(C) 2013 Carole C. Baldwin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A second species of the blenniiform genus Haptoclinus is described from deep reefs off Curaçao, southern Caribbean. Haptoclinus dropi sp. n. differs from the northwestern Caribbean Haptoclinus apectolophus Böhlke and Robins, 1974, in having 29 total dorsal-fin elements—III-I-XIII, 12 (vs. 31—III-I-XIV, 13 or III-I-XIII, 14); 19 anal-fin soft rays (vs. 20-21); 12 pectoral-fin rays (vs. 13); 12 precaudal vertebrae (vs. 13); and the first dorsal-fin spine longer than the second (vs. the second longer than the first). It further differs from Haptoclinus apectolophus in lacking scales (vs. three-quarters of body densely scaled), in having a distinctive pattern of spotting on the trunk and fins in preservative (vs. no spotting), and in lacking a fleshy flap on the anterior rim of the posterior nostril (vs. flap present). Color in life is unknown for Haptoclinus apectolophus, and the color description presented for the new species constitutes the first color information for the genus. Familial placement of Haptoclinus remains questionable, but the limited relevant information obtained from morphological examination of the new species provides additional support for a close relationship with the Chaenopsidae. Haptoclinus dropi represents one of numerous new teleost species emerging from sampling to 300 m off Curaçao as part of the Smithsonian Institution’s Deep Reef Observation Project (DROP).
Blenniiformes, submersible, Substation Curaçao, Haptoclinus apectolophus, Deep Reef Observation Project (DROP)
Diving to 300 m off Curaçao in the southern Caribbean using Substation Curaçao’s (http://www.substation-Curacao.com) manned submersible Curasub is expanding our knowledge of the deep-reef Caribbean fish fauna. Targeted fish specimens are collected with the sub’s two flexible, hydraulic arms, one of which is equipped with a quinaldine-ejection system and the other with a suction hose. Occasionally, small, inconspicuous, non-targeted fishes are collected along with the target specimens. One bycatch specimen collected between 157 and 167 m represents a new species and the second known species referable to the blenniiform genus Haptoclinus Böhlke and Robins, 1974. Haptoclinus apectolophus Böhlke and Robins, 1974 was described based on two specimens that were trawled from depths of 174−366 m at Arrowsmith Bank in the northwestern Caribbean. The new species is similar to Haptoclinus apectolophus in having the dorsal fin consisting of four parts (three spinous, one soft) and represents a southern range extension for the genus of 9° latitude and an eastern range extension of 17° longitude. In this paper we describe the new species, compare it with Haptoclinus apectolophus, and comment on the familial placement of Haptoclinus.
The specimen was collected by submersible using the fish anesthetic quinaldine pumped from a reservoir through a tube attached to one hydraulic arm and a suction hose (that uses the same pump as the anesthetic-delivery apparatus) attached to the other arm. The latter empties into a vented plexiglass cylinder attached to the outside of the sub. At the surface, the fish was measured, photographed, tissue sampled (right eye removed), and preserved. It was later photographed to document preserved pigment pattern and x-rayed with a digital radiography system. Counts and measurements included in the description are those described for Haptoclinus apectolophus by
urn:lsid:zoobank.org:act:3091C7AF-C686-4317-AD7E-43D8C8D86357
http://species-id.net/wiki/Haptoclinus_dropi
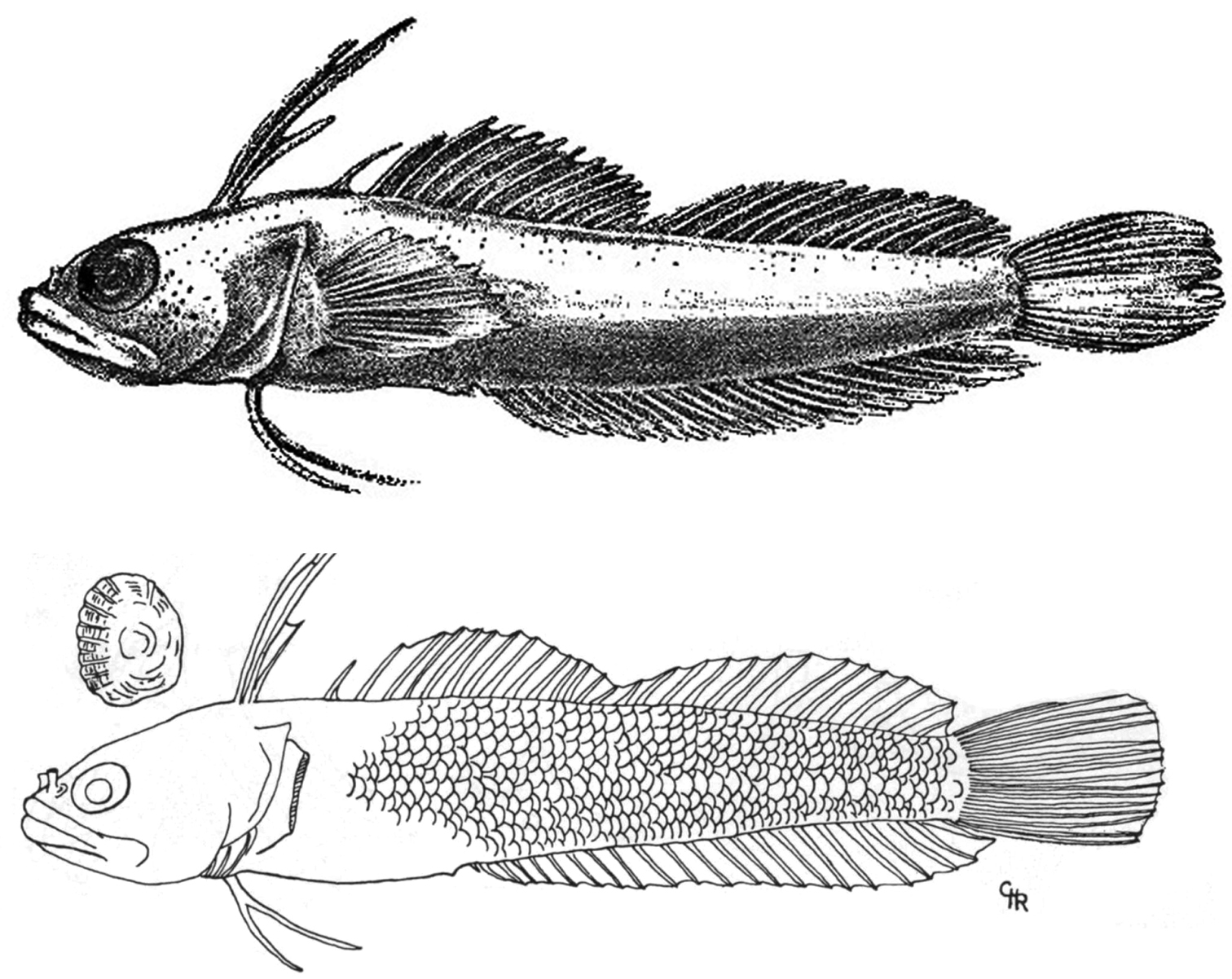
Figs 1–2 Four-fin blennyCuraçao, southern Caribbean
USNM 414915, 21.5 mm SL, female, Curasub submersible, sta. 12-7, southern Caribbean, Curaçao, east of downline off Substation Curaçao dock, near 12°05.069'N, 68°53.886'W, 157−167 m, quinaldine, 13 Aug 2012, D. R. Robertson, A. Schrier, B. Brandt, C. Castillo.
A species of Haptoclinus distinguished from its congener by the following combination of characters: dorsal-fin elements III-I-XIII, 12; anal-fin soft rays 19; pectoral-fin rays 12; precaudal vertebrae 12; first dorsal-fin spine longer than second dorsal-fin spine; scales absent; posterior nostril without fleshy flap; and trunk, dorsal- and anal fins with spotted pigment pattern in preservative.
Dorsal-fin elements: III-I-XIII, 12; anal-fin elements II, 19; ultimate pterygiophore of dorsal and anal fins supporting a single segmented soft ray. Pectoral-fin rays 12, 12. Pelvic-fin rays I, 3. Segmented caudal-fin rays 7+6, procurrent caudal-fin rays 6+5. All fin rays unbranched. Vertebrae 12+24=36. Three anal-fin pterygiophores anterior to first haemal spine.
Measurements (in mm): head length 6.6, snout length 1.3, eye diameter 1.0, body depth at fourth dorsal-fin spine 3.5, depth at caudal peduncle 1.0, greatest head width 5.4, body width at anus 2.0, width of bony interorbital 0.4, length of upper jaw 2.6, length of caudal peduncle 2.0, distance from snout to origin of dorsal fin 4.1, distance from snout to upper pectoral-fin base 5.9, distance from snout to insertion of pelvic fin 4.4, distance from snout to origin of anal fin 9.2, length of first dorsal-fin spine 4.1, length of second dorsal-fin spine 3.8, length of third dorsal-fin spine 1.5, length of fourth dorsal-fin spine 0.7, length of longest pectoral-fin ray 4.2, length of pelvic fin 4.1, length of longest caudal-fin ray 3.4.
Body without scales or scale pockets. Small, pointed teeth present in both jaws and on vomer and palatines; teeth uniserial except on anterior portion of premaxilla and dentary, where several teeth form an inner row. Anterior nostril with long tube; posterior nostril a simple opening with minute fleshy rim anteriorly but no fleshy flap; posterior nostril situated closer to eye than to anterior nostril. Mouth terminal and jaws equal. Upper edge of maxilla not sheathed by lacrimal when mouth closed; maxilla reaching vertical through posterior margin of orbit. Gill membranes broadly joined across but free of isthmus. Dorsal margin of upper lip free and continuous across tip of snout. Head lacking cirri. Pores of cephalic lateralis system as drawn for Haptoclinus apectolophus (
Dorsal fin originating on head about half way between verticals through posterior margins of eye and operculum; fin terminating slightly anterior to vertical through base of ultimate anal-fin ray. Small membrane connecting last dorsal- and anal-fin rays to caudal peduncle. First dorsal-fin spine longest, reaching base of seventh spine when depressed. Fourth dorsal-fin spine short, separated by gaps from anterior three and posterior thirteen spines. Low membrane connecting last dorsal-fin spine to first segmented soft ray. First anal-fin spine shorter than second. Membranes of pectoral fin notched; in dorsal portion of fin (dorsal to longest ray), membranes extending from distal tip of one fin ray to distal tip of adjacent fin ray; in ventral portion of fin, membranes extending from distal tip of one fin ray dorsally to point well proximal of distal tip of adjacent fin ray. Ninth pectoral-fin ray (from top of fin, fourth from bottom) longest (broken on left side of holotype), this ray on right side of body reaching posteriorly to vertical through base of third segmented anal-fin ray. Pelvic fin reaching posteriorly to anus when straightened; innermost (third) pelvic soft ray very small, about half length of small (0.5-mm long), pelvic-fin spine. Caudal fin truncate.
Color Prior to Preservation (Fig. 2).—When photographed against a white background (Fig. 2, top), the following visible on the fresh holotype: ground color of body pale grey; side of belly with rectangular-shaped patch of orange-brown pigment with indistinct whitish diagonal bar across center; spinal column with series of eight internal, irregular orange blotches; dorsalmost region of trunk (beneath dorsal fin) and ventralmost region of trunk (above anal fin) each with row of eight to nine orange/brown spots; head grey, densely speckled with fine black melanophores; nape orange-brown, with several yellow blotches; iris orange, grading to yellowish inner ring; yellow bar extending anteroventrally from anteroventral corner of orbit to anterolateral aspect of upper jaw and anterior tip of lower jaw; operculum pale orange, with two yellow-orange spots on lower edge; anterior dorsal finlet (spines I-III) creamy yellow, with four irregular dark brown horizontal cross-bars; second dorsal finlet (spine IV) translucent; remainder of dorsal fin translucent, with two or three irregular rows of round orange-brown spots on both spinous and soft portions; anal fin translucent, with two rows of round orange-brown spots;caudal fin translucent, with row of round, mostly orange spots along dorsal and ventral fin margins and two vertical rows of such spots across posterior third of fin pectoral and pelvic finswithout obvious pigment. When photographed against a black background (Fig. 2, bottom), the following also visible on the fresh holotype: series of long, yellow/white, roughly vertical bars on dorsal fin—one on second finlet (spine IV), three on main portion of spinous dorsal fin, four on soft dorsal fin; bars extending onto dorsal portion of trunk as small white blotches; a series of tiny white spots beneath dorsal-fin base just ventral to white blotches; row of small white spots on trunk just above anal-fin base between dark spots, several extending onto rear of anal fin as short white bars; thin white bar across caudal-fin base; several white spots on outer portion of upper caudal lobe.
Color in Alcohol (Fig. 1).—Trunk pale, central region with midlateral row of four small, rounded blotches of melanophores; additional small blotch present at center of posterior end of caudal peduncle; eight internal blotches of pigment present on dorsal portion of trunk beneath dorsal fin: first blotch beneath origin of main portion of spinous dorsal fin (spine V); last blotch on caudal peduncle; eight similar internal blotches present on ventral portion of trunk above anal fin, posterior markings darker and including a few external melanophores. Head tan, covered entirely with fine melanophores. First dorsal finlet with four dark blotches on membrane between first and second spines; some of this pigment extending onto membrane between second and third spines; second dorsal finlet (spine IV) unpigmented; remainder of fin with two or three rows of small, rounded spots. Anal fin with rounded spots in single row on most of fin, posterior portion of fin with two rows; spots in distal row smaller than those in proximal row. Caudal fin with small pigment blotch on bases of dorsal procurrent rays and another on bases of ventral procurrent rays; remainder of fin mostly pale except with several small, dark markings on dorsal portion of dorsal lobe. Pectoral fin pale, with a few dark spots on membrane between lowermost third and fourth rays. Pelvic fin pale.
Haptoclinus dropi, sp. n., holotype, USNM 414915, 21.5 mm SL, female. Both photographs were taken after the fish was in preservation for several months, the top image against a white background, the bottom against a black background. Photographs by Ian Silver-Gorges.
Haptoclinus dropi, sp. n., holotype, USNM 414915, 21.5 mm SL, female. Both photographs were taken soon after the fish was captured, the top image against a white background, the bottom against a black background.
Haptoclinus apectolophus, holotype, ANSP 121251, 25.2 mm SL, male. Modified from
Known only from Curaçao, southern Caribbean.
The specific name is in reference to the acronym for the Smithsonian Institution’s Deep Reef Observation Project (DROP), which is treated here as a noun in the genitive case. Haptoclinus dropi is the first of numerous new species that will be described from DROP submersible research in the southern Caribbean.
“Four-fin blenny” is in reference to the configuration of the dorsal fin.
For comparative purposes, counts and measurements of the holotypes of Haptoclinus dropi and Haptoclinus apectolophus (Fig. 3) are given in Table 1, along with distinguishing features of general morphology. Haptoclinus dropi has two fewer dorsal-fin elements than Haptoclinus apectolophus (29 vs. 31), the differences occurring in the third spinous dorsal finlet and soft dorsal fin (III-I-XIII, 12 in Haptoclinus dropi, III-I-XIII, 14 or III-I-XIV, 13 in Haptoclinus apectolophus). Haptoclinus dropi also has one or two fewer soft anal-fin rays (19 vs. 20-21), one fewer pectoral-fin ray (12 vs. 13), and one fewer precaudal vertebra (12 vs. 13). The shape of the first dorsal finlet is different in the two species because of differences in relative sizes of the first three dorsal-fin spines: the first dorsal-fin spine is the longest of the three elements in Haptoclinus dropi (length of first three dorsal spines 18, 7 and 3% SL, respectively); the second dorsal spine is longest in Haptoclinus apectolophus (length of first three dorsal-fin spines 22, 26, and 12% SL, respectively).
Haptoclinus dropi and Haptoclinus apectolophus have very different preserved pigment patterns. In Haptoclinus dropi, the trunk is uniformly pale with a row of external blotches along the lateral midline, a row of mostly internal blotches just beneath the dorsal fin, and a row of mostly internal blotches just above the anal fin. In Haptoclinus apectolophus, there is much more pigment on the ventral portion of the body than there is dorsally, and there are no obvious internal or external blotches of pigment. In Haptoclinus dropi, the first dorsal finlet has four dark blotches, the fourth dorsal-fin spine is unpigmented, and the remainder of the fin is pale with two or three rows of small dark spots. In Haptoclinus apectolophus, the first dorsal finlet is uniformly dark and both the spinous and soft portions of the dorsal fin are peppered with fine melanophores in no apparent pattern. The anal fin is uniformly pale with one or two rows of small dark spots in Haptoclinus dropi. In Haptoclinus apectolophus, the basal three-quarters of that fin are heavily and uniformly pigmented, and the distal quarter is pale. The caudal fin has dark spots dorsally in Haptoclinus dropi, and the pectoral fin has a few dark spots ventrally. In Haptoclinus apectolophus, the caudal and pectoral fins lack melanophores. Haptoclinus dropi differs from Haptoclinus apectolophus in other minor ways: Haptoclinus dropi lacks a fleshy flap on the posterior nostril (vs. fleshly flap extending from anterior margin and covering anterior half of nostril) and has a more slender body (body depth 16.3% SL at the fourth dorsal spine vs. 17.9% SL, depth at caudal peduncle 6.6% SL vs. 9.1% SL).
Counts and measurements of the holotypes of Haptoclinus dropi, sp. n., and Haptoclinus apectolophus and distinguishing characters of general morphology. Data for Haptoclinus apectolophus are from
| Haptoclinus dropi | Haptoclinus apectolophus | |
|---|---|---|
| Catalog number | USNM 414915, Holotype | ANSP 121251, Holotype |
| SL | 21.5 | 25.2 |
| Dorsal-fin elements | III-I-XIII, 12 | III-I-XIV, 13 |
| Total dorsal-fin elements | 29 | 31 |
| Anal-fin elements | II, 19 | II, 20 |
| Pectoral-fin rays | 12/12 | 13/13 |
| Pelvic-fin rays | I, 3 | I, 3 |
| Segmented caudal-fin rays | 7 + 6 | 7 + 6 |
| Procurrent caudal-fin rays | 6 + 5 | 6 + 5 |
| Vertebrae | 12 + 24 | 13 + 24 |
| Head Length | 30.7 | 29.8 |
| Snout Length | 6.0 | 6.7 |
| Eye Diameter | 6.6 | 7.9 |
| Body depth at 4th dorsal spine | 16.3 | 17.9 |
| Depth at caudal peduncle | 6.6 | 9.1 |
| Greatest head width | 25.1 | 15.9 |
| Body width at anus | 9.3 | 10.3 |
| Bony interorbital width | 1.9 | 2.3 |
| Upper jaw length | 12.1 | 15.1 |
| Caudal-peduncle length | 9.3 | 8.7 |
| Snout to origin of dorsal fin | 19.1 | 21.4 |
| Snout to upper pectoral-fin base | 27.4 | 29.8 |
| Snout to insertion of pelvic fin | 20.5 | 24.2 |
| Snout to origin of anal fin | 42.8 | 46.8 |
| First dorsal-fin spine length | 19.1 | 22.2 |
| Second dorsal-fin spine length | 17.7 | 26.2 |
| Third dorsal-fin spine length | 7.0 | 11.9 |
| Fourth dorsal-fin spine length | 3.3 | 8.3 |
| Longest pectoral-fin ray | 19.5 | 23.0 |
| Pelvic-fin length | 19.1 | 21.4 |
| Longest caudal-fin ray | 21.5 | 20.2 |
| Body squamation | None | Posterior 3/4 scaled |
| Spotted pigment pattern | Present | Absent |
| Posterior nostril | No fleshy flap | Fleshy flap anteriorly |
The configuration of the dorsal fin in Haptoclinus, in which the anterior spinous finlet is separated from the main spinous portion by a gap that contains a single isolated spine (the fourth)—thus resulting in a dorsal fin that consists of four parts, is unique among blenniiforms (
Familial placement of Haptoclinus is uncertain.
The Chaenopsidae were not defined phylogenetically until
Morphological examination of Haptoclinus dropi enables us to add
Haptoclinus apectolophus, holotype, ANSP 121251, 25.2 mm SL, male; paratype, ANSP 121252, cleared and stained (disarticulated and in poor condition). Radiograph of holotype examined on the ANSP website: http://clade.ansp.org/ichthyology/FTIP/view.php?mode=details&id=121251.
We thank Adriaan (Dutch) Schrier, Cristina Castillo, Bruce Brandt, Barbara van Bebber, Laureen Schenk, Lee Weigt, and Amy Driskell for assistance in the field; Sandra Raredon and Ian Silver-Gorges for providing the radiograph and images of the preserved holotype; and Mark Sabaj Pérez for sending the type material of Haptoclinus apectolophus on loan to the first author. Funding for the Smithsonian Institution’s Deep Reef Observation Project is provided internally by the Consortium for Understanding and Sustaining a Biodiverse Planet and by National Geographic Society’s Committee for Research and Exploration (Grant #9102-12). Victor Springer, Philip Hastings, and Jeffrey Williams provided helpful comments on drafts of this manuscript.