(C) 2012 Magdalini Christodoulou. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Atyaephyra de Brito Capello, 1867 was described from the Mediterranean region almost 200 years ago. Since then, the genus has been recorded from various freshwater habitats in Europe, North Africa and the Middle East. Despite its long history, the taxonomic status of Atyaephyra species remains confusing and uncertain. Consequently numerous specimens from the known range of Atyaephyra were analysed using morphological characters and mitochondrial COI sequences in an attempt to clarify the taxonomy of this genus. The present study recognises seven Atyaephyra species, more than twice as many as previously recorded (three), four of which are considered as new. The new species are described, additional information to the original descriptions are provided for the remaining three taxa, while neotypes of Atyaephyra desmarestii Millet, 1831 and Atyaephyra stankoi Karaman, 1972 are designated to stabilize their taxonomy. Non-overlapping distinguishing morphological characters are used to discriminate the examined material into five species, e.g., Atyaephyra desmarestii, Atyaephyra stankoi, Atyaephyra orientalis Bouvier, 1913, Atyaephyra thyamisensis sp. n., Atyaephyra strymonensis sp. n. In addition, the genetic analysis supports the existence of multiple phylogenetic clades in the broader Mediterranean area and distinguishes two new cryptic species, namely Atyaephyra tuerkayi sp. n. and Atyaephyra acheronensis sp. n. The geographic distribution of these species is confirmed and their phylogenetic relationships are described.
Atyidae, Atyaephyra, new species, cryptic species, COI, freshwater shrimp, molecular data, morphology, taxonomy
Atyidae is one of the most diverse shrimp families comprising at least 469 valid species (
Atyaephyra is the most widespread atyid taxon in the Mediterranean region with its native range spanning from the Middle East to North Africa, a large part of Southern Europe and to some Mediterranean islands (Corsica, Sardinia, Sicily) (d’
Atyaephyra was first reported in the Mediterranean region almost 200 years ago (
In the beginning of the 20th century,
Subsequent studies (
Recently,
After examining two mitochondrial genes (COI, 16S) from specimens collected mainly from the western Mediterranean area, Garcia Muñoz et al. (2009) proposed the existence of two species: Atyaephyra desmarestii, distributed in West Europe and North Africa and Atyaephyra stankoi Karaman, 1972 distributed in Greek freshwaters which was elevated from the subspecies to the species level. Furthermore, the authors argued about the existence of a third genetically distinguished group, Atyaephyra mesopotamica Al-Adhub, 1987 (or Atyaephyra orientalis Bouvier, 1913), without confirming its status as a distinct species. In addition, they synonomised Atyaephyra rosiana, as described by
A comprehensive revision of synonyms of the Atyaephyra, at species level, has been provided by
This eventful taxonomic history, and the high intra- and inter-specific morphological variability observed among the Atyaephyra taxa make the recognition of discrete species intricate. Also, the wide distribution of the genus and the apparent isolation between populations may support the existence of new non-described species. Therefore the lack of any study including material covering all the known distribution of the genus provoked the present current multidisciplinary study.
In an attempt to recognize and delimit species within Atyaephyra, samples covering the known distribution of the genus were analysed, using morphological and molecular methods to evaluate the consensus of groupings as inferred by both datasets. In the last decade molecular data have been widely used in conjunction with decapod morphology, and have been instrumental in discriminating cryptic or sibling species (e.g.
This study specifically aims to: (a) test the status of the species already recognized based on morphological and molecular data; (b) describe new species based on morphological and molecular data; (c) provide knowledge on the current geographic distribution of the Atyaephyra species; (d) describe the phylogenetic relationships of new and previously described species based on COI gene.
Material and methods Abbreviations usedMMNH: Macedonian Museum of Natural History, Skopje, F.Y.R.O.M.; ZMAUTH: Zoological Museum of the Department of Biology, Aristotle University of Thessaloniki, Greece; MNHN: Muséum National d’Histoire Naturelle, Paris, France; NHM: Natural History Museum, London, England; NMW: Naturhistorisches Museum Wien, Austria; OUMNH: Oxford University Museum of Natural History, England; SMF: Senckenberg Research Institute and Natural History Museum, Frankfurt, Germany and NHMC: Natural History Museum of Crete, Greece; CL: carapace length (measured from the posterior margin of the orbit to the posterior margin of the carapace); stn: station; ovig: ovigerous.
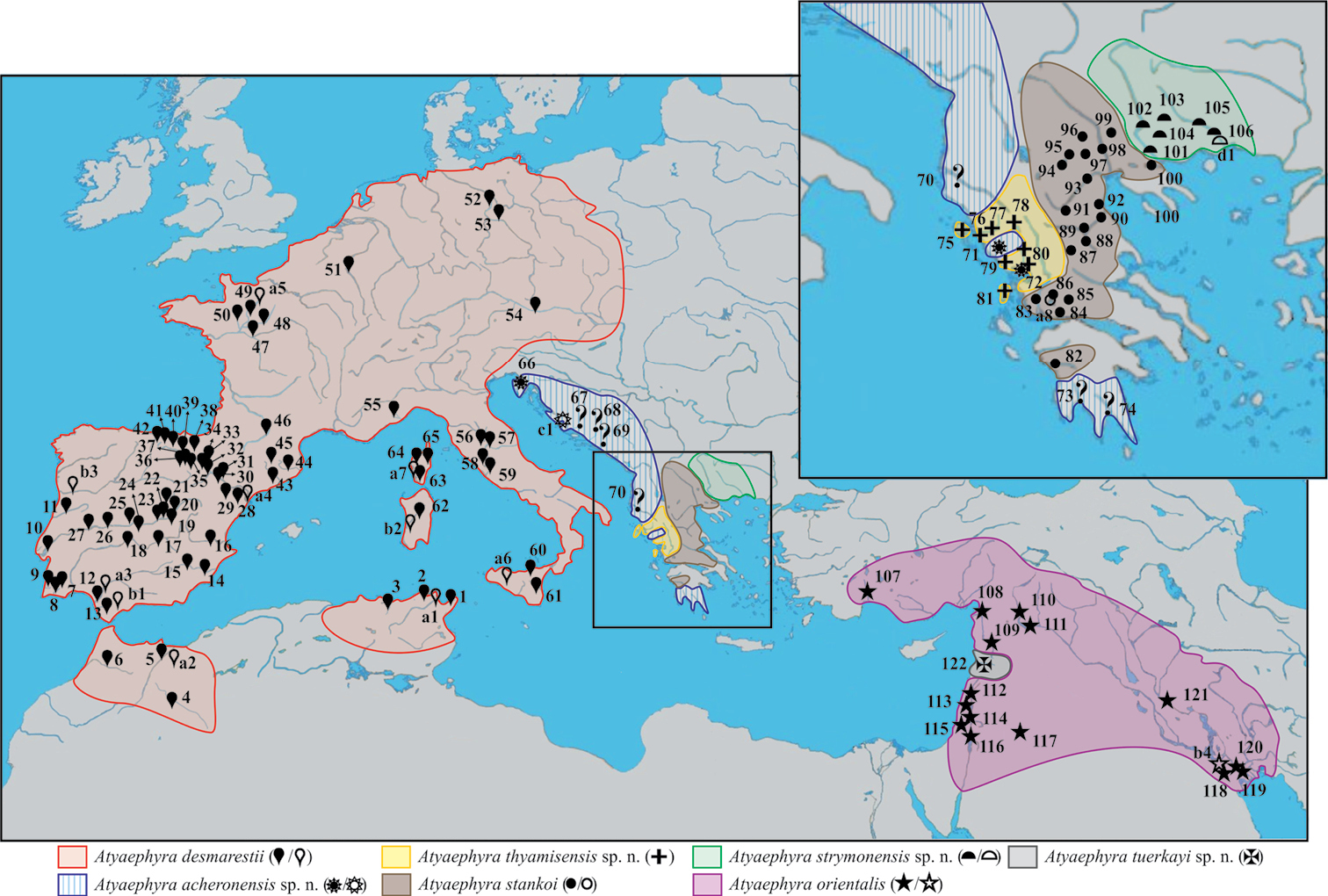
Morphological analysesSpecimens were collected with a hand dredge over the period 2000–2012 from numerous river catchments in Greece, while additional material from the rest of the Mediterranean region was either offered or loaned by researchers and Museum collections. Samples were loaned or offered from the following museums: NHM, NMW, MNHN, MMNH, ZMAUTH, OUMNH and SMF. In total 1, 082 adult individuals (Atyaephyra acheronensis sp. n.: 4, Atyaephyra desmarestii: 431, Atyaephyra thyamisensis sp. n.: 194, Atyaephyra orientalis: 111, Atyaephyra stankoi: 106, Atyaephyra strymonensis sp. n.: 92, Atyaephyra tuerkayi sp. n.: 2; furthermore 112 and 30 additional individuals were examined pending their assignment to Atyaephyra acheronensis and Atyaephyra tuerkayi respectively) were examined from 122 different stations (49 river basins, 20 countries) spanning throughout the known distribution of the genus Atyaephyra from Middle East to North Africa and Europe (Fig. 1). Part of this examined material has been included in the studies of Kinzelbach and Koster (1987) and
Map showing the sampling localities of Atyaephyra and the geographic distribution of the genus in Europe, Middle East and North Africa. Numbers 1–122, next to a solid symbol, indicate the different rivers, lakes or barrages from where samples were collected. Letters a–d, next to an open symbol, represent localities reported in the published sources of sequences. The symbols correspond to different Atyaephyra species. Question marks indicate station’s unsure placement inside Atyaephyra acheronensis (the clarification of their position will have to await the sequencing) while the general distribution of Atyaephyra acheronensis shown is only speculation.
All data (e.g. taxon descriptions, figures, characters measured) underlying this publication can also be accessed on Atyaephyra Scratchpad (http://atyaephyra.myspecies.info/ ). Scratchpads (http://scratchpads.eu ) is a Virtual Research Environment, that enable taxonomists to collaborate in the production of websites documenting the diversity of life (
Genomic DNA was extracted exclusively from abdominal tissue using ammonium acetate protocol (provided by Poulakakis N, NHMC, University of Crete, Greece). Abdominal tissue was dissolved in 600μl extraction buffer (0.05M Tris-HCl pH 7.5, 1mM EDTA pH 8.0, 0.15M NaCl, 0.3% sodium dodecyl sulfate, and 0.6μg/μl proteinase K) and incubated in a shaking waterbath at 56°C overnight. Following the incubation, 340μl of 4M ammonium acetate were added to each sample and incubated at room temperature for 60 min. Samples were mixed several times during this period by inversion. The solution was centrifuged at 18, 000g for 20 min and supernatant was transferred to 2.0ml centrifuge tubes and 1ml of absolute ethanol was added to each sample. The tubes were inverted several times and centrifuged at 18, 000g for 30 min. Following the removal of ethanol samples were dried overnight. DNA pellet was diluted by adding 50μl ddH2O and incubated at 4°C overnight. A fragment of the 5' region of mitochondrial (mtDNA) cytochrome c oxidase subunit I (COI) gene was amplified using the polymerase chain reaction (PCR). Two pairs of primers were used for each DNA extract, following the technique of nested PCR. Different combinations of primers were used as first pair: (a) LCO-1490 (5'-GGTCAACAAATCATAAAGATATTGG-3';
In some cases after the nested PCR a re-amplification was made using a modified Band-stab PCR protocol (
Thirty-seven new COI sequences were generated (GenBank accession numbers JX289898–JX289919, JX289921–JX289933, JX289935–JX289936; Table 1). Our dataset was supplemented with eight COI sequences of Atyaephyra from the study of Garcia Muñoz et al. (2009), one from
Atyaephyra specimens and COI sequences accession numbers listed by area and species. The sex and the CL are given for each specimen sequenced in parenthesis (first column). Museum accession numbers are given in parentheses (second column). GenBank accession numbers of published sequences, used in this study, are provided with their corresponding studies indicated by the letters a–e [a:
| Specimen | Sampling site | Station number in Fig. 1 | GenBank accession no. COI |
|---|---|---|---|
| Atyaephyra desmarestii | |||
| Leb1 (♀, CL: 6.6 mm) | Tunisia, Lebna Barrage, 21.3.2010, coll. S. Dhaouadi-Hassen | 1 | JX289898 |
| Met1 (♀, CL: 6.8 mm) | Tunisia, Ben Metir Barrage, 22.2.1974 (NHM 1515–1540.22.2.74) | 2 | JX289899 |
| Moul1 (♀, CL: 6.1 mm) | Morocco, Moulouya River, 11.4.2011, coll. M. Melhaoui | 5 | JX289900 |
| Krum2 (♀, CL: 6.9 mm) | Morocco, Krumane River, 22.7.1952, coll. J. Phillipson (NHM 1953.12.2.12–15) | 6 | JX289901 |
| Bord2 (♀, CL: 5.7 mm) | Portugal, Bordeira River, 5.3.1985, coll. J. Paula (NHM 1986.261) | 9 | JX289902 |
| Sint1 (♀, CL: 7.0 mm) | Portugal, Tagus Basin, Colares River, 1880 (NHM 1880.36) | 10 | JX289903 |
| Mon1 (♀, CL: 7.2 mm); Mon2 (♀, CL: 6.8 mm) |
Portugal, Mondego Basin, Ceira River, 24.5.2010, coll. V. Ferreira | 11 | JX289904; JX289905 |
| Vet1 (♀, CL: 8.0 mm) | Spain, Guadalquivir Basin, Guadiamar River, 8.5.2006, coll. C. Lejeusne | 12 | JX289906 |
| Mu1 (♀, CL: 6.9 mm) | Spain, Segura Basin, Mundo River, 27.9.2001, coll. J.L. Moreno Alcaraz | 15 | JX289907 |
| Vb1 (♀, CL: 6.1 mm) | Spain, Guadiana Basin, Vado Blanco River, 3.10.2001, coll. J.L. Moreno Alcaraz | 17 | JX289908 |
| Ta1 (♀, CL: 7.8 mm) | Spain, Tagus Basin, Tajuna River, 7.8.2001, coll. J.L. Moreno Alcaraz | 20 | JX289909 |
| Er1 (♀, CL: 8.2 mm) | Spain, Ebro Basin, Erro River, 25.5.2007, coll. J. Oscoz | 38 | JX289910 |
| Fl1 (♂, CL: 5.3 mm) | Spain, Catalan Basin, Fluvia River, 4.2.2005, coll. M.L. Zettler | 44 | JX289911 |
| Gar2 (♀, CL: 6.0 mm) | France, Garrone River, 25.8.2004, coll. R. Liasko and S. Combes | 46 | JX289912 |
| Sart1 (♀, CL: 7.0 mm) | France, Loire Basin, Sarthe River, 20.9.2000, coll. P. Noél | 48 | JX289913 |
| May2 (♀, CL: 5.6 mm) | France, Loire Basin, Mayenne River, 20.9.2000, coll. P. Noél | 49 | JX289914 |
| Hav1 (♀, CL: 6.3 mm) | Germany, Elbe Basin, Havel River, 26.8.2005, coll. M.L. Zettler | 53 | JX289915 |
| Dan1 (♀, CL: 7.4 mm) | Austria, Danube River, 8.10.1998, coll. Zipek and Melcher (NMW 18315) | 54 | JX289916 |
| Sim3 (♀, CL: 6.5 mm) | Sicily, Simeto River, 1.9.1978, coll. C. Froglia | 61 | JX289917 |
| Riz1 (♂, CL: 5.8 mm) | Corsica, Rizzanese River, 13.8.2003, coll. M.L. Zettler | 64 | JX289918 |
| Br1 (♀, CL: 7.9 mm) | Corsica, Bravone River, 16.8.2003, coll. M.L. Zettler | 65 | JX289919 |
| Tunisia, Medjerda River | a1 | FJ594343 | |
| Morocco, Zegzel River | a2 | FJ594340 | |
| Spain, Guadalquivir River | a3 | FJ594339 | |
| Spain, Ebro River | a4 | FJ594342 | |
| France, Loire Basin, Mayenne River | a5 | FJ594341 | |
| Sicily, Frattina River | a6 | FJ594344 | |
| Corsica, Liamone River | a7 | FJ594345 | |
| Guad1 | Spain, Guadalhorce River, coll. C.N. Sánchez | b1 | JX853921 |
| Cog1 | Sardinia, Coghinas River, coll. M. Jowers | b2 | JX853920 |
| Dour1 | Portugal, Douro River, coll. M. Fidalgo | b3 | JX289920 |
| Atyaephyra acheronensis sp. n. | |||
| Drag1 (♂, CL: 5.1 mm) | Slovenia, Dragonja River, Aug.1971 | 66 | JX289921 |
| Ach1 (♀ ovig., CL: 5.9 mm) | Greece, Acherontas River, 15.4.2012, coll. Ch. Anastasiadou (NHM 2012.1493) | 71 | JX289922 |
| Lour1 (♀, CL: 7.6 mm); Lour2 (♀ ovig., CL: 7.0 mm) |
Greece, Louros River, 15.4.2012, coll. Ch. Anastasiadou | 72 | JX289923; JX289924 |
| Croatia, Krka River | c1 | DQ320047 | |
| Atyaephyra thyamisensis sp. n. | |||
| Lour3 (♀, CL: 7.4 mm) | Greece, Louros River, 15.4.2012, coll. Ch. Anastasiadou | 72 | JX289925 |
| Lef2 (♂, CL: 5.7 mm) | Greece, Lefkada Island, Vardas River, 2.10.1932, coll. Beier (NHMW 466) | 81 | JX289926 |
| Atyaephyra stankoi | |||
| Doir2 (♀, CL: 5.0 mm) | Greece–F.Y.R.O.M., Doirani Lake, 26.10.1994, coll. S. Jovanovich | 99 | JX289927 |
| Greece, Lisimakhia River | a8 | FJ594346 | |
| Atyaephyra strymonensis sp. n. | |||
| Myl1(♀, CL: 5.2 mm); Myl2 (♀, CL: 5.3 mm) |
Greece, Strymonas Basin, Mylopotamos Springs, 23.5.2011, coll. M. Christodoulou and M.S. Kitsos | 102 | JX289928; JX289929 |
| Greece, Nestos River | d1 | DQ641570 | |
| Atyaephyra orientalis | |||
| Kar2 (♀, CL: 4.5 mm) | Turkey, Orontes Basin, Karasu River, 22.9.1982, coll. R.K. Kinzelbach (SMF 12174) | 108 | JX289930 |
| Or2 (♀, CL: 5.0 mm) | Syria, Orontes River, 30/31.3.1979, coll. R.K. Kinzelbach (SMF 12050) | 109 | JX289931 |
| Euph2 (♀, CL: 4.7 mm) | Syria, Euphrates River, 17.8.1978, coll. R.K. Kinzelbach (SMF 12188) | 110 | JX289932 |
| Shat2 (♀, CL: 5.3 mm) | Iraq, Euphrates–Tigris Basin, Shatt Al-Arab River, 2011, coll. M.D. Naser | 120 | JX289933 |
| AlH1 | Iraq, Euphrates–Tigris Basin, Al-Huaizah Marshes, coll. M.D. Naser | b4 | JX289934 |
| Atyaephyra tuerkayi sp. n. | |||
| Nah1 (♀, CL: 6.2 mm); Nah2(♀, CL: 7.1 mm) |
Syria: Nahr Al-Kabir River, 5.3.1979, coll. R.K. Kinzelbach (SMF 43020-1) | 122 | JX289935; JX289936 |
| Outgroups | |||
| Dugastella valentina | Spain | d2 | DQ641569 |
| Dugastella marocana | Morocco | a9 | FJ594347 |
| Paratya curvirostris | New Zealand (North Island), Marawara Stream | e1 | AY661487 |
COI sequences were aligned using FSA (Fast Statistical Alignment) (
Phylogenetic inference analyses were conducted using Neighbor Joining (NJ), Maximum Likelihood (ML), and Bayesian Inference (BI) methods. The nucleotide substitution model selected by jModeltest [Tamura-Nei, 1993 (TrN) + gamma (G)] was applied to the data matrix in all analyses. A NJ tree was produced with the software MEGA where branch support was assessed with 1, 000 bootstrap replicates. ML estimates were made using PhyML online web server (
Out of the 51 Atyaephyra COI sequences 35 distinct haplotypes were distinguished. Shared haplotypes were observed among individuals in close geographical proximity. Of the 600 nucleotide sites examined, 237 were variable of which 197 were parsimony informative (14% in the first, 2% in second, and 84% in third codon position). The nucleotide substitution model that best fits our data according to both AIC and BIC criteria is Tamura and Nei (1993) + gamma (G) based on which Atyaephyra sequence divergence ranged from 0% to 25.7%.
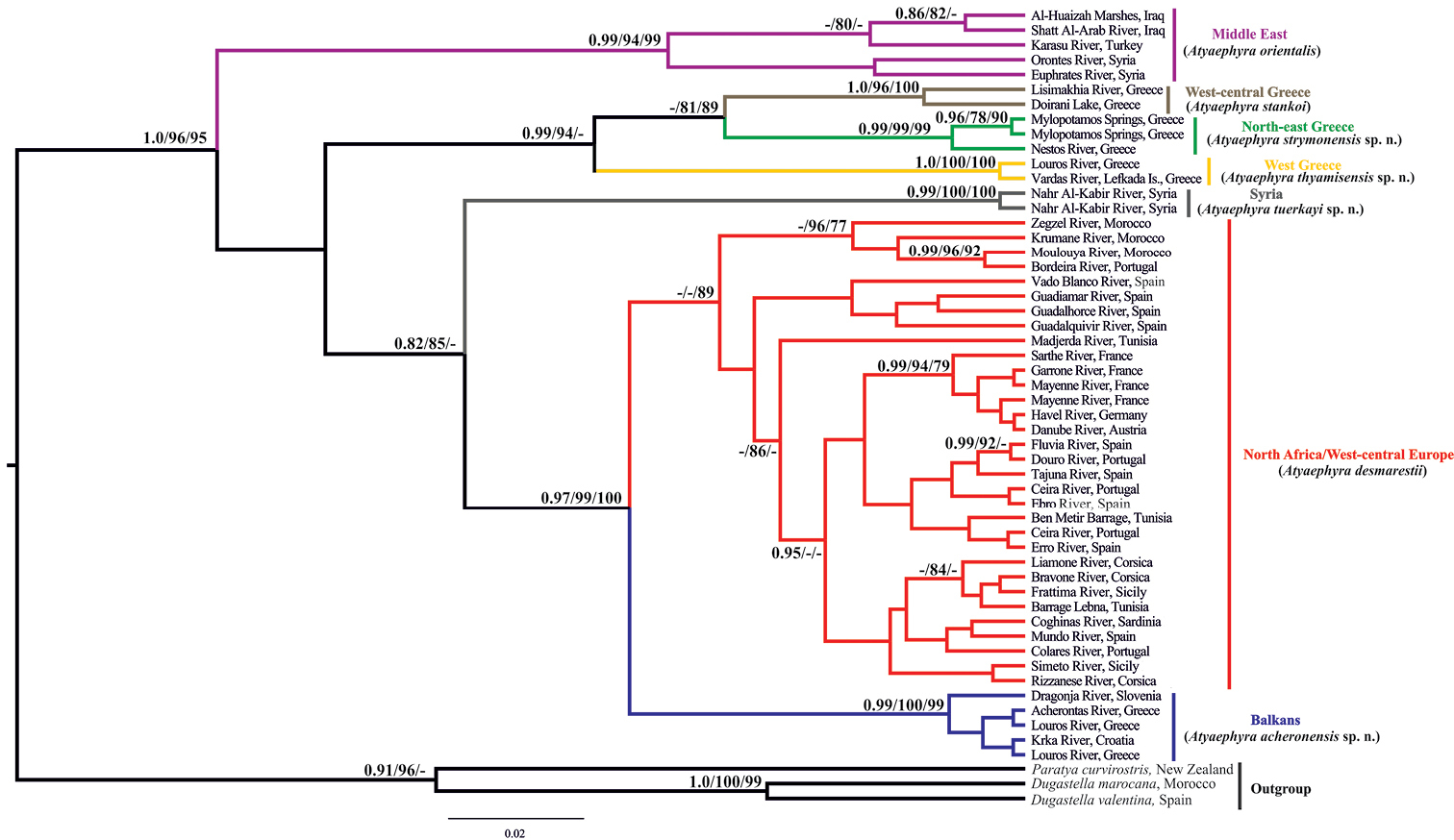
All employed methods yielded consistent tree topologies (Fig. 2). The monophyly of the genus is highly supported in all methodologies (BI posterior probability: 1.0, ML SH-like value: 96, NJ bootstrap value: 95).
Bayesian inference phylogenetic tree of Atyaephyra based on COI dataset. Numbers on nodes indicate Bayesian Inference posterior probabilities, Maximum Likelihood SH-like branch support and Neighbor Joining bootstrap respectively. Only values above 0.75 and 75% are shown. Colours correspond to those used in Figure 1.
In all phylogenetic analyses four main and well-supported phylogroups were identified, corresponding to different groups of species designated by morphology (presented in the next section) and/or well defined geographic regions throughout the Mediterranean region (Fig. 2). The first phylogroup comprises specimens from the Middle East which were classified to the nominal species, Atyaephyra orientalis by morphology. Specimens from the topotypical populations of the subspecies Atyaephyra desmarestii orientalis (Orontes River, Syria) and Atyaephyra desmarestii mesopotamica (Shatt Al-Arab River, Iraq) were also included. However, present data do not allow for within clade fine scale resolution. The mean genetic distances between the Middle East phylogroup (Atyaephyra orientalis) and the other groups/subgroups were very high ranging from 18.7% to 24.5% while the average intraspecific distance was 5.8% (Table 2).
Nucleotide mean distances (% Tamura-Nei 1993 + G model) of cytochrome c oxidase I (COI) within (first column) and among the Atyaephyra species. The range of pairwise distances is given in parenthesis.
| Within species | Atyaephyra desmarestii | Atyaephyra acheronensis sp. n. | Atyaephyra thyamisensis sp. n. | Atyaephyra stankoi | Atyaephyra strymonensis sp. n. | Atyaephyra orientalis | |
|---|---|---|---|---|---|---|---|
| Atyaephyra desmarestii | 0.016; (0.000–0.048) |
||||||
| Atyaephyra acheronensis sp. n. | 0.001; (0.000–0.003) |
0.083; (0.059–0.116) |
|||||
| Atyaephyra thyamisensis sp. n. | 0.000; (0.000) |
0.239; (0.206–0.271) |
0.238; (0.233–0.251) |
||||
| Atyaephyra stankoi | 0.024; (0.024) |
0.236; (0.204–0.261) |
0.232; (0.215–0.241) |
0.167; (0.163–0.176) |
|||
| Atyaephyra strymonensis sp. n. | 0.003; (0.000–0.005) |
0.233; (0.201–0.273) |
0.219; (0.205–0.234) |
0.182; (0.166–0.194) |
0.119; (0.117–0.119) |
||
| Atyaephyra orientalis | 0.058; (0.009–0.102) |
0.222; (0.192–0.287) |
0.238; (0.216–0.256) |
0.187; (0.169–0.200) |
0.226; (0.190–0.244) |
0.245; (0.219–0.270) |
|
| Atyaephyra tuerkayi sp. n. | 0.000; (0.000) |
0.230; (0.208–0.260) |
0.222; (0.215–0.232) |
0.257; (0.237–0.278) |
0.232; (0.215–0.242) |
0.254; (0.243–0.267) |
0.197; (0.172–0.221) |
The second phylogroup which is strongly supported by both BI and ML methodologies while in NJ yielded lower bootstrap values (BI posterior probability: 0.99, ML SH-like value: 94, NJ bootstrap value: 65) includes sequences exclusively from Greek populations. The Greek phylogroup is further subdivided into three well supported groups. The first subgroup corresponds to the nominal species, Atyaephyra stankoi, found in West-central Greece. It is worth noticing that specimens from the type locality (Doirani Lake) of Atyaephyra desmarestii stankoi are also included. The remaining Greek specimens are grouped in two well defined subgroups, one distributed in North-east Greece while the other is located in West Greece (Fig. 1). The mean genetic divergence among the three subgroups ranges from 11.9% to 18.2%, while the mean genetic distances within subgroups varied from 0% to 2.4% (Table 2).
The third phylogroup contains specimens from the Syrian River Nahr Al-Kabir and it is strongly supported in all methodologies (BI posterior probability: 0.99, ML SH-like value: 100, NJ bootstrap value: 100). The mean genetic distances between the Syrian subgroup and the other groups/subgroups were very high ranging from 19.7% to 25.7% (Table 2).
The fourth phylogroup which is well supported by BI, ML and NJ (BI posterior probability: 0.97, ML SH-like value: 99, NJ bootstrap value: 100) includes specimens originating from West-central Europe, North Africa and the Balkans. Within this phylogroup, specimens from Croatia, Slovenia and Greece form a distinct highly supported subgroup (BI posterior probability: 0.99, ML SH-like value: 100, NJ bootstrap value: 99). The remaining specimens within the phylogroup i.e. specimens from West-central Europe and North Africa, although classified as Atyaephyra desmarestii (nominal species) by morphology (discussed in the next section) do not constitute a well supported subgroup except in NJ analysis where it is relatively well supported (NJ bootstrap value: 89). Sequences from the topotypical populations of the Atyaephyra desmarestii (Mayenne and Sarthe River), and Atyaephyra rosiana described by de Brito Capello (Ceira River, tributary of Mondego River) were included in this subgroup as well as a sequence acquired from river Bordeira (Portugal) which is near to São Barnabé River from where Atyaephyra rosiana was re-described by
The present study recognises five well defined by morphology species of Atyaephyra: Atyaephyra desmarestii (Millet, 1831), Atyaephyra stankoi Karaman, 1972, Atyaephyra orientalis Bouvier, 1913 and two new species, Atyaephyra thyamisensis sp. n. and Atyaephyra strymonensis sp. n. Neotypes are designated for Atyaephyra desmarestii and Atyaephyra stankoi in an attempt to stabilize their taxonomy. In addition, two cryptic species are defined by the molecular analysis. Descriptions are provided for all these species.
Taxonomy Family Atyidae de Haan, 1849 (in de Haan, 1833–1850)Atyaephyra rosiana de Brito Capello, 1867: 6–7, Pl. 1, Figs 1A–E [type locality: Coimbra, Portugal]; by monotypy.
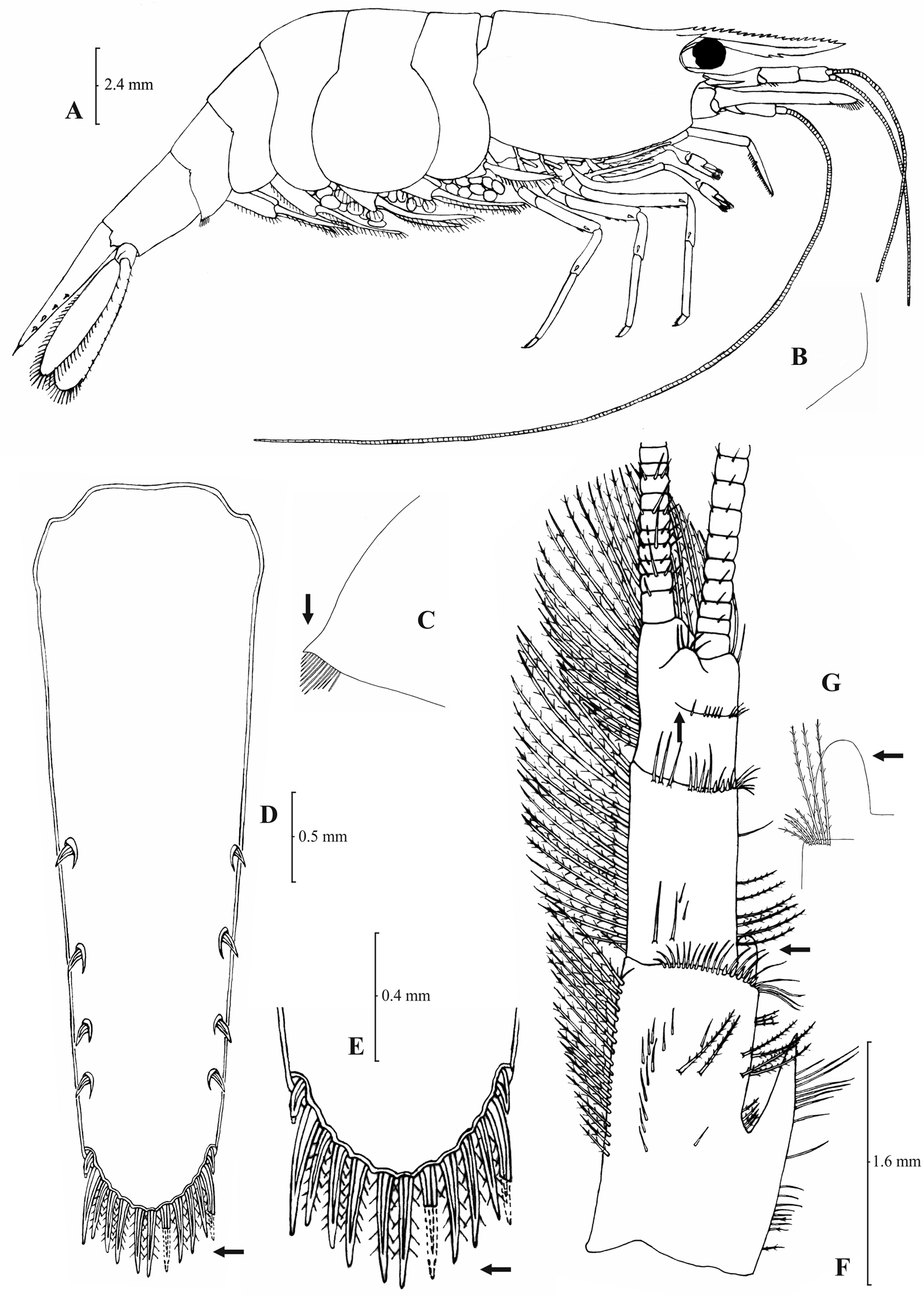
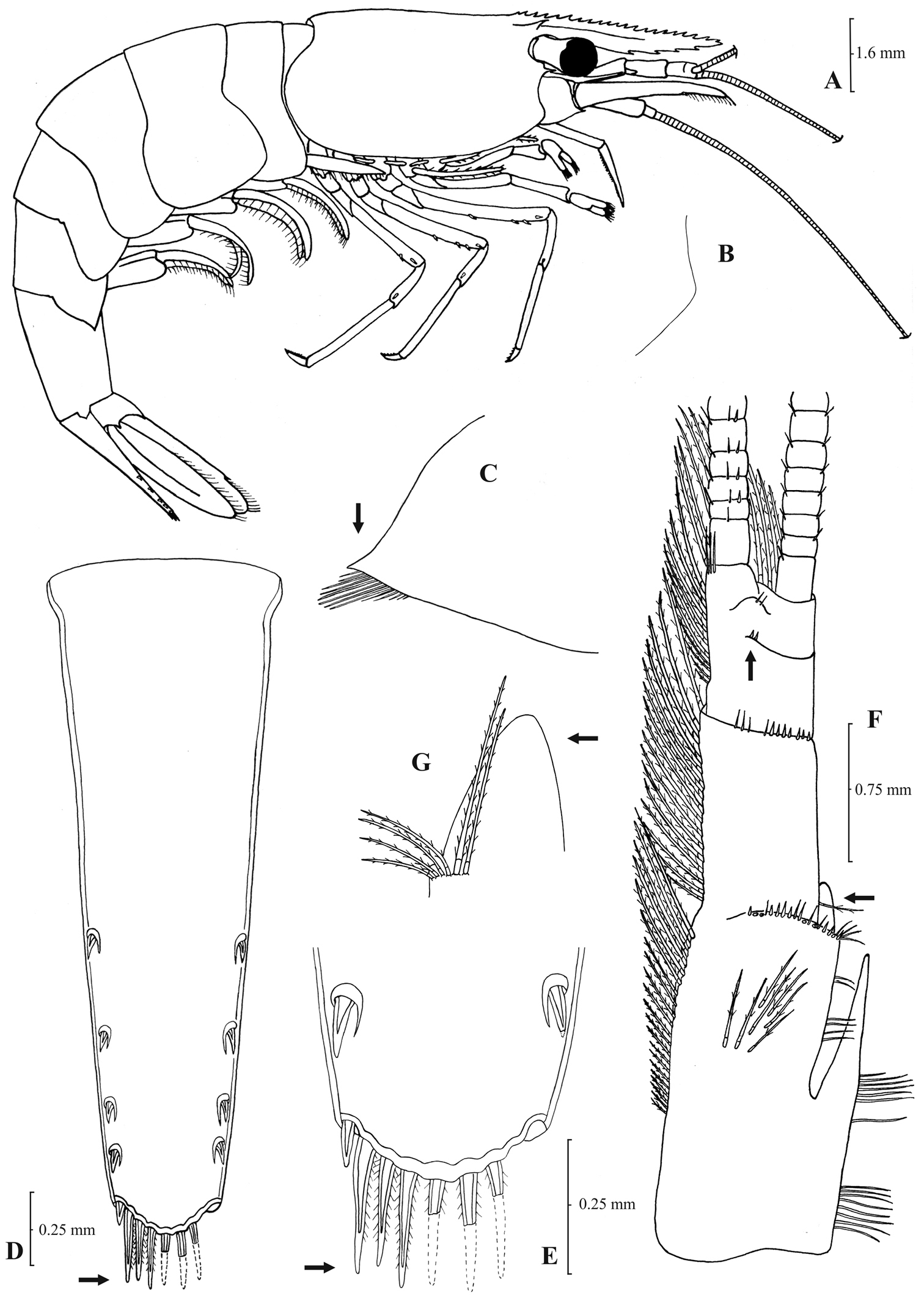
Carapace with supraorbital and antennal tooth. Rostrum long and armed up to the tip. Eyes well developed, pigmented. Exopods present only on the two first pairs of pereiopods, carpus of first and second pair of pereiopods with a distal excavation. Uropod diaeresis with a single spine (rarely two). Appendix masculina of male second pleopod long, sub-cylindrical and armed with numerous spiniform setae. Eggs small to medium, size 0.40–0.75 × 0.25–0.5 mm.
http://species-id.net/wiki/Atyaephyra_desmarestii
Type material. Neotype: 1 ovig. ♀ (CL 7.1 mm), MNHN-IU-2009-2270 (ex MNHN-Na480), Maine-et-Loire, France [here designated].
Tunisia: 8 ♀♀ (1 ovig.) (CL 5.4–7.4 mm), Barrage Lebna (Fig. 1, stn 1), 21.3.2010, coll. S. Dhaouadi-Hassen; 2 ♀♀ (CL 6.0–6.8 mm), NHM 1515–1540.22.2.74, Ain Draham, Barrage Ben Metir (Fig. 1, stn 2), 22.2.1974. Algeria: 1 ♂ (CL 5.1 mm), NHM 1955.5.3.15–18, Algiers, Seybouse River (Fig. 1, stn 3), 3.5.1955; 11 ♀♀ (6 ovig.) (CL 5.0–8.0 mm) and 1 ♂ (CL 5.2 mm), NHM 1949.5.2.1–12, Beni Abbes, Saoura River (Fig. 1, stn 4), 2.5.1949, coll. H. Munro Fox. Morocco: 4 ♀♀ (1 ovig.) (CL 5.5–6.5 mm) and 1 ♂ (CL 5.0 mm), Moulouya River (Fig. 1, stn 5), 11.4.2011, coll. M. Melhaoui; 1 ♀ (CL 6.9 mm) and 4 ♂♂ (CL 5.2–5.6 mm), NHM 1953.12.2.12–15, Krumane River (Fig. 1, stn 6), 22.7.1952, coll. J. Phillipson. Portugal: 21 ♀♀ (12 ovig.) (CL 5.8–7.3 mm) and 11 ♂♂ (CL 5.0–5.7 mm), Algarve, São Barnabé River (Odelouca River) (Fig. 1, stn 7), 23.7.1988, coll. C. d' Udekem d' Acoz; 7 ♀♀ (6 ovig.) (CL 6.2–7.7 mm) and 5 ♂♂ (CL 5.0–5.2 mm), NHM 1971.105, Portimao, Odelouca River (Fig. 1, stn 8), 1970; 18 ♀♀ (4 ovig.) (CL 5.5–8.0 mm) and 3 ♂♂ (CL 5.0–5.1 mm), NHM 1986.261, Bordeira River (Fig. 1, stn 9), 5.3.1985, coll. J. Paula; 5 ♀♀ (4 ovig.) (CL 7.0–8.1 mm) NHM 1880.36, Sintra, Colares River (Fig. 1, stn 10), 1880; 15 ♀♀ (3 ovig.) (CL 5.8–7.9 mm) and 5 ♂♂ (CL 5.3–6.1 mm), Coimbra, Ceira River (Fig. 1, stn 11), 24.5.2010, coll. V. Ferreira. Spain: 2 ♀ (CL 6.5–8.0 mm), Veta la Arena, Guadiamar River (Fig. 1, stn 12), 8.5.2006, coll. C. Lejeusne; 5 ♀♀ (CL 6.1–6.7 mm) and 17 ♂♂ (CL 5.0–6.5 mm), Cadiz, Guadalete River (Fig. 1, stn 13), 2000, coll. A. Rodriguez; 3 ♀♀ (CL 5.1–6.3 mm), Segura River (Fig. 1, stn 14), 28.9.2001, coll. J.L. Moreno Alcaraz; 10 ♀♀ (1 ovig.) (CL 6.1–7.5 mm) and 1 ♂ (CL 5.5 mm), Mundo River (Fig. 1, stn 15), 18/27.9.2001, coll. J.L. Moreno Alcaraz; 2 ♀♀ (CL 6.6–7.7 mm) and 1 ♂ (CL 5.5 mm), Villalva de la Sierra, Jucar River, 40°07.99'N, 02°08.38'W (Fig. 1, stn 16), 16.8.2001, coll. J.L. Moreno Alcaraz; 7 ♀♀ (CL 5.1–6.4 mm) and 1 ♂ (CL 5.3 mm), Ossa de Montiel, Vado Blanco River, 38°54.60'N, 02°48.03'W (Fig. 1, stn 17), 3.10.2001, coll. J.L. Moreno Alcaraz; 3 ♀♀ (CL 5.7–6.5 mm), El Torno, Bullaque River, 39°14.36'N, 04°15.57'W (Fig. 1, stn 18), 11.10.2001, coll. J.L. Moreno Alcaraz; 2 ♀♀ (CL 7.2–7.7 mm), Canavera, Guadiella River, 40°25.36'N, 02°28.95'W (Fig. 1, stn 19), 14.8.2001, coll. J.L. Moreno Alcaraz; 3 ♀♀ (CL 6.2–8.0 mm), Abanades, Tajuna River (Fig. 1, stn 20), 7.8.2001, coll. J.L. Moreno Alcaraz; 3 ♀♀ (1 ovig.) (CL 6.3–7.2 mm) and 6 ♂♂ (CL 5.5–6.5 mm), Henares River, (Fig. 1, stn 21), 1.8.2001, coll. J.L. Moreno Alcaraz; 1 ovig. ♀ (CL 7.4 mm), Naharros, Canamares River, 41°09.10'N, 02°55.14'W (Fig. 1, stn 22), 30.7.2001, coll. J.L. Moreno Alcaraz; 2 ovig. ♀♀ (CL 7.3–7.8 mm), Puebla de Valles, Jarama River (Fig. 1, stn 23), 31.7.2001, coll. J.L. Moreno Alcaraz; 1 ♀ (CL 5.9 mm) and 1 ♂ (CL 5.1 mm) La Guardia, Cedron River, 39°48.26'N, 03°20.33'W (Fig. 1, stn 24), 6.9.2001, coll. J.L. Moreno Alcaraz; 1 ♀ (CL 5.2 mm), Escalona, Alberche River, 40°09.45'N, 04°25.04'W (Fig. 1, stn 25), 27.8.2001, coll. J.L. Moreno Alcaraz; 1 ♀ (CL 5.1 mm) and 2 ♂♂ (CL 5.3–5.7 mm), Tietar River (Fig. 1, stn 26), 28.8.2001, coll. J.L. Moreno Alcaraz; 9 ♀♀ (1 ovig.) (CL 5.1 mm) and 1 ♂ (CL 5.0 mm), Tagus River (Fig. 1, stn 27), 14.8.2001 and 5.9.2001, coll. J.L. Moreno Alcaraz; 1 ♂ (CL 5.5 mm), Calanda, Guadalope River (Fig. 1, stn 28), 25.5.2004, coll. J. Oscoz; 1 ♀ (CL 7.2 mm) and 1 ♂ (CL 5.1 mm), Escatron, Martin River (Fig. 1, stn 29), 24.5.2001, coll. J. Oscoz; 1 ♀ (CL 5.6 mm) and 3 ♂♂ (CL 5.3–5.6 mm), Murillo de Gallego, Gallego River (Fig. 1, stn 30), 7.8.2007, coll. J. Oscoz; 1 ovig. ♀ (CL 6.5 mm), Gurrea de Gallego, Soton River (Fig. 1, stn 31), 14.6.2006, coll. J. Oscoz; 1 ♂ (CL 6.2 mm), Lumbier, Irati River (Fig. 1, stn 32), 8.7.2005, coll. J. Oscoz; 2 ovig. ♀♀ (CL 6.9–7.5 mm) and 4 ♂♂ (CL 5.2–5.8 mm), Aspurz, Salazar River (Fig. 1, stn 33), 3.7.2007, coll. J. Oscoz; 1 ovig. ♀ (CL 6.5 mm) and 1 ♂ (CL 5.2 mm), Ripodas, Areta River (Fig. 1, stn 34), 3.7.2007, coll. J. Oscoz; 5 ♀♀ (4 ovig.) (CL 5.0–7.5 mm) and 2 ♂♂ (CL 5.6 mm), Castejon, Alfaro, Tudela, Ebro River (Fig. 1, stn 35), 11/12.7.2007, coll. J. Oscoz; 6 ♀♀ (5 ovig.) (CL 7.0–8.6 mm), San Adrian, Ega River (Fig. 1, stn 36), 27.6.2007, coll. J. Oscoz; 1 ovig. ♀ (CL 7.3 mm) and 2 ♂♂ (CL 5.2–5.5 mm), Marcilla, Aragon River (Fig. 1, stn 37), 28.6.2007, coll. J. Oscoz; 2 (1 ovig.) ♀♀ (CL 8.2–8.5 mm) and 2 ♂♂ (CL 5.6–6.5 mm), Urroz, Erro River (Fig. 1, stn 38), 25.5.2007, coll. J. Oscoz; 1 ovig. ♀ (CL 7.5 mm) and 2 ♂♂ (CL 5.8–6.0 mm), Mendigorria, Salado River (Fig. 1, stn 39), 14.6.2007, coll. J. Oscoz; 1 ovig. ♀ (CL 7.6 mm), Puentelarreina, Arga River (Fig. 1, stn 40), 20.6.2007, coll. J. Oscoz; 1 ♀ (CL 7.2 mm), Iraneta, Arakil River (Fig. 1, stn 41), 20.6.2007, coll. J. Oscoz; 1 ♀ (CL 7.4 mm), Palazuelos, Jerea River (Fig. 1, stn 42), 1.6.2004, coll. J. Oscoz; 3 ovig. ♀♀ (CL 7.3–8.0 mm) and 2 ♂♂ (CL 5.3–5.5 mm), NHM 1955.10.5.2–6 and NHM 1957.8.12.69–75, Barcelona, Llobregat River (Fig. 1, stn 43), 5.10.1955 and 12.8.1955; 8 ♂♂ (CL 5.2–6.1 mm), Bascara, Fluvia River (Fig. 1, stn 44), 4.2.2005, coll. M.L. Zettler; 3 ♀♀ (CL 5.6–6.6 mm), NHM 1955.10.5.8–10, Gerona, Lake of Banyoles (Fig. 1, stn 45), 5.10.1955. France: 30 ♀♀ (18 ovig.) (CL 5.0–7.0 mm) and 20 ♂♂ (CL 5.0–5.2 mm), Merville, Garrone River (Fig. 1, stn 46), 25.8.2004, coll. R. Liasko and S. Combes; 2 ♀♀ (CL 5.5–6.5 mm), NHM 1955.5.3.11–14, Maine et Loire, Loire River (Fig. 1, stn 47), 3.5.1955; 2 ♀♀ (CL 6.6–7.0 mm), Angers, Sarthe River (Fig. 1, stn 48), 20.9.2000, coll. P. Noél; 2 ♀♀ (CL 5.1–5.6 mm), Mayenne River (Fig. 1, stn 49), 20.9.2000, coll. P. Noél; 3 ♀♀ (CL 6.3–6.5 mm), NMW 467, Rennes, Vilaine River (Fig. 1, stn 50), coll. G. Laponge. Belgium: 31 ♀♀ (8 ovig.) (CL 5.2–8.3 mm) and 7 ♂♂ (CL 5.0–6.0 mm), Ombret, Meuse River, (Fig. 1, stn 51), 3.8.1979, coll. C. d' Udekem d' Acoz. Germany: 1 ♂ (CL 5.2 mm) Berlin, Tegel Lake, 52°34.98'N, 13°16.44'E (Fig. 1, stn 52), 13.9.1995, coll. K. Rudolph and M.L. Zettler; 4 ♀♀ (CL 5.7–7.0 mm) and 1 ♂ (CL 5.0 mm), Havel River (Fig. 1, stn 53), 52°23.82'N, 12°17.04'E, 26.8.2005 (Saxony–Anhalt) and 52°29.82'N, 12°24.30'E, 27.8.2005 (Brandenburg), coll. M.L. Zettler. Austria: 1 ♀ (CL 7.4 mm), NMW 18315, Danube River (Fig. 1, stn 54), 8.10.1998, coll. Zipel and Melcher. Italy: 2 ♂♂ (CL 5.0–5.7 mm), Centa River (Fig. 1, stn 55), 28.5.1989, coll. C. Froglia; 4 ♀♀ (CL 5.3–5.8 mm) and 1 ♂ (CL 5.6 mm), Nestore River (Fig. 1, stn 56), 11.11.1974, coll. C. Froglia; 2 ♂♂ (CL 5.2–5.6 mm), Ponte Nuovo, Chiascio River, (Fig. 1, stn 57), 9.9.1975, coll. Cianficoni; 2 ovig. ♀♀ (CL 7.0–7.5 mm) and 1 ♂ (CL 5.2 mm), Nera River (Fig. 1, stn 58), 5.6.1971, coll. Moretti; 5 ♀♀ (CL 6.2–6.8 mm) and 7 ♂♂ (CL 5.0–6.3 mm), Tiber River (Fig. 1, stn 59), 10.10.1975 (Nestore), 14.10.1975 (Orte), 13.11.1975 (Umbertide), coll. Cianficoni. Sicily: 1 ovig. ♀ (CL 7.5 mm) and 4 ♂♂ (CL 5.4–5.9 mm), San Bartolomeo, Rosmarino River (Fig. 1, stn 60), 13.5.1986, coll. C. Froglia; 2 ♀♀ (CL 5.8–6.4 mm) and 1 ♂ (CL 5.5 mm), Simeto River (Fig. 1, stn 61), 1.9.1978, coll. C. Froglia. Sardinia: 7 ♀♀ (4 ovig.) (CL 5.5–7.2 mm) and 2 ♂ (CL 5.0 mm), unknown locality (Fig. 1, stn 62), 13.9.1977, coll. Cav; 2 ♀ (CL 6.7–7.6 mm) and 1 ♂ (CL 5.6 mm), unknown locality, coll. R.B. Manning. Corsica: 3 ♀♀ (1 ovig.) (CL 6.3–6.9 mm) and 1 ♂♂ (CL 5.0 mm), Favello, Taravo River (Fig. 1, stn 63), 10.8.2003, coll. M.L. Zettler; 5 ♂♂ (CL 5.0–5.8 mm), Propriano, Rizzanese River (Fig. 1, stn 64), 13.8.2003, coll. M.L. Zettler; 2 ♀♀ (CL 7.2–7.9 mm) and 4 ♂♂ (CL 5.3–6.0 mm), Bravone, Bravone River, 42°12.36'N, 09°32.10'E (Fig. 1, stn 65), 16.8.2003, coll. M.L. Zettler.
Rostrum long, dorsal margin straight or slightly curved in the middle and pointed upwards, 3.79–8.70, mostly (82% of the individuals examined) 4.64–6.50, × as long as high, shorter, equal to, or longer than scaphocerite. From 17 to 36 (21–28 in 86% of the individuals examined) pre orbital teeth on dorsal margin of rostrum arranged to tip. One to five, most frequently (90% of the individuals examined) 2–4, post orbital teeth and 1–13, most often (88% of the individuals examined) 4–9, teeth on ventral margin of rostrum. Carapace smooth with pterygostomial angle not protruding, rounded (
Atyaephyra desmarestii is a large sized species with maximum carapace length to be 6.8 mm in ♂♂, 8.5 mm in ♀♀ and 8.6 mm in ovig. ♀♀.
Atyaephyra desmarestii can be differentiated from all other species of Atyaephyra by molecular characters, as demonstrated by the phylogenetic analysis of mtDNA COI sequences. Furthermore, 22 haplotypes from 30 different localities found in Atyaephyra desmarestii were not shared by any other species of the genus. Finally, it differs from all the other species in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1 (Genbank accession number JX289920), position 213: cytosine (C), position 234: cytosine (C) and position 444: adenine (A).
Atyaephyra desmarestii is found in freshwater habitats of North Africa and West-central Europe (see material examined and Fig. 1).
Atyaephyra desmarestii has been exhaustively described and illustrated by
In light of the current revision of the species complex across Europe, North Africa and the Middle East, a nomenclatorial problem exists with the nomen, Atyaephyra desmarestii var. occidentalis
Atyaephyra desmarestii can be distinguished among other characters from Atyaephyra stankoi, Atyaephyra orientalis and Atyaephyra thyamisensis sp. n. by the presence of 0–8 mesial spines (
http://species-id.net/wiki/Atyaephyra_orientalis
Figs 3–4Turkey: 3 ♀♀ (CL 4.8–5.0 mm), Antalya, Kirkgoz Spring (Fig. 1, stn 107), 21.6.2006, coll. M. Özbek; 7 ♀♀ (CL 4.5–5.5 mm), SMF 12174, Akbez, Karasu River (Fig. 1, stn 108), 22.9.1982, coll. R.K. Kinzelbach. Syria: 10 ♀♀ (3 ovig.) (CL 5.0–6.0 mm) and 4 ♂♂ (CL 4.0–5.0 mm), SMF 12050, below the dam of Ascharna, Orontes River (Fig. 1, stn 109), 30/31.3.1979, coll. R.K. Kinzelbach; 34 ♀♀ (15 ovig.) (CL 4.1–4.8 mm), SMF 12188, north of M’adan, Euphrates River (Fig. 1, stn 110), 17.8.1978, coll. R.K. Kinzelbach; 3 ♀♀ (2 ovig.) (CL 4.5–5.6 mm), SMF SYR8, Euphrates River (Fig. 1, stn 111), 15/16.6.1998, coll. R. Beck. Israel: 3 ♀♀ (2 ovig.) (CL 4.7–5.3 mm) and 2 ♂♂ (CL 3.9–4.0 mm), SMF IES 1189, Te’o Spring (Fig. 1, stn 112), 16.2.1977; 9 ♀♀ (CL 4.3–6.0 mm) and 4 ♂♂ (CL 3.9–4.0 mm), Hula Lake (Fig. 1, stn 113), 29.1.1981, coll. D. Eurth; 2 ovig. ♀♀ (CL 3.8–3.9 mm), NHM 1913.7.24.3–12, Kinneret Lake (Fig. 1, stn 114), 24.7.1913; 1 ♀ (CL 3.9 mm), Samakh, Kinneret Lake, 6.5.1986, coll. R. Ortal; 1 ♀ (CL 4.4 mm), Zaki River (Fig. 1, stn 115), 6.5.1986, coll. R. Ortal; 1 ♀ (CL 4.0 mm), Jordan River (Fig. 1, stn 116), 6.5.1981, coll. R. Ortal; 1 ♀ (CL 4.2 mm) and 1 ♂ (CL 3.8 mm), NHM 1938.1.26.8.12, Jordan River, 26.1.1938. Jordan: 2 ♀♀ (1 ovig.) (CL 4.0–4.9 mm), SMF 12057, Al-Azraq Oasis (Fig. 1, stn 117), 24.3.1977, coll. H. Damian. Iraq: 12 ♀♀ (CL 5.6–6.8 mm) and 3 ♂♂ (CL 4.5–4.8 mm), Basrah, Garmat Ali marsh (Fig. 1, stn 118), 24.2.1987, coll. A.H.Y. Al-Adhub; 1 ♀ (CL 5.2 mm), NHM 1919.11.14.5–20, Basrah, Shatt Al-Arab River (Robat creek) (Fig. 1, stn 119), 14.11.1919, coll. Capt. Boulenger; 1 ♂ (CL 4.2 mm), NHM 1919.4.28.2–3, Basrah, Shatt Al-Arab River (Robat creek), 28.4.1919, coll. P.J. Barraud; 4 ♀♀ (1 ovig.) (CL 5.2–5.5 mm) and 1 ♂ (CL 4.8 mm), Basrah, Shatt Al-Arab River (Fig. 1, stn 120), 2011, coll. M.D. Naser; 1 ovig. ♀, NHM 1919.11.12.11, Amarah, Tigris River (Fig. 1, stn 121), 12.11.1919, coll. J.O. Cooper Esq.
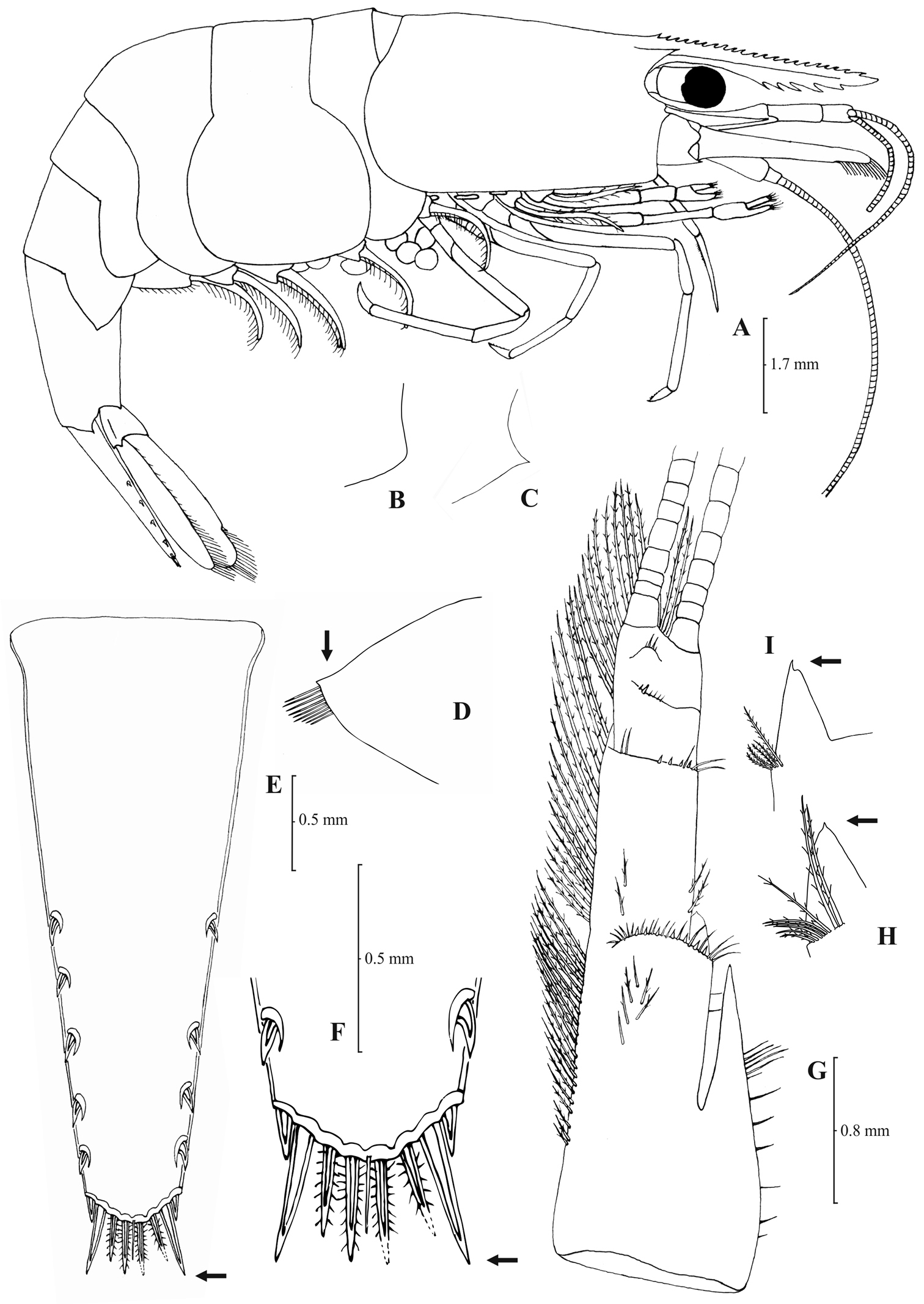
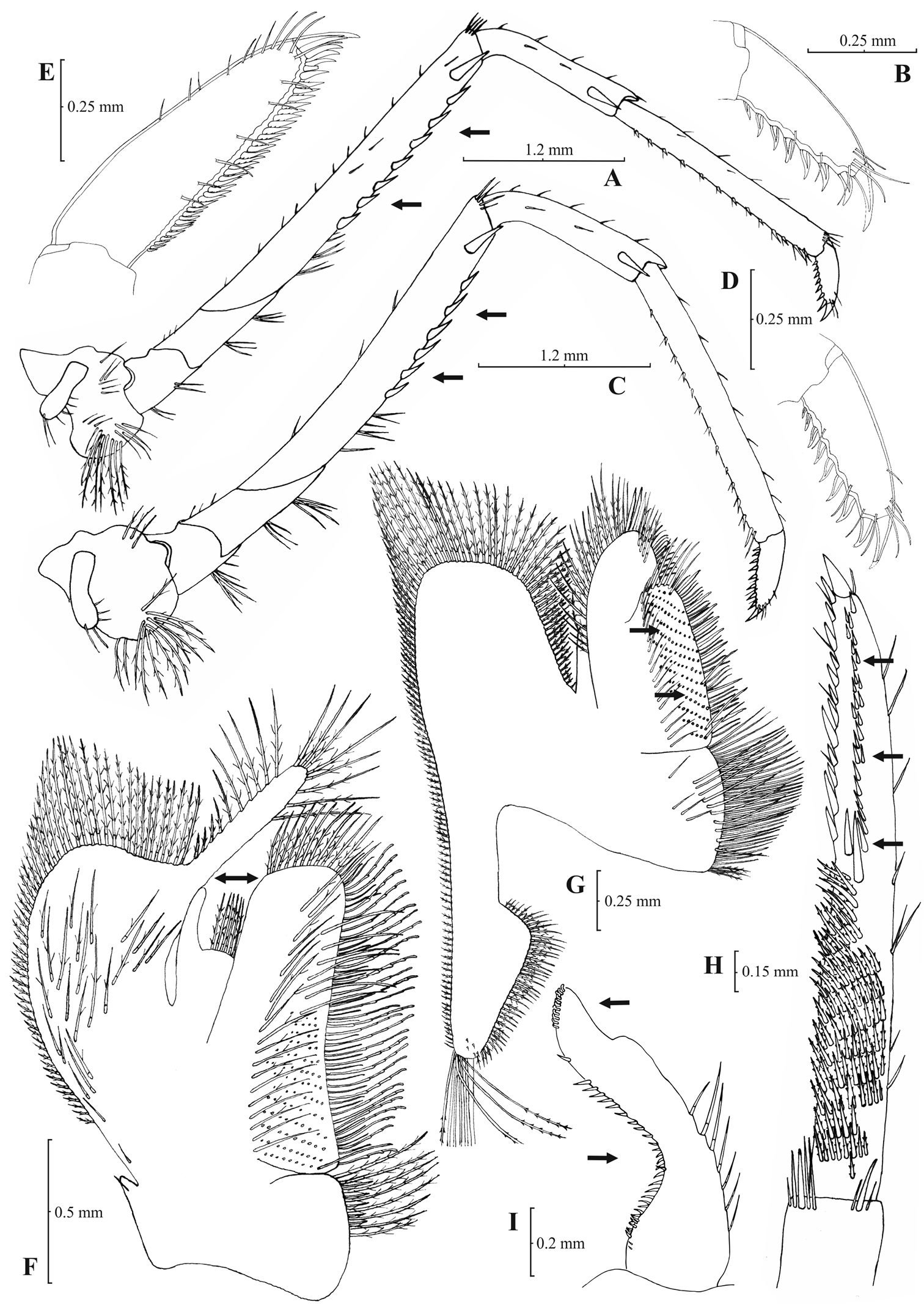
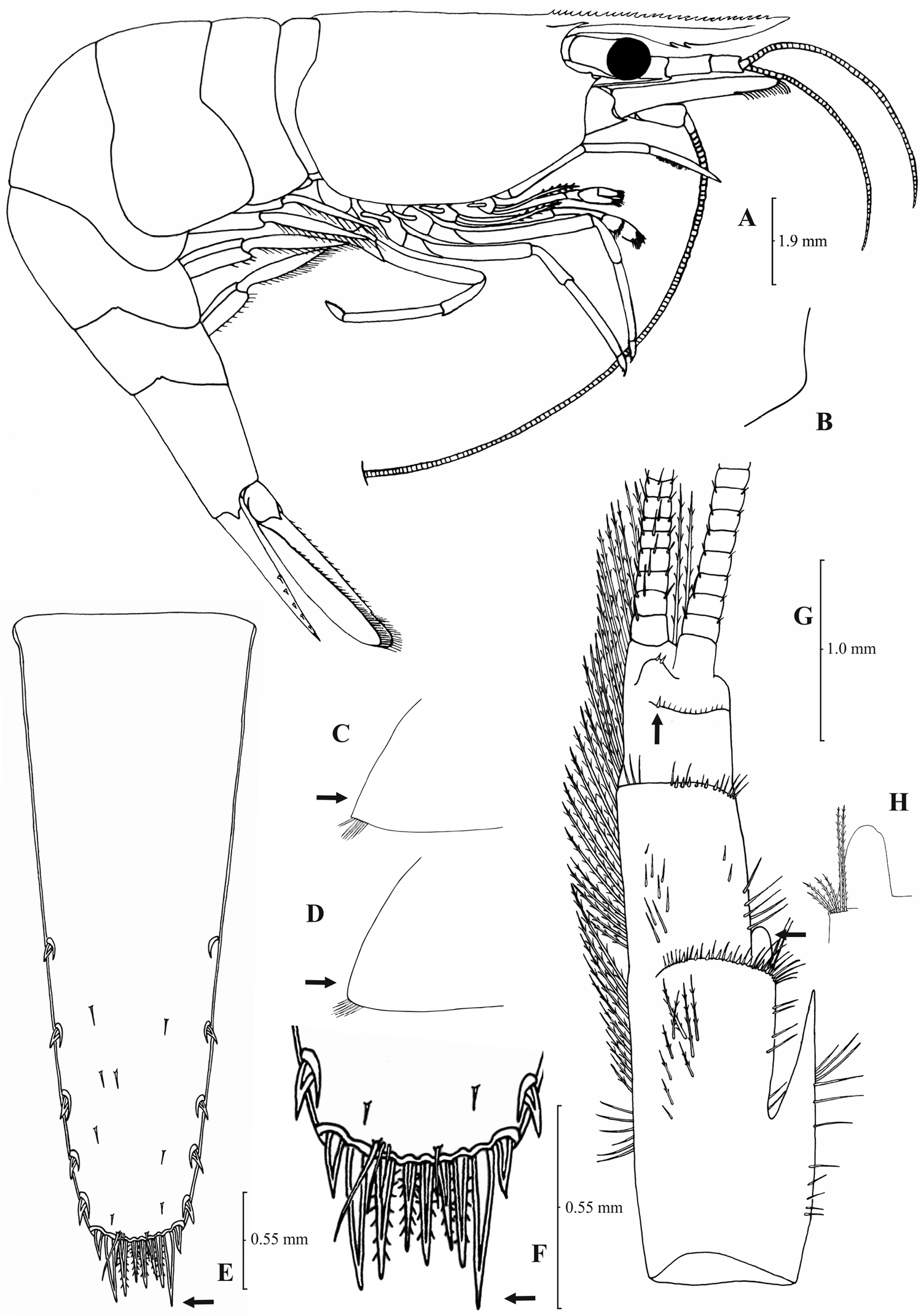
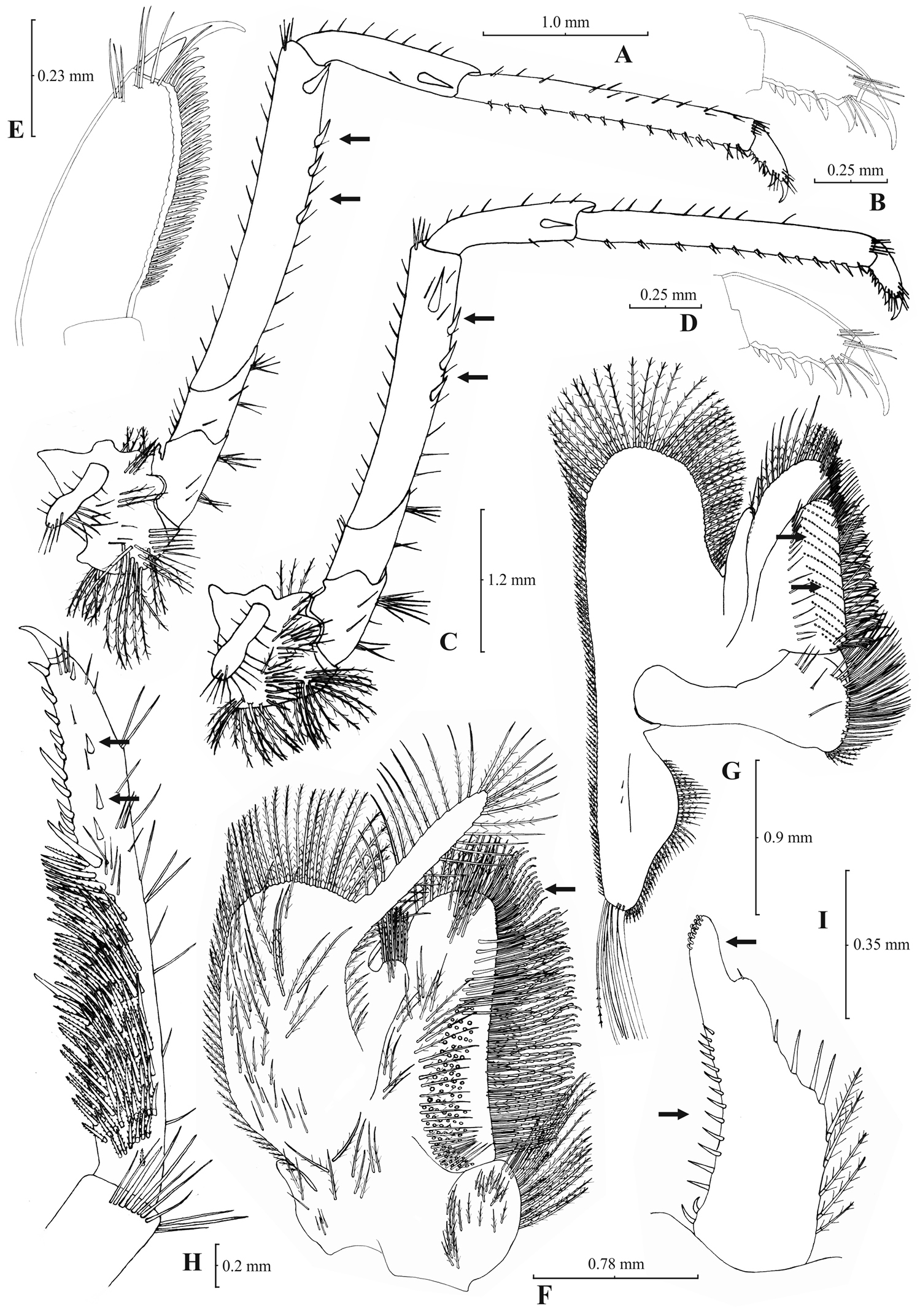
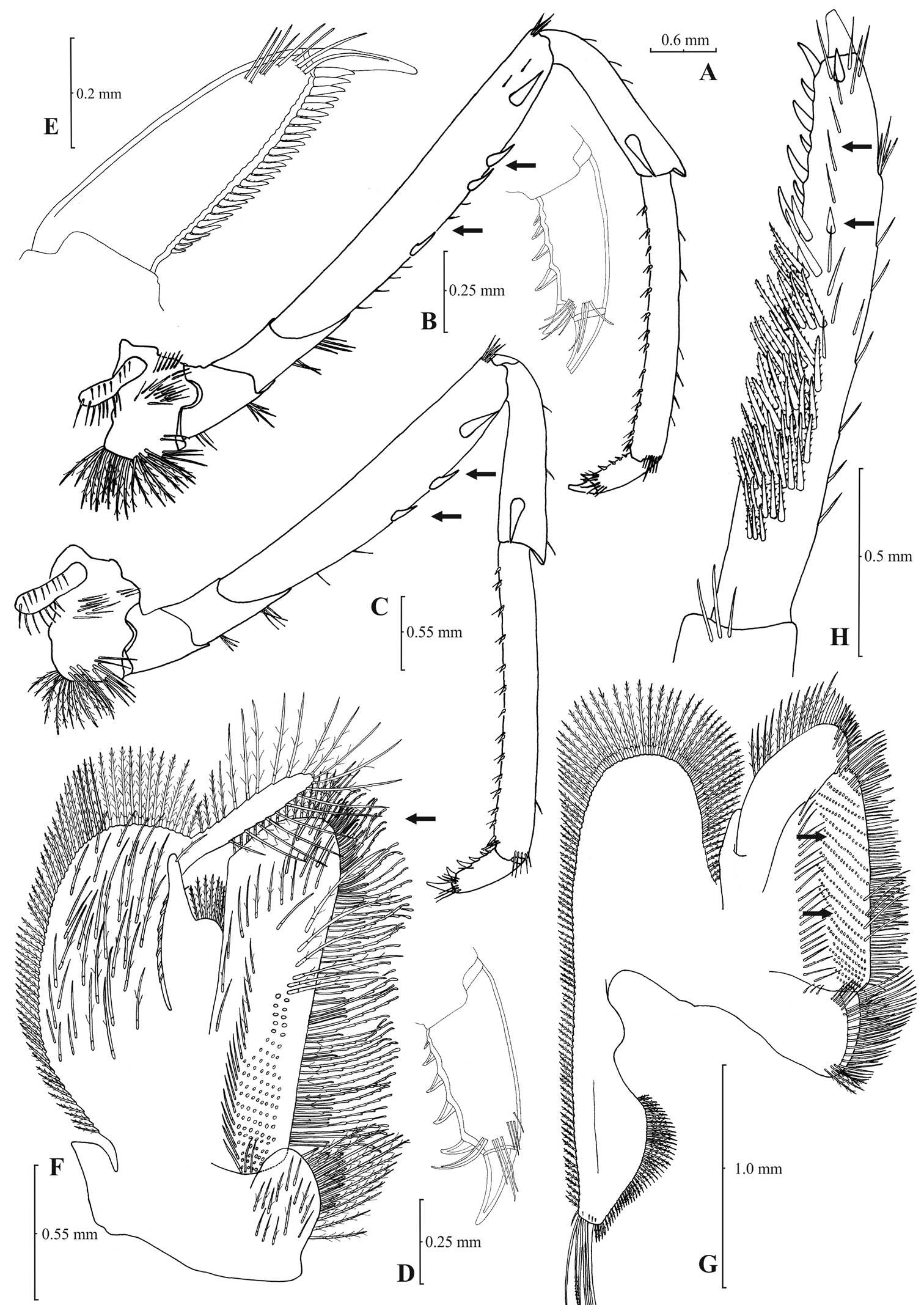
Rostrum long, slender, dorsal margin straight, slightly or strongly curved in the middle and pointed upwards or downwards, 6.0–10.0, most frequently (91% of the individuals examined) 6.5–9.25, × as long as high, shorter or equal to, or longer than scaphocerite (longer in 71% of the individuals examined). 14–29 (18–23 in 80% of the individuals) pre orbital teeth on dorsal margin of rostrum arranged to tip. 0–3, most often (85%) 1–3, post-orbital teeth. 3–13 teeth, mostly (96%) 4–10, arranged on ventral margin of rostrum (Fig. 3A). Carapace smooth with pterygostomial angle not protruding and rounded or bluntly produced (Figs 3B–C). Pleuron of fifth abdominal segment pointed ending in an acute or an obtuse posterior angle (Fig. 3D). Telsonwith 3–6, predominantly (93%) 4–5, pairs of dorsal spines arranged in curved fashion (Fig. 3E). Distal border of telson with 7–12, most often (91%) 8–10, spines (4–5 pairs) arranged in a fork-like or a fan-like way. Outermost pair of spines shortest, similar to dorsal spines, adjacent pair stronger terminating beyond, along with or before (beyond and along with in 64% of the individuals) the inner finely setulose pairs (Figs 3E–F). Basal segment of antennular peduncle with long stylocerite, with its tip failing to reach, reaching or overreaching the distal end of basal segment. Anterolateral lobe of basal segment short and pointed (Figs 3H–I). Distal segment of antennular peduncle with 0–3, most often (93%) 1–2, spines (Fig. 3G). Basal lower endite of maxilla densely covered with long simple setae arranged in 11–16 (12–15 in 93% of the individuals) oblique parallel rows. Endite of maxilla 1.75–2.20, mostly (93%) 1.81–2.07, × as long as basal lower endite (Fig. 4G). Basal endite of first maxilliped failing or reaching to distal end of exopod distal margin (Fig. 4F). Distal one-third of terminal segment of third maxilliped bearing 10–36 (14–31 in 84% of the individuals), mesial spines and one subdistal lateral spine near the base of larger terminal spine (Fig. 4H). Armature along flexor margin of dactylus of third and fourth pereiopod consisting of 6–11 (7–10 in 97% of the individuals) and 7–11 (8–10 in 89% of the individuals) spines (including terminal spine) respectively (Figs 4B, 4D). Merus of third and fourth pereiopod with 6–10 (7–9 in 85% of the individuals) and 5–9 (6–7 in 83% of the individuals) spines respectively (Figs 4A, 4C). Dactylus of fifth pereiopod with 33–55 (36–49 in 83% of the individuals) spines arranged in comb-like fashion on flexor margin (Fig. 4E). Endopod of first male pleopod expanded proximally with a distal portion stout and not tapering, often, with a, large protruding lobe in its outer subdistal part. Endopod with 13–38 spines arranged on a strongly curved inner margin and 5–8 setae arranged on outer margin (Fig. 4I,
Atyaephyra orientalis Bouvier, 1913, adult ovig. ♀ (SMF 12050): A entire individual B detail ofpterygostomial boarder C detail ofpterygostomial boarder (adult ♀, SMF 12050) D right pleuron of fifth abdominal segment E telson F distal margin of telson G right antennular peduncle H right antennular lobe I right antennular lobe (adult ♀, SMF 12050).
Atyaephyra orientalis Bouvier, 1913, adult ovig. ♀ (SMF 12050): A right third pereiopod B dactylus of third pereiopod C right fourth pereiopod D dactylus of fourth pereiopod E dactylus of right fifth pereiopod F right first maxilliped G right maxilla H rightterminal segment of third maxilliped. Adult ♂ (SMF 12050): I right endopod of first male pleopod.
Atyaephyra orientalis is a small-medium sized species of Atyaephyra, with maximum carapace length to be 4.8 mm in ♂♂, 6.8 mm in ♀♀ and 5.5 mm in ovig. ♀♀.
Atyaephyra orientalis can be differentiated from all other species of Atyaephyra by molecular characters, as demonstrated by the phylogenetic analysis of mtDNA COI sequences. Additionally, 5 haplotypes, each from a different location, found in Atyaephyraorientalis were not shared by any other species of the genus. It also differs from all the other species in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1, position 273: guanine (G), position 276: guanine (G) and position 369: cytosine (C).
Atyaephyra orientalis is found in freshwater habitats of Middle East, from Turkey to Iraq (see material examined and Fig. 1).
Atyaephyra orientalis appears to be morphologically more similar to Atyaephyra stankoi and Atyaephyra thyamisensis sp. n. by sharing characters such as the presence of numerous mesial spines (10–38) on terminal segment of third maxilliped (Figs 4H, 6H, 8H). It also shares in common with the other two species the presence of fewer rows of setae (12–16) on basal lower endite of maxilla, the endite of maxilla being 1.75–2.24 × as long as basal lower endite (Figs 4G, 6G, 8G) and basal endite of first maxilliped failing or reaching to distal end of exopod distal margin (Figs 4F, 6F, 8F). Atyaephyra orientalis can be separated from Atyaephyra thyamisensis sp. n. and Atyaephyra stankoi by the presence of a pointed antennular lobe (Figs 3H–I) (vs. round in Atyaephyra stankoi and Atyaephyra thyamisensis sp. n. Figs 5H, 7H). Further, Atyaephyra orientalis can be distinguished by the strongly curved and distally stout and not tapering endopod of male first pleopod (Fig. 4I) (vs. slightly curved and distally more or less elongated but always tapering in Atyaephyra stankoi, Fig. 6I; slightly or strongly curved but always its distal part is elongated and tapering (ribbon shaped) in Atyaephyra thyamisensis sp. n., Fig. 8I). Atyaephyra orientalis differs from the other four species of Atyaephyra in having 10–36 spines on terminal segment of third maxilliped (Fig. 4H) (vs. 0–8 in Atyaephyra desmarestii, Atyaephyra strymonensis sp. n., Atyaephyra acheronensis sp. n. and Atyaephyra tuerkayi sp. n. Figs 10H, 12H, 14H).
http://species-id.net/wiki/Atyaephyra_stankoi
Figs 5–6Type material. Neotype: NHM 2012.1475, adult ♀ (CL 6.0 mm), Greece–F.Y.R.O.M., Doirani Lake, (Fig. 1, stn 99), among aquatic plants, 9.11.1992, coll. S. Jovanovich and E. Stojkoska [here designated].
Greece: 4 ♀♀ (CL 5.4–5.9 mm), Peloponnesus, Alfeios River (Fig. 1, stn 82), 24.9.2001, coll. Ch. Anastasiadou; 4 ♀♀ (CL 5.4–5.7 mm), Aitoloacarnania, Ozeros Lake (Fig. 1, stn 83), 22.11.2001, coll. Ch. Anastasiadou; 2 ovig. ♀♀ (CL 5.5–7.0 mm), Aitoloakarnania, Aitoliko, Acheloos River (Fig. 1, stn 84), 4.4.2002, coll. Ch. Anastasiadou; 3 ♀♀ (CL 5.0–5.5 mm), Aitoloakarnania, Trichonida Lake (Fig. 1, stn 85), 22.10.2001, coll. Ch. Anastasiadou; 4 ♀♀ (CL 5.1–6.5 mm) Aitoloacarnania, Lysimachia Lake (Fig. 1, stn 86), 22.11.2001, coll. Ch. Anastasiadou; 1 ♀ (CL 6.9 mm) and 2 ♂♂ (CL 5.1–5.3 mm), Thessalia, Tavropou Lake (Fig. 1, stn 87), 14.11.2001, coll. Ch. Anastasiadou; 17 ♀♀ (CL 6.0–8.0) and 2 ♂♂ (CL 5.0 mm), Thessalia, Enipeas River (Fig. 1, stn 88), 14.10.2001, coll. Ch. Anastasiadou; 3 ♀♀ (CL 6.5–7.6 mm) and 1 ♂ (CL 5.5 mm), ZMAUTH G1-910, Thessalia, Mati Tyrnavou Lake (Fig. 1, stn 89), 15.11.1977, coll. A. Koukouras; 1 ♀ (CL 6.8 mm) and 1 ♂ (CL 5.2 mm) Thessalia, Pineios River (Fig. 1, stn 90), 15.11.2001, coll. Ch. Anastasiadou; 1 ♀ (CL 7.0 mm), Thessalia, Lithaios River (Fig. 1, stn 91), 14.11.2001, coll. Ch. Anastasiadou; 5 ♀♀ (CL 6.0–7.0 mm) and 1 ♂ (CL 5.0 mm), Thessalia, Gritsas River (Fig. 1, stn 92), 15.11.2001, coll. Ch. Anastasiadou; 3 ♀♀ (CL 6.0–6.7 mm), Macedonia, Aliakmonas River (Fig. 1, stn 93), 9.9.1974 and 26.11.1978; 4 ♀♀ (2 ovig.) (CL 5.7–6.8 mm), ZMAUTH G1-1005, Macedonia, Vegoritida Lake (Fig. 1, stn 94), 17.6.1968; 4 ♀♀ (1 ovig.) (CL 5.5–6.3 mm), ZMAUTH G1-1018, Thessalia, Agra Lake (Fig. 1, stn 95), 17.6.1968, coll. P. Economides; 12 ♀♀ (CL 5.5–7.0 mm) and 3 ♂♂ (CL 5.0–5.5 mm), Thessalia, Edessaios River (Fig. 1, stn 96), 19.10.2001, coll. Ch. Anastasiadou; 5 ♀♀ (CL 5.0–5.5 mm) and 1 ♂ (CL 5.0 mm), Thessalia, Kariotissa, Moglenitsa River (Fig. 1, stn 97), 18.10.2001, coll. Ch. Anastasiadou; 4 ♀♀ (CL 6.0–7.0 mm) and 1 ♂ (CL 5.0 mm), ZMAUTH G1-988, Macedonia, Axios River (Fig. 1, stn 98), 16.7.1971, coll. P. Economides; 11 ♀♀ (CL 5.9–7.3 mm) and 1 ♂ (CL 5.1 mm), Macedonia, Richios River (Fig. 1, stn 100), 26.10.01, coll. Ch. Anastasiadou; Greece–F.Y.R.O.M.: 4 ♀♀ (CL 5.0–5.7 mm), Doirani Lake, (Fig. 1, stn 99), 9.11.1992, coll. S. Jovanovich and E. Stojkoska.
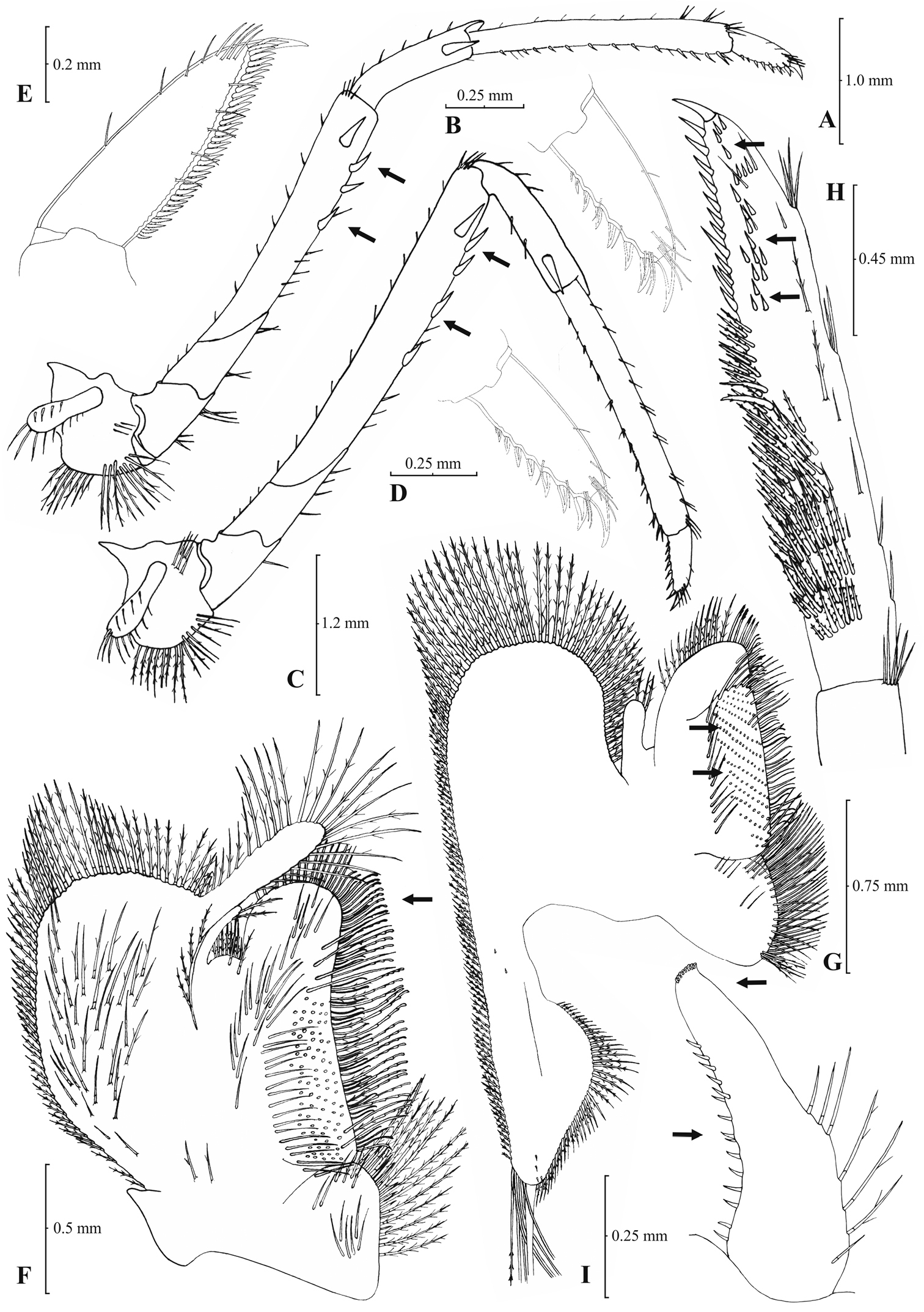
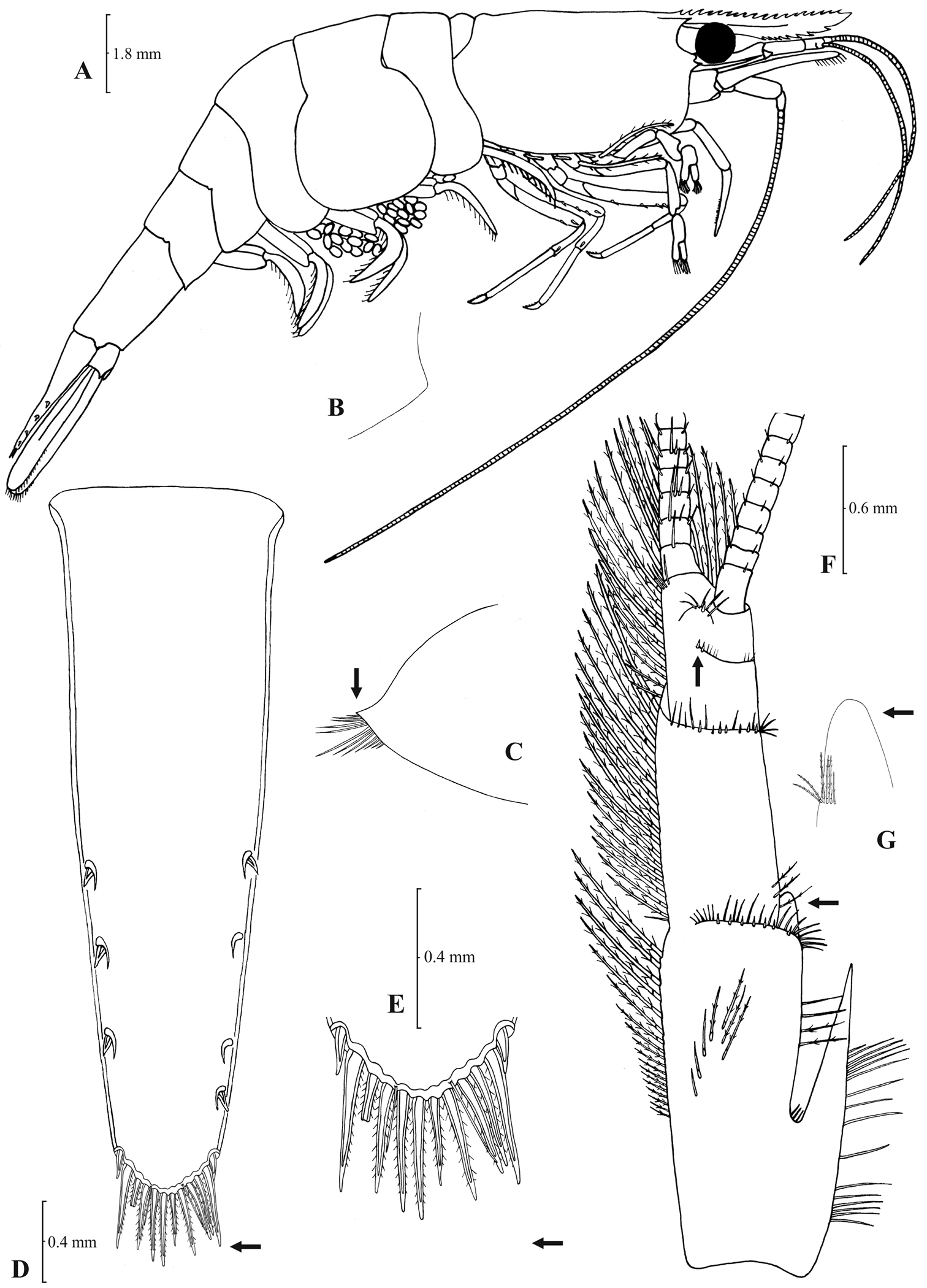
Rostrum long, slender, dorsal margin straight or slightly curved in the middle and pointed upwards, 6.12–8.67, mostly (83% of the examined individuals) 6.25 to 7.54, × as long as high, shorter, equal to, or longer than scaphocerite (longer in 76% of the individuals examined). From 17 to 28 (19–27 in 91% of the individuals) pre orbital teeth on dorsal margin of rostrum arranged up to tip. 0–3, predominantly (96%) 1–3, post-orbital teeth. 2–8, most often (96%) 2–6, teeth arranged on ventral margin of rostrum (Fig. 5A). Carapace smooth with pterygostomial angle not protruding, rounded (Fig. 5B). Pleuron of fifth abdominal segment usually pointed ending in an obtuse (ending in an acute angle in 11% of the individuals) posterior angle (Figs 5C–D). Telsonwith 3–6, most often (93%) 5–6, pairs of dorsal spines arranged in curved fashion (Fig. 5E). Distal border of telson with 6–11, mostly (87%) 8–10, spines (3–6 pairs), arranged in a fork-like pattern. Outermost pair of spines shortest, similar to dorsal spines, adjacent pair stronger terminating beyond (or along with) the inner finely setulose pairs (Figs 5E–F). Basal segment of antennular peduncle with long stylocerite, with its tip failing to reach, reaching or overreaching the distal end of basal segment. Anterolateral lobe of basal segment short and rounded (Fig. 5H). Distal segment of antennular peduncle with 1–4, mostly (93%) 1–3, spines (Fig. 5G). Basal lower endite of maxilla densely covered with long simple setae arranged in 12–16, (13–15 in 89% of the individuals), oblique parallel rows. Endite of maxilla 1.78–2.08, mostly (89%) 1.84–1.99, × as long as basal lower endite (Fig. 6G). Basal endite of first maxilliped failing or reaching to distal end of exopod (Fig. 6F). Distal one-third of terminal segment of third maxilliped bearing 11–35, frequently (85%) 16–28, mesial spines and one subdistal lateral spine near the base of larger terminal spine (Fig. 6H). Armature along flexor margin of dactylus of third and fourth pereiopod consisting of 7–11 (7–9 in 98% of the individuals) and 7–10 (7–9 in 98% of the individuals) spines (including terminal spine) respectively (Figs 6B, 6D). Merus of third and fourth pereiopod with 3–8 (4–6 in 83% of the individuals examined) and 2–6 (3–5 in 88% of the individuals) spines respectively (Figs 6A, 6C). Dactylus of fifth pereiopod with 26–47, most often (80%) 32–41, spines arranged in comb-like fashion on flexor margin (Fig. 6E). Endopod of first male pleopod expanded proximally and with a distal portion either elongated (ribbon shape) or more stout but always tapering. Endopod with 13–17 spines arranged on a slightly curved inner margin and 7–12 setae arranged on the outer margin (Fig. 6I). 96–195 eggs of 0.6–0.7 × 0.4 mm in size.
Atyaephyra stankoi Karaman, 1972. Neotype, adult ♀ (NHM 2012.1475): A entire individual B right detail ofpterygostomial boarder C right pleuron of fifth abdominal segment D right pleuron of fifth abdominal segment (adult ♀) E telson F distal margin of telson G right antennular peduncle H right antennular lobe.
Atyaephyra stankoi Karaman, 1972. Neotype, adult ♀ (NHM 2012.1475): A rightthird pereiopod B dactylus of third pereiopod C rightfourth pereiopod D dactylus of fourth pereiopod E dactylus of fifth pereiopod F rightfirst maxilliped G rightmaxilla H right terminal segment of third maxilliped. Adult ♂ (ZMAUTH G1 988): I right endopod of first male pleopod.
Atyaephyra stankoi is a large sized species with maximum carapace length of 5.50 mm in ♂♂, 7.60 mm in ♀♀ and 6.8 mm in ovig. ♀♀.
Atyaephyra stankoi can be distinguished from all other species of Atyaephyra by molecular characters, as shown by the phylogenetic analysis of mtDNA COI sequences, such as the two unique Atyaephyra stankoi haplotypes. Furthermore, it differs from all the other species in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1, position 192: cytosine (C), position 282: adenine (A), position 320: cytosine (C), position 342: cytosine (C) and position 423: cytosine (C).
Atyaephyra stankoi is found in freshwater habitats in the mainland of West-central Greece and South F.Y.R.O.M. (see material examined and Fig. 1).
Efforts made to trace Karaman’s type material in the MMNH were unsuccessful. According to the director of the Museum, Dr Petkovski S. (pers. comm.), Karaman’s material is considered lost after a fire that took place in the Museum.
A neotype for Atyaephyra stankoi is proposed for reasons of taxonomic clarification and stability, as foreseen by Art. 75 (ICZN, 1999). The neotype will contribute to the stability of the taxonomic status of the species and avoid further confusion due to nomenclature (see also Atyaephyra desmarestii remarks). Furthermore, it incorporates novel characteristics that distinguish it from the remaining Atyaephyra species such as: having 11–35 mesial spines on terminal segment of third maxilliped, basal endite of first maxilliped failing or reaching to distal end of exopod, distal boarder of telson with spines arranged in a fork-like pattern, a rounded antennular lobe, a pterygostomial angle not protruding, and a slightly curved and distally more or less elongated but always tapering endopod of male first pleopod. The name-bearing types are considered lost while the neotype has been collected from Doirani Lake, the same locality from where
Atyaephyra stankoi is similar to Atyaephyra thyamisensis sp. n. in having: 11–38 mesial spines on terminal segment of third maxilliped (Figs 6H, 8H), 12–16 rows of setae on basal lower endite of maxilla (Figs 6G, 8G), 3–6 pairs (mostly 4–5) of spines on distal boarder of telson with the second pair to be the strongest and terminating beyond (or along with) the other pairs arranged in a fork-like pattern (Figs 5E–F, 7E–F), a rounded antennular lobe (Figs 5H, 7H) and the basal endite of first maxilliped failing or reaching to distal end of exopod (Figs 6F, 8F). Atyaephyra stankoi differs from Atyaephyra thyamisensis sp. n. in not having a sharply protruding pterygostomial angle (Figs 5B, 7B). Atyaephyra stankoi can be distinguished from Atyaephyra orientalis by the presence of a rounded antennular lobe (Fig. 5H) (vs. pointed in Atyaephyra orientalis; Figs 3H–I). Further, Atyaephyra stankoi can be distinguished by the slightly curved and distally more or less elongated but always tapering endopod of male first pleopod (Fig. 6I) (vs. strongly curved and distally stout and not tapering in Atyaephyra orientalis; Fig. 4I). Atyaephyra stankoi can be separated from Atyaephyra desmarestii, Atyaephyra strymonensis, Atyaephyra acheronensis and Atyaephyra tuerkayi by the presence of numerous mesial spines (11–35) on terminal segment of third maxilliped (Fig. 6H) (vs 0–8 mesial spines; Figs 10H, 12H, 14H).
urn:lsid:zoobank.org:act:E57CE407-D38C-4EF2-B4AC-C0B9BEE6EFB1
http://species-id.net/wiki/Atyaephyra_thyamisensis
Figs 7–8Type material. Holotype: NHM 2012.1476, adult ovig. ♀ (CL 7.1 mm), Greece, Epirus, Thyamis River, 39°32.26'N, 20°09.76'E (Fig. 1, stn 76), among aquatic plants, 19.3.2005, coll. Ch. Anastasiadou; Allotype: NHM 2012.1477, adult ♂ (CL 5.3 mm), same data collection as holotype; Paratypes: NHM 2012.1478–1483, 4 ♀♀ (3 ovig.) (CL 6.0–6.8 mm) and 2 ♂♂ (CL 5.0–5.3 mm) same data collection as holotype. NHM 2012.1484–1485, 2 ♀ (CL 6.5–7.4 mm), Greece, Epirus, Louros River, 39°03.14'N, 20°46.26'E (Fig. 1, stn 72), among aquatic plants, 25.3.2012, coll. Ch. Anastasiadou. OUMNH.ZC 2012-08-001, 4 ♀♀ (2 ovig.) (CL 6.0–7.8 mm) and 2 ♂ (CL 5.2 mm) same data collection as holotype. SMF 43022, 4 ♀♀ (2 ovig.) (CL 5.8–7.1 mm) and 2 ♂♂ (CL 5.0–5.2 mm) same data collection as holotype. NHMW 25453, 4 ♀♀ (2 ovig.) (CL 5.5–7.5 mm) and 1 ♂♂ (CL 5.0 mm) same data collection as holotype
Greece: 2 ♀♀ (CL 5.2–5.5 mm), NHMW 462, Corfu Island (Fig. 1, stn 75), 1.9.1937, coll. Stephanides; 13 ♀♀ (1 ovig.) (CL 5.3–8.1 mm) and 8 ♂♂ (CL 5.2–6.2 mm), Epirus, Thyamis River (Fig. 1, stn 77), 20.5.2000 and 26.10.01, coll. Ch. Anastasiadou; 20 ♀♀ (15 ovig.) (CL 6.5–7.5 mm) and 3 ♂♂ (CL 5.0–5.7 mm), Epirus, Pamvotida Lake (Fig. 1, stn 78), 24.3.2006, coll. Ch. Anastasiadou; 20 ♀♀ (CL 5.0–7.0) and 8 ♂♂ (CL 5.0–5.5), Epirus, Ziros Lake (Fig. 1, stn 79), 28.10.2001, coll. Ch. Anastasiadou; 20 ♀♀ (CL 5.8–8.5 mm) and 4 ♂♂ (CL 5.2–6.4 mm), ZMAUTH D-334, Epirus, Filipiada, Louros River (Fig. 1, stn 80), 20.10.1977, coll. P. Economides; 15 ♀♀ (CL 5.5–8.0) and 6 ♂♂ (CL 5.0–6.0), Louros River (Fig. 1, stn 80), 28.10.2001, coll. Ch. Anastasiadou; 8 ovig. ♀♀ (CL 6.4–8.0 mm) and 6 ♂♂ (CL 5.3–6.2 mm), NHMW 465, Lefkada Island, Kaligoni, Vardas River (Fig. 1, stn 81), Aug.1929, coll. Beier; 3 ovig. ♀♀ (CL 7.3–8.0 mm) and 3 ♂♂ (CL 5.0–5.9 mm), NHMW 466, Lefkada Island, Kaligoni, Vardas River (Fig. 1, stn 81), 2.10.1932, coll. Beier.
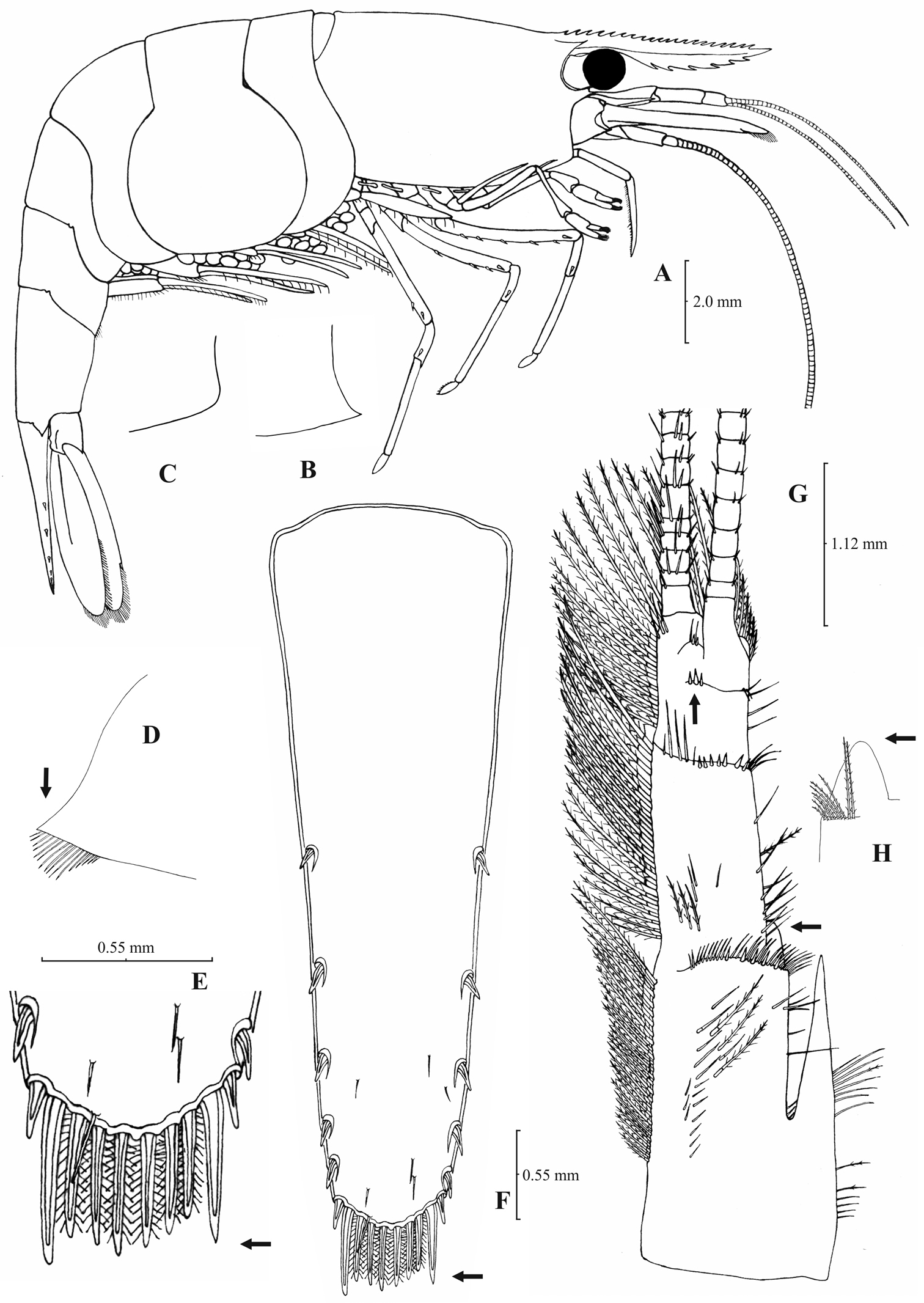
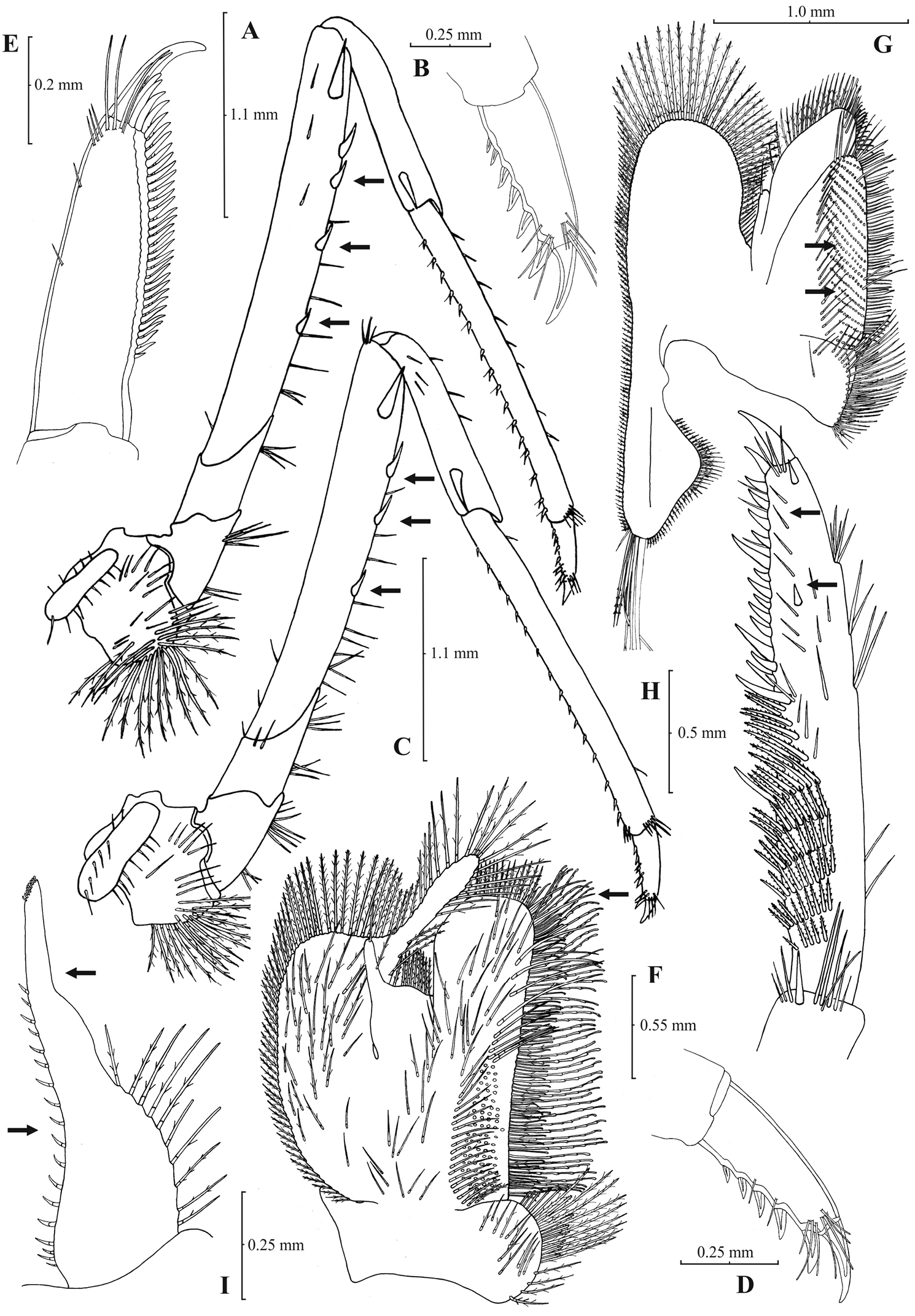
Rostrum long, slender, dorsal margin straight or slightly curved in the middle and pointed upwards, shorter, equal to, or longer than scaphocerite, 6.0–9.50, most often (84% of the examined individuals) 6.33 to 8.76, × as long as high. 18–27 (18–24 in 91% of the individuals) pre orbital teeth on dorsal margin arranged up to tip of rostrum. 0–2, predominantly (84%) 1–2, post-orbital teeth. 4–10 teeth, most often (87%) 5–8, arranged on ventral margin of rostrum (Fig. 7A). Carapace smooth with pterygostomial angle bluntly produced (Fig. 7B). Pleuron of fifth abdominal segment pointed with an acute posterior angle (Fig. 7D). Telsonwith 5–8, mostly (97%) 5–7, pairs of dorsal spines arranged in curved fashion (Fig. 7E). Distal border of telson with 8–12, mostly (86%) 8–10, spines (4–6 pairs) arranged in fork-like pattern. Outermost pair of spines shortest, similar to dorsal spines, adjacent pair stronger terminating beyond (or along with) the finely setulose inner pairs (Figs 7E–F). Basal segment of antennular peduncle with long stylocerite, with its tip reaching or overreaching the distal end of basal segment. Anterolateral lobe of basal segment short and round (Fig. 7H). Distal segment of antennular peduncle with 1–6, frequently (92%) 2–4, spines (Fig. 7G). Basal lower endite of maxilla densely covered with long simple setae arranged in 12–16 (13–15 in 80% of the individuals), oblique parallel rows. Endite of maxilla 1.84–2.24, mostly (93%) 1.89–2.05, × as long as basal lower endite (Fig. 8G). Basal endite of first maxilliped failing or reaching to distal end of exopod (Fig. 8F). Distal third of terminal segment of third maxilliped bearing 13–38 (19–30 in 88% of the individuals) mesial spines and one subdistal lateral spine near the base of larger terminal spine (Fig. 8H). Armature along flexor margin of dactylus of third and fourth pereiopod consisting of 6–9 (7–9 in 97% of the individuals) and 6–10 (7–9 in 97% of the individuals) spines respectively (Figs 8B, 8D). Merus of third and fourth pereiopod with 3–7 (4–6 in 93% of the individuals) and 2–6 (4–5 in 96% of the individuals) spines respectively (Figs 8A, 8C). Dactylus of fifth pereiopod with 28–43, usually (82%) 32–40, spines arranged in comb-like fashion on flexor margin (Fig. 8E). Endopod of first male pleopod expanded proximally and with a distal portion elongated (ribbon shaped) and tapering. Endopod with 14–21 spines arranged on a slightly or strongly curved inner margin and 12–18 setae arranged on outer margin (Fig. 8I). 172–465 eggs of 0.60–0.7 × 0.40–0.45 mm in size.
Atyaephyra thyamisensis sp. n. Holotype, adult ovig. ♀ (NHM 2012.1476): A entire individual B detail ofleftpterygostomial boarder C detail ofrightpterygostomial boarder D right pleuron of fifth abdominal segment E telson F distal margin of telson G right antennular peduncle H right antennular lobe.
Atyaephyra thyamisensis sp. n. Holotype, adult ovig. ♀ (NHM 2012.1476): A right third pereiopod B dactylus of third pereiopod C rightfourth pereiopod D dactylus of fourth pereiopod E right dactylus of fifth pereiopod F rightfirst maxilliped G right maxilla H right terminal segment of third maxilliped. Allotype, adult ♂ (NHM 2012.1477): I rightendopod of first male pleopod.
Atyaephyra thyamisensis sp. n. is a large sized species with a maximum carapace length of 6.4 mm in ♂♂, 8.0 mm in ♀♀ and 8.1 mm in ovig. ♀♀.
Atyaephyra thyamisensis sp. n. is different from all the other species of Atyaephyra by molecular characters, as shown by the phylogenetic analysis of mtDNA COI sequences. The one haplotype found was unique in the genus. Furthermore, it differs from all the other species in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1, position 172: cytosine (C), position 207: cytosine (C), position 249: guanine (G), position 258: cytosine (C), position 324: guanine (G), position 348: guanine (G) and position 387: cytosine (C).
Atyaephyra thyamisensis sp. n. is named after the Thyamis River, Greece, the type locality.
Atyaephyra thyamisensis sp. n. is found in fresh water habitats of North-west Greece as well as in the islands Corfu and Lefkada (see material examined and Fig. 1).
Remarks: Atyaephyra thyamisensis can be discriminated from Atyaephyra stankoi by the presence of a sharply protruding pterygostomial angle (Fig. 7B). It should be noted that this character has been observed to be missing from one side (either the left or the right) in some very large sized individuals (Fig. 7C). This character is shared by Atyaephyra orientalis (present in some populations) along with the presence of numerous spines (10–38) on terminal segment of third maxilliped (Figs 4H, 8H) and the presence of fewer rows of setae (12–16) on basal lower endite of maxilla (Figs 4G, 8G). The two species can be distinguished by the presence of a rounded antennular lobe in Atyaephyra thyamisensis (Figs 7G–H) (vs. pointed in Atyaephyra orientalis; Figs 3G–I). Further, Atyaephyra thyamisensis can be distinguished by the slightly or strongly curved endopod of first male pleopod having its distal part always elongated and tapering (ribbon shaped; Fig. 8I) (vs. strongly curved and distally stout and not tapering in Atyaephyra orientalis; Fig. 4I). Atyaephyra thyamisensis can be separated easily from the remaining three species of Atyaephyra by the presence of numerous mesial spines (13–38; Fig. 8H) on terminal segment of third maxilliped (vs. 0–8 mesial spinesin Atyaephyra desmarestii, Atyaephyra strymonensis, Atyaephyra acheronensis and Atyaephyra tuerkayi; Figs 10H, 12H, 14H).
urn:lsid:zoobank.org:act:A0C25BDC-4FB3-4C41-A507-5FA0BF6BCFC7
http://species-id.net/wiki/Atyaephyra_strymonensis
Figs 9–10Type material. Holotype: NHM 2012.1486, adult ovig. ♀ (CL 7.0 mm), Greece, Macedonia, Mylopotamos Springs (Strymonas River), 41°08.90'N, 24°04.29'E (Fig. 1, stn 102), among aquatic plants, 23.5.2011, coll. M. Christodoulou and M.S. Kitsos. Allotype: NHM 2012.1487, adult ♂ (CL 5.0 mm), same data collection as holotype. Paratypes: NHM 2012.1488–1492, 4 ♀♀ (CL 5.2–7.0 mm) and 1 ♂ (CL 5.0 mm) same data collection as holotype. OUMNH.ZC 2012-08-002 4 ♀♀ (1 ovig.) (CL 5.2–7.0 mm) and 1 ♂ (CL 5.0 mm) same data collection as holotype; SMF 43023 2 ♀♀ (CL 6.7–7.2 mm) and 1 ♂ (CL 5.0 mm) same data collection as holotype; NHMW 25454, 2 ♀♀ (CL 6.1–7.3 mm) same data collection as holotype.
Greece: 3 ♀♀ (CL 5.4–6.0 mm) Macedonia, Strymonas River (Fig. 1, stn 101), 1.10.2001, coll. Ch. Anastasiadou; 20 ♀♀ (13 ovig.) (CL 6.3–7.9 mm), Macedonia, Mylopotamos Springs (Fig. 1, stn 102), 4.4.2001, coll. Ch. Anastasiadou; 9 ♀♀ (CL 5.5–7.1 mm) and 5 ♂♂ (CL 5.1–5.3 mm) Macedonia, Agias Varvaras Springs (Fig. 1, stn 103), 4.4.2001, coll. Ch. Anastasiadou; 11 ♀♀ (4 ovig.) (CL 6.0–7.4 mm) and 3 ♂♂ (CL 5.1–5.3 mm), Macedonia, Kefalariou Springs (Fig. 1, stn 104), 4.5.2001, coll. Ch. Anastasiadou; 2 ♀♀ (CL 6.3 mm) and 2 ♂♂ (CL 5.3–5.6 mm), Thrace, Paradeisos, Nestos River (Fig. 1, stn 105), ZMAUTH G1-1024, 6.7.1972, coll. P. Economides; 14 ♀♀ (CL 5.5–7.3 mm) and 6 ♂♂ (CL 5.1–5.5 mm) Thrace, Kyrnos, Nestos River (Fig. 1, stn 106), 30.9.2002, coll. Ch. Anastasiadou.
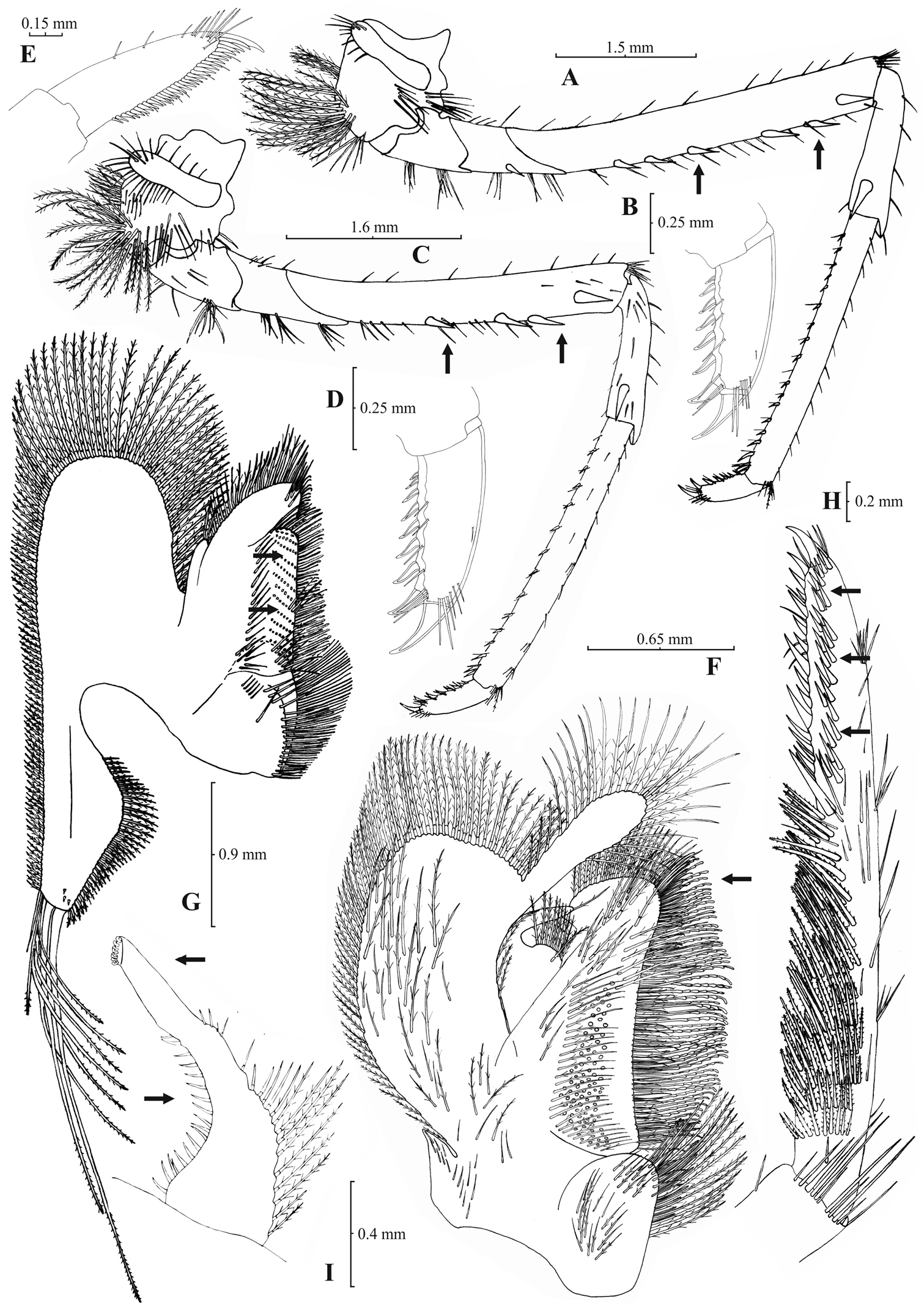
Rostrum long, slender, dorsal margin straight or slightly curved in the middle and pointed upwards, 5.89–8.80, mostly (92% of the individuals examined) 6.75–8.80, × as long as high, shorter, equal to, or longer than scaphocerite. 10–29, frequently (92%) 14–23, pre orbital teeth on dorsal margin of rostrum arranged up to tip. Rostrum without post-orbital teeth, leaving a short unarmed proximal gap. With maximally five teeth, mostly (91%) up to three, arranged on ventral margin of rostrum (Fig. 9A). Carapace smooth with pterygostomial angle, not protruding, rounded (Fig. 9B). Pleuron of fifth abdominal segment pointed with an acute posterior angle (Fig. 9C). Telsonwith 2–7, predominantly (97%) 3–4, pairs of dorsal spines arranged in curved fashion (Fig. 9D). Distal border of telson with 11–15, usually (96%) 12–14, spines (6–8 pairs), arranged in fan-like way. Outermost pair of spines shortest, similar to dorsal spines, adjacent pair stronger terminating before the finely setulose inner pairs (Figs 9D–E). Basal segment of antennular peduncle with long stylocerite, with its tip failing to reach or reaching the distal end of basal segment. Anterolateral lobe of basal segment short and round (Fig. 9G). Distal segment of antennular peduncle with 0–1 but mostly (87%) with no spines (Fig. 9F). Basal lower endite of maxilla densely covered with long simple setae arranged in 12–17 (14–16 in 90% of the individuals), oblique parallel rows. Endite of maxilla 1.77–1.95, mostly (89%) 1.78–1.91, × as long as basal lower endite (Fig. 10G). Basal endite of first maxilliped failing, reaching or overreaching the distal end of exopod (reaching the end in 65% of the individuals) (Fig. 10F). Distal one-third of terminal segment of third maxilliped bearing 1–7 mesial spines and one subdistal lateral spine near the base of larger terminal spine (Fig. 10H). Armature along flexor margin of dactylus of third and fourth pereiopod consisting of 6–8 (7–8 in 96% of the individuals) and 7–8 spines (including terminal spine) respectively (Figs 10B, 10D). Merus of third and fourth pereiopod with 3–6 (3–5 in 90% of the individuals) and 3–5 spines respectively (Figs 10A, 10C). Dactylus of fifth pereiopod with 25–37, mostly (87%) 30–35, spines arranged in comb-like fashion on flexor margin (Fig. 10E). Endopod of first male pleopod expanded proximally and with a distal portion elongated and tapering, often, with a small, protruding lobe in its outer subdistal part. Endopod with 14–23 spines arranged on a slightly curved inner margin and 9–15 setae arranged on outer margin (Fig. 10I). 210–250 eggs of 0.50–0.70 × 0.40–0.50 mm in size.
Atyaephyra strymonensis sp. n. Holotype, adult ovig. ♀ (NHM 2012.1486): A entire individual B detail of rightpterygostomial boarder C right pleuron of fifth abdominal segment D telson E distal margin of telson F right antennular peduncle G right antennular lobe.
Atyaephyra strymonensis sp. n. Holotype, adult ovig. ♀ (NHM 2012.1486): A rightthird pereiopod B dactylus of third pereiopod C right fourth pereiopod D dactylus of fourth pereiopod E dactylus of fifth pereiopod F rightfirst maxilliped G right maxilla H rightterminal segment of third maxilliped. Allotype, adult ♂ (NHM 2012.1487): I right endopod of first male pleopod.
Atyaephyra strymonensis sp. n. is a large sized species with maximum carapace length to be 5.6 mm in ♂♂, 7.9 mm in ♀♀ and 7.5 mm in ovig. ♀♀.
Atyaephyra strymonensis sp. n. is unique in the genus in having 2 haplotypes not found in any of the other species. Also, it differs from all the other species in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1, position 201: cytosine (C), position 252: guanine (G), position 303: cytosine (C), position 309: thymine (T), position 318: guanine (G), position 319: adenine (A), position 367: thymine (T), position 393: cytosine (C) and position 453: thymine (T).
Atyaephyra strymonensis sp. n. is named after the Strymon (Strymonas) River, Greece, the type locality.
Atyaephyra strymonensis sp. n. is found in North-western Greece in the Rivers Strymon and Nestos (see material examined and Fig. 1).
Atyaephyra strymonensis sp. n. is unique in the combination of the following characters: (a) absence of post orbital teeth (Fig. 9A), (b) leaving a short unarmed proximal gap on dorsal surface of rostrum (Fig. 9A), (b) having a round anterolateral lobe on basal segment of antennular peduncle (Figs 9F–G), (c) having a not protruding, rounded pterygostomial angle (Fig. 9C), (d) endite of maxilla 1.77–1.95 × as long as basal lower endite (Fig. 10G) and having 1–7 mesial spines in the terminal segment of third maxilliped (Fig. 10H). Atyaephyra strymonensis is similar to Atyaephyra desmarestii, Atyaephyra acheronensis and Atyaephyra tuerkayi in having fewer spines in the terminal segment of third maxilliped. However Atyaephyra strymonensis differs by the absence of post-orbital teeth, leaving a short unarmed proximal gap on dorsal surface of rostrum and by the endite of maxilla being 1.77–1.95 × as long as basal lower endite (vs. 1.49–1.71). Atyaephyra strymonensis differs from Atyaephyra stankoi, Atyaephyra thyamisensis and Atyaephyra orientalis in having fewer mesial spines in the terminal segment of third maxilliped.
urn:lsid:zoobank.org:act:EBF698A2-82F9-49E8-89DA-8C4EB7588939
http://species-id.net/wiki/Atyaephyra_acheronensis
Figs 11–12Type material. Holotype: NHM 2012.1493, 1 ovig. ♀ (CL 5.9 mm), Greece, Epirus, Acherontas River, 39°13.96'N, 20°29.11'E (Fig. 1, stn 71), among aquatic plants, 15.4.2012, coll. Ch. Anastasiadou (Sequenced specimen: Ach1).
Greece: 1 ♀ (CL 7.6 mm) (Sequenced specimen: Lour1) and 1 ovig. ♀ (CL 7.0 mm) (Sequenced specimen: Lour2), Greece, Epirus, Louros River, 39°03.14'N, 20°46.26'E (Fig. 1, stn 72), 15.4.2012, coll. Ch. Anastasiadou; Slovenia: 1 ♂ (CL 5.1 mm), Dragonja River (Fig. 1, stn 66), Aug.1971 (Sequenced specimen: Drag1).
Rostrum long, dorsal margin straight, 6.28–6.66 × as long as high, equal to or longer than scaphocerite. 19–26 pre orbital teeth on dorsal margin of rostrum arranged up to tip. With 1–3 post orbital teeth and 3–8 teeth on ventral margin of rostrum (Fig. 11A). Carapace smooth with pterygostomial angle not protruding, rounded (Fig. 11B). Pleuron of fifth abdominal segment pointed with an acute posterior angle (Fig. 11C). Telsonwith four pairs of dorsal spines arranged in curved fashion (Fig. 11D). Distal border of telson with 12–15 spines (6–8 pairs) arranged in a fan-like pattern. Outermost pair of spines shortest, similar to dorsal spines, adjacent pair stronger terminating before the finely setulose, inner pairs (Figs 11D–E). Antennulary stylocerite with its tip failing to reach or reaching distal margin of basal peduncle segment. Anterolateral lobe of basal segment short and round (Fig. 11G). Distal segment of antennular peduncle with 1–2 spines (Fig. 11F). Basal lower endite of maxilla densely covered with long simple setae arranged in 18–20 oblique parallel rows. Endite of maxilla 1.56–1.65 × as long as basal lower endite (Fig. 12G). Basal endite of first maxilliped reaching clearly beyond distal end of exopod (Fig. 12F). Distal one-third of terminal segment of third maxilliped bearing 1–5 mesial spines and one subdistal lateral spine near the base of larger terminal spine, interpretable as dactylus (Fig. 12H). Armature along flexor margin of dactylus of third and fourth pereiopod consisting of 5–7 and 6–7 spines respectively (Figs 12B, 12D). Merus of third and fourth pereiopod with 4–6 and 3–4 spines respectively (Figs 12A, 12C). Armature along flexor margin of dactylus of fifth pereiopod consisting of 27–38 spines (Fig. 12E). Endopod of first male pleopod expanded proximally and with a distal portion elongated (ribbon shaped) and tapering. Endopod with 18 spines arranged on a slightly curved inner margin and 12 setae arranged on outer margin (Fig. 12I). 579–1117 eggs of 0.40–0.55 × 0.25–0.35 mm in size.
Atyaephyra acheronensis sp. n. Holotype, adult ovig. ♀ (NHM 2012.1493): A entire individual B detail of rightpterygostomial boarder C right pleuron of fifth abdominal segment D telson E distal margin of telson F right antennular peduncle G right antennular lobe.
Atyaephyra acheronensis sp. n. Holotype, adult ovig. ♀ (NHM 2012.1493): A rightthird pereiopod B dactylus of third pereiopod C right fourth pereiopod D dactylus of fourth pereiopod E dactylus of fifth pereiopod F rightfirst maxilliped G right maxilla H rightterminal segment of third maxilliped. Adult ♂: I right endopod of first male pleopod.
Atyaephyra acheronensis sp. n. is a large sized species with maximum carapace length to be 5.1 mm in ♂♂, 7.6 mm in ♀♀ and 7.0 mm in ovig. ♀♀.
Molecular information based on the COI sequences provides compelling evidence that is a well defined species.Atyaephyra acheronensis sp. n. is unique in Atyaephyra in having 2 haplotypes not shared by any other species. Furthermore, it differs from all its congeners in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1, position 255: adenine (A) and position 318: cytosine (C). Finally, the mean genetic distances between Atyaephyra acheronensis and the remaining Atyaephyra species range from 8.3% to 23.8% (Table 2).
Atyaephyra acheronensis sp. n.is named after the Acheron (Acherontas) River, Greece, the type locality.
Atyaephyra acheronensis sp. n. is found in freshwater habitats of Croatia (Krka River), Slovenia (Dragonja River) and Greece (Acherontas River and Louros River) (see material examined and Fig. 1). Although this study was based on a limited number of specimens, it is postulated that Atyaephyra acheronensis sp. n. occurs in more rivers covering an area ranging from Croatia to Greece.
In addition to the type- and non type-material we investigated the morphology of the following specimens originating from the Balkan Peninsula: 6 ♀♀ collected from Dragonja River (Fig. 1, stn 66), Slovenia; 3 ♀♀ collected from Jadro River (Fig. 1, stn 67), NHMW 460 and 4 ♀♀ (3 ovig.) and 1 ♂ from Ombla River (Fig. 1, stn 69), NHMW 459, Croatia; 2 ♂♂ collected from Krupa River (Fig. 1, stn 68), NHMW 458, Bosnia and Herzegovina; 9 ♀♀ and 12 ♂♂ from Aoos River (Fig. 1, stn 70), Albania; 47 ♀♀ (13 ovig.) and 9 ♂♂ from Acherontas River (Fig. 1, stn 71), Greece, 10 ♀♀ and 2 ♂♂ collected from Louros River (Fig. 1, stn 72), Greece, 2 ♀♀ from Pamisos River (Fig. 1, stn 73), Greece, 4 ♀♀ and 1 ♂ sampled from Evrotas River (Fig. 1, stn 74), NHM 1987.93, Greece. However, without sequencing the individuals, their placement to Atyaephyra acheronensis sp. n. can’t be made with certainty.
Out of the 135 characters examined (see Appendix: Table 1) there were no morphological features distinguishing Atyaephyra acheronensis sp. n. from Atyaephyra desmarestii and Atyaephyra tuerkayi sp. n. Nevertheless, Atyaephyra acheronensis sp. n. presents a more limited variability in the values of its morphological characters than Atyaephyra desmarestii. Atyaephyra acheronensis sp. n. can easily be distinguished from Atyaephyra orientalis, Atyaephyra stankoi and Atyaephyra thyamisensis by the presence of fewer mesial spines (1–5) on terminal segment of third maxilliped (Fig. 12H) (vs. 10–38 in Atyaephyra orientalis, Atyaephyra stankoi and Atyaephyra thyamisensis; Figs 4H, 6H, 8H) and by the basal endite of first maxilliped overeaching distal end of exopod (Fig. 12F) (vs. failing to reach or reaching distal end in Atyaephyra orientalis, Atyaephyra stankoi and Atyaephyra thyamisensis; Figs 4F, 6F, 8F). Atyaephyra acheronensis sp. n. can be separated from Atyaephyra strymonensis by the presence of 1–3 post orbital rostral teeth (Fig. 11A) (vs. no post orbital teeth present leaving short unarmed proximal gap in Atyaephyra strymonensis; Fig. 9A) and by the endite of maxilla being 1.56–1.65 × as long as basal lower endite (Fig. 12G) (vs. 1.77–1.95 in Atyaephyra strymonensis; Fig. 10G).
urn:lsid:zoobank.org:act:94C1EC2A-1667-4456-8721-D10F03CDF4E6
http://species-id.net/wiki/Atyaephyra_tuerkayi
Figs 13–14Type material. Holotype: adult ♀ (CL 6.2 mm), SMF 43020, Syria, Nahr Al-Kabir River (Fig. 1, stn 122), at bridge near the coastal road, 5.3.1979, coll. R.K. Kinzelbach (Sequenced specimen: Nah1); Paratype: 1 ♀ (CL 7.1 mm), SMF 43021 same data as the holotype (Sequenced specimen: Nah2).
Rostrum long, dorsal margin slightly curved in the middle and pointed upwards 6.43–6.66 × as long as high, shorter than or equal to scaphocerite. 19–23 pre orbital teeth on dorsal margin of rostrum arranged up to tip. With two post orbital teeth and 4–7 teeth on ventral margin of rostrum (Fig. 13A). Carapace smooth with pterygostomial angle not protruding, rounded (Fig. 13B). Pleuron of fifth abdominal segment pointed with an acute posterior angle (Fig. 13C). Telsonwith four pairs of dorsal spines arranged in curved fashion (Fig. 13D). Distal border of telson with 9 spines (5 pairs) arranged in fan-like pattern. Outermost pair of spines shortest, similar to dorsal spines, adjacent pair stronger terminating before the finely setulose, inner pairs (Fig. 13E). Antennulary stylocerite with its tip failing to reach or reaching distal margin of basal peduncle segment. Anterolateral lobe of basal segment short and round (Fig. 13G). Distal segment of antennular peduncle with 1–2 spines (Fig. 13F). Basal lower endite of maxilla densely covered with long simple setae arranged in 18–20 oblique parallel rows. Endite of maxilla 1.58–1.59 × as long as basal lower endite (Fig. 14G). Basal endite of first maxilliped reaching clearly beyond distal end of exopod (Fig. 14F). Distal one-third of terminal segment of third maxilliped bearing 1–6 mesial spines and one subdistal lateral spine near the base of larger terminal spine (Fig. 14H). Armature along flexor margin of dactylus of third and fourth pereiopod consisting of 6–7 and 6–7 spines respectively (Figs 14B, 14D). Merus of third and fourth pereiopod with 4 and 3 spines respectively (Figs 14A, 14D). Armature along flexor margin of dactylus of fifth pereiopod consisting of 28 spines (Fig. 14E).
Atyaephyra tuerkayi sp. n. Holotype, adult ♀ (SMF 43020): A entire individual B detail of rightpterygostomial boarder C right pleuron of fifth abdominal segment D telson E distal margin of telson F right antennular peduncle G right antennular lobe.
Atyaephyra tuerkayi sp. n. Holotype, adult ♀ (SMF 43020): A rightthird pereiopod B dactylus of third pereiopod C right fourth pereiopod D dactylus of fourth pereiopod E dactylus of fifth pereiopod F rightfirst maxilliped G right maxilla H rightterminal segment of third maxilliped.
Atyaephyra tuerkayi is a large sized species with maximum carapace length to be 7.1 mm for ♀♀
A haplotype found in Atyaephyra tuerkayi sp. n. is not shared by any other species of Atyaephyra. Additionally, it differs from all the other species in the following nucleotide positions in the COI gene of Atyaephyra desmarestii specimen Dour1, position 174: guanine (G), position 207: adenine (A), position 246: adenine (A), position 318: thymine (T), position 321: adenine (A), position 339: adenine (A), position 357: cytosine (C), position 372: thymine (T), position 399: thymine (T), position 417: adenine (A) and position 441: cytosine (C). Finally, the mean genetic distances between Atyaephyra tuerkayi and the other species were ranging from 19.7% to 25.7% (Table 2).
Atyaephyra tuerkayi sp. n. is named after Professor Michael Türkay, in appreciation of his contribution to the study of Decapoda.
Atyaephyra tuerkayi sp. n. is found in the Nahr Al-Kabir River situated between Syria and Lebanon (see material examined and Fig. 1).
In addition to the type-material we investigated the morphology of the 23 female individuals (6 ovig.) and 7 males originating from Nahr Al-Kabir River (Fig. 1, stn 122; SMF 12189, SMF 12191, SMF 12192). All the individuals examined (including the sequenced ones) were morphologically identical. However, their placement to Atyaephyra tuerkayi, sp. n. has still to await sequencing. Since no male or ovigerous individual was sequenced observation regarding the form of the endopod of first male pleopod and number of eggs carried by the female were not included in the description. But observations were made in other individuals of the same sample and population and thus given here: endopod of first male pleopod expanded proximally and with a distal portion elongated and tapering, endopod with 9–16 spines arranged on a slightly curved inner margin and 9–11 setae arranged on outer margin. 430–450 eggs of 0.45–0.50 × 0.30–0.35 mm in size. Maximum carapace length to be 5.7 mm for ♂♂, 7.9 mm for ♀♀ and 7.6 mm for ovig. ♀♀.
Out of the 135 characters examined (see Appendix: Table 1) there were no morphological features distinguishing Atyaephyra tuerkayi sp. n. from Atyaephyra desmarestii and Atyaephyra acheronensis sp. n. However, Atyaephyra tuerkayi sp. n. can easily be distinguished from Atyaephyra orientalis, Atyaephyra stankoi and Atyaephyra thyamisensis by the presence of fewer mesial spines (Fig. 14H) (1–6) on terminal segment of third maxilliped (vs. 10–38 in Atyaephyra orientalis, Atyaephyra stankoi and Atyaephyra thyamisensis; Figs 4H, 6H, 8H) and by the basal endite of first maxilliped overreaching distal end of exopod (Fig. 14F) (vs. failing to reach or reaching distal end in Atyaephyra orientalis, Atyaephyra stankoi and Atyaephyra thyamisensis; Figs 4F, 6F, 8F). Atyaephyra tuerkayi sp. n. can be separated from Atyaephyra strymonensis by the presence of 1–3 post orbital rostral teeth (Fig. 13A) (vs. no post orbital teeth present leaving short unarmed proximal gap in Atyaephyra strymonensis; Fig. 9A) and by the endite of maxilla being 1.58–1.59 × as long as basal lower endite (Fig. 14G) (vs 1.77–1.95 in Atyaephyra strymonensis; Fig. 10G).
Given the highly structured nature of freshwater habitats and the limited potential for dispersal of the freshwater species (mainly due to natural barriers) in combination with the wide distribution of Atyaephyra in the Mediterranean region, a hypothesis under which several species are expected to be harbored in the genus seemed highly possible.
However, until recently, Atyaephyra was considered as a monotypic genus. Over the last 100 years many authors (
In the latest revision of the Atyaephyra (Garcia Muñoz et al. 2009), which was based on the genetic information deriving from two mitochondrial genes (COI, 16S), two species were recognized while a third was proposed but without confirming it. In the current study seven species are defined, based both on morphological and molecular data. This difference in numbers is attributed to the limited geographical focus of the former study, which was primarily carried out on material collected from the Western Mediterranean area.
After an exhaustive study of a large number of specimens from 20 different countries and a thorough examination of more than 135 morphological characters, including somatometric distances, new characters were found which could differentiate species or groups of species within the Atyaephyra. One of these characters is the number of mesial spines on the terminal segment of the third maxilliped according to which two main groups can be distinguished. The first group is characterized by 10–38 mesial spines and comprises three species, Atyaephyra thyamisensis sp. n., Atyaephyra stankoi, Atyaephyra orientalis whereas the second by 1–8 mesial spines including the remaining four, namely Atyaephyra desmarestii, Atyaephyra acheronensis sp. n., Atyaephyra strymonensis sp. n.and Atyaephyra tuerkayi sp. n.
The species included in the first group can subsequently be distinguished by a series of features, e.g. presence-absence of a protruding pterygostomial angle, shape of antennular lobe and shape of endopod of first male pleopod. Atyaephyra thyamisensis sp. n., Atyaephyra stankoi and Atyaephyra orientalis are morphologically and phylogenetically well defined. In the phylogenetic tree they represent three well supported clades (16.7%–22.6% divergent from each other). In the second group, Atyaephyra strymonensis sp. n. is also a well defined species morphologically and can be distinguished from the remaining members by a combination of characters such as the lack of post orbital teeth, presence of a short unarmed proximal gap on rostrum and ratio of basal lower endite of maxilla in relation to the whole maxilla endite. The genetic divergence observed between Atyaephyra strymonensis sp. n. and its closest congeners by morphology is quite high (21.9%–25.4%). Thus, both morphological and molecular data show congruent patterns and jointly support its recognition as a distinct species within the genus. In addition, although Atyaephyra strymonensis sp. n. seems to be morphologically closer to the members of the second group e.g. Atyaephyra desmarestii, Atyaephyra acheronensis sp. n., Atyaephyra tuerkayi sp. n., genetically it is more closely related to the other two species of the first group from Greece (e.g. Atyaephyra thyamisensis sp. n.and Atyaephyra stankoi)with which it forms a strongly supported phylogroup (genetic divergence range: 11.9%–18.2%).
No diagnostic morphological characters were found to distinguish the species Atyaephyra desmarestii, Atyaephyra acheronensis sp. n. and Atyaephyra tuerkayi sp. n. from each other, a fact which is mainly caused by the high morphological variability observed in Atyaephyra desmarestii. However, their genetic distinctiveness coupled with their discrete geographical distribution provides enough evidence to distinguish the three species as distinct taxa.
The range of genetic divergence observed between the specimens of Atyaephyra desmarestii and of Atyaephyra acheronensis sp. n. (TrN distances: 5.9%–11.6%, Uncorrected p-distances: 5.3%–8.7%) is comparable to those found for other cryptic or sibling species of freshwater shrimps (e.g.
Furthermore, the fact that no haplotypes were shared between Atyaephyra desmarestii and Atyaephyra acheronensis sp. n. would suggest that the populations of shrimps from both species, although recently evolved, had independent evolutionary histories for a relatively long period of time. Additional support, although further research is still needed, comes from their geographical distribution since Atyaephyra desmarestii and Atyaephyra acheronensis sp. n. seems to be allopatric. Atyaephyra acheronensis is found in the western Balkan Peninsula, ranging from Croatia to Greece. In Greece, this species is found only on the west side of the mainland reaching most probably as far as South Peloponnese although with a remarkable fragmented distribution. In comparison Atyaephyra desmarestii is distributed in West-central Europe and North Africa. It should be noted here that the native distribution of Atyaephyra desmarestii is limited to Southern Europe and its presence in North-Central Europe up to the Danube River is believed to have been caused by its dispersal through the canals that were opened to connect the main rivers of Europe (
The monophyly of the species Atyaephyra desmarestii, although supported by NJ, was poorly or not supported at all by BI and ML analyses, respectively. In the study of Garcia Muñoz et al. (2009) the monophyly of this species, based on the COI sequences, was strongly supported. This difference should be attributed to the larger number of sequences used in this study. Atyaephyra desmarestii (Millet, 1831) does not comprise a strongly supported genetically distinct group and appears as a not well resolved part of the phylogeny. However, the genetic distances observed within this group are quite small in comparison with the other Atyaephyra species and this in combination with the morphological data supports the consideration of all the populations inside this group as one taxonomic entity. More sequence and morphological data, especially from the area of South Portugal and Morocco (the monophyly of the species is strongly supported once the sequences originating from Morocco and South Portugal material are removed), as well as other molecular markers are needed in order for the relationships within Atyaephyra desmarestii to be clarified.
In the southwestern part of the Mediterranean area, only two species of Atyaephyra have been described to date: Atyaephyra desmarestii and Atyaephyra rosiana. These two species had been considered synonyms until
The Tunisian populations, on the other hand, are more closely related to the western European ones, probably due to the past connections through the Sicily Strait with European populations (
The second cryptic species Atyaephyra tuerkayi sp. n. has been found only in the River Nahr Al-Kabir which is located along the borders of Lebanon with Syria. Atyaephyra tuerkayi sp. n. is completely isolated geographically from the other two morphologically closest to it species, Atyaephyra desmarestii and Atyaephyra acheronensis sp. n. In fact Atyaephyra tuerkayi sp. n. is surrounded by Atyaephyra orientalis populations which show a wide distribution from Turkey to Iraq. Atyaephyra tuerkayi sp. n. is genetically well discriminated from Atyaephyra desmarestii and Atyaephyra acheronensis (genetic distances are 23.0% and 22.2% respectively) as well as from Atyaephyra orientalis that is found in the adjacent areas (genetic distance is 19.7%). The genetic distances are among the highest observed between Atyaephyra species and by far exceed currently published records of intra-population variability of other fresh water decapods (e.g.
In the area of the Middle East, two subspecies were previously described, Atyaephyra desmarestii orientalis Bouvier, 1913 and Atyaephyra desmarestii mesopotamica Al-Adhub, 1987. However, no observable morphological characters where found that could differentiate them (see remarks of Atyaephyra orientalis). Furthermore, although the genetic distances within the Atyaephyra orientalis phylogroup were high (0.9%–10.2%) no firm conclusion could be drawn whether the hypothesis of multiple species is valid or not. Sequences from Orontes River (topotypical location of Atyaephyra desmarestii orientalis) and from Shatt Al-Arab River (topotypical location of Atyaephyra desmarestii mesopotamica) presented a noticeable mean genetic divergence (5.0%) but still not strong enough to support the hypothesis of different species. Detailed future studies on the morphological and genetic variability within the samples of Atyaephyra distributed throughout the Middle East will help clarify the relationships between the populations in this region, however given the present data, only one species is considered to exist, Atyaephyra orientalis.
Four species (Atyaephyra acheronensis sp. n., Atyaephyra thyamisensis sp. n., Atyaephyra stankoi, and Atyaephyra strymonensis sp. n.) were found to co-exist in Greece with well defined and clearly separated distributions. Only two species (Atyaephyra acheronensis sp. n. and Atyaephyra thyamisensis sp. n.) were found to co-exist in the same river (River Louros, Epirus). Multiple individuals collected from the Louros estuary and further upstream, dating back to 1977 until 2001 were examined. These specimens were all identified as Atyaephyra thyamisensis sp. n. However, in a recent sample (2012) both species were found. Probably, this could be attributed to fish transfers or translocation where shrimps could have accidentally been introduced. Additionally, the distance between the estuaries of the Rivers Louros and Acherontas is less than 30 km making human mediated dispersal, between the two watersheds, highly possible. Furthermore, numerous translocations of fish were made within Greece over the last 70 years (
It is surprising that four out of the seven Atyaephyra species examined for the present study are recorded from Greece and three of these are endemic. Greece is considered to be a faunal and floral biodiversity hot spot within the Mediterranean region where freshwater fauna is not an exception (
The importance of morphology versusmolecular data in order to resolve the phylogeny of a taxon still provides a forum for scientific debate (
We are very grateful to the following researchers who provided us with material either as loan or as a gift: Dr Chrysa Anastasiadou (University of Ioannina, Greece), Prof. Sonia Dhaouadi-Hassen (University of Carthage, Tunisia), Dr Cedric d’ Udekem d’ Acoz (Royal Belgian Institute of Natural Sciences, Belgium), Prof. Manuel Graça and Dr Veronica Ferreira (University of Coimbra, Portugal), Dr Carlo Froglia (Italy), Mr Ahmad Ghane (Inland Waters Aquaculture Research Centre, Iran), Dr Jure Jugovic (University of Ljubljana, Slovenia), Dr Christophe Lejeusne (Doñana Biological Station, Spain), Prof. Mohammed Melhaoui (University of Oujda, Morocco), Prof. Jose Luis Moreno Alcaraz (University of Castilla-La Mancha, Spain), Mr Murtada D. Naser (University of Basrah, Iraq), Dr Javier Oscoz (University of Navarra, Spain), Ass. Prof. Murat Özbek (Ege University, Turkey), Dr Michael L. Zettler (Leibniz Institute for Baltic Sea Research, Germany), Mrs Niovi Christodoulou and Mr Andreas Savvides (Wageningen University, Netherlands). Also, we are very grateful to the following researchers that allowed us access to Museum collections: Dr Paul F. Clark (NHM), Dr Sammy De Grave (OUMNH), Dr Peter Dworschak (NMW), Dr Pier Noél (MNHN), Dr Svetozar Petkovski (MMNH) and Professor Michael Türkay (SMF). Furthermore, we will like to thank Dr Timothy J. Page (Griffith University, Australia) for offering us four COI sequences and Ass. Prof. Nikos Poulakakis (University of Crete, Greece) for supplying us with the DNA extraction protocol. The authors will like to thank Dr Sammy De Grave and Dr Arthur Anker for their help in finding Bouvier’s material in NHMN. Specifically we will like to thank Dr Arthur Anker who went through the collection as well as for taking the photos of Atyaephyra desmarestii and Atyaephyra rosiana material. The senior author will like to thank Dr De Grave for his useful comments and help through some difficult issues dealing with the nomenclature code of zoology. For their helpful comments and critical reading we will like to thank Dr Paul Clark and Dr Christos Arvanitidis. We will also like to thank Mrs Kristin Pietratus (SMF), Mrs Miranda Lowe (NHM), Mrs Paula Martin-Lefevre and Dr Laure Corbari (MNHN) for their help with the loaned material from Museum collections. We also acknowledge the help of Stelios Derivianakis, Katerina Oikonomaki and Vaso Terzoglou in the genetic lab. The senior author will like to thank Synthesys: the European Union-funded Integrated Activities grant that supported her for a monthly visit to the NHM. Also the senior author will like to specially thank Dr Paul Clark for his supervision. Finally, the authors, gratefull aknowledge the three reviewers for their very usufull comments and suggestions that improved significantly the manuscript. The senior author will like to acknowledge the State Scholarships Foundation for supporting partially her studies. This study was partially funded by MARBIGEN: “Supporting research potential for MARine BIodiversity and GENomics in the Eastern Mediterranean” and ViBRANT: “Virtual Biodiversity Research and Access Network for Taxonomy”.
List of morphological characters studied and photos of Atyaephyra desmarestii and "Atyaephyra rosiana" material examined by Bouvier (1913). (doi: 10.3897/zookeys.229.3919.app) File format: Mictosoft Office Document (docx).
Explanation note: A list of 135 morphological characters (67 meristic and 68 somatometric distances) examined in Atyaephyra species is given. Schematic drawings of Atyaephyra appendages showing the studied somatometric characters are supplied. Furthermore, photos of Atyaephyra desmarestii and Atyaephyra rosiana material, collected from Maine et Loire (France) and Coimbra (Portugal) respectively, examined by Bouvier (1913) are given.