(C) 2012 Fang Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Prior to this study, only Megapulvinaria maxima (Green) was known from China. However, a new species Megapulvinaria beihaiensis Wang & Feng, sp. n. is described below and Megapulvinaria maxima is redescribed. A key is provided for the five species now placed in this genus.
Hemiptera, Coccoidea, soft scale, taxonomy, China
Soft scale or Coccidae is the third largest family after Diaspididae and Pseudococcidae within the superfamily Coccoidea (
The genus Megapulvinaria was erected by
Previously, only Megapulvinaria maxima was known from China but a new species has now been discovered. The adult female of Megapulvinaria maxima is redescribed, the adult female of the new species Megapulvinaria beihaiensis Wang & Feng sp. n. is described and a key is provided for separation of the five species now known in this genus.
Materials and methodsSpecimens were slide mounted using the method recommended by
All specimens are deposited in the Entomological Museum of Northwest A & F University, Yangling, Shaanxi, China (NWAFU).
Checklist of known species of the genus Megapulvinaria YoungMegapulvinaria maxima (Green, 1904); China (Guangxi, Yunnan, Taiwan), Thailand, India, Indonesia, Philippines, Sri Lanka, Vietnam, Papua New Guinea, Chuuk Islands.
Megapulvinaria burkilli (Green, 1908); India.
Megapulvinaria orientalis (Reyne, 1963); Thailand.
Megapulvinaria maskelli (Olliff, 1891); Australia.
Megapulvinaria beihaiensis sp. n.; China (Guangxi).
Taxonomyhttp://species-id.net/wiki/Megapulvinaria
Adult female. Body elongate oval to broad oval; stigmatic clefts distinct. Dorsum. Dorsal setae spinose or conical. Dorsal submarginal tubercles absent. Preopercular pores present or absent. Dorsal tubular ducts present or absent. Eyespots generally displaced onto dorsum (marginal on Megapulvinaria maxima). Anal plates together quadrate, each plate with 2 spinose and/or truncate setae along inner margin, a similar seta on apex and a spinose seta present in discal position (possibly on outer margin of Megapulvinaria maskelli). Anal ring with 6 setae. Margin. Marginal setae stout, apex truncate or bidentate, and with 2 types present, one shorter and broader than other (about same length and one slightly broader than other both in Megapulvinaria maskelli and Megapulvinaria beihaiensis); broader setae on head and posterior margins of abdomen (0–3 broader setae present between two stigmatic clefts in Megapulvinaria beihaiensis). Stigmatic clefts deep or shallow, each with 3–12 stigmatic spines. Venter. Antennae 7–9 (mostly 8) segmented. Legs well-developed, each with a tibio-tarsal articulation and an articulatory sclerosis, each claw with a denticle on the widest part. Pregenital setae 2 pairs. Spiracular disc-pores each mainly with 5 loculi. Pregenital disc-pores each mainly with 10 loculi, restricted to abdominal segments. Ventral tubular ducts of three types, with a submarginal band of small tubular ducts; median area of head, thorax, and anterior 1–3 abdominal segments with large ducts each with both outer and inner ductules broad or stout (anterior submargin and all median area in Megapulvinaria maskelli); posterior abdominal segments of moderately tubular ducts.
Oriental and Australian regions.
| 1 | Dermal areolations absent | Megapulvinaria burkilli (Green) |
| – | Dermal areolations present | 2 |
| 2 | Anal plates with dorsal reticulations | 3 |
| – | Anal plates without dorsal reticulations | 4 |
| 3 | With only 3 stigmatic spines in each stigmatic cleft | Megapulvinaria maskelli (Olliff) |
| – | With more than 3 stigmatic spines in each stigmatic cleft | Megapulvinaria beihaiensis sp. n. |
| 4 | With only 1 pair of interantennal setae present; all of lateral stigmatic spines about same length | Megapulvinaria orientalis (Reyne) |
| – | With 2–5 pairs of interantennal setae present; not all of lateral stigmatic spines about same length | Megapulvinaria maxima (Green) |
http://species-id.net/wiki/Megapulvinaria_maxima
Figure 15 adult females, CHINA, Yunnan, Jingdong, 18. x. 1976 on Pigeonpea (Cajanus cajan (L.) Millsp., Leguminosae), Xiao-Ze Chen (NWAFU).
The measurements are based on all 5 specimens.
Adult female. Mounted material. Body elongate oval, about 4.2–6.2 mm long and 2.7–3.8 mm wide. Anal cleft approximately 1/7 of the body length. Stigmatic clefts deep.
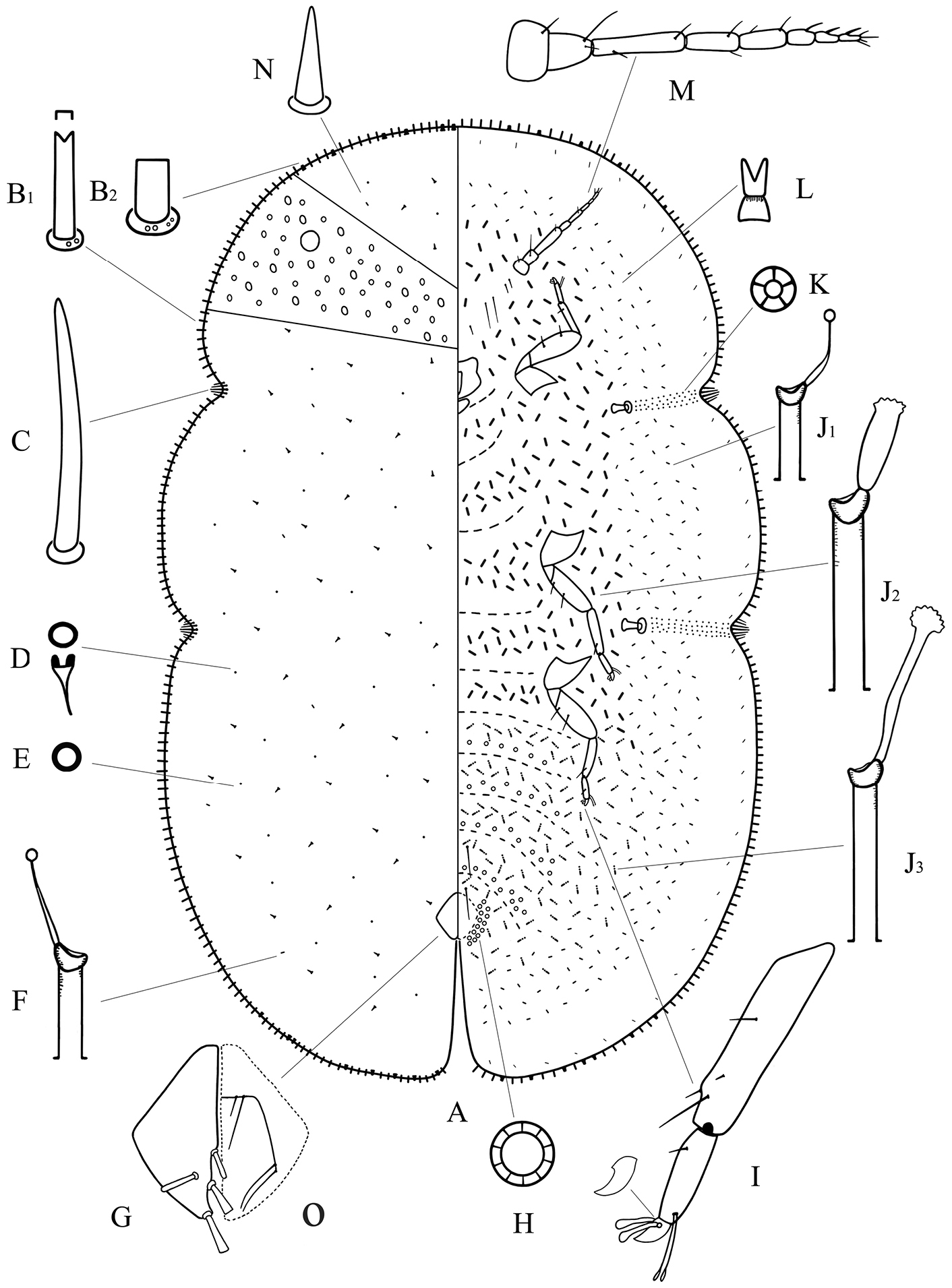
Dorsum. Derm membranous. Dermal areolations well developed, each with 1 or 2 dorsal microducts. Dorsal setae conical, with a well-developed basal socket, each 8–16 µm long, scattered throughout. Dorsal simple pores each with a slightly sclerotized margin, randomly distributed. Dorsal microducts each with a very short outer ductule and a longer, fairly broad inner filamentous ductule, sparsely located in dorsal areaolations. Dorsal tubular ducts each with a short outer ductule and a fine inner ductule with a minute terminal gland, sparsely distributed. Preopercular pores absent. Anal plates together quadrate; posterior margin slightly longer than anterior margin, outer angle slightly obtuse; each plate with a large cylindrical seta in discal position, each 34–50 µm long, a large spatulate seta apically, each 52–64 µm long, and with 2 spinose and/or spatulate setae along posterior 1/3rd of inner margin, each 40–56 µm long. Ano-genital fold with 1 pair of long setae and 1 pair of short setae along anterior margin and 2 or 3 pairs lateral margin. Anal ring subcircular, with 2 or 3 rows of translucent pores and 6 anal ring setae. Eyespots present some way onto dorsum, each 80–96 µm wide.
Margin.Marginal setae of 2 types: 1) large and stout setae, each 17–38 µm long, with nearly parallel sides, and with either a truncate or a bifid apex, all with well-developed basal sockets, each socket with 1 or 2 small pores; with 96–110 setae between anterior clefts, 36–46 setae on each side between stigmatic clefts, and 84–98 setae between each posterior stigmatic cleft and anal cleft; and 2) quite broad and short setae, each 14–24 µm long, with parallel sides and a truncate, flattened apex, and with a larger basal socket about twice as broad as that of type 1), each socket with 3–8 small pores; latter type of marginal setae only distributed on anterior and posterior ends, with 16–22 setae anteriorly on head and prothorax, 5–12 setae on either side of abdomen near anal cleft. Stigmatic clefts deep; stigmatic spines bluntly spinose and mostly straight, with 4–8 spines in each anterior cleft and 5–10 in each posterior cleft; length of each 42–96 µm, with median 1–3 spines much longer than the lateral spines.
Venter. Derm membranous. Antennae 8 segmented, each 505–586 µm long; third segment longest; with 2 pairs of long setae and 1–3 pairs of short interantennal setae. Clypeolabral shield 198–232 µm long, 205–240 µm wide; labium 90–106 µm long, 113–144 µm wide. Legs well-developed, each with a tibio-tarsal articulation and articulatory sclerosis; claws with a denticle on widest part, claw digitules broad and expanded apically, tarsal digitules slender, knobbed and longer than claw digitules; trochanter+femur 239–405 µm, tibia 180–245 µm and tarsus 96–122 µm. With 2 pairs of long pregenital setae present in both segments VI & VII; submarginal setae present in a single row; other setae slender, each 4–10 µm long, quite sparsely distributed. Spiracles normal, spiracular disc-pores each with 5 loculi, present in a broad band between stigmatic cleft and each spiracle. Pregenital disc-pores each mainly with 10 loculi, present around the vulva and on posterior 4 abdominal segments. Ventral microducts scattered. Ventral tubular ducts of 3 types present: 1) a duct with a short outer ductule and a fine inner filament, with a minute terminal gland, present in a complete submarginal band; 2) a duct with outer and inner ductules both broad and with a well-developed terminal gland, present medially on head, thorax and anterior 1 or 2 abdominal segments; and 3) a duct with a moderately long outer ductule and a thin inner ductule slightly longer than outer ductule, with a flower-shaped terminal gland, present medially on posterior abdominal segments and extending and mingling with marginal band of type 1) ducts.
Adult female of Megapulvinaria maxima Green, A body derm B1, B2 two kinds of marginal setae C stigmatic spine D dorsal microduct E dorsal pore F dorsal tubular duct G anal plates O ano-genital fold H pregenital disc-pore I tibio-tarsus of hind leg J1, J2, J3 ventral tubular ducts K spiracle disc-pore L ventral microduct M antenna N dorsal seta.
China (Guangxi, Yunnan, Taiwan), Thailand, India, Indonesia, Philippines, Sri Lanka, Vietnam, Papua New Guinea, Chuuk Islands.
Since
This species is close to Megapulvinaria burkilli (Green) (data from Green, 1908), but it can be distinguished from the latter by the following features (character states of Megapulvinaria burkilli in brackets): (1) the much larger body size in comparison to the latter (4 mm long, 2 mm wide); and (2) with well-developed dermal areolations present (absent).
urn:lsid:zoobank.org:act:56E4CA5F-6C56-431C-AD2B-6A54776BC16B
http://species-id.net/wiki/Megapulvinaria_beihaiensis
Figure 2Holotype: adult female. CHINA, Guangxi, Beihai, Haibin Park. 26. vii. 2010, on Cinnamomum sp., (Lauraceae), Bin Zhang (NWAFU)
3 adult females, the data same as holotype.
The measurements are based on all 4 specimens.
Description. Adult female. Unmounted material. Adult female yellowish brown or dark brown, elongate oval and with a longitudinal dorsal ridge in dorsal straight median area (materials examined were all immersed in 75% ethanol, and the ovisac was not seen). The specimens collected on the lamina of the host plant.
Mounted material. Body elongate oval, about 2.1–3.2 mm long, 1.3–1.7 mm wide. Anal cleft approximately 1/8 of the body length. Stigmatic clefts deep.
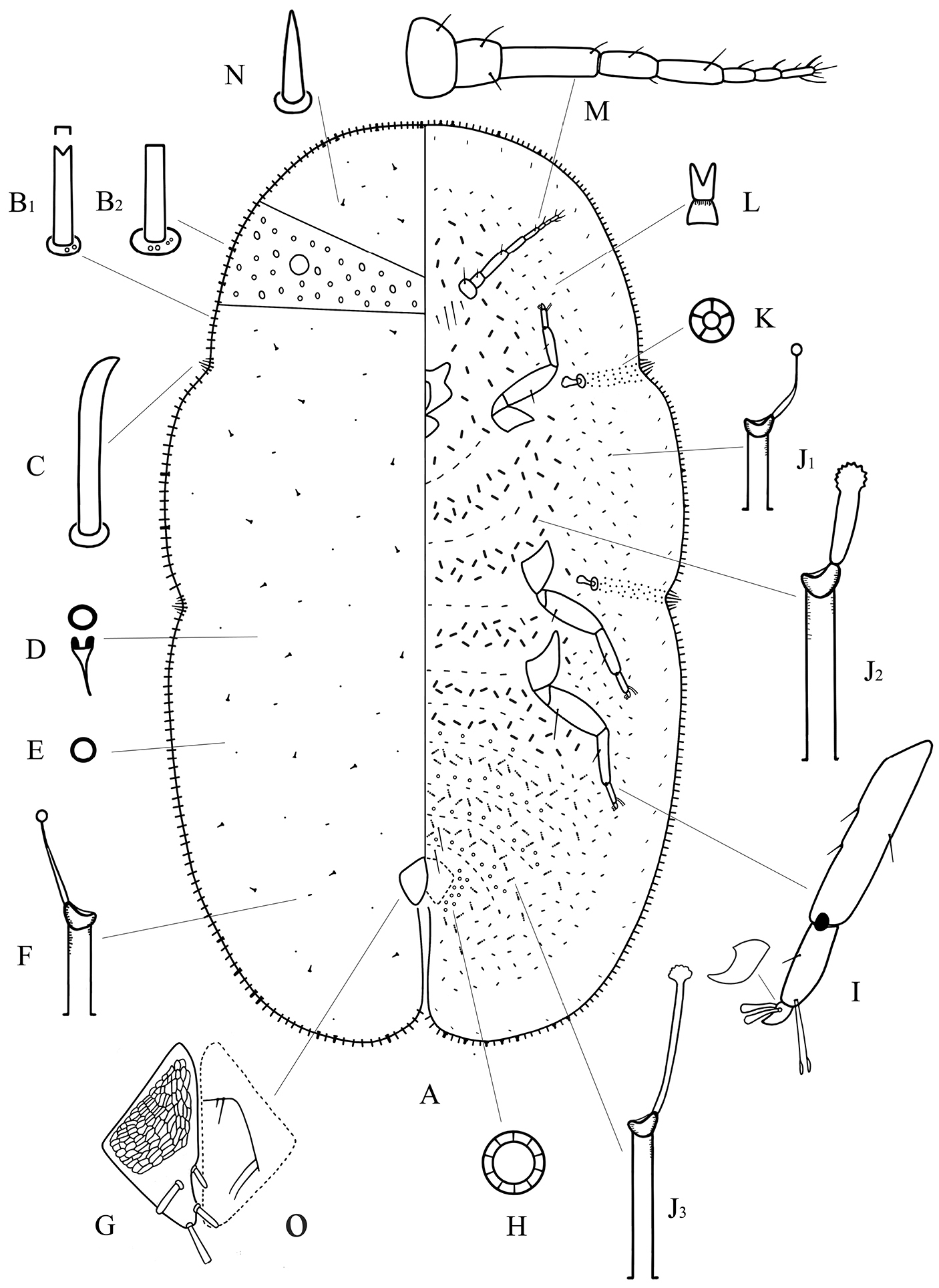
Dorsum. Derm membranous. Dermal areolations well-developed, each with a dorsal microduct. Dorsal setae conical, with a well-developed basal socket, each 6–11 µm long, scattered throughout. Dorsal simple pores each with a slightly sclerotized margin, randomly distributed. Dorsal microducts each with a very short outer ductule and a long, fairly broad inner filamentous ductule, sparsely located in each dorsal areaolation. Dorsal tubular ducts each with a short outer ductule and a fine inner ductule with a minute terminal gland, sparsely distributed. Preopercular pores absent. Anal plates together quadrate, dorsal surface with reticulations on anterior two-thirds; posterior margin subequal to or slightly longer than anterior margin, outer angle a right-angle; each plate with a blunt spinose seta in discal position, each 34–42 µm long, a large spinose or spatulate seta apically, each 48–54 µm long, and with 2 spinose setae along posterior 1/3rd of the inner margin, each 32–44 µm long, length of plates 146–167 µm, width of single plate 74–88 µm. Ano-genital fold with 1 pair of long setae and 1 pair of short setae along anterior margin and 2 or 3 pairs lateral margin. Anal ring subcircular, with 2 or 3 rows of translucent pores and 6 anal ring setae. Eyespots present some way onto dorsum, each 42–60 µm wide.
Margin. Marginal setae of 2 types: 1) stout setae, each 18–30 µm long; each seta with nearly parallel sides and with either a truncate or a bifid apex, all with well-developed basal sockets, each socket with 1 or 2 small pores; with 101–111 setae between anterior clefts, 34–42 setae on each side between stigmatic clefts, and 74–85 setae between each posterior stigmatic cleft and anal cleft; 2) quite strong setae, subequal in length with type 1) but slightly broader; each seta with parallel sides, with a truncate and flattened apex, and with a large basal socket about twice as broad as that of type 1), each socket with 2–8 small pores; with 10–16 setae anteriorly on head and prothorax, 0–3 setae between stigmatic clefts, and 4–10 setae on either side of abdomen near anal cleft. Stigmatic clefts deep; stigmatic spines bluntly spinose and mainly curved apically, with 4 or 5 spines in each anterior cleft and 5–8 spines in each posterior cleft; length of each 34–62 µm, and the median 1–3 spines longer than the lateral spines.
Venter. Derm membranous. Antennae 8 segmented, each 346–378 µm long, the third segment longest; with 2 pairs of long setae and 2 or 3 pairs of short interantennal setae. Clypeolabral shield 138–160 µm long, 160–172 µm wide; labium 96–112 µm long, 84–112 µm wide. Legs well-developed, each with a tibio-tarsal articulation and articulatory sclerosis; claws with a denticle on widest part, claw digitules both broad and expanded apically; tarsal digitules slender, knobbed and longer than claw digitules; trochanter+femur 212–245 µm, tibia 136–188 µm and tarsus 54–75 µm. With 2 pairs of long pregenital setae present in both segments VI & VII; submarginal setae present in a single row; other setae slender, 6–20 µm long, quite sparsely distributed. Spiracles normal; spiracular disc-pores each mainly with 5 loculi, present in a broad band between stigmatic cleft and each spiracle. Pregenital disc-pores each mainly with 10 loculi, present around the vulva and on posterior 5 abdominal segments but becoming progressively less frequent anteriorly. Ventral microducts scattered. Ventral tubular ducts of 3 types present: 1) a duct with a short outer ductule and a fine inner filament with a minute terminal gland, present in a complete submarginal band; 2) a duct with a broad outer ductule, a stout inner ductule (as broad as outer ductule in some specimens) and with a well-developed terminal gland, present medially on thorax and anterior abdominal segments; and 3) a duct with a moderately long outer ductule, a thin inner ductule slightly longer than outer ductule, with a flower-shaped terminal gland, present medially on posterior abdominal segments and extending and mingling with marginal band of type 1) ducts.
Adult female of Megapulvinaria beihaiensis sp. n., A body derm B1, B2 two kinds of marginal setae C stigmatic spine D dorsal microduct E dorsal pore F dorsal tubular duct G anal plates O ano-genital fold H pregenital disc-pore I tibio-tarsus of hind leg J1, J2, J3 ventral tubular ducts K spiracle disc-pore L ventral microduct M antenna N dorsal seta.
China (Guangxi).
The specific epithet is taken from the type locality Beihai.
This new species resembles Megapulvinaria maskelli (Olliff) (data from
Megapulvinaria maskelli, currently only known from the Australian region, is the only non-Oriental species in this genus and has some distinctive characteristics within Megapulvinaria. It differs from other species in having: (1) only 3 stigmatic spines in each stigmatic cleft; (2) eyespots located on margin; and (3) the discal setae possibly on outer margin of anal plates.
We are grateful to Dr. Kumar Avasthi (Vaish College, Department of Zoology, India) for providing related papers. We wish to thank Dr. John Richard Schrock (Emporia State University, USA) for his very kind linguistic help with this manuscript. This study is supported by the National Natural Science Foundation of China (Grant No. 30870324).