(C) 2012 Mostafa R. Sharaf. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The ant subfamily Aenictinae is recorded for the first time from the Kingdom of Saudi Arabia and for the second time from the Arabian Peninsula. A new species Aenictus arabicus sp. n., is described from the worker caste. Aenictus arabicus belongs to the Aenictus wroughtonii-group and appears to be most closely related to Aenictus rhodiensis Menozzi, but can be easily distinguished from the latter by the following characters: overall smaller size; cephalic index (head width/head length) small; occipital corners in lateral view rounded; antennal scape when laid back surpassing approximately two-thirds of head length; funicular segments 2–8 each at least 2× as long as broad; subpetiolar process well developed; petiole and postpetiole distinctly imbricate; gaster and clypeus entirely yellow, teeth of mandibles reddish- brown. Aenictus arabicus was collected from leaf litter, next to a tree of Psidium guajava L. The new species also is similar to Aenictus sagei and Aenictus wroughtonii. Affinities and a key to related species of the species group are given.
Aenictus, taxonomy, Arabian Peninsula, Saudi Arabia, Al Sarawat Mountains, ants, Palaearctic region
The subfamily Aenictinae Emery, 1901, was elevated to the rank of subfamily by
The subfamily Aenictinae is characterized by having:- a waist of two segments, with the spiracle of the postpetiole set behind the midlength of the tergite; all gastral spiracles circular; and the first gastral segment with a narrow, neck-like constriction behind the articulation with the postpetiole, 8-10 antennal segments, the frontal lobes reduced with the antennal sockets completely exposed, and the promesonotal suture absent (
Since
Ten species of Aenictus have been reported from the Palaearctic, nine of which are distributed in the Southwestern part of the region, Morocco in the west to Afghanistan in the east (
Here the subfamily Aenictinae is recorded for the first time from Saudi Arabia and for the second time from the Arabian Peninsula. A new species, Aenictus arabicus sp. n., is described based on the worker caste. The queen and male are unknown. A key to the related species within the Aenictus wroughtonii-group is given.
Materials and methodsThe following abbreviations are used for particular morphological features and metrics:
TL Total length; the outstretched body length from the mandibular apex to the gastral apex.
HW Head width; the maximum width of the head in full-face view.
HL Head length; the maximum length of the head, excluding the mandibles.
CI Cephalic index (HW × 100/HL).
SL Scape length, excluding condylar bulb.
SI Scape index (SL × 100/HW).
ML Mesosoma length; the length of the mesosoma in lateral view, from the point at which the pronotum meets the cervical shield to the posterior base of the propodeal lobes or teeth.
PRW Pronotal width; the maximum pronotal width in dorsal view.
PL Petiole length; the maximum length of petiole measured in dorsal view, from the anterior margin to the posterior margin.
PW Petiole width; the maximum petiolar width measured in dorsal view.
PPL Postpetiole length; the maximum postpetiolar length measured in dorsal view.
PPW Postpetiole width; the maximum postpetiolar width measured in dorsal view.
All measurements are expressed in millimeters. Images were taken with a scanning electron microscope ((SEM) JSM-6380 LA).
Depositories of type materialBMNH Natural History Museum, London, United Kingdom.
CASC California Academy of Science Collection, San Francisco, California, USA.
KSMA King Saud Museum of Arthropods, King Saud University, Riyadh, Kingdom of Saudi Arabia (Holotype depository).
MCZC Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA.
MHNG Muséum ďHistoire Naturelle, Geneva, Switzerland.
NHMB Naturhistorisches Museum, Basel, Switzerland.
SEMC Division of Entomology (Snow Entomological Collections), University of Kansas Natural History Museum, Lawrence, Kansas, USA.
WMLC World Museum Liverpool, Liverpool, United Kingdom.
Resultsurn:lsid:zoobank.org:act:347C091D-1E98-4765-AEF5-10C4CACE8DDE
http://species-id.net/wiki/Aenictus_arabicus
Figs 1–12Saudi Arabia, Al Baha-Mukhwah Aqaba RD, 19.IV.2012, 20.00000°N, 41.43758°E, 1300 m, 19.IV.2012 (M. R. Sharaf leg.); deposited in the KSMA.
21 workers, same data as holotype; 1 deposited in MHNG (Dr Bernhard Merz); 1 deposited in NHMB (Mrs. Isabelle Zürcher-Pfander); 2 deposited in CASC (Dr Brian Fisher); 2 deposited in MCZC (Prof. E. O. Wilson); 2 deposited in SEMC (Prof. Michael S. Engel); 1 deposited in WMLC (Mr. Tony Hunter), 1 deposited in BMNH (Mr. Barry Bolton); the remaining specimens in KSMA (M. R. Sharaf).
Holotype: TL 3.0, HL 0.65, HW 0.52, SL 0.50, PRW 0.35, ML 0.95, PL 0.22, PW 0.15, PPL 0.17, PPW 0.15. Indices: SI 96, CI 80.
TL 2.75-3.12, HL 0.60-0.72, HW 0.42-0.55, SL 0.40-0.52, PRW 0.20-0.35, ML 0.77-1.00, PL 0.22-0.27, PW 0.12-0.15, PPL 0.15-0.20, PPW 0.12-0.17. Indices: SI 77-104, CI 70-92. (n=11).
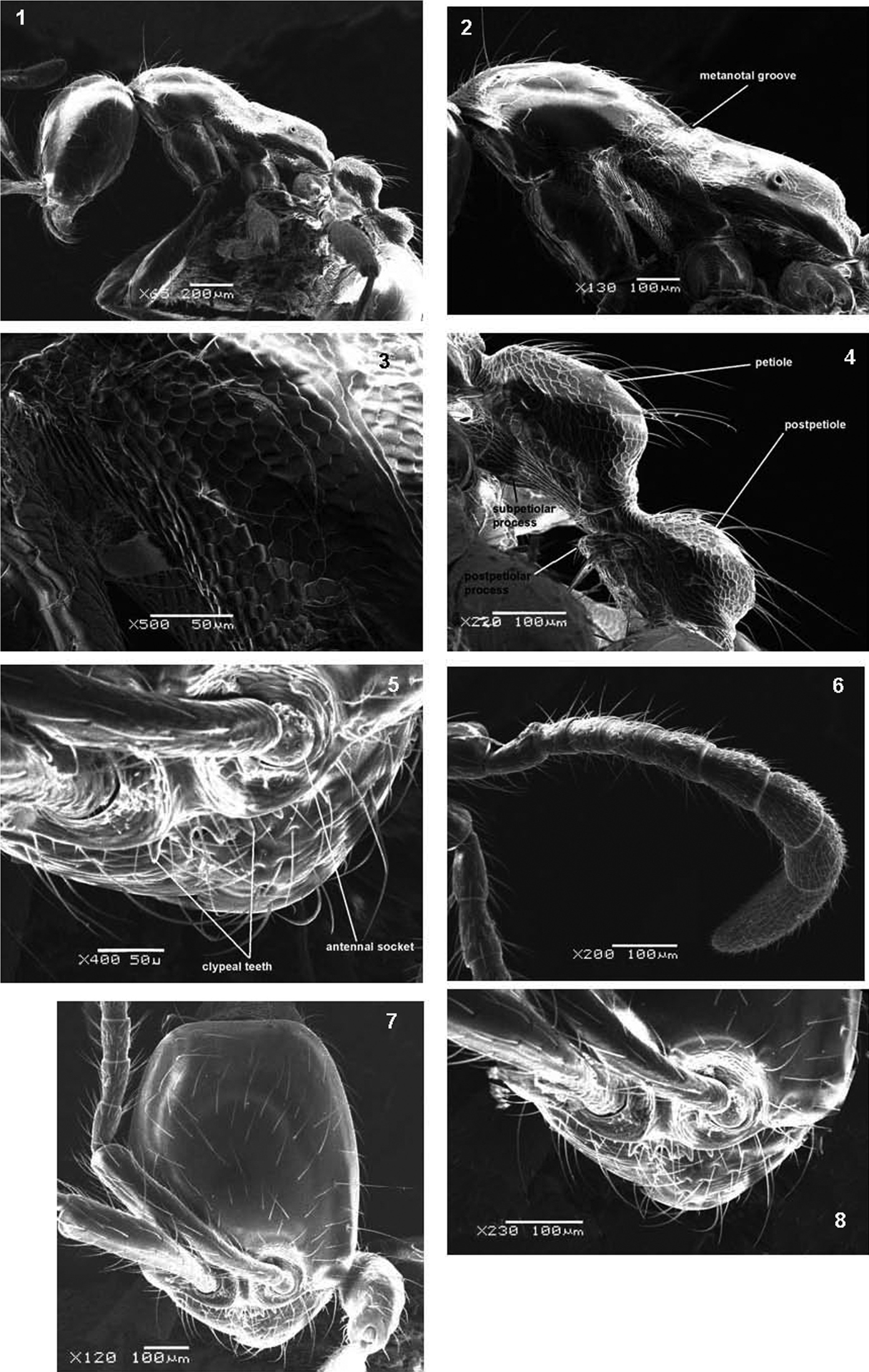
Head entirely smooth and shining. In full-face view head distinctly longer than broad, with convex sides and nearly straight posterior margin; occipital corners in lateral view rounded; anterior clypeal margin with six small denticles; masticatory margin of mandibles armed with a large apical tooth followed by five smaller subequal teeth and a relatively larger basal tooth; when laid back, antennal scapes surpassing about two thirds of head length; all funicular segments at least twice as long as broad; terminal funicular segment about 2.5 × as long as the proceeding segment; mandibles dull with longitudinal striations; whole head dorsum and antennae with stiff scattered long hairs. Mesosoma in dorsal view broader anteriorly than posteriorly; promesonotum in profile distinctly convex, bearing many pairs of hairs; metanotal groove distinct; mesopleuron faintly but distinctly imbricate; propodeum bare or in some individuals with very sparse decumbent pubescence; propodeal dorsum long, about 4× as long as declivity; propodeum in profile slightly lower than promesonotum and almost flat dorsally; propodeal junction rounded. Petiole longer than broad in dorsal view with node clearly convex in lateral view; subpetiolar process triangular with convex ventral margin and blunt anteriorly. Postpetiole distinctly smaller than petiole, its node roundly convex, and its anteroventral edge sharp and bearing many hairs; both petiole and postpetiole distinctly imbricate and equipped dorsally with several pairs of backward directed long hairs. Gaster smooth and shining with abundant pairs of hairs. Color uniformly yellow.
SEM of Aenictus arabicus sp. n. paratype 1 body in profile 2 mesosoma in profile 3 imbricate sculpture of mesopleuron 4 petiole and postpetiole in profile 5 antennal sockets and anterior clypeal margin 6 antenna 7 head in full-face view 8 anterior part of head.
Automontage of Aenictus arabicus sp. n. paratype 9 body in profile 10 body in dorsal view 11 head in full-face view 12 label. ( CASENT0280972).
This species is named after the type locality.
Affinities. Aenictus arabicus is similar to Aenictus rhodiensis Menozzi, 1936 from Greece; and Aenictus sagei and Aenictus wroughtonii described by Forel from India. All the three species are members of the Aenictus wroughtonii-group as defined by
Comparing Aenictus arabicus with Aenictus rhodiensis, both species have a similar general morphology, notably the shape of the mesosoma, petiole and postpetiole, a similar body pilosity; also both have a peculiar subpetiolar process which is somewhat wide and blunt anteriorly and the anterior clypeal margin is equipped with six small denticles. From the more accurate description in Aktaç et al.(2004), Aenictus arabicus can be separated readily from Aenictus rhodiensis. The former has a small relatively long, narrow head (HL 0.60-0.72, HW 0.42-0.55, CI 70-92) and long scapes, when laid back surpassing about two-thirds of the head length (SI 77-104) while the latter has a shorter head (HL 1.23, HW 1.02) and shorter scapes, which just surpass the midpoint of the head. Aenictus arabicus has a nearly straight posterior margin of the head whereas it is weakly concave in Aenictus rhodiensis. The funicular segments 2-8 are at least twice as long as broad in the former, while they are as long as broad in the latter. Aenictus arabicus has an entirely yellow clypeus and reddish-brown mandibular teeth while the sides of the clypeus and mandibular teeth are reddish brown in Aenictus rhodiensis. The gaster of Aenictus arabicus is entirely yellow, whereas in Aenictus rhodiensis, the middle of the third gastral tergite has two longitudinal brownish lines which diverge forward, sometimes reducing to small points. Aenictus dlusskyi Arnoldi, known only from the type series from Armenia, also resembles Aenictus arabicus but is of a similar size to rhodiensis (
Comparing Aenictus arabicus with the Asian species Aenictus sagei (CASENT0281958) and Aenictus wroughtonii (lectotype images are given in Jaitronget al.2010: 35), Aenictus arabicus has the anterior clypeal margin bearing six small denticles; Aenictus sagei has 9-10 denticles; whereas Aenictus wroughtonii has 8-10 denticles. In addition, Aenictus arabicus hasthe subpetiolar process well developed, triangular, with convex ventral margin and blunt anteriorly and body pilosity fewer and shorter; Aenictus sagei has a weakly developed subpetiolar process, with its ventral outline nearly straight; its anteroventral corners obtusely angulate and body pilosity distinctly long and abundant (length of the longest pronotal hair 0.20–0.25 mm, Jaitronget al.2010); whereas Aenictus wroughtonii has an undeveloped subpetiolar process, with its ventral outline feebly convex and without anterior angle and relatively sparse standing hairs which are shorter than in Aenictus sagei.
Habitat and biologyAl-Baha Province is divided by massive and steep rocky mountains into the lowland coastal plain to the west, known as “Tihama”, and the mountainous area ranging 1500 - 2450 m above sea level to the east, known as “Al-Sarat or Al-Sarah” which forms part of Al-Sarawat Mountains. The type locality (Fig. 13) is a small farm at the beginning of a narrow valley isolated between the mountains and the plain with a few native shrubs and trees at 1300 m. The farm is planted with Annona squamosa L. (Annonaceae), Prunus persica (L.), Prunus Amigdalus (Mill.) (Rosaceae), Psidium guajava L. (Family: Myrtaceae), Zea mays ssp. mays L. (Family: Poaceae), in addition to banana, and mango. The new species was found foraging on the ground under leaf litter and next to a tree of Psidium guajava L. The soil, at the time of collection was well saturated through irrigation and accumulation of organic matter.
Type locality, Al Bahah, Mukhwah Aqaba RD. (photo M. R. Sharaf).
The climate in Al-Baha Province is greatly influenced by its varying topography. It is generally moderate in summer and cold in winter with average temperatures ranging between 12–23 °C. In Tihama, the climate is hot in summer, warm in spring and mild in winter, with humidity ranging between 52%–67%, and a rainfall less than 100 mm annually. While in the mountainous area, Al-Sarah, The climate is greatly different from that in Tihama although they are separated by no more than 30 km. The weather is cooler in summer and winter due to its high altitude. Al-Sarah is exposed to the formation of clouds and fog, and this often happens in winter because of air masses coming from the Red Sea, accompanied by thunderstorms. In spring and summer, the climate is mild and pleasant. Also, rainfall is higher with falls in the range of 229–581 mm. The average rain falls throughout the whole province is 100–250 mm annually
The presence of an Aenictus speciesin the Southwestern part of Saudi Arabia is not surprising as the area is regarded as being Afrotropical (
Despite the Afrotropical nature of the type locality we found it important to give a key to the closely related species in the Aenictus wroughtonii-group.
Key to species of the Aenictus wroughtonii-group related to Aenictus arabicus based on worker| 1 | Subpetiolar process almost absent, anteroventrally not angulate (India) | Aenictus wroughtonii |
| – | Subpetiolar process present, its anteroventral corners angulate | 2 |
| 2 | Anterior clypeal margin bearing 9-10 denticles; subpetiolar process weakly developed (India) | Aenictus sagei |
| – | Anterior clypeal margin bearing six denticles; subpetiolar process well developed | 3 |
| 3 | Funicular segments 2–8 as long as broad; middle of third gastral tergite with two longitudinal brownish lines, sometimes reducing to small points; scapes when laid back just surpass the midpoint of head (Greece) | Aenictus rhodiensis |
| – | Funicular segments 2–8 at least twice as long as broad; gaster entirely yellow; scapes when laid back surpassing about two-thirds of head length (Saudi Arabia) | Aenictus arabicus sp. n. |
This project was supported by King Saud University, Deanship of Scientific research, College of Food and Agriculture Sciences, Research Center. The authors are indebted to Barry Bolton, Brian Taylor, Boris Kondratieff and Michael S. Engel for their useful comments. We thank Brian Fisher and two anonymous reviewers for valuable suggestions. Special thanks to Lutfy El-Juhany (King Saud University) for identifying the plants from the type locality, Mahmoud Abdel-Dayem for technical assistance, Michele Esposito and Estella Ortega for taking automontage photos and Omer Hamid for SEM work.