal segment with 5 setae | Clausidium caudatum (Say, 1818) |
| – | Antennary distal segment with 7 setae | Clausidium searsi C. B. Wilson, 1937 |
| 3 | P2 and P3 enp-2 with 1 seta | 4 |
| – | P2 and P3 enp-2 with 2 setae | 5 |
| 4 | P1 exp-3 with 5 elements in total | Clausidium saldanhae Kensley, 1974 |
| – | P1 exp-3 with 7 elements in total | Clausidium tenax Humes, 1949 |
| 5 | P4 enp-2 with 1 seta | 6 |
| – | P4 enp-2 with 2 setae | 7 |
| 6 | Maxilliped enp-2 with 5 elements in total; antenna enp-2 with 3 setae | Clausidium vancouverense (Haddon, 1912) |
| – | Maxilliped enp-2 with 6 elements in total; antenna enp-2 with 4 setae | Clausidium rodriguesi sp. n. |
| 7 | P2 and P3 enp-3 with 5 elements in total | Clausidium apodiforme (Phillippi, 1839) |
| – | P2 and P3 enp-3 with 6 elements in total | 8 |
| 8 | P4 exp-3 with 9 elements in total | Clausidium travancorense Pillai, 1959 |
| – | P4 exp-3 with 8 elements in total | 9 |
| 9 | P1 exp-3 with 4 elements in total | Clausidium dissimile C. B. Wilson, 1921 |
| – | P1 exp-3 with 5 elements in total | Clausidium chelatum Pillai, 1959 |
| – | P1 exp-3 with 7 elements in total | Clausidium senegalense Humes, 1957 |
We would like to thank Prof. Dr. Sérgio de Almeida Rodrigues (im memoriam) and Prof. Dr. Gisela Shimizu (Universidade de São Paulo - USP) for providing us with the material studied and Prof. Dr. Roberto Shimizu (Universidade de São Paulo - USP) for helping us with bibliographic references for Brazilian callianassids. We are also grateful to the reviewers for their helpful and detailed comments.