(C) 2013 Leontine E. Becking. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Species of the genus Placospongia are common within the tropical Indo-West Pacific, demonstrating a wide variety of colors and either branching or encrusting growth forms. A revision of Indo-West Pacific Placospongia was undertaken based on a redescription of the holotypes of species of Placospongia from the Indian Ocean and Western Pacific and an examination of an additional 103 specimens of Placospongia ssp. collected from Indonesia (including
Sponge, Indonesia, marine lake, coral reef, mangrove, anchialine pool, ITS, COI
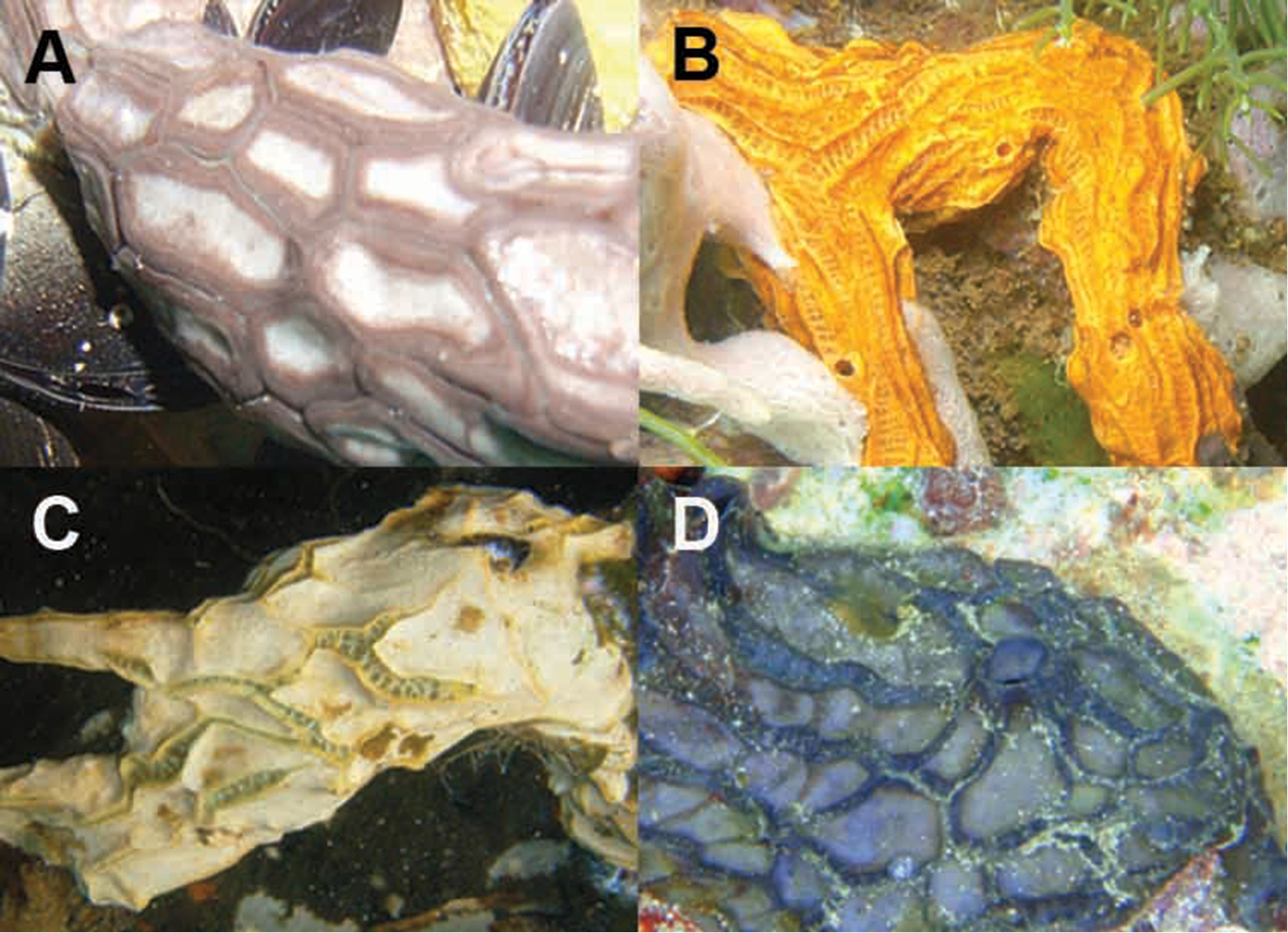
Species of the genus Placospongia in the tropical Indo-West Pacific occur in a wide variety of habitats such as marine lakes, coral reefs and mangroves. They may display a variety of colors and growth forms, from encrusting to branching (Figs 1, 2). Generally only two species have been recorded in species checklists within the Indo-West Pacific (e.g.
The taxonomic literature records six valid species of Placospongia worldwide, of which there are three from the Indian Ocean and Western Pacific: Placospongia carinata (type locality “South Sea”, presumably in the Pacific), Placospongia labyrinthica
The objectives of the present study were to revise the genus Placospongia in the Indo-West Pacific by examining the holotypes of Placospongia melobesioides, Placospongia carinata, Placospongia mixta, as well as 103 specimens of Placospongia spp. that were collected from Indonesia (including the Vosmaer & Vernhout material), Singapore, Seychelles, Madagascar, and Micronesia. In order to obtain a full view of the species from the Western Pacific and Indian Ocean the holotypes of the temperate species Geodinella anthosigma, and Placospongia labyrinthica were also examined. Subsequently it was determined if growth form and color can be used as diagnostic characteristics to identify different species of Placospongia in the field. Finally, the aim was to provide species names to the five clades of Indo-Pacific Placospongia as published by
Specimens from Indonesia were collected via snorkeling in marine lakes and mangroves, and scuba diving in reefs. For a detailed description of marine lakes in Indonesia see
Measurements of spicules of Placospongia carinata, Placospongia melobesioides, Placospongia mixta, and Placospongia santodomingoae sp. n. Sample location, growth form, color and spicule measurements provided per specimen. Spicule dimensions are based on 25 measurements and given in the text as minimum-average-maximum. Spheraster measurements in Placospongia melobesioides based on less than ten measurements, due to low of abundance in specimens.
| tylostyle blunt end | tylostyle sharp end | selenaster | spheraster | streptaster | microrhabd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| growthform | color live | length | max width | head width | length | max width | head width | length | width | diameter | total length | length ray | length | width | |
| Placospongia carinata | |||||||||||||||
| R122b-86g-BK1390 (holotype) | 500-710.4-800 | 10-13.4-15 | 10-15.3-18 | 140-317.4-450 | 5-8.4-12.5 | 8-9.3-13 | 80-90-98 | 60-71.3-85 | 23-33.8-43 | 8-11.6-15 | 8-12.0-18 | 2.5 | |||
| RMNH POR. 4482 | branching | orange | 660-726-800 | 10-12.3-15 | 10-14.5-18 | 180-263-410 | 3-5-7.5 | 8-7.5-8 | 65-71.5-75 | 50-58.5-65 | 15-34-48 | 10-13.0-15 | 8-11.7-15 | 2.5 | |
| RMNH POR. 4483 | encrusting | light brown | 610-703.8-800 | 10-13.1-15 | 13-14.9-18 | 190-286.7-470 | 5-6.4-10 | 5-8.6-13 | 60-80-85 | 60-62.9-70 | 20-33.7-40 | 10-13.2-15 | 8-11.9-18 | 2.5 | |
| RMNH POR. 4484 | encrusting | cream | 560-709.16-920 | 8-11.7-18 | 10-13.9-18 | 175-267.1-550 | 3-4.4-10 | 5-6.4-13 | 50-61.8-70 | 35-47.4-55 | 25-29.7-35 | 8-11.0-15 | 10-13.3-18 | 2.5 | |
| RMNH POR. 4485 | branching | dark brown | 550-761.2-930 | 10-14-18 | 13-15.5-18 | 210-295.2-450 | 3-5.6-8 | 5-7.6-10 | 28-63-73 | 38-50-58 | 20-27.6-38 | 5-9.0-13 | 5-9.4-13 | <2.5 | |
| RMNH POR. 744 | encrusting | purple | 450-748.6-980 | 8-11.1-13 | 10-13.2-15 | 195-256.8-550 | 5-6.2-10 | 5-6.7-8 | 60-66.3-70 | 50-55.6-65 | 25-29.9-38 | 10-12.918 | 8-10.8-13 | <2.5 | |
| RMNH POR. 754 | encrusting | white | 540-705.8-830 | 10-12.8-15 | 13-15.2-18 | 280-355.5-500 | 5-7.0-10 | 5-8.6-13 | 55-67.7-75 | 45-51.8-55 | 25-30.9-38 | 8-9.5-13 | 8-12.3-18 | 2.5 | |
| RMNH POR. 755 | encrusting | cream | 560-764.7-910 | 8-12.2-15 | 10-14.7-18 | 250-311.8-360 | 5-7.3-8 | 5-8.2-10 | 55-61.1-65 | 38-47.5-55 | 30-32.9-38 | 8-9.8-13 | 8-10.2-13 | 2.5 | |
| ZMA Por. 10727 | encrusting | - | 620-738.7-840 | 8-11-13 | 13-15.5-18 | 240-258.3-270 | 3-3.3-5 | 3-4.6-8 | 50-58.8-78 | 35-42.5-63 | 25-27.6-38 | 8-11.1-15 | 8-8.1-10 | <2.5 | |
| ZMA Por. 9189 | branching | - | 550-703.3-820 | 10-12.8-15 | 13-15-18 | 210-318.8-410 | 5-7.5-10 | 5-9.7-13 | 63-72.2-78 | 50-56.8-65 | 30-35-48 | 8-10.7-15 | 8-9.2-13 | 2.5 | |
| Placospongia melobesioides | |||||||||||||||
| BMNH52.4.1.14 (holotype) | branching | dark brown | 670-879.6-1010 | 10-13.2-18 | 10-16.3-20 | 205-293.4-420 | 5-9.9-13 | 5-9.9-13 | 58-63.1-68 | 45-51.7-68 | 15-16.8-18 | ||||
| RMNH POR. 4495 | encrusting | dark brown | 480-717.6-1040 | 5-9.5-15 | 8-10.3-15 | 190-297.6-370 | 3-5.8-8 | 3-6.1-8 | 45-56.6-70 | 30-41.6-50 | |||||
| RMNH POR. 4496 | branching | dark brown | 580-778.4-900 | 8-11.7-15 | 10-14.1-18 | 230-272.8-400 | 5-7.4-10 | 8-9.1-10 | 45-60-75 | 35-45-63 | |||||
| RMNH POR. 4497 | branching | dark brown | 620-745.2-860 | 10-12.2-15 | 13-14.8-18 | 250-320.8-450 | 5-8.8-10 | 5-9.4-13 | 63-70.8-83 | 45-59.6-65 | |||||
| RMNH POR. 3935 | encrusting | dark brown | 460-660.9-760 | 10-11.6-15 | 10-13.7-18 | 210-325.8-450 | 3-7.4-13 | 3-8.3-13 | 45-63.9-70 | 38-51.3-60 | 15-20 | ||||
| RMNH POR. 3166 | encrusting | dark brown | 460-704.8-810 | 8-11.4-13 | 10-13.2-15 | 200-288-470 | 3-9.5-13 | 5-10.8-15 | 60-63.6-70 | 50-50.2-55 | |||||
| RMNH POR. 3976 | branching | dark brown | 600-793.6-910 | 10-12-15 | 13-14-18 | 190-321.2-450 | 5-8.5-13 | 5-9.6-13 | 48-66.8-75 | 48-55.2-65 | |||||
| RMNH POR. 3977 | branching | brown | 510-683.6-780 | 10-11.5-13 | 13-13.9-15 | 200-326-450 | 5-7.5-10 | 8-9.5-13 | 58-63.3-68 | 40-46-53 | |||||
| RMNH POR. 758 | branching | purple | 630-853.2-1020 | 10-13.3-15 | 13-15.8-18 | 210-253.2-310 | 5-9.5-13 | 8-11.8-15 | 50-55.2-62.5 | 35-42.3-50 | 15 | ||||
| RMNH POR. 757 | branching | white | 550-829.2-960 | 10-13.3-16 | 13-15.8-18 | 260-302.1-370 | 8-9.6-13 | 10-11.2-15 | 55-60.4-65 | 43-48.0-53 | |||||
| RMNH POR. 2464 | branching | - | 710-933.4-1080 | 12.5-15-17.5 | 13-15.7-20 | 240-326.7-330 | 5-9.2-13 | 5-10.8-15 | 67.5-81-87.5 | 60-72.5-85 | |||||
| ZMA Por. 10459 | branching | brown | 520-670.8-820 | 7.5-11.4-12.5 | 10-13.4-17.5 | 310-362.5-430 | 5-8.8-10 | 5-10.1-13 | 62.5-68.9-72.5 | 50-55.5-65 | |||||
| Placospongia mixta | |||||||||||||||
| ZMB3204 (holotype) | encrusting | - | 355-672.4-940 | 8-12.1-18 | 8-15.6-20 | 165-226.4-275 | 3-6.1-8 | 3-7.8-10 | 55-69.8-75 | 43-55.4-73 | 20-25-30 | 15-23.9-33 | 3-7.6-13 | 5-7.1-10 | <2.5 |
| RMNH POR. 4112 | encrusting | red | 480-870-1040 | 10-12.7-15 | 13-15.8-28 | 210-288-410 | 5-6.2-10 | 5-7.2-10 | 50-66.6-75 | 38-50.7-58 | 18-20.2-25 | 18-23.7-35 | 5-6.4-10 | 5-6.4-10 | <2.5 |
| RMNH POR. 4113 | encrusting | cream | 550-817.6-1030 | 10-13.1-15 | 13-15.6-18 | 160-260-350 | 5-7.3-10 | 5-8.2-12.5 | 62.5-66-70 | 45-53-57.5 | 20-22.1-25 | 20-24.8-30 | 5-5.7-8 | 5-7.5-10 | 2, 5 |
| RMNH POR. 742 | branching | red | 550-759.2-850 | 10-11.9-15 | 10-14.9-20 | 120-230-380 | 3-5.9-10 | 3-7.6-10 | 50-65.4-73 | 33-46.5-56 | 22-23.4-25 | 15-22.2-35 | 2-5.7-8 | 5-7.4-10 | <2.5 |
| RMNH POR. 4489 | encrusting | cream | 630-886.6-1010 | 10-12.9-15 | 13-15.4-19 | 175-221.5-320 | 3-3.9-8 | 2-7.2-10 | 60-68-75 | 43-50.8-58 | 18-20.6-25 | 20-26.1-35 | 8-10.8-15 | 8-8.5-10 | <2.5 |
| RMNH POR. 4490 | encrusting | cream | 510-727.6-970 | 8-13.120 | 13-16.3-23 | 150-240-310 | 3-5.3-8 | 2-6.4-8 | 55-70.4-83 | 40-53.3-65 | 13-20.5-25 | 15-21.7-30 | 5-6.4-13 | 8-9.2-13 | <2.5 |
| RMNH POR. 4491 | encrusting | brown | 780-1001.4-1200 | 10-14.8-18 | 15-17.5-20 | 240-284-350 | 5-6.3-8 | 5-8.3-10 | 60-71-75 | 48-57.5-63 | 18-23-25 | 20-27.3-35 | 5-7-10 | 5-6.3-8 | 2, 5 |
| RMNH POR. 4492 | encrusting | white | 610-995.8-1250 | 10-16-20 | 13-19-25 | 260-274-290 | 8-9-10 | 8-9-10 | 58-71-78 | 45-54.6-70 | 15-20.2-25 | 18-24.8-33 | 10-11.2-15 | 5-8.6-18 | <2.5 |
| RMNH POR. 3158 | encrusting | cream | 550-990-1210 | 13-16.9-20 | 13-17.5-20 | 130-267.8-400 | 5-8.8-15 | 8-9-10 | 65-71-75 | 50-56.5-63 | 23-23.8-25 | 23-28.4-35 | 5-8.7-13 | 5-6.6-8 | <2.5 |
| RMNH POR. 745 | encrusting | red | 760-914.1-1030 | 13-17-23 | 10-18-25 | 250-366.6-480 | 3-8-13 | 3-9-13 | 45-73.6-80 | 45-60-70 | 20-23.9-25 | 20-23.7-30 | 3-6.4-9 | 5-7.5-10 | <2.5 |
| RMNH POR. 4493 | encrusting | brown | 460-761.6-1070 | 10-14.6-23 | 13-17.38-25 | 220-323.6-430 | 8-9.1-13 | 10-11.3-15 | 73-80.3-85 | 53-65.3-73 | 20-26.5-30 | 18-23.4-30 | 15-8.1-10 | 8-8.7-13 | <2.5 |
| RMNH POR. 4494 | encrusting | brown | 540-758-900 | 10-12.2-18 | 10-13.8-20 | 180-216.9-350 | 3-3.3-5 | 4-4.4-8 | 50-59.1-68 | 35-42.3-58 | 15-20.9-28 | 23-26.9-30 | 8-10.4-13 | 8-8.5-10 | <2.5 |
| Placospongia santodomingoae sp.n. | |||||||||||||||
| RMNH POR. 4486 (holotype) | branching | brown | 430-605.6-660 | 13-15.5-20 | 13-18.1-23 | 240-261.3-290 | 5-7.2-8 | 5-8.8-10 | 80-84.8-90 | 60-67.3-75 | 8-12.3-18 | 2.5-2.7-3.5 | |||
| RMNH POR. 4487 | branching | orange | 530-652.4-740 | 13-16-20 | 15-18.0-23 | 220-274.7-310 | 5-8.2-13 | 8-9.5-15 | 63-82.9-93 | 60-66.3-73 | 5-10.5-20 | 2.5-2.6-3.5 | |||
| RMNH POR. 4488 | branching | orange | 480-633.2-760 | 15-17.2-20 | 18-19.6-23 | 190-273.2-380 | 5-7.9-10 | 8-10.3-13 | 80-87-93 | 58-69-75 | 8-13.5-18 | 2.5-2.9-3.5 | |||
DNA extractions were made with Qiagen DNEasy animal blood and tissue extraction kit following the manufacturer’s protocol. The polymerase chain reaction (PCR) reaction volume was 25 μ l and contained 5 μ l Phire ® Hot Start reaction buffer, 1 unit Hotstart Phire® Hot Start DNA polymerase (Finnzymes), 2 μ l 1 mM dNTPs (Gibco), 1 μ l DNA template (5-20 ng) and 0.625 μ l of 10mM each primer. The standard DNA-barcoding fragment of the mitochondrial cytochrome oxidase subunit I (COI) fragment was amplified by using a specific forward primer designed by the author for Placospongia P-COI-F: GCA GG ATG ATA GGA ACA GGW TTT AG and the degenerated reverse primer from
Abbreviations used in this manuscript: Naturalis Biodiversity Center, Leiden, The Netherlands (RMNH POR.), the Zoological Museum of the University of Amsterdam (ZMA Por.), Zoologisches Museum für Naturkunde an der Universität Humboldt zu Berlin, Berlin, Germany (ZMB), The Natural History Museum, London, United Kingdom (BMNH).
Placospongia melobesioides Gray, 1867 by monotypy
Encrusting to branching growth forms. Small encrustations of 3 cm2 to large surfaces of >2m2 to branching individual with total size of up to 45cm in length and branch diameter between 0.25-1.5cm. Total size of specimens is hard to establish as parts of the body may be encrusting within cracks. Dried material is hard, alcohol preserved and live specimens remain compressible as the choanosome is of more pliant material than the cortex. The surface is made up of smooth cortical plates separated by contractible grooves which form a kind of network on the surface while these are firmly closed in preserved specimens. See
Skeleton. The cortical plates consist of densely packed selenasters and can also contain auxiliary microscleres. Developmental stages of selenasters occur throughout the choanosome. Tylostyle tracts support the margins of the cortical plates. In branching specimens radial tylostyle tracts run from the centre core (consisting of densely packed selenaster) to the cortical plates, in encrusting specimens tracts run in direction from substrate to cortex. The sharp ends of the smaller tylostyles are projected beyond the cortex surface. Microscleres occur in the cortex and scattered in choanosomal skeleton. For a detailed description of external morphology and anatomy see
Spicules. Megascleres are tylostyles in two size classes, microscleres are selenasters, and can include choanosomal and ectosomal spirasters (slender-spined streptasters and acanthose microrhabds), spherasters, and/or spherules. Selenasters often remain pigmented after treatment with bleach or nitric acid.
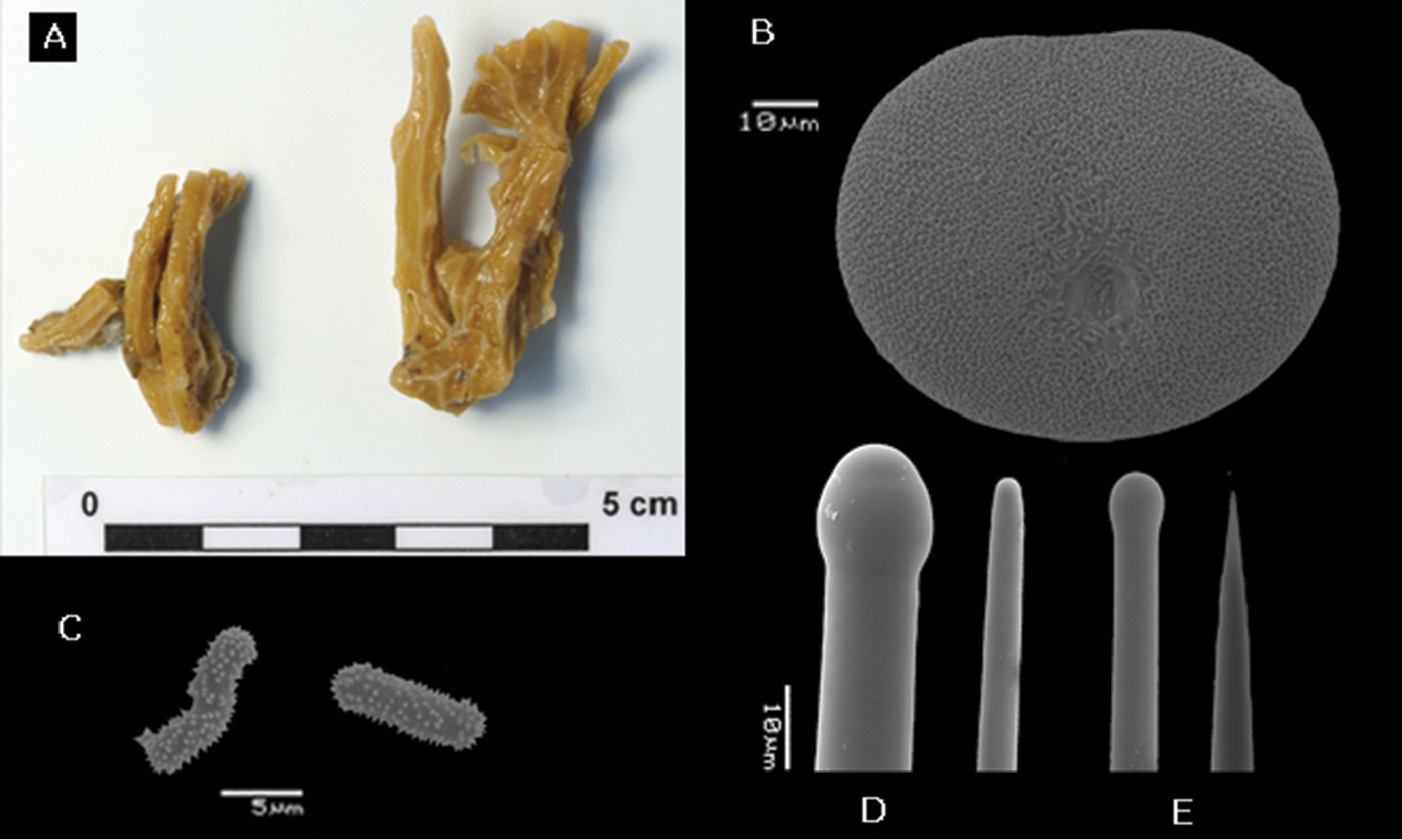
In situ underwater images of Placospongia spp. in Indonesia, displaying natural variation in color and growth form of live specimens. A Placospongia mixta (by L.E. Becking) B Placospongia carinata (by L.E. Becking) C Placospongia carinata (by L.E. Becking) D Placospongia melobesioides (by N.J. de Voogd).
Gradation of external coloration in preserved specimens. A Placospongia mixta RMNH POR. 4492 B Placospongia mixta RMNH POR. 4113 C Placospongia carinata RMNH POR. 4482 D Placospongia carinata RMNH POR. 4483 E Placospongia mixta RMNH POR. 3979 F Placospongia melobesioides RMNH POR. 4114.
http://species-id.net/wiki/Placospongia_anthosigma
Figure 3Holotype. NSMT-Po R288 (National Museum of Nature and Science, Tokyo, Japan), Japan, Kannonzuka-dashi, Amadaiba, Sagami Bay, 62–67m. depth.
HolotypeNSMT-Po R288 encrusting specimen in three pieces of 1–2cm2 and 5mm thick, beige to pink in alcohol (Figure 3A).
Spicules. Megascleres large tylostyles with blunt point 520-797-930 × 15-18-20 × 18-20-23 μ m, small tylostyles with blunt point 250-320-410 × 10-12-18 × 13-14-18 μ m; microscleres selenasters 85-90-98 × 70-73-80 μ m, spherasters 15-19-25 μ m, stout spirasters with two or three contortions and acanthose spines spirally placed on shaft 8-11-18 × 3-4.5-5 μ m (Fig. 3)
Skeleton. As description of genus with addition that spirasters form a layer over and amidst the selenaster cortex and are also prevalent in choanosomal tissue. Spherasters amidst selenaster cortex and dispersed in choanosome.
Placospongia anthosigma holotype (NSMT-Po R288) A type specimen (image taken from website database of the Museum of Nature and Science, Tokyo, Japan) B selenaster C large tylostyle (head and blunt end) D spheraster E spirasters referred to as ‘anthosigma’ by
Typelocality Sagami Bay, Eastern Japan, presently not recorded from any other locality.
On rock substrate in deep temperate waters.
Originally described by
http://species-id.net/wiki/Placospongia_carinata
Figures 4, 5Holotype. “South Sea”: BMNH R1228 - 86g - Bk.1390 (slide), R1275 - PE01 - Bk1390 (slide).
Vosmaer & Vernhout (1902), Siboga expedition: RMNH POR. 755; RMNH POR. 754; RMNH POR. 744. Other material: RMNH POR. 4484, RMNH POR. 3943, RMNH POR. 3944, RMNH POR. 4485, RMNH POR. 3945, RMNH POR. 3946, RMNH POR. 3947, RMNH POR. 3948, RMNH POR. 3949, RMNH POR. 3950; RMNH POR. 3951, RMNH POR. 3952, RMNH POR. 3953, RMNH POR. 3954, RMNH POR. 3955, RMNH POR. 4482, RMNH POR. 3956, RMNH POR. 3957, RMNH POR. 4483, RMNH POR. 3958; ZMA Por. 8813ZMA Por. 09578; ZMA Por. 11367, ZMA Por. 16584, ZMA Por. 10727, ZMA Por. 1818, ZMA Por. 10481, ZMA Por. 20735; ZMA POR.9189. (See Table 2 for full details per specimen)
Location details of reviewed specimens of Placospongia carinata.
| registration number | fieldcode | country | province | region | island | locality | habitat | latitude | longitude | depth (m.) | date | collector |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMNH POR. 744 | #1500 | Indonesia | Moluccas | W of Aru | Kur | benthic hard | 20-40 | 6.xii.1899 | Siboga expedition | |||
| RMNH POR. 754 | #1458 | Philippines | Sulu Sea | Ubian islands | anchorage off North Ubian | lithothamnion | 06°7.5'N, 120°26'E | 23 | 28.vi.1899 | Siboga expedition | ||
| RMNH POR. 755 | #1848 | Indonesia | West Papua | Raja Ampat | Misool | sand, stones | 02°28'.5S, 131°3'.3E | 32 | 20.viii.1899 | Siboga expedition | ||
| RMNH POR. 3943 | #KKB/mol716 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3944 | #KKB/mol754 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3945 | #KKB/mol780 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3946 | #KKB/mol810 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3947 | #KKB/mol814 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3948 | #KKB/mol825 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3949 | #KKB/mol713 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3950 | #KKB/mol1068 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3951 | #MA/mol700 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3952 | #MA/mol975 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3953 | #MA/mol947 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3954 | #MA/mol1055 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3955 | #MA/mol1012 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3956 | #MA/mol1001 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3957 | #MA/mol1009 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3958 | #MA/mol1500 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4482 | #MA/mol1061 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4483 | #MA/LE172 | Indonesia | East Kalimantan | Berau | Maratua | Haji Buang lake | marine lake | 02°12'31.2"N, 118°35'46.8"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4484 | #KKB/mol110 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4485 | #KKB/mol763 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| ZMA Por. 1818 | Indonesia | Maluku | Banda islands | Banda anchorage | reef | 04°32'23.3"S, 129°54'28.8"E | 9-45 | 22.xi.1899 | Siboga expedition | |||
| ZMA Por. 9578 | Singapore | Pulau Salu | reef | 01°12'59.0"N, 103°42'25.2"E | 2 | 22.xii.1977 | H. Moll | |||||
| ZMA Por. 8813 | Indonesia | Nusa Tenggara | Komodo | NE cape | reef | 08°28'60.0"S, 119°34'4.8"E | 30 | 19.ix.1984 | R.W.M. van Soest (Snellius II Expedition) | |||
| ZMA Por. 9189 | India | Laccadive Islands | Agatti | 20-25 | 1987 | National Institute of Oceanography | ||||||
| ZMA Por. 10481 | Seychelles | Mahé | Mahé | SE coast, near Pointe Cocos | reef | 35-45 | 24.xii.1992 | R.W.M. van Soest | ||||
| ZMA Por. 10727 | Seychelles | Mahé | Mahé | NE Point | reef | 04°34'59.9"S, 055°28'0.1"E | 1 | 14.xii.1992 | R.W.M. van Soest | |||
| ZMA Por. 11367 | Seychelles | Mahé | N of Aride | reef | 04°10'59.9"S, 055°40'0.1"E | 40 | 19.xii.1992 | R.W.M. van Soest | ||||
| ZMA Por. 16584 | Seychelles | Mahé | Mahé | SW coast, Baie Lazare, Anse Gaulettes | reef | 04°10'59.9"S, 055°40'0.1"E | 1-4 | 6.xii.1992 | R.W.M. van Soest | |||
| ZMA Por. 20735 | Seychelles | Mahé | reef | 1992 | R.W.M. van Soest | |||||||
Reviewed material is encrusting and/or branching. External morphology follows the description of the genus. Color of live specimens can be purple brown, chocolate brown, milk coffee brown, orange brown, orange, cream, or white (Fig. 1, 2). Color of choanosome is pale beige. After preservation in ethanol specimens retain some color of the live coloration.
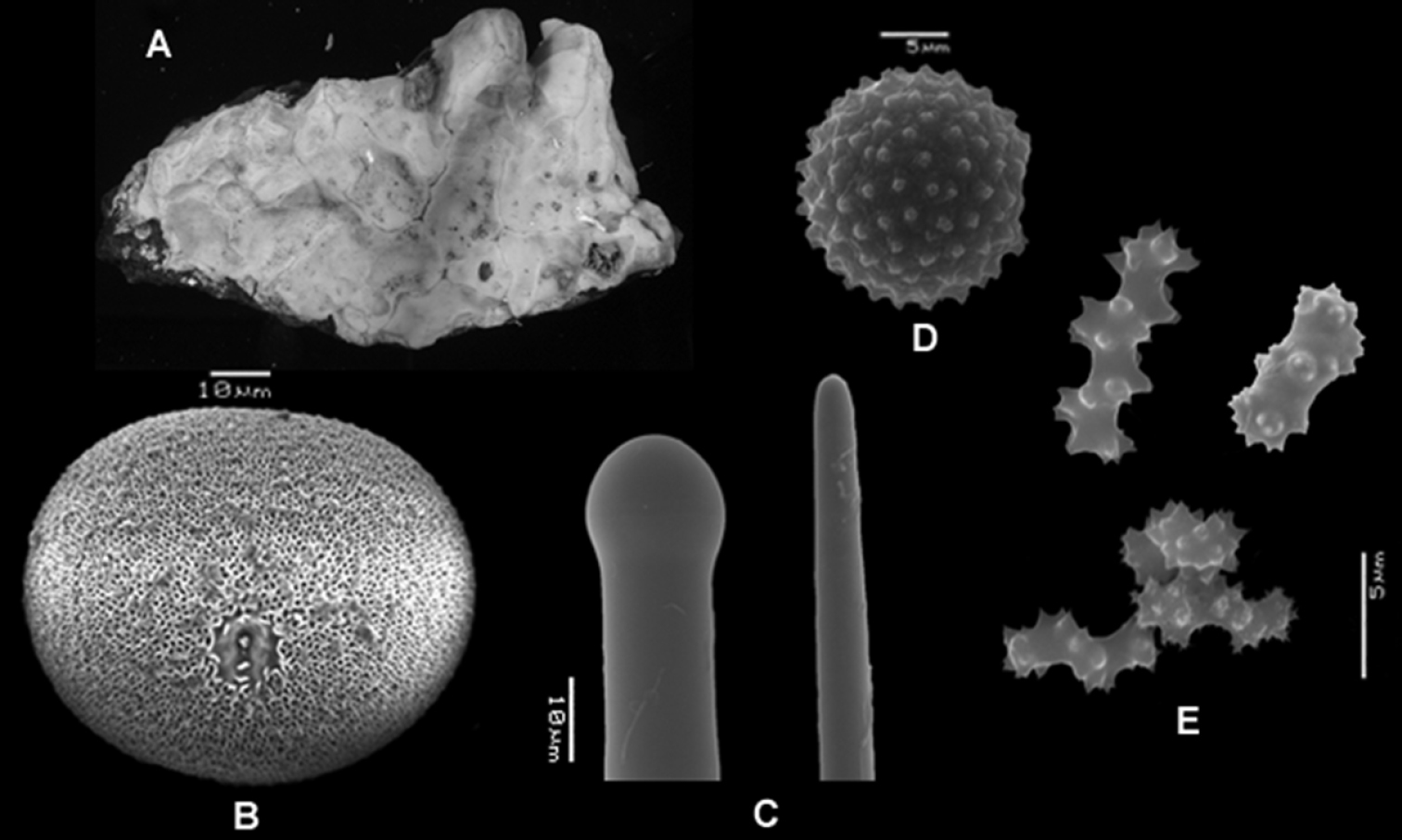
Spicules. Holotype slide with spicules R1228-86g-Bk.1390 (BMNH) and slide with thick section R1275-PE01-Bk1390 (BMNH) (Fig. 4): megascleres large straight tylostyles with blunt ends 500-7 10-820 × 10-13-15 × 10-15-18 μm, small straight tylostyles with sharp ends 140-317-450 × 5-8-25 × 8-9-13 μm; microscleres selenasters 80-90-98 μm, streptasters with varying number of (spined) rays (5-10) with bifurcating endings or tufts 23-34-43 × 8-15 μm, acantho microrhabds 8-12-18 × 1-2.5 μm, spherasters absent. The range within the examined material (Table 1 & Fig. 5): megascleres large tylostyles 540-990 × 8-18 × 10-18 μ m, small tylostyles 175-550 × 3-10 × 3-13 μ m; microscleres selenasters 50-85 × 35-70 μ m, streptasters 15-48 × 5-18 μ m, acanthose microrhabds 5-18 × 1-2.5 μ m, spherasters absent.
Skeleton. As description of genus with addition that microrhabds form a layer over and amidst the selenaster cortex and are also prevalent in choanosomal tissue. Spirasters scattered in choanosome.
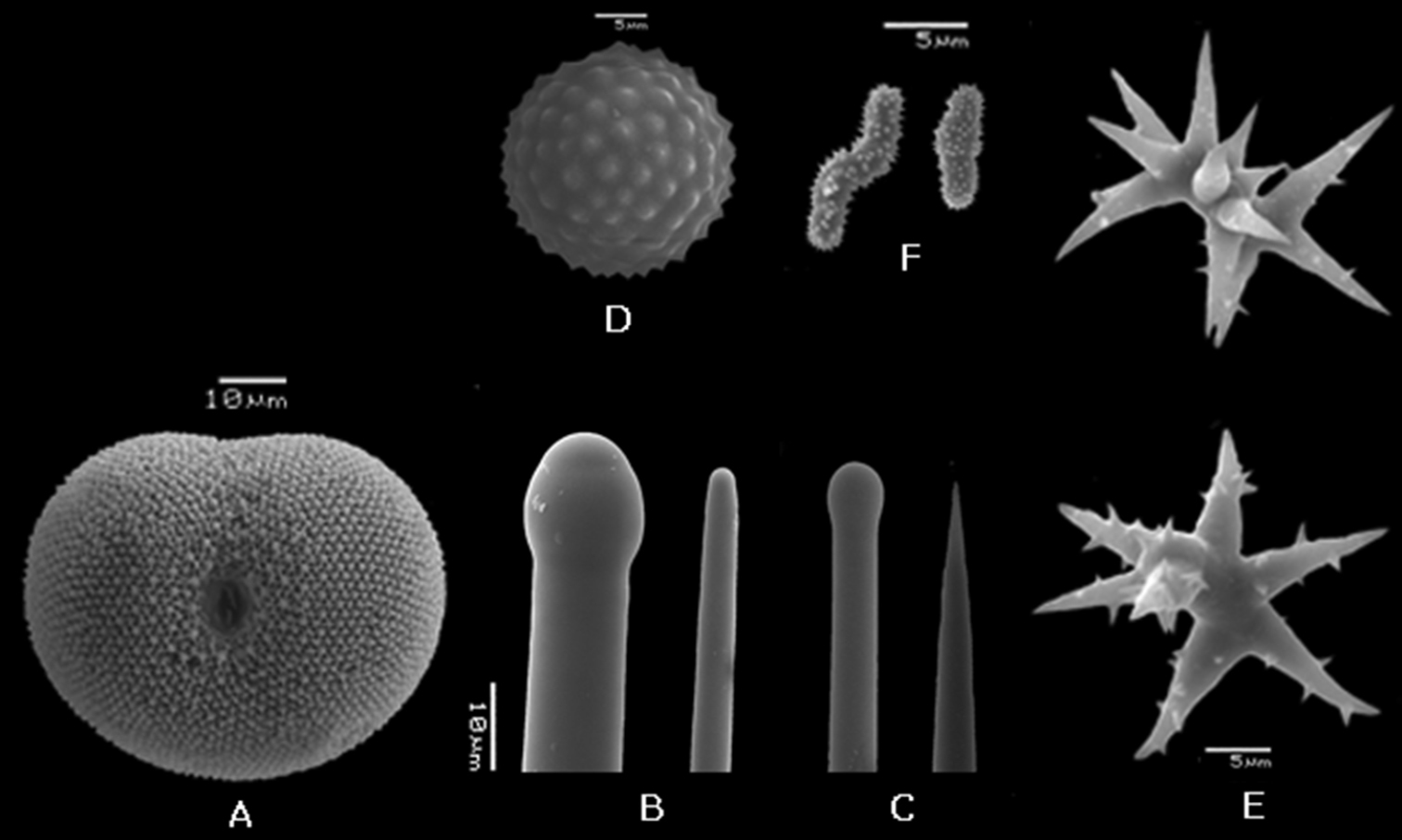
Placospongia carinata slide of holotype (BMNH, R1228, 86g, Bk.1390; R1275, PE01, Bk1390). A large tylostyle (scale=200 μ m) B small tylostyle (scale=50 μ m) C selenaster (scale=50 μ m) D close up of large tylostyle (scale=50 μ m) E close up of small tylostyle F streptasters (scale=50 μ m) G acanthose microrhabds H original slide of thick section of holotype I original slide of spicules of holotype.
Placospongia carinata (RMNH POR. 4483). A selenaster B large tylostyle (head and blunt end) C small tylostyle (head and hastate end) D streptasters, E. acanthose microrhabds.
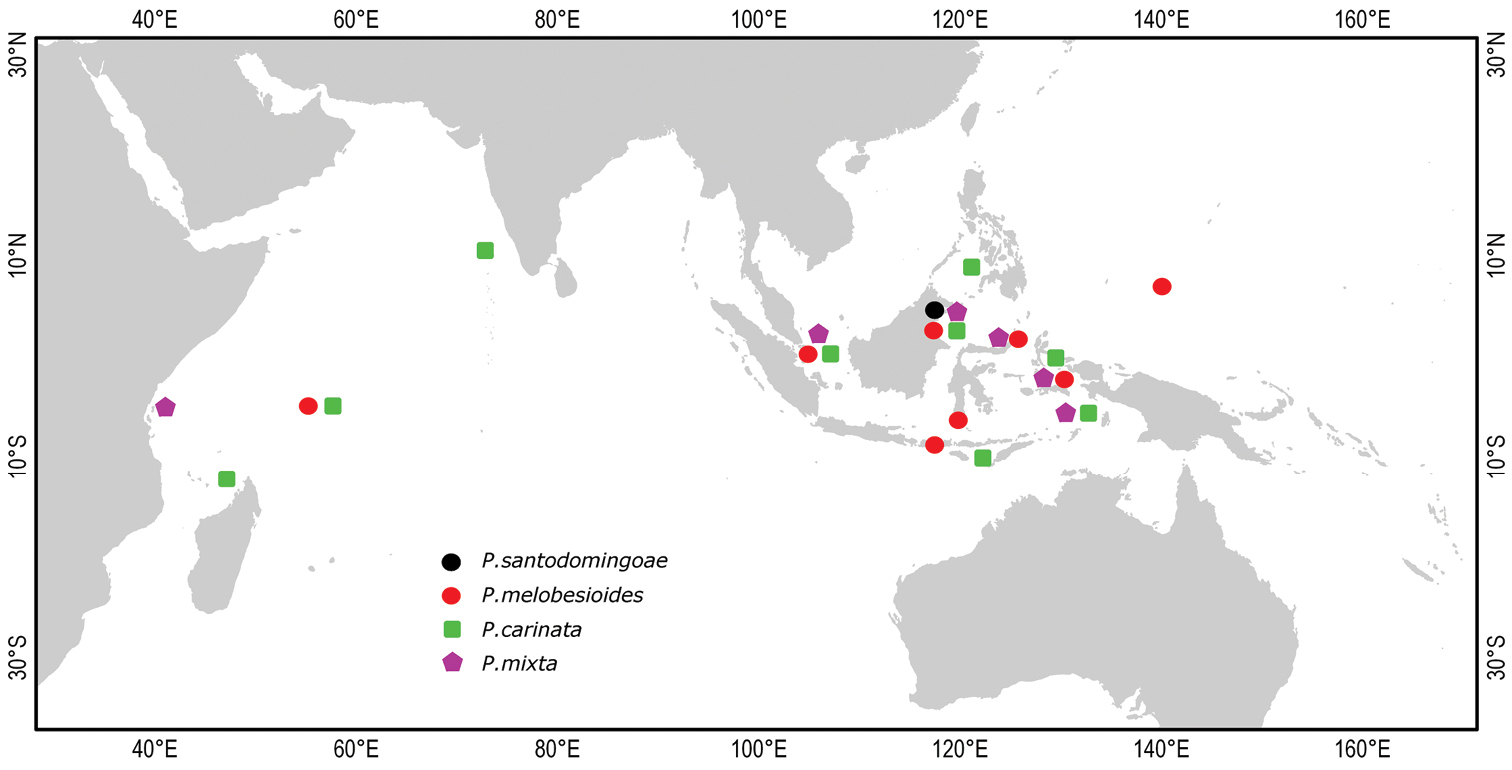
East African coast to eastern Indonesia (Fig. 9, Table 2). Originally described from the ‘South Sea’, presumably the South Pacific Ocean. This has been interpreted by some (
Depth 0–45m. In Indonesia rarely found in reef environment, but high abundance in marine lakes. Possibly higher prevalence in reefs in Eastern Africa, based on the ZMA Por. collection from the Seychelles and the publication from Madagascar (
The Bowerbank description from 1858 should be considered as the original description of ‘Geodia carinata’, now accepted as Placospongia carinata, with plates XXV fig. 19 and XXVI fig. 10 representing the streptasters (“arborescent elongo-subsphero-stella”). Subsequently in 1874 Bowerbank published a more extensive description of “Geodia carinata” including a drawing of the streptasters (fig. 3, p.299) and spined microrhabds (“minute multiangulated cylindrical retentive spicula”, fig. 2, p.299) that he described as characteristic of the species. In neither publication registration numbers were provided, however. The habitus drawing in fig. 5, p.299 of Bowerbank publication in 1874 is identical to the specimen BMNH95.6.7.1 that I received from the BMNH after requesting the holotype for Placospongia carinata. In addition, I received the slides of spicules (codes: R1228, 86g, Bk.1390) and of the thick cut (codes: R1275, PE01, Bk1390) that were labeled to belong to the holotype (Fig. 5). Upon inspection I discovered that the specimen BMNH 95.6.7.1 is in fact a Placospongia melobesioides, while the two slides do indeed represent Placospongia carinata containing the characteristic streptasters with bifurcating endings and the microrhabds as indicated in the Bowerbank images and in the images taken from these slides in Fig. 5. The slides clearly do not come from the specimen BMNH 95.6.7.1. In the 16 years between
http://species-id.net/wiki/Placospongia_melobesioides
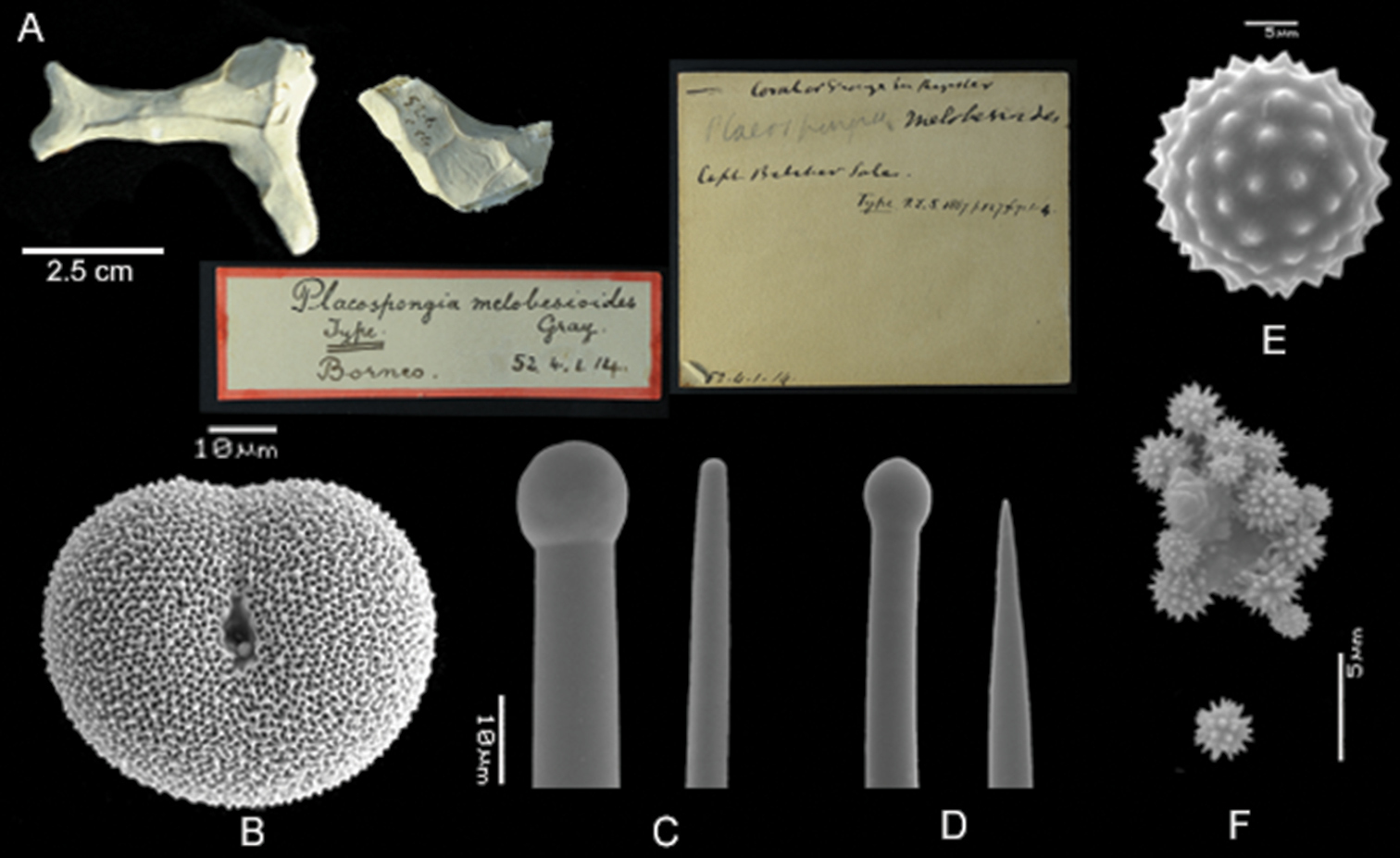
Figure 6Holotype. BMNH 52.4.1.14, Indonesia, Borneo island.
Vosmaer & Vernhout (1902), Siboga expedition: RMNH POR. 756, RMNH POR. 761, RMNH POR. 758, RMNH POR. 757, RMNH POR. 760, RMNH POR. 759. Other material:: RMNH POR. 4497, RMNH POR. 4496, RMNH POR. 4495, RMNH POR. 4114, RMNH POR. 3978, RMNH POR. 3977, RMNH POR. 3976, RMNH POR. 3942, RMNH POR. 3941, RMNH POR. 3940, RMNH POR. 3939, RMNH POR. 3938, RMNH POR. 3937, RMNH POR. 3935, RMNH POR. 3934, RMNH POR. 3933, RMNH POR. 3932, RMNH POR. 3177, RMNH POR. 3166, RMNH POR. 3154, RMNH POR. 2464, RMNH POR. 2463, ZMA Por. 13097, ZMA Por. 10459 (See Table 3 for full details per specimen)
Location details of reviewed specimens of Placospongia melobesioides.
| registration number | fieldcode | country | province | region | island | locality | habitat | latitude | longitude | depth (m.) | date | collector |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMNH POR. 761 | #1033 | Indonesia | S of Moluccas | sand & rock | 04°12'S, 129°20.4'E | 45 | 1899 | Siboga expedition | ||||
| RMNH POR. 756 | #660 | Indonesia | Nusa Tenggara | N of Sumbawa | sand & rock | 07°12.6'S, 118°7.7'E | 36 | 14.ii.1900 | Siboga expedition | |||
| RMNH POR. 757 | #1849 | Indonesia | Moluccas | SE of Misool | Banda islands | sand & rock | 32 | 1899 | Siboga expedition | |||
| RMNH POR. 758 | #1847 | Indonesia | Moluccas | SE of Misool | Banda islands | sand & rock | 32 | 1899 | Siboga expedition | |||
| RMNH POR. 759 | #1853 | Indonesia | Moluccas | SE of Misool | Banda islands | sand & rock | 32 | 1899 | Siboga expedition | |||
| RMNH POR. 760 | #1851 | Indonesia | Moluccas | SE of Misool | Banda islands | sand & rock | 32 | 1899 | Siboga expedition | |||
| RMNH POR. 2463 | #Sin05/270306/025 | Singapore | Semaku | Pulau Semakau NW side | reef | 01°13'70"N, 103°45'61"E | 10-12 | iii.2006 | N.J. de Voogd | |||
| RMNH POR. 2464 | #Sin05/270306/026 | Singapore | Semaku | Pulau Semakau NW side | reef | 01°13'70"N, 103°45'61"E | 10-12 | iii.2006 | N.J. de Voogd | |||
| RMNH POR. 3154 | #LEMD05/30 | Indonesia | North Sulawesi | Bunaken | Pangalisang | reef | 01°37'26"N, 124°46'55"E | 9 | 24.ix.2006 | L.E.Becking | ||
| RMNH POR. 3166 | #LEMD13/69 | Indonesia | North Sulawesi | Bunaken | Pangalisang | reef | 01°37'26"N, 124°46'55"E | 19 | 28.ix.2006 | L.E.Becking | ||
| RMNH POR. 3177 | #LEMD22/87 | Indonesia | North Sulawesi | Bunaken | Likuan2 | reef | 01°35'78"N, 124°46'06"E | 21 | 13.x.2006 | L.E.Becking | ||
| RMNH POR. 3932 | #KKB/mol866 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3933 | #KKB/mol766 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3934 | #KKB/mol767 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3935 | #BER113/mol689 | Indonesia | East Kalimantan | Berau | Maratua | NE Maratua | reef | 02°17'32.3"N, 118°35'26.1"E | 5-10 | 15.viii.2008 | N.J. de Voogd | |
| RMNH POR. 3937 | #BER107/mol604 | Indonesia | East Kalimantan | Berau | Sangalaki | E Sangalaki | reef | 02°05'36.6"N, 118°24'15.2"E | 5-10 | 15.viii.2008 | L.E.Becking | |
| RMNH POR. 3938 | #BER107/mol608 | Indonesia | East Kalimantan | Berau | Sangalaki | E Sangalaki | reef | 02°05'36.6"N, 118°24'15.2"E | 5-10 | 15.viii.2008 | L.E.Becking | |
| RMNH POR. 3939 | #BER108/mol601 | Indonesia | East Kalimantan | Berau | Sangalaki | W Sangalaki | reef | 02°05'07.7"N, 118°23'28.0"E | 5-10 | 15.viii.2008 | L.E.Becking | |
| RMNH POR. 3940 | #P-YAP1 | Micronesia | Yap | Yap | reefflat in front of mangrove | 09°31'36.7"N, 138°07'48.7"E | 1-3 | 28.viii.2010 | L.E.Becking | |||
| RMNH POR. 3941 | #P-YAP2 | Micronesia | Yap | Yap | reefflat in front of mangrove | 09°31'36.7"N, 138°07'48.7"E | 1-3 | 28.viii.2010 | L.E.Becking | |||
| RMNH POR. 3942 | #P-YAP3 | Micronesia | Yap | Yap | reefflat in front of mangrove | 09°31'36.7"N, 138°07'48.7"E | 1-3 | 28.viii.2010 | L.E.Becking | |||
| RMNH POR. 3976 | #PM-TER02 | Indonesia | Moluccas | Ternate | reef | 5-10 | xi.2009 | N.J. de Voogd | ||||
| RMNH POR. 3977 | #PM-TER08 | Indonesia | Moluccas | Ternate | reef | 5-10 | xi.2009 | N.J. de Voogd | ||||
| RMNH POR. 3978 | #PM-TER12 | Indonesia | Moluccas | Ternate | reef | 5-10 | xi.2009 | N.J. de Voogd | ||||
| RMNH POR. 4114 | #KKB/mol795 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4495 | #KKB/mol1075 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4496 | #KKB/mol776 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4497 | #BER107/mol603 | Indonesia | East Kalimantan | Berau | Sangalaki | E Sangalaki | reef | 02°05'36.6"N, 118°24'15.2"E | 5-10 | 15.viii.2008 | L.E.Becking | |
| ZMA Por. 10459 | Seychelles | Mahé | Mahé | NE coast, North East Point | reef | 04°34'59.9"S, 055°28'0.1"E | 5 | 8.xii.1992 | R.W.M. van Soest | |||
| ZMA Por. 10496 | Seychelles | Mahé | Mahé | North East Point | reef | 04°34'59.9"S, 055°28'0.1"E | 14.xii.1992 | R.W.M. van Soest | ||||
| ZMA Por. 13097 | Indonesia | South Sulawesi | Spermonde archipelago | Samalona | reef | 5-30 | 27.iv.1997 | N.J. de Voogd | ||||
Holotype BMNH 52.4.1.14 dry, chalky white angular branches, hard. Other examined material encrusting to branching, hard, thicker specimens slightly compressible. External morphology follows the description of the genus. Size ranging between 5-50 cm, though encrusting specimens may be larger growing within crevices. Ectosome color in life ranging from purple, dark black brown, chocolate brown, orange brown to light beige (Fig. 1, 2). Choanosome pale beige. After preservation color of ectosome is similar to live color.
Spicules. Holotype BMNH 52.4.1.14 (Fig. 6): Megascleres large straight tylostyles with blunt ends 670-880-1010 × 10-13-18 × 10-16-20 μm, small concave to straight tylostyles with sharp ends 205-293-420 × 5-10-13 × 5-10-13 μm. Microscleres selenasters 58-63-68 × 45-52-68 μm, spherasters 15-17-18 μm (five measurements, not abundant), spherules 1-2-3 μ m. The range within the examined material (Table 1): large tylostyles 460-1040 × 5-16 × 8-18 μ m, small tylostyles 190-470 × 3-13 × 3-15 μ m, selenasters 45-83 × 30-65 μ m, spherules 1-3 μ m, spherasters only found in singles in some individuals 15-20 μ m. Streptasters and microrhabds absent.
Skeleton. As description of genus with addition of sporadic spherasters lodged amidst selenasters in cortex and high abundance of spherules in choanosome and cortex.
Placospongia melobesioides holotype (BMNH 52.4.1.14). A Holotype with two labels B selenaster C large tylostyle (head and blunt end) D small tylostyle (head and hastate end) E spheraster F spherules.
Depth: 0-45m. Reefs, rocky shores, reefflats, mangroves, and marine lakes.
Type locality: Borneo. Distribution from Seychelles to Micronesia (Fig. 9, Table 3). Possibly further east to Central Pacific.
In the original description by
http://species-id.net/wiki/Placospongia_mixta
Figure 7Holotype. ZMB 3204, Indonesia, Moluccas, Ternate.
Vosmaerand & Vernhout (1902), Siboga expedition: RMNH POR. 753, RMNH POR. 751, RMNH POR. 745, RMNH POR. 742. Other material: RMNH POR. 4494, RMNH POR. 4493, RMNH, POR. 4492, RMNH POR. 4491, RMNH POR. 4490, RMNH POR. 4489, RMNH POR. 4113, RMNH POR. 4112, RMNH, POR. 3979, RMNH POR. 3975, RMNH POR. 3974, RMNH POR. 3973, RMNH POR. 3972, RMNH POR. 3971, RMNH POR. 3970, RMNH POR. 3969, RMNH POR. 3968, RMNH POR. 3967, RMNH POR. 3966, RMNH POR. 3965, RMNH POR. 3964, RMNH POR. 3963, RMNH POR. 3962, RMNH POR. 3961, RMNH POR. 3960, RMNH POR. 3959, RMNH, POR. 3163, RMNH POR. 3158, RMNH POR. 3157, RMNH POR. 3155, RMNH POR. 3148, ZMA Por. 10495, ZMA Por. 896 (See Table 4 for full details per specimen)
Location details of reviewed specimens of Placospongia mixta.
| registration number | fieldcode | country | province | region | island | locality | habitat | latitude | longitude | depth | date | collector |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMNH POR. 753 | #311 | Indonesia | West Papua | E. of Misool | sand & rock | 01°42.5'S, 130°47.5'E | 32 | 20.viii.1899 | Siboga expedition | |||
| RMNH POR. 751 | #1857 | Indonesia | West Papua | E. of Misool | sand & rock | 01°42.5'S, 130°47.5'E | 32 | 20.viii.1899 | Siboga expedition | |||
| RMNH POR. 745 | #577 | Indonesia | South Sulawesi | N. of Kabia | Saleyer anchorage | sand & rock | 36 | 20.viii.1899 | Siboga expedition | |||
| RMNH POR. 742 | #163a | Indonesia | Moluccas | Aru | Pearl Banks, anchorage off Pulu Jedan | reef | 13 | 23.xii.1899 | Siboga expedition | |||
| RMNH POR. 3148 | #LEMD04/21 | Indonesia | North Sulawesi | Bunaken | Likuan 2 | reef | 01°35'78"N, 124°46'06"E | 15 | 24.ix.2006 | L.E. Becking | ||
| RMNH POR. 3155 | #LEMD06/32 | Indonesia | North Sulawesi | Lembeh Strait | Nudi Reed Reed | reef | 01°24'06"N, 125°12'22"E | 21 | 25.ix.2006 | L.E. Becking | ||
| RMNH POR. 3157 | #LEMD08/39 | Indonesia | North Sulawesi | Lembeh Strait | Nudi Fols | reef | 01°27'26"N, 125°13'05"E | 6 | 25.ix.2006 | L.E. Becking | ||
| RMNH POR. 3158 | #LEMD08/42 | Indonesia | North Sulawesi | Lembeh Strait | Nudi Fols | reef | 01°27'26"N, 125°13'05"E | 8 | 25.ix.2006 | L.E. Becking | ||
| RMNH POR. 3163 | #LEMD11/52 | Indonesia | North Sulawesi | Bunaken | 0.5-1km W. of Park administration office | reef | 01°36'57"N, 124°45'41"E | 8 | 27.ix.2006 | L.E. Becking | ||
| RMNH POR. 3959 | #KKB/mol827 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3960 | #KKB/mol829 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3961 | #KKB/mol851 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 3962 | #BER111/mol1203 | Indonesia | East Kalimantan | Berau | Kakaban | SW Kakaban | reef | 02°08'07.5"N, 118°30'23.3"E | 10 | 17.viii.2008 | N.J. de Voogd | |
| RMNH POR. 3963 | #BER111/1209 | Indonesia | East Kalimantan | Berau | Kakaban | SW Kakaban | reef | 02°08'07.5"N, 118°30'23.3"E | 10 | 17.viii.2008 | N.J. de Voogd | |
| RMNH POR. 3964 | #BER111/1213 | Indonesia | East Kalimantan | Berau | Kakaban | SW Kakaban | reef | 02°08'07.5"N, 118°30'23.3"E | 10 | 17.viii.2008 | N.J. de Voogd | |
| RMNH POR. 3965 | #BER111/mol1219 | Indonesia | East Kalimantan | Berau | Kakaban | SW Kakaban | reef | 02°08'07.5"N, 118°30'23.3"E | 10 | 17.viii.2008 | N.J. de Voogd | |
| RMNH POR. 3966 | #RAJ23/mol195 | Indonesia | West Papua | Raja Ampat | Gam | Ctenophore lake | marine lake | 00°27'17.5"S, 130°29'33.8"E | 0-2 | xi.2007 | L.E. Becking | |
| RMNH POR. 3967 | #RAJ23/mol187 | Indonesia | West Papua | Raja Ampat | Gam | Ctenophore lake | marine lake | 00°27'17.5"S, 130°29'33.8"E | 0-2 | xi.2007 | L.E. Becking | |
| RMNH POR. 3968 | #RAJ64/mol429 | Indonesia | West Papua | Raja Ampat | Waigeo | Teluk Mayabilit | reef | 00°18'17.0"S, 130°54'15.6"E | 10 | xii.2007 | L.E. Becking | |
| RMNH POR. 3969 | #RAJ64/mol430 | Indonesia | West Papua | Raja Ampat | Waigeo | Teluk Mayabilit | reef | 00°18'17.0"S, 130°54'15.6"E | 10 | xii.2007 | L.E. Becking | |
| RMNH POR. 3970 | #RAJ64/mol431 | Indonesia | West Papua | Raja Ampat | Waigeo | Teluk Mayabilit | reef | 00°18'17.0"S, 130°54'15.6"E | 10 | xii.2007 | L.E. Becking | |
| RMNH POR. 3971 | #RAJ64/mol432 | Indonesia | West Papua | Raja Ampat | Waigeo | Teluk Mayabilit | reef | 00°18'17.0"S, 130°54'15.6"E | 10 | xii.2007 | L.E. Becking | |
| RMNH POR. 3972 | #RAJ64/mol433 | Indonesia | West Papua | Raja Ampat | Waigeo | Teluk Mayabilit | reef | 00°18'17.0"S, 130°54'15.6"E | 10 | xii.2007 | L.E. Becking | |
| RMNH POR. 3973 | #RAJ39/mol249 | Indonesia | West Papua | Raja Ampat | Fam | rocky shore | 00°36'01.5"S, 130°45'08"E | 0-1 | xi.2007 | L.E. Becking | ||
| RMNH POR. 3974 | #RAJ39/mol250 | Indonesia | West Papua | Raja Ampat | Fam | rocky shore | 00°36'01.5"S, 130°45'08"E | 0-1 | xi.2007 | L.E. Becking | ||
| RMNH POR. 3975 | #RAJ39/mol254 | Indonesia | West Papua | Raja Ampat | Fam | rocky shore | 00°36'01.5"S, 130°45'08"E | 0-1 | xi.2007 | L.E. Becking | ||
| RMNH POR. 3979 | #KKB/mol 779 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4112 | #P-TER11 | Indonesia | Moluccas | Ternate | reef | xi.2009 | N.J. de Voogd | |||||
| RMNH POR. 4113 | #P-TER22 | Indonesia | Moluccas | Ternate | reef | xi.2009 | N.J. de Voogd | |||||
| RMNH POR. 4489 | #KKB/mol721 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4490 | #KKB/mol830 | Indonesia | East Kalimantan | Berau | Kakaban | Kakaban lake | marine lake | 02°08'57.3"N, 118°31'26.4"E | 0-2 | ix.2008 | L.E.Becking | |
| RMNH POR. 4491 | #BER109/mol629 | Indonesia | East Kalimantan | Berau | lighthouse near Berau river | reef | 02°09'49.9"N, 118°10'12.8"E | 10 | 16.viii.2008 | L.E.Becking | ||
| RMNH POR. 4492 | #BER111/mol666 | Indonesia | East Kalimantan | Berau | Kakaban | SW Kakaban | reef | 02°08'07.5"N, 118°30'23.3"E | 10 | 17.viii.2008 | N.J. de Voogd | |
| RMNH POR. 4493 | #RAJ64/mol428) | Indonesia | West Papua | Raja Ampat | Waigeo | Teluk Mayabilit | reef | 00°18'17.0"S, 130°54'15.6"E | 10 | xii.2007 | L.E. Becking | |
| RMNH POR. 4494 | #RAJ23/mol199 | Indonesia | West Papua | Raja Ampat | Gam | Ctenophore lake | marine lake | 00°27'17.5"S, 130°29'33.8"E | 0-2 | xi.2007 | L.E. Becking | |
| ZMA Por. 896 | Indonesia | South Sulawesi | SW Salayer | reef N of Pulau Bahuluang | reef | 06°27'00"S, 120°25'48"E | 10-45 | 30.ix.1984 | R.W.M. van Soest (Snellius Expedition II) | |||
| ZMA Por. 10495 | Seychelles | Mahé | Mahé | SE coast near Pointe Cocos | 04°45'00"S, 055°32'60"E | 35-45 | 24.xii.1992 | R.W.M. van Soest | ||||
Holotype ZMB 3204 encrusting, size 5 × 2.5 cm and thickness 1–5 mm (as described by Thiele, now very small fragment), white after preservation in alcohol. The majority of the reviewed material is encrusting with a thickness of 4-10mm, but branching specimens also occur. External morphology follows the description of the genus. Color of the ectosome can be red, orange, brown orange, dark brown, chocolate brown, milk coffee brown, cream, or white (Fig. 1, 2). Color of choanosome is pale beige. After preservation in ethanol color is similar to live specimens, but lighter shade.
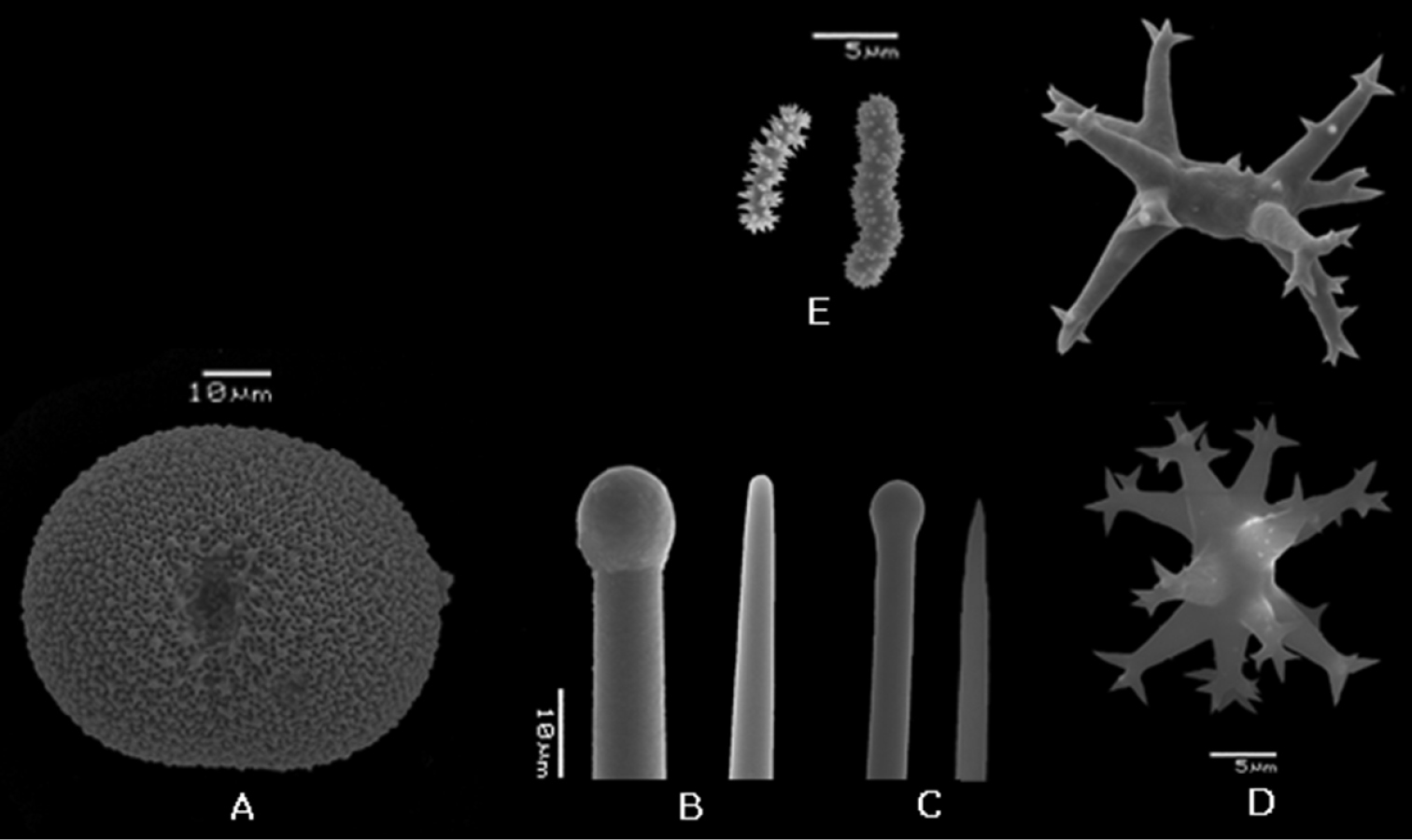
Spicules. Holotype ZMB 3204 (Fig. 6) Megascleres large straight tylostyles with blunt/rounded point 355-672-940 × 7.5-12-17.5 × 7.5-16-20 μm, small straight tylostyles with sharp point 165-226-275 × 2.5-6-7.5 × 2.5-8-10 μm; microscleres selenasters 55-70-75 × 42.5-55-72.5 μm, spherasters (abundant) 20-25-30 μm, streptasters typically with well developed axis and with 4-9 rays with hastate tips, rays are smooth or can be spined, but do not have bifurcations of the tips 15-24-32.5 × 2.5-8-12.5 μm; acanthose microrhabs with straight or zig-zag axis 5-7-10 × <2.5 μ m.The range within the examined material (Table 1): large tylostyles 460-1250 × 8-23 × 10-25 μ m, small tylostyles 120-430 × 3-15 × 2-15 μ m, selenasters 50-85 × 22-73 μ m, spherasters 13-30 μ m, streptasters 15-35 × 2-15 μ m, rays 5-18 × 1-2.5 μ m.
Skeleton. As description of genus with addition that microrhabds form a layer over and amidst the selenaster cortex and are also prevalent in choanosomal tissue. Streptasters scattered in choanosome. Spherasters amidst selenasters in cortex and scattered in choanosome.
Placospongia mixta holotype (ZMB 3204). A selenaster B large tylostyle (head and blunt end) C small tylostyle (head and hastate end) D spheraster E streptasters F microacanthose microrhabds.
East African coast to eastern Indonesia (Fig. 9, Table 4). Possibly further east to Central Pacific.
Depth 0–45m. Common in reefs, also occurs in marine lakes.
In 1900 Thiele described a new species named Placospongia mixta, which was originally identified as Placospongia melobesioides by
urn:lsid:zoobank.org:act:3C4F2599-15C0-4075-BD3B-8C6439C8F821
http://species-id.net/wiki/Placospongia_santodomingoae
Figure 8RMNH POR. 4486, Indonesia, East Kalimantan province, Maratua island, Buli Halo anchialine pool, 02°11'16.4"N, 118°37'06.4"E, 0–1m. depth, xi.2008, coll. N.K. Santodomingo & Estradivari, #BER128/mol1147. Paratypes. RMNH POR. 4487, Indonesia, East Kalimantan province, Maratua island, Buli Halo anchialine pool, 02°11'16.4"N, 118°37'06.4"E, 0–1m. depth, xi.2008, coll. N. K. Santodomingo & Estradivari; RMNH POR. 4488, Indonesia, East Kalimantan province, Maratua island, Buli Halo anchialine pool, 02°11'16.4"N, 118°37'06.4"E, 0–1m. depth, xi.2008, coll. N. K. Santodomingo & Estradivari, #BER128/1156.
Holotype and paratypes are branching and encrusting, size 8cm in length. Total size of specimens in situ is hard to establish as parts of the body may be encrusting within cracks. Alcohol preserved and live specimens are hard but slightly compressible. The surface is made up with typical Placospongia cortical plates separated by contractible grooves which form a network on the surface. Oscules are present in the grooves. Live color of holotype was dark brown, the paratypes were orange, and these colors were mostly retained after alcohol preservation (Fig. 8A).
Spicules. Holotype (Fig. 8) megascleres large straight tylostyles with blunt point 430-605.5-660 × 13-15.5-20 × 13-18.1-23 μm, small straight tylostyles with sharp point 240-261.3-290 × 5-7.2-8 × 5-8.8-10 μm; microscleres selenasters 80-84.8-90 × 60-67.3-75 μm, acanthose microrhabds 8-12.3-18 × 2.5-2.7-3.5 μm. Range of the paratypes (Table 1) large straight tylostyles with blunt point 430-760 × 13-20 × 15-23 μm, small straight tylostyles with sharp point 190-380 × 5-13 × 8-15 μm, microscleres selenasters 63-93 × 58-75 μ m, acanthose microrhabds 5-20 × 2.5-3.5 μ m.
Skeleton. The cortical plates consist of densely packed selenasters, microrhabds form a layer over and amidst this selenaster cortex and are also prevalent in choanosomal tissue. Developmental stages of selenasters occur throughout the choanosome. Tylostyle tracts support the margins of the cortical plates in radial tracts from the centre core (consisting of densely packed selenaster) to the cortical plates. The sharp ends of the smaller tylostyles can be projected beyond the cortex surface.
Placospongia santodomingoae sp. n. (RMNH POR. 4486). A ethanol preserved specimen B selenaster C large tylostyle (head and blunt end) D small tylostyle (head and hastate end) E microrhabds.
Presently only recorded from Buli Halo anchialine pool on Maratua island, Berau, East Kalimantan, Indonesia (Fig. 9). For a full description of the pool, see
Distribution of Placospongia spp. in the Indo-West Pacific. Location of symbols is approximate.
Depth 0–2m. occurs in anchialine pool, can be exposed to air during low tide and can tolerate great fluctuations in salinity (from 24 to 33 ‰).
Named in honor of Nadiezhda K. Santodomingo, the collector of the types, for her years of tireless work in marine science including anchialine research.
Placospongia santodomingoae sp. n. is similar to Placospongia carinata, yet lacks streptasters and has shorter tylostyles. Placospongia santodomingoae sp. n. likewise differs from Placospongia mixta by the absence of streptasters as well as the absence of spherasters. Placospongia santodomingoae sp. n. differs from Placospongia anthosigma by the absence of anthosigma, and by having hastate endings of the smaller tylostyles.
http://species-id.net/wiki/Geodia_labyrinthica
Holotype. BMNH 02.11.16.1, South Africa, East London Coast, 33°06'30"S, 028°11'E.
Spicules. Megascleres styles, oxea; microscleres sterrasters, chiasters
This species was originally described as ‘Placospongia labyrinthica’, butdoes not have the characteristic cortical plates of Placospongia. The specimen furthermore has sieve pores, sterrasters with star-like plates, euasters, styles and oxea characteristic of the Geodiidae. In the original description,
| 1 | Streptasters absent | 2 |
| – | Streptasters present | 3 |
| 2 | Spherules present | Placospongia melobesioides |
| – | Spherules absent | 4 |
| 3 | Streptasters have rays with birfurcating ends | Placospongia carinata |
| – | Streptasters have rays with hastate ends, spherasters present | Placospongia mixta |
| 4 | Spherasters present, microrhabds with short spines spirally places on shaft | Placospongia anthosigma |
| – | Spherasters absent, acanthose microrhabds present | Placospongia santodomingoae sp.n. |
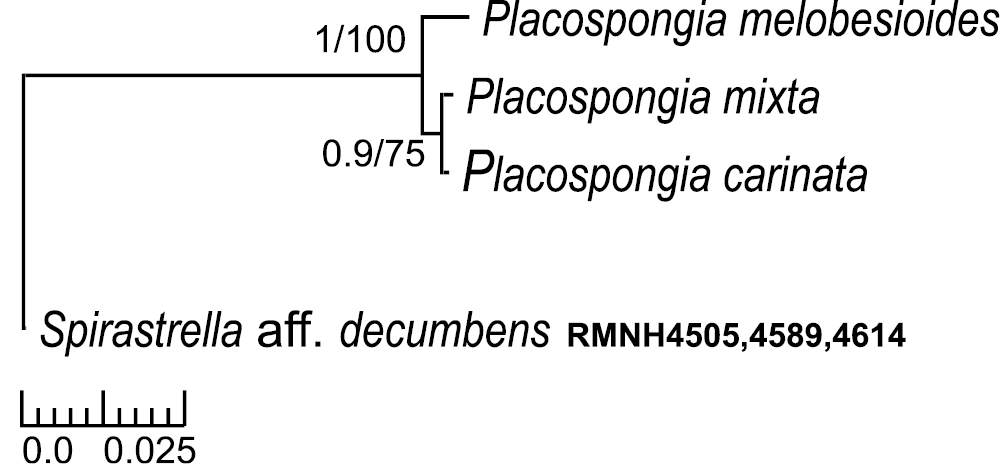
All sequences were submitted to GenBank with accession numbers KC848421 - 41 (Table 5). Final alignments (excluding primers) were obtained for the sponge Placospongia of 581 bp for COI with three genetic variants (28 individuals) and 13 polymorphic sites. The three genetic variants correspond to the three species Placospongia melobesioides, Placospongia mixta, and Placospongia carinata that represent monophyletic groups which are strongly supported by both Bayesian and maximum likelihood inference methods (Fig. 10). There was no intra-specific variation within each species, regardless of geographic locality. The inter-specific p- distances ranged between 0.5-2.1% (Table 6).There were 11 substitutions between Placospongia melobesioides and Placospongia carinata, 12 substitutionsbetween Placospongia melobesioides and Placospongia mixta, and three substitutions between Placospongia mixta and Placospongia carinata. The specimens of Placospongia carinata and of Placospongia santodomingoae sp. n. had identical genotypes for COI. No molecular work could be done on the dried holotype of Placospongia anthosigma and fresh material was not available.
Final alignments (excluding primers) of 720 bp were obtained for ITS with 18 genetic variants from the present study (22 individuals). An additional 27 genetic variants from GenBank (for GenBank accession numbers see Fig. 11) were included in the phylogenetic analysis. The ITS sequences represented five clades that were strongly supported by both Bayesian and maximum likelihood inference methods (Fig. 11). These five divergent clades (see Table 7 for uncorrected p- distances) correspond to the clades C3, C4, C5, C6, and C9 as presented by the study of
Bayesian/maximum likelihood phylograms of Cytochrome Oxidase I (COI) sequences from Indo-Pacific Placospongia spp. See Table 5 for GenBank accession numbers. Only posterior probabilities of >90 and maximum likelihood values of >70 indicated. Scale bar indicates substitutions/site.
Bayesian/maximum likelihood phylograms of genotypes of the internal transcribed spacer region of nuclear ribosomal operons (ITS) of Indo-Pacific Placospongia spp. found in this study and related species from the same genus collected from GenBank. Clades C3, C4, C5, C6 & C9 refer to the clades presented in the study by
Specimens of Placospongia studied for DNA analysis. Genbank accession numbers provided for sequences of Cytochrome Oxidase I (COI) and internal transcribed spacer region (ITS).
| Registration number | Species | COI | ITS |
|---|---|---|---|
| RMNH POR. 4482 | Placospongia carinata | KC848441 | KC848429 |
| RMNH POR. 4483 | Placospongia carinata | KC848441 | KC848427 |
| RMNH POR. 4484 | Placospongia carinata | KC848441 | KC848428 |
| RMNH POR. 4485 | Placospongia carinata | KC848441 | KC848429 |
| ZMA Por. 10727 | Placospongia carinata | KC848441 | - |
| ZMA Por. 11367 | Placospongia carinata | KC848441 | - |
| RMNH POR. 2464 | Placospongia melobesioides | KC848439 | - |
| RMNH POR. 3942 | Placospongia melobesioides | KC848439 | KC848422 |
| RMNH POR. 3976 | Placospongia melobesioides | KC848439 | - |
| RMNH POR. 4114 | Placospongia melobesioides | KC848439 | KC848426 |
| RMNH POR. 4495 | Placospongia melobesioides | KC848439 | KC848436 |
| RMNH POR. 4496 | Placospongia melobesioides | KC848439 | KC848436 |
| RMNH POR. 4497 | Placospongia melobesioides | KC848439 | KC848437 |
| RMNH POR.3166 | Placospongia melobesioides | KC848439 | KC848422 |
| ZMA Por. 10459 | Placospongia melobesioides | KC848439 | KC848438 |
| RMNH POR. 3158 | Placospongia mixta | KC848440 | KC848421 |
| RMNH POR. 3960 | Placospongia mixta | KC848440 | KC848423 |
| RMNH POR. 3979 | Placospongia mixta | KC848440 | - |
| RMNH POR. 4113 | Placospongia mixta | KC848440 | KC848425 |
| RMNH POR. 4489 | Placospongia mixta | KC848440 | - |
| RMNH POR. 4490 | Placospongia mixta | KC848440 | KC848433 |
| RMNH POR. 4491 | Placospongia mixta | KC848440 | KC848433 |
| RMNH POR. 4492 | Placospongia mixta | KC848440 | KC848434 |
| RMNH POR. 4493 | Placospongia mixta | KC848440 | KC848435 |
| RMNH POR. 4494 | Placospongia mixta | KC848440 | KC848435 |
| RMNH POR. 4486 | Placospongia santodomingoae sp. n. | KC848441 | KC848430 |
| RMNH POR. 4487 | Placospongia santodomingoae sp. n. | KC848441 | KC848431 |
| RMNH POR. 4488 | Placospongia santodomingoae sp. n. | KC848441 | KC848432 |
The number of base differences per site from averaging over all Cytochrome Oxidase I (COI) sequence pairs between Placospongia spp. groups are shown (uncorrected p-distances). Standard error estimate (s) are shown above the diagonal in italic. The analysis involved 30 nucleotide sequences. There was no within-group difference. Spirastrella aff. decumbens was used as outgroup in the phylogenetic inference (see Fig. 10).
| %p-distance COI | Placospongia melobesioides | Placospongia mixta | Placospongia carinata | Spirastrella aff. decumbens |
|---|---|---|---|---|
| Placospongia melobesioides | * | 0.6 | 0.6 | 1.3 |
| Placospongia mixta | 2.1 | * | 0.3 | 1.2 |
| Placospongia carinata | 1.9 | 0.5 | * | 1.3 |
| Spirastrella aff. decumbens | 12.2 | 11.5 | 11.7 | * |
The number of base differences per site from averaging over all internal transcribed spacer (ITS) sequence pairs between Placospongia spp. groups are shown (uncorrected p-distances). Standard error estimate (s) are shown above the diagonal. All positions with less than 5% site coverage were eliminated. Black cursive along the diagonal indicates within-group uncorrected p- distance. The analysis involved 73 nucleotide sequences. C9, C5, C6, C4, C3 refer to five clades in the Indo-West Pacific Placospongia as presented in Fig. 11.
| %p-distance ITS | Placospongia melobesioides | Placospongia mixta | Placospongia carinata | Placospongia santodomingoae sp. n. | C9 | C5 | C6 | C4 | C3 |
|---|---|---|---|---|---|---|---|---|---|
| Placospongia melobesioides | 0.1 | 1.3 | 1.4 | 1.4 | 0.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| Placospongia mixta | 13.8 | 0.7 | 0.9 | 0.9 | 1.2 | 0.2 | 0.5 | 0.9 | 0.9 |
| Placospongia carinata | 14.7 | 6.3 | 0.4 | 0.2 | 1.3 | 0.9 | 0.9 | 0.2 | 0.9 |
| Placospongia santodomingoae sp. n. | 13.2 | 5.8 | 0.6 | 1.6 | 1.3 | 0.9 | 0.9 | 0.3 | 0.9 |
| C9 | 0.9 | 13.5 | 14.6 | 13.6 | 0.1 | 1.2 | 1.2 | 1.2 | 1.2 |
| C5 | 13.5 | 0.9 | 6.6 | 6.1 | 12.9 | 0.7 | 0.5 | 0.8 | 0.9 |
| C6 | 14 | 2.2 | 6.4 | 6.1 | 13.2 | 2.2 | 0.1 | 0.8 | 0.8 |
| C4 | 14.8 | 6.3 | 0.5 | 0.9 | 14.3 | 6.3 | 6 | 0.4 | 0.8 |
| C3 | 15.2 | 7.1 | 6.1 | 5.9 | 14.5 | 6.9 | 6.3 | 5.6 | 0.9 |
In the Indo-West Pacific at least five species of the genus Placospongia can be identified based on spicule morphology: Placospongia anthosigma, Placospongia carinata, Placospongia mixta, Placospongia melobesioides, and Placospongia santodomingoae sp. n. Placospongia melobesioides, Placospongia carinata, and Placospongia mixta can be distinguished with the DNA barcode marker (COI) and a nuclear marker (ITS). The species Placospongia santodomingoae sp. n. and Placospongia carinata have the same sequence of COI. The sequence variation of COI in sponges can be low (e.g.
A molecular phylogeny using the internal transcribed spacer region (ITS) showed that there were five distinct clades within the genus Placospongia in the Indo-West Pacific (clades C3, C4, C5, C6 & C9) (
Each of the five species of the genus Placospongia in the Indo-West Pacific can be distinguished based on the composition and morphology of spicules. The external morphology, however, does not allow species distinction. The most common species from the tropical Indo-West Pacific (Placospongia melobesioides, Placospongia mixta, and Placospongia carinata) can have both encrusting and branching growth forms displaying a variety of colors from white to dark brown. The only observed consistent pattern was that all the red specimens belonged to Placospongia mixta, while all the dark black-brown specimens belonged to Placospongia melobesioides.These two colors may be useful for field identifications, yet both species can also display the range of other colors (white, cream, beige, light brown) as well. The density of canals/ridges (or size of cortical plates) appears to be related to environment as this is higher in specimens from high sediment locations such as the marine lakes than in specimens from the reefs (Fig. 1, 2). Within each species there is also some natural variation in the range of tylostyle length and spicule morphology. The streptaster morphology varies within species and even within individuals. Within one individual the number of rays can vary from 4-10 (Figs 3, 4) and between individuals the decoration and size of spines can be diverse. For example the streptasters of Placospongia carinata specimens from Haji Buang marine lake are micro-acanthose while the specimens from other locations are not. Spherasters are always present and abundant in Placospongia mixta and Placospongia anthosigma, but are in low abundances or absent in Placospongia melobesioides, as has been indicated previously by
Placospongia melobesioides and Placospongia mixta are common in the reef environment. Most of the collected material from the reefs in Indonesia were one of these two species. Placospongia carinata appears to be rare in the reefs, in Indonesia at least, while it is highly abundant in the marine lakes Haji Buang and Kakaban in East Kalimantan, Indonesia. Placospongia santodomingoae n.sp. is restricted to an anchialine pool. Placospongia anthosigma was not found in any of the examined collections from the tropical Western Pacific, this species is restricted to more temperate and deeper waters. Placospongia melobesioides is indicated in the Systema Porifera to have a distribution from the Indo-West Pacific to the Tropical Atlantic (
Future biodiversity surveys and species checklists both in the Atlantic as well as in the Pacific are advised to check the spicule morphology of Placospongia specimens in order to identify species, as the external morphology and color will not give an indication to the number of species. The different Placospongia spp. can occupy the same type of habitats in the tropics. An example of such sympatry is represented in Kakaban lake where in the 4 km2 area of the marine lake the three common tropical species of Placospongia co-exist side by side. Neglecting to review the spicule morphology would mean possibly missing the true diversity of species that are present in the location of study.
N.J. de Voogd, R.W.M. van Soest, and three reviewers provided valuable comments on the original manuscript. For the loan of the type-specimens I thank: E. Sherlock (British Museum of Natural History), H. Namikawa (National Museum of Nature and Science, Tokyo, Japan), and C. Eckert (Zoologisches Museum für Naturkunde). I am also grateful to the following people for their help in various ways in field- or labwork logistics: B.W Hoeksema, Suharsono, Y. Tuti, Y. Ise, S. Hoshino, Y. Nakao, N. K. Santodomingo, Estradivari, Bahruddin, M. Erdmann, M. Ammer, D. Erpenbeck, S. Menken, H. Breeuwer, J. van Ooyen, E. Beglinger, E. Kruidenier, and the staff of Papua Diving, of Nabucco Island Dive Resort, of Derawan Dive Resort, and of WWF/TNC Berau office. The present study was funded by the Netherlands Organisation for Scientific Research (NWO), division Earth and Life Sciences (ALW IPJ-07002, # 817.01.008; ALW-Rubicon # 825.12.007). Fieldwork in Indonesia was made possible through additional financial support of World Wildlife Foundation Netherlands-INNO Fund, the Schure-Beijerinck-Popping Fund of the Royal Dutch Academy of Science (KNAW), the Treub-Maatschappij Fund, the Lerner-Gray Fund for Marine Research (American Natural History Museum), the Leiden University Fund (LUF) /Slingelands, Singapore Airlines, the A.M. Buitendijk Fund and the J.J. ter Pelkwijk Fund. I thank the Indonesian Institute of Sciences (LIPI) and the Indonesian State Ministry of Research and Technology (RISTEK) for providing research permits in Indonesia.