(C) 2014 Oliver Schweiger. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Schweiger O, Harpke A, Wiemers M, Settele J (2014) CLIMBER: Climatic niche characteristics of the butterflies in Europe. ZooKeys 367: 65–84. doi: 10.3897/zookeys.367.6185 Resource ID: GBIF key: http://www.gbif.org/dataset/e2bcea8c-dfea-475e-a4ae-af282b4ea1c5
Resource Citation: Helmholtz Centre for Environmental Research - UFZ (2013). CLIMBER: Climatic niche characteristics of the butterflies in Europe. 397 records, Online at http://ipt.pensoft.net/ipt/resource.do?r=climber, version 1.3 (released on 3/12/2013), Resource ID: GBIF key: http://www.gbif.org/dataset/e2bcea8c-dfea-475e-a4ae-af282b4ea1c5, Data Paper ID: doi: 10.3897/zookeys.367.6185
Detailed information on species’ ecological niche characteristics that can be related to declines and extinctions is indispensable for a better understanding of the relationship between the occurrence and performance of wild species and their environment and, moreover, for an improved assessment of the impacts of global change. Knowledge on species characteristics such as habitat requirements is already available in the ecological literature for butterflies, but information about their climatic requirements is still lacking. Here we present a unique dataset on the climatic niche characteristics of 397 European butterflies representing 91% of the European species (see Appendix). These characteristics were obtained by combining detailed information on butterfly distributions in Europe (which also led to the ‘Distribution Atlas of Butterflies in Europe’) and the corresponding climatic conditions. The presented dataset comprises information for the position and breadth of the following climatic niche characteristics: mean annual temperature, range in annual temperature, growing degree days, annual precipitation sum, range in annual precipitation and soil water content. The climatic niche position is indicated by the median and mean value for each climate variable across a species’ range, accompanied by the 95% confidence interval for the mean and the number of grid cells used for calculations. Climatic niche breadth is indicated by the standard deviation and the minimum and maximum values for each climatic variable across a species’ range. Database compilation was based on high quality standards and the data are ready to use for a broad range of applications.
It is already evident that the information provided in this dataset is of great relevance for basic and applied ecology. Based on the species temperature index (STI, i.e. the mean temperature value per species), the community temperature index (CTI, i.e. the average STI value across the species in a community) was recently adopted as an indicator of climate change impact on biodiversity by the pan-European framework supporting the Convention on Biological Diversity (Streamlining European Biodiversity Indicators 2010) and has already been used in several scientific publications. The application potential of this database ranges from theoretical aspects such as assessments of past niche evolution or analyses of trait interdependencies to the very applied aspects of measuring, monitoring and projecting historical, ongoing and potential future responses to climate change using butterflies as an indicator.
Climate change, climate warming, CTI, global change, global warming, modelling, risk, trend, STI, Europe, butterflies, Lepidoptera, Papilionidae, Pieridae, Lycaenidae, Riodinidae, Nymphalidae, Hesperiidae
Global change seriously threatens biodiversity at all organisational levels ranging from genetic diversity, performance and occurrence of single species, taxonomic, phylogenetic and functional diversity of communities and species assemblages to properties of whole ecosystems including the provision of ecosystem services for human well-being (
One of the most important ecological characteristics to assess how species react to climate change obviously is the climatic niche. While knowledge on particular species characteristics such as habitat requirements is already available for some species groups, crucial publicly available information about climatic requirements is still lacking for the majority of the species. Here we present a unique dataset on climatic niche characteristics of 397 (91%) butterfly species in Europe, which have been shown to be particularly sensitive to changing climates (
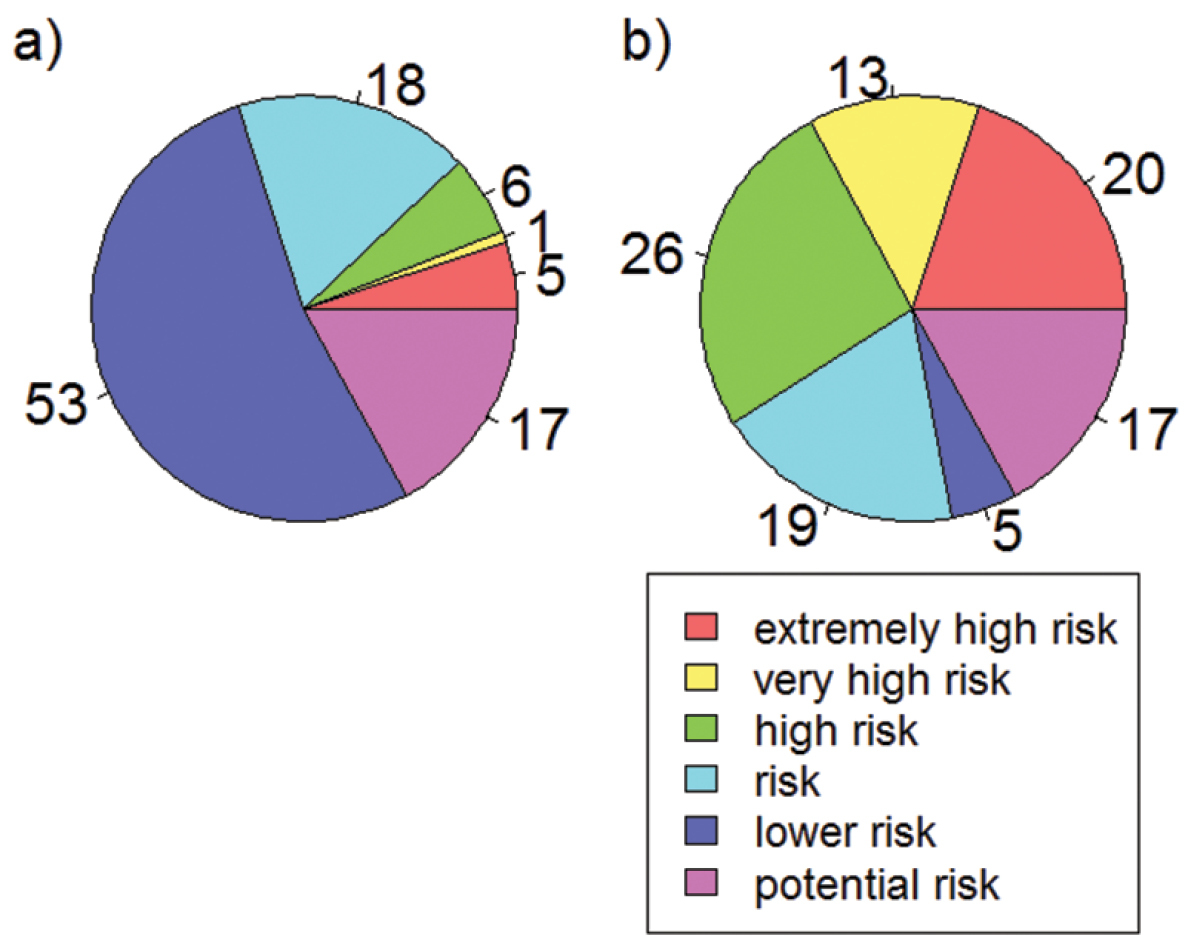
Proportion of species (%) with different climatic risk status after
Based on detailed data on the distribution of European butterflies, which also led to the ‘Distribution Atlas of European Butterflies’ (
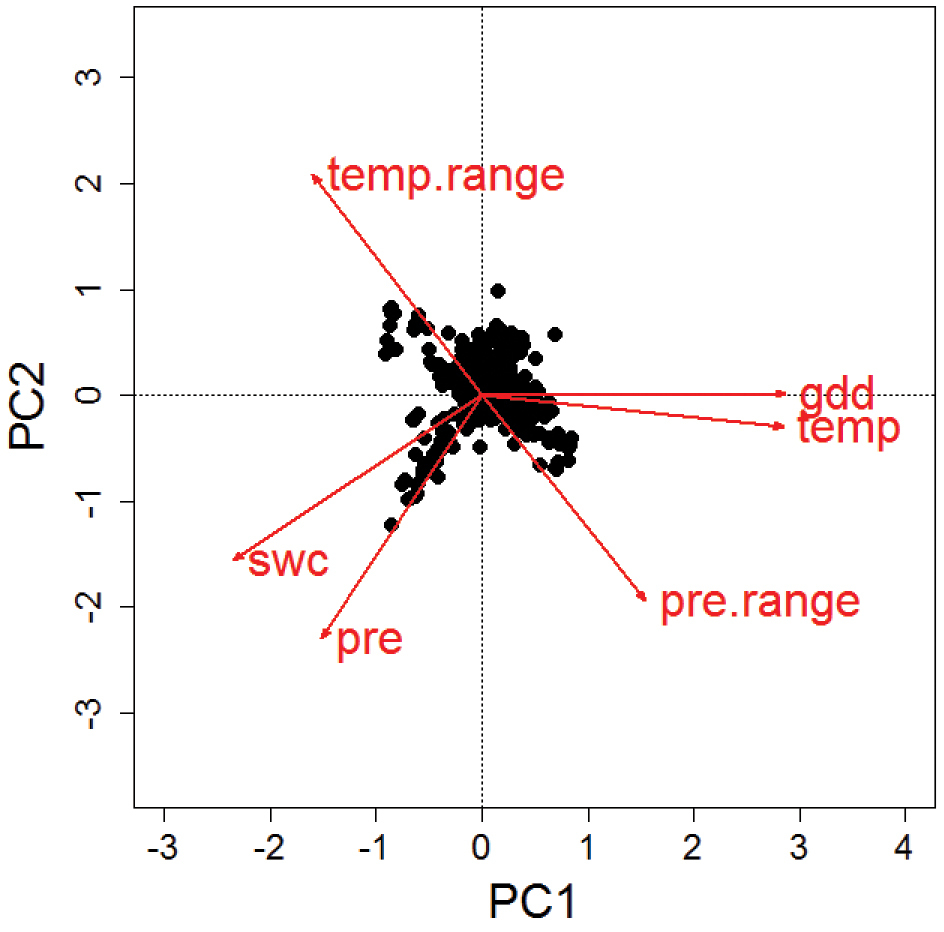
Results from a principal component analysis of the species-specific mean values of six different climate variables. Mean values per species have been calculated based on the observed records per 50 km × 50 km CGRS grid cell across a species’ European distribution. PC1 explained 58% and PC2 32% of the variability. Niche characteristics according to annual temperature (temp) and growing degree days until August (gdd) are highly correlated. Also, the two measures of water availability, annual precipitation (pre) and soil water content (swc) show some similarity, while the indicators of annual range in precipitation (pre.range) and temperature (temp.range) are negatively correlated. In spite of these similarities, aspects of energy, water and their annual variability can be assessed independently with a choice of at least three of the indicators.
By combining a comprehensive database on the distribution of European butterflies with publicly available climatic data in combination with a constantly high level of quality control at crucial steps of the data generation, CLIMBER represents a unique and ready-to-use dataset for a broad variety of potential applications. Analysis of phylogenetic signals in the climatic niche characteristics, for instance, can be used to assess past niche evolution which can lead to projections of potential future risks in the face of rapid climate change (for a comparable analysis for birds see
STI values for European butterflies can be of great value for governmental and non-governmental conservation organisations (
For the description of the metadata we followed the standards suggested by
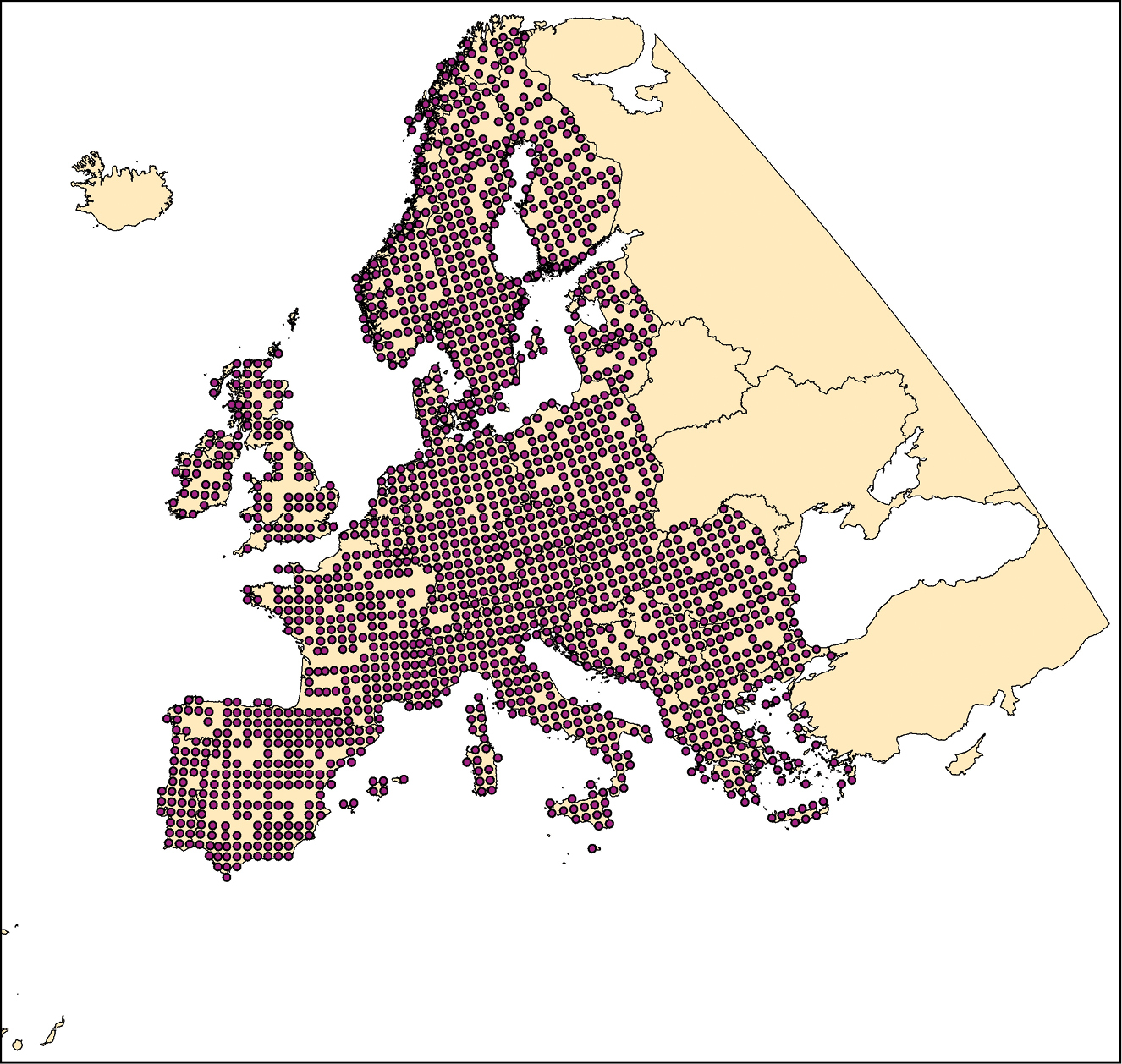
Climatic niche characteristics are provided for all butterfly species occurring within a European window of 11°W, 32°E longitude and 34°N, 72°N latitude (Fig. 3). Resolution of butterfly distribution and corresponding climate data used to calculate climatic niche characteristics corresponds to the 50 km × 50 km Common European Chorological Grid Reference System (CGRS; http://www.eea.europa.eu/data-and-maps/data/common-european-chorological-grid-reference-system-cgrs).
Geographic coverage used for the calculation of the climatic species characteristics. Purple dots indicate 50 km × 50 km CGRS grid cells with available species records.
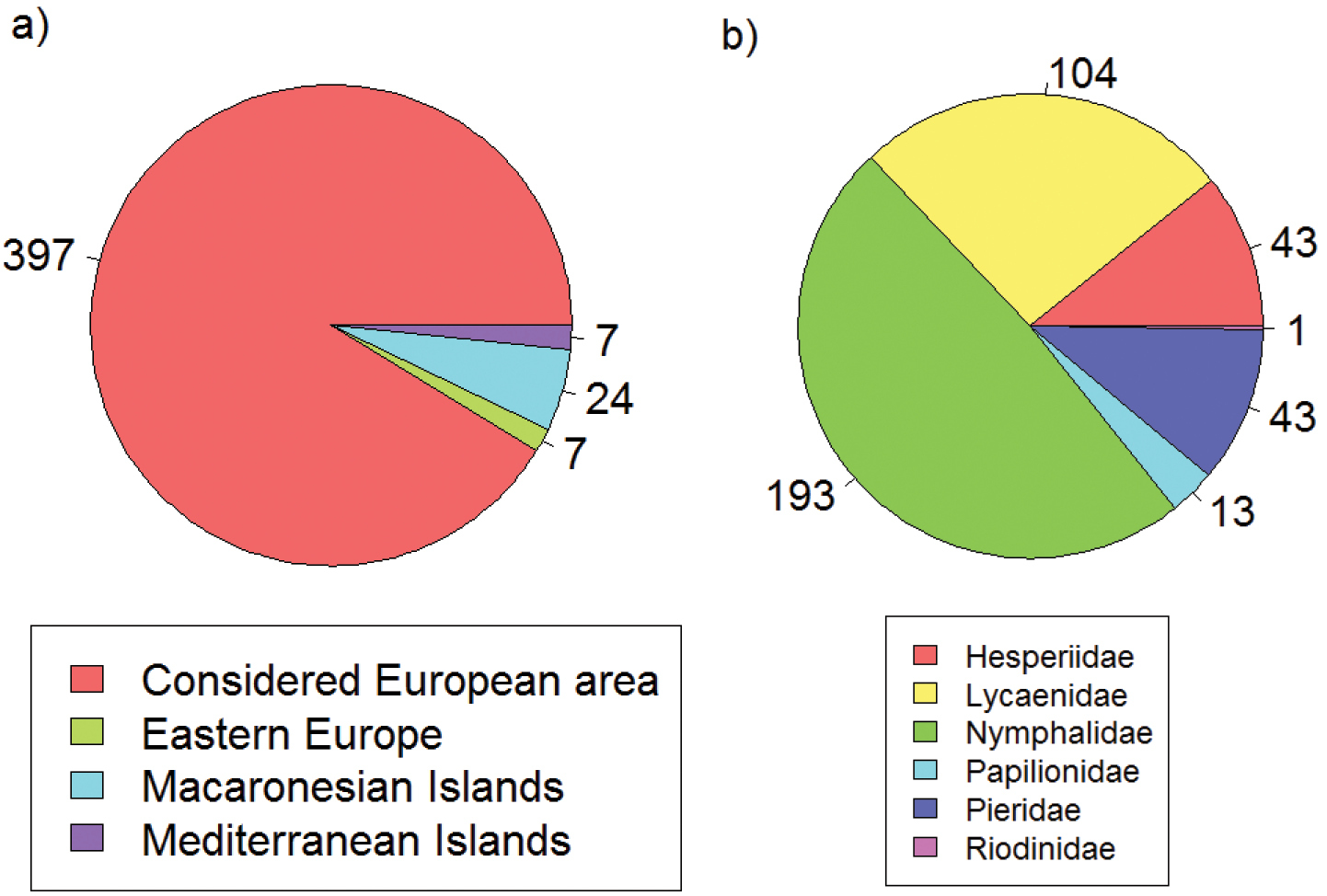
The geographic window excludes data from the Atlantic islands under European administration (the Azores, Madeira and Canary Islands) as well as Cyprus and Iceland. Due to low levels of recording, data from Belarus, Ukraine, Moldova, and Russia were also excluded. Additionally, no climate data were available for two species with extremely local distributions on the Pontine Islands and the Greek island of Nissiros. These restrictions led to the exclusion of 38 of the European butterfly species listed in
Species occurring in Europe and listed in
| Species | European range |
|---|---|
| Azanus ubaldus (Stoll, 1782) | Canary Islands |
| Catopsilia florella (Fabricius, 1775) | Canary Islands |
| Chazara persephone (Hübner, [1805]) | Ukraine |
| Chilades galba (Lederer, 1855) | Cyprus |
| Cigaritis acamas (Klug, 1834) | Cyprus |
| Cyclyrius webbianus (Brulle, 1839) | Canary Islands |
| Euchloe eversi Stamm, 1963 | Canary Islands |
| Euchloe grancanariensis Acosta, 2008 | Canary Islands |
| Euchloe hesperidum Rothschild, 1913 | Canary Islands |
| Glaucopsyche paphos Chapman, 1920 | Cyprus |
| Gonepteryx cleobule (Hübner, 1825) | Canary Islands |
| Gonepteryx eversi Rehnelt, 1974 | Canary Islands |
| Gonepteryx maderensis Felder, 1863 | Madeira |
| Gonepteryx palmae Stamm, 1963 | Canary Islands |
| Hipparchia azorina (Strecker, 1899) | Azores |
| Hipparchia bacchus Higgins, 1967 | Canary Islands |
| Hipparchia cypriensis (Holik, 1949) | Cyprus |
| Hipparchia gomera Higgins, 1967 | Canary Islands |
| Hipparchia maderensis (Bethune-Baker, 1891) | Madeira |
| Hipparchia sbordonii Kudrna, 1984 | Pontine Islands |
| Hipparchia tamadabae Owen & Smith, 1992 | Canary Islands |
| Hipparchia tilosi (Manil, 1984) | Canary Islands |
| Hipparchia wyssii (Christ, 1889) | Canary Islands |
| Hypolimnas misippus (Linnaeus, 1764) | Canary Islands |
| Maniola cypricola (Graves, 1928) | Cyprus |
| Maniola halicarnassus Thomas, 1990 | Nissiros Island |
| Neolycaena rhymnus (Eversmann, 1832) | Ukraine |
| Pararge xiphia (Fabricius, 1775) | Madeira |
| Pararge xiphioides Staudinger, 1871 | Canary Islands |
| Pieris cheiranthi (Hübner, 1808) | Canary Islands |
| Pieris wollastoni Butler, 1866 | Madeira |
| Polyommatus corydonius (Herrich-Schäffer, 1852) | Ukraine |
| Polyommatus damocles (Herrich-Schäffer, 1844) | Ukraine |
| Polyommatus damone (Eversmann, 1841) | Ukraine |
| Pseudochazara euxina (Kusnezov, 1909) | Ukraine |
| Thymelicus christi Rebel, 1894 | Canary Islands |
| Tomares callimachus (Eversmann, 1848) | Ukraine |
| Vanessa vulcania (Godart, 1819) | Canary Islands & Madeira |
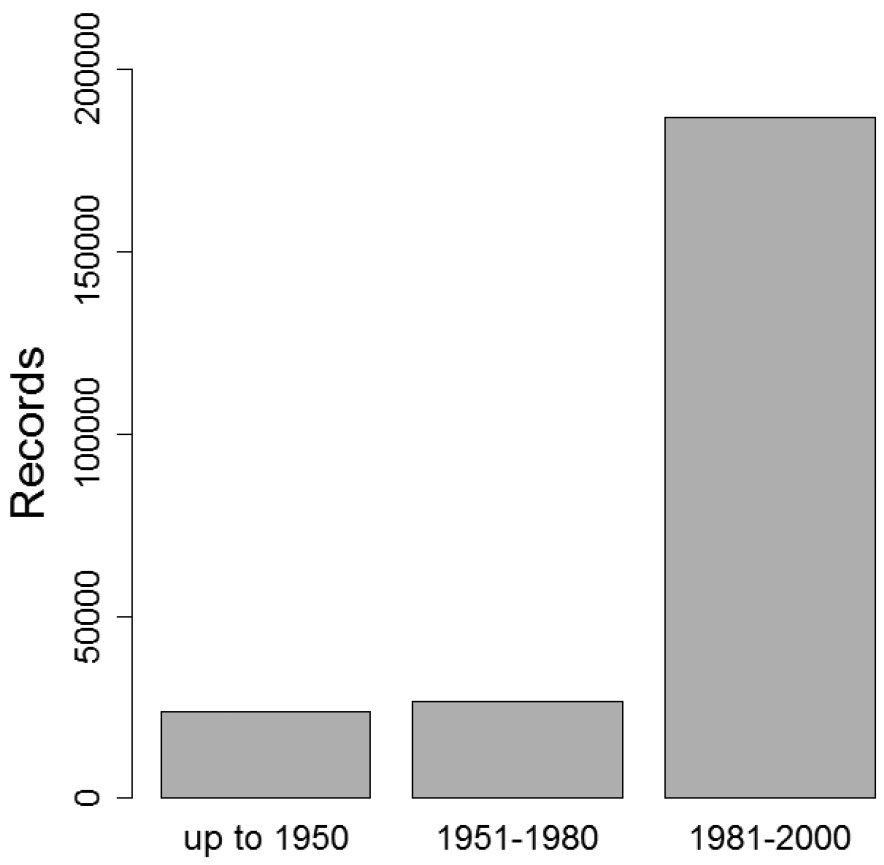
Only butterfly distribution data from the period of 1981 to 2000 were considered due to low sampling intensity in earlier periods (Fig. 4) and to minimize errors due to ongoing range shifts as a response to recent climate change.
Temporal availability of records and corresponding sampling intensity. Only the period of 1981–2000 has been considered in CLIMBER.
The taxonomic coverage spans all butterfly species within the selected geographic window (397 species) and represents 91% of all European species (Fig. 5). Thirty-eight species from less well sampled Eastern European countries, Atlantic and small Mediterranean islands have not been considered (Fig. 5a). The taxonomy of European butterfly species follows
Taxonomic coverage according to the entire European butterfly fauna (a) and families (b). Values indicate number of species.
Corrected species names (cf.
| Corrected species names |
|---|
| Anthocharis damone Boisduval, 1836 |
| Apatura metis Freyer, 1829 |
| Argynnis elisa Godart, 1823 |
| Aricia morronensis Ribbe, 1910 |
| Cacyreus marshalli Butler, 1898 |
| Colias aurorina Herrich-Schäffer, 1850 |
| Erebia ottomana Herrich-Schäffer, 1847 |
| Maniola chia Thomson, 1987 |
| Maniola halicarnassus Thomson, 1990 |
| Melitaea asteria Freyer, 1828 |
| Melitaea varia Meyer-Dür, 1851 |
| Pararge xiphioides Staudinger, 1871 |
| Plebejus trappi (Verity, 1927) |
| Pseudochazara amymone Brown, 1976 |
| Pseudochazara orestes Prins & Poorten, 1981 |
Aricia artaxerxes (Fabricius, 1793) and Aricia montensis Verity, 1928 are treated in CLIMBER as distinct species with parapatric distributions (see
For the local Macedonian endemic Pseudochazara amymone Brown, 1976 no data were available for the considered time period. After its first discovery in Greece in 1975, the species was not reliably recorded again until its recent rediscovery in Southern Albania (
The following species in our database actually comprise records of more than one species, most of which were recognized only recently, and are difficult or impossible to distinguish without genitalia examination or molecular methods.
•Carcharodus alceae (Esper, 1780) probably contains data of the sibling species Carcharodus tripolinus (Verity, 1925) from the Southern Iberian Peninsula, differing only in genitalia characters.
•Leptidea sinapis (Linnaeus, 1758) is a complex of three sibling species, and includes data of Leptidea juvernica Williams, 1946, and Leptidea reali Reissinger, 1990 (
•Lycaena tityrus (Poda, 1761) includes data of Lycaena bleusei Oberthür, 1884 from Central Spain and Central Portugal, which appears to be a distinct species according to unpublished molecular data.
•Melitaea athalia (Rottemburg, 1775) includes the Southwest European Melitaea nevadensis Oberthür, 1904 (syn. celadussa Fruhstorfer, 1910) which might only be a subspecies of the former. Molecular data are inconclusive regarding the taxonomic status of these parapatric taxa.
•Melitaea phoebe (Goeze, 1779) recently turned out to be a complex of at least two largely sympatric species with distinctive larval colouration, and our data probably include records of Melitaea ornata Christoph, 1893 (syn. telona Fruhstorfer, 1908 and emipunica Verity, 1919) (see
•Polyommatus icarus (Rottemburg, 1775) includes data of Polyommatus celina (Austaut, 1879), which was recognized as a distinct species from North Africa and the Canary Islands by molecular methods (
•Pontia daplidice (Linnaeus, 1758) includes the data of the sibling species Pontia edusa (Fabricius, 1777), a parapatric taxon, which can only be distinguished by molecular methods (
Climatic niche characteristics of the butterflies in Europe are based on their European distribution. Butterfly distributions were available from about 7000 georeferenced localities and about 200, 000 database records. These records were stored in a database and constituted also the basis for ‘The Distribution Atlas of European Butterflies’ (
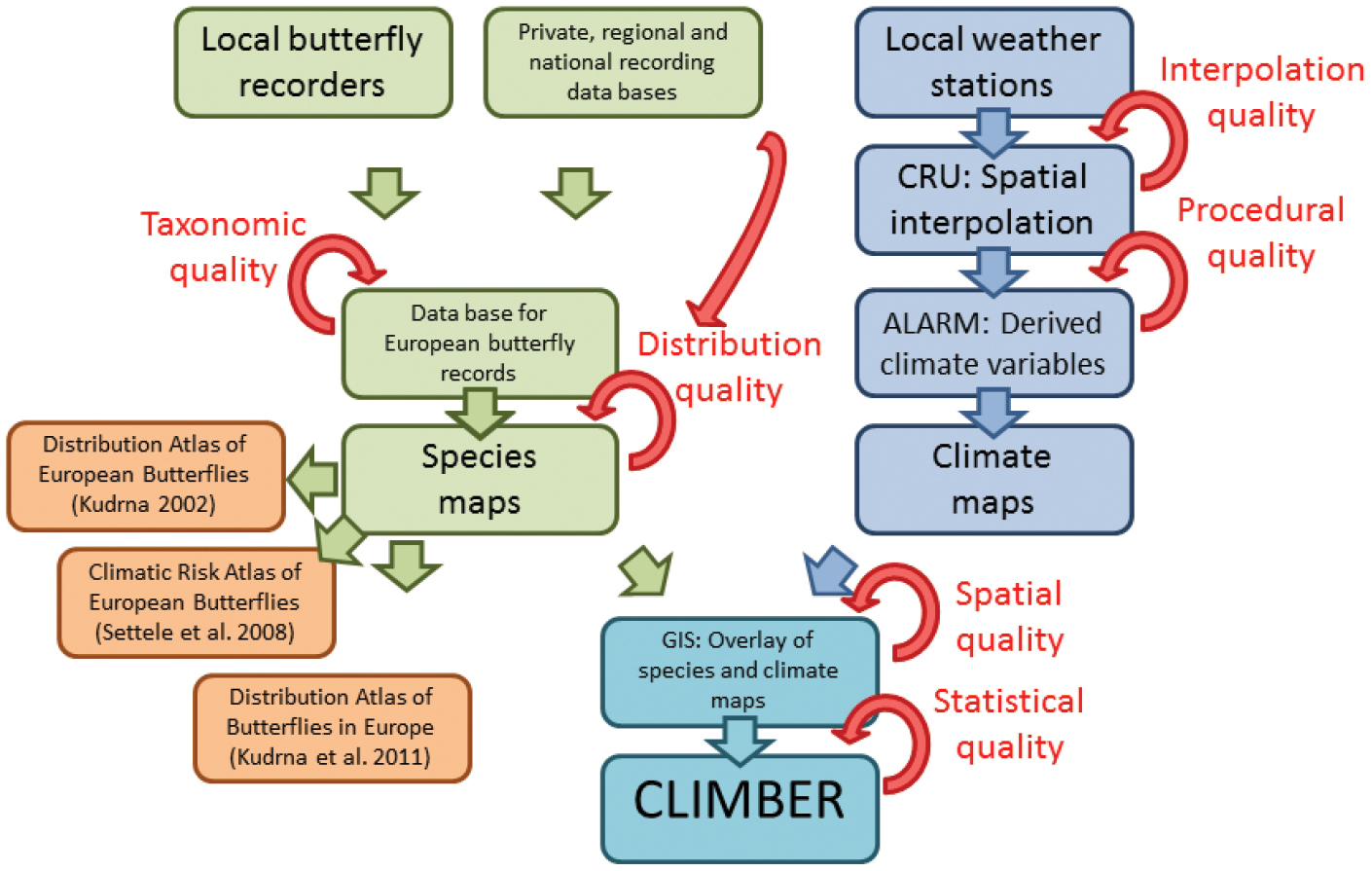
Work flow and data sources for the generation of CLIMBER. Butterfly distribution data are based on a database which combines information from local recorders and private, regional and national databases. Thereof, species distributional maps have been developed. Together with maps of original and derived climate variables, based on interpolated data from local weather stations, species distribution-climate relationships have been assessed in a GIS. Based on these relationships several statistics describing the climatic characteristics of 397 European butterfly species have been developed and stored in CLIMBER. Several steps of quality control ensure a high level of data accuracy. CRU; Climate Research Unit, University of East Anglia (http://www.cru.uea.ac.uk/). ALARM; EU, FP6 project ‘Assessing Large Scale Risks for Biodiversity with Tested Methods’ (http://www.alarmproject.net/climate/climate/).
We used monthly, interpolated climate data (publicly available at http://www.alarmproject.net/climate/climate), originally provided via the ALARM project (
Climatic niche characteristics were calculated per butterfly species according to the climatic conditions across their respective ranges, i.e. across all grid cells in which a particular species occurs (see
Several steps of quality control ensure a high level of data accuracy (Fig. 6). During the step of compiling butterfly records for Europe, taxonomic experts addressed problems of potential misidentification, synonymy and the taxonomic concept. Once the species distribution maps had been produced, internal and external control ensured the elimination of obviously wrong records (species outside their natural range). Climate data are based on original climate variables from the Climate Research Unit (CRU) of the University of East Anglia and derived climate variables generated by the ALARM project. Both, CRU and ALARM ensured a high level of internal and external quality control. Data quality for the calculation of the climatic niche characteristics for each butterfly species is high (about 200, 000 records for butterfly distribution; well recognised and commonly accepted climate data). Additionally, we provide the number of grid cells which have been used to calculate the climatic species characteristics and the standard deviation to assess uncertainty of the measures.
Data set version: v1.3
Latest update: 18.10.2013.
Metadata status: Metadata are complete and stored with the data.
Copyright restrictions: None.
Proprietary restrictions: This dataset is freely available for non-commercial scientific use.
Citation: Data users must cite this Data Paper properly in any publication that results from an analysis using the provided data as a whole or in parts as: Schweiger O, Harpke A, Wiemers M, Settele J (2013). CLIMBER: Climatic niche characteristics of the butterflies in Europe. ZooKeys 367: 65–84. doi: 10.3897/zookeys.367.6185
In addition to the Data Paper the resource should be cited as: Helmholtz Centre for Environmental Research - UFZ (2013). CLIMBER: Climatic niche characteristics
of the butterflies in Europe. 397 records, Online at http://ipt.pensoft.net/ipt/resource.do?r=climber, version 1.3 (released on 3/12/2013), Resource ID: GBIF key: http://www.gbif.org/dataset/e2bcea8c-dfea-475e-a4ae-af282b4ea1c5, Data Paper ID: doi: 10.3897/zookeys.367.6185
oration: Data users might consider collaboration and/or co-authorship with the data owners.
Storage location: http://ipt.pensoft.net/ipt/resource.do?r=climber
File name: CLIMBER.v.1.3.csv
Size: 398 rows, 67 columns; 183 kB.
Format and storage mode: ASCII csv, semicolon-delimited; decimal separator: ‘.’.
Header information: First row provides variable names.
Alphanumeric attributes: Mixed.
Special characters: Missing values are indicated by NA.
Climatic niche characteristics are based on nine climate variables (Table 3). All climate variables represent average values for the period of 1971–2000. Seven statistics are available for each climate variable (Table 4).
Climatic variables used for the assessment of climatic niche characteristics of the butterflies in Europe.
| Name | Definition | Unit | Interpretation |
|---|---|---|---|
| range.size | Distributional range size as number of occupied grids | Grid cells | Sample size |
| temp | Mean annual temperature | °C | Temperature (STI) |
| range.ann.temp | Annual range in monthly temperature (warmest month - coldest month) | °C | Continentality |
| precip | Annual precipitation sum | mm | Precipitation |
| range.ann.precip | Annual range in monthly precipitation sum (wettest month - driest month) | mm | Oceanity |
| gdd.feb | Accumulated growing degree days above 5°C from January to February | °C | Temperature corrected for metabolic activity preconditions |
| gdd.apr | Accumulated growing degree days above 5°C from January to April | °C | Temperature corrected for metabolic activity preconditions |
| gdd.june | Accumulated growing degree days above 5°C from January to June | °C | Temperature corrected for metabolic activity preconditions |
| gdd.aug | Accumulated growing degree days above 5°C from January to August | °C | Temperature corrected for metabolic activity preconditions |
| swc | Soil water content of the upper horizon (0.5 m) | No unit (0-1) | Water availability |
Statistics available for each climate variable describing the niche position and breadth for the butterflies in Europe.
| Name | Definition | Interpretation |
|---|---|---|
| mean | Mean value of climate variable across the species’ range | ‘Optimal’ climatic conditions; niche position |
| ci.95.low | Lower 95% confidence interval for the mean | Uncertainty of the mean |
| ci.95.up | Upper 95% confidence interval for the mean | Uncertainty of the mean |
| min | Minimum value of the climate variable across the species range | Lower climatic limit |
| max | Maximum value of the climate variable across the species range | Upper climatic limit |
| sd | Standard deviation of the climate variable across the species range | Niche breadth |
We also provide an estimation of species range size (range.size) to assess the number of grid cells used for calculating the climatic species characteristics. For a detailed description of swc see section Climate data. Annual measures are calculated over full 12 month periods, while accumulated growing degree days have been calculated for four periods from January to February, April, June and August to cover a variety of phenological aspects and life cycle stages.
Species range refers to the distributional range according to the 50 km × 50 km CGRS grid cells in which a species was recorded.
Most of the distributional data were compiled by Dr. Otakar Kudrna, especially within the framework of
Database of the climatic niche characteristics of the butterflies in Europe (CLIMBER). (doi: 10.3897/zookeys.367.6185.app) File format: Comma-separated values file (csv).