(C) 2012 Augusto Loni. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The finding of Zombrus bicolor (Enderlein) (Hymenoptera: Braconidae: Doryctinae) in a Tuscan vineyard of the Siena province (Italy) represents the first record of this species in western Europe. A female was captured in summer 2009 with a malaise trap located in an organic vineyard. Until this finding, the species was recorded only in the Oriental regions of continental China, Taiwan, Korea and Japan and, very recently, in the eastern and southern parts of the Palaearctic region.
Vineyard, new finding, wood borer parasitoid, exotic species, Anoplophora chinensis
A single female of Zombrus bicolor (Enderlein) (Hymenoptera: Braconidae: Doryctinae) was collected in June 2009 in a malaise trap located in a vineyard (cv Sangiovese) of an organic farm in the Montalcino district (Siena-Tuscany-Italy, 43°05.21N, 11°28.51E). The trap was installed at ca 180 m above sea level in a typical rural Tuscan landscape, with vineyards included in rolling, gentle hills surrounded by cultivated grassland areas, woods and strips of shrubs, and wild bushes of deciduous trees. Until the current finding, Zombrus bicolor had only been reported as an Eastern Palaearctic and Oriental species, based on the description of specimens collected in Taiwan, China, Mongolia, Japan, Korea and, very recently, in Kyrgyzstan and in the European part of Russia (Astrakhan province) (
The type specimens of Zombrus bicolor are two males stored in the collection of the Museum of the Zoology Institute in Warsaw (Poland). These males were collected by H. Sauter in 1907 in Takao (Formosa), the old name of the current city of Kaohsiung in south-western Taiwan, and subsequently described by
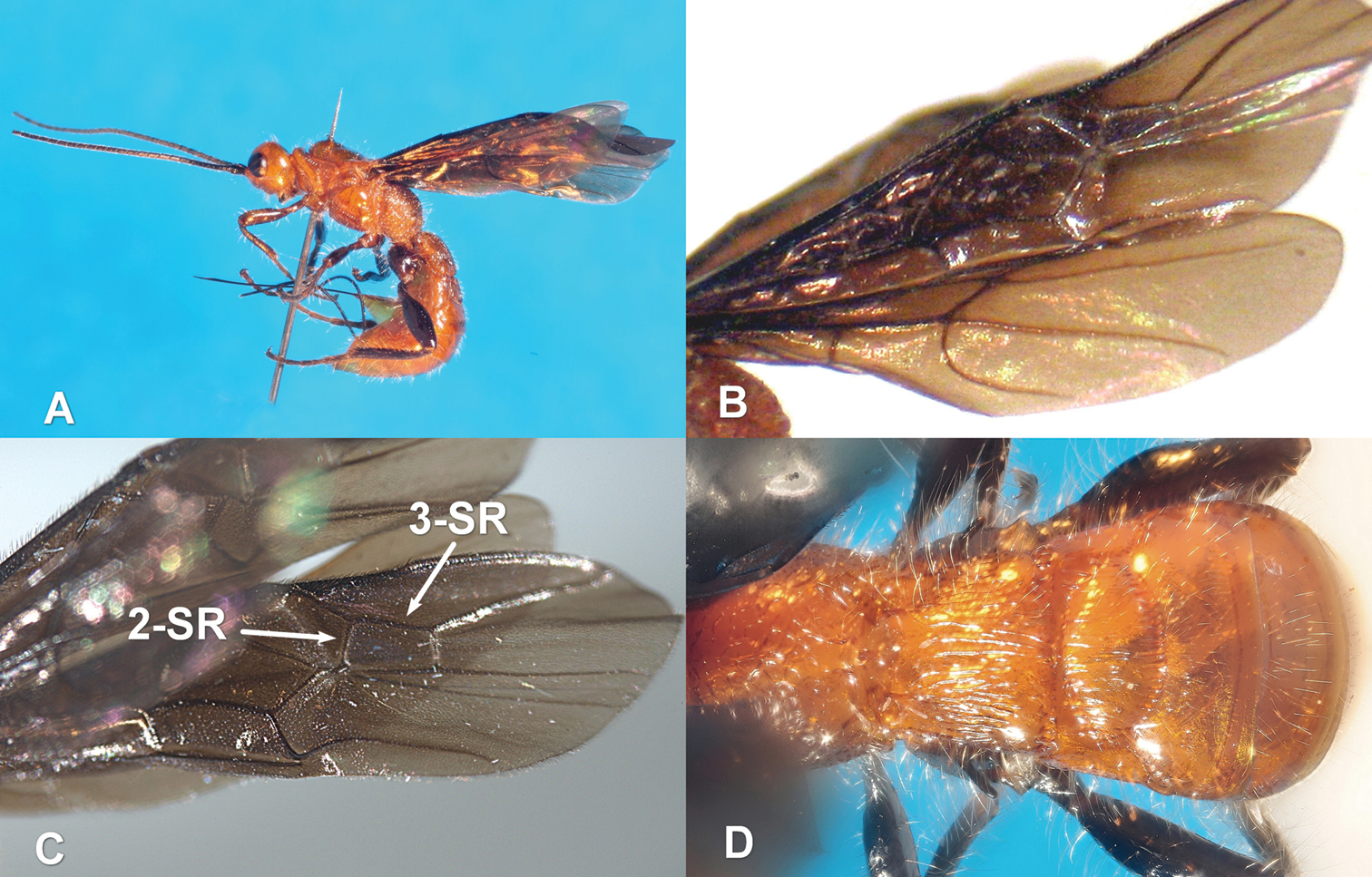
Zombrus bicolor female: A body lateral view B fore and hind wings C detail on the fore wing D abdominal terga 1-3 dorsal view.
Zombrus bicolor (Fig. 1A) belongs to the subtribe Odontobraconina of the tribe Holcobraconini, a monophyletic group including six genera and currently divided in the three subtribes Holcobraconina, Odontobraconina and Ivondroviina, (
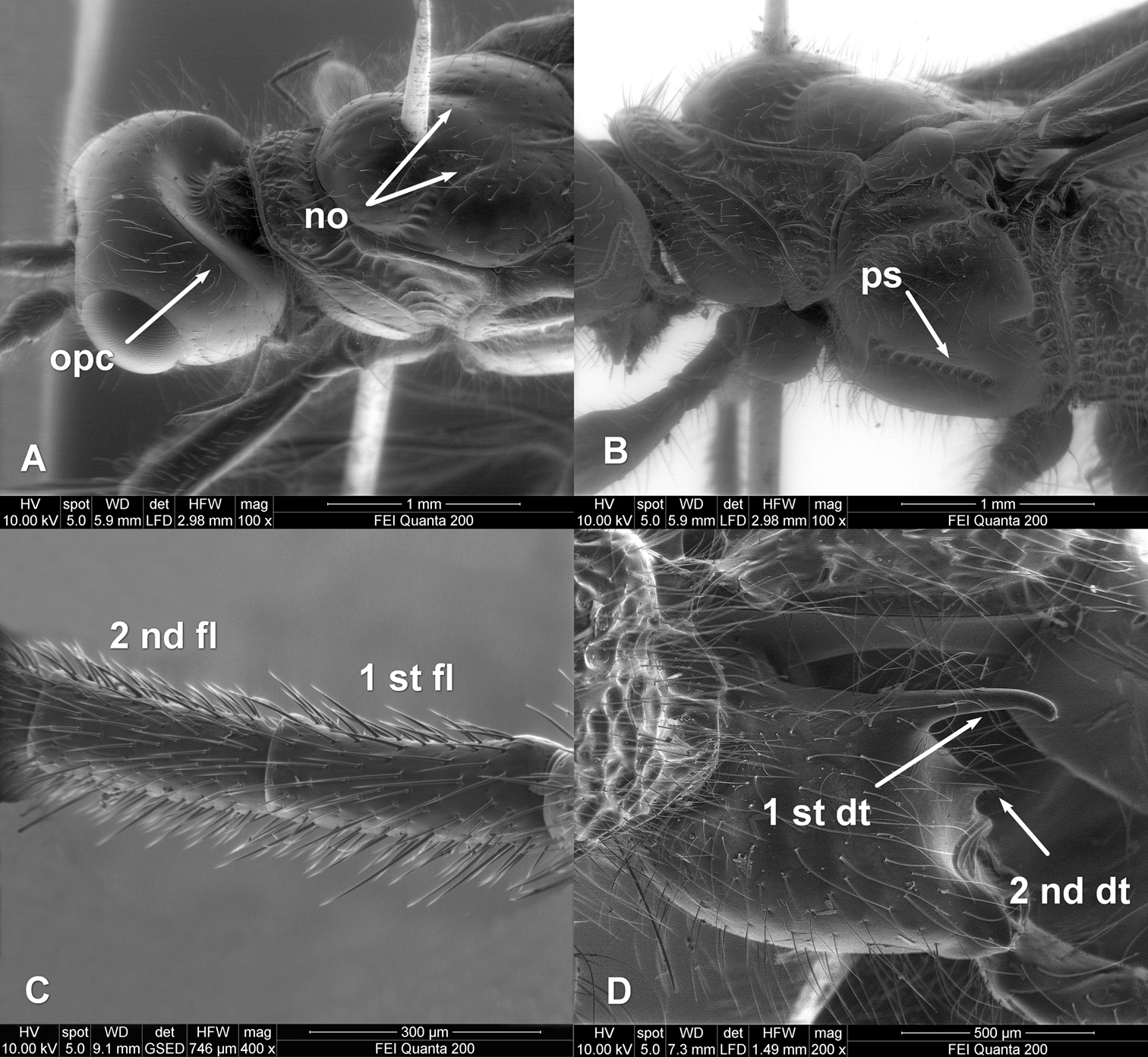
According to these authors, the typical morphological features of Zombrus bicolor, which distinguish this species from the other Palaearctic species of Zombrus, are the completely dark fore wings (Fig. 1B), the body covered with long and dense setae (Fig. 1 D, Fig. 2 A, B, D) and the presence of the occipital carina dorsally and, partly, laterally (Fig. 2A).
Zombrus bicolor female, SEM micrographs details on: A head and mesoscutum B mesopleuron C first and second flagellomeres D hind coxa. Abbreviations: 2-SR and 3-SR sectio radii veins of fore wing opc = occipital carina no = notauli ps = precoxal sulcus 1st and 2ndfl = first and second flagellomeres 1st and 2nddt = first and second dorsal teeth of hind coxa. Morphological terminology is used according to
All the features of the specimen we collected in Italy match well with those reported by
Our finding could represent one of the many cases of accidental introduction of exotic insects that have occurred in Italy in the last decades. At European level as well, the accidental introduction of new insect species from their original areas has become more and more frequent and alarming. Intensification of plant trade with geographically distant commercial partners and global warming, that allows aliens to establish successfully in new ecosystems, are considered the main causes of the occurrence of invasive species.
As an important crossroad in the Mediterranean basin, Italy seems particularly suitable to the introduction and settlement of alien species, also because its landmass extends over a wide range of latitudes climatically suitable to harbour subtropical species in the South and insects coming from temperate and continental zones in the northern and central regions of the peninsula.
It has been estimated that about 200 exotic species have been introduced and established in Italy since 1970 (
Importantly, our finding concerns not a pest but a parasitoid, which in this case could have followed his victim/s in the transfer from one continent to another. This is permissible every time that the natural enemy is a cryptic species, such as an endoparasitoid, or an ectoparasitoid living in concealed galleries excavated by larvae of wood boring insects.
Zombrus bicolor is known as a solitary, larval parasitoid of many wood boring beetles (
Though data are not available at the moment on the presence of Anoplophora chinensis in the areas where we collected Zombrus bicolor, we cannot exclude that the braconid, in its movement to West Europe, had been carried by these or other specific hidden hosts.
We are grateful to Dr. S.A. Belokobylskij (Zoological Institute, Russian Academy of Sciences, St Petersburg) for his useful suggestions.