(C) 2013 Mansour Aliabadian. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Aliabadian M, Beentjes KK, Roselaar CS, van Brandwijk H, Nijman V, Vonk R (2013) DNA barcoding of Dutch birds. In: Nagy ZT, Backeljau T, De Meyer M, Jordaens K (Eds) DNA barcoding: a practical tool for fundamental and applied biodiversity research. ZooKeys 365: 25–48. doi: 10.3897/zookeys.365.6287
The mitochondrial cytochrome c oxidase subunit I (COI) can serve as a fast and accurate marker for the identification of animal species, and has been applied in a number of studies on birds. We here sequenced the COI gene for 387 individuals of 147 species of birds from the Netherlands, with 83 species being represented by > 2 sequences. The Netherlands occupies a small geographic area and 95% of all samples were collected within a 50 km radius from one another. The intraspecific divergences averaged 0.29% among this assemblage, but most values were lower; the interspecific divergences averaged 9.54%. In all, 95% of species were represented by a unique barcode, with 6 species of gulls and skua (Larus and Stercorarius) having at least one shared barcode. This is best explained by these species representing recent radiations with ongoing hybridization. In contrast, one species, the Lesser Whitethroat Sylvia curruca showed deep divergences, averaging 5.76% and up to 8.68% between individuals. These possibly represent two distinct taxa, S. curruca and S. blythi, both clearly separated in a haplotype network analysis. Our study adds to a growing body of DNA barcodes that have become available for birds, and shows that a DNA barcoding approach enables to identify known Dutch bird species with a very high resolution. In addition some species were flagged up for further detailed taxonomic investigation, illustrating that even in ornithologically well-known areas such as the Netherlands, more is to be learned about the birds that are present.
Aves, conservation, cytochrome c oxidase subunit I , COI, taxonomy
DNA barcoding is used as an effective tool for both the identification of known species and the discovery of new ones (
Initially, DNA barcodes were proposed for the Animal Kingdom in 2003, when Hebert and colleagues tested a single gene barcode to identify species and coined the term ‘DNA barcoding’ (
Birds are among the best-known classes of animals and thus provide a taxonomically good model for analyzing the applicability of DNA barcoding. In the last seven years some 30 scientific papers have been published on the DNA barcoding of bird species, which combined have been cited 500 times (V. Nijman, unpubl. data April 2013). Most of the studies have shown that from this small fragment of DNA, individuals have been identified down to species level for 94% of the species in Scandinavian birds (
Here we explore the efficiency of identifying species using DNA barcoding from a large set of sympatric bird species in the Netherlands. Compared to previous studies on birds, our study area covers a very small geographic area, allowing to directly test the functionality of DNA barcoding ‘in one’s backyard’.
The Netherlands is a small, densely populated country in northwestern Europe, with a land surface area of some 34 000 km2, and ornithologically it is arguable one of the best-covered countries (
The tissue samples were subsampled and subjected to DNA extraction using DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer’s protocol. PCR and sequencing reactions were performed, mainly following the same protocols described in
Sequences shorter than 500 bp and containing more than 10 ambiguous nucleotides were excluded from the analyses. All sequences have been deposited in GenBank (Accession numbers KF946551 to KF946937). A full list of the museum vouchers, for all specimens applied in this study, is provided in Appendix – Table 1.
For all sequence comparisons, the Kimura 2-parameter (K2P) model was used, because it is shown to be the best metric to compare closely related taxa (
For a group of birds that expressed a larger than expected intraspecific variation, the Sylvia warblers, we created a phylogenetic tree and created a haplotype network. We chose GTR+G+I as the best-fitting model of nucleotide substitution based on its Akaike’s information criterion as implemented in JModelTest v0.1.1 (
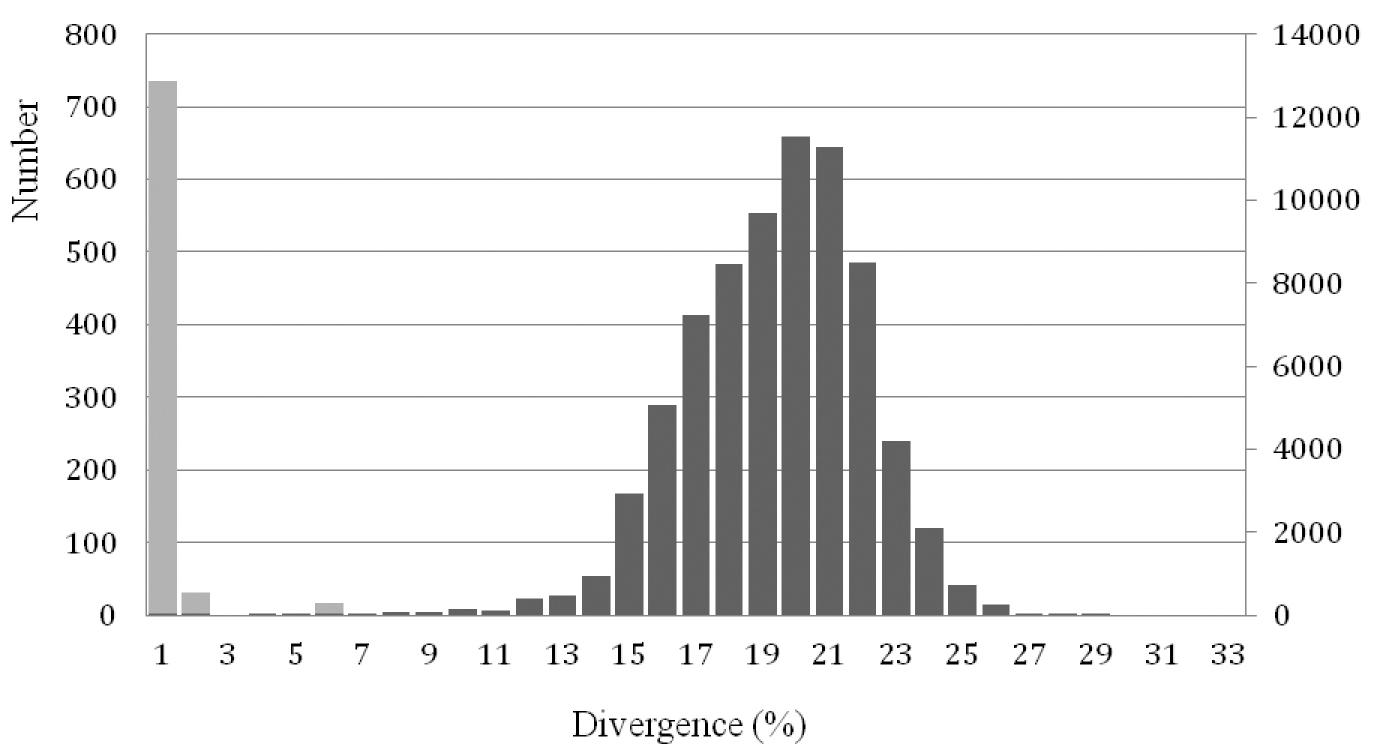
A total of 387 sequences for 141 species (representing at least 158 taxa) were retrieved, including 52% of the breeding bird species in the Netherlands (Supplementary table 1). The average number of sequences per species was 3.36 (range 1-13), with 83 species (59%) represented by more than two sequences. The mean K2P-divergence within species bears no significant relationship with sample sizes, i.e. number of sequences per species (R2 = 0.001, p = 0.465). The mean intraspecific K2P-distance was 0.29% (range 0-8.68%) some 30 times lower than the mean intrageneric K2P-distances (9.54%, range 0-27.71%) (Table 1, Figure 1).
Comparisons of K2P-pairwise distances based on the COI gene of 141 species of birds from the Netherlands, showing a clear barcoding gap. Interspecific distances are indicated with light grey bars and intraspecific distances with dark grey bars. Left Y-axis: numbers of intraspecific comparisons; Right Y-axis: numbers of interspecific comparisons.
Comparisons of K2P-pairwise distances within various taxonomic levels for 83 species of birds from the Netherlands for which two or more sequences were available. Distances are expressed in percentages.
| Individuals | Taxa | Comparisons | Distances | |||
|---|---|---|---|---|---|---|
| Minimum | Mean ± S.E.M. | Maximum | ||||
| Within Species | 340 | 83 | 805 | 0 | 0.294±0.001 | 8.683 |
| Within Genera | 203 | 23 | 794 | 0 | 9.544±0.004 | 15.849 |
| Within Families | 282 | 20 | 2519 | 5.809 | 14.467±0.001 | 20.473 |
In general, 95% of species (134 species) showed a unique DNA barcode (these included the 58 species for which we only sequenced single individuals), while six congeneric species shared the same barcode and the mean intraspecific distance of them fell well below the threshold of species based on distance-based criterion (
Bird species (Charadriiformes) from the Netherlands with one or more shared DNA barcodes (K2P-distances of 0%). For a detailed breakdown of the individual samples involved see Appendix – Table 2.
| Family | Species | Nearest species | Mean K2P-distance (%) |
|---|---|---|---|
| Laridae | Herring Gull Larus argentatus | Yellow-legged Gull Larus michahellis | 0.14 |
| Lesser Black-backed Gull Larus fuscus | Caspian Gull Larus cachinnans | 0 | |
| Iceland Gull Larus glaucoides | Caspian Gull Larus cachinnans | 0 | |
| Glaucous Gull Larus hyperboreus | Yellow-legged Gull Larus michahellis | 0.58 | |
| Yellow-legged Gull Larus michahellis | Caspian Gull Larus cachinnans | 0 | |
| Stercorariidae | Great Skua Stercorarius skua | Pomarine Skua Stercorarius pomarinus | 0.30 |
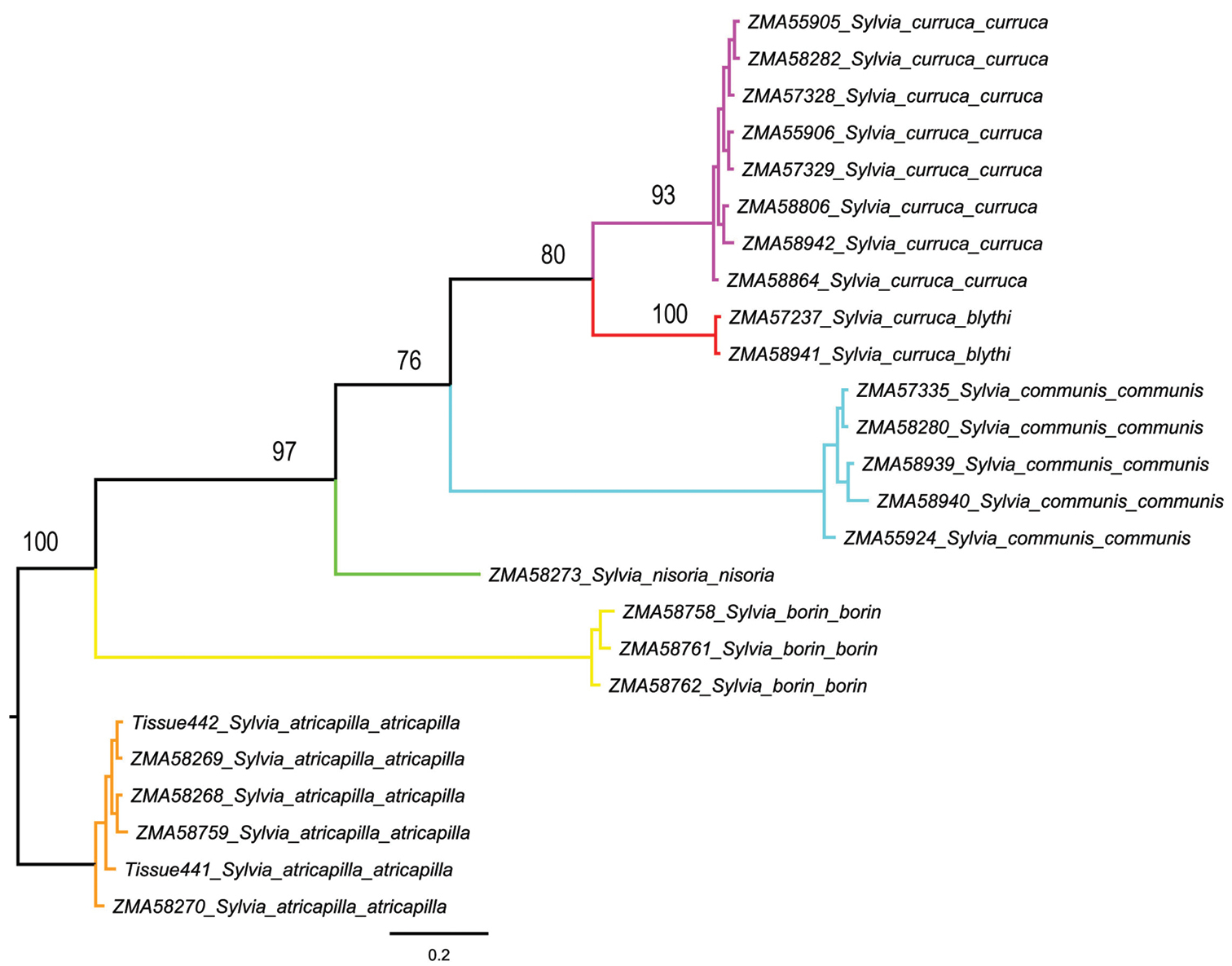
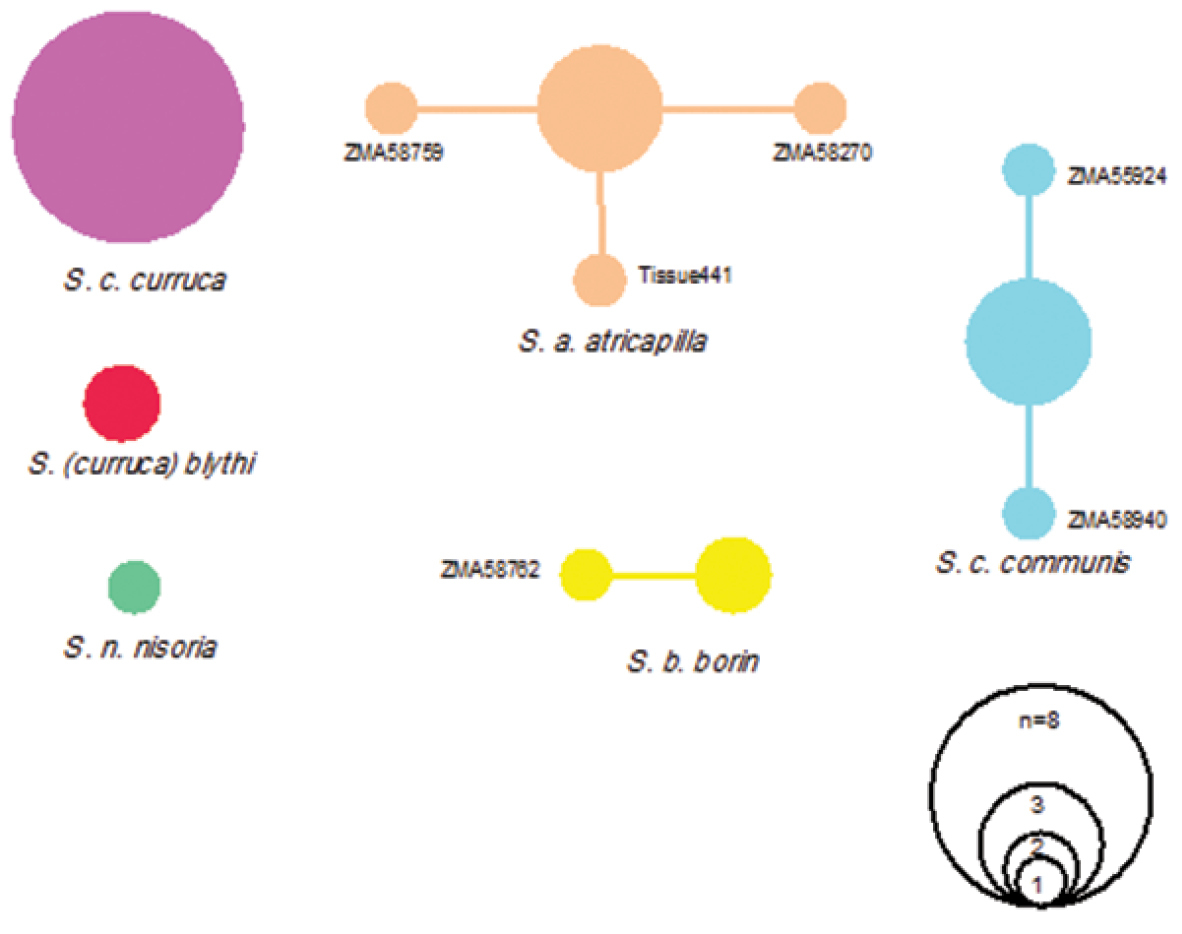
Although most species possessed low intraspecific distances, one species showed high intraspecific K2P-distances clearly above the threshold of 2 to 3 per cent sequence divergence in our data set. This is the Lesser Whitethroat Sylvia curruca, with a mean interspecific divergence of 5.76% and a maximum interspecific distance of 8.68%. Two subspecies occur in the Netherlands, i.e. the Western Lesser Whitethroat Sylvia curruca curruca and, as a migrant, the Northeastern Lesser Whitethroat Sylvia curruca blythi. Both are morphologically somewhat distinct, with compared to the nominate Sylvia curruca blythi having a paler top of the head, separated from face by a white supercilium, and geographically the nominate occupies the western part of the species range and Sylvia curruca blythi the eastern part. A maximum likelihood tree for these two taxa based on K2P-model is presented in Figure 2. Two different haplotype networks, one each for Sylvia curruca curruca and Sylvia curruca blythi were recovered by TCS (Figure 3), and given the large genetic distances between their haplotypes, the two taxa are not included in the same haplotype network.
Phylogenetic relationships of two putative subspecies of Lesser Whitethroat, i.e. the Western Lesser Whitethroat Sylvia curruca curruca and the Northeastern Lesser Whitethroat Sylvia curruca blythi from the Netherlands, based on analysis of 694 bp of the mitochondrial cytochrome c oxidase subunit I gene (COI). Bootstrap values are given for the maximum likelihood (ML) analysis.
Haplotype networks constructed with statistical parsimony based on 694 bp of the mitochondrial cytochrome c oxidase subunit I gene (COI) of the Sylvia group (25 individuals). Each circle represents one haplotype; size of circles is proportional to haplotype frequency.
We here present the results of a modest effort to barcode the avifauna of the Netherlands. In terms of DNA barcoding of birds, the Netherlands form the southernmost part of one of the most densely sampled regions globally (
Recently,
The mean intraspecific divergences found in the birds of the Netherlands (0.29%, based on 147 species) is congruent with that of for instance Argentina (0.24%, 500 species), North America (0.23%, 643 species) and the Holarctic (0.24%, 566 species) (
Most DNA barcoding studies of birds flag a small number of deep divergences (e.g.
Those cases where we found species sharing the same DNA barcodes were small in number but not insignificant. Seven of the eight cases involved closely related gulls with partially overlapping ranges, or allopatric distributions, that are part of a recent Holarctic radiation (
We conclude that DNA barcoding approach makes it possible to identify known Dutch bird species with a very high resolution. Although some species were flagged for further detailed taxonomic investigation, our study reaffirms once more that a short segment of COI gene can be used to handle large number of taxa and aid in detecting overlooked taxa and hybridizing species with low deep barcode divergences.
We thank Tineke G Prins, involved in the sampling and administering of the bird specimens over the years in the Zoological Museum Amsterdam, for her commitment and hard work, and Miguel Vences, formerly of the Zoological Museum Amsterdam and currently at the Technical University Braunschweig, as the initiator of this project. Hans Breeuwer, Betsy Voetdijk, Peter Kuperus, and Lin Dong are thanked for their support and advice in the molecular laboratory of the Evolutionary Biology Department, University of Amsterdam. Finally, we thank the editors of this special issue for their patience, guidance and support, and two sets of reviewers for constructive comments: combined their efforts greatly improved the quality and clarity of the work. Our molecular work is funded in part by the Fonds Economische Structuurversterking. We dedicate this paper to the memory of Jan Wattel, former curator of birds at the Zoological Museum Amsterdam, who passed away in March 2013.
List of all Dutch birds that have been sequenced in this study, with voucher numbers and collection localities. Note that specimens from which only tissue samples have been taken have not been given a collection number, sine loco refers to specimens collected in the Netherlands but without a precise named collection locality. Localities in the province of Friesland are listed with their Dutch name first, followed by their Frisian name. Coordinates are given in decimal degrees.
| Species or subspecies | ZMA number | Preparation | Locality | Coordinates | Access numbers |
|---|---|---|---|---|---|
| Accipiter gentilis gentilis | ZMA58297 | skin | Zaandam | 52.25N, 4.49E | KF946551 |
| Accipiter gentilis gentilis | ZMA58724 | skin | De Rips | 51.32N, 5.48E | KF946552 |
| Accipiter nisus nisus | ZMA58243 | skin | Malden | 51.47N, 5.52E | KF946553 |
| Accipiter nisus nisus | ZMA58245 | skin | Helden | 51.21N, 5.55E | KF946554 |
| Accipiter nisus nisus | ZMA58246 | skin | Reuver | 51.17N, 6.04E | KF946555 |
| Accipiter nisus nisus | ZMA58247 | skin | Culemborg | 51.55N, 5.15E | KF946556 |
| Accipiter nisus nisus | ZMA58248 | skin | Amsterdam | 52.21N, 4.53E | KF946557 |
| Accipiter nisus nisus | ZMA58741 | skin | Amsterdam | 52.21N, 4.53E | KF946558 |
| Accipiter nisus nisus | ZMA58742 | skin | Montfort | 51.07N, 5.56E | KF946559 |
| Accipiter nisus nisus | ZMA58743 | skin | Belfeld | 51.18N, 6.08E | KF946560 |
| Accipiter nisus nisus | ZMA58744 | skin | Laren | 52.11N, 6.22E | KF946561 |
| Accipiter nisus nisus | ZMA58745 | skin | Almere | 52.22N, 5.13E | KF946562 |
| Accipiter nisus nisus | ZMA58746 | skin | Venlo | 51.21N, 6.11E | KF946563 |
| Acrocephalus palustris | ZMA56679 | skin | Harderbroek reserve | 52.22N, 5.35E | KF946564 |
| Acrocephalus palustris | ZMA58811 | skin | Castricum | 52.32N, 4.36E | KF946565 |
| Acrocephalus schoenobaenus | ZMA58278 | skin | Almere | 52.22N, 5.13E | KF946566 |
| Acrocephalus schoenobaenus | ZMA58809 | skin | Almere | 52.22N, 5.13E | KF946567 |
| Acrocephalus schoenobaenus | ZMA58810 | skin | Castricum | 52.32N, 4.36E | KF946568 |
| Acrocephalus schoenobaenus | ZMA58862 | skin | Wassenaar | 53.08N, 5.53E | KF946569 |
| Acrocephalus scirpaceus scirpaceus | ZMA58277 | skin | Oostvaardersdijk | 52.29N, 5.23E | KF946570 |
| Acrocephalus scirpaceus scirpaceus | ZMA58725 | skin | Schermerhorn | 52.36N, 4.54E | KF946571 |
| Acrocephalus scirpaceus scirpaceus | ZMA58727 | skin | Lelystad | 52.29N, 5.24E | KF946572 |
| Acrocephalus scirpaceus scirpaceus | ZMA58728 | skin | Lelystad | 52.29N, 5.24E | KF946573 |
| Acrocephalus scirpaceus scirpaceus | ZMA58729 | skin | Castricum | 52.32N, 4.36E | KF946574 |
| Acrocephalus scirpaceus scirpaceus | ZMA58863 | skin | Lauwersmeer | 53.22N, 6.14E | KF946575 |
| Acrocephalus scirpaceus scirpaceus | ZMA58937 | skin | Lelystad | 52.29N, 5.24E | KF946576 |
| Acrocephalus scirpaceus scirpaceus | ZMA58938 | skin | Purmerend | 52.28N, 4.58E | KF946577 |
| Aegithalos caudatus europaeus | ZMA57353 | skin | Westenschouwen | 51.41N, 3.42E | KF946578 |
| Aegithalos caudatus europaeus | ZMA57354 | skin | Westenschouwen | 51.41N, 3.42E | KF946579 |
| Aegithalos caudatus europaeus | ZMA57356 | skin | Hilversum | 52.13N, 5.09E | KF946580 |
| Aegithalos caudatus europaeus | ZMA58804 | skin | Castricum | 52.32N, 4.36E | KF946581 |
| Alcedo atthis ispida | ZMA56216 | skin | Haelen | 51.13N, 5.56E | KF946582 |
| Alcedo atthis ispida | ZMA57341 | skin | Purmerland | 52.28N, 4.55E | KF946583 |
| Alcedo atthis ispida | ZMA57342 | skin | Alkmaar | 52.38N, 4.44E | KF946584 |
| Alcedo atthis ispida | ZMA57343 | skin | Utrecht | 52.03N, 5.08E | KF946585 |
| Alcedo atthis ispida | ZMA58869 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946586 |
| Alle alle alle | ZMA58842 | skin | Amsterdam | 52.21N, 4.53E | KF946587 |
| Alle alle alle | ZMA58917 | skin | Amsterdam | 52.21N, 4.53E | KF946588 |
| Alle alle alle | ZMA58918 | skin | Den Helder | 52.55N, 4.46E | KF946589 |
| Anas acuta | ZMA58228 | skin | Vlieland Island | 53.15N, 4.59E | KF946590 |
| Anas strepera strepera | ZMA58913 | skin | Driebond Polder | 53.11N, 6.37E | KF946591 |
| Anthus spinoletta spinoletta | ZMA58279 | skin | Lelystad | 52.29N, 5.24E | KF946592 |
| Anthus spinoletta spinoletta | ZMA64552 | skin | Castricum | 52.32N, 4.36E | KF946593 |
| Anthus trivialis trivialis | Tissue553 | DNA sample | Castricum | 52.32N, 4.36E | KF946594 |
| Apus apus apus | ZMA58717 | skin | Tegelen | 51.19N, 6.09E | KF946595 |
| Ardea cinerea cinerea | Tissue434 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946596 |
| Ardea cinerea cinerea | Tissue435 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946597 |
| Asio flammeus flammeus | ZMA58253 | skin | Texel Island | 53.04N, 4.43E | KF946598 |
| Asio otus otus | Tissue455 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946599 |
| Asio otus otus | ZMA58233 | skin | Purmerend | 52.28N, 4.58E | KF946600 |
| Asio otus otus | ZMA58234 | skin | Zutphen | 52.07N, 6.12E | KF946601 |
| Athene noctua vidalii | ZMA58493 | skin | Heerhugowaard | 52.4N, 4.51E | KF946602 |
| Athene noctua vidalii | ZMA58294 | skin | Blerick | 51.21N, 6.08E | KF946603 |
| Bombycilla garrulus garrulus | ZMA56300 | skin | Amsterdam | 52.21N, 4.53E | KF946604 |
| Bombycilla garrulus garrulus | ZMA56301 | wings | Texel Island | 53.04N, 4.43E | KF946605 |
| Bombycilla garrulus garrulus | ZMA58301 | wings | Hellendoorn | 52.23N, 6.26E | KF946606 |
| Bombycilla japonica | ZMA58302 | skin | Amsterdam | 52.21N, 4.53E | KF946607 |
| Buteo buteo buteo | Tissue461 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946608 |
| Buteo buteo buteo | ZMA58238 | skin | Wieringermeer | 52.54N, 5.01E | KF946609 |
| Buteo buteo buteo | ZMA58239 | skin | De Rips | 51.32N, 5.48E | KF946610 |
| Buteo buteo buteo | ZMA58781 | wing | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946611 |
| Buteo buteo buteo | ZMA58828 | skin | Wartena | 52.12N, 4.3E | KF946612 |
| Buteo buteo buteo | ZMA58920 | wings | Rolde | 52.58N, 6.38E | KF946613 |
| Calidris alpina alpina | ZMA58700 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946614 |
| Calonectris diomedea borealis | ZMA57255 | skin | Lith | 51.47N, 5.26E | KF946615 |
| Carduelis cannabina cannabina | ZMA58911 | skin | Noordijk | 52.08N, 6.34E | KF946616 |
| Carduelis carduelis | ZMA58866 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946617 |
| Carduelis chloris chloris | ZMA57337 | skin | Cadier en Keer | 50.49N, 5.46E | KF946618 |
| Carduelis chloris chloris | ZMA58947 | skin | Goor | 52.14N, 6.34E | KF946619 |
| Carduelis flammea cabaret | ZMA57248 | skin | Kennemerduinen | 52.42N, 4.58E | KF946620 |
| Carduelis flammea cabaret | ZMA58283 | skin | Westenschouwen | 51.41N, 3.42E | KF946621 |
| Carduelis flammea flammea | ZMA57251 | skin | Kennemerduinen | 52.42N, 4.58E | KF946622 |
| Carduelis flammea flammea | ZMA64564 | skin | Castricum | 52.32N, 4.36E | KF946623 |
| Carduelis flavirostris | ZMA57253 | skin | Castricum | 52.32N, 4.36E | KF946624 |
| Carduelis flavirostris | ZMA57254 | skin | Castricum | 52.32N, 4.36E | KF946625 |
| Carduelis spinus | ZMA55904 | skin | Nijverdal | 52.22N, 6.28E | KF946626 |
| Carduelis spinus | ZMA57256 | skin | Westenschouwen | 51.41N, 3.42E | KF946627 |
| Carduelis spinus | ZMA58286 | skin | Hellendoorn | 52.23N, 6.26E | KF946628 |
| Certhia brachydactyla megarhyncha | ZMA57322 | skin | Hellendoorn | 52.23N, 6.26E | KF946629 |
| Certhia brachydactyla megarhyncha | ZMA57323 | skin | Lekkerkerk | 51.53N, 4.41E | KF946630 |
| Certhia brachydactyla megarhyncha | ZMA57325 | skin | Wageningen | 51.58N, 5.38E | KF946631 |
| Certhia brachydactyla megarhyncha | ZMA57326 | skin | Zeist | 52.05N, 5.16E | KF946632 |
| Certhia brachydactyla megarhyncha | ZMA57327 | skin | Heiloo | 52.36N, 4.44E | KF946633 |
| Certhia brachydactyla megarhyncha | ZMA58805 | skin | Castricum | 52.32N, 4.36E | KF946634 |
| Certhia brachydactyla megarhyncha | ZMA58949 | skin | Lekkerkerk | 51.53N, 4.41E | KF946635 |
| Certhia brachydactyla megarhyncha | ZMA64563 | skin | Castricum | 52.32N, 4.36E | KF946636 |
| Charadrius hiaticula | Tissue452 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946637 |
| Circus aeruginosus aeruginosus | ZMA58780 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946638 |
| Circus aeruginosus aeruginosus | ZMA58826 | skin | Eibergen | 52.06N, 6.37E | KF946639 |
| Circus aeruginosus aeruginosus | ZMA58874 | wings | Zuid-Flevoland | 52.26N, 5.16E | KF946640 |
| Coccothraustes coccothraustes | ZMA56212 | skin | Laag Keppel | 51.59N, 6.13E | KF946641 |
| Corvus corax corax | ZMA57144 | skin | Appelscha/Appelskea | 52.55N, 5.2E | KF946642 |
| Coturnix coturnix coturnix | ZMA58775 | skin | Deventer | 52.15N, 6.11E | KF946643 |
| Coturnix coturnix coturnix | ZMA58776 | skin | Het Bildt | 53.17N, 5.4E | KF946644 |
| Cuculus canorus canorus | ZMA56681 | skin | Bergen | 52.4N, 4.41E | KF946645 |
| Cuculus canorus canorus | ZMA64549 | skin | Alkmaar | 52.38N, 4.44E | KF946646 |
| Delichon urbicum | ZMA56215 | skin | Sea | KF946647 | |
| Delichon urbicum urbicum | ZMA55919 | skin | Nieuwegein | 52.01N, 5.05E | KF946648 |
| Delichon urbicum urbicum | ZMA58300 | wings | Lage Zwaluwe | 51.42N, 4.42E | KF946649 |
| Delichon urbicum urbicum | ZMA58870 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946650 |
| Dendrocopos major pinetorum | ZMA58803 | skin | Oudkerk/Aldtsjerk | 53.15N, 5.53E | KF946651 |
| Dryocopus martius martius | ZMA58766 | skin | Tegelen | 51.19N, 6.09E | KF946652 |
| Emberiza citrinella citrinella | ZMA57257 | skin | Westenschouwen | 51.41N, 3.42E | KF946653 |
| Emberiza melanocephala | ZMA56996 | skin | Bovenkerk | 52.17N, 4.49E | KF946654 |
| Emberiza pusilla | ZMA58859 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946655 |
| Emberiza pusilla | ZMA58860 | skin | Vlieland Island | 53.15N, 4.59E | KF946656 |
| Emberiza schoeniclus schoeniclus | ZMA58857 | skin | Noordpolderzijl | 53.25N, 6.34E | KF946657 |
| Emberiza schoeniclus schoeniclus | ZMA58858 | skin | Oostvaardersdijk | 52.29N, 5.23E | KF946658 |
| Erithacus rubecula rubecula | Tissue436 | DNA sample | Castricum | 52.32N, 4.36E | KF946659 |
| Erithacus rubecula rubecula | Tissue437 | DNA sample | Castricum | 52.32N, 4.36E | KF946660 |
| Erithacus rubecula rubecula | ZMA58274 | skin | Bloemendaal | 52.24N, 4.33E | KF946661 |
| Erithacus rubecula rubecula | ZMA58740 | skin | Doldersum | 52.52N, 6.17E | KF946662 |
| Falco columbarius aesalon | ZMA58840 | skin | Texel Island | 53.04N, 4.43E | KF946663 |
| Falco columbarius aesalon | ZMA60127 | skin | Spaarndam | 52.24N, 4.41E | KF946664 |
| Falco peregrinus peregrinus | ZMA58872 | skin | Haarlem | 52.23N, 4.37E | KF946665 |
| Falco subbuteo subbuteo | ZMA56231 | skin | Zundert | 51.28N, 4.38E | KF946666 |
| Falco subbuteo subbuteo | ZMA56232 | skin | Heerhugowaard | 52.4N, 4.51E | KF946667 |
| Falco subbuteo subbuteo | ZMA58241 | skin | Hoogland | 52.1N, 5.21E | KF946668 |
| Falco subbuteo subbuteo | ZMA58242 | skin | Texel Island | 53.04N, 4.43E | KF946669 |
| Falco subbuteo subbuteo | ZMA58841 | skin | Amsterdam | 52.21N, 4.53E | KF946670 |
| Falco tinnunculus tinnunculus | Tissue456 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946671 |
| Falco tinnunculus tinnunculus | ZMA58296 | skin | Zaandam | 52.25N, 4.49E | KF946672 |
| Falco tinnunculus tinnunculus | ZMA58752 | skin | Maasbree | 51.21N, 6.03E | KF946673 |
| Falco tinnunculus tinnunculus | ZMA58754 | skin | Boekend | 51.22N, 6.06E | KF946674 |
| Falco tinnunculus tinnunculus | ZMA58774 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946675 |
| Falco tinnunculus tinnunculus | ZMA58837 | skin | Westzaan | 52.26N, 4.46E | KF946676 |
| Falco tinnunculus tinnunculus | ZMA58838 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946677 |
| Falco tinnunculus tinnunculus | ZMA58839 | wings | Reutum | 52.23N, 6.5E | KF946678 |
| Falco vespertinus | ZMA58773 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946679 |
| Ficedula hypoleuca muscipeta | ZMA55913 | skin | Otterlo | 52.04N, 5.5E | KF946680 |
| Ficedula hypoleuca muscipeta | ZMA57239 | skin | Markelo | 52.14N, 6.3E | KF946681 |
| Ficedula hypoleuca muscipeta | ZMA57320 | skin | Garderen | 52.12N, 5.43E | KF946682 |
| Ficedula hypoleuca | ZMA58865 | skin | Eemshaven | 53.26N, 6.52E | KF946683 |
| Fratercula arctica grabae | ZMA56226 | skin | Texel Island | 53.04N, 4.43E | KF946684 |
| Fratercula arctica grabae | ZMA58226 | skin | Texel Island | 53.04N, 4.43E | KF946685 |
| Fratercula arctica grabae | ZMA58227 | skin | Hondsbossche Zeewering | 52.44N, 4.38E | KF946686 |
| Fringilla coelebs coelebs | ZMA58948 | skin | Goor | 52.14N, 6.34E | KF946687 |
| Fringilla montifringilla | Tissue449 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946688 |
| Fulmarus glacialis auduboni | ZMA56235 | wings | Hondsbossche Zeewering | 52.44N, 4.38E | KF946689 |
| Fulmarus glacialis glacialis | ZMA60119 | skin | Neeltje Jans | 51.37N, 3.41E | KF946690 |
| Fulmarus glacialis glacialis | ZMA60120 | skin | Texel Island | 53.04N, 4.43E | KF946691 |
| Fulmarus glacialis glacialis | ZMA60121 | skin | Hondsbossche Zeewering | 52.44N, 4.38E | KF946692 |
| Fulmarus glacialis glacialis | ZMA60123 | skin | Ameland Island | 53.27N, 5.39E | KF946693 |
| Fulmarus glacialis glacialis | ZMA60124 | skin | Ameland Island | 53.27N, 5.39E | KF946694 |
| Fulmarus glacialis glacialis | ZMA60125 | skin | Hondsbossche Zeewering | 52.44N, 4.38E | KF946695 |
| Fulmarus glacialis glacialis | ZMA60126 | skin | Petten | 52.46N, 4.38E | KF946696 |
| Fulmarus glacialis | ZMA58737 | skin | Vlieland Island | 53.15N, 4.59E | KF946697 |
| Gallinula chloropus chloropus | Tissue105 | DNA sample | Wijde Wormer | 52.28N, 4.53E | KF946698 |
| Gallinula chloropus chloropus | Tissue110 | DNA sample | Wijde Wormer | 52.28N, 4.53E | KF946699 |
| Garrulus glandarius glandarius | ZMA58306 | wings | Amsterdam | 52.21N, 4.53E | KF946700 |
| Gavia immer | Tissue214 | DNA sample | Bergen aan Zee | 52.39N, 4.37E | KF946701 |
| Haematopus ostralegus ostralegus | Tissue458 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946702 |
| Haematopus ostralegus ostralegus | Tissue459 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946703 |
| Hirundo rustica rustica | Tissue450 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946704 |
| Hirundo rustica rustica | Tissue451 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946705 |
| Hirundo rustica rustica | ZMA56214 | skin | Amstelveen | 52.18N, 4.53E | KF946706 |
| Hirundo rustica rustica | ZMA58289 | skin | Appelscha/Appelskea | 52.55N, 5.2E | KF946707 |
| Hirundo rustica rustica | ZMA58290 | skin | Appelscha/Appelskea | 52.55N, 5.2E | KF946708 |
| Hirundo rustica rustica | ZMA58696 | skin | Rijswijk | 51.57N, 5.21E | KF946709 |
| Hirundo rustica rustica | ZMA58802 | skin | Noordbergum/Noardburgum | 53.13N, 6E | KF946710 |
| Jynx torquilla torquilla | ZMA56213 | skin | Aarle-Rixtel | 51.3N, 5.39E | KF946711 |
| Jynx torquilla torquilla | ZMA57330 | skin | Limmen | 52.34N, 4.41E | KF946712 |
| Jynx torquilla torquilla | ZMA58303 | wings | Belfeld | 51.18N, 6.08E | KF946713 |
| Jynx torquilla torquilla | ZMA58873 | skin | Wilnis | 52.11N, 4.54E | KF946714 |
| Larus argentatus argenteus | ZMA58921 | wings | Eemshaven | 53.26N, 6.52E | KF946715 |
| Larus argentatus | Tissue433 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946716 |
| Larus cachinnans | ZMA64547 | skin | Vlieland Island | 53.15N, 4.59E | KF946717 |
| Larus fuscus graelsii | Tissue432 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946718 |
| Larus fuscus intermedius | Tissue327 | DNA-sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946719 |
| Larus fuscus intermedius | ZMA55932 | skin | Neeltje Jans | 51.37N, 3.41E | KF946720 |
| Larus fuscus intermedius | ZMA56230 | skin | Europoort | 51.56N, 4.05E | KF946721 |
| Larus fuscus intermedius | ZMA58834 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946722 |
| Larus glaucoides glaucoides | ZMA58836 | wings | Texel Island | 53.04N, 4.43E | KF946723 |
| Larus hyperboreus | ZMA56221 | skin | Texel Island | 53.04N, 4.43E | KF946724 |
| Larus melanocephalus | ZMA57226 | skin | Wijdenes | 52.37N, 5.1E | KF946725 |
| Larus michahellis michahellis | ZMA58835 | skin | Afsluitdijk | 52.57N, 5.04E | KF946726 |
| Limosa lapponica lapponica | ZMA58202 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946727 |
| Limosa lapponica lapponica | ZMA58203 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946728 |
| Limosa lapponica taymyrensis | ZMA58204 | skin | Paesens | 53.24N, 6.06E | KF946729 |
| Limosa lapponica taymyrensis | ZMA58205 | skin | Paesens | 53.24N, 6.06E | KF946730 |
| Limosa lapponica taymyrensis | ZMA58206 | skin | Paesens | 53.24N, 6.06E | KF946731 |
| Limosa lapponica taymyrensis | ZMA58207 | skin | Paesens | 53.24N, 6.06E | KF946732 |
| Limosa lapponica taymyrensis | ZMA58208 | skin | Paesens | 53.24N, 6.06E | KF946733 |
| Limosa lapponica taymyrensis | ZMA58782 | wings | Castricum | 52.32N, 4.36E | KF946734 |
| Limosa lapponica taymyrensis | ZMA58783 | wings | Castricum | 52.32N, 4.36E | KF946735 |
| Limosa limosa limosa | Tissue457 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946736 |
| Limosa limosa limosa | ZMA57227 | skin | Holysloot | 52.24N, 5.01E | KF946737 |
| Limosa limosa limosa | ZMA58229 | skin | Waterland | 52.07N, 4.19E | KF946738 |
| Limosa limosa limosa | ZMA58230 | skin | Edam | 52.32N, 5.01E | KF946739 |
| Limosa limosa limosa | ZMA58231 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946740 |
| Limosa limosa limosa | ZMA58232 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946741 |
| Locustella luscinioides luscinioides | ZMA64557 | skin | Castricum | 52.32N, 4.36E | KF946742 |
| Locustella naevia naevia | ZMA56675 | skin | Almere | 52.22N, 5.13E | KF946743 |
| Locustella naevia naevia | ZMA56678 | skin | Almere | 52.22N, 5.13E | KF946744 |
| Locustella naevia naevia | ZMA57235 | skin | Westenschouwen | 51.41N, 3.42E | KF946745 |
| Locustella naevia naevia | ZMA58812 | skin | Castricum | 52.32N, 4.36E | KF946746 |
| Locustella naevia naevia | ZMA58936 | skin | Hondsbossche Zeewering | 52.44N, 4.38E | KF946747 |
| Locustella naevia naevia | ZMA60132 | skin | Kennemerduinen | 52.42N, 4.58E | KF946748 |
| Locustella naevia naevia | ZMA60133 | skin | Kennemerduinen | 52.42N, 4.58E | KF946749 |
| Locustella naevia naevia | ZMA64556 | skin | Castricum | 52.32N, 4.36E | KF946750 |
| Loxia curvirostra curvirostra | ZMA57246 | skin | Eesveen | 52.5N, 6.06E | KF946751 |
| Loxia curvirostra curvirostra | ZMA57247 | skin | Leersum | 52.01N, 5.25E | KF946752 |
| Luscinia megarhynchos megarhynchos | ZMA58798 | skin | Amsterdam | 52.21N, 4.53E | KF946753 |
| Lymnocryptes minimus | ZMA55930 | skin | Heerhugowaard | 52.4N, 4.51E | KF946754 |
| Lymnocryptes minimus | ZMA58293 | skin | Uitgeest | 52.31N, 4.42E | KF946755 |
| Milvus milvus milvus | ZMA58307 | wings | Grolloo | 52.55N, 6.39E | KF946756 |
| Milvus milvus milvus | ZMA58824 | wings | Susteren | 51.03N, 5.52E | KF946757 |
| Milvus milvus milvus | ZMA58825 | skin | Heurne | 51.54N, 6.34E | KF946758 |
| Motacilla alba yarrellii | ZMA58946 | skin | Haastrecht | 51.59N, 4.46E | KF946759 |
| Motacilla cinerea cinerea | ZMA57241 | skin | Westenschouwen | 51.41N, 3.42E | KF946760 |
| Motacilla cinerea cinerea | ZMA58266 | skin | Westenschouwen | 51.41N, 3.42E | KF946761 |
| Motacilla cinerea cinerea | ZMA58267 | skin | Westenschouwen | 51.41N, 3.42E | KF946762 |
| Motacilla cinerea cinerea | ZMA58945 | skin | Westenschouwen | 51.41N, 3.42E | KF946763 |
| Muscicapa striata striata | ZMA57336 | skin | Ilpendam | 52.27N, 4.56E | KF946764 |
| Numenius arquata arquata | Tissue431 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946765 |
| Numenius arquata arquata | ZMA58765 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946766 |
| Numenius arquata arquata | ZMA58829 | skin | Heemskerk | 52.3N, 4.36E | KF946767 |
| Oenanthe oenanthe leucorhoa | ZMA58868 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946768 |
| Oenanthe oenanthe oenanthe | ZMA58275 | skin | Hondsbossche Zeewering | 52.44N, 4.38E | KF946769 |
| Oenanthe oenanthe oenanthe | ZMA58800 | skin | Noordbergum/Noardburgum | 53.13N, 6E | KF946770 |
| Oriolus oriolus oriolus | ZMA58288 | skin | Heteren | 51.57N, 5.45E | KF946771 |
| Oriolus oriolus oriolus | ZMA58305 | wings | Zundert | 51.28N, 4.38E | KF946772 |
| Pandion haliaetus haliaetus | ZMA58823 | wing | Vlieland Island | 53.15N, 4.59E | KF946773 |
| Panurus biarmicus biarmicus | ZMA57318 | skin | Oostvaardersdijk | 52.29N, 5.23E | KF946774 |
| Panurus biarmicus biarmicus | ZMA58262 | skin | Lelystad | 52.29N, 5.24E | KF946775 |
| Panurus biarmicus biarmicus | ZMA58263 | skin | Lelystad | 52.29N, 5.24E | KF946776 |
| Panurus biarmicus biarmicus | ZMA58264 | skin | Lelystad | 52.29N, 5.24E | KF946777 |
| Panurus biarmicus biarmicus | ZMA58265 | skin | Lelystad | 52.29N, 5.24E | KF946778 |
| Panurus biarmicus biarmicus | ZMA58854 | skin | Oostvaardersdijk | 52.29N, 5.23E | KF946779 |
| Panurus biarmicus biarmicus | ZMA58855 | skin | Oostvaardersdijk | 52.29N, 5.23E | KF946780 |
| Panurus biarmicus biarmicus | ZMA58856 | skin | Oostvaardersdijk | 52.29N, 5.23E | KF946781 |
| Parus ater ater | Tissue555 | DNA sample | Castricum | 52.32N, 4.36E | KF946782 |
| Parus ater ater | ZMA56219 | skin | Huizen | 52.17N, 5.14E | KF946783 |
| Parus ater ater | ZMA57242 | skin | Arnhem | 51.58N, 5.53E | KF946784 |
| Parus ater ater | ZMA57243 | skin | Amsterdam | 52.21N, 4.53E | KF946785 |
| Parus ater ater | ZMA58867 | skin | Amsterdam | 52.21N, 4.53E | KF946786 |
| Parus ater ater | ZMA64562 | skin | Castricum | 52.32N, 4.36E | KF946787 |
| Parus caeruleus caeruleus | Tissue438 | DNA sample | Castricum | 52.32N, 4.36E | KF946788 |
| Parus caeruleus caeruleus | Tissue439 | DNA sample | Castricum | 52.32N, 4.36E | KF946789 |
| Parus caeruleus caeruleus | Tissue440 | DNA sample | Castricum | 52.32N, 4.36E | KF946790 |
| Parus caeruleus caeruleus | ZMA58944 | wing | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946791 |
| Parus cristatus mitratus | ZMA56677 | skin | Nijverdal | 52.22N, 6.28E | KF946792 |
| Parus cristatus mitratus | ZMA57245 | skin | Hoog Buurlo | 52.1N, 5.5E | KF946793 |
| Parus major major | ZMA58796 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946794 |
| Parus major major | ZMA58797 | skin | Castricum | 52.32N, 4.36E | KF946795 |
| Parus palustris palustris | ZMA57244 | skin | Castricum | 52.32N, 4.36E | KF946796 |
| Parus palustris palustris | ZMA64561 | skin | Goor | 52.14N, 6.34E | KF946797 |
| Passer domesticus domesticus | ZMA58799 | skin | Cadier en Keer | 50.49N, 5.46E | KF946798 |
| Passer domesticus domesticus | ZMA60138 | skin | Lekkerkerk | 51.53N, 4.41E | KF946799 |
| Passer montanus montanus | ZMA58851 | skin | Zuidhorn | 53.14N, 6.23E | KF946800 |
| Passer montanus montanus | ZMA58852 | skin | Zuidhorn | 53.14N, 6.23E | KF946801 |
| Passer montanus montanus | ZMA58853 | skin | Zuidhorn | 53.14N, 6.23E | KF946802 |
| Passer montanus montanus | ZMA58950 | skin | Zuidhorn | 53.14N, 6.23E | KF946803 |
| Perdix perdix perdix | ZMA58738 | skin | Texel Island | 53.04N, 4.43E | KF946804 |
| Perdix perdix perdix | ZMA58739 | skin | Petten | 52.46N, 4.38E | KF946805 |
| Pernis apivorus | ZMA58827 | wings | Vledder | 52.53N, 6.13E | KF946806 |
| Phalacrocorax aristotelis aristotelis | ZMA58224 | skin | Wijk aan Zee | 52.28N, 4.34E | KF946807 |
| Philomachus pugnax | ZMA56680 | skin | Graftermeer polder | 52.33N, 4.48E | KF946808 |
| Philomachus pugnax | ZMA58250 | skin | Lelystad | 52.29N, 5.24E | KF946809 |
| Phoenicopterus chilensis | ZMA56683 | skin | Ransdorp | 52.23N, 4.59E | KF946810 |
| Phoenicurus phoenicurus phoenicurus | ZMA55914 | skin | Westenschouwen | 51.41N, 3.42E | KF946811 |
| Phylloscopus collybita collybita | ZMA55917 | skin | Nijverdal | 52.22N, 6.28E | KF946812 |
| Phylloscopus collybita collybita | ZMA55918 | wings | Leveroy | 51.14N, 5.5E | KF946813 |
| Phylloscopus collybita collybita | ZMA56217 | skin | Hoogland | 52.1N, 5.21E | KF946814 |
| Phylloscopus trochilus | ZMA58284 | skin | Lelystad | 52.29N, 5.24E | KF946815 |
| Phylloscopus trochilus | ZMA58710 | skin | Almere | 52.22N, 5.13E | KF946816 |
| Phylloscopus trochilus | ZMA58713 | skin | Egmond aan Zee | 52.37N, 4.38E | KF946817 |
| Phylloscopus trochilus | ZMA58714 | skin | Lekkerkerk | 51.53N, 4.41E | KF946818 |
| Phylloscopus trochilus | ZMA58715 | skin | Texel Island | 53.04N, 4.43E | KF946819 |
| Phylloscopus trochilus | ZMA58716 | skin | Castricum | 52.32N, 4.36E | KF946820 |
| Phylloscopus trochilus | ZMA58861 | skin | Castricum | 52.32N, 4.36E | KF946821 |
| Phylloscopus trochilus | ZMA58933 | wings | Goor | 52.14N, 6.34E | KF946822 |
| Phylloscopus trochilus | ZMA58934 | skin | Eemshaven | 53.26N, 6.52E | KF946823 |
| Picus viridis viridis | ZMA58718 | skin | Breda | 51.33N, 4.46E | KF946824 |
| Picus viridis viridis | ZMA58719 | skin | Haaksbergen | 52.08N, 6.4E | KF946825 |
| Picus viridis viridis | ZMA58720 | skin | Alkmaar | 52.38N, 4.44E | KF946826 |
| Picus viridis viridis | ZMA58721 | skin | Roggel | 51.17N, 5.54E | KF946827 |
| Picus viridis viridis | ZMA58722 | skin | Bergen | 52.4N, 4.41E | KF946828 |
| Plectrophenax nivalis insulae | ZMA56672 | skin | Castricum | 52.32N, 4.36E | KF946829 |
| Pluvialis apricaria | ZMA58213 | skin | Winsum | 53.09N, 5.38E | KF946830 |
| Pluvialis apricaria | ZMA58214 | skin | Winsum | 53.09N, 5.38E | KF946831 |
| Pluvialis apricaria | ZMA58215 | skin | Dronrijp/Dronryp | 53.11N, 5.4E | KF946832 |
| Pluvialis squatarola squatarola | ZMA56224 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946833 |
| Pluvialis squatarola squatarola | ZMA56225 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946834 |
| Puffinus gravis | ZMA64542 | skin | Sexbierum/Seisbierrum | 53.14N, 5.28E | KF946835 |
| Pyrrhula pyrrhula europoea | ZMA56673 | skin | Castricum | 52.32N, 4.36E | KF946836 |
| Pyrrhula pyrrhula europoea | ZMA58793 | skin | Castricum | 52.32N, 4.36E | KF946837 |
| Pyrrhula pyrrhula europoea | ZMA58794 | skin | Castricum | 52.32N, 4.36E | KF946838 |
| Pyrrhula pyrrhula europoea | ZMA58795 | skin | Castricum | 52.32N, 4.36E | KF946839 |
| Pyrrhula pyrrhula europoea | ZMA60137 | wings | Kennemerduinen | 52.42N, 4.58E | KF946840 |
| Rallus aquaticus aquaticus | ZMA58763 | skin | Lauwersmeer | 53.22N, 6.14E | KF946841 |
| Recurvirostra avosetta | ZMA58216 | skin | Petten | 52.46N, 4.38E | KF946842 |
| Regulus ignicapilla ignicapilla | Tissue448 | DNA sample | Castricum | 52.32N, 4.36E | KF946843 |
| Regulus ignicapilla ignicapilla | ZMA57360 | skin | Zundert | 51.28N, 4.38E | KF946844 |
| Regulus ignicapilla ignicapilla | ZMA58807 | skin | Castricum | 52.32N, 4.36E | KF946845 |
| Regulus ignicapilla ignicapilla | ZMA58808 | skin | Castricum | 52.32N, 4.36E | KF946846 |
| Regulus regulus regulus | ZMA64560 | skin | Castricum | 52.32N, 4.36E | KF946847 |
| Riparia riparia riparia | ZMA58871 | skin | Zeewolde | 52.21N, 5.34E | KF946848 |
| Saxicola rubetra | ZMA60131 | skin | Kennemerduinen | 52.42N, 4.58E | KF946849 |
| Saxicola rubetra | ZMA64555 | skin | Castricum | 52.32N, 4.36E | KF946850 |
| Somateria mollissima mollissima | ZMA58912 | skin | Lauwersoog | 53.24N, 6.12E | KF946851 |
| Stercorarius longicaudus | ZMA58779 | wings | Afsluitdijk | 52.57N, 5.04E | KF946852 |
| Stercorarius longicaudus | ZMA64546 | skin | Petten | 52.46N, 4.38E | KF946853 |
| Stercorarius parasiticus | ZMA56229 | skin | Vlieland Island | 53.15N, 4.59E | KF946854 |
| Stercorarius parasiticus | ZMA56684 | wings | Terschelling Island | 53.26N, 5.29E | KF946855 |
| Stercorarius parasiticus | ZMA58778 | skin | Den Oever | 52.56N, 5.02E | KF946856 |
| Stercorarius parasiticus | ZMA58830 | skin | Den Helder | 52.55N, 4.46E | KF946857 |
| Stercorarius pomarinus | Tissue211 | DNA sample | Texel Island | 53.04N, 4.43E | KF946858 |
| Stercorarius pomarinus | ZMA55929 | skin | Hondsbossche Zeewering | 52.44N, 4.38E | KF946859 |
| Stercorarius skua skua | ZMA64545 | skin | Egmond aan Zee | 52.37N, 4.38E | KF946860 |
| Sterna albifrons albifrons | ZMA58832 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946861 |
| Sterna hirundo hirundo | ZMA58915 | skin | Eemshaven | 53.26N, 6.52E | KF946862 |
| Sterna paradisaea | ZMA58831 | skin | Amsterdam | 52.21N, 4.53E | KF946863 |
| Streptopelia decaocto decaocto | ZMA58923 | wing | Hoogkerk | 53.12N, 6.3E | KF946864 |
| Streptopelia turtur turtur | ZMA58757 | skin | Texel Island | 53.04N, 4.43E | KF946865 |
| Sylvia atricapilla atricapilla | Tissue441 | DNA sample | Castricum | 52.32N, 4.36E | KF946866 |
| Sylvia atricapilla atricapilla | Tissue442 | DNA sample | Castricum | 52.32N, 4.36E | KF946867 |
| Sylvia atricapilla atricapilla | ZMA58268 | skin | Bloemendaal | 52.24N, 4.33E | KF946868 |
| Sylvia atricapilla atricapilla | ZMA58269 | skin | Bloemendaal | 52.24N, 4.33E | KF946869 |
| Sylvia atricapilla atricapilla | ZMA58270 | skin | Bloemendaal | 52.24N, 4.33E | KF946870 |
| Sylvia atricapilla atricapilla | ZMA58759 | skin | Cadier en Keer | 50.49N, 5.46E | KF946871 |
| Sylvia borin borin | Tissue443 | DNA sample | Castricum | 52.32N, 4.36E | KF946872 |
| Sylvia borin borin | ZMA58758 | skin | Groningen | 53.14N, 6.35E | KF946873 |
| Sylvia borin borin | ZMA58761 | skin | Almere | 52.22N, 5.13E | KF946874 |
| Sylvia borin borin | ZMA58762 | skin | Purmerend | 52.28N, 4.58E | KF946875 |
| Sylvia communis communis | ZMA55924 | wing | Asten | 51.21N, 5.48E | KF946876 |
| Sylvia communis communis | ZMA57335 | skin | Almere | 52.22N, 5.13E | KF946877 |
| Sylvia communis communis | ZMA58280 | skin | Breda | 51.33N, 4.46E | KF946878 |
| Sylvia communis communis | ZMA58939 | skin | Castricum | 52.32N, 4.36E | KF946879 |
| Sylvia communis communis | ZMA58940 | skin | Bloemendaal | 52.24N, 4.33E | KF946880 |
| Sylvia curruca blythi | ZMA58941 | skin | Houten | 52.01N, 5.1E | KF946881 |
| Sylvia curruca blythi | ZMA57237 | skin | Rotterdam | 51.57N, 4.32E | KF946882 |
| Sylvia curruca curruca | ZMA55905 | skin | Westenschouwen | 51.41N, 3.42E | KF946883 |
| Sylvia curruca curruca | ZMA55906 | skin | Amsterdam | 52.21N, 4.53E | KF946884 |
| Sylvia curruca curruca | ZMA57328 | skin | Almere | 52.22N, 5.13E | KF946885 |
| Sylvia curruca curruca | ZMA57329 | skin | Texel Island | 53.04N, 4.43E | KF946886 |
| Sylvia curruca curruca | ZMA58282 | skin | Zeewolde | 52.21N, 5.34E | KF946887 |
| Sylvia curruca curruca | ZMA58806 | skin | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946888 |
| Sylvia curruca curruca | ZMA58864 | skin | Eemshaven | 53.26N, 6.52E | KF946889 |
| Sylvia curruca curruca | ZMA58942 | skin | Bloemendaal | 52.24N, 4.33E | KF946890 |
| Sylvia nisoria nisoria | ZMA58273 | skin | Westenschouwen | 51.41N, 3.42E | KF946891 |
| Tringa ochropus | ZMA64544 | skin | Castricum | 52.32N, 4.36E | KF946892 |
| Tringa totanus totanus | ZMA58212 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946893 |
| Troglodytes troglodytes troglodytes | Tissue447 | DNA sample | Castricum | 52.32N, 4.36E | KF946894 |
| Troglodytes troglodytes troglodytes | ZMA58281 | skin | Bloemendaal | 52.24N, 4.33E | KF946895 |
| Turdus iliacus iliacus | ZMA58287 | skin | Bloemendaal | 52.24N, 4.33E | KF946896 |
| Turdus merula merula | ZMA56669 | skin | Haarlem | 52.23N, 4.37E | KF946897 |
| Turdus merula merula | ZMA56670 | skin | Bergen | 52.4N, 4.41E | KF946898 |
| Turdus merula merula | ZMA57345 | skin | Zwolle | 52.3N, 6.06E | KF946899 |
| Turdus merula merula | ZMA58731 | skin | Alkmaar | 52.38N, 4.44E | KF946900 |
| Turdus merula merula | ZMA58732 | skin | Maasbree | 51.21N, 6.03E | KF946901 |
| Turdus merula merula | ZMA58733 | skin | Maasbree | 51.21N, 6.03E | KF946902 |
| Turdus merula merula | ZMA58734 | skin | Steijl | 51.2N, 6.07E | KF946903 |
| Turdus merula merula | ZMA58736 | skin | Schiermonnikoog Island | 53.29N, 6.11E | KF946904 |
| Turdus philomelos philomelos | Tissue453 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946905 |
| Turdus philomelos philomelos | Tissue454 | DNA sample | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946906 |
| Turdus torquatus torquatus | ZMA56222 | skin | Texel Island | 53.04N, 4.43E | KF946907 |
| Turdus torquatus torquatus | ZMA56671 | skin | Castricum | 52.32N, 4.36E | KF946908 |
| Turdus torquatus torquatus | ZMA58693 | skin | Apeldoorn | 52.1N, 5.58E | KF946909 |
| Turdus torquatus torquatus | ZMA58694 | skin | Vlieland Island | 53.15N, 4.59E | KF946910 |
| Turdus torquatus torquatus | ZMA58695 | skin | Zuilichem | 51.48N, 5.07E | KF946911 |
| Turdus torquatus torquatus | ZMA64554 | skin | Texel Island | 53.04N, 4.43E | KF946912 |
| Turdus viscivorus viscivorus | ZMA60130 | skin | Kennemerduinen | 52.42N, 4.58E | KF946913 |
| Tyto alba alba | ZMA56233 | skin | Burgerbrug | 52.45N, 4.42E | KF946914 |
| Tyto alba guttata | ZMA56682 | skin | Wierden | 52.22N, 6.34E | KF946915 |
| Tyto alba guttata | ZMA58235 | skin | Texel Island | 53.04N, 4.43E | KF946916 |
| Tyto alba guttata | ZMA58236 | skin | Ouderkerk aan de Amstel | 52.17N, 4.56E | KF946917 |
| Tyto alba guttata | ZMA58843 | skin | Westzaan | 52.26N, 4.46E | KF946918 |
| Tyto alba guttata | ZMA58844 | skin | Zaanstreek | 52.28N, 4.44E | KF946919 |
| Tyto alba guttata | ZMA58845 | skin | Roodkerk/Readtsjerk | 53.15N, 5.55E | KF946920 |
| Tyto alba guttata | ZMA58846 | skin | Garijp/Garyp | 53.1N, 5.57E | KF946921 |
| Tyto alba guttata | ZMA58847 | skin | Middenmeer | 52.48N, 4.59E | KF946922 |
| Tyto alba guttata | ZMA58848 | wings | Leeuwarden/Ljouwert | 53.13N, 5.45E | KF946923 |
| Tyto alba guttata | ZMA58919 | skin | Texel Island | 53.04N, 4.43E | KF946924 |
| Tyto alba guttata | ZMA64550 | skin | Purmerend | 52.28N, 4.58E | KF946925 |
| Tyto alba guttata | ZMA64551 | skin | Goor | 52.14N, 6.34E | KF946926 |
| Uria aalge albionis | ZMA56227 | skin | Amsterdam | 52.21N, 4.53E | KF946927 |
| Uria aalge albionis | ZMA58218 | skin | Vlieland Island | 53.15N, 4.59E | KF946928 |
| Uria aalge albionis | ZMA58916 | skin | Petten | 52.46N, 4.38E | KF946929 |
| Vanellus vanellus | ZMA58784 | wing | Valkenburg | 52.09N, 4.25E | KF946930 |
| Vanellus vanellus | ZMA58785 | wing | Valkenburg | 52.09N, 4.25E | KF946931 |
| Vanellus vanellus | ZMA58786 | wing | Valkenburg | 52.09N, 4.25E | KF946932 |
| Vanellus vanellus | ZMA58787 | wing | Valkenburg | 52.09N, 4.25E | KF946933 |
| Vanellus vanellus | ZMA58788 | wing | Valkenburg | 52.09N, 4.25E | KF946934 |
| Vanellus vanellus | ZMA58789 | wing | Valkenburg | 52.09N, 4.25E | KF946935 |
| Vanellus vanellus | ZMA58790 | wing | Valkenburg | 52.09N, 4.25E | KF946936 |
| Vanellus vanellus | ZMA58791 | wing | Valkenburg | 52.09N, 4.25E | KF946937 |
Bird species (gulls Larus and skuas Stercorarius) from the Netherlands with low (< 1.1%) K2P-mean intraspecific distances.
| Collection number and species | Collection number and species | Distance (%) | ||
|---|---|---|---|---|
| #ZMA58835 | Larus michahellis | #Tissue327 | Larus fuscus | 0 |
| #ZMA58835 | Larus michahellis | #Tissue432 | Larus fuscus | 0 |
| #ZMA58835 | Larus michahellis | #ZMA55932 | Larus fuscus | 0 |
| #ZMA58835 | Larus michahellis | #ZMA56230 | Larus fuscus | 0 |
| #ZMA64547 | Larus cachinnans | #Tissue327 | Larus fuscus | 0 |
| #ZMA64547 | Larus cachinnans | #Tissue432 | Larus fuscus | 0 |
| #ZMA64547 | Larus cachinnans | #ZMA55932 | Larus fuscus | 0 |
| #ZMA64547 | Larus cachinnans | #ZMA56230 | Larus fuscus | 0 |
| #ZMA64547 | Larus cachinnans | #ZMA58835 | Larus michahellis | 0 |
| #ZMA58921 | Larus argentatus | #ZMA55932 | Larus fuscus | 0.14 |
| #ZMA58921 | Larus argentatus | #ZMA58835 | Larus michahellis | 0.14 |
| #ZMA58921 | Larus argentatus | #Tissue432 | Larus fuscus | 0.15 |
| #ZMA58921 | Larus argentatus | #ZMA56230 | Larus fuscus | 0.15 |
| #ZMA64547 | Larus cachinnans | #ZMA58834 | Larus fuscus | 0.15 |
| #ZMA64547 | Larus cachinnans | #ZMA58921 | Larus argentatus | 0.15 |
| #ZMA58921 | Larus argentatus | #Tissue327 | Larus fuscus | 0.16 |
| #ZMA55932 | Larus fuscus | #Tissue433 | Larus argentatus | 0.29 |
| #ZMA58835 | Larus michahellis | #Tissue433 | Larus argentatus | 0.29 |
| #Tissue433 | Larus argentatus | #Tissue432 | Larus fuscus | 0.30 |
| #ZMA56230 | Larus fusca | #Tissue433 | Larus argentatus | 0.30 |
| #ZMA64545 | Stercorarius skua | #ZMA55929 | Stercorarius pomarinus | 0.30 |
| #ZMA58836 | Larus glaucoides | #Tissue432 | Larus fuscus | 0.31 |
| #ZMA58836 | Larus glaucoides | #ZMA55932 | Larus fuscus | 0.31 |
| #ZMA58836 | Larus glaucoides | #ZMA56230 | Larus fuscus | 0.31 |
| #ZMA58836 | Larus glaucoides | #ZMA58835 | Larus michahellis | 0.31 |
| #ZMA64547 | Larus cachinnans | #Tissue433 | Larus argentatus | 0.31 |
| #ZMA64547 | Larus cachinnans | #ZMA58836 | Larus glaucoides | 0.31 |
| #Tissue433 | Larus argentatus | #Tissue327 | Larus fuscus | 0.32 |
| #ZMA58836 | Larus glaucoides | #Tissue327 | Larus fuscus | 0.32 |
| #ZMA64545 | Stercorarius skua | #Tissue211 | Stercorarius pomarinus | 0.43 |
| #ZMA58835 | Larus michahellis | #ZMA58834 | Larus fuscus | 0.45 |
| #ZMA58836 | Larus glaucoides | #ZMA58834 | Larus fuscus | 0.46 |
| #ZMA58921 | Larus argentatus | #ZMA58836 | Larus glaucoides | 0.46 |
| #ZMA56221 | Larus hyperboreus | #ZMA55932 | Larus fuscus | 0.58 |
| #ZMA58835 | Larus michahellis | #ZMA56221 | Larus hyperboreus | 0.58 |
| #ZMA56221 | Larus hyperboreus | #Tissue432 | Larus fuscus | 0.60 |
| #ZMA56230 | Larus fuscus | #ZMA56221 | Larus hyperboreus | 0.60 |
| #ZMA58921 | Larus argentatus | #ZMA58834 | Larus fuscus | 0.60 |
| #ZMA64547 | Larus cachinnans | #ZMA56221 | Larus hyperboreus | 0.61 |
| #ZMA58836 | Larus glaucoides | #Tissue433 | Larus argentatus | 0.62 |
| #ZMA56221 | Larus hyperboreus | #Tissue327 | Larus fuscus | 0.64 |
| #ZMA58921 | Larus argentatus | #ZMA56221 | Larus hyperboreus | 0.73 |
| #ZMA58834 | Larus fuscus | #Tissue433 | Larus argentatus | 0.75 |
| #ZMA56221 | Larus hyperboreus | #Tissue433 | Larus argentatus | 0.87 |
| #ZMA58836 | Larus glaucoides | #ZMA56221 | Larus hyperboreus | 0.93 |
| #ZMA58834 | Larus fuscus | #ZMA56221 | Larus hyperboreus | 1.06 |