(C) 2013 Jing-Fu Tsai. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The soapberry bug, Jadera haematoloma (Herrich-Schäffer, 1847) (Insecta: Hemiptera: Heteroptera: Rhopalidae: Serinethinae), a species native in tropical and subtropical regions of the New World and accidentally introduced to Hawaii, is reported for the first time from Asia (Taiwan). This record represents the first occurrence of the species in Asia. Stable populations composed of hundreds of specimens were found in seven localities of Kaohsiung City and one locality in Tainan City, and a single specimen was observed in Chiayi County. Aggregating adults and larvae fed in large numbers on the sapindacean plants Cardiospermum halicacabum L. and Koelreuteria elegans (Seem.) A. C. Smith ssp. formosana (Hayata) F. G. Meyer. Diagnostic characters of adults and larvae of Jadera haematoloma are discussed. A review of its bionomics and a bibliography are provided. Initial observations on the populations in southern Taiwan are presented. The species is potentially invasive, and further extension of its range is anticipated in Southeast Asia.
Hemiptera, Jadera haematoloma, alien species, invasion, rapid evolution, Sapindaceae, Asia
Soapberry bugs (Hemiptera: Heteroptera: Rhopalidae: Serinethinae) are seed predators feeding exclusively on members of the soapberry family (Sapindaceae). The subfamily contains three genera: Leptocoris Hahn, 1833 (more than 40 species) is found throughout the tropical and subtropical regions of the Old World (
The best-known species of Jadera is Jadera haematoloma (Herrich-Schäffer, 1847), commonly called the soapberry bug or the red shouldered bug. It is widely distributed in tropical and subtropical regions of North, Central and northern South America (
A single individual of Jadera haematoloma was found in Dagangshan Scenic Area, Alian District, Kaohsiung City, southern Taiwan on 31 August 2012 by Y.X. Hsieh and J.X. Fang. Subsequent targeted search in the region resulted in discovery of populations at seven localities. These represent the first occurrences of this species and the genus Jadera in Asia. We provide the first records of Jadera haematoloma with data on its distribution, population and host plants in Taiwan, present the diagnostic characters allowing its recognition, document the immature stages, and provide a bibliography and a review of the bionomics, ecology, and distribution of this species.
Jadera haematoloma (Herrich-Schäffer, 1847)
Leptocoris haematoloma Herrich-Schäffer, 1847: 103. Syntype (s): Mexico; lost? (Göllner-Scheiding 1975: 57).
Lygaeus marginalis Walker, 1872: 45. Lectotypus (
Serinetha haematoloma: Dallas 1852: 463 (record),
Lygaeus (Serinetha) haematolomus:
Jadera haematoloma:
Pyrrhotes haematoloma:
Leptocoris haematoloma:
The genus Jadera can be recognized within Serinethinae by the long bucculae which approach base of head posteriorly; in the two other genera of the subfamily, Leptocoris and Boisea, they are short, at most extending to middle of ventral surface of head (
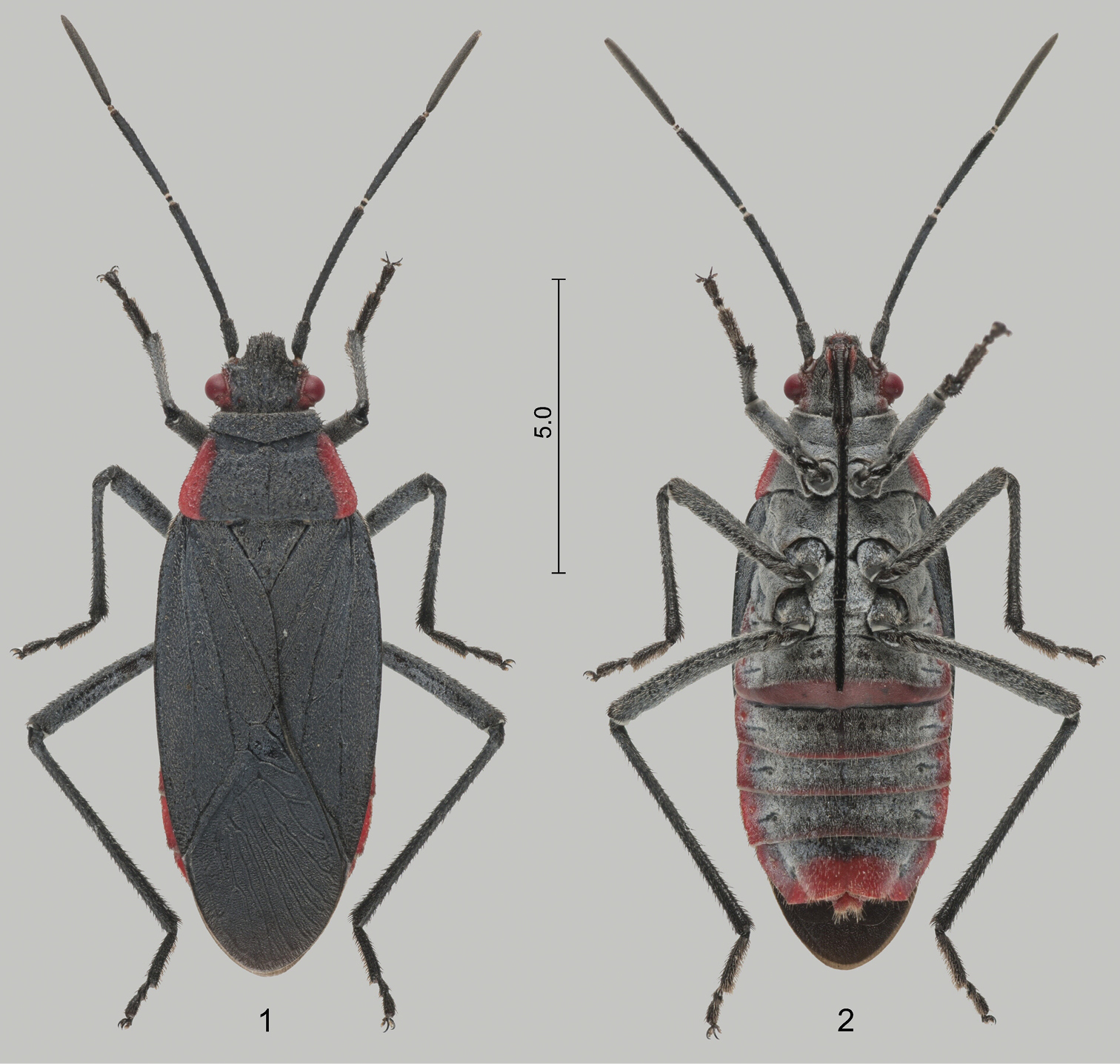
Jadera haematoloma is a medium-sized species within the genus (9.5–14.5 mm) readily recognized by its colour (Figs 1–5): dorsal ground colour black, head with a narrow red stripe along each eye, and pronotum broadly margined with bright red laterally; abdominal venter black, lateral margins, posterior margin of sternite VI and posterior third of sternite VII broadly red (occasionally more extensively red). Only two other species of the genus have a uniformly black dorsum with contrasting red lateral margins of the pronotum: Jadera pyrrholoma Stål, 1870 and Jadera diaphona Göllner-Scheiding, 1982. The South American Jadera pyrrholoma differs from Jadera haematoloma, among others, by its considerably greater size (14.0–18.5 mm) and its uniformly red abdomen. The Central American Jadera diaphona is similar to Jadera haematoloma, but it has a uniformly orange abdominal venter. Detailed morphological redescriptions of Jadera haematoloma and other congeners were provided by
Female of Jadera haematoloma 1 dorsal view 2 ventral view. Scale bar in mm.
Brachypterous males of Jadera haematoloma, with different wing shapes, dorsal views. Scale bar in mm.
Body size. Both males and females in regions of southcentral USA (Oklahoma) are significantly smaller than those in tropical areas (southern Florida) (
Colour pattern varies only slightly within a population. Caribbean specimens (Bahamas, Cuba) usually have broader vitta along the lateral margin of pronotum, red pattern is present on thoracic pleuron, and the apex of the clypeus also is red (
Wing polymorphism. Usually macropterous (Figs 1–2); approximately 20% of the population in the southern USA is brachypterous (
Frequency of wing morphs is under complex genetic and physiological regulation. Crossing experiments indicate a polygenic inheritance of wing morphs (
Length of labium. The labium is significantly longer in macropterous specimens (
Male diploid chromosome number is 13 (10A+2m+X0) (
Jadera haematoloma colonizes various habitats where host plants are available and can be found in city parks and other human-dominated environments (
As all other members of the subfamily Serinethinae, Jadera haematoloma is an oligophagous seed-predator that develops exclusively on plants of the soapberry family (Sapindaceae s. lato, including the former Hippocastanaceae and Aceraceae). All of its hosts belong to the subfamily Sapindoideae. In contrast to several other congeners, which are restricted to members of the tribe Paullinieae, Jadera haematoloma also feeds on plants of the subfamilies Sapindeae (Sapindus) and Koelreuteriae (Koelreuteria) (Table 1) (
Host plants of Jadera haematoloma at different localities and reports of aggregation behaviour or mass occurrence based on literature data.
| Host plant | Locality | Aggregation | References |
|---|---|---|---|
| Sapindus saponaria L. | Hawaii |
|
|
| Sapindus saponaria L. var. drummondii (Hook. & Arn.) L.D. Benson | Arizona | + |
|
| Kansas | – |
|
|
| Oklahoma | + |
|
|
| Sapinus oahuensis Hillebr. ex Radlk. | Hawaii | + |
|
| Sapindus mukorossi Gaertn. | USA |
|
|
| Koelreuteria paniculata Laxm. | Florida | + |
|
| Georgia |
|
||
| Missouri |
|
||
| New Mexico |
|
||
| Oklahoma | + |
|
|
| Koelreuteria elegans (Seem.) A.C. Smith | Florida | + |
|
| Koelreuteria elegans subsp. formosana (Hayata) F.G. Meyer | Hawaii | + |
|
| Koelreuteria bipinnata Franch. | USA |
|
|
| Koelreuteria sp. (unspecified) | North Carolina |
|
|
| Cardiospermum halicacabum L. | Texas |
|
|
| Mississippi |
|
||
| Louisiana |
|
||
| Hawaii | + |
|
|
| Bahamas |
|
||
| Cardiospermum corindum L. | Florida | + |
|
| Mexico |
|
||
| Cardiospermum grandiflorum Sw. | California |
|
|
| Hawaii |
|
||
| Serjania brachycarpa A.Gray ex Radlk. | Texas | + |
|
In the southwestern USA its primary native host plant is the western soapberry (Sapindus saponaria var. drummondii), but it also can be found in large numbers on the littlefruit slipplejack (Serjania brachycarpa). Within its native area it also successfully colonizes several sapindaceous trees introduced to that region, e.g. large aggregations are commonly found on the goldenrain tree (Koelreuteria paniculata) and the Chinese rain tree (Koelreuteria elegans), which are native to eastern Asia and introduced in the southern part of the United States (
In Florida the bug is common on the native balloon vine (Cardiospermum corindum) and also feeds on the introduced Sapindus mukorossii but avoids a native congener, Sapindus saponaria (
Jadera haematoloma occasionally has been reported from plants belonging to other families, e.g. from Ficus brevifolia Nutt. and unspecified species of Ficus (Moraceae) (
Under laboratory conditions, Jadera haematoloma cultures can be maintained for several generations on seeds of Koelreuteria paniculata and water; seeds of Cardiospermum corindum and Cardiospermum grandiflorum were also successfully used for such purposes (
Occasionally the bugs feed on various disabled or freshly dead arthropods (
It feeds exclusively on the mature and nearly mature seeds of host plants (
The bugs cannot access seeds of Sapindus oahuensis through the fleshy, hardened drupe; therefore, it feeds only on the pericarp (
In several cases length of the labium differs significantly between populations on native host plants and nearby populations on introduced host plants. In some populations the change in the average length of the labium can be nearly 25%. The increase or decrease in the length of the labium is consistent with the difference in fruit size and morphology of the native and introduced hosts (
Feeding and reproductive adults and larvae form prominent, mixed-instar aggregations on host plants, most commonly on the trunks and on fallen seeds (
The structure of aggregations formed by diapausing adults in the canopies of goldenrain trees (Koelreuteria sp.) in Florida was studied by
Data are available only from the USA (
In Oklahoma (where the population feeds on Koelreuteria paniculata and Sapindus saponaria var. drummondii with seeds ripening in late July–August and mid-August–September, respectively) reproduction is highly seasonal. Adults and larvae overwinter in dense clusters, mostly on the ground among leaf litter. They leave their refugia around February or March, and overwintered females generally oviposit in March; then the overwintering adults decline in May and June. Adults of the new generation start to emerge in late July; mating and oviposition continue until early October. In October, while food is still available, they enter diapause (
In Florida (where the population feeds on Cardiospermum corindum with most seeds ripening in May and in November–December) it breeds year round. Adults start to feed and reproduce in late April and May, with bugs (mainly adults of the new generation) entering a starvation diapause in early summer when the seed base is exhausted. A second reproductive period follows from November until January. From January, as food again becomes unavailable, they enter starvation diapause, spending the period in clusters, mostly on herbaceous plants (
Individuals are inactive but the moulting of larvae is continuous during diapause in both populations (
Mean adult development time does not different between sexes (
Data are available only from the USA. Adult sex ratio in populations in humid parts of the southcentral region (Oklahoma) is generally strongly male-biased (ranging from 1:1 to 5:1, average 2.73±0.95 males per female), while in populations in tropical areas (southern Florida), it is close to 1:1 (
Mating behaviour was studied in detail by
Average duration of copulations of virgin females is significantly shorter than those of the same females during subsequent copulations. Under laboratory conditions, duration of the copulation tends to be greater in groups where sex ratios are more male biased because of intense male–male competition (
Eggs generally are laid in a hole about 1 cm deep, which the female digs with its fore legs in dry soil close to the host tree. After completing oviposition, the female covers the eggs with soil using its fore legs (
Egg clutches typically are laid at 1- to 2-day intervals for 2–3 weeks; a clutch contains 1–20 eggs (14±4.1 in average) in Oklahoma (
If the male departs it remains sexually active, and often mounts the next available female encountered. Most females also copulate with several males (
In Oklahoma, females produce significantly more and smaller eggs than those from southern Florida (
The conspicuous aggregations of the red larvae are aposematic. Laboratory experiments with toads (various Bufo spp., Bufonidae) and blue jays (Cyanocitta cristata (Linnaeus, 1758), Corvidae), as well as field observations on Mantidae, showed that after having tasted larvae these predators avoided other larvae. Although adults also are distasteful, their effectiveness alone in causing avoidance is uncertain (
In U.S. populations there is little or no predation on the bugs (
Pinching the bugs causes them to discharge haemolymph from the rostrum and intersegmentally, and also to emit secretions from the scent glands (
The haemolymph of Jadera haematoloma sequesters glycosides. These are not truly cyanogenic; HCN is released from crushed individuals only if they were reared on Cardiospermum grandiflorum and if β-glucosidase is added (
Stridulation was recorded and documented by
Large populations around habitations may alarm people (
The distribution range of Jadera haematoloma is determined by the geography of its native and introduced host plant species (
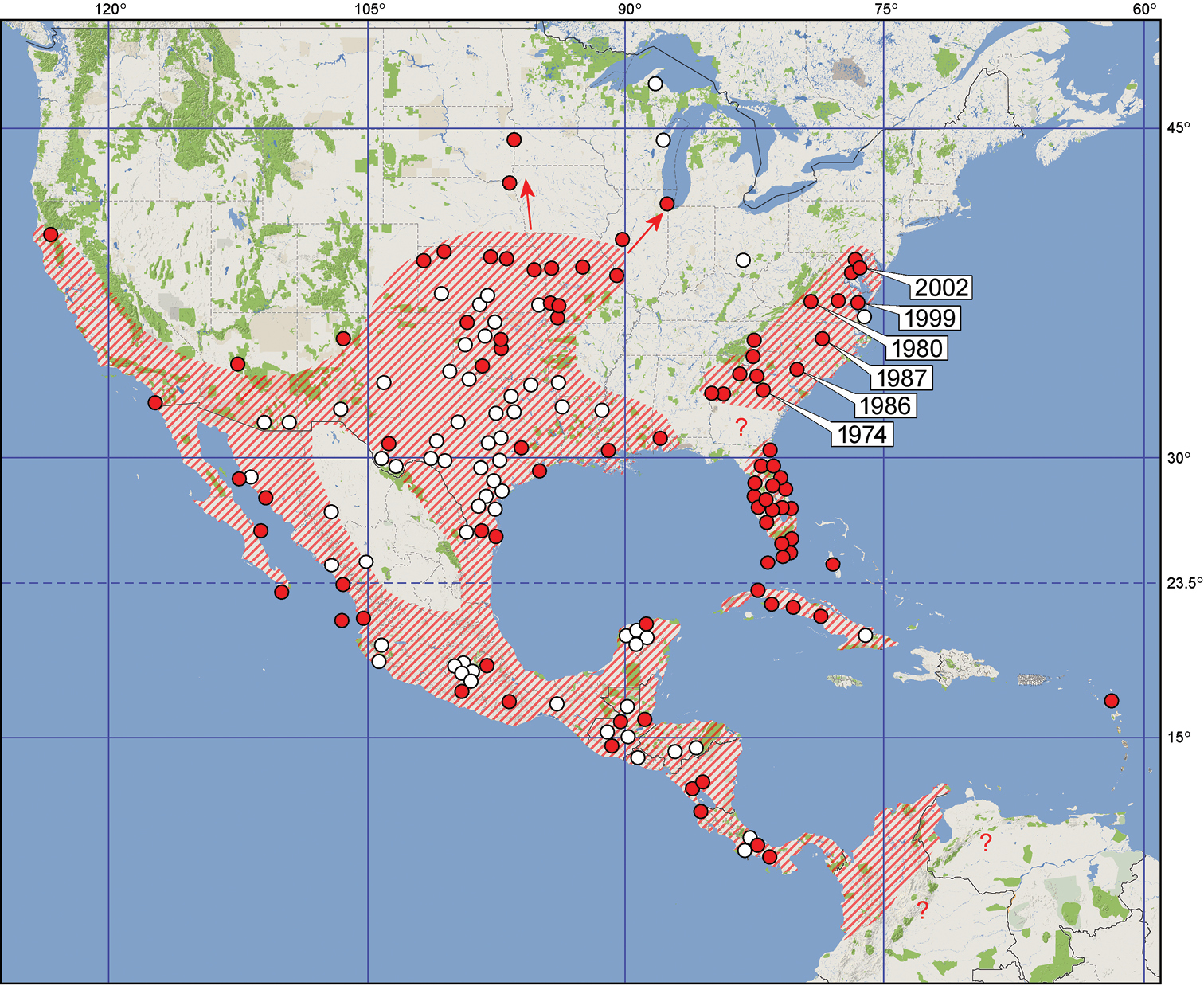
Distribution of Jadera haematoloma in the Americas. Red dots represent literature records, white dots represent localities mapped by
Although Jadera haematoloma is common in the peninsular part of Florida, it does not enter the ‘panhandle’; therefore, this population is apparently disjunct from that of the southcentral USA (cf.
Records from northern South America are scarce, but most likely its area is bordered in the south by the Northern Andes.
Records from the sub-Amazonian South America, e.g. southern Brazil (
Jadera haematoloma colonizes several islands of the Caribbean. The single record from Antigua is based on an incompletely coloured specimen, the record therefore is uncertain (
Because several sapindaceans are cultivated widely as ornamental trees, eventual introduction of Jadera haematoloma likely will result in the colonization of new areas.
USA. California: Coronado Is. (
Populations of Jadera haematoloma were observed at 7 sites in Kaohsiung City, southern Taiwan (J.F. Tsai, Y.X. Hsieh, November 2012–January 2013) and at one site in Tainan City (January 2013, U. Ong). Single individuals were recorded from two additional localities.
Specimens were examined using a SteREO Discovery.V20 microscope with a PlanApo S 1.0x FWD 60mm objective. Measurements of larvae were taken using a calibrated Leica stage micrometer (10310345); they were preserved by freezing in order to maintain their shape. Photographs were taken with Nikon D300 and Canon EOS 5D digital cameras equipped with AF-S Nikkor 60mm micro-lens and MPE-65 mm lens, respectively.
Measurements of populations of specimens were compared using non-parametric Wilcoxon–Mann–Whitney two-sample rank-sum test; all presented U and p values were obtained using this test.
Plant names are used following the online database of the International Plant Names Index (www.ipni.org, accessed December 2012).
Voucher specimens of Jadera haematoloma collected during the present study have been deposited in the following public collections: National Museum of Natural Science, Taichung, Taiwan; Taiwan Forestry Research Institution, Taipei, Taiwan; Department of Entomology, National Taiwan University, Taipei, Taiwan; Department of Entomology, National Chung Hsing University, Taichung, Taiwan; Taiwan Agricultural Research Institute, Taichung, Taiwan; Department of Plant Medicine, National Pingtung University of Science and Technology, Neipu, Taiwan; Department of Entomology, Nankai University, Tianjin, China; Hungarian Natural History Museum, Budapest, Hungary; Department of Entomology, National Museum, Prague, Czech Republic.
Single individuals of Jadera haematoloma were observed at the following localities:
Kaohsiung City: Alian District, Dagangshan Scenic Area, at Huashi Pavilion, on the way to Chaofeng Temple, 30.viii.2012, Y.X. Hsieh, J.X. Fang. A secondary forest with Bauhinia variegata L. (Fabaceae) as dominant, mixed with several cultivated plants, most importantly Broussonetia papyrifera (L.) Vent. (Moraceae), Mallotus japonicus Müll.Arg. and Bischofia javanica Blume (both Euphorbiaceae). The specimen was observed on Miscanthus sp. (Poaceae).
Chiayi County: Zhuchi Township, Shihjhuo (23°28'27"N, 120°42'03"E), 1300 m a.s.l., 5.xi.2012, S.F. Yang. Digital photo of a single specimen was taken and provided to us by S.F. Yang.
Populations of Jadera haematoloma were collected or observed at the following sites (Table 2):
Site 1. Kaohsiung City: Cishan, Ci-nan Third Road (22°49'23"N, 120°27'44.17"E), 30.xi.2012, Y.X. Hsieh, J.F. Tsai (Fig. 21). Around a lychee (Litchi chinensis Sonn., Sapindaceae) orchard. The orchard was bordered by a chain-link fence climbed by several plants, the dominant among them was the heartseed vine (Cardiospermum halicacabum), mixed with some Passiflora foetida L. (Passifloraceae), Mikania micrantha Kunth and Bidens pilosa L. var.radiata Sch.Bip. (both Asteraceae).The fallen leaves of the litchee trees were removed from under the trees and moved to the margin of the orchard under the fence. Several macropterous and brachypterous adults and larvae of all instars were observed to actively walk on and in the leaf litter and feed on Cardiospermum halicacabum.
Site 2. Kaohsiung City: Cishan, a residential area along Ci-ping First Road (22°52'56"N, 120°29'46"E), 2.xii.2012, Y.X. Hsieh. An empty yard with two patches of Cardiospermum halicacabum. Adults with first and fourth instar larvae were observed.
Site 3. Kaohsiung City: Ciaotou, corner of Gong-yuan Road and Ciao-chung Street (22°45'23"N, 120°18'30"E), 30.xi.2012, Y.X. Hsieh, J.F. Tsai. A vegetable garden bordered by a plastic mesh fence fixed to cemented pillars, climbed by Cardiospermum halicacabum only, with a layer of dead cucurbitacean leaves under the fence (the garden was apparently used for growing melon earlier). Adults and larvae (first to third instars) were observed mainly on the heartseed vine, only a few specimens in the leaf litter under the plant. Several mating pairs and brachypterous individuals were found.
Site 4. Kaoshiung City: Ciaotou, at the junction of Shu-he Road and Tong-shu Road (22°45'17"N, 120°18'16"E), 30.xi.2012, Y.X. Hsieh, J.F. Tsai. A fallow ground owned by Taiwan Sugar Corporation, with some herbs on the ground, among them Cardiospermum halicacabum. Several adults, including mating pairs were observed on 16.xi.2012 by Y.X. Hsieh, but the abundance of adults was very low two weeks later: only 7 adults were collected; however, about a hundred larvae were found.
Site 5. Kaohsiung City: Ciaotou (22°44'27"N, 120°19'24"E), 30.xi.2012, Y.X. Hsieh, J.F. Tsai. A flower farm of Taiwan Sugar Corporation; a public recreation farm with several cultivated vegetables, flowers and trees. Several adults and 2nd–4th instar larvae were collected in an old-growth patch of Koelreuteria elegans subsp. formosana with a thick layer of fallen leaves and seed pods under the trees. Several dozens of adults were collected by Y.H. Hsieh at the same locality on 3.xii.2012. One month later (14.i.2013, Y.X. Hsieh) hundreds of adults (clearly more males than females), including several mating pairs, and larvae of all instars forming aggregations near the base of the trunks were observed. Careful searching on all dates yielded no brachypterous individuals.
Site 6. Kaoshiung City: Nanzih (22°43'51"N, 120°20'08"E), 3.xii.2012, Y.X. Hsieh. A patch of Koelreuteria elegans subsp. formosana trees (with mature fruits in this season) planted along the street, opposite the building of Kaohsiung High Administrative Court. Adults were actively walking and feeding on the seeds on and among the fallen leaves and fruits under the tree.
Site 7. Kaohsiung City: Nanzih. Kaohsiung Metropolitan Park (22°43'57.08"N, 120°19'0.71"E), 10.xii.2012, Y.X. Hsieh. A patch of Koelreuteria elegans subsp. formosana trees (with mature fruits) close to the baseball field. Several mating pairs were observed on the trunk of the trees near the ground; all females were gravid. A careful search yielded no larvae or brachypterous adults.
Site 8. Tainan City: East District, near Sheng-chen Road (22°57'55.28"N, 120°13'23.33"E), 13.i.2013, U. Ong. A large fallow ground owned by Taiwan Sugar Corporation, with a large number of Cardiospermum halicacabum mixed with Bidens pilosa var. radiata. A large number of adults, including several mating pairs, and larvae were observed feeding on Cardiospermum halicacabum and nectar of Bidens pilosa. Brachypters were much more abundant than macropters.
Collected individuals of Jadera haematoloma in the investigated sites of Kaohsiung City and Tainan City (for description of the sites see text).
| male | female | host | |||
|---|---|---|---|---|---|
| brachypterous | macropterous | brachypterous | macropterous | ||
| Site 1 | Cardiospermum halicacabum | ||||
| 21.xi.2012 | 3 | 6 | |||
| 22.xi.2012 | 3 | 5 | |||
| 26.xi.2012 | 1 | 1 | 2 | ||
| 30.xi.2012 | 3 | 25 | 2 | 14 | |
| Site 3 | Cardiospermum halicacabum | ||||
| 22.xi.2012 | 4 | 3 | 1 | 2 | |
| 30.xi.2012 | 2 | 7 | 2 | 5 | |
| Site 4 | Cardiospermum halicacabum | ||||
| 16.xi.2012 | 4 | 6 | |||
| 30.xi.2012 | 3 | 1 | 1 | 2 | |
| Site 5 | Koelreuteria elegans subsp. formosana | ||||
| 30.xi.2012 | 9 | 2 | |||
| 3.xii.2012 | 39 | 17 | |||
| 14.i.2013 | 42 | 14 | |||
| Site 6 | Koelreuteria elegans subsp. formosana | ||||
| 3.xii.2012 | 3 | 1 | |||
| Site 7 | Koelreuteria elegans subsp. formosana | ||||
| 10.xii.2012 | 6 | 6 | |||
| Site 8 | Cardiospermum halicacabum | ||||
| 13. i.2013 | 20 | 5 | 12 | 3 | |
Colour. Only slight variation in the colour was observed. In males middle portion of abdominal sternites II–VI was usually black, but several specimens, especially females, had sternites III–VI more or less broadly margined with red posteriorly (Fig. 2) as reported by
Body measurements. Adults (n = 187) from various localities in Kaohsiung were measured (Table 3). Body length of males was significantly smaller than females in both the macropterous (U = 16.74, p < 0.001) and brachypterous (U = 6.45, p < 0.001) specimens. Width of pronotum of males was also significantly smaller than that of females in both the macropterous (U = 16.93, p < 0.001) and brachypterous (U = 6.52, p < 0.001) individuals. Humeral width of pronotum of macropterous specimens was significantly larger than that of brachypterous individuals in both males (U = 5.26, p < 0.001) and females (U = 4.30, p < 0.001).
Measurements (in mm) and relative length of the labium of macropterous and brachypterous adults.
| body length | width of pronotum |
relative length of labium |
|||
|---|---|---|---|---|---|
| range | average, SD | range | average, SD | ||
| males | |||||
| macropterous (N = 101) | 9.37–12.01 | 10.60±0.56 | 2.64–3.30 | 2.98±0.16 | II-P to IV-P |
| brachypterous (N = 8) | 8.58–9.10 | 8.71±0.29 | 2.51–2.90 | 2.74±0.14 | III-A to IV-A |
| females | |||||
| macropterous (N = 68) | 10.16–12.80 | 11.67±0.66 | 2.90–3.96 | 3.30±0.20 | III-M to IV-P |
| brachypterous (N = 7) | 9.24–10.82 | 9.67±0.60 | 2.90–3.56 | 3.19±0.23 | III-P |
1 The position of the apex of the labium in respect to the abdominal sternites is given; Roman numerals refer to the segmental homology; A = anterior half; P = posterior half.
2 II-P: 5, III-A: 43, III-P: 38, IV-A: 8, IV-P: 1. Five specimens excluded.
3 III-A: 6, III-P: 1, IV-A: 1.
4 III-M: 26, III-P: 15, IV-A: 16, IV- P: 3. Eight specimens excluded.
Adults collected on two different host plants (Cardiospermum halicacabum, Koelreuteria elegans subsp. formosana) at various sites in Kaohsiung were compared (Table 4). Males collected on Cardiospermum halicacabum were slightly smaller on average than those collected on Koelreuteria elegans subsp. formosana, but neither the difference in total length (U = 0.69, p = 0.488), nor length measured from apex of the clypeus to the apex of abdomen (U = 0.93, p = 0.353) was statistically significant. Females collected on Cardiospermum halicacabum were slightly larger on average than those collected on Koelreuteria elegans subsp. formosana (U = 0.93, p = 0.353) in respect to total length, but the relationship was opposite (U = 1.89, p = 0.059) when measuring from apex of the clypeus to the apex of abdomen; these differences are also not statistically significant. The fact that on one of the host plants the mean total lengths of specimens of one sex were greater than those of the opposite sex also suggests that there is no substantial difference in the body size of individuals from the two host plants. Measurements to apex of abdomen might reveal comparative differences in food level, hydration or reproductive condition of females. However, because it is very plastic, it is not as useful a measure for assessing developmental or genetic size differences among adults within or between populations.
Variation in the relative length of labium. The same specimens as in the previous paragraph were examined (Table 3). The apex of the labium in resting position attains at least the posterior margin of sternite II (♂) or the middle of abdominal sternite III (♀), and in extreme cases it approaches the posterior margin of abdominal sternite IV (♂, ♀). Both macropterous and brachypterous females had a relatively longer labium on average than the males. In both sexes macropterous individuals had a relatively longer labium on average than brachypterous individuals of the same sex. The relative length of the labium seems to be slightly longer in both males and females of the populations on Cardiospermum halicacabum than those on Koelreuteria elegans subsp. formosana (Table 4), but no conclusion can be drawn for our data and careful testing is needed based on absolute lengths.
Body size (in mm) and relative length of the labium in specimens of different sex collected from different host plants (all macropterous).
| Cardiospermum halicacabum | Koelreuteria elegans subsp. formosana | |||||
|---|---|---|---|---|---|---|
| body length (head–wing) | body length (head–abdomen) | labium | body length (head–wing) | body length (head–abdomen) |
labium |
|
| males | 10.56±0.58 (9.50–12.01) N = 47 |
8.93±0.34 (8.32–9.50) N = 49 |
II-P (N = 2) III-A (N = 14) III-P (N = 17) IV-A (N = 7) IV-P (N = 1) |
10.64±0.55 (9.37–11.88) N = 54 |
9.02±0.40 (8.05–9.90) N = 49 |
II-P (N = 3) III-A (N = 29) III-P (N = 21) IV-A (N = 1) |
| females | 11.72±0.70 (10.16–12.80) N = 43 |
9.88±0.63 (8.84–11.22) N = 39 |
III-A (N = 1) III-P (N = 18) IV-A (N = 14) IV-P (N = 1) |
11.59±0.59 (10.16–12.41) N = 25 |
10.19±0.53 (9.37–10.96) N = 19 |
III-A (N = 12) III-P (N = 10) IV-A (N = 2) IV-P (N = 1) |
1 The position of the apex of the labium in respect to the abdominal segments (sternites) is given; Roman numerals refer to the segmental homology; A = anterior half; P = posterior half.
Wing polymorphism. At most sites macropterous (Figs 1–2) and brachypterous (3–5) specimens were observed and collected too. Forty-four adults were counted at site 1 on 30.xi.2012; 5 (11.4%) were brachypterous. On one occasion (site 8, 13.i.2013) the vast majority (32 of 40, 80%) of the observed specimens were brachypterous (Table 2). No conspicuous difference was observed in the frequency of brachypterous individuals between the two sexes. Brachypterous specimens were observed only on Cardiospermum halicacabum and never on Koelreuteria elegans subsp. formosana (Table 2).
Slight variability was observed in the development of the fore wing of the brachypterous individuals. The apex of the wing can reach the anterior (Fig. 5) or posterior portion (Figs 3–4) of abdominal sternite VI; in some individuals the membrane is rather broad and subtriangular (Fig. 3), shorter and broadly rounded in others (Fig. 4), and in others is reduced to a narrow band (Fig. 5).
Larvae of Rhopalidae can readily be recognized using the family keys of
Colour. Body bright red (1st instar, Figs 7–8), or bright red with prothorax, pterothoracic tergum and pleuron reddish gray, pterothoracic sternum reddish (2nd–5th instars, Figs 9–20); antenna and legs pale (1st instar) to dark gray (2nd–5th instars), with more or less reddish shade, especially in younger instars, extremities of antennal segments usually more distinctly red at intersegmental articulations.
Integument and vestiture. Smooth, subshining, weakly sclerotized (1st instar) or dull, head, prothorax, pterothoracic tergum and pleuron more strongly sclerotized than abdomen, dorsal surface of head and thorax pruinose, ventral surface of head and prothorax together with thoracic pleuron and all coxae more strongly pruinose (2nd–5th instars); body sparsely covered with strong, stiff, almost bristle-like, pale (1st instar) or black (2nd–5th instar) setae.
Head and cephalic appendages. Head pentagonal; dorsal surface provided with several setae, with a series of discontinuously arranged setae along dorsal margin of eye, ventral surface without setae; vertex rounded and convex; V-shaped ecdysial suture distinct;clypeus simple, elevated above level of mandibular plates, pilose, with a tuft of setae at apex, apically broadened;mandibular plate thick, with a row of seate dorsally, laterally straight, not reaching apex of clypeus; antennifer situated in front of eye, visible in dorsal view, antenniferous tubercle distinct, with a tuft of setae;buccula undeveloped (1st–5th instars); eye rounded, prominent, distinctly separated from pronotum by a relatively long postocular margin provided with a single series of setae.Antenna with segments I–III subcylindrical, segment IV distinctly spindle-shaped in younger (1st–2nd) instars, gradually becoming subcylindrical in older (3rd–5th) instars.Labium and its individual segments of variable length; segment I slightly shorter than remaining segments, reaching or slightly surpassing posterior margin of eye but never reaching base of head (1st–5th instars); segment IV distinctly longer than remaining segments (1st–5th instars); labium of newly hatched larvae reaching apex of abdomen (Fig. 8), relative length of labium gradually becoming shorter from 1st to 5th instar, but individual variability great (Table 7).
Thorax and thoracic appendages.Prothorax: pronotum broader than long, more or less trapeziform, with distinct anterior collar (1st–5th instars), humeri rounded, not protruded; prothoracic acetabula open posteriorly; mesonotum rectangular (1st–2nd instars), slightly expanded laterally (3rd instar), with well-developed mesothoracic wing pads reaching posterior margin of abdominal tergite I to middle of tergite II (4th instar) or posterior margin of tergite II to posterior half of tergite IV (5th instar); scutellar pad distinct in 4th and 5th instars; mesosternum flat; metanotum simple (1st–3rd instars) or with well developed metathoracic wing pads (4th–5th instars); metasternum large, subhexagonal, plate-like. Legs simple, setose, distance between fore and mid legs greater than that of mid and hind legs; distance between fore coxae much smaller that of mid coxae, distance between hind coxae somewhat greater than that of mid coxae.
Abdomen composed of 11 visible segments (tergites I and II distinct, sternite I absent); venter distinctly more convex than dorsum. Dorsal abdominal scent glands with two single minute openings situated between tergites IV/V and V/VI, intersegmental suture between tergites IV and V nearly straight, that between tergites V and VI deeply curved anteriad along midline; therefore, tergite V short along midline and gland openings situated close to each other; spiracles II–VIII situated posterolaterally on the respective sternites; trichobothrial formula 0-0-0-3-3-2 (sternites II–VII) in all stages; trichobothria on sternites III and IV situated submedially (rarely 3+4 trichobothria present on sternite IV), trichobothria of sternites V–VII situated on anterior portion of respective sternites, arranged transversely; genital segment distinguishable in 5th instars of both sexes: posterior margin of sternite VIII with slight (4th instar) to deep (5th instar) incision along midline, sternite IX depressed in female, abdominal sternite IX undivided (4th–5th instars), much swollen (5th instar) in male; ring-like segment XI usually exposed.
Diagnostic characters for older larvae (3rd–5th instars) of Jadera haematoloma and two sympatric serinethines, Leptocoris augur and Leptocoris vicinus.
| Jadera haematoloma (Herrich-Schäffer, 1847) | Leptocoris augur (Fabricius, 1781) | Leptocoris vicinus (Dallas, 1852) | |
|---|---|---|---|
| 1 | Body bright red, head and thorax darker and conspicuously pruinose especially ventrally (Figs 7–20). | Body bright orange (Fig. 31: arrow, 34: arrow), head and thorax somewhat darker, body with weak or indistinct pruinosity. | Body colour similar to Jadera haematoloma but frequently darker red, body without pruniosity. |
| 2 | Mandibular plates broadly rounded distally, portion of head anteriad of antenniferous tubercles broadly truncate anteriorly. | Mandibular plates strongly narrowed distally, portion of head anteriad of antenniferous tubercles broadly rounded anteriorly. | |
| 3 | With a single, broadly interrupted series of setae along dorsal margin of eye. | With a single, uninterrupted series of setae along dorsal margin of eye. | |
| 4 | Ecdysial suture of head V-shaped. | Ecdysial suture of head rather U-shaped, with its contralateral branches less diverging. | |
| 5 | Postocular portion of head of somewhat angulate lateral outline in dorsal view, provided with a single series of setae at each side, without protuberance. | Postocular portion of head of rounded lateral outline in dorsal view, provided with at least two irregular series of setae or irregular pilosity at each side, with a pair of blunt, angular protuberance dorsolaterally. | |
| 6 | Apex of labial segment I reaching posterior margin of eye. | Apex of labial segment I reaching base of head. | Apex of labial segment I extending to postocular part of head, approaching base of head. |
| 7 | All legs uniformly grey to black. | Coxae red to brownish, remaining segments of legs chestnut-coloured to black. | Coxae brownish red, remaining segments of legs black. |
| 8 | Intersegmental suture IV/V almost straight. | Intersegmental suture IV/V slightly curved posteriad at middle. | Intersegmental suture IV/V strongly curved posteriad at middle. |
| 9 | Openings of dorsal abdominal scent glands of segments IV and V close to each other. | Openings of dorsal abdominal scent glands of segments IV and V far from each other. | Openings of dorsal abdominal scent glands of segments IV and V rather close to each other. |
Larvae of Jadera haematoloma, dorsal (7, 9, 11, 13) and ventral (8, 10, 12, 14) views 7–8 first instar (freshly hatched) 9–10 second instar (freshly moulted) 11–12 second instar (old) 13–14 third instar (old). Scale bar in mm.
Larvae of Jadera haematoloma, dorsal (15, 17, 19) and ventral (16, 18, 20) views 15–16 fourth instar (old) 17–18 fifth instar, male (old) 19–20 fifth instar, female (old). Scale bar in mm.
Provided in Table 6.
Measurements of larval instars (in mm) and relative lengths of their mesothoracic wing pads collected at site 4. Abbreviations: L1–L5 = 1st–5th larval instars, l = length, w = width.
| instar |
body length |
head length | head width | head w: l | pronotum width | pronotum w: head w |
mesothoracic wing pad |
|
|---|---|---|---|---|---|---|---|---|
| L1 | total | 1.92±0.23 (1.72–2.38) |
0.63±0.04 (0.57–0.70) |
0.67±0.02 (0.66–0.70) |
1.00–1.21 | 0.75±0.02 (0.74–0.78) |
1.09–1.13 | absent |
|
young (N = 6) |
1.72±0.03 (1.72–1.80) |
0.59±0.03 (0.57–0.66) |
0.68±0.02 (0.66–0.70) |
1.00–1.21 | 0.75±0.02 (0.74–0.78) |
1.09–1.13 | ||
|
old (N = 4) |
2.17±0.14 (2.09–2.38) |
0.67±0.02 (0.66–0.70) |
0.67±0.02 (0.66–0.70) |
1.00–1.03 | 0.75±0.02 (0.74–0.78) |
1.09–1.13 | ||
| L2 | total | 3.19±0.38 (2.67–3.73) |
0.80±0.04 (0.74–0.82) |
0.87±0.03 (0.82–0.90) |
1.05–1.17 | 0.97±0.04 (0.90–1.03) |
1.05–1.14 | absent |
|
young (N = 5) |
2.75±0.11 (2.67–2.87) |
0.82±0 (0.82–0.82) |
0.88±0.02 (0.86–0.90) |
1.05–1.10 | 0.98±0.02 (0.94–0.98) |
1.05–1.14 | ||
|
old (N = 10) |
3.41±0.23 (3.08–3.73) |
0.79±0.04 (0.74–0.80) |
0.87±0.03 (0.82–0.90) |
1.05–1.17 | 0.96±0.04 (0.90–1.03) |
1.05–1.14 | ||
| L3 | total | 4.51±0.51 (3.63–5.48) |
0.94±0.04 (0.86–0.99) |
1.15±0.04 (1.06–1.25) |
1.13–1.38 | 1.24±0.08 (1.06–1.45) |
1.00–1.19 | minute primordia |
|
young (N = 16) |
3.94±0.30 (3.63–4.49) |
0.94±0.04 (0.86–0.99) |
1.14±0.04 (1.12–1.22) |
1.13–1.29 | 1.22±0.08 (1.12–1.39) |
1.00–1.19 | ||
|
old (N = 18) |
4.83±0.25 (4.36–5.48) |
0.94±0.05 (0.86–0.99) |
1.15±0.05 (1.06–1.25) |
1.13–1.38 | 1.27±0.09 (1.06–1.45) |
1.00–1.17 | ||
| L4 | total | 5.92±0.79 (4.56–7.36) |
1.14±0.07 (1.04–1.28) |
1.49±0.07 (1.36–1.68) |
1.20–1.46 | 1.66±0.11 (1.44–1.92) |
1.03–1.21 | I-P to II-M |
|
young (N= 10) |
4.96±0.36 (4.56–5.68) |
1.10±0.06 (1.04–1.20) |
1.47±0.07 (1.36–1.60) |
1.20–1.46 | 1.61±0.08 (1.44–1.68) |
1.03–1.21 | I-P to II-M | |
|
old (N = 19) |
6.37±0.45 (5.44–7.36) |
1.15±0.06 (1.04–1.28) |
1.49±0.07 (1.40–1.68) |
1.20–1.46 | 1.68±0.12 (1.48–1.92) |
1.05–1.17 | I-P | |
| L5 | total | 8.10±1.06 (6.05–10.23) |
1.25±0.12 (1.1–1.43) |
1.87±0.09 (1.76–2.04) |
1.33–1.64 | 2.32±0.12 (2.20–2.53) |
1.18–1.29 | II-P to IV-P |
|
young (N = 9) |
6.97±0.78 (6.05–8.14) |
1.36±0.08 (1.21–1.43) |
1.89±0.10 (1.76–2.04) |
1.33–1.55 | 2.35±0.12 (2.20–2.53) |
1.18–1.29 | III-P to IV-P | |
|
old (N = 19) |
8.58±0.75 (7.37–10.23) |
1.20±0.10 (1.1–1.43) |
1.86±0.08 (1.76–1.98) |
1.38–1.64 | 2.31±0.11 (2.20–2.53) |
1.18–1.29 | II-P to III-P | |
1 6 individuals of young 3rd, 1 individual of young 4th, and 1 individual of young 5th instars were excluded from measuring the body length because of their shrunken abdomen.
2 The position of the apex of the metathoracic wing pad in respect to the abdominal tergites is given; Roman numerals refer to the segmental homology; A = anterior half; P = posterior half.
The body is short and oval in newly hatched larvae (Figs 7–8). Abdomen of older first instar larvae is considerably extended because of feeding, the body therefore more elongate; shape of older larvae gradually becoming more similar to that of adult (Figs 9–20). Second to fifth instar larvae undergo in rather conspicuous changes during each developmental stage (cf. Tables 6–7): larvae of each instar soon after moulting are brighter red, the body appearing smaller because of the shorter abdomen; therefore, the labium is apparently longer in relation to the abdominal sternites. After moulting, the body appears less bright (a dust-like substance on the thorax makes it appear pruinose) and the abdomen extends so it becomes longer and the labium appears relatively shorter when compared to the body length. These changes are demonstrated in two specimens of second instar larvae in Figs 9–10 (freshly moulted) and 11–12 (older). Because of the extension of the abdomen, a small difference can be observed in the relative length of the mesothoracic wing pads of the fourth and fifth instars.
Variation of the relative length of the labium in different instar larvae (numbers of examined individuals). The position of the apex of the labium in respect to the abdominal sternites is given; Roman numerals refer to the segmental homology; A = anterior half; P = posterior half.
| II | III | IV | V | VI | VII | VIII | IX | X | XI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | A | P | A | P | A | P | A | P | P | |||||
| L1 (newly hatched) (N= 6) | 6 | |||||||||||||
| L1 (old) (N= 4) | 1 | 3 | ||||||||||||
| L2 (young) (N = 5) | 5 | |||||||||||||
| L2 (old) (N = 10) | 1 | 4 | 2 | 3 | ||||||||||
| L3 (young) (N = 10) | 4 | 4 | 1 | 1 | ||||||||||
| L3 (old) (N = 18) | 3 | 7 | 3 | 4 | 1 | |||||||||
| L4 (young) (N = 7) | 1 | 1 | 3 | 1 | 1 | |||||||||
| L4 (old) (N = 19) | 6 | 4 | 7 | 2 | ||||||||||
| L5 (young) (N = 9) | 1 | 2 | 2 | 3 | 1 | |||||||||
| L5 (old) (N = 19) | 5 | 8 | 4 | 2 | ||||||||||
Several adults and larvae were observed feeding on the ripe fruits of Cardiospermum halicacabum (sites 1–4) and Koelreuteria elegans subsp. formosana (sites 5–7).
At sites 1, 4, 5, 6 and 7, the thick layer of dead leaves accumulated below the host plant offered an ideal microhabitat for adults and larvae. First and second larval instars were never observed on the plants; they hid among the leaf litter (Fig. 27) and fed mostly on fallen, mature fruits (with brownish pericarp), which were open (Fig. 30). At site 4 most of the first to third instar larvae aggregated within the ripe and open fruits. Third instar and older larvae were more vagile than the first two instars; they walked around and frequently climbed and formed aggregations on the stem of the heartseed vine, on the trunk of Koelreuteria elegans subsp. formosana (commonly hiding in the crevices), and occasionally on the shadow side of cement pillars around the plants.
Jadera haematoloma on and around its host plant (Cardiospermum halicacabum) (site 1). 21 Chain-link fence with heartseed vine 22 Adult male feeding on a fruit (arrow: stylet with its basal portion ensheathed in the concavity of labrum) 23 Adult male feeding on a seed 24 fruit damaged by Jadera haematoloma (arrows: feeding scars) 25 seed damaged by Jadera haematoloma (arrow: feeding cone) 26 fourth instar larva feeding on the stalk of a fruit 27 larvae walking and feeding on a leaf stem of heartseed vine among leaf litter.
Jadera haematoloma on and around its host plant (Cardiospermum halicacabum). 28 A mating couple, the female (in the right) feeding on fruit of the host plant 29 Adult feeding on a flower of Cardiospermum halicacabum 30 second instar larva feeding on seed of the host plant 31 Adults feeding on a fourth instar larva of Leptocoris augur (arrow: another fourth instar larva of Leptocoris augur) 32 fifth instar larvae in the fruit of Cardiospermum halicacabum 33 a male guarding a gravid female 34 aggregation of a brachypterous male, a fourth instar larva (arrow) of Leptocoris augur and two fourth instar larvae of Jadera haematoloma.
During feeding, stylets of adults and fourth and fifth instar larvae penetrated deeply into fruit through the pericarp (Figs 22: arrow, 28), and reached the seeds. Brown spots appeared on fruits where it was damaged by the feeding of adults or older larvae (Fig. 24). All larval instars accessed seeds by climbing into the fruit through an opening or injury to the pericarp (Fig. 32), or consumed fallen seeds; several adults also fed similarly. Adults and all (including first) larval instars were frequently sucked the fruit stalks (Fig. 26). Adults and at least older larvae commonly drank nectar from flowers of Cardiospermum halicacabum (Fig. 29) too. Frequent nectar consumption from flowers of Bidens pilosa var. radiata, an asteracean weed, was observed at site 8 (U. Ong, pers. comm.).
As in several other Pentatomomorpha, especially those consuming seeds or feeding from other hard surfaces, the feeding is of the stylet-sheath type (
A female of Jadera haematoloma attacking a female of Leptocoris augur (site 1). In Fig. 36 arrow shows the feeding cone.
Dozens of specimens were kept for several days in captivity on seeds of Cardiospermum halicacabum, but no cannibalism was observed. Nevertheless, several instances of zoophagy on other rhopalid species were observed; these are discussed below under ‘Competitors’.
At some places the abundance of Jadera haematoloma was rather high on and around its host plant. At site 1 an hour of searching along the fence (in an area of about 10 × 1 m, cf. Fig. 21) yielded 43 adults and dozens of larvae. A few minutes of searching resulted in hundreds of larvae at site 4; aggregations composed by about 10–30 specimens were observed at this locality.
In the time of the observations (between 6.xi.2012 and 14.i.2013) specimens were still walking, running and feeding actively, and did not show any sign of diapause. Several mating pairs were found (6 at site 1, 2 at site 3, 5 at site 4, 6 at site 7, several at site 8) (Fig. 28). Many of the copulating females were gravid, with greatly enlarged abdomens (Fig. 33); a large number of gravid females was found on Koelreuteria elegans subsp. formosana during December 2012 and January 2013.
Several dozen larvae were observed and approximately one hundred were captured. At site 1 (30.ix) only first to third instars were found; careful searching did not yield any older instars. All larval instars were observed at site 4 on the same day, forming aggregations of dozens of larvae at ground level.
The largest number of adults was collected at site 1 (30.xi.2012) and at site 5 (3.xii.2012 and 14.i.2013). Estimating from this limited sample, all of these populations were apparently distinctly male-biased, with a ratio of 1.75:1 (28 ♂♂, 16 ♀♀) in the first case, 2.4:1 (38 ♂♂, 16 ♀♀) in the second case, and 3:1 (42 ♂♂, 14 ♀♀) in the third case.
In all localities Jadera haematoloma co-occurred with Leptocoris augur (Fabricius, 1781), a taxonomically closely related serinethine species native and rather abundant in Taiwan. In all of the above localities Leptocoris augur was estimated to be clearly more abundant than Jadera haematoloma. Individuals of both species frequently occur within the same aggregation (Fig. 34).
According to our subjective observations, adults and especially larvae of Jadera haematoloma are more vagile than Leptocoris augur. Although first and second instars usually do not walk much, they ran quickly when approached, and they were distinctly quicker than larvae of Leptocoris augur of the same instar. The difference in older instars also was evident.
Leptocoris augur was observed to feed on both Cardiospermum halicacabum and Koelreuteria elegans subsp. formosana in a manner similar to that described above for Jadera haematoloma; nectar feeding in Leptocoris augur also was observed. In some cases direct interference between individuals of the two species was observed. At site 2, adults and larvae were observed to feed on a freshly moulted adult of Leptocoris augur (Fig. 31). A similar phenomenon was observed in a plastic container where the two species were reared together: a freshly moulted adult of Leptocoris augur was attacked and consumed by six larvae (representing all larval instars) of Jadera haematoloma. At site 1 a female of Jadera haematoloma was observed to approach a female of Leptocoris augur, climb its back, and penetrate its labium into its neck (Figs 35–36). They remained in this position for about 15 minutes; after that the individual of Leptocoris augur was still alive but it stopped moving and died a few hours later.
At site 5 another related native rhopalid having similar host plants and habits, Leptocoris vicinus (Dallas, 1852) was observed. Adults and all larval instars of Leptocoris vicinus were found on the ground, but in smaller numbers than Jadera haematoloma and Leptocoris augur.
Our field observations indicate that Jadera haematoloma has probably already established in southern Taiwan. Because of the large number of adults, high frequency of mating pairs, presence of several gravid females, and most importantly the large numbers of all larval instars, it is apparent that strong, reproducing populations are present in southern Taiwan. The number and the condition of the observed populations suggest that Jadera haematoloma was not introduced in 2012, but at least one or two years earlier. From the current geographic distribution within Taiwan it seems probable that the species entered through the seaport of Kaohsiung, the largest harbour of the country where most of Taiwan’s marine import and export passes.
Apparently the populations in Taiwan have a host range similar to those in North America. Cardiospermum halicacabum and Koelreuteria elegans subsp. formosana were identified as host plants of Jadera haematoloma in Taiwan; both plants previously were reported as hosts in the continental USA, the Caribbean and Hawaii (Table 1). Frequent nectar consumption from host flowers and Bidens pilosa var. radiata was observed.
Little is known about the bug’s phenology in Taiwan. Active, reproducing populations fed on both Cardiospermum halicacabum and Koelreuteria elegans subsp. formosana from late November to mid-January. Because of the subtropical and tropical climate of Taiwan, no winter diapause is expected. Because fruits of balloon vine are available year round in Taiwan, and seeds of Koelreuteria elegans subsp. formosana also are available until late March (
At least several populations in Taiwan seem more or less male-biased and show variation similar to those in the southern USA. Females are significantly larger than males in both wing morphs and macropterous morphs are significantly larger than brachypters, which is similar to the North American populations (
11.4% of the individuals in the population at site 1 observed on 30.xi.2012 were brachypterous; this ratio is about 20% in the southern USA (
Jadera haematoloma occurs in the same habitats and uses the same food in the same manner as do Leptocoris augur and Leptocoris vicinus, two taxonomically closely related native rhopalid species in Taiwan. Mixed-species aggregations of Jadera haematoloma and one or both of the native species were commonly observed at several localities. Although no interspecific competition between Jadera haematoloma and other hemipterans was reported in North America (
Koelreuteria elegans subsp. formosana originally was found mainly at lower altitudes (
Several of the sapindacean plants that have already been reported as host plants of Jadera haematoloma in the USA also occur in southeast China (
We are grateful to Jian-Xiang Fang, Uika Ong, and Sheng-Fu Yang (Taiwan) for help in field observations and photographing and for providing specimens; to Ursula Göllner-Scheiding (Humboldt University, Berlin) for discussion about the South American records of Jadera haematoloma; to Harry Brailovsky (Universidad Nacional Autónoma de México) for sharing several unpublished records of this species from Mexico and for his permission to use them in this paper; to Scott P. Carroll (University of California, Davis) and an anonymous reviser for valuable comments to the manuscript; to Petr Kment (National Museum, Prague), Felipe Moreira (Universidade Federal do Rio de Janeiro), and David Rider (North Dakota State University, Fargo) for providing references, to Jung-Tai Chao, Shen-Shan Lu (Taiwan Forestry Research Institute, Taipei) and Shiang-Rong Wei (Taipei) for various help. The third author receives support from the China Postdoctoral Science Foundation (grant No. 20110490769).