(C) 2013 Michael Schmitt. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Schmitt M, Frank M (2013) Notes on the ecology of rolled-leaf hispines (Chrysomelidae, Cassidinae) at La Gamba (Costa Rica). In: Jolivet P, Santiago-Blay J, Schmitt M (Eds) Research on Chrysomelidae 4. ZooKeys 332: 55–69. doi: 10.3897/zookeys.332.5215
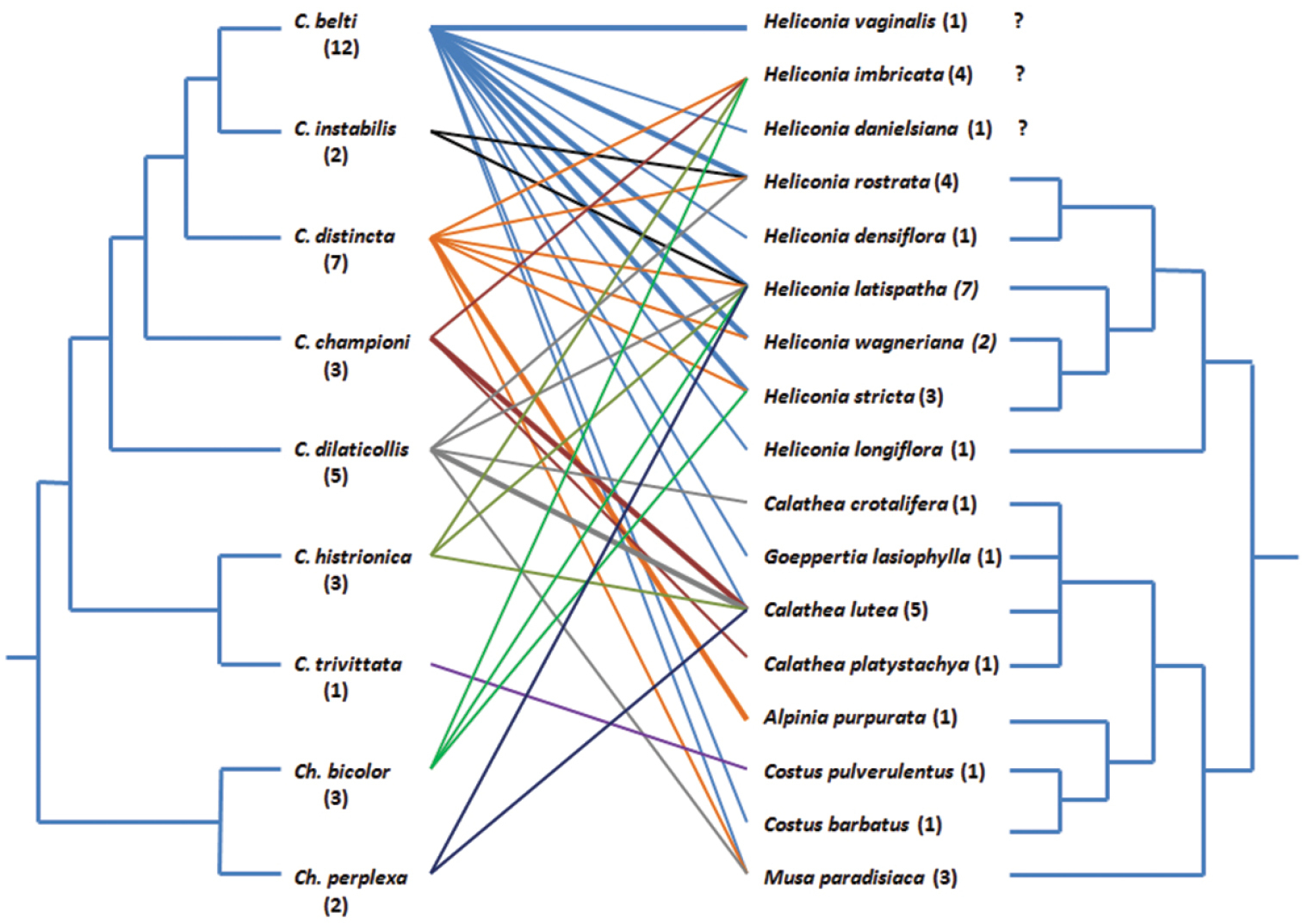
A total of 301 adult hispine beetles of the genera Cephaloleia and Chelobasis were found in rolled leaves of plants of 17 species of Zingiberales (families Costaceae, Heliconiaceae, Maranthaceae, Musaceae, and Zingiberaceae) during a field study at La Gamba, Golfito region, Costa Rica. Of these beetles, Cephaloleia belti was recorded from 12 potential host plant species, C. distincta from 7, C. dilaticollis from 5, C., Chelobasis bicolor, C. championi, and C. histrionica from 3, Chelobasis perplexa and C. instabilis from 2, whereas C. trivittata from only one. Of the plant species, Heliconia latispatha had 7 beetle species in its leaf rolls, Calathea lutea had 5, H. imbricata and H. rostrata had 4, H. stricta and Musa paradisiaca had 3, H. wagneriana had 2, while on H. vaginalis, H. danielsiana, H. densiflora, H. longiflora, Calathea crotalifera, C. platystachya, Goeppertia lasiophylla, Alpinia purpurata, Costus pulverulentus and Costus barbatus, H. densiflora, H. vaginalis, and H. danielsana only hispines of one species were found.
Cephaloleia belti occurred together with beetles of six other hispine species, whereas Cephaloleia trivittata never shared a leaf roll with another hispine species. The remaining beetle species aggregated with one to four other hispines. Adults of C. belti and C. championi were frequently seen, occasionally also with C. dilaticollis, C. histrionica, and Chelobasis perplexa, to co-occur with the carabid Calophaena ligata in the same leaf roll without any sign of interspecific aggression.
A comparison of host choices and the phylogeny of the hispines and of their host plants revealed no signs that beetles used species level phylogenetic relationships within the Zingiberales to select food plants. Obviously, within this plant order, rolled-leaf hispines choose their plant hosts in a nearly opportunistic manner. Seemingly, they use differences among plants at higher taxonomic levels but within the Zingiberales, the availability of young – rolled – leaves might be the actual decisive factor.
Insecta, Coleoptera, Chrysomelidae, Cephaloleia, Chelobasis, Zingiberales, Costaceae, Heliconiaceae, Maranthaceae, Musaceae, Zingiberaceae, synecology, host plant, Costa Rica
Since the nineteenth century it has been known to science that beetles of a (probably monophyletic:
Our primary aim was to assess the number of rolled-leaf hispines species and their abundances in the area of the biological field station “La Gamba” in the Golfito region of Costa Rica and to compare our findings with those from the “La Selva” biological station (
All field work was performed at the La Gamba biological station, Costa Rica (Puntarenas), 8 km NNW of the city of Golfito, 8°42'61"N, 83°12'97"W, 70 m a.s.l., and ca. 8 km off the coast of the Golfo Dulce. The station is located at the edge of the Piedras Blancas National Park and is run by the Verein zur Förderung der Tropenstation La Gamba (society for the furtherance of the La Gamba tropical field station), based at the University of Vienna (Austria) (www.lagamba.at). Numerous plants of the order Zingiberales grow in the 2200 m2-garden of the station. Most individual plants are accurately identified to species and labelled. Botanists from the department of Tropical Ecology and Animal Biodiversity at the University of Vienna are responsible for the scientific supervision of the station, and the accurate identification of the plants in the garden. The station is situated between secondary and primary forest areas to the west, south, and east, and adjoins agriculturally managed areas, mostly pastures and oil palm plantations, to the north. Several trails through the forest allow access to sites inside the forest, e.g. to clearings where the host plants in this study were most abundant.
As no other plants at La Gamba formed rolled young leaves, we censused only Zingiberales plants in the station’s park and along trails for rolled leaves at 15 day intervals within the months of January through April, 2009. We unrolled 120 rolled leaves and recorded the macrofauna found in them. The hispine leaf beetles and other arthropods were collected from the leaves and taken to the station. We also kept records of findings of hispine larvae, eggs, and feeding tracks. In some cases we took photographs as exemplars. Some of the hispines were killed and mounted for identification, others were stored in ethanol. The whole material is still with the senior author for further examination of the non-hispine species. It will be deposited at the Museo Zoológico of the Universidad de Costa Rica at San Pedro, voucher specimens will be deposited at Zoologisches Forschungsmuseum Alexander Koenig, Bonn (Germany).
We identified the beetles by comparison with identified specimens in the collection at the Instituto Nacional de Biodiversidad (INBio) at Santo Domingo de Heredia and by means of published keys and original descriptions (
We found 301 individuals of nine species of hispines, all from two genera, Cephaloleia Chevrolat, 1837 and Chelobasis Grey, 1832. These were the Cephaloleiini Cephaloleia belti Baly, 1885, Cephaloleia championi Baly, 1885 (Fig. 1), Cephaloleia dilaticollis Baly, 1858, Cephaloleia distincta Baly, 1885, Cephaloleia histrionica Baly, 1885, Cephaloleia instabilis Baly, 1885, Cephaloleia trivittata Baly, 1885 and the Arescini Chelobasis bicolor Gray, 1832 (Fig. 2) and Chelobasis perplexa Baly, 1858. They were collected from 17 identified and at least two unidentified Zingiberales species: Alpinia purpurata (Zingiberaceae), Calathea crotalifera, Calathea lutea, Calathea platystachya, and Goeppertia lasiophylla (Marantaceae), Costus barbatus and Costus pulverulentus (Costaceae), Heliconia danielsiana, Heliconia densiflora, Heliconia imbricata, Heliconia latispatha, Heliconia longiflora, Heliconia rostrata, Heliconia stricta, Heliconia vaginalis, and Heliconia wagneriana (Heliconiaceae), and Musa paradisiaca (Musaceae). The numbers of the collected beetles and the respective potential host plants are given in Table 1. The beetle records are unequally distributed over their potential host plants. Of all species found in more than 20 individuals and on more than one potential host plant, a marked majority of records are from one or few of their potential host plants.
Cephaloleia championi from an unrolled Heliconia-leaf at La Gamba. M.Schmitt phot.
Chelobasis bicolor, La Gamba. M. Frank phot.
Numbers of collected hispines and their potential host plants at La Gamba
| Cephaloleia belti | Cephaloleia championi | Cephaloleia dilaticollis | Cephaloleia distincta | Cephaloleia histrionica | Cephaloleia instabilis | Cephaloleia trivittata | Chelobasis bicolor | Chelobasis perplexa | |
|---|---|---|---|---|---|---|---|---|---|
| Alpinia purpurata (Zingiberaceae) | 14 | ||||||||
| Calathea crotalifera (Marantaceae) | 8 | ||||||||
| Calathea lutea (Marantaceae) | 2 | 33 | 15 | 6 | 1 | ||||
| Calathea platystachya (Marantaceae) | 3 | ||||||||
| Costus barbatus (Costaceae) | 1 | ||||||||
| Costus pulverulentus (Costaceae) | 3 | ||||||||
| Goeppertia lasiophylla (Heliconiaceae) | 1 | ||||||||
| Heliconia danielsiana (Heliconiaceae) | 1 | ||||||||
| Heliconia densiflora (Heliconiaceae) | 2 | ||||||||
| Heliconia imbricata (Heliconiaceae) | 1 | 1 | 6 | 1 | |||||
| Heliconia latispatha (Heliconiaceae) | 57 | 3 | 3 | 4 | 3 | 4 | 3 | ||
| Heliconia longiflora (Heliconiaceae) | 4 | ||||||||
| Heliconia rostrata (Heliconiaceae) | 36 | 4 | 1 | 1 | |||||
| Heliconia stricta (Heliconiaceae) | 12 | 1 | 1 | ||||||
| Heliconia vaginalis (Heliconiaceae) | 2 | ||||||||
| Heliconia wagneriana (Heliconiaceae) | 41 | 3 | |||||||
| Musa paradisiaca (Musaceae) | 2 | 1 | 1 | ||||||
| Zingiberales indet. | 9 | 1 | 5 | ||||||
| Totals | 170 | 37 | 31 | 25 | 16 | 4 | 8 | 6 | 4 |
The unidentified Zingiberales grew outside the station garden and lacked inflorescences. We could not identify them using
We opposed a molecular cladogram of the genus Cephaloleia (from
Food web of the rolled-leaf hispines of La Gamba and their possible food plants based on the data in Table 1, drawn by hand using MS Powerpoint. Bold lines indicate more than ten beetle records on the respective plant. Numbers in parentheses give the number of plant or beetle “partners”, respectively. Beetle cladogram after
Rolled-leaf hispines, with the exception of Cephaloleia trivittata, co-occurred at La Gamba with at least one other species in the same leaf roll, Cephaloleia histrionica with only one other species. Cephaloleia belti was found in the same leaf roll together with Cephaloleia championi, Cephaloleia dilaticollis, Cephaloleia distincta, Cephaloleia instabilis, Chelobasis bicolor and Chelobasis perplexa. The remaining hispines shared leaf rolls with two to four other hispine species. The numbers differed considerably, as shown in Table 2. Statistical tests – we used Chi2 - could only be performed for the four most abundant species, as of the remaining species we found too few individuals.
Co-occurrence of rolled-leaf hispines in the same leaf roll at La Gamba.
| Found in the same leaf roll together with: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cephaloleia belti | Cephaloleia championi | Cephaloleia dilaticollis | Cephaloleia distincta | Cephaloleia histrionica | Cephaloleia instabilis | Cephaloleia trivittata | Chelobasis bicolor | Chelobasis perplexa | a | b | |
| Cephaloleia belti (n = 170) | 120 | 1 | 17 | 26 | 10 | 15 | 8 | Chi2: 286.7 p: <0.001 | Chi2: 9.878 p: 0.003 | ||
| Cephaloleia championi (n = 37) | 6 | 17 | 12 | 8 | 1 | Chi2: 6.581 p: 0.087 | Chi2: 1.884 p: 0.227 | ||||
| Cephaloleia dilaticollis (n = 31) | 15 | 11 | 14 | 1 | 1 | Chi2: 0.650 p: 0.778 | Chi2: 3.600 p: 0.080 | ||||
| Cephaloleia distincta (n = 25) | 11 | 1 | 15 | 2 | 2 | 1 | Chi2: 17.20 p: 0.001 | Chi2: 0.000 p: 1.000 | |||
| Cephaloleia histrionica (n = 16) | 4 | 12 | Chi2: n.a. p: n.a. | Chi2: n.a. p: n.a. | |||||||
| Cephaloleia instabilis (n = 4) | 4 | 3 | Chi2: n.a. p: n.a. | Chi2: n.a. p: n.a. | |||||||
| Cephaloleia trivittata (n = 8) | 8 | Chi2: n.a. p: n.a. | Chi2: n.a. p: n.a. | ||||||||
| Chelobasis bicolor (n = 6) | 4 | 3 | 1 | Chi2: n.a. p: n.a. | Chi2: n.a. p: n.a. | ||||||
| Chelobasis perplexa (n = 4) | 3 | 1 | 1 | 2 | Chi2: n.a. p: n.a. | Chi2: n.a. p: n.a. | |||||
Numbers in the cells indicate the numbers of beetles given in the first column co-occurring with beetles of species given in the same horizontal row, e.g.: 26 of the 170 Cephaloleia belti were found together with Cephaloleia distincta. As in several cases beetles of more than two species were found in one leaf roll, the checksums in these cases are higher than the total numbers given in the first column. White cells mark the exclusively conspecific aggregations, pink cells indicate observed records, yellow and blue cells mean that these theoretically possible co-occurrences have not been found in the present study.
Column a: Chi2 and p-value for an aggregation of the species in the line with conspecifics or with any other species.
Column b: Chi2 and p-value for an aggregation of the species in the line with the pooled other hispines.
Individuals of the ground beetle, Calophaena ligata Bates, 1883 (Carabidae: Harpalinae, Fig. 4) (29 individuals) were found on Calathea lutea exclusively, co-occurring with Cephaloleia belti (11), Cephaloleia championi (19), Cephaloleia dilaticollis (7), Cephaloleia histrionica (4), and Chelobasis perplexa (1). Four Calophaena ligata-individuals were found in a single leaf roll without any hispine company. These beetles always sat on the inner surface of the leaf roll and were never found between two layers of a roll. We could never observe them feeding inside the leaf roll, nor could we find them on an uncoiled Zingiberales leaf at daylight.
Calophaena ligata on an uncoiled Calathea lutea-leaf. M. Schmitt phot.
Our small set of observations show that in the use of host plants there are generalists and specialists among the hispine beetles found in the rolled leaves of Zingiberales at La Gamba. This is in general concordance with earlier investigations (
It is evident that of all Zingiberales species at La Gamba, Heliconia latispatha harboured the greatest number of rolled-leaf hispine species (7), followed by Calathea lutea (5) and Heliconia imbricata and Heliconia rostrata (4 each). The species richness of Heliconia latispatha is well documented (
Due to limitations in sample size, the outstanding case of Cephaloleia trivittata on Costus pulverulentus does not convincingly demonstrate a high degree of specialisation, especially since we found five Cephaloleia trivittata individuals on unidentified Zingiberaceae. We can interpret this finding at best as an indication of a possible specialist among the species investigated.
Another remarkable observation is that Cephaloleia distincta obviously prefers Alpinia purpurata but uses sporadically up to six other Zingiberales species. This could mean that Cephaloleia distincta has a high potential to shift host plants when necessary. Differing from
As Fig. 3 shows, there is seemingly no phylogenetic pattern in the hispine-Zingiberales relations at La Gamba. Even if we consider only those relations based on more than ten beetle records per plant species, indicated by the bold lines in the figure, phylogenetic relatedness of the plants or the beetles involved does not seem to play any role. Otherwise, we would have found that closely related beetles use closely related plants. It may well be that phylogenetic factors determine food plant choice on a broader scale so that a phylogenetic pattern, as
We could observe feeding only occasionally. Therefore, it is by no means certain that the associations we report here represent indeed trophic interactions.
The other possible meaningful result is that we found four adult beetles of three species in banana leaf rolls. Banana (Musa x paradisiaca) was introduced to this area of Central America by man ca. 120 years ago (
Although it was already reported by Baly in 1885 that Cephaloleia-individuals were found “often in company with species of Calophaena (Carabidae)” (p. 8), it was to our knowledge not mentioned in the modern papers on the biology of rolled-leaf hispines. We found 29 individuals of Calophaena ligata in the lumen of Calathea-lutea-leaf-rolls. Obviously, they co-existed harmoniously with the hispines in their leaf rolls (Cephaloleia belti, Cephaloleia championi, Cephaloleia dilaticollis, Cephaloleia histrionica, and Chelobasis perplexa). The genus Calophaena belongs to the tribe Harpalini, which comprises many phytophagous species (see, e.g.,
After all, it is interesting to note that Calophaena-individuals have exclusively been found on Calathea lutea plants. Daniel Blanke reported in his unpublished diploma thesis (“Autökologie der Laufkäfer der Gattung Calophaena – Coleoptera, Carabidae – im Piedras Blancas Nationalpark, Costa Rica”, University of Bonn 2010, supervised by M.S.) that he had found 389 individuals of this species at La Gamba, of which 387 were discovered on Calathea lutea. He saw them moving around on lower leaf surfaces and gnawing at the base of leaves at dark. He speculates that the ground beetles take up flavonoids from the plant and use them to produce their aposematic colouration (see Fig. 4). This idea appears plausible since Calathea lutea-leaves are outstandingly rich among Zingiberales in flavonoid content (
The core conclusions from our results are: The rolled-leaf hispines at La Gamba have been found on Zingiberales, as already known from other regions in Central America (La Selva, Panama). However, we did not systematically check other plants. Among the beetles we collected were some with a broader spectrum of potential host plants, above all Cephaloleia belti, while other species live on fewer plant species or even only on one (Cephaloleia trivittata on Costus pulverulentus). However, the many observations of few individuals or singletons of a beetle species on several Zingiberales when the majority of their conspecifics was found on one or only a few other Zingiberales underpin the statement of
We thank the Alexander-Koenig-Gesellschaft (Bonn, Germany) for financial support of the junior author, the staff at the Tropenstation La Gamba for friendly co-operation, the colleagues at the University of Vienna (Austria) responsible for the Tropenstation (Werner Huber, Daniel Schaber, Anton Weissenhofer) for kind practical assistance, Gabriele Uhl (Greifswald, Germany) for carefully checking and improving the manuscript and her help with the statistics, and three anonymous reviewers for highly constructive comments.
1 Contribution to the 8th International Symposium on the Chrysomelidae, held August 23, 2012, in Daegu, South Korea