(C) 2013 Jiří Kolibáč. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Kolibáč J (2013) Trogossitidae: A review of the beetle family, with a catalogue and keys. ZooKeys 366: 1–194. doi: 10.3897/zookeys.366.6172
The family Trogossitidae (Coleoptera: Cleroidea) is reviewed to species level. Keys to its genera, tribes and subfamilies are presented for the first time. All known species and subspecies are listed, together with complete taxonomic references back to 1910, the date of issue of the last catalogue of Trogossitidae. Higher taxa reviews are accompanied by remarks on phylogeny, distribution and biology as well as a brief description of adults and larvae. All known fossil records of Trogossitidae are reviewed and discussed. The work includes maps of distribution, colour photographs of generic representatives, morphological illustrations, SEM photographs and phylogenetic trees.
Coleoptera, Cleroidea, Trogossitidae, key, catalogue
The main purpose of the work is to introduce some modern order into current knowledge of the family Trogossitidae and extend knowledge of this relatively small but fascinating group of beetles, especially to both amateur entomologists and professional “non-cleroid” workers. It is deliberately written as a “compilation” of papers on the topic to date, especially because some of them were published in journals and books that are not easily accessible to all, and to bring various fragmented sources together.
Because of the character and purpose of the work, I have tried to avoid introducing any new thoughts and systematic changes, apart from a few minor ones mentioned in the “New taxonomic acts” section. A catalogue of species lies at the core of the work. I have not repeated references included in Coleopterorum Catalogus of Temnochilidae by
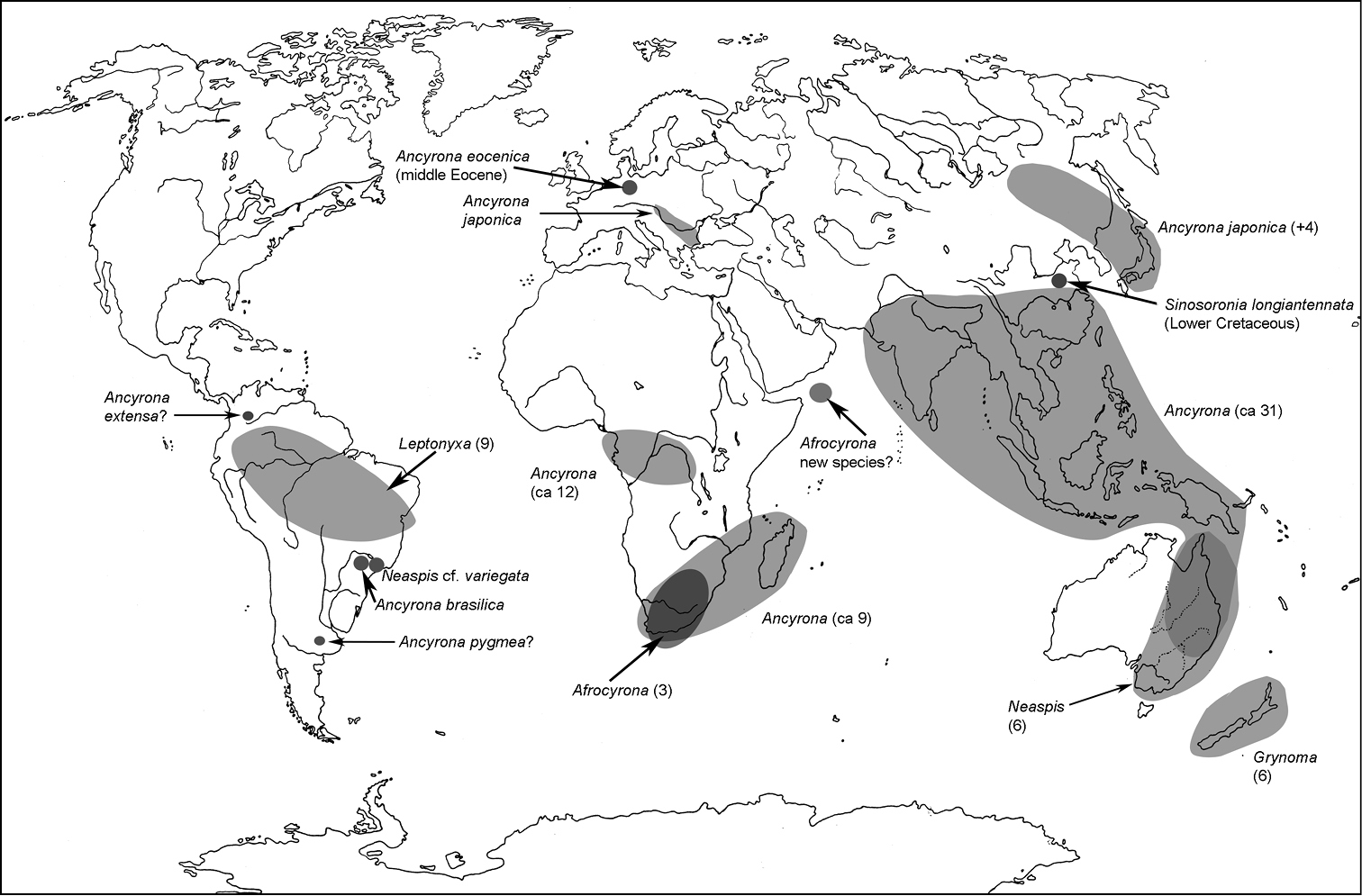
The systematics of Trogossitidae is still in its infancy. There remains a great deal of work to be done in the higher taxonomy, as well as with regard to generic limits, especially in widespread, species-rich genera. Ancyrona is a good example of such a genus, distributed from tropical Africa, the Palaearctic, south-eastern Asia to Australia. Tenebroides and Temnoscheila are further complex taxa, each with more than a hundred described species distributed in both North and South America. On the other hand, there also exists a relatively rich modern material of trogossitids to be collected in various parts of the world, certainly containing plenty of new species. Unfortunately, only a few people are seriously interested in the family and only a few of them, in turn, try to gather and publish further information. Therefore, another purpose of the book is to encourage interest in this highly interesting group of beetles.
Keys to higher taxa may be considered a further major element of this contribution. With the exception of those for subfamilies, these have not been published to date. Although it is not always easy to recognize some species-rich and variable trogossitid genera, I have done my best to use simple and easily-visible features in the keys.
† Meligethiellinae Kireichuk & Ponomarenko, 1990 is resurrected. The subfamily is removed from synonymy with Peltinae: Thymalini and shifted from Cleroidea to Cucujoidea sensu lato (including genera † Meligethiella Medvedev, 1969 and † Ostomalynus Kireichuk & Ponomarenko, 1990; genus † Juralithinus Kireichuk & Ponomarenko, 1990 is classified within Trogossitidae: Peltinae incertae sedis).
† Meligethiella Medvedev, 1969 is removed from Trogossitidae and Cleroidea (species † Meligethiella glabra Kireichuk & Ponomarenko, 1990, † Meligethiella kovalevi Kireichuk & Ponomarenko, 1990, † Meligethiella soroniiformis Medvedev, 1969).
† Ostomalynus Kireichuk & Ponomarenko, 1990 is removed from Trogossitidae and Cleroidea (species † Ostomalynus ovalis Kireichuk & Ponomarenko, 1990).
† Peltocoleops Ponomarenko, 1990 is removed from Trogossitidae and Cleroidea and classified as Coleoptera incertae sedis (species † Peltocoleops onokhojensis Ponomarenko, 1990).
Tenebroides bipustulatus (Fabricius, 1801) (var. impressifrons Reitter, 1875 syn. n.).
Tenebroides bonvouloiri Léveillé, 1889 (var. chontalensis Sharp, 1891 syn. n.).
Tenebroides maroccanus Reitter, 1884 (var. baillioti Léveillé, 1903 syn. n.).
The superfamily Cleroidea was established by
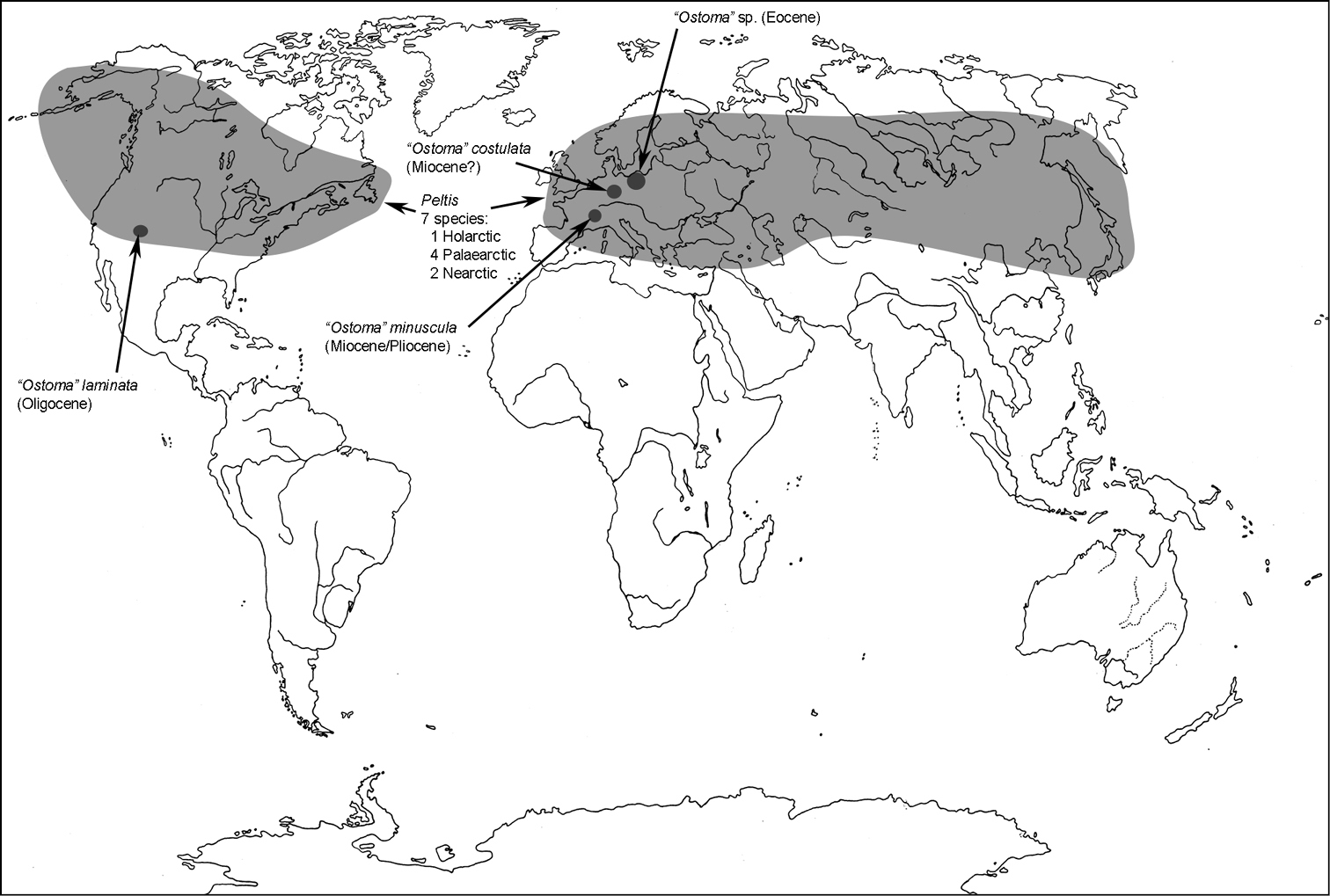
Major classifications of Trogossitidae.
| Trogositidae = Helotinae + Trogositinae (Nemozomini incl. present Egoliini; Trogositini; Leperini incl. present Calityini; Peltini incl. present Decamerini, Thymalini, Ancyronini) (Phloiophilus not addressed in the paper) | |
| Temnochilidae = Nemosominae (incl. present Egoliini) + Temnochilinae + Leperininae (incl. present Calityini) + Ostominae (incl. present Decamerini, Lophocaterini, Ancyronini) + Lycoptis (genus incertae sedis) | |
| Malacodermata: Cantharidae: Dasytinae: Phloiophilus | |
| Clavicornia: Ostomidae | |
| Crowson 1964 | Phloiphilidae (included in Cleroidea by |
| Peltidae = Decamerinae + Peltinae (incl. present Calityini) + Egoliinae | |
| Trogossitidae = Lophocaterinae + Trogossitinae | |
| Peltidae = Egoliinae + Decamerinae + Peltinae + Rentoniinae (Rentoniini, Protopeltini) (Trogossitidae and Calitys not treated in the paper) | |
| Phloiophilidae | |
| Trogossitidae = Trogossitinae + Calitinae + Egoliinae | |
| Peltidae = Decamerinae + Peltinae + Protopeltinae + Rentoniinae | |
| Lophocateridae (incl. present Lophocaterini, Ancyronini, Lycoptis) | |
| Trogossitidae = Trogossitinae + Peltinae (incl. present Decamerini, Peltini, Thymalini, Lophocaterinae, Calityini) | |
| Trogossitidae = Peltinae + Lophocaterinae + Larinotinae (incl. present Colydiopeltini) + Protopeltinae + Decamerinae + Rentoniinae + Calitinae + Egoliinae + Trogossitinae | |
| Trogossitidae = Trogossitinae (Calitiyni, Larinotini, Egoliini, Gymnochilini, Trogossitini) + Peltinae (Peltini, Thymalini incl. Rentoniinae and Protopeltinae, Colydiopeltini, Decamerini, Ancyronini, Lophocaterini) Phloiophilidae not addressed in the paper. | |
| Trogossitidae = Trogossitinae (Calityini, Larinotini, Egoliini, Gymnochilini, Trogossitini, †Lithostomatini) + Lophocaterinae (Decamerini, Lophocaterini, Ancyronini) + Peltinae (Peltini, Phloiophilini, Colydiopeltini, Thymalini incl. Rentoniinae and Protopeltinae) |
Distribution abbreviations: AD = author of description, AL = A. Léveillé, JK = J. Kolibáč, JRB = J. R. Barron, RAC = R. A. Crowson, varA= other authors.
More than seven years have passed since I formulated theses on the higher classification of Trogossitidae. Although some opinions about the phylogeny have changed and the systematic placement of some genera has recently been called into question, the main purpose of the keys is confined to identification of the trogossitid genera. The keys are given for extant subfamilies, tribes and genera. Extinct taxa are listed in relevant sections, together with their descriptions and remarks on their classification. Generic names in parentheses in particular descriptions denote a similar character state occuring in another genus or genera.
The morphological descriptions of particular genera are largely based on several hundred detailed ink-drawings that have already been published by myself (
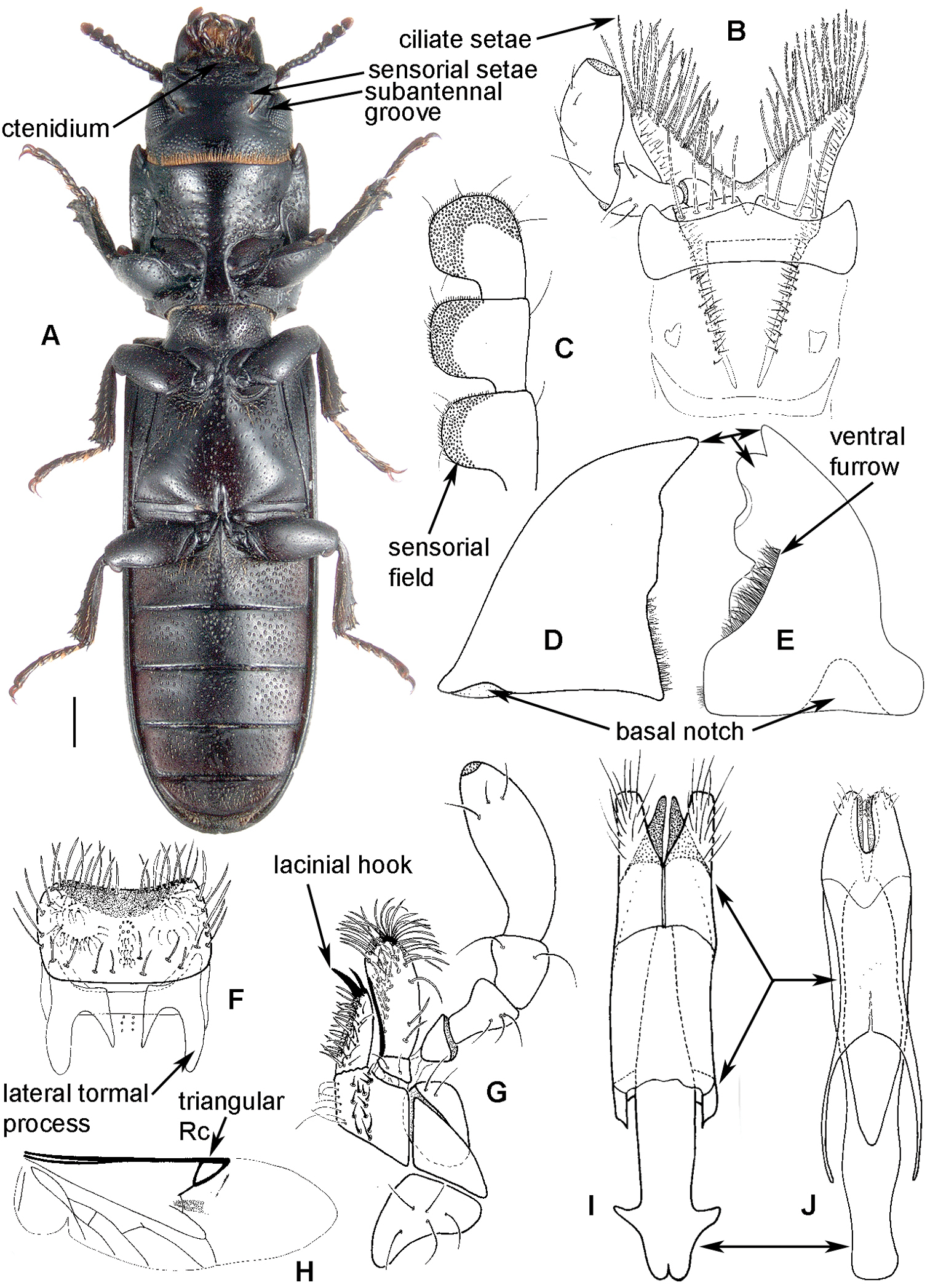
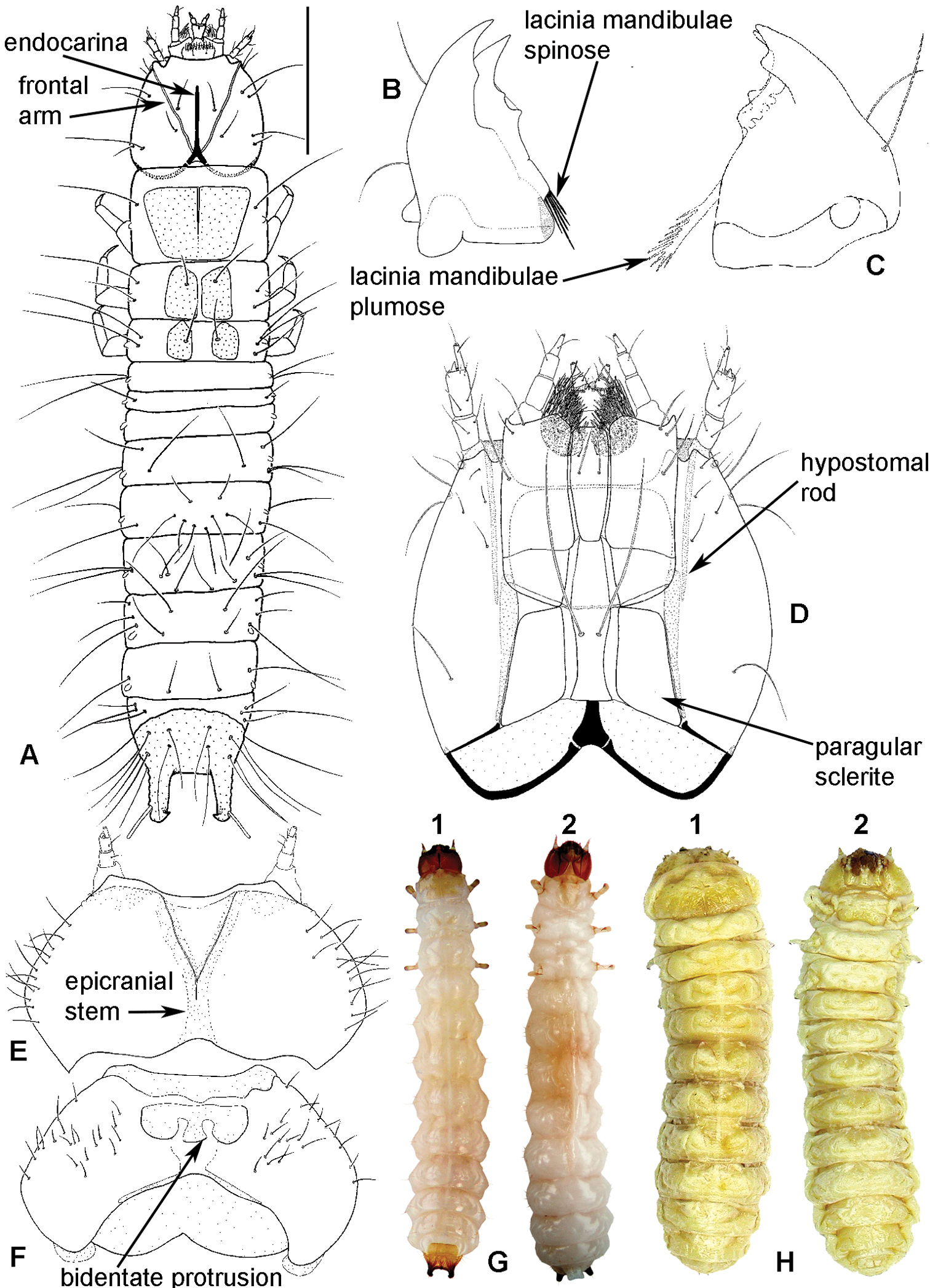
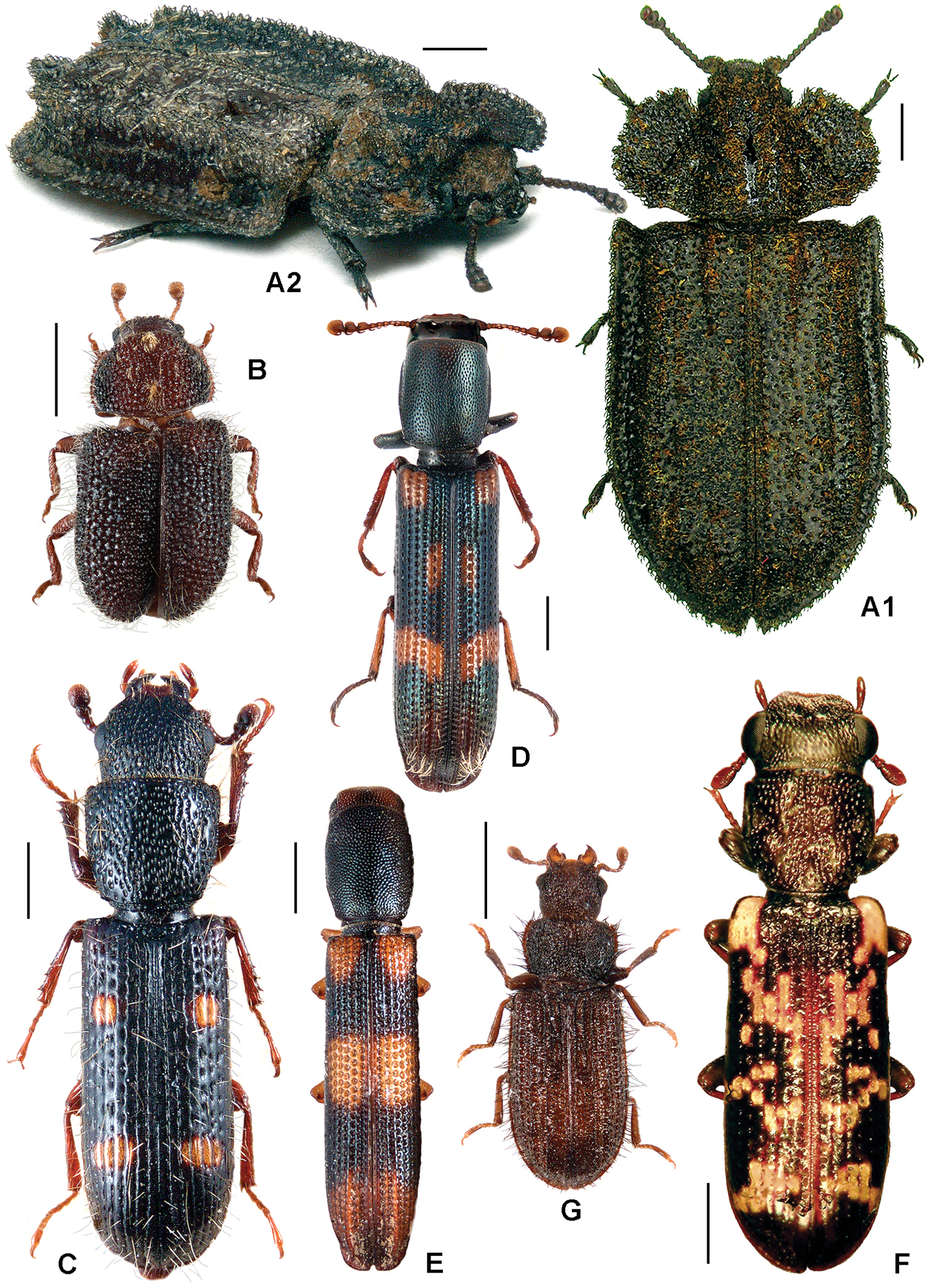
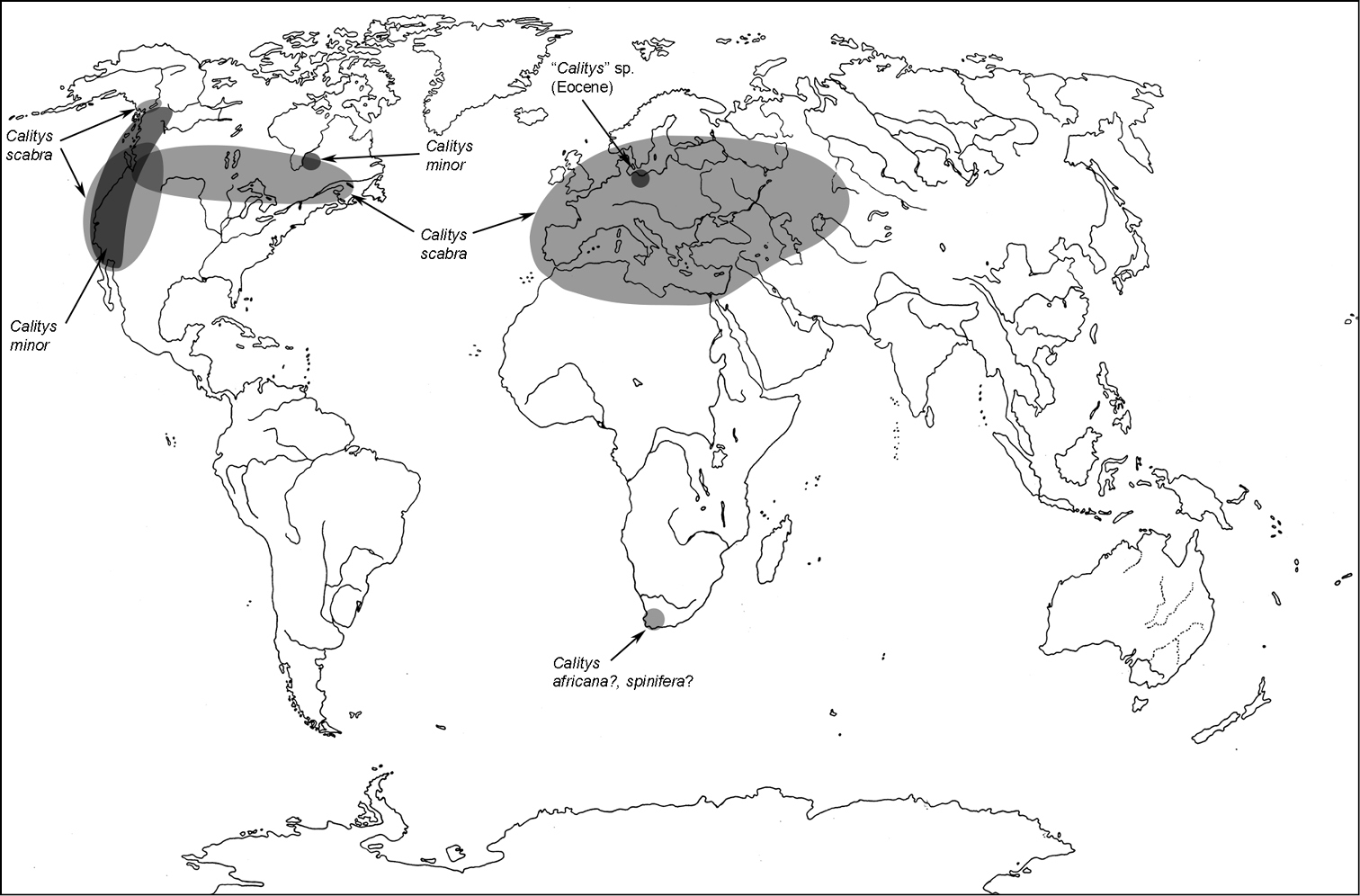
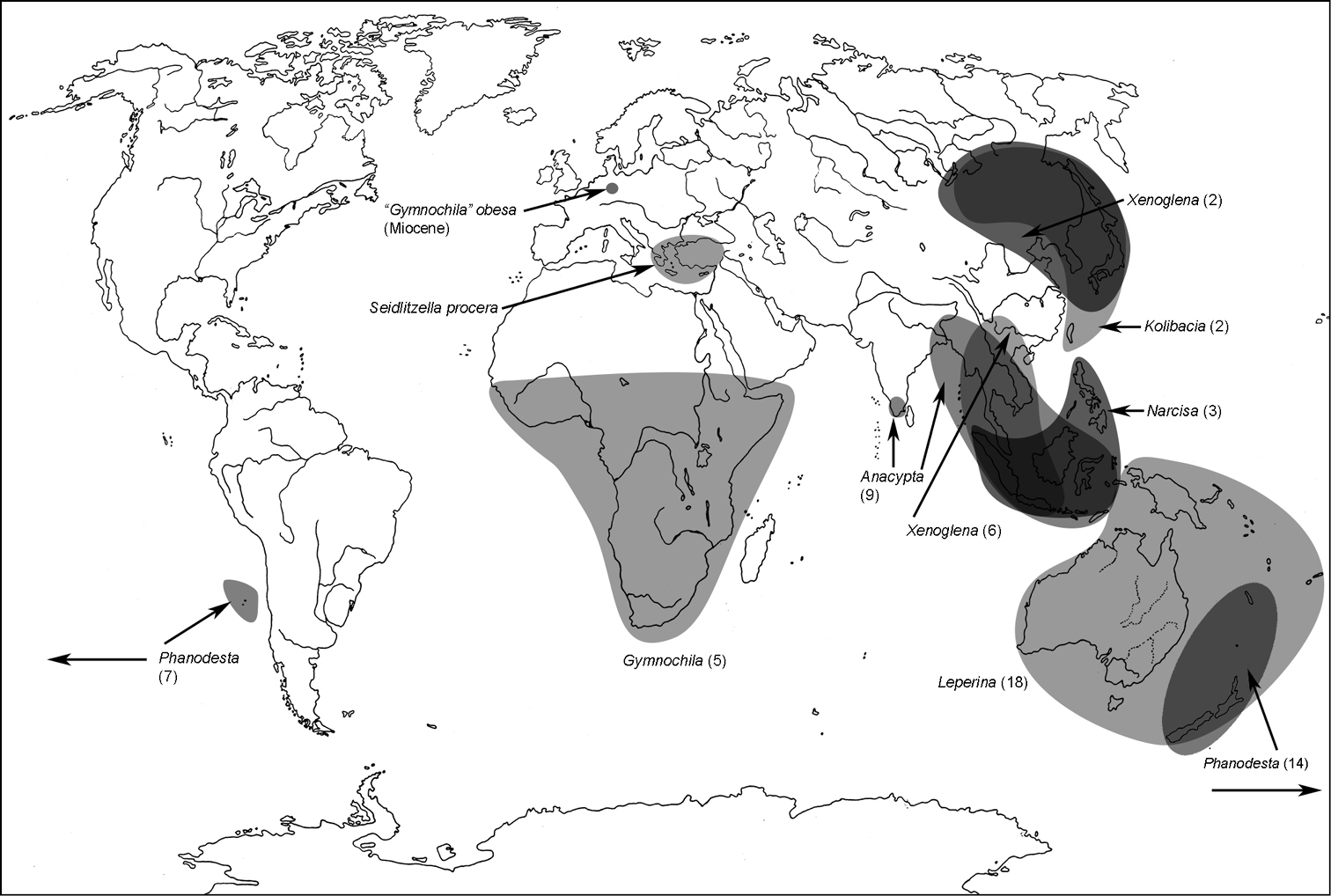
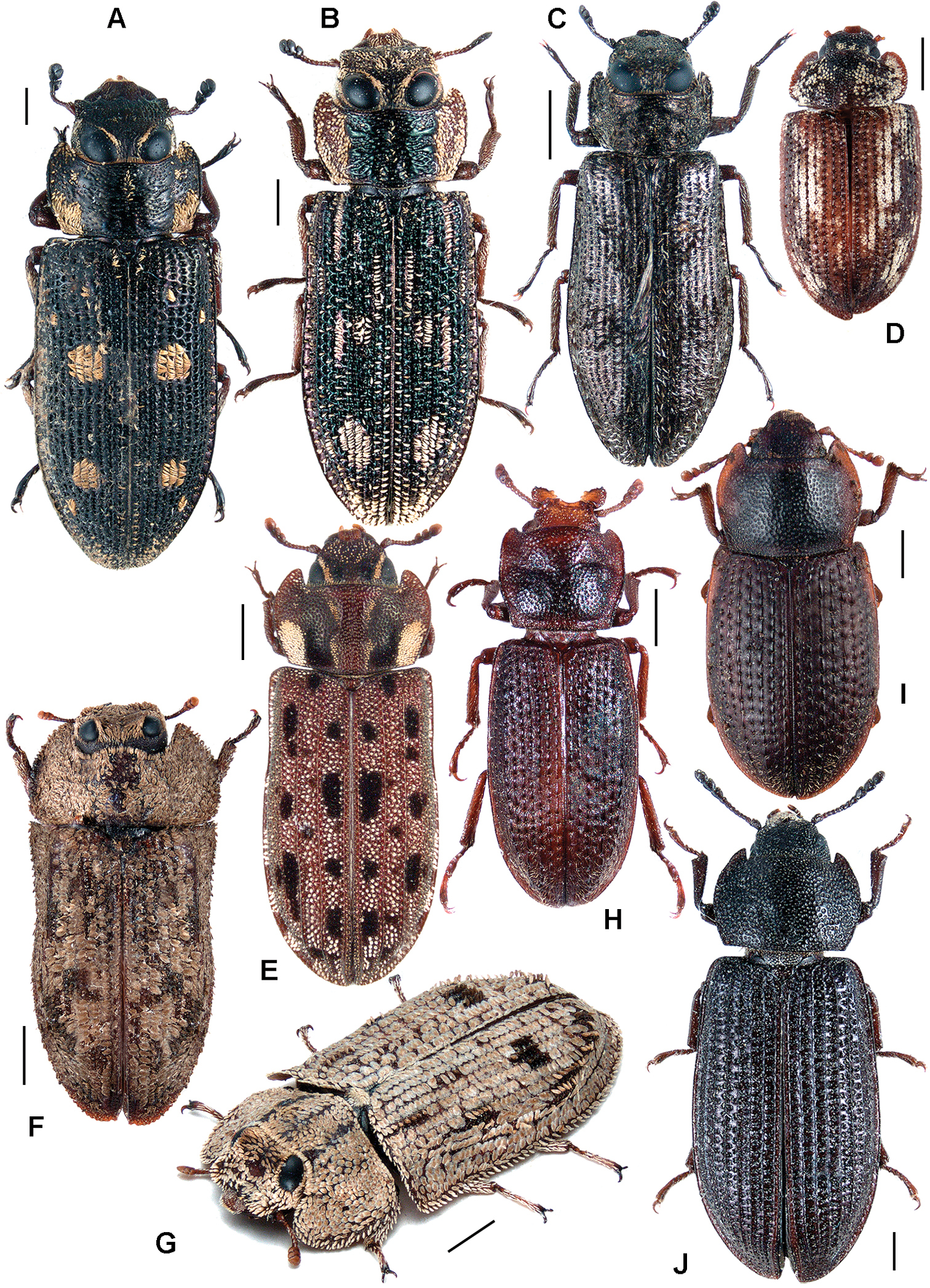
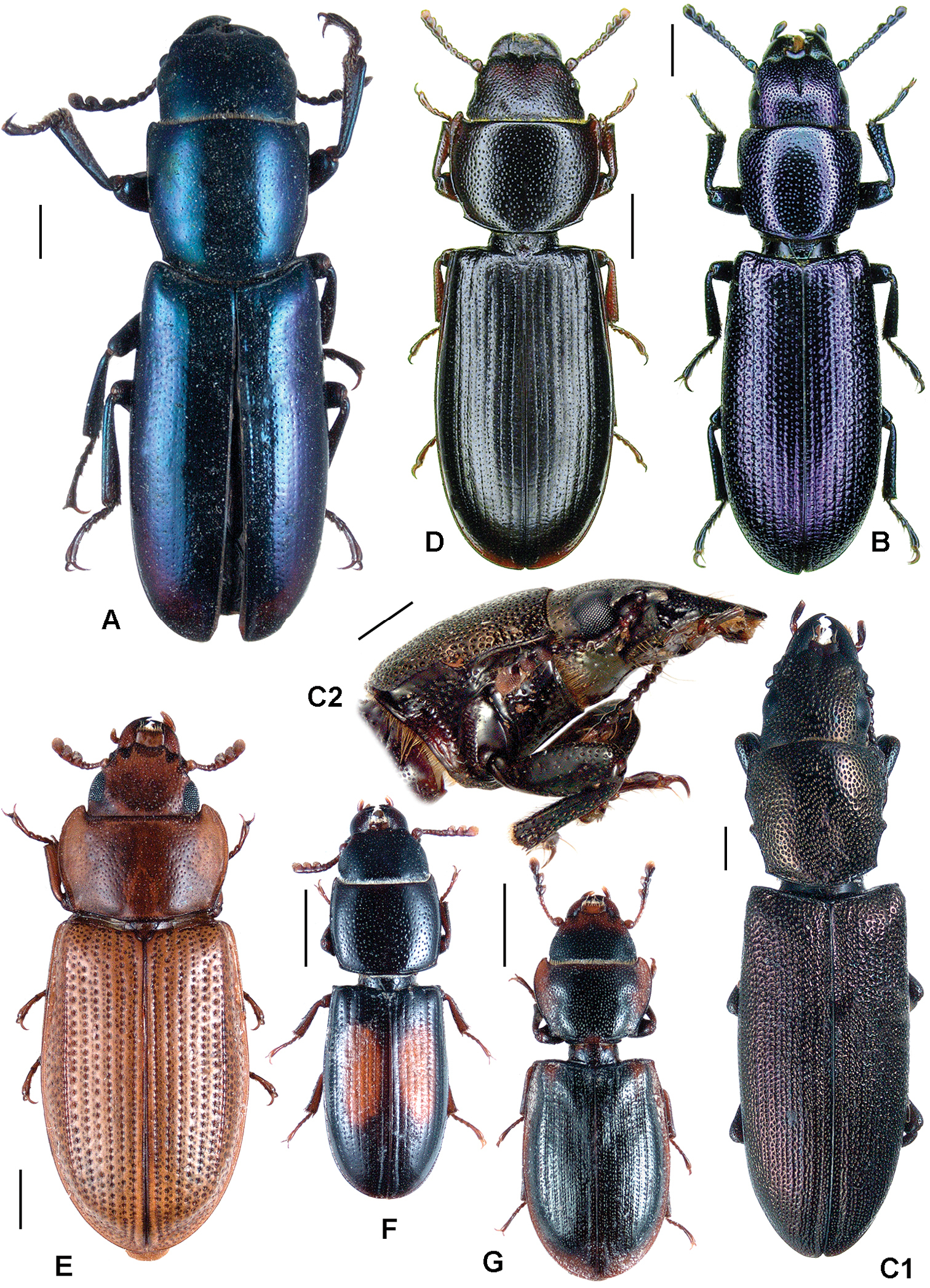
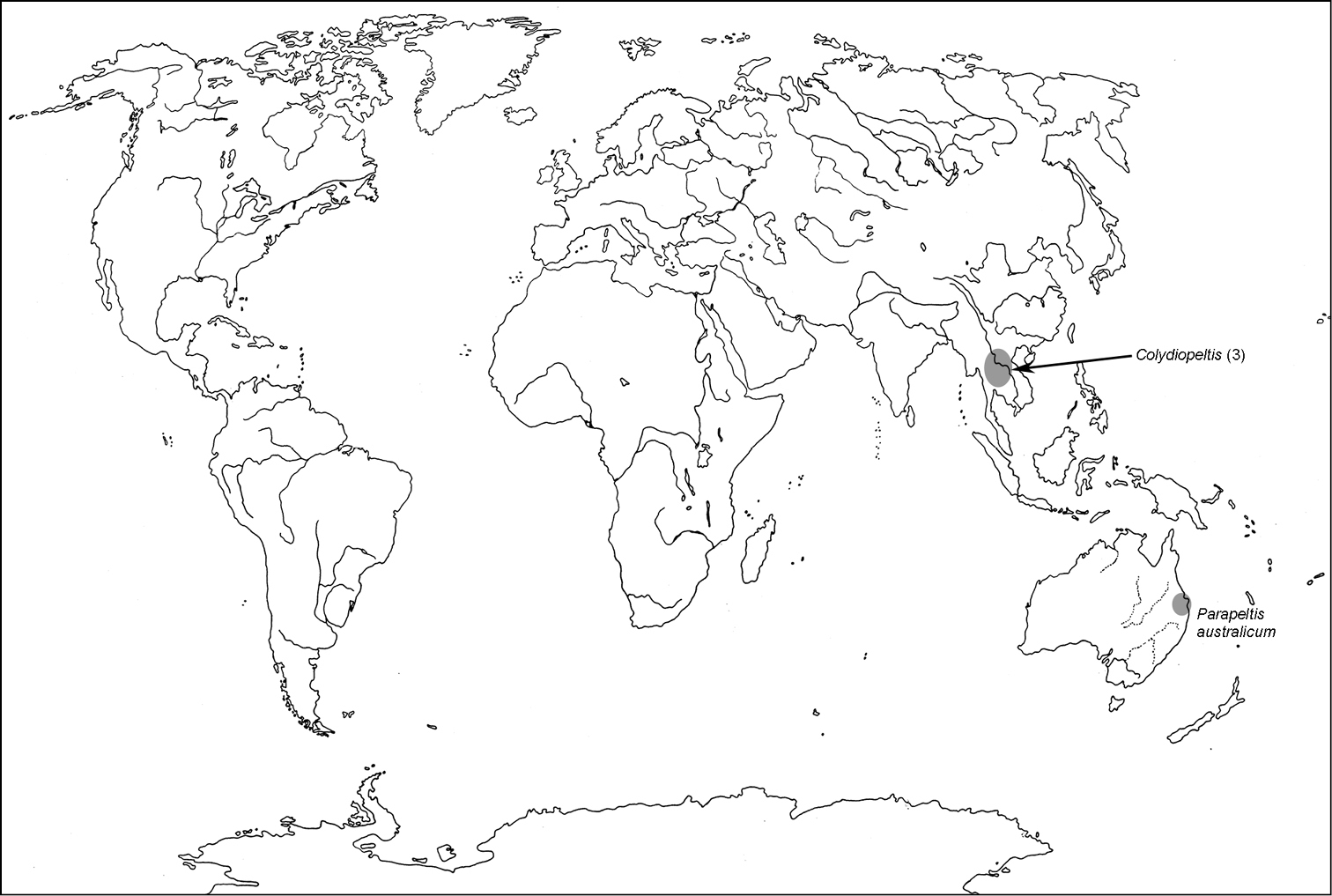
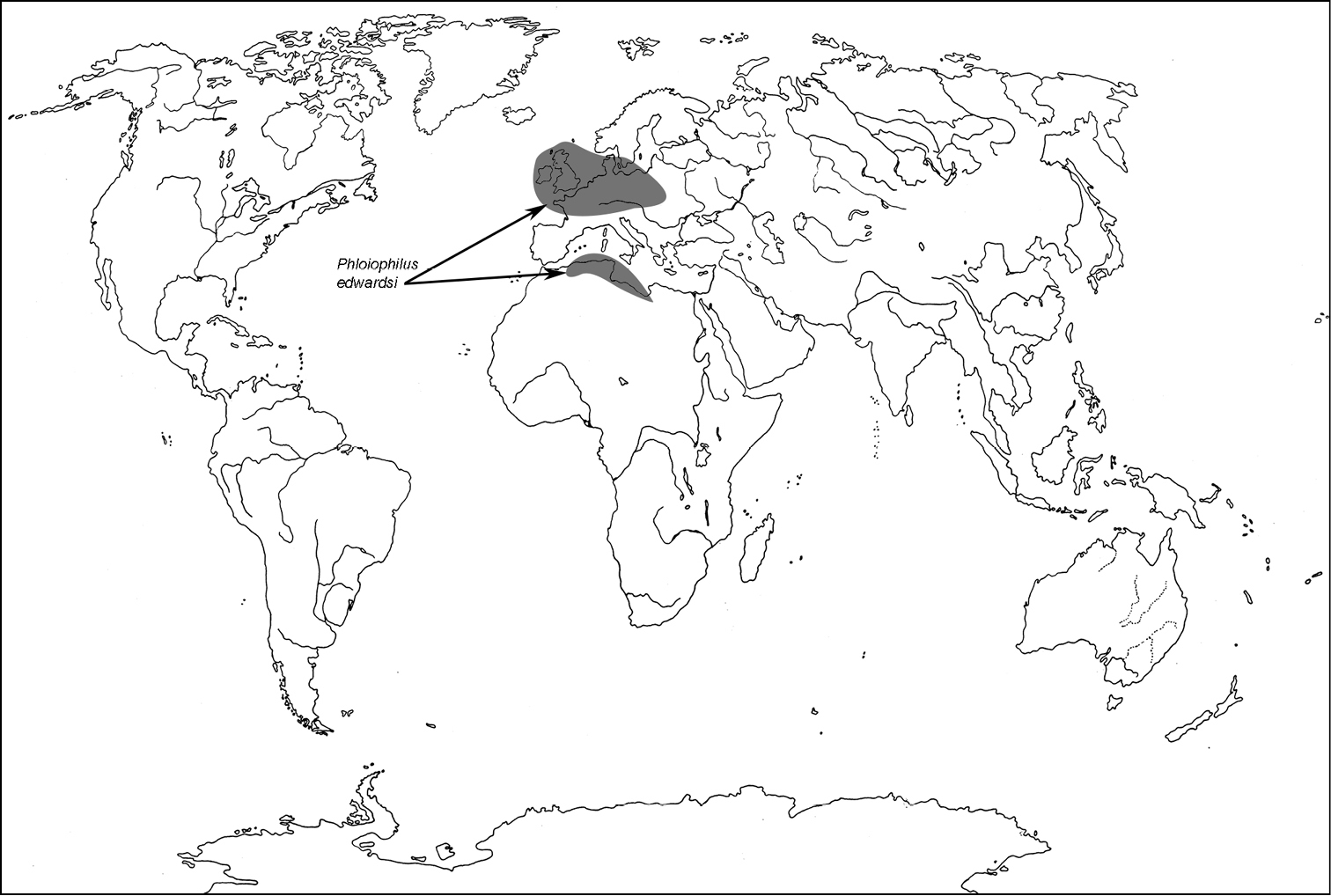
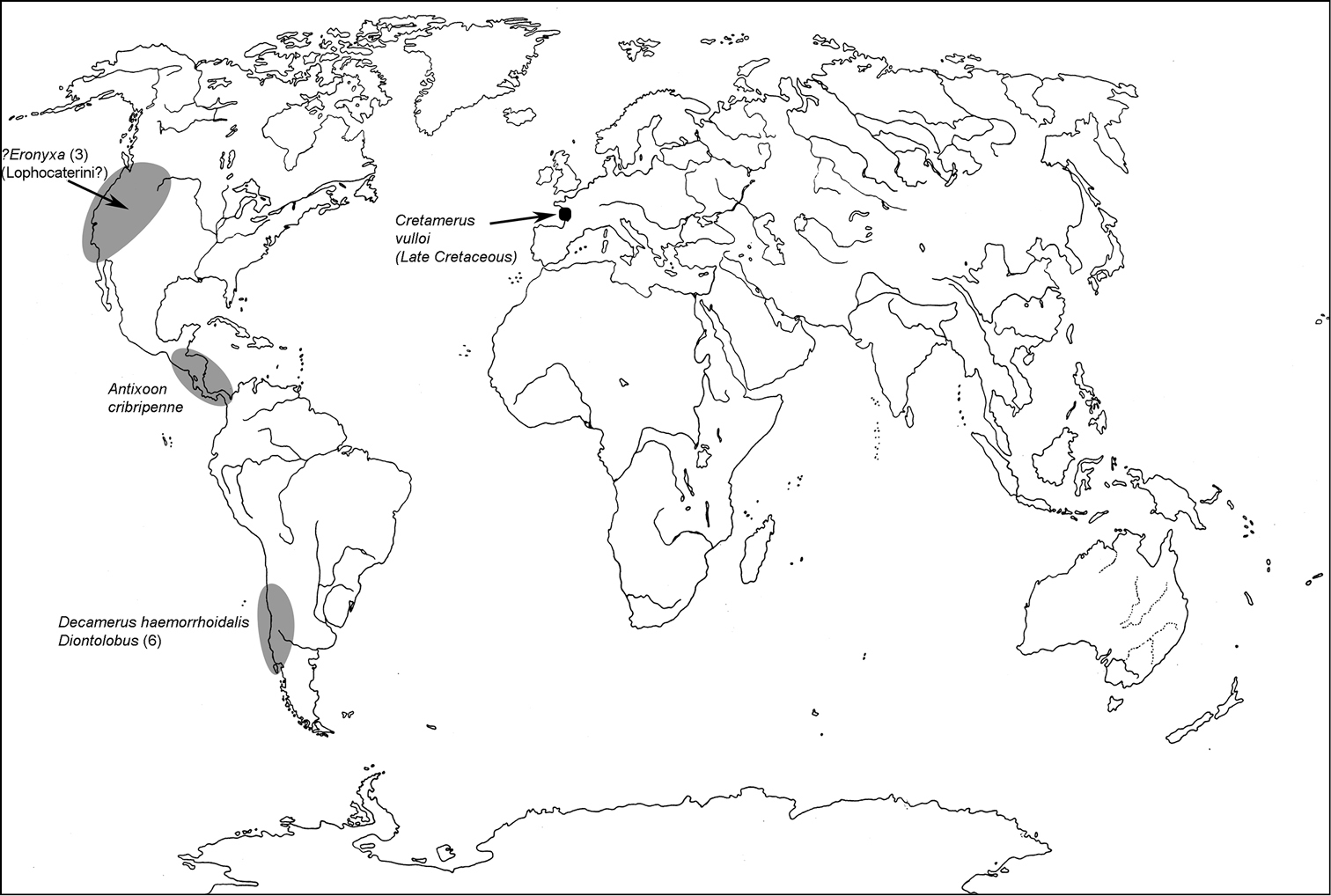
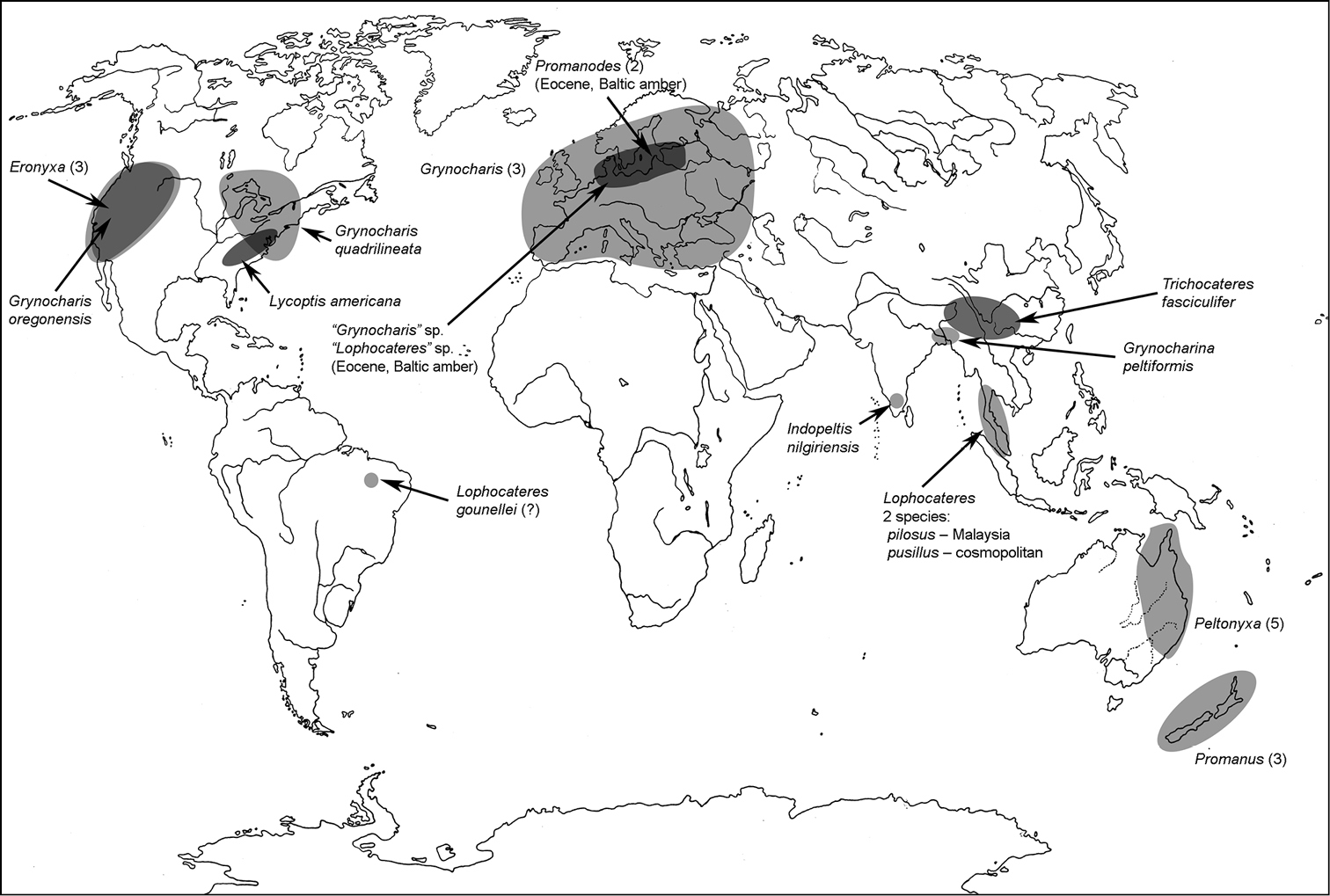
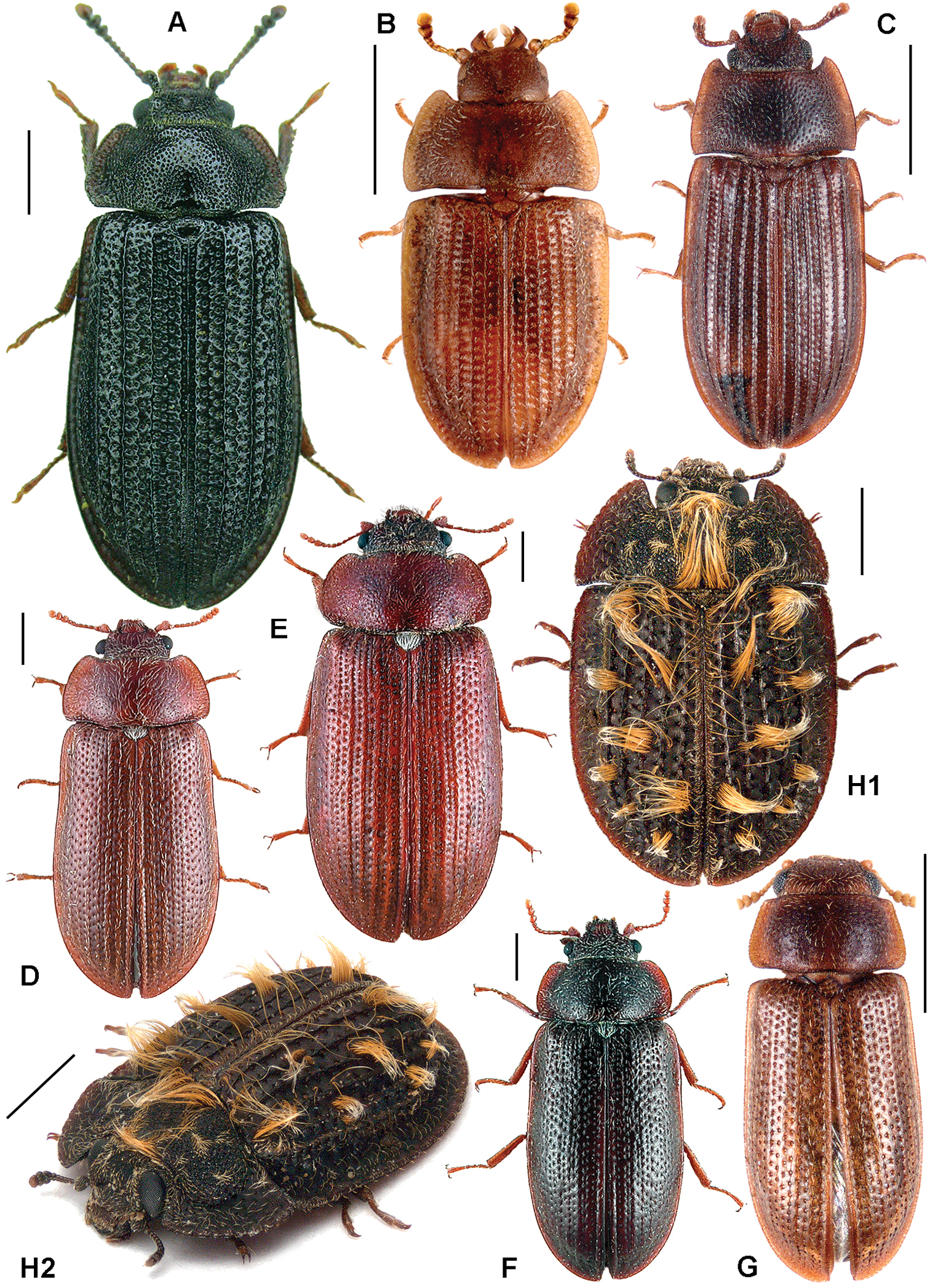
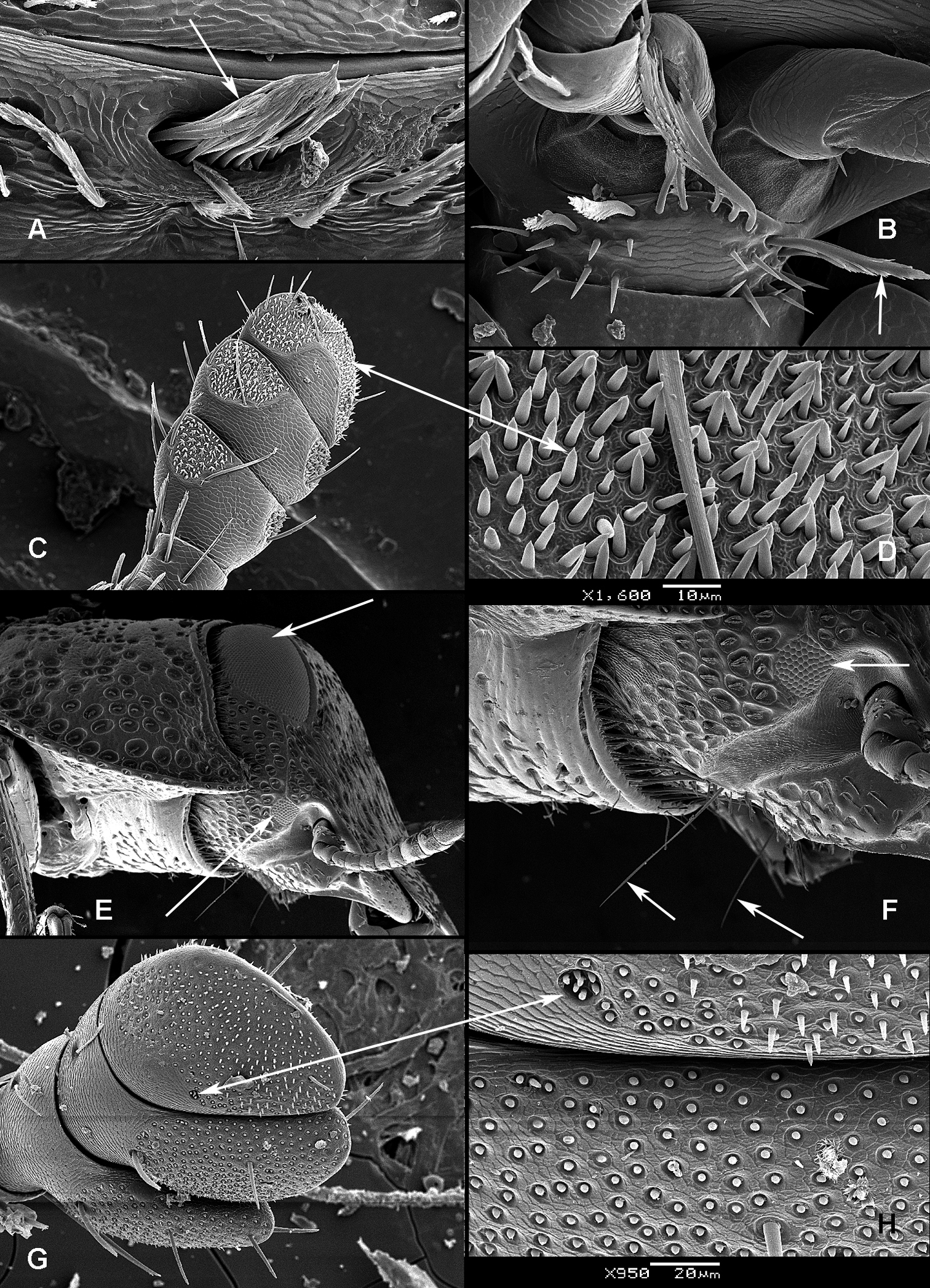
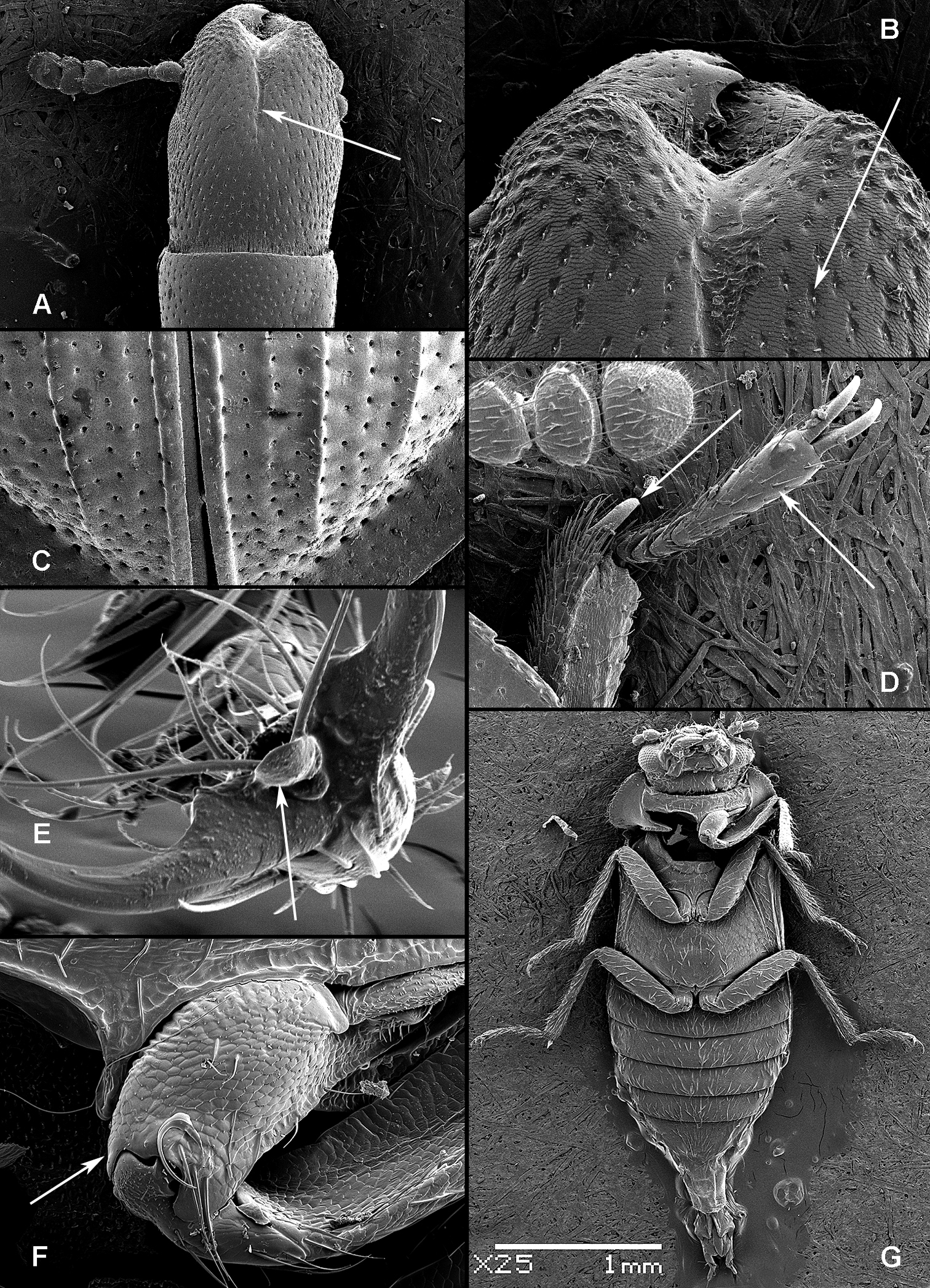
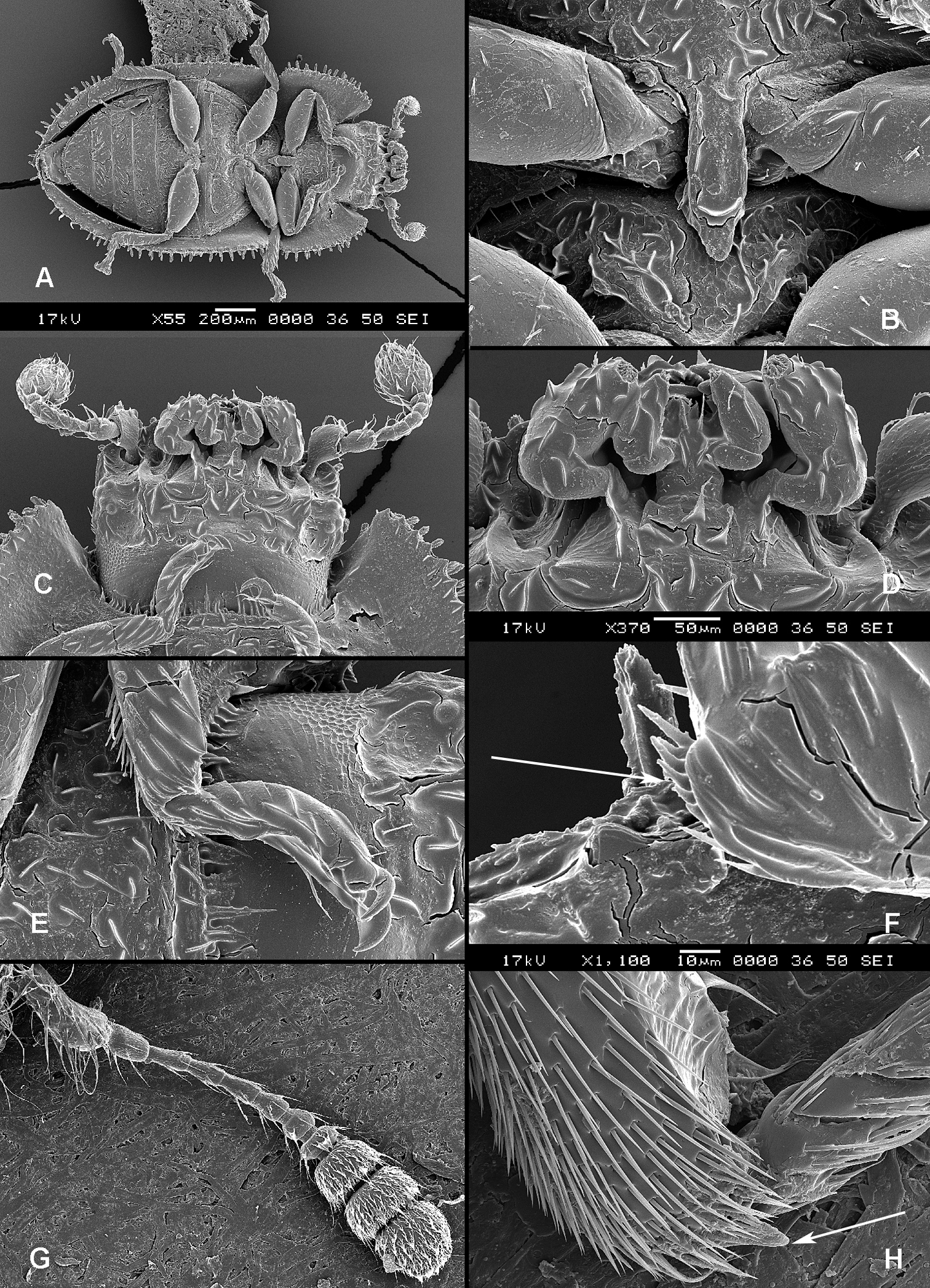
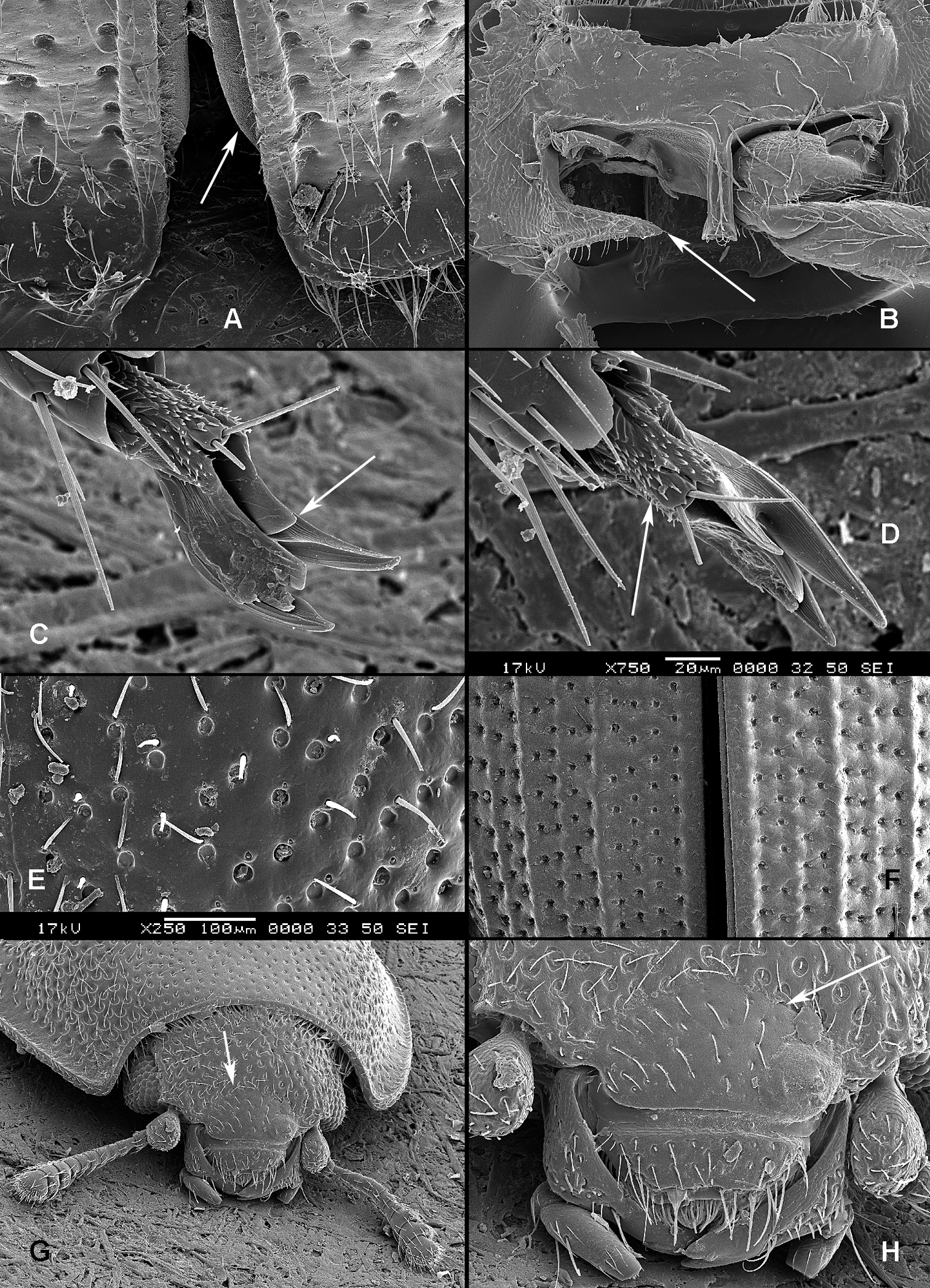
All scale bars in plates (Figs 1–12) express one millimetre. Beetles in colour plates (Figs 3–12) are pictured in approximate proportion (“large species” are larger than “small species”). White arrows in SEM photographs (Figs 13–18) denote important characters further mentioned in relevant captions. Numbers in parentheses in maps of distribution (Maps 1–13) denote the number of species within the given genus.
http://species-id.net/wiki/Trogossitidae
(Figs 1, 2). Adults (Fig. 1) (according to
Adult morphology: A Alindria sp. from Laos, ventral surface B Leipaspis lauricola, labium C Airora cylindrica, antennal club D Corticotomus cylindricus, mandible dorsally E Airora cylindrica, mandible ventrally F Acalanthis quadrisignata, labium G Acalanthis quadrisignata, maxilla H Acalanthis quadrisignata, wing I Peltonyxa deyrollei, tegmen composed of 3 parts J Airora cylindrica, tegmen composed of 2 parts.
Larval morphology: A Tenebroides “fuscus”, dorsal surface B Tenebroides “fuscus”, mandible ventrally C Lophocateres pusillus, mandible ventrally D Tenebroides “fuscus”, head ventrally E–F Peltis ferruginea, head capsule (E dorsally F ventrally) G Ancyrona diversa (1 dorsally 2 ventrally) H Peltis ferruginea (1 dorsally 2 ventrally).
Larvae (Fig. 2) (according to
Identification of the trogossitid subfamilies using the various determination keys published by a range of authors tends to be a complicated and frustrating process. Unfortunately, my “lumping” of nine former subfamilies (e.g.,
Similar ways of life (members of the both subfamilies tend to be predatory), reductions of morphological structures common to the whole order Coleoptera (e.g. wing venation, lateral edge of pronotum, mola), mosaic character patterns and probably some underlying synapomorphies complicate the definition of subfamilies even other higher taxa in Trogossitidae, in much the same way as they do in the related family Cleridae. The key that follows is therefore not based on absolutely inclusive synapomorphies. The most important, clearly-visible characters appear in bold type.
| 1 | Adult: labium with rigid ligula; epipharynx mostly with cordate sclerite along apex of labrum; antennal club mostly conspicuously asymmetrical, terminal antennomeres (antennal club) mostly with sensorial fields; front coxal cavities externally closed; body cylindrical or oval but not conglobate; end of elytral suture with distinct interlocking mechanism (“elytral lock”). Larva: head capsule with distinct endocarina, gular sutures and hypostomal rods; frontal arms mostly straight; gular region mostly with paragular sclerites. Mainly predatory, rarely fungivorous or phytophagous (e.g. feeding on grains) | Trogossitinae |
| – | Adult: labium with membranous ligula; epipharynx without cordate sclerite; antennal club weakly asymmetrical or symmetrical, terminal antennomeres (antennal club) without distinct sensorial fields; front coxal cavities mostly externally open (except for Lophocaterinae: Decamerini); body often oval and flat (but sometimes also convex or conglobate); elytral suture without distinct interlocking mechanism. Larva: head capsule mostly without endocarina; gular sutures and hypostomal rods reduced; frontal arms often curved; gular region without paragular sclerites | 2 |
| 2 | Adult: frontoclypeal suture absent or inconspicuous; gular sutures wide, subparallel; eyes moderate, not distinctly elevate; antennal club symmetrical; radial cell oblong, moderate; tibial spines along sides mostly reduced; body flat, convex or conglobate. Larva: lacinia mandibulae tridentate, absent or minute. Fungivorous or phytophagous | Peltinae |
| – | Adult: frontoclypeal suture present, sometimes distinctly emarginate (or concave); gular sutures wide, convergent at apex; eyes almost elevate, laterally situated; antennal club weakly asymmetrical; radial cell moved downwards, towards wing centre, sometimes small or reduced; tibial spines along sides present; body always flat. Larva: lacinia mandibulae plumose, always distinct. Primitive members fungivorous or phytophagous, advanced ones floricolous or predatory | Lophocaterinae |
Latreille, P. A. 1802: 110.
See “Family Trogossitidae” section for further references.
| 1 | Elytral interlocking mechanism absent or weak; antennal club loose, symmetrical; dorsal surface flat, with tufts of setae and tubercles. Fungivorous | Calityini |
| – | Elytral interlocking mechanism present; antennal club asymmetrical (or compact and symmetrical); dorsal surface convex, with scales or regularly pubescent or bare. Predatory, rarely phytophagous | 2 |
| 2 | Middle coxal cavities closed; dorsal surface mostly with very long hairs | 3 |
| – | Middle coxal cavities open; dorsal surface bare, with sparse pubescence or with scales | 4 |
| 3 | Antennal club compact and symmetrical; tibiae with reduced apical spurs; gular sutures wide, convergent at apex. Larva without paragular sclerites | Larinotini |
| – | Antennal club asymmetrical; tibiae with conspicuous apical spurs; gular sutures narrow, subparallel at apex. Known larvae with paragular sclerites | Egoliini |
| 4 | Eyes more/less dorsally situated, some genera with 2 pairs of eyes; body surface distinctly regularly sculptured or covered with scales or with short, thick setae; elytra with distinct carinae; anterior margin of pronotum always deeply emarginate | Gymnochilini |
| – | Eyes laterally situated, rather flat, always only single pair of eyes present; body surface finely punctate or wrinkled, without scales or thick setae; elytra without carinae or with weak carinae; anterior margin of pronotum emarginate or not | Trogossitini |
Calitys Thomson, 1859
Bouchard, P. et al. 2011: 57. Crowson, R. A. 1970: 13 (referred as Calitinae subfam.nov.). Ślipiński, S. A. 1992: 442 (Calitinae). Lawrence, J. F. & Newton, A. F., Jr. 1995: 869 (Calitinae). Kolibáč, J. 2006: 117 (Calityni Winkler, 1922; sic!) (diagnosis, new status). Kolibáč, J. 2007a: 364. Kolibáč, J. & Leschen, R. A. B. 2010: 242
The position of the single genus Calitys within the trogossitid system has changed many times over the past century or so. It has been classified within either Peltinae or Trogossitinae (compare, for example,
http://species-id.net/wiki/Calitys
Figs 3, 13; Map 1Hispa scabra Thunberg, 1784 [by original designation and monotypy]
Barron, J. R. 1971: 18. Crowson, R. A. 1964a: 296. Kolibáč, J. 2005: 49 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364. Kolibáč, J. 2008: 118–119 (phylogeny). Kolibáč, J. et al. 2005: 129 (key). Léveillé, A. 1910: 24. Reitter, E. 1911: 6, 7 (Calytis Thomson, 1859: misspelling; see Barron, J. R. 1971: 19). Reitter, E. 1922: 66. Spahr, U. 1981: 74 (amber and copal fossils)
Nosodes LeConte, 1861 [type species: no species included; see
Barron, J. R. 1971: 19. Reitter, E. 1876: 43
Peltidea Motchulsky, 1858 [type species: Peltidea dentata Fabricius, 1787]
Barron, J. R. 1971: 18. (nomen oblitum)
(Calitys scabra). Body size: about 10.0 mm. Adult: Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination deep. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection curved downwards, processes with bridge (Peltis). Ligula: ciliate setae absent. Ligula rigid, strongly retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 11-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron wide. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales present. Wing: radial cell oblong (or reduced), wedge cell small (Peltis), cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale present. Tegmen composed of three parts.
Larva: Frontal arms weakly curved. Epicranial stem absent. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes absent. Paragular sclerites present. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae absent. Mola reduced. Maxillary palpi 3-segmented. Cardo-Stipes not fused. Cardo: size much smaller than stipes. Ligula present. Labial palpi 2-segmented. Antennal joints 1 and 2 elongate. Sensory appendix very small. Thoracic sclerites pattern (dorsally) 1-2-2. Thoracic sclerites pattern (ventrally) 0+0+0. Trochanter oblong. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
A Calitys scabra B Larinotus umblicatus C Acalanthis quadrisignata D Calanthosoma flavomaculata E Calanthosoma (syn. Marnia) sipolisi F Egolia variegata G Necrobiopsis tasmanicus.
Fungivorous. Live under bark of old coniferous trees (fir, pine) and on tree fungi. Calitys scabra was observed together with its larvae, for example, in the old stump of a fir Abies alba in Slovakia (J. Vávra, pers. observ.).
Two species Holarctic. Two more species also reported from South Africa, of which Calitys spinifera is unknown to me. I studied a single Calitys africana non-type specimen in the Musée d’Histoire Naturelle in Geneva in 2003. It does not belong to Cleroidea.
A distribution of the tribe Calityini.
Calitys africana Boheman, 1848; Caffraria (AL)
Léveillé, A. 1910: 24. Kolibáč, J., 2003: unpublished observation of non-type specimen in MHN Geneve: not Cleroidea. Reitter, E. 1876: 44. (Nosodes africana)
Calitys minor Hatch, 1962; USA, Canada (JRB)
Barron, J. R. 1971: 23. Hatch, M. H. 1962: 189
Calitys scabra Thunberg, 1784; Europe, Siberia to Far East, North Africa(?), USA, Canada (varA)
Léveillé, A. 1910: 24. Barron, J. R. 1971: 19. Barron, J. R. 1971: 20 (syn. Bolitophagus silphides Newman, 1838, synonymized by whom?). Barron, J. R. 1971: 20 (syn. Peltis serrata LeConte, 1859, synonymized by whom?). Barron, J. R. 1971: 20 (syn. Silpha dentata Fabricius, 1787, synonymized by whom?). Borowiec, L. 1983: 11. Brustel, H. 2009: 227–232 (biology). Burakowski, B. et al. 1986: 118. Dajoz, R. 1997: 44 (ecology). Hansen, S. O. & Borgersen, B. 1991: 40 (distribution in Norway). Klausnitzer, B. 1976: 5. Klausnitzer, B. 1996: 155 (larva). Klausnitzer, B. 1978: 176. Kolibáč, J. 1993a: 20 (key). Kolibáč, J. 1993b: 90. Kolibáč, J. 2005: 49 (redescription). Kolibáč, J. 2006: 106 (larva). Kolibáč, J. 2007a: 364. Kolibáč, J. et al. 2005: 132 (key). Mitter, H. 1998: 560. Pileckis, S. & V. Monsevičius 1995: 273. Reitter, E. 1876: 44 (Nosodes). Vogt, H. 1967: 16
Calitys spinifera Reitter, 1877; Cap (AL)
Léveillé, A. 1910: 24
Calitys sp.
Beutel, R. G. & Pollock, D. A. 2000: 826 (larva, morphology). Beutel, R. G. & Ślipiński, S. A. 2001: 219
† “Calitys” sp.
Larsson, S. G. 1978: 150 (fossil, Baltic amber)
Larinotus Carter & Zeck, 1937
Bouchard, P. et al. 2011: 57. Lawrence, J. F. & Newton, A. F., Jr. 1995: 868 (Larinotinae). Kolibáč, J. 2006: 118 (Larinotini) (diagnosis, stat. n.). Kolibáč, J. 2008: 118–119 (phylogeny). Kolibáč, J. & Leschen, R. A. B. 2010: 242
The subfamily Larinotinae was originally established for the genera Colydiopeltis, Parapeltis and Larinotus (
The closed mesocoxal cavities are the single apomorphy distinguishing Larinotini from adult Egoliini. Larval characters connecting Larinotus with Peltinae are: (1) cranium with median endocarina, (2) gula with anterior apodemes, (3) strongly reduced urogomphi (minute, apices downturned). However, character states 1 and 2 are considered plesiomorphies while the third character state (reduction of urogomphi) could be a tendency observed in various cleroids living in tightly-confined surroundings (e.g. Peltis, Cleridae: Dermestoides).
http://species-id.net/wiki/Larinotus
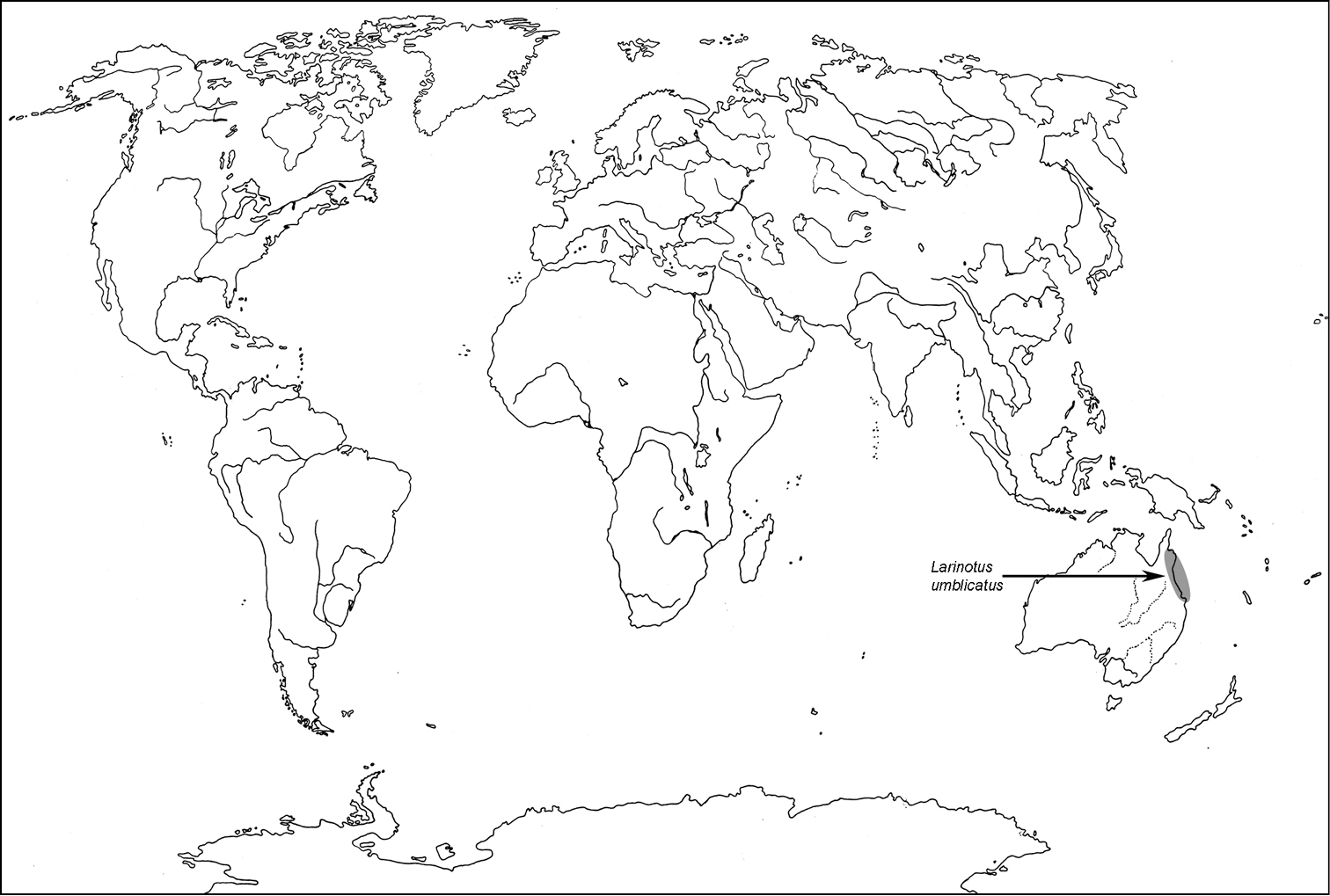
Fig. 3; Map 2Larinotus umblicatus Carter & Zeck, 1937 [by monotypy]
Kolibáč, J. 2005: 62 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Ślipiński, S. A. 1992: 455 (redescription)
Nebophilus Crowson, 1970 [type species: Nebophilus hirsutus Crowson, 1970; designated by author]
Crowson, R. A. 1970: 14. Lawrence, J. F. 1980: 307 (synonymized with Larinotus umblicatus)
(see also
Larva: Frontal arms weakly curved. Epicranial stem reduced. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes present. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: five. Mandible apical teeth number: two, horizontally situated. Lacinia mandibulae with several small spines. Mola absent. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes not fused. Cardo: size much smaller than stipes. Ligula absent. Labial palpi 2-segmented. Prementum in single part, anterior margin projecting. Torma: two separate lateral sclerites. Antennal joints 1, 2 transverse. Sensory appendix larger than half of joint 3. Thoracic sclerites pattern (dorsally) 0+0+0. Thoracic sclerites pattern (ventrally) 0+0+0. Trochanter triangular. Abdominal segment IX not divided. Tergite IX depressed. Urogomphi minute; median process absent.
The larvae and adults were found together by J. Doyen “in rotten wood beneath resupinate fungi” (
Larinotus umblicatus Carter & Zeck, 1937; Australia: Queensland (varA)
Carter, H. J. & Zeck, E. H. 1937: 186. Crowson, R. A. 1970: 16 (Nebophilus hirsutus). Lawrence, J. F. 1980: 307 (syn. Nebophilus hirsutus Crowson, 1970; synonymized by author). Ślipiński, S. A. 1992: 455 (redescription adult, description larva). Kolibáč, J. 2005: 62
Egolia Erichson, 1842
Arias, E. et al. 2009: 37. Bouchard, P. et al. 2011: 57. Crowson, R. A. 1964a: 287 (Egoliinae). Lawrence, J. F. & Newton, A. F., Jr. 1995: 869 (Egoliinae). Ślipiński, S. A. 1992: 442 (Egoliinae). Kolibáč, J. 2006: 106, 119 (larval morphology; stat. n.; phylogeny). Kolibáč, J. 2008: 118–119 (phylogeny)
This tribe exhibits several primitive features (for example, mandibles with distinct mola) and belongs among the basal groups of Trogossitinae. While earlier entomologists always classified Acalanthis, Egolia and Calanthosoma together with Nemozoma and allied genera (for example, in the “Nemozomini” of
| 1 | Pronotum distinctly elongate; antennae 11-segmented, club 3-segmented with sensorial fields; elytra bare, long hairs at elytral apex only | Calanthosoma |
| – | Pronotum cordate or transverse; antennae 8- or 10-segmented, club 1- or 2-segmented without sensorial fields; elytra and pronotum with long hairs or, rarely, perfectly bare | 2 |
| 2 | Pronotum transverse; frontoclypeal suture present; antennae 8-segmented, club 1-segmented (composed of 3 united segments) | Necrobiopsis |
| – | Pronotum cordate; frontoclypeal suture absent; antennae 10-segmented, club 2-segmented | 3 |
| 3 | Dorsal surface bare | Egolia |
| – | Dorsal surface with long, pale hairs | 4 |
| 4 | Head, pronotum and elytra black or elytral apex dark blue; each elytron with two orange spots or with two transverse bands composed of light pubescence | Acalanthis |
| – | Head, pronotum and elytra brown; elytra with X-shaped yellowish spot in anterior half and transverse yellowish stripe in apical half | Paracalanthis |
http://species-id.net/wiki/Acalanthis
Figs 1, 3, 13; Map 3Acalanthis quadrisignata Erichson, 1844 [by monotypy]
Léveillé, A. 1910: 4. Arias, E. et al. 2009: 39. Crowson, R. A. 1964a: 299 (larva, described as Phanodesta). Crowson, R. A. 1970: 13. Kolibáč, J. 2005: 40. Kolibáč, J. 2006: 106 (larva; see
An antennal apex I figured (
Body size: 6.0–9.0 mm. Body shape elongate. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate Eyes number: two. Epicranial acumination moderate. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola reduced but present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge present. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, weakly retroflex, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 10-segmented, Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum cordate. Prepectus absent. Middle coxal cavities closed. Elytra: long hairs present. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell triangular, wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
Larva: Frontal arms V-shaped. Epicranial stem reduced. Endocarina present. Antennal joints 1 and 2 elongate. Thoracic sclerites pattern (dorsally) 1-2-2. Tergite IX depressed. Urogomphi present, hooked; median process absent.
Predatory.
Temperate forests of central Chile and Argentina (
A distribution of the tribe Egoliini.
Acalanthis mirabilis Reitter, 1876; Chile (AL, varA)
Léveillé, A. 1910: 4. Arias, E. et al. 2009: 40
Acalanthis quadrisignata Erichson, 1844; Chile (AL, varA)
Léveillé, A. 1910: 4. Arias, E. et al. 2009: 39. Kolibáč, J. 1999b: 12. Kolibáč, J. 2005: 40 (redescription)
Acalanthis semimetallica Fairmaire, 1861 (Clerus); Chile (AL, varA)
Léveillé, A. 1910: 4. Arias, E. et al. 2009: 41
http://species-id.net/wiki/Calanthosoma
Fig. 3; Map 3Calanthosoma flavomaculatum Reitter, 1876 [by monotypy]
Léveillé, A. 1910: 4.
Marnia Léveille, 1889 [Type species: Marniasipolisi Léveillé, 1889; designated by
Léveillé, A. 1910: 14. Kolibáč, J. 2005: 47 (synonymized with Calanthosoma)
A single genus, it lives in tropical South America beyond the temperate “Gondwanan” distribution of the other egoliine genera. Calanthosoma shows a character set intermediate between Egoliini and Trogossitini. Longitudinal wrinkles on the dorsal surface of head and pronotum, long hairs at apex of elytra, mandibles with mola, and lacinia with distinct hooked spine at apex are typical of the egoliins; however, the genus shares ciliate labial setae and terminal antennomeres with sensorial fields with most of trogossitins. Nevertheless, most of apomorphic characters are clearly egoliine and I have no doubts about its classification within that tribe. Common trogossitine features of Calanthosoma may provide evidence of a common ancestor for Egoliini and Trogossitini.
The synonymization of Marnia with Calanthosoma is beyond doubt; the representatives of the genera are very similar.
Body size: about 9.0 mm. Body shape elongate. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum of males: ctenidium present. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: one. Galea: shape clavate. Galea: ciliate setae present. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, vertically situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow not ciliate. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, weakly retroflex, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum cordate. Prepectus absent. Middle coxal cavities closed. Elytra: long hairs present. Epipleuron moderate. Elytral interlocking mechanism present, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
Unknown, probably predatory.
Only a few specimens is known to date: Venezuela, Brazil, Antilles.
Calanthosoma flavomaculatum Reitter, 1876; Antillae, Venezuela (AL, JK)
Léveillé, A. 1910: 4. Kolibáč, J. 2005: 47 (redescription). Reitter, E. 1876: 11
Calanthosoma grouvellei Léveille, 1899 (Marnia); Brazil (AL)
Léveillé, A. 1910: 14
Calanthosoma sallei Léveille, 1889 (Marnia); Venezuela (AL)
(J. Kolibáč, unpublished note: maybe synonymous with Calanthosoma flavomaculatum)
Calanthosoma sipolisi Léveille, 1889 (Marnia); Brazil: Minas Geraes (AL)
Léveillé, A. 1910: 14. Kolibáč, J. 2005: 47
http://species-id.net/wiki/Egolia
Fig. 3; Map 3Egolia variegata Erichson, 1842 [by monotypy]
Léveillé, A. 1910: 4. Crowson, R. A. 1970: 13. Kolibáč, J. 2005: 54. Kolibáč, J. 2006: 111 (phylogeny). Reitter, E. 1876: 8
Body size: about 9.0 mm. Body shape elongate. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge present. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, weakly retroflexed, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 10-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum cordate. Prepectus absent. Middle coxal cavities closed. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell triangular, wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts.
Larva: Frontal arms V-shaped. Epicranial stem absent. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes absent. Paragular sclerites present. Hypostomal rods absent. Stemmata number: 3. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae absent. Mola absent. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala bilobed. Mala: bidentate protrusion absent. Cardo-Stipes not fused. Cardo: size much smaller than stipes. Ligula absent. Labial palpi 2-segmented. Prementum consists of two parts. Torma: single compact plate. Antennal joints 1 and 2 elongate. Sensory appendix medium-sized (reaching half-way along joint 3). Thoracic sclerites pattern (dorsally) 1-2-2. Trochanter triangular. Abdominal segment IX not divided. Tergite IX depressed. Urogomphi present, hooked; median process absent.
Probably predatory.
Australia: Tasmania; Tahiti?
Egolia variegata Erichson, 1842; Tasmania, Tahiti (AL)
Léveillé, A. 1910: 4. Kolibáč, J. 2005: 54 (redescription). Reitter, E. 1876: 8
http://species-id.net/wiki/Necrobiopsis
Fig. 3; Map 3Necrobiopsis tasmanicus Crowson, 1964 [by original designation and monotypy]
Arias, E. et al. 2009: 39. Crowson, R. A. 1970: 14. Kolibáč, J. 2005: 71 (redescription). Kolibáč, J. 2006: 111 (phylogeny)
Body size: 3.0–4.5 mm. Body shape convex (not conglobate). Gular sutures narrow, subparallel at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: two. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, vertically situated. Mola reduced but present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 8-segmented, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities closed. Elytra: long hairs present. Epipleuron thin. Elytral interlocking mechanism present, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell triangular, wedge cell absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale present. Tegmen composed of two parts.
Unknown in Necrobiopsis tasmanicus. The recently described Chilean species Necrobiopsis shangrila wascollected by canopy fogging in Nothophagus forests, including fogging of Cyttaria fungi on Nothophagus (
Tasmania, central Chile (Region VIII, Biobío).
Necrobiopsis tasmanicus Crowson, 1964; Tasmania (RAC)
Crowson, R. A. 1964a: 293. Arias, E. et al. 2009: 38. Kolibáč, J. 2005: 71 (redescription)
Necrobiopsis shangrila Arias, Ślipiński, Lawrence & Elgueta, 2009; Chile (AD)
Arias, E. et al. 2009: 39
http://species-id.net/wiki/Paracalanthis
Map 3Paracalanthis binnaburrense Crowson, 1970 [by original designation and monotypy]
Kolibáč, J. 2005: 74. Kolibáč, J. 2006: 116 (phylogeny)
Body size: about 12.0 mm. Body shape elongate. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Antenna 10-segmented. Front coxal cavities externally closed, internally open. Pronotum cordate. Middle coxal cavities closed. Elytra: long hairs present. Epipleuron thin, carinae reduced. Elytral punctation regular, scales absent. Front tibiae: spines along side moderate. Hooked spur absent in middle and hind tibiae. Claws: denticle absent.
Unknown. Collected “from decayed log” (
Australia: Queensland.
Paracalanthis binnaburrense Crowson, 1970; Australia: Queensland (RAC)
Crowson, R. A. 1970: 14, 16 (larva). Kolibáč, J. 2005: 74. Kolibáč, J. 2006: 108 (larva)
Gymnochila Erichson, 1844 (= Gymnocheilis Dejean, 1835)
Bouchard, P. et al. 2011: 57. Burakowski, B. et al. 1986: 118 (Gymnochilinae). Kolibáč, J. 2006: 119 (diagnosis, stat. n.). Kolibáč, J. 2007a: 364. Kolibáč, J. 2008: 118–119 (phylogeny). Leschen, R. A. B. & Lackner, T. 2013: 283
Five genera of Gymnochilini (namely Anacypta, Gymnocheilis, Narcisa, Xenoglena, Leperina) form, beyond doubt, a monophyletic group. Gymnochilins constitute an advanced group of Trogossitinae, adapted to a predatory way of life. They are rapid flyers, dwelling on fallen logs and hunting for bostrichids, scolytids and other insects, strongly resembling the jewel beetles (Buprestidae) in their body shape and movement. Two distinctly separated pairs of eyes in most of them and their ability to jump (Anacypta) characterize the tribe as one of the most advanced of all trogossitids. Some remarks about the independent status of the tribe Gymnochilini with regard to Trogossitini are made below, in the section relating the latter tribe.
The inclusion of Phanodesta from Juan Fernandez Isl. was more or less confirmed by two separate character analyses (
In both analyses by
| 1 | Head with 1 pair of eyes. Ventral part of cranium with long setae at sides. Radial cell triangular and moved down, or reduced. Elytra with conspicuous carinae | 2 |
| – | Head with 2 pairs of eyes. Ventral part of cranium without long setae at sides. Radial cell mostly oblong. Elytral carinae reduced or inconspicuous | 5 |
| 2 | Body bare, without setae. Window punctures absent. Unicolorous, black species | Seidlitzella |
| – | Body surface with vestiture consisting of scales or setae or both. Window punctures absent or present. Dorsal side brown or black-brown, with colour patterns formed by vestiture | 3 |
| 3 | Dorsal vestiture consisting of scales. Elytral carinae not beaded; intercarinal space punctate; window punctures present. Mucro absent | 4 |
| – | Dorsal vestiture consisting of setae, scales or both. Elytral carinae usually beaded; intercarinal space apunctate; window punctures absent. Mucro absent or weakly developed | Phanodesta |
| 4 | Elytral intercarinal space bipunctate; window punctures simple. Protibial edge smooth. Mandibles with mola | Kolibacia |
| – | Elytral intercarinal space multipunctate; window punctures tuberculate. Protibial edge spinate. Mandibles without mola | Leperina |
| 5 | Body rather flat, compact; smaller species (about 4–8 mm) | 6 |
| – | Body rather cylindrical, elongate; larger species (about 6–20 mm) | 7 |
| 6 | Body perfectly covered with large scales | Narcisa |
| – | Body without scales or pubescence, with metallic lustre | Anacypta |
| 7 | Body with colour pattern composed of scales and short thick setae; antennal club large | Gymnocheilis |
| – | Body without scales, spots on pronotum and elytra formed by short setae; antennal club smaller | Xenoglena |
http://species-id.net/wiki/Anacypta
Figs 4, 14; Map 4Nitidula punctata Fabricius, 1801 [designated by
Kolibáč, J. 2005: 44 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364
Acrops Dalman, 1824 [Type species: Acrops metallica Dalman, 1824 (= Nitidula punctata Fabricius, 1801)]
Léveillé, A. 1910: 23
Body size: about 4.5–7.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium present. Antennal groove present. Eyes: size large, dorsal. Eyes number: four. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape clavate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, weakly retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 10-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 present. Front tibiae: spines along side reduced. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
A Anacypta sp., Vietnam B Anacypta punctata C Anacypta sp., Laos D Gymnocheilis subfasciata E Gymnocheilis sp., Ghana F Gymnocheilis varia G Kolibacia regularis H Kolibacia squamulata I Leperina cirrosa.
Predatory. Adults run rapidly on logs and branches of fallen trees, hunting for prey. If disturbed, they fly very quickly. Some beetle collectors remark (P. Pacholátko, pers. comm.; experience from southern India) that some species can even jump(!) before they fly off.
South-eastern Asia including Indonesia, Laos, Vietnam (numerous modern unpublished records). Often recorded together with species of Xenoglena.
A distribution of the tribe Gymnochilini.
Anacypta birmanica Léveillé, 1888; Burma (AL)
Léveillé, A. 1910: 23 (Acrops)
Anacypta cicatricosa Reitter, 1880; „Himalaya” (AL)
Léveillé, A. 1910: 23 (Acrops). Léveillé, A. 1910: 23 (Anacypta cicatricosa var. rugosa Léveillé, 1899). Kolibáč, J. 2007a: 364 (syn. Anacypta rugosa Léveillé, 1899)
Anacypta cyanea Léveillé, 1899; Perak (AL)
Léveillé, A. 1910: 23 (Acrops)
Anacypta feai Léveillé, 1888; Tenasserim (AL)
Léveillé, A. 1910: 23 (Acrops)
Anacypta gambeyi Léveillé, 1890; “Cochinchina” (AL)
Léveillé, A. 1910: 23 (Acrops)
Anacypta higonia Lewis, 1888; Japan (AL, varA)
Léveillé, A. 1910: 23 (Acrops). Kolibáč, J. 2007a: 364. Nakane, T. et al. 1963: 181 (Acrops)
Anacypta perraudierei Léveillé, 1905; Tonkin (AL)
Léveillé, A. 1910: 23 (Acrops)
Anacypta punctata Fabricius, 1801; Sumatra, Moluccae, Borneo (AL)
Léveillé, A. 1910: 23
Léveillé, A. 1910: 23 (syn. buprestoides Weber, 1801); (Sumatra, Moluccae) (AL)
Léveillé, A. 1910: 23 (syn. metallica Dalman, 1824); (Borneo) (AL)
Léveillé, A. 1910: 23 (syn. punctata var. dohrni Reitter, 1876). Kolibáč, J. 2005: 44 (redescription)
Anacypta weyersi Léveillé, 1900; Sumatra (AL)
Léveillé, A. 1910: 23 (Acrops)
http://species-id.net/wiki/Gymnocheilis
Figs 4, 13, 14; Map 4[Type species: Peltis squamosa Gray in
Léveillé, A. 1910: 22 (Gymnochila). Kolibáč, J. 2005: 60 (Gymnochila, redescription). Kolibáč, J. 2006: 111 (Gymnochila, phylogeny). Reitter, E. 1876: 37 (Gymnochila). Leschen, R. A. B. & Lackner, T. 2013: 279, 289
Lepidopteryx Hope, 1840 [Type species: Peltis squamosa Gray in
Gymnochila Erichson, 1844 [Type species: Trogosita vestita Griffith, 1832 (= Gymnochila varia Fabricius, 1801); by monotypy]
Yves Bousquet (pers. comm., 2010) kindly checked the nomenclatoral problem of Leperina that also bears upon the name of Gymnocheilis: “... the type species of Lepidopteryx is Trogositas quamosa Gray, 1832 described from ‘Melville’s Island’, not Lepidopteryx squamosa Hope, 1840. In his second and third catalogues, Dejean proposed the genus Gymnocheilis for squamosa Gray. So Lepidopteryx Hope, 1840 is a senior synonym of Gymnochila Erichson, 1844, which in turn is a junior synonym of Gymnocheilis Dejean, 1835.” The correct name Gymnocheilis has already been used by
Body size: about 9.0–14.0 mm. Body shape elongate. Gular sutures reduced. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum of males: ctenidium present. Submentum: ctenidium present. Antennal groove present. Eyes: size large, dorsal. Eyes number: four. Epicranial acumination deep. Lacinial hooks absent. Galea: shape clavate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge denticulate. Mandibular apical teeth number: one. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch deep. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities closed. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales present. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 present. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts.
Probably predatory. Sometimes collected at light.
Africa south of the equator.
Gymnocheilis lepidoptera Reitter, 1876; Abyssinia (AL)
Léveillé, A. 1910: 22. Reitter, E. 1876: 39
† Gymnocheilis obesa Heer, 1862; Germany: Öhningen; Tertiary: Middle Eocene (varA)
Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 23
Gymnocheilis rugosa Thunberg, 1808; Guinea (AL)
Gymnocheilis sparsuta J. Thomson, 1858; Gabon (AL)
Léveillé, A. 1910: 22. (syn. Gymnochila angulicollis J. Thomson, 1858). Kolibáč, J. 2005: 60 (redescription). Reitter, E. 1876: 38
Gymnocheilis subfasciata J. Thomson, 1858; Gabon (AL)
Léveillé, A. 1910: 22. Reitter, E. 1876: 39
Gymnocheilis varia Fabricius, 1801; South Africa (AL)
Léveillé, A. 1910: 22 (syn. Gymnochila adspersa Boheman, 1848) (Caffraria)
Léveillé, A. 1910: 22 (syn. Gymnochila laticollis Boheman, 1848) (Caffraria)
Léveillé, A. 1910: 22 (syn. Gymnochila squamosa Gray in Griffith 1832) (Cap)
Léveillé, A. 1910: 22. (syn. Gymnochila vestita Fabricius, 1844) (Cap, South Africa)
Kolibáč, J. 2005: 60 (redescription). Reitter, E. 1876: 38
http://species-id.net/wiki/Kolibacia
Fig. 4; Map 4Leperina tibialis Reitter, 1889 [designated by
Schawaller, W. 1993: 4 (Leperina and Seidlitzella, Palaearctic species). Yoshitomi, H. & Lee, C.-F. (in press)
(according to
Larva (according to
The adults and larvae are probably predatory. An adult Kolibacia squamulata was, for example, found in rotten birch (
Russian Far East, Mongolia, North Korea, North-eastern China, Japan: Hokkaido, Tsushima.
Kolibacia okinawana Yoshitomi & Lee (in press); Japan: Okinawa, Amami-Ôshima (AD)
Yoshitomi, H. & Lee, C.-F. (in press)
Kolibacia regularis Grouvelle, 1913; Taiwan, Vietnam (varA)
Grouvelle, A. 1913: 46 (Leperina). Yoshitomi, H. & Lee, C.-F. (in press; comb. Kolibacia, key)
Kolibacia squamulata Gebler, 1830 (Peltis); Russian Far East, Mongolia, North Korea, Northeastern China (varA, JK)
Léveillé, A. 1910: 22. Esaki, T. et al. 1951: 1061 (Lepidopteryx squamulosa). Kolibáč, J. 2006: 107 (Lepidopteryx squamulosa). Kolibáč, J. 2007a: 364. Löbl & Smetana 2010: 26 (Lepidopteryx). Mamaev, B. M. 1976: 1652 (larva, Lepidopteryx). Nakane, T. et al. 1963: 181 (Lepidopteryx squamulosa). Nikitsky, N. B. 1992: 81 (Lepidopteryx squamulosa). Leschen, R. A. B. & Lackner, T. 2013: 288. (comb. Kolibacia). Schawaller, W. 1993: 3 (key, Leperina). Yoshitomi, H. & Lee, C.-F. (in press; key)
Kolibacia tsushimana Nakane, 1985; Japan: Tsushima (varA)
Nakane, T. 1985: 162 (Lepidopteryx squamulata tsushimana). Kolibáč, J. 2009: 128 (Lepidopteryx squamulata tsushimana). Miyatake, M. 1985: 148 (Leperina tsushimana). Leschen, R. A. B. & Lackner, T. 2013: 288. (comb. Kolibacia squamulata tsushimana). Yoshitomi, H. & Lee, C.-F. (in press; Kolibacia tsushimana; key)
Kolibacia tibialis Reitter, 1889 (Leperina); Japan: Hokkaido (varA)
Léveillé, A. 1910: 22. Esaki, T. et al. 1951: 1061. Kolibáč, J. 2007a: 364. Löbl & Smetana 2010: 26 (Lepidopteryx). Nakane, T. et al. 1963: 181. Nikitsky, N. B. 1992: 81. Leschen, R. A. B. & Lackner, T. 2013: 288. (comb. Kolibacia). Yoshitomi, H. & Lee, C.-F. (in press; key)
http://species-id.net/wiki/Leperina
Figs 4, 5; Map 4Trogosita decorata Erichson, 1842 [designated by
Léveillé, A. 1910: 21. Crowson, R. A. 1964a: 299. Kolibáč, J. 2005: 65 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364. Kolibáč, J. 2009: 127 (Lepidopteryx). Leschen, R. A. B. & Lackner, T. 2013: 289. Matthews, E. G. 1992: 3 (key, Lepidopteryx). Reitter, E. 1876: 35
Onyschomorpha Arrow, 1900 [Type species: Onyschomorpha marmorata Arrow, 1900; synonymized by Kolibáč, J. 2005: 65]
Léveillé, A. 1910: 23 (Onyschomorpha). Kolibáč, J. 2007a: 364 (Onyschomorpha, as a synonym)
There has been a lack of clarity about the names Leperina and Lepidopteryx in the last decade or so. Leperina tended to be used by European authors while their overseas, mainly antipodean, colleaguespreferred Lepidopteryx. I referred – correctly – to the genus as Leperina in 2005, 2006 and in Catalogue of Palaearctic Beetles edited by I. Löbl and A. Smetana (
Most recently,
(according to
A description by
A Xenoglena sp.1, Vietnam B Xenoglena sp.2, Vietnam C Xenoglena quadrisignata (cf. yunnanensis) D Leperina (syn. Onyschomorpha) marmorata E Leperina decorata F Narcisa decidua G Narcisa sp., Seram H Phanodesta cribraria, “Chili” I Phanodesta sp., Juan Fernandez Isl. J Seidlitzella procera.
Both larva and adult are predatory. Leperina decorata and Leperina monilata were found in stems of Eucalyptus obliqua, where they were preying on larvae of Epithora dorsalis (
Australia incl. Tasmania, New Zealand, New Caledonia, New Guinea.
Leperina adusta Pascoe, 1860; Melbourne (AL)
Léveillé, A. 1910: 21
Leperina burnettensis MacLeay, 1871; Gayndah (AL)
Léveillé, A. 1910: 21
Leperina cincta Léveillé, 1889; New Zealand (AL)
Léveillé, A. 1910: 21
Leperina cirrosa Pascoe, 1860; Moreton Bay (AL)
Léveillé, A. 1910: 21. Hawkeswood, T. J. 1991: 159 (biology, Lepidopteryx). Hawkeswood, T. J. 1992: 229 (biology, Lepidopteryx)
Leperina conspicua Olliff, 1885; Australia (AL)
Léveillé, A. 1910: 21
Leperina decorata Erichson, 1842; Tasmania (AL)
Léveillé, A. 1910: 21. Kolibáč, J. 2005: 65 (redescription)
Léveillé, A. 1910: 21 (syn. Leperina gayndahensis MacLeay, 1871); (Gayndah)
Leperina fraterna Olliff, 1885; Australia (AL)
Léveillé, A. 1910: 21
Leperina lacera Pascoe, 1860; Viti Isl. (AL)
Léveillé, A. 1910: 21. Léveillé, A. 1910: 21 (syn. Leperina signoreti Reitter, 1876). Kukalová-Peck, J. & Lawrence, J. F. 1993: 243 (morphology of wing)
Leperina lichenea Fauvel, 1866; New Caledonia (AL)
Léveillé, A. 1910: 21
Leperina lifuana Fauvel, 1903; Lifu Isl. (AL)
Léveillé, A. 1910: 21
Leperina loriae Léveillé, 1893; New Guinea (AL)
Léveillé, A. 1910: 21
Leperina marmorata Arrow, 1900 (Onyschomorpha); Christmas Isl. (AL)
Léveillé, A. 1910: 23 (Onyschomorpha Arrow, 1900)
Leperina mastersi MacLeay, 1869; Gayndah (AL)
Léveillé, A. 1910: 21
Leperina monilata Pascoe, 1872 (= moniliata Blackburn, 1902); Australia (AL)
Léveillé, A. 1910: 22. Reitter, E. 1876: 31 (Peltis)
Leperina opatroides Léveillé, 1884; New Guinea (AL)
Léveillé, A. 1910: 22
Leperina seposita Olliff, 1885; Australia (AL)
Léveillé, A. 1910: 22
Leperina spercheoides Léveillé, 1878; New Caledonia (AL)
Léveillé, A. 1910: 22
Leperina turbata Pascoe, 1863; Australia (AL)
Léveillé, A. 1910: 22. Léveillé, A. 1910: 22 (syn. Leperina fasciculata Redtenbacher, 1868)
Narcisa decidua Pascoe, 1863 [by monotypy]
Léveillé, A. 1910: 23. Kolibáč, J. 2005: 69 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Reitter, E. 1876: 43
The genus is apparently related to Anacypta and Xenoglena. The body is larger than in Anacypta but not so slender as in Xenoglena, moreover perfectly covered in scales. Further to the three species described, I have also encountered some undescribed species, all of them distributed in the Indonesian islands.
Body size: about 7.0–9.0 mm. Body shape flat. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium present. Antennal groove present. Eyes: size large, dorsal. Eyes number: four. Epicranial acumination absent. Lacinial hooks absent. Galea: shape clavate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, vertically situated. Mola absent. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales present. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 present. Front tibiae: spines along side reduced. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts.
Unknown, probably predatory like Anacypta.
Indonesia (species that remain formally undescribed are known from Sulawesi and other islands).
Narcisa bimaculata Gestro, 1879; Sumatra (AL)
Léveillé, A. 1910: 23
Narcisa decidua Pascoe, 1863; Batchian (AL)
Léveillé, A. 1910: 23. Kolibáč, J. 2005: 69 (redescription). Reitter, E. 1876: 43
Narcisa lynceus Olliff, 1883; Borneo (AL)
Léveillé, A. 1910: 23
http://species-id.net/wiki/Phanodesta
Fig. 5; Map 4Phanodesta cordaticollis Reitter, 1876 [designated by
Léveillé, A. 1910: 20. Crowson, R. A. 1964a: 299. Kolibáč, J. 2005: 77 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Leschen, R. A. B. & Lackner, T. 2013: 290
As mentioned previously, six Phanodesta species from Juan Fernandez Island have been considered peculiar wingless beetles, with a form of elytral sculpture that is unknown in other trogossitids. Two analyses have shown a relationship for the genus within Gymnochilini, perhaps as a strongly-derived descendent of Australian Leperina (
(according to
A description by
Probably predatory. Adultsmay be collected at night on fungi and on the trunks of dead, dying and live trees, or on the ground (
Chile: Juan Fernandez Island; New Caledonia, New Zealand mainland and offshore islands, Lord Howe Island.
Phanodesta argentea Montrouzier, 1860 (Nitidula); New Caledonia (AL)
Léveillé, A. 1910: 21 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 300 (comb. from Leperina; maybe conspecific with Leperina spercheoides)
Phanodesta brevipennis Reitter, 1876; Chile: Juan Fernandez Island (AL)
Léveillé, A. 1910: 20. Reitter, E. 1876: 33. Leschen, R. A. B. & Lackner, T. 2013: 300
Phanodesta brounii Pascoe, 1880; New Zealand (AL)
Léveillé, A. 1910: 21 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 291 (comb. from Leperina)
Phanodesta carinata Leschen & Lackner, 2013; New Zealand (AD)
Leschen, R. A. B. & Lackner, T. 2013: 292
Phanodesta cribraria Blanchard, 1851 (Toxicum); Chile: Juan Fernandez Island (AL)
Léveillé, A. 1910: 20 (syn. Phanodesta cordaticollis Reitter, 1876). Léveillé, A. 1910: 21 (syn. Phanodesta picea Germain, 1855). Kolibáč, J. 2005: 77 (redescription). Leschen, R. A. B. & Lackner, T. 2013: 300. Reitter, E. 1876: 32 (Phanodesta cordaticollis Reitter, 1876)
Phanodesta cribrata Germain, 1855; Chile: Juan Fernandez Island (AL)
Léveillé, A. 1910: 21 (syn. Phanodesta angulata Reitter, 1876). Leschen, R. A. B. & Lackner, T. 2013: 300. Reitter, E. 1876: 33 (Phanodesta angulata)
Phanodesta francoisi Léveillé, 1909; New Caledonia (AL)
Léveillé, A. 1910: 21 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 300 (comb. from Leperina; combination based on an image of the type)
Phanodesta guerini Montrouzier, 1860 (Nitidula); New Caledonia (AL)
Léveillé, A. 1910: 21 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 300 (comb. from Leperina). Reitter, E. 1876: 35 (Phanodesta)
Phanodesta iohowi Germain, 1898; Chile: Juan Fernandez Island (AL)
Léveillé, A. 1910: 21 (Phanodesta johowi). Leschen, R. A. B. & Lackner, T. 2013: 300
Phanodesta manawatawhi Leschen & Lackner, 2013; New Zealand (AD)
Leschen, R. A. B. & Lackner, T. 2013: 293
Phanodesta oculata Leschen & Lackner, 2013; New Zealand (AD)
Leschen, R. A. B. & Lackner, T. 2013: 295
Phanodesta nigrosparsa White, 1846 (Gymnocheila); New Zealand (AL)
Léveillé, A. 1910: 22 (Leperina). Klimaszewski, J. & Watt, J. C. 1997: 43 (Lepidopteryx). Leschen, R. A. B. & Lackner, T. 2013: 294 (comb. from Leperina). Reitter, E. 1876: 35 (Phanodesta)
Phanodesta pubescens Germain, 1898; Chile: Juan Fernandez Island (AL)
Léveillé, A. 1910: 21. Leschen, R. A. B. & Lackner, T. 2013: 300
Phanodesta pudica Olliff, 1889 (Ostoma); Lord Howe Island (varA)
Léveillé, A. 1910: 32 (Ostoma incertae sedis). Leschen, R. A. B. & Lackner, T. 2013: 299 (comb. from Ostoma)
Phanodesta robusta Pic, 1924; Chile: Juan Fernandez Island (varA)
Pic, M. 1924: 378
Phanodesta shandi Broun, 1909; New Zealand (varA)
Broun, 1909: 307 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 296 (comb. from Leperina)
Phanodesta signoreti Montrouzier, 1860 (Leperina); New Caledonia (AL)
Léveillé, A. 1910: 22 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 300 (comb. from Leperina)
Phanodesta sobrina White, 1846 (Gymnocheila); New Zealand (AL)
Léveillé, A. 1910: 22 (Leperina). Brookes, A. 1932: 28 (Leperina interrupta; syn. by Leschen, R. A. B. & Lackner, T. 2013: 296). Léveillé, A. 1910: 22 (syn. Leperina fasciolata Blanchard, 1853). Léveillé, A. 1910: 22 (syn. Leperina nigro-sparsa Blanchard, 1853; homonym with Leperina nigrosparsa White, 1846). Leschen, R. A. B. & Lackner, T. 2013: 296 (comb. from Leperina). Reitter, 1876: 35 (Phanodesta). Sharp, 1877: 266 (Leperina farinosa; syn. by Leschen, R. A. B. & Lackner, T. 2013: 296)
Phanodesta tepaki Leschen & Lackner, 2013; New Zealand (AD)
Leschen, R. A. B. & Lackner, T. 2013: 297
Phanodesta variegata Germain, 1855; Chile: Juan Fernandez Island (AL)
Léveillé, A. 1910: 21. Léveillé, A. 1910: 21. (syn. Phanodesta costipennis Reitter, 1876). Leschen, R. A. B. & Lackner, T. 2013: 300. Reitter, E. 1876: 34 (Phanodesta costipennis)
Phanodesta wakefieldi Sharp, 1877 (Leperina); New Zealand (AL)
Léveillé, A. 1910: 22 (Leperina). Leschen, R. A. B. & Lackner, T. 2013: 298 (comb. from Leperina)
http://species-id.net/wiki/Seidlitzella
Fig. 5; Map 4Peltis procera Kraatz, 1858 [by monotypy]
Kolibáč, J. 2005: 82 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 365. Schawaller, W. 1993: 2 (synonymized with Leperina). Leschen, R. A. B. & Lackner, T. 2013: 279, 289
Cymba Seidlitz, 1875 (homonym) [type species: Peltis procera Kraatz, 1858; by original designation and monotypy]
Léveillé, A. 1910: 20. Kolibáč, J. 2007a: 365. Reitter, E. 1876: 30. Schawaller, W. 1993: 2
Peltocymba Reitter, 1920 [type species: Peltis procera Kraatz, 1858; by original designation and monotypy]
Kolibáč, J. 2007a: 365. Schawaller, W. 1993: 2
Body size: 11.0–19.0 mm. Body shape elongate. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum of males: ctenidium present. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, vertically situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, weakly retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present or absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of two parts. Coxitae undivided.
Predatory. Adults found on logs of various trees (e.g. the fir Abies cilicia), larvae found under pine bark (
Greece, Cyprus, Turkey.
Seidlitzella procera Kraatz, 1858; Greece, Cyprus, Turkey (JK)
Léveillé, A. 1910: 20 (Cymba). Kolibáč, J. 2005: 82 (redescription). Kolibáč, J. 2007a: 365. Reitter, E. 1876: 31 (Cymba). Schawaller, W. 1993: 4 (larva)
http://species-id.net/wiki/Xenoglena
Fig. 5; Map 4Xenoglena deyrollei Reitter, 1876 [by monotypy]
Léveillé, A. 1910: 23. Kolibáč, J. 2005: 85 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364. Nikitsky, N. B. 1992: 81
Outer habitus resembles the jewel beetles (Buprestidae), especially those of the genus Chrysobothris. Anacypta asahinai Kono, 1938 was combined with Xenoglena by
Body size: about 7.0–10.0 mm. Body shape elongate. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium present. Antennal groove present. Eyes: size large, dorsal. Eyes number: four. Epicranial acumination absent. Lacinial hooks absent. Galea: shape clavate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, vertically situated. Mola reduced but present. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales present. Wing: radial cell oblong (or reduced), wedge cell absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side reduced. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Coxitae undivided.
Predatory. Adults dwell on fallen trees and dry branches, hunting for xylophagous insects. They fly and run at great speed and appear very like some jewel beetles in body shape.
Indonesia, Malayan Peninsula, Russian Far East, Japan, northern China. A large body of material of perhaps-undescribed species is known to me from northern Laos.
Xenoglena asahinai Kono, 1938: 227 (Acrops); Japan (varA)
Nakane, T. et al. 1963: 181 (Acrops). Kolibáč, J. 2009: 128 (comb. with Xenoglena)
Note: maybe synonym of Xenoglena quadrisignata; opinion by
Xenoglena chrysobothroides Léveillé, 1897; Malacca (AL)
Léveillé, A. 1910: 23
Xenoglena deyrollei Reitter, 1876; Java (AL)
Léveillé, A. 1910: 23. Kolibáč, J. 2005: 87 (redescription). Reitter, E. 1876: 41
Xenoglena fryi Léveillé, 1899; Perak (AL)
Léveillé, A. 1910: 23
Xenoglena quadrisignata Mannerheim, 1852; Siberia, Mongolia, Far East, Japan, North Korea, China: Northeast Territory (varA)
Léveillé, A. 1910: 22 (Gymnochila). Kolibáč, J. 2005: 86 (redescription). Kolibáč, J. 2007a: 364. Nikitsky, N. B. 1992: 81. Reitter, E. 1876: 40 (Gymnochila)
Xenoglena tetrasigma Léveillé, 1878; Malacca (AL)
Léveillé, A. 1910: 23
Xenoglena vicina Léveillé, 1897; Malacca (AL)
Léveillé, A. 1910: 23
Xenoglena yunnanensis Léveillé, 1907; China: Yunnan (AL)
Léveillé, A. 1910: 23. Kolibáč, J. 2007a: 364
Trogossita Olivier, 1790 (= Temnoscheila Westwood, 1830)
Burakowski, B. et al. 1986: 115 (Nemosomatinae). Kolibáč, J. 2006: 120 (diagnosis, stat. n.). Kolibáč, J. 2007a: 364 (phylogeny). Kolibáč, J. 2008: 118–119 (phylogeny)
Two character analyses of Trogossitini (
There are also some genera that are not included in the two character analyses because of insufficient data sets, namely Dupontiella, Elestora, Eupycnus, Euschaefferia, and Parallelodera. The classification of all these rather advanced genera within Trogossitini is undeniable, apart from the monotypic Elestora which is obviously related to Melambia, for which the systematics are quite complicated and in need of revision.
Most of the members of Trogossitini lead the kind of life typical of predatory Cleridae, especially of the subfamilies Clerinae and Tillinae. Adults hunt for xylophagous insects (e.g. Curculionidae: Scolytinae, Bostrichidae) on branches and logs while larvae dwell and hunt under bark or in galleries. However, some trogossitine adults live in insect galleries together with their larvae (e.g. Nemozoma). The trogossitins are not as efficient in the air as the gymnochilins, and neither do they move so swiftly on the ground.
| 1 | Frons with conspicuous sharp longitudinal groove | 2 |
| – | Frons without groove, with shallow depression at most | 3 |
| 2 | Anterior part of cranium (frons) with two large horn-like processes; body extremely elongate, small (about 3–6 mm) | Nemozoma |
| – | Anterior part of cranium (frons) without distinct processes; body not extremely elongate, larger (about 7–25 mm) | Temnoscheila |
| 3 | Pronotum conspicuously elongate, weakly narrowed at base; body elongate and cylindrical | 4 |
| – | Pronotum transverse or quadrate or narrowed at base; elytra widest in apical third and somewhat flattened | 8 |
| 4 | Pronotum somewhat cordate; elytra with carinae; large species (about 10–35 mm) | Alindria |
| – | Sides of pronotum nearly parallel; elytra without carinae; smaller species (about 2–15 mm) | 5 |
| 5 | Outer margins of all tibiae with large spines; antennae reach backwards anterior margin of pronotum; larger species (about 7–15 mm) | Airora |
| – | Outer margin of tibiae with 2–3 spines at apex at most; antennae reach back to beyond anterior margin of pronotum; smaller species (about 2–5 mm) | 6 |
| 6 | Pronotum conspicuously narrowed (constricted) at base | Dupontiella |
| – | Pronotum not narrowed at base, oblong | 7 |
| 7 | Pronotum with distinctly raised lateral margins; submentum distinctly separated from gula in front, outer angles not prominent; at least front tibiae with spines at apex | Corticotomus |
| – | Pronotum without distinctly raised lateral margins, apical angles obliterated; submentum not distinctly separated from gula in front, outer angles prominent and produced apically at least to base of mandibles; tibiae without spines | Euschaefferia |
| 8 | Elytra with conspicuous carinae and regular punctation | 9 |
| – | Elytra without carinae, with regular sculpture only | 10 |
| 9 | Dorsal body surface distinctly flattened; very wide, black species, elytra with four striking orange spots; mesonotum with long orange hairs | Elestora |
| – | Dorsal body surface not distinctly flattened, almost cylindrical; elongate, almost cylindrical, unicolorous (black or brown) species without colour pattern | Melambia |
| 10 | Body including head and pronotum distinctly elongate; pronotum constricted at base | Leipaspis |
| – | Body not so elongate; sides of pronotum subparallel or cordate | 11 |
| 11 | Tarsal pattern 4-4-4: 1st tarsomere coalescent with 2nd tarsomere in all pairs of legs; elytra rather convex | Parallelodera |
| – | Tarsal pattern 5-5-5; elytra rather flattened | 12 |
| 12 | All tibiae with about 3-6 conspicuous spines along outer margin; pronotum subparallel, elongate; labrum retracted, hardly visible; body more coarsely sculptured | Eupycnus |
| – | All tibiae with about 2–4 spines along outer margin; pronotum cordate, approximately as long as wide; labrum distinctly visible; body sculpture finer | Tenebroides |
Trogossita cylindrica Serville, 1828 [designated by
Léveillé, A. 1910: 7. Barron, J. R. 1971: 64. Kolibáč, J. 2005: 41 (redescription). Kolibáč, J. 2006: 111 (phylogeny)
Temnochilodes Léveillé, 1890 [type species: Temnochilodes dugesi Léveillé, 1890]
Léveillé, A. 1910: 9. Kolibáč, J. 2005: 41 (synonymized)
Body size: about 7.0–16.0 mm. Body shape elongate. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination deep. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, vertically situated. Mola reduced but present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, strongly retroflexed, deeply emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum elongate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side large. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of two parts. Coxitae undivided.
Larva: Frontal arms V-shaped. Epicranial stem reduced. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes absent. Paragular sclerites present. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae absent. Mola absent. Maxillary palpi 3-segmented. Cardo: size much smaller than stipes. Labial palpi 2-segmented. Prementum in single part. Antennal joints 1 and 2 elongate. Sensory appendix very small. Thoracic sclerites pattern (dorsally) 1-2-2. Thoracic sclerites pattern (ventrally) 3+1+1. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
A Alindria spectabilis B Alindria elongata C Alindria sp., Thailand D Airora cylindrica E Airora (syn. Temnochilodes) dugesi F Corticotomus (syn. Colydobius) divisus G Elestora fulgurata.
Predatory. In USA, adults dwell mostly on branches and logs of various species of pine. Some specimens were also collected from the bush Cercidiumcorreyanum and some emerged from the fungus Fomesapplanatus. Airora minuta adults were observed preying on the bark beetle Hylocurus. (All
Airora aequalis Reitter, 1876; Canada, USA, Mexico (JRB)
Barron, J. R. 1971: 65 (syn. Airora bicolor Casey, 1916; synonymized by
Airora apicalis Reitter, 1876; Colombia (AL)
Léveillé, A. 1910: 7
Airora bituberculata Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 7
Airora canescens Reitter, 1876; Central America (AL)
Léveillé, A. 1910: 7
Airora centralis Sharp, 1891; Mexico, Guatemala, Panama (AL)
Léveillé, A. 1910: 7
Airora cylindrica Serville, 1828; Canada, USA, Mexico (JRB)
Léveillé, A. 1910: 7. Barron, J. R. 1971: 65 (syn. Airora nigellus Melsheimer, 1846; synonymized by whom?). Barron, J. R. 1971: 65 (syn. Airora teres Melsheimer, 1846; synonymized by author). Böving, A. G. & Craighead, F. C. 1931: 273 (larva). Léveillé, A. 1910: 7 (Airora teres Melsheimer, 1846 = syn. Airora aequalis Reitter, 1877; synonymized by
Airora decipiens Léveillé, 1899; Mexico (AL)
Léveillé, A. 1910: 7
Airora dugesi Léveillé, 1890; Mexico (AL)
Léveillé, A. 1910: 9 (Temnochilodes). Kolibáč, J. 2005: 43 (redescription, combination)
Airora ferruginea Léveillé, 1905; Venezuela (AL)
Léveillé, A. 1910: 7
Airora grouvellei Léveillé, 1889; Colombia (AL)
Léveillé, A. 1910: 7
Airora humeralis Léveillé, 1894; Brazil (AL)
Léveillé, A. 1910: 7
Airora longicollis Guérin, 1846; Central and South America (AL)
Léveillé, A. 1910: 7 (syn. Airora clivinoides Reitter, 1876; synonymized by author?)
Airora mathani Léveillé, 1878; Bolivia (AL)
Léveillé, A. 1910: 7
Airora minuta Schaeffer, 1918; USA: Arizona, California (JRB)
Barron, J. R. 1971: 69
Airora modesta Léveillé, 1907; Venezuela (AL)
Léveillé, A. 1910: 7
Airora parallelicollis Léveillé, 1894; Brazil, Venezuela (AL)
Léveillé, A. 1910: 7
Airora pollens Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 7
Airora procera Reitter, 1876; Bolivia, Paraguay (AL)
Léveillé, A. 1910: 7
Airora striatopunctata Reitter, 1876; West Indies, Brazil (AL)
Léveillé, A. 1910: 7
Airora suturata Léveillé, 1894; Brazil (AL)
Léveillé, A. 1910: 7
Airora vicina Léveillé, 1903; Brazil (AL)
Léveillé, A. 1910: 7
Airora wagneri Léveillé, 1907; Argentina (AL)
Léveillé, A. 1910: 8
Airora yucatanica Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 8
http://species-id.net/wiki/Alindria
Figs 1, 6; Map 5Trogossita grandis Serville, 1828 [designated by
Léveillé, A. 1910: 8. Kolibáč, J. 2005: 43 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364
Body size: about 11.0–34.0 mm. Body shape elongate. Gular sutures reduced. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum of males: ctenidium present. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination deep. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, horizontally situated. Mola reduced but present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, strongly retroflexed, deeply emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell triangular, wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 present. Front tibiae: spines along side large. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale absent. Tegmen composed of two parts. Coxitae undivided.
Predatory. Biology unknown; adults are sometimes collected at light.
Disjunctive distribution: most species distributed in tropical Africa and Madagascar; about four south-eastern Asian species are also congeneric. Two unidentified specimens from Argentina (Természettudományi Múzeum Budapest) may be mislabelled.
Alindria alluaudi Léveillé, 1894; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria alutacea Murray, 1867; Old Calabar (AL)
Léveillé, A. 1910: 8
Alindria angusta Léveillé, 1898; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria auberti Léveillé, 1905; China: Sichuan (AL)
Léveillé, A. 1910: 8. Kolibáč, J. 2007a: 364
Alindria australis Péringuey, 1885; South Africa: Cap, Transvaal (AL)
Léveillé, A. 1910: 8
Alindria bicolor Basilewsky, 1956; Rwanda (AD)
Basilewsky, P. 1956: 392
Alindria bouvieri Léveillé, 1898; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria chevrolati Reitter, 1876; Senegal (AL)
Léveillé, A. 1910: 8
Alindria cribrosicollis Léveillé, 1888; Tenasserim (AL)
Léveillé, A. 1910: 8
Alindria cyaneicornis Fairmaire, 1887; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria docorsei Léveillé, 1901; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria elongata Guérin, 1846; Guinea (AL)
Léveillé, A. 1910: 8
Alindria grandis Serville, 1825; Senegal, Cap (varA)
Léveillé, A. 1910: 8 (syn. Alindria cylindrica Olivier, 1792; synonymized by author?). Kolibáč, J. 2005: 43 (redescription). Léveillé, A. 1910: 8 (syn. Alindria ingenicula Gistl & Bromme, 1850; synonymized by author?). Léveillé, A. 1910: 8 (syn. Alindria major Guérin, 1825; syn. by author?)
Alindria lesnei Léveillé, 1907; East Africa (AL)
Léveillé, A. 1910: 8
Alindria orientalis Redtenbacher, 1844; India: Kashmir (AL)
Léveillé, A. 1910: 8
Léveillé, A. 1910: 8 (syn. Alindria parallela Léveillé, 1889; synonymized by
Kolibáč, J. 2007a: 364 (syn. Alindria parallela Léveillé, 1889; synonymized by author)
Alindria ornata Léveillé, 1898; Congo (AL)
Léveillé, A. 1910: 8
Alindria ruandana Basilewsky, 1956; Rwanda (AD)
Basilewsky, P. 1956: 390
Alindria sedilloti Léveillé, 1881; Madagascar (AL)
Léveillé, A. 1910: 8. Léveillé, A. 1910: 8 (syn. Alindria sikorai Kuwert, 1891; synonymized by author?)
Alindria sericea Léveillé, 1898; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria spectabilis Klug, 1833; Madagascar (AL)
Léveillé, A. 1910: 8
Alindria virescens Léveillé, 1907; “India” (varA)
Léveillé, A. 1910: 9. Kolibáč, J. 2007a: 364
http://species-id.net/wiki/Corticotomus
Figs 1, 6; Map 5Corticotomus basalis Sharp, 1891 [designated by
Léveillé, A. 1910: 7. Downie, N. M. & Arnett, R. H. Jr. 1996: 936 (key). Kolibáč, J. 2005: 50 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Lepesme, P. & Paulian, R. 1944: 138 (Colydobius Sharp, 1891) (key). Léveillé, A. 1910: 20 (Colydobius Sharp, 1891)
Colydobius Sharp, 1891 [type species: Colydobius divisus Sharp, 1891; designated by
Léveillé, A. 1910: 20. Kolibáč, J. 2005: 50 (synonymized)
Leveillesoma Lepesme & Paulian, 1944 [type species: Nemosomia fulva Léveillé, 1905; by original designation]
Lepesme, P. & Paulian, R. 1944: 139. Kolibáč, J. 2005: 50 (synonymized)
Parafilumis Casey, 1916 [type species: Parafilumis estriata Casey, 1916; by original designation and monotypy]
Barron, J. R. 1971: 53 (synonymized)
Body size: about 3.0–5.0 mm. Body shape elongate. Gular sutures narrow, subparallel at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination deep. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae present. Mediostipes-Lacinia not fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, horizontally situated. Mola reduced but present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae present. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite sickle shaped. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum elongate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell open (outer vein present), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side large. Hooked spur present. Claws: denticle absent. Spiculum gastrale absent. Tegmen composed of two parts. Coxitae undivided.
Predatory. North American species have been both reared and collected from various species of pine and willow, also from Rhus, Pseudotsuga, and other trees (
Distributed from Brazil to Canada. One species, unknown to me, is described from Chile.
Corticotomus apicalis Van Dyke, 1944; Western USA (JRB)
Barron, J. R. 1971: 60. Dajoz, R. 1997: 42 (biology). Van Dyke, E. C. 1944: 151
Corticotomus basalis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 7. Kolibáč, J. 2005: 50
Corticotomus bicolor Léveillé, 1895; Chile (AL)
Léveillé, A. 1910: 7
Corticotomus californicus Van Dyke, 1915; Western USA (JRB)
Barron, J. R. 1971: 60 (syn. Parafilumis estriata Casey, T. L. 1916: 207, 283; synonymized by whom?). Van Dyke, E. C. 1915: 28. Schaeffer, C. F. A. 1920: 193
Corticotomus caviceps Fall, 1910; Western Canada and USA (JRB, varA)
Barron, J. R. 1971: 58. Dajoz, R. 1997: 41 (diagnosis). Barron, J. R. 1971: 58 (syn. Corticotomus laeviventris Casey, 1916; synonymized by whom?). Fall, H. C. 1910: Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str. caviceps Fall, 1910)
Corticotomus cylidricus LeConte, 1863; Eastern USA: Iowa (JRB)
Léveillé, A. 1910: 5 (Nemosoma). Barron, J. R. 1971: 57 (syn. Corticotomus cylindricus var. texanus Schaeffer, 1918). Schaeffer, C. F. A. 1920: 193 (Corticotomus cylindricus subsp. texanus Schaeffer, C. F. A. 1918: 192). Barron, J. R. 1971: 56. Van Dyke, E. C. 1915: 26. Kolibáč, J. 2005: 51 (redescription). Böving, A. G. & F. C. Craighead, 1931: 273 (larva). Reitter, E. 1876: 14 (Nemosoma). Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str.)
Corticotomus depressus Schaeffer, 1918; Eastern USA (JRB)
Barron, J. R. 1971: 54. Schaeffer, C. F. A. 1918: 192
Corticotomus divisus Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 20 (Colydobius). Kolibáč, J. 2005: 51 (combination)
Corticotomus dufaui Léveillé, 1907; Guadeloupe (AL)
Léveillé, A. 1910: 20. Kolibáč, J. 2005: 52 (redescription)
Corticotomus fulva Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 4 (Nemosomia). Kolibáč, J. 2005: 50 (combination). Lepesme, P. & Paulian, R. 1944: 139 (Nemosomia) (combined with Leveillesoma)
Corticotomus parallelum Melsheimer, 1844; Eastern USA (JRB)
Léveillé, A. 1910: 6 (Nemosoma). Barron, J. R. 1971: 55. Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma). Reitter, E. 1876: 14 (Nemosoma)
Corticotomus quadrimaculatus Léveillé, 1894; Brazil (AL)
Léveillé, A. 1910: 7
Corticotomus sharpi Léveillé, 1905; Mexico (AL)
Léveillé, A. 1910: 7
Corticotomus signatus Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 20
Corticotomus testaceus Dajoz, 1997; USA: Arizona (AD)
Dajoz, R. 1997: 40
http://species-id.net/wiki/Cretocateres
Fig. 19; Map 5Cretocateres mongolicus Ponomarenko, 1986 [by monotypy and author’s designation]
Kolibáč, J. 2006: 116 (classification). Ponomarenko, A. G. 1986: iii (1–101) (Lophocateridae). Ponomarenko, A. G. 1990: 73, 74. Kolibáč, J. 2006: 116 (Trogossitidae: Trogossitini). Ponomarenko, A. G. & Kireichuk, A. G. (2005–2008): http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm (Peltidae). Schmied, H. et al. 2009: 24
Body size: probably about 3.0 mm (no scale provided). Body elongate, terminal antennomeres conspicuously asymmetrical. Very similar to Siberian Thoracotes (see
Mesozoic: Lower Cretaceous; West Mongolia.
† Cretocateres mongolicus Ponomarenko, 1986; W Mongolia; Lower Cretaceous (varA)
Ponomarenko, A. G. 1986: iii (1–101) (Lophocateridae). Ponomarenko, A. G. 1990: 73, 74. Kolibáč, J. 2006: 116 (Trogossitidae: Trogossitini). Ponomarenko, A. G. & Kireichuk, A. G. (2005–2008): http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm (Peltidae). Schmied, H. et al. 2009: 24
http://species-id.net/wiki/Dupontiella
Figs 20–23; Map 5Dupontiella ichneumonoides Spinola, 1844 [designated by
Léveillé, A. 1910: 6. Kolibáč, J. 2005: 54. Kolibáč, J. 2006: 116 (phylogeny). Reitter, E. 1876: 15
Body size: about 4.0–5.0 mm. The genus was originally described in Cleridae.
The 11-segmented antennae with distinct 3-segmented asymmetrical club, as well as perhaps a tri-sinuate anterior margin to the frons, probably indicate proper classification within Trogossitinae, perhaps related to Nemozoma and allied genera. Spinola’s note “[...] les mandibles de la forme ordinaire, la face antérieurement arrondie et non tri-échancrée[...]” could mean that the mandibles are unidentate. Some species, possibly only specimens of Corticotomus, are the only known trogossitids with mandibles that may be unidentate (
Venezuela.
Dupontiella fasciatella Spinola, 1844; Venezuela: Caracas (AL)
Léveillé, A. 1910: 6. Reitter, E. 1876: 16
Dupontiella ichneumonoides Spinola, 1844; Venezuela: Caracas (AL)
Léveillé, A. 1910: 6. Kolibáč, J. 2005: 54. Reitter, E. 1876: 15
http://species-id.net/wiki/Elestora
Fig. 6; Map 5Elestora fulgurata Pascoe, 1868 [by monotypy]
Léveillé, A. 1910: 20. Kolibáč, J. 2005: 55. Kolibáč, J. 2006: 116 (phylogeny). Reitter, E. 1876: 30
The phylogeny of the genus is unclear and in need of revision, together with Melambia. Elestora could be related to some species of the latter genus (see “Remarks” on Melambia). I presume a classification of Elestora within the trogossitins rather than with the gymnochilins.
Body size: 15.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum of males: ctenidium present. Antennal groove present. Eyes: size flat. Eyes number: two. Mandibular apical teeth number: one. Labrum-Cranium not fused. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 present. Front tibiae: spines along side moderate. Hooked spur absent in middle and hind tibiae. Claws: denticle absent.
Probably predatory. A specimen was observed on a smouldering log at the margin of a tropical forest clearing in Laos. Its light orange spots on the elytra (much duller in a few museum specimens) perfectly imitate wood embers. Very rapid flier.
Distributed from Malaysia to northern Laos but perhaps very rare.
Elestora fulgurata Pascoe, 1868; Malaysia: Penang, Laos (varA)
Léveillé, A. 1910: 20. Kolibáč, J. 2005: 55. Kolibáč, J. 2006: 152. Reitter, E. 1876: 30
http://species-id.net/wiki/Eotenebroides
Map 5Eotenebroides tumoculus Ren, 1995
Ren, D. 1995: 89 [species description in Chinese only]. Kolibáč, J. & Huang, D.-Y. 2008: 141 (Trogossitini)
“Head triangular, longer than wide. Eyes distinct, larger, situated laterally at median. Last 3 segments of antennae not thickened. Basal part of lateral margin of pronotum not narrowed. Scutellum larger. Coxa of middle and hind legs very close. Elytra not longer than abdomen, surface with 5 visible longitudinal streaks. Length 6.1 mm, width 2.4 mm.” (
“Head: head slightly longer than wide; mandible robust, slightly curved; antenna with 11 segments, scapus a littler longer than others, length of other segments similar to one another, last three segments not thickened; eyes large, around 1/3 as long as head (excluding mandible), not projecting, clearly separated from front edge of pronotum. Thorax: pronotum longer than wide, anterior edge concave, anterio-lateral angles projecting, sharp, posterio-lateral angles rounded, posterior edge straight, as long as anterior edge; connection of pronotum with elytra forming a neck; scutellum large, triangular; metepisternum visible, not making contact with mid-coxa; front coxae transverse, well-separated, femur thick; middle coxae rounded, close to one another, femur thicker than tibia; hind coxae nearly rounded, close to one another, hind femur as wide as middle femur. Elytra: elytra does not reach end of abdomen, its base as wide as pronotum, equipped with 5 longitudinal ridges. Abdomen: 6 sternites visible.” (
China: SW of Beijing, Chongqing reservoir; Mesozoic: Lower Cretaceous, Lushangfen formation.
† Eotenebroides tumoculus Ren, 1995; China: SW of Beijing; Lower Cretaceous (AD)
Ren, D. 1995: 88 [species description in Chinese only]. Kolibáč, J. & Huang, D.-Y. 2008: 141
Eupycnus lentus Sharp, 1891 [by monotypy]
Léveillé, A. 1910: 14. Kolibáč, J. 2005: 56 (redescription). Kolibáč, J. 2006: 116 (phylogeny)
Body size: about 7.5 mm. Body shape elongate. Gular sutures wide, convergent at apex. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination moderate. Mandibular apical teeth number: two, vertically situated. Labrum-Cranium not fused. Ligula: ciliate setae present. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum subquadrate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell open (outer vein present), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side large. Hooked spur present in all tibiae. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale present. Tegmen composed of three parts.
A Eupycnus lentus B Melambia crenicollis C Melambia striata D Leipaspis lauricola E Nemozoma cornutum F Nemozoma elongatum G Nemozoma caucasicum H Parallelodera quadraticollis I Temnoscheila pini.
Predatory. The single described species probably has the same way of life as Tenebroides species.
Mexico. One undescribed species is known to me from Cuba.
Eupycnus lentus Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 14. Kolibáč, J. 2005: 56 (redescription)
http://species-id.net/wiki/Euschaefferia
Map 5Stenodema hicoriae Schaeffer, 1918 [by original designation and monotypy]
Barron, J. R. 1971: 62. Kolibáč, J. 2005: 57. Kolibáč, J. 2006: 116 (phylogeny)
Pseudocotomus Van Dyke, 1944 [type species: Pseudocotomus mclayi Van Dyke, 1944; by original designation]
Van Dyke, E. C. 1944: 153. Barron, J. R. 1971: 62 (synonymized)
Stenodema Schaeffer, 1918 (preoccupied) [type species: Stenodema hicoriae Schaeffer, 1918; by original designation]
Barron, J. R. 1971: 62. Leng, C. W. 1920: 193. Schaeffer, C. F. A. 1918: 193
Body size: 2.0–3.0 mm. Body shape elongate. Frons: longitudinal groove or depression absent. Labrum-Cranium not fused. Antenna 11-segmented. Antennal club asymmetrical. Pronotum elongate. Elytra: long hairs absent, carinae reduced. Elytral punctation irregular, scales absent. Front tibiae: spines along side reduced. Hooked spur absent in middle and hind tibiae. Claws: denticle absent.
Predatory. It was recorded from the galleries of the bark beetle Pseudothysanoes burtoni and has also been reared on the plant Vachellia farnesiana (
Belt of the southern states of the USA.
Euschaefferia hicoriae Schaeffer, 1918; USA: Texas, North Carolina (JRB)
Barron, J. R. 1971: 63. Kolibáč, J. 2005: 57. Schaeffer, C. F. A. 1918: 193
Euschaefferia mclayi Van Dyke, 1944; USA: California (JRB)
Barron, J. R. 1971: 63 (comb). Van Dyke, E. C. 1944: 153. (Pseudocotomus)
http://species-id.net/wiki/Leipaspis
Figs 1, 7; Map 5Leipaspis lauricola Wollaston, 1862 [designated by
Léveillé, A. 1910: 14 (Lipaspis). Kolibáč, J. 2005: 63 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364. Reitter, E. 1876: 27 (Lipaspis)
Body size: about 8.5–9.5 mm. Body shape elongate. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae present. Mediostipes-Lacinia not fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae present. Ligula rigid, not retroflexed, deeply emarginate. Hypopharyngeal sclerite sickle shaped. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum elongate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Coxitae divided.
Predatory. The species was collected on the stems of various plants, such as Euphorbia sp., Pinus sp., Suadea vermiculata, Arthrocnemum fruticosum, Laurus nobilis, and Nicotiana glauca, probably preying on anobiids (Xestobium) or other xylophagous insects.
Canary Islands.
Leipaspis caulicola Wollaston, 1862;
Léveillé, A. 1910: 14 (Lipaspis). Plata-Negrache, P. & Prendes-Ayala, C. 1981: 227. Reitter, E. 1876: 27 (Lipaspis)
Leipaspis caulicola caulicola Wollaston, 1862; Canary Islands (varA)
Kolibáč, J. 2007a: 364
Leipaspis caulicola oceanica Wollaston, 1865; Madeira: Selvagens (varA)
Erber, D. & Wheater, C. P. 1987: 166 (Leipaspis caulicola var. oceanica Wollaston, 1865). Kolibáč, J. 2007a: 364 (subspecies)
Leipaspis lauricola Wollaston, 1862; Canary Islands (varA)
Léveillé, A. 1910: 14 (Lipaspis). Kolibáč, J. 2005: 63. Reitter, E. 1876: 27 (Lipaspis)
Leipaspis lauricola lauricola Wollaston, 1862; Canary Isl: La Palma, Tenerife (varA)
Plata-Negrache, P. & C. Prendes-Ayala 1981: 229. Kolibáč, J. 2007a: 364
Leipaspis lauricola gomerensis Plata & Prendes, 1981 Canary Isl.: La Gomera, El Hierro (varA)
Kolibáč, J. 2007a: 364. Machado, A. & Oromí, P. 2000: ii. Plata-Negrache, P. & Prendes-Ayala, C. 1981: 229
Leipaspis pinicola Wollaston, 1862; Canary Islands (varA)
Léveillé, A. 1910: 14 (Lipaspis). Kolibáč, J. 2007a: 364. Plata-Negrache, P. & Prendes-Ayala, C. 1981: 229. Reitter, E. 1876: 27
http://species-id.net/wiki/Melambia
Fig. 7; Map 5Trogossita gigas Fabricius, 1798 [designated by
Léveillé, A. 1910: 9. Kolibáč, J. 2005: 68 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364. Mamaev, B. M. 1976: 1652 (larva). Reitter, E. 1876: 24
The placement of the genus in Trogossitini should be revised because my analysis of 2008 disclosed a possible relationship of some its species with Gymnochilini. There are distinct differences in the body shape among the numerous species of Melambia, for example between Melambia grandis (a robust species with a cordate pronotum) and Melambia orientalis (an elongate species with pronotum shaped somewhat like that of Tenebroides). Consideration of a re-classification of Seidlitzella within Gymnochilini, similar in habitus to Melambia, needs species revision and new phylogenetic analysis with reference to Trogossitini and Gymnochilini, including special attention to the related trogossitine genus Alindria. As a preliminary opinion, I assume that both genera, Melambia and Alindria, form a basal group of Trogossitini.
Body size: about 20.0–30.0 mm. Body shape elongate. Gular sutures reduced. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum of males: ctenidium present. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape clavate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, vertically situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, weakly retroflexed, deeply emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism present, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale absent. Tegmen composed of two or three parts.
Larva: Frontal arms V-shaped. Epicranial stem present. Endocarina present. Stemmata number: five. Maxillary palpi 3-segmented. Palpifer absent. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes not fused. Cardo: size much smaller than stipes. Ligula absent. Labial palpi 2-segmented. Prementum in single part, anterior margin with notch. Thoracic sclerites pattern (dorsally) 2-0-0. Abdominal segment IX not divided. Tergite IX flat. Urogomphi minute; median process absent.
Predatory. According to
South-eastern, southern and central Asia; also several species in Africa from Egypt to South Africa. Such a disjunctive distribution is possible, but the African species need to be checked because of possible confusion with the similar genus Alindria.
Melambia cardoni Léveillé, 1908; India: “Bengalia” (AL)
Léveillé, A. 1910: 9
Melambia cordicollis Reitter, 1876; Philippines (AL)
Léveillé, A. 1910: 9. Reitter, E. 1876: 25
Melambia crenicollis Guérin, 1846; India: “Bengalia” (AL)
Léveillé, A. 1910: 9
Melambia funebris Pascoe, 1862; Cambodia (AL)
Léveillé, A. 1910: 9. Reitter, E. 1876: 25
Melambia gautardi Tournier, 1872; Egypt (AL)
Léveillé, A. 1910: 9. Kolibáč, J. 2007a: 364. Reitter, E. 1876: 26
Melambia gigas Fabricius, 1798; Guinea, Senegal (AL)
Léveillé, A. 1910: 9. Kolibáč, J. 2005: 68 (redescription). Reitter, E. 1876: 25
Melambia maura Pascoe, 1862; South Africa: “N’gami”, Mauritania (varA)
Léveillé, A. 1910: 9. Mateu, J. 1972: 547
Melambia memnonia Pascoe, 1862; Sri Lanka (AL)
Léveillé, A. 1910: 9
Melambia opaca Reitter, 1876; South Africa: Cap (AL)
Léveillé, A. 1910: 9. Reitter, E. 1876: 25
Melambia pumila Léveillé, 1885; Burma (AL)
Léveillé, A. 1910: 9
Melambia striata Olivier, 1790; Senegal (varA)
Léveillé, A. 1910: 9. Mateu, J. 1972: 547. Reitter, E. 1876: 24
Melambia subcyanea Gerstaecker, 1871; Zanzibar (AL)
Léveillé, A. 1910: 9. Reitter, E. 1876: 26
Léveillé, A. 1910: 9 (syn. Melambia coeruleata Fairmaire, 1882) Somalia (AL)
Melambia tekkensis Koenig, 1889; „Transcaspia”, Turkmenistan (varA)
Léveillé, A. 1910: 9. Kolibáč, J. 2007a: 364. Mamaev, B. M. 1976: 1650 (larva)
http://species-id.net/wiki/Nemozoma
Figs 7, 15; Map 5Dermestes elongatus Linnaeus, 1760 [by monotypy]
Léveillé, A. 1910: 5. Barron, J. R. 1971: 45. Crowson, R. A. 1964a: 299. Kolibáč, J. 2005: 72 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 364. Kolibáč, J. et al. 2005: 31+135 (key). Lepesme, P. & Paulian, R. 1944: 139 (subgen. Nemosoma s.str.). Lucht, W. 1998: 207 (key). Mamaev, B. M. 1976: 1652 (larvae). Nikitsky, N. B. 1974: 563. Reitter, E. 1876: 13
Aponemosoma Lepesme & Paulian, 1944 (subgenus) [type species: Nemosoma caucasicum Ménétries, 1832; by original designation]
Barron, J. R. 1971: 45 (synonymized). Kolibáč, J. 2007a: 364. Lepesme, P. & Paulian, R. 1944: 139 (Nemosoma subgen. Aponemosoma)
Cylidrella Sharp, 1891 [type species: Cylidrellamollis Sharp, 1891; by monotypy]
Léveillé, A. 1910: 6. Barron, J. R. 1971: 52. Kolibáč, J. 2005: 72 (synonymized). Kolibáč, J. 2007a: 364
Monesoma Léveillé, 1894 (subgenus) [type species: Nemosoma cornutum Sturm, 1826; by original designation]
Léveillé, A. 1910: 6 (subgenus). Barron, J. R. 1971: 45 (=Sturmia Ragusa, 1892). Kolibáč, J. 2007a: 364. Lepesme, P. & Paulian, R. 1944: 139 (synonymized)
Nemosomia Reitter, 1876 [type species: Nemosomia vorax Reitter, 1876; by original designation]
Léveillé, A. 1910: 4. Barron, J. R. 1971: 45. Kolibáč, J. 2007a: 364. Lepesme, P. & Paulian, R. 1944: 139 (subgenus). Reitter, E. 1876: 11
Paranemosoma Lepesme & Paulian, 1944 (subgenus) [type species: Nemosoma punctatum Léveillé, 1886; by original designation]
Lepesme, P. & Paulian, R. 1944: 139. Kolibáč, J. 2007a: 364
Sturmia Ragusa, 1892 [type species: Nemosoma cornutum Sturm, 1826; by original designation]
Barron, J. R. 1971: 45 (preoccupied, synonymized with Monesoma). Kolibáč, J. 2007a: 364
Pseudalindria Fall, 1910 [type species: Pseudalindria fissiceps Fall, 1910; by original designation]
Barron, J. R. 1971: 45. Fall, H. C. 1910: 126. Kolibáč, J. 2007a: 364
Body size: about 2.5–5.0 mm. Body shape elongate. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression present. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae present. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, vertically situated. Mola absent. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae present. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented or 10-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum elongate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell open (outer vein present), wedge cell absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side large. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
Larva: Frontal arms V-shaped. Epicranial stem reduced. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes absent. Paragular sclerites present. Stemmata number: five. Mandibular apical teeth number: one tooth. Lacinia mandibulae with several small spines. Mola absent. Maxillary palpi 3-segmented. Palpifer absent. Pedunculate seta present. Mala simple. Mala: bidentate protrusion absent. Cardo: size much smaller than stipes. Ligula absent. Labial palpi 2-segmented. Prementum in single part, anterior margin with notch. Antennal joints 1 and 2 elongate. Sensory appendix medium sized (to half of joint 3). Thoracic sclerites pattern (dorsally) 1-2-2. Thoracic sclerites pattern (ventrally) 3+1+1. Trochanter triangular. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
Predatory. Adults live together with larvae under the bark of deciduous and coniferous trees and shrubs, hunting especially for bark beetles.
From Brazil to Canada, Europe including European part of Russia, Near East (Turkey, Syria), North Africa. No records are known from Asia east of Caucasus.
Nemozoma alasanicum Fursov, 1930; Georgia (varA)
Fursov, N. I. 1930: 183. Lepesme, P. & Paulian, R. 1944: 140 (subgen. Nemosoma s. str.)
Nemozoma attenuatum Van Dyke, 1915; USA: California, Washington (JRB)
Barron, J. R. 1971: 47. Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str.). Sakamoto, J. M. 2007: 342 (distribution). Van Dyke, 1915: 26
Nemozoma brasiliense Léveillé, 1900; Brazil: Jatahy (varA)
Lepesme, P. & Paulian, R. 1944: 138 (combined from Monesoma)
Nemozoma breviatum Peyerimhoff, 1918; Algeria (varA)
Peyerimhoff, P. M., de 1918: 329. Hallan, J. 2007–2012: http://insects.tamu.edu (syn. of Nemozoma elongatum?). Kolibáč, J. 2009: 128
Note: Brustel, H. (pers. comm., 2011) newly recorded 15 specimens from North Africa; he compared Palaearctic species and found Nemozoma breviatum valid.
Nemozoma caucasicum Ménétries, 1832; Austria, Poland, SW Russia, Slovakia, Ukraine, „Caucasus” (varA)
Léveillé, A. 1910: 6 (Monesoma). Hilszczanski, J. 2006: 29. Klausnitzer, B. 1996: 148 (larva). Kolibáč, J. 1993b: 90. Kolibáč, J. 1993a: 19, 21. Kolibáč, J. 2007a: 364 (syn. Nemozoma fasciicole Hampe, 1864). Lepesme, P. & Paulian, R. 1944: 141 (Aponemosoma). Mamaev, B. M. 1976: 1653 (larva). Milkowski, M. & Wojas, T. 2008: 172 (distribution). Nikitsky, N. B. 1974: 566 (larva). Pankow, W. 2010: 87 (distribution). Reitter, E. 1876: 13
Kolosov, ? 1931: 116 (syn. Nemosoma curtulum Fursov, 1930); „Chernyi les” (varA)
Fursov, N. I. 1930: 182 (Nemosoma curtulum Fursov, 1930; synonymized by Kolosov 1931: 116 after
Nemozoma championi Wickham, 1916; Western USA: Colorado, New Mexico (JRB)
Barron, J. R. 1971: 52 (Cylidrella). Wickham, 1916: 147
Nemozoma cornutum Sturm, 1826; SW Russia, Ukraine, „Caucasus” (varA)
Léveillé, A. 1910: 6. Klausnitzer, B. 1996: 148 (larva). Kolibáč, J. 2007a: 364. Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str.). Mamaev, B. M. 1976: 1654 (larva). Moragues, G. 1981: 262–263 (distribution). Nikitsky, N. B. 1974: 566 (larva). Reitter, E. 1876: 14. Sarikaya, O. & Avci, M. 2009: 253–264 (biology)
Nemozoma cupressi Van Dyke, 1944; USA: Nothern coast of California (JRB)
Barron, J. R. 1971: 51. Van Dyke, E. C. 1944: 147, 149
Nemozoma cylindricolle Fursov, 1930; Georgia (varA)
Fursov, N. I. 1930: 182. Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str.)
Nemozoma elongatum Linnaeus, 1761; Europe, Syria, Turkey, Tunisia (JK)
Léveillé, A. 1910: 5. Baader, E. J. 1989: 1 (biology). Bahillo de la Puebla P. & López-Colón, J. I. 2004: 129. Baier, P. 1994: 51 (biology). Baier, P. 1991: 421 (biology). Borowiec, L. 1983: 8. Burakowski, B., Mroczkowski, M. & Stefanska, J. 1986: 115. Conrad, R. 1995: 190–195 (contribution). Cunev, J. 1999: 76. Dippel, C. 1991: 473 (biology). Dippel, C. 1995: 67 (biology). Dippel, C. 1996: 391 (biology). Dippel, C. et al. 1997: 161 (biology). Gobbi, G. 1983: 52. Gueorguiev, B. et al. 2003: 107 (distribution). Heuer, H. & Vite, J. P. 1984a: 214 (biology). Heuer, H. & Vite, J. P. 1984b: 586 (biology). Hallan, J. 2007–2012: 4 (syn. Nemosoma breviatum Peyrimhoff, 1918?). Klausnitzer, B. 1976: 5. Klausnitzer, B. 1978: 176. Klausnitzer, B. 1996: 146 (larva). Kolibáč, J. 1993a: 21. Kolibáč, J. 1993b: 90. Kolibáč, J. 1996: 473. Kolibáč, J. 2005: 72 (redescription). Kolibáč, J. 2007a: 365 (syn. Nemozoma fasciatum Panzer, 1796; in Colydium). Kolibáč, J. 2007a: 364 (syn. Nemozoma corsicum Reitter, 1876). Kolibáč, J. 2007a: 365 (syn. Nemozoma siculum Ragusa, 1891). Kolibáč, J. 2007a: 365 (syn. Nemozoma syriacum Pic, 1901). Kolibáč, J. 2007a: 365 (syn. Nemozoma elongatum var. tuniseum Pic, 1900). Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str.). Mamaev, B. M. 1976: 1654 (larva). Mitter, H. 1998: 559. Nikitsky, N. B. 1974: 566 (larva). Peyrimhoff, 1918: 329 (Nemozoma breviatum; valid species: H. Brustel, pers. comm. 2011). Pileckis, S. & V. Monsevičius 1995: 271. Reitter, E. 1876: 13 (syn. Nemozoma corsicum Ratti, E. 1997: 179. Reitter, 1876; synonymized by Léveillé 1889: 8). Skatulla, U. & Feicht, E. 1992: 4 (biology). Szafraniec, S. 1997: 207 (distribution). Vogt, H. 1967: 15. Wigger, H. 1993: 68 (biology). Wigger, H. 1994: 8 (biology). Wigger, H. 1996: 55 (biology)
Nemozoma fissiceps Fall, 1910; USA: California, Oregon (JRB)
Barron, J. R. 1971: 49. Fall, H. C. 1910: 127
Nemozoma gounellei Léveillé, 1894; Brazil (AL)
Léveillé, A. 1910: 6
Nemozoma landesi Léveillé, 1901; Martinica (AL)
Léveillé, A. 1910: 4. Lepesme, P. & Paulian, R. 1944: 139 (subgen. Nemosomia)
Nemozoma maculata Dajoz, 1991; USA (AD)
Dajoz, R. 1991: 245 (Cylidrella)
Nemozoma mollis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 6 (Cylidrella). Kolibáč, J. 2005: 73 (redescription, combination)
Nemozoma picta Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 4 (Nemosomia)
Nemozoma pujoli Léveillé, 1902; Brazil (AL)
Léveillé, A. 1910: 4
Nemozoma punctatum Léveillé, 1888; Brazil (AL)
Léveillé, A. 1910: 6. Lepesme, P. & Paulian, R. 1944: 140
Nemozoma punctulata Van Dyke, 1920; Canada: British Columbia to USA: California (JRB)
Barron, J. R. 1971: 49. Lepesme, P. & Paulian, R. 1944: 140. Van Dyke, E. C. 1916: 71, 72 (described as punctatum). Van Dyke, E. C. 1920: 85 (jun. homonym; renamed)
Nemozoma schwarzi Schaeffer, 1918; USA: Arizona, California (JRB)
Barron, J. R. 1971: 48. Lepesme, P. & Paulian, R. 1944: 140 (Nemosoma s.str.). Schaeffer, C. F. A. 1918: 191 (subgen. Monesoma)
Nemozoma signatum Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 6
Nemozoma simoni Léveillé, 1889; Venezuela (AL)
Léveillé, A. 1910: 4 (Nemosomia). Lepesme, P. & Paulian, R. 1944: 140
Nemozoma vorax Reitter, 1876; Columbia (AL)
Léveillé, A. 1910: 4 (Nemosomia). Lepesme, P. & Paulian, R. 1944: 141. Reitter, E. 1876: 12 (Nemosomia)
http://species-id.net/wiki/Parallelodera
Fig. 7; Map 5Parallelodera quadraticollis Fairmaire, 1881 [by monotypy]
Léveillé, A. 1910: 8. Kolibáč, J. 2005: 74 (redescription). Kolibáč, J. 2006: 116 (phylogeny)
Body size: about 11.0–12.0 mm. Body shape elongate. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression present. Cranium ventrally: tufts of long setae at sides present. Submentum of males: ctenidium absent. Antennal groove present. Eyes: size flat. Eyes number: two. Mandibular apical teeth number: two, vertically situated. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum subquadrate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell open (outer vein present), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side large. Hooked spur present in all tibiae. Claws: denticle absent.
In the light of a presumable relationship with Airora and Temnoscheila, I suggest a predatory way of life.
Pacific islands as listed with the particular species.
Parallelodera luteicornis Fairmaire, 1881; Fiji, Viti Isl. (AL)
Léveillé, A. 1910: 8. Kolibáč, J. 2005: 75
Parallelodera parallelus Fairmaire, 1850; New Caledonia, Madera, Nuka-Hiva, Tahiti (JK)
Léveillé, A. 1910: 18 (Tenebroides). Léveillé, A. 1910: 18 (syn. Tenebroides serratus Wollaston, 1854). Kolibáč, J. 2005: 75 (combination)
Parallelodera quadraticollis Fairmaire, 1881; Fiji, Viti Isl. (AL)
Léveillé, A. 1910: 8. Kolibáč, J. 2005: 74 (redescription)
http://species-id.net/wiki/Temnoscheila
Figs 7, 8; Map 5Trogossita caerulea Olivier, 1790 [by monotypy]
Downie, N. M. & Arnett, R. H., Jr. 1996: 937 (key). Kolibáč, J. 2005: 83 (redescription). Kolibáč, J. 2006: 109 (review of larvae), 111 (phylogeny). Kolibáč, J. 2007a: 365. Spahr, U. 1981: 74 (amber and copal fossils)
Temnochila Erichson, 1844 (unjustified emendation by
Léveillé, A. 1910: 9. Barron, J. R. 1971: 70. Kolibáč, J. 2007a: 365 (unjustified emendation). Nikitsky, N. B. 1992: 80
Trogossita Olivier, 1790 [type species: Trogossita caerulea Olivier, 1790]
Barron, J. R. 1971: 70. Crowson, R. A. 1964a: 299. Mamaev, B. M. 1976: 1652 (larva). Matthews, E. G. 1992: 3. Reitter, E. 1876: 26 (Trogosita)
Body size: about 9.0–26.0 mm. Body shape elongate. Gular sutures reduced. Frontoclypeal suture absent. Frons: longitudinal groove or depression present. Cranium ventrally: tufts of long setae at sides present. Submentum of males: ctenidium present. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination deep. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae present. Mediostipes-Lacinia not fused. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, vertically situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae absent. Ligula rigid, weakly retroflexed, deeply emarginate. Hypopharyngeal sclerite sickle shaped. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum elongate. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell triangular, wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Coxitae undivided.
Larva: Frontal arms V-shaped. Epicranial stem reduced. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes absent. Paragular sclerites present. Hypostomal rods present. Stemmata number: five. Mandibular apical teeth number: two, horizontally even, vertically situated. Lacinia mandibulae with several small spines. Mola absent. Maxillary palpi 3-segmented. Palpifer absent. Pedunculate seta present. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes partially fused. Cardo: size much smaller than stipes. Ligula present. Labial palpi 2-segmented. Prementum in single part, anterior margin projecting. Torma: two separate lateral sclerites. Antennal joints 1 and 2 elongate. Sensory appendix very small. Thoracic sclerites pattern (dorsally) 1-2-2. Thoracic sclerites pattern (ventrally) 3+1+1. Trochanter oblong. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
A Temnoscheila smaragdina B Temnoscheila caerulea C Temnoscheila punctatissima D Tenebroides mauritanicus E Tenebroides ruber F Tenebroides bipustulatus G Tenebroides fossulatus.
Predatory. Adults hunt for xylophagous beetles on logs and branches of various trees and shrubs. Larvae live mostly under bark but sometimes dwell on the surface of wood as well.
The bulk of species are distributed in the two Americas. Only a few species spread through to the Palaearctic region. Several species tend to cosmopolitanism (Temnoscheila caerulea, Temnoscheila virescens).
Temnoscheila acuta LeConte, 1858; Texas (JRB)
Léveillé, A. 1910: 10 (Temnochila). Barron, J. R. 1971: 85 (Temnochila)
Temnoscheila aenea Olivier, 1790; Brazil, Porto Rico (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila aerea LeConte, 1858; Guatemala (JRB)
Léveillé, A. 1910: 10 (Temnochila). Barron, J. R. 1971: 78 (Temnochila). Barron, J. R. 1971: 78 (syn. Temnochila virescens var. nyentia Dow, 1912). Dow, R. P. 1912: 70
Temnoscheila alticola Sharp, 1891
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila aureola Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 10
Temnoscheila aurora Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila barbata LeConte, 1863; “Cap S. Lucas” (JRB)
Léveillé, A. 1910: 10 (Temnochila). Barron, J. R. 1971: 73 (Temnochila)
Temnoscheila bedeli Léveillé, 1889; Venezuela (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila belti Sharp, 1891; Nicaragua (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila biolleyi Léveillé, 1903; Costa Rica (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila boboensis Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 10
Temnoscheila boliviensis Léveillé, 1903; Bolivia (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila borrei Reitter, 1875; Antilles, Colombia (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila brevior Léveillé, 1889; Colombia (AL)
Léveillé, A. 1910: 10 (Temnochila)
Temnoscheila caerulea Olivier, 1790; South Europe, Southwestern Asia, North Africa, China (JK)
Léveillé, A. 1910: 10. Bahillo de la Puebla P. & López-Colón, J. I. 1999: 13. Sarikaya, O. & Avci, M. 2009: 129. Borowiec, L. 1983: 10. Burakowski et al. 1986: 116. Crowson, R. A. 1972: 339 (Trogossita). Gobbi, G. 1983: 51 (Temnochila). Klausnitzer, B. 1976: 5 (Temnochila). Kolibáč, J. 1993a: 21 (Temnochila). Kolibáč, J. 1993b: 90 (Temnochila). Mitter, H. 1998: 560 (Temnochila). Kolibáč, J. 2005: 83 (redescription)
Kolibáč, J. 2007a: 36 (syn. Temnoscheila gemella Bedel, 1900; described as species; synonymized by
Kolibáč, J. 2007a: 365 (syn. Temnoscheila pini Brullé, 1838); Canary Isl. (JK)
Kolibáč, J. 2007a: 365 (syn. Temnoscheila rogenhoferi Reitter, 1875) „India or.” (JK)
Klausnitzer, B. 1978: 176 (Temnochila). Léveillé, A. 1910: 11 (Temnochila caerulea var. asiatica Léveillé, 1908); Yunnan (AL)
Léveillé, A. 1910: 10 (syn. Temnochila rogenhoferi Reitter, 1875)
Léveillé, A. 1910: 11 (Temnochila var. gemella Bedel, 1900) Algeria (AL)
Mamaev, B. M. 1976: 1655 (Trogossita) (larva). Pajares, J. A. et al. 2004: 633 (biology). Whitehouse, N. J. 1997: 293 (biology). Vogt, H. 1967: 15 (Temnochila)
Temnoscheila caerulea pini Brullé, 1838; Canary Isl. (varA)
Plata-Negrache, P. & C. Prendes-Ayala 1981: 226
Temnoscheila chalcea Kirsch, 1873; Peru, America centr. (AL)
Léveillé, A. 1910: 10
Temnoscheila championi Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 10
Temnoscheila chevrolati Reitter, 1875; Brazil: „Cayenna”, America centr. (AL)
Léveillé, A. 1910: 10
Temnoscheila chiriquensis Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 10
Temnoscheila chlorodia Mannerheim, 1843; Western USA, Western Canada (JRB)
Barron, J. R. 1971: 82 (syn. Temnochila prosternalis Schaeffer, 1918; synonymized by
Boone, C. K. S. et al. 2008: 411 (biology). Dahlsten, D. L. et al. 2004: 1554 (biology). DeMars, C. J. Jr. et al. 1986: 881 (biology). Dominguez-Sanchez, B. et al. 2008: 175 (biology). Gaylord, M. L. W. et al. 2008: 57 (biology). Goheen, D. J. et al. 1985: 1535 (biology). Fettig, Ch. J. & Dabney, Ch. P. 2006: 75 (biology). Fettig, Ch. J. et al. 2004: 490 (biology). Fettig, Ch. J. et al. 2005: 748 (biology). Fettig, Ch. J. et al. 2007: 141 (biology). Léveillé, A. 1910: 13 (Temnoscheila virescens). Marsden, M. A. et al. 1981: 1 (biology). Miller, D. R. et al. 1997: 2013 (biology). Ross, D. W. & Daterman, G. E. 1998: 500 (biology). Swezey, S. L. & Dahlsen, D. L. 1982: 142 (biology). Williams, K. K. et al. 2009: 351 (biology). Zhou, J. et al. 2001: 993 (biology)
Temnoscheila chrysosterna Reitter, 1875; Brazil: „Cayenna” (AL)
Léveillé, A. 1910: 10
Temnoscheila colossus Serville, 1828; Colombia, Brazil: „Cayenna” (AL)
Léveillé, A. 1910: 11
Temnoscheila corinthia Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 11
Temnoscheila costaricensis Sharp, 1891; Costarica (AL)
Léveillé, A. 1910: 11
Temnoscheila curta Léveillé, 1889; Cayenna (AL)
Léveillé, A. 1910: 11
Temnoscheila davidi Léveillé, 1898; Ecuador (AL)
Léveillé, A. 1910: 11
Temnoscheila dendrobia Gistl & Bromme, 1850; Colombia (AL)
Léveillé, A. 1910: 11
Temnoscheila derasa Sharp, 1891; Mexico, Guatemala (AL)
Léveillé, A. 1910: 11
Temnoscheila diffinis Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 11
Temnoscheila digitata Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 11
Temnoscheila doumerci Serville, 1828; Brazil: „Cayenna” (AL)
Léveillé, A. 1910: 11
Temnoscheila dryadis Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 11
Temnoscheila ebenina Blanchard, 1875; Bolivia, Uruguay (AL)
Léveillé, A. 1910: 11
Temnoscheila edendata Schaeffer, 1918; USA: Arizona, California, Mexico: Baja (JRB)
Barron, J. R. 1971: 76 (syn. Temnochila sonorana Barrett, 1932: 171; synonymized by
Schaeffer, C. F. A. 1918: 194
Temnoscheila exarata Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 11
Temnoscheila festiva Serville, 1828; Brazil (AL)
Léveillé, A. 1910: 11 (syn. Temnochila splendens Gray in
Temnoscheila foveicollis Reitter, 1875; Brazil: „Cayenna”, „Para” (AL)
Léveillé, A. 1910: 11
Temnoscheila fraudulenta Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 11
Temnoscheila fulgidovittata Blanchard, 1875; Bolivia (AL)
Léveillé, A. 1910: 11
Temnoscheila geminata Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 11
Temnoscheila gigantea Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 11
Temnoscheila gloriosa Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 11
Temnoscheila grandis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 11
Temnoscheila grilloi Léveillé, 1905; Brazil: „Paraná” (AL)
Léveillé, A. 1910: 11
Temnoscheila grouvellei Léveillé, 1889; America centr. (AL)
Léveillé, A. 1910: 12
Temnoscheila guatemalana Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 12
Temnoscheila hubbardi Léveillé, 1889; USA: Florida (JRB)
Léveillé, A. 1910: 12. Barron, J. R. 1971: 75
Temnoscheila insignis Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 12. Reitter, E. 1875: 10 (homonym with Temnoscheila insignis Heer, 1868)
† Temnoscheila insignis Heer, 1868 (Trogosita); Tertiary: Eocene; Greenland (JRB)
Barron, J. R. 1971: 120
Temnoscheila iris Reitter, 1875; America centr. (AL)
Léveillé, A. 1910: 12
Temnoscheila jansoni Léveillé, 1889; Brazil: “Minas Geraes” (AL)
Léveillé, A. 1910: 12
Temnoscheila japonica Reitter, 1875; Japan, North Korea, Russian Far East, Northeastern China (JK)
Léveillé, A. 1910: 12. Esaki, T. et al. 1951: 1060. Inouye, M. & Nobuchi, A. 1957: 194 (Temnochila) (larva). Klausnitzer, B. 1996: 146 (larva). Kolibáč, J. 2007a: 365. Mamaev, B. M. 1976: 1655 (Trogossita) (larva). Nakane, T. K. et al. 1963: 181. Nikitsky, N. B. 1992: 80
Temnoscheila jekeli Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 12 (syn. Temnochila sennevillei Léveillé, 1878)
Temnoscheila kirschi Reitter, 1875; Colombia: Bogota (AL)
Léveillé, A. 1910: 12
Temnoscheila laevicollis Reitter, 1875; Brazil: „Cayenna” (AL)
Léveillé, A. 1910: 12
Temnoscheila laticollis Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 12
Temnoscheila lebasi Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 12
Temnoscheila leveillei Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 12
Temnoscheila lucens Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 12
Temnoscheila metallica Percheron, 1835?; Mexico (AL)
Léveillé, A. 1910: 12 (syn. Temnochila mexicana Reitter, 1875; synonymized by Salle 1877)
Temnoscheila mirabilis Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 12
Temnoscheila miranda Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 12
Temnoscheila nigritarsis Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 12
Temnoscheila obscura Reitter, 1875; North America? (AL)
Léveillé, A. 1910: 12
Temnoscheila obsoleta Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 12
Temnoscheila obtusicollis Reitter, 1875; Venezuela (AL)
Léveillé, A. 1910: 12
Temnoscheila olivacea Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 12
Temnoscheila olivicolor Léveillé, 1889; Ecuador (AL)
Léveillé, A. 1910: 12
Temnoscheila omolopha Barron, 1971; USA: Arizona, New Mexico (JRB)
Barron, J. R. 1971: 77
Temnoscheila parva Léveillé, 1889; Santo Domingo (AL)
Léveillé, A. 1910: 12
Temnoscheila patricioi Kirsch, 1881; „S. Thomé Isl.” (AL)
Léveillé, A. 1910: 12
Temnoscheila peruviana Léveillé, 1907; Peru (AL)
Léveillé, A. 1910: 12
Temnoscheila planicollis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 12
Temnoscheila planipennis Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 12 (syn. Temnochila metallica Reitter, 1875; synonymized by whom?)
Temnoscheila polita Chevrolat, 1833; America centr. (AL)
Léveillé, A. 1910: 12
Temnoscheila pollens Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 12
Temnoscheila polygonalis Léveillé, 1899; Brazil (AL)
Léveillé, A. 1910: 12
Temnoscheila portoricensis Léveillé, 1907; Porto Rico (AL)
Léveillé, A. 1910: 12
Temnoscheila praeterita Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 12
Temnoscheila punctatissima Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 12
Temnoscheila punicea Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 12
Temnoscheila quadricollis Reitter, 1875; America centr. (AL)
Léveillé, A. 1910: 13
Temnoscheila querula Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 13
Temnoscheila reitteri Kirsch, 1885; Colombia (AL)
Léveillé, A. 1910: 13
Temnoscheila reversa Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 13
Temnoscheila rhyssa Barron, 1971; USA: California, Idaho (JRB)
Barron, J. R. 1971: 77
Temnoscheila rugulosa Kirsch, 1873; Peru (AL)
Léveillé, A. 1910: 13
Temnoscheila sallei Léveillé, 1889; Guatemala, Yucatan (AL)
Léveillé, A. 1910: 13
Temnoscheila salvini Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 13
Temnoscheila sculpturata Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 13
Temnoscheila sharpi Léveillé, 1894; Bogota (AL)
Léveillé, A. 1910: 13
Temnoscheila smithi Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 13
Temnoscheila splendida Gory, 1831; Brazil: Cayenna (AL)
Léveillé, A. 1910: 13
Temnoscheila steinheili Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 13
Temnoscheila stipes Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 13
Temnoscheila subcylindrica Léveillé, 1907; Brazil (AL)
Léveillé, A. 1910: 13
Temnoscheila sulcifrons Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 13
Temnoscheila sulcisternum Léveillé, 1889; Jamaica (AL)
Léveillé, A. 1910: 13
Temnoscheila suturata Reitter, 1875; Mexico, Brazil (AL)
Léveillé, A. 1910: 13
Temnoscheila telemanensis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 13
Temnoscheila tristis Mulsant & Rey, 1853; Italia, Argentina, Colombia, Brazil (JK)
Léveillé, A. 1910: 13 (syn. Temnochila cribricollis Reitter, 1875; synonymized by whom?). Kolibáč, J. 2007a: 365
Temnoscheila urbensis Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 13
Temnoscheila varians Guérin, 1846; Brazil: Cayenna (AL)
Léveillé, A. 1910: 13
Temnoscheila variicolor Léveillé, 1889; Colombia (AL)
Léveillé, A. 1910: 13
Temnoscheila virescens Fabricius, 1775; Guayana, Central America, USA, introduced to Australia (varA)
Léveillé, A. 1910: 13. Abbott, I. 1993: 35 (biology). Barron, J. R. 1971: 79. Barron, J. R. 1971: 80 (syn. Temnochila cyanea Reitter, 1875; syn. by Léveillé, 1888). Barron, J. R. 1971: 80 (syn. Temnochila cyanea Reitter, 1875; may be synonym of Temnoscheila chlorodia! Note
Temnoscheila yuccae Crotch, 1874; USA: California, Nevada; Mexico: Baja (JRB)
Léveillé, A. 1910: 13. Barron, J. R. 1971: 74
Temnoscheila sp.
Beutel, R. G. & Ślipiński, S. A. 2001: 219 (phylogeny, morphology). Costa, C. et al. 1988: 177 (larva)
http://species-id.net/wiki/Tenebroides
Figs 2, 8; Map 5Tenebrio mauritanicus Linnaeus, 1758 [designated by Westwood 1838]
Léveillé, A. 1910: 14. Barron, J. R. 1971: 88. Crowson, R. A. 1964a: 299. Downie, N. M. & Arnett, R. H. Jr. 1996: 937 (key). Kobayashi, K. 1980: 1–6 (ecology). Kolibáč, J. 2005: 84 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. et al. 2005: 30 + 134 (key). Mamaev, B. M. 1976: 1652 (larva). Matthews, E. G. 1992: 3. Merkl, O. 1993: 12 (key). Nikitsky, N. B. 1992: 80. Ratti, E. 1997: 178 (Tenebroides sp.). Reitter, E. 1876: 28. Spahr, U. 1981: 74 (amber and copal fossils). Uchida, A. 1980: 61–73 (biology)
Trogossita Olivier, 1790 [type species: Trogossita caerulea Olivier, 1790]
Barron, J. R. 1971: 88 (list of references)
Body size: about 3.5–12.0 mm. Body shape elongate. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove present. Eyes: size flat. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae present. Mediostipes-Lacinia fused together. Palpifer: outer edge denticulate. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite present. Lateral tormal process: projection curved downwards, processes not connected (Airora). Ligula: ciliate setae present. Ligula rigid, weakly retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club asymmetrical, sensorial fields present. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism present, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell open (outer vein present), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
Larva: Frontal arms V-shaped. Epicranial stem reduced. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes absent. Paragular sclerites present. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae with several small spines. Mola absent. Maxillary palpi 3-segmented. Palpifer absent. Pedunculate seta present. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes partially fused. Cardo: size much smaller than stipes. Ligula present. Labial palpi 2-segmented. Prementum in single part, anterior margin with notch. Torma: two separate lateral sclerites. Antennal joints 1 and 2 elongate. Sensory appendix medium sized (to half of joint 3). Thoracic sclerites pattern (dorsally) 1-2-2. Thoracic sclerites pattern (ventrally) 3+1+1. Trochanter oblong. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
The species of the genus are mostly predatory, occasionally feeding on grains (e.g. the cadelle beetle, Tenebroides mauritanicus, which is adapted to a synanthropic way of life and is a serious pest of stored grain). It is probably originally European. The wild population, sometimes designated as the separate species Tenebriodes fuscus Goeze, 1777, lives in forests and its adults and larvae may be found under the bark of deciduous trees, where they feed on other insects. Supposed differences between adults of the both species can be summarized as follows: (1) Tenebriodes fuscus – antennae dilated from antennomere 6; elytra weakly glabrous, densely transversely wrinkled; frons narrower than that in the following species. (2) Tenebroides mauritanicus – antennae dilated from antennomere 8; elytra rather dull, sparsely transversely wrinkled; frons wider than that in Tenebriodes fuscus.
The bulk of species is distributed in the two Americas. Only a few species live in the Palaearctic region. Synanthropic Tenebroides mauritanicus is cosmopolitan.
Tenebroides aenelpennis Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 14
Tenebroides aeneus Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 14
Tenebroides albomaculatus Reitter, 1875; Colombia, America centr. (AL)
Léveillé, A. 1910: 14
Tenebroides albonotatus Reitter, 1875; Brazil: Cayenna (AL)
Léveillé, A. 1910: 15
Tenebroides alticola Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 15
Tenebroides alutaceus Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 15
Tenebroides americanus Kirby, 1837; USA, Canada (JRB)
Léveillé, A. 1910: 15 (syn. Tenebroides castaneus Melsheimer, 1844; synonymized by LeConte 1863). Barron, J. R. 1971: 103 (syn. Trogosita castanea Melsheimer, 1844). Barron, J. R. 1971: 103 (syn. Trogosita nigrita Horn, 1862; synonymized by LeConte 1863)
Tenebroides anceps Léveillé, 1889; America centr. (AL)
Léveillé, A. 1910: 15
Tenebroides antennalis Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 15
Tenebroides auriculatus Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 15
Tenebroides australis Boisduval, 1835; Tasmania (?) (AL)
Léveillé, A. 1910: 15
Tenebroides bimaculatus Melsheimer, 1844; USA, „Pennsylvania” (JRB)
Léveillé, A. 1910: 15. Barron, J. R. 1971: 102
Tenebroides bipustulatus Fabricius, 1801; Brazil: Cayenna (AL)
Léveillé, A. 1910: 15 (syn. var. impressifrons Reitter, 1875: “Amer. mer.”; syn. n.)
Tenebroides boggianii Léveillé, 1905; Paraguay (AL)
Léveillé, A. 1910: 15
Tenebroides bonvouloiri Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 15 (var. chontalensis Sharp, 1891: Nicaragua; syn. n.)
Tenebroides brevis Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 15
Tenebroides breviusculus Reitter, 1875; America centr., Brazil (AL)
Léveillé, A. 1910: 15
Tenebroides brunneovittatus Léveillé, 1894; America centr., Brazil (AL)
Léveillé, A. 1910: 15
Tenebroides brunneus Léveillé, 1889; Brazil: Cayenna (AL)
Léveillé, A. 1910: 15
Tenebroides bugnioni Léveillé, 1903; Colombia (AL)
Léveillé, A. 1910: 15
Tenebroides carbonarius Léveillé, 1889; Brazil: Cayenna (AL)
Léveillé, A. 1910: 15
Tenebroides carinatus Léveillé, 1894; Brazil (AL)
Léveillé, A. 1910: 15
Tenebroides celatus Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 15
Tenebroides chevrolati Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 15
Tenebroides circumcinctus Léveillé, 1889; America centr. (AL)
Léveillé, A. 1910: 15
Tenebroides collaris Sturm, 1807; Canada: Ontario, E USA to Michigan and E Texas (JRB)
Léveillé, A. 1910: 15 (syn. Tenebroides nigripennis Sturm, 1826). Barron, J. R. 1971: 97 (Trogosita nigripennis Sturm, 1826; nomen nudum). Barron, J. R. 1971: 97 (Trogosita nigripennis Dejean, 1836; nomen nudum)
Tenebroides complicatus Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 15
Tenebroides cordicollis Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 15
† Tenebroides corrugata Wickham, 1913; USA: Colorado, Florissant; Early Oligocene (JRB)
Barron, J. R. 1971: 120. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 23. Wickham, H. F. 1913: 291
Tenebroides corticalis Melsheimer, 1844; Guatemala, Mexico, USA, Canada to Alaska (JRB)
Léveillé, A. 1910: 15 (syn. Tenebroides dubius Melsheimer, 1844; synonymized by
Tenebroides crassicornis Horn, 1862; Western states of Canada, USA (JRB)
Léveillé, A. 1910: 15. Barron, J. R. 1971: 95 (syn. Trogosita californica Horn, 1862; synonymized by LeConte 1863). Barron, J. R. 1971: 95 (syn. Trogosita pleuralis Horn, 1862; synonymized by LeConte 1863). Léveillé, A. 1910: 18. (Tenebroides pleuralis Horn, 1862: California)
Tenebroides cribratus Léveillé, 1894; Mexico (AL)
Léveillé, A. 1910: 15
Tenebroides cucujoides Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 15
Tenebroides delicatus Léveillé, 1899; Brazil (AL)
Léveillé, A. 1910: 16
Tenebroides depressior Palisot de Beauvois, 1811 (Trogossita) incertae sedis; N America (JRB)
Léveillé, A. 1910: 16. Barron, J. R. 1971: 119 (Trogossita depressior Palisot de Beauvois, 1811; incertae sedis)
Tenebroides depressus Guérin, 1846; Brazil, America centr. (AL)
Léveillé, A. 1910: 16
Tenebroides difficilis Léveillé, 1889; Honduras (AL)
Léveillé, A. 1910: 16
Tenebroides dilatatus Erichson, 1847; Peru (AL)
Léveillé, A. 1910: 16
Tenebroides donckieri Léveillé, 1902; Brazil (AL)
Léveillé, A. 1910: 16
Tenebroides elongatulus Jacquelin du Val, 1857; Cuba (AL)
Léveillé, A. 1910: 16
† Tenebroides emortua Germar, 1849; Germany: Orsberg; Tertiary: Upper Oligocene (varA)
Mörs, T. 1995: ii. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 23
† Tenebroides eocenica Meunier, 1921; Germany: Messel; Tertiary: middle Eocene (varA)
Meunier, F. 1921: ii. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 23
Tenebroides excellens Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 16
Tenebroides explanatus Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 16
Tenebroides facilis Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 16
Tenebroides farmairei Léveillé, 1889; Tonga-Tabu (AL)
Léveillé, A. 1910: 16
Tenebroides fenestratus Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 16
Tenebroides flaviclavis Reitter, 1875; Cuba (AL)
Léveillé, A. 1910: 16
Tenebroides floridanus Schaeffer, 1918; USA: Florida, Louisiana, centr. America (JRB)
Barron, J. R. 1971: 111. Schaeffer, C. F. A. 1918: 199
Tenebroides fossulatus Léveillé, 1899; Bolivia (AL)
Léveillé, A. 1910: 16
Tenebroides fryi Léveillé, 1898; Brazil (AL)
Léveillé, A. 1910: 16
Tenebroides fulgens Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 16
Tenebroides fulvolineatus Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 16
Tenebroides germaini Léveillé, 1895; Bolivia (AL)
Léveillé, A. 1910: 16
Tenebroides godmani Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 16
Tenebroides gounellei Léveillé, 1889; Brazil: “Minas Geraes” (AL)
Léveillé, A. 1910: 16
Tenebroides gracilipes Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 16
Tenebroides harpaloides Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 16
Tenebroides helophorus Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 16
Tenebroides humeralis Léveillé, 1889; Colombia (AL)
Léveillé, A. 1910: 16
Tenebroides importunus Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 16
Tenebroides incertus Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 16
† Tenebroides insignis Heer, 1883; Greenland: Atanrkerdluk; Mesozoic: Upper Cretaceous, Maastrichtian (varA)
Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 23
Tenebroides insinuans Walker, 1858; Ceylon (?) (AL)
Léveillé, A. 1910: 16
Tenebroides instabilis Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 16
Tenebroides iteratus Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 16
Tenebroides jatahyensis Léveillé, 1902; Brazil (AL)
Léveillé, A. 1910: 16
Tenebroides latens Wollaston, 1862; Canary Isl: Teneriffa (JK)
Léveillé, A. 1910: 16. Kolibáč, J. 2007a: 365. Plata-Negrache, P. & Prendes-Ayala, C. 1981: 230
Tenebroides laticollis Horn, 1862; Eastern USA, Canada (JRB)
Léveillé, A. 1910: 16. Barron, J. R. 1971: 105 (syn. Trogosita obscura Horn, 1862; synonymized by Leconte 1863)
Tenebroides latus Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 17
Tenebroides lineolatus Reitter, 1877; Colombia (AL)
Léveillé, A. 1910: 17
Tenebroides litigiosus Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 17
Tenebroides longicornis Léveillé, 1889 – Brazil (AL)
Léveillé, A. 1910: 17
Tenebroides longulus Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 17
Tenebroides lucidus Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 17
Tenebroides marginatus Palisot de Beauvois, 1811; S and middle E USA (JRB)
Léveillé, A. 1910: 17. Barron, J. R. 1971: 99 (syn. Tenebroides cucujiformis Horn, 1862; synonymized by LeConte 1863). Barron, J. R. 1971: 99. Léveillé, A. 1910: 15 (Tenebroides cucujiformis Horn, 1862). Léveillé, A. 1910: 16 (homonym Tenebroides marginatus Latreille, 1833 replaced by Tenebroides latreillei Léveillé, 1889: “Amer. aequin.”; synonymized by
Tenebroides marginicollis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 17
Tenebroides maroccanus Reitter, 1884; N Africa, Azores, Corsica, Spain (JK)
Léveillé, A. 1910: 17 (var. baillioti Léveillé, 1903: Madrid; syn. n.). Audisio, P. et al. 1995: 14. Kolibáč, J. 2007a: 365. Villemant, C. & Ramzi, H. 1997: 441 (biology)
Tenebroides marseuli Reitter, 1875; St. Catharina (AL)
Léveillé, A. 1910: 17
Tenebroides mauritanicus Linnaeus, 1758 (Tenebrio); cosmopolitan; type locality: “Algiriae” (varA)
(syn. Lucanus dubius Scriba, 1790; Carabus bucephalus Herbst, 1783; Lucanus fuscus Goeze, 1777; Lucanus fuscus Preyssler, 1790; Platycerus fuscus Geoffroy, 1762; Platycerus striatus Fourcroy, 1785; Tenebrio caraboides Linnaeus, 1758: type locality: “Europa”; Tenebrio piceus Schaller, 1783; Tenebrio planus Quensel, 1790; Tenebroides complanatus Piller & Mitterpacher, 1783; Tenebrio piceus Schaller, 1783; Trogosita affinis White, 1846)
Léveillé, A. 1910: 17 (syn. Tenebrioides fuscus Goeze, 1777). Léveillé, A. 1910: 17 (var. nitidus Horn, 1862: 83 synonymized by Kolibáč 2007). Arbogast, R. T. & Byrd, R. V. 1982: 61 (biology). Audisio, P. et al. 1995: 14 (Tenebroides fuscus Goeze, 1777). Bahillo de la Puebla P. & López-Colón, J. I. 1999: 13. Bahillo de la Puebla P. & López-Colón, J. I. 1999: 13 (Tenebroides fuscus Goeze, 1777). Bahillo de la Puebla P. & López-Colón, J. I. 2004: 129. Sarikaya, O. & Avci, M. 2009: 129 (Tenebroides fuscus Goeze, 1777). Barron, J. R. 1971: 92–93. Barron, J. R. 1971: 93 (syn. Trogosita nitida Horn, 1862; synonymized by LeConte 1863). Borowiec, L. 1983: 11. Borowiec, L. 1983: 11 (Tenebroides fuscus Goeze, 1777). Burakowski, B. et al. 1986: 117. Burakowski, B. et al. 1986: 117 (Tenebroides fuscus Goeze, 1777). Dziadik-Turner, C. et al. 1981: 546 (biology). Esaki, T. et al. 1951: 1061. Faustini, D. I. & D. G. H. Halstead 1982: 45. Gollkowski, V. 1988: 42. Herger, P. 1998: 105 (Tenebroides fuscus Goeze, 1777; distribution). Holzer, E. 1995: 30. Huber, Ch. & Kobel, E. 1994: 1. Kampsu, G. 2005: 17 (biology). Klausnitzer, B. 1976: 8. Klausnitzer, B. 1976: 8 (Tenebrioides fuscus Goeze, 1777). Klausnitzer, B. 1978: 176. Klausnitzer, B. 1996: 146. Klausnitzer, B. 1996: 151 (Tenebroides fuscus Goeze, 1777). Kolibáč, J. 1993a: 21. Kolibáč, J. 1993a: 21 (Tenebroides fuscus Goeze, 1777). Kolibáč, J. 1993b: 90. Kolibáč, J. 1993b: 90 (syn. Tenebroides fuscus Goeze, 1777). Kolibáč, J. 1996b: 473. Kolibáč, J. 1999b: 12. Kolibáč, J. 2005: 84 (redescription). Kolibáč, J. 2006: 109 (Tenebroides fuscus Goeze, 1777; larva). Kolibáč, J. 2007a: 365 (Tenebroides fuscus Goeze, 1777). Kolibáč, J. 2007a: 365 (syn. Tenebroides nitidus Horn, 1862). Kolibáč, J. 2007a: 365. Kolibáč, J. 2007a: 365 (syn. Tenebroides piceus Dalla Torre, 1879). Mamaev, B. M. 1976: 1654. Matthews, E. G. 1992: 3. Mitter, H. 1998: 560. Mitter, H. 1998: 560 (Tenebroides fuscus Goeze, 1777). Nakane, T. et al. 1963: 181. Nikitsky, N. B. 1992: 80. Pileckis, S. & V. Monsevičius 1995: 272. Plata-Negrache, P. & Prendes-Ayala, C. 1981: 230. Pospischil, R. 2003: 4 (biology). Purrini, K. & Ormieres, R. 1979: 437. Ratti, E. 1997: 178. Šefrová, H. & Laštůvka, Z. 2005: 162. Szwalko, P. & Kubisz, D. 1994: 46 (Tenebroides fuscus Goeze, 1777; distribution). Teson, A. & Dagoberto, E. L. 1979: 127 (biology). Vogt, H. 1967: 16. Vogt, H. 1967: 16 (Tenebriodes fuscus Preyssler, 1790). Leschen, R. A. B. & Lackner, T. 2013: 301 (syn. Trogosita affinis White, 1846; New Zealand)
Tenebroides metallescens Reitter, 1875
Léveillé, A. 1910: 18; Brazil (AL)
Tenebroides moerens Sharp, 1891; Panama (AL)
Léveillé, A. 1910: 18
Tenebroides mordax Sharp, 1891; Costarica (AL)
Léveillé, A. 1910: 18
Tenebroides murinus Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 18
Tenebroides muticus Palisot de Beauvois, 1811 (incertae sedis); N America (JRB)
Léveillé, A. 1910: 18. Léveillé, A. 1888: 439 (syn. Trogosita punctata Dejean, 1836). Barron, J. R. 1971: 120 (Trogosita punctata Dejean, 1836; nomen nudum). Barron, J. R. 1971: 119. (incertae sedis, syn. with Tenebroides nanus Melsheimer, 1844 by Horn 1862)
Tenebroides nanus Melsheimer, 1844; E USA, Mexico (JRB)
Léveillé, A. 1910: 18. Barron, J. R. 1971: 98. Barron, J. R. 1971: 119. (syn. Tenebroides muticus Palisot de Beauvois, 1811 syn. by Horn 1862). Klausnitzer, B. 1996: 150. Kolibáč, J. 2006: 109
Tenebroides nemosomiaeformis Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 18
Tenebroides nigrocyaneus Léveillé, 1905; Paraguay (AL)
Léveillé, A. 1910: 18
Tenebroides nigroviridis Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 18
Tenebroides oblongus Sharp, 1891; Mexico, Panama (AL)
Léveillé, A. 1910: 18
Tenebroides obtusus Horn, 1862; Eastern coastal states of USA (JRB)
Léveillé, A. 1910: 18. Barron, J. R. 1971: 108
Tenebroides occidentalis Fall, 1910; W Canada and USA, Mexico (JRB)
Barron, J. R. 1971: 117. Fall, H. C. 1910: 128
Tenebroides ocularis Lewis, 1894; Japan (AL)
Léveillé, A. 1910: 18
Tenebroides opacus Reitter, 1875 (incertae sedis); N America (?), Colombia (JRB)
Léveillé, A. 1910: 18. Barron, J. R. 1971: 120 (incertae sedis)
Tenebroides ornatus Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 18
Tenebroides passeti Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 18
Tenebroides patruelis Reitter, 1875 (incertae sedis); Brazil, “Carol. mer.” (JRB)
Léveillé, A. 1910: 18. Barron, J. R. 1971: 120 (incertae sedis)
Tenebroides politus Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 18
Tenebroides pollens Sharp, 1891; America centr. (AL)
Léveillé, A. 1910: 18
Tenebroides pulchellus Reitter, 1875; “Nov. Grenada” (AL)
Léveillé, A. 1910: 18
Tenebroides pumilus Léveillé, 1889; Colombia (AL)
Léveillé, A. 1910: 18
Tenebroides punctatolineatus Fairmaire, 1850; Polynesia (?) (AL)
Léveillé, A. 1910: 18
Tenebroides punctulatus Reitter, 1875; Cuba, Portorico (AL)
Léveillé, A. 1910: 18
Tenebroides pusillimus Mannerheim, 1843 (incertae sedis); USA: Alaska (JRB)
Léveillé, A. 1910: 18. Barron, J. R. 1971: 120 (incertae sedis)
Tenebroides quadridens Palisot de Beauvois, 1811; “Oware” (?) (AL)
Léveillé, A. 1910: 18
Tenebroides quadriguttatus Reitter, 1875; Brazil, Argentina (AL)
Léveillé, A. 1910: 18
Tenebroides rectus Wollaston, 1862; Canary Isl.: Lanzarote (JK)
Léveillé, A. 1910: 18. Kolibáč, J. 2007a: 365. Plata-Negrache, P. & Prendes-Ayala, C. 1981: 230
Tenebroides reflexus Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 18
Tenebroides reitteri Léveillé, 1889; Panama, Brazil (AL)
Léveillé, A. 1910: 18
Tenebroides repetitus Sharp, 1891; Mexico, Guatemala (AL)
Léveillé, A. 1910: 19
Tenebroides ritsemae Léveillé, 1889; Colombia, Costarica (AL)
Léveillé, A. 1910: 19
Tenebroides ruber Reitter, 1875; America centr., Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides rubromarginatus Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides ruficollis Reitter, 1875; Bogota (AL)
Léveillé, A. 1910: 19
Tenebroides rufipes Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides rufiventris Reitter, 1875; Colombia, Argentina (AL)
Léveillé, A. 1910: 19
Tenebroides rufolimbatus Léveillé, 1889; Mexico (AL)
Léveillé, A. 1910: 19
Tenebroides rugosipennis Horn, 1862; E USA to Arizona (JRB)
Léveillé, A. 1910: 19. Barron, J. R. 1971: 108 (syn. Tenebroides arizonensis Schaeffer, 1918; synonymized by
Tenebroides sallei Sharp, 1891; Mexico (AL)
Léveillé, A. 1910: 19
Tenebroides scaberrimus Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides schaufussi Reitter, 1875; Venezuela: Caracas (AL)
Léveillé, A. 1910: 19
Tenebroides sculpturatus Reitter, 1875; Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides semicylindricus Horn, 1862; Eastern coast of USA to Mexico (JRB)
Barron, J. R. 1971: 109 (syn. Tenebrioides subaenea Reitter, 1875; synonymized by whom?). Barron, J. R. 1971: 109 (syn. Tenebroides foveatus Blatchley, 1917; synonymized by
Tenebroides semicylindricus Horn, 1862
Léveillé, A. 1910: 19
Tenebroides sennevillei Léveillé, 1889; America centr. (AL)
Léveillé, A. 1910: 19
Tenebroides sericatus Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 19
Tenebroides serraticollis Léveillé, 1907; Argentina (AL)
Léveillé, A. 1910: 19
Tenebroides sharpi Léveillé, 1891; Panama (AL)
Léveillé, A. 1910: 19
Léveillé, A. 1910: 19 (syn. Tenebroides bimaculatus Sharp, 1891; syn by
Tenebroides similis Léveillé, 1905; Brazil
Léveillé, A. 1910: 19
Tenebroides sinuatus LeConte, 1861; W USA, Canada; to Kansas, Montana, Texas (JRB)
Léveillé, A. 1910: 19 (syn. Tenebroides sinuatus var. californicus Horn, 1862; synonymized by
Tenebroides sonorensis Sharp, 1891; SW USA, Mexico, Cuba (?) (JRB)
Léveillé, A. 1910: 19 (distribution in Cuba). Barron, J. R. 1971: 112 (syn. Tenebroides debilis Fall, 1910; synonymized by whom?). Dajoz, R. 1997: 42 (ecology)
Tenebroides soror Jacquelin du Val, 1857; USA: Florida, Bahamas, N Cuba (JRB)
Léveillé, A. 1910: 19. Barron, J. R. 1971: 101
Tenebroides spectator Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 19
Tenebroides steinheili Reitter, 1875; Colombia (AL)
Léveillé, A. 1910: 19
Tenebroides stultus Léveillé, 1907; Argentina (AL)
Léveillé, A. 1910: 19
Tenebroides sublaevis Palisot de Beauvois, 1811; ‘Oware’ (AL)
Léveillé, A. 1910: 19
Note: species not mentioned by
Tenebroides subnigra Palisot de Beauvois, 1811 (incertae sedis); USA: Pennsylvania (JRB)
Léveillé, A. 1910: 19 (Tenebroidessubniger; misspelling). Barron, J. R. 1971: 119 (incertae sedis)
Tenebroides subplanus Reitter, 1875; Mexico (AL)
Léveillé, A. 1910: 19
Tenebroides subruber Léveillé, 1899; Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides subvirescens Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 19
Tenebroides sulcifrons Jacquelin du Val, 1857; Cuba (AL)
Léveillé, A. 1910: 19
† Tenebroides tenebrioides Germain, 1837; Germany: Rott, Siebengebirge; Tertiary: Upper Oligocene (varA)
Léveillé, A. 1910: 19. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 23
Tenebroides tenuistriatus Fall, 1910; USA: Colorado, New Mexico, Arizona; Mexico (JRB)
Barron, J. R. 1971: 114
Tenebroides transversicollis Jacquelin du Val, 1857; Cuba (AL)
Léveillé, A. 1910: 20
Tenebroides turkestanicus Ballion, 1870; Central Asia (JK)
Léveillé, A. 1910: 20. Kolibáč, J. 2006: 109. Kolibáč, J. 2007a: 365. Mamaev, B. M. 1976: 1654 (larva)
Tenebroides undulatus Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 20
Tenebroides viridescens Léveillé, 1889; Brazil (AL)
Léveillé, A. 1910: 20
Tenebroides yucatanicus Léveillé, 1889; Yucatan, Honduras (AL)
Léveillé, A. 1910: 20
Tenebroides zapotensis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 20
Tenebroides zunilensis Sharp, 1891; Guatemala (AL)
Léveillé, A. 1910: 20
http://species-id.net/wiki/Thoracotes
Figs 24, 25; Map 5Thoracotes dubius Handlirsch, 1906 [by monotypy]
Kolibáč, J. 2006: 121 (classification). Ponomarenko, A. G. 1985: 68. Ponomarenko, A. G. 1990: 74. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24
(Thoracotes dubius): “Ein 8 mm langer Käfer von ähnlicher Gestalt wie Parnidium Geinitzi. Der Prothorax is aber anders geformt un nähert sich mehr der Kreisform, Auch der Kopf scheint anders gewesen zu sein. Flügeldecken punktiert, 3, 5 mal so lang als breit. Geinitz vergleicht diese Form mit Latridiites Schaumi, mit dem sie allerdings auch einige Ähnlichkeit hat.”
This is potentially the oldest fossil record of Trogossitidae. Unfortunately, its description is inadequate and the illustration very poor. It is unclear to me why two more Mesozoic species from Russia were assigned to this dubious genus. It was not indicated in the original papers describing the two new species if the type of Thoracotes dubius was studied in situ (
(Thoracotes glabrus, translation from Russian). “Head sligthtly longer than wide, narrowed in front of eyes; eyes relatively large, situated at sides of head; cheeks[genae] short; temples [tempora] slightly shorter than eyes. The first antennomere large, second transverse, third 1.5 times longer than second, fourth to seventh as long as wide, eighth longer than wide, nineth to eleventh asymmetric. Pronotum with lateral margins rounded, its corners not acute, 1.5 times longer than wide. Front coxae not large, spherical, with exposed trochantins. Prosternal process short and blunt. Middle coxae transverse, nearly touching each other. Metathorax trapezoidal, narrowed anteriad, its length 1.8 times lesser than wide at hind [basal] margin. Hind coxae touch each other, transverse, not concave posteriorly [sic] and without coxal plates, at lateral sides shorter than at mid-point. Last abdominal sternite distinctly longer than penultimate one. Ovipositor long, with sclerotized palpifers [sic] and pairs of appendages [coxitae] with pubescent cerci [styli]. Legs relatively long, tibiae and tarsi thin. Elytra smooth. Length of beetle 3.6, width 1.6; length of elytra 2.3 mm.” “The beetle of Pavlovka distinctly smaller (length 3.0, width 1.0 mm).” (
Germany: Dobbertin in Mecklenburg, Russia: central Siberia; Transbaikalia: Pavlovka, Onokhoy; Mesozoic: from Lower to Upper Jurassic.
† Thoracotes dubius Handlirsch, 1906; Germany; Lower Jurassic: Upper Lias (varA)
Kolibáč, J. 2006: 121 (classification). Schmied, H. et al. 2009: 24
† Thoracotes glabrus Ponomarenko, A., 1990; Russia: Transbaikalia; Upper Jurassic (varA)
Ponomarenko, A. G. 1990: 74. Kolibáč, J. 2006: 121. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24
† Thoracotes sibiricus Ponomarenko, 1985; Russia: Siberia; Middle Jurassic (varA)
Ponomarenko, A. G. 1985: 68. Kolibáč, J. 2006: 121. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24
Lithostoma Martynov, 1926 [by monotypy and author’s designation]
Kolibáč, J. & Huang, D.-Y. 2008: 142. Yu, Y. et al. 2012: 250
This fossil differs from all other Mesozoic Trogossitidae described to date. If
On the other hand, the concept of Lithostomatini was justifiably called into question by
http://species-id.net/wiki/Lithostoma
Map 6Lithostoma expansum Martynov, 1926 [by monotypy]
Kolibáč, J. & Huang, D.-Y. 2008: 142 (Remarks, English description and translation of Russian description). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 26
(translation from Russian). “Head free, dilated between the eyes [Russ.: and narrowed backwards]; mandibulae strong, sharply bent inwards, with perhaps two teeth at the apices; antennae resembling those in living Ostomatinae [Russ.: in Ostoma and other genera]; basal joint large, bulbous anteriorly, second much smaller, third still smaller, short, fourth joint of the same width but elongated, 5–7th joints elongated but becoming gradually thicker towards the tip [Russ.: joints 10 and 11 not preserved], last 8–11 joints only slightly thicker, without distinct apical club (clavus). Pronotum broadening posteriorly, apparently furnished with marginal dilatations [Russ.: as in Ostoma]; covered all over with numerous point-like pits. Elytra broad, rounded at postero-lateral margins [Russ.: as in Ostoma], with perhaps 8 longitudinal stripes, each containing two rows of raised black points; intermediate narrow stripes barely elevated; both marginal dilatations also with pits and points [Russ.: Body size about 6 mm.].” (
English text: “Head and antennae as in generic description; sides of pronotum convex, points distinct; elytra broad, rounded at the postero-lateral margins; marginal dilatations rather broad; the dividing stripes not elevated. Length of the body 6 mm.” (
Translation of Russian text: “Head free, strongly projecting anteriorly (partly artificial condition in compressed specimen). Mandibles robust, left mandible bidentate (right inconspicuous). Antennae as described above, each joint weakly dilated at apex; each dilated area with thin [sic], dark, round rim. Final two joints torn off, only a trace of joint 10 present. Pronotum widened towards base, with shallow punctures interspersed with small tubercles. Elytra wide, rounded apically and dorsally [sic]; flattened sides well-developed and probably lighter than dark brown convex portion of elytra. Elytra, including flattened sides, with rows of well-developed small, black tubercles [orig.: “convex punctures”]; carinae among them not higher than tubercles. Length of body from anterior margin of labrum to apex of elytra – 6 mm.” (
The Russian and English texts vary somewhat from one another; the Russian is more comprehensive.
Southern Kazakhstan: paper-shales near Galkino (approximately 42.15N, 70.02E), Chimkentskaya oblast district. Mesozoic: Upper Jurassic, probably Oxfordian.
A distribution of the tribe Lithostomatini and the genus † Juralithinus (Peltinae incertae sedis).
† Lithostoma expansum Martynov, 1926; Kazakhstan; Upper Jurassic (varA)
Martynov, A. V. 1926: 13 (in Russian), 32 (in English). Kolibáč, J. & Huang, D.-Y. 2008: 143 (Remarks, English description and translation of Russian description). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 26
| 1 | Antenna 8-segmented; body surface with stout scale-like setae; wingless species with convex body | Colydiopeltini |
| – | Antenna 10- or 11-segmented; body surface without scales or scale-like setae; winged or wingless species with flat or convex or conglobate body | 2 |
| 2 | Body flat, relatively large (more than 8 mm). Larva without distinct urogomphi | Peltini |
| – | Body convex or conglobate, smaller (mostly less than 7 mm). Larva with distinct urogomphi | 3 |
| 3 | Adult: mandible without mola, with membranous penicillus; front coxae distinctly projecting. Larva: mandible without mola, with tridentate lacinia mobilis | Phloiophilini |
| – | Adult: mandible with mola, membranous penicillus absent; front coxae indistinctly projecting. Larva: mandible with mola, without distinct lacinia mobilis | Thymalini |
http://species-id.net/wiki/Juralithinus
Map 6Juralithinus gracilidorsum Kireichuk & Ponomarenko, 1990 [by monotypy and original designation]
Kolibáč, J. 2006: 126 (Thymalini). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24
The genus was originally described in Peltidae and classified within the extinct subfamily Meligethiellinae along with Meligethiella and Ostomalynus (
(translation from Russian). “Diagnosis: Body conspicuously longitudinal, with prothorax relatively elongate and narrow, coxae narrowly separated in all pairs of legs; metathorax without femoral lines [paracoxal sutures? – they are drawn in l.c., Fig. 1a]; elytra distinctly wider than pronotum; first visible abdominal sternite approximately as large as the second to fourth, the last sternite longer.”
(translation from Russian). “Elongate-oval beetle [sic]. Head with moderately protruded bidentate mandibles, very small transverse mentum and developed gular sutures. Pronotum with anterior margin deeply emarginate and widely rounded anterior corners. Transverse front coxae strongly [distinctly] narrowly separated, process between them not observed. Mesothorax convex at centre, middle coxae narrowly separated. Mesonotum wide, with widely rounded scutellum. Metathorax with conspicuous discrimen and paracoxal sutures, outer apices of metepisterna strongly anteriorly projecting. Hind coxae oval, very narrowly separated and obliquely situated towards centre. Elytra, with medium sized epipleurons, seem not to cover abdomen perfectly; their apices possibly artificially [sic] broken off. Femora medium-dilated, all with oval outlines. Tibiae weakly dilated towards apices, in middle, strangely enough, relatively thin with elongate apodemes [orig.: arrows] at apex. Hind tarsi relatively long, comprised of simple segments [i.e., without lobes]. Length – 9.3; width – 4.6; length of elytra – 2.3 [mm].”
Kazakhstan: Chimkentskaya obl., Mikhailovka; Mesozoic: Upper Jurassic, Karabastayskaya formation.
† Juralithinus gracilidorsum Kireichuk & Ponomarenko, 1990; Kazakhstan; Upper Jurassic (varA)
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 80. Kolibáč, J. 2006: 126. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24
http://species-id.net/wiki/Sinopeltis
Map 6Sinopeltis jurassica Yu, Leschen, Ślipiński, Ren & Pang, 2012
This genus, containing two species, has only recently been established. The fossils are well preserved, with both part and counterpart. The species are relatively large, body shape perfectly appropriate to Peltinae or Lophocaterinae. The eyes are distinctly elevate, much more so than those in extant Ancyronini. The 3-segmented antennal club of Sinopeltis jurassica is “weakly asymmetrical” (quite symmetrical in the original picture), that of Sinopeltis amoena is “strongly asymmetrical” (inconspicuously so in the picture). The mesocoxae appear contiguous (unknown state in Trogossitidae) in Sinopeltis jurassica, whereas they are narrowly separated in Sinopeltis amoena. Both discrepancies mentioned may be the results of different positions of body parts (coxae, antennae) assumed during fossilization. Unfortunately, neither the ends of the tibiae nor the tarsi and mouthparts are described in either species, which leads to a lack of direct evidence for classification. Both Sinopeltis jurassica and Sinopeltis amoena are about 165 million years old and belong, if their classification is correct, together with species of Thoracotes, among the oldest known fossil members of Trogossitidae.
“Body broadly ovoid and parallel-sided. Antenna distinctly clubbed with antennomeres symmetrical [S. jurassica: “weakly asymmetrical”; S. amoena: “strongly asymmetrical”]; antennal insertions visible in dorsal view. Eyes convex. Frontoclypeal suture present. Antennal grooves present and parallel. Anterior pronotal angles well developed and subrounded. Mesoventrite not vaulted. Mesocoxae widely separated [see “note” in Remarks]. Metaventrite lacking axillary space and metakatepisternal suture. Metacoxae not excavate and narrowly separated. Elytra with seriate punctation (in one fossil). Abdominal ventrite 1 about as long as 2, intercoxal process narrow. Body length 7.5–7.6 mm, width 4.5–4.8 mm.” (Diagnosis of the genus according to
China: Inner Mongolia, Daohugou. Middle Jurassic, Jiulongshan formation.
† Sinopeltis jurassica Yu, Leschen, Ślipiński, Ren & Pang, 2012; China: Inner Mongolia; Middle Jurassic (AD)
Yu, Y., Leschen, R. A. B., Ślipiński, A., Ren, D. & Pang, H. 2012: 247
† Sinopeltis amoena Yu, Leschen, Ślipiński, Ren & Pang, 2012; China: Inner Mongolia; Middle Jurassic (AD)
Yu, Y., Leschen, R. A. B., Ślipiński, A., Ren, D. & Pang, H. 2012: 247
Peltis O. F. Müller, 1764
Hunt, T. et al. 2007: 1915 (Peltinae) (molecular phylogeny). Kolibáč, J. 2006: 125 (diagnosis, phylogeny). Kolibáč, J. 2007a: 366. Lawrence, J. F. & Newton, A. F., Jr. 1995: 868 (Kirby, W. 1837: 104 is considered the author of the family rank name). Ślipiński, S. A. 1992: 442 (Peltinae)
The single genus Peltis exhibits a noteworthy mixture of the both advanced and primitive morphological features. However, some of the derived, especially larval, character states may considered as various kinds of reduction. A few species of Peltis are highly adapted to a hidden way of life under tree bark or in rotten wood: they are flattened and slow-moving, their robust mandibles have distinct mola, and the larval urogomphi are strongly reduced. The outer appearance is similar to that in Calitys, while some details of both adult and larval morphology (for example the gular appendages in the larval cranium) resemble Thymalus. The synonymization of Zimioma with Peltis is undeniable. Apart from body size, there is no morphological character to distinguish between the two genera.
http://species-id.net/wiki/Peltis
Figs 2, 9, 15; Map 7Silpha grossa Linnaeus, 1758 [designated by
Bacianskas, V. 2009: 30 (biology). Barron, J. R. 1971: 24. Barron, J. R. 1996: 193 (Nearctic species). Kolibáč, J. 2005: 76. Kolibáč, J. 2006: 111. Kolibáč, J. 2007a: 366. Noreika, N. 2009: 68 (distribution). Spahr, U. 1981: 74 (amber and copal fossils)
Gaurambe C. G. Thomson, 1862 [type species: Silpha ferruginea Linnaeus, 1758]
Barron, J. R. 1971: 24. Kolibáč, J. 2007a: 366. Lafer, G. Sh. 1992: 83. Silfverberg, H. 1978: 117
Ostoma Laicharting, 1781 [type species: Silpha ferruginea Linnaeus, 1758]
Léveillé, A. 1910: 30. Downie, N. M. & Arnett, R. H. Jr. 1996: 935 (key). Barron, J. R. 1971: 23. Crowson, R. A. 1964a: 295 (Peltis Kugel, 1791). Kolibáč, J. 2005: 76 (synonymized). Kolibáč, J. 2007a: 366. Larsson, S. G. 1978: 150 (fossil, Baltic amber). Lafer, G. Sh. 1992: 83. Reitter, E. 1876: 61
Zimioma Gozis, 1886 [type species: Silpha grossa Linnaeus, 1758]
Léveillé, A. 1910: 29 [Ostoma (Zimioma) Gozis, 1886]. Kolibáč, J. 2007a: 366
Body size: about 7.5–23.0 mm. Body shape flat. Gular sutures wide, subparallel. Frontoclypeal suture broadly emarginate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: one. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection curved downwards, processes with bridge (Peltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell small (Peltis), cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae divided.
Larva: Frontal arms Y-shaped. Epicranial stem present. Endocarina absent. Gular sutures inconspicuous. Gula: anterior apodemes present. Paragular sclerites absent. Hypostomal rods present. Stemmata number: 3. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae absent. Mola reduced. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion present. Cardo-Stipes not fused. Cardo: size much smaller than stipes. Ligula present. Labial palpi 2-segmented. Prementum in two parts, anterior margin even. Torma: two separate lateral sclerites. Antennal joints 1 and 2 elongate. Sensory appendix very small. Thoracic sclerites pattern (dorsally) 0+0+0. Thoracic sclerites pattern (ventrally) 0+0+0. Trochanter oblong. Abdominal segment IX not divided. Tergite IX flat. Urogomphi minute; median process absent.
A Peltis (syn. Zimioma, Ostoma) grossa B Peltis (syn. Ostoma) ferruginea C Colydiopeltis loebli D Colydiopeltis compactum E Colydiopeltis burckhardti F Parapeltis australicum G Protopeltis viridescens H cf. Globorentonium plaumanni, Brazil (1–3: three different specimens from the same locality).
Fungivorous. The adults live under the bark of dead or dying deciduous and coniferous trees and feed on fungi. Larvae can be found in rotten or decaying wood, for example at the base of old trees or inside stumps.
Holarctic temperate zones: North America from Arizona to Alaska, Europe including British Isles and Scandinavia, Siberia, Korea, Japan.
A distribution of the tribe Peltini.
Peltis columbiana Casey, 1924; widespread USA, Canada (from Alaska) (JRB)
Barron, J. R. 1971: 30
† Peltis constulata Heyden, 1862; Germany: Rott, Siebengebirge; Tertiary: Upper Oligocene (varA)
Ponomarenko, A. G. & Kireichuk A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24
Peltis ferruginea Linnaeus, 1758; Europe, Siberia, Japan, North Korea, USA, Canada (JK)
Léveillé, A. 1910: 30 (Ostoma). Bahillo de la Puebla P. & López-Colón, J. I. 1999: 13 (Ostoma). Sarikaya, O. & Avci, M. 2009: 129 (Ostoma)
Barron, J. R. 1971: 28 (syn. Ostoma cassidoides Lepechin, 1774); Russia (JRB)
Barron, J. R. 1971: 28 (syn. Ostoma cimicoides Degeer, 1774; not binominal). Barron, J. R. 1971: 28 (Ostoma)
Barron, J. R. 1971: 28 (syn. Ostoma fraternus Randall, 1838); USA: Maine (JRB)
Barron, J. R. 1971: 28 (syn. Ostoma nigricans Dalla Torre, 1879); Oberösterreich (JRB)
Barron, J. R. 1971: 28 (syn. Ostoma nigrina Casey, 1916); Canada: British Columbia (JRB)
Barron, J. R. 1971: 28 (syn. Ostoma rubicunda Laicharting, 1781); Germania (JRB)
Barron, J. R. 1971: 28 (syn. Ostoma septentrionalis Randall, 1838); USA: Maine (JRB)
Borowiec, L. 1983: 13 (Ostoma). Burakowski, B. et al. 1986: 122 (Ostoma)
Klausnitzer, B. 1976: 7 (Ostoma). Klausnitzer, B. 1996: 155 (Ostoma, larva). Kolibáč, J. 1993a: 21 (Ostoma). Kolibáč, J. 1993b: 90 (Ostoma). Kolibáč, J. 1999b: 12 (Ostoma). Kolibáč, J. 2006: 108 (larva). Kolibáč, J. 2007a: 366 (syn. Peltis cimicoides DeGeer, 1774). Kolibáč, J. 2007a: 366 (syn. Peltis fraterna Randall, 1838). Kolibáč, J. 2007a: 366 (syn. Peltis nigricans Dalla Torre, 1879). Kolibáč, J. 2007a: 366 (syn. Peltis nigrina Casey, 1916). Kolibáč, J. 2007a: 366 (syn. Peltis rubicunda Laicharting, 1781). Kolibáč, J. 2007a: 366 (syn. Peltis septentrionalis Randall, 1838). Krasutskii, B. V. 1996: 274 (Ostoma). Lafer, G. Sh. 1992: 84 (Ostoma). Mitter, H. 1998: 561 (Ostoma). Nakane, T. et al. 1963: 181 (Ostoma). Pileckis, S. & V. Monsevičius 1995: 272 (Ostoma). Reitter, E. 1876: 62 (Ostoma). Vogt, H. 1967: 17 (Ostoma)
Peltis gigantea Reitter, 1882; East Siberia, Far East, Japan, China: Northeast Territory (JK)
Léveillé, A. 1910: 29 (Ostoma subgen. Zimioma). Esaki, T. et al. 1951: 1062 (Ostoma). Kolibáč, J. 2007a: 366. Lafer, G. Sh. 1992: 84 (Zimioma). Nakane, T. et al. 1963: 181 (Zimioma)
Peltis grossa Linnaeus, 1758; Europe (JK)
Léveillé, A. 1910: 29 (Ostoma subgen. Zimioma)
Léveillé, A. 1910: 30 (syn. Ostoma (Zimioma) lunata Fabricius, 1787); Europe (AL)
Bahillo de la Puebla P. & López-Colón, J. I. 1999: 13 (Zimioma). Sarikaya, O. & Avci, M. 2009: 129 (Ostoma). Borowiec, L. 1983: 12. Burakowski, B. et al. 1986: 121 (Zimioma). Cunev, J. 1999: 76. Fjellberg, A. & Hansen, S. O. 1997: 77 (biology). Karalius, S. & Monsevičius, V. 1992: 5 (distribution). Klausnitzer, B. 1976: 7 (Zimioma). Klausnitzer, B. 1978: 176. Klausnitzer, B. 1996: 156 (larva). Kolibáč, J. 1993a: 21. Kolibáč, J. 1993b: 90. Kolibáč, J. 2005: 139 (redescription). Kolibáč, J. 2007a: 366. Krasutskii, B. V. 2006: 763 (biology). Mamaev, B. M. 1976: 1656 (larva). Mitter, H. 1998: 560 (Zimioma). Pileckis, S. & Monsevičius, V. 1995: 272. Ratti, E. 1997: 178. Reitter, E. 1876: 62 (Ostoma). Šablevičius, B. & Ferenca, R. 1995: 146. Svitra, G. & Aliukonis, A. 2009: 72 (distribution, biology). Vogt, H. 1967: 17 (Zimioma)
Peltis jakowlewi Semenov, 1898; Russia: South European Territory (JK)
Léveillé, A. 1910: 30 (Ostoma subgen. Zimioma). Kolibáč, J. 2007a: 366
† Peltis laminata Wickham, 1910; USA: Colorado, Florissant; Tertiary: Lower Oligocene (JRB, varA)
Barron, J. R. 1971: 120. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 24. Wickham, H. F. 1910: 48
† Peltis minuscula Pilton, 1935; France: Cantal; Tertiary: Upper Miocene, Messinian (varA)
Deuve, P. 1988: ii (exact reference unknown). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Pilton, 1935: ii (exact reference unknown). Schmied, H. et al. 2009: 24
Peltis pippingskoeldi Mannerheim, 1852; western states of USA, Canada: British Columbia (JRB)
Léveillé, A. 1910: 31 (Ostoma). Barron, J. R. 1971: 25 (Ostoma). Dajoz, R. 1997: 44 (Ostoma) (biology). Reitter, E. 1876: 62 (Ostoma)
Peltis valida Lewis, 1894; Japan (JK)
Léveillé, A. 1910: 30 (Ostoma subgen. Zimioma). Kolibáč, J. 2007a: 366
Colydiopeltis Ślipiński, 1992
Kolibáč, J. 2006: 126
A record by Ivan Löbl and Daniel Burckhardt (Muséum d’Histoire Naturelle Genève) of three minute wingless Colydiopeltis species from a forest litter in Thailand, together with their scientific processing (
| 1 | Rather elongate species, pronotum narrower than elytra; front coxal cavities externally closed; mandible with two apical teeth | Parapeltis |
| – | More compact species, pronotum at base as wide as elytra; front coxal cavities externally open; mandible with one apical tooth | Colydiopeltis |
http://species-id.net/wiki/Colydiopeltis
Figs 9, 16; Map 8Colydiopeltis burckhardti Ślipiński, 1992 [by original designation]
Kolibáč, J. 2005: 50. Kolibáč, J. 2006: 111 (phylogeny)
Body size: 1.5–2.0 mm. Body shape convex (not conglobate). Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: two or 1. Galea: shape very small. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: one. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge present. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection curved upwards (Colydiopeltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 8-segmented, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron wide. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation regular, scales present. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale present. Tegmen composed of three parts. Coxitae undivided.
Unknown. Species of the genus were collected from forest litter by mass-sampling methods in the dry season.
Colydiopeltis burckhardti Ślipiński, 1992; Thailand: Chiang Mai (AD)
Ślipiński, S. A. 1992: 449. Kolibáč, J. 2005: 49
Colydiopeltis compactum Ślipiński, 1992; Thailand: NE of Bangkok (AD)
Ślipiński, S. A. 1992: 450
Colydiopeltis loebli Ślipiński, 1992; Thailand: Phetchaburi, Kanchanburi (AD)
Ślipiński, S. A. 1992: 451
http://species-id.net/wiki/Parapeltis
Fig. 9; Map 8Parapeltis australicum Ślipiński, 1992 [by original designation and monotypy]
Kolibáč, J. 2005: 75. Kolibáč, J. 2006: 111 (phylogeny)
Body size: 1.2 mm. Body shape convex (not conglobate). Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Pubescence above mola or cutting edge present. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection curved upwards (Colydiopeltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 8-segmented, sensorial fields absent. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales present. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Spiculum gastrale present. Tegmen composed of three parts.
Unknown. Collected from leaf litter in Eucalyptus woodland.
Australia: Queensland.
Parapeltis australicum Ślipiński, 1992; Australia: Queensland
Ślipiński, S. A. 1992: 454. Kolibáč, J. 2005: 75
Phloiophilus Stephens, 1830
Bouchard, P. et al. 2011: 56 (as Phloiophilidae). Klausnitzer, B. 1996: 145. Kolibáč, J. 1987: 110. Kolibáč, J. 2004a: 242. Kolibáč, J. 2008: 123 (phylogeny; stat. n. sub Trogossitidae). Lohse, G. A. 1979: 83 (as Phloeophilinae; sub Melyridae). Lawrence, J. F. 1982: 519 (morphology, systematics). Lawrence et al. 1993: CD-ROM (morphology of larvae). Lawrence et al. 1999a: CD-ROM (morphology of larvae). Lawrence et al. 1999b: CD-ROM (morphology of larvae).
Phloiophilus has been classified within Dasytidae or Melyridae sensu lato (
http://species-id.net/wiki/Phloiophilus
Figs 10, 15; Map 9Phloiophilus edwardsii Stephens, 1830 [by monotypy]
Lohse, G. A. 1979: 83 (Phloeophilus, sub Melyridae). Majer, K. 1986: 114. Kolibáč, J. 2008: 105
Body size: about 3.0 mm. Body shape convex (not conglobate). Gular sutures wide, subparallel. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size moderate. Eyes number: two. Epicranial acumination absent. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge present. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Tegmen composed of only one part. Coxitae divided.
Larva: Frontal arms curved (cucujoid). Epicranial stem reduced. Endocarina present. Gular sutures inconspicuous. Gula: anterior apodemes present. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally even, vertically situated. Lacinia mandibulae tridentate. Mola absent. Maxillary palpi 3-segmented. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion present. Ligula present. Labial palpi 2-segmented. Antennal joints 1, 2 transverse. Sensory appendix larger than half of joint 3. Thoracic sclerites pattern (dorsally) 1-0-0. Thoracic sclerites pattern (ventrally) 1+1+1. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
A Thymalus limbatus B Phloiophilus edwardsi C Eronyxa marginicollis D Decamerus haemorhoidalis E Diontolobus punctatipennis F Afrocyrona ciskeiensis G Afrocyrona dwesae H Grynoma sp., New Zealand I Grynoma diluta.
Fungivorous.
Phloiophilus edwardsii Stephens, 1830; Austria, Belgium, Czechia, Denmark, France, Great Britain, Germany, Hungary, Ireland, the Netherlands, Poland, North Africa (JK)
Audisio, P. et al. 1995: 14. Beutel, R. G. & Pollock, D. A. 2000: 826 (larva, morphology). Crowson, R. A. 1964b: 151 (biology). Gurlich, S. et al. 1995: 49. Klausnitzer, B. 1996: 161. Kolibáč, J. 1999: 12. Kolibáč, J. 2008: 120. Lohse, G. A. 1979: 83 (Phloeophilus, sub Melyridae). Majer, K. 1986: 114. Mayor, A. 2007: 363 (syn. Phloiophilus bimaculatus Stephens, 1830; synonymized by whom?). Mayor, A. 2007: 364 (syn. Phloiophilus cooperi Stephens, 1830; synonymized by whom?). H. Vogt 1967: 13. Wielink van, P. et al. 2010: 17 (biology)
Thymalus Latreille, 1802
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 79 (Meligethiellinae, new Mesozoic subfamily). Kolibáč, J. 2006: 126 (diagnosis, stat. n.). Kolibáč, J. 2007a: 366. Krivosheina, N. P. & Mamaev, B. M. 1981: 50. Lawrence, J. F. & Newton, A. F. Jr. 1995: 868 (Protopeltinae; Rentoniinae). Ślipiński, S. A. 1992: 442 (Protopeltinae, Rentoniinae)
Meligethiellinae Kireichuk & Ponomarenko, 1990
Kolibáč, J. 2006: 126 (synonymized). revalid. subfam. Note: Removed from synonymy; see the “Remarks” section for explanation of this synonymy
Protopeltini Crowson, 1966; Protopeltinae Crowson, 1970
Kolibáč, J. 2006: 126 (synonymized)
Rentoniini Crowson, 1966; Rentoniinae Crowson, 1966 (Rentoniinae = Protopeltini + Rentoniini; all
Kolibáč, J. 2006: 126 (synonymized)
A recent and extensive morphological study by
Since the extinct Mesozoic genera Meligethiella and Ostomalynus are excluded from Thymalini, Peltinae and Trogossitidae herein, the synonymization of the subfamily Meligethiellinae is not valid henceforth and the taxon should not be further listed in synonymy of the tribe Thymalini.
(Globorentonium Lawrence & Ślipiński, 2013 not included)
| 1 | Body convex but not conglobate; elytra with conspicuous punctation | 2 |
| – | Body conglobate; elytra without sculpture (smooth) or with very fine irregular punctures or shagreened | 3 |
| 2 | Body extremely bulged; head including eyes covered by pronotum when viewed from above (only clypeus and labrum visible); larger species (about 4–7 mm) | Thymalus |
| – | Body convex but not extremely bulged; head protruding (not covered by pronotum when viewed from above); smaller species (about 2.5 mm) | Protopeltis |
| 3 | Elytra with light and dark spots; pubescence formed by short decumbent and long erect hairs | Australiodes |
| – | Elytra unicolorous, without spots; pubescence absent or made up of short hairs only | 4 |
| 4 | Elytra smooth, without pubescence, strongly involute; middle tibiae with row of spines at apex; wingless | Rentonellum |
| – | Elytra pubescent, not involute; middle tibiae probably without row of spines at apex; mostly winged | 5 |
| 5 | Middle coxal cavities widely separated; ventrites II–V with deeply transversely impressed anterior margins; last antennal segment transverse and truncate at apex; pubescence of elytra with shot-silk effect | Parentonium |
| – | Middle coxal cavities narrowly separated; ventrites II–V with or without weakly impressed anterior margins; last antennal segment not truncate at apex; pubescence of elytra sparse, directed backwards, without shot-silk effect | 6 |
| 6 | Head strongly transverse; front margin of clypeus with marked emargination; first ventrite with median keel at least in front; ventrites II–IV each with transverse line of strong backward-projecting setae near its hind border | Rentonidium |
| – | Head not transverse or only slightly so; front margin of clypeus almost straight; first ventrite without median keel; ventrites II–IV without such apical lines of setae | Rentonium |
http://species-id.net/wiki/Australiodes
Map 10Clambus vestitus Broun, 1886 [by monotypy]
Crowson, R. A. 1966: 120 (transferred from Liodidae to Trogossitidae: Peltinae). Kolibáč, J. 2005: 47. Kolibáč, J. 2006: 116 (phylogeny)
Body size: 1.4 mm. Body shape conglobate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Eyes number: two. Lacinial hooks absent. Galea: shape partially fused with lacinia. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Labrum-Cranium not fused. Front coxal cavities externally open, internally closed. Pronotum subquadrate. Elytra: long hairs absent. Tegmen composed of a single unit (
“Maxilla with galea and lacinia largely fused; erect setae among pubescence of upper surface; metendosternite with elongate oblique arms, without lamina; elytra with pattern of light and dark patches” (ex
Adults were collected by
New Zealand: Port Nicholson, Wellington; Waipoua State Forest, Northland; Western Hills, Whangarei.
A distribution of the tribe Thymalini including Rentonium-group.
Australiodes vestitus Broun, 1886; New Zealand (varA)
Crowson, R. A. 1966: 120. Endrödy-Younga, S. 1960: 239 (Liodidae). Kolibáč, J. 2005: 47
http://species-id.net/wiki/Globorentonium
Figs 9, 17; Map 10Globorentonium globulum Lawrence & Ślipiński, 2013 [by original designation]
The genus has been just recently described, therefore, it is not included in a generic key to Thymalini. A key to the all genera of Rentonium-group (denoted as Rentoniini Crowson, 1966) is provided by authors (
Australia: Victoria, New South Wales, Tasmania; Brazil: Santa Catarina.
Globorentonium globulum Lawrence & Ślipiński, 2013; Australia: Victoria, NSW, Tasmania (AD)
Lawrence, J. F. & Ślipiński, S. A. 2013: 260
Globorentonium lescheni Lawrence & Ślipiński, 2013; Australia: NSW (AD)
Lawrence, J. F. & Ślipiński, S. A. 2013: 264
Globorentonium plaumanni Lawrence & Ślipiński, 2013; Brazil: Santa Catarina (AD)
Lawrence, J. F. & Ślipiński, S. A. 2013: 268
http://species-id.net/wiki/Parentonium
Map 10Rentonium magnum Crowson, 1966 [by original designation]
Kolibáč, J. 2005: 76. Kolibáč, J. 2006: 116 (phylogeny)
Body size: 1.3–2.0 mm. Body shape conglobate. Gular sutures wide, subparallel. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum of males: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks absent. Galea: shape partially fused with lacinia. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge denticulate. Front coxal cavities externally open, internally closed. Pronotum transverse. Elytra: long hairs absent. Epipleuron wide. Elytral interlocking mechanism absent, scales absent. Front tibiae: spines along side reduced. Claws: denticle absent.
Parentonium australicum was found under the bark of a fallen Nothofagus trunk at an altitude of about 1200 m. Parentonium magnum was collected in mixed leaf-litter at about 250 m (
Australia: Queensland, Lamington National Park; New Zealand: Grampian Hill, Nelson.
Parentonium australicum Crowson, 1970; Australia: Queensland (RAC)
Crowson, R. A. 1970: 6
Parentonium magnum Crowson, 1966; New Zealand (RAC)
Crowson, R. A. 1966: 121 (Rentonium). Crowson, R. A. 1970: 6 (Parentonium, combination). Kolibáč, J. 2005: 76
http://species-id.net/wiki/Protopeltis
Fig. 9; Map 10Grynoma viridescens Broun, 1886 [by original designation]
Crowson, R. A. 1964a: 295. Crowson, R. A. 1966: 120. Kolibáč, J. 2005: 79. Kolibáč, J. 2006: 111 (phylogeny)
Body size: about 2.5 mm. Body shape flat. Gular sutures wide, subparallel. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Lacinial hooks: three. Galea: shape elongate. Galea: ciliate setae absent. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge present. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Ligula: ciliate setae absent. Ligula membranous, not retroflexed. Antenna 11-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell oblong (or reduced), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Claws: denticle absent. Tegmen composed of two parts.
Larva: Frontal arms curved (cucujoid). Epicranial stem present. Endocarina present. Gular sutures conspicuous, parallel. Gula: anterior apodemes present. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae tridentate. Mola absent. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion present. Cardo-Stipes not fused. Cardo: size much smaller than stipes. Labial palpi 2-segmented. Prementum in single part, anterior margin even. Antennal joints 1, 2 transverse. Sensory appendix larger than half of joint 3. Thoracic sclerites pattern (dorsally) 1-2-2. Thoracic sclerites pattern (ventrally) 1+0+0. Trochanter triangular. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
Larvae of Protopeltis viridescens were collected, according to Crowson’s note, at “fungusy bark of dead Nothofagus”. The adult gut he examined contained “abundant fungal material”, determined as “probably Hymenochaete sp.” (
New Zealand (for example, Arthur’s Pass National Park).
Protopeltis pulchella Broun, 1915; New Zealand (RAC)
Broun, T. 1915: 314 (Promanus). Crowson, R. A. 1964a: 289
Protopeltis viridescens Broun, 1886; New Zealand (RAC)
Léveillé, A. 1910: 29 (Grynoma). Crowson, R. A. 1964a: 290 (larva). Iablokoff-Khnzorian, S. M. 1975: 147. Kolibáč, J. 2005: 78. Kolibáč, J. 2006: 109
http://species-id.net/wiki/Rentonellum
Figs 9, 17; Map 10Rentonellum apterum Crowson, 1966 [by original designation and monotypy]
Kolibáč, J. 2005: 80. Kolibáč, J. 2006: 111 (phylogeny)
Body size: about 1.0 mm. Body shape conglobate. Gular sutures wide, subparallel. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks absent. Galea: shape partially fused with lacinia. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 10-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally closed. Pronotum transverse. Prepectus absent. Middle coxal cavities closed. Elytra: long hairs absent. Epipleuron wide. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Coxitae undivided.
Rentonellum apterum was found in Nothophagus and Podocarpus leaf-litter at an altitude of 900 m (
New Zealand; Brazil: “Nova Teutonia”.
Rentonellum apterum Crowson, 1966; New Zealand (RAC)
Crowson, R. A. 1966: 120. Kolibáč, J. 2005: 80
Rentonellum loebli Kolibáč, 2005; Brazil: “Nova Teutonia” (JK)
Kolibáč, J. 2005: 80
Note: It has been recently proposed that Rentonellum loebli might be a ciide rather than a rentoniine species (
http://species-id.net/wiki/Rentonidium
Figs 9, 17; Map 10Rentonidium costiventris Crowson, 1966 [by original designation and monotypy]
Kolibáč, J. 2005: 81. Kolibáč, J. 2006: 116 (phylogeny)
(according to
Collected in “male flower of Pinus insignis” (
New Zealand: Northland, Waipoua State Forest.
Rentonidium costiventris Crowson, 1966; New Zealand (RAC)
Crowson, R. A. 1966: 123. Kolibáč, J. 2005: 81
http://species-id.net/wiki/Rentonium
Figs 9, 17; Map 10Rentonium daldiniae Crowson, 1966 [by original designation and monotypy]
Crowson, R. A. 1970: 6. Kolibáč, J. 2005: 81 (redescription). Kolibáč, J. 2006: 111 (phylogeny)
Body size: 1.3–1.5 mm. Body shape conglobate. Gular sutures wide, subparallel. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Mandibular apical teeth number: one. Mola reduced but present. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 10-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally closed. Pronotum transverse. Epipleuron thin. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell oblong (or reduced), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Spiculum gastrale present. Tegmen composed of only a single unit.
Larva: Frontal arms V-shaped. Epicranial stem absent. Endocarina present. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae with several small spines. Mola absent. Maxillary palpi 2-segmented. Labial palpi 1-segmented. Torma: two separate lateral sclerites. Antennal joints 1, 2 transverse. Sensory appendix larger than half of joint 3. Thoracic sclerites pattern (dorsally) 1-2-2. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
The single known larva described (Crowson speculated that it might be identified as Rentonium daldiniae) was extracted from a forest litter sample (mainly Nothofagus) at an elevation of about 500m (
New Zealand: Canterbury, Waimate. South Chile: Isla Bertrand.
Rentonium chilense Crowson, 1970; Chile (RAC)
Crowson, R. A. 1970: 7. Kolibáč, J. 2005: 81 (redescription)
Rentonium daldiniae Crowson, 1966; New Zealand (RAC)
Crowson, R. A. 1966: 121, 123 (supposed larva). Crowson, R. A. 1970: 7. Kolibáč, J. 2005: 81 (redescription). Kolibáč, J. 2006: 109
http://species-id.net/wiki/Thymalus
Figs 10, 16, 18; Map 10Peltis brunnea Thunberg, 1794 (= Cassida limbata Fabricius, 1787) [by original designation and monotypy]
Léveillé, A. 1910: 32. Barron, J. R. 1971: 35. Crowson, R. A. 1964a: 296. Kolibáč, J. 2005: 85 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 366. Nikitsky, N. B. et al. 1998: 29 (key). Lafer, G. Sh. 1992: 83. Reitter, E. 1876: 64
Thymalops Iablokoff-Khnzorian, 1962 [Type species: Cassida limbata Fabricius, 1787]
Barron, J. R. 1971: 35. Iablokoff-Khnzorian, S. M. 1962: 421
Comparing the larvae as well as adults of Thymalus and Protopeltis, I found some interesting similarities, which led me to consideration of their phylogenetic relationship. Later character analysis (
Body size: 4.3–7.5 mm. Body shape convex (not conglobate). Gular sutures wide, subparallel. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: two. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 11-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally closed. Pronotum transverse. Prepectus absent. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron wide. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell present, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side reduced. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts.
Larva: Frontal arms V-shaped. Epicranial stem reduced. Endocarina absent. Gular sutures inconspicuous. Gula: anterior apodemes present. Paragular sclerites absent. Hypostomal rods present. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae with several small spines. Mola absent. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion present. Cardo-Stipes partially fused. Cardo: size much smaller than stipes. Ligula present. Labial palpi 2-segmented. Prementum in single part, anterior margin even. Torma: two separate lateral sclerites. Antennal joints 1, 2 transverse. Sensory appendix larger than half of joint 3. Thoracic sclerites pattern (dorsally) 2-0-0. Thoracic sclerites pattern (ventrally) 0+0+0. Trochanter triangular. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
The beetles are not associated with any particular tree species and are found on both deciduous and coniferous trees. Thymalus limbatus is known from the trunks of birch, beech, linden, and spruce, mostly under bark. It is assumed that the larvae feed on fungi in rotten or decaying wood (
Holoarctic: Northern states of USA, Canada, Europe, North Africa, Siberia to China and Japan. Some specimens, probably a new species, have recently been collected in Chinese Sichuan and Yunnan and also in northern Thailand.
Thymalus aubei Léveillé, 1877; “Batum”, Caucasus (varA)
Léveillé, A. 1910: 32. Klausnitzer, B. 1996: 156. Barron, J. R. 1971: 36 (syn. Thymalus aubei Léveillé, 1877 with Thymalus marginicollis Chevrolat, 1842). Barron, J. R. 1971: 36 (syn. Thymalus fulgidus var. aubei Léveillé, 1877 with Thymalus marginicollis Chevrolat, 1842). Kolibáč, J. 2007a: 366 (syn. Thymalus subtilis Reitter, 1889). Lafer, G. Sh. 1992: 86 (Thymalus subtilis Reitter, 1889). Léveillé, A. 1910: 33 (Thymalus subtilis Reitter, 1889). Nikitsky, N. B. et al. 1998: 28 (syn. Thymalus subtilis Reitter, 1889; lectotype designated). Nikitsky, N. B. & Semenov, V. B. 2001: 49
Thymalus chinensis Fairmaire, 1900; China: Fujian (JK)
Léveillé, A. 1910: 32. Kolibáč, J. 2007a: 366
Thymalus laticeps Lewis, 1894; Japan (varA)
Léveillé, A. 1910: 32. Esaki, T. et al. 1951: 1063. Kolibáč, J. 2007a: 366. Lafer, G. Sh. 1992: 86. Nakane, T. et al. 1963: 181
Thymalus limbatus Fabricius, 1787; Europe, North Africa: Tunisia (JK)
Léveillé, A. 1910: 32. Alexander, K. N. A. 1996: 90 (biology). Bahillo de la Puebla, P. & López-Colón, J. I. 1999: 13. Bahillo de la Puebla, P. & López-Colón, J. I. 2004: 129. Bercedo, P. et al. 2006: 180 (distribution). Borowiec, L. 1983: 15. Burakowski, B. et al. 1986: 123. Cunev, J. 1999: 76. Franz, H. 1981: 51–52 (distribution). Klausnitzer, B. 1976: 8. Klausnitzer, B. 1978: 176. Klausnitzer, B. 1996: 155. Kolibáč, J. 1993a: 20. Kolibáč, J. 1993b: 90. Kolibáč, J. 2002: 55 (larva). Kolibáč, J. 2005: 85 (redescription). Kolibáč, J. 2006: 110 (larva). Kolibáč, J. 2007a: 366 (distribution). Kolibáč, J. 2007a: 366 (syn. brunneus Thunberg, 1794). Kolibáč, J. 2007a: 366 (syn. rubiginosus Gmelin, 1790). Krasutskii, B. V. 1996: 274. Mitter, H. 1998: 561. Pileckis, S. & Monsevičius, V. 1995: 273. Ratti, E. 1997: 178. Reitter, E. 1876: 64. Theunert, R. 2006: 113–114 (distribution). Vogt, H. 1967: 18
Thymalus marginicollis Chevrolat, 1842; Canada, USA (JRB)
Barron, J. R. 1971: 36 (syn. Thymalus aubei Léveillé, 1877). Barron, J. R. 1971: 36 (syn. Thymalus fulgidus Erichson, 1844). Barron, J. R. 1971: 36 (syn. Thymalus fulgidus var. aubei Léveillé, 1877). Böving, A. G. & Craighead, F. C. 1931: 273 (larva). Dajoz, R. 1997: 44 (biology). Reitter, E. 1876: 64 (Thymalus fulgidus Erichson, 1844: “Amer. bor.”)
Thymalus oblongus Reitter, 1889; Russia: North and Central Europea teritorries, Sweden, East Siberia (JK)
Léveillé, A. 1910: 33. Lafer, G. Sh. 1992: 86. Kolibáč, J. 2007a: 366. Krasutskii, B. V. 2006: 763 (biology). Nikitsky, N. B. et al. 1998: 28 (lectotype designated)
Thymalus parviceps Lewis, 1894; Japan (varA)
Léveillé, A. 1910: 33. Esaki, T. et al. 1951: 1063. Kolibáč, J. 2007a: 366. Lafer, G. Sh. 1992: 86. Nakane, T. et al. 1963: 181
Thymalus punctidorsum Latreille, 1894; Japan (varA)
Léveillé, A. 1910: 33. Kolibáč, J. 2007a: 366. Lafer, G. Sh. 1992: 86. Nakane, T. et al. 1963: 181
The subfamily Lophocaterinae was established by
| 1 | Tarsal claws with denticle. Larva: median process between urogomphi absent | Decamerini |
| – | Tarsal claws without denticle. Larva: median process between urogomphi present | 2 |
| 2 | Penicillus mostly composed of tuft of long setae. Frontoclypeal suture absent or weak and straight | Ancyronini |
| – | Penicillus membranous or composed of short, fine setae. Frontoclypeal suture conspicuous, mostly broadly emarginate | Lophocaterini |
http://species-id.net/wiki/Cretamerus
Map 11† Cretamerus vulloi Peris, Kolibáč & Delclòs, (in press) [by monotypy and author’s designation]
The fossil is the oldest known record confirmed for the entire superfamily Cleroidea on the European continent. Due to the fine state of preservation, certain morphological character states of the fossil were inserted into a character matrix of Trogossitidae genera. The resulting tree reveals the basal position of Cretamerus vulloi within the lophocaterine clade. It may form an extinct branch of the recent Decamerini.
In view of its fine state of preservation, certain morphological character states of the fossil to be inserted into a character matrix of Trogossitidae genera. The resulting tree reveals the basal position of Cretamerus vulloi within the lophocaterine clade. It may form an extinct branch of the recent Decamerini.
Body very small, less than 2 mm. Antenna 10-segmented, 3-segmented club nearly symmetrical. Prothorax with dentate lateral margin; procoxal cavities externally closed. Elytra without carinae, punctation irregular or less than obviously regular. Tibiae with two straight (i.e. not hooked) spurs. Abdomen with six visible ventrites.
France: Charente-Maritime (Fouras/Bois-Vert); Cretaceous: early Cenomanian.
A distribution of the tribe Decamerini including the newly described genus † Cretamerus. A position of Eronyxa is indicated.
† Cretamerus vulloi Peris, Kolibáč & Delclòs (in press); France; Cretaceous (AD)
Peris D., Kolibáč J. & Delclòs X. (in press): iii
Decamerus Solier, 1849
Barron, J. R. 1975: 12 (syn. Decamerinae = Peltinae). Kolibáč, J. 2006: 127 (diagnosis, stat. n.). Lawrence, J. F. & Newton, A. F., Jr. 1995: 868 (Decamerinae). Ślipiński, S. A. 1992: 442, 460 (key) (Decamerinae)
Since it was established (
| 1 | Front coxal cavities completely externally closed; tarsal claws split into two almost identical parts | Antixoon |
| – | Front coxal cavities externally open or not completely closed; tarsal claws with large denticle (but shorter than outer claw) | 2 |
| 2 | Antenna 10-segmented; tegmen composed of one part; front coxal cavities open (or closed to 3/4) | Decamerus |
| – | Antenna 11-segmented; tegmen composed of three parts; front coxal cavities nearly closed | Diontolobus |
Antixoon cribripenne Gorham, 1886 (sub Melyridae) [by monotypy] (not included in
Crowson, R. A. 1964a: 291 (key). Kolibáč, J. 2005: 47. Kolibáč, J. 2006: 116 (phylogeny)
In consideration of a recent re-classification of Eronyxa within Lophocaterini (
Body size: about 3.0 mm. Body shape flat. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Antennal groove absent. Eyes number: two. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally closed. Pronotum transverse. Elytra: long hairs absent. Claws: denticle distinct.
Floricolous: “collected in the flowers of bushes in more or less open country”
(Map 11.) Central America: Panama.
Antixoon cribripenne Gorham, 1886; Central America (Panama) (RAC)
Gorham, H. S. 1886: 332
http://species-id.net/wiki/Decamerus
Figs 10, 18; Map 11Decamerus haemorrhoidalis Solier, 1849 [by monotypy]
Léveillé, A. 1910: 28. Crowson, R. A. 1964a: 291. Kolibáč, J. 2005: 52 (redescription). Kolibáč, J. 2006: 111 (phylogeny)
Peltostoma Reitter, 1877 [Type species: Peltostoma unguicularis Reitter, 1877; by monotypy]
Léveillé, A. 1910: 28 (synonymized?). Reitter, E. 1877: 173
Body size: about 3.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projections extending laterally and downwards (Eronyxa). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 10-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle distinct. Parasternites number along ventrites III–VII: absent. Spiculum gastrale absent. Tegmen composed of only a single part.
The species occur on the flowers of various bushes. Floricolous, found together with Diontolobus.
Central part of Chile, approximately regions IV to VI.
Decamerus haemorrhoidalis Solier, 1849; Chile (AL)
Léveillé, A. 1910: 28. Kolibáč, J. 2005: 52 (redescription). Reitter, E. 1877: 174 (syn. Peltostoma unguicularis Reitter, 1877; synonymized by
http://species-id.net/wiki/Diontolobus
Fig. 10; Map 11Diontolobus punctipennis Solier, 1849 [by monotypy]
Léveillé, A. 1910: 27. Crowson, R. A. 1964a: 291. Kolibáč, J. 2005: 53 (redescription). Kolibáč, J. 2006: 111 (phylogeny)
Micropeltis Redtenbacher, 1867 [Type species: Micropeltis serraticollis Redtenbacher, 1867; by monotypy]
Léveillé, A. 1910: 27 (synonymized?). Reitter, E. 1876: 58
Body size: about 3.0–4.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks absent. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge present. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection curved upwards (Colydiopeltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 11-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally closed, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle distinct. Parasternites number along ventrites III–VII: absent. Spiculum gastrale absent. Tegmen composed of three parts.
Larva: Frontal arms curved (cucujoid). Epicranial stem absent. Endocarina absent. Gular sutures conspicuous, parallel. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: five. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae plumose. Mola present. Maxillary palpi 3-segmented. Pedunculate seta absent. Mala simple. Cardo-Stipes not fused. Ligula present. Labial palpi 2-segmented. Prementum in single part. Antenna 1st transverse, 2nd elongate. Sensory appendix medium sized (to half of joint 3). Thoracic sclerites pattern (dorsally) 0+0+0. Abdominal segment IX not divided. Tergite IX flat. Urogomphi present, hooked; median process absent.
The species occur on flowers. Pollen grains were found in the gut of Diontolobus punctipennis (
As same as in Decamerus, the central part of Chile, approximately regions IV to VI.
Diontolobus costulata Reitter, 1876; Chile (AL)
Léveillé, A. 1910: 27. Reitter, E. 1876: 60 (Micropeltis costulata Reitter, 1876)
Diontolobus flavolimbata Reitter, 1877; Chile (AL)
Léveillé, A. 1910: 27
Diontolobus inaequalis Reitter, 1877; Chile (AL)
Léveillé, A. 1910: 28
Diontolobus incostata Reitter, 1876; Chile (AL)
Léveillé, A. 1910: 28. Reitter, E. 1876: 59 (Micropeltis incostata Reitter, 1876)
Diontolobus lanuginosa Léveille, 1895; Chile (AL)
Léveillé, A. 1910: 28
Diontolobus punctipennis Solier, 1849; Chile (AL)
Léveillé, A. 1910: 28. Kolibáč, J. 2005: 53 (redescription). Reitter, E. 1876: 59 (syn. Micropeltis serraticollis Redtenbacher, 1867, synonymized by
Diontolobus punctipennis var. lateritius Fairmaire, 1883: 488
Diontolobus sp. (supposed larva); Chile (RAC)
Crowson, R. A. 1964a: 291. Kolibáč, J. 2006: 106
Ancyrona Reitter, 1876 [designated by
Kolibáč, J. 2007a: 365. Kolibáč, J. & Zaitsev, A. A. 2010: 59 (phylogeny, larval morphology)
Ancyronini are undeniably related to Lophocaterini. As observations made upon the recently recorded Ancyrona diversa larva have shown (
| 1 | Head quite hypognathous; tarsal pattern 4-4-4 or 5-5-5; body convex | 2 |
| – | Head quite prognathous; tarsal pattern 5-5-5; body more/less flattened | 3 |
| 2 | Antennae 10- or 11-segmented with loose 3-segmented club; mandible with penicillus formed by membranous appendage with short setae; eyes distinctly elevate | Afrocyrona |
| – | Antennae 10-segmented with compact 2-segmented club, segments 9 and 10 coalescent; mandible with penicillus composed of tuft of long setae; eyes not very elevate | Neaspis |
| 3 | Eyes distinctly elevate; labrum sometimes fused with cranium (males); lacinia without hooked spurs; mediostipes fused with lacinia | 4 |
| – | Eyes not very elevate; labrum free; lacinia with hooked pigmented spurs; mediostipes not fused with lacinia | Ancyrona |
| 4 | Mandible enlarged, male mandible monstrous; galea and lacinia without spines | Leptonyxa |
| – | Mandible not enlarged; lacinia with several pale spines | Grynoma |
http://species-id.net/wiki/Afrocyrona
Fig. 10; Map 12Afrocyrona dwesae Kolibáč, 2007 [designated by
Body size: 2.9–5.0 mm. Body shape flat. Gular sutures narrow, subparallel at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 11-segmented or 10-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales present. Wing: radial cell oblong (or reduced) or cell moved down, often small, wedge cell absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Tarsal pattern 4-4-4 or 5-5-5. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of two parts. Coxitae divided.
The species were collected by beating branches and sifting rotten wood and litter. Detritus and some insect remnants were found in the gut of Afrocyrona dwesae, whereas only insect fragments were found in the gut of Afrocyrona ciskeiensis. The species are probably predatory and partly or occasionally fungivorous. Afrocyrona dwesae may be unable to fly.
Three described species are known from South Africa: Eastern Cape and Transvaal. One or two more species have been recently found in the island of Sokotra.
A distribution of the tribe Ancyronini.
Afrocyrona ciskeiensis Kolibáč, 2007; South Africa (JK)
Kolibáč, J. 2007b: 61
Afrocyrona dwesae Kolibáč, 2007; South Africa (JK)
Kolibáč, J. 2007b: 63
Afrocyrona gussmannae Kolibáč, 2007; South Africa (JK)
Kolibáč, J. 2007b: 64
http://species-id.net/wiki/Ancyrona
Figs 2, 11; Map 12Ancyrona lewisi Reitter, 1876 [designated by Kolibáč 1993]
Léveillé, A. 1910: 25. Lafer, G. Sh. 1992: 83. Matthews, E. G. 1992: 3 (key, Australia). Kolibáč, J. 2005: 45. Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 365. Kolibáč, J. & Zaitsev, A. A. (2010): 55 (larva)
Latolaeva Reitter, 1876 (Type species: Latolaeva ferrarii Reitter, 1876; designated by
Léveillé, A. 1910: 25. Kolibáč, J. 2005: 63. Kolibáč, J. 2006: 111 (phylogeny).
Body size: 3.1–8.0 mm. Body shape flat. Gular sutures narrow, subparallel at apex. Frontoclypeal suture present or broadly emarginate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 10-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales present. Wing: radial cell oblong (or reduced) or cell moved down, often small, wedge cell absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of two or three parts. Coxitae undivided.
Larva Ancyrona diversa Pic, 1921 (
A Ancyrona (syn. Latolaeva) bivittata B Ancyrona vicina C Ancyrona kosnovskorum (= fairmairei?) D Ancyrona sp., lewisi-group, Malaysia E Ancyrona japonica, Slovakia F Ancyrona gabonica G Leptonyxa fairmairei H Neaspis variegata.
Predatory. Adults can be beaten from dry branches or individually collected on fallen timber, where they hunt for other insects.
Africa in the south of Sahara, eastern Asia (from India to Japan and Russian Far East), Australia: Queensland, New South Wales (types of Ancyrona aegra, Ancyrona amica, Ancyrona latebrosa, Ancyrona laticeps, Ancyrona vesca checked), New Guinea (including undescribed species). Strangely enough, one Japanese species, Ancyrona japonica, was introduced to Europe and first recorded in Hungary (“Visegrad 1904”). It has now been collected several times in adjacent Slovakia, on branches of decaying oak. Two Tertiary fossil species are known from European Eocene.
In a review of Afrocyrona and Ancyrona, I divided the latter genus in several informal species groups (
Species. Ancyrona lewisi, Ancyrona shibatai, Ancyrona haroldi, and numerous undescribed species from south and eastern Asia and New Guinea.
Distribution. Japan, China, southeastern Asia.
Diagnosis. Small, flat, broad, oval species. Dorsal surface with colour pattern formed by scales or rigid decumbent setae.
Species. Ancyrona gabonica, Ancyrona ferrarii, Ancyrona vicina, Ancyrona bivittata, Ancyrona feai, Ancyrona incensa, Ancyrona martini, Ancyrona plana, and numerous undescribed species from eastern Asia and tropical Africa. Australian species Ancyrona aegra, Ancyrona amica, Ancyrona latebrosa, Ancyrona laticeps, Ancyrona vesca probably also belong to the group.
Distribution. Tropical Africa, south-eastern Asia and probably Australia.
Diagnosis. Extremely flat, broad, oval species without scales and lacking decumbent pubescence on dorsal surface; dorsal side quite bare or with inconspicuous or erect pubescence. Some species with longitudinal colour stripes on elytra; stripes not formed by pubescence or scales. Antennal club very loose, nearly serrate. Tegmen composed of two or three parts.
Species. Ancyrona japonica, Ancyrona diversa.
Distribution. Japan, northern China, Ussuri. Ancyrona japonica also spread to south-eastern and central Europe (from Bulgaria through Hungary to Slovakia); first European record in 1904.
Diagnosis. Rather elongate unicolorous species with long, soft pubescence on dorsal surface. Tegmen composed of 1 or 2 parts (parameral piece fused with phallobase), male abdominal segments IX–X more or less reduced.
Species. Ancyrona colobicoides, Ancyrona fairmairei, Ancyrona kosnovskorum (syn. fairmairei?), Ancyrona minor.
Distribution. Madagascar.
Diagnosis. Flat, elongate species with conspicuously elevate eyes and compact 3-segmented club. Yellowish elytra with dark black-brown pattern (stripes, spots), pubescence short and decumbent. Primitive tegmen composed of three parts. Elytra with distinct carinae. One specimen known to me from Madagascan copal (Cap d’Ambre).
Species. Ancyrona endroedyi, Ancyrona muellerae, Ancyrona caffra(?).
Distribution. South Africa.
Diagnosis. Body quite elongate (as in japonica group), with long, soft, erect hairs or short, decumbent, scale-like setae. Dorsal surface unicolorous or with pattern formed by pubescence. Radial cell completely or partly reduced. Tegmen composed of two or three parts. Elytra with more or less conspicuous carinae.
Ancyrona aegra Olliff, 1885; “Nov. Gal. mer.” (AL)
Léveillé, A. 1910: 25
Ancyrona amica Olliff, 1885; S Australia (AL)
Léveillé, A. 1910: 25
Ancyrona andrewesi Léveillé, 1907; India (AL)
Léveillé, A. 1910: 25
Ancyrona aurora Léveillé, 1899; Congo gall. (AL)
Léveillé, A. 1910: 25
Ancyrona bivittata Léveillé, 1899; Cameroon (AL)
Léveillé, A. 1910: 25 (Latolaeva bivittata Léveillé, 1899). Kolibáč, J. 2007b: 55 (comb.; transferred from Latolaeva to gabonica-group)
Ancyrona blaisei Léveillé, 1907; Tonkin (AL)
Léveillé, A. 1910: 25
Ancyrona bouchardi Léveillé, 1902; Sumatra (AL)
Léveillé, A. 1910: 25
Ancyrona brasilica Perty, 1830; Brazil: Minas Geraes (AL)
Léveillé, A. 1910: 25 (Latolaeva brasilica Perty, 1830)
Note: Cleroidea? (see
Ancyrona brunnea Léveillé, 1905; India (AL)
Léveillé, A. 1910: 25
Ancyrona brunneolimbata Léveillé, 1905; Fernando Po (AL)
Léveillé, A. 1910: 25
Ancyrona caffra Reitter, 1876; Cap (AL)
Reitter, E. 1876: 52. Léveillé, A. 1910: 25
Ancyrona cassidoides Reitter, 1876; Malacca (AL)
Léveillé, A. 1910: 25 (Latolaeva cassidoides Reitter, 1876). Reitter, E. 1876: 50 (Latolaeva cassidoides Reitter, 1876)
Ancyrona ciliata Murray, 1867; Old Calabar (Nigeria) (AL)
Léveillé, A. 1910: 25 (transferred from Peltis to Ancyrona). Reitter, E. 1876: 53 (Peltis)
Ancyrona colobicoides Fairmaire, 1868; Madagascar (AL, confirmed JK)
Léveillé, A. 1910: 30 (Ostoma (s.str.)). Kolibáč, J. 2007b: 57 (comb.; transferred from Ostoma to colobicoides-group)
Note: maybe conspecific with Ancyrona kosnovskorum.
Ancyrona congolensis Léveille, 1905; Congo gall. (AL)
Léveillé, A. 1910: 26
Ancyrona crenata Murray, 1867; Old Calabar (Nigeria) (AL)
Léveillé, A. 1910: 26. Reitter, E. 1876: 54 (Peltis)
Ancyrona diversa Pic, 1921; E Siberia (Ussuri) (JK)
Pic, M. 1921: 1 (Ostoma). Kolibáč, J. 1993a: 17 (comb.; transferred from Ostoma to Ancyrona). Kolibáč, J. 1999b: 12 (morphology). Kolibáč, J. 2007a: 365 (distribution). Kolibáč, J. 2007b: 56 (shifted to japonica-group). Kolibáč, J & Zaitsev, A. A. 2010: 53 (larva)
Ancyrona elongata Léveille, 1905; India (AL)
Léveillé, A. 1910: 26
Ancyrona endroedyi Kolibáč, 2007; SA: Transvaal (JK)
Kolibáč, J. 2007b: 58
† Ancyrona eocenica Schmied, Wappler & Kolibáč, 2009; Germany; Tertiary: middle Eocene
Schmied, H. et al. 2009: 18
Ancyrona extensa Reitter, 1877; Bolivia: Bogota (AL)
Léveillé, A. 1910: 26
Ancyrona fairmairei Léveillé, 1903; Madagascar (AL, confirmed JK)
Léveillé, A. 1910: 30 (Ostoma (s.str.)). Kolibáč, J. 2007b: 58 (comb.; transferred from Ostoma to colobicoides-group)
Ancyrona feai Léveillé, 1905; Congo gall., Cameroon (AL)
Léveillé, A. 1910: 26. Kolibáč, J. 2007b: 55 (shifted to Ancyrona gabonica-group)
Ancyrona ferrarii Reitter, 1876; Batchian Ins. (AL)
Léveillé, A. 1910: 25 (Latolaeva). Kolibáč, J. 2005: 63 (Latolaeva; redescription). Kolibáč, J. 2007b: 55 (comb.; transferred from Latolaeva to gabonica-group). Reitter, E. 1876: 50 (Latolaeva)
Ancyrona francoisi Léveillé, 1907; Tonkin (AL)
Léveillé, A. 1910: 26
Ancyrona fryi Léveillé, 1899; Assam, Perak, Sumatra (AL)
Léveillé, A. 1910: 26
Ancyrona gabonica Léveillé, 1899; Congo gall. (AL)
Léveillé, A. 1910: 26. Kolibáč, J. 2007b: 55 (shifted to gabonica-group)
Ancyrona gestroi Reitter, 1880; Australia, New Guinea (AL)
Léveillé, A. 1910: 26
Ancyrona grouvellei Léveille, 1899; Détr. Torres (AL)
Léveillé, A. 1910: 26
Ancyrona haroldi Reitter, 1877; Japan (AL, confirmed JK)
Léveillé, A. 1910: 26. Esaki, T. et al. 1951: 1062. Kolibáč, J. 2007a: 365. Kolibáč, J. 2007b: 54 (shifted to lewisi-group). Nakane, T. et al. 1963: 181
Ancyrona higonia Lewis, 1894; Japan (AL)
Nakane, T. et al. 1963: 182 (Ostoma)
Note: combined with Ancyrona here according to a photograph in Nakane, T. et al. (1963).
Ancyrona horni Léveillé, 1902; Sri Lanka (AL)
Léveillé, A. 1910: 26
Ancyrona incensa Oliff, 1883; Malacca (AL)
Léveillé, A. 1910: 25 (Latolaeva). Kolibáč, J. 2007b: 55 (comb.; transferred from Latolaeva to gabonica-group
Ancyrona indica Léveillé, 1907; India (AL)
Léveillé, A. 1910: 26
Ancyrona japonica Reitter, 1889; Asia: Japan; Europe: Bulgaria?, Czechia, Hungary (JK)
Reitter, E. 1889: 15 (Ostoma). Léveillé, A. 1910: 31 (Ostoma). Esaki, T. et al. 1951: 1062 (Grynocharis;comb.). Kolibáč, J. 1993a: 18 (comb.; transferred from Grynocharis to Ancyrona). Kolibáč, J. 1993b: 90 (check-list). Kolibáč, J. 1999: 12 (morphology). Kolibáč, J. 2007a: 365 (distribution). Kolibáč, J. 2007b: 56 (shifted to japonica-group). Lafer, G. Sh. 1992: 84 (Grynocharis). Nakane, T. et al. 1963: 182 (Grynocharis)
Ancyrona javanica Léveille, 1905; Java (AL)
Léveillé, A. 1910: 26
Ancyrona kosnovskorum Kolibáč, 2005; Madagascar (JK)
Kolibáč, J. 2005: 46. Kolibáč, J. 2007b: 58 (shifted to colobicoides-group)
Note: maybe synonym of Ancyrona colobicoides.
Ancyrona lanuginosa Motschulsky, 1863; Sri Lanka (AL)
Léveillé, A. 1910: 26. Reitter, E. 1876: 52
Ancyrona latebrosa Olliff, 1885; Queensland (AL)
Léveillé, A. 1910: 26
Ancyrona laticeps Olliff, 1885; Queensland, Nov. Gall. mer. (=NSW?) (AL)
Léveillé, A. 1910: 26
Ancyrona lewisi Reitter, 1876; Japan (AL)
Léveillé, A. 1910: 26. Kolibáč, J. 1993a: 16. Kolibáč, J. 2005: 45. Kolibáč, J. 2007b: 54 (shifted to lewisi-group). Lafer, G. Sh. 1992: 84. Reitter, E. 1876: 52
Ancyrona maculipennis Kraatz, 1878; S Africa (AL)
Léveillé, A. 1910: 26
Ancyrona martini Léveille, 1899; Natal (AL)
Léveillé, A. 1910: 26. Kolibáč, J. 2007b: 55 (shifted to gabonica-group)
Ancyrona minor Fairmaire, 1900; Madagascar (AL)
Léveillé, A. 1910: 31 (Ostoma (s.str.)). Kolibáč, J. 2007b: 58 (comb.; transferred from Ostoma to colobicoides-group)
Ancyrona muellerae Kolibáč, 2007; SA: Transvaal (JK)
Kolibáč, J. 2007b: 59 (Ancyrona endroedyi-group)
Ancyrona nigrita J. Thomson, 1858; Gabon (AL)
Léveillé, A. 1910: 26. Reitter, E. 1876: 53 (Peltis)
Ancyrona obscura Léveille, 1899; Sumatra, Ternate (AL)
Léveillé, A. 1910: 26
Ancyrona orbicularis Léveille, 1894; Sumatra, Ternate (AL)
Léveillé, A. 1910: 26
Ancyrona ovalis M’Leay, 1825; Borneo, Java (AL)
Léveillé, A. 1910: 25. Reitter, E. 1876: 49 (comb.; transferred from Peltis to Latolaeva)
Ancyrona plana Léveille, 1902; E Africa (AL)
Léveillé, A. 1910: 26. Kolibáč, J. 2007b: 55 (shifted to gabonica-group)
Ancyrona pryeri Olliff, 1883; Borneo (AL)
Léveillé, A. 1910: 26
Ancyrona pygmea Léveille, 1907; Argentina (?) (AL)
Léveillé, A. 1910: 26
Ancyrona quadrimaculata Reitter, 1877; Malacca (AL)
Léveillé, A. 1910: 25
Ancyrona reitteri Olliff, 1883; New Guinea, Ins. Aru (AL)
Léveillé, A. 1910: 26
Ancyrona rufolineata Léveille, 1899; Cameroon (AL)
Léveillé, A. 1910: 26
Ancyrona shibatai Nakane, 1963; Japan (varA)
Nakane, T. 1963: 46. Kolibáč, J. 2007a: 365. Kolibáč, J. 2007b: 54 (shifted to lewisi-group). Nakane, T. et al. 1963: 182
Ancyrona simoni Reitter, 1880; Ashantee (AL)
Léveillé, A. 1910: 26
Ancyrona soror Léveille, 1902; Sumatra (AL)
Léveillé, A. 1910: 26
Ancyrona subrotundata Motschulsky, 1863; Sri Lanka (AL)
Léveillé, A. 1910: 26. Reitter, E. 1876: 53 (Ostoma)
Ancyrona vesca Olliff, 1885; Australia (AL)
Léveillé, A. 1910: 27
Ancyrona vicina Léveille, 1899; Cameroon (AL)
Léveillé, A. 1910: 27. Kolibáč, J. 2007b: 55 (shifted to gabonica-group)
Grynoma diluta Sharp, 1877 [designated by
Léveillé, A. 1910: 29. Crowson, R. A. 1964a: 299 (as Grynoma Broun). Kolibáč, J. 2005: 59. Kolibáč, J. 2006: 111 (phylogeny)
Body size: about 5.0–5.5 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 10-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts.
Larva: Paragular sclerites absent. Cardo-Stipes not fused. Cardo: size nearly as large as stipes. Torma H-shaped. Abdominal segment IX transversely divided. Tergite IX flat. Urogomphi present, hooked; median process present.
New Zealand.
Grynoma albosparsa Broun, 1909; New Zealand (AL)
Léveillé, A. 1910: 29
Grynoma ambiguum Broun, 1880; New Zealand (AL)
Léveillé, A. 1910: 21 (Leperina ambigua). Leschen, R. A. B. & Lackner, T. 2013: 301 (comb. from Leperina)
Grynoma diluta Sharp, 1877; New Zealand (AL)
Léveillé, A. 1910: 29. Kolibáč, J. 2005: 59 (redescription)
Grynoma fusca Sharp, 1877; New Zealand (AL)
Léveillé, A. 1910: 29
Grynoma regularis Sharp, 1882; New Zealand (AL)
Léveillé, A. 1910: 29
Grynoma varians Broun, 1893; New Zealand (RAC)
Crowson, R. A. 1964a: 297 (adult and larva). Kolibáč, J. 2006: 107 (larva)
Note: The species was omitted by
http://species-id.net/wiki/Leptonyxa
Fig. 11; Map 12Leptonyxa brevicollis Reitter, 1876 [designated by
Léveillé, A. 1910: 27. Kolibáč, J. 2005: 66. Kolibáč, J. 2006: 111 (phylogeny)
I have studied only two species of Leptonyxa, Leptonyxa germaini and Leptonyxa fairmairei. The mandibles of the males are so peculiar that I hesitated over whether the genus truly belongs in Trogossitidae (
Body size: about 7.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides present. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks absent. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) absent. Pubescence above mola or cutting edge present. Ventral furrow present. Basal notch shallow or absent. Labrum-Cranium fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite absent. Antenna 10-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: absent. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
Nothing is known of the biology of these rare species. The last record known to me was made by a flight interception trap in Brazil. They are probably predatory.
Tropical South America: Bolivia, Colombia, Brazil.
Leptonyxa boliviensis Léveillé, 1895; Bolivia (AL)
Léveillé, A. 1910: 27
Leptonyxa brevicollis Reitter, 1876; Colombia (AL)
Reitter, E. 1876: 54. Léveillé, A. 1910: 27. Kolibáč, J. 2005: 66 (redescription)
Leptonyxa costipennis Reitter, 1876; Brazil (AL)
Reitter, E. 1876: 55. Léveillé, A. 1910: 27
Leptonyxa fairmairei Léveillé, 1892; Brazil (AL)
Léveillé, A. 1910: 27. Kolibáč, J. 2006: 152
Leptonyxa germaini Léveillé, 1895; Bolivia (AL)
Léveillé, A. 1910: 27
Leptonyxa grouvellei Léveillé, 1895; Brazil (AL)
Léveillé, A. 1910: 27
Leptonyxa ornata Léveillé, 1895; Brazil: Bahia (AL)
Léveillé, A. 1910: 27
Leptonyxa sedilloti Léveillé, 1888; Colombia (AL)
Léveillé, A. 1910: 27
Leptonyxa variegata Léveillé, 1907; Brazil (AL)
Léveillé, A. 1910: 27
http://species-id.net/wiki/Neaspis
Fig. 11; Map 12Neaspis villosa Pascoe, 1872 [by monotypy]
Léveillé, A. 1910: 25. Matthews, E. G. 1992: 4. Kolibáč, J. 2005: 70. Kolibáč, J. 2006: 111 (phylogeny). Reitter, E. 1876: 47
Body size: about 4.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size moderate. Eyes number: two. Epicranial acumination absent. Lacinial hooks absent. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula rigid, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 10-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Middle coxal cavities closed. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation regular, scales present. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
The species are probably predatory. According to
The genus is autochthonous in Australia; Neaspis squamata from the Phillippines is probably mislabelled, misidentified or introduced (I did not examine the species). Recently, I studied a specimen of Neaspis cf. variegata collected in Brazil by Wygodzinski (coll. National Museum Prague), labelled as “Corcovado, Rio D.F.” (= Rio de Janeiro, former Districto Federal). This record is believed to be plausible. The presence (either autochthonous distribution or introduction) might be the grounds for certain improbable descriptions of South American Ancyrona species.
Neaspis pusilla Blackburn, 1891; South Australia (AL)
Léveillé, A. 1910: 25
Neaspis sculpturata Reitter, 1876; Australia (AL)
Léveillé, A. 1910: 25. Reitter, E. 1876: 48
Neaspis serrata Léveillé, 1907; Queensland (AL)
Léveillé, A. 1910: 25
Neaspis squamata Escherich, 1822; Philippines: Luzon (AL)
Léveillé, A. 1910: 25. Reitter, E. 1876: 49
Note: doubtful record.
Neaspis variegata MacLeay, 1873; Australia, one specimen from Brazil (varA)
Léveillé, A. 1910: 25. Kolibáč, J. 2005: 70 (redescription). Reitter, E. 1876: 47 (Neaspis subtrifasciata Reitter, 1876)
Neaspis villosa Pascoe, 1872; Australia (AL)
Léveillé, A. 1910: 25. Reitter, E. 1876: 48
http://species-id.net/wiki/Sinosoronia
Map 12Sinosoronia longiantennata Zhang, 1992 [by monotypy]
Kolibáč, J. 2006: 136 (Trogossitidae incertae sedis). Kolibáč, J. & Huang, D.-Y. 2008: 145 (Ancyronini). Ponomarenko, A. G. & Kireichuk, A. G. (2005–2008): http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm (Peltidae). Zhang, J.-F. 1992: 333 [in Chinese], 336 [in English]. Schmied, H. et al. 2009: 26
Sinosoronia might be related to another Mesozoic genus, Peltocoleops. The latter genus was described as “Cleroidea incertae sedis” (
The “posterior femur” in the original description is probably the hind coxa. The long antenna with a loose club resembles that of species of the Ancyrona gabonica species-group, while a similar shape of the pronotum may be found in the colobicoides species-group. Such an extremely small size of body is not known in recent Ancyronini but occurs in an concurrently described species from the late middle Eocene (
“Brown in colour. Head about as long as wide. Mandibles large but dentes indistinguishable. Eyes circular, expanded laterally but exterior margin ill-preserved. Antennae 1.2 times as long as head and pronotum together, several basal segments ill-preserved except for the thickened scape, each flagellum cylindrical, about twice as long as wide, club elongate, nearly one-third the length of antenna, slightly thickened apically. Pronotum 2.1 times as broad as long; anterior margin arched, its median part straight, curved forwards laterally, lateral margins arched, posterior margin sinuate, and closely connected to elytra. Scutellum about as long as wide. Elytra smooth, not striated, exterior and interior margins slightly arched, shoulder rounded, its terminal part distinctly exceeding apex of abdomen, each elytron 2.6 times as long as wide. Middle and posterior femora seemingly clubbed, both tibiae and tarsi absent. Total length 2.3 mm, width 1.3 mm.” (
China: Shandong province; Mesozoic: Lower Cretaceous, Laiyang formation.
† Sinosoronia longiantennata Zhang, 1992; China: Shandong; Lower Cretaceous: Laiyang formation (varA)
Kolibáč, J. 2006: 136. Kolibáč, J. & Huang, D.-Y. 2008: 145. Ponomarenko, A. G. & Kireichuk, A. G. (2005–2008): http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 26. Zhang, J.-F. 1992: 333 [in Chinese], 336 [in English]
Lophocateres Olliff, 1883
Barron, J. R. 1971: 11, 12 (syn. Lophocateridae = Peltinae). Barron, J. R. 1975: 1119. Burakowski, B. et al. 1986: 119 (Lophocateridae). Kolibáč, J. 2006: 128 (diagnosis, stat. n.). Kolibáč, J. 2007a: 365. Kolibáč, J. 2010: 35. Lafer, G. Sh. 1992: 83 (key). Lawrence, J. F. & Newton, A. F., Jr. 1995: 868 (Lophocateridae). Lucht, W. 1998: 207 (key). Ślipiński, S. A. 1992: 442 (Lophocaterinae)
Lycoptini Casey, 1890 (Type genus: Lycoptis Casey, 1890)
Kolibáč, J. 2006: 128 (synonymized)
The main issue to be addressed for Lophocaterini is their possible paraphyly in relation to Ancyronini. The whole clade (lophocaterins + ancyronins) is monophyletic but the lophocaterins might be paraphyletic (i.e., non-holophyletic in the traditional Hennigian meaning) because ancyronins can only be advanced members of more primitive lophocaterins. See also “Remarks” in the Ancyronini section. Further, more detailed study is required to resolve the question. The generic composition of Decamerini and its position within Lophocaterinae should be examined along – not, however, before an associated larva of the decamerins is known.
(after
| 1 | Elytra with irregular punctation; lateral margins of pronotum broadly explanate, lateral edge sparsely denticulate | Eronyxa |
| – | Elytra regularly punctate; lateral margins of pronotum narrowly explanate, lateral edge almost entirely evenly rounded or densely denticulate | 2 |
| 2 | Antenna 7- or 9-segmented | 3 |
| – | Antenna 11-segmented | 5 |
| 3 | Antenna 7-segmented, club 1-segmented; mandible with mola | Lycoptis |
| – | Antenna 9-segmented, club 2- or 3-segmented; mandible without mola | 4 |
| 4 | Antennal club 2-segmented; mandible with prostheca near base of mandible formed by tuft of long setae; submental area lacking concave or depressed area; wing with oblong radial cell | Grynocharina |
| – | Antennal club 3-segmented; mandible without penicillus or prostheca; submental area concave; wing with small triangular radial cell displaced downwards | Peltonyxa |
| 5 | Lateral edge of pronotum densely denticulate; lacinia with one pigmented spine | Indopeltis |
| – | Lateral edge of pronotum evenly rounded or at most finely undulating; lacinia with two or three pigmented spines | 6 |
| 6 | Elytra with inconspicuous carinae; mola absent; ligula deeply emarginate; probably predatory. Larva: sensory appendix very short | Promanus |
| – | Elytra with conspicuous carinae; mola or remnant of mola present; ligula deeply or weakly emarginate. Mode of life: predatory (Trichocateres), herbivorous (Lophocateres), fungivorous (Grynocharis). Larva (Lophocateres, Grynocharis): length of sensory appendix about half or more than half that of antennal segment 3 | 7 |
| 7 | Elytra with six carinae; tegmen without projecting phallobasic apodeme; lacinia with three pigmented, hooked spines; small species (less than 3 mm) | Lophocateres |
| – | Elytra with five or four distinct carinae; tegmen with projecting phallobasic apodeme; different pattern of pigmented lacinial spines; larger species (above 5 mm) | 8 |
| 8 | Elytra with four distinct (higher) carinae and another three to four lower carinae among them; pronotum and elytra without tufts of long hairs, with short decumbent or semi-erect pubescence only, or without conspicuous pubescence; lacinia with two pigmented, hooked spines; tibial apical spur pattern 2-2-2; larger species (about 4.5–10.5 mm) | Grynocharis |
| – | Elytra with only five distinct carinae; pronotum and elytra with tufts of long, yellow-orange hairs; lacinia with three pigmented spines at apex in pattern 1+2, apical spine large and hooked, two other spines much smaller; tibial apical spur pattern 1-1-1; smaller species (about 5 mm) | Trichocateres |
http://species-id.net/wiki/Eronyxa
Fig. 10; Map 11, 13Ostomodes dohrni Reitter, 1876 [by monotypy]
Barron, J. R. 1971: 38. Reitter, E. 1876: 57. Kolibáč, J. 2005: 55 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2010: 35 (key). Léveillé, A. 1910: 28
Ostomodes Reitter, 1877 (Type species: Ostomodes dohrni Reitter, 1876)
Barron, J. R. 1971: 38. Léveillé, A. 1910: 28.
Grynocharis (pars.)
Barron, J. R. 1971: 38. Van Dyke, E. C. 1916: 73
The genus was formerly classified within Decamerini (
Body size: about 3.5 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: two. Galea: shape very small. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projections extending laterally and downwards (Eronyxa). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite absent. Antenna 11-segmented. Antennal club symmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation irregular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 present, cross vein AA1+2-3+4 absent. Front tibiae: spines along side large. Hooked spur absent, apical spurs not hooked or weakly hooked. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale absent. Tegmen composed of two parts. Coxitae divided.
Larva: Frontal arms V-shaped. Epicranial stem absent. Endocarina present. Gular sutures conspicuous, convergent. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: two. Mandibular apical teeth number: two, horizontally even, vertically situated. Lacinia mandibulae plumose. Mola absent. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes partially fused. Ligula present. Labial palpi 2-segmented. Prementum in two parts. Torma H-shaped. Antennal joints 1 and 2 elongate. Sensory appendix very small. Thoracic sclerites pattern (dorsally) 1-0-0. Thoracic sclerites pattern (ventrally) 1+0+0. Trochanter triangular. Abdominal segment IX transversely divided. Tergite IX flat. Urogomphi present, hooked; median process present.
Eronyxa expansus was collected under the bark of Libocedrus (= Calocedrus) decurrens. The larva probably feeds on Xylococculus macrocarpae (
Western states of USA (California, Idaho, Nevada, Oregon) and Canada (British Columbia).
A distribution of the tribe Lophocaterini.
Eronyxa angustus Casey, 1916; USA: California, Idaho, Nevada, Oregon (JRB)
Barron, J. R. 1971: 42. Kolibáč, J. 2005: 55 (redescription)
Eronyxa expansus Van Dyke, 1916; USA: California (JRB)
Barron, J. R. 1971: 38. Kolibáč, J. 2006: 107 (larva). Leschen, R. A. B. 2000: 920 (biology). Tait, S. M. et al. 1990: 13 (larva)
Eronyxa pallida Motschulsky, 1863; Canada: British Columbia, USA: California, Oregon (JRB)
Léveillé, A. 1910: 28. Barron, J. R. 1971: 39 (syn. Grynocharis pilosula Crotch, 1873; synonymized by whom?). Barron, J. R. 1971: 39 (syn. Ostomodes dohrni Reitter, 1877; synonymized by
http://species-id.net/wiki/Grynocharina
Fig. 12; Map 13Grynocharina peltiformis Reitter, 1877 [by monotypy]
Léveillé, A. 1910: 24. Kolibáč, J. 2005: 57 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2010: 35 (key)
Only a single male specimen of the monotypic genus is known. Grynocharina peltiformis was originally placed in a basal position on the lophocaterine tree (
Body size: 3.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture present. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: three. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) long setae. Pubescence above mola or cutting edge absent. Ventral furrow present, not ciliate. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection curved upwards (Colydiopeltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite H-shaped. Antenna 9-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron thin. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell oblong (or reduced), wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale present. Tegmen composed of three parts.
Unknown.
Burma (Myanmar).
Grynocharina peltiformis Reitter, 1877; Burma (AL)
Léveillé, A. 1910: 24. Kolibáč, J. 2005: 57 (redescription)
http://species-id.net/wiki/Grynocharis
Figs 12, 18; Map 13Silpha oblonga Linnaeus, 1758 [by original designation and monotypy]
Léveillé, A. 1910: 31. Barron, J. R. 1971: 32. Kolibáč, J. 2005: 58 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 365. Kolibáč, J. 2010: 35 (key). Lafer, G. Sh. 1992: 84. Larsson, S. G. 1978: 150 (Baltic amber fossil). Spahr, U. 1981: 74 (amber and copal fossils)
Gaurambe Thomson, 1859
Barron, J. R. 1971: 32 (syn. Gaurambe Thomson, 1859; misapplied)
Body size: about 5.5–8.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture broadly emarginate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination deep. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola reduced but present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow present, not ciliate. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection curved downwards, processes with bridge (Peltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Spiculum gastrale present. Tegmen composed of three parts.
Larva: Frontal arms V-shaped. Epicranial stem absent. Endocarina present. Stemmata number: two. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae plumose. Mola absent. Maxillary palpi 3-segmented. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes not fused. Cardo: size nearly as large as stipes. Ligula present. Labial palpi 2-segmented. Prementum in single part. Antennal joints 1 and 2 elongate. Sensory appendix medium sized (to half of joint 3). Thoracic sclerites pattern (dorsally) 1-2-2. Abdominal segment IX transversely divided. Tergite IX flat. Urogomphi present, hooked; median process present.
A Grynocharis oblonga B Grynocharina peltiformis C Lophocateres pusillus D Promanus auripilis E Promanus subcostatus F Promanus depressus G Peltonyxa sp., Australia, NSW H Trichocateres fasciculifer.
Adults and larvae of Grynocharis oblonga live under bark or bark scales and in rotten wood of deciduous and coniferous trees (willow, birch, spruce, fir); they are fungivorous. In the USA, the species have been collected on Libocedrus decurrens and Populus (
Europe including Russia to the Urals, Caucasus; USA excluding central and southern states, Canada: south-western and south-eastern states.
Grynocharis caucasica Motschulsky, 1863; Caucasus (JK)
Léveillé, A. 1910: 31 (Ostoma). Kolibáč, J. 2007a: 366 (nomen dubium)
Grynocharis oblonga Linnaeus, 1758; all Europe to Russia (varA)
Léveillé, A. 1910: 31 (Ostoma (subgen. Grynocharis)). Bahillo de la Puebla, P. & López-Colón, J. I. 2004: 129. Borowiec, L. 1983: 13. Burakowski, B. et al. 1986: 119. Conrad, R. 1995: 190. Gobbi, G. 1996: 65. Klausnitzer, B. 1976: 8. Klausnitzer, B. 1978: 178. Klausnitzer, B. 1996: 163. Kolibáč, J. 1993a: 21. Kolibáč, J. 1993b: 90. Kolibáč, J. 2005: 58 (redescription). Kolibáč, J. 2006: 107 (larva, phylogeny). Kolibáč, J. 2007a: 365 (distribution). Lafer, G. Sh. 1992: 84. Lemdahl, G. 2001: 39 (biology). Mitter, H. 1998: 561. Nilsson, S. G. 1997: 1 (biology). Pileckis, S. & Monsevičius, V. 1995: 272. Reitter, E. 1876: 63 (Ostoma). Vogt, H. 1967: 18
Grynocharis oregonensis Schaeffer, 1918; USA, Canada: western states (JRB)
Léveillé, A. 1910: 31 (Ostoma (subgen. Grynocharis) oregonensis Crotch, 1873). Barron, J. R. 1971: 34. Dajoz, R. 1997: 44 (biology)
Grynocharis pubescens Erichson, 1844; Georgia, South European Territory of Russia, „Caucasus”, Crimea (JK)
Léveillé, A. 1910: 31 (Ostoma (subgen. Grynocharis)). Lafer, G. Sh. 1992: 84. Kolibáč, J. 2006: 107. Kolibáč, J. 2007a: 365. Mamaev, B. M. 1976: 1656 (larva). Reitter, E. 1876: 63 (Ostoma)
Grynocharis quadrilineata Melsheimer, 1844; NE USA, Canada: Ontario, Quebec (JRB)
Léveillé, A. 1910: 31 (Ostoma (subgen. Grynocharis) marginata Melsheimer, 1844). Barron, J. R. 1971: 33 (syn. Grynocharis marginata Melsheimer, 1844, synonymized by Lacordaire 1854?). Reitter, E. 1876: 63 (Ostoma)
http://species-id.net/wiki/Indopeltis
Map 13Indopeltis nilgiriensis Crowson, 1966 [by original designation and monotypy]
Kolibáč, J. 2005: 61 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2010: 35 (key)
Body size: about 5.5 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture absent. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: one. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge present. Ventral furrow present. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection not developed (all remaining). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 11-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Spiculum gastrale present. Tegmen composed of three parts.
Unknown;
India: Tamil Nadu, Nilgiri Hills.
Indopeltis nilgiriensis Crowson, 1966; South India: Tamil Nadu (RAC)
Crowson, R. A. 1966: 126. Kolibáč, J. 2005: 61 (redescription). Kolibáč, J. 2006: 111 (phylogeny)
http://species-id.net/wiki/Lophocateres
Figs 2, 12, 18; Map 13Lophocateres nanus Olliff, 1883 [by monotypy] (= Lophocateres pusillus Klug, 1833)
Léveillé, A. 1910: 27. Barron, J. R. 1971: 42. Crowson, R. A. 1964a: 299. Kolibáč, J. 2005: 67 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2007a: 366. Kolibáč, J. 2010: 35 (key). Lafer, G. Sh. 1992: 84. Larsson, S. G. 1978: 150 (fossil, Baltic amber). Spahr, U. 1981: 74 (amber and copal fossils)
Body size: about 2.5–4.0 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture broadly emarginate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination moderate. Lacinial hooks: three. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge present. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection curved upwards (Colydiopeltis). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, weakly emarginate. Hypopharyngeal sclerite consisting of two separate parts. Antenna 11-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: one. Spiculum gastrale absent. Tegmen composed of three parts. Coxitae undivided.
Larva: Frontal arms V-shaped. Epicranial stem absent. Endocarina present. Gular sutures conspicuous, convergent. Gula: anterior apodemes absent. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: two. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae plumose. Mola absent. Maxillary palpi 3-segmented. Palpifer present. Pedunculate seta absent. Mala simple. Mala: bidentate protrusion absent. Cardo-Stipes not fused. Cardo: size nearly as large as stipes. Ligula present. Labial palpi 2-segmented. Prementum in single part, anterior margin even. Torma H-shaped. Antennal joints 1, 2 transverse. Sensory appendix larger than half of joint 3. Thoracic sclerites pattern (dorsally) 0+0+0. Thoracic sclerites pattern (ventrally) 2+0+0. Trochanter triangular. Abdominal segment IX transversely divided. Tergite IX flat. Urogomphi present, hooked; median process present.
Lophocateres pusillus lives in storage facilities (warehouses, stores, barns, larders) and feeds on grains. The biology of wild populations is unknown.
Lophocateres pusillus is cosmopolitan, although the centre of its distribution, is in south-eastern Asia. The species Lophocateres gounellei is probably a misidentification or a synonym of Lophocateres pusillus. Some new species are known to me from Malaysia.
Lophocateres gounellei Léveillé, 1905; Brazil (AL)
Léveillé, A. 1910: 27
Note: dubious species – probably synonym of Lophocateres pusillus or different genus.
Lophocateres pilosus Olliff, 1883; Malaysia: Penang (AL)
Léveillé, A. 1910: 27
Lophocateres pusillus Klug, 1833; cosmopolitan (origin in SE Asia) (varA)
Léveillé, A. 1910: 27 (syn. Lophocateres africanus Motschulsky, 1863); Algeria (AL)
Léveillé, A. 1910: 27 (as Lophocateres nanus Olliff, 1883); Borneo (AL)
Léveillé, A. 1910: 27 (syn. Lophocateres yvani Allibert, 1847). Bahillo de la Puebla, P. & López-Colón, J. I. 2004: 129. Barron, J. R. 1971: 43 (syn. Lophocateres nanus Olliff, 1883). Barron, J. R. 1971: 43 (syn. Peltis africanus Motschulsky, 1863). Barron, J. R. 1971: 43 (syn. Peltis yvani Allibert, 1847). Borowiec, L. 1983: 14. Burakowski, B. et al. 1986: 119. Chang, T-C. & Liu, T.-Y. 1981: 116 (biology). Ghosh, S. & Haldar, D. P. 1989: 49 (biology). Ghosh, S. & Saha, K. 1992a: 181 (biology). Ghosh, S. & Saha, K. 1992b: 613 (biology). Ghosh, S. & Saha, K. 1995: 207 (biology). Halstead, D. G. H. 1968: 197 (biology). Klausnitzer, B. 1976: 7. Klausnitzer, B. 1978: 177. Klausnitzer, B. 1996: 163 (larva). Kolibáč, J. 1993a: 21. Kolibáč, J. 1999b: 12. Kolibáč, J. 2005: 67 (redescription). Kolibáč, J. 2006: 107 (larva). Kolibáč, J. 2007a: 366 (distribution). Lafer, G. Sh. 1992: 86. Nakane, T. et al. 1963: 182. Vogt, H. 1967: 16. Reitter, E. 1876: 63 (Ostoma yvani)
Peltis americana Motschulsky, 1863 [by monotypy]
Léveillé, A. 1910: 33. Barron, J. R. 1971: 120. Barron, J. R. 1975: 1117. Kolibáč, J. 2005: 67 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2010: 35 (key)
Body size: 1.9–2.2 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture broadly emarginate. Frons: longitudinal groove or depression absent. Submentum: ctenidium absent. Antennal groove absent. Eyes: size large, lateral. Eyes number: two. Lacinial hooks: three. Galea: shape elongate. Galea: ciliate setae absent. Mediostipes-Lacinia partially fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola present. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Ligula: ciliate setae absent, not retroflexed, deeply emarginate. Antenna 7-segmented, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Spiculum gastrale present. Tegmen composed of two parts.
Lycoptis americana is a very rare beetle, probably fungivorous.
USA: Georgia, Maryland, North Carolina, South Carolina (
Lycoptis americana Motschulsky, 1863; USA: Georgia, Maryland, N Carolina, S Carolina (JRB)
Léveillé, A. 1910: 27 (Lophocateres americanus Motschulsky, 1863). Léveillé, A. 1910: 33 (Lycoptis villosa Casey, 1890). Barron, J. R. 1971: 120 (syn. Lycoptis villosa Casey, 1890). Barron, J. R. 1971: 120 (Peltis americana Motschulsky, 1863; comb.). Barron, J. R. 1975: 1120. Kolibáč, J. 2005: 67 (redescription)
http://species-id.net/wiki/Peltonyxa
Figs 1, 12; Map 13Peltonyxa deyrollei Reitter, 1876 [by monotypy]
Léveillé, A. 1910: 24. Kolibáč, J. 2005: 76 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2010: 35 (key). Matthews, E. G. 1992: 3
Floricateres Crowson, 1970 [Type species: Floricateres pusillus Crowson, 1970]
Crowson, R. A. 1970: 10. Kolibáč, J. 2005: 76 (syn. Floricateres)
Body size: about 3.5 mm. Body shape flat. Gular sutures wide, subparallel. Frontoclypeal suture broadly emarginate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum of males: ctenidium present. Antennal groove present. Eyes: size moderate. Eyes number: two. Epicranial acumination moderate. Lacinial hooks absent. Galea: shape very small. Galea: ciliate setae absent. Mediostipes-Lacinia not fused. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) absent. Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch moderate. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection reduced or absent (Promanus). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 9-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae conspicuous or reduced. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Spiculum gastrale present. Tegmen composed of three parts. Coxitae divided.
Peltonyxa pusillus was collected on the flowers of Bursaria (
Australia: South Australia, New South Wales, Victoria.
Peltonyxa australis Blackburn, 1891; S Australia (AL)
Léveillé, A. 1910: 24
Peltonyxa deyrollei Reitter, 1876; Australia (AL)
Léveillé, A. 1910: 24. Kolibáč, J. 2005: 76. Reitter, E. 1876: 46
Peltonyxa invalida Blackburn, 1902; Australia („Nov. Gall. mer.”) (AL)
Léveillé, A. 1910: 24
Peltonyxa pubescens Blackburn, 1891; Australia: Victoria (AL)
Léveillé, A. 1910: 24
Peltonyxa pusillus Crowson, 1970; Australia: NSW (RAC)
Crowson, R. A. 1970: 11 (Floricateres)
Promanus depressus Sharp, 1877 [by monotypy]
Léveillé, A. 1910: 29. Crowson, R. A. 1964a: 298. Kolibáč, J. 2005: 78 (redescription). Kolibáč, J. 2006: 111 (phylogeny). Kolibáč, J. 2010: 35 (key). Kolibáč, J. et al. 2010: 36
Body size: 6.8–8.8 mm. Body shape flat. Gular sutures wide, convergent at apex. Frontoclypeal suture broadly emarginate. Frons: longitudinal groove or depression absent. Cranium ventrally: tufts of long setae at sides absent. Submentum: ctenidium absent. Antennal groove present. Eyes: size large, lateral. Eyes number: two. Epicranial acumination absent. Lacinial hooks: two. Galea: shape sub-clavate. Galea: ciliate setae absent. Mediostipes-Lacinia fused together. Palpifer: outer edge even. Mandibular apical teeth number: two, horizontally situated. Mola absent. Penicillus (at base) present (fine, often membranous). Pubescence above mola or cutting edge absent. Ventral furrow absent. Basal notch shallow or absent. Labrum-Cranium not fused. Epipharyngial sclerite absent. Lateral tormal process: projection projection reduced or absent (Promanus). Ligula: ciliate setae absent. Ligula membranous, not retroflexed, deeply emarginate. Hypopharyngeal sclerite H-shaped. Antenna 11-segmented. Antennal club weakly asymmetrical, sensorial fields absent. Front coxal cavities externally open, internally open. Pronotum transverse. Prepectus present. Middle coxal cavities open. Elytra: long hairs absent. Epipleuron moderate. Elytral interlocking mechanism absent, carinae reduced. Elytral punctation regular, scales absent. Wing: radial cell moved down, often small, wedge cell absent, cross vein MP3-4 absent, cross vein AA1+2-3+4 absent. Front tibiae: spines along side moderate. Hooked spur present. Claws: denticle absent. Parasternites number along ventrites III–VII: two. Coxitae divided.
Larva: Frontal arms V-shaped. Epicranial stem absent. Endocarina present. Gular sutures conspicuous, convergent. Gula: anterior apodemes absent. Paragular sclerites absent. Hypostomal rods absent. Stemmata number: two. Mandibular apical teeth number: two, horizontally situated. Lacinia mandibulae plumose. Mola absent. Maxillary palpi 3-segmented. Pedunculate seta present. Mala: bidentate protrusion absent. Cardo-Stipes not fused. Cardo: size nearly as large as stipes. Labial palpi 2-segmented. Prementum in single part, anterior margin even. Antennal joints 1 and 2 elongate. Sensory appendix very small. Abdominal segment IX transversely divided. Tergite IX flat. Urogomphi present, hooked; median process present.
The adults and larvae are predatory.
New Zealand.
Promanus auripilis Broun, 1893; New Zealand (AL)
Léveillé, A. 1910: 29. Kolibáč, J. et al. 2010: 36 (redescription)
Promanus depressus Sharp, 1877; New Zealand (AL)
Léveillé, A. 1910: 29. Crowson, R. A. 1964a: 298 (larva). Kolibáč, J. 2005: 78 (redescription). Kolibáč, J. et al. 2010: 36
Promanus subcostatus Broun, 1909; New Zealand (AL)
Léveillé, A. 1910: 29. Kolibáč, J. et al. 2010: 36 (redescription)
http://species-id.net/wiki/Promanodes
Map 13Promanodes serafini Kolibáč, Schmied, Wappler & Kubisz, 2010 [by monotypy and author’s designation]
Schmied, H. et al. 2009: 105 (distribution). Kolibáč, J. 2011: 58
Body size: 3.1–5.1 mm. Procoxal cavities nearly closed; maxillary palps with securiform terminal joint; extraordinarily elongate terminal segment of labial palps (distinctly longer or as long as two preceding segments together); slender and elongate tarsi in all pair of legs (approximately as long as tibiae); tibiae without “hooked” apical spine; procoxal cavities nearly closed or maybe perfectly closed in the new species; six visible abdominal ventrites; 10-segmented antennae; flat body; antennal club large and loose, 3-segmented; mesocoxae weakly transverse; elytra with distinct carinae. (Genus diagnosis after
The new genus is very similar to the recent Promanus, the both genera share deep and incurvate frontoclypeal suture, distinctly elevated eyes, weakly or no way projecting anterior pronotal corners, elytra with weak or inconspicuous carinae widest at about 2/3 of length, radial cell obliquely situated, dorsal body surface sparsely pubescent or nearly bare, femora conspicuously clavate, very small trochanters, and especially abdomen with six visible ventrites. Body length 3–5 mm. (According to
(Map 13.) Baltic amber: Poland, East Baltic coast(?); Tertiary: Eocene.
† Promanodes alleni Kolibáč, 2011; Baltic amber: East Baltic coast(?); Tertiary: Eocene (JK)
Kolibáč, J. 2011: 59
† Promanodes serafini Kolibáč, Schmied, Wappler & Kubisz, 2010; Baltic amber: Poland; Tertiary: Eocene (varA)
Schmied, H. et al. 2009: 105 (distribution). Kolibáč, J. et al. 2010: 36. Kolibáč, J. 2011: 59
http://species-id.net/wiki/Trichocateres
Fig. 12; Map 13Trichocateres fasciculifer Kolibáč, 2010 [by monotypy and author’s designation]
Body size: 5.2–5.5 mm. With general characteristics of the tribe Lophocaterini (body oval, frontoclypeal suture deeply arcuate, antennal club weakly asymmetrical, mandibular mola present, base of mandible with membranous appendage/penicillus, prostheca composed of tuft of setae, lacinia with spines). It is most closely related to Lophocateres, Indopeltis and Grynocharis (mandibular mola present, elytral carinae well-developed).
Similarities of Trichocateres with Lophocateres and Indopeltis: the mandible with membranous penicillus and distinct prostheca; the wing venation with cross-veins MP3-4 and AA1+2-3+4 absent; and the metendosternite with robust stalk and widely separated anterior tendons. It resembles Indopeltis in aedeagus with projecting phallobasic apodeme, eyes similarly shaped, relatively large and situated dorsally, and lateral edge of pronotum undulating, whereas Lophocateres parallels include labrum with tormal processes branched at base and maxilla with mediostipes not fused with lacinia. Excluding the characters mentioned, Trichocateres differs from all three abovementioned genera chiefly in tibial spur pattern 1-1-1, two sharp grooves in prosternal process and tufts of long hairs on elytra and pronotum. (Genus diagnosis after
The circumstances of collection are not exactly known; the specimens were knocked down from branches or fallen timber. Remnants of insect cuticle were found in the gut of the Assam specimen, the remains of an insect larva in the gut of the Laos specimen.
India: Assam, northern Laos.
Trichocateres fasciculifer Kolibáč, 2010; NE India: Assam, N Laos (JK)
Kolibáč, J. 2010: 36
Calitys africana Boheman, 1848 (Cucujoidea?)
Léveillé, A. 1910: 24
Note: I studied only one non-type specimen determined as Calitys africana in the Museé d’Histoire Naturelle in Geneva. The specimen does not belong in Cleroidea
Latolaeva brasilica Perty, 1830 (Cucujoidea?)
Reitter, E. 1876: 51
Note. I have not studied any specimens of the species. The autochthonous distribution of Latolaeva or Ancyrona in South America is unprobable, although introduction is possible. Species of the two genera could be misidentified for an autochthonous or introduced Australian Neaspis or Peltonyxa (see “Distribution” for Neaspis)
Ostoma australis Boisduval, 1835
Léveillé, A. 1910: 31
Ostoma higonia Lewis, 1894
Léveillé, A. 1910: 31
Ostoma pubescens Escherich, 1822
Léveillé, A. 1910: 32
http://species-id.net/wiki/Anhuistoma
Anhuistoma hyla Lin, 1985 [designated by author and by monotypy]
Kolibáč, J. & Huang, D.-Y. 2008: 136 (Coleoptera incertae sedis)
This beetle was originally described in Trogossitidae and later removed from the superfamily as Coleoptera incertae sedis.
“A broadly elliptic beetle of small size; pronotum broadly hemiorbicular (hemispherical?); elytra short and broad, ornamented with many longitudinal striae; legs short, three pairs of coxae clearly separated, fore-coxa transverse, mid-coxa rounded, both posterior coxae transverse and connected with each other; abdomen with 5 visible sternites.” (
“The body of a small beetle with head and legs missing, 3.5 mm long and 2.3 mm wide. Body broadly elliptic. Pronotum in hemiorbicular (hemispherical?) form, slightly broader than long; anterior margin of pronotum concave, posterior margin of pronotum as wide as the anterior. Anterior coxa transverse and disjointed. Mid-thorax slightly smaller than metathorax. Both mid-coxae rounded and disjointed from one another. Posterior coxae transversely connected. Elytra much broader at base, gradually narrowing toward apex, rounded at apical angle; surface covered with several longitudinal striae. Abdomen with 5 visible sternites.” (
† Anhuistoma hyla Lin, 1985; China: Anhui province; Mesozoic: Lower-Middle Jurassic
Lin, Q.-B. 1985: 309 (Trogossitidae). Kolibáč, J. 2006: 136 (Trogossitidae incertae sedis). Kolibáč, J. & Huang, D.-Y. 2008: 137 (Coleoptera incertae sedis)
http://species-id.net/wiki/Peltocoleops
Peltocoleops onokhojensis Ponomarenko, 1990 (by monotypy and original designation)
Kolibáč, J. 2006: 129 (Lophocaterini). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm (Peltidae). Schmied, H. et al. 2009: 25 (Trogossitidae: Peltinae)
The genus was established in Cleroidea, with no indication of an exact family classification (
(translation from Russian). “Medium sized, oval, flat beetle. Head short, gular sutures divergent backwards [sic], antennae long with indistinct symmetrical club. Pronotum wide and short, its base not narrower than elytra, front coxae large, transverse, not projecting, with exposed trochantin, prosternal process extending behind coxae, very slightly widened. Middle coxal cavities closed by mesepimeron. Middle coxal cavities large, rounded, with exposed trochantin. Metathorax transverse, weakly convex along join of legs [sic], hind trochantins [sic] oblique. Abdomen with 5 visible sternites, their bases bordered (?). Legs relatively long, outer side of middle tibiae with row of firm spines.”
(translation from Russian). “Head twice shorter than wide, tempora shorter than eyes, gula shorter than wide. Antennomere 6 reaches backwards base [sic] of pronotum. Pronotum evenly rounded anteriorly, 2.5 times shorter than wide, anterior margin straight, about twice narrower than basal margin. Prothorax short, shorter than front coxae, prosternal process distinctly runs behind coxae. Middle coxae relatively widely separated. Metathorax about 2.5 times shorter than wide along its basal margin. Hind coxa is longest along middle of body and 3.5 times shorter than wide [sic], coxae distinctly shortened at lateral end. The first and the last abdominal sternites conspicuously longer than others. Elytra widest at basal portion, glabrous. Middle femora scarcely project out of body outline, tibiae widened, with longitudinal sulcus with row of firm spines (or setae) along outer side, tibiae longer than femora.” “Length 7.2, width 4.1, length of elytra 5.2 mm.”
Russia: Transbaikalia, Onokhovo, Onokhovsky Graben, Baleysky distr., Chitinskaya obl.; Mesozoic: Early Cretaceous, Lower Neocomian, Leskovskaya Beds.
† Peltocoleops onokhojensis Ponomarenko, 1990; Russia: Transbaikalia; Early Cretaceous, Lower Neocomian (varA)
Ponomarenko, A. G. 1990: 73. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 26
Because Meligethiellinae is excluded from Cleroidea herein, the synonymization of the subfamily with Peltinae: Thymalini is no longer valid. Meligethiellinae should be classified within traditional Cucujoidea and it is probably related to the extant Nitidulidae. However, this issue lies beyond the scope of this communication and should be addressed by those working on Cucujoidea.
http://species-id.net/wiki/Meligethiella
Meligethiella soroniiformis Medvedev, 1969 (by monotypy and original designation)
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 81 (Peltidae: Meligethiellinae). Kolibáč, J. 2006: 126 (Thymalini). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 25
The genus was originally described in Nitidulidae (
(translation from Russian). “Diagnosis: Body broadly oval, head not distinctly retracted into pronotum, pronotum just about narrower than elytra, 3–4 times shorter than elytra, middle coxae widely separated; mesothorax with groove for apex of prosternal process; elytra perfectly cover abdominal apex, smooth or with irregular punctation; femoral lines in metathorax [paracoxal sutures?] mostly well-developed; first visible abdominal sternite longer than following one. [Measurements 3.7–4.7 mm.]”
Russia: Bouriatskaya Autonomnaya Respublika; W Transbaikalia – Chitinskaya oblast; Kazakhstan: Chimkentskaya oblast, Baissa. Mesozoic: Late Jurassic: Karabastayskaya formation; Lower Cretaceous: Turginskaya formation; middle Neocomian: Zazinskaya formation.
† Meligethiella glabra Kireichuk & Ponomarenko, 1990; Russia: Transbaikalia, Chitinskaya obl.; Lower Cretaceous, Turginskaya formation (varA)
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 82. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 25
† Meligethiella kovalevi Kireichuk & Ponomarenko, 1990; Kazakhstan: Chimkentskaya obl.; Late Jurassic: Karabastayskaya formation (varA)
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 81. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 25
† Meligethiella soroniiformis Medvedev, 1969; Russia: Bouriatskaya Autonom. Rep., Baissa; Lower Cretaceous: Zazinskaya formation (varA)
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 81. Medvedev, L. N. 1969: 120. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 25
http://species-id.net/wiki/Ostomalynus
Ostomalynus ovalis Kireichuk & Ponomarenko, 1990 (by monotypy and original designation)
Kolibáč, J. 2006: 126 (Thymalini). Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 25
The genus was described in Meligethiellinae by Kireichuk & Ponomarenko (1990) and latter shifted to Thymalini by (
“Body oviform, strongly narrowed posteriorly. Pronotum short, five times shorter than elytra, prothorax very short, nearly lenticular, with strong longitudinal keel along centre, middle coxae widely separated, anterior part of mesothorax with groove for apex of prosternal process, elytra reaching behind end of abdomen, dorsally with thin lines beneath them longitudinal rows of punctures can be visible. [Measurements: length 5.3, width 2.8 mm.]”
Russia: Transbaikalia, Chitinskaya oblast, Nerchinsko-Zavodskoy district, Pavlovka village. Mesozoic: Early Cretaceous, Lower Neocomian, Gidarinyskaya formation.
† Ostomalynus ovalis Kireichuk & Ponomarenko, 1990; Russia: Transbaikalia; Lower Cretaceous, Gidarinyskaya formation
Kireichuk, A. G. & Ponomarenko, A. G. 1990: 83. Ponomarenko, A. G. & Kireichuk, A. G. 2005–2008: http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm. Schmied, H. et al. 2009: 25
http://species-id.net/wiki/Palaeoendomychus
The author (
“Body minute and compact, oval, hairless. Head deeply sunk into pronotum. Eyes rather large, widely separated, slightly prominent. Antennae short, flagellum cylindrical. Pronotum transverse, short (Chin.: raised at centre), without membrane [sic] in front, but with lateral sides (Chin. “edges”) broadly flattened, posterior angles prominent. Scutellum small, narrowly triangular, distinctly longer than broad. Elytra wide, dehiscent, as wide at base as pronotum, and both closely connected to one another, humeral angles rounded, terminal angles prominent, surface with striae. Legs normal, with tarsi short and narrow (Chin. 3 tarsomeres visible), first and second tarsal segments triangular, each about as long as wide, third cylindrical, noticeably longer than wide.” (
Note: The Chinese and English texts are somewhat different from one another. Important items from the Chinese are translated in parentheses.
† Palaeoendomychus gymnus Zhang, 1992; China: Shandong; Mesozoic: Lower Cretaceous
Zhang, J.-F. 1992: 337 (Endomychidae). Kolibáč, J. 2006: 136 (Trogossitidae incertae sedis). Kolibáč, J. & Huang, D.-Y. 2008: 139 (Cucujoidea incertae sedis). Ponomarenko, A. G. & Kireichuk A. G. (2005–2008): http://www.zin.ru/animalia/Coleoptera/rus/paleosy2.htm (Trogossitidae). Schmied, H. et al. 2009: 26
Genus Paralindria Olliff, 1883
[Type species: Paralindria bipartita Olliff, 1883]
Olliff, S. 1883b: 57 (“... be placed between [Alindria] and Melambia.”). Reitter, E. 1890: 264 [“Paralindria Oliff, 1883 = Serrotibia Reitt. (Stett. Ztg. 1877, 339)]
Paralindria bipartita Olliff, 1883; Ecuador
Reitter, E. 1890: 264 [“Paralindria bipartita Oliff. = Serrotibia bicolor Reitt., 1. c. pag. 341”]
Note. The synonymy is widely accepted and the genus Serrotibia is classified within Salpingidae at present.
Genus Peltoschema Reitter, 1880
[Type species: Peltoschema filicorne Reitter, 1880]
Reitter, E. 1880: 4 (Trogossitidae). Léveillé, A. 1910: 28 (Trogossitidae)
Note. The classification of the genus within Chrysomelidae has been well known for a long time, e.g.
Genus Peltastica Mannerheim, 1852
[Type species: Peltastica tuberculata Mannerheim, 1852]
Léveillé, A. 1910: 28 (Trogossitidae). Reitter, E. 1876: 60 (Trogossitidae)
Peltastica tuberculata Mannerheim, 1852; Alaska (AL)
Léveillé, A. 1910: 28 (Trogossitidae). Reitter, E. 1876: 60 (Trogossitidae)
Peltastica amurensis Reitter, 1879; Siberia (AL)
Léveillé, A. 1910: 28 (Trogossitidae)
Peltastica reitteri Lewis, 1884; Japan (AL)
Léveillé, A. 1910: 28 (Trogossitidae)
Note. The classification of the genus within Derodontidae has been well known for a long time.
Helota MacLeay, 1825; eastern Asia
Reitter, E. 1876: 5 (Trogositidae: subfamilia Helotidae; sic!)
? Nemozoma nigripenne Reitter, 1876; Colombia (varA)
Léveillé, A. 1910: 6 (Nemosoma (subgen. Monesoma)). Reitter, E. 1876: 14 (Nemozoma nigripennis)
syn. Thione championi Sharp, 1899?
Lepesme, P. & Paulian, R. 1944: 138. According to their notes, they studied the holotype of Nemosoma (Monesoma) nigripenne Lév. [sic] and consider it “[...] est d’apres le type, un Cucujidae et est identique a Thione Championi Sharp”. The matter may be unfamiliar to monotomid workers as I have found no remarks on this synonymy in the literature.
Genus Syntelia Westwood, 1864
[Type species: Syntelia indica Westwood, 1864] (genus originally described in Trogossitidae)
Reitter, E. 1876: 23 (Trogossitidae). Westwood, J. O. 1864: 11. (Trogossitidae)
Syntelia davidis Fairmaire, 1889; China: Sichuan
Syntelia histeroides Lewis, 1882; Russia: Far East, Japan
Syntelia indica Westwood, 1864; Nepal, Northeast India
Reitter, E. 1876: 23 (Trogossitidae). Westwood, J. O. 1864: 11
Syntelia mexicana Westwood, 1864; Mexico: Oaxaca
Reitter, E. 1876: 23 (Trogossitidae). Westwood, J. O. 1864: 11
Syntelia westwoodi Salle, 1873; Mexico
Reitter, E. 1876: 23 (Trogossitidae)
Genus Holopleuridia Reitter, 1876
[Type species: Holopleuridia maculosa Reitter, 1876]
Kolibáč, J. 2005: 87 (transferred to Tenebrionoidea). Léveillé, A. 1910: 27 (Trogossitidae). Reitter, E. 1876: 56 (Trogossitidae)
Holopleuridia maculosa Reitter, 1876; “La Luzera” (Colombia?)
Reitter, E. 1876: 56 (Trogossitidae)
Recently, several important studies have centred upon the phylogeny of Cucujiformia, including Cleroidea and Trogossitidae.
The most recent system of Trogossitidae is based on my morphological studies of 2005 (adults) and 2006 (larvae). The most important result is a confirmation of both Crowson’s ideas on the relationship of Calitys with Trogossitinae and Protopeltis with the Rentonium-group. On the other hand, his opinion about the relationship between the lophocaterins and the trogossitins is called in question. However, it is correct to say that larval characters actually connect the two groups while the majority of adult characters tend to defeat them in favour of a sister relationship of the lophocaterins and trogossitins. Both major clades were analyzed separately under equal weights and then with the use of successive reweighting (
I employed 31 traditional morphological characters (16 adult, 15 larval) for 15 taxa (8 cleroid, 6 cucujoid, 1 derodontoid) in the analysis of 2008. Five cucujoid families (minus Phloeostichidae: Hymaea) were found to be monophyletic as well as Cleroidea. The group of cucujoid families (Cerylonidae, Coccinelidae, Endomychidae, Cucujidae, Byturidae, Phloeostichidae) were found to be paraphyletic with regard to the monophyletic Cleroidea. Trogossitidae were also found to be paraphyletic in this analysis. However, Phloiophilus was placed within a branch composed of trogossitid representatives, probably as a sister group of Thymalus. In the following analysis, Phloiophilus was analysed together with 43 trogossitid genera (88 characters of which 56 were adult and 32 larval). A strict consensus tree of 48 equally parsimonious trees indicates a possible relation of Phloiophilus with Peltinae and justifies independent subfamilial status for Lophocaterinae (the tree is reproduced herein, Fig. 26). It must be pointed out that 32 of 48 trees supported a sister relationship between the lophocaterins and the peltins. Only 16 trees supported a relationship between the lophocaterins and the trogossitins. This is perfectly consistent with the results of
The most extensive modern paper on the beetle phylogeny based on morphological data is that by
A work by
The most updated studies are those by the Tree of Life team and R. A. B. Leschen and co-authors, unpublished as yet. Their preliminary, mutually different, results were presented in the XXIVth International Congress of Entomology in Daegu (
Although the outcomes of the works above differ in details, major ideas may be summarized as follows:
- The superfamily Cleroidea is a monophyletic group.
- A part of the traditional Cucujoidea is probably paraphyletic with regard to Cleroidea. Cleroidea will probably, therefore, be extended to include several other “cucujoid” families.
- Lophocaterinae probably constitutes a sister group to Peltinae.
- Trogossitinae, as a basal group, may form a sister taxon to the major bulk of Cleroidea (although none of the molecular studies included the Metaxinidae-Chaetosomatidae-Thanerocleridae cluster, which might be in sister relationship with the trogossitines). This thesis, however, can hardly be justified by just the morphological evidence. If the subfamily Trogossitinae is actually confirmed paraphyletic, it should be classified at family rank again, as well as Peltidae composed of Peltinae and Lophocaterinae.
- The exact position of Phloiophilus is uncertain; it is a basal group of Cleroidea, probably related to Trogossitidae sensu lato.
A–B Calitys scabra (A head and pronotum B elytral apex with interlocking mechanism) C–D Acalanthis quadrisignata (C apex of protibia with hooked spur and lateral spines D sculpture of frons with punctures conjoined into wrinkles) E–H Gymnocheilis sp., Cameroon (E head F protibia G elytral apex H detail of scales).
A–F Anacypta sp., Assam (A ctenidium B labium with ciliate setae C antennal club with sensorial fields D detail of sensillae E head laterally with divided eye F detail of sensorial setae and ventral eye) G–H Gymnocheilis sp., Cameroon (G antennal club H detail of sensillae).
A–B Nemozoma elongatum (A cranium with longidutinal groove B detail of elongate punctures) C–D Peltis ferruginea (C elytral apex D antennal club, hooked spur and protarsus with large 5th tarsomere) E–G Phloiophilus edwardsi (E metatarsal claws and empodium F projecting procoxae G ventral surface).
A–F Colydiopeltis compactum (A ventral surface B procoxal area C head ventrally D detail of mouthparts E protarsus F apex of metatibia with row of spines) G–H Thymalus limbatus (G antenna H apex of protibia with straight spur).
A–B “Rentonellum” loebli (A ventral surface B prosternal intercoxal process) C cf. Globorentonium plaumanni, Brazil (C ventral surface D pro- and mesothorax ventrally E head ventrally F detail of mouthparts G protibia with tarsus H mesotibia with tarsus).
A Thymalus limbatus, elytral apex with interlocking mechanism B–E Decamerus haemorhoidalis (B prothorax with partially closed procoxal cavities C metatarsal claws with denticle D protarsus with projecting empodium E irregular punctation of elytra) F Grynocharis oblonga, elytral sculpture G–H Lophocateres pusillus (G head in frontal view with deeply emarginate frontoclypeal suture H detail).
Cretocateres mongolicus Ponomarenko, 1986. A reproduction of the original table.
A facsimile of
A facsimile of
A facsimile of
A facsimile of
A facsimile of
A facsimile of
A phylogenetic tree of Trogossitidae adapted and modified from original drawing by
I am indebted to Ottó Merkl (Hungarian Natural History Museum, Budapest), Jean J. Menier and Nicole Berti (National Museum of Natural History, Paris), Jane Beard and Max Barclay (Natural History Museum, London), Lee Herman (American Museum of Natural History, New York), Ivan Löbl (Natural History Museum, Geneva), Josef Jelínek and Vladimír Švihla (National Museum, Prague) and many other curators for their generosity with time, help and hospitality.
I would also like to thank Petr Banař (Moravian Museum, Brno) for the SEM photographs (Figs 13–18), Alexey A. Zaitsev for the photo of the Ancyrona larva (Fig. 2G), and Jiří Ch. Vávra (Ostrava museum, Ostrava) who allowed me to take photos in his collection.
I am further obliged to Ivan Löbl (Geneva) and Rich Leschen (Auckland) for their critical notes on a draft of the manuscript.
As ever, I am grateful to Tony Long (Svinošice, Czech Republic) for working up the English.
This publication appears through financial support provided to the Moravian Museum by the Ministry of Culture of the Czech Republic, as part of its long-term conceptual development program for research institutions (ref. MK000094862).