(C) 2012 James K. Liebherr. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Seven species of Mecyclothorax Sharp from Moorea, Society Islands are newly described; Mecyclothorax perraulti sp. n., Mecyclothorax pahere sp. n., Mecyclothorax menemene sp. n., Mecyclothorax mahatahi sp. n., Mecyclothorax popotioaoa sp. n., Mecyclothorax mapo sp. n., and Mecyclothorax fatata sp. n. These constitute the first Mecyclothorax species described from Moorea, and the first carabid beetle species shown to be geographically restricted to that island. Each of the newly described species is most similar to a different species on the island of Tahiti, suggesting that none of the seven Moorean taxa are evolutionary end-products of autochthonous speciation within Moorea. The occurrence of precinctive Mecyclothorax species on both Moorea and Tahiti demonstrates that radiation of Mecyclothorax in the Society Islands has been facilitated by speciation events implicating both islands. Whether this speciation has been preceded by vicariance or dispersal is discussed, with the generality of a dispersal hypothesis tested using information from Society Island Nabidae (Hemiptera). Salient morphological characters for taxa in the Society and Hawaiian Islands are compared to those representing a broad survey of southwest Pacific Mecyclothorax spp. This comparison supports the independent founding of each radiation in the Societies and Hawaii from an Australian ancestral propagule, likely drawn from the ecologically general, geographically widespread Mecyclothorax punctipennis (Macleay).
French Polynesia, Moriomorphini, adaptive radiation, biogeography, colonization
The genus Mecyclothorax Sharp is distributed throughout Australia and associated landmasses and islands including New Guinea, the Greater Sundas of Java and Borneo, Lord Howe and Norfolk Islands, and St. Paul and Amsterdam Islands of the Indian Ocean (
This paper extends the comparison of the Hawaiian and Tahitian Mecyclothorax radiations by describing the first collections of Mecyclothorax species from a second Society Island, Moorea. Whereas Tahiti, including the volcanoes Tahiti Nui and Tahiti Iti, or Presqu’île de Taiarapu, encompasses 1040 km2, with highest elevations of 2241 m and 1332 m respectively on the two constituent volcanoes, Moorea has a much smaller land area of 142 km2 and a peak elevation of 1207 m at the summit of Mont Tohiea (Fig. 1). Rainfall is also less abundant on Moorea, though the 5000 mm/yr recorded precipitation (
Mont Tohiea, Moorea, east face. Collections of Mecyclothorax spp. have been limited to the highest portions of the summit ridge, from 1100 m to the summit, elevation 1207 m.
Type specimens of Tahitian Mecyclothorax species used in the diagnosis of the new species were borrowed from the Naturhistorisches Museum, Basel (NHMB) and the Muséum national d’Histoire naturelle, Paris (MNHN). Primary type specimens of the new Moorean species, and associated allotypic paratypes where available, are deposited in the MNHN and incorporated into the Georges G. Perrault collection of Tahitian Carabidae. Other institutional depositories include: Cornell University Insect Collection (CUIC); Essig Museum of Entomology Collection (EMEC); U.S. National Museum of Natural History (NMNH).
Laboratory techniquesSpecimens were killed in ethyl acetate impregnated killing jars, maintained under that atmosphere for 24 hr, and then transferred to 70% ethanol for transport to the laboratory, or preserved directly in 100% ethanol. The former specimens were pointed or platen-mounted from ethanol. The latter specimens were maintained in ethanol at -16˚ C and examined under ethanol at room temperature, or removed from ethanol and pointed when a specimen was required for a holotype or allotype specimen. Description of characters was based only on air-dried specimens viewed using a dissecting microscope under bidirectional halogen light.
Labeling is presented verbatim for holotype and associated allotype specimens. Individual lines on labels are separated by a single slash ( / ), and separate labels are indicated by a double slash ( // ). Data for the other paratypes is presented in a standardized, condensed format organized chronologically by collection. Where information is repeated in adjacent, subsequent collections, that field is removed from the data string and is to be interpolated from the previous paratype data entry. Paratypes labeled with an MBIO#### lot number were retained in 100% ethanol and returned to EMEC for ensuing DNA extraction.
Dissection, clearing and staining techniques follow
Descriptive conventions build upon data reported by
Various ratios are used to characterize shapes or relative dimensions. The ocular ratio is the measurement across the outer surface of the compound eyes divided by the minimum distance across the frons between the eyes. The ocular lobe ratio is the distance from the anterior to posterior margin of the eye measured from directly above, divided by the distance from the anterior margin of the eye to the groove at the juncture of the gena and ocular lobe using the same viewpoint. Various body meaurements are presented as ratios, with the measurements including: APW, anterior pronotal width, i.e. the width between the most anteriormost pronotal margins at the front angles; MPW, maximum pronotal width; BPW, basal pronotal width measure across the hind angles; PL, median pronotal length; HuW, humeral width, or distance between the anteriormost points along the basal groove-humeral juncture, i.e., the humeral angle; MEW, maximum elytral width. In order to present infraspecific variation in body shape, a maximum of five specimens, where available, were measured to compose these ratios. All available specimens were scanned and the largest individual, the smallest individual, and representatives of both sexes were included in the sample of five. Each of these specimens was labeled with a small number label that corresponds to its entry in the character matrix (unpubl. data). This sampling produced a range of ratios, with the smallest and largest individuals often producing the most disparate ratios. The number of sampled individuals, which may range from 1 to 5, is presented as (n = X).
The configuration of the antennae—moniliform, submoniliform, filiform, or elongate filiform—is categorized using a ratio of the dimensions of the eighth antennomere; length from basal juncture with seventh antennomere to apex divided by the maximal breadth, excluding the dense setal pelage. The configuration of the metathorax is quantified by the metepisternum width/length ratio. Width is measured perpendicular to the longitudinal body axis from the lateral edge adjacent to elytral epipleuron, to the medial juncture of mesepimeron, metasternum and metepisternum. Length is measured as the length of the medial edge from mesepimeron to juncture with metepimeron.
Elytral setation is described based on the dorsal setal positions in the third elytral interval relative to overall elytral length as measure in the standardized body length. The lateral elytral setae of the ninth interval, just laterad the eighth stria, are arrayed in an anterior series starting laterad the humerus, and an apical series that terminates just anterad the subapical sinuation. The two series are presented as A + B, with variation in setal number among individuals reported in parentheses; (B – C). When a particular setal count varied, and one state was observed only rarely, the rarely observed setal number is presented alone in parentheses; i.e., B(C).
Coloration is graded relatively from flavous (i.e. yellow without melanization), to rufoflavous, and then to rufobrunneous. Colors darker than rufobrunneous may entail dominant reddish coloration, thereby leading to rufous, dark rufous, and rufopiceous, or the colors may be dominated by browns, leading from rufobrunneous to brunneous, to rufopiceous. The darkest coloration observed is piceous, or shiny coal black. These base colors may be modified by a darker cast; incomplete melanization of the surface near setae or in thicker portions of the cuticle.
Description of microsculpture follows the general terms used in
Features of the male aedeagal internal sac were described based on the homology system of
Moriomorphini Sloane, 1890: 646 (sensu
Subtribe Moriomorphina Sloane, 1890: 646.
Melisoderides Sloane, 1898: 470 (synonymy
Subtribe Amblytelina Blackburn, 1892: 85 (type genus Amblytelus Erichson).
Meonides Sloane, 1898: 470 (synonymy
Tropopterides Sloane, 1898: 470 (synonymy
Mecyclothoracitae Jeannel, 1940: 97 (synonymy
Mecyclothorax Sharp was classified in the subtribe Amblytelina based on the shared presence of elongate, apically narrowed male parameres that bear setae along the ventral margin of the right paramere and at the apex of the left paramere (
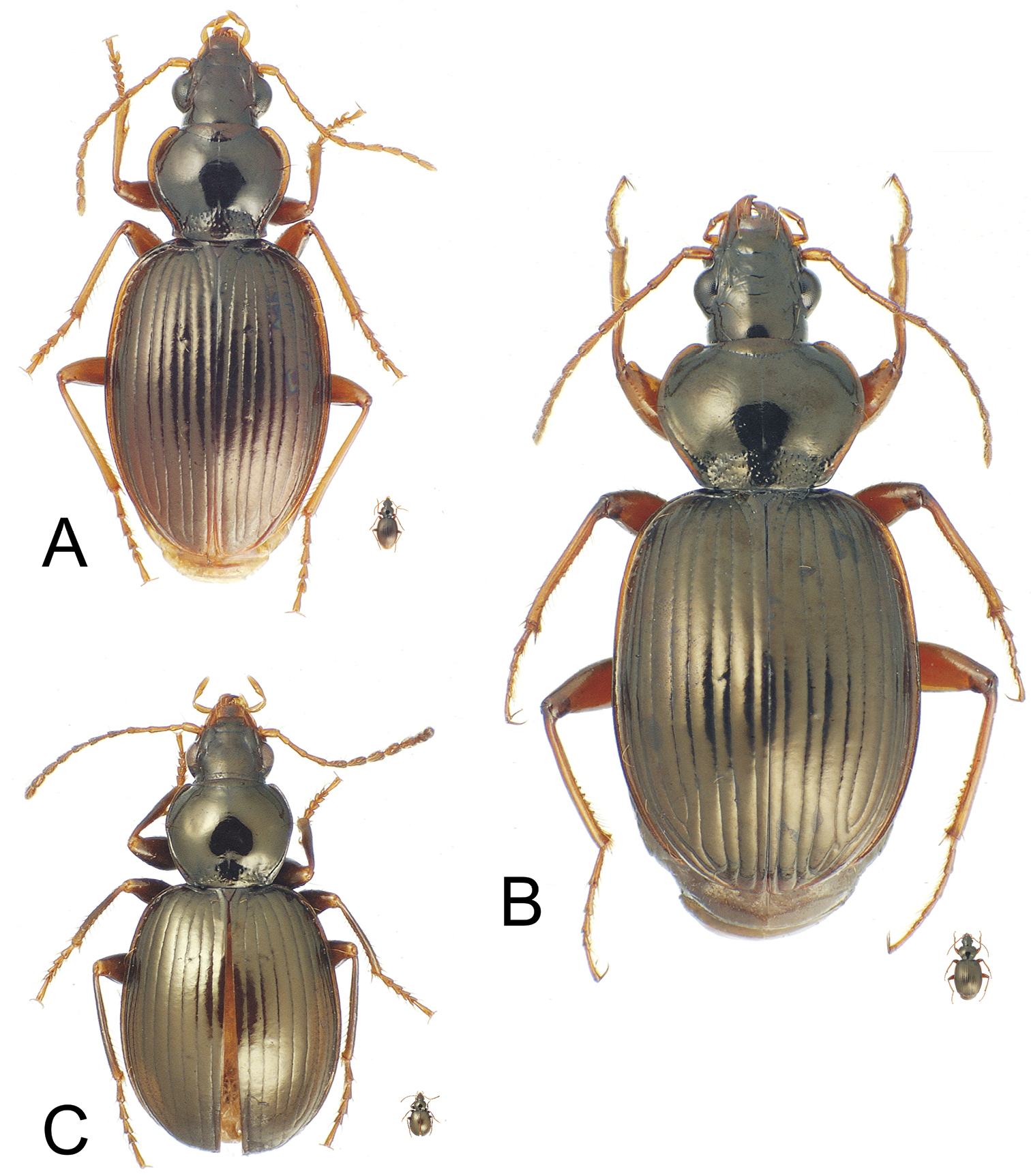
Mecyclothorax spp., dorsal view; silhouette to lower right of each habitus photo indicates actual size of beetle specimen at printed journal page size A Mecyclothorax perraulti male holotype B Mecyclothorax pahere male holotype C Mecyclothorax menemene male holotype.
Mecyclothorax spp., dorsal view; silhouette to lower right of each habitus photo indicates actual size of beetle specimen at printed journal page size A Mecyclothorax mahatahi female holotype B Mecyclothorax popotioaoa male holotype C Mecyclothorax mapo male paratype (CUIC) D Mecyclothorax fatata female paratype (CUIC).
| 1 | Pronotal lateral margin explanate throughout pronotal length, translucent | 2 |
| – | Pronotal lateral margin narrowly reflexed, edge narrowly upturned or beaded adjacent to pronotal lateral seta | 3 |
| 2 | Pronotal margin broadly explanate entire pronotal length, sinuate anterad hind angle | Mecyclothorax perraulti sp. n. |
| – | Pronotal margin broadest near posterior angle, narrowed anterad toward position of lateral seta, not sinuate anterad the broadly rounded hind angle | Mecyclothorax pahere sp. n. |
| 3 | Pronotum cordate, margin distinctly sinuate anterad hind angle | 4 |
| – | Pronotum ovate, margin straight to slightly convex anterad obtuse hind angle | Mecyclothorax menemene sp. n. |
| 4 | Both anterior and posterior supraorbital setae present, a thin carina present between dorsoanterior margin of eye and anterior seta | 5 |
| – | Posterior supraorbital seta present, anterior supraorbital seta absent, only a low broad convexity mesad dorsoanterior margin of eye | Mecyclothorax mahatahi sp. n. |
| 5 | Pronotum bisetose, both lateral and basal setae present; pronotum moderately cordate, MPW/BPW ratio 1.52–1.64, pronotal lateral margins subparallel to slightly divergent anterad obtuse-rounded hind angles | 6 |
| – | Pronotum unisetose, only the lateral seta present, hind angle glabrous; pronotum distinctly cordate, MPW/BPW ratio 1.67– 1.76, pronotal lateral margins convergent for ~1/9 pronotal length anterad sharply right hind angle | Mecyclothorax popotioaoa sp. n. |
| 6 | Body size larger, standardized body length 4.7–5.0 mm; a single dorsal elytral seta present at ~0.25 distance from base of scutellum to elytral apex; both apical and subapical elytral setae present | Mecyclothorax mapo sp. n. |
| – | Body size smaller, standardized body length 3.8–4.4 mm; two dorsal elytral setae positioned at ~0.32–0.34 and ~0.66–0.68 distance from base of scutellum to elytral apex; apical elytral seta (apex 2nd stria) present, subapical elytral seta (in 7th stria) absent | Mecyclothorax fatata sp. n. |
Mecyclothorax gourvesi species group
Diagnosis.
urn:lsid:zoobank.org:act:F7C6AC8A-46B8-4E9C-9B58-645A839318C8
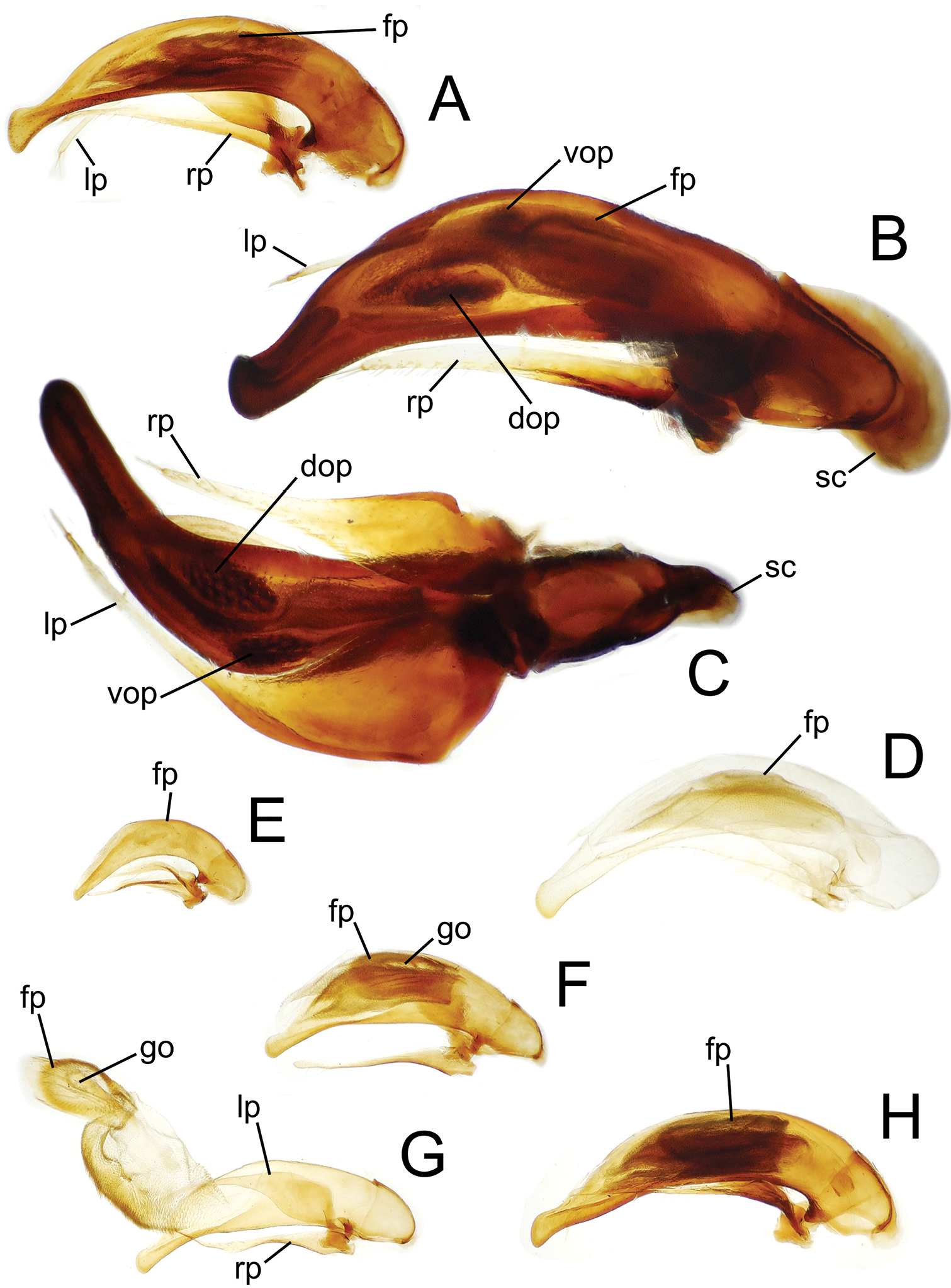
Consistent with species group membership, the pronotal lateral margins are broadly explanate and translucent, with the basolateral margin sinuate anterad the projected, nearly right hind angles (Fig. 2A). The elytral lateral margin is also broadly expanded and translucent, with the margin upraised just laterad the angulate humerus. Mecyclothorax perraulti is most similar to Mecyclothorax gourvesi, though the pronotal base is narrower relative to pronotal maximum width in Mecyclothorax perraulti (MPW/BPW = 1.49–1.52), versus the basally broader pronotum of Mecyclothorax gourvesi (MPW/BPW = 1.37–1.39). The male aedeagal median lobe of Mecyclothorax perraulti has a broadened, adze-shaped apex, with the apical face straighter than the dorsal and ventral margins (Fig. 4A). Individuals of Mecyclothorax perraulti are also larger than those of Mecyclothorax grouvesi; standardized body length 5.8–6.2 mm versus a body length of 5.5 mm for the latter (
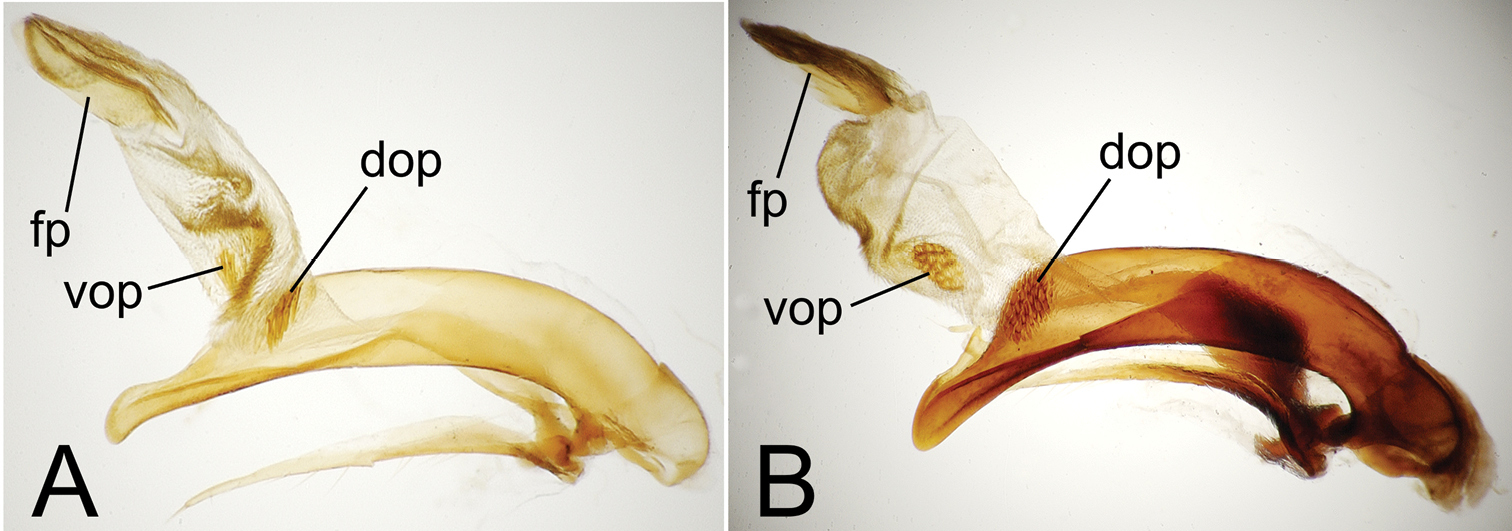
Male aedeagal median lobe and associated parameres. A Mecyclothorax perraulti, right lateral view B Mecyclothorax pahere, right lateral vew C Mecyclothorax pahere, euventral view D Mecyclothorax menemene, right lateral view (teneral specimen) E Mecyclothorax popotioaoa , right lateral view F Mecyclothorax mapo, right lateral view G Mecyclothorax mapo, right lateral view, internal sac everted H Mecyclothorax fatata, right lateral view. Abbreviations: dop dorsal ostial microtrichial patch fp flagellar plate go gonopore lp left paramere rp right paramere sc sagittal crest vop ventral ostial microtrichial patch.
Head capsule with broadly excavate, straight frontal grooves, bordered laterally by a finely raised carina just mesad the anterior supraorbital seta, the mesal face indistinctly wrinkled between the eyes; neck not depressed dorsally between the eyes, frons flat in lateral view; ocular lobe distinctly projected, the posterior margin abruptly meeting gena behind eye, a fine, shallow groove at ocular lobe-genal juncture; ocular ratio 1.48–1.58 (n = 4); ocular lobe ratio 0.79–0.85 (n = 4); anterior margin of labrum broadly, shallowly emarginate, emargination about 1/9 labral length; antennomeres 1–3 glabrous except for apical setae; antennae filiform, antennomere 8 length 2.4× greatest width; mentum tooth with sides defining an acute angle, the apex tightly rounded. Prothorax moderately transverse, MPW/PL = 1.24–1.31 (n = 4); median base slightly depressed relative to disc, 25–27 small, isolated punctures each side of depressed area; basal margin broadly convex between laterobasal depressions, nearly straight medially; median longitudinal impression fine and shallow on disc, a lenticular broadening at front of median base; anterior transverse impression finely incised throughout breadth, shallower medially; anterior callosity slightly convex, crossed with indistinct longitudinal wrinkles; front angles protruded, subangulate medially adjacent to head, broadly convex laterally; distance between front angles less than between hind angles, APW/BPW 0.87–0.94 (n = 4); lateral marginal depression canaliculate where the convex pronotal disc meets the explanate, angularly upraised lateral margin; laterobasal depression a linear extension of the narrow, deep lateral depression; proepisternum with ~7 indistinct punctulae along hind margin, smaller irregularities along posterior marginal bead of proepimeron; prosternal process broad, slightly depressed anterad coxal cavities, convex posteriorly at juncture with posterior face. Elytra subquadrate, MEW/HuW = 2.29–2.37 (n = 4); disc flat medially, markedly sloped laterad interval 7 to near vertical juncture with lateral marginal depression; basal groove indistinctly though broadly recurved to angulate humerus; parascutellar seta present; parascutellar striole 6–7 punctate, continuously depressed between punctures; sutural interval elevated to sutural juncture, more convex than intervals 2–4; sutural and second striae subequally depressed at elytral apex; striae 1–7 minutely punctate basally, smooth apically, intervals distinctly convex; eighth interval distinctly carinate laterad stria 7 in apical half of elytron, the lateral portions of interval 8 depressed enhancing carina and change in discal curvature; 2 dorsal elytral setae, positioned at 0.24–0.25× and 0.57–0.61× elytral length, each seta in an indistinct depression that spans half or less the width of interval 3; both apical and subapical setae present; lateral elytral setae 7 + 6; elytral marginal depression broadly reflexed, translucent posterolaterad humerus; slightly broader laterad anterior lateral elytral setal series, narrowed apically to a beadlike margin anterad subapical sinuation; subapical sinuation abrupt, internal plica evident beneath sinuation in ectal view. Mesepisternum distinctly punctate anteriorly, ~18 evident punctures in 2–3 rows; metepisternum moderately foreshortened, width to length ratio 0.67; mesepisternal/mesepimeral suture distinct; metathoracic flight wing a narrow, elongate vestigium, length 3× width, remnants of veins C, R, M, and Cu discernible. Abdomen with visible ventrites 1–4 irregularly wrinkled laterally, ventrites 3–6 with round depressions laterally; suture between visible ventrites 2 and 3 effaced laterally. Legs moderately elongate, ratio of metatarsomere 1 length to metatibia length 0.23; metatarsomere 4 lobate, length including outer apical lobe 1.5× median tarsomere length; metatarsomere 4 with apical setae only, subapical setae absent; metatarsal dorsolateral sulci very shallow, obsolete, tarsomere dorsum broadly convex. Microsculpture of frons obsolete, surface glossy, shallow isodiametric sculpticells in transverse rows on neck; pronotal disc with mesh of transverse sculpticells 2–3× broad as long; pronotal median base covered with irregular, swirling isodiametric and transverse sculpticells between punctures; elytral disc with transverse microsculpture, sculpticells connected into a mesh, to parallel, unconnected transverse lines; metasternum covered with distinct transverse mesh; basal 2 abdominal ventrites covered with swirling isodiametric and transverse sculpticells. Coloration a somber reddish brown; head capsule rufous with a piceous cast; antennomere 1 rufoflavous, 2–11 rufobrunneous; pronotal disc rufopiceous, base, apex and lateral margins rufobrunneous; proepipleuron rufoflavous, proepisternum rufous; elytral disc rufobrunneous with purplish reflection due to transverse microsculpture; sutural interval paler, rufous basally, rufoflavous apically; elytral lateral margins rufoflavous, lateral marginal depression flavous inside dark margin; elytral apex broadly, slightly paler, rufoflavous; elytral epipleuron rufoflavous, metepisternum rufobrunneous with a piceous cast; abdominal ventrites 1–3 rufobrunneous, 4–6 paler, rufoflavous; metafemur rufoflavous with medial brunneous cloud; metatibia rufoflavous with brunneous cast.
Male Genitalia. (n = 1). Aedeagal median lobe narrowed dorsoventrally in apical half, apex dorsoventrally expanded to an adze-like tip (Fig. 4A), the apical face of tip much less convex than dorsal and ventral margins; flagellar plate large, length 0.5× distance from parameral articulation to apical face of tip; right paramere extended to 0.8× distance from parameral articulation to apical face, left paramere extended nearly to tip; internal sac with broad dense ventral microtrichial field, and small, lightly spinose dorsal ostial microtrichial patch (assessed in uneverted condition).
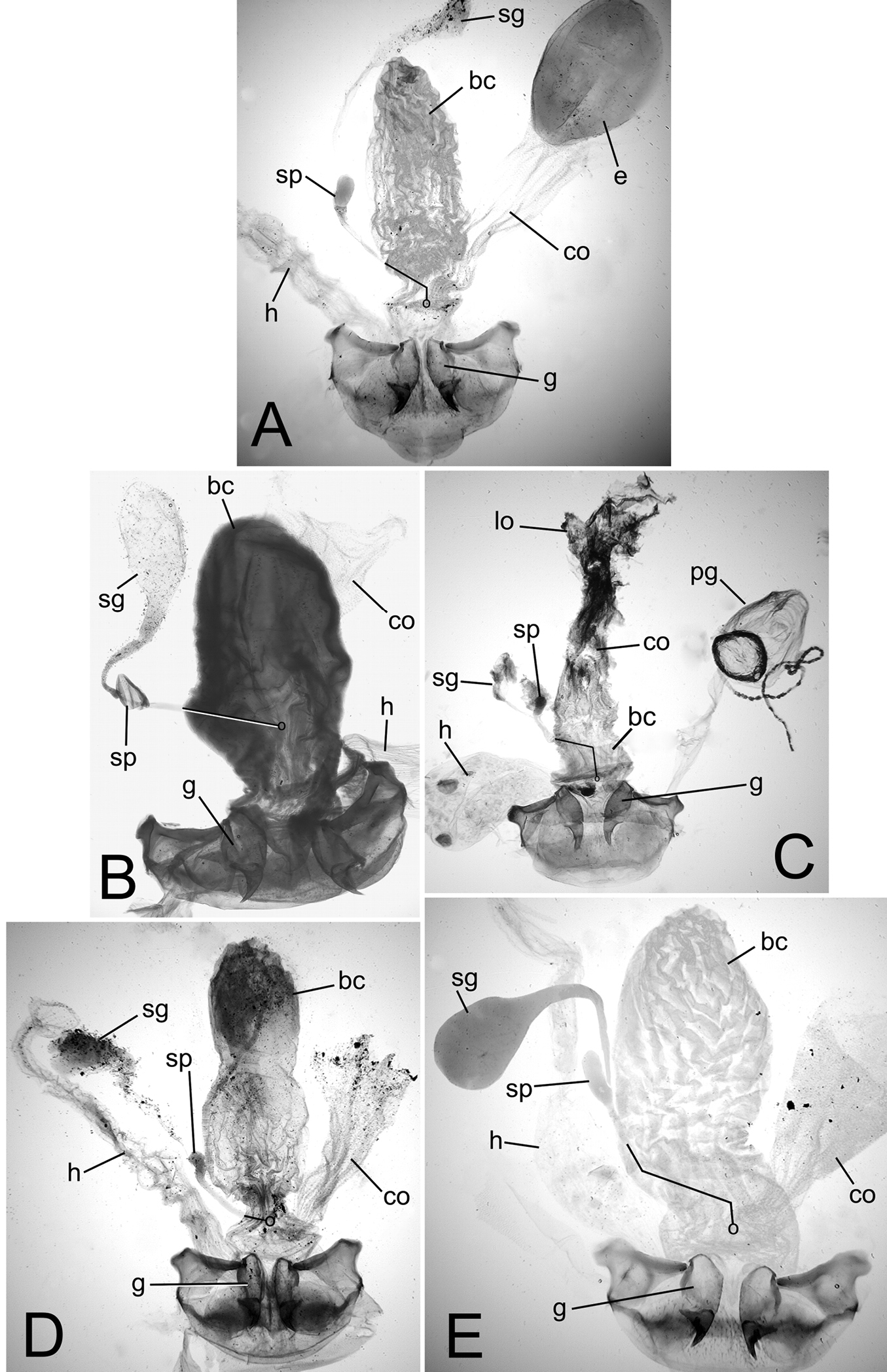
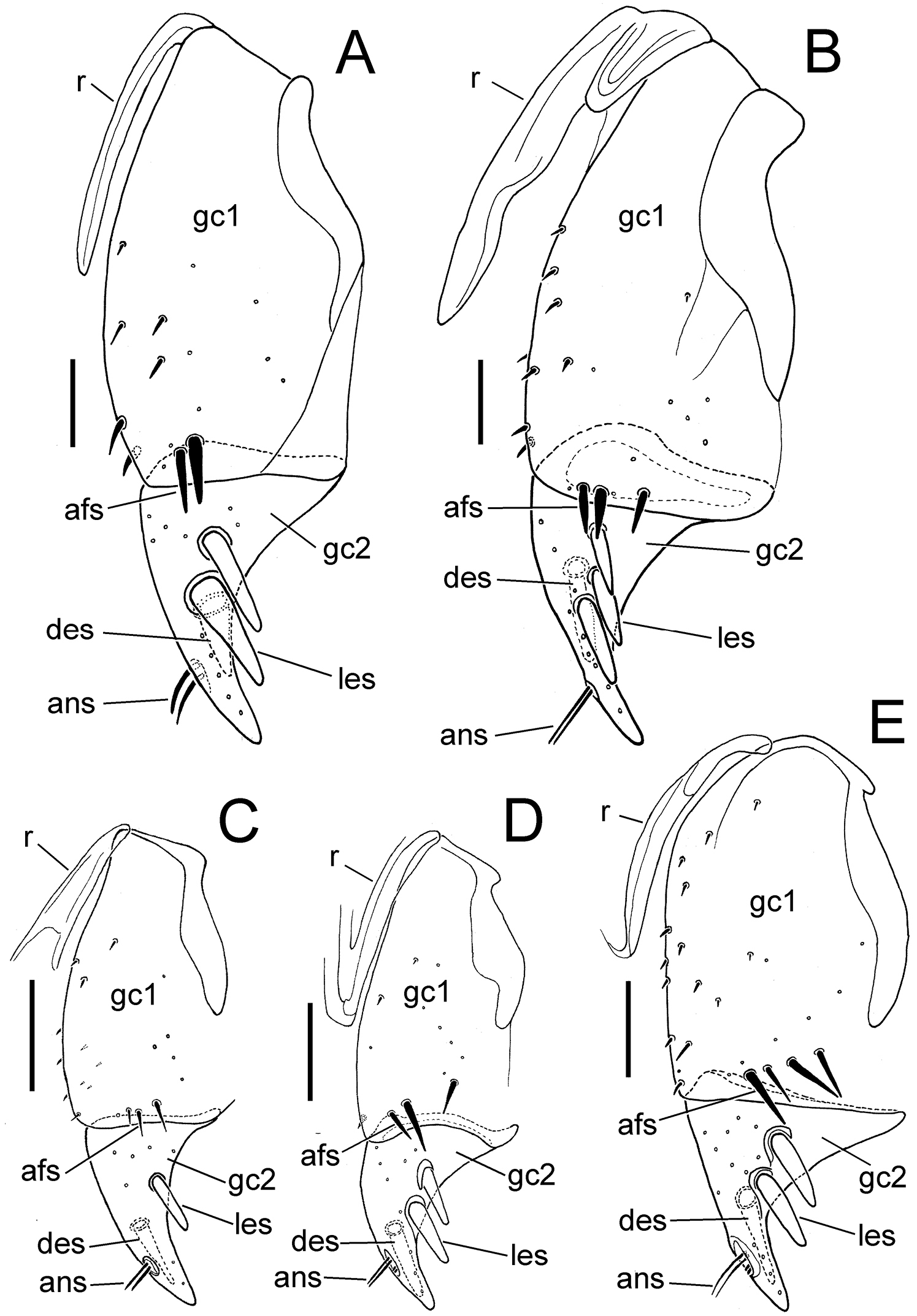
Female Reproductive Tract. (n = 1). Bursa copulatrix columnar, elongate, length 3× maximum breadth in slide-mounted specimen (Fig. 5A); bursa membranous, lightly sclerotized based on amount of Chlorazol Black staining; spermatheca reniform, spermathecal gland duct long enough so that gland extends beyond apex of bursa; basal gonocoxite 1 with apical fringe of 2–3 setae, 6–7 smaller setae along mesal margin (Fig. 6A); apical gonocoxite 2 subacuminate, apex tightly rounded, basolateral area moderately expanded; 2 lateral ensiform setae, the apical seta stouter, 1 dorsal ensiform setae, and 2 apical nematiform setae.
Female reproductive tract and associated abdominal structures, ventral view. Black line indicates position of spermathecal duct dorsad bursa copulatrix or common oviduct; circle at end of line indicates position of juncture of spermathecal duct and dorsal wall of bursa. A Mecyclothorax perraulti B Mecyclothorax pahere C Mecyclothorax popotioaoa D Mecyclothorax mapo E Mecyclothorax fatata. Abbreviations: bc bursa copulatrix co common oviduct e egg g gonocoxa h rectum of hindgut lo lateral oviduct pg pygidial gland reservoir sg spermathecal gland sp spermatheca.
Holotype male (MNHN), labeled: FRENCH POLYNESIA: Moorea / Toheia, off trail beneath ridge / 26-ix-2009 el. 1145 m C. Ewing/-17.55152 -149.82147 pyr. fog / unknown tree MBIO6551 // HOLOTYPE / Mecyclothorax / perraulti / J.K. Liebherr 2012 (black-bordered red label)
Allotype female (MNHN), labeled: FRENCH POLYNESIA / Moorea Tohiea summit / 12-IX-2006 lot 07 / S17°33.03', W149°49.33' / el. 1150-1200 m beating / flowering Myrsine after / dark J.K. Liebherr // ALLOTYPE / Mecyclothorax / perraulti / J.K. Liebherr 2012 (black-bordered red label)
SOCIETY ISLANDS. Moorea: Tohiea summit, 1120 m el., S17°33.07', W149°49.38', 12-ix-2006 lot 05, beating rotten Freycinetia and ferns, Liebherr (CUIC, 1); 1097 m el., S17°33.05', W149°49.28', 13-ix-2006 lot 02, Berlese extraction of fern litter, Ewing (CUIC, 1); muddy gulch on trail, 1170 m el., S17°33.08', W149°49.31', 25-ix-2009, pyrethrin fog mossy tree, MBIO 5853, Ewing (EMEC, 1).
This species epithet honors the memory of Georges G. Perrault, the principal describer and reviser of the Tahitian Mecyclothorax fauna.
Individuals of this species have been found from the summit at 1207 m elevation down to 1100 m. Specimens have been found microsympatrically in rotten Freycinetia stalks with Mecyclothorax mapo, and in association with Mecyclothorax mapo and Mecyclothorax fatata on flowering Myrsine at night, and in a pyrethrin fog sample of a mossy tree trunk. An individual of this species was the lone carabid beetle recovered from a Berlese extraction sample of leaf litter taken at 1100 m.
Mecyclothorax altiusculus species group
Diagnosis. All species of this group as first defined by
urn:lsid:zoobank.org:act:52B4B915-D08B-4409-A118-4901879032DE
This species plus Mecyclothorax altiusculus, Mecyclothorax pseudaltiusculus Perrault, and Mecyclothorax paraltiusculus Perrault share a bisetose, trapezoidal pronotum with rounded hind angles, and a setal formula of 2122, however individuals of this new species are larger; standardized body length 7.5–7.9 mm. The pronotum is also more transverse; MPW/PL = 1.35–1.36 (n = 2). The striae are deep and distinctly punctate in their basal half, and the convex intervals are covered with dense transverse microsculpture consisting of a mixture of parallel transverse lines and transverse-mesh sculpticells 2–4× broad as long. The mesal face of the male metatibia is lined with pectinate swellings at the points of articulation of the mesal longitudinal setal series (Fig. 2B). Of the three species listed above, Mecyclothorax paraltiusculus is most similar, attaining a similar body size—7.0 mm—and possessing elytral microsculpture consisting of a mixture of transverse lines and transverse mesh.
Head capsule withbroad, shallow frontal grooves, the frontal surface transversely wrinkled between the grooves, and a broad, low convexity bordering the groove mesad the anterior supraorbital seta; dorsally the head capsule is flat from the frons to the pronotum; ocular lobe moderately prominent and largely covered by eyes, the posterior portion of lobe obtusely joined to gena, the juncture marked by fine, shallow groove; ocular ratio 1.47, ocular lobe ratio 0.79–0.83; labral anterior margin broadly emarginate 0.2× length; antennomeres glabrous except for apical setae; antennae elongate filiform, antennomere 8 length 3× greatest width; mentum tooth with sides defining an acute angle, the apex rounded. Prothorax transverse, the basolateral margins straight to slightly concave due to the upcurved margin anterad the rounded hind angles, MPW/BPW = 1.55–1.60 (n = 2); median base slightly depressed medially, moreso laterally, with more than 40 small punctures each side; basal margin broadly, slightly convex between laterobasal depressions; median longitudinal impression obsolete but traceable on disc, present as a lenticular depression at front of median base; anterior transverse impression deep, finely incised, with 7–8 elongate punctures each side bordered by longitudinal carinae that span impression from disc to anterior callosity; anterior callosity moderately convex, covered with indistinct longitudinal wrinkles; front angles protruded, broadly rounded, APW/BPW = 0.95–0.96 (n = 2); lateral marginal depression broadly explanate, translucent, edge upturned anteriorly, more beadlike near lateral seta; laterobasal depression a broadened continuation of the lateral depression, surface punctured as median base, deepest portion meeting posterior margin at lateral edge of basal margin convexity; proepisternum with 7 distinct punctulae along hind margin, ~10 smaller punctures along posterior marginal bead of proepimeron; prosternal process broad, slightly depressed anteriorly between coxal cavities, convex posterad at juncture with posterior face. Elytra broad, subquadrate, MEW/HuW = 2.32–2.35 (n = 2); disc convex medially, sides sloped to nearly vertical; basal groove moderately curved to subangulate humerus, elytral margin at humerus only slightly upraised; parascutellar seta present; parascutellar striole defined by 4 separated punctures, the striole depressed between punctures; sutural intervals elevated to meet at suture, only slightly more convex than distinctly convex discal intervals; striae 1–8 deep, finely impressed, and distinctly punctate in basal half; all striae deep and smooth at elytral apex; eighth interval upraised in a narrow bulbous carina laterad subapical seta, the interval’s outer face nearly vertical; 2 dorsal elytral setae (one individual with 3 setae on one elytron) positioned at 0.22× and 0.52–0.60× elytral length (asymmetrical third seta at 0.75× elytral length), each seta within evident depressions that span ⅔ width of interval 3; both apical and subapical elytral setae present; lateral elytral setae 7 + (5–6); elytral marginal depression moderately broad throughout length, translucent, reduced to beadlike margin only just anterad abruptly concave subapical sinuation. Mesepisternum distinctly punctate anteriorly, ~19 deep punctures in 2–3 rows; metepisternum short, width to length ratio 0.87; metepisternal-metepimeral suture varied, a distinct suture in one individual, an indistinct, broad depression in the second; metathoracic flight wing an elongate straplike vestigium, length 4× width, and apical ¼ of wing length surpassing hind margin of metathorax, rudimentary R and M veins evident. Abdomen with irregular wrinkles on visible ventrites 1–4, and indistinct round depressions laterally on ventrites 4–6; suture between visible ventrites 2 and 3 complete. Legs with metatarsomere 1 moderate, length 0.19× length of metatibia; metatarsomere 4 with short apical lobes, maximal tarsomere length 1.2× median tarsomere length; metatarsomere 4 with very short subapical setae and longer apical setae; metatarsal dorsolateral sulci very shallow, obsolete, dorsum broadly convex. Microsculpture on frons and vertex a transverse mesh, sculpticell breadth 2× length; pronotal disc with obsolete microsculpture, indistinct transverse mesh with sculpticells 3–4× broad as long visible near edge of light reflections; pronotal median base with evident transverse-mesh microsculpture between punctures, sculpticell breadth 2–3× length; elytral disc covered with a mixture of transverse lines and transverse mesh with sculpticell breadth 2–4× length; elytral apex covered with transverse mesh, sculpticell breadth 2–4× length; metasternum glossy with obsolete transverse sculpticells; laterobasal abdominal ventrites glossy with shallow, swirling isodiametric and transverse mesh sculpticells. Coloration of frons and vertex piceous; antennomeres 1–4 rufoflavous to brunneous, segments 5–11 slightly darker; pronotal disc rufopiceous with metallic silvery reflection; reflexed pronotal margins translucent brunneous; proepipleuron rufo-brunneous, proepisternum rufopiceous; elytral disc rufopiceous with cupreous reflection; sutural interval concolorous basally, rufous apically; reflexed elytral margin brunneous, elytral apex narrowly, indistinctly paler, brunneous; elytral epipleuron rufobrunneous, metepisternum rufopiceous; abdominal ventrites glossy rufopiceous, apical ventrite narrowly rufobrunneous; metafemur and metatibia rufobrunneous.
Male genitalia. (n = 1). Aedeagal median lobe broadest dorsoventrally near midlength, apex hooklike, with small dorsal toothlike expansion (Fig. 4B); median lobe curved to right in ventral view (Fig. 4C), the apex elongated beyond apical margin of ostium; internal sac with well-developed dorsal and ventral ostial microtrichial patches; flagellar plate moderately large, length 0.3× distance from apex to parameral articulation; parameres broad basally, right paramere extended 0.7× distance from parameral articulation to apex (Fig. 4B), left paramere longer, extended 0.9× distance to apex (Fig. 4C).
Female reproductive tract. (n = 1). Bursa copulatrix columnar, heavily sclerotized, the surface leathery, bursal length 2× maximal width in slide-mounted dissection (Fig. 5B); spermathecal duct entering on dorsal bursal wall apicad position along length of bursal juncture with common oviduct; spermathecal gland duct short, little longer than spermatheca, the glandular reservoir elongate and gradually widened in diameter from duct; basal gonocoxite 1 with 3–4 apical fringe setae, and 8–10 setae along ventromesal margin (Fig. 6B); apical gonocoxite 2 broad basally with narrow, acuminate tip, and 2–3 lateral ensiform setae, 1 dorsal ensiform seta, and 2 apical nematiform setae.
Female left gonocoxa, ventral view; scale bar = 0.05 mm. A M. perraulti B M. pahere C Mecyclothorax popotioaoa D Mecyclothorax mapo E Mecyclothorax fatata. Abbreviations: afs apical fringe setae of gonocoxite 1 ans apical nematiform seta des dorsal ensiform seta gc1 basal gonocoxite 1 gc2 apical gonocoxite 2 les lateral ensiform seta(e) r ramus.
Holotype male (MNHN), labeled: FRENCH POLYNESIA: Moorea / Tohiea, muddy gulch along trail / 24-ix-2009 el. 1150 m C. Ewing / -17.55130 -149.82178 pyr. fog / mossy tree trunk MBIO 5860 // HOLOTYPE / Mecyclothorax / pahere / J.K. Liebherr 2012 (black-bordered red label).
Allotype female (MNHN), labeled: FRENCH POLYNESIA: Moorea / Tohiea, muddy gulch along trail / 25-ix-2009 el. 1150 m C. Ewing / -17.55130 -149.82178 pyr. fog / mossy tree trunk MBIO 5859 // ALLOTYPE / Mecyclothorax / pahere / J.K. Liebherr 2012 (black-bordered red label).
The species epithet is the Tahitian word pahere, or comb in English, either the noun or verb form (Wahlroos, 2002), and being indeclinable, is to be treated as a noun in apposition. The name is indicative of the metatibial comb in the male, formed by the evaginated bosslike articulatory processes associated with with the mediolongitudinal series of tibial setae.
Distribution and habitat. The allotype female was collected in a pyrethrin fog sample from a mossy tree trunk along with one individual each of Mecyclothorax fatata and Mecyclothorax mapo. The holotype male comprised the only beetle collected in a similar situation the day earlier.
urn:lsid:zoobank.org:act:2D31BAF6-A9E3-4344-BE4D-32658F0D209E
This species shares an ovoid, bisetose pronotum, and indistinctly striate elytra with Mecyclothorax jarrigei Perrault, however the striae are even less developed in Mecyclothorax jarrigei, and individuals of that species at 6.2 mm are larger than the unique specimen of this new species; standardized body length 5.2 mm. This species exhibits a setal formula of 2121 as in Mecyclothorax jarrigei; setae in the new species include both discal elytral setae and the apical elytral seta positioned just laterad the apex of the second stria. The elytra are exceedingly convex in the unique holotype (Fig. 2C), perhaps due to the slightly teneral nature of the specimen leading to apical distortion of the elytra. The dense transverse microsculpture on the elytra results in a silvery metallic reflection.
Head capsule frontal grooves smooth, with deepest portions sinuously curved mesad anterior margin of eye, broadest near frontoclypeal suture, and with low rounded carina mesad anterior supraorbital seta; dorsum of head slightly concave between anterior portions of eyes, neck slightly convex; ocular lobe broadly protruded, the posterior portion very obtusely meeting gena at very shallow ot obsolete groove; eyes small, about 16 ommatidia along a horizontal diameter commencing posterad ventral edge of antennal articulatory socket; ocular ratio 1.36, ocular lobe ratio 0.75; labral anterior margin straight; antennomeres 1–3 glabrous except for apical setae; antennae robust, submoniliform, antennomere 8 length 1.9× greatest width; mentum tooth with sides defining acute angle, apex rounded. Prothorax subovoid, hind angle definable by abrupt change in curvature of pronotal margin, but basolateral margin convex anterad angle, MPW/PL = 1.17 (n = 1); median base slightly depressed relative to disc, ~10 punctures of varying depth each side; basal margin slightly convex between laterobasal depressions; median longitudinal impression obsolete on disc, indicated by darker color of endocarina, present as a narrow elongate depression on front of median base; anterior transverse impression very fine and shallow, obsolete medially, finely incised mesad front angles; anterior callosity narrow, slightly elevated but flat, crossed by irregular longitudinal wrinkles; front angles slightly protruded, rounded, distance between somewhat greater than basal pronotal width, APW/BPW = 1.08; lateral marginal depression very narrow laterally, margin beaded, slightly broader with edge little upturned at front angles, gradually broadened toward laterobasal depression, edge upturned basally; laterobasal depression a broadened continuation of lateral depression, deepest portions mostly smooth but with minute irregularities; proepisternum with ~7 indistinct punctulae along hind margin, proepimeron with minute irregularities along marginal collar; prosternal process broad, slightly depressed mesad anterior margins of procoxal cavities, convex posterad at juncture with posterior face. Elytra subovoid, disc upraised above position of scutellum, MEW/HuW = 2.39; basal groove moderately curved to meet subangulate humerus; parascutellar seta present; parascutellar striole shallow, broadly impressed, smooth; sutural interval slightly elevated at sutural juncture, but not more convex than interval 2 laterally; striae 1–8 of same depth in basal half, shallow but complete, generally smooth but with minute irregularities associated with slight change of direction of deepest portions; at elytral apex, sutural striae 1 and 7 deeply incised, stria 2 shallow, almost discontinuous, striae 3 and 4 shallow but continuous; eighth interval convex apically, the convexity oriented laterally above subapical sinuation; 2 dorsal elytral setae at 0.25× and 0.60× elytral length, set in a moderate impression that spans ⅔ width of interval 3; apical elytral seta present, subapical seta absent; lateral elytral setae 7 + 6; elytral marginal depression narrow but margin narrowly upturned at humerus, depression somewhat broader with more gently upturned edge at midlength, margin becoming less upturned without bead anterad subapical sinuation; subapical sinuation shallow, only slightly changing curvature of apical marginal bead. Mesepisternum punctate anteriorly, ~18 punctures in 2–3 rows; mesepisternum longer than broad, width to length ratio 0.77; metepisternum separated from metepimeron by distinct suture; metathoracic flight wing vestigium apex extended to hind margin of metathorax, venation not assessed. Abdomen with irregular wrinkles laterally on visible ventrites 1–4, indistinct rounded impressions laterally on ventrites 4–6; suture between ventrites 2 and 3 complete laterally. Metatarsomere 4 with emarginate apical margin, overall length including apical lobes 1.4× median tarsomere length; metatarsomere 4 with short subapical and longer apical setae; metatarsal dorsolateral sulci shallow, lateral, the median surface broadly convex; male metatibia with four rounded projections at bases of four apical setae in mesal longitudinal series. Microsculpture of frons, obsolete, surface glossy, neck with indistinct isodiametric sculpticells in transverse rows; pronotal disc covered with shallow transverse mesh, sculpticell breadth 2–4× length; pronotal median base with shallow, swirling isodiametric and transverse sculpticells between punctures; elytral disc covered with mixture of well-developed transverse mesh, sculpticell breadth 2–4× length, and transverse lines; eytral apex with transverse mesh, sculpticells 2–4× broad as long; metasternum with distinct transverse-mesh microsculpture; laterobasal abdominal ventrites covered with swirling isodiametric and transverse mesh. Coloration of head capsule rufous with piceous cast; antennomeres 1–2 rufoflavous, 3–11 darker, brunneous; pronotal disc rufobrunneous medially, darker than head laterally on disc, lateral marginal depression concolorous, only extreme basal and apical margins paler, rufoflavous; proepipleuron dark rufoflavous, proepisternum rufobrunneous; elytral disc rufobrunneous with silvery reflection, sutural interval concolorous; elytral marginal depression narrowly translucent, slightly paler than disc, apex not paler than more basal portions; elytral epipleuron, metepisternum, and abdominal ventrites concolorous, rufobrunneous; metafemur and metatibia brunneous medially, apices paler, rufoflavous.
Male Genitalia. (n = 1). Aedeagal median lobe evenly narrowed dorsoventrally from midlength to broadly rounded apex, the apex slightly expanded on ventral margin (Fig. 4D); flagellar plate smaller, length 0.26× distance from parameral articulation to apex (assessed in uneverted, teneral dissection); internal sac dorsal surface apparently covered with field of fine spicules, neither dorsal nor ventral ostial microtrichial patch apparent; both parameres extended apically about 0.8× distance from parameral articulation to apex.
Holotype male (MNHN), labeled: French Polynesia: Moorea / Tohiea summit el. 1130 m / 12-IX-2006 lot10 / 17°33.07'S, 149°49.38'W / pyrethrin fog mossy logs / D.A. Polhemus// HOLOTYPE / Mecyclothorax / menemene / J.K. Liebherr 2012.
The species epithet is the Tahitian word menemene, i.e. round or spherical in English (Wahlroos, 2002), denoting the rounded pronotum and broadly rounded, convex elytra of this species. Being indeclinable, the epithet is to be treated as a noun in apposition.
The unique holotype of this species was collected from a rotten log situated in a deep, wet gulch, in association with four individuals of Mecyclothorax mapo and one of Mecyclothorax popotioaoa.
Mecyclothorax globosus species group
Diagnosis. Species in this group are characterized by cordate pronota, the basolateral margin distinctly sinuate anterad the projected hind angle, with the lateral marginal depression very narrow and of even breadth throughout the length of the pronotum. The elytra are very convex, with the eighth interval nearly vertical at the juncture with the elytral lateral marginal depression (Perrault, 1986, 1988). Individuals of the included species are smaller; body lengths range 3.3–5.0 mm (Perrault, 1989).
urn:lsid:zoobank.org:act:A5DBBF5A-AAF8-487C-90B1-20CDD9E34633
This species shares reduced setation, setal formula 1111 (Fig. 3A), with four other species in the group; Mecyclothorax sabulicola Britton, Mecyclothorax ataraensis Perrault, Mecyclothorax taiarapu Perrault, and Mecyclothorax cupripennis Perrault. Of these, Mecyclothorax cupripennis shares reduced microsculpture with this species, the pronotal disc lacking any discernible sculpticells. The two species differ in elytral microsculpture. This new species is characterized by a glossy elytral integument, with only sporadic small patches of indistinct isodiametric sculpticells in transverse rows, whereas Mecyclothorax cupripennis is characterized by presence of a more regular, though shallow, transverse mesh on the discal elytral intervals; the sculpticells consistently visible outside the reflection of bright, direct microscope light. Body size is similar for the two species; standardized body length of the new species is 3.7 mm, that for Mecyclothorax cupripennis 3.5 mm (measurement made on male specimen, CUIC).
Head capsule withsinuous frontal grooves, closest at frontoclypeal suture and defining a lyre shape, adjacent area of frons broadly depressed mesad anterior margin of eye, base of frontal groove separated from eye by a broad, low convexity; dorsum of head flat on frons in lateral view, neck convex; anterior supraorbital seta absent, posterior seta situated at dorsal terminus of broad shallow groove between ocular lobe and gena; eyes and ocular lobe little protruded, posterior portion of lobe meeting gena at about 135˚ angle; compound eye with 10 ommatidia on horizontal diameter defined by lower margin of antennal articulatory socket; ocular ratio 1.37, ocular lobe ratio 0.67; labral anterior margin broadly, shallowly emarginate ¹⁄₁₂ length; antennomeres 1–3 glabrous escept for apical setae; antennae moniliform, antennomere 8 length subequal to greatest width; mentum tooth with sides defining acute angle, apex tightly rounded. Prothorax cordate, disc convex, basolateral margins convergent anterad acute, projected hind angles, MPW/BPW = 1.53, MPW/PL = 1.18 (Fig. 3A); median base slightly depressed relative to disc, moreso laterally, with ~13 larger isolated punctures each side; basal margin broadly convex between laterobasal depressions; median longitudinal impression absent on basal half of disc, obsolete and traceable anterad due to indistinct transverse wrinkles at position of impression; anterior transverse impression shallow, broad, smooth medially, finely incised in lateral ⅔ of breadth; anterior callosity slightly convex, smooth; front angles slightly protruded, tightly rounded, APW/BPW = 0.99 (n = 1); lateral marginal depression very narrow, slightly broader at front angle, edge beaded throughout; laterobasal depression a deep continuation of lateral depression, bordered anteromesally by punctate median base, and laterally and posteriorly by raised marginal bead at hind angle; proepisternum with 5 minute punctures along hind margin; prosternal process narrowly depressed medially, broadly upraised each side between coxae. Elytra subovoid, MEW/HuW = 2.14 (n = 1), middle of disc flat, intervals 2–8 increasingly depressed to near vertical juncture with lateral marginal depression; basal groove distinctly curved forward to angulate humerus that lies distinctly anterad base of scutellum; parascutellar seta present, immediately adjacent to parascutellar striole; parascutellar striole finely incised, smooth anterad, 1–2 small punctures near apex; sutural interval coplanar with stria 2; striae 1–6 shallow, complete, with very small punctures at strial depth, the punctures less distinct in lateral striae; sutural stria 1 deepest at elytral apex, striae 2, 3, and 7 shallow but complete apically; discal elytral intervals slightly convex; interval 8 narrowly subcarinate laterad apex of stria 7, slightly more convex than more mesal intervals; a single dorsal elytral seta at 0.24× elytral length set in small setal depression spanning less than half of interval 3; apical elytral seta present, subapical seta absent; lateral elytral setae 7 + 6; elytral marginal depression moderately narrow, margin upraised near humerus, beadlike only near subapical sinuation; subapical sinuation deep, abruptly excavate anteriorly. Mesepisternum punctate anteriorly, with ~14 punctures in 2–3 rows; metepisternum short, anterior and mesal edges subequal, width to length ratio 0.8; metepisternum separated from metepimeron by a distinct suture. Abdomen irregularly wrinkled on lateral portions of visible ventrites 1–4, indistinct rounded depressions laterally on ventrites 4–6; suture between ventrites 2 and 3 effaced laterally. Legs with short, stout tarsomeres, metatarsomere 4 overall length subequal to breadth, length including apical lobes 1.2× median tarsomere length; metatarsomere 4 with both apical and subapical setae; metatarsal dorsolateral sulci deep, lateral, median broadly convex. Microsculpture of frons obsolete, surface glossy, indistinct transverse mesh in deepest portions of frontal grooves; pronotal disc glossy with indistinct elongate transverse mesh—sculpticell breadth 3–4× length—visible near edge of areas of reflected light; pronotal median base glossy except for obsolete transverse mesh near discal margin; elytral disc mostly glossy, patches of transverse mesh, breadth 3–4× length, visible near striae; elytral apex glossy, transverse mesh visible at apical margin; metasternum with evident transverse mesh; laterobasal abdominal ventrites with swirling isodiametric and transverse sculpticells. Coloration of head capsule a glossy rufopiceous; antennomere 1 flavous, 2–3 rufoflavous, 4–11 rufobrunneous; pronotal disc glossy rufopiceous, pronotal lateral margins concolorous, base and apex slightly paler at edge; proepipleuron rufopiceous along edge, mediolongitudinally rufoflavous, rufobrunneous along margin with rufobrunneous proepisternum; elytral disc glossy rufopiceous; scutellum and base of sutural interval dark rufous, apex of sutural interval broadly rufobrunneous; elytral margin concolorous with disc near elytral base, lateral marginal depression paler, rufoflavous behind; elytral apex broadly, slightly paler, brunneous; elytral epipleuron paler, rufoflavous dorsally, rufobrunneous ventrally to match metepisternum; abdominal visible ventrite 1 rufobrunneous; ventrites 2–3 rufopiceous medially, brunneous laterally along with ventrites 4–6; apical ventrite 6 rufoflavous in apical ⅓; metafemur rufoflavous; metatibia rufoflavous with brunneous cast.
Female reproductive tract. The unique female holotype was not dissected.
Holotype female (MNHN), labeled: FRENCH POLYNESIA: Moorea / Tohiea, off trail beneath ridge / 25-ix-2009 el. 1145 m C. Ewing / -17.55152 -149.82147 pyr. fog / unknown tree MBIO5551 // HOLOTYPE / Mecyclothorax / mahatahi / J.K. Liebherr 2012 (black-bordered red label).
The species epithet is a compounding of maha, Tahitian for the number four, and tahi, Tahitian for one (Wahlroos, 2002), indicative of the reduced setation in this species resulting in the setal formula of 1111. As tahi is indeclinable, the epithet is to be treated as a noun in apposition.
The unique specimen was collected in pyrethrin fog sample of a mossmat in association with one specimen of Mecyclothorax perraulti.
urn:lsid:zoobank.org:act:CD0E0395-531F-4DD2-B68C-8761EA70D2F5
Within the Mecyclothorax globosus group, this is the only species for which individuals lack dorsal elytral setae, resulting in a setal formula of 2101; one individual of the five type specimens has an asymmetrically positioned dorsal elytral seta at 0.24× length on the left elytron, however this is considered a variant condition not characterizing the species. This species is also characterized by shallow elytral striae (Fig. 3B), and much reduced microsculpture across the entire dorsum. The most similar species in the group, Mecyclothorax hemisphaericus Perrault, shares much reduced elytral striae, though in this species they are nearly obsolete. Mecyclothorax hemisphaericus also exhibits reduced setation, though presence of a single dorsal elytral seta results in a setal formula of 2111. The new species can also be distinguished from Mecyclothorax hemisphaericus by the more narrowly ovoid elytra, MEW/MPW = 1.43–1.48 (n = 4), versus more broadly ovoid elytra in Mecyclothorax hemisphaericus, MEW/MPW = 1.61 (n = 2 paratypes, MNHN). Individuals of both species are of similar size; standardized body length for this species is 3.7–4.0 mm, versus 3.5–3.9 mm for Mecyclothorax hemisphaericus, as determined from two examined paratypes (MNHN).
Head capsule withslightly sinuous frontal grooves, the two grooves approaching each other mesad a broad convexity near frontoclypeal suture, frons mesad grooves depressed and transversely wrinkled, groove bordered laterally by thin carina mesad anterior supraorbital seta; dorsum of head flat on frons in lateral view, neck convex; ocular lobe little projected, posterior portion meeting gena at >135˚, a narrow, shallow groove at juncture; eyes slightly more convex than ocular lobe, 14–15 ommatidia along horizontal diameter oriented to ventral margin of antennal articulatory socket; ocular ratio 1.36–1.42 (n = 4), ocular lobe ratio 0.74–0.76 (n = 4); labral anterior margin broadly, shallowly emarginate ¹�₆ of length; antennomeres 1–3 glabrous except for apical setae; antennae submoniliform, antennomere 8 length 1.75× greatest breadth; mentum tooth with sides defining an acute angle, apex tightly rounded. Pronotum narrow, little transverse, distinctly cordate, the basolateral margins subparallel to slightly convergent for 1/9 pronotal length anterad projected, slightly obtuse hind angles (Fig. 3B), MPW/BPW = 1.67–1.76 (n = 4), MPW/PL = 1.12–1.16 (n = 4); median base slightly depressed relative to disc, 12–14 large punctures each side; basal margin broadly convex between hind angles; median longitudinal impression very shallow, finely incised, but traceable across disc, briefly extended as an elongate puncture at front of median base; anterior transverse impression very shallow, smooth, obsolete medially, finely incised in outer half of breadth each side; anterior callosity slightly convex, smooth but with very minute longitudinal wrinkles; front angles not protruded anterad, the margin perpendicular to longitudinal axis and curving posterad in a tight curve, APW/BPW = 1.13–1.19 (n = 4); lateral marginal depression very narrow, pronotal margin beaded throughout length to hind angle and posterad laterobasal depression; laterobasal depressions ill defined, punctate, coplanar with lateral portion of median base; proepisternum with ~10 very fine irregularities along hind margin; prosternal process narrowly depressed medially, sides broadly upraised mesad coxal cavities. Elytra narrowly subovoid (Fig. 3B), disc convex, sides distinctly sloped to near vertical; basal groove evenly curved anterad to angulate humerus, MEW/HuW = 2.2–2.3 (n = 4); parascutellar seta present; parascutellar striole 3–4 punctate, shallow but continuous; sutural interval slightly elevated at sutural juncture basally, moreso apically; striae 1–6 shallow on disc, indistinctly punctate, stria 7 obsolete but traceable, smooth; striae 1 and 7 deep and smooth apically, striae 2–6 shallower but traceable, though juncture of 5 and 6 is deeper; discal intervals slightly convex, lateral intervals less upraised, though convex due to curvature of elytron; interval 8 distinctly bulbous laterad stria 7, broadly subcarinate dorsal subapical sinuation; apical elytral seta present, subapical seta absent; lateral elytral setae (5–6) + (4–5); elytral marginal depression narrow with little upraised margin at humerus, slightly broader laterally, margin beaded anterad subapical sinuation. Mesepisternum densely punctate anteriorly, ~14 large punctures in 2–3 rows; metepisternum slightly longer than broad, width to length ratio 0.75; metepisternum separated from metepimeron by distinct suture. Abdomen with visible ventrites 1–5 irregularly wrinkled laterally, ventrites 3–6 with rounded depressions laterally; suture between visible ventrites 2 and 3 effaced laterally. Metatarsomere 4 indistinctly lobate, overall length including lobes 1.5× median tarsomere length, both subapical and apical setae present; metatarsal dorsolateral sulci very shallow, lateral, median surface of tarsomere broadly convex. Microsculpture obsolete on frons, surface glossy, shallow isodiametric sculpticells in transverse rows on neck; pronotal disc glossy, micro- sculpture obsolete but indistinct transverse sculpticells, 3–4× broad as long, discernible just outside areas of light reflection; pronotal median base with indistinct isodiametric mesh between punctures; elytral disc glossy, obsolete transverse mesh visible along edge of reflected light; elytral apex with shallow isodiametric and transverse sculpticells; metasternum with distinct transverse mesh; laterobasal abdominal ventrites covered with swirling isodiametric and transverse sculpticells. Coloration of head capsule rufous with slight piceous cast; antennomeres 1–3 rufoflavous, 4–11 slightly darker, the apical antennomeres rufobrunneous; pronotal disc dark rufous, pronotal margins with piceous cast, basal and apical edge slightly paler, rufobrunneous where narrowed in thickness; proepipleuron rufoflavous medially, dark dorsally, rufous with piceous cast ventrally to match proepisternum; elytral disc dark rufous, sutural interval rufous basally, rufoflavous apically; elytral marginal depression concolorous at humerus, rufoflavous in deepest portion laterally and to subapical sinuation; elytral apex broadly slightly paler, rufobrunneous; elytral epipleuron rufoflavous ventrad dark margin, rufobrunneous ventrally, metepisternum slightly darker, rufous with piceous cast; abdomen dark rufous basally, ventrites 4–6 paler, rufobrunneous, apical ventrite with apical third paler, rufoflavous; metafemur rufoflavous; metatibia rufoflavous with brunneous cast.
Male genitalia. (n = 1). Aedeagal median lobe evenly curved and of subequal diameter in basal half, narrowed apically to tightly rounded apex that extends little beyond apical ostial margin (Fig. 4E); internal sac lightly sclerotized, only flagellar plate visible in uneverted dissection, length of plate 0.33× distance from parameral articulation to apex; right paramere short, apex extended toward apex 0.7× distance from parameral articulation to apex, left paramere longer, extended 0.9× that distance.
Female reproductive tract. (n = 1). Bursa copulatrix very short, present as a very short lobe situated dorsad the broad common oviduct (Fig. 5C); bursal apex extended beyond evident transverse fold at base of oviduct the same distance as that from transverse fold to a line drawn between bases of basal gonocoxites; bursal surface lightly sclerotized, as membranous as surface of median oviduct; spermatheca reniform, spermathecal gland connected to base of spermatheca by a short duct; basal gonocoxite 1 with apical fringe of 3–4 setae, and 10–11 smaller setae along mesal margin, spanning ventromedial to dorsomedial surfaces of gonocoxite (Fig. 6C); apical gonocoxite 2 narrow basally with tightly rounded apex, single dorsal and lateral ensiform setae and 2 apical nematiform setae.
Holotype male (MNHN) labeled: French Polynesia: Moorea / Tohiea summit el. 1080- / 1120 m 12-IX-2006 lot 09 / 17°33.07'S, 149°49.38'W / beating dead fern fronds / C.P. Ewing // HOLOTYPE / Mecyclothorax / popotioaoa / J.K. Liebherr 2012 (black-bordered red label).
Allotype female (MNHN): French Polynesia: Moorea / Tohiea summit el. 1120 m / 12-IX-2006 lot 08 / 17°33.07'S, 149°49.38'W / beating ferns C.P. Ewing // ALLOTYPE / Mecyclothorax / popotioaoa / J.K. Liebherr 2012 (black-bordered red label).
SOCIETY ISLANDS. Moorea: Tohiea summit, 1120 m el., S17°33.07', W149°49.38', 12-ix-2006 lot08, beating ferns, Ewing (CUIC, 1); 1120 m el., S17°33.07', W149°49.38' 12-ix-2006 lot 10, pyrethrin fog mossy log, deep gulch, Polhemus (NMNH, 1); gulch S of summit, 1160-1180 m el., S17°33.03', W149°49.36', 24-ix-2009, on fern frond, gulch wall, MBIO 5852, Ewing & Yang (EMEC, 1).
The species epithet is a compounding of the Tahitian word popoti, beetle or cockroach, and oaoa, the Tahitian adjective narrow (Wahlroos, 2002), signifying the constricted pronotal base and narrow body of adult beetles of this species. As oaoa is indeclinable, the epithet is to be treated as a noun in apposition.
Distribution and habitat. Four of the five specimens recorded for the species have been collected on fern fronds, either living or dead, in all such instances associated with individuals of Mecyclothorax mapo. The fifth specimen was collected in association with Mecyclothorax mapo and the single known Mecyclothorax menemene by pyrethrin fogging of a mossy log complex in a deep, wet gulch.
Mecyclothorax viridis species group
Diagnosis.
urn:lsid:zoobank.org:act:6E037A49-8874-4791-BCC4-F3FD8EC5556F
This species shares transverse-line elytral microsculpture and deep punctate elytral striae with Mecyclothorax castaneus, and individuals are of similar body size; standardized body length for this species 3.8–4.4 mm versus 3.8 mm for Mecyclothorax castaneus. The pronotum is of similar dimensions in the two species, with MPW/PL = 1.14–1.17 (n = 5) in this species, versus a ratio of 1.19 in Mecyclothorax castaneus (Perrault, 1986). The species differ in setation, with this species consistently characterized by two discal elytral setae, and therefore a setal formula of 2221, versus Mecyclothorax castaneus where one of the two type specimens had two discal setae on one elytron, whereas the other three elytra of the two beetles were unisetose. In addition, the pronotal base of this species is relatively broader, MPW/BPW = 1.52–1.64 (n = 5), versus a narrower base and greater ratio of 1.70 for Mecyclothorax castaneus.
Head capsule withfrontal grooves nearly straight on lateral margins, depressed area of frons triangular with broadest portion at frontoclypeal suture, groove terminated posteriorly at thin carina mesad anterior supraorbital seta; dorsum of head flat on frons in lateral view, neck convex; ocular lobe broadly convex, little protruded from head capsule, hind portion meeting gena at 135˚ angle, at a fine groove bordered posterad by fine carina; compound eye with 15–16 ommatidia along a horizontal diameter defined by lower margin of antennal articulatory socket; ocular ratio 1.40–1.49 (n = 5), ocular lobe ratio 0.73–0.83 (n = 5); labral anterior margin nearly straight, only slightly emarginate; antennomeres 1–3 glabrous except for apical setae; antennae submoniliform, antennomere 8 length 1.6× greatest breadth; mentum tooth sides defining an acute angle, apex subacuminate. Pronotum quadrisetose, cordate (Fig. 3C), margin variously, slightly convergent, subparallel, or slightly divergent anterad obtuse hind angles; median base depressed relative to disc, margined anteriorly by a row of punctures, some elongate, 13–14 punctures each side of base, punctures sparser medially; basal margin convex between the laterobasal depressions; median longitudinal impression fine, shallow but continuous on disc, continued as fine impression onto front of median base; anterior transverse impression broad, shallow, smooth, finely incised only mesad front angle; anterior callosity only slightly convex, with very fine and shallow longitudinal wrinkles, variously restricted to front margin of pronotum to crossing callosity and anterior transverse impression; front angles slightly protruded, tightly rounded, apical and basal pronotal widths subequal, APW/BPW = 1.0–1.09 (n = 5); lateral marginal depression very narrow, edge beaded throughout most of length, except at front angle where margin is slightly upturned to flat, and along basolateral sinuation where margin is broadly upturned; laterobasal depression defined by linear mesal extension of lateral depression and lateral raised basolateral margin, punctures of median base not reaching deepest linear portion; proepisternum with 6 distinct punctulae along hind margin, ~5 smaller punctures along marginal collar of proepimeron; prosternal process narrowly depressed between broadly upraised lateral areas between procoxae, convex posterad at juncture with posterior face. Elytra subovoid, sides broadly convex; disc convex with sides sloping to near vertical juncture with lateral marginal depression; basal groove short, distinctly, anteriorly curved to proximate, angulate humeri, MEW/HuW = 2.29–2.43 (n = 5); parascutellar seta present; parascutellar striole 3–4 punctate, irregularly depressed or not between punctures; sutural interval dorsally expanded to meet at a sutural callous that extends to apex; striae 1–4 distinctly impressed on disc, elongately punctate at depth, striae 5–6 shallower but also elongately punctate, stria 7 reduced to series of isolated punctures; sutural stria 1 deep and smooth apically, stria 7 nearly as deep apicad subapical sinuation, 2–6 shallow, continuous, with rudimentary punctures in deeper portions; intervals 2–6 slightly convex on disc; interval 8 bulbously carinate laterad stria 7 dorsad subapical sinuation, the interval nearly vertical in orientation laterad its midpoint; two dorsal elytral setae in impressions that span ½ to ⅔ of interval 3, positioned at 0.32–0.34× and 0.66–0.68× elytral length; apical elytral seta present, subapical seta absent; lateral elytral setae 6 + 5; elytral marginal depression narrow, edge thick and upturned at humerus, more thinly upturned laterally, beadlike anterad subapical sinuation; subapical sinuation shallowly excavate, broad. Mesepisternum anteriorly with 6 large, isolated punctures in 1–2 rows; metepisternum longer than wide, width to length ratio 0.72; metepisternum separated from metepimeron by distinct suture; metathoracic wing vestigium an elongate strap, 1.57× long as wide that extends from base to 0.73× length of metanotum, rudiments of R and M veins visible on strap. Abdomen with irregular wrinkles laterally on visible ventrites 1–4, rounded depressions laterally on ventrites 3–6; suture between visible ventrites 2 and 3 laterally effaced. Metatarsomere 4 triangular in dorsal view, lobate, length including lobes 1.6× median tarsomere length, subapical and apical setae present; metarsal dorsolateral sulci shallow, lateral, tarsomeres broadly convex medially. Microsculpture of frons reduced, surface glossy, shallow transverse mesh visible adjacent to area of reflected light; pronotal disc glossy, shallow, indistinct transverse mesh, sculpticells 3–4× broad as long, visible adjacent to area of reflected light; pronotal median base glossy between punctures; elytral disc with distinct, subiridescent transverse mesh, sculpticells 3–4× broad as long, mixed with less cross–connected transverse lines; elytral apex with transverse mesh, sculpticells 2–3× broad as long; metasternum glossy with obsolete transverse mesh; laterobasal abdominal ventrites glossy, covered with shallow swirling isodiametric and transverse mesh. Coloration of head capsule rufobrunneous with piceous cast; antennomeres 1–2 flavous, 3–4 rufoflavous, 5–11 slightly darker; pronotal disc rufobrunneous, anterior transverse impression rufopiceous; proepipleuron rufoflavous, proepisternum rufobrunneous; elytral disc rufobrunneous with silvery to bluish reflection; sutural interval rufous basally, rufoflavous apically; elytral lateral marginal depression concolorous with disc at humerus, increasingly paler to rufoflavous anterad subapical sinuation; elytral apex narrowly rufoflavous anterad incised portion of stria 7; elytral epipleuron rufoflavous, metepisternum rufobrunneous; abdomen rufobrunneous, lateral margins concolorous to darker, with piceous cast, abdominal apical ventrite rufoflavous in apical ¼; metafemur flavous with medial brunneous cast; metatibia flavous.
Male genitalia. (n = 3). Aedeagal median lobe broad in basal ⅔ of length, narrowed apically to broadly rounded apex with blunt apical face, ventral portion of median lobe straight (Fig. 4F); internal sac ventrally covered with dense microspiculate field, distinct ventral or dorsal microtrichial patches absent; flagellar plate large, length 0.57× distance between parameral articulation and apex, gonopore visible on middle of dorsal surface, longitudinally radiate sclerotic ridges on inner, ventral, surface of plate; right paramere narrowly elongate (Fig. 4G), tip extended 0.85× distance from parameral articulation to apex, left paramere slightly longer, extended 0.90× such distance.
Female reproductive tract. (n = 1). Bursa copulatrix constricted basally apicad juncture with common oviduct (Fig. 5D), bursa columnar, length 3× greatest breadth in slide–mounted dissection, surface thin, membranous based on staining with Chlorazol Black; spermatheca reniform, spermathecal duct heavily sclerotized, inflexible; spermathecal gland attached to spermatheca by an elongate duct; basal gonocoxite 1 with 2–3 apical fringe setae, and 2–3 smaller setae along the mesal surfaces of coxite (Fig. 6D); gonocoxite 2 broad basally, subacuminate apically, the lateral margin broadly concave; 2 lateral ensiform setae, 1 dorsal ensiform seta, and 2 apical nematiform setae present on gonocoxite 2.
Holotype male (MNHN), labeled: French Polynesia: Moorea / Tohiea summit el. 1125– / 1200 m 12–IX–2006 lot 01 / 17°33.03'S, 149°49.33'W / beating ferns & Myrsine / J.K. Liebherr // HOLOTYPE / Mecyclothorax / mapo / J.K. Liebherr 2012 (black–bordered red label).
Allotype female (MNHN), labeled as holotype but with black–bordered red ALLOTYPE label.
SOCIETY ISLANDS. Moorea: Tohiea summit, 1125–1200 m el., S17°33.03', W149°49.33', 12–ix–2006 lot 01, beating Myrsine + ferns, Liebherr (CUIC, 8); 1125 m el., S17°33.07', W149°49.38', 12–ix–2006 lot 02, pyrethrin fog Weinmannia moss + roots, Liebherr (CUIC, 3); 1120 m el., S17°33.07', W149°49.38', 12–ix–2006 lot 03, beating Dicranopteris ferns, Liebherr (CUIC, 5); lot 05, beating rotten Freycinetia, Liebherr (CUIC, 4); 1150–1200 m el., S17°33.03', W149°49.33', 12–ix–2006 lot 07, beating flowering Myrsine at night, Liebherr (CUIC, 12; NMNH, 2); 1120 m el., S17°33.07', W149°49.38', 12–ix–2006 lot 08, beating ferns, Ewing (EMEC, 2); 1120 m el., S17°33.07', W149°49.38', 12–ix–2006 lot 09, dead fern fronds, deep gulch, Ewing (CUIC, 2; EMEC, 2); 1120 m el., S17°33.07', W149°49.38', 12–ix–2006 lot 10, pyrethrin fog mossy log, deep gulch, Polhemus (NMNH, 4); gulch S of summit 1160–1180 m el., S17°33.03', W149°49.36', 24–ix–2009 on fern frond, gulch wall, MBIO 5852, Ewing & Yang (EMEC, 5); muddy gulch on trail, 1150 m el., S17°33.08', W149°49.31', 24–ix–2009, pyrethrin fog mossy tree trunk, MBIO 5856, Ewing (CUIC, 1; EMEC, 1); summit along ridge to west, 1190–1207 m el., S17°33.04', W149°49.34', 24–ix–2009, beating Myrsine, MBIO 5857, Ewing (CUIC, 1; EMEC, 6); muddy gulch on trail, 1150 m el., S17°33.08', W149°49.31', 24–ix–2009, beating Angiopteris evecta, MBIO 5854, Stavrinides (EMEC, 2); 1170 m el., S17°33.08', W149°49.31', 25–ix–2009, pyrethrin fog mossy tree, MBIO 5853, Ewing (EMEC, 2); 1150 m el., S17°33.08', W149°49.31', 25–ix–2009, pyrethrin fog mossy tree trunk, MBIO 5859, Ewing (EMEC, 1); gulch S of summit, 1150–1170 m el., S17°33.03', W149°49.36', 26–ix–2009, on fern frond steep gulch, MBIO 5861, Ewing (EMEC, 3).
Given that this species is most similar to Mecyclothorax castaneus, the common name of the Tahitian chestnut tree, Inocarpus fagifer (Parkinson) (Fabaceae)—i.e. mapo (Wahlroos, 2002)—was chosen for the species epithet. The epithet is to be treated as a noun in apposition.
This species has been found in a variety of situations on Mont Tohiea, accounting for 68 of the 90 specimens of Mecyclothorax collected on or near the summit. Specimens have been found by sampling ferns, Angiopteris, rotten Freycinetia, Myrsine foliage and flowers, and moss–covered Wienmannia trunks and roots. In keeping with this species’ numerical dominance, it has been collected in association with all other Mecyclothorax spp. known from Mont Tohiea.
urn:lsid:zoobank.org:act:14EE6095-7917-4D0F-8D25-42F7C8763199
This species shares upturned pronotal margins with a visible lateral depression (Fig. 3D) and regular transverse–mesh elytral microsculpture with Mecyclothorax ata Perrault. Individuals of the two species are of similar body size; standardized body length 4.7–5.0 for this species versus 4.5 mm for Mecyclothorax ata. However this species deviates from Mecyclothorax ata by presence of only the anterior elytral seta resulting in a setal formula of 2211 versus 2221 for Mecyclothorax ata. The pronotal base is also more constricted in this species, MPW/BPW = 1.53–1.62 (n = 5) versus a ratio of 1.49 in Mecyclothorax ata (
Head capsule gracile, elongate, frontal grooves subparallel at thin carina posteriorly, mesad anterior supraorbital seta, convergent anterad, clypeo–ocular prolongation broadly convex, frons between groove densely covered with fine transverse wrinkles; dorsum of head flat on frons in lateral view, neck convex; ocular lobe protruded, posterior portion meeting gena at broad, moderately deep groove; compound eye slightly protruded from ocular lobe, slightly convex dorsally laterad supraorbital seta, more than 20 ommatidia along diameter defined by lower margin of antennal articulatory socket; ocular ratio 1.48–1.60, ocular lobe ratio 0.79–0.86; labral anterior margin broadly shallowly emarginate 1/9 length; antennomeres 1–3 mostly glabrous except for apical seta, antennomere 3 with a few very short setae on posterior surface of shaft; antennae moderately elongate, antennomere 8 length 1.8× maximum breadth; mentum tooth sides defining an acute angle, apex tightly rounded. Pronotum smoothly cordate, basolateral margins nearly subparallel anterad rounded obtuse hind angles, distinctly divergent just anterad basal pronotal setae; pronotum somewhat transverse, MPW/PL = 1.15–1.20 (n = 5); median base distinctly depressed relative to disc, punctures arrayed along margin of base and disc, 13–14 punctures of various sizes each side; basal margin slightly convex between laterobasal depressions; median longitudinal impression very fine and shallow but complete on disc, prolonged as longitudinal crease on median base; anterior transverse impression broad and shallow, smooth, finely incised only mesad front angles; anterior callosity flat, little upraised, densely covered with shallow longitudinal wrinkles; front angles very slightly protruded anterad, broadly rounded, distances between front and hind angles subequal, APW/BPW = 0.99–1.05 (n = 5); lateral marginal depression bordered by upraised lateral margin at lateral pronotal seta, broader with margin less upraised at front angle, broader basally joining laterobasal depression which is broader, the surface irregularly punctured; proepisternum with 7 distinct punctulae along hind margin, proepimeron with about 10 very small punctures along marginal collar; prosternal process narrowly depressed medially, sides broadly upraised between procoxae, surface convex posterad at juncture with posterior face. Elytra ellipsoid, humeri proximate, disc convex medially, surface sloping laterally to near vertical juncture with lateral marginal depression; basal groove briefly curved to angulate humerus, MEW/HuW = 2.43–2.67 (n = 5); parascutellar seta present; parascutellar striole 3–4 punctate, surface not depressed between punctures; sutural intervals elevated to meet at suture, more convex, callouslike apically; striae 1–6 punctate, the punctures expanding strial width, but striae shallow between punctures, and striae progressively shallower laterally, stria 7 obsolete, indicated by irregularly evident shallow, longitudinal punctulae; intervals on disc flat; sutural stria 1 and stria 7 deep, well defined at apex, striae 2–6 very shallow, difficult to trace; mesal margin of interval 8 protruded as a distinct carina apicad the subapical sinuation; anterior dorsal elytral setae in small impression spanning ½ of interval 3, the setae situated at 0.25–0.26× elytral length; apical elytral seta present, subapical seta absent; lateral elytral setae 7 + 6; elytral marginal depression moderately narrow, edge upturned at humerus, depression broader and edge more upturned laterally to elytral midlength, then depression narrowed, margin beadlike anterad subapical sinuation; subapical sinuation very shallow and brief, nearly obsolete. Mesepisternum with single dorsoventral row of 5–6 large punctures; metepisternum moderately elongate, width to length ratio 0.68; metepisternum separated from metepimeron by distinct suture; metathoracic flight wing vestigium an elongate strap, length 3× width, the apical half of strap extended beyond hind margin of metanotum, with rudiments of wing veins C, R, M, and Cu visible in vestigium. Abdomen with visible ventrites 1–4 irregularly wrinkled laterally, ventrites 3–6 with rounded depressions laterally; suture between visible ventrites 2 and 3 effaced laterally. Metatarsomere 4 emarginate apically, short apical lobes present, overall tarsomere length 1.5× median tarsomere length; metatarsomere 4 with long apical and very short subapical setae that are situated along dorsal margin of tarsal apical lobes; metatarsal dorsolateral sulci shallow and lateral, tarsomere dorsum broad, nearly flat. Microsculpture on frons a regular transverse mesh, sculpticell breadth 2–3× length, neck with isodiametric sculpticells in transverse rows; pronotal disc with a mixture of transverse lines and transverse mesh with sculpticell breadth 3–4× length, the surface subiridescent due to microsculpture; pronotal median base covered with dense, swirling isodiametric and transverse sculpticells between punctures; elytral disc with distinct transverse mesh, sculpticell breadth 2–4× length, the surface subiridescent; elytral apex with transverse mesh, sculpticells 2–4× broad as long, the sculpticells slightly upraised; metasternum with distinct elongate transverse mesh; laterobasal abdominal ventrites with glossy surface, swirling isodiametric and transverse sculpticells plainly visible. Coloration of head capsule rufobrunneous with a piceous cast; antennomeres 1–2 flavous, 3–11 rufoflavous; pronotal disc rufobrunneous with silvery metallic reflection, lateral margins, base and anterior callosity darker than disc, with piceous cast; proepipleuron rufoflavous, proepisternum rufobrunneous; elytral disc rufobrunneous with silvery reflection; sutural interval concolorous with disc basally paler, rufoflavous apically; elytral marginal depression concolorous with disc at humerus, depressed area rufoflavous from midlength to subapical sinuation; elytral epipleuron rufoflavous, metepisternum rufobrunneous; abdomen rufobrunneous, broadly paler apically to rufoflavous apex of visible ventrite 6; metafemur rufoflavous; metatibia rufoflavous with brunneous cast.
Male genitalia. (n = 2). Aedeagal median lobe of equal diameter in basal ⅔ of length, narrowed to a bluntly rounded, ventrally expanded apex (Fig. 4H); internal sac with melanized microspicules on surface, appearing dark in uneverted dissection; flagellar plate large, melanized, length 0.5× distance from parameral articulation to apex; right paramere narrowly elongate, apex extended 0.85× distance from parameral articulation to apex, left paramere longer, apex extended 0.90× such distance.
Female reproductive tract. (n = 1). Bursa copulatrix columnar, length 2.4× greatest width in microslide–mounted dissection (Fig. 5E), bursal surface lightly sclerotized based on staining with Chlorazol Black; spermatheca reniform, spermathecal duct thin and lighly sclerotized; spermathecal gland bulbous, apparently filled with material that did not clear in 10% KOH in dissected individual, attached to spermatheca by short duct; basal gonocoxite 1 with apical fringe of 4 setae (Fig. 6E), 13–14 small setae arrayed across medial portion of coxite; apical gonocoxite 2 broad basolaterally, the lateral margin broadly excavate, the apex subacuminate; 2 lateral and 1 dorsal ensiform setae, and 2 apical nematiform setae present.
Variation. Marginal setation of the apical visible ventrite in males is unstable in this species. Of the four male specimens, one individual has 4 terminal abdominal setae along the apical margin of visible ventrite 6, 2 on each side (EMEC); one has 3 apical setae, 1 on the right and 2 on the left (CUIC); and two others exhibit the usual 2 setae, 1 each side (MNHN, EMEC).
Holotype male (MNHN), labeled: FRENCH POLYNESIA: / Moorea Tohiea summit / 12–IX–2006 lot 07 / S17°33.03', W149°49.33' / el. 1150–1200 m beating / flowering Myrsine after / dark J.K. Liebherr // HOLOTYPE / Mecyclothorax / fatata / J.K. Liebherr 2012 (black–bordered red label).
Allotype female (MNHN), labeled as holotype but with black–bordered red ALLOTYPE label.
SOCIETY ISLANDS. Moorea: Tohiea summit, 1125–1200 m el., S17°33.03', W149°49.33', 12–ix–2006 lot 01, beating Myrsine + ferns, Liebherr (CUIC, 2); 1125 m el., S17°33.07', W149°49.38', 12–ix–2006 lot 02, pyrethrin fog Weinmannia, moss + roots, Liebherr (CUIC, 1); summit along ridge to west, 1190–1207 m el., S17°33.04', W149°49.34', 24–ix–2009, beating Myrsine, MBIO 5857, Ewing (EMEC, 1); muddy gulch on trail, 1170 m el., S17°33.08', W149°49.31', 25–ix–2009, pyrethrin fog mossy tree, MBIO 5853, Ewing (CUIC, 1); 1150 m el., S17°33.08', W149°49.31', 25–ix–2009, pyrethrin fog mossy tree trunk, MBIO 5859, Ewing (EMEC, 1).
Because this species is most similar to Mecyclothorax ata, the Tahitian epithet fatata, near or nearly (
All six collections and eight specimens of Mecyclothorax fatata were made in association with the numerically dominant Mecyclothorax mapo. In two instances Mecyclothorax perraulti was also present in the sample. Five of the eight Mecyclothorax fatata specimens were collected from Myrsine, one from moss–covered Weinmannia, and two others from unidentified trees.
The greatest similarity of the seven Moorean Mecyclothorax spp. to seven different Tahitian Mecyclothorax spp. (Table 1) points to independent biogeographic relationships—i.e. independent speciation events—for the seven sister–species pairs. This pattern suggests that all seven speciation events have occurred such that the descendant species occupy allopatric distributions on either side of the Moorea Channel. Isolation by this Channel leading to speciation could have conceivably involved three biogeographic phenomena: 1, vicariance between Moorea and Tahiti based on subsidence of intermediate land areas and associated oceanic incursion; 2, dispersal of an ancestral population from Tahiti to Moorea; 3, dispersal of an ancestral population from Moorea to Tahiti. The first option involving subsidence of an ancient mountain range was favored by
Moorean Mecyclothorax spp. and hypothesized Tahitian adelphotaxa based on greatest morphological similarity, plus distributional range in Tahiti of hypothesized adelphotaxa. The distributional ranges Aorai and Marau are the ridges culminating in those peaks to the south. Taiarapu represents species collected in the Mts. Teatara, Presqu’île de Tairarapu.<br/>
| Moorean species | Tahitian adelphotaxon | Adelphotaxon range |
|---|---|---|
| Mecyclothorax perraulti | Mecyclothorax gourvesi | Aorai + Marau |
| Mecyclothorax pahere | Mecyclothorax paraltiusculus | Taiarapu |
| Mecyclothorax menemene | Mecyclothorax jarrigei | Aorai |
| Mecyclothorax mahatahi | Mecyclothorax cupripennis | Taiarapu |
| Mecyclothorax popotioaoa | Mecyclothorax hemisphaericus | Marau |
| Mecyclothorax mapo | Mecyclothorax castaneus | Marau |
| Mecyclothorax fatata | Mecyclothorax ata | Aorai |
Taking up the hypotheses that the biogeographic relationships of the Moorean and Tahitian taxa are due to dispersal, we can ask whether a consistent biogeographic relationhip between Mont Tohiea and an area of endemism in Tahiti is observed. If Mecyclothorax beetles dispersed between these islands, they necessarily did so over water, not in the air, as all Mecyclothorax spp. on both islands possess vestigial flight wings and a truncated metathoracic flight apparatus evolutionarily associated with winglessness (
The generality of the proposed, most prevalent dispersal pattern can be tested using information from other taxa. To date the best example of such a test involves the Moorean Nabidae, which comprise three micropterous species, all related to different micropterous species in Tahiti (
At present we have no evidence that Moorean Mecyclothorax have undergone autochthonous speciation in Moorea.
The most speciose radiations of Mecyclothorax spp. are concentrated in the Society and Hawaiian island chains. Is this shared level of extreme diversity indicative of phylogenetic affinity for these two radiations? The most generalized species in the genus are Australian.
A–C Mecyclothorax spp., females, dorsal view. A Mecyclothorax punctipennis, Mt. Buffalo S.P., Victoria, Australia; proposed adelphotaxon to both the Society Island and Hawaiian Mecyclothorax radiations B Mecyclothorax wallisi, female, Aorai, Tahiti; member of Mecyclothorax striatopunctatus species group C Mecyclothorax montivagus, Haleakala, Maui; proposed as closest extant relative to founding Hawaiian Mecyclothorax species D–F Elytral apex, dorsal view; sa, subapical elytral seta D Mecyclothorax punctipennis E Mecyclothorax wallisi F Mecyclothorax montivagus; subapical seta absent from right elytron.
Mecyclothorax spp., male aedeagal median lobe and parameres, internal sac everted. A Mecyclothorax punctipennis. B Mecyclothorax montivagus. Abbreviations: dop dorsal ostial microtrichial patch; fp flagellar plate; vop ventral ostial microtrichial patch.
Based on comparison of the seven new Moorean species plus all Tahitian species described by G.G. Perrault (MNHN) to representatives of all Australian and New Zealand species (
If Mecyclothorax punctipennis spawned the colonizing propagules that founded radiations on both Hawaiian and Society archipelagoes, were these radiations founded in a stepping–stone like manner from a source in the southwest Pacific? Such a stepping-stone pattern of colonization has been shown for the dominant Hawaiian Ohi`a, Metrosideros polymorpha (Myrtaceae) and allied species, with dispersal stemming from New Zealand and Lord Howe Island to the Marquesas and the Societies, and ultimately to the Hawaiian Islands (
Independent colonization of the Societies and Hawaii by propagules derived from populations of Mecyclothorax punctipennis suggests several ancillary conclusions. First, both colonizing events would seem to have involved propagules derived from one of the currently most common carabid beetle species in Australia, Mecyclothorax punctipennis. The numerical dominance and ecological plasticity of this species, which occurs from dry Xanthorrhea-Eucalyptus forest at sea level to open montane snow gum (Eucalyptus pauciflora Sieber) forests at over 1500 m elevation (unpubl. data), enhances the probability that were a propagule produced from this species, the colonizers would possess substantial genetic variability to allow survival as ecological pioneers across the range of habitats occupied by the beetles in Australia. This range would include the present–day leeward montane shrubland habitat of Mecyclothorax montivagus in Maui.
Second, if Mecyclothorax punctipennis were the source of colonizing propagules, colonization of both the Society and Hawaiian Islands must have occurred recently. Present evidence for the Society radiation, with species restricted to Moorea and Tahiti, would place the age of origin of the fauna at no more than 2.25 Ma were the radiation founded on Moorea, or 1.75 Ma if founded on Tahiti (
Finally, as the Tahitian and Hawaiian radiations have the same, extant sister species as their adelphotaxon, intense acceleration of the speciation rate is demonstrable in both island archipelagoes. This accelerated diversification must be directly attributable to life on these isolated volcanic islands. The subtropical Hawaiian and Society volcanic islands developed forests quickly, as orographic rainfall synergized nutrient transfer to forest plants within thousands of years of plant colonization (
I thank Curtis P. Ewing for access to specimens he collected during both the first expedition to the summit of Mont Tohiea in 2006 as well as during his second trip there in 2009. Thierry Deuve (MNHN) and Michel Brancucci (NHMB) loaned material that proved essential to diagnosing the new species. TD hospitably permitted me free access to the Georges Perrault collection of Tahitian Carabidae, thereby facilitating enhancement of that collection’s diversity. This project was supported by N.S.F. award DEB–0451971, Survey of terrestrial arthropods of French Polynesia; R.G. Gillespie principal investigator. I thank the Delegation a la recherché (de la Polynésie Française), Dr. Priscille Frogier directeur, for permission to collect in Tahiti and Moorea. Jean–Yves Meyer coordinated the Mont Tohiea expedition and provided much appreciated field support in Tahiti. Dan Polhemus (NMNH) provided funding for helicopter support to the summit of Mont Tohiea. Elin Claridge, Richard B. Gump Moorea Field Station, went above and beyond to collect valuable specimens from Pito Hiti. This contribution is dedicated to the memory of Georges G. Perrault, who documented the incredible diversity of the Tahitian Mecyclothorax, thereby providing the essential information to justify continued study of this fascinating radiation.