(C) 2013 Shih-Wei Su. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The photosymbiotic ascidian fauna at Changi Beach, Pulau Semakau, Sentosa and St. John’s Island, Singapore were surveyed. A total of five species, Diplosoma simile, Lissoclinum bistratum, Lissoclinum punctatum, Lissoclinum timorense and Trididemnum cyclops, were recorded, with Lissoclinum timorense and Trididemnum cyclops being newly recorded in Singapore. However, no photosymbiotic species were found at Changi Beach probably due to the polluted waters in the region. Coastal development has caused Singapore waters to become turbid, leading to decrease in suitable habitats for photosymbiotic ascidians. Clean waters in Pulau Semakau probably provide a better environment for the growth of photosymbiotic ascidians and this area has a greater variety of these ascidians than the other areas in Singapore. Each of the five species has also been recorded in the Ryukyu Archipelago (Japan) and three species (Diplosoma simile, Lissoclinum bistratum and Trididemnum cyclops) have also been recorded in Taiwan.
Algal symbiosis, Ascidian, Biogeography, Coral reefs, Didemnidae
Photosymbioses have been known in some colonial ascidians of the family Didemnidae in tropical and subtropical waters. Photosymbionts such as Prochloron and Synechocystis are cyanobacteria (see
The biogeographic survey of photosymbiotic didemnids in Ryukyus has recorded the current distribution range for each species. To date, at least 20 photosymbiotic species are known to be distributed in Japan, mainly in the Ryukyu Archipelago (
Singapore is positioned at the equator and there are only a few reports on the ascidian fauna in Singapore waters (e.g.,
During 2009-2011, we surveyed the photosymbiotic ascidians at Changi Beach, Pulau Semakau, Sentosa and St. John’s Island, Singapore in collaboration with the Tropical Marine Science Institute, National University of Singapore. Herein, five photosymbiotic didemnid species were reported as additions to the marine benthic fauna of Singapore.
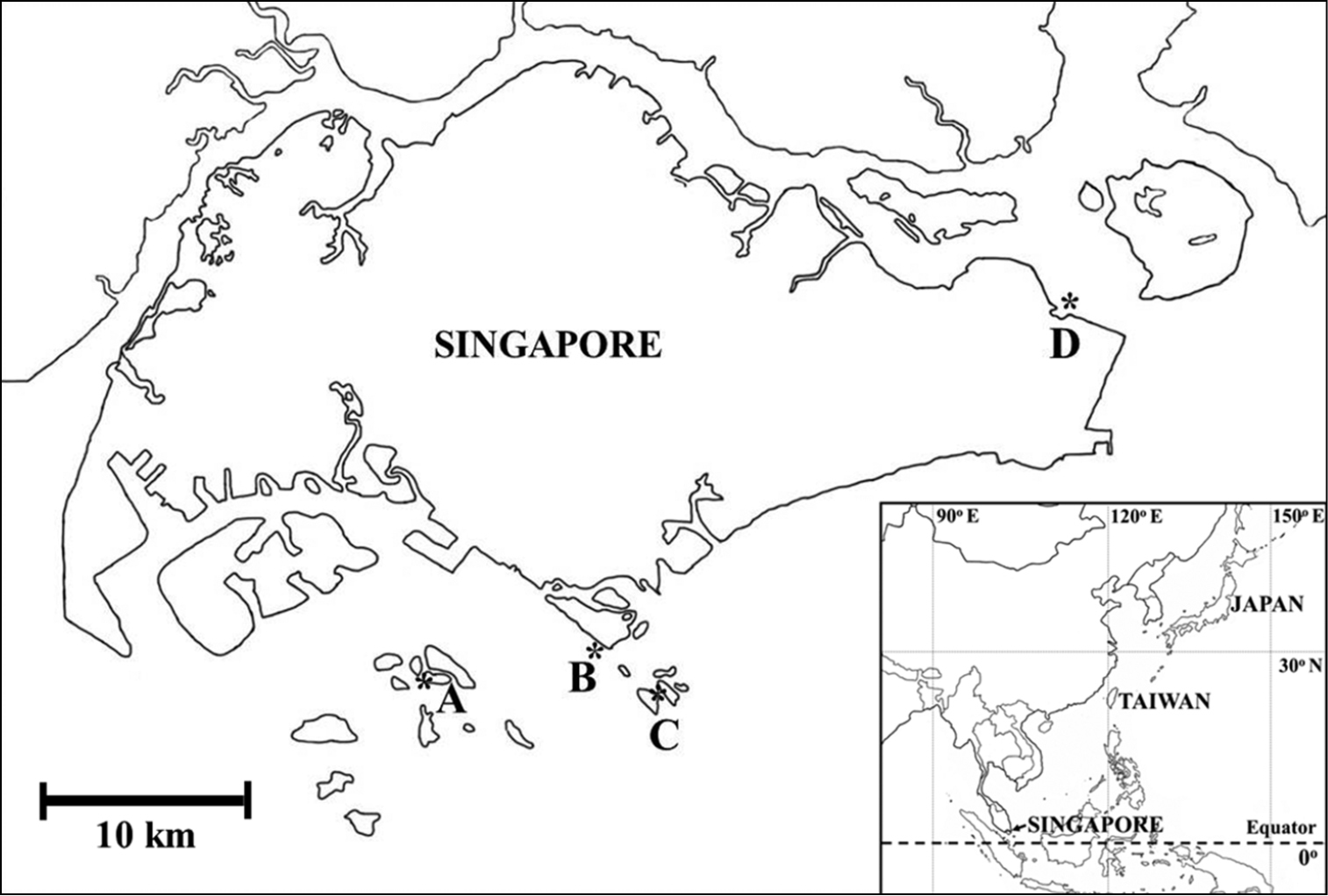
Ascidian colonies were collected by snorkeling in the shallow subtidal zone. Samples were photographed in situ before being collected. Collection sites were as follows: Changi Beach, Pulau Semakau, Sentosa, and St. John’s Island (Fig. 1). The habitats consisted of sand beach, seagrass meadow, coral rubble, sheltered beach and coral reefs. The Changi Beach is a beach park located at the northeastern of Singapore. The park is approximately 3.3 km long with stretches of sandy beaches and a lot of seagrass. Pulau Semakau is located at the south of the main island of Singapore. There is a vast seagrass meadow and a wide zone of coral rubble with various marine lives and an enormous area rich in wildlife. Sentosa is a popular island resort in Singapore that includes a 2 km long sheltered beach. St. John’s Island is one of the Southern Islands in Singapore. The hilly island is transformed into a tranquil getaway. Coral reefs and seagrass bed scatter in the zone. Specimens were anesthetized with menthol and 0.37 M MgCl2 for approximately 2 h, and then fixed with 10% formalin-seawater. The fixed colonies were dissected under a binocular stereomicroscope and a compound microscope equipped with differential interference contrast optics. In some photomicrographs, several images were combined to increase the depth of field using the post-processing image software Helicon Focus Pro 4.2.2 (Helicon Soft, Ltd., Kharkov, Ukraine). Taxa were mainly identified following
Collection sites of photosymbiotic ascidians in Singapore. A Pulau Semakau B Sentosa C St. John‘s Island and D Changi Beach.
The present report describes the occurrence of didemnid ascidians harboring prokaryotic algae from Pulau Semakau, Sentosa and St. John’s Island, Singapore. In total, five species were collected, two of which were new records for Singapore. Their occurrences at each site and dates are listed in Table 1. No photosymbiotic species were found at Changi Beach.
Distribution records of photosymbiotic didemnid ascidians in Singapore.
| Location | Changi Beach | Pulau Semakau | St. John’s Island | Sentosa | |||
|---|---|---|---|---|---|---|---|
| Date | 2011 Nov | 2009 Dec | 2010 May | 2011 Nov | 2010 May | 2011 Nov | 2010 April |
| Trididemnum cyclops | + | ||||||
| Diplosoma simile | + | + | + | + | |||
| Lissoclinum bistratum | + | + | + | + | |||
| Lissoclinum punctatum | + | + | + | ||||
| Lissoclinum timorense | + | + | + | ||||
ZRC-TUN-0012 (St. John’s Island, subtidal at depth 0.5 m).
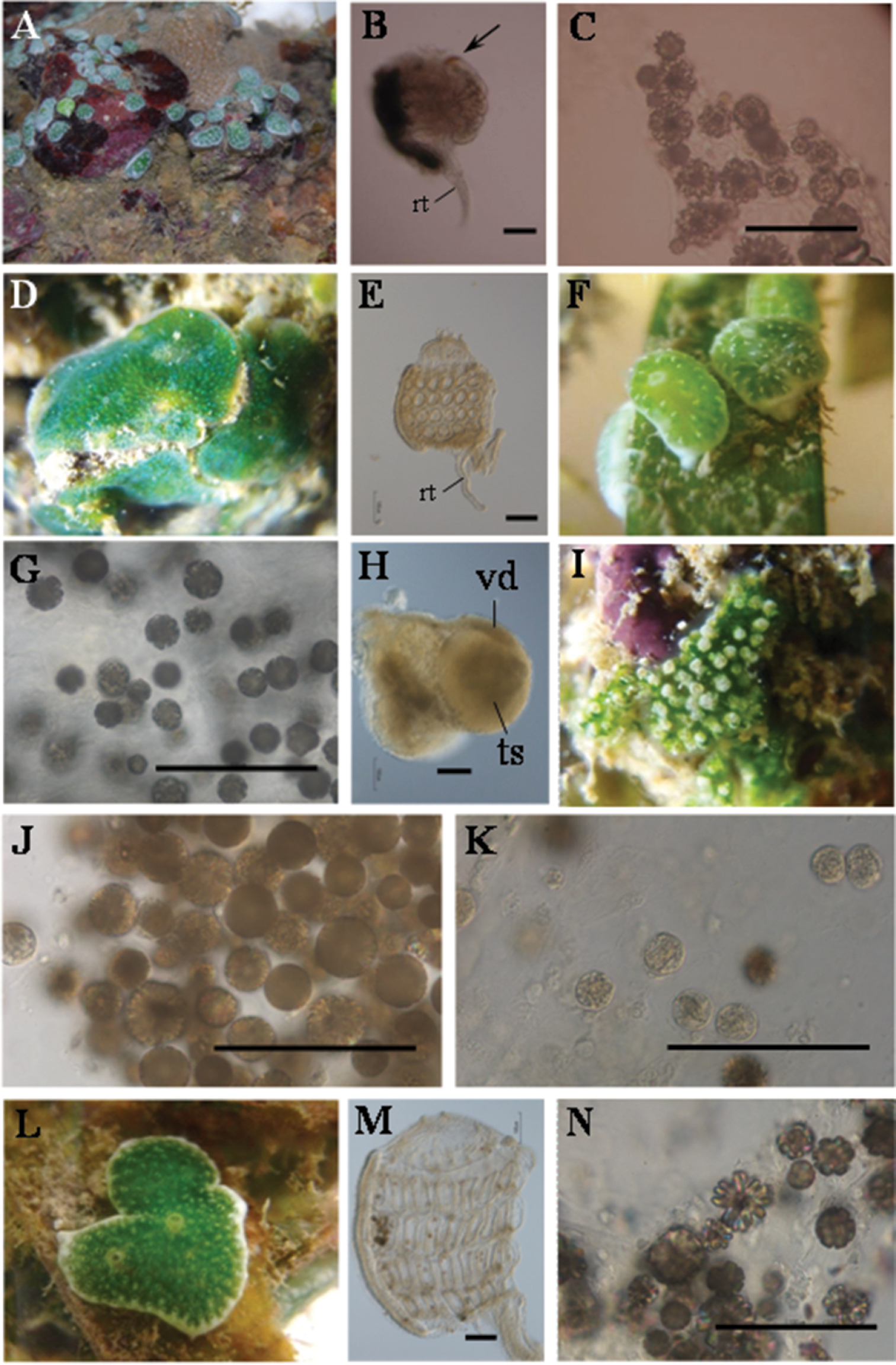
Colonies are oval or irregularly shaped cushions of 2-6 mm on the long axis (Fig. 2A). Each zooid has a black dot, due to a pigment mass at the top of the endostyle (Fig. 2B). Berry-like spicules are distributed in the colonial margin and basal tunic, while they are rarely found in the surface tunic. Spicules are up to 40 mm in diameter (Fig. 2C). The biased distribution of the spicules allows the symbionts to receive sunlight for photosynthesis.
Photosymbiotic ascidians collected. A Trididemnum cyclops, St. John’s Island, Singapore (Depth = 0.5 m). Colonies are 2–6 mm on the long axis B Thorax of Trididemnum cyclops. Arrows indicatethe endostylarpigment cap. Scale bar = 100 mm. C Tunic spicules in the tunic of Trididemnum cyclops. Scale bar = 100 mm D Diplosoma simile, Pulau Semakau, Singapore (Depth = 0.5 m). Colonies are approximately 15 mm in diameter E Thorax of Diplosoma simile (left view). Scale bar = 100 mm F Lissoclinum bistratum, Pulau Semakau, Singapore (Depth = 0.5 m). Colonies are approximately 5 mm in diameter G Tunic spicules in the tunic of Lissoclinum bistratum. Scale bar = 100 mm H A testis with an uncoiled vas deferens of Lissoclinum bistratum. Scale bar = 100 mm I Lissoclinum punctatum, Pulau Semakau, Singapore (Depth = 0.5 m). Colonies are approximately 10 mm in diameter J Tunic spicules in the tunic of Lissoclinum punctatum. Scale bar = 100 mm K Tunic phycocytes of Lissoclinum punctatum. Scale bar = 100 mm L Lissoclinum timorense, Pulau Semakau, Singapore (Depth = 0.5 m). Colonies are approximately 10 mm in diameter M Thorax of Lissoclinum timorense (left view) N Tunic spicules in the tunic of Lissoclinum timorense. Scale bar = 100 mm. rt, retractor muscle; ts, testis; vd, vas deferens.
NMNS-7027-001, NMNS-7027-002, ZRC-TUN-0001 and ZRC-TUN-0015 (Pulau Semakau, subtidal at depth 0.5 m), ZRC-TUN-0011 (St. John’s Island, subtidal at depth 0.5 m), ZRC-TUN-0009 (Sentosa, subtidal at depth 0.5 m)
Colonies are irregularly shaped sheets about 2 mm thick without spicules (Fig. 2D). They are entirely green due to the Prochloron cells in the common cloacal cavities. The thorax has four stigmatal rows: there are six stigmata in the first (top), second, and third row and five stigmata in the fourth row (bottom). The retractor muscle emerges from the bottom of the thorax (Fig. 2E). Testis and/or egg are found in some zooids, and vas deferens is uncoiled. Kott (1982) reported this species from Singapore. This is one of the most common species in the didemnid-Prochloron obligate symbioses in the tropical Pacific, and it has also been recently found in Caribbean Panama (
NMNS-7027-003, NMNS-7027-004, ZRC-TUN-0002, ZRC.TUN.0004, ZRC-TUN-0007, ZRC-TUN-0008 and ZRC-TUN-0014 (Pulau Semakau, subtidal at depth 0.5 m), ZRC-TUN-0010 (Sentosa, subtidal at depth 0.5 m).
Colonies are oval cushions of 4 mm on the long axis (Fig. 2F). The photosymbiont Prochloron gives the colonies a green color, while the colonial margin and bottom are white due to dense aggregations of globular spicules in the tunic (Fig. 2G). The thorax has four stigmatal rows. It is difficult to count accurately the number of stigmata owing to the distortion of thoraxes caused by the shrinkage of zooids upon fixation. There are about seven stigmata in each row. Some zooids have a testis with an uncoiled vas deferens (Fig. 2H). Kott (1982) reported this species from Singapore.
NMNS-7027-007, ZRC-TUN-0005 and ZRC-TUN-0016 (Pulau Semakau, subtidal at depth 0.5 m), ZRC-TUN-0013 (St. John’s Island, subtidal at depth 0.5 m)
Colonies are irregularly shaped sheets about 2 mm thick (Fig. 2I). Globular spicules aggregate around each zooid, which is enclosed in a capsule of white spicules (Fig. 2J). Many Prochloron cells are distributed in both cloacal cavities and tunic cells (tunic phycocytes) (Fig. 2K; also see
NMNS-7027-005, NMNS-7027-006, ZRC-TUN-0003, ZRC-TUN-0006 and ZRC-TUN-0017 (Pulau Semakau, subtidal at depth 0.5 m)
Colonies are irregularly shaped sheets about 2–5 mm thick (Fig. 2L). The colonies are green due to Prochloron cells distributed in the common cloacal cavities, while the colonial margin and bottom are white due to the dense distribution of stellate and globular spicules. In the five zooids we examined, the thorax had four stigmatal rows: there were seven stigmata in the first row (top), eight in the second row, seven in the third row, and five or six in the fourth row (bottom) (Fig. 2M). Gonads are not found in the present specimens. There are globular spicules in the tunic (Fig. 2N). The presence of stellate spicules easily distinguishes the present species from Lissoclinum bistratum, which lacks these spicules. However,
Five photosymbiotic ascidians were recorded in the present survey, including three species previously observed by Kott (1982) and two new records in Singapore. There were four species in Pulau Semakau, three species on St. John’s Island and two species in Sentosa, but no photosymbiotic species were found at Changi Beach. The five species listed here might be far from the entire coverage of the photosymbiotic ascidian fauna in Singapore, because the present survey was conducted over a very short period of time and at only four sites. It is expected that more species still remain to be recorded.
Once, there were over 60 offshore islands and patch reefs around Singapore, most of which were situated south of mainland Singapore. However, since the mid 1970s, Singapore has been undergoing coastal reclamation. As its population grows until more than four million, the Singapore government faces problem in providing ample land. Some of offshore islands in Singapore have been deformed or enlarged by some coastal reclamation projects. Many of the coral reef organisms were smothered by reclamation, while others were severely affected by the resulting increase in water turbidity. The high turbidity of waters restricts light penetration, and determines the maximum distribution depth for corals and photosymbiotic ascidians. Visibility has been reduced from 10 m in the 1960s to 2 m or less to date (Coral Reefs of Singapore, http://coralreef.nus.edu.sg/). As a result, up to 60% of the live coral cover has been lost in Singapore since 1986 (
The coastal environment of Singapore is limited and currently severely affected by coastal development and the port industry, which is one of the biggest economic businesses in the country. Harbor limits occupy most of the territorial waters, and reclamation has transformed considerably almost the entire southern and northeastern coasts of the main island (Chou and Goh 1998). Most of the coastal waters are filled with suspended particles that block photosynthetic activities of marine organisms. When these particles sink, they settle over sessile organisms, such as photosymbiotic ascidians, and adversely affect their metabolism and growth (
More than 20 photosymbiotic species are known to be distributed in Japan, mainly in the Ryukyu Archipelago (
Distribution records of photosymbiotic didemnid ascidians in Japan, Taiwan and Singapore.
| Location | Japan | Taiwan | Singapore |
|---|---|---|---|
| Didemnum molle | + | + | |
| Diplosoma aggregatum | + | + | |
| Diplosoma gumavirens | + | + | |
| Diplosoma ooru | + | + | |
| Diplosoma simile | + | + | + |
| Diplosoma simileguwa | + | + | |
| Diplosoma variostigmatum | + | ||
| Diplosoma virens | + | + | |
| Diplosoma watanabei | + | ||
| Lissoclinum bistratum | + | + | + |
| Lissoclinum midui | + | ||
| Lissoclinum patella | + | ||
| Lissoclinum punctatum | + | + | |
| Lissoclinum timorense | + | + | |
| Lissoclinum triangulum | + | ||
| Trididemnum clinides | + | + | |
| Trididemnum cyclops | + | + | + |
| Trididemnum miniatum | + | ||
| Trididemnum nubilum | + |
(
The authors are indebted to Dr. Tan Koh Siang, Head of Marine Biology and Ecology Group, Tropical Marine Science Institute, National University of Singapore, and Dr. Li-Lian Liu, Professor of Institute of Marine Biology, National Sun Yat-sen University, Taiwan, who encouraged us and supported our survey. The present study was partly supported by Pilot Overseas Internships, sponsored by the Ministry of Education Taiwan. The collection of material in the subtidal of Singapore was granted by the National Biodiversity Centre, Singapore (NP/RP 11-091).