(C) 2014 Giovanni Amori. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Amori G, Aloise G, Luiselli L (2014) Modern analyses on an historical data set: skull morphology of Italian red squirrel populations. ZooKeys 368: 79–89. doi: 10.3897/zookeys.368.4691
Recent molecular evidence suggests that Sciurus vulgaris populations from Calabria (southern Italy) are distinct from those occurring in northern and central Italy. Here, we re-analyzed using multivariate and univariate techniques an historical dataset provided by Cavazza (1913), who documented measurements for the now extinct squirrel population from Campania. Both univariate and multivariate analyses confirmed that the sample from Calabria was homogenous and relatively distinct compared to the rest of the squirrel samples.
Morphometrics, red squirrel, Italy, historical dataset
The Eurasian red squirrel, Sciurus vulgaris Linnaeus, 1758, is characterized by great variability in fur coloration, which led to the description of more than 40 subspecies throughout its wide geographic distribution across the Eurasian continent (
- Sciurus vulgaris fuscoater Altum, 1876 (European form occurring in the Alps and in the northern Apennines), characterized by relatively small size and a strong degree of coat-colour polymorphism both within and between populations;
- Sciurus vulgaris italicus Bonaparte, 1838 (endemic to Central Italy), also characterized by relatively small size, albeit bigger than the previous subspecies. This subspecies shows some degree of coat colour polymorphism, with the dark brown morph dominant in mountainous forests at higher altitudes. The populations of the southern tip of the range are black (subspecies Sciurus vulgaris alpinus, sensu
Costa 1839 ); - Sciurus vulgaris meridionalis Lucifero, 1907 (endemic to the most southern Apennines), with uniform fur colour, always having black dorsal fur with grey shades on the sides, a black tail, and a contrasting white belly. It is also the largest Italian subspecies (
Wauters and Martinoli 2008 ).
Although widespread in Italy, this species’ distribution is associated with forested areas, and affected by their fragmentation (
Recent molecular data (
In this paper, we reanalyzed Cavazza’s original dataset using modern statistical multivariate analyses with the aim to evaluate whether morphometric and genetic data agree with respect to patterns of geographic differentiation in Italian squirrel populations.
We used the data reported in
Map of Italy showing the localities where squirrels were collected according to
Skull measurements (in mm). OTU = Operational Taxonomic Unit; A = Alps; B = North and Central Italy; C = Abruzzo and Campania; D = Calabria (from
| ID | OTU | Skull length | Skull width | Skull height | Mandible length | Interorbital width | Locality | sex |
|---|---|---|---|---|---|---|---|---|
| 17Alpf | A | 50.1 | 28.5 | 19.6 | 27.3 | 18.3 | AlpiCentrali | f |
| 8Apf | A | 51.9 | 29.6 | 23.4 | 28.0 | 19.2 | Lanzo | f |
| 7Alpm | A | 49.7 | 28.3 | 21.0 | 28.2 | 18.8 | Lanzo | m |
| 12Aplf | A | 51.8 | 30.0 | 21.0 | 28.2 | 19.0 | Porlezza | f |
| 7Alpf | A | 51.3 | 29.0 | 23.3 | 28.8 | 18.9 | Lanzo | f |
| 3Alpf | A | 52.0 | 29.0 | 22.0 | 28.9 | 20.0 | AlpiPiem. | f |
| 9Alpf | A | 51.2 | 29.1 | 21.3 | 28.9 | 18.4 | Porlezza | f |
| 5Alpm | A | 49.6 | 29.0 | 20.0 | 29.0 | 19.2 | Biellese | m |
| 13Alpm | A | 51.5 | 31.2 | 21.6 | 29.0 | 20.0 | Porlezza | m |
| 2Alpf | A | 52.6 | 29.6 | 22.0 | 29.0 | 20.0 | AlpiPiem. | f |
| 4Alpf | A | 51.8 | 29.0 | 21.0 | 29.0 | 19.8 | Biellese | f |
| 15Alpf | A | 52.6 | 30.6 | 19.9 | 29.0 | 20.0 | SopraLugano | f |
| 18Alpf | A | 51.7 | 29.8 | 21.0 | 29.0 | 18.2 | AlpiCentrali | f |
| 25Alpf | A | 50.1 | 28.7 | 21.0 | 29.0 | 19.0 | Cadore | f |
| 26Alpf | A | 51.2 | 29.6 | 22.0 | 29.0 | 19.4 | Cadore | f |
| 9Alpm | A | 51.8 | 30.6 | 21.0 | 29.1 | 19.5 | Lanzo | m |
| 16Alpf | A | 55.0 | 31.2 | 21.0 | 29.1 | 18.6 | AlpiCentrali | f |
| 1Alpf | A | 53.0 | 30.8 | 22.0 | 29.2 | 21.0 | AlpiPiem. | f |
| 5Alpf | A | 50.8 | 28.9 | 22.6 | 29.2 | 18.7 | Lanzo | f |
| 13Alpf | A | 55.9 | 31.0 | 22.6 | 29.2 | 20.2 | Buggiolo | f |
| 21Alpf | A | 53.0 | 30.0 | 21.2 | 29.2 | 19.0 | AlpiCentrali | f |
| 2Alpm | A | 57.3 | 32.0 | 21.0 | 29.3 | 20.0 | AlpiPiem. | m |
| 10Alpm | A | 52.2 | 31.0 | 21.0 | 29.3 | 20.0 | Lanzo | m |
| 14Alpm | A | 49.9 | 27.8 | 20.8 | 29.3 | 18.3 | Porlezza | m |
| 6Alpf | A | 52.0 | 29.8 | 23.0 | 29.3 | 19.1 | Lanzo | f |
| 14Alpf | A | 52.6 | 30.3 | 21.8 | 29.3 | 21.0 | SopraLugano | f |
| 20Alpf | A | 51.8 | 29.9 | 21.2 | 29.3 | 19.0 | AlpiCentrali | f |
| 19Alpf | A | 52.0 | 30.0 | 21.3 | 29.4 | 19.5 | AlpiCentrali | f |
| 12Alpm | A | 52.0 | 30.2 | 21.6 | 29.5 | 20.0 | Lanzo | m |
| 3Alpm | A | 50.0 | 29.2 | 20.4 | 29.6 | 19.2 | AlpiPiem. | m |
| 6Alpm | A | 52.7 | 30.3 | 22.2 | 29.8 | 19.8 | Lanzo | m |
| 1Alpm | A | 53.0 | 32.2 | 21.2 | 30.0 | 20.7 | AlpiPiem. | m |
| 18Alpm | A | 52.8 | 32.0 | 21.0 | 30.0 | 20.0 | SopraLugano | m |
| 4Alpm | A | 53.0 | 33.0 | 21.3 | 31.0 | 20.0 | Biellese | m |
| 26Alpm | B | 51.5 | 30.3 | 21.0 | 28.9 | 19.3 | Cadore | m |
| 25Alpm | B | 51.6 | 30.8 | 20.8 | 29.0 | 19.0 | Cadore | m |
| 1Lomm | B | 52.2 | 29.4 | 22.6 | 29.0 | 18.8 | Lombardia | m |
| 2Emim | B | 52.6 | 29.0 | 22.2 | 29.0 | 19.4 | Emilia | m |
| 3Emif | B | 50.1 | 29.0 | 21.8 | 29.0 | 18.6 | Emilia | f |
| 9Tosf | B | 53.2 | 30.2 | 22.1 | 29.0 | 18.8 | Toscana | f |
| 10Tosf | B | 52.8 | 29.7 | 22.9 | 29.0 | 18.0 | Toscana | f |
| 4Emif | B | 51.1 | 29.3 | 22.0 | 29.1 | 18.9 | Emilia | f |
| 5Emif | B | 52.0 | 29.0 | 22.0 | 29.1 | 18.7 | Emilia | f |
| 11Tosm | B | 52.0 | 30.0 | 22.0 | 29.2 | 18.6 | Toscana | m |
| 1Ligf | B | 51.2 | 28.3 | 22.0 | 29.2 | 18.3 | Liguria | f |
| 6Emif | B | 52.2 | 29.1 | 22.3 | 29.3 | 18.6 | Emilia | f |
| 7Emim | B | 52.7 | 30.1 | 22.3 | 29.5 | 19.0 | Emilia | m |
| 3Emim | B | 53.7 | 29.6 | 23.0 | 29.6 | 19.9 | Emilia | m |
| 8Emim | B | 52.7 | 30.2 | 22.2 | 29.6 | 18.9 | Emilia | m |
| 10Tosm | B | 53.2 | 30.1 | 22.0 | 29.6 | 19.0 | Toscana | m |
| 9mim | B | 52.7 | 30.3 | 22.2 | 29.8 | 19.0 | Emilia | m |
| 1Alpm | B | 52.9 | 30.1 | 21.3 | 30.0 | 19.8 | AlpiCentrali | m |
| 20Alpm | B | 52.3 | 30.1 | 20.6 | 30.0 | 18.9 | AlpiCentrali | m |
| 22Alpm | B | 50.0 | 31.0 | 22.0 | 30.0 | 18.8 | AlpiCentrali | m |
| 6Emim | B | 53.5 | 34.3 | 22.1 | 30.0 | 18.0 | Emilia | m |
| 12Tosf | B | 53.0 | 30.8 | 22.9 | 30.0 | 18.3 | Toscana | f |
| 13Tosf | B | 52.3 | 30.3 | 22.8 | 30.0 | 18.7 | Toscana | f |
| 18Tosm | B | 52.2 | 31.0 | 22.3 | 30.2 | 18.7 | Toscana | m |
| 21Alpm | B | 53.1 | 32.0 | 21.2 | 30.3 | 19.7 | AlpiCentrali | m |
| 17Tosm | B | 53.0 | 32.0 | 22.0 | 30.3 | 18.0 | Toscana | m |
| 13Tosm | B | 52.0 | 30.0 | 20.8 | 30.6 | 18.2 | Toscana | m |
| 11Tosf | B | 52.0 | 31.5 | 23.0 | 31.0 | 18.7 | Toscana | f |
| 12Tosm | B | 55.0 | 31.9 | 21.0 | 31.2 | 19.0 | Toscana | m |
| 3Napf | C | 52.3 | 29.7 | 24.1 | 28.9 | 18.6 | Napoletano | f |
| 4Napf | C | 54.6 | 29.9 | 25.0 | 29.0 | 19.0 | Napoletano | f |
| 3Napm | C | 52.8 | 28.9 | 22.9 | 29.2 | 19.0 | Napoletano | m |
| 2Napf | C | 54.3 | 29.8 | 24.9 | 29.4 | 18.9 | Napoletano | f |
| 4Napm | C | 55.0 | 29.8 | 22.8 | 29.5 | 19.6 | Napoletano | m |
| 2Napm | C | 55.2 | 31.3 | 24.0 | 30.0 | 20.0 | Napoletano | m |
| 2Calf | D | 56.3 | 33.6 | 22.7 | 31.8 | 19.1 | Calabria | f |
| 3Calm | D | 56.0 | 33.9 | 22.4 | 32.2 | 19.0 | Calabria | f |
| 1Calm | D | 56.0 | 33.5 | 22.6 | 33.9 | 20.7 | Calabria | f |
| 1Calf | D | 57.2 | 33.4 | 22.8 | 33.9 | 19.2 | Calabria | f |
| 2Calm | D | 54.5 | 32.9 | 22.3 | 34.1 | 20.2 | Calabria | f |
Univariate measurements were log-transformed in order to achieve normality and then compared across groups by one-way Analysis of Variance (ANOVA). In this analysis, the same four groups as defined by
Specimens were divided into four Operational Taxonomic Units (hereby OTUs), according to their geographical provenance and corresponding to the Italian subspecies. These four OTUs followed exactly the subdivisions made by
We studied the dispersion of specimens in multivariate space with Principal Components Analysis (PCA) using the covariance matrix (
The original dataset reported by
Mean and dispersion measures of the five skull variables analyzed in this study (original dataset from
| Mean (S.D.) | Range | |
|---|---|---|
| Skull length | 52.61 (1.70) | 49.6–57.3 |
| Skull width | 30.37 (1.41) | 27.8–34.3 |
| Skull height | 21.92 (1.04) | 19.6–25.0 |
| Mandible length | 29.63 (1.18) | 27.3–34.1 |
| Interorbital length | 19.21 (0.70) | 18.0–21.0 |
Mean and dispersion measures of the five skull variables analyzed in this study (original dataset from
| Mean | SD | |
|---|---|---|
| A (n = 34) | ||
| Skull length | 52.05 | 1.64 |
| Skull width | 30.03 | 1.19 |
| Skull height | 21.42 | 0.88 |
| Mandible length | 29.12 | 0.62 |
| Interorbital length | 19.46 | 0.74 |
| B (n = 29) | ||
| Skull length | 52.37 | 1.02 |
| Skull width | 30.32 | 1.20 |
| Skull height | 21.97 | 0.68 |
| Mandible length | 29.67 | 0.63 |
| Interorbital length | 18.22 | 0.49 |
| C (n = 6) | ||
| Skull length | 54.03 | 1.20 |
| Skull width | 29.90 | 0.77 |
| Skull height | 23.95 | 0.94 |
| Mandible length | 29.33 | 0.39 |
| Interorbital length | 19.18 | 0.51 |
| D (n = 5) | ||
| Skull length | 56.00 | 0.97 |
| Skull width | 33.46 | 0.36 |
| Skull height | 22.56 | 0.20 |
| Mandible length | 33.18 | 1.08 |
| Interorbital length | 19.64 | 0.76 |
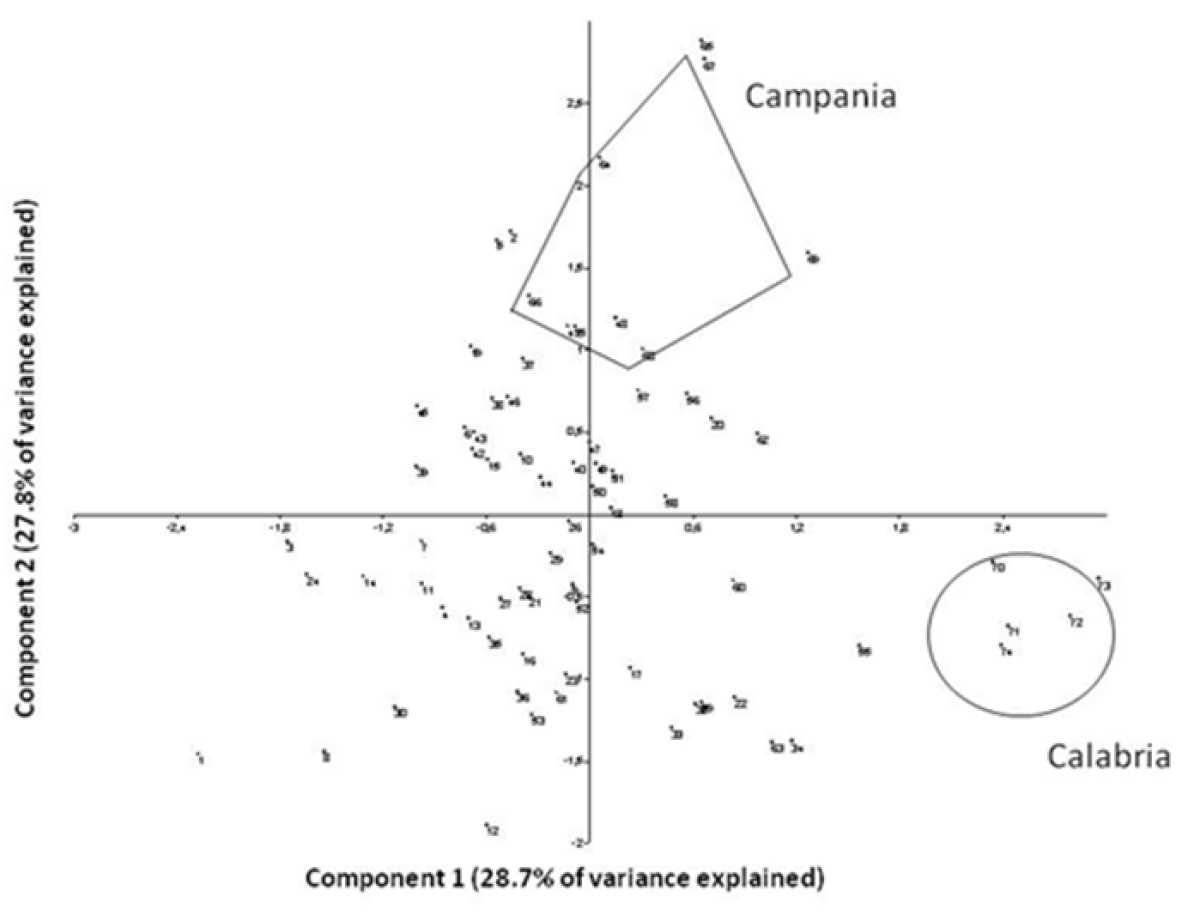
Both sets of multivariate analyses revealed that the sample from Calabria was homogenous and relatively distinct compared to the rest of the squirrel samples (Figures 2 and 3). In the PCA (variance explained by the first two axes: 56.5%; with axis 1 explaining 28.7% and axis 2 explaining 27.8% of the total variance; see Table 4 for the loadings) there was a trend suggesting clinal variation from the Alps to Campania, with Calabria specimens, while distinct, being more similar to those of Campania than to those of northern Italy (Figure 2). The Campania group showed less variance (Levene’s test; F = 6.67, P < 0.03) compared to the rest of the central and northern Italian samples in the PCA than in the neighbor joining analysis (Figure 3).
PCA of skull measurements (VARIMAX rotation applied) based on
Neighbor joining dendrogram of skull measurements (with 10, 000 bootstraps) based on
Loadings of the PCA as in Figure 2.
| Component 1 | Component 2 | |
|---|---|---|
| Skull length | 0.876 | 0.154 |
| Skull width | 0.882 | -0.159 |
| Skull height | 0.341 | 0.836 |
| Mandible length | 0.842 | -0.034 |
| Interorbital length | 0.432 | -0.684 |
Both multivariate and univariate tests identified some morphometric differentiation among different squirrel populations that were previously highlighted by the molecular results of
On the other hand, Calabria specimens do appear to be quite distinct from the rest of the Italian squirrels in size (Figure 2), though we note that our analyses involve quite small sample sizes (
Our approach in this paper highlights the lasting value of historical publications on biodiversity, especially when they present data on populations which are now extinct. These often overlooked publications – such as Cavazza’s, published in Italian in a regional journal – can be important sources of data that can be re-analysed, for renewed insight, using modern statistical tools.
This paper was edited by Russell L. Burke (Hofstra University, New York). We thank A. Cardini and an anonymous referee for very helpful comments on the submitted draft.