(C) 2012 M. Antonio Todaro. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In previous papers, faunistic and preliminary taxonomic data on the gastrotrich communities along the coastline of the Brazilian states of São Paulo and Rio de Janeiro were reported; among the over 40 records, the occurrence of several species new to science was highlighted. One of such new taxa is described here based on observation carried out on living and SEM prepared specimens. Pseudostomella dolichopoda sp. n. (Gastrotricha: Thaumastodermatidae) is the only species in the genus that attains 420 µm in total length, is covered by pentancres and possesses, among others, caudal pedicles up to 45 µm in length. Additional differences with co-generic taxa characterized by a pentancrous covering are discussed. Furthermore, a key to the described Pseudostomella species of the world based on easily discernible traits, visible in both living and formalin-fixed specimens, is provided.
Gastrotrichs, Brazil, São Paulo, biodiversity, taxonomy, new species, key
The study is part of a larger research program aimed at shedding light on the diversity of marine invertebrates of the northern coasts of the State of São Paulo, Brazil (see
Sandy sediment from the littoral and/or sublittoral site of 23 locations along the northern coasts of the State of São Paulo was collected during several field trips between 20 April and 3 May 2002. Littoral samples were taken during low tide, by digging three 30 cm-deep holes, ca 5 m apart from each other, at mid-water mark, and collecting the sand, about 500 ml, from the wall using a steel spoon. Sublittoral sand was taken by scooping the top sediment layers with a 500 ml plastic jar; sediment below 4 m water depth was collected by SCUBA diving. After collection, samples from each site were taken as soon as possible to the São Paulo University’s CEBIMar laboratory in São Sebastião. A general account on the visited locations including geographic coordinates and physical and chemical characteristics of the microhabitats are reported in
Using the same techniques, additional samples were taken in September 2003 from six beaches, four new and two already investigated (see
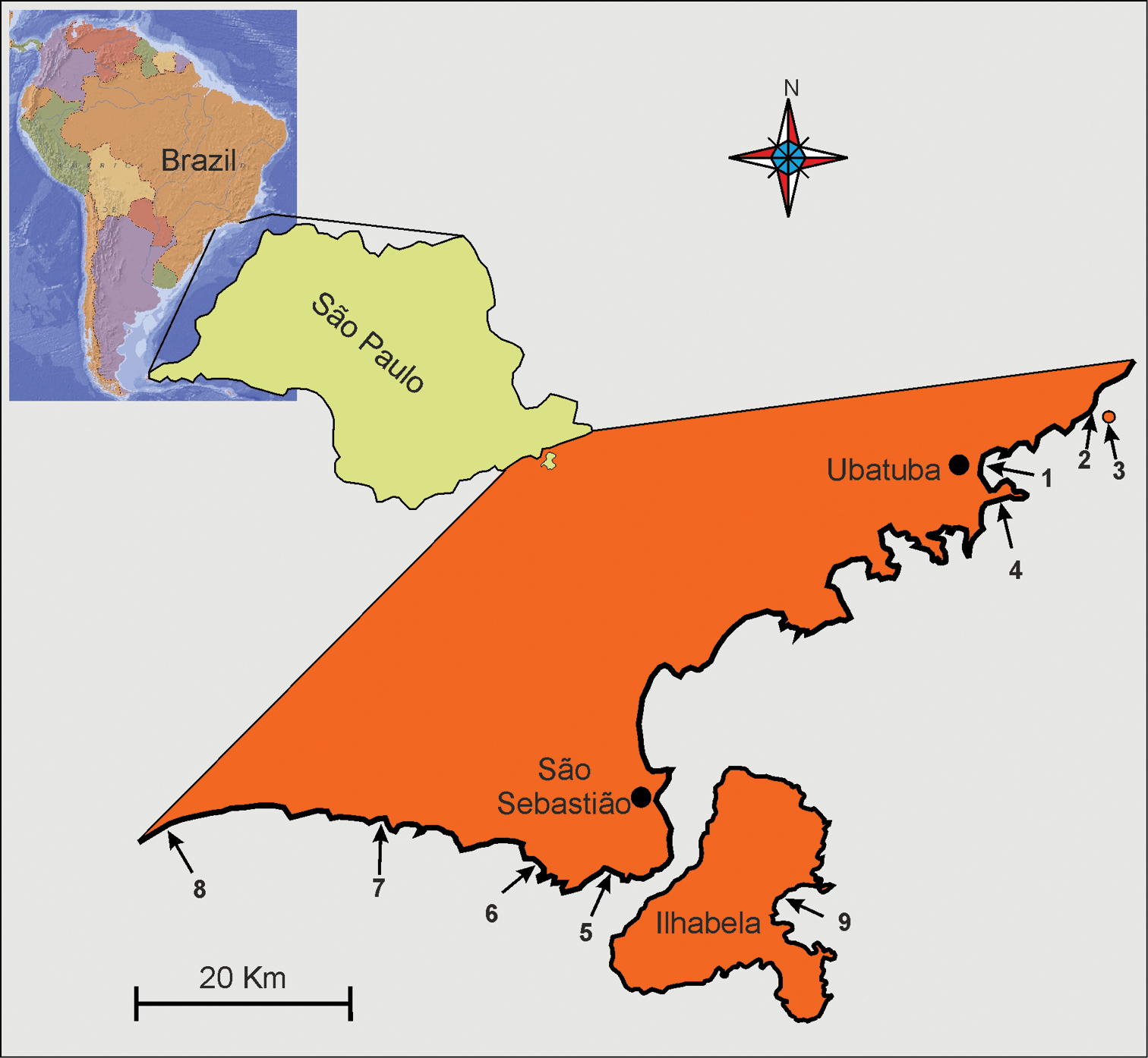
Localities along the cost of the State of São Paulo (Brazil) where Pseudostomella dolichopoda sp. n. was found. 1 praia Grande 2 praia Prumirim 3 Ilha do Prumirim 4 praia do Tenório (Ubatuba district) 5 praia de Guaecá 6 praia de Santiago 7 praia do Saí 8 praia Preta e Conchas(São Sebastião district) 9 praia de Castelhanos (Ilhabela island). For geographic coordinates and characteristics of the microhabitat see
The description of the new species follows the convention of
Granulometric analysis of the substrata was carried out according to
Abbreviations are as follows: TL, total body length; PhL, pharynx length; PhIJ, pharyngeo-intestinal junction; TbA, adhesive tubes of the anterior series; TbDL, adhesive tubes of the dorsolateral series; TbL, adhesive tubes of the lateral series; TbVL, adhesive tubes of the ventrolateral series; TbP, adhesive tubes of the posterior series.
The rationale for the key to the ecological characteristics of the species, according to
Abundance of a species among other species of a sample - Rare, less than 1% of a sample; scarce, 3–5% of a sample; numerous, 10–20% of a sample (often a sub-dominant); prevalent, more than 30% of a sample (usually dominant or co-dominant).
Taxonomic account Order Macrodasyida Remane, 1925 [Rao & Clausen, 1970] Family Thaumastodermatidae Remane, 1927 Subfamily Thaumastodermatinae Remane, 1927 Genus Pseudostomella Swedmark, 1956urn:lsid:zoobank.org:act:89445CF5-147A-4B27-A403-0DD50268E442
http://species-id.net/wiki/Pseudostomella_dolichopoda
Figures 2–4Brazil, State of São Paulo, Praia Grande of Ubatuba (23°23'04.4"S; 45°03'49.9"W). At Mid-Water Mark, in fine (mean grain size, 0.160 mm ) moderately well sorted (sorting, 0.70 mm) siliceous sand, and at 1.5 m water depth in fine (mean grain size, 0.153 mm), moderately sorted (sorting, 0.54 mm), siliceous sand. Values of salinity, temperature and pH of the interstitial water at date of sampling 35.1 psu, 26.3° C and 7.92 respectively; from the same beach additional specimens were collected on 7 September 2003. Other location in the State - Ubatuba: praia Prumirim (sl=sublittoral) also in 2003, Ilha do Prumirim (2003, l=littoral), praia do Tenório (2003, sl); São Sebastio: praia de Guaecá (sl), praia de Santiago (sl), praia do Saí (l, sl), praia Preta e Conchas (sl); Ilhabela: praia de Castelhanos (sl) (see Fig. 1 and also
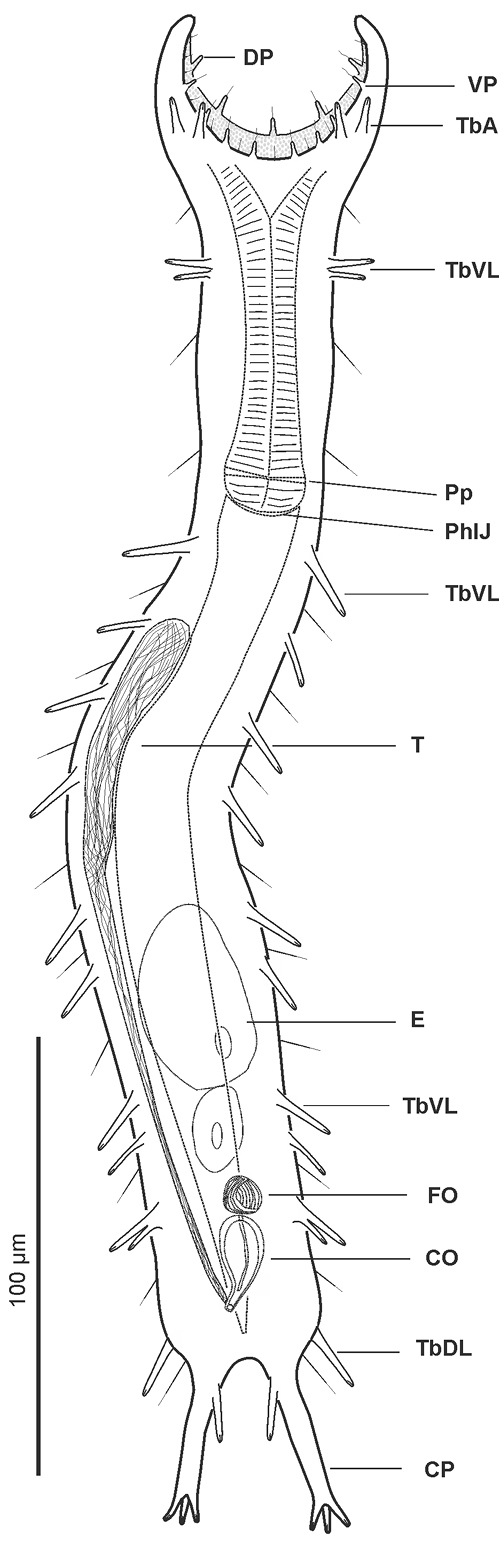
Pseudostomella dolichopoda sp. n. schematic drawing. Habitus as seen from the ventral side. CO caudal organ CP caudal pedicle DP dorsal papillae E egg FO frontal organ PhIJ pharyngeo-intestinal junction Pp pharyngeal pores T testicle TbA anterior adhesive tubes TbDL dorsolateral adhesive tubes TbL lateral adhesive tubes TbVL ventrolateral adhesive tubes VP ventral papillae.
Holotype, the 358 μm long adult specimen shown in Figure 3.
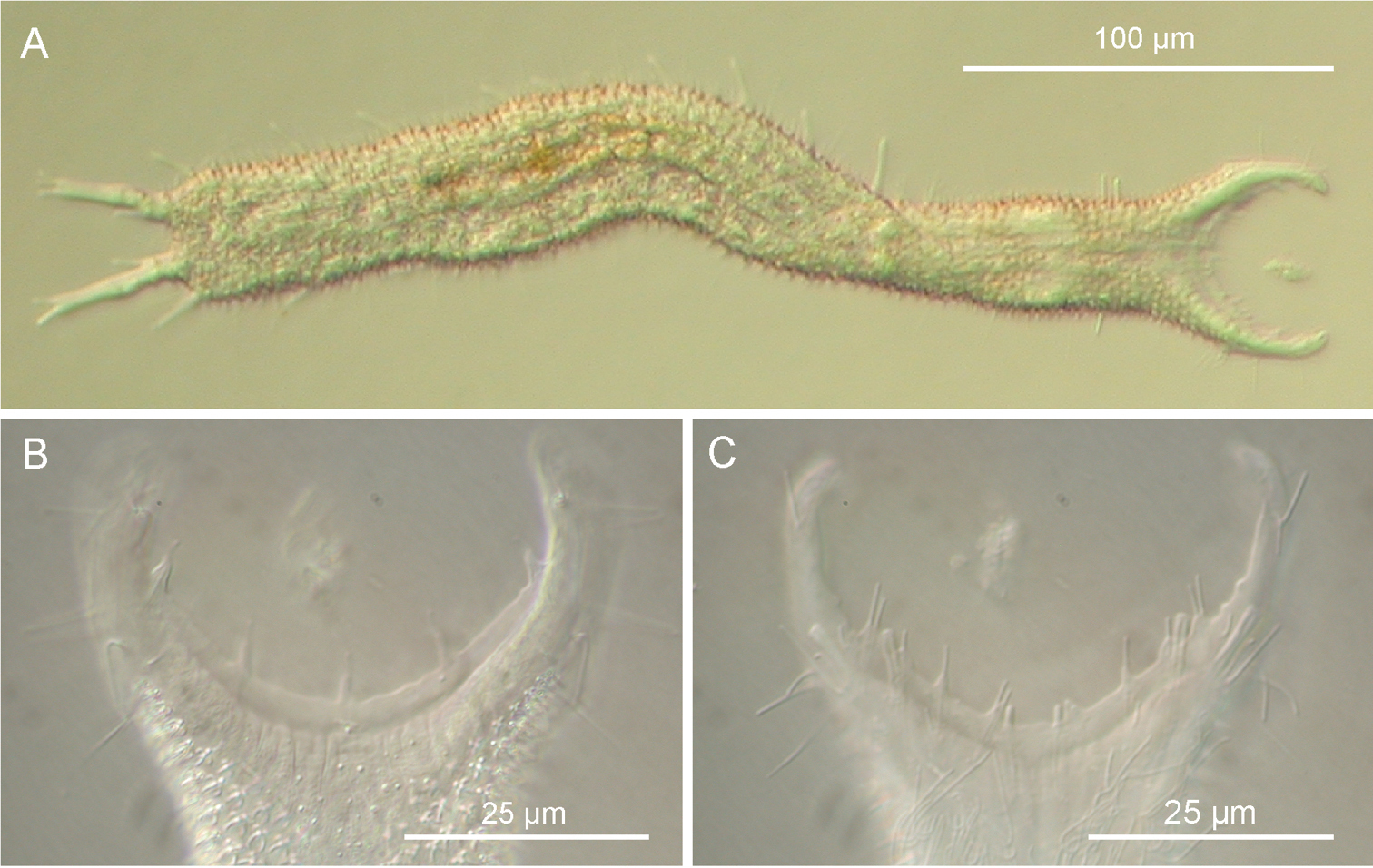
Pseudostomella dolichopoda sp. n. DIC photomicrographs. A habitus B close-up of the anterior region, dorsal view C Close-up of the anterior region, ventral view.
Pseudostomella dolichopoda sp. n. SEM photomicrographs. A habitus, dorsal view B habitus, ventral view; C close-up of the anterior region, dorsal view D close-up of the anterior region, ventrolateral view E close-up of the posterior region, dorsal view F close-up of the posterior region, ventral view, arrow shows the two ventrolateral adhesive tubes borne from a common base.
Fifteen adult specimens(including the holotype) collected by the author from different localities (see below); ten specimens were observed alive and are not longer extant, while five were prepared for SEM survey and are kept in the meiofauna collection of the author (Ref. n. 2002-BR-01-05).
Frequency of occurrence – common in sediment of the northern sites, usual in southern sites but occasional in locations facing the São Sebastião channel and on Ilhabela. Abundance - prevalent to numerous in sub-littoral sediment, scarce in littoral sediments where found.
A Pseudostomella with an adult length to 425 μm; pharynx length to 90 μm, with pharyngeal pores at base. Pharyngeo-intestinal junction (PhIJ) at U32; body slender, with graceful lines and elongate, furcate caudum. Head with mid-sized fleshy preoral palps curving around forward; palps showing few sensory hairs and provided with 5 and 8 papillae on the dorsal and ventral border respectively. Sensory hair sparce but evenly spaced on the body, forming lateral columns from about U12.5 to U85; glands barely visible, asymmetrically scattered along most of the length of the body. Cuticular armature of small, delicate pentancres on whole dorsal and ventrolateral surface, except for a bare, roughly T-shaped area posterior to the palps. Adhesive tubes: TbA, 2 per side in a row U7.8; TbDL, 1 per side, robust, inserting on lateral margin of the posterior trunk region at U83.5; TbVL, 11 per side, 2 smaller ones in the anterior pharyngeal region, roughly at U16, 7 of slightly variable size, irregularly spaced in the intestinal region from U33.5 to U71.5, the remainder 2 originate from a common base at U76.2; TbP, 4 per side, 2 + 1 at the end of each foot of the furcated caudum and the other one flanking each foot medially. Ventral locomotor cilia: a continuous field of transverse rows covering sparsely the entire surface from U12 to U35; the field splits in the anterior intestinal region to form paired lateral tufts that extend onto the ano-genital area at U84. Reproductive system: testis on the right body side, caudal organ inverted pyriform, at U78; frontal organ bladder-like, at U74.5; maturing eggs mid-dorsally above the posterior half of the intestine.
The specific name alludes to the extraordinary length of the caudal pedicles (dólicos Gr., long, and poús, podos Gr., foot).
The description is mainly based on the adult specimen, 358 µm in total length, shown in Figure 3. Body somewhat slender, little swollen in the posterior pharyngeal region and at the base of the 43 µm-long caudal pedicles. Pharynx 81 µm in length, measured from the ventral border of the oral opening to the pharyngeo-intestinal junction; pharyngeal pores near the base, at U29.5; pharyngeo-intestinal junction at U32; widths of neck\PhIJ\trunk\caudal base 30\29.5\40\31 µm at U15\U31\U51\U82, respectively. Head with well developed, fleshy preoral palps, incurving ventromedially; the dorsal border projecting just beyond the ventral. Sensory hairs and papillae occur on dorsal and ventral borders of the preoral palps; hairs are scattered on the dorsal, lateral and ventral surface of the palps; dorsally there are five papillae, nearly same in length (8–10 µm), symmetrically arranged along the inner border of the palps in a 2 + 1 + 2 pattern; ventrally, there are eight papillae, 5–8 µm in length, symmetrically arranged more centrally about the inner border of the palps in a 4 + 4 pattern; all papillae bearing one or two, short sensory hairs at their tip; other hairs form lateral columns that are evenly spaced from U12.5 to U85; individual hairs are 12–15 µm in length. Glands barely visible, variable in shape (oval to oblong) and size (4-8 µm in diameter), asymmetrically scattered along most of the length of the body.
Cuticular armature. Small sized pentancres with delicate, curved grasping tines, as tall as wide (3 × 3 µm – 5 × 5 µm) on whole dorsal and ventrolateral surface, except for a bare, roughly T-shaped area posterior to the palps; posteriorly most ancres extend onto the caudum.
Adhesive tubes. TbA, 2 per side (7-8 µm in length) in a row at U7.8; TbDL, 1 per side (15-18 µm in length), robust, inserting on the lateral margin of the posterior trunk region at U83.5; TbVL, 11 per side, 2 smaller ones (9-11 µm in length) in the anterior pharyngeal region, roughly at U16, 7 of slightly variable size and length (11-16 µm in length), irregularly spaced in the intestinal region from U33.5 to U71.5, the remainder 2, of unequal length (10 and 14 µm), originate from a common base at U76.2; TbP, 4 per side, 2 + 1 (5-6 µm in length) at the end of each foot of the furcated caudum and the other one (10 µm in length) flanking each foot medially.
Ventral locomotor cilia. A continuous field of transverse rows covering sparsely the entire surface from U12 to U35; the field splits in the anterior intestinal region to form paired lateral tufts that extend onto the ano-genital area at U84. Reproductive system: testis on the right body side, caudal organ inverted pyriform (11 × 22 µm), at U78; frontal organ bladder-like (9 µm in diameter), at U74.5; maturing eggs mid-dorsally above the posterior half of the intestine.
Measurement and variability. Body length of 15 living specimens ranged from 340-425 µm (mean = 388 µm, SD = 25.4 µm) all of them were mature (i.e., showed at least the testicles filled with sperm). Strange enough all of the SEM prepared specimens resulted of smaller size (i.e., less then 300 µm) even though only specimens appearing larger under the dissecting microscope were selected for this scope; these measurements are well below the 5.5% length reduction allowed for fixed specimens (cf.
Prior to the present study, the total number of Pseudostomella species known was 15 including one described by
Gastrotricha Thaumastodermatidae, including species of Pseudostomella, are characterized by peculiar cuticular armatures made up of sculptured plates, spines or a combination of both. Species of the genera Acanthodasys, Pseudostomella, Tetranchyroderma and Thaumastoderma bear peculiar pronged scales called ancres: uniancres, triacres, tetrancres and pentancres depending on number of prongs.
Acanthodasy and Thaumastoderma species bear ancres of a single type only, uniancres and tetrancres respectively whereas Pseudostomella and Tetranchyroderma bear tri- tetra- or pentancres depending on species. In the latter two taxa the type of pronged cuticular armature has been regarded as the single most important taxonomic trait to classify species (e.g.,
Based on the type of ancres, the new species approaches the latter four taxa. However, Pseudostomella cataphracta, is unique in that it shows a group of four ventral adhesive tubes per side that is missing in the other species, while Pseudostomella etrusca is peculiar in thatit possesses a robust dorsal adhesive tubes inserted at base of each oral palp, a trait lacking in the other taxa. Pseudostomella dolichopoda sp. n. differs from Pseudostomella cheraensis mainly in virtue of its larger size (up 425 µm vs. up to 295 µm), presence of two adhesive tubes in the anterior pharyngeal region and on the presence of two adhesive tubes originated from a common base located in the posterior intestinal region; finally, Pseudostomella dolichopoda sp. n. differs from Pseudostomella sp1 because, among others, it has larger size (425 µm vs. 350 µm), possesses longer caudal pedicles (43 µm vs. 23 µm), bears two pairs of anterior adhesive tubes vs. a single pair present of Somali species, and because of the presence of the two adhesive tubes originate from a common base located in the posterior intestinal region.
| 1 | Cuticular armature of triancres | 2 |
| – | other | 6 |
| 2 | Five dorsal papillae on the prebuccal apparatus | 3 |
| – | seven dorsal papillae on the prebuccal apparatus | 4 |
| 3 | Four TbA per side; foot-like TbV present (3 tubes per side) | Pseudostomella megapalpator Hochberg, 2002 |
| – | five TbA per side; foot-like TbV absent | Pseudostomella klauserae Hochberg, 2002 |
| 4 | Scales of a triancre arise from a common, forked shaft | Pseudostomella plumosa Ruppert, 1970 |
| – | each scale shaft arises independently from the base | 5 |
| 5 | Scales of triancres foliate (=scaled triancres | Pseudostomella faroensis Clausen, 2004 |
| – | scales of triancres needle-like | Pseudostomella triancra Hummon, 2008 |
| 6 | Cuticular armature of tetrancres | 7 |
| – | cuticular armature of pentancres | 12 |
| 7 | Five dorsal papillae on the prebuccal apparatus | 8 |
| – | seven dorsal papillae on the prebuccal apparatus | 9 |
| 8 | Five TbA per side; pedicles of 7 tubes (0:3:4); copulatory organ pyriform; bare area on the dorsal side, posterior to the prebuccal apparatus present | Pseudostomella longifurca Chang & Lee, 2002 |
| – | two TbA per side; pedicles of 5 tubes (1:3:1); bare area absent | Pseudostomella indica Rao, 1970 |
| 9 | copulatory organ tube-like | Pseudostomella koreana Chang & Lee, 2002 |
| – | copulatory organ pyriform | 10 |
| 10 | Bare area on the dorsal side, posterior to the prebuccal apparatus present | Pseudostomella roscovita Swedmark, 1956 |
| – | Bare area absent | 11 |
| 11 | TbL, 3 pairs; body short (about 200 µm in lenght) | Pseudostomella malayica Renaud-Mornant, 1967 |
| – | TbL, 8 pairs; body elongate (about 500 µm in length) | Pseudostomella andamanica Rao, 1993 |
| 12 | Five dorsal papillae | 13 |
| – | seven dorsal papillae; 3 TbA per side; pedicles of 5 tubes (1:3:1) each; foot-like TbV present (4 tubes per side) | Pseudostomella cataphracta Ruppert, 1970 |
| 13 | Four TbA; single dorsal tube protruding from base of preoral palps present | Pseudostomella etrusca Hummon, Todaro & Tongiorgi, 1993 |
| – | Two TbA; dorsal tube absent | 14 |
| 14 | Adhesive tubes along the pharyngeal region absent | Pseudostomella cheraensis Priyalakshmi, Menon & Todaro, 2007 |
| – | Adhesive tubes along the pharyngeal region present | 15 |
| 15 | Single pair of adhesive tubes along the pharyngeal region; dorsal cuticular covering complete.. | Pseudostomella sp.1 [Valbonesi & Luporini, 1984] |
| – | Two pairs of adhesive tubes along the pharyngeal region; presence of a bare area on the dorsal side, posterior to the prebuccal apparatus | Pseudostomella dolichopoda sp. n. |
This work was mainly supported by the State of São Paulo Research Foundation (FAPESP) within the BIOTA/FAPESP - The Biodiversity Virtual Institute Program. I’m grateful to Carlos Rocha, for inviting me to perform the research. Many thanks are due to Alvaro E. Migotto and the stuff of CEBIMar for the invaluable assistance received during the stay at the São Bebastião laboratory. Funding for the study was partially met by the BIOTOME project (UNIMORE).