(C) 2012 Ehsan Rakhshani. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The species of Adialytus Förster in Iran are taxonomically studied and new data on distribution and host associations are presented. The existence of a species complex, in the case of Adialytus ambiguus (Haliday), and the morphological variability in commonly used taxonomic characters has been discussed. In total, four valid species belonging to the genus Adialytus including Adialytus ambiguus (Haliday), Adialytus salicaphis (Fitch), Adialytus thelaxis (Starý) and Adialytus veronicaecola (Starý) have been identified and recorded from Iran. Also, we recognized two additional phenotypes: “Adialytus arvicola” (Starý) and “Adialytus cf. ambiguus” (Haliday). These phenotypes and Adialytus veronicaecola are newly recorded from Iran in association with Sipha and Aphis species, respectively. An illustrated key for identification of the species and two variable phenotypes is presented.
Adialytus, taxonomy, host aphid associations, species complex
The genus Adialytus Förster is morphologically very close to the genus Lysiphlebus Förster from which it can be differentiated by the absence of M & m-cu and r-m veins in the fore wing. It was classified as a subgenus of Lysiphlebus (
There was considerable ambiguity about Lysiphlebus confusus Tremblay & Eady and Adialytus ambiguus. The first species name was selected by
Here we review the species of Adialytus in Iran, together with new data on their host associations and distribution. In addition, the possible existence of species complexes and morphological variability within genus are discussed.
Material and methodsSamples of different host plants including wild and cultivated trees, shrubs and herbs bearing the aphid colonies were gently cut off and placed inside the semi-transparent plastic boxes. The collected material were subsequently transferred to the laboratory and kept under controlled conditions with temperature range of 24–28°C and RH: 60±5%, for 2-3 weeks until the emergence of the adult parasitoids. The rearing boxes were inspected daily to prevent the activity of emerging hyperparasitoids. Once detected, they were immediately removed from the rearing boxes. The emerged parasitoids were also carefully collected using an aspirator and dropped into 75% ethanol for further examination. A few specimens from each sample were carefully dissected and mounted in slides using a Hoyer medium. The ratio measurements were based on these slide-mounted specimens using an ocular micrometer. Additional material from European and central Asian countries were also used for comparison of the morphological variation. The characters of flagellar segments, clypeus, fore wing, first metasomal tergit (=petiole) and female genitalia were used for comparison and differentiation of the species, as well as to find the reliable characters for identification key. The external morphology was studied using a NIKON Eclips E200 microscope equipped with a SONY DSC digital camera.
The morphological terminology for parasitoids used in this paper follows
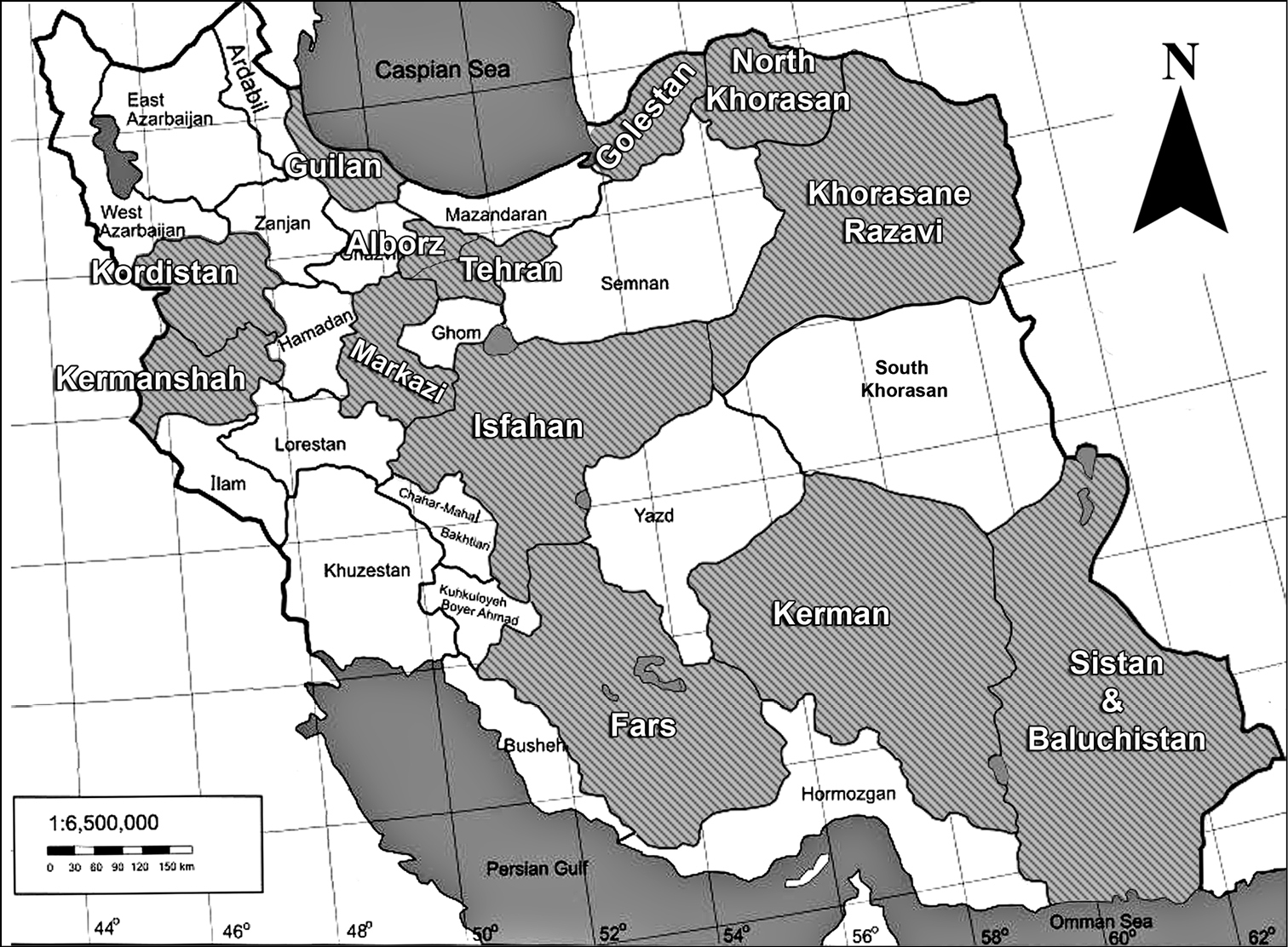
Map of the sampling areas at various parts of Iran, indicating 13 provinces.
Four valid species of the genus Adialytus, as well as two additional phenotypes: “Adialytus arvicola” (Starý) and “Adialytus cf. ambiguus” (Haliday) (Table 1) were collected and identified in association with 14 aphid species on 15 host plants. Many specimens of Adialytus ambiguus (Haliday) were inconsistently different from examined specimens which originated in other countries. We categorized these specimens as “Adialytus cf. ambiguus”. Adialytus veronicaecola (Starý) and “Adialytus arvicola”(Starý) arenewly recorded from Iran. The latter species was reared from Sipha aphids which were also the specific hosts for Adialytus ambiguus. We found significant differences between the Adialytus ambiguus and “Adialytus arvicola” phenotype, based on the characters of fore wing, flagellar segments, hind legs, petiole (Table 2) and coloration. Additionally, a comparison with type specimens of Adialytus arvicola from the Czech Republic (
A list of aphid-parasitoid associations.
| Aphid family | Aphid species | Parasitoid species |
|---|---|---|
| Chaitophorinae | Sipha maydis Passerini | Adialytus cf. ambiguus (Haliday)Adialytus arvicola (Starý) |
| Sipha elegans del Guercio | Adialytus ambiguus (Haliday)Adialytus cf. ambiguus (Haliday)Adialytus arvicola (Starý) | |
| Sipha flava (Forbes) | Adialytus arvicola (Starý) | |
| Chaitophorus spp. | Adialytus salicaphis (Fitch) | |
| Thelaxinae | Thelaxes suberi (del Guercio) | Adialytus thelaxis (Starý) |
| Aphidiinae | Aphis craccivora Koch | Adialytus veronicaecola (Starý)<br/> |
| Aphis gossypii Glover | ||
| Aphis sp. |
The morphometric and meristic data for different characters of Adialytus species (Female) in Iran.
| F1†l/w‡ | F2 l/w | F3 l/w | F4 l/w | F1/F2 length | F1/F3 length | F1/F4 length | F1LP§ | F2LP | Pt#l/w | R1§§/Pt length | Setae on Clypeus | Petiole l/w | Ovipo-sitor sheathl/w | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adialytus ambiguus | 2.10–2.30 | 2.40–2.60 | 1.90–2.10 | 1.80–2.00 | 1.00–1.10 | 0.90–1.00 | 0.90–1.00 | 0 | 1–2 | 2.90–3.00 | 1.30–1.40 | 4–5 | 1.80–2.00 | 2.90–3.20 |
| Adialytus cf. ambiguus | 2.60–2.85 | 2.70–2.90 | 2.70–2.85 | 1.80–2.00 | 0.90–1.00 | 0.90–1.10 | 0.90–1.10 | 0–1 | 2–3 | 2.85–3.10 | 0.90–1.10 | 4–5 | 1.80–2.00 | 2.80–3.20 |
| Adialytus arvicola | 2.50–2.80 | 2.10–2.45 | 2.20–2.40 | 1.70–1.90 | 0.90–1.10 | 0.90–1.10 | 0.95–1.20 | 0–1 | 2–4 | 3.00–3.20 | 0.70–0.80 | 6–8 | 2.00–2.20 | 2.80–3.10 |
| Adialytus salicaphis | 2.70–2.90 | 2.60–2.90 | 2.50–2.80 | 2.30–2.50 | 1.00–1.20 | 0.90–1.10 | 1.00–1.20 | 3–5 | 3–5 | 3.25–3.35 | 0.90–1.00 | 8–10 | 2.20–2.40 | 2.40–2.50 |
| Adialytus thelaxis | 1.60–1.70 | 1.50–1.60 | 1.50–1.60 | 1.60–1.70 | 1.00–1.20 | 1.00–1.20 | 0.90–1.10 | 3–5 | 4–6 | 2.80–3.10 | 0.90–1.00 | 8–10 | 1.80–2.00 | 2.60–2.70 |
| Adialytus veronicaecola | 2.00–2.20 | 1.90–2.00 | 1.90–2.00 | 2.05–2.15 | 1.00–1.10 | 1.00–1.10 | 1.00–1.10 | 0–1 | 0–1 | 3.00–3.20 | 0.60–0.70 | 6–8 | 1.90–2.20 | 2.15–2.30 |
†: F1–F4: Flagellomers 1–4‡l/w: Length/width ratio§ LP: Longitudinal placodes§§ R1: Radial vein 1 (= metacarpus)#: Pterostigma
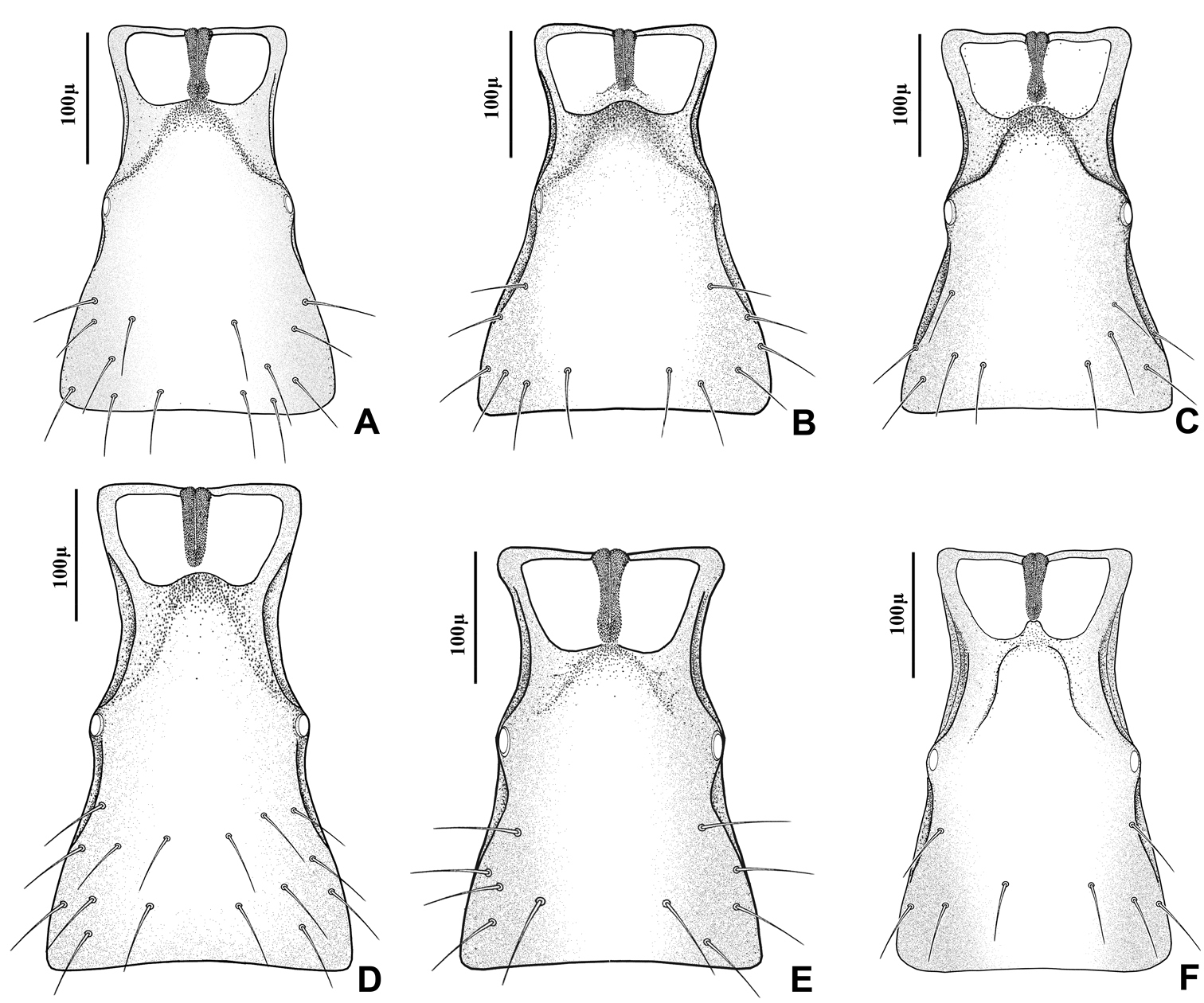
| 1 | Ovipositor sheath considerably elongated, lengh/width ratio of 2.80–3.20 (Figs 6A–C) | 2 |
| – | Ovipositor sheath stout, length/width ratio of 2.20–2.70 (Figs 6D–F) | 4 |
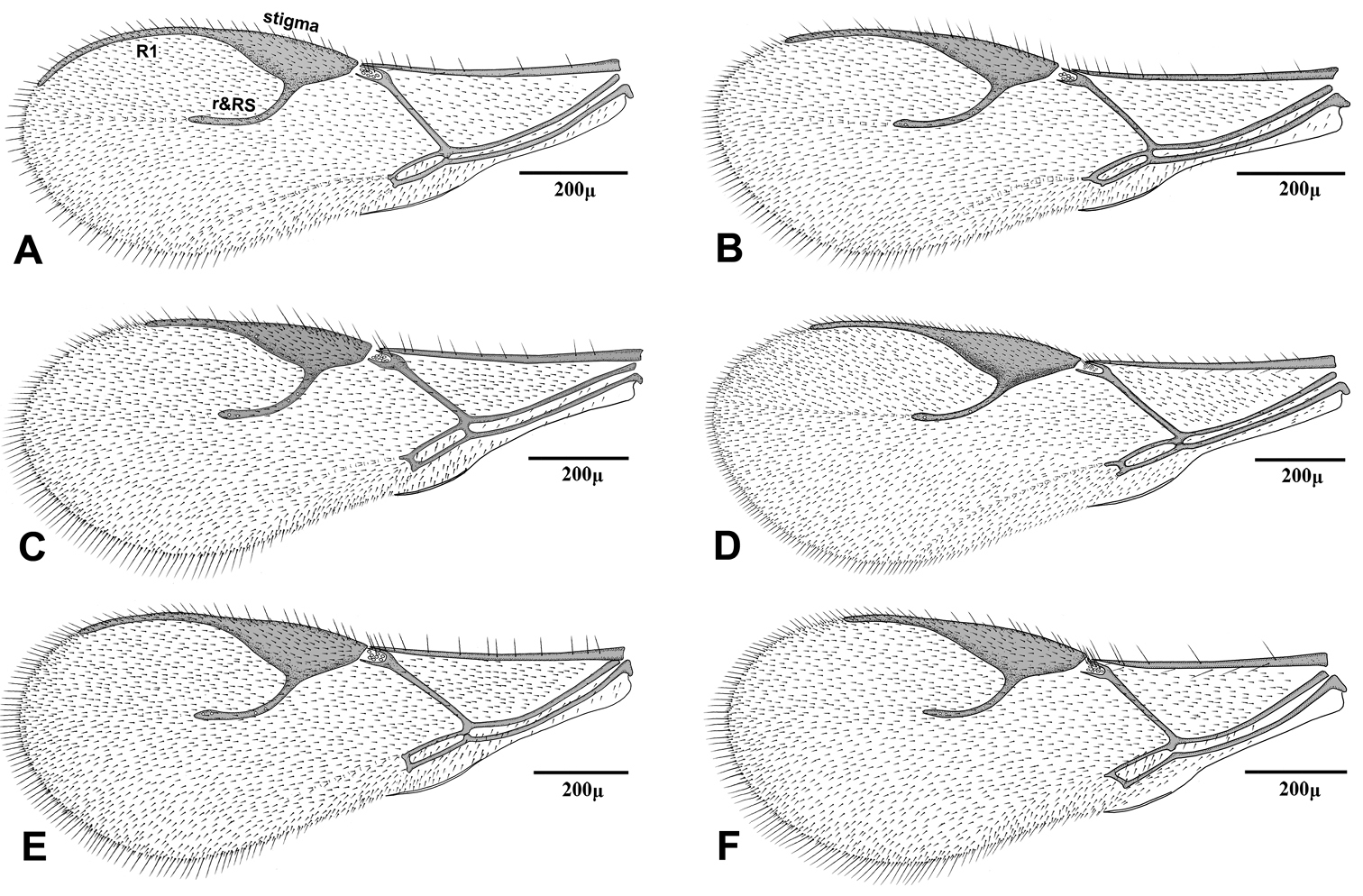
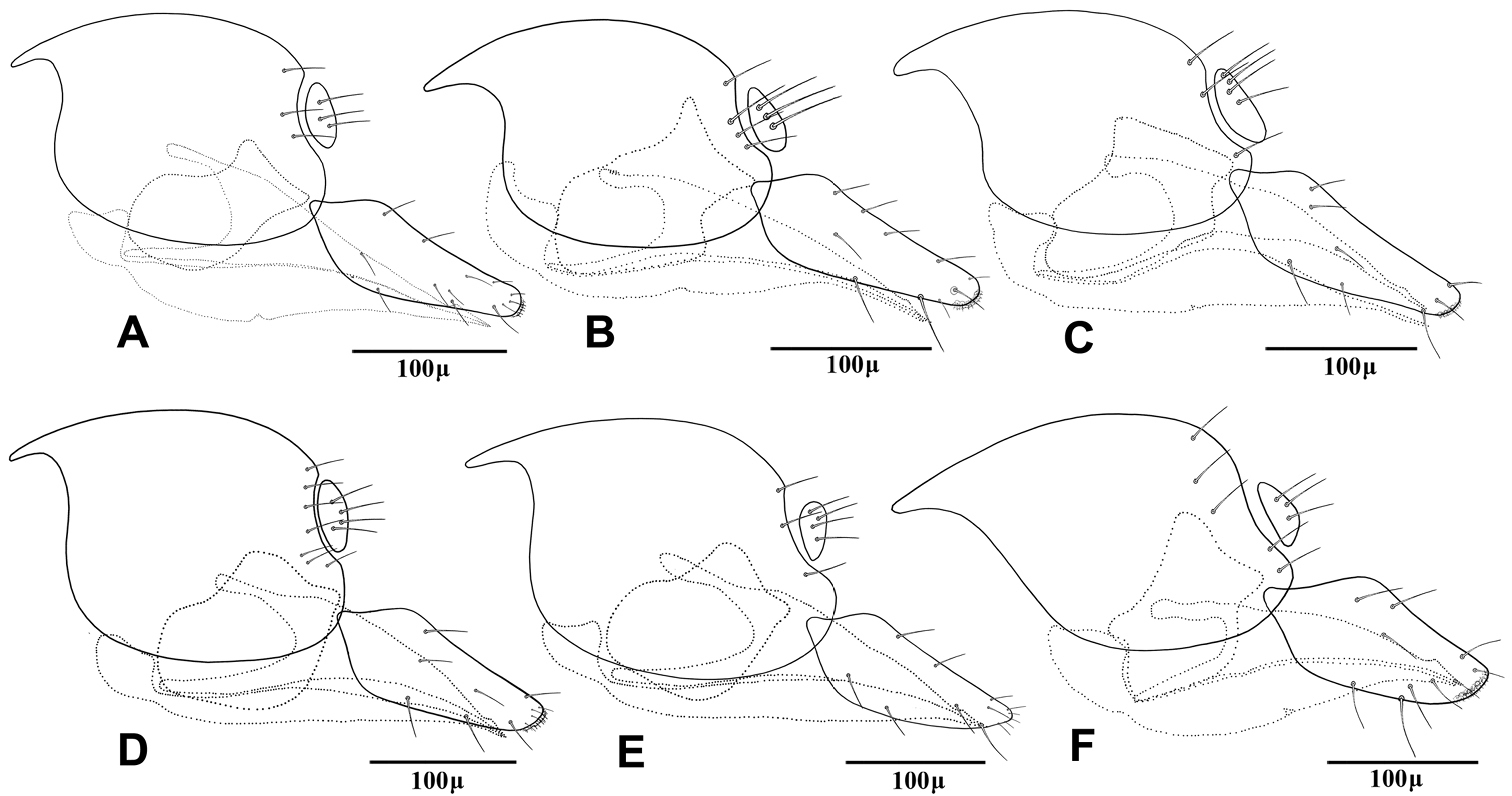
| 2 | Vein R1 (= metacarpus) of fore wing 0.7–0.8 × as long as pterostigma (Fig 3C) | “Adialytus arvicola” (Starý) |
| – | Vein R1 of fore wing subequal (Fig 3B) or considerably longer (Fig 3A) than pterostigma | 3 |
| 3 | Vein R1 of fore wing 1.3–1.4 × as long as pterostigma, reaching apex of wing (Fig 3A) | Adialytus ambiguus (Haliday) |
| – | Vein R1 of fore wing 0.9–1.1 × as long as pterostigma, not reaching apex of wing (Fig 3B) | “Adialytus cf. ambiguus” (Haliday) |
| 4 | Flagellar segments (Fig 2E) subquadrate, slightly longer than their maximum width, l/w ratio of 1.5–1.6. Flagellar segments (Fig 2E) and hind femur (Fig. 4E) covered with long and prevalently erect setae. Ovipositor sheath sharply angular (Fig 6E) | Adialytus thelaxis (Starý) |
| – | Flagellar segments (Figs 2D, 2F) cylindrical, considerably longer than their maximum width, l/w ratio of 2.0–2.9. Flagellar segments and hind femur covered with adpressed (Figs 2F, 4F) or semi-erect (Fig 2D, 4D) setae. Ovipositor sheath roundly angular (Figs 6D, 6F) | 5 |
| 5 | First metasomal tergite (petiole) elongate, 2.2–2.4 × as long as wide at level of spiracles (Fig 5D). Flagellar segments covered with prevalently semi-erect setae which are equal to diameter of segment. Flagellomere 1 bearing 3–4 longitudinal placodes (Fig 2D). Hind femur covered with prevalently semi-erected setae (Fig 4D) | Adialytus salicaphis (Fitch) |
| – | First metasomal tergite (petiole) short, 1.9–2.1X as long as wide at spiracles (Fig 5F). Flagellar segments covered with adpressed setae which are distinctly shorter than diameter of segment. Flagellomere 1 with 0–1 longitudinal placode (Fig 2F). Hind femur covered with short adpressed setae (Fig. 4F) | Adialytus veronicaecola (Starý) |
http://species-id.net/wiki/Adialytus_ambiguus
Figs 2A, 3A, 4A, 5A, 6A1♂ 1♀, Sipha elegans del Guercio on Triticum aestivum, FA, Shiraz (29°34'22"N, 52°41'58"E, 1489 m), 27.IV.2005, 1♂ 1♀, coll.: E. Rakhshani.
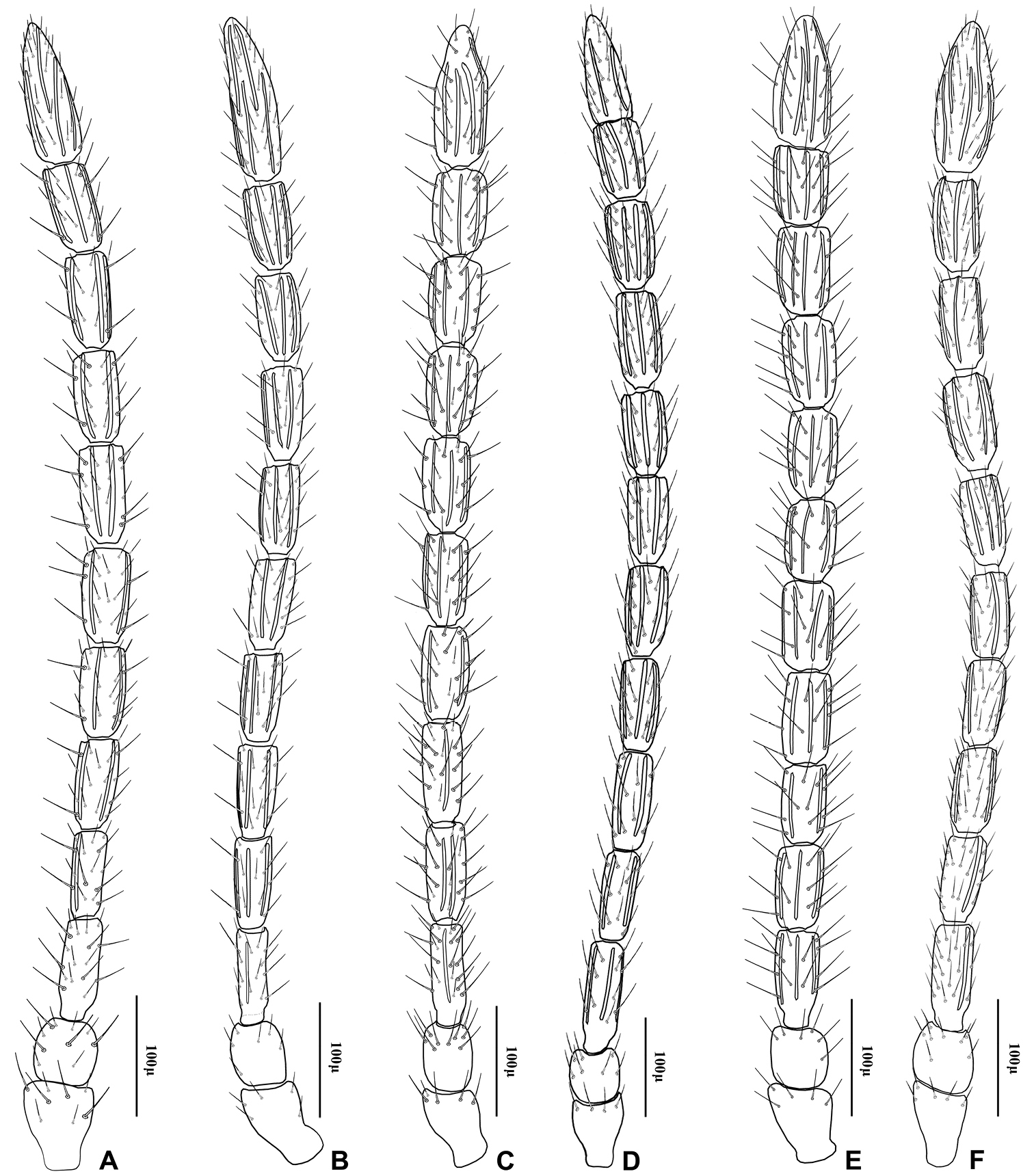
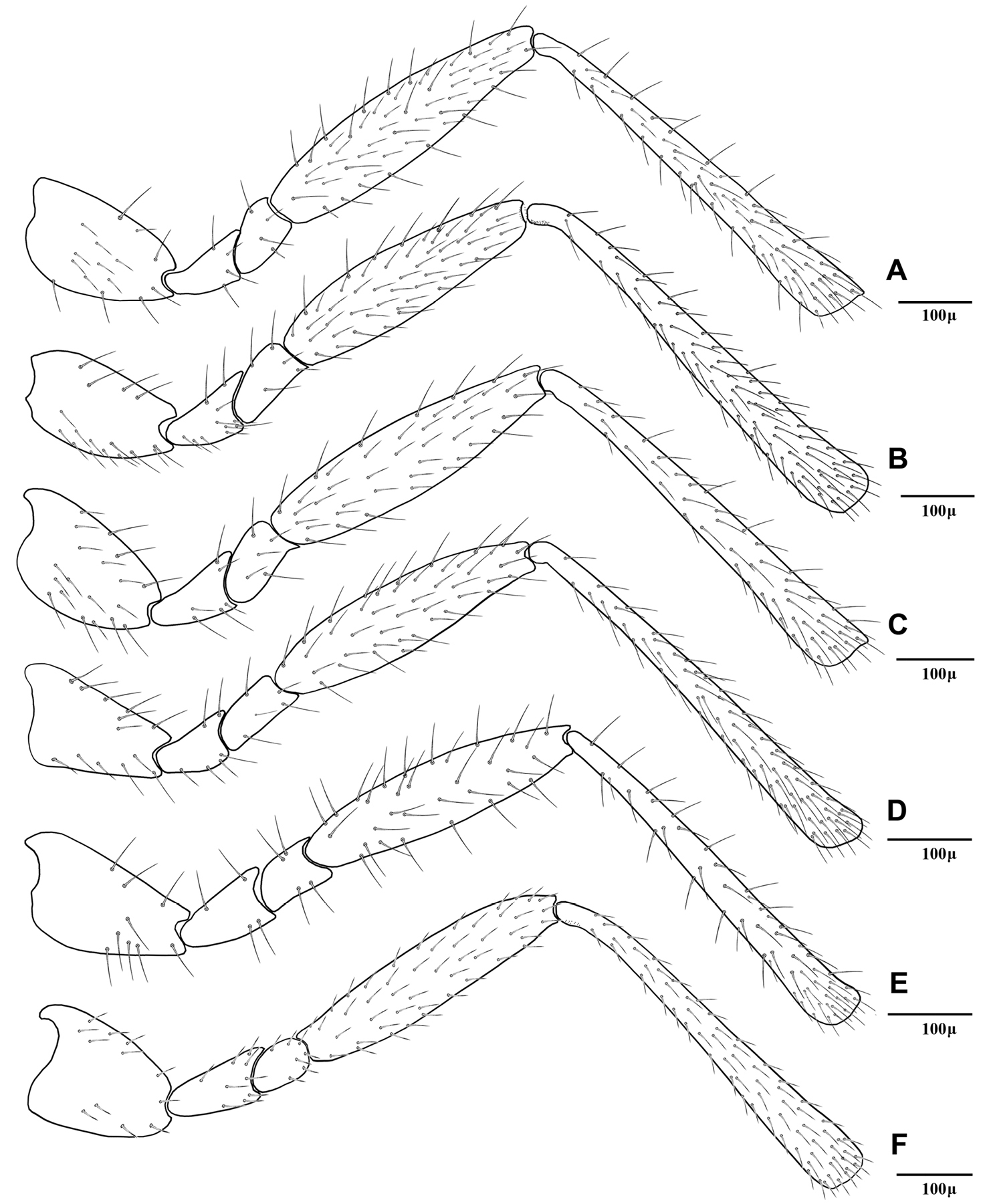
This species is closely related to other parasitoids of Sipha aphids, in its elongated ovipositor sheath (Fig 6A) and triangular shape of petiole which bears anterior and spiracular tubercles (Fig 5A). It can be differentiated from other species in having an extremely long vein R1 (= metacarpus) (Fig 3A). The hind femur and tibia are covered with both short and prevalently erect long setae (Fig. 4A).
22♂ 20♀, Sipha maydisPasserini on Bromus tectorum, NK, Gharemeidan (37°25'42"N, 56°33'19"E, 1544 m), 14.V.2008, 15♂ 18♀, coll. S. Kazemzadeh; Sipha elegansdel Guercio on Gastridium phleoides, IS, Nazhvan (32°38'25"N, 51°35'48"E, 1582 m), 05.IX.2011, 7♂ 2♀, coll. E. Nader.
The specimens normally run to Adialytus ambiguus according to the general characters of the first metasomal tergite (Fig 5B), ovipositor sheath (Fig 6B), the flagellomeres (Fig 2B) and the setae on the hind femur (Fig 4B). It can be differentiated from Adialytus ambiguus by having the shorter vein R1 that is 0.9–1.1 × as long as pterostigma that does not reach the apex of the fore wing (Fig 3B). It can be separated from Adialytus arvicola (Fig 3C), by its longer vein R1.
http://species-id.net/wiki/Adialytus_arvicola
Figs 2C, 3C, 4C, 5C, 6C38♂ 63♀, Sipha flava(Forbes) on Agropyrum repens, KE, Kermanshah (34°19'33"N, 47°05'53"E, 1322 m), 25.VI.2011, 22♂ 55♀, coll. Y. Nazari; Sipha maydisPasserini on Avena fatua, KE, Kermanshah (34°19'33"N, 47°05'53"E, 1322 m), 11.VI.2011, 2♂, coll. Y. Nazari; on Bromus tectorum, KE, Sanandaj (35°17'52"N, 46°59'59"E, 1517 m), 16.V.2005, 1♂, coll. E. Rakhshani; on Cynodon dactylon, KN, Kerman (30°14'28"N, 57°07'20"E, 1775 m), 22.XI.2007, 6♂ 2♀, coll. H. Barahoei; on Sorghum halepense, KE, Kermanshah (34°19'35"N, 47°06'00"E, 1320 m), 11.VI.2011, 2♂ 3♀, coll.: Y. Nazari; Sipha elegans del Guercio on Triticum aestivum, KR, Mashhad (36°15'22"N, 59°28'42"E, 1164m), 12.IV.2012, 5♂ 3♀, coll. J. Karimi.
Generally this species can be confused with other Adialytus species on Sipha aphids, but it is immediately distinguishable by its very short vein R1 (0.7–0.8 × as long as pterostigma) (Fig 3C). Also, its petiole has much stronger anterior and spiracular tubercles (Fig 5C). Most of the metasoma is yellowish, while in other Adialytus species it is uniformly brown to dark brown.
http://species-id.net/wiki/Adialytus_salicaphis
Figs 2D, 3D, 4D, 5D, 6D138♂ 223♀, Chaitophorus euphraticus Hodjat on Populus euphratica, SB, Zahedan (29°23'27"N, 60°48'49"E, 1498 m), 24.III.2003, 3♂ 7♀, coll. E. Rakhshani; Chaitophorus remaudierei Pintera on Salix alba, KD, Marivan (35°31'33"N, 46°09'21"E, 1293 m), 08.X.2004, 4♂ 6♀, coll. E. Rakhshani; Chaitophorus salijaponicus niger Mordvilko on Salix alba, FA, Sepidan (30°15'55"N, 51°58'43"E, 2244 m), 23.V.2009, 7♂ 9♀, coll. S. Taheri; NK, Shirvan, 24.VI.2008, 32♂ 54♀, coll. S. Kazemzadeh; NK, Esfarayen (37°05'12"N, 57°30'39"E, 1293 m), 17.V.2008, 8♂ 13♀, coll. S. Kazemzadeh; Chaitophorus populialbae (Boyer de Fonscolombe) on Populus alba, AL, Karadj (35°44'45"N, 51°10'07"E, 1296 m), 09.X.2002, 16♂ 29♀, coll. E. Rakhshani; Chaitophorus populeti(Panzer) on Populus nigra, TH, Tehran (35°47'52"N, 51°24'08"E, 1650 m), 09.XI.2002; 32♂ 48♀ coll. E. Rakhshani; Chaitophorus leucomelasKoch on Populus nigra, KN, Lalezar (29°31'05"N, 56°48'59"E, 2845 m), 09.X.2007, 5♂ 15♀, coll. H. Barahoei; AL, Karadj (35°55'06"N, 51°05'04"E, 1875 m) 27.VI.2003; 11♂ 18♀, coll. E. Rakhshani; on Populus sp. FA, Sepidan (30°15'55"N, 51°58'43"E, 2244 m), 22.V.2009, 8♂ 12♀, coll.: S. Taheri; Chaitophorus vitellinae(Schrank) on Salix alba, MA, Mahallat (33°53'12"N, 50°27'31"E, 1652 m), 22.IV.2005, 5♂ 4♀, coll.: E. Rakhshani; Chaitophorussp., on Populus alba, NK, Shirvan (37°23'35"N, 57°54'40"E, 1082 m), 24.V.2008, 7♂ 8♀, coll. S. Kazemzadeh.
Adialytus salicaphis differs from other congeners in having very elongated first metasomal tergite (petiole) (Fig 5D), and short and dense marginal setae of the fore wing (Fig 3D). It can also be differentiated from Adialytus arvicola by the number of longitudinal placodes on flagellomere 1 (3–5 in Adialytus salicaphis vs. 0–1 in Adialytus arvicola). The specimens of Adialytus salicaphis associated with Salix spp., especially those reared from Chaitophorus salijaponicus niger on Salix alba, were slightly different from the specimens that reared from Chaitophorus spp. on Populus. The major differences were the lesser number of setae on the clypeus (4–5 vs. 8–10), lesser longitudinal placodes on the first flagellomere (1–2 vs. 3–5) and predominantly adpressed and short setae on the flagellomeres and hind femur compared with the long semi-erect to erect setae among the short setae in specimens from Populus.
http://species-id.net/wiki/Adialytus_thelaxis
Figs 2E, 3E, 4E, 5E, 6E11♂ 26♀, Thelaxes suberi (del Guercio) on Quercus sp., GN, Rasht (37°17'24"N, 49°35'43"E, -4 m), 24.V.2004, 4♂ 3♀, coll.: E. Rakhshani; on Quercus castanifolia, GL, Gorgan (36°47'33"N, 54°27'02"E, 340 m), 06.IV.2010, 7♂ 23♀, coll. A. Sargazi.
This species can be easily separated from other congeners by having mainly erect long setae on the flagellomeres (Fig 2E) and the hind femur (Fig 4E). The setae on the postero-dorsal aspect of petiole are similar (Fig 5E). Additionally, Adialytus thelaxis is the only species with a sharply pointed ovipositor sheath (Fig 6E).
http://species-id.net/wiki/Adialytus_veronicaecola
Figs 2F, 3F, 4F, 5F, 6F2♂ 3♀, Aphis craccivoraKoch on Phaseolus vulgaris, IS, Flavarjan (32°30'56"N, 51°29'02"E, 1618 m), 2♀, coll. E. Nader; Aphis sp. on Rubia tinctorum, IS, Mobarakeh (32°30'56"N, 51°30'17"E, 1658 m), 13.XI.2010, 1♂ 1♀, coll. E. Nader; Aphis gossypiiGlover on Cucurbita pepo, IS, Ghahderijan (32°36'18"N, 51°28'25"E, 1611 m), 05.XI.2010, 1♂, coll. E. Nader.
This species is unique in that it was reared from Aphis species. According to the general characters of the fore wing (Fig 3F), petiole or first metasomal tergite (Fig 5F) and the ovipositor sheath (Fig 6F) it is closely related to Adialytus salicaphis from which it can be immediately distinguished in having prevalently short and adpressed setae on the flagellomeres (Fig 2F) and hind femur (Fig 4F). It can also be differentiated from Adialytus salicaphis by having lesser longitudinal placodes on flagellomeres 1 and 2 (0–1 in Adialytus veronicaecola vis 3–5 in Adialytus salicaphis). In addition, Adialytus veronicaecola differs from the other species in having a stout ovipositor sheath with a strongly convex postero-dorsal outline (Fig 6F).
Antenna of Adialytus species A Adialytus ambiguus B Adialytus cf. ambiguus C Adialytus arvicola D Adialytus salicaphis E Adialytus thelaxis F Adialytus veronicaecola.
Fore wing of Adialytus species A Adialytus ambiguus B Adialytus cf. ambiguus C Adialytus arvicola D Adialytus salicaphis E Adialytus thelaxis F Adialytus veronicaecola.
Hind leg of Adialytus species, excluding tarsomeres A Adialytus ambiguus B Adialytus cf. ambiguus C Adialytus arvicola D Adialytus salicaphis E Adialytus thelaxis F Adialytus veronicaecola.
Petiole or first metasomal tergite of Adialytus species A Adialytus ambiguus B Adialytus cf. ambiguus C Adialytus arvicola D Adialytus salicaphis E Adialytus thelaxis F Adialytus veronicaecola.
Female genitalia of Adialytus species A Adialytus ambiguus B Adialytus cf. ambiguus C Adialytus arvicola D Adialytus salicaphis E Adialytus thelaxis F Adialytus veronicaecola.
In a biological aspect, the host range pattern of Adialytus species can be used as an appropriate criterion supporting its generic status as separate from, but closely related to the genus Lysiphlebus Förster. Species of the genus Lysiphlebus are mostly parasitoids of the genera Aphis and Brachycaudus (
Adialytus veronicaecola was originally described from Kazakhstan (
It is yet unclear which “phenotype” of Adialytus ambiguus was used for the phylogenetic analyses (
In general, identification of the Adialytus species merely based on the morphological characters is rather difficult, since they are very similar and even these characters may be contributed to intraspecific variation. Nevertheless, the host range patterns which are mostly specific can be greatly useful for separation of most species, excluding taxa in the Adialytus ambiguusspecies complex, which have almost the same host range. Further investigations based on the geometric morphometric analysis, as well as suitable molecular markers might reveal the exact identity of the above-mentioned taxa and status “Adialytus arvicola” and “Adialytus cf. ambiguus”. Furthermore, a re-classification at a tribal level is necessary to reconstruct the relationships between two groups of Adialytus species and their position compared to the genus Lysiphlebus.
This study was supported by the grant No. 86-19, University of Zabol, Iran. The contributions by P. Starý and Ž. Tomanović were partially funded by the Entomology Institute project AV0Z50070508 (Academy of Sciences of the Czech Republic), and by the grant of the Ministry of Education and Science of the Republic of Serbia (43001), respectively.