(C) 2013 Josiah H. Townsend. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of palm-pitviper of the genus Bothriechis is described from Refugio de Vida Silvestre Texíguat in northern Honduras. The new species differs from congeners by having 19 dorsal scale rows at midbody, a bright green dorsal coloration in adults, the prelacunal scale fused to the second supralabial, and in representing a northern lineage that is sister to Bothriechis lateralis, which is distributed in Costa Rica and western Panama and is isolated from the new taxon by the Nicaraguan Depression. This represents the 15th endemic species occurring in Refugio de Vida Silvestre Texíguat, one of the richest herpetofaunal sites in Honduras, itself being the country with the highest degree of herpetofaunal endemism in Central America. We name this new species in honor of a Honduran conservationist slain in fighting against illegal logging, highlighting the sacrifices of rural activists in battling these issues and the critical importance of conservation in these areas.
Bothriechis guifarroi sp. n. , Bothriechis lateralis, Bothriechis marchi, Central America, conservation, cryptic species, endemic, Honduras, Pico Bonito National Park, Texíguat Wildlife Refuge
In the past decade, a steady stream of taxonomic discoveries have come out of the Chortís Highlands of Mesoamerica, a biogeographic region found to the south and east of the tectonic boundary between the Chortís and Mayan Blocks and north of the Nicaraguan Depression (
Our knowledge of the taxonomic diversity of Mesoamerican pitvipers has also greatly increased since the turn of the century (e.g.
The green palm-pitvipers (genus Bothriechis) of Mesoamerica have long been a source of taxonomic uncertainty and confusion (
Bothriechis marchi sensu lato is known from localities in the Cordillera Nombre de Dios, Cordillera de Merendón, and Sierra de Sulaco of Honduras and adjacent areas of Guatemala, with Bothriechis thalassinus being found in the Cordillera de Merendón of Guatemala and Honduras, Cerro Santa Bárbara and nearby highland forests in Honduras, Cerro del Mono in eastern Guatemala, and the highlands around the El Salvador-Guatemala-Honduras border area (
Two expeditions in 2010 provided the first herpetofaunal inventory of the extensively forested windward portions of Refugio de Vida Silvestre Texíguat, one of the most endemism-rich highland forests in Mesoamerica (
The type series was collected during sampling in the vicinity of La Liberación (15.53°N, 87.29°W; camp established at 1, 030 m elevation) during 10–21 June (11 participants; 1, 320 person-hours sampling) and 26 July–2 August 2010 (13 participants; 880 person hours). Tissue samples were preserved in SED buffer (20% DMSO, 0.25 M EDTA, pH 7.5, NaCl saturated;
Genomic DNA was isolated from muscle tissue taken from eleven specimens of Bothriechis using a Qiagen DNeasy extraction kit and protocol. Four mitochondrial gene fragments (NADH dehydrogenase subunit 4 (ND4), cytochrome b (cyt b), 12S rRNA, and 16S rRNA) were independently PCR-amplified as described in multiple studies (
Previously published sequences of Bothriechis were downloaded from GenBank and combined with new sequence data generated in this study (Table 1). Representatives of two Mesoamerican genera from the diverse sister clade to Bothriechis (which contains Atropoides [Mesoamerica], Bothriopsis [South America], Bothrocophias [South America], Bothrops [Mexico to South America], Cerrophidion [Mesoamerica], and Porthidium [Mexico to South America]) were selected for use as outgroups to root our Bothriechis phylogeny (
Taxa, vouchers, locality data, and GenBank accession numbers for sequences used in this study. Novel sequences from this study are indicated in boldface; country codes used as follows: CR = Costa Rica; EC = Ecuador; GT = Guatemala; HN = Honduras; MX = Mexico; NI = Nicaragua.
| Taxon | Locality | Voucher | GenBank Accession Numbers | |||
|---|---|---|---|---|---|---|
| ND4 | cyt b | 12S | 16S | |||
| Atropoides mexicanus | HN: Atlántida: Texíguat | USNM 578906 | KC847289 | KC847271 | KC847268 | KC847255 |
| Bothriechis aurifer | GT: | UTA R-35031 | DQ305483 | DQ305466 | DQ305425 | DQ305448 |
| Bothriechis bicolor | GT: | UTA R-34156 | DQ305484 | DQ305467 | DQ305426 | DQ305449 |
| Bothriechis guifarroi | HN: Atlántida: Texíguat | CM 156870 | — | KC847280 | — | KC847260 |
| HN: Atlántida: Texíguat | MVZ 269305 | — | KC847279 | — | KC847258 | |
| HN: Atlántida: Texíguat | USNM 579873 | KC847286 | KC847274 | KC847267 | KC847262 | |
| HN: Atlántida: Texíguat | USNM 579874 | KC847288 | KC847282 | KC847266 | KC847263 | |
| HN: Atlántida: Texíguat | USNM 579875 | KC847287 | KC847281 | KC847265 | KC847264 | |
| HN: Atlántida: Texíguat | USNM 579876 | — | KC847276 | — | — | |
| HN: Atlántida: Texíguat | USNM 579877 | — | KC847278 | — | — | |
| HN: Atlántida: Texíguat | USNM 579878 | — | KC847277 | — | KC847261 | |
| HN: Atlántida: Texíguat | UTA R-60303 | — | KC847275 | — | KC847259 | |
| Bothriechis lateralis | CR: Acosta | MZUCR-11155 | U41873 | AY223588 | AF057211 | AF057258 |
| Bothriechis marchi | GT: Zacapa: Cerro del Mono | UTA R-52959 | DQ305486 | DQ305469 | DQ305428 | DQ305451 |
| HN: Cortés: Sierra de Omoa | MVZ 263604 | — | KC847283 | — | — | |
| Bothriechis nigroviridis | CR: San Gerondo de Dota | MZUCR-11151 | AY223635 | AY223589 | AF057212 | AF057259 |
| Bothriechis rowleyi | MX: Cerro Baúl | JAC 13295 | DQ305485 | DQ305468 | DQ305427 | DQ305450 |
| Bothriechis schlegelii | CR: Cariblanco de Sarapiquí | MZUCR-11149 | AY223636 | AY223590 | AF057213 | AF057260 |
| EC: Pichincha | FHGO Live coll | AF292611 | AF292573 | — | — | |
| HN: Cortés: Yojoa | UF 157577 | KC847285 | KC847272 | KC847270 | KC847257 | |
| NI: Jinotega: Bosawas | UF 166874 | KC847284 | KC847273 | KC847269 | KC847256 | |
| Bothriechis supercilliaris | CR: San Vito | — | DQ305487 | DQ305470 | DQ305429 | DQ305452 |
| Bothriechis thalassinus | GT: Zacapa | UTA R-52958 | DQ305482 | DQ305465 | DQ305424 | DQ305447 |
| Cerrophidion wilsoni | HN: Olancho: Botaderos | UTA R-52953 | JQ724172 | JQ724159 | JQ724146 | JQ627132 |
Bayesian inference (BI) and maximum likelihood (ML) were implemented to reconstruct phylogenies for the Bothriechis ingroup taxa. To identify appropriate models of nucleotide substitution for both analyses, we used the program MrModeltest v2.2 (
Phylogenetic analyses using BI were conducted with MrBayes v3.0b4 (
Phylogenetic relationships were inferred using ML as implemented in RAxML 7.2.8 (
Results from a priori model selections based on Akaike information criterion (AIC) conducted in MrModeltest 2.2 (
| Partition | Total characters | Parsimony-informative characters | Best-fit model |
|---|---|---|---|
| ND4 1st pos | 222 | 39 | GTR+Γ |
| ND4 2nd pos | 222 | 9 | GTR+I |
| ND4 3rd pos | 222 | 128 | GTR+Γ |
| Cyt b 1st pos | 231 | 33 | GTR+Γ |
| Cyt b 2nd pos | 231 | 12 | HKY+I |
| Cyt b 3rd pos | 231 | 134 | GTR+I+Γ |
| 12S | 409 | 60 | GTR+Γ |
| 16S | 495 | 39 | GTR+I |
We examined 34 preserved specimens of Bothriechis for this study (Appendix). Definitions of scale counts and morphological features follow
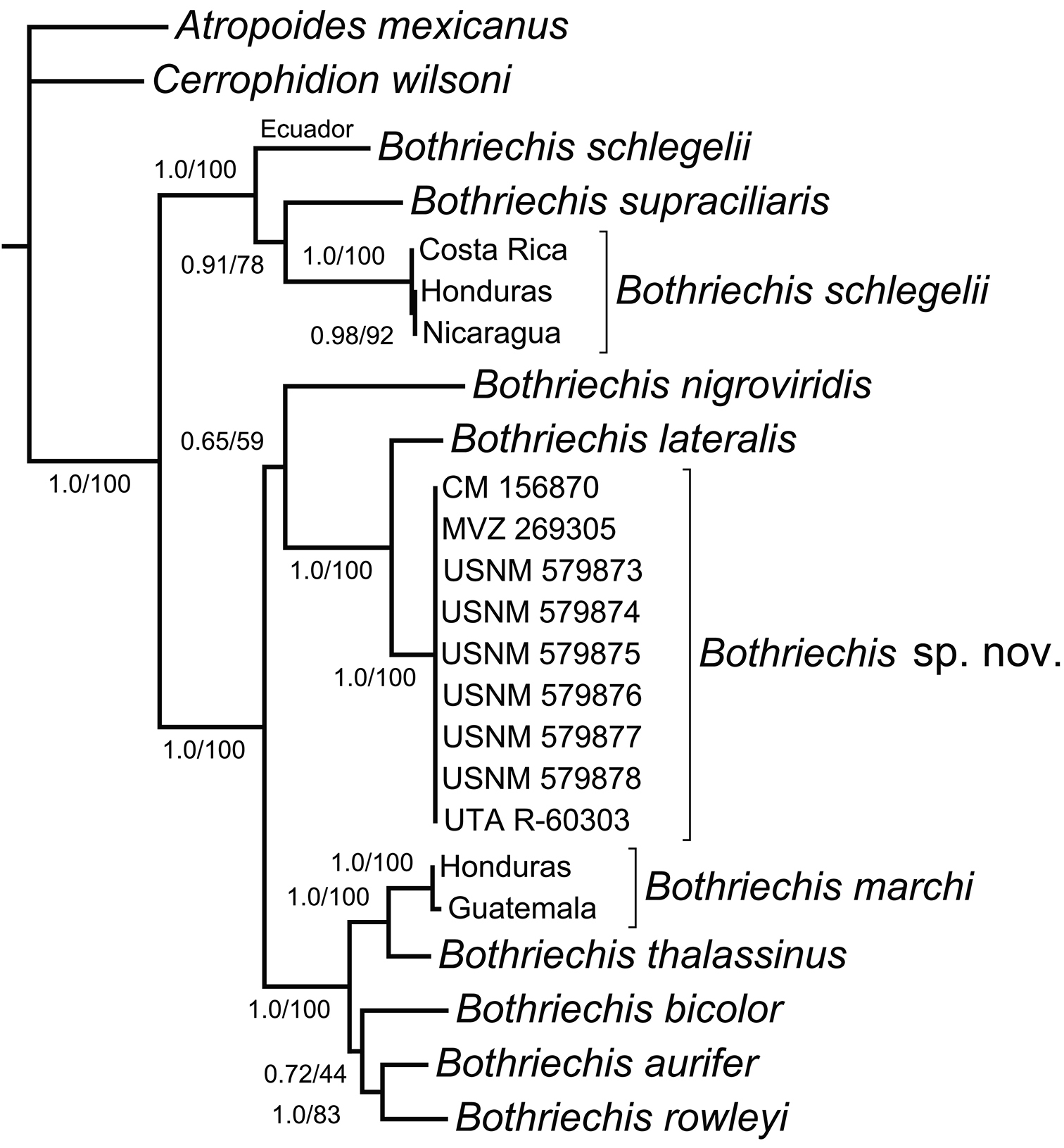
Bayesian and Maximum Likelihood phylogenetic analyses produced congruent topological results. Our phylogeny (Fig. 1) is generally congruent with those of
Based on the phylogenetic results, we examined morphological variation among populations of Bothriechis marchi sensu lato, which confirm the evolutionary and taxonomic distinctiveness of the Texíguat population as well as its apparent morphological affinity with a specimen from Parque Nacional Pico Bonito, approximately 75 km to the east of Refugio de Vida Silvestre Texíguat. We present the following description of this relict northern lineage as a new species.
Phylogeny of palm-pitvipers (genus Bothriechis), showing strong support for a species-level clade of the Texíguat population (Bothriechis sp. n.) that is sister to Bothriechis lateralis. The tree was estimated from a Bayesian 50% majority-rule consensus composed from a concatenated mitochondrial dataset (ND4, cyt b, 12S, and 16S; total of 2263 bp). Numbers at nodes represent values of Bayesian posterior probabilities (PP, left) and Maximum Likelihood bootstraps (BS, right). Nodes supported by ≥ 95% PP and ≥ 70 BS are considered highly supported.
urn:lsid:zoobank.org:act:1FC0661F-B08B-4D0D-85CC-DAEAB502D0DB
http://species-id.net/wiki/Bothriechis_guifarroi
Figs 2–3, 5–6UTA R-60303 (Figs 2, 3), an adult male from La Liberacíon (Fig. 4A, C), 15.5302°N, 87.2939°W (DD), 1, 015 m elevation, Refugio de Vida Silvestre Texíguat, Departamento de Atlántida, Honduras, collected 25 July 2010 by the field team of E. Aguilar, A. Contreras, L. Gray, L.A. Herrera-B., M. Medina-Flores, A. Portillo, A. Stubbs, and J. H. Townsend. Original field number JHT 3243. Genbank accession numbers: 16S (KC847259), cyt b (KC847275).
HONDURAS: Departamento de Atlántida: Refugio de Vida Silvestre Texíguat: adult female (USNM 579875) collected 19 June 2010, two adult females (USNM 579876–77) collected 29 July 2010, and two unsexed neonates collected 18 June 2010 (USNM 579873–74), all from Cerro El Chino (Fig. 4B), 15.5225°N, 87.2802°W (DD), 1, 360–1, 450 m elevation, southeast of La Liberación. Two males (CM 156870 and MVZ 269305) collected 28 July 2010 from a ridge-top trail above La Liberación, 15.5418°N, 87.2891°W (DD), 1, 290 m elevation. One male (USNM 579878) collected 30 July 2010 from La Liberación (Fig. 4A, C), 15.5302°N, 87.2939°W (DD), 1, 015 m elevation.
HONDURAS: Departamento de Atlántida: AMNH 46949 from “Tela, ” collected sometime before April 1932; USNM 319942 from Quebrada de Oro, Parque Nacional Pico Bonito. Departamento de Yoro: Refugio de Vida Silvestre Texíguat: USNM 337488–89 from 2.5 airline km north-northeast of La Fortuna. See Remarks.
Bothriechis guifarroi is distinguished from all nine congeners by the following combination of features: dorsal scales in 19-19-15 rows; ventrals in males 162–166 (163.8), in females 158–166 (164.0), 162–166 (164.0) in neonates; subcaudals in males 60–68 (63.0), in females 60–63 (61.0), 62–68 (65.0) in neonates; intersupraoculars (3–7); superciliary scales absent; prelacunal scale fused to second supralabial on both sides; two known color patterns in juveniles, one brown (with a pale paraventral stripe and a series of short darker dorsal blotches and a dark brown postocular stripe bordered by yellow on its lower edge) and the other green (with a series of pale blue blotches and a deep blue postocular stripe bordered by pale blue on its lower edge); dorsal coloration in adults green with pale blue trim on anterior edges of dorsal scales, and pale blue postocular stripe with green along the keels in center of stripe; and iris pale green, pale gray, or pale tan.
Bothriechis guifarroi can be distinguished from the other members of the genus Bothriechis as follows (Bothriechis guifarroi features indicated first, those for species compared next): Bothriechis aurifer (distributed at moderate and intermediate elevations from extreme east-central Chiapas, Mexico, to east-central Guatemala) — adult color pattern (green vs. black-bordered yellow blotches on green background and prominent black postocular stripe) and juvenile color pattern (green with pale blue blotches or brown with pale paraventral stripe and dark dorsal blotches vs. pale lime green with black-bordered yellow blotches); Bothriechis bicolor (occurring marginally at low upward to intermediate elevations from southeastern Chiapas, Mexico, to south-central Guatemala) — number of dorsal scales at midbody (19 vs. 21) and condition of prelacunal and second supralabial scales (fused vs. separate); Bothriechis lateralis (moderate to marginally high elevations from northwestern Costa Rica to western Panama) — number of dorsal scale rows at midbody (19 vs. modal number of 23), adult color pattern (green vs. green with pale paravertebral bars and paraventral stripe), and juvenile color pattern (bi-morph pattern of green with blue dorsal blotching or brown with pale paraventral stripe and short unicolor dark blotches vs. uni-morph pattern of brown ground color with pale paraventral stripe and short bicolor dark and pale blotches); Bothriechis marchi (found marginally at low elevations up to intermediate elevations in northwestern Honduras and adjacent Guatemala) — condition of prelacunal and second supralabial scales (fused vs. separate), number of subcaudals in females (60–63 vs. 46–57); Bothriechis nigroviridis (moderate to intermediate elevations from north-central Costa Rica to west-central Panama) — adult color pattern (patternless green vs. green with very heavy black mottling), juvenile color pattern (green with pale blue blotches or brown with pale paraventral stripe and a series of short darker dorsal blotches vs. green with heavy black mottling), iris color (pale green, pale gray, or pale tan vs. almost black), numbers of ventral scales in both sexes (162–166 and 158–166 vs. 143–158 and 134–158), numbers of subcaudal in both sexes (60–68 and 60–63 vs. 49–56 and 44–58), and condition of prelacunal and second supralabial scales (fused vs. separate); Bothriechis rowleyi (moderate to intermediate elevations from extreme southeastern Oaxaca to northwestern Chiapas, Mexico) — condition of prelacunal and second supralabial scales (fused vs. almost always separate), iris color (pale green, pale gray, or pale tan vs. yellow), and juvenile color pattern (green with pale blue blotches or brown with pale paraventral stripe and a series of short darker dorsal blotches vs. pale yellowish green with brown or purple dorsal blotches); Bothriechis schlegelii (low to intermediate elevations from northwestern Chiapas, Mexico, southward through Central America and into northwestern South America as far as extreme western Venezuela and extreme northern Peru) — lack of superciliary scales in the former and their presence in the latter, number of supralabials (10–12, usually 10 vs. 7–10, usually 8), number of midbody dorsal scale rows (19 vs. 21–25, usually 23), and adult color pattern (green vs. extremely variable color and pattern involving ground color of yellow, pink, brown, gray, or green and dorsal blotching of a sizable array of colors, but sometimes absent; contrasting postocular stripe absent vs. present); Bothriechis supraciliaris (moderate to intermediate elevations from southwestern Costa Rica to west-central Panama) — lack of superciliary scales in the former and their presence in the latter, number of midbody dorsal scale rows (19 vs. 21–23, usually 23), number of ventral scales in both sexes (162–166 and 158–166 vs. 145–150 and 141–148), number of subcaudal scales in both sexes (60–68 and 60–63 vs. 48–54 and 45–52), and adult color pattern (green vs. extremely variable color and pattern involving ground color of shades of green, brown, or maroon and dorsal blotching of an array of colors contrasting with that of the ground color; contrasting postocular stripe absent vs. present); Bothriechis thalassinus (moderate to intermediate elevations from extreme eastern Guatemala and extreme northwestern El Salvador to western Honduras) — number of midbody dorsal scale rows (19 vs. 21–23, usually 21), and condition of prelacunal and second supralabial scales (fused vs. separate).
An adult male (Figs 2, 3) with hemipenes partially everted, left removed;rostral broader than high (4.38 × 3.26 mm); 2 internasals anteriorly; 2/2 canthals; 4 posterior intercanthals; supraoculars slightly more than two times as long as broad; 5 intersupraoculars; many scales on head of large size, including large, flat frontal and parietal scales; interrictals 25; single loreal, longer than high, bounded by upper two preoculars, canthal above, prelacunal and prefoveals below, and nasal; prefoveals 3/3, subfoveals 1/1; prelacunal and second supralabial fused; preoculars 3/3, upper largest, middle large and in contact with supralacunal; suboculars 2/2; postoculars 2/2; supralabials 10/10; mental broader than long (4.39 x 3.29 mm); infralabials 11/11; chin shields contacting first four pairs of infralabials; gulars between chin shields and first preventral 6/4; dorsal scale rows 19-19-15; preventrals 2; ventrals 161; cloacal scute undivided; 65 undivided subcaudals; tail spine short and blunt.
Photographs in life of the adult male holotype of Bothriechis guifarroi (UTA R-60303), with lateral and dorsal views of the head. Photographs by JHT.
Dorsal, lateral, and ventral aspects of the head of the holotype of Bothriechis guifarroi (UTA R-60303). Photographs by RCJ.
Habitat in the vicinity of the type locality of Bothriechis guifarroi, La Liberación, Refugio de Vida Silvestre Texíguat, Honduras; A riparian vegetation along the Río Jilamito, 1, 015 m elevation B small seepage pond near the top of Cerro El Chino, 1, 380 m elevation C premontane rainforest with the clearing aroundLa Liberación (1, 030 m elevation) visible in the foreground. Photographs by JHT.
Paratypes of Bothriechis guifarroi in life; A USNM 579874, green-phase juvenile B USNM 579873, brown-phase juvenile C close-up of head of USNM 579874 D close-up of head of USNM 579873 E USNM 579875, female paratype photographed in situ at Cerro El Chino, 1, 420 m elevation. Photographs by JHT.
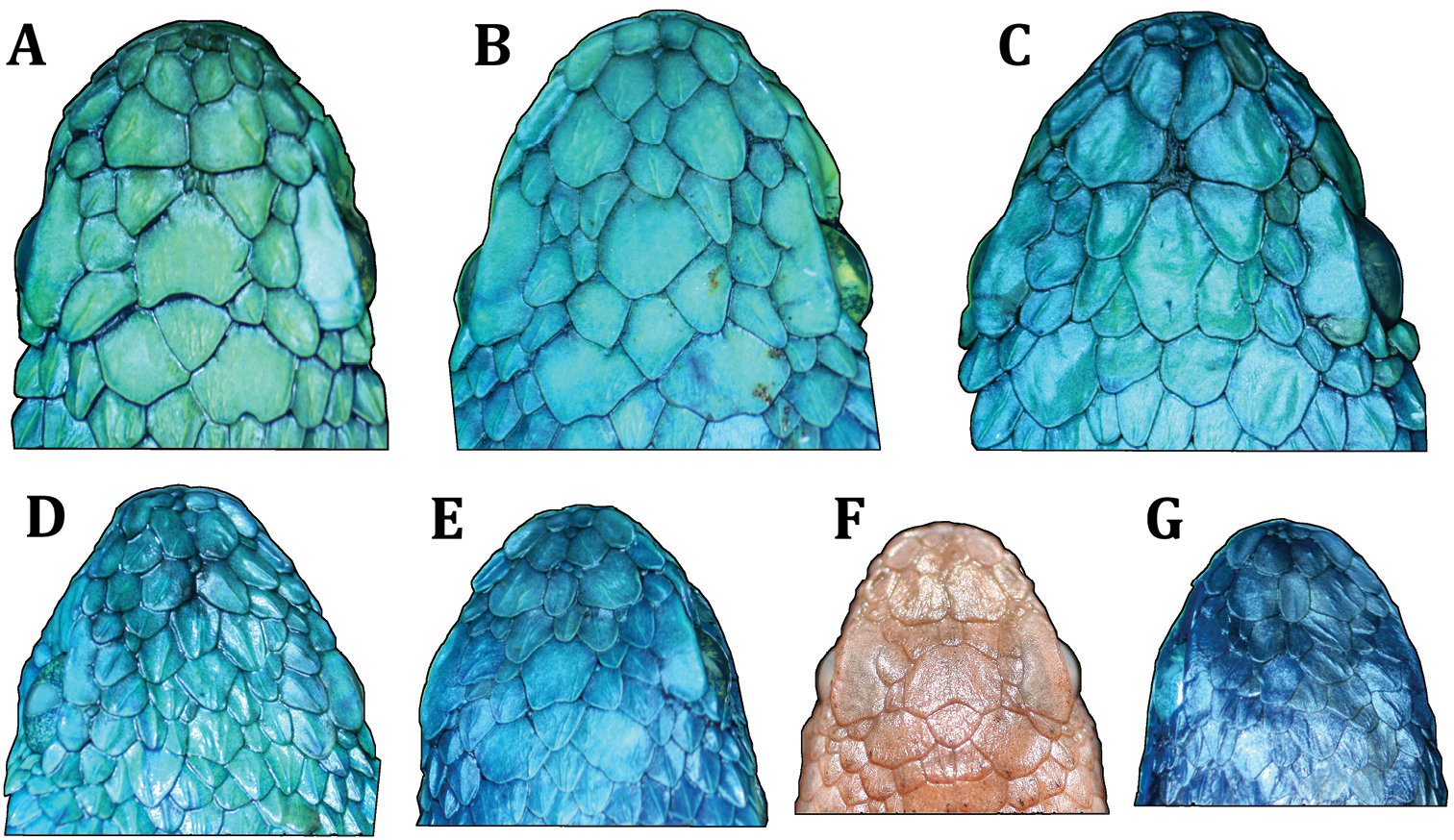
Variation in dorsal head scales among paratypes of Bothriechis guifarroi; A USNM 579877, adult female B MVZ 269305, adult male C USNM 579876, adult female D USNM 579875, adult female E USNM 579878, adult male F USNM 579873, neonate G USNM 579874, neonate. Photographs by JHT.
Total length 734 mm; tail length 136 mm, comprising 18.5% of total length; head 29.8 mm from front face of rostral to posterior end of mandible; head 19 mm at broadest point; neck 7 mm directly behind jaws.
The partially everted left hemipenis of the holotype is described. Hemipenis at least 20 mm in total length and 13 mm in maximum width at level of crotch; on sulcate side base with several rows of small spines (< 0.5 mm) followed by rows of larger spines and hooks extending for 5 mm, largest protruding ca. 3.5 mm; asulcate side with minutely spined base up to 7 mm before level of bilobation; numerous small mesial spines (< 0.5 mm) arranged in rows present for 4 mm to the calyces, with peripheral section of each lobe containing nine spines and hooks (≥ 2 mm), five of which border lower rim of calyces; calyces follow spines and hooks distally; calyces scalloped, at least 10 rows at least 7 mm to apex of hemipenis on asulcate side; sulcus spermaticus deep and bifurcating ca. 4 mm before site of bilobation and extending upwards through spines and calyces likely to tip of each lobe; sulcus spermaticus bordered by two, occasionally three, columns of minute scales to the beginning of calyces, which form the border likely to the apex of the lobes. Although the majority of the hemipenial characters of this specimen are reported, the lack of a fully everted hemipenis leaves information on the total length and the nature of the calyces incomplete.
Middorsal scales of the holotype Yellowish Spectrum Green (Color 128), fading to Light Grass Green (Color 109) laterally and becoming Chartreuse (Color 89) ventrolaterally, with Medium Greenish Yellow (Color 88) ventral scales; dorsal body scales edged anteriorly in Light Caribbean Blue (Color 163), with up to approximately one-fourth of the anterior end of some scales edged in blue; skin concealed between dorsal scales Spectrum Violet (Color 186); postoccipital stripe Light Caribbean Blue (Color 163), with keel and adjacent portion of three scales that lie within the postoccipital stripe Light Emerald Green (Color 142); terminal portion of tail Plumbeous (Color 295); iris Pale Bluish Gray (Color 287) with fine black reticulations most heavily concentrated around the pupil.
Scales on dorsal surfaces of the head and body blue-green, becoming more green laterally and yellow-green to yellow ventrally. Tail is mostly green with some grayish blue-green at the dorsal base. Pupil is cloudy and pale, surrounded by lime green iris heavily speckled with black.
We discuss scutellational variation in the three adult male paratypes first, the three adult females next, and finally the two unsexed neonates. Scutellation varies as follows (range followed by mean): ventrals (162–166 [164.3], 158–166 [161.7], 162 and 166); subcaudals (60–68 [64.0], 60–63 [61.0], 62 and 68); ventrals + subcaudals (222–233 [228.3], 221–226 [222.7], 228 and 230); cloacal scute entire in all specimens; dorsal scale row formula 19-19-15, with the reduction to 15 rows occurring at ventrals 114–162; supralabials (10–11 [10.2], 10—11 [10.2], 10–10 and 12–11); infralabials (10–12 [11.0], 10–13 [11.7], 11–11 and 12–11); preoculars 2–2 in all specimens, except 3-3 in CM 156870; postoculars 2–2 in all specimens, except 3–2 in MVZ 269305 and 4–4 in CM 156870; suboculars 2–3 [2.5], 2–3 [2.7], 2–2 and 3–4; relative tail length (0.184–0.223 [0.199], 0. 167–0.182 [0.176], 0.179 and 0.194).
Two juvenile color patterns are present in this species (Fig. 5), one we refer to as a “green phase”, the other as a “brown phase.” Both juvenile phases have distinctively colored tail-tips, presumably used in caudal luring, and well-differentiated postocular stripes. The green phase (USNM 579874) has a Chartreuse (Color 89) dorsal ground color with Light Turquoise Green (Color 146) edging on the dorsal scales as well as on a series of irregular middorsal blotches, a Pale Green (Color 99) venter, and a Chartreuse (Color 89) head with a Jet Black (Color 300) postocular stripe, bordered above and below by Light Turquoise Green (Color 146); tip of tail Cobalt Blue (Color 180); the iris is Pale Neutral Gray (Color 296) with fine darker reticulations. The brown phase (USNM 579873) has a Robin Rufous (Color 29) ground color middorsally and anteriorly, becoming Salmon Color (Color 58) laterally and posteriorly, with a series of irregular Ferrunginous (Color 35) middorsal blotches, a Pale Buff (Color 1) ventral surface of head and venter becoming gradually darker (Pale Pinkish Buff [Color 3]) posteriorly, a Dark Salmon Color (Color 59) head with a Chestnut (Color 30) postocular stripe, bordered above and below by Light Buff (Color 2); tip of tail Sepia (Color 286); a Pale Buff (Color 1) paraventral stripe is present on the lower half of the first dorsal scale row and the lateral edge of the ventrals; dark speckling along lateral edge of the ventrals; the iris is Chamois (Color 84) with fine darker reticulations.
The type series of Bothriechis guifarroi demonstrates considerable variation in the condition and shape of the scales on the dorsal surface of the head (Fig. 6), a characteristic often considered diagnostic among Bothriechis (
The specific name guifarroi is a patronym used to honor our colleague and friend, Honduran environmental leader Mario Guifarro of Olancho. Don Mario fearlessly led grassroots efforts to stop illegal logging in the indigenous Tawahka territory of eastern Honduras, despite repeated assassination attempts and threats on his own life and those of his compatriots. Don Mario was murdered on 15 September 2007, ironically Honduras’ Independence Day, while leading a mission to demarcate the boundaries of the Tawahka-Asangni Biosphere and stave off further illegal deforestation. On 21 July 2008, the only witness to Mario’s assassination, his son Shamir Guifarro Ramírez, was also murdered, along with Mario’s father-in-law, Henry Arturo Chacón, and mother-in-law, Nelda Ochoa, after they were followed out of the city of Juticalpa by unknown assailants.
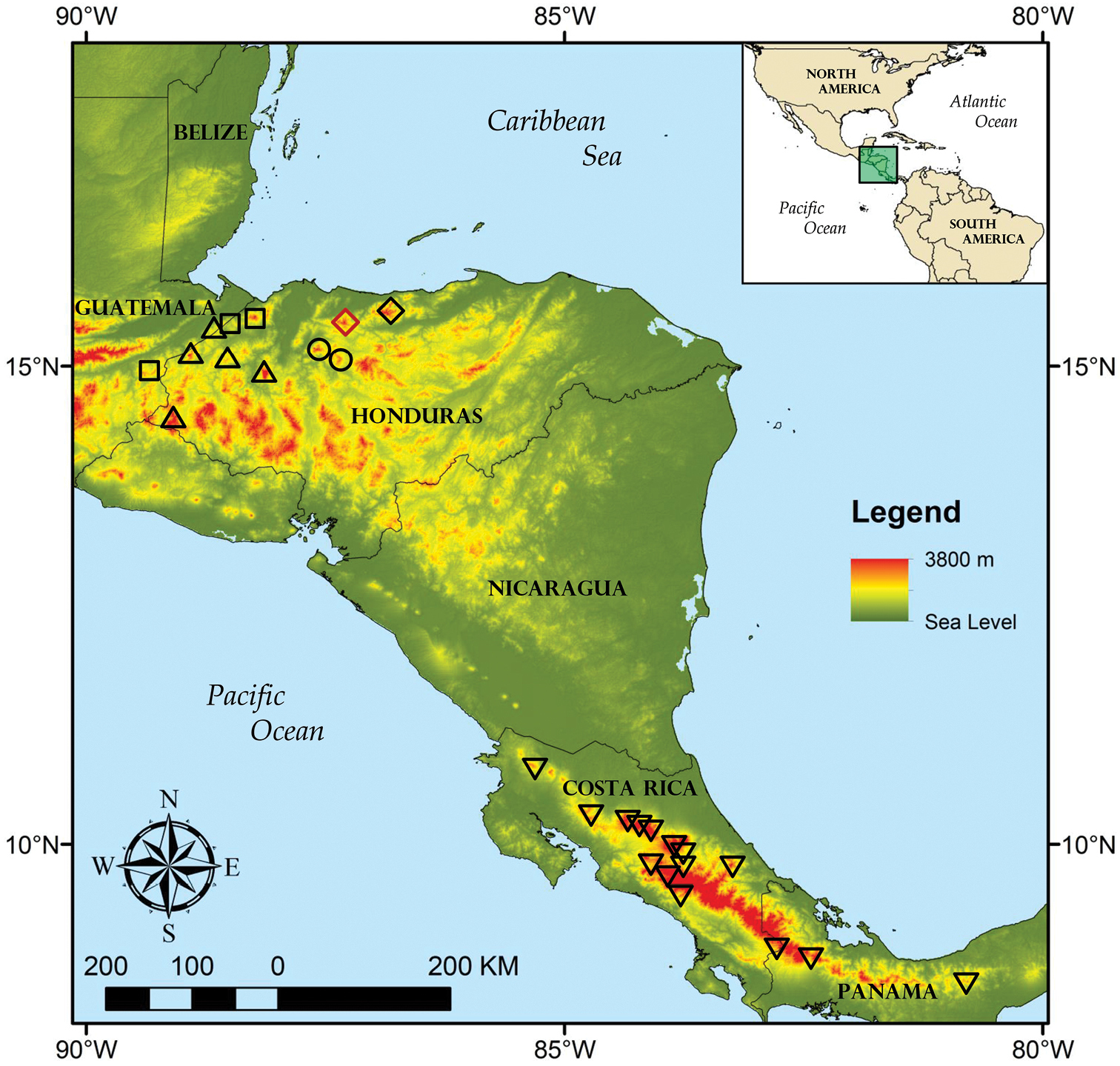
Populations genetically confirmed to represent Bothriechis guifarroi are found between 1, 015–1, 450 m elevation in the western portion of the Cordillera Nombre de Dios, Department of Atlántida, Honduras, within the boundaries of Refugio de Vida Silvestre Texíguat (Fig. 7). These localities lie within the Premontane Wet Forest and peripherally in the Lower Montane Wet Forest formations of
Geographic distribution of selected Bothriechis species and populations discussed in the text; localities are based on data published herein and those of
The holotype was found coiled at 2130h approximately 2.5 m above the ground among old leaf sheaths in the crown of a medium-sized understory palm in gallery forest alongside the Río Jilamito (Fig. 4A). Anurans of the genera Duellmanohyla and Ptychohyla were abundant in the immediate vicinity of the holotype. Two adult males (CM 156870 and MVZ 269305) were collected along a ridge on the north side of La Liberacíon on the night of 28 July 2010. CM 156870 was active on a small tree from 0.5–1.5 m above the ground; the second snake (MVZ 269305) was sitting coiled on the ground at the edge of the trail, and attempted to escape by crawling across the path when we approached. In the immediate vicinity of these snakes were numerous Craugastor rostralis active on the ground and Bolitoglossa sp. active on low vegetation. Two neonates were collected on the same night (2100–2200 h) on 18 June 2010, and an adult female (USNM 579875) was collected the next night, in an area of elfin forest at 1, 380 m on the ridge called Cerro El Chino (Fig. 4B) above the remote ranch locality La Liberación (at 1, 030 m). The brown-phase neonate (USNM 579873) was found atop a large, similarly-colored dead palm frond, while the green-phase neonate (USNM 579874) was sitting in essentially the same ambush position as USNM 579873, but on top of a living green frond. Amphibian species collected in the immediate vicinity of Bothriechis guifarroi include Bolitoglossa sp., Nototriton sp., Craugastor rostralis, Plectrohyla chrysopleura, and Ptychohyla spinipollex. Twelve Bolitoglossa sp. were encountered the same night as the two neonates, all while active on or around dead and living palm fronds in the immediate vicinity of the neonates.
We tentatively refer four additional specimens to Bothriechis guifarroi: two from a locality on the leeward side of Refugio de Vida Silvestre Texíguat (USNM 337488–89, from 2.5 airline km NNE of La Fortuna, Dept. Yoro, 1, 550 m elevation), one from the central portion of the Cordillera Nombre de Dios (USNM 319942, from Quebrada de Oro in Parque Nacional Pico Bonito, Dept. Atlántida, 1, 090 m elevation), and one from “Tela” (AMNH 46949). All four specimens also have fused prelacunals and second supralabials on both sides. USNM 319942 was collected as a juvenile and raised in captivity (
AMNH 46949 was collected sometime during or before 1932 by Douglas March of the Lancetilla Serpentarium, just outside of the seaside city of Tela. While the “Tela” locality is considered erroneous (
Conservation status of Bothriechis guifarroi. With the description of Bothriechis guifarroi, there are now at least three species of palm pitvipers endemic to the Chortís Highlands, including Bothriechis marchi and Bothriechis thalassinus, with the potential for additional undescribed taxonomic diversity pending phylogenetic evaluation of allopatric populations in central Honduras. Based on the IUCN Red List criteria (2012), Bothriechis guifarroi should be classified as Critically Endangered (B1ab[iii]+2ab[iii]) due to its limited known area of occurrence and the potential for anthropogenic damage to its habitat. According to the algorithm developed by
The vicinity of the type locality of Bothriechis guifarroi is part of the relatively large and intact premontane rainforests and cloud forests of Refugio de Vida Silvestre Texíguat, one of the most important areas of herpetofaunal endemism in Mesoamerica (
Herpetofaunal Endemism in the Chortís Highlands. Whereas Nuclear Central America has long been accepted as a region of high biodiversity and endemicity, some observers have further recognized the western and eastern portions of this highland block as distinct biogeographic entities (
The majority of the geographical extent of the Chortís Highlands is found within the political boundaries of Honduras, the country with the highest degree of herpetofaunal endemism of any Central American nation (
Bothriechismarchi and the status of populations from Yoro. We recognize Bothriechis marchi sensu stricto as occurring in localities in the Cordillera de Merendón in the Honduran departments of Cortés and Santa Bárbara along the border with Guatemala, as well as for at least one isolated locality in eastern Guatemala (Fig. 7). This Guatemalan locality, Cerro del Mono in Departamento de Zacapa, previously was the source of the only sequenced sample assigned to Bothriechis marchi (UTA R-52959;
The type localities of Bothriechis marchi and Bothriechis thalassinus are both in the Sierra de Caral, within approximately 20 km of one another on opposite sides of the Guatemala/Honduras border (
Paraphyly in Bothriechis marchi sensu lato in terms of populations from the Cordillera de Merendón and the Cordillera Nombre de Dios, the latter now known to represent Bothriechis guifarroi, calls into question the taxonomic status of populations from isolated localities in the Sierra de Sulaco in Departmento de Yoro (Fig. 7). These populations are represented in collections by one specimen from Cerro de Pajarillos (USNM 561085), three specimens from the Montaña de Mataderos (FMNH 21777, MCZ R-38785–86), 14 specimens from “Portillo Grande” near Montaña Macuzal (FMNH 34732–35, 35895, 35999–601, 37217, 38542, 41621; MCZ R-38789–91), and two specimens from “Subirana Valley” (FMNH 21892, MCZ R-38788). Four of these specimens examined by us for this paper (MCZ R-38785–86, 38790–91) differ from Bothriechis guifarroi in having 21–19–15 dorsal scale rows (versus 19–19–15) and having varying conditions of fusion of the prelacunal and second supralabial (fused on both sides in MCZ R-38790, separate on both sides in MCZ R-38786, and fused on one side and separate on the other in MCZ R-38785 an R-38791). Given the considerable phylogenetic diversification presented by analysis of Bothriechis guifarroi and Bothriechis marchi s. s., we cannot justify assignment of the Yoro populations to either taxon in the absence of molecular characterization of those populations. Therefore, we tentatively refer to the Yoro populations as Bothriechis species inquirenda pending collection of fresh material and phylogenetic characterization. Efforts to secure this material are currently underway.
Evolutionary and biogeographic implications. The phylogenetic position of Bothriechis guifarroi has significant implications for our understanding of the biogeography and evolution of palm-pitvipers.
The Cordillera Nombre de Dios, which includesRefugio de Vida Silvestre Texíguat and Parque Nacional Pico Bonito, is home to a distinct endemic biota that, in many cases, bears little resemblance to the endemic communities found in other nearby cloud forests (
Research was carried out under permits issued by ICF (Resolución DE-MP-086-2010 and Dictamen DVS-ICF-045-2010). We are indebted to the following individuals and organizations for supporting our work in Refugio de Vida Silvestre Texíguat: Allan J. Fuentes (PROLANSATE); Alcalde Adolfo Pagoada-Saybe (Municipalidad de Arizona), Saíd Laínez, Iris Acosta, Roberto Downing, Andrés Alegría (Instituto Nacional de Conservación y Desarrollo Forestal, Áreas Protegidas y Vida Silvestre [ICF]); guardabosques Efrain Aguilar (San José de Texíguat), Alfonso Contreras (Mezapita), and Alionso Portillo (Jilamito Nuevo); and José Dubón, majordomo at La Liberación. We thank Benjamin K. Atkinson, Cesar A. Cerrato, Levi N. Gray, Luis A. Herrera, Paul House, Mayron McKewy Mejía, Ciro Navarro-Umaña, Alexander L. Stubbs, and Hermes Vega-Rodríguez for their valued assistance and hard work in the field during 2010. Fieldwork was supported in part by a grant to Kirsten E. Nicholson (Central Michigan University; National Science Foundation DEB-0949359). For facilitating deposition of the type series, we thank Jonathan Campbell, Carl Franklin, and Eric Smith (UTA), Roy McDiarmid, Steve Gotte, and James Poindexter (USNM), Carol Spencer, Jim McGuire, and Ted Papenfuss (MVZ), and José Padial and Stephen Rogers (CM). We would also like to thank Sean M. Rovito for sharing data and a tissue sample of a Bothriechis marchi collected from the Sierra de Omoa, and Eric N. Smith for sharing his insights about highland Bothriechis in eastern Guatemala. William Brenneman (IUP) provided helpful comments on a draft of this manuscript, and Ileana Luque-Montes created the map used for Figure 7.
Specimens examined; museum acronyms follow
Bothriechis guifarroi (12) – Honduras: ATLÁNTIDA: Quebrada de Oro, USNM 319942; “Tela, ” AMNH 46949; La Liberación, CM 156870, MVZ 269305, USNM 579873–78, UTA R-60303. YORO: 2.5 airline km NNE of La Fortuna, USNM 337488–89.
Bothriechis marchi (19) – Honduras: CORTÉS: “near Cofradía, ” MCZ 28014; “La Cumbre, ” AMNH 46954–57, MCZ 32029–31; Sierra de Omoa, MCZ 33334–36, 33560–64, USNM 83454. SANTA BÁRBARA: “Quimistán, ” MCZ 27260, 27510; “between Cofradía and Quimistán, ” MCZ 27567–68, UMMZ 90677 (was MCZ 27569); “Santa Bárbara, ” MCZ 28014.
Bothriechis thalassinus (3) – Honduras: COPÁN: Quebrada Grande, KU 203094; OCOTEPEQUE: 21.7 km east of Nuevo Ocotepeque, LSUMZ 23821; SANTA BÁRBARA: southeastern slope of Cerro Santa Barbara, LSUMZ 11638.
Bothriechis sp. inquirenda (4) – Honduras: YORO: Montañas de Mataderos, MCZ 38785–86; Portillo Grande, MCZ R-38790–91.