(C) 2012 Stephen D. Cairns. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The 120 presently recognized genera and seven subgenera of the azooxanthellate Scleractinia are keyed using gross morphological characters of the corallum. All genera are illustrated with calicular and side views of coralla. All termes used in the key are defined in an illustrated glossary. A table of all species-level keys, both comprehensive and faunistic, is provided covering the last 40 years.
Azooxanthellate, Illustrated Key, Genera, Glossary, Scleractinia
The ready identification of azooxanthellate Scleractinia (determined herein by depth of occurrence and previously published observations) to the genus and species levels has been hampered by a lack of a comprehensive key to the genera as well as a lack of species level keys. For instance, the last comprehensive set of keys to the genera was published by
Previously published keys to azooxanthellate taxa, divided as comprehensive keys to all taxa with in a monophyletic taxon, and partial (faunistic) keys of species. Taxa listed alphabetically by taxon name. Tabular keys (T) are included.
| Comprehensive keys | |
|---|---|
| Anthemiphyllia, species (T) |
|
| Asterosmilia, species (T) |
|
| Aulocyathus, species |
|
| Caryophyllia, species (T) |
|
| Caryophyllia, species |
|
| Conocyathus, species |
|
| Crispatotrochus, species |
|
| Deltocyathus, species |
|
| Dendrophylliidae, genera (T) |
|
| Flabellidae, genera (T) |
|
| Guyniidae, genera (T) |
|
| Javania, species |
|
| Micrabaciidae, genera |
|
| Placotrochides, species |
|
| Scleractinia, families and genera |
|
| Stephanophyllia, species |
|
| Trochocyathus (Aplocyathus), species (T) |
|
| Turbinoliidae, genera |
|
| Faunistic keys | |
| Astrangia, E. Pacific |
|
| Azooxanthellate Scleractinia, Antarctica |
|
| Azooxanthellate Scleractinia, E. Gulf of Mexico |
|
| Azooxanthellate Scleractinia, New Zealand |
|
| Azooxanthellate Scleractinia, NE Pacific |
|
| Azooxanthellate Scleractinia, NW Pacific |
|
| Azooxanthellate Scleractinia, S. Australia |
|
| Azooxanthellate Scleractinia, Cold Temp. NE Atl. |
|
| Azooxanthellate Scleractinia, Brazil |
|
| Balanophyllia, W. Atlantic |
|
| Balanophyllia, Japan |
|
| Balanophyllia, W. Atlantic (T) |
|
| Caryophyllia, New Zealand |
|
| Caryophyllia, W. Atlantic |
|
| Caryophyllia, W. Pacific |
|
| Caryophyllia and Premocyathus, Japan |
|
| Conotrochus and Trochocyathus, Japan |
|
| Culicia, Australia |
|
| Deltocyathus, W. Atlantic |
|
| Deltocyathus, W. Pacific |
|
| Dendrophyllia, Japan |
|
| Flabellum, New Zealand |
|
| Flabellum, Japan |
|
| Fungiacyathus, W. Pacific (T) |
|
| Fungiacyathus, Japan |
|
| Heterocyathus, W. Pacific |
|
| Heterocyathus, Japan |
|
| Heteropsammia, W. Pacific |
|
| Heteropsammia, Japan |
|
| Madracis, W. Atlantic |
|
| Paracyathus and Polycyathus, Japan |
|
| Trochocyathus, W. Pacific |
|
| Truncatoflabellum, W, Pacific |
|
| Truncatoflabellum, SW Indian Ocean |
|
| Truncatoflabellum, Australia (T) |
|
| Truncatoflabellum, Japan |
|
| Tubastraea, Red Sea |
|
| Tubastraea, Galapagos |
|
| Tubastraea, Japan |
|
| Turbinoliidae, Japan |
|
Some genera are keyed two or even three times because of the variation within those genera regarding the characters used in the key. In theory, all variations of that genus will be correctly keyed. Although most couplets are dichotomous, some are polychotomous, such as the columella or colony shape, which allows the reader to clearly see the multiple states of a particular character.
Although it would be desirable to follow the generic key with keys to all of the approximately 720 azooxanthellate species, it is a simple fact that not many species level keys have been published. Those that have been published in the last 35 years are listed in Table 1, separated as to whether they are keys to all of the taxa within a monophyletic taxon (comprehensive) or to a more limited fauna of a region (faunistic). Keys made before 1970 were found to be, in general, not up to date and are thus not included. It should be noted that fully one-third of the genera (40) are monotypic, and thus do not require a key following a correct genus identification, and another 22 genera have but two species. Finally, although they do not include keys, the treatises of
Other sources of useful taxonomic information include a list of all extant Recent scleractinian species as of 1999 (
Geographic ranges within brackets in the key are not meant to be considered as distinguishing characters, but simply informational, which may nonetheless hint at an incorrect identification. Abbreviations: Ant. = Antarctic or Subantarctic, Atl. = Atlantic, IP = Indo Pacific, IWP = Indo-West Pacific, Pac. = Pacific, SubAnt = Subantarctic; Cosmopolitan implies occurrence in all three oceans as well as Subantarctic and/or Antarctic. Museums and Institutions acronyms: AM = Australian Museum (Sydney); AU = Auckland University Museum (Auckland); CSIRO = Commonwealth Scientific and Industrial Research Organisation (Hobart); JCU = James Cook University (Townsville); MNHN = Muséum national d’Histoire naturelle (Paris); SBMNH = Santa Barbara Natural History Museum (Santa Barbara); SIO = Scripps Institute of Oceanography (San Diego); NZOI = New Zealand Oceanographic Institution (now the National Institute of Water and Atmospheric Research) (Wellington); USNM = United States National Museum (now the National Museum of Natural History, Smithsonian) (Washington, D.C.); YPM = Yale Peabody Museum (New Heaven).
Useful sources for more information about definitions of terms used in the glossary include:
(An asterisk indicates genera that have azooxanthellate and zooxanthellate representatives)
| 1a | Corallum colonial | 2 |
| 1b | Corallum solitary | 43 |
| 2a | Corallum free of attachment (recumbent, usually curved with a broken or open base, or globular) | 3 |
| 2b | Corallum firmly attached (arborescent, bushy, encrusting, or reptoid) | 5 |
| 3a | Corallum recumbent (composed of a large primary corallite from which smaller buds originate); no sipunculid commensalism | 4 |
| 3b | Corallum globular; pores in lateral base of colony associated with commensal sipunculid | [IWP] Heteropsammia* (in part) Plate 1, Figures A–B |
| 4a | Corallum not porous (solid); septa arranged normally | [Atl. + IP] Anomocora Plate 1, Figures C–D |
| 4b | Corallum, especially septa porous; septa arranged in a Pourtalès Plan | [Atl. + IWP] Eguchipsammia Plate 1, Figures E–F |
| 5a | Corallum arborescent or bushy | 6 |
| 5b | Corallum encrusting or reptoid | 27 |
| 6a | Branching intratentacular | 7 |
| 6b | Branching extratentacular | 9 |
| 7a | Equal distomadeal budding | 8 |
| 7b | Unequal monostomaeous budding | [Cosmopolitan] Lophelia Plate 1, Figures G–H |
| 8a | Texture of corallum rough (like sandpaper), resulting from a porous theca; septa arranged in a weak Pourtalès Plan | [W. Pac.] Dichopsammia Plate 1, Figures I–J |
| 8b | Texture of corallum smooth or costate, solid; septa arranged normally | Cosmopolitan] Solenosmilia Plate 1, Figures K–L |
| 9a | Septal symmetry decameral or octameral, septa in only one cycle; columella styliform | [Atl. + IP] Madracis* (in part) Plate 2, Figures A–B |
| 9b | Septal symmetry hexameral, septa arranged in multiple cycles; columella papillose, fascicular or absent | 10 |
| 10a | Texture of theca and septa rough (like sandpaper), resulting from a porous theca | 11 |
| 10b | Texture of theca smooth, granular, or ridged (solid) | 14 |
| 11a | Septa arranged in a Pourtalès plan | 12 |
| 11b | Septa arranged normally | 13 |
| 12a | Corallum small (bushy), most corallites budding from a common basal coenosteum or from the edge zone of corallites that originate from the basal coenosteum | [Atl. + Pac.] Cladopsammia Plate 2, Figures C–D |
| 12b | Corallum large (bushy to arborescent), with multiple successive generations of budding forming an erect colony | [Atl. + IP] Dendrophyllia Plate 2, Figures E–F |
| 13a | Corallum porosity only apparent near calicular edge; found in deep-water: 110-2165 m | [Atl. + IWP] Enallopsammia Plate 2, Figures G–H |
| 13b | Corallum porosity uniform: shallow-water: 0-110 m | [Atl. + IP] Tubastraea (in part) Plate 2, Figures I–J |
| 14a | Columella absent | 15 |
| 14b | Columella present (papillose, trabecular or fascicular) | 16 |
| 15a | Corallum large (arborescent), with numerous budding cycles, adjacent corallites often linked with hollow, tubular coenosteal bridges; tabular endothecal dissepiments common | [I–P + Subant.] Goniocorella Plate 2, Figures K–L |
| 15b | Corallum a small bush, corallites originating from a common basal coenosteum or from the sides of other corallites and from relatively few budding cycles; endothecal dissepiments not prominent | [E. Atl. + New Zealand] Hoplangia (in part) Plate 5, Figures L–M |
| 16a | Columella fascicular | 17 |
| 16b | Columella papillose or trabecular | 18 |
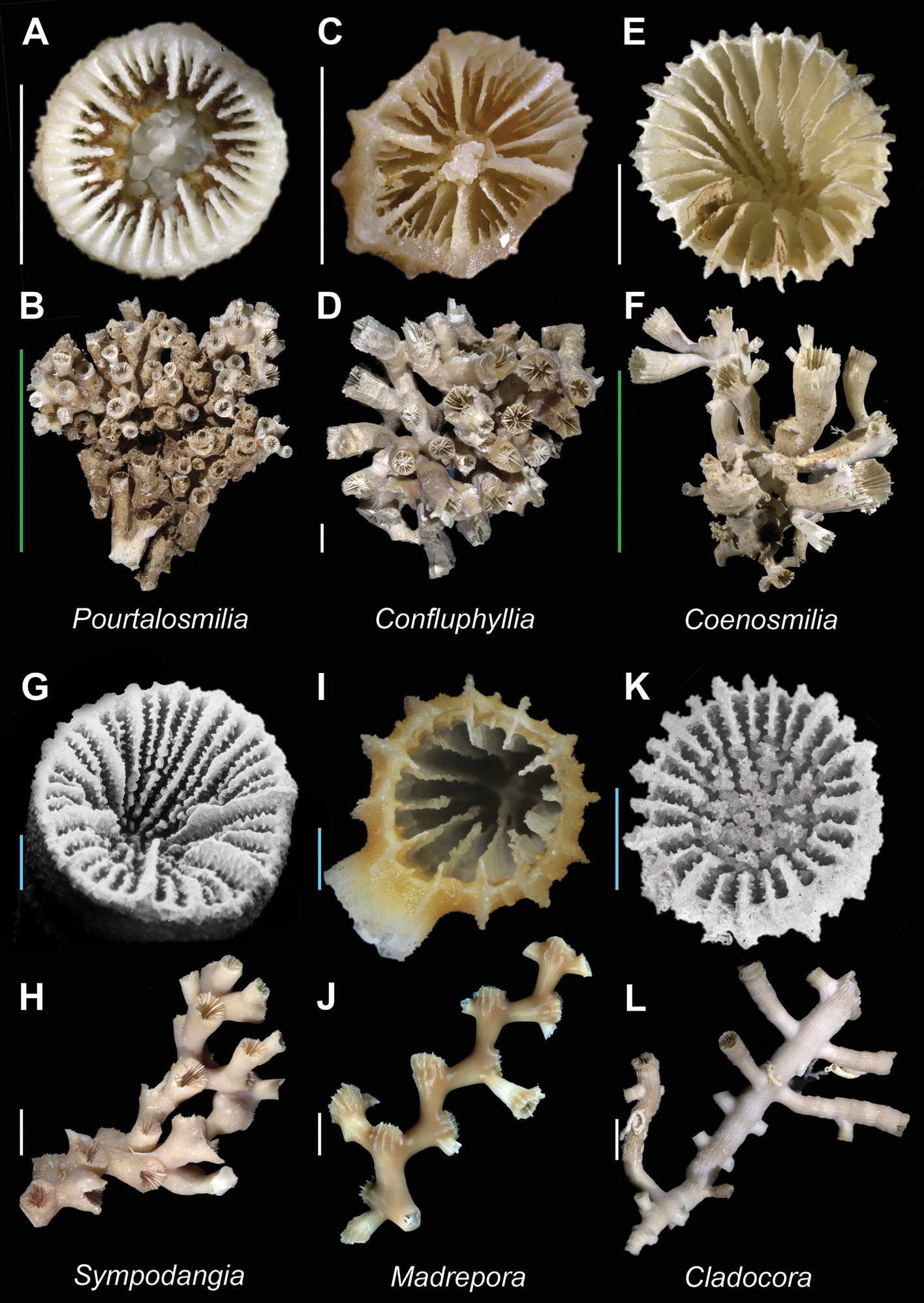
| 17a | Pali before septa of third cycle (P3) | [N. Atl.] Pourtalosmilia Plate 3, Figures A–B |
| 17b | Pali absent | [W. Pac.] Confluphyllia Plate 3, Figures C–D |
| 18a | Columella trabecular, composed of slender ( flattened laths); corallum never with more than 4 generations of budding | [Atl. + W. Pac.] Coenosmilia Plate 3, Figures E–F |
| 18b | Columella papillose (composed of rods); corallum composed of many generations of budding | 19 |
| 19a | Axial septal edges dentate | [W. Pac.] Sympodangia Plate 3, Figures G–H |
| 19b | Axial septal edges smooth | 20 |
| 20a | Pali absent | [Cosmopolitan] Madrepora (in part) Plate 3, Figures I–J |
| 20b | Pali present | 21 |
| 21a | Pali arranged in multiple crowns before septa of all but last cycle; axial edge of septa minutely dentate | 22 |
| 21b | Pali arranged in two crowns before S2 and S3 or S1-3; axial edges of septa smooth | 24 |
| 22a | Coenosteum costate | [Atl. + Pac.] Cladocora Plate 3, Figures K–L |
| 22b | Coenosteum not costate | 23 |
| 23a | Axial corallite associated with each branch | [SW Pac.] Petrophyllia Plate 4, Figures A–B |
| 23b | Axial corallites absent | [Atl. + Pac.] Oculina* Plate 4, Figures C–D |
| 24a | P1-3 arranged in two palar crowns | [IWP] Cyathelia Plate 4, Figures E–F |
| 24b | One palar crown of P2 or P3 | 25 |
| 25a | Only P2 present | 26 |
| 25b | Only P3 present | [SW Atl. + E. Pac.] Bathelia Plate 4, Figures G–H |
| 26a | Columella massive | [SE Atl.] Sclerhelia Plate 4, Figures I–J |
| 26b | Columella rudimentary | [Cosmopolitan] Madrepora (in part) Plate 4, Figures K–L |
| 27a | Septal symmetry decameral or octameral, septa in only one cycle; columella styliform | [Atl. + IP] Madracis* (in part) Plate 5, Figures A–B |
| 27b | Septal symmetry hexameral, septa arranged in multiple cycles; columella papillose, fascicular, spongy, lamellar or absent | 28 |
| 28a | Texture of corallum rough (like sandpaper), resulting from a porous theca | 29 |
| 28b | Texture of corallum smooth or costate, solid | 31 |
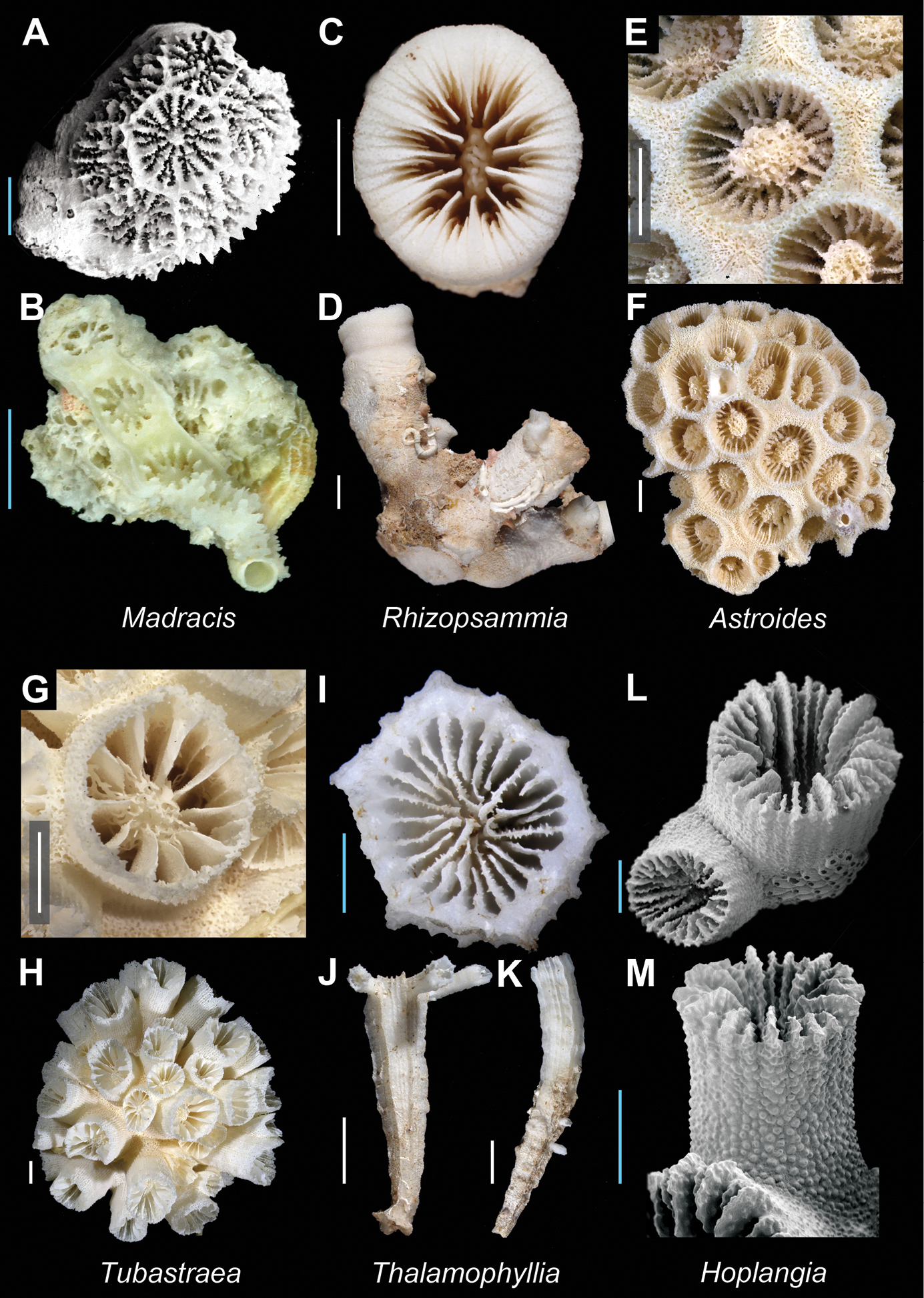
| 29a | Corallum increases by stoloniferous budding (reptoid), the connection among corallites often obscured, thus sometimes appearing to be solitary; Pourtalès Plan present | [W. Atl. + IP] Rhizopsammia Plate 5, Figures C–D |
| 29b | Corallum increases by budding from a common basal coenosteum, the connection among polyps quite evident; septa normally inserted | 30 |
| 30a | Columella massive; epitheca surrounds each corallite | [E. Atl.] Astroides Plate 5, Figures E–F |
| 30b | Columella of moderate to small size; epitheca lacking | [Atl. + IP] Tubastraea (in part) Plate 5, Figures G–H |
| 31a | Columella absent | 32 |
| 31b | Columella present | 33 |
| 32a | Corallites united by thin basal stolons (reptoid) | [Atl. + IWP] Thalamophyllia Plate 5, Figures I–K |
| 32b | Corallites bud from a common basal coenosteum | [E. Atl. + New Zealand] Hoplangia (in part) Plate 5, Figures L–M |
| 33a | Axial edges of some or all cycles of septa finely dentate or beaded | (Rhizangiidae) 34 |
| 33b | Axial edges of all septa smooth | 38 |
| 34a | Thin epitheca encircles corallites; axial edges of S1-2 smooth, sometimes lobate (but inner edges of S3-4 dentate) | 35 |
| 34b | Epitheca absent; axial edges of all septa dentate | 36 |
| 35a | Corallite base polycyclic; one crown of large P3 | [Atl.+ Pac.] Colangia Plate 6, Figures A–B |
| 35b | Corallite base monocyclic; pali, if present, of uniform size | [IP] Culicia Plate 6, Figures C–D |
| 36a | Corallite base polycyclic; pali absent | [IP] Oulangia Plate 6, Figures E–F |
| 36b | Corallite base monocyclic; pali before septa of all but last cycle | 37 |
| 37a | Corallum stoloniferous (reptoid) or cerioid; peritheca absent | [Atl. + IP] Astrangia* Plate 6, Figures G–H |
| 37b | Corallum massive (subramose); peritheca unite corallites | [Indian] Cladangia Plate 6, Figures I–J |
| 38a | Pali or paliform lobes on axial edges of septal of all but last cycle | 39 |
| 38b | Pali or paliform lobes present only on septa of penultimate cycle (usually P3) | 41 |
| 39a | Corallum stoloniferous (reptoid) | [IWP] Rhizosmilia Plate 6, Figures K–L |
| 39b | Corallites bud from a common basal coenosteum | 40 |
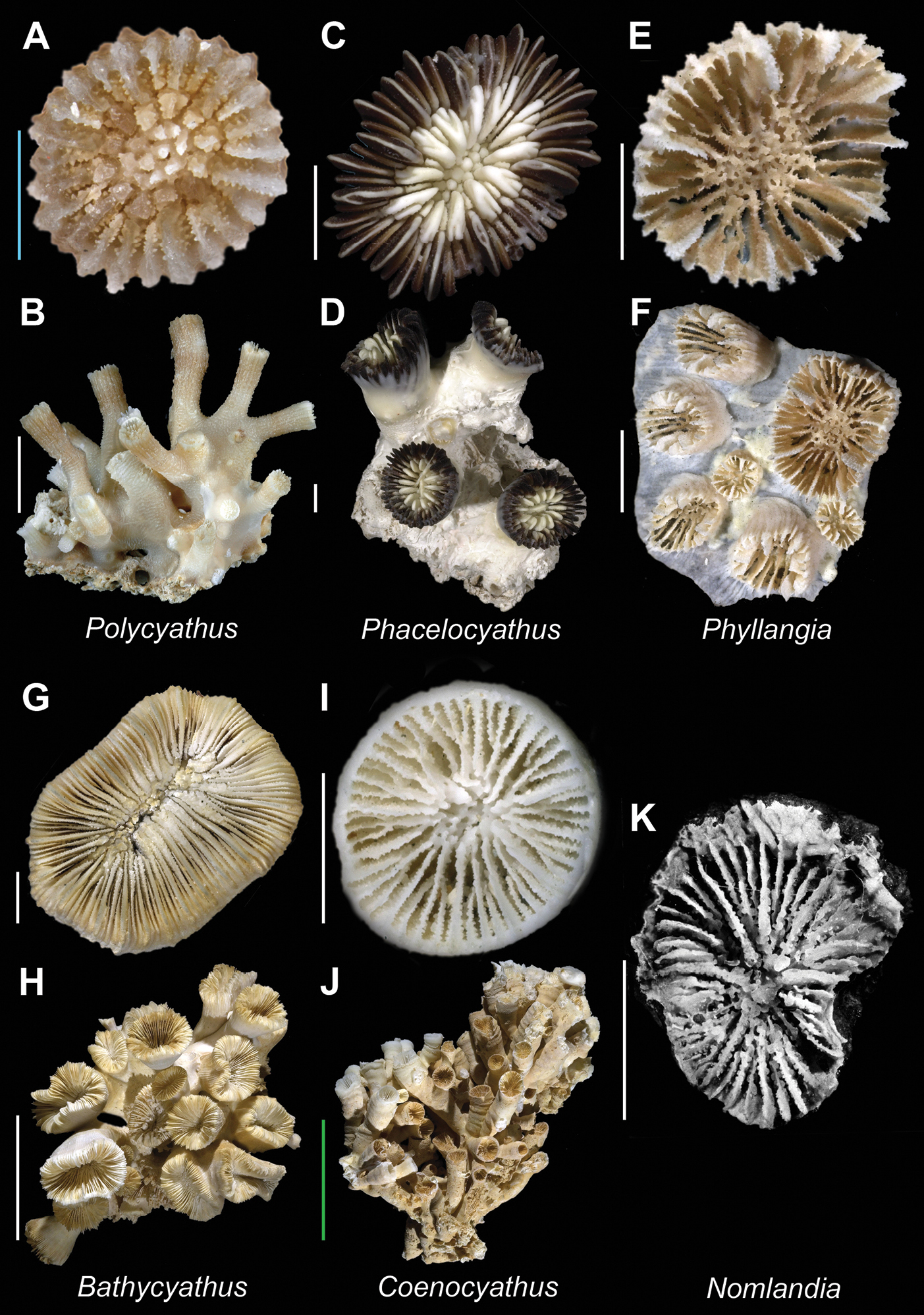
| 40a | Corallites monocyclic; pali before septa of all but last cycle, and all of approximately the same size | [IWP] Polycyathus Plate 7, Figures A–B |
| 40b | Corallites polycyclic; pali before septa of all but last cycle, those of P3 crown much larger than others | [W. Atl.] Phacelocyathus Plate 7, Figures C–D |
| 41a | Columella fascicular | 42 |
| 41b | Columella trabecular | [Atl. + IP] Phyllangia Plate 7, Figures E–F |
| 42a | Occurrence of pali variable: usually P4, occasionally also P3, occasionally absent | [E. Pac.] Bathycyathus Plate 7, Figures G–H |
| 42b | Pali in one crown before septa of third cycle (P3) | [Atl. + Pac.] Coenocyathus Plate 7, Figures I–J |
| 43a | Corallum firmly attached (fixed) | 44 |
| 43b | Corallum unattached (free) | 67 |
| 44a | Theca granular, the granules usually occurring on longitudinally oriented costae | 45 |
| 44b | Theca smooth (epithecate or stereome-reinforced), sometimes with fine transverse ridges encircling the theca | 53 |
| 44c | Theca and septa porous, although in some genera a smooth epitheca may cover the basal portion of the corallum | 61 |
| 44d | Theca absent (corallum discoidal) | [E. Pac.] Nomlandia Plate 7, Figure K |
| 45a | Columella papillose | 46 |
| 45b | Columella fascicular | 51 |
| 45c | Columella absent | 52 |
| 45d | Columella labyrinthiform | [Atl. + IP] Labyrinthocyathus Plate 8, Figures A–B |
| 46a | Pali or paliform lobes absent; base polycyclic | [W. Atl. + W. Pac.] Oxysmilia Plate 8, Figures C–D |
| 46b | Pali or paliform lobes present; base monocyclic | 47 |
| 47a | Coralla usually arranged in pseudocolonial assemblages | [W. Pac.] Lochmaeotrochus Plate 8, Figures G–H |
| 47b | Coralla discrete | 48 |
| 48a | Pali before S1-2 (P1, P2), indistinguishable from columellar elements | [W. Atl. + IWP] Monohedotrochus Plate 8, Figures E–F |
| 48b | Pali before septa of all but last cycle; palar crowns discrete | 49 |
| 49a | Multiple slender paliform lobes on axial edge of every lower cycle septum, not arranged in crowns | [Atl. + IP] Paracyathus Plate 8, Figures I–J |
| 49b | Two crowns of discrete pali or paliform lobes (P1+P2 and P3), only one palus or paliform lobe per septum | 50 |
| 50a | True pali present, the P1-2 smaller than P3 but not significantly | [Atl. + IP] Trochocyathus (Trochocyathus) (in part) Plate 8, Figures K–L |
| 50b | Paliform lobes present, the P1-2 much smaller than the broad P3 | [W. Atl. + W. Pac.] Vaughanella Plate 9, Figures A–B |
| 51a | Pali before septa of penultimate cycle | [Cosmopolitan] Caryophyllia (Caryophyllia) (in part) Plate 9, Figures C–D |
| 51b | Pali absent | [Cosmopolitan] Crispatotrochus Plate 9, Figures E–F |
| 52a | Corallum base monocentric; epitheca lacking; calice elliptical in outline; menianes lacking | [Cosmopolitan] Desmophyllum Plate 9, Figures G–H |
| 52b | Corallum polycentric; transverse epithecal bands near corallum base; calicular outline modified by calicular extensions; menianes on septal faces | [W. Pac.] Dactylotrochus Plate 9, Figures I–J |
| 53a | Columella absent or simply a rudimentary fusion of lower axial edges of major septa deep in fossa | 54 |
| 53b | Columella present (papillose, fascicular or labyrinthiform) | 57 |
| 54a | Pedicel reinforced (thickened) with stereome deposits | [Cosmopolitan] Javania Plate 9, Figures K–L |
| 54b | Pedicel reinforced with hollow rootlets, most easily seen in cross section of base or pedicel, or in a damaged corallum | 55 |
| 55a | Rootlets non-contiguous with pedicel, 2-20 adventitious rootlets anchoring the corallum | [IWP] Rhizotrochus Plate 10, Figures A–B |
| 55b | Rootlets (symmetrical or asymmetrical in placement) contiguous with pedicel, forming an integral part of the lower corallum | 56 |
| 56a | Calicular edge jagged | [W. Atl. + IP] Polymyces Plate 10, Figures C–D |
| 56b | Calicular edge smooth | [E. Atl. + W. Pac.] Monomyces Plate 10, Figures E–F |
| 57a | Columella papillose | 58 |
| 57b | Columella fascicular | 60 |
| 57c | Columella labyrinthiform | [W. Pac.] Stolarskicyathus Plate 10, Figures G–I |
| 58a | Corallum base polycyclic; no notch between upper outer edges of septa and theca | 59 |
| 58b | Base monocyclic, but may have an accessory basal rootlet; septal notch present | [W. Atl. + IWP] Gardineria Plate 10, Figures J–K |
| 59a | Pali before septa of penultimate cycle | [Atl. + E. Pac.] Concentrotheca Plate 10, Figures L–M |
| 59b | Paliform lobes present before septa of S1-2 (P1-2) | [E. Atl. + E. Pac.] Ceratotrochus Plate 11, Figures A–B |
| 59c | Pali before septa of all but last cycle in two crowns | [Atl. + Pac.] Tethocyathus Plate 11, Figures C–D |
| 60a | Corallum cylindrical and very small (calicular diameter less than 2 mm); a row of thecal spots or pores present in every interseptal region; octameral septal symmetry; only 1 columellar element | [Atl. + IWP] Guynia Plate 11, Figures E–G |
| 60b | Corallum trochoid and larger (adult calicular diameter over 10 mm); thecal spots and pores lacking; hexameral symmetry; numerous columellar elements | [IWP] Conotrochus (in part) Plate 11, Figures H–I |
| 61a | Septa arranged in a Pourtalès Plan | 62 |
| 61b | Septa arranged normally | 63 |
| 62a | Corallum base polycyclic; theca costate | [Cosmopolitan] Balanophyllia (Balanophyllia)* Plate 11, Figures J–K |
| 62b | Corallum base monocyclic; theca hispid (not costate) | [W. Atl. + SW Pac.] Thecopsammia Plate 11, Figures L–M |
| 63a | Columella absent or rudimentary | 64 |
| 63b | Columella spongy | 65 |
| 64a | Corallum trochoid; theca costate | [W. Atl.] Trochopsammia Plate 12, Figures A–B |
| 64b | Corallum subcylindrical (sometimes scolecoid); theca uniformly hispid (not costate) | [S. Africa] Pourtalopsammia Plate 12, Figures C–D |
| 65a | Costae absent; axial edges of all septa smooth; no endothecal dissepiments | [W. Atl.] Bathypsammia Plate 12, Figures E–F |
| 65b | Costae granular or hispid; axial edges of higher cycle septa dentate to laciniate; endothecal dissepiments present in an elongate corallum | 66 |
| 66a | Columella not discrete (merging with lower axial edges of septa); costae weakly granular … | [IP] Endopsammia Plate 12, Figures G–H |
| 66b | Columella discrete; costae hispid | [E. Atl. + IWP] Leptopsammia Plate 12, Figures I–J |
| 67a | Corallum unattached (free) in every growth stage (lacking transverse division) | 68 |
| 67b | Corallum undergoes transverse division, resulting in a free anthocyathus stage with a basal scar, but with a fixed anthocaulus stage | 102 |
| 68a | Corallum conical (ceratoid, trochoid or turbinate) | 69 |
| 68b | Corallum bowl-shaped | 87 |
| 68c | Corallum cupolate (theca horizontal with no surrounding vertical theca) | 91 |
| 68d | Corallum cuneiform | (Turbinoliidae, in part) 97 |
| 68e | Corallum globular (pores in base of corallum associated with commensal sipunculid) | 101 |
| 68f | Corallum cylindrical | [Atl. + IWP + Ant.] Stenocyathus Plate 12, Figures K–L |
| 69a | Columella papillose | 70 |
| 69b | Columella rudimentary or absent | 78 |
| 69c | Columella fascicular | 83 |
| 69d | Columella trabecular | 86 |
| 69e | Columella styliform | [SW Pac.] Turbinolia Plate 13, Figures A–B |
| 69f | Columella spongy | [W. Atl. + IWP] Balanophyllia (Eupsammia) Plate 13, Figures C–D |
| 70a | Pali or paliform lobes present | 71 |
| 70b | Pali and paliform lobes absent | [IWP] Foveolocyathus Plate 13, Figures E–F |
| 71a | Pali before septa of second cycle (P2) | 72 |
| 71b | Pali or paliform lobes before septa of all but last cycle | 77 |
| 71c | Pali or paliform lobes before septa of third cycle (P3) | E. Atl. + IWP + Ant.] Paraconotrochus Plate 13, Figures G–H |
| 72a | Theca bears numerous linear rows of spots, pits or thecal perforations | 73 |
| 72b | Theca solid, not bearing spots, pits or perforations | 75 |
| 73a | Theca perforate | [W. Atl. + W. Pac.] Trematotrochus Plate 13, Figures I–J |
| 73b | Theca bears linearly arranged spots or pits | 74 |
| 74a | A row of pits occurs in each interseptal space on inner theca; costae granular | [W. Pac.] Endocyathopora Plate 13, Figures K–L |
| 74b | A row of white spots occurs in each interseptal space on outer theca; theca smooth (epithecate) or covered with hispid spines | [W. Atl.] Pourtalocyathus Plate 14, Figures A–B |
| 75a | Theca bears serrate costae | 76 |
| 75b | Theca smooth (epithecate) | [SW Pac.] Lissotrochus Plate 14, Figures C–D |
| 76a | Theca covered with twice as many costae as septa | [SW Pac.] Pleotrochus Plate 14, Figures E–F |
| 76b | Costae and septa of equal number | [W. Atl. + W. Pac.] Cryptotrochus Plate 14, Figures G–H |
| 77a | Pali discrete, pairs of P3 fused into chevrons within each system; no parricidal budding | [W. Pac.] Notocyathus Plate 14, Figures I–J |
| 77b | Multiple paliform lobes on all septa; parricidal budding common | [IWP] Thrypticotrochus Plate 14, Figures K–L |
| 78a | Theca smooth (epithecate), costae not present | 79 |
| 78b | Theca granular, costae present (twice the number of septa) | 82 |
| 79a | Rows of thecal spots visible on theca | 80 |
| 79b | Thecal spots lacking | 81 |
| 80a | Twelve contiguous rootlets present in pedicel; parricidal budding absent | [W. Pac.] Pedicellocyathus Plate 15, Figures A–C |
| 80b | Rootlets lacking; parricidal budding from parent fragment common | [Atl.] Schizocyathus Plate 15, Figures D–E |
| 81a | Calicular edge smooth | [Cosmopolitan] Flabellum (Flabellum) Plate 15, Figures F–G |
| 81b | Calicular edge jagged | [Cosmopolitan] Flabellum (Ulocyathus) Plate 15, Figures H–I |
| 82a | Theca perforate; septa hexamerally arranged in 3 or 4 cycles | [IWP] Conocyathus Plate 15, Figures J–K |
| 82b | Theca imperforate; only10 septa (6+4) | [SW. Pac.] Holcotrochus Plate 15, Figures L–M |
| 83a | Pali before septa of penultimate cycle (usually P3) | 84 |
| 83b | Pali absent | [E. Pac.] Pseudocyathoceras Plate 16, Figures A–B |
| 84a | Thecal edge spines or crests present | 85 |
| 84b | Thecal edge spines and crests absent | [Cosmopolitan] Caryophyllia (Caryophyllia)(in part) Plate 16, Figures C–D |
| 85a | Base of corallum usually open, as though broken from parent through asexual budding | [Atl. + IWP] Premocyathus Plate 16, Figures E–F |
| 85b | Base of corallum intact | [W. Pac.] Caryophyllia (Acanthocyathus) Plate 16, Figures G–H |
| 86a | Theca costate; septal notch absent | [Atl. + IWP] Dasmosmilia Plate 16, Figures I–J |
| 86b | Theca smooth; septal notch present | [E. Atl. + IWP + Ant.] Aulocyathus Plate 16, Figures K–M |
| 87a | Paliform lobes on septa of all cycles; septal edges smooth | 88 |
| 87b | Pali before septa of all but last cycle; septal edges smooth | 90 |
| 87c | Pali before septa of third cycle (P3); septal edges smooth | [W. Pac.] Ericiocyathus Plate 17, Figures A–B |
| 87d | Pali and paliform lobes absent; septal edges coarsely dentate | [W. Atl. + IWP] Anthemiphyllia (in part) Plate 17, Figures C–D |
| 88a | Lower outer edge of corallum bears tubercles or spines on the C1 or C1-2 | 89 |
| 88b | Tubercles and spines absent | [Atl. + IWP] Stephanocyathus (Stephanocyathus) Plate 17, Figures E–F |
| 89a | Six long C1 spines on lower outer edge of corallum | [IWP] Stephanocyathus (Acinocyathus) Plate 17, Figures G–H |
| 89b | Twelve to 18 short spines or tubercles on lower outer edge of corallum | [W. Atl. + IWP] Stephanocyathus (Odontocyathus) Plate 17, Figures I–J |
| 90a | Six C1 spines on lower outer edge of corallum | [W. Pac.] Trochocyathus (Aplocyathus) Plate 17, Figures K–M |
| 90b | Costal spines absent | [Atl. +Pac.] Deltocyathoides Plate 18, Figures A–C |
| 91a | Costae alternate in position with septa; higher cycle septa increase by bifurcation; thecal base perforate | (Micrabaciidae) 92 |
| 91b | Costae continuous with septa; higher cycle septa increase by adding additional cycles; base imperforate | 95 |
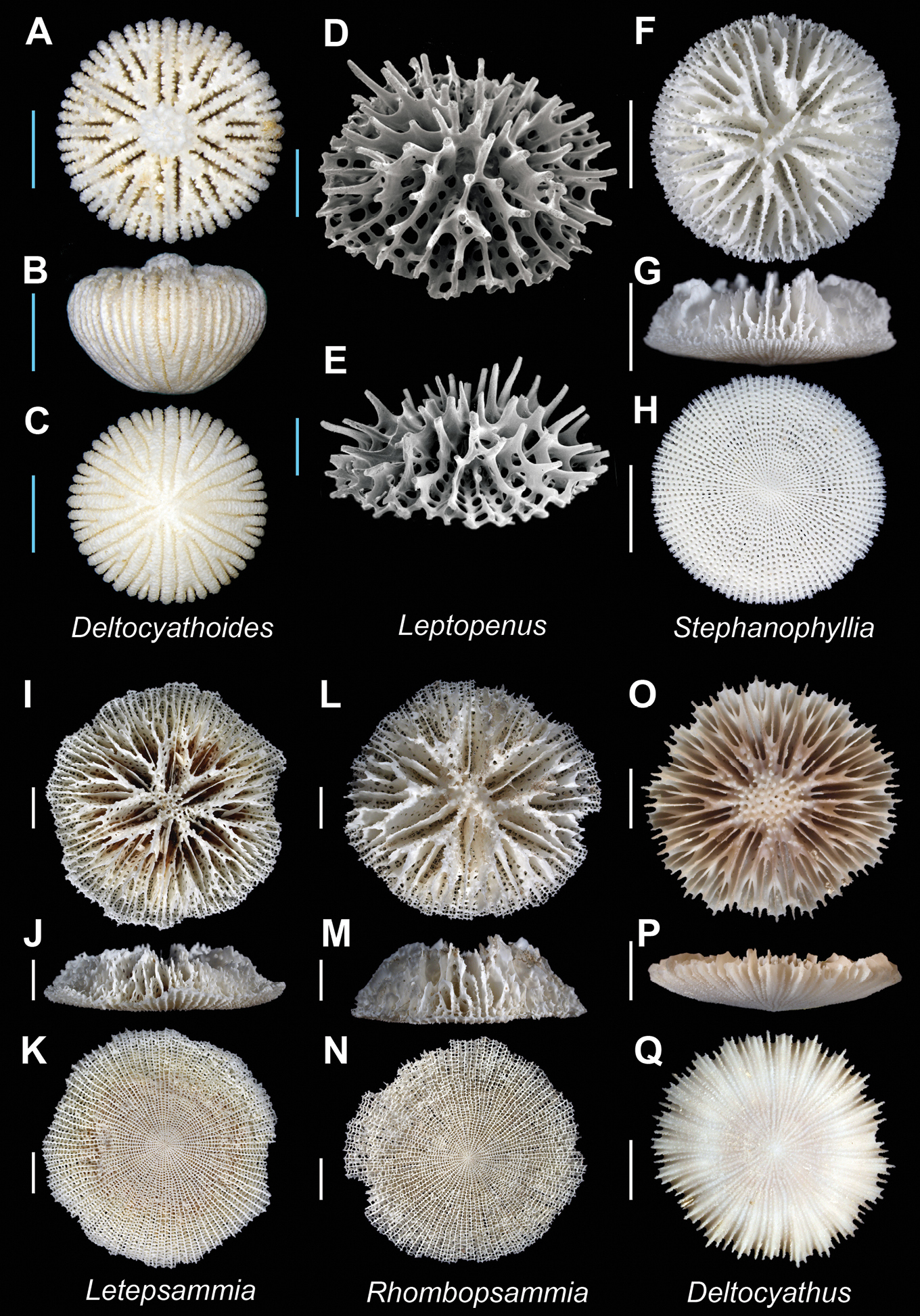
| 92a | Septa rudimentary, composed of a series of tall spines | [Cosmopolitan] Leptopenus Plate 18, Figures D–E |
| 92b | Septa lamellar | 93 |
| 93a | Marginal shelf present; columella spongy | 94 |
| 93b | Marginal shelf absent; columella solid | [IWP] Stephanophyllia Plate 18, Figures F–H |
| 94a | Septa highly porous | [IWP] Letepsammia Plate 18, Figures I–K |
| 94b | Septa essentially imperforate, porous only at points at which septa bifurcate | [IWP] Rhombopsammia Plate 18, Figures L–N |
| 95a | Synapticular platelets absent; corallum robust; upper septal edges smooth | [Atl. + IP] Deltocyathus Plate 18, Figures O–Q |
| 95b | Synapticular platelets brace adjacent septa; corallum fragile; upper septal edges bear slender elongate spines | 96 |
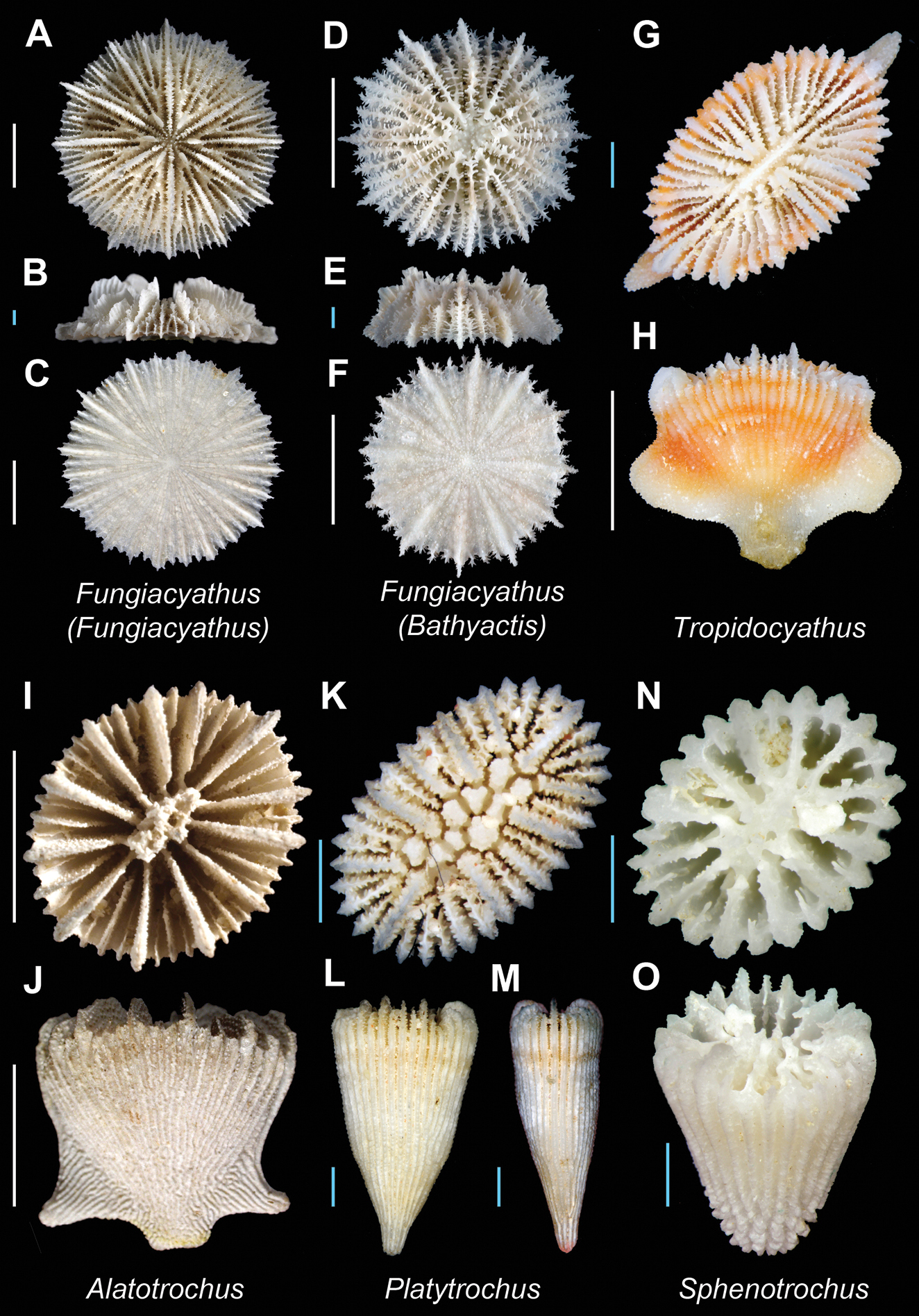
| 96a | Corallum with five cycles of septa (96 septa) | [Atl. + IWP. + Ant.] Fungiacyathus (Fungiacyathus) Plate 19, Figures A–C |
| 96b | Corallum with four cycles of septa (48 septa) | [Cosmopolitan] Fungiacyathus (Bathyactis) Plate 19, Figures D–F |
| 97a | Thecal edge crests present | 98 |
| 97b | Thecal edge crests absent | 100 |
| 98a | Pali absent | 99 |
| 98b | Pali present, before septa of all but last cycle | [IWP] Tropidocyathus Plate 19, Figures G–H |
| 99a | Twice as many costae as septa | [W. Pac.] Alatotrochus Plate 19, Figures I–J |
| 99b | Equal number of costae and septa | [IWP] Platytrochus Plate 19, Figures K–M |
| 100a | Columella lamellar; pali absent | [Atl. + Pac.] Sphenotrochus Plate 19, Figures N–O |
| 100b | Columella papillose; pali before septa of all but last cycle | [IWP] Cyathotrochus Plate 20, Figures A–B |
| 101a | Theca imperforate (although septa may be perforate) | [IWP + W. Atl.] Heterocyathus* Plate 20, Figures C–E |
| 101b | Theca and septa perforate | [IWP] Heteropsammia* (in part) Plate 20, Figures F–H |
| 102a | Columella papillose | 103 |
| 102b | Columella spongy | 108 |
| 102c | Columella a solid fusion in center of calice | 109 |
| 102d | Columella absent | 110 |
| 102e | Columella fascicular | 111 |
| 102f | Columella lamellar | [IWP] Placotrochus Plate 20, Figures I–J |
| 102g | Columella trabecular | [Atl. + IWP] Placotrochides Plate 20, Figures N–O |
| 103a | Pali before septa of all but last cycle | 104 |
| 103b | Pali before S1-2 (P1-2) | 106 |
| 103c | Pali before S2 (P2) | [SW Pac.] Kionotrochus Plate 21, Figures A–B |
| 103d | Pali absent | [W. Atl. + IWP] Anthemiphyllia (in part) Plate 21, Figures C–E |
| 104a | Six long C1 spines on lower outer edge of corallum | [W. Pac.] Bourneotrochus Plate 21, Figures F–H |
| 104b | Thecal spines absent, although corallum may bear thecal edge crests | 105 |
| 105a | Corallum small (calicular diameter usually less than 5 mm); higher cycle septa bend toward and fuse with adjacent lower cycle septa | [Atl. + W. Pac.] Peponocyathus Plate 21, Figures I–K |
| 105b | Corallum larger (calicular diameter usually over 10 mm); higher cycle septa independent | [W. Pac.] Trochocyathus (Trochocyathus) (in part) Plate 21, Figures L–N |
| 106a | Septa alternate in position with costae; thecal spots absent | 107 |
| 106b | Septa correspond to costae; lines of thecal spots present | [W. Pac.] Temnotrochus Plate 21, Figures O–P |
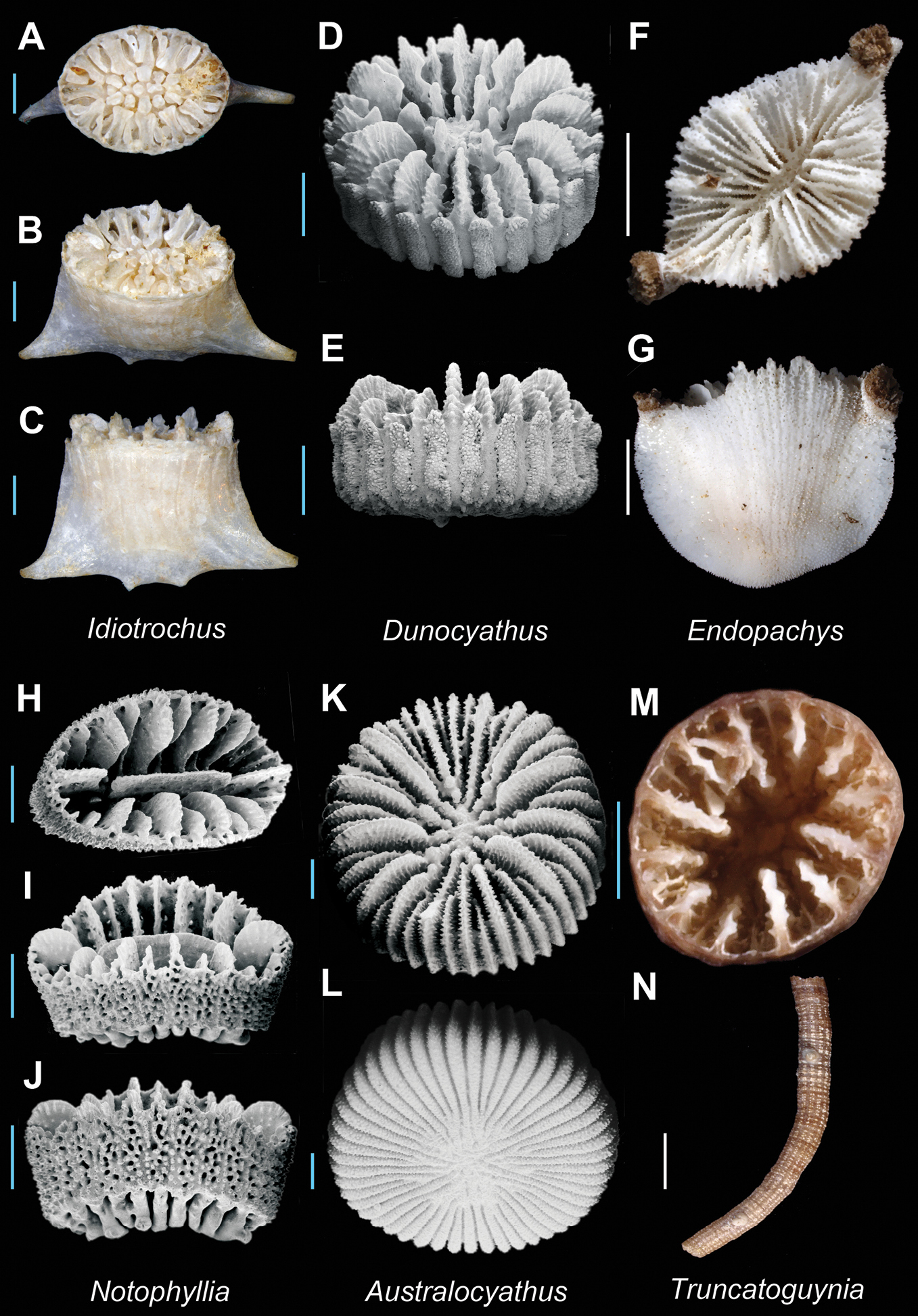
| 107a | Corallum cuneiform in shape, sometimes with basal thecal spurs (fish-tail morphology) | [W. Pac.] Idiotrochus Plate 22, Figures A–C |
| 107b | Corallum (anthocyathus) discoidal to bowl-shaped, without thecal spurs | .[W. Pac.] Dunocyathus Plate 22, Figures D–E |
| 108a | Septa arranged in a Pourtalès Plan; thecal edges often crested | [IP] Endopachys Plate 22, Figures F–G |
| 108b | Septa normally arranged; thecal edges rounded | [SW Pac.] Notophyllia Plate 22, Figures H–J |
| 109a | Multiple paliform lobes on septa of all but last cycle; thecal spots absent; corallum discoidal to cupolate in shape | [Indian] Australocyathus Plate 22, Figures K–L |
| 109b | Pali and paliform lobes absent; linear rows of thecal spots present; corallum compressed-cylindrical | [W. Pac.] Truncatoguynia Plate 22, Figures M–N |
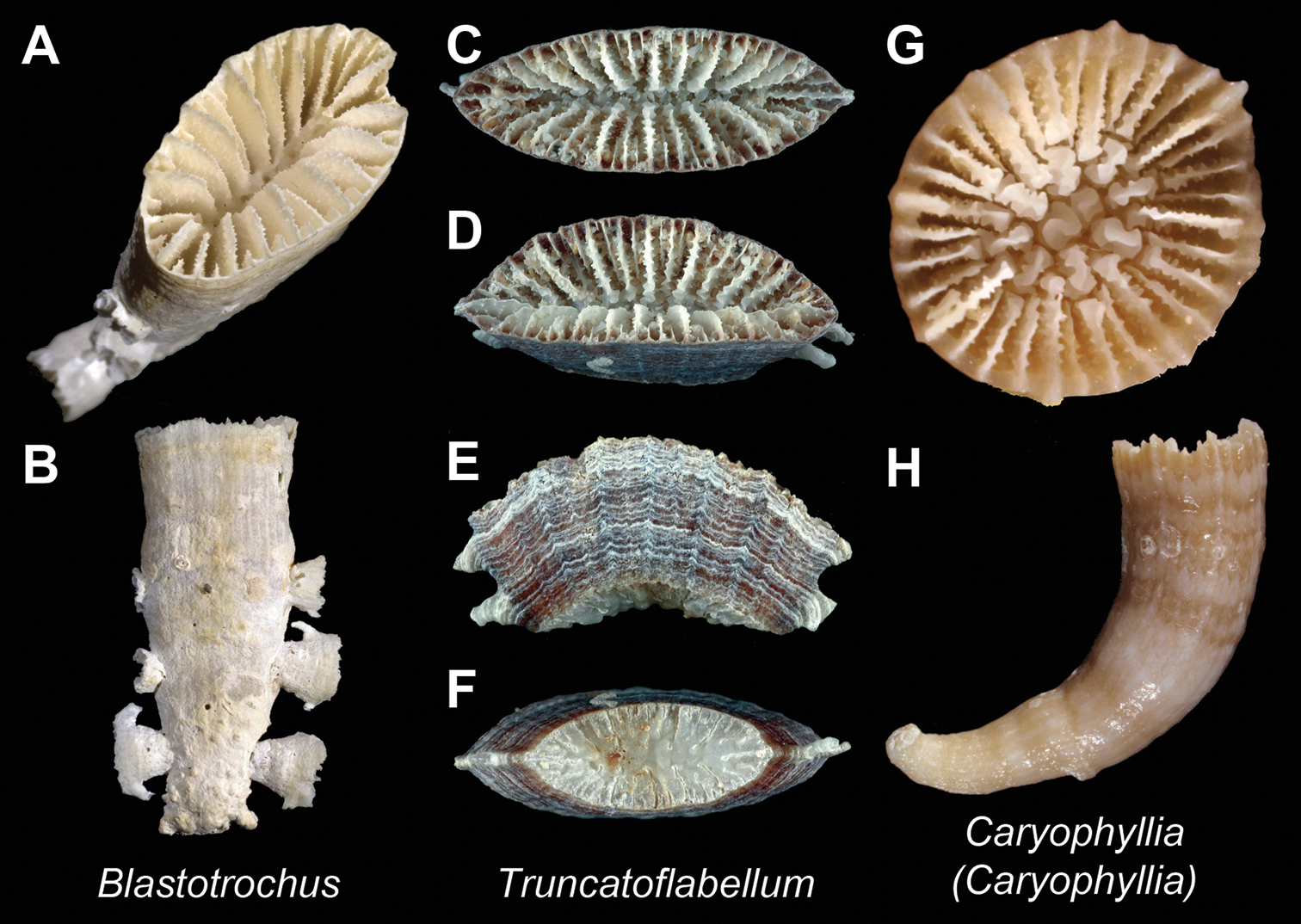
| 110a | Buds propagate from thecal edges of corallum | [W. Pac.] Blastrotrochus Plate 23, Figures A–B |
| 110b | No budding from thecal edges | [E. Atl. +IWP + Ant.] Truncatoflabellum Plate 23, Figures C–F |
| 111a | Pali before third cycle septa; theca costate | [Cosmopolitan] Caryophyllia (Caryophyllia) (in part) Plate 23, Figures G–H |
| 111b | Paliform lobes before second cycle septa; epithecate | [SW Pac.] Falcatoflabellum Plate 20, Figures K-M |
We wish to thank Anna Maria Addamo for showing us the need for writing such a paper, and Helmut Zibrowius for supplying images of the genus Cladangia. MVK is also thankful to Philippe Bouchet, Bertrand Richer de Forges, and MNHN and IRD- Nouméa staff and collaborators for their great effort in collecting and preserving deep-water scleractinians used to illustrate many genera in the present study. MVK is supported by the São Paulo Research Foundation (FAPESP).
Anthocaulus: See Transverse Division.
Anthocyathus: See Transverse Division.
Apozooxanthellate: Species that have facultative symbiotic relationships with unicellular photosynthetic dinoflagellates (Symbiodinium spp.).
Axial Corallite: See Corallite.
Axial Septal Margin: See Septum.
Azooxanthellate: Species that do not have symbiotic relationships with unicellular photosynthetic dinoflagellates (Symbiodinium spp.).
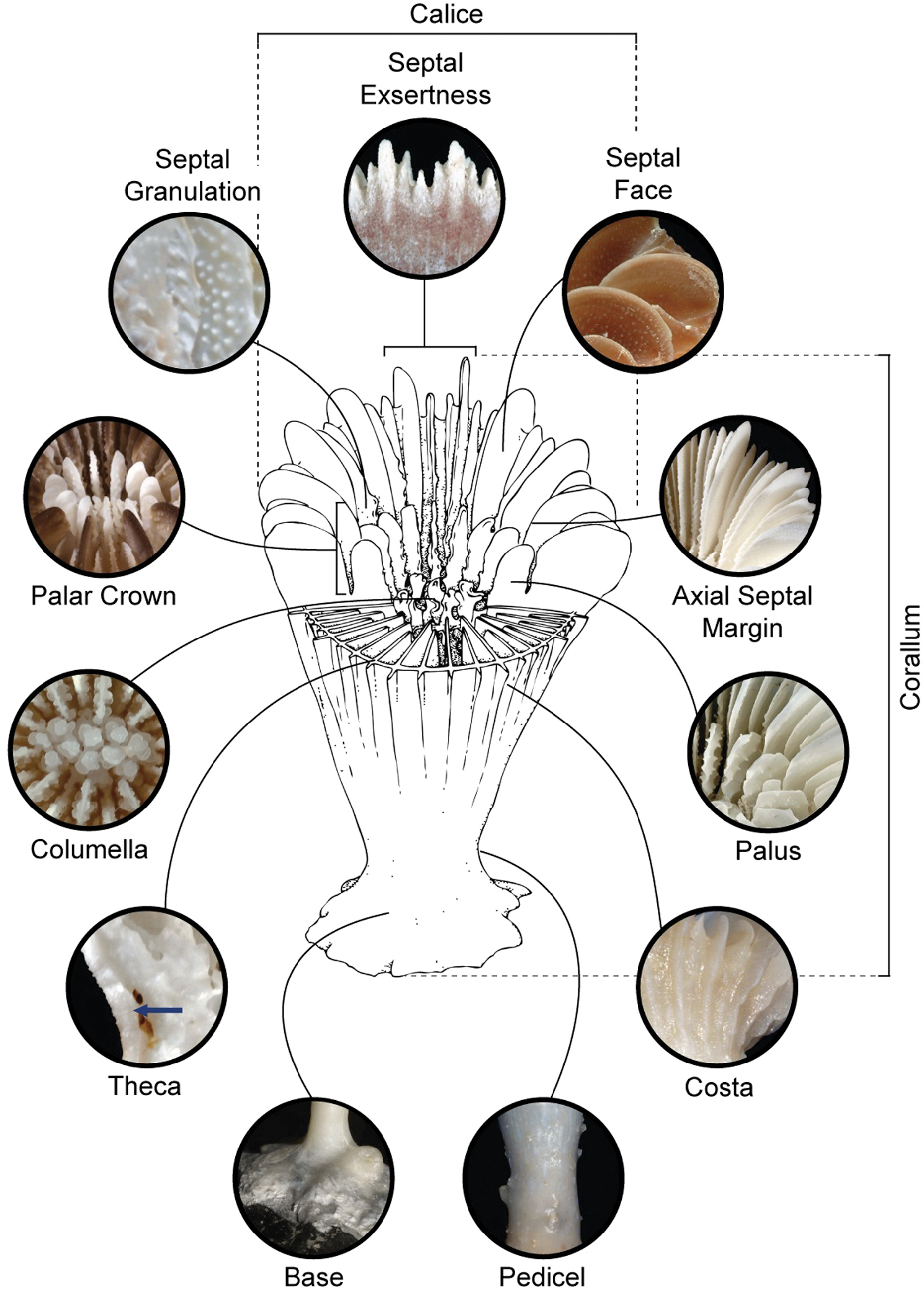
Base (Figure 1): The lower several millimeters of a solitary corallum, usually solid and composed of an accretion of thecal layers (a monocyclic base) (e.g. Plate 24, Fig. B), but in some genera composed of concentric rings of partitioned chambers, called a polycyclic base (e.g. Plate 24, Fig. C) (
Budding: The process of asexual reproduction that adds new mouths (or polyps) to a corallum, often resulting in a colony. Intratentacular budding adds new polyps to the oral disc inside the ring of tentacles surrounds its mouth (e.g. Plate 1, Fig. K–L). Extratentacular budding adds new polyps outside the ring of tentacles (e.g. Plate 2, Fig. H). (The third form of asexual reproduction is transverse division -
Calice (Figure 1) (pl. Calices): The skeletal analog of the polyp, cupping the polyp from below, and consisting of the septa, and, if present, the columella and pali (e.g. Plate 1, Figs C, E, G; Plate 9, Figs A, C, E, G, I, K).
Ceratoid Corallum: See Solitary Corallum.
Coenosteum: The skeletal structure found between the individual corallites of a colonial corallum, including the costae, and various kinds of dissepiments; sometimes called peritheca (e.g. Plate 24, Fig. E).
Colonial Corallum: See Corallum.
Columella (Figure 1): An axial structure of diverse shape and composition that projects from the center of a calice. If in the shape of a single lamella (called lamellar) (e.g. Plate 20, Fig. I), if a maze of interconnected lamellae (labyrinthiform) (e.g. Plate 8, Fig. A), if a set of twisted lamellae (fascicular) (e.g. Plate 20, Figs C, E), if a simple rod (styliform) (e.g. Plate 13, Fig. A), if a group of rods (papillose) (e.g. Plate 8, Fig. K), if a fine porous mass (spongy) (e.g. Plate 12, Fig. G), and if an irregular group of twisted elements (trabecular) (e.g. Plate 16, Fig. K).
Conical Corallum: See Solitary Corallum.
Corallite: The vertical, usually cylindrical, structure produced by an individual polyp, consisting of endothecal dissepiments and the calice at the upper end (e.g. Plate 1, Fig. F). If a corallite occurs at the tip of a colony’s branch, it is termed an axial corallite (e.g. Plate 24, Fig. F).
Corallum (Figure 1) (pl. Coralla): The aragonitic calcium carbonate skeleton of a scleractinian coral. If the coral has only one mouth (or calice), it is termed solitary (e.g. Plate 10, Figs A–M; Plate 17, Figs A–M), if polystomatous (or more than one calice), then a colonial (e.g. Plate 2, Fig. A–L; Plate 3, Figs A–L).
Costae (Figure 1) (sing. Costa; adj. Costate): Continuation of a septum on the outside of the corallite wall, often as a ridge or low linear mound (e.g. Plate 24, Figs A, D, G, M).
Crest: See Edge Spine.
Crown (Figure 1): See Palus.
Cuneiform Corallum: See Solitary Corallum.
Cupolate Corallum: See Solitary Corallum.
Cycle: See Septum.
Cylindrical Corallum: See Solitary Corallum.
Discoidal Corallum: See Solitary Corallum.
Dissepiments: Thin horizontal (tabular dissepiments, e.g., Plate 2, Fig. K) or blister-like plates that form within a corallite (endothecal) or beneath the coenosteum outside corallites (exothecal), which separate the polyp from the lower part of the corallum that it no longer occupies.
Distomodeal Budding: A mode of intratentacular budding in which two polyps (or calices) develop within the common tentacular ring (e.g., Plate 1, Figs I–K).
Edge Spine/Crest/Spur: The external thecal edges of a solitary coral, those associated with the principal septa, sometimes bears a low thin crest, or a series of hollow spines. If the crest is limited to the basal portion of the corallum and project outward in the shape of a fish tail, they may be called spurs (e.g. Plate 24, Figs H–M).
Edge Zone: The fold of the polyp body that extends over the edge of the theca (e.g. Plate 25, Fig. A).
Endothecal: See Dissepiments.
Epitheca: Thin, external, smooth or wrinkled, non-trabecular sheath surrounding individual corallites, formed by centripetal (inward) growth (e.g. Plate 10, Fig. I, K; Plate 11, Figs D, I; Plate 12, Fig. F). Tectura is very similar in outward appearance by originates by centrifugal (outward) growth (e.g. Plate 25, Fig. B) (
Exothecal: See Dissepiments.
Extratentacular Budding: See Budding.
Fascicular Columella: See Columella.
Free: An unattached corallum (e.g. Plate 14, Figs A–L; Plate 17, Figs A–M; Plate 18, Figs A–Q).
Globular Corallum: See Solitary Corallum.
Imperforate Theca: See Theca.
Intratentacular Budding: See Budding.
Labyrinthiform Columella: See Columella.
Lamellar Columella: See Columella.
Marginal Shelf: A low rim encircling a solitary corallum composed of greatly reduced septa and costae, or costal spines (
Menianes: Short ledge-like features on septal faces formed by aligned lateral extensions of trabeculae (e.g. Plate 25, Fig. C).
Monocyclic Base: See Base.
Monostomaeous/Monostomatous: A single-mouthed corallum, i.e., a solitary form (e.g. Plate 8, Figs A–F).
Normal Arrangement of Septa: Arrangement of septa within a calice in which the septa are independent and all aligned with the center of the calice. (See Pourtalès Plan) (e.g. Plate 8, Fig. E; Plate 9, Fig. E).
Paliform Lobes: Small, flattened lobes on the axial septal edge of various cycles, often more than one per septum, and part of the septum to which they are attached (e.g., Plate 17, Fig. I).
Palus (Figure 1) (pl. Pali): Small flattened lobes on the axial septal edge of various cycles, always one per septum, and not part of the septum to which it is attached but ontogenetically different. Groups of pali occurring on the same cycle of septa and thus stand at the same distance from the center of the calice are called crowns of pali (e.g. Figure 1).
Papillose Columella: See Columella.
Parricidal Budding: A mode of intratentacular budding in which new polyps are generated from the inner surface of a fragment of a parent corallum that has longitudinally split apart (e.g. Plate 16, Fig. J, L–M).
Pedicel (Figure 1): The stem-like region of a solitary coral just above the base and below the calicular surface.
Perforate Theca: See Theca.
Peritheca: See Coenosteum.
Polycyclic Base: See Base.
Polystomaeous/Polystomatous: See Corallum.
Pourtalès Plan: A form of septal arrangement in which the axial edges of pairs of higher cycle septa bend in front of and unite before their adjacent lower cycle septum (e.g. Plate 25, Fig. E). See
Reptoid Budding: A type of extratentacular budding in which polyps are asexually generated from a thin, reticulate, encrusting ribbon (similar to stoloniferous budding) (e.g. Plate 6, Fig. B; Plate 7, Fig. F).
Scolecoid Corallum: See Solitary Corallum.
Septum (pl. Septa): Radially arranged longitudinal partitions of a corallite (Figure 1), usually arranged in hexameral symmetry. Septa are added in cycles, the first cycle composed of 6 septa, the second also of 6, the third of 12, the fourth of 24, the fifth of 48, etc. resulting in corallites consisting of 6, 12, 24, 48, or 96, etc. septa per calice. Septa can bear smooth, dentate, or laciniate axial margin (Figure 1).
Solitary Corallum: Solitary coralla exist in a variety of shapes, the shape being one of the criteria used to differentiate genera and species. Many solitary coralla are shaped as an inverted cone (conical), and may be attached and straight or free and usually curved. If the edges of the cone diverge at a hypothetical basal angle of 10–40°, this corallum is called ceratoid (e.g. Plate 10, Figs H–I), if the angle is 40–60°, then trochoid (e.g. Plate 8, Fig. F), if the angle is 60–80°, turbinate (e.g. Plate 13, Fig. H), and if the angle is160–180° and the corallum is low, discoidal (e.g. Plate 18, Fig. P). Coralla may also be cylindrical (e.g. Plate 22, Fig. N), and if the cylinder is irregular in shape, scolecoid (e.g. Plate 12, Fig. D). Others are wedge-shaped (cuneiform) or bowl-shaped (e.g. Plate 17, Figs F, H, J, M). Still others have a flat base with a convex upper surface (cupolate) (e.g. Plate 19, Figs A–F) and others are simply onion-shaped or irregular (globular) (e.g. Plate 18, Fig. B).
Spongy Columella: See Columella.
Stereome: A general term for thick calcareous deposits, generally thickening various parts of the corallum.
Stoloniferous Budding: A type of extratentacular budding in which polyps are asexually generated from a thin, elongate, encrusting coenenchymal ribbon, the connecting ribbon often obscured by encrusting organisms (e.g. Plate 5, Fig. B; Plate 6, Fig. D).
Styliform Columella: See Columella.
Synapticular Plate: Ribbons of calcium carbonate linking adjacent fungiacyathid septa, first appearing as vertical rods midway between septa, later bifurcate, the two ends fusing to adjacent septal faces (T- or Y-shaped) (
Trabecular Columella: See Columella.
Tabular Endothecal Dissepiment: See Dissepiment.
Tectura: See Epitheca.
Theca (Figure 1): The skeletal sides, or walls, of solitary coralla or corallites of colonial corals, that enclose the polyps. If the theca is solid, it is termed imperforate (e.g. Plate 9, Figs D, L); if the theca is porous, perforate (e.g. Plate 22, Figs I–J).
Thecal spots/pores: Some genera have longitudinal series of small pores aligned with the interseptal spaces of various cycles, termed thecal pores. In other genera, these analogous structures do not penetrate the theca but are visible only as slightly differently colored spots of a constructional consistently different from the remaining theca, these termed spots (e.g. Plate 11, Figs F–G; Plate 22, Fig. N).
Thecal spur: See Edge Spine.
Trabecular Columella: See Columella.
Transverse Division: One of the three main methods of asexual reproduction among the Scleractinia (
Trochoid Corallum: See Solitary Corallum.
Turbinate Corallum: See Solitary Corallum.
Zooxanthellate: Species that have symbiotic relationships with unicellular photosynthetic dinoflagellates (Symbiodinium spp.).
Cutaway diagram of a species of Caryophyllia illustrating the basic morphological features of an attached, solitary scleractinian (Modified from
Heteropsammia cochleaA (USNM 97652) and B (USNM 73772): Calicular and lateral view respectively; Anomocora gigas (MNHN uncatalogued, Terrasses stn. CP3091) C and D Calicular and lateral view respectively; Eguchipsammia fistula (USNM uncatalogued, Norfolk 2 stn. 2024) E and F Calicular and colony view respectively; Lophelia pertusa(USNM 1071877) G and H Calicular and colony view respectively; Dichopsammia granulosa(USNM 15847, holotype) I and J Calicular and colony view respectively; Solenosmilia variabilis(USNM 47426) K and L Distal branch and colony view respectively. Scale bars: blue = 1 mm; white = 5 mm; green = 50 mm. Bold face indicates type species for the genus.
Madracis asperulaA (SEM, USNM 99068) and B (USNM 99056): Calicular and colony view respectively; Cladopsammia sp. (USNM uncatalogued, Norfolk 2 stn. 2023)C and D Calicular and colony view respectively; Dendrophyllia alcocki (USNM uncatalogued, Norfolk 2 stn. 2135)E and F Calicular and colony view respectively; Enallopsammia rostrata (USNM uncatalogued, Norfolk 2 stn. DW 2056) G and H Calicular and colony (calicular and acalicular side) view respectively; Tubastraea coccinea(USNM 46973) I Calicular view; Tubastraea diaphana (USNM 83677) J Colony view; Goniocorella dumosa(USNM 47505) K and L Calicular and colony view respectively. Scale bars: blue = 1 mm; white = 5 mm; green = 50 mm. Bold face indicates type species for the genus.
Pourtalosmilia anthophyllitesA (USNM 1174947) and B (USNM 117494): Calicular and colony view respectively; Confluphyllia juncta(USNM 97316, paratype) C and D Calicular and colony view respectively; Coenosmilia arbuscula(USNM 97312) E and F Calicular and colony view respectively; Sympodangia albatrossi (USNM 97308, holotype) G (SEM) and H Calicular and colony view respectively; Madrepora oculata(MNHN uncatalogued, Halipro 2 stn. BT104) I and J Calicular and colony view respectively; Cladocora debilis K (USNM 10452, SEM) and L (USNM 62351): Calicular and colony view respectively. Scale bars: blue = 1 mm; white = 5 mm; green = 50 mm. Bold face indicates type species for the genus.
Petrophyllia rediviva (USNM 82696) A and B Distal branch and colony view respectively; Oculina virgosa (MNHN uncatalogued, SMIB 5 stn. DW101) C and D Calicular and colony view respectively; Cyathelia axillaris(USNM 92665)E and F Calicular and colony view respectively; Bathelia candida(USNM 47512) G and H Calicular and colony view respectively; Sclerhelia hirtella(MNHN Michellin collection) I and J Calicular and colony view respectively; Madrepora minutiseptum (MNHN uncatalogued, SMIB 5 stn. DW101) K and L Calicular and colony view respectively. Scale bars: blue = 1 mm; white = 5 mm; green = 50 mm. Bold face indicates type species for the genus.
Madracis pharensis A (USNM 96676, SEM): Colony view; Madracis sp. B (MNHN uncatalogued, New Caledonia): Colony view; Rhizopsammia sp. (USNM uncatalogued, Bathus 4 stn. DW 941) C and D Calicular and colony view respectively; Astroides calycularis(USNM 78767) E and F Calice detail and colony view respectively; Tubastraea coccinea(USNM 46973) G and H Calicular and colony view respectively; Thalamophyllia tenuescens I (MNHN uncatalogued, Bathus 3 stn. CH802), J and K (Norfolk 2 stn. 2095): Calicular and corallum views respectively; Hoplangia durotrix(AU 6097) L (SEM) and M (SEM): Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm; green = 50 mm. Bold face indicates type species for the genus.
Colangia immersa (USNM 73917) A and B Calicular and colony view respectively; Culicia stellata(MNHN uncatalogued, New Caledonia) C and D Calicular and stolon connection view respectively; Oulangia bradleyi (USNM 92371)E and F Calicular and lateral view respectively; Astrangia danae (USNM 78507, SEM) G Calicular view; Astrangia poculata(USNM 80350, neotype) G and H and colony view respectively; Cladangia exusta (YPM 1359, syntype ?) I and J Calicular and colony view respectively; Rhizosmilia sagamiensis (USNM uncatalogued, Norfolk 2 stn. 2124) K and L Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Polycyathus sp. (MNHN uncatalogued, Beryx 11 stn. DW11) A and B Calicular and colony view respectively; Phacelocyathus flos(USNM 46077)C and D Calicular and colony view respectively; Phyllangia americana(USNM 80881)E and F Calicular and colony view respectively; Bathycyathus chilensis(USNM 100711) G and H Calicular and colony view respectively; Coenocyathus anthophyllites (USNM 48694)I and J Calicular and colony view respectively; Nomlandia californica(SBMNH 35560, holotype)K Calicular view (after
Labyrinthocyathus limatulus (USNM uncatalogued, Bathus 4 stn. DW 936)A and B Calicular and lateral view respectively; Oxysmilia corrugata (USNM uncatalogued, Norfolk 2 stn. DW2125)C and D Calicular and lateral view respectively; Monohedotrochus circularis (USNM uncatalogued, Norfolk 2 stn. DW2124)E and F Calicular and lateral view respectively; Lochmaeotrochus oculeus(USNM uncatalogued, Musorstom 6 stn. DW394)G and H Calicular and “aggregation” view respectively; Paracyathus sp. (MNHN uncatalogued, Ebisco stn. DW2555) I and J Calicular and lateral view respectively; Trochocyathus efateensis (USNM uncatalogued, Bathus 4 stn. DW818) K and L Calicular and lateral view respectively. Scale bars represent 5 mm. Bold face indicates type species for the genus.
Vaughanella concinna (USNM uncatalogued, Norfolk 2 stn. DW2070) A and B Calicular and lateral view respectively; Caryophyllia (Caryophyllia) diomedeae (USNM uncatalogued, Norfolk 2 stn. DW2086) C and D Calicular and lateral view respectively; Crispatotrochus rubescens (USNM uncatalogued, Bathus 3 stn. CP833)E and F Calicular and lateral view respectively; Desmophyllum dianthus(USNM uncatalogued, Halipro 1 stn. CP877) G and H Calicular and lateral view respectively; Dactylotrochus cervicornis(USNM uncatalogued, SMIB 10 stn. DW208) I and J Calicular and lateral view respectively; Javania insignis(USNM uncatalogued, Norfolk 2 stn. DW2023) K and L Calicular and lateral view respectively. Scale bars represent 5 mm. Bold face indicates type species for the genus.
Rhizotrochus typus(USNM uncatalogued, Norfolk 2 stn. DW2024)A and B Calicular and lateral view respectively; Polymyces wellsi C (MNHN uncatalogued, Ebisco stn. DW2618) and D (MNHN uncatalogued, New Caledonia): Calicular and lateral view respectively; Monomyces pygmaea(USNM 48561) E and F Calicular and lateral view respectively; Stolarskicyathus pocilliformis(MNHN uncatalogued, Ebisco stn. DW2573) G, H, and I Calicular, oblique, and lateral view respectively; Gardineria hawaiiensis(USNM uncatalogued, Norfolk 2 stn. DW2086) J and K Calicular and lateral view respectively; Concentrotheca laevigata(USNM 80748) L and M Calicular and oblique view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Ceratotrochus magnaghii (USNM 48780) A and B Calicular and lateral view respectively; Tethocyathus virgatus (USNM uncatalogued, SMIB 10 stn. DW205) C and D Calicular and lateral view respectively; Guynia annulata(MNHN uncatalogued, Biogeocal stn. DW253) E, F and G Calicular, lateral, and oblique view respectively; Conotrochus funicolumna (USNM uncatalogued, Bathus 4 stn. CP967) H and I Calicular and lateral view respectively; Balanophyllia (Balanophyllia) laysanensis (MNHN uncatalogued, Musorstom 6 stn. DW407) J and K Calicular and lateral view respectively; Thecopsammia socialis(USNM 61828) L and M Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Trochopsammia infundibulum(USNM 46722)A and B Calicular and lateral view respectively; Pourtalopsammia togata(USNM 91792) C and D Calicular and lateral view respectively; Bathypsammia tintinnabulum (USNM 14569)E and F Calicular and lateral view respectively; Endopsammia philippensis(USNM 83006) G and H Calicular and lateral view respectively; Leptopsammia stokesiana(USNM 78603) I and J Calicular and lateral view respectively; Stenocyathus vermiformis(USNM uncatalogued, Norfolk 2 stn. ?) K and L Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
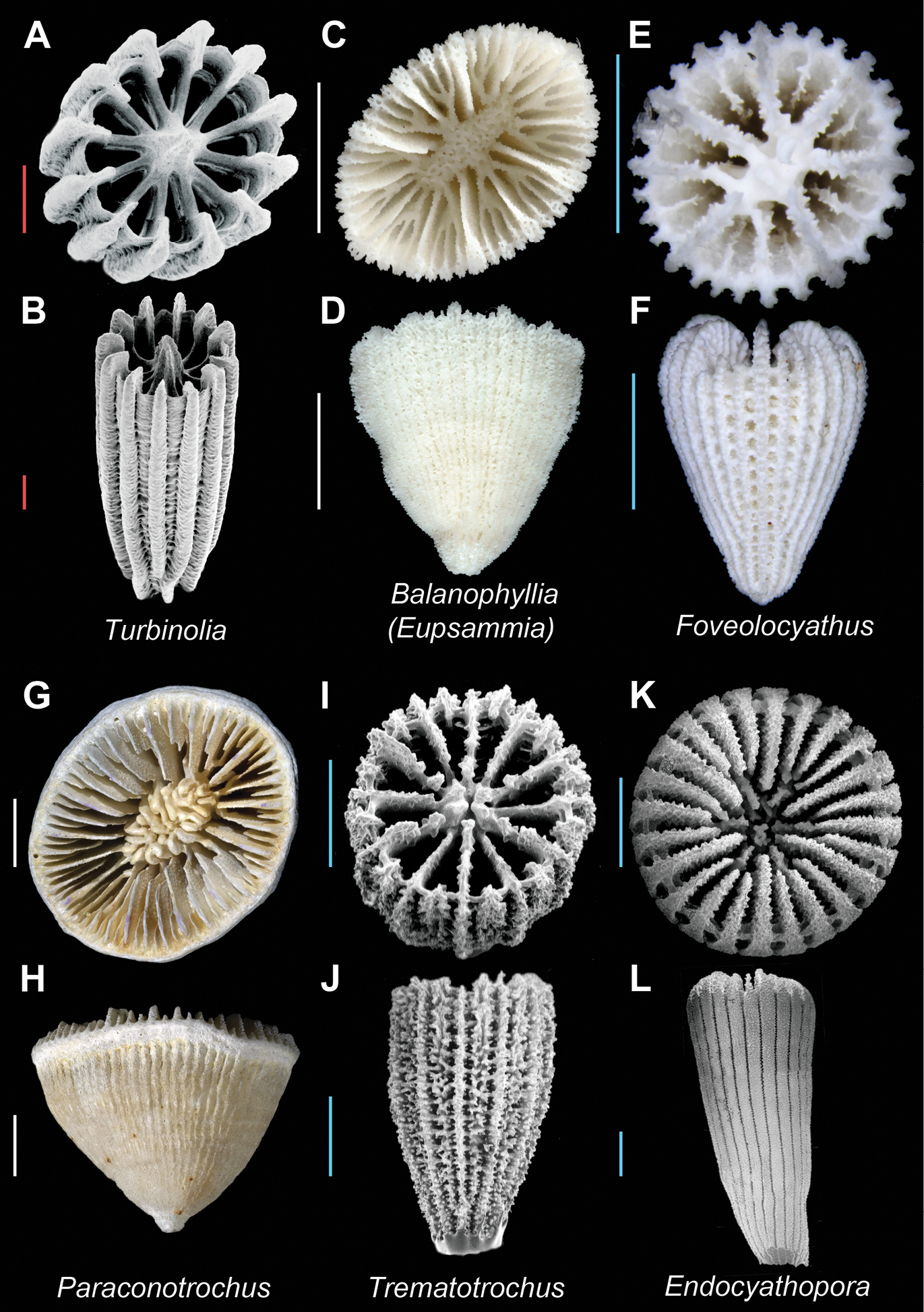
Turbinolia stephensoni (USNM 80014) A (SEM) and B (SEM): Calicular and oblique view respectively; Balanophyllia (Eupsammia) carinata (MNHN uncatalogued, Chalcal stn. D22) C and D Calicular and lateral view respectively; Foveolocyathus parkeri (MNHN uncatalogued, Musorstom 5 stn. 280)E and F Calicular and lateral view respectively; Paraconotrochus zeidleri(USNM 85677, paratype) G and H Calicular and lateral view respectively; Trematotrochus corbicula (USNM 46477) I (SEM) and J (SEM): Calicular and lateral view respectively; Endocyathopora laticostata (USNM 81894) K (SEM) and L (SEM): Calicular and lateral view respectively. Scale bars: red = 0.25 mm; blue = 1 mm; white = 5 mm; green = 50 mm. Bold face indicates type species for the genus.
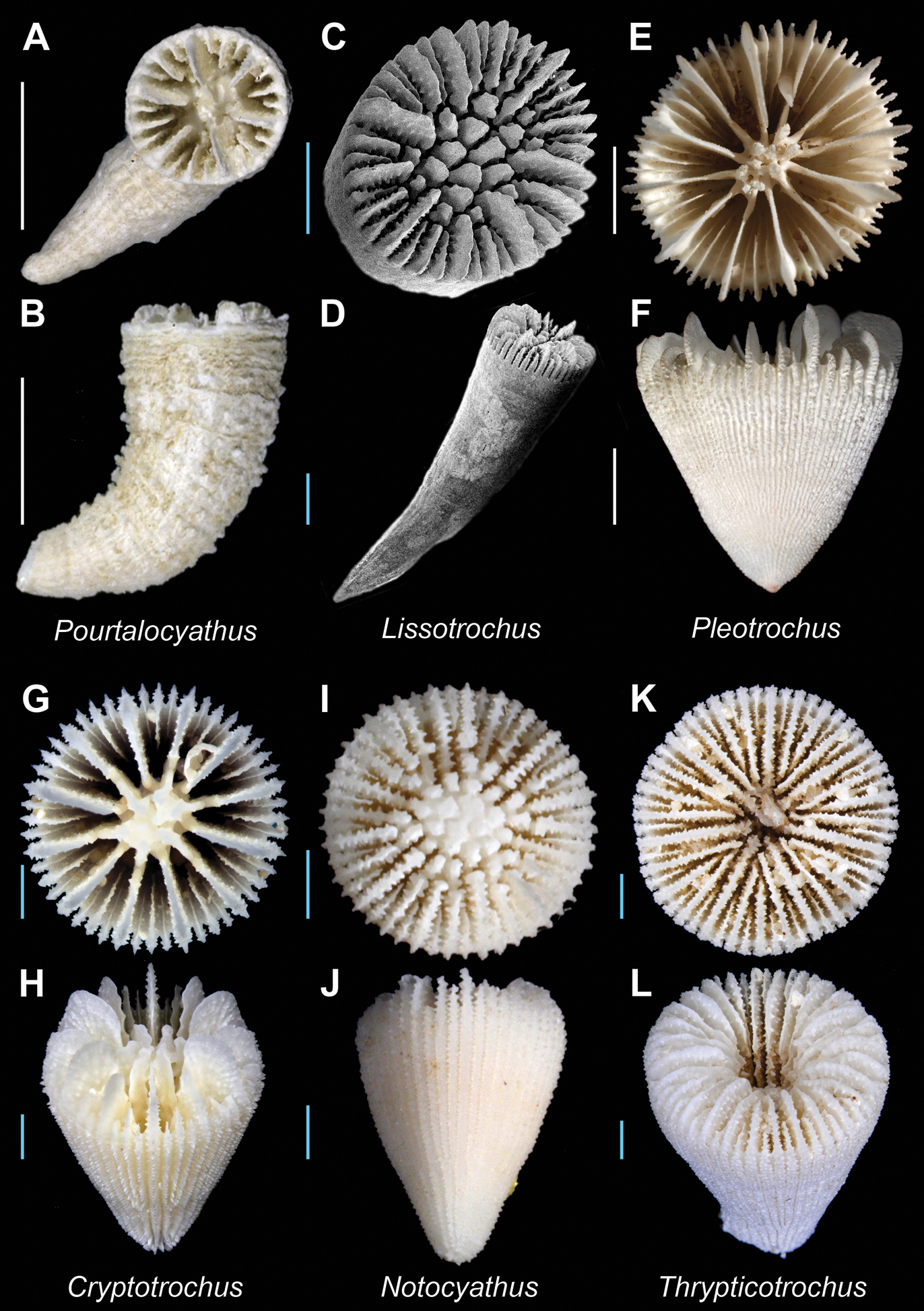
Pourtalocyathus hispidus(USNM 61928)A and B Calicular and lateral view respectively; Lissotrochus curvatus(AM G16745) C (SEM) and D (SEM): Calicular and oblique view respectively; Pleotrochus venustus(USNM uncatalogued, Norfolk 2 stn. DW 2104) E and F Calicular and lateral view respectively; Cryptotrochus sp. (MNHN uncatalogued, Ebisco stn. DW2603) G and H Calicular and oblique view respectively; Notocyathus venustus (USNM uncatalogued, Bathus 4 stn. DW 958) I and J Calicular and lateral view respectively; Thrypticotrochus petterdi(MNHN uncatalogued, Ebisco stn. DW2561) K and L Calicular and oblique view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
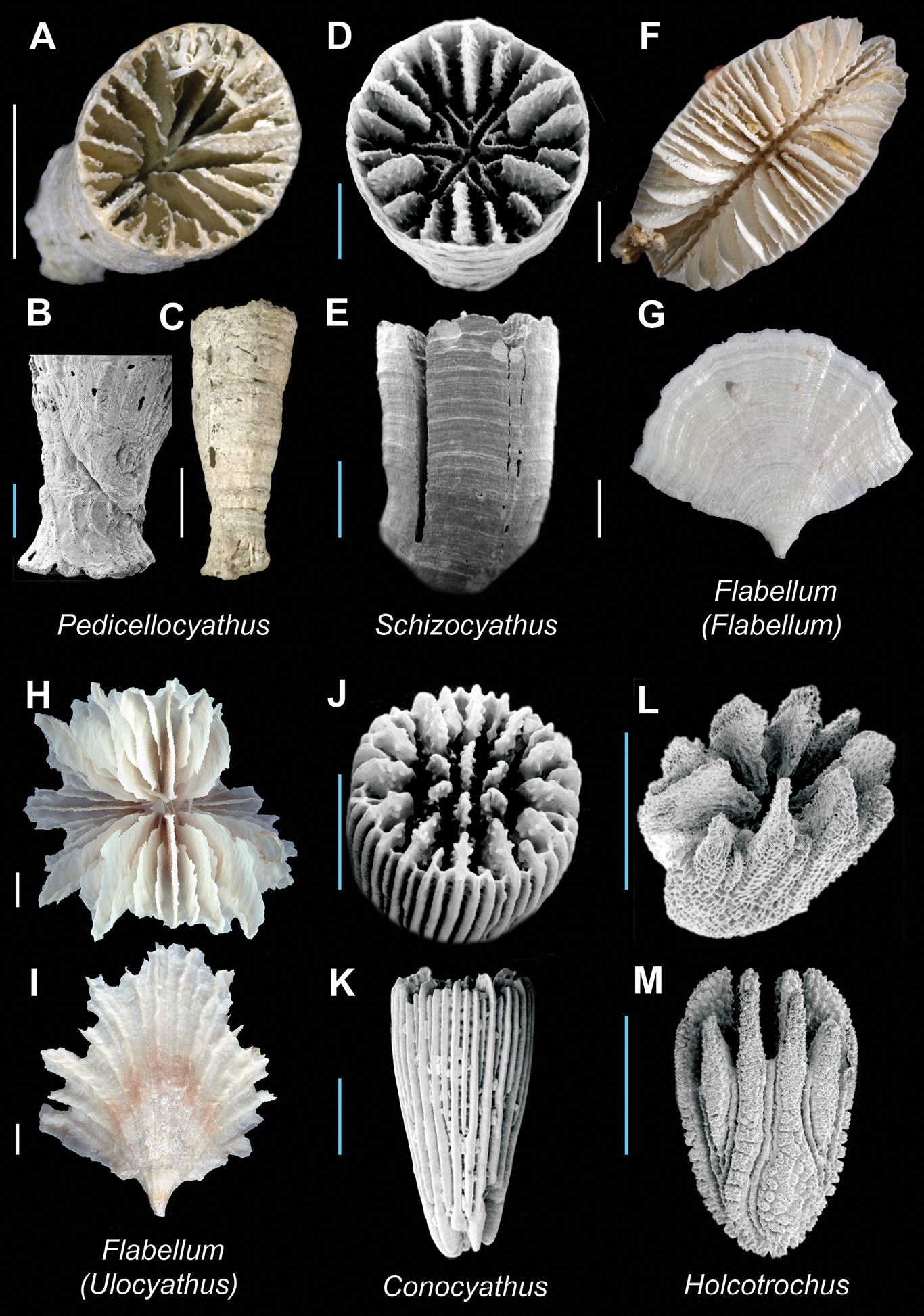
Pedicellocyathus keyesi(USNM 94268, paratype) A, B (SEM), and C Calicular, pedicel detail, and lateral view respectively; Schizocyathus fissilis(USNM 61747) D (SEM) and E (SEM): Calicular and lateral view respectively; Flabellum (Flabellum) politum (USNM uncatalogued, Bathus 4 stn. DW933) F and G Calicular and lateral view respectively; Flabellum (Ulocyathus) messum (MNHN uncatalogued, Bathus 1 stn. DW661) H and I Calicular and lateral view respectively; Conocyathus zelandiae (USNM 85713) J (SEM) and K (SEM): Oblique and lateral view respectively; Holcotrochus scriptus (USNM 85687) L (SEM) and M (SEM): Oblique and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
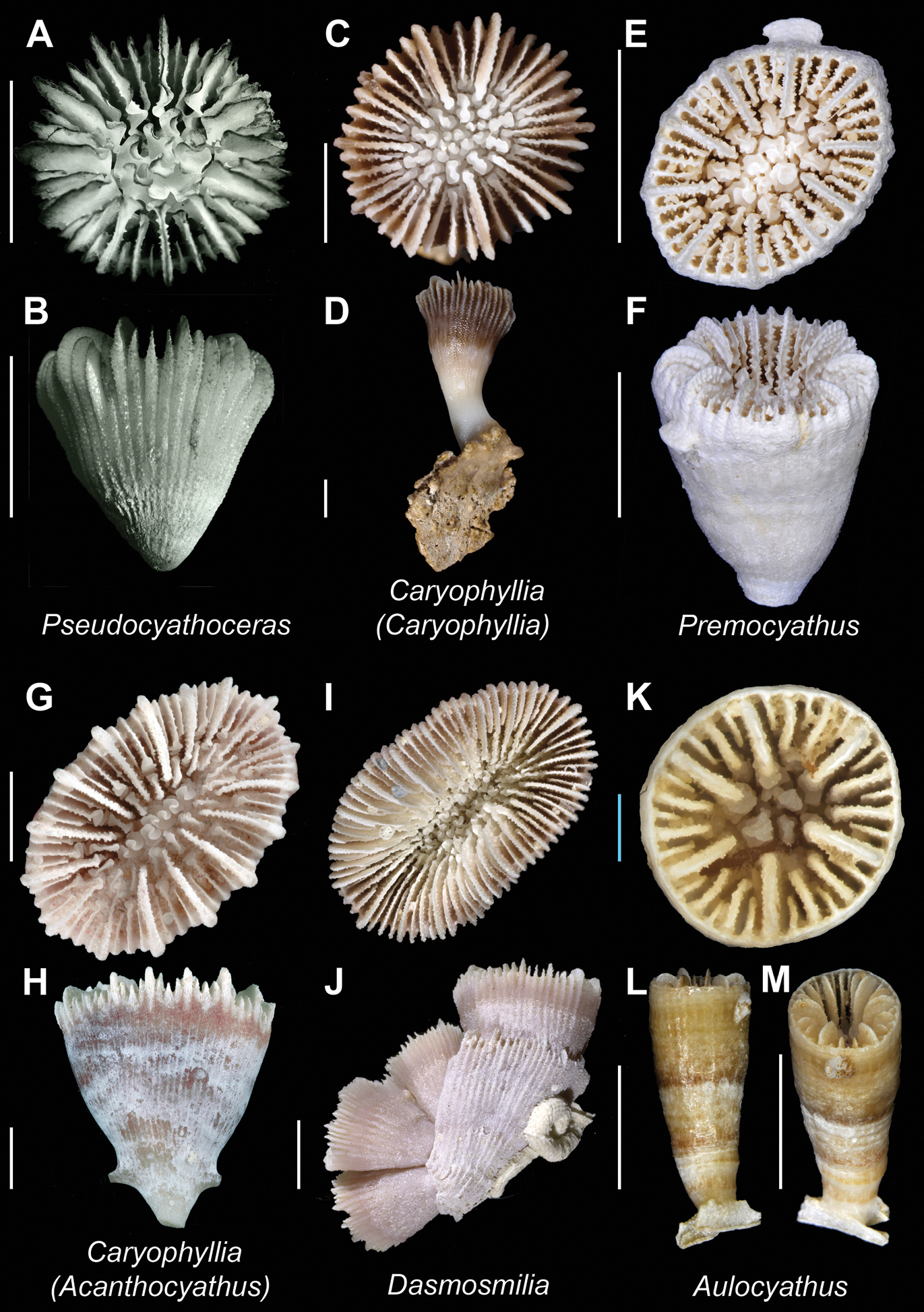
Pseudocyathoceras avis(USNM 46962, holotype)A and B Calicular and lateral view respectively; Caryophyllia (Caryophyllia) quadragenaria (USNM uncatalogued, PrFO, New Caledonia) C and D Calicular and oblique view respectively; Premocyathus dentiformis(MNHN uncatalogued, Ebisco stn. DW2573) E and F Calicular and oblique view respectively; Caryophyllia (Acanthocyathus) grayi(MNHN uncatalogued, Ebisco stn. DW2559) G and H Calicular and lateral view respectively; Dasmosmilia lymani(USNM 82997) I and J Calicular and lateral (aggregation) view respectively; Aulocyathus juvenescens(MNHN uncatalogued, Lifou 2000 stn. DW37) K, L, and M Calicular, lateral, and oblique view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Ericiocyathus echinatus(USNM 97169, holotype)A and B Calicular and lateral view respectively; Anthemiphyllia patera costata (USNM uncatalogued, Norfolk 2 stn. 2066) C and D Calicular and lateral view respectively; Stephanocyathus (Stephanocyathus) regius (USNM uncatalogued, Bathus 3 stn. 858) E and F Calicular and lateral view respectively; Stephanocyathus (Acinocyathus) spiniger(USNM uncatalogued, Bathus 3 stn. CP877) G and H Calicular and lateral view respectively; Stephanocyathus (Odontocyathus) coronatus (USNM uncatalogued, Bathus 4 stn. CP950) I and J Calicular and lateral view respectively; Trochocyathus (Aplocyathus) brevispina (MNHN uncatalogued, Musorstom 8 stn. DW960) K, L, and M Calicular, oblique, and lateral view respectively. Scale bars represent 5 mm. Bold face indicates type species for the genus.
Deltocyathoides orientalis(MNHN uncatalogued, Bathus 3 stn. DW829) A, B and C Calicular, lateral, and base view respectively; Leptopenus discus(SIO Co-1271) D (SEM) and E (SEM): Oblique views respectively; Stephanophyllia complicata (USNM uncatalogued, New Caledonia) F, G, and H Calicular, lateral, and base view respectively; Letepsammia formosissima(USNM uncatalogued, Norfolk 2 stn. DW2032) I, J, and K Calicular, lateral, and base view respectively; Rhombopsammia niphada (USNM uncatalogued, Norfolk 2 stn. DW2069) L, M, and N Calicular, lateral, and base view respectively; Deltocyathus rotulus (MNHN-Scl.2008-0004) O, P, and Q Calicular, lateral, and base view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Fungiacyathus (Fungiacyathus) paliferus (USNM uncatalogued, Bathus 3 stn. DW 887) A, B and C Calicular, lateral, and base view respectively; Fungiacyathus (Bathyactis) variegatus (MNHN uncatalogued, Lagoon NO stn. DC933) D, E, and F Calicular, lateral, and base view respectively; Tropidocyathus lessoni(MNHN uncatalogued, Musorstom 8 stn. DW1105) G and H Calicular and lateral view respectively; Alatotrochus rubescens(USNM uncatalogued, Bathus 4 stn. DW 908) I and J Calicular and lateral view respectively; Platytrochus hastatus (MNHN uncatalogued, Ebisco stn. DW2559) K, L, and M Calicular and lateral (GCD and LCD) views respectively; Sphenotrochus hancocki (MNHN uncatalogued, Ebisco stn. DW2617) N and O Calicular and oblique view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Cyathotrochus pileus(USNM uncatalogued, Bathus 3 stn. CP833) A and B Calicular and lateral view respectively; Heterocyathus aequicostatus(USNM uncatalogued, Bathus 4 stn. DW933) C, D, and E Calicular, lateral, and base view respectively; Heteropsammia cochlea(USNM uncatalogued, Bathus 3 stn. DW894)F, G, and H Calicular, lateral, and base view respectively; Placotrochus laevis(USNM 81989) I and J Calicular and lateral view respectively; Falcatoflabellum raoulensis(MNHN uncatalogued, Ebisco stn. DW2603) K, L, and M Calicular, lateral, and oblique view respectively; Placotrochides scaphula(MNHN uncatalogued, Chalcal stn. DW75) N and O Calicular and oblique view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Kionotrochus suteri(NZOI F915) A (SEM) and B (SEM): Calicular and lateral view respectively; Anthemiphyllia dentata (USNM uncatalogued, Bathus 4 stn. DW914) C, D, and E Calicular, lateral, and base view respectively; Bourneotrochus stellulatus(USNM uncatalogued, Bathus 3 stn. DW877) F, G, and H Calicular, lateral, and base view respectively; Peponocyathus folliculus(MNHN uncatalogued, Norfolk 1 stn. DW1697) I, J, and K Calicular, lateral, and base view respectively; Trochocyathus (Trochocyathus) discus (MNHN uncatalogued, Biocal stn. DW46) L, M, and N Calicular, lateral, and base view respectively; Temnotrochus kermadecensis(MNHN uncatalogued, Musorstom 5 stn. DW328) O and P Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Idiotrochus emarciatus(MNHN uncatalogued, Ebisco stn. DW2632) A, B, and C Calicular, oblique, and lateral view respectively; Dunocyathus parasiticus(USNM 85697) D (SEM) and E (SEM): Oblique and lateral view respectively; Endopachys grayi (USNM uncatalogued, Norfolk 2 stn. DW2158)G and H Calicular and lateral view respectively; Notophyllia recta (USNM 85752) H, I, and J Calicular, oblique, and lateral view respectively; Australocyathus vincentinus(USNM 85699) K and L Oblique views respectively; Truncatoguynia irregularis(USNM uncatalogued, Norfolk 2 stn. DW2117) M and N Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
Blastotrochus nutrix(USNM 97553)– A and B Calicular and lateral view respectively; Truncatoflabellum sp. (MNHN uncatalogued, Concalis stn. DW2934) C, D, E, and F Calicular, oblique, lateral, and basal scar view respectively; Caryophyllia (Caryophyllia) abrupta (MNHN-Scl.2009-0067) G and H Calicular and lateral view respectively. Scale bars: blue = 1 mm; white = 5 mm. Bold face indicates type species for the genus.
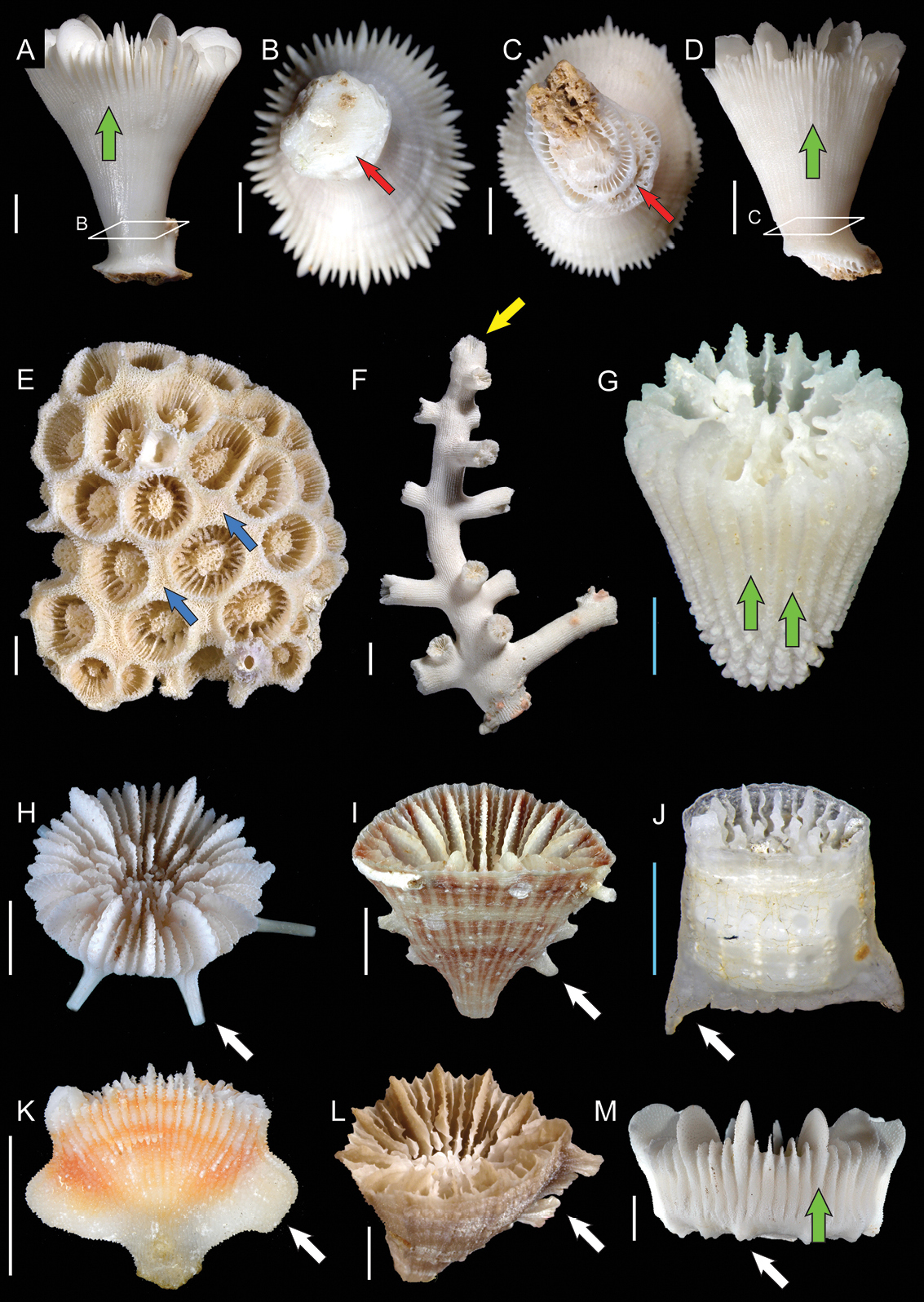
A and B Caryophyllia ralphae (MNHN-Scl.2009-0077, A lateral view and B pedicel section): Green and red arrows indicating costae and monocyclic base respectively C and D Rhizosmilia robusta (USNM uncatalogued, Norfolk 2 stn. DW2114 C pedicel section and D lateral view): Red and green arrows indicating polycyclic base and costae respectively E Astroides calycularis (USNM 78767, colony view): Blue arrows indicating coenosteum F Dendrophyllia ijimai (USNM uncatalogued, Bathus 4 stn. DW933, lateral colony view): Yellow arrow indicating the axial polyp G Sphenotrochus hancocki (MNHN uncatalogued, Ebisco stn. DW2617, oblique view): Green arrows indicating costae H Trochocyathus hastatus (MNHN uncatalogued, Ebisco stn. DW2497, oblique view): White arrow indicating costal spines I Truncatoflabellum vigintifarium (MNHN uncatalogued, Ebisco stn. DW2578, oblique view): White arrow indicating lateral edge spines J Idiotrochus emarciatus (MNHN uncatalogued, Ebisco stn. DW2632, lateral view): White arrow indicating lateral edge spines (fish tail) K Tropidocyathus lessoni (MNHN uncatalogued, Musorstom 8 stn. DW1105, lateral view): White arrow indicating alate edge crests L Caryophyllia unicristata (MNHN-Scl.2009-0094, oblique view): White arrow indicating very sinuous lateral crest M Stephanocyathus weberianus (MNHN uncatalogued, Musorstom 5 stn. DW313, lateral view): White and green arrows indicating tubercles and costae respectively; Scale bars: blue = 1 mm; white = 5 mm.
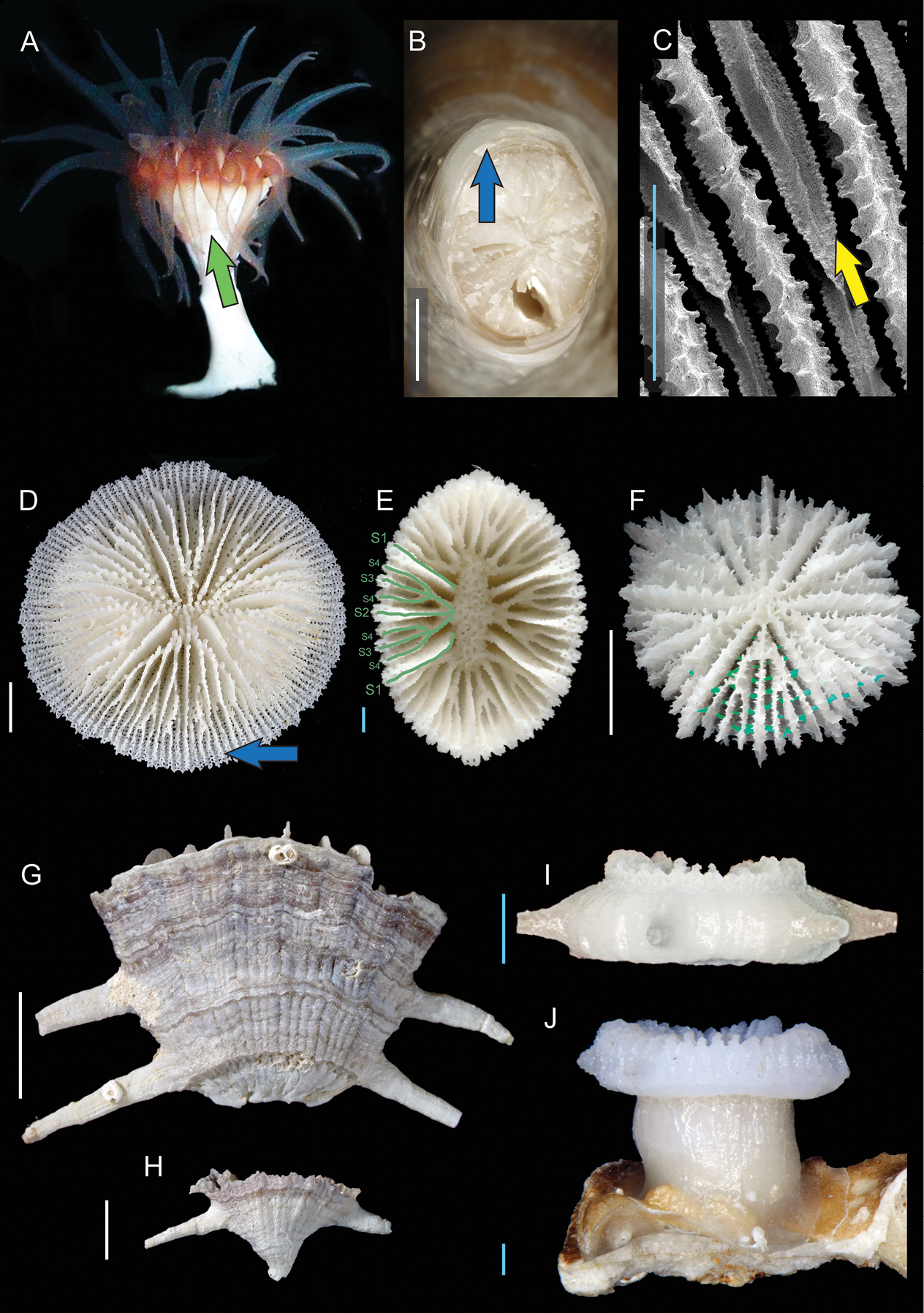
A Indeterminate Caryophylliina (lateral view of live specimen, Roatan, Honduras ~200 m deep): Green arrow indicating the edge zone B Javania sp. (USNM uncatalogued, Norfolk 2 stn. CH 2115, broken pedicel section): Blue arrow indicating tectura layers C Leptoseris gardineri (JCU uncatalogued, Australia, septal detail [SEM]): Yellow arrow indicating meniane D Letepsammia franki (MNHN uncatalogued, Musorstom 6 stn. CP464, oblique view): Blue arrow indicating marginal shelf E Balanophyllia carinata (MNHN uncatalogued, Chalcal stn. D22, calicular view): Green diagram indicating a complete septal system arranged in a Pourtalès Plan configuration F Fungiacyathus sp. (MNHN uncatalogued, Biocal stn. CP17, oblique view): Synapticular plates highlighted in green G and H Truncatoflabellum candeanun (CSIRO uncatalogued, SS102005 stn. 170-086, lateral views) G anthocyathus and H anthocaulus I and J Bourneotrochus stellulatus (MNHN uncatalogued, Musorstom 4 stn. DW162, lateral views) I anthocyathus and J a specimen undergoing transverse division. Scale bars: blue = 1 mm; white = 5 mm.