(C) 2012 Raquel López-Antoñanzas. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Cannomys and Rhizomys are the sole living genera of the tribe Rhizomyini (Rhizomyinae, Spalacidae, Rodentia), known in the fossil record since the Late Miocene. The dental morphology of fossil Rhizomyini has been described in detail but until recently such descriptions were unavailable for extant species. A detailed account of the morphology and dental wear pattern of the cheek teeth of Cannomys badius is provided here based on the examination of museum specimens. Three stages of wear are recognized. Cannomys shares with Rhizomys the synapomorphy of having a mesolophid that is a long continuation of the protoconid on the first lower molar. There are significant differences between these taxa, such as the much smaller size of the cheek teeth and the trilophodont dental pattern of the M2, M3, and m2 in Cannomys.
Rhizomyinae, Rhizomyini, Cannomys, dental wear pattern
The subfamily Rhizomyinae (Spalacidae, Rodentia) is known in the fossil record since the Oligocene. It is represented by three modern genera: the Asian bamboo rats Rhizomys (a trispecific genus) and Cannomys (monospecific) and the African mole rats Tachyoryctes. The latter genus is considered bispecific by some authors (
Cannomys badius was originally named as Rhizomys badius by
The dental morphology of all living Rhizomyinae other than Cannomys badius, has been described in detail recently (Tachyoryctes;
All the specimens of Cannomys badius housed in the Laboratoire de Zoologie-Mammifères et Oiseaux of the Muséum national d’Histoire naturelle, Paris, France (MNHN) and in the Institut für Systematische Zoologie-Zoologische Sammlung of the Museum für Naturkunde, Berlin, Germany (ZMB) have been examined. MNHN individuals come from Thailand and Nepal, whereas those at ZMB are from Myanmar.
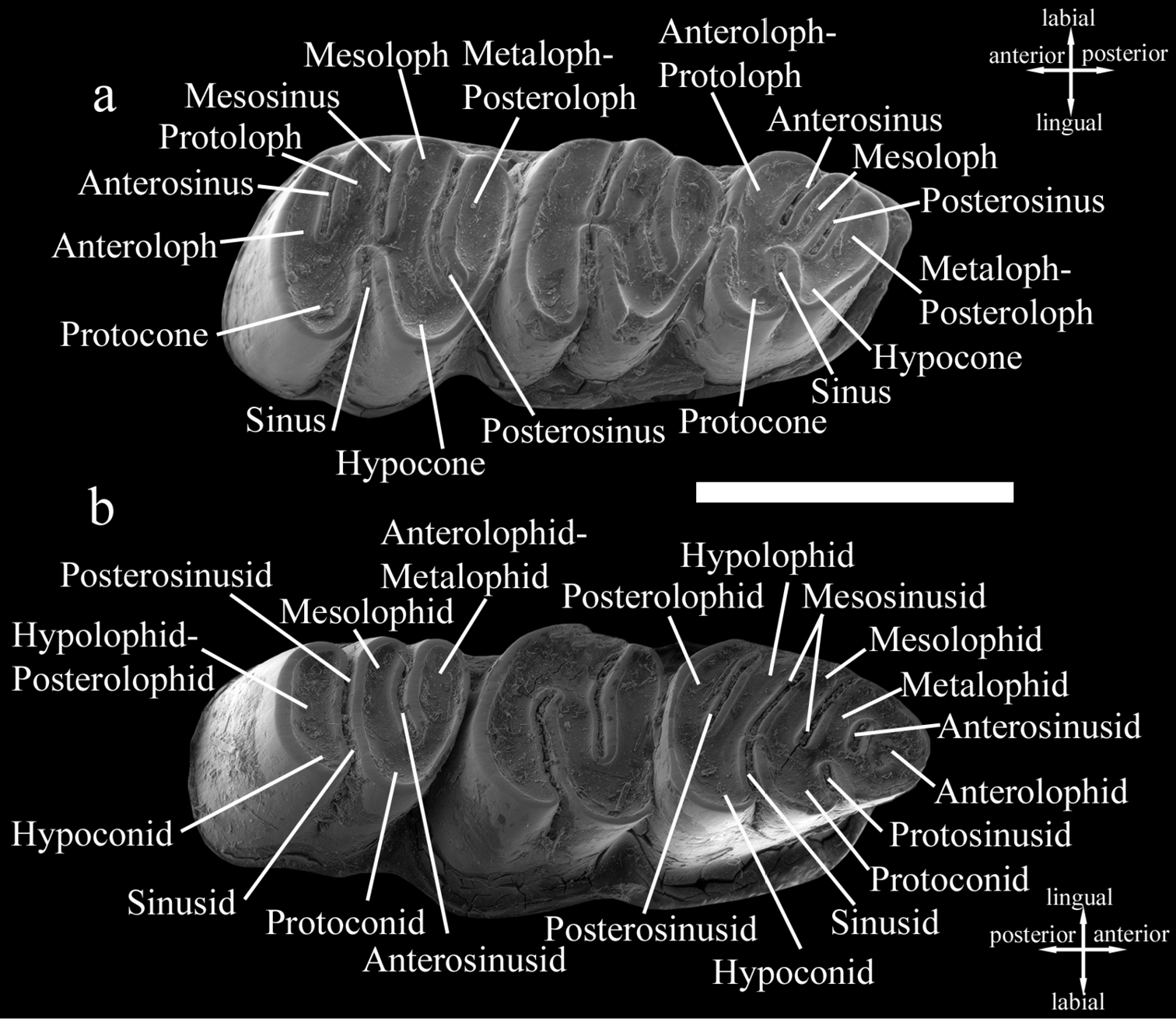
First, second, and third lower molars are designated as m1, m2, and m3, respectively, and first, second, and third upper molars as M1, M2, and M3, respectively. The terminology used in the tooth descriptions follows the rodent dental terminology of
Dental terminology used in this paper. Cannomys badius. a Left upper cheek teeth b Right lower cheek teeth. Scale bar equals 5 mm.
Incisors of Cannomys badius are strongly proodont, flattened anteriorly, lack major ornamentation and have the enamel pigmented orange.
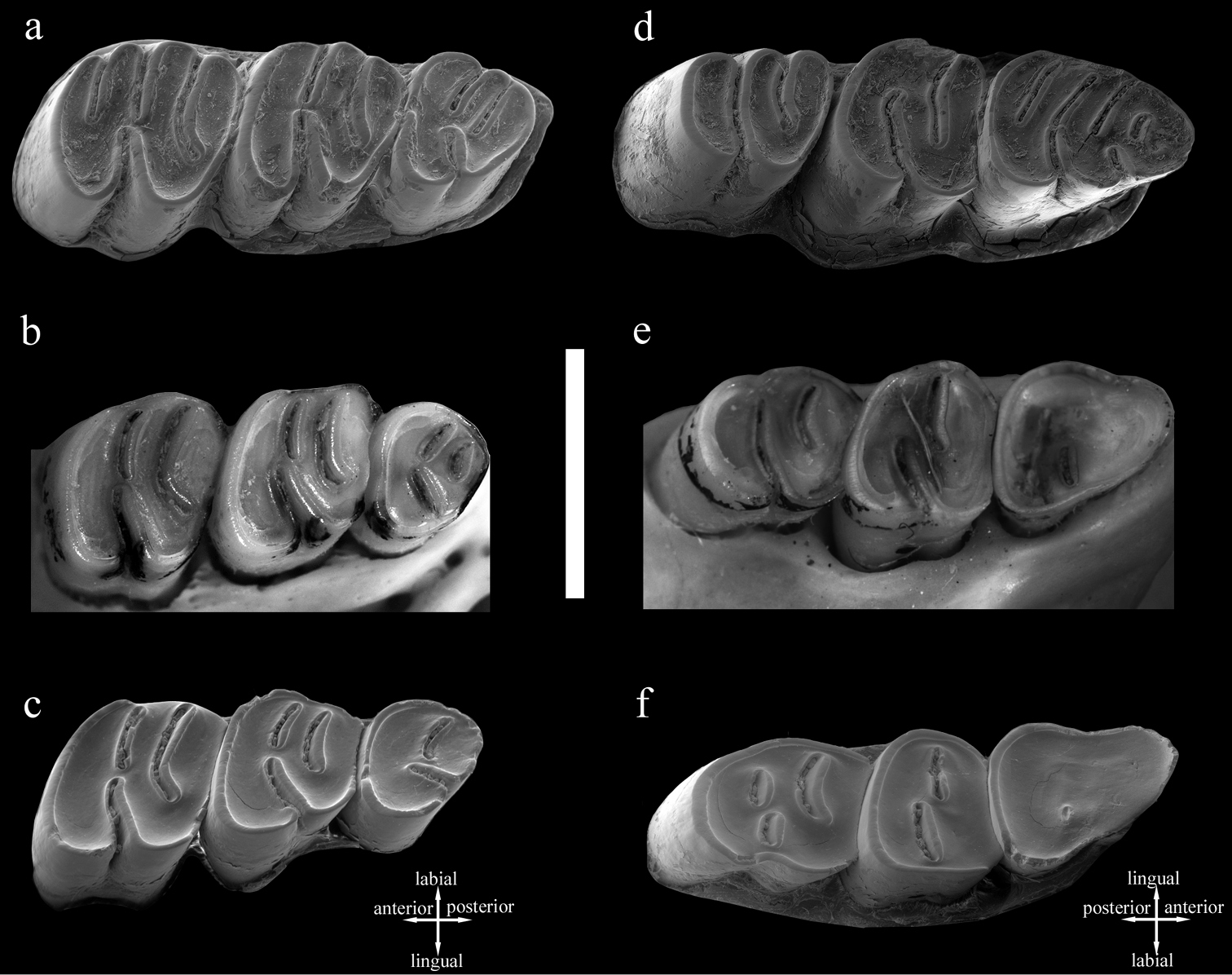
The upper molars of Cannomys badius show unilateral hypsodonty, with crowns higher lingually than labially. m1 has four roots, the anterolabial one being the most developed. Its occlusal outline is square. In early wear (e.g., MNHN C.G. 2000-271; Fig. 2a), it has four transverse lophs (anteroloph, protoloph, mesoloph, and metaloph-posteroloph) and all labial and lingual sinuses are open. In later wear (e.g., ZMB 44769 and MNHN C.G. 2000-761; Fig. 2b–c), the number of lophs is reduced to three as the anteroloph and protoloph combine and join at the margin of the tooth, isolating two transversely elongated enamel lakes, whereas the lingual sinus remains open. The latter, narrow and short, is directed toward the middle enamel lake. The connection between the anterior and posterior parts of the tooth persists through wear.
The occlusal outline of m2is square, with its posterior side more reduced than the anterior one. This tooth is much shorter than m1. In early wear (e.g., MNHN C.G. 1860-382; Fig. 2a), it has three transverse lophs (anteroloph-protoloph, mesoloph, and metaloph-posteroloph). The sinus is directed toward the anterosinus. All reentrants remain open. After moderate wear (e.g., ZMB 44769, Fig. 2b), the sinus becomes narrower and the anterosinus and posterosinus are closed-off, isolating two enamel lakes. Late in wear (e.g., MNHN C.G. 2000-761; Fig. 2c), the morphology of M2 is quite similar to that in the previous wear stage. However, the posterior part of the tooth becomes more reduced and, even though the lingual sinus remains open, it turns out to be more anterolabially directed due to the labial displacement of the hypocone. The connection between the anterior and posterior parts of the tooth persists through wear.
Cannomys has a reduced m3. In early wear (e.g., MNHN C.G. 1860-382; Fig. 2a), it is morphologically similar to m2 but with its posterior part smaller due to the more labial position of the hypocone. This tooth is trilophodont, with anteroloph-protoloph, mesoloph, and metaloph-posteroloph. After moderate wear (e.g., ZMB 44769; Fig. 2b), the anteroloph-protoloph is nearly connected to the mesoloph and the metaloph-posteroloph joins the mesoloph, isolating a labial circular enamel lake. The hypocone is much more labially displaced and the sinus much more anterolabially oriented. Therefore, the posterior part of the tooth becomes much reduced. In late wear (e.g., MNHN C.G. 2000-761; Fig. 2c), the first and second lophs combine and the anterior enamel lake disappears. At this stage of wear only a labial enamel lake persists and the hypocone is located on the posterior margin of the tooth.
The lower molars are lower crowned than the upper molars. As for the lower jaw bone, the mandibular foramen is located well caudal to the posterior margin of m3 (a little dorsal to m3, at the level of the tip of the coronoid process), whereas the mental foramen is situated rostrally to the anterior border of m1 (approximately on the midline of the dentary).
The occlusal outline of m1 is triangular, with its anterior part much narrower than its posterior. In early wear (e.g., MNHN C.G. 1860-382; Fig. 2d), it shows a pentalophodont dental pattern with anterolophid, metalophid, mesolophid, hypolophid, and posterolophid. The metalophid joins lingually the anterolophid and labially the protoconid, isolating a small and oval anterior enamel lake. The mesolophid is a long continuation of the protoconid. The anterior part of the tooth is isolated from the rest of the crown by a long sinusid. The latter results from the junction of the sinusid (or labial reentrant) with the mesosinusid (or middle labial reentrant). All lophids join the lingual margin of the tooth, isolating four transversely elongated enamel lakes. The m1 shows two open labial sinusids (protosinusid and sinusid). After moderate wear (e.g., ZMB 44768; Fig. 2e), all reentrants are closed-off, the protosinusid disappears, and the anterior part of the tooth is deprived of any enamel lake. However, two enamel lakes persist posteriorly. In late wear (e.g., MNHN C.G. 2000-761; Fig. 2f), the occlusal surface is completely flat and generally devoid of enamel lakes although a tiny and circular posterolabially located enamel lake may persist. The enamel has disappeared from the anterior border of the tooth, but it persists posteriorly.
The m2 has four roots, the posterior ones being the most developed. Its occlusal outline is square and it is anteroposteriorly compressed. In early wear (e.g., MNHN C.G. 1860-382, Fig. 2d); this tooth has three lophs (anterolophid-metalophid, mesolophid, and hypolophid-posterolophid). The mesolophid is short and joins labially the anterior lophid (through the protoconid) and lingually the posterior one. The sole lingual reentrant is closed-off, isolating a labial enamel lake, which is elongated. The narrow and posterolingually directed sinusid is open. After moderate wear (e.g., ZMB 44768, Fig. 2e); the morphology of the tooth is similar to that of the preceding stage. In late wear (e.g., MNHN C.G. 2000-761; Fig. 2f), the sinusid is closed off and the tooth shows both a labial and a lingual enamel lake. The anterior side of the tooth loses the enamel.
In early wear (e.g., MNHN C.G. 1860-382; Fig. 2d), m3 has three lophids (anterolophid-metalophid, mesolophid, and hypolophid-posterolophid). The first lophid joins the second one through the protoconid. The posterior lophid is isolated from the rest of the crown by a long reentrant. At this stage of wear, all reentrants are open. After moderate wear (e.g., ZMB 44768, Fig. 2e), the lingual reentrants are closed-off. The tooth has an elongate anterior enamel lake and a long sinusid. In late wear (e.g., MNHN C.G. 2000-761; Fig. 2f), the lingual reentrant is closed-off, isolating two enamel lakes, and the anterior enamel lake persists.
Dental wear pattern in Cannomys badius. a–c Upper molars: a Stage of wear 1, juvenile individual, left maxilla with M1-M3 in occlusal view (MNHN C.G. 1860-382) b Stage of wear 2, left maxilla with M1-M3 (ZMB 44769) c Stage of wear 3, left maxilla with M1-M3 in occlusal view (MNHN C.G. 2000-761). d–f Lower molars: d Stage of wear 1, juvenile individual, right hemimandible with m1-m3 in occlusal view (MNHN C.G. 1860-382), e Stage of wear 2, right hemimandible with m1-m3 in occlusal (ZMB 44768) f Stage of wear 3, left hemimandible with m1-m3 in occlusal (reversed) (MNHN C.G. 2000-761). Scale bar equals 5 mm.
The examination of the cheek teeth in various specimens of Cannomys badius has allowed determining the changes undergone by the dental pattern during wear. Three fundamental stages of wear have been recognized.
As postulated by
I sincerely thank C. Denys (Muséum national d’Histoire naturelle, Paris) and F. Mayer and N. Lange (Museum für Naturkunde, Berlin) for making available the rhizomyine material under their care. P. Jenkins (Natural History Museum, London) kindly answered all my inquiries. L. J. Flynn (Harvard University, Cambridge MA, USA), K. P. Aplin and K. M. Helgen (Smithsonian Institution, Washington DC, USA) helped to improve various aspects of this paper. M. Furió, A. García and L. Tormo (Museo nacional de Ciencias naturales-CSIC, Madrid) kindly took the SEMs.
My sojourns in Berlin and Paris were funded by the SYNTHESYS Project (http://www.synthesys.info/ ), which is financed by the European Community Research Infrastructure Action under the FP7 “Capacities” Program, and by the EDIT Gender Action Plan, respectively. I am currently supported by the Ramón y Cajal Program and the research project CGL2011-24829.