(C) 2013 Michel P. Valim. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We describe and illustrate three new species of chewing lice in the genus Philopteroides parasitic on passerines (Order Passeriformes, families Acanthizidae, Rhipiduridae and Petroicidae) from New Zealand. They are: Philopteroides pilgrimi sp. n. from Gerygone igata igata; Philopteroides fuliginosus sp. n. from Rhipidura fuliginosa placabilis and Rhipidura fuliginosa fuliginosa; and Philopteroides macrocephalus sp. n. from Petroica macrocephala macrocephala and Petroica macrocephala dannefaerdi. The identity of Docophorus lineatus Giebel, 1874 is discussed based on its morphology and host association. We also transfer Tyranniphilopterus beckeri to the genus Philopteroides, and provide a key to identify adults of 12 of the 13 species now included in Philopteroides.
Philopteroides, Philopteridae, Phthiraptera, lice, new species, new combination, key to species, Passeriformes, New Zealand
The genus Philopteroides was erected by
Species of the Philopterus-complex are relatively sedentary lice belonging to the docophorid ecotype (
Specimens examined from New Zealand hosts belong to the Museum of New Zealand Te Papa Tongarewa, Wellington, N.Z. (MONZ), except for some paratypes deposited in the Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil (MZUSP). The remaining material examined is held in the Naturhistorischen Museum, Rudolstadt, Thüringen, Germany (NHMR). The lice were treated and mounted on slides following the Canada balsam technique (
All measurements are in millimeters, taken from digitalized images from slide-mounted specimens using the software Leica Application Suite (LAS), and identified by the following abbreviations: as3, length of the anterior setae 3; ADPL, anterior dorsal plate length (taken at middle line); ADPLL, anterior dorsal plate lateral length (taken from the base of anterior dorsal setae – ads, to lateral apices of the plate); ADPW, anterior dorsal plate width (taken at its widest point); ANW, width of anterior notch (taken between the bases of as3); AW, maximum width of the abdomen (taken at level of segment V); EWG, external width of genital chamber; GL, male genitalia length; GW, male genitalia width (taken at the basal plate); HL, head length (excluding hyaline membrane); IWG, internal width of genital chamber; POL, preantennal length (taken from the base of the conus to the bases of as3, obliquely to the head axis); POW, preantennal width (taken between bases of the coni); PTW, pterothorax width; PTL, pterothorax length; PW, prothorax width; TL, total length; TPVL, tergal plate V length; TRL, trabecula length; TRW, trabecula width; TW, temporal width.
The sternal abdominal setae are given in sequence from the left to the right side and named by the letters: “L” meaning long and flexible, and “S” meaning short and spine-like. Thus, the first letter indicates the outermost seta on the left side and the last letter the outermost on the right side. For example: LSLS-SLSL if the pattern is symmetrical, or LSLS-L-SLSL if asymmetrical.
The minute spine-like and the long trichoid setae present on each side of the pterothorax are not included in the number of pterothoracic setae. The chaetotaxy of the abdominal tergocentral setae does not include the postspiracular setae, except for tergite II where postspiracular setae, if present, can not be distinguished from the remaining setae. We regard as pleural setae those situated on the lateral sides of the tergo-pleurites, and as sternal setae those next to the sternites. Some species have ventral setae between the innermost pleural and the outermost sternal setae, which we regard as additional setae.
The nomenclature of head features and setae follows
Philopteroides novaezelandiae Mey, 2004 (by original designation).
Two species-groups:
beckeri species-group: two species
mitsusui species-group: ten species
Passeriformes, suborder Acanthisitti (Acanthisittidae), and suborder Passeri (Acanthizidae, Meliphagidae, Monarchidae Nectariniidae, Petroicidae, Platysteiridae, Pycnonotidae, Rhipiduridae).
Africa (Senegal, Uganda), Asia (India, Vietnam, Taiwan), Oceania (Micronesia, New Zealand).
In addition to those characters mentioned by
Member of the Philopterus-complex by presence of well-developed trabeculae. Anterior dorsal head plate with posterior median projection well developed and sclerotized, but without antero-lateral projections. Hyaline membrane and anterior head plates deeply concave forming an “osculum” (mitsusui group) (Figs 11–12, 34, 36); some species with wide frons (beckeri group) (Figs 9–10). Hyaline membrane deeply or slightly concave, arising from the level of the tips of the marginal carinae or above the anterior setae 3 (as3), with a conspicuous median sclerotization and without additional setae. Marginal carina not interrupted laterally, but with a conspicuous lateral suture on the dorsal surface, at the level of the posterior dorsal sub-medial setae (d.sm.s.). Conus ranging from much reduced to well developed. Marginal temporal setae 2 (m.t.s.2) and pre-ocular setae (p.o.s.) median to short. Prothoracic dorsal setae close to the middle of the segment, and to its posterior margin. Pleuro-tergal plates II–IV without postero-lateral projections (‘posterior heads’), but few species with at most a slightly pronounced angle on segment II, but not on III or IV. Spine-like setae present on some of the sternites II–VI.
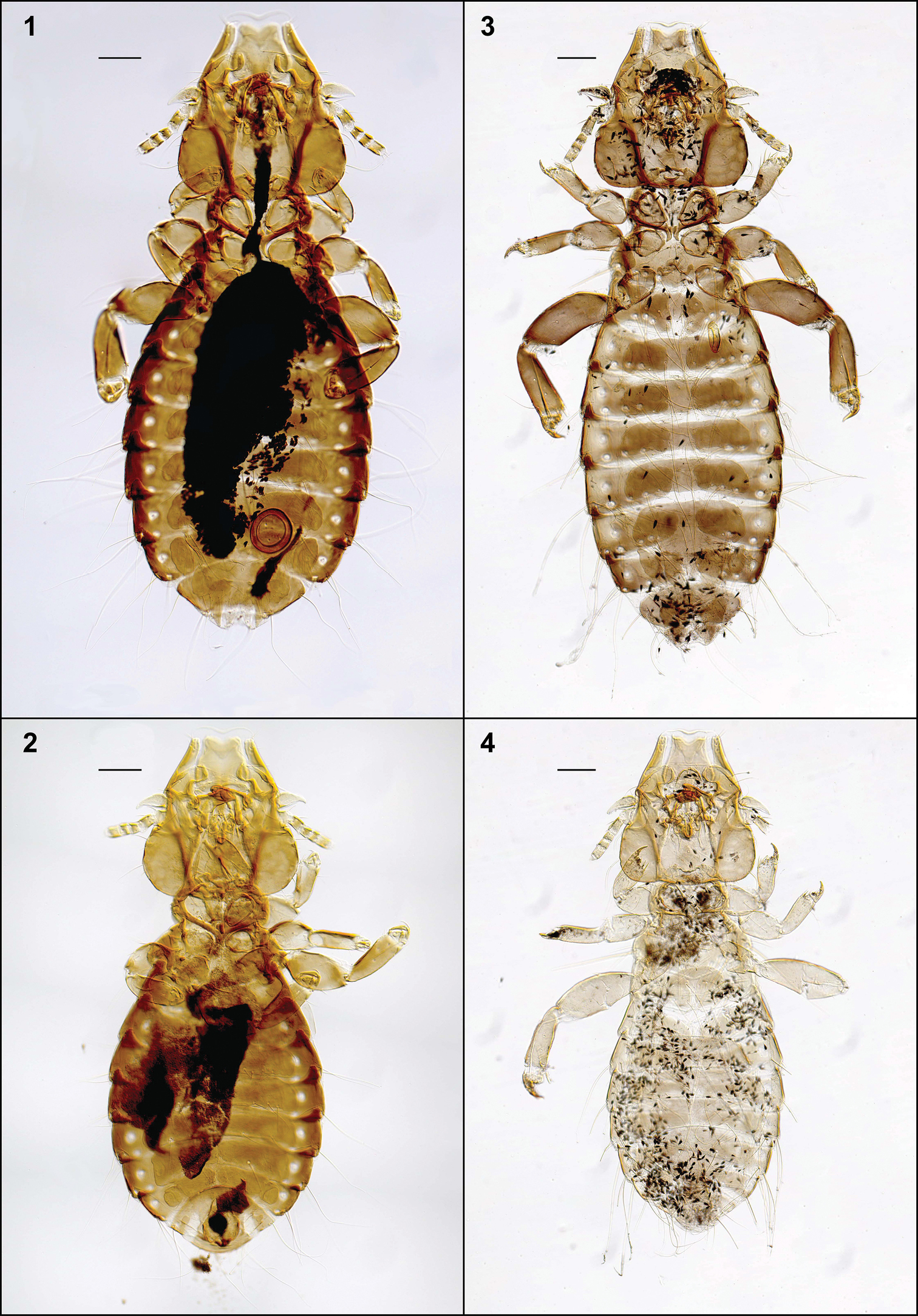
Habitus, ventral views: Philopteroides beckeri female paratype (1); Philopteroides beckeri male holotype (2); Philopteroides pilgrimi female holotype (3); Philopteroides pilgrimi male paratype (4). Scale bars = 0.1 mm
Habitus, ventral views: Philopteroides fuliginosus female holotype (5); Philopteroides fuliginosus male paratype (6); Philopteroides macrocephalus female holotype (7); Philopteroides macrocephalus male paratype (8). Scale bars = 0.1 mm
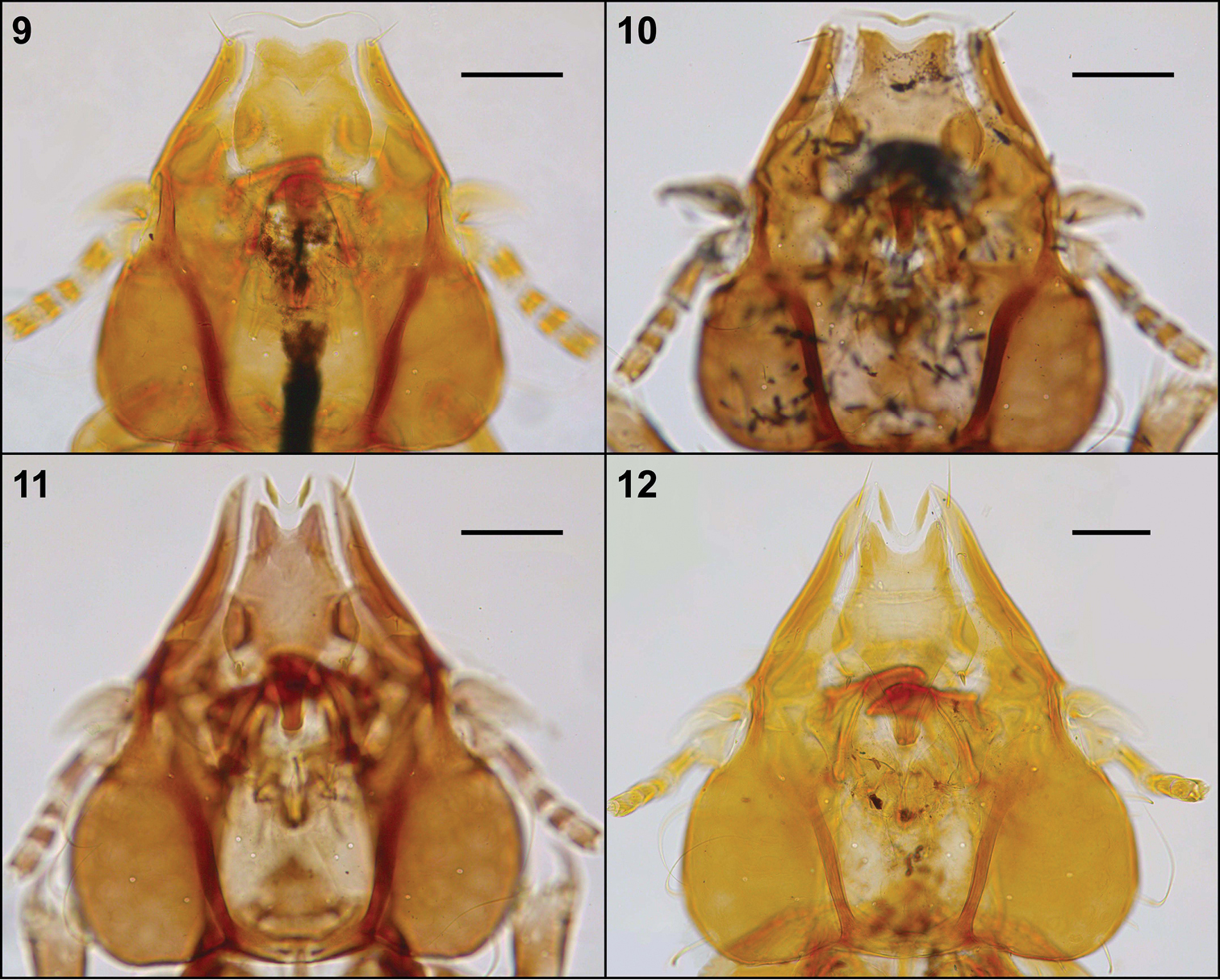
Head, dorsal view: Philopteroides beckeri female paratype (9); Philopteroides pilgrimi female holotype (10); Philopteroides fuliginosus female (11); Philopteroides macrocephalus female holotype (12). Scale bars = 0.1 mm
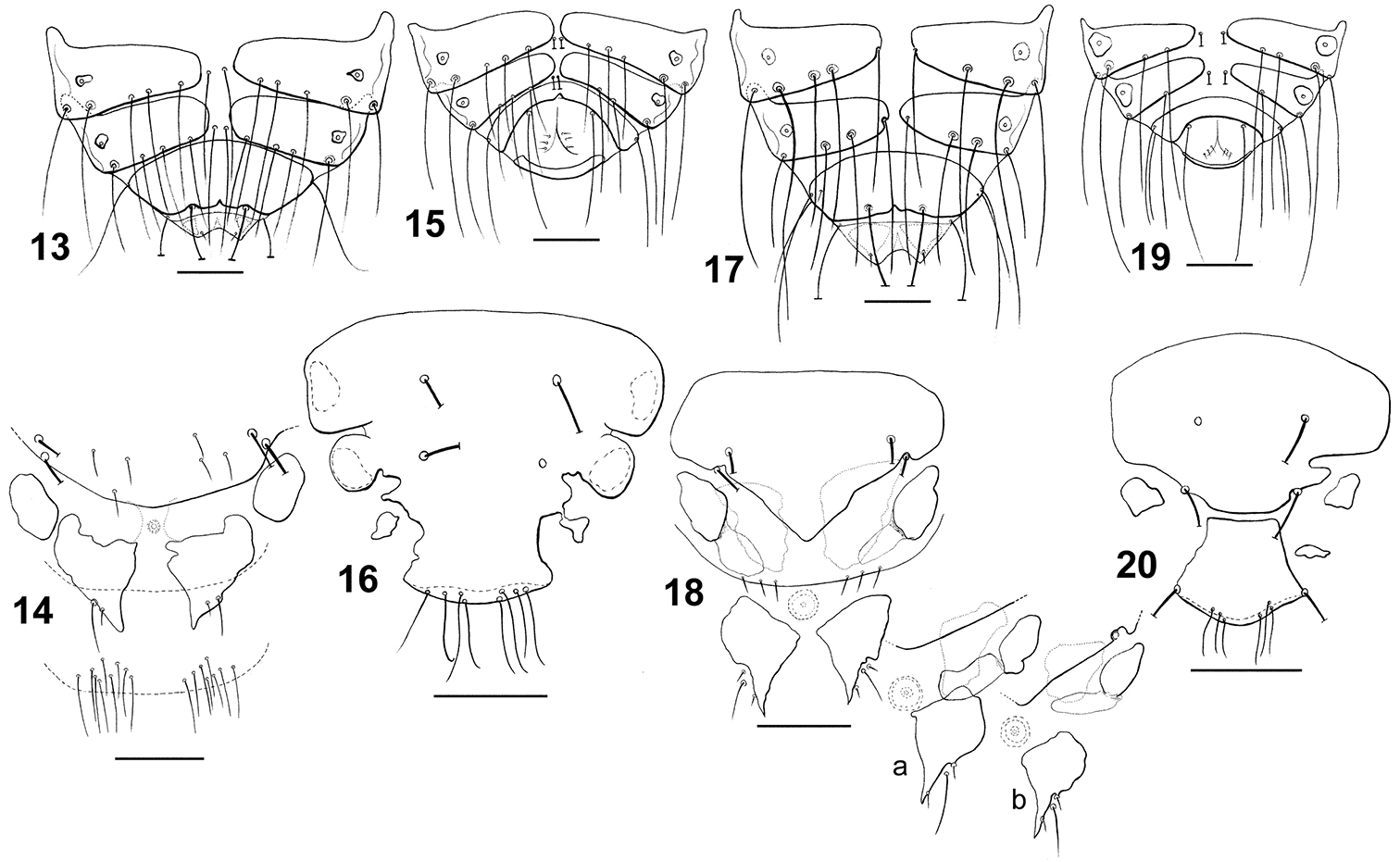
Dorsal terminalia: Philopteroides beckeri female (13); Philopteroides beckeri male (15); Philopteroides pilgrimi female (17); Philopteroides pilgrimi male (19). Female ventral terminalia: Philopteroides beckeri (14); Philopteroides pilgrimi (18); Philopteroides pilgrimi intraspecific variation (18a, b). Male subgenital plate: Philopteroides beckeri (16); Philopteroides pilgrimi (20). Scale bars = 0.1 mm.
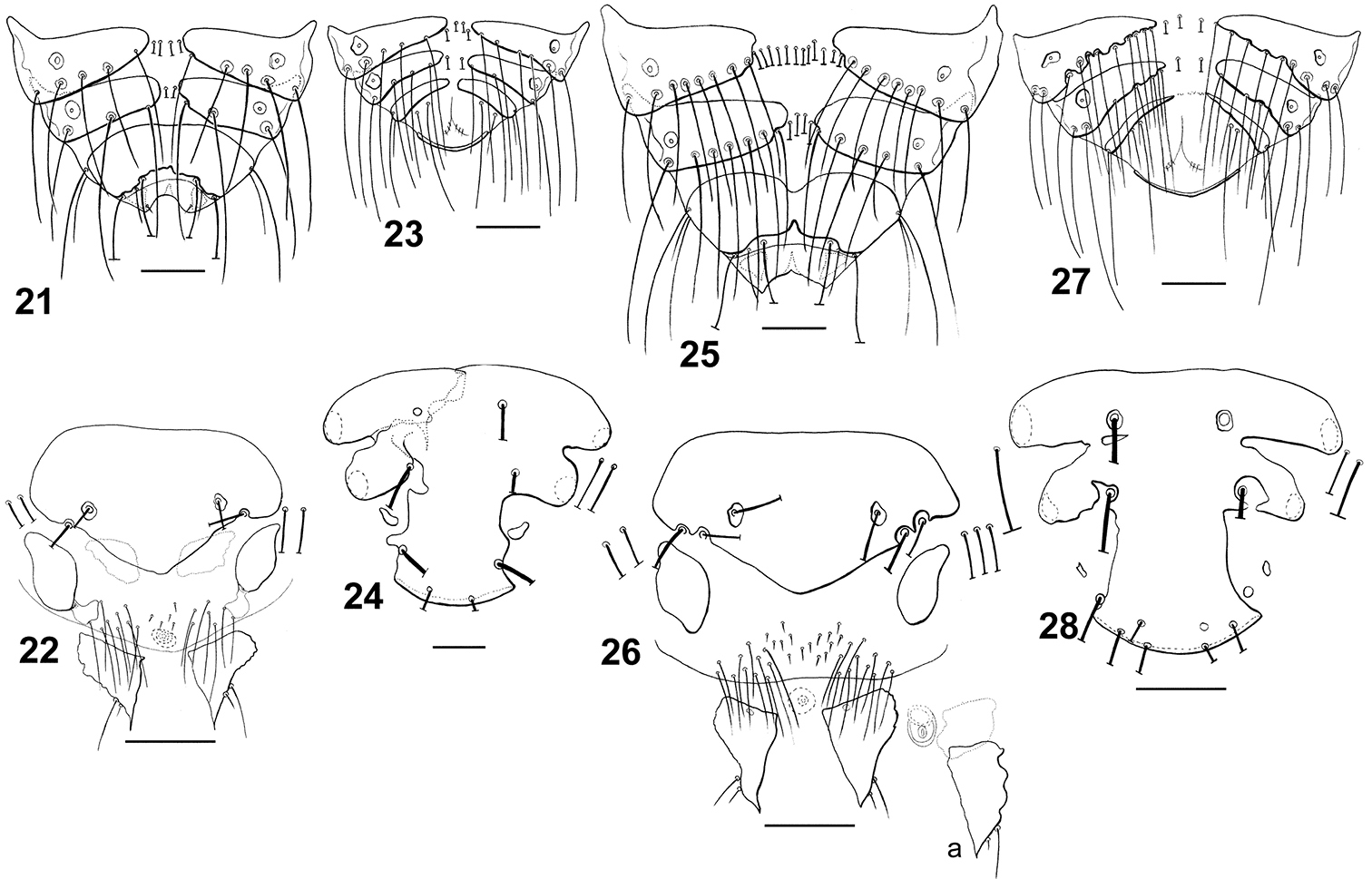
Dorsal terminalia: Philopteroides fuliginosus female (21); Philopteroides fuliginosus male (23); Philopteroides macrocephalus female (25); Philopteroides macrocephalus male (27). Female ventral terminalia: Philopteroides fuliginosus (22); Philopteroides macrocephalus (26); Philopteroides macrocephalus intraspecific variation (26a). Male subgenital plate: Philopteroides fuliginosus (24); Philopteroides macrocephalus (28). Scale bars = 0.1 mm.
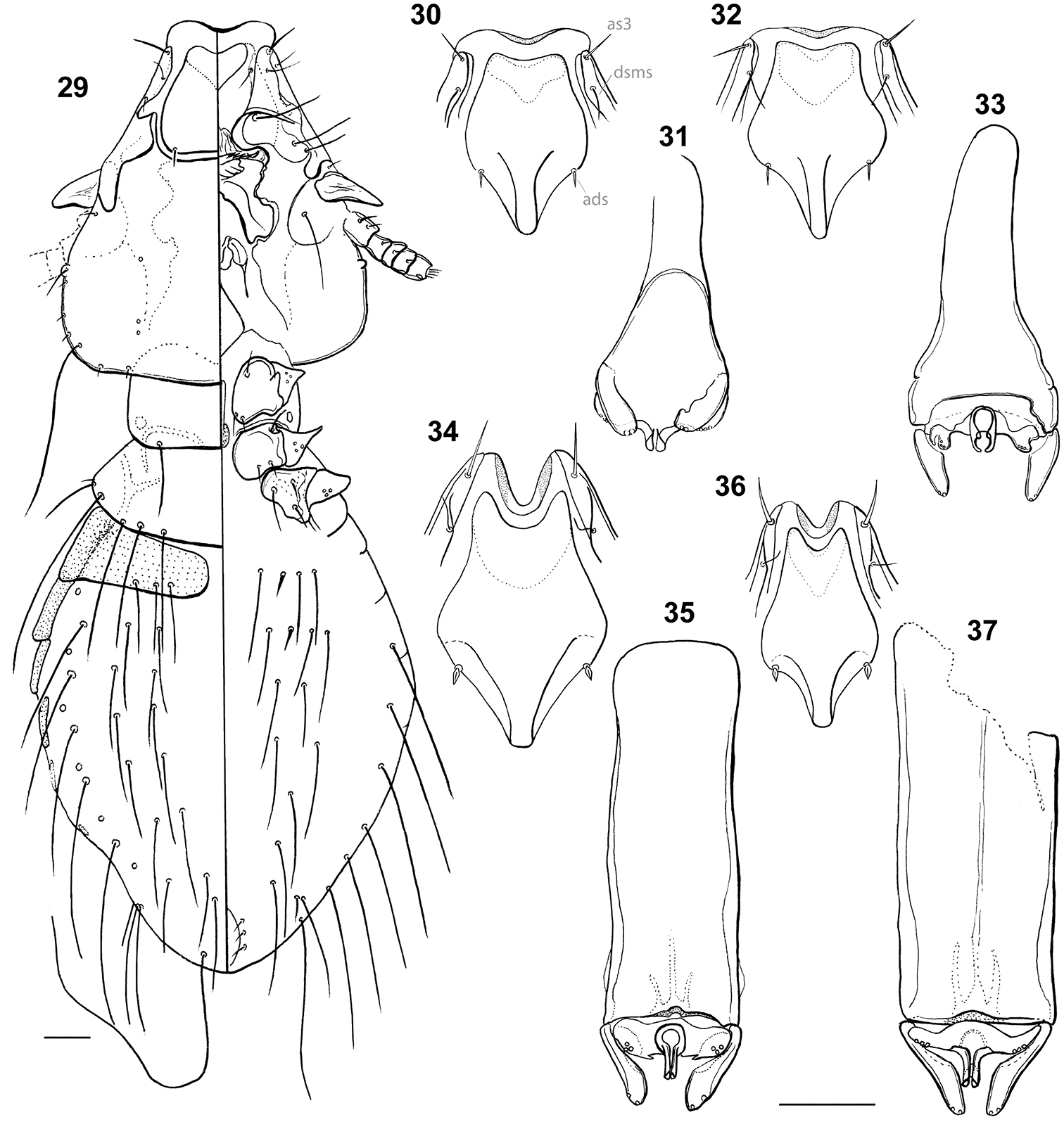
Habitus: Philopteroides beckeri nymph II, dorso-ventral view (29). Hyaline margins and anterior dorsal plates: Philopteroides beckeri (30); Philopteroides pilgrimi (32); Philopteroides fuliginosus (34); Philopteroides macrocephalus (36). Male genitalia: Philopteroides beckeri (31); Philopteroides pilgrimi (33); Philopteroides fuliginosus (35); Philopteroides macrocephalus (37). Scale bars = 0.05 mm
Docophorus lineatus Giebel, 1874 was included in Philopteroides by
http://species-id.net/wiki/Philopteroides_lineatus
Arachnothera longirostra (Latham, 1790) – Little spiderhunter (Nectariniidae). See note below.
Unknown. The original description does not include a type locality. There are 13 subspecies of Arachnothera longirostra distributed throughout the Indo-Malayan region (
Considering current louse species descriptions,
In his original description,
Regarding the host-louse association of Docophorus lineatus,
http://species-id.net/wiki/Philopteroides_beckeri
Figs 1–2, 9, 13–16, 29–31Platysteira cyanea nyansae Neumann, 1905 – Brown-throated wattle-eye. (Platysteiridae)
Uganda.
This species was recently described in detail and, therefore, it is not necessary to redescribed it again. We only include habitus images of the holotype male and one paratype female (Figs 1–2), not figured in the original description by
All species of the genus Tyranniphilopterus Mey, 2004 have the following characters, which are lacking in “Tyranniphilopterus” beckeri: (1) hyaline margin arises at a level below the as3; (2) conspicuous antero-lateral projections on anterior dorsal head plate; (3) prothoracic dorsal setae located on the posterior-lateral angles of the segment, not on its posterior margin.; (4) pleuro-tergal plates II–III with well developed postero-lateral projections. Instead, “Tyranniphilopterus” beckeri has the features which define Philopteroides. These morphological features justify placing it in the latter genus, as Philopteroides beckeri (Mey, 2004) new combination.
Furthermore, the hosts of all species of Tyranniphilopterus – except for “Tyranniphilopterus” beckeri – belong to the passerine suborders Tyranni and Passeri, and are confined to the Americas, while the hosts of all species of Philopteroides belong to the passerine suborders Passeri and Acanthisitti distributed in Africa, Asia and Oceania. Hence, the geographical distribution of its host is further evidence that placing “Tyranniphilopterus” beckeri in the genus Philopteroides is correct.
Head, thorax and abdomen as in Fig. 29. Head sub-conical, marginal carina entire laterally, with well developed anterior dorsal and ventral head plates. Anterior setae 3 (as3) rigid, 0.04 in length. Dorsal head plate with convex lateral margins and almost straight posterior margin, bearing rigid anterior dorsal head setae (ads), as the adults. Anterior ventral plate cordiform. Marginal temporal setae 3 (m.t.s.3) very long, other temporal setae short to minute. Each of the pair of posterior setae on pronotum located half way between the middle of the segment and its lateroposterior angle. Only four long pterothoracic setae, as in the second nymphal stage of most species of Philopteridae (
Holotype ♂ (NHMR #4322.c) and 1♀ paratype (NHMR #4322.b), ex Platysteira cyanea nyansae; UGANDA: Paraa, Murchison Falls, National park, 22.X.1998, P. Becker col.
1 nymph II (NHMR #4322.b), mounted on the same slide as the female paratype.
urn:lsid:zoobank.org:act:22B8C261-0710-4C23-85B6-54EA58333235
http://species-id.net/wiki/Philopteroides_pilgrimi
Figs 3–4, 10, 17–20, 32–33Gerygone igata igata (Quoy & Gaimard, 1830) – Grey warbler (Acanthizidae)
New Zealand.
Female. Habitus as in Fig. 3 and head as in Fig. 10. Anterior setae 3 (as3) rigid, 0.04–0.05 long, anterior dorsal head setae (ads) minute, 0.02 long. Hyaline membrane with shallow concavity and thin sclerotization; anterior dorsal plate slightly concave and ventral head plate deeply concave (Fig. 32). Pterothorax with 6–8 posteromarginal setae on each side. Tergo-pleural plate II with a reduced postero-lateral projection overlapping segment III, tergo-pleural plate III without projection. Tergocentral setae: II, 5–6 (plus 2 anterior setae); III, 6; IV, 5–6; V, 5–6; VI, 4–6; VII, 5–6; VIII, 5. Tergites VII–IX+X as in Fig. 17. Abdominal sternal setae: segment II, LS-SL; III, LS-SL; IV, SS-SS (rarely LS on one side only); V, SS-SS; VI, LL-LL (one female with one S on one side only). Paratergal setae (all long): II–III, 0; IV–V, 2; VI–VIII, 3. Tergites VI–VIII with an innermost seta (included in the paratergal count), lateral to postspiracular seta. Sternites III–VI well-developed as large, rectangular plates. Vulva with 3 medium long setae each side. Subgenital plate, inner genital sclerite, subvulval sclerites, and vulvar margin as in Fig. 18, and intraspecific variation of two additional females as in Figs 18a and 18b. Measurements (n = 8): HL 0.39–0.43, ANW 0.13–0.15, POL 0.16–0.17, POW 0.28–0.31, ADPL 0.21–0.22, ADPLL 0.14, ADPW 0.13–0.16, TRL 0.09–0.10, TRW 0.03–0.04, TW 0.35–0.41, PW 0.22–0.28, PTL 0.13–0.14, PTW 0.29–0.35, TPVL 0.19–0.25, AW 0.44–0.54, EWG 0.06–0.08; IWG 0.03–0.04, TL 1.35–1.65.
Male. Similar to female, except in dimensions and morphology of terminalia (Figs 4, 19, 20). Pterothorax with 5–6 posteromarginal setae on each side. Tergocentral setae: II, 6 (plus 2 anterior setae); III, 6; IV, 6; V, 6; VI, 6; VII, 6; VIII, 4. Dorsal terminalia as in Fig. 19. Sternal setae as for female. Paratergal setae (all long): II–III, 0; IV–V, 2; VI–VIII, 3. Sternal plates well developed and entire on segments III–VI; subgenital plate with 4 long setae as in Fig. 20, the anterior pair on the plate, and the posterior on the plate margin. Genitalia: length of parameres 0.40 (Fig. 33). Measurements (n = 1): HL 0.38, ANW 0.14, POL 0.15, POW 0.28, ADPL 0.18, ADPLL 0.13, ADPW 0.14, TRL 0.09, TRW 0.03, TW 0.36, PW 0.21, PTL 0.15, PTW 0.31, TPVL 0.22, AW 0.47, GL 0.19, GW 0.08, TL 1.30.
Holotype ♀ (MONZ AI. 030137), ex Gerygone igata igata; NEW ZEALAND, no other data. Paratypes: 1♂, 2♀ (MONZ AI.017303) 1♀ (MZUSP #2885), same data as holotype; 2♀ (MONZ AI.017299), same host species, NEW ZEALAND: Orongorongo Valley, 20.V.1976, B.M. Fitzgerald col.; 1♀ (MONZ AI.017301), same host species, NEW ZEALAND: Kowhai Bush, Kaikoura, 15.XI.1976, B. Gill col.; 1♀ (MONZ AI.017302), same host species, NEW ZEALAND: Orongorongo Valley, 12.V.1977, B.M. Fitzgerald col..
This species is named in memory of the late Professor Robert L.C. Pilgrim (1921–2010), for his outstanding contribution to knowledge of ectoparasitic insects, and for his long friendship with RLP (
Morphologically close to Philopteroides beckeri, especially by features of the head. In addition to the key characters mentioned below, the habitus of both species is distinct (compare Figs 1 and 3; 2 and 4, respectively). Both sexes of Philopteroides pilgrimi can be distinguished by (1) the presence of spine-like setae on sternite V (absent in Philopteroides beckeri); (2) female tergites IX+X with long innermost setae situated on the tergal plate (Fig. 17) (on soft tegument in Philopteroides beckeri, Fig. 13); (3) female subgenital plate without medial setae (Fig. 18) (against three pairs of setae on each side, as in Philopteroides beckeri, Fig. 14); (4) shape of sub-vulval sclerites (compare Figs 14 and 18); (5) male subgenital plates (compare Figs 16 and 20); and (6) male genitalia (compare Figs 31 and 33).
mitsusui species-group
The approximately triangular shape of the head is the distinctive character in the ten species included in this group. The preantennal region is longer (POL 0.22–0.29) and narrower (ANW 0.10–0.12) than in the beckeri species-group, and the hyaline margin is deeply concave at midline. Conus well-developed.
Philopteroides mitsusui (Uchida, 1948)
Bitrabeculus mitsusui Uchida, 1948: 321, fig. 7.
Philopterus mitsusui (Uchida, 1948);
Philopteroides mitsusui (Uchida, 1948);
Type host. Myzomela rubratra dichromata Wetmore, 1919 – Micronesian honeyeater (Meliphagidae)
Distribution. Pohnpei I., Caroline Islands, Micronesia.
Philopteroides kayanobori (Uchida, 1948)
Bitrabeculus kayanobori Uchida, 1948: 322, fig. 8.
Philopterus kayanobori (Uchida, 1948);
Philopteroides kayanobori (Uchida, 1948);
Type host. Spizixos semitorques cinereicapillus Swinhoe, 1871 – Collared finchbill bulbul (Pycnonotidae)
Distribution. Taiwan.
Philopteroides sclerotifrons (Tandan, 1955)
Philopterus sclerotifrons Tandan, 1955: 417, figs 1–7.
Philopteroides sclerotifrons (Tandan, 1955);
Type host. Cinnyris asiaticus asiaticus (Latham, 1790) – Purple sunbird (Nectariniidae)
Distribution. India.
Philopteroides novaezelandiae Mey, 2004
Philopteroides novaezelandiae Mey, 2004: 174, figs 21–22c, d.
Type host. Acanthisitta chloris chloris (Sparrman, 1787) – Rifleman (Acanthisittidae)
Distribution. South Island, New Zealand.
Philopteroides xenicus Mey, 2004
Philopteroides xenicus Mey, 2004: 176, fig. 22a, b, e.
Type host: Xenicus longipes longipes (Gmelin, 1789) – Bush wren (Acanthisittidae)
Distribution. South Island, New Zealand.
Philopteroides cucphuongensis Mey, 2004
Philopteroides cucphuongensis Mey, 2004: 176, fig. 23, table 2.
Type host: Pycnonotus finlaysoni eous Riley, 1940 – Stripe-throated bulbul (Pycnonotidae)
Distribution. Vietnam.
Philopteroides flavala Najer & Sychra, 2012
Philopteroides flavala Najer & Sychra, 2012a: 39, figs 1, 2A–G, 5A–B.
Type host: Hemixos flavala Blyth, 1845 – Ashy bulbul (Pycnonotidae)
Distribution. Vietnam.
Philopteroides terpsiphoni Najer & Sychra, 2012
Philopteroides terpsiphoni Najer & Sychra, 2012b: 95, figs 6–12.
Type host: Terpsiphone viridis (Statius Müller, 1776) – African paradise-flyctacher (Monarchidae)
Distribution. Senegal.
urn:lsid:zoobank.org:act:70A9F313-D518-4143-A683-E76F8168787B
http://species-id.net/wiki/Philopteroides_fuliginosus
Figs 5–6, 11, 21–24, 34–35Rhipidura fuliginosa placabilis Bangs, 1921 – New Zealand fantail (Rhipiduridae)
New Zealand.
Female. Habitus as in Fig. 5 and head as in Fig. 11. Anterior setae 3 (as3) rigid, 0.04–0.05 long; anterior dorsal head setae (ads) peg-like, 0.02 long. Hyaline membrane with deep concavity and thick sclerotization of its margin, 0.03–0.04 long; anterior margin of the anterior dorsal plate deeply concave and ventral head plate more concave than the dorsal one (Fig. 34). Pterothorax with 8 posteromarginal setae on each side. Tergo-pleural plate II with a reduced postero-lateral projection overlapping segment III, tergo-pleural plate III without any projection. Tergocentral setae: II, 12–13 (plus 2 anterior setae); III, 15–16; IV, 13–18; V, 16–19; VI, 16–17; VII, 12–14; VIII, 6–12. Tergites VII–IX+X as in Fig. 21. Sternal setae (intraspecific variation in parentheses): II, SL-SL (plus 1–2 long setae laterad to sternal plate); III, LL-SLL (LSL-SLL); IV, LLSLL-LLSLL (LLSLL-LSLLL); V, LLSLL-LLSLL (LLSLL-LSLLL); VI, LLLL-LLLL (LLLLL-LLLL). Segments IV-VII with 1–4 long additional setae situated on soft tegument, between the sternal plate and the pleural setae. Paratergal setae (all long): II–III, 0; IV–V, 3; VI, 4 (rarely 3 on one side only); VII, 3–4; VIII, 1–2. Segments VI–VIII with an innermost dorsal seta on each tergite (included in the paratergal count), besides the postspiracular seta. Sternites III–VI with well-developed, large plates, roughly rectangular. Vulva with 7–9 medium long setae each side, and 4–6 small setae on the middle of the vulvar margin. Subgenital plate, inner genital sclerite, subvulval sclerites, and vulvar margin as in Fig. 22. Measurements (n = 8): HL 0.45–0.48, ANW 0.09–0.10, POL 0.20–0.22, POW 0.31–0.32, ADPL 0.18–0.20, ADPLL 0.15–0.16, ADPW 0.13–0.14, TRL 0.09–0.10, TRW 0.03–0.04, TW 0.43–0.46, PW 0.25–0.26, PTL 0.16–0.18, PTW 0.35–0.37, TPVL 0.21–0.23, AW 0.53–0.60, EWG 0.05–0.06; IWG 0.03–0.04, TL 1.37–1.49.
Male. Similar to female, except in dimensions and morphology of terminalia (Fig. 6). Pterothorax with 7–9 posteromarginal setae on each side. Tergocentral setae: II, 11–14 (plus 2 anterior setae); III, 10–18; IV, 10–17; V, 9–19; VI, 10–18; VII, 11–13; VIII, 7–8. Tergites VII–IX+X as in Fig. 23. Paratergal setae (all long): II–III, 0; IV–V, 2–4; VI–VII, 3–4; VIII, 2. Sternal plates well developed and entire on segments III–VI; subgenital plate with 4 proximal long setae and 2 distal postero-lateral long setae (Fig. 24). Genitalia: length of parameres 0.50 (Fig. 35). Measurements (n = 8): HL 0.42–0.46, ANW 0.09–0.10, POL 0.20–0.21, POW 0.28–0.30, ADPL 0.18–0.19, ADPLL 0.14–0.15, ADPW 0.11–0.12, TRL 0.09–0.10, TRW 0.03–0.05, TW 0.40–0.42, PW 0.24–0.25, PTL 0.14–0.16, PTW 0.32–0.35, TPVL 0.20–0.21, AW 0.47–0.53, GL 0.21–0.23, GW 0.06–0.07, TL 1.12–1.24.
Holotype ♀ (MONZ AI. 030138), ex Rhipidura fuliginosa placabilis, NEW ZEALAND: Otaihanga, Paraparaumu, WN, 23.III.1996, N. Hyde col. Paratypes: 2♂ (MONZ AI.017297), 1♂, 1♀ (MZUSP #2886), same data as holotype; 2♂, 1♀ (MONZ AI.017295), same host species, NEW ZEALAND: Wallaceville Animal Research Centre, Upper Hutt, 4.IV.1974, D.M. Pearce col.; 1♂ (MONZ AI.017296), same host species, NEW ZEALAND: Little Barrier I., 1.IX.1977, C.R. Veitch col.; 1♂ (MONZ AI.017298), same host species, NEW ZEALAND: Days Bay, Wellington, 8.II.2003, E.W. Dawson col.
1♂ (MONZ AI.017290), ex Rhipidura fuliginosa fuliginosa (Sparrman, 1787), NEW ZEALAND: Jackson Bay, Westland, 6.VII.1969, W. Spiekman col.; 1♀ (MONZ AI.017291), NEW ZEALAND: Coutts I., Canterbury, 22.XII.1970, J.R. Jackson col.; 1♂, 1♀ (MONZ AI.017292), NEW ZEALAND: Nelson, 8.V.1972, B.A. Holloway col.; 2♂, 2♀ (MONZ AI.017293), NEW ZEALAND: Nelson, 22.V.1972, G. Kuschel col.; 1♀ (MONZ AI.017294), NEW ZEALAND: Franz Joseph Glacier, 1973–1974, P. Fletcher col.
The s pecies epithet is a noun in apposition derived from the species name of the host, and also refers to the dark colour of the lice.
Morphologically close to Philopteroides macrocephalus by having: anterior dorsal head setae (ads) peg-like; segment III without pleural setae; segment IV with 2 pleural setae; sternite VI without spine-like setae; setae of the male subgenital plate situated on the plate. However, both sexes of Philopteroides fuliginosus can be distinguished from those of Philopteroides macrocephalus by: (1) the hyaline margin with a shallower indentation (0.03–0.04mm) at its midline (compare Figs 11 and 12; 34 and 36); (2) the female tergal chaetotaxy and shape of tergites IX+X (Fig. 21); (3) the male subgenital plate (Fig. 24); and (4) the male genitalia (Fig. 34).
urn:lsid:zoobank.org:act:968250A7-D7C5-470A-8BFE-5EBA96AC5314
http://species-id.net/wiki/Philopteroides_macrocephalus
Figs 7–8, 12, 25–28, 36–37Petroica macrocephala macrocephala (Gmelin, 1789) – New Zealand tit or tomtit (Petroicidae)
New Zealand.
Female. Habitus as in Fig. 7, and head as in Fig. 12. Anterior setae 3 (as3) rigid, peg-shaped, 0.05–0.06 long; anterior dorsal head setae (ads) peg-like, 0.02 long. Hyaline membrane with deep concavity and thin sclerotization, 0.06–0.08 long; anterior dorsal plate slightly concave and ventral head plate deeply concave (Fig. 36). Pterothorax with 9 posteromarginal setae on each side. Tergo-pleural plate II with a much reduced postero-lateral projection overlapping segment III; tergo-pleural plate III without any projection. Tergocentral setae: II, 17–19 (plus 2 anterior setae); III, 23–25; IV, 25–27; V, 26–27; VI, 24–26; VII, 23–25; VIII, 12–14. Tergites VII–IX+X as in Fig. 25. Sternal setae: II, SL-LS; III, LLLLSL-LSLLLL; IV, LLLLSLL-LLSLLLL; V, LLLLLSLL-LLSLLLLL; VI, LLLLL-LLLLL (some specimens show slight variations from this symmetrical pattern). Segments II–VII with 3–5 additional long setae situated on the soft tegument, between the sternal plate and the pleural setae. Paratergal setae (all long): II–III, 0; IV, 5–6; V, 4–5; VI–VII, 4; VIII, 2. Tergites VI–VII with an innermost dorsal seta (included in the paratergal count), besides the postspiracular setae (not included in the count). Sternites III–VI with large, well-developed plates, roughly rectangular. Vulva with 9–11 medium to long setae each side, and 14–15 small setae in the middle of the vulvar margin. Subgenital plate, inner genital sclerite, subvulval sclerites, and vulvar margin as in Fig. 26, plus a variation of an additional female in Fig. 26a. Measurements (n = 7): HL 0.59–0.62, ANW 0.10–0.12, POL 0.28–0.30, POW 0.40–0.41, ADPL 0.24–0.25, ADPLL 0.20–0.21, ADPW 0.18–0.19, TRL 0.11–0.13, TRW 0.04–0.05, TW 0.58–0.64, PW 0.30–0.36, PTL 0.21–0.25, PTW 0.44–0.48, TPVL 0.25–0.27, AW 0.72–0.79, EWG 0.05–0.06, IWG 0.03–0.04, TL 1.85–2.13.
Male. Similar to female, except in dimensions and morphology of terminalia (Fig. 8). Pterothorax with 7–9 posteromarginal setae on each side. Tergocentral setae: II, 17–18 (plus 2 anterior setae); III, 17–19; IV, 22–24; V, 18–20; VI, 17–20; VII, 16–18; VIII, 10–12. Paratergal setae (all long): II–III, 0; IV–V, 4 (rarely 2 setae on one side only); VI–VII, 4–5 (rarely 3 setae on one side only); VIII, 2. Sternal plates III–VI well developed and entire; 4 long setae on the subgenital plate with only the anterior pair situated on the plate (Fig. 28). Genitalia: length of parameres 0.50 (Fig. 37). Measurements (n = 9): HL 0.55–0.57, ANW 0.10–0.12, POL 0.27–0.28, POW 0.37–0.39, ADPL 0.23–0.25, ADPLL 0.27–0.28, ADPW 0.16–0.17, TRL 0.10–0.12, TRW 0.04–0.05, TW 0.51–0.55, PW 0.28–0.30, PTL 0.17–0.20, PTW 0.42–0.44, TPVL 0.25–26, AW 0.62–0.65, GL 0.27–0.32, GW 0.07–0.08, TL 1.49–1.61.
Holotype ♀ (MONZ AI.030122), ex Petroica macrocephala macrocephala, NEW ZEALAND: Haast Pass, 30.IX.1969, C.N. Challies col. (University of Canterbury). Paratypes: 3♂, 3♀ (MONZ AI.017278 & AI.017279), 1♂, 1♀ (MZUSP #2887), same data as holotype.
2♀ (MONZ AI.017281), ex Petroica macrocephala dannefaerdi (Rothschild, 1894), NEW ZEALAND: Penguin Creek, Snares Island, 29.III.1972, D.S. Horning & C.J. Horning cols.; 1♂ (MONZ AI.017282), NEW ZEALAND: Boat Harbour, Snares Is, 22.II.1975, H.A. Best col.; 1♂ (MONZ AI.017283), NEW ZEALAND: Station Point, Snares Island, 25.II.1977, D.S. Horning col.; 1♂ (MONZ AI.017284), NEW ZEALAND: Tern Point, Snares Islands, 10.I.1987, A. Tennyson col.; 1♂ (MONZ AI.017280), NEW ZEALAND: Snares Is, no date, CMu Av. 4635. 1♂ (MONZ AI.017277), ex Petroica macrocephala, NEW ZEALAND: Springs Junction, Maruia, 20.VIII.1966, J.R. Jackson col.
The s pecies epithet is a noun in apposition derived from the species name of the host, and also refers to the larger head of this louse species.
Morphologically close to Philopteroides fuliginosus by the features mentioned under that species above. However, both sexes of Philopteroides macrocephalus can be distinguished from those of Philopteroides fuliginosus by (1) the hyaline margin with a deeper incision (0.06–0.08mm) at its midline (compare Figs 11 and 12, Figs 34 and 36); (2) the female tergal chaetotaxy and shape of tergites IX+X (compare Figs 21 and 25); (3) the male subgenital plate (compare Figs 24 and 28); and (4) the male genitalia (compare Figs 35 and 37).
Key to the species of Philopteroides Mey, 2004 (adults only) *
*except Philopteroides lineatus (Giebel, 1874)
| 1 | Preantennal region short (POL ≤0.15–0.17) and broad (ANW ≥0.13–0.15); hyaline margin with a shallow concavity at midline (Figs 9–10, 30, 32) | beckeri species-group, 2 |
| – | Preantennal region long (POL ≥0.20–0.28) and narrow (ANW ≤0.10–0.12); hyaline margin with a deep concavity at midline (Figs 11–12, 34, 36) | mitsusui species-group, 3 |
| 2 | Sternite V without spine-like setae. Females: posterior setae on tergite IX+X situated outside plate (Fig. 13); subgenital plate with median setae (Fig. 14) | Philopteroides beckeri (Mey, 2004) |
| – | Sternite V with at least one pair of spine-like setae. Females: posterior setae on tergite IX+X situated on plate (Fig. 17); subgenital plate without median setae (Fig. 18) | Philopteroides pilgrimi Valim & Palma, sp. n. |
| 3 | Pleural setae present on segment III | Philopteroides kayanobori (Uchida, 1948) |
| – | Pleural setae absent on segment II | 4 |
| 4 | Pleural segment IV with only 1 setae on each side | Philopteroides mitsusui (Uchida, 1948) |
| – | Pleural segment IV with 2 or more setae on each side | 5 |
| 5 | Anterior dorsal setae (ads) peg-like (Figs 34, 36) | 6 |
| – | Anterior dorsal setae (ads) thin and rigid (Figs 30, 32) | 10 |
| 6 | Sternite VI with at least one pair of spine-like setae; without additional setae between pleural and sternal setae; sternites sexually dimorphic | Philopteroides terpsiphoni Najer & Sychra, 2012 |
| – | Sternite VI without spine-like setae; with additional setae between pleural and sternal setae, situated on soft tegument; sternites not dimorphic | 7 |
| 7 | Males: anterior setae of the subgenital plate situated outside plate | 8 |
| – | Males: anterior setae of the subgenital plate situated on plate | 9 |
| 8 | Sternite V with at least one pair of spine-like setae. Male genitalia with a large trapezoidal medial sclerite on basal plate (as in fig. 22e in |
Philopteroides xenicus Mey, 2004 |
| – | Sternite V without spine-like setae. Male genitalia with a small roughly oval medial sclerite on basal plate (as in fig. 22d in |
Philopteroides novaezelandiae Mey, 2004 |
| 9 | Hyaline margin with a deep incision (0.06–0.08mm) at its midline (Figs 12, 36). Female tergite IX+X with an anterior and a posterior notch (Fig. 25). Lateral margin of the male subgenital plate with deep incisions (Fig. 28); male genitalia as in Fig. 37 | Philopteroides macrocephalus Valim & Palma, sp. n. |
| – | Hyaline margin with a shallow incision (0.03–0.04mm) at its midline (Figs 11, 34). Female tergite IX+X without anterior notch and with an uneven posterior margin (Fig. 21). Lateral margin of the male subgenital plate with shallow incisions (Fig. 24); male genitalia as in Fig. 35 | Philopteroides fuliginosus Valim & Palma, sp. n. |
| 10 | Females without central sternal plates, only with lateral rounded sternal plates on III–VI. Males without lateral rounded sternal plates | Philopteroides sclerotifrons (Tandan, 1955) |
| – | Females with central sternal plates, including lateral rounded sternal plates on III–VI (occasionally fused with the central plates). Males with lateral rounded sternites on at least segment III | 11 |
| 11 | With lateral rounded sternal plates on segment II. Females with a central sclerite plus two rounded lateral ones (separated from each other) on sternite VI | Philopteroides flavala Najer & Sychra, 2012 |
| – | Without lateral sternal plates on segment II. Females with a unique central sclerite, and only vestiges of lateral rounded sternites (fused to the central sclerite) on sternite VI | Philopteroides cucphuongensis Mey, 2004 |
We thank Dr Eberhard Mey (Naturhistorischen Museum, Rudolstadt, Thüringen, Germany) for the loan of the types of Tyranniphilopterus beckeri deposited in NHMR, and for his assistance in the interpretation of the original German description of Docophorus lineatus in