(C) 2012 Beatriz Yáñez-Rivera. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
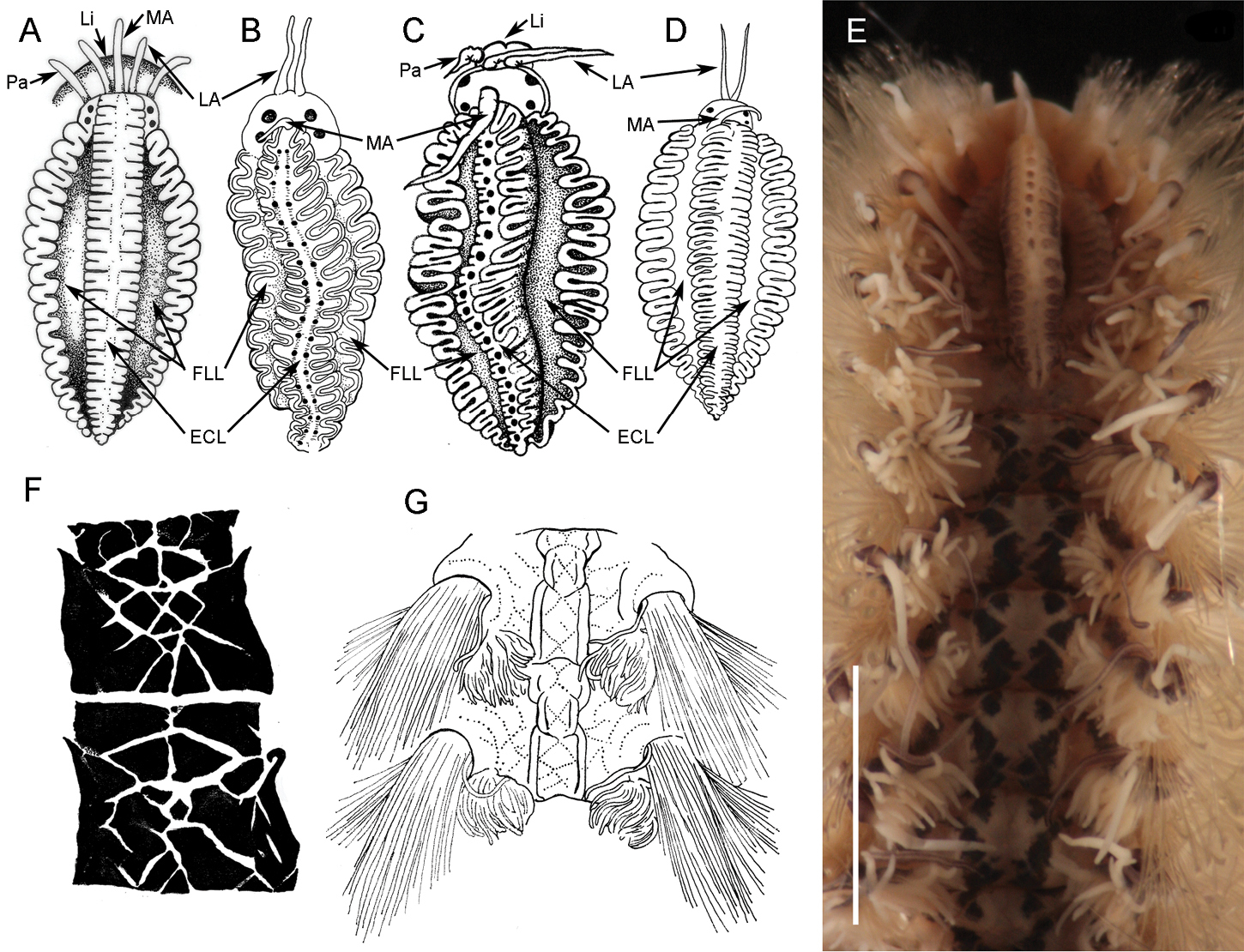
The species of the genus Notopygos Grube, 1855 are characterized by an ovate body, a prominent caruncle with three lobes, dendritic branchiae, and double dorsal cirri. Twenty-two species belonging to Notopygos have been described, mostly from the Indo-Pacific region. In America, few species are frequently recorded: Notopygos crinita Grube, 1855 from St. Helena Island (Atlantic) and Notopygos ornata Grube and Ørsted in
Amphiamerican, DNA barcoding, pigmentation pattern, taxonomy, polychaete
Polychaetes belonging to the family Amphinomidae are commonly known as fireworms (
Notopygos was erected by
Twenty-two species of the genus have been described, most of them from the Indo-Pacific region. The original descriptions of several species are incomplete, since relevant features such as the beginning of branchiae, position of the anal opening and folds of the caruncle were omitted. Consequently, in some cases it is difficult to delimit and identify the species (
Notopygos crinita is widely distributed in the Western Atlantic. In addition, there are reports of the species in the central Mediterranean where it was considered to be alien (
During a revision of preserved material from museum collections and newly collected material from the barcode project “Mexican Barcode of Life, MEXBOL”, we found an adult form of Notopygos megalops and an undescribed species from reef zones in the Caribbean. Herein, Notopygos megalops is reestablished and a new species of Notopygos is described. In addition, DNA barcoding was used to complement the morphological approach in order to explain the records of Notopygos ornata in the Atlantic and to better differentiate the new species, since both have a similar pigmentation pattern.
MethodsReviewed materials belong to the following collections: The Natural History Museum, London (BMNH); Reference Collection (ECOSUR) and collection of ethanol-fixed specimens (ECOSUR-OH) of El Colegio de la Frontera Sur, Chetumal; Marine Invertebrate Museum, Rosenstiel School, University of Miami (UMML); and National Museum of Natural History, Smithsonian Institution, Washington (USNM).
Material from ECOSUR-OH was collected by snorkelling in the reef lagoons; worms were removed from coralline rocks and fixed in 96% ethanol. To observe the morphological attributes, we used Shirlastain A and methyl green to bring out details. Specimens were measured to record the width (in the widest part without chaetae), the body length (from prostomium to pygidium), appendage length, caruncle length and width, branchial filaments by branchiae and distal lobes length and width. All of the appendages and caruncle were measured directly under the microscope with a miniscale (0.1 mm divisions). Taxonomical features were illustrated with microphotography using a Cannon Rebel EOS and line drawing to caruncle and pigmentation pattern. Semi-permanent slides of chaetae from chaetigers 1, 3, 10 and 15 (often including some additional) were prepared to describe the chaetal features. Chaetae were measured with a calibrated microscope scale and SEM analyses were performed.
DNA barcoding for Notopygos ornata and the new species followed standard protocols (
Sequences were aligned with ClustalW interface MEGA version 5 (
urn:lsid:zoobank.org:act:6D3A9313-282E-4297-87B3-FDD4E6756370
http://species-id.net/wiki/Notopygos_caribea
Figures 2 , 4c, fHolotype [ECOSUR 0145 (ECOSUR-OH-0213)] Xahuayxhol, Quintana Roo, México, 18°30'30"N, 87°44'02"W, August 2004, 1 m, reef lagoon, coralline rock, Coll. LFCP. Paratypes (3) ECOSUR 0146, 0147, 0148] the same data as for holotype (Fig. 1).
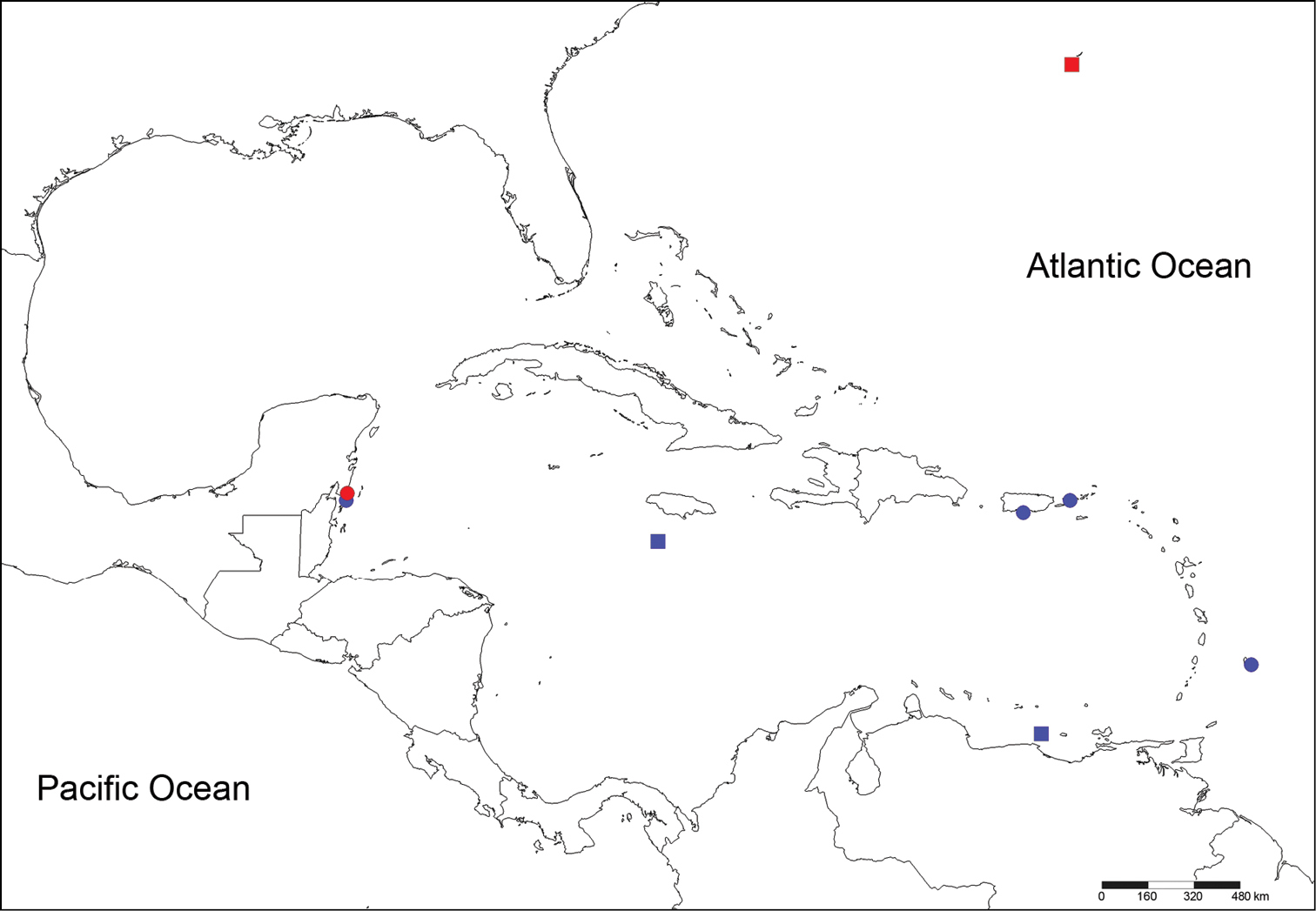
Localities from material reviewed. Circles: Notopygos caribea sp. n., squares: Notopygos megalops, red: type material, blue: additional material.
[ECOSUR P2641] Xcalak, Quintana Roo, México, sta. 4, 18°16'57"N, 87°49'8"W, in nightlight lift-net, August 2005, Coll. L. Vazquez. [USNM 15921] Sail Rock, Off Saint Thomas, 37 m, coral; [USNM 20296] Pillars of Hercules, English Harbor, Antigua; [USNM 15859] Puerto Rico; [USNM 20273] Barbados (Fig. 1); all as Notopygos crinita.
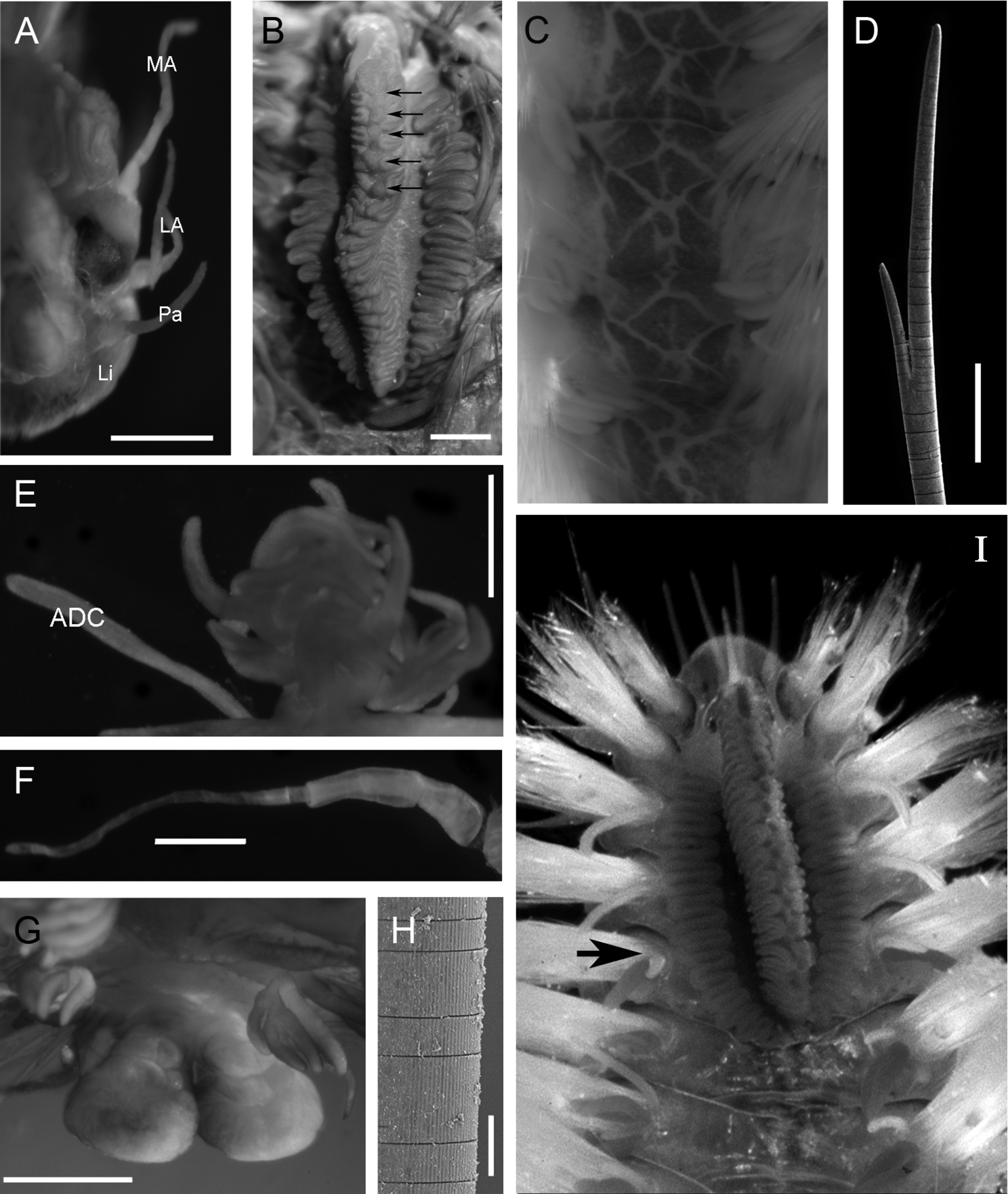
Holotype (ECOSUR 0145) mature male, complete, with 30 chaetigers, 3 cm total length, 1 cm wide. Body fusiform, orange to light brown, with reddish-brown branchiae. Pigmentation pattern complex, triangular and rhomboid forms covering the dorsum (Figs 2C, 4F). Prostomium semicircular with four eyes in pigmented strip, anterior eyes twice size of posterior ones (Fig. 2A). Median antenna in central position on prostomium, long and slender (1.1 mm long, Figs 2A, 4C); pair of lateral antennae placed on anterior prostomial margin, size similar to median antenna (1 mm long). Lips with lateral palps, shorter than lateral antennae (0.7 mm). Mouth ventral between peristomium and chaetiger 3.
Caruncle oval (2.9 mm long, 1.7 mm wide), with an elevated central lobe with about 20 folds (Fig. 4C). Row of circular projection protruding between each pair of folds in middle of caruncle (Figs 2B, 4C). Lateral lobes flattened with pigmented base and folding edge with 19 and 22 folds (Fig. 4C).
Branchiae from chaetiger 5 (Fig. 2I), present throughout body. Each branchia with main short stem, branching in several filaments of various thicknesses and lengths (Fig. 2E). First branchia with 11 branchial filaments, second branchia with about 20, middle one with about 40, posterior branchiae with 35–45 filaments up to the last chaetiger, where filaments are fewer. The large number of filaments causes a secondary ramification without a defined pattern.
Parapodia biramous, notopodium with double cirri and neuropodium with single ventral cirrus. Notopodial cirri differing; accessory cirrus (= branchial cirrus) simple with similar length along body (1.0–1.2 mm); dorsal cirrus with short, thick cirrophore (0.5 mm) in first chaetigers, subsequent ones with long slender cirrophore (1 mm) and cirrostyle (1.5 mm, Fig. 2F). Ventral cirri similar along body, cirrophore short (0.2 mm) and cirrostyle long (1 mm), decreasing towards last chaetigers.
Noto- and neurochaetae all asymmetrically furcated, slender (<0.03 mm), ratio of difference between short and long tines varies from three to four times (Fig. 2D). First chaetigers with some notochaetae with extra long tines, 10 to 30 times longer than short tines. Both neuro- and notochaetae include short and long types; shorter chaetae on exterior edge of chaetal lobe. Chaetae of first chaetigers with serrated margin. Some chaetae with an external “hard cover” that easily breaks up, giving the impression of being articulated (Fig. 2H).
Anus dorsal, on chaetiger 23. Posterior end margin with pair of distal lobes (0.5 mm long, 0.5 mm wide in the widest part, Fig. 2G).
Gametes: Gametes are located in the coelom. Oocytes are 40–57 μm in diameter (mean: 28.7±8.3 μm, n=20, one paratype female). Spermatozoids have a spherical head (~ 3 μm), ect-aquasperm type, aggregated in a mass (holotype).
Variation: Material examined varied in total length from 1.0 to 2.1 cm, in width from 0.3 to 0.5 cm, chaetigers from 23 to 26, and varies in the following features. Prostomium: median antenna similar length to lateral ones (from 0.4 mm to 1 mm), palps shorter (0.3–0.7 mm). In some worms the pigmented strip on the prostomium continues to the buccal lips, around the palps, and even onto the ventral body with a dark region, although in holotype this pigmentation is faded. Caruncle fold number varies from 13 in smallest to 20; in all specimens number of folds between elevated lobe and laterals is very similar (±2). Pigmented circular projections in mid-caruncle faded in some preserved specimens. Number of branchial filaments is size-dependent, smallest specimens having only three to nine, largest specimens having up to 25 filaments in median region. Branchiae from chaetiger 5 in both juvenile and adults.
Notopygos caribea sp. n. A Prostomium, dorsal view B Caruncle, arrow showing circular projection in the middle of the caruncle C Pigmentation pattern between chaetiger 9–12 D Notochaeta from chaetiger 10 E Branchia and accessory dorsal cirrus from chaetiger 15 F Main dorsal cirrus from chaetiger 15 G Distal lobes H Chaetal fragmentation I Anterior part of live specimen from Guana Island, BVI (photo: Leslie Harris), arrows showing branchiae beginning. Holotype: A, B, E–G, paratype: C, D, H. ADC accessory dorsal cirrus LA lateral antennae Li lips MA median antenna Pa palps. Scale bar: A, B, E-G= 0.5 mm, D= 100 μm.
The specific name refers to the distribution area of the species.
Greater Caribbean basin in shallow waters, related to coralline areas, particular associations are unknown.
Notoygos caribea sp. n. is characterized by the complex pigmentation pattern covering the dorsum with triangular and rhomboid forms (Figs 2C, 4F), and by other features such as branchiae beginning on chaetiger 5, anus on chaetiger 23, and a prominent caruncle with a median keel with a series of highlighted points arranged in a a longitudinal row of circles. The juvenile specimen also shows the coloration pattern and the distinctive caruncle. The coloration pattern on the caruncle of some specimens is faded; however, it is possible to distinguish the serial projections on the mid-caruncle.
The most common species recorded in the Atlantic is Notopygos crinita. This species was briefly described by
Material from Puerto Rico on corals revised by
In the Indo-Pacific region, eight Notopygos species have branchiae beginning on chaetiger 5 (Table 1):
Comparison of relevant features in some Notopygos species. For branchiae start and anus position, the numbers indicate chaetigers.
| Species | Branchiae beginning | Caruncle | Pigmentation pattern | Anus location | Distribution |
|---|---|---|---|---|---|
| Notopygos crinita Grube, 1855 | 5 | Ovoid, crenulated, ornamented with elevated median lobe | – | 21+ Ehlers, 1887 (intersegment 21–22) | Atlantic St Helena |
|
Notopygos ornata Grube & Ørsted in |
4 | About 20 folds in the elevated lobe with a row of ovals in the middle. Lateral lobes with pigmented areas (Fig. 4E) | Complex Triangular and rhomboid forms in symmetric pattern, 50% of cover (Figs 4E; 5) | 24+ Monro, 1933 (23) | Eastern Pacific |
| Notopygos megalops McIntosh, 1885 | 6 | About 6 folds in the elevated lobe with a row of rectangles in the middle. Narrow lateral lobes (Fig. 3B)+ (folded structure) | Only in the cirrophore | 18–19+ (undescribed) | Greater Caribbean |
| Notopygos rayneri (Baird, 1870) | 5 | Three lobes strongly wrinkled. The central lobe detached. | Complex Dorsum violet with white lines crossing in diverse directions | 22 | Indo-Pacific |
| Notopygos flavus Haswell, 1878 | 5 | Elongated and sinuous. Details undescribed. | Lack of pigmentation | undescribed | Indo-Pacific |
| Notopygos variabilis Potts, 1909 | 5 | Three lobes with slack arrangement. Lateral lobes with pigmented areas (Fig. 4A) | Orange spots like chessboard (live), Unpigmented in preserved material. | 22–25 | Indian Ocean |
| Notopygos sibogae Horst, 1911 | 5 | Lateral lobes with a dark tone and 16–17 folds. Central lobe undescribed | Each segment colorless with an area having triangular shape. Violet band around the notopodium and only secondary cirri violet | 23 | Indo-Pacific |
| Notopygos cirratus Horst, 1911 | 5 | Lateral lobes with 11 folds without pigmentation. Central lobe undescribed | Each segment has three areas. Grey with a dark band around the base of each notopodium and violet cirrophore | Intersegment 23–24 on a papilla | Indo-Pacific |
| Notopygos gigas Horst, 1911 | 5 | Lateral lobes with 30 folds and pigmented areas. Central lobe undescribed. | Violet or brown in the middle of dorsum with several white lines. Branchiae and two dorsal cirri pigmented. | 25 | Indo-Pacific |
| Notopygos horsti Monro, 1924 | 5 | 18 folds in the elevated lobe with two rows of small circles. Lateral lobes with light pigmented areas (Fig. 4B) | Dorsum marbled with a dark purple pigment, which covers the basal branchiae portion | Intersegment 22–23 | Indo-Pacific |
| Notopygos andrewsi Monro, 1924 | 5 | More than 25 folds in the elevated lobe, without pigmented areas (Fig. 4D) | Dorsum with crossed lines and raised longitudinal ridges (Fig. 4G) | 24 | Indo-Pacific |
| Notopygos caribea sp. n. | 5 | About 20 folds in the elevated lobe, with a row of circles in the middle. Lateral lobes with pigmented areas (Figs 2B, I, 4C) | Complex Triangular and rhomboid forms, 90% of cover (Figs 2C, I, 4F, 5) | 23 | Greater Caribbean |
+ Modified after original description; in parenthesis, original data.
1) Notopygos rayneri (Baird, 1870) from north-eastern Australia also has a complex pigmentation pattern but with white lines crossing in various directions, whereas Notopygos caribea sp. n. lacks white lines even in live specimens (Figs 2I, 5). 2) Notopygos flavus Haswell, 1878 from northern Australia lacks any pigmentation pattern. 3) Notopygos variabilis Potts, 1909 from the Maldives differs from Notopygos caribea sp. n. mainly in caruncle shape (Fig. 4A) and pigmentation pattern of live specimens, which is like a chessboard with orange spots (original description lacking illustration of pigmentation pattern). 4) Notopygos sibogae Horst, 1911 from Indonesia differs in that the pigmentation pattern includes a violet band around the notopodium and violet secondary cirri (original description lacking illustration of pigmentation pattern). 5) Notopygos cirratus Horst, 1911 from the Philippines differs in the pigmentation pattern grey with a dark band around the base of each notopodium and violet cirrophore, and in the intersegmentary location of the anus above an elevation. 6) Notopygos gigas Horst, 1911 from the south coast of Timor differs in the dissimilar anus location and branchiae shape, being three large stems in Notopygos gigas, while in Notopygos caribea sp. n. the branchial ramification lacks a defined pattern. 7) Notopygos horsti Monro, 1924 from northern Australia differs from Notopygos caribea sp. n. by having two series of circular projections on the caruncle median lobe (Fig. 4B) instead of only one (Fig. 4C) and by the intersegmental anus position. 8) Notopygos andrewsi Monro, 1924 from Christmas Island has a pigmentation pattern with crossed lines and raised longitudinal ridges and caruncle without pigmentation (Fig. 4D, G), different from Notopygos caribea sp. n.
In addition,
http://species-id.net/wiki/Notopygos_megalops
Figure 3Holotype (juvenile) [BMNH 1885.12.1.12] Bermuda, 32°07' N, 65°04'W, Sta. 36, “Challenger”, April 1873, 55 m. Broken into two parts, damaged (Fig. 1).
[UMML 22.909] Near Jamaica, 17°27'N; 78°10'W, Sta. 1256, “R/V Pillsbury”, July 1970, 590 m. [UMML 22.903] Venezuela, 10°57'N, 66°18'W, Sta. 739, “R/V Pillsbury”, July 1968, 257m, juvenile (Fig. 1).
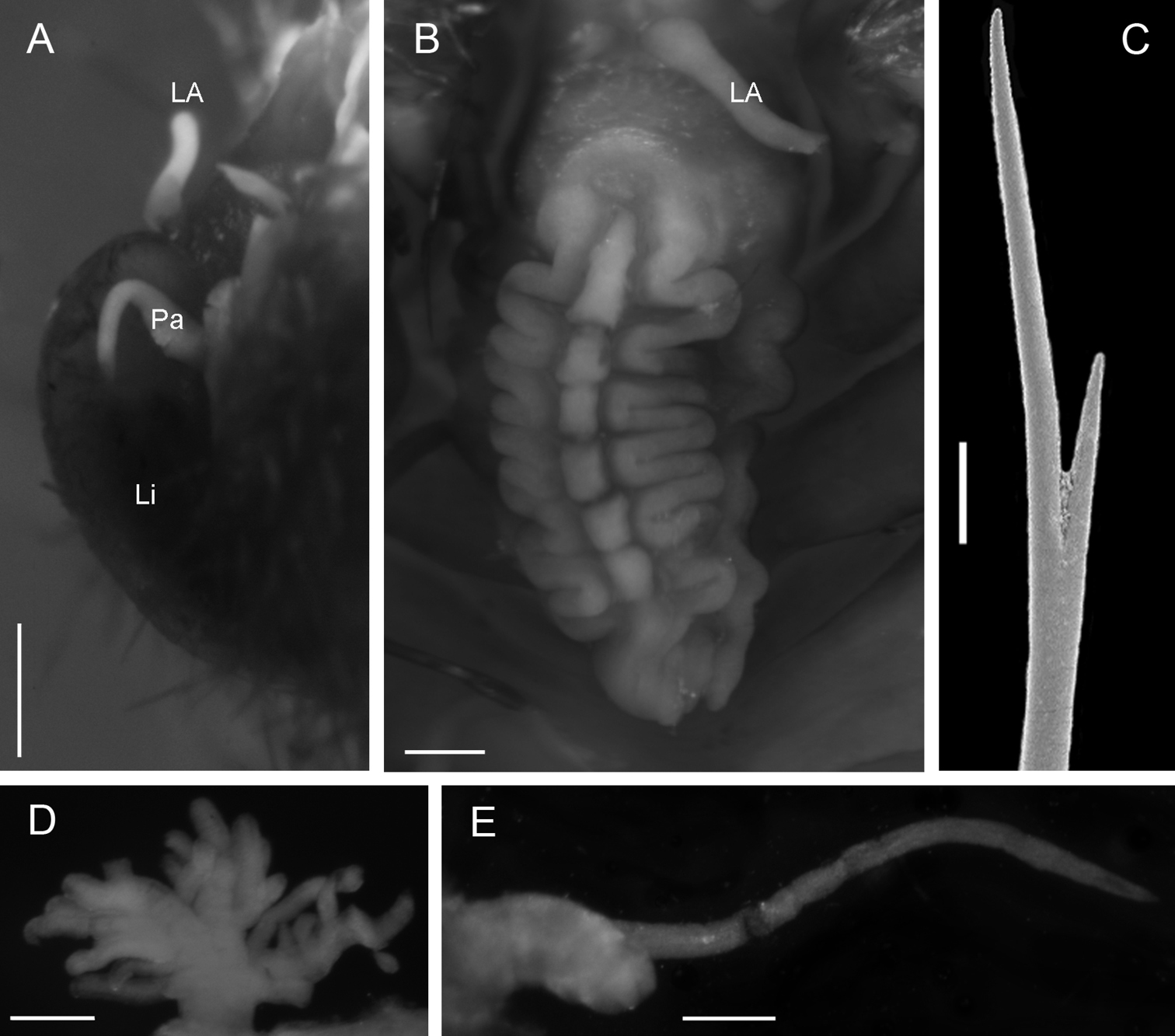
Adult specimen (UMML 22.909) complete with 23 chaetigers, damaged, gut exposed, broken, posterior end in poor condition; body fusiform; 3 cm total length, 1 cm wide in the widest part. Prostomium semicircular without pigmented areas, four eyes. Median antenna lost, in central position of prostomium, lateral antennae and palps of similar length (0.7 mm). With stain, ventral surface of buccal lips conserved the stain displaying a glandular zone (Fig. 3A). Mouth placed ventrally in chaetiger 3.
Caruncle oval (1.9 mm length, 0.8 mm wide), elevated lobe with about seven folds in the middle, a rectangular projection between each fold pair. Lateral lobes narrow, with a slightly folded edge, without pigmentation (Fig. 3B).
Branchiae from chaetiger 6, present throughout body. Each branchia as a tuft of slender branchial filaments. First branchiae with a main stem, with seven branchial filaments; in median chaetigers with a main short stem, branching in four stems with five to seven branchial filaments each (Fig. 3D).
Parapodia biramous, notopodium with double cirri and neuropodium with single ventral cirrus. Accessory cirrus simple, long (2 mm); main cirrus with robust short cirrophore (0.5 mm length) and slender and long cirrostyle (1.5–2.0 mm) in all chaetigers (Fig. 3E). Ventral cirri of similar length (1 mm) along the body, last one smallest (0.7 mm).
Chaetae in noto- and neuropodia of two sizes, short and long. All neurochaetae slender (<0.04 mm wide), long notochaetae (Fig. 3C) twice as thick as short notochaetae. All chaetae asymmetrical furcates; ratio of difference between small and large tines is similar in all chaetae, varying from three to four times.
Anus dorsal in the intersegment 18–19. Posterior end margin with pair of short distal lobes.
Gametes: Unknown.
Notopygos megalops McIntosh, 1885. A Prostomium B Caruncle C Notochaeta from chaetiger 15 D Branchia chaetiger 10 E Main dorsal cirrus from chaetiger 15. LA lateral antennae Li lips Pa palps. Scale bar: A, B, D, E= 0.5 mm, C= 100 μm.
Caruncles and pigmentation pattern of some Notopygos species. A Caruncula of Notopygos variabilis B Caruncula of Notopygos horsti C Caruncula of Notopygos caribea sp. n. D Caruncula of Notopygos andrewsi E Caruncula and pigmentation pattern of Notopygos ornata from Mexican Pacific F Pigmentation pattern of Notopygos caribea sp. n. between chaetigers 6–7G Pigmentation pattern of Notopygos andrewsi anterior chaetigers. Redrawn from original descriptions: A, B, D, G. ECL elevated central lobe FLL flattened lateral lobe LA lateral antennae Li lips MA median antenna Pa palps.Scale bar: 3.5 mm.
Greater Caribbean, 55 to 590 m.
Notopygos megalops is characterized by branchiae beginning on chaetiger 6, with four main stems on median chaetigers; anus dorsal in the intersegment 18–19 and a short caruncle in comparison with other Notopygos species. The caruncle has a wide median lobe with seven wide folds and narrow lateral lobes.
The main attributes that permit us to associate the juvenile described in the original description with the adult forms are the caruncle, the position of the first branchiae, branchial branching, largest notochaeta and the stout cirrophore.
Only two species of the genus have branchiae beginning on chaetiger 6: Notopygos megalops and Notopygos hispidus Potts, 1909 from the Seychelles. The latter has a complex pigmentation pattern, and a well-developed caruncle with expanded lateral lobes, with about 20 folds and continuous projection between folds in the elevated lobe. In addition, the anus is on chaetiger 21. We consider that there are sufficient features to distinguish Notopygos megalops from the other Notopygos species; thus, we regard it as a valid species.
The caruncle in juvenile specimens is not completely developed; however, the branchiae beginning on chaetiger 6 and the large size of the notochaetae permit species identification.
Amphinomid species have been reported from both the Atlantic and Pacific oceans of America; however, some misidentifications could have occurred because there is a lack of complete species descriptions, illustrated guides and identification keys. This is the case for Notopygos ornata:
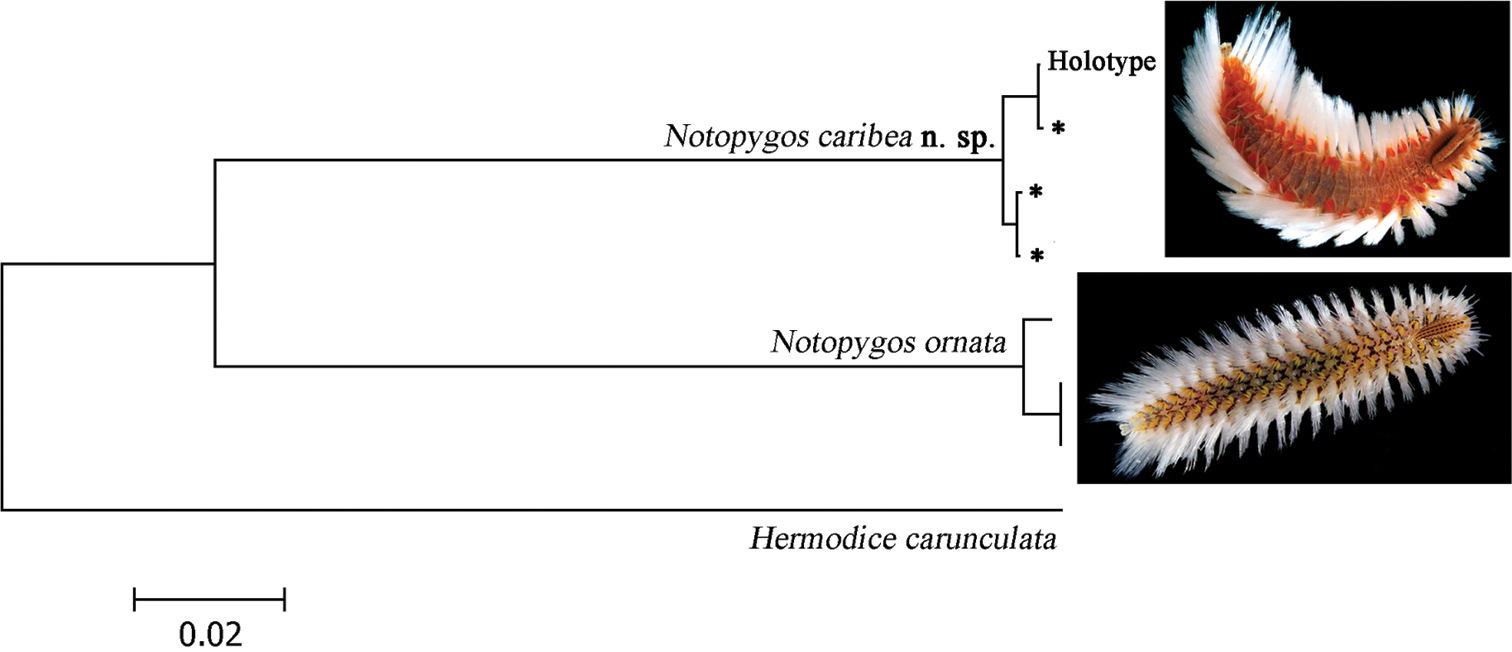
Eight nucleotide sequences between 618–690 bp of the section of COI gene were obtained to calculate genetic divergence (4: Notopygos caribea sp. n., 3: Notopygos ornata and 1: Hermodice carunculata). The K2P distance between Notopygos caribea sp. n. and Notopygos ornata shows a genetic divergence around 11% (Fig. 5). Intraspecific genetic divergence in Notopygos caribea sp. n. was around 0.8% and for Notopygos ornata was 0.7%. Previous studies considered that sequences divergences among related polychaete species average from 8.4% to 21% (
Neighbor-joining tree of COI sequences of two Notopygos species (K2P). * sequences from topotype specimens (photos: Leslie Harris).
Recently, a study of populations of Eurythoe complanata from the Caribbean, South America and Eastern Pacific has shown high levels of genetic divergence indicating three cryptic species, but the morphological evidence only recognizes one (
The COI genetic divergence between Notopygos caribea sp. n. and Notopygos ornata is smaller than in another trans-isthmian amphinomids; in Eurythoe, the genetic divergence between Pacific and Atlantic clades was 22%; however, the divergence between the two Atlantic species was 10% (
The morphological features and the sequence divergence are congruent; both show sufficient differences to distinguish the Notopygos species. Other polychaetes, such as in Eunice species, show a similar divergence value (12.9%), which has been supported by morphological features (
This contribution was partially supported by CONACyT (61609) and is part of the PhD thesis of BYR (PCMyL–UNAM). Barcoding of Life Data System provided the sequences from Mexican material through Manuel Elías (ECOSUR) as part of Mexican Barcode of Life (MEXBOL). Emma Sherlock (BMNH) supplied photographs of the holotype of Notopygos megalops. Nancy Voss (RSMAS–MBF) supplied the material from UMML. Leslie Harris (LACM–AHF) supplied photographs of the live specimens. Lourdes Vazquez (ECOSUR) supplied additional material from the new species. We thank Marcelo Silva (CCBAS–UAA) for SEM assistance and the librarian Clara Ramirez (ICMyL–UNAM). Nuria Méndez (ICMyL–UNAM), Sergio I. Salazar-Vallejo (ECOSUR) and two anonymous reviewers made useful recommendations to improve the manuscript. We also thank Ann Grant and Jacqueline Partridge for the revision of the English manuscript.