(C) 2013 Wilson R. Lourenço. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
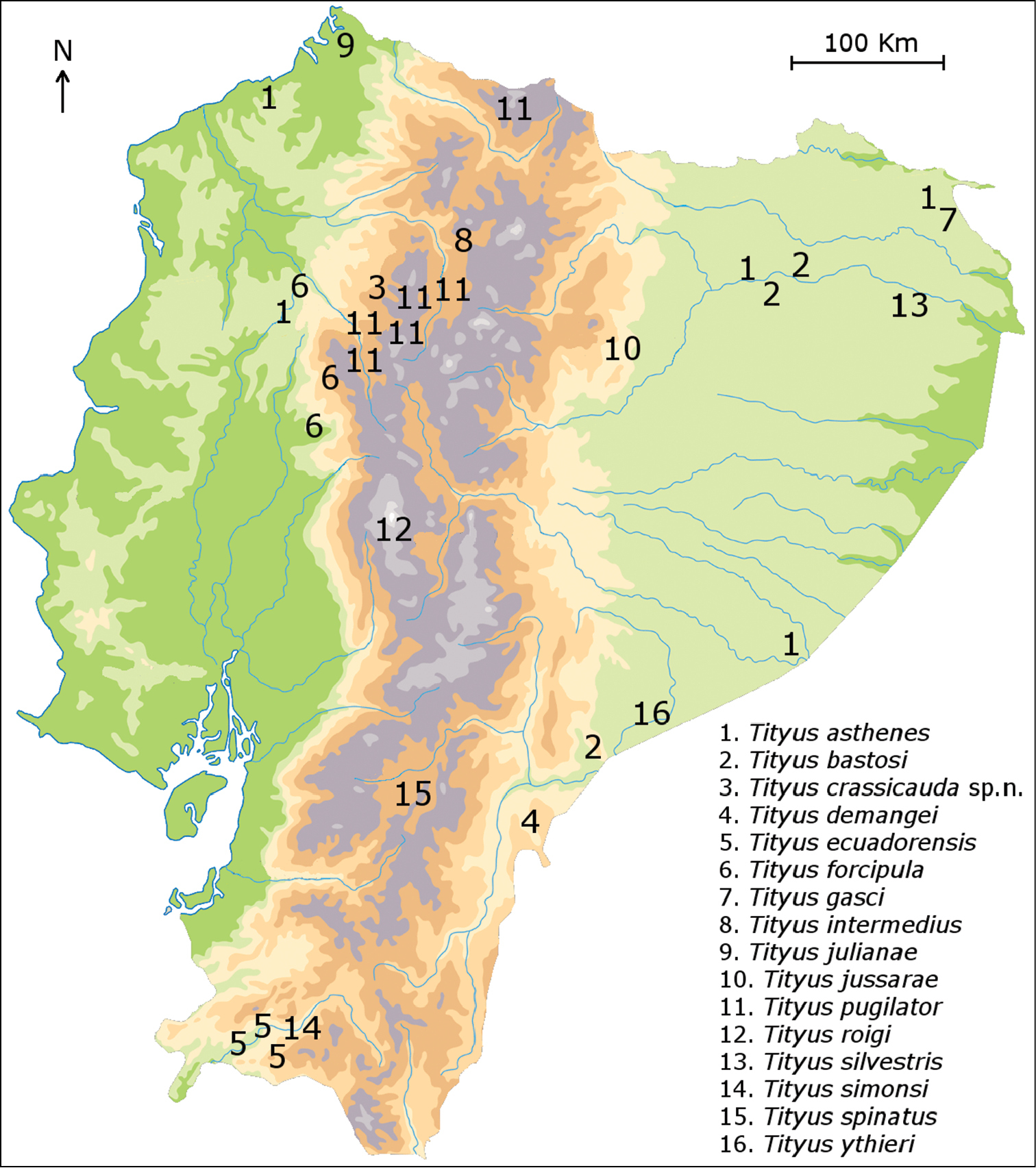
A new species of Tityus, subgenus Atreus (Scorpiones: Buthidae) is described from the Province of Pichincha in the Ecuadorian Andes. Ecuadorian scorpion fauna remains one of the less well studied among those of South America. Nevertheless, some comments are addressed about its remarkable diversity and high level of endemic elements.
Scorpiones, Buthidae, Tityus, Ecuador, New Species, diversity
The scorpion fauna inhabiting the regions between Southern Colombia and the Ecuadorian Andes has attracted the attention of experts since the middle of the 19th century (e.g.
Although studies by
Illustrations and measurements were made with the aid of a Wild M5 stereo-microscope with a drawing tube (camera lucida) and an ocular micrometer. Measurements follow
urn:lsid:zoobank.org:act:2F53526B-67DB-419D-8F64-650AF221B19E
http://species-id.net/wiki/Tityus_crassicauda
Figs 1–18Ecuador, Pichincha Province, Tandayapa, 2200 m, VIII/2012 (G. Onore & I. Tapia leg.). Inside of Lauraceae rotten logs.
Male holotype and female paratype. Deposited in the Muséum national d’Histoire naturelle, Paris.
Specific name refers to the strongly enlarged posterior metasomal segments.
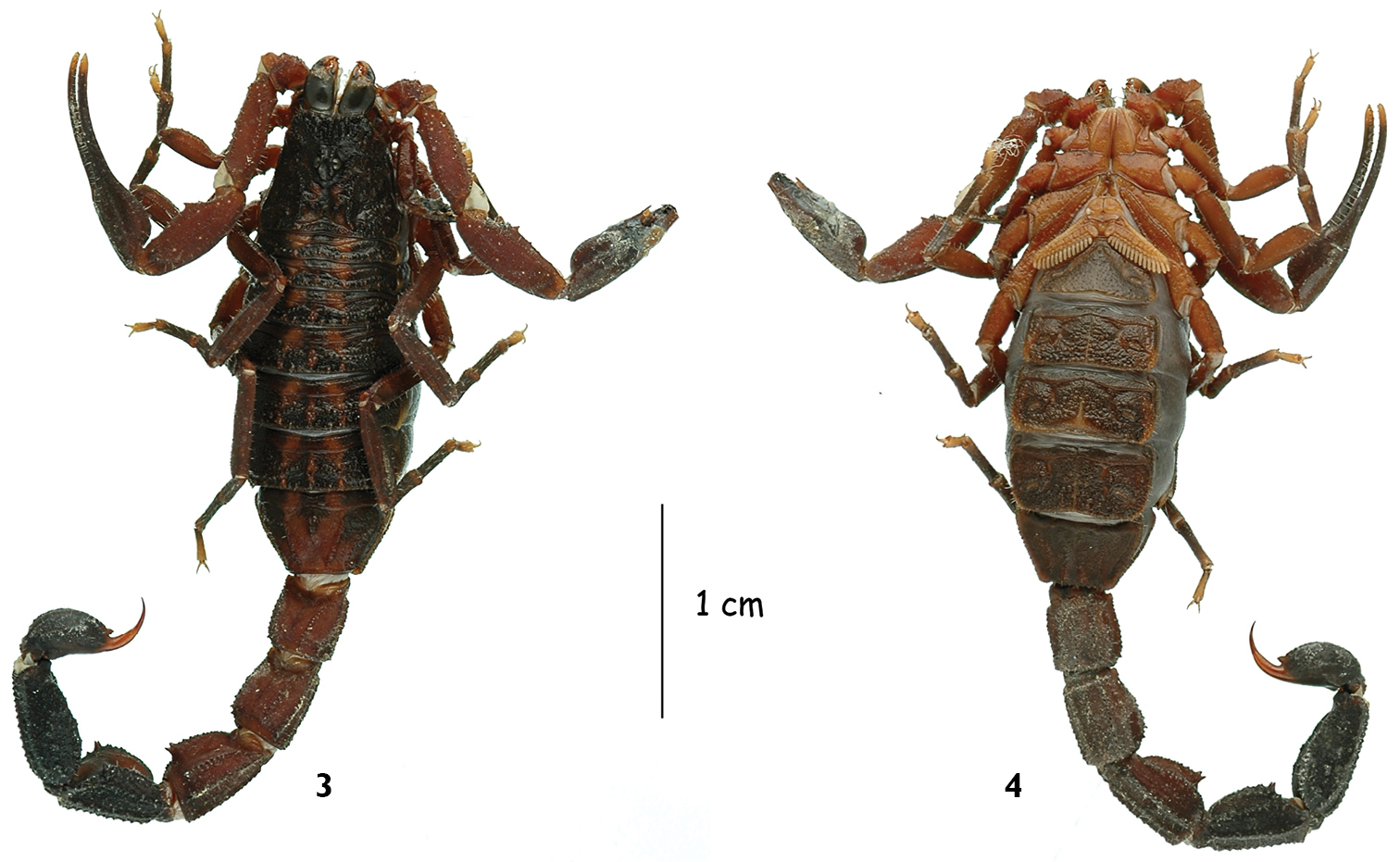
Scorpions of medium size in relation to other species within the genus. Total length in male and female, 51.9 and 50.5 mm, respectively. General coloration reddish-brown with darker blackish zones; carapace and tergites with three longitudinal brownish stripes, separated by yellow zones. Metasomal segments flattened, comparatively to other species of the group; segments IV and V very strongly enlarged, especially in the male holotype. Dorsal carinae of metasomal II to IV terminating distally with two very strong spinoid granules, more distinct on the female paratype. Pectines small with 15–15 teeth on male and 15–14 on female; basal piece of the middle lamella strongly dilated on female, and oval in shape. Cutting edges of fixed and movable fingers of pedipalp chela with 12–13 and 12–14 rows of granules on the male and female, respectively.
Relationships. The new species is clearly allied to Tityus forcipula (Gervais, 1843) and other associated species such as Tityus spinatus Pocock, 1898 and Tityus cuellari Lourenço, 1994, species also known from the Colombian and Ecuadorian Andes and, previously defined as part of the Tityus forcipula complex (see
Description based on male holotype and female paratype (measurements in Table 1).
Coloration. Reddish-brown with darker, blackish areas. Prosoma: carapace reddish-brown, with yellowish zones between posterior median carinae. Mesosoma: reddish-brown with three brown to blackish longitudinal stripes extending from the posterior zone of the carapace and over tergites I to VII. Metasomal segments I to III reddish-brown, with dark to blackish carinae; IV and V reddish-brown dorsally, blackish laterally and ventrally. Vesicle: brownish-black; aculeus yellowish. Venter is reddish-brown, with some yellowish zones. Chelicerae yellowish-brown, with a very dark thread of variegated spots; fingers dark; teeth reddish. Pedipalps: reddish; fingers blackish, with extremities yellowish. Legs reddish-yellow to reddish-brown, with some diffuse and discrete fuscous spots; tarsi yellowish.
Measurements (in mm) of male holotype and female paratype of Tityus crassicauda sp. n., and of male and female of Tityus forcipula from Pichincha.
| Tityus crassicauda sp. n. | Tityus forcipula | |||
| ♂ | ♀ | ♂ | ♀ | |
| Total length (including telson) | 51.9 | 50.5 | 58.4 | 57.8 |
| Carapace | ||||
| length | 5.7 | 5.7 | 6.4 | 6.3 |
| anterior width | 3.9 | 3.9 | 4.5 | 4.4 |
| posterior width | 6.5 | 6.9 | 7.4 | 7.8 |
| Mesosoma length: | 13.3 | 14.9 | 13.6 | 17.8 |
| Metasomal segment I: | ||||
| length | 4.1 | 3.7 | 4.3 | 4.4 |
| width | 3.7 | 3.5 | 4.2 | 3.7 |
| Metasomal segment II: | ||||
| length | 4.9 | 4.2 | 5.5 | 4.6 |
| width | 4.0 | 3.3 | 4.3 | 3.8 |
| Metasomal segment III: | ||||
| length | 5.3 | 4.6 | 6.2 | 5.4 |
| width | 4.3 | 3.5 | 4.8 | 3.9 |
| Metasomal segment IV: | ||||
| length | 5.9 | 5.2 | 7.2 | 5.9 |
| width | 5.4 | 4.0 | 5.3 | 4.5 |
| Metasomal segment V: | ||||
| length | 6.5 | 6.1 | 8.3 | 6.8 |
| width | 5.8 | 4.3 | 5.3 | 4.8 |
| depth | 3.2 | 2.6 | 4.3 | 3.6 |
| Telson length: | 6.2 | 6.1 | 6.9 | 6.6 |
| Vesicle: | ||||
| width | 3.0 | 2.6 | 3.3 | 3.2 |
| depth | 2.5 | 2.3 | 2.7 | 2.4 |
| Pedipalp Femur: | ||||
| length | 6.0 | 5.5 | 6.8 | 6.5 |
| width | 1.7 | 1.8 | 2.0 | 2.0 |
| Pedipalp Patella: | ||||
| length | 6.8 | 6.1 | 7.7 | 7.1 |
| width | 2.5 | 2.2 | 2.8 | 2.4 |
| Pedipalp Chela: | ||||
| length | 11.6 | 10.2 | 13.2 | 12.1 |
| width | 3.4 | 2.2 | 4.1 | 2.5 |
| depth | 3.5 | 2.0 | 3.9 | 2.6 |
| Movable finger: | ||||
| length | 7.6 | 7.1 | 8.2 | 8.2 |
Morphology. Carapace moderately to strongly granular; anterior margin with a moderately marked concavity. Anterior median superciliary and posterior median carinae moderate to strong. Furrows moderately to strongly deep. Median ocular tubercle distinctly anterior to the centre of carapace. Eyes separated by a little more than one ocular diameter. Three pairs of lateral eyes. Sternum triangular. Mesosomal tergites moderately granular. Median carina strong on all tergites. Tergite VII pentacarinate. Venter: genital operculum divided longitudinally, forming two semi-oval plates. Pectines small; pectinal tooth count 15–15 in male holotype and 15–14 in female paratype; basal piece of the middle lamellae of the female pectines oval shaped and strongly dilated. Sternites very strongly granular, in particular on male holotype; spiracles moderately elongated; VII with four moderately to strongly marked carinae. Metasomal segments strongly flattened dorsally; IV and V very strongly enlarged, especially in the male holotype; segments I and II with ten carinae; III and IV with eight carinae, crenulate; V with five carinae. Dorsal carinae on segments II to IV with two very strong spinoid granules, distally. Lateral inframedian carinae on segment I complete, crenulate; on II almost complete; absent from III and IV. Ventrolateral carinae strong, crenulated; ventral submedian carinae strongly crenulate. Intercarinal spaces moderately granular. Segment V with weak dorsolateral carinae; ventrolateral and ventromedian carinae strong, crenulate. Lateral intercarinal spaces moderately to strongly granular. Telson moderately granular, with a long, strongly curved aculeus. Dorsal surface smooth; ventral surface weakly granular; subaculear tooth strong and spino-rhomboidal in shape. Cheliceral dentition characteristic of the family Buthidae (Vachon, 1963); movable finger with two basal teeth well distinct; ventral aspect of both fingers and manus with long, dense setae. Pedipalps: femur pentacarinate; patella with seven carinae; chela with nine carinae, more distinct on female paratype; all faces with only a few granules; smooth. Fixed and movable fingers of chela with respectively 12 and13 oblique rows of granules on the male holotype and 12–14 on the female paratype. Trichobothriotaxy; orthobothriotaxy A-α (alpha) (
Tityus (Atreus) crassicauda sp. n., male holotype, dorsal and ventral aspects.
Tityus (Atreus) crassicauda sp. n., female paratype, dorsal and ventral aspects.
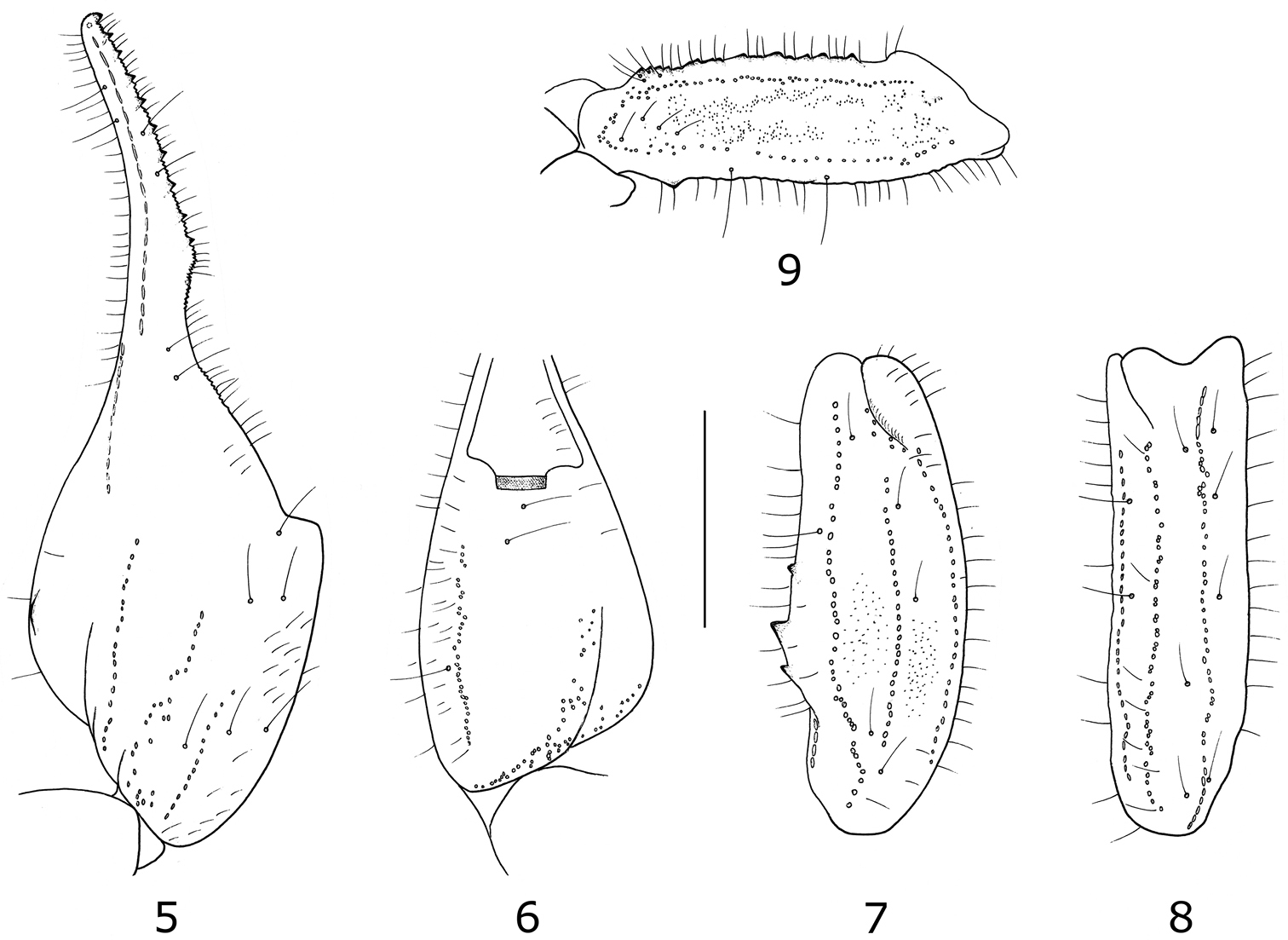
Tityus (Atreus) crassicauda sp. n. Trichobothrial pattern of male holotype 5–6 Chela dorso-external and ventral aspects 7–8 Patella, dorsal and external aspects 9 Femur, dorsal aspect (scale bar = 3 mm).
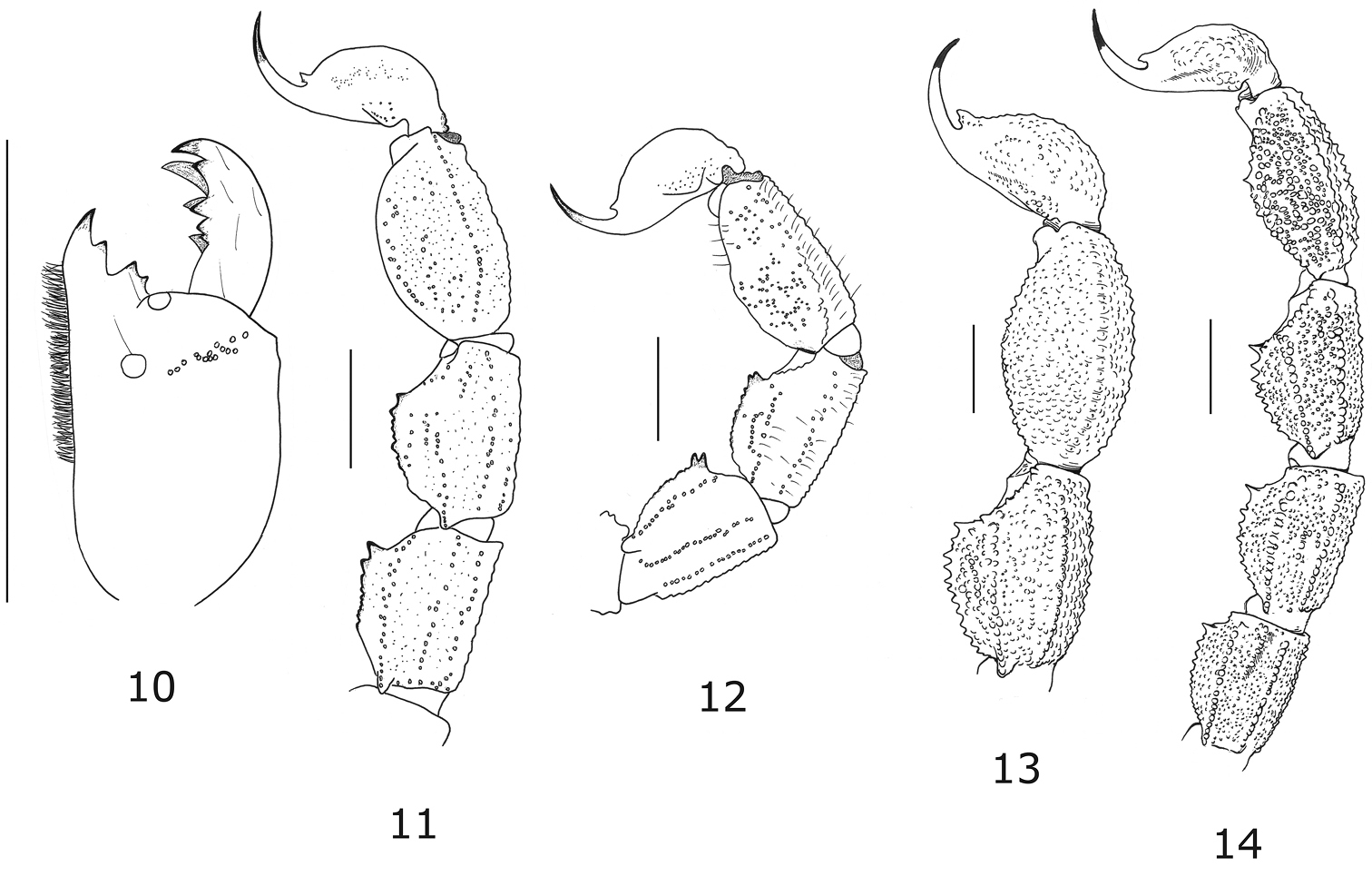
Tityus (Atreus) crassicauda sp. n. 10 Right chelicera, dorsal aspect (male holotype) 11–12 Metasomal segments III-V and telson, lateral aspect (male holotype and female paratype) 13 Segments IV, V and telson for Tityus (Atreus) forcipula, male holotype 14 Segments II to V and telson for Tityus (Atreus) spinatus, female holotype (scale bars = 3 mm).
Tityus (Atreus) crassicauda sp. n. Male holotype alive in the field.
The natural habitat of Tityus (Atreus) crassicauda sp. n. 16 General view of the Andean Mountains 17 Detail of the vegetation cover.
As already outlined by
The number of endemic taxa in this region is very high - more than 80% - and is comparable to the number of endemic taxa found in other previously studied regions such as Baja California (about 75%) (
The scorpion fauna of the Ecuadorian Andes is characterized by an outstanding concentration of species belonging to genera such as Tityus and Teuthraustes Simon, 1878 (Chactidae). Within each genus, the species show to be closely related. The speciation pattern of the scorpions corresponds to the explosive model proposed by
We are most grateful to Michael M. Webber, University of Nevada, Las Vegas for her comments and review of the manuscript and to two anonymous referees for useful comments and suggestions; to Elise-Anne Leguin, MNHN, Paris for the preparation of some photos and to Peter Schwendinger of the Geneva Museum for the loan of specimens of Tityus forcipula. We also want to express our sincere gratitude to Prof. G. Onore for providing the collected specimens.