(C) 2012 Bert W. Hoeksema. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The coral species Leptoseris troglodyta sp. n. (Scleractinia, Agariciidae) is described as new to science. It is the first known azooxanthellate shallow-water agariciid and is recorded from the ceilings of caves at 5-35 m depth in West Pacific coral reefs. The corals have monocentric cup-shaped calices. They may become colonial through extramural budding from the basal coenosteum, which may cause adjacent calices to fuse. The size, shape and habitat of Leptoseris troglodyta are unique compared to other Leptoseris species, many of which have been recorded from mesophotic depths. The absence of zooxanthellae indicates that it may survive well in darkness, but endolithic algae in some corals indicate that they may be able to get some light. The presence of menianes on the septal sides, which may help to absorb light at greater depths in zooxanthellate corals, have no obvious adaptive relevance in the new species and could have been inherited from ancestral species that perhaps were zooxanthellate. The new species may be azooxanthellate as derived through the loss of zooxanthellae, which would be a reversal in Leptoseris phylogeny.
Cavernicolous, colonial, dwarfism, extramural budding, monocentric, skiophilous, solitary, troglobiotic
Reef-dwelling species of the genus Leptoseris Milne-Edwards and Haime, 1849 (Scleractinia: Agariciidae) consist of foliaceous corals that are common in poorly illuminated environments, such as the deepest parts of reef slopes and vertical rocky walls with crevices, caves, tunnels and overhangs (
Because some Leptoseris species inhabit deep and poorly accessible habitats, they may not all be well known. An example is the recently discovered Leptoseris kalayaanensis Licuanan & Aliño, 2009, which so far has only been recorded from rocky substrates at 13–28 m depth in the South China Sea basin (
The Agariciidae were not known to include true deep-sea species but according to recent phylogenetic studies, the solitary attached deep-water coral Dactylotrochus cervicornis (Moseley, 1881), which was originally classified with the Caryophylliidae, is also a member of the Agariciidae (
Because Dactylotrochus cervicornis is predomintly from deep water, it is considered the first known extant azooxanthellate agariciid. Dactylotrochus cervicornis holds a basal position in a recent phylogeny reconstruction of extant Agariciidae and because extinct solitary agariciids from the Middle Cretaceous were also solitary, it is assumed that the ancestor of the Agariciidae, which nowadays predominantly consist of colonial and zooxanthellate species, was also solitary and azooxanthellate (
In the present paper a new agariciid coral species is described that is entirely azooxanthellate and dwells on ceilings of caves in steep reef slopes and walls. No co-occurrence with any zooxanthellate scleractinians was observed. Although its calices are relatively small, cup-shaped and predominantly monocentric, it resembles species of Leptoseris, which otherwise is known to consist of zooxanthellate species with polycentric (“circumoral”) calices (
Specimens were observed, photographed and collected while diving with the help of SCUBA. All specimens were encountered below 5 m depth on the ceilings of caves inside steep reef slopes and walls, usually in areas with limestone outcrops. The caves measured one to several meters in width and several meters in length, enabling easy access and maneuvering for observations. Use of an underwater torch was indispensable to locate the corals. Collected specimens were soaked in fresh water or in sodium hypochlorite solution for cleaning. They were deposited in the Coelenterata collection (RMNH Coel.) of Naturalis Biodiversity Center, Leiden (formerly known as Rijksmuseum van Natuurlijke Historie). Specimens from Cebu were already available in the RMNH collection before they were photographed and collected for the present research. SEM photographs were taken with a Jeol 6480LV electron microscope operated at 10 kV.
Systematic section Order Scleractinia Bourne, 1905 Family Agariciidae Gray, 1847 Genus Leptoseris Milne Edwards & Haime, 1849urn:lsid:zoobank.org:act:DB802B45-E18D-4F55-9D23-5401660DD94E
http://species-id.net/wiki/Leptoseris_troglodyta
Figures 1–8Holotype. RMNH Coel. 40138 (1 specimen, dry, Figure 3a), Palau, W of Ulong Island (Rattakadokoru Island), W off barrier reef, 07°18'40"N, 134°13'30"E, Tsey’s tunnel ceiling at 32 m depth, 28 July 2002, coll. B.W. Hoeksema. Paratypes: RMNH Coel. 40139 (15 specimens, dry), same collection data (Figures 2d, 3b–d).
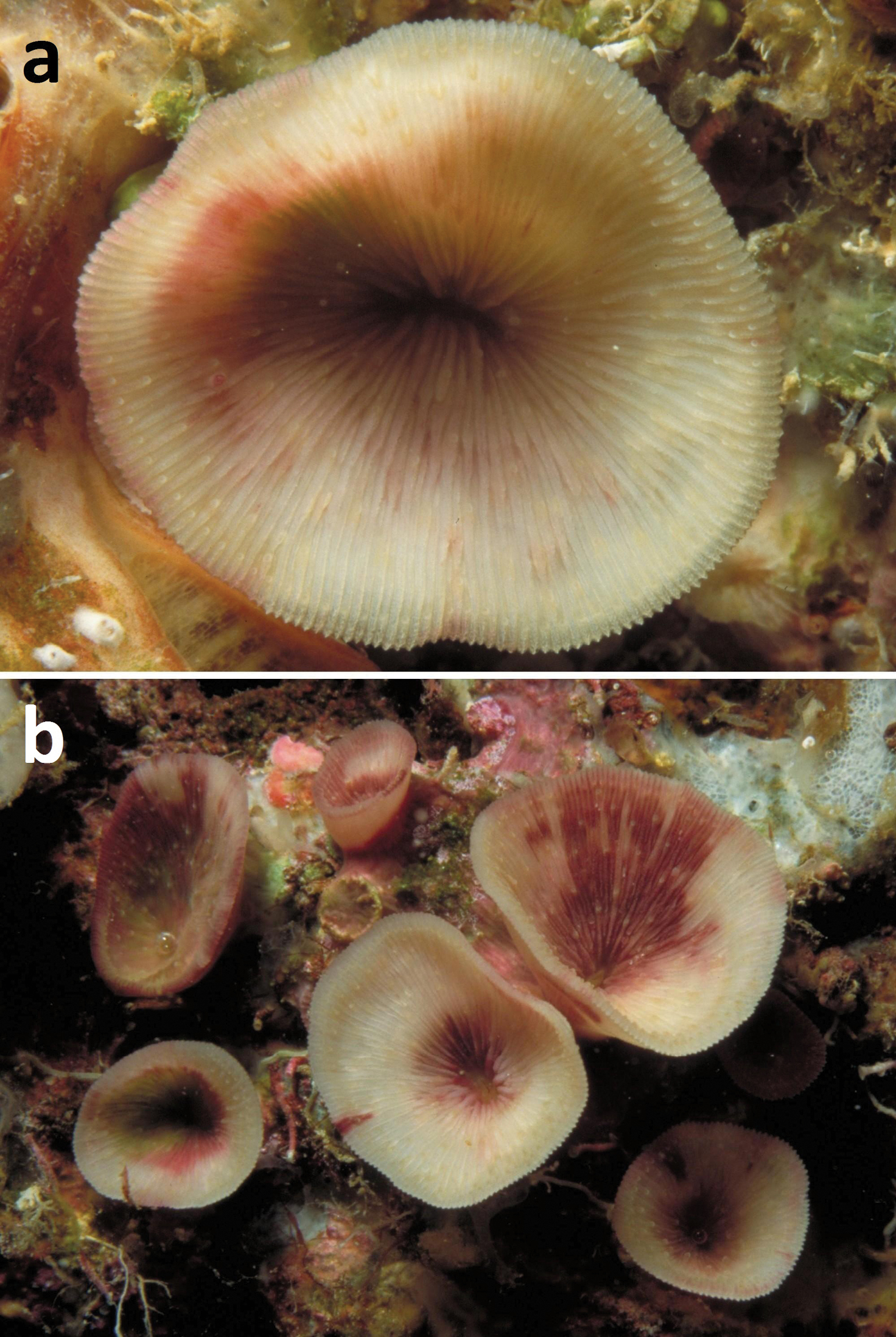
Living specimens of Leptoseris troglodyta sp. n. a Philippines, Cebu Strait, W of Bohol, NW of Cabilao Island, 10–30 m depth (7 November 1999) b Indonesia, NE Kalimantan, Berau Islands, S of Derawan Island, 7–10 m depth (4 October 2003).
Living specimens of Leptoseris troglodyta sp. n. a Philippines, Cebu Strait, W of Bohol, NW of Cabilao Island, 10–30 m depth (7 November 1999) b Indonesia, Tukang Besi Islands (Wakatobi), Binongko, 20 m depth (10 May 2003) c Indonesia, North Sulawesi, S of Bunaken Island, 17 m depth (19 December 2008; photo B.T. Reijnen) d Palau, W of Ulong Island (Rattakadokoru Island), W off barrier reef, 32 m depth (28 July 2002) e Papua New Guinea, Misima Island, 6–10 m depth (31 May 1998; photo G. Paulay) f Guam, Blue Hole, 35 m depth (1 June 2000; photo G. Paulay).
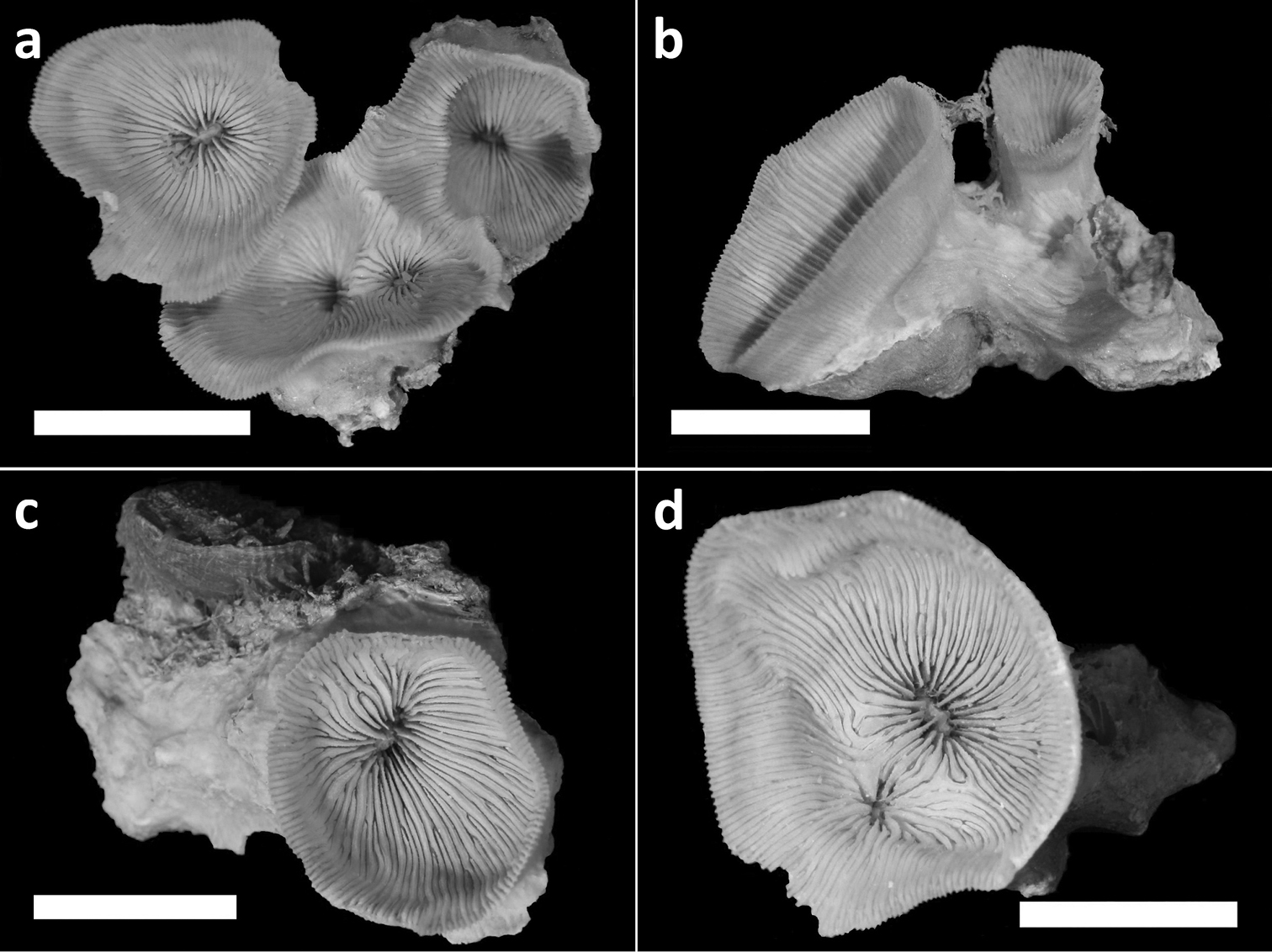
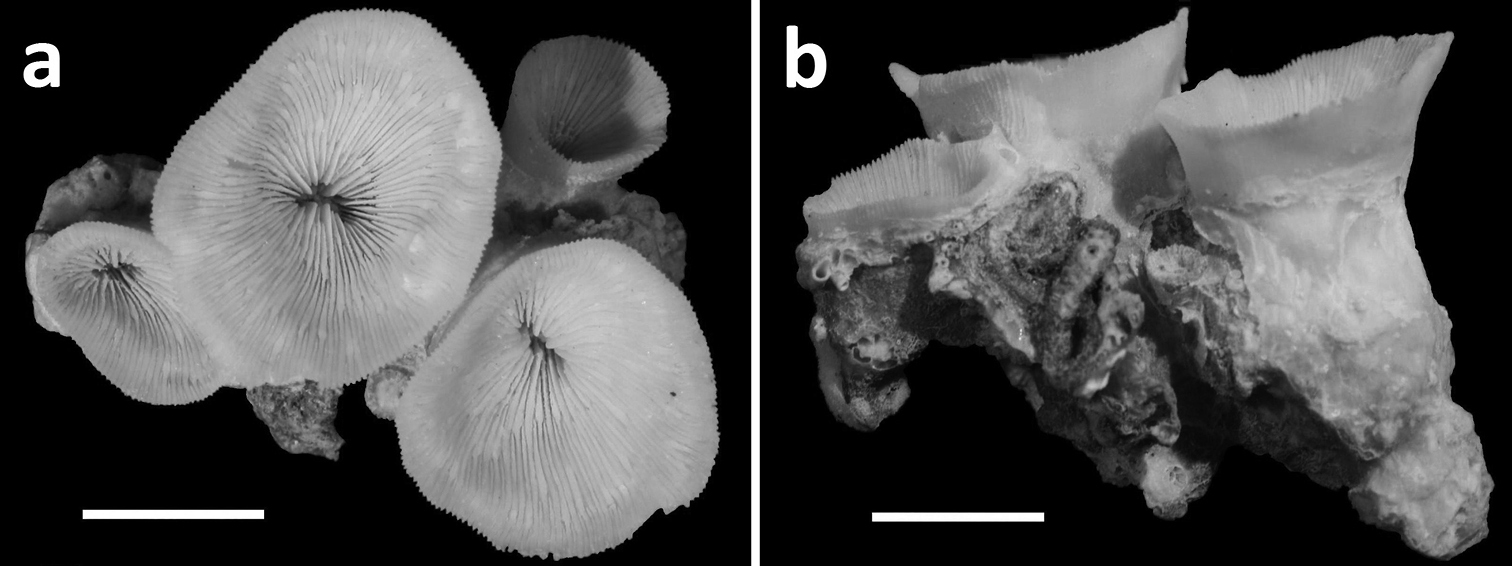
Holotype (RMNH Coel. 40138) and three paratypes (RMNH Coel. 40139) of Leptoseris troglodyta sp. n.from Palau. Scale bars: 1 cm. a Holotype consists of four calices: one (most left) has fused mid-height its calyx with two totally fused calices (centre), while another (right) has fused only with its corallum margin to those at the centre and the rest of its calyx has remained separate b Paratype: two separate calyces c Paratype: single calyx d Paratype: two fused calices. Scale bars: 1 cm.
Philippines. RMNH Coel. 24187 (3 specimens, dry), RMNH Coel 24195 (36 specimens, dry), Cebu Strait, W of Bohol, NW side of Cabilao Island, 09°53'12"N, 123°45'32"E, vertical wall with caves, 10–30 m depth, 7 and 17 November 1999, coll. B.W. Hoeksema (Figures 1a, 2a, 7, 8). RMNH Coel. 40151 (2 specimens, dry), Philippines, Cebu, Mactan Island, Lapu-Lapu City, Marigondon Cave, 10°15'33"N, 123°59'07"E, ceiling of cave at 25-30 m depth, May 1981, coll. M.B. Best (Figure 6). Indonesia. RMNH Coel. 40152 (7 specimens, dry), Tukang Besi Islands (Wakatobi), Binongko, SW Bay, 05°59'47"S, 124°02'55"E, cave in steep wall 20 m depth, 10 May 2003, coll. B.W. Hoeksema (Figures 2b, 4–5). RMNH Coel. 40150 (25 specimens, ethanol), Tukang Besi Islands (Wakatobi), NE Hoga Island, 05°28'S, 123°47'E, cave in steep reef slope 5 m depth, 12 July 2011, coll. B.W. Hoeksema. RMNH Coel. 40137 (2 specimens, ethanol), North Sulawesi, South of Bunaken Island, Alung Banua village, 01°37'07"N, 124°45'30"E, tunnel in reef wall 17 m depth, 19 December 2008, coll. B.T. Reijnen and S.E.T van der Meij (Figure 2c).
Indonesia, NE Kalimantan, Berau Islands, S of Derawan Island, jetty Derawan Dive Resort, 02°17'03"N, 118°14'49"E, ceiling of caves, 7–10 m deep (4 October 2003; Figure 1b). Papua New Guinea, Off SE point, Misima Island, Pt. Ebola, 6–10 m depth (31 May 1998; Figure 2e). Guam, Blue Hole, 35 m depth (1 June 2000; Figure 2f).
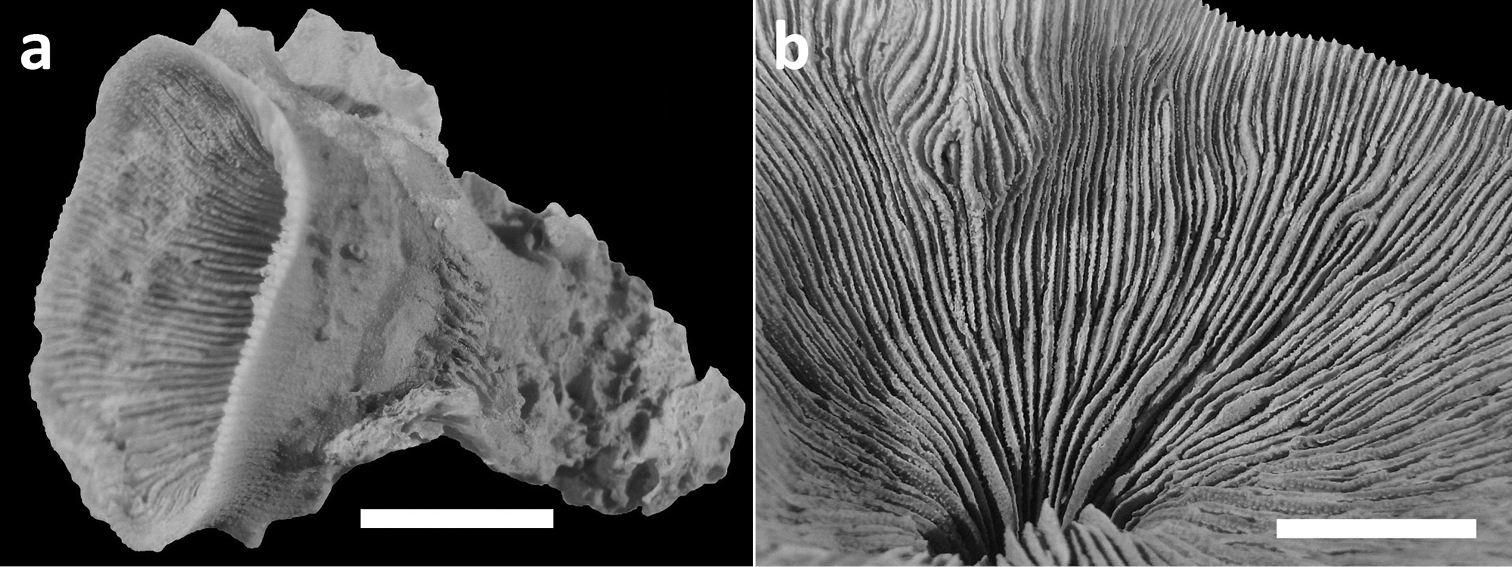
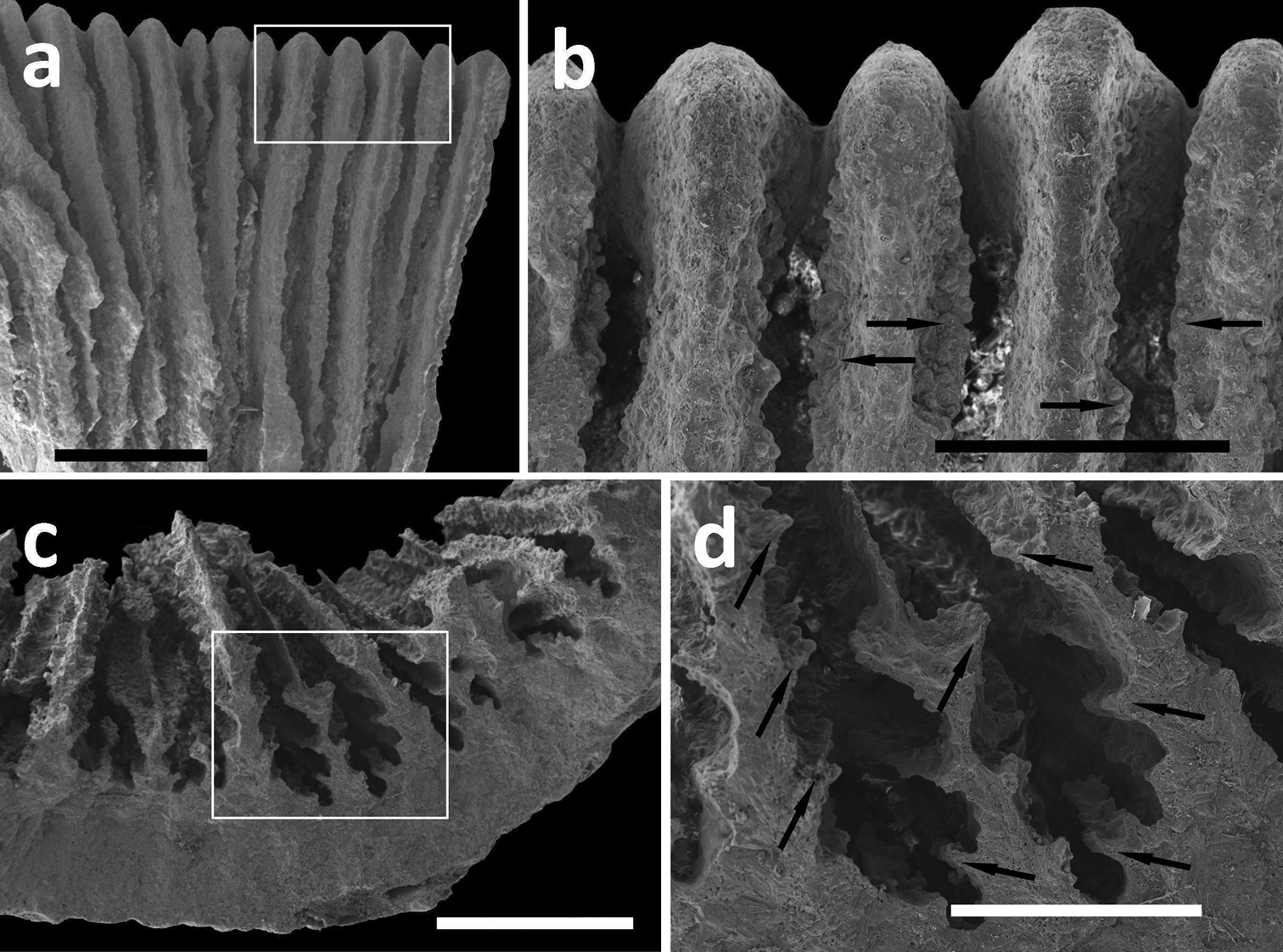
Corallum attached and solitary or colonial by budding from basal coenosteum (Figures 3–4, 6–8). Calices predominantly monocentric, very thin, cup-shaped to foliaceous, height < 15 mm, outline irregularly circular, Ø < 30 mm (Figures 1–4, 6–8). They are usually separate from each other above the interconnecting basal plate (Figures 3b, 6, 7), but can also be fused at their margins or lateral sides (Figures 3a, 8b). Corallum wall massive. Costae equal and well defined, with small spiny protuberances (Figures 4a, 7a, 8d). Septa approximately equal in size, with smooth upper edges and parallel ridges (menianes) on their sides (Figures 3, 5, 8). Septal sides may show evenly distributed granulations where menianes are absent (Figures 8b–c). Columella nearly solid (Figures 3a, 3c–d, 8a–b). Living animals azooxanthellate; corals are white, or partly green or red (Figures 1–2), owing to the presence of endolithic algae (
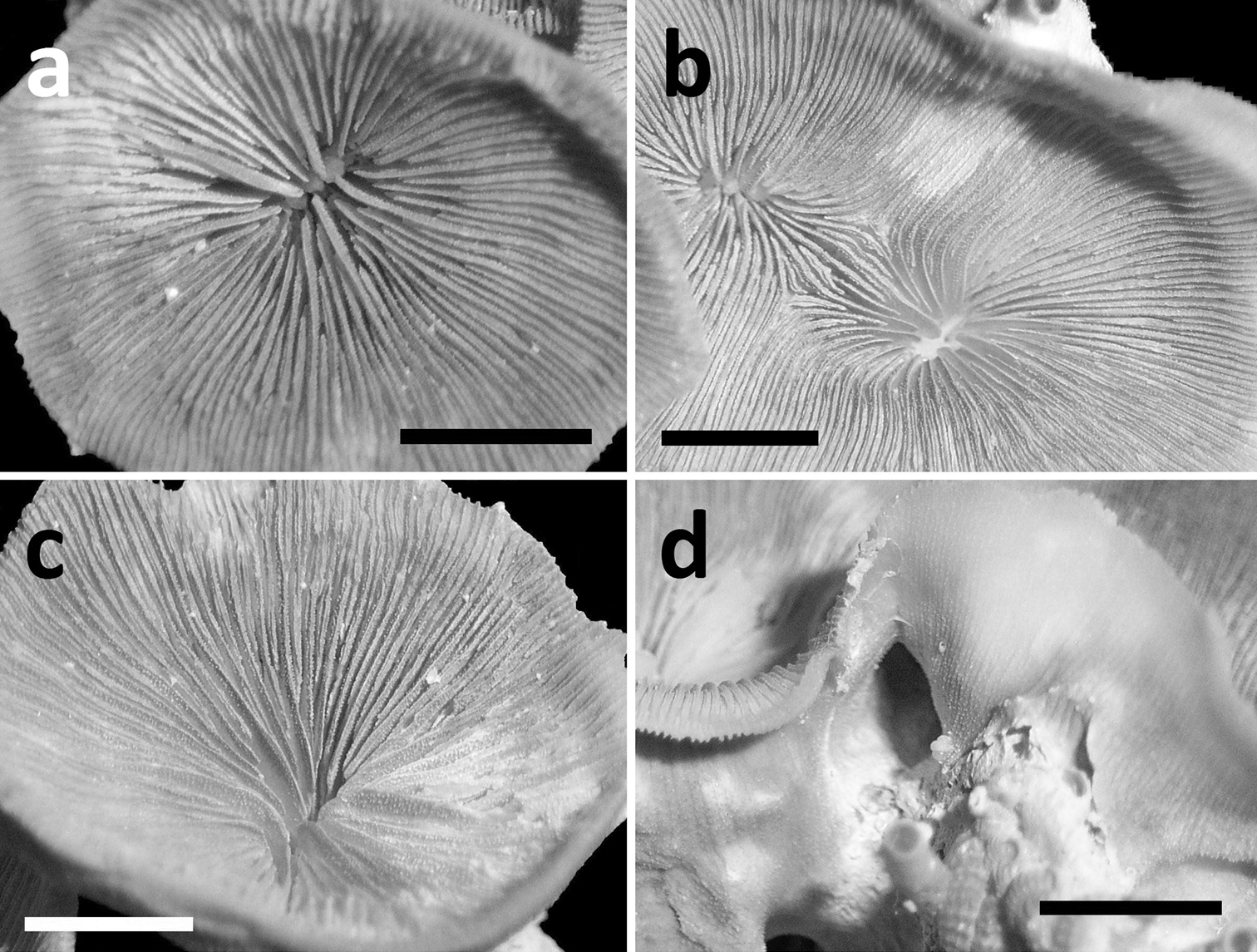
Specimens of Leptoseris troglodyta sp. n.from Indonesia, Wakatobi (RMNH Coel. 40152) a Single specimen from the side showing cup-shaped calyx shape and costae with fine granular spines (scale bar: 1 cm) b Close-up of large calyx showing septa with wide menianes along their sides (see Figure 5; scale bar: 5 mm).
SEM photographs of Leptoseris troglodyta sp. n. from Wakatobi, Indonesia (RMNH Coel. 40152). a Upper side of septa showing wide menianes (scale bar: 1 mm; insert: Figure 5b) b Close-up (insert) of Figure 5a (arrows: menianes; scale bar: 0.5 mm) c Cross-section of septa showing multiple menianes along their sides (scale bar: 0.5 mm; insert: Figure 5d) d Close-up (insert) of Figure 5c (arrows: menianes; scale bar: 0.5 mm).
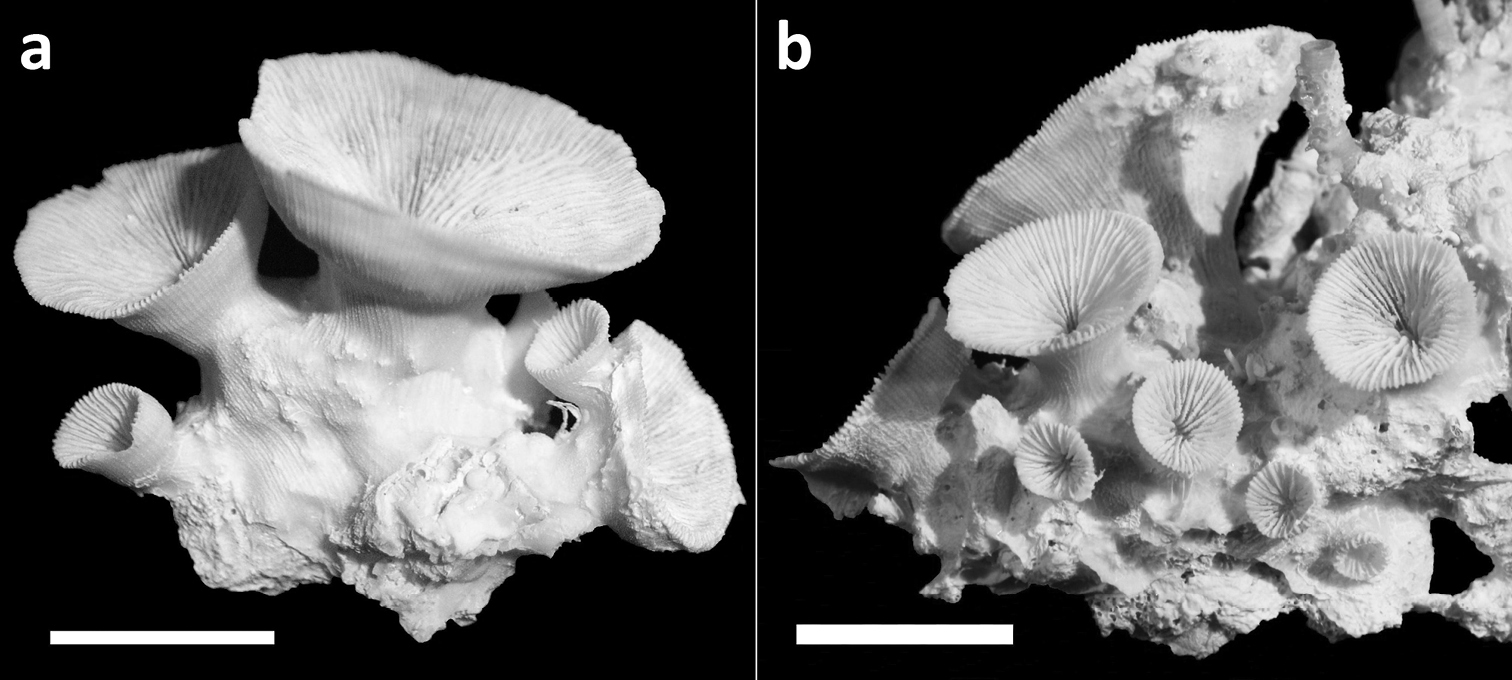
Specimens of Leptoseris troglodyta sp. n. from the Philippines, Cebu, Mactan Island, (RMNH Coel. 40151). Scale bars: 1 cm. a Upper side of a specimen showing four separate calices b Three calices that are partly fused at their sides.
Specimens of Leptoseris troglodyta sp. n.from the Philippines, W of Bohol, NW side of Cabilao Island (RMNH Coel 24195). Scale bars: 1 cm. a Cluster of calices formed by extra-calicular budding showing costae with small granular spines b Idem.
Specimens of Leptoseris troglodyta sp. n. from the Philippines, W of Bohol, NW side of Cabilao Island (RMNH Coel 24195). Scale bars: 5 mm a Single calyx from above, showing nearly solid columella in the fossa b Two totally fused monocentric calices showing, each with its own fossa c Upper side of calyx showing clearly visible granulation on septal sides d Calyx showing solid corallum wall covered by costae with fine granular spines.
Corals cave-dwelling, azooxanthellate. Calices small, cup-shaped, monocentric or fused, forming buds at basal coenosteum.
The epithet troglodyta (noun) means cave dweller in Latin, derived from ancient Greek for “one who dwells in holes”.
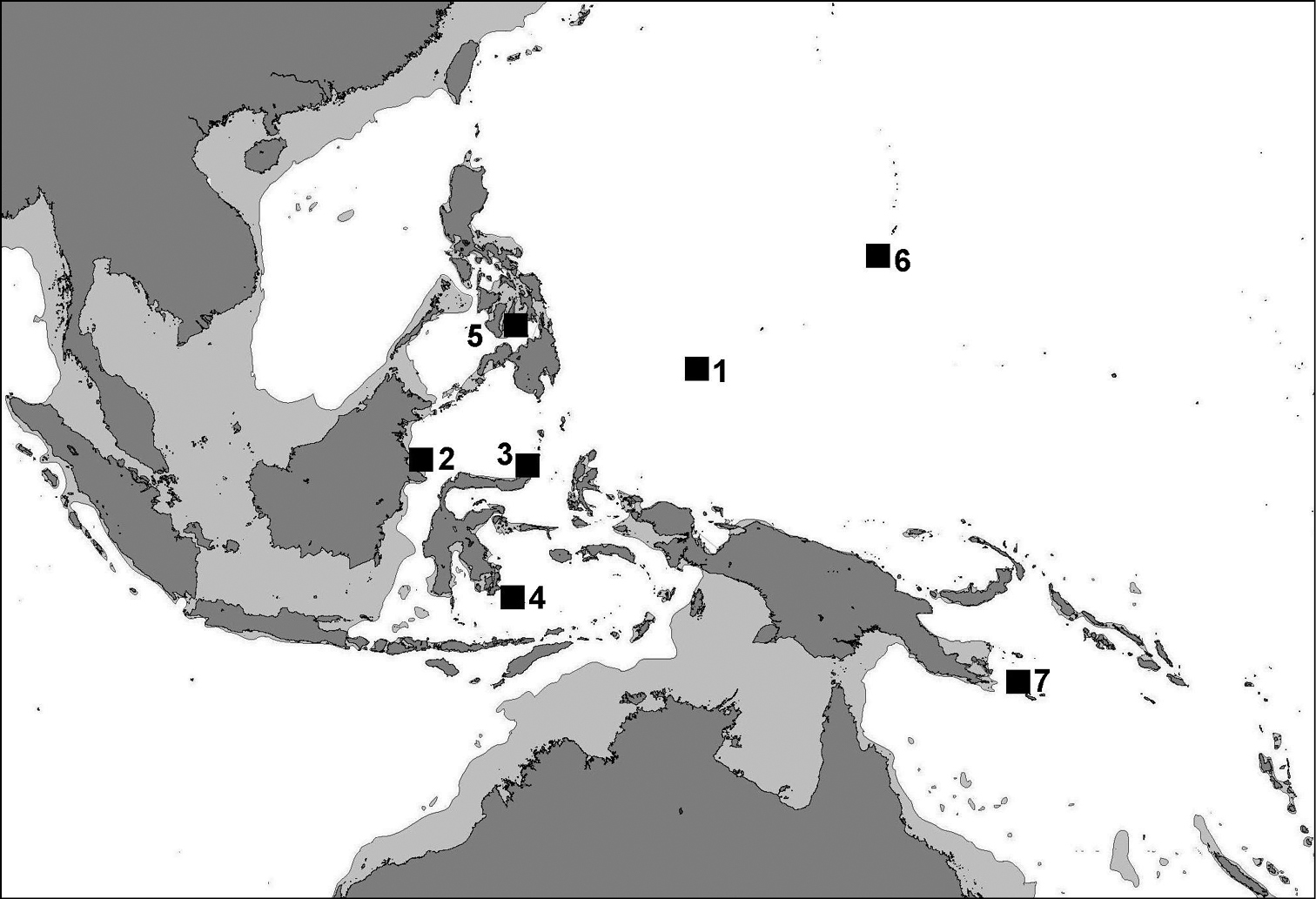
Records are from coral reefs, usually in areas with limestone outcrops: Indonesia (East Kalimantan, North Sulawesi, Southeast Sulawesi), the central Philippines (Cebu, Bohol), Palau, eastern Papua New Guinea, and the Marianas (Guam) (Figure 9).
Distribution map ofLeptoseris troglodyta sp. n. showing records at (1) Palau, (2) East Kalimantan, (3) North Sulawesi, (4) Wakatobi, (5) Bohol, (6) Guam, (7), eastern Papua New Guinea.
Leptoseris troglodyta sp. n. has a habitat and growth form unlike any other known Leptoseris; its corals are cavernicolous and azooxanthellate, and have small, monocentric calices that may multiply by extramural budding and fuse. Other Leptoseris species are polycentric by circumoral and marginal budding (
The new species lacks pigments of its own, like many cavernicolous (= troglobiotic) animals. Although there are no zooxanthellae in its soft tissue, it usually contains green or red shade-adapted endolithic cyanobacteria imbedded in the skeleton, which have also been reported from Leptoseris fragilis Milne-Edwards and Haime, 1849 (
Despite its unique small monocentric corallites and lack of zooxanthellae, the new species is classified with Leptoseris because of similarly shaped septa and costae, and by its thin, cup-shaped or foliaceous coralla somewhat resembling those of Leptoseris fragilis. The latter species features corals that can be monocentric, but its calices grow larger (Ø > 50 mm) and may eventually form secondary stomata by intracalicular, circumoral budding. Other extinct agariciid genera predominantly consist of encrusting and massive, polystomatous species (
Support from molecular analyses (Stefani et al. in prep) would be needed to justify the position of the new species in a separate genus instead of Leptoseris. In that case, it could be appropriate to classify it with Trochoseris Milne Edwards & Haime, 1849, an extinct genus (Mid Cretaceous – Oligocene) consisting of corals that are solitary, attached and turbinate or trochoid (
The corals have a basic growth form like that of Cladopsammia gracilis (
Leptoseris troglodyta is the first extant shallow-water agariciid known known to be reef-dwelling and azooxanthellate. The extinct agariciid genera, Trochoseris Milne Edwards & Haime, 1849 and Vaughanoseris Wells, 1934, also consists of monocentric species; the first being attached and turbinate or trochoid, and the second being free-living and discoid (
Coincidently, the Fungiidae show many examples of solitary free-living species that resemble the extinct Vaughanoseris. So, regarding their lifestyle, Vaughanoseris species could be reef-dwelling and zooxanthellate as well. Moreover, the attached, monocentric dendrophylliid Balanophyllia europaea (Risso, 1826) is an example of a single zooxanthellate species (
The deep-water species Dactylotrochus cervicornis and the cave-dweller Leptoseris troglodyta posses parallel ridges on the sides of their septa, which are called menianes (
All live specimens of Leptoseris troglodyta, which were observed in their habitat (Figures 1–2), were clearly azooxanthellate by lacking a brown colour, like completely bleached corals (
The deep-water species Dactylotrochus cervicornis and the cave-dweller Leptoseris troglodyta both live in dark environments. The latter has been observed to be azooxanthellate in caves at various localities. Dactylotrochus cervicornis specimens may have to be examined for the absence of zooxanthellae to be sure that this species is always azooxanthellate. If so, its menianes have no use in connection to light absorption, like in Leptoseris troglodyta. Leptoseris troglodyta shows that the evolutionary relation between scleractinian reef corals and their algal symbionts is not fixed and that it may be difficult to deduct such a relation based on coral growth forms and their possible position in phylogeny reconstructions, especially if no molecular data can be obtained, as is the case for fossil corals. According to a recent molecular study, coloniality may have become lost at least six times and symbiosis with zooxanthellae at least three times in the phylogeny of the Scleractinia (
Leptoseris troglodyta sp. n. is the first reef-dwelling agariciid coral without zooxanthellae. As a small cave-dwelling species it can live without sunlight. It has not been observed to co-occur with any zooxanthellate scleractinians (only azooxanthellate species), although in small and poorly illuminated cavities a variety of zooxanthellate scleractinians can be discerned (
I want to thank the following people, organizations and institutes who assisted during the fieldwork or other parts of the research: Dr Pat Colin and Ms Lori Colin (Coral Reef Research Foundation, Palau), Dr Filipina B. Sotto and Dr Thomas Heeger (University of San Carlos, Cebu City); Dr Arjan Gittenberger (Naturalis and Leiden University), The Nature Conservancy, Operation Wallacea (Wakatobi, Indonesia), Research Centre for Oceanography, Indonesian Institute of Sciences (PPO-LIPI, Jakarta). Fieldwork was funded by the Schure-Beijerinck-Popping Fonds. Mr Bastian T. Reijnen and Ms Sancia E.T. van der Meij supplied material and photos from North Sulawesi. Dr Gustav Paulay generously provided photos and accompanying data of specimens from Guam and Papua New Guinea. Dr Leen P. van Ofwegen took the SEM photographs. I am grateful to Dr Francesca Benzoni, Dr Marcelo Kitahara, and various other members of the Scleractinia Systematics Working Group (SSWG) for their suggestions given during discussions. I thank Ms Zarinah Waheed and two reviewers, Dr Zena Dinesen and Dr Marcelo Kitahara, for constructive remarks on early drafts of the manuscript.