(C) 2012 Michael G. Rix. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The assassin spiders of the family Archaeidae from tropical north-eastern Queensland are revised, with eight new species described from rainforest habitats of the Wet Tropics bioregion and Mackay-Whitsundays Hinterland: Austrarchaea griswoldi sp. n., Austrarchaea hoskini sp. n., Austrarchaea karenae sp. n., Austrarchaea tealei sp. n., Austrarchaea thompsoni sp. n., Austrarchaea wallacei sp. n., Austrarchaea westi sp. n. and Austrarchaea woodae sp. n. Specimens of the only previously described species, Austrarchaea daviesae Forster & Platnick, 1984, are redescribed from the southern Atherton Tableland. The rainforests of tropical eastern Queensland are found to be a potential hotspot of archaeid diversity and endemism, with the region likely to be home to numerous additional short-range endemic taxa. A key to species complements the taxonomy, with maps, natural history information and conservation assessments provided for all species.
New species, taxonomy, conservation, Wet Tropics, rainforests, Palpimanoidea
Few families of Australian spiders are as distinctive or as enigmatic as the ‘assassin spiders’ of the family Archaeidae, renowned for their unique cephalic morphology, strange araneophagic biology, great phylogenetic antiquity and relictual biogeography across the Southern Hemisphere. Although once considered to be one of the rarest of spiders families – and certainly one of the least understood in terms of taxonomy, phylogeny and biology – recent and ongoing research in the U.S.A., South Africa and Australia has shed increasing light on this remarkable lineage of spiders (see
Australian Archaeidae have been progressively revised over the last two years, with 26 new species described since 2011, taking the total number of currently described Australian species to 30 (
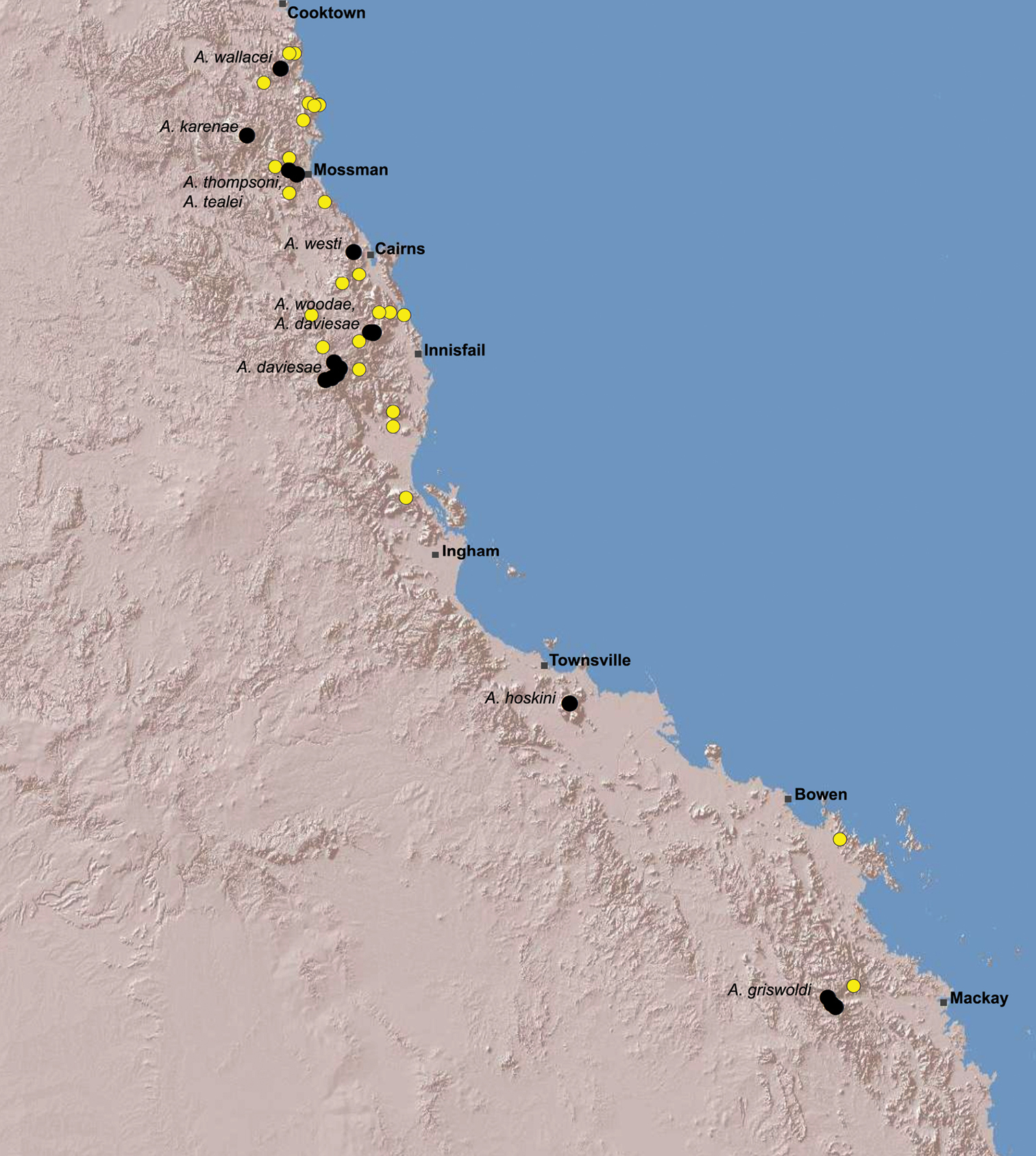
The current paper – the third and final in a series revising the Archaeidae of Australia – presents a taxonomic revision of the assassin spiders from tropical north-eastern Queensland, including those species from the Mackay-Whitsundays Hinterland and the Wet Tropics bioregion, between Cooktown and Townsville (Fig. 2, 25). This revision takes the total number of described Australian Archaeidae to 38 species, with the genus Austrarchaea now including 27 described short-range endemic species.
Material and methodsAll taxa were described and illustrated from specimens stored in 75% or 95% ethanol. Digital images were taken using a Leica MZ16A binocular microscope and a Leica DM2500 compound microscope, with auto-montage images captured using Leica DFC500 mounted cameras with Leica Application Suite Version 3.6.0 software. Male left pedipalps were dissected prior to imaging and bulbs were aligned for standardised comparison in the ventral and retrolateral positions illustrated. Female genitalia were dissected and cleared in a 10% lactic acid plus 90% glycerol solution, prior to mounting on temporary glass slides and imaging in a postero-ventral position (Fig. 14G; see also
Measurements are in millimetres (rounded to the nearest hundredth of a millimetre) and were taken using an ocular graticule on a Leica M80 binocular microscope. Left legs were removed from specimens prior to taking measurements and imaging lateral body profiles. Lateral profile images were standardised for inter-specific comparison by vertically aligning the centre of each left anterior median eye with the lower anterior margin of the carapace (above the labrum) (
Abbreviations used in the text are as follows:
CH/CL Carapace height (CH) to carapace length (CL) ratio
F1/CL Femur I length (F1) to carapace length (CL) ratio
HPC Highest point of pars cephalica
HT 1-4 Abdominal hump-like tubercles 1-4
SEM Scanning electron micrograph/s
TS 1-3 Tegular sclerites 1-3
Specimens described in this study are lodged at the following institutions:
AMS Australian Museum, Sydney (G. Milledge)
ANIC Australian National Insect Collection, Canberra (B. Halliday)
CASENT California Academy of Sciences, San Francisco (C. Griswold, A. Carmichael)
QMB Queensland Museum, Brisbane (R. Raven, O. Seeman)
WAM Western Australian Museum, Perth (MSH, J. Waldock)
Taxonomy Family Archaeidae Koch & Berendt, 1854http://species-id.net/wiki/Austrarchaea
Archaea nodosa Forster, 1956, by original designation.
Diagnosis. Species of Austrarchaea can be distinguished from all southern Australian species of Zephyrarchaea by the significantly taller carapace (CH/CL ratio ≥ 2.0), by the presence of accessory setae on the distal bulge of the male cheliceral paturon, and by the fusion of the two conductor sclerites on the male pedipalp (
For a full generic description see
Species of Austrarchaea occur in mesic habitats throughout eastern Queensland and New South Wales (Fig. 3), usually in montane rainforests (Figs 1E-F), but also in lowland rainforests or wet eucalypt forests on or adjacent to the Great Dividing Range (
Nineteen described species – Austrarchaea alani Rix & Harvey, 2011, Austrarchaea aleenae Rix & Harvey, 2011, Austrarchaea binfordae Rix & Harvey, 2011, Austrarchaea christopheri Rix & Harvey, 2011, Austrarchaea clyneae Rix & Harvey, 2011, Austrarchaea cunninghami Rix & Harvey, 2011, Austrarchaea daviesae Forster & Platnick, 1984, Austrarchaea dianneae Rix & Harvey, 2011, Austrarchaea harmsi Rix & Harvey, 2011, Austrarchaea helenae Rix & Harvey, 2011, Austrarchaea judyae Rix & Harvey, 2011, Austrarchaea mascordi Rix & Harvey, 2011, Austrarchaea mcguiganae Rix & Harvey, 2011, Austrarchaea milledgei Rix & Harvey, 2011, Austrarchaea monteithi Rix & Harvey, 2011, Austrarchaea nodosa (Forster, 1956), Austrarchaea platnickorum Rix & Harvey, 2011, Austrarchaea raveni Rix & Harvey, 2011, Austrarchaea smithae Rix & Harvey, 2011 – plus the eight new species from north-eastern Queensland: Austrarchaea griswoldi sp. n., Austrarchaea hoskini sp. n., Austrarchaea karenae sp. n., Austrarchaea tealei sp. n., Austrarchaea thompsoni sp. n., Austrarchaea wallacei sp. n., Austrarchaea westi sp. n. and Austrarchaea woodae sp. n.
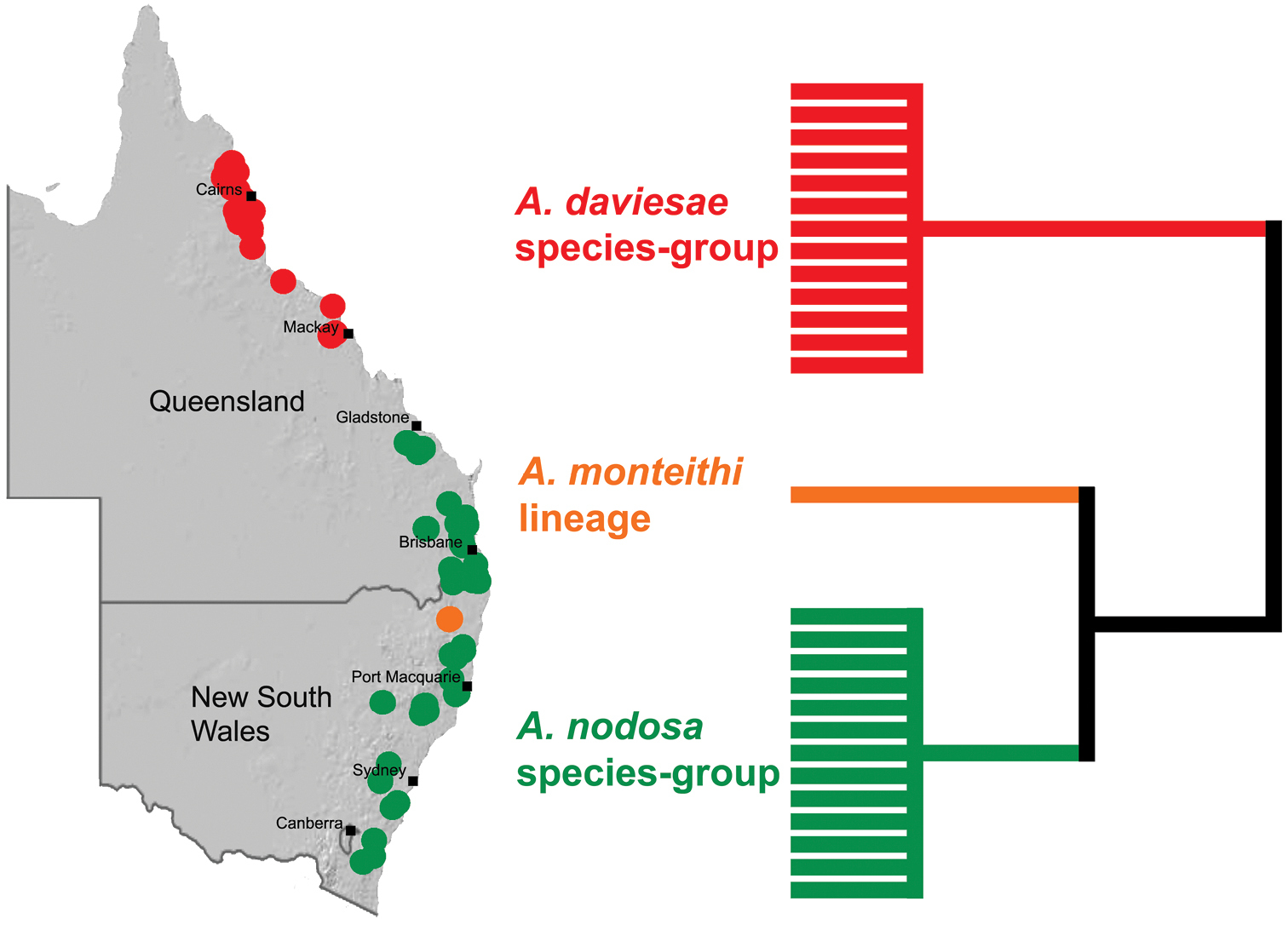
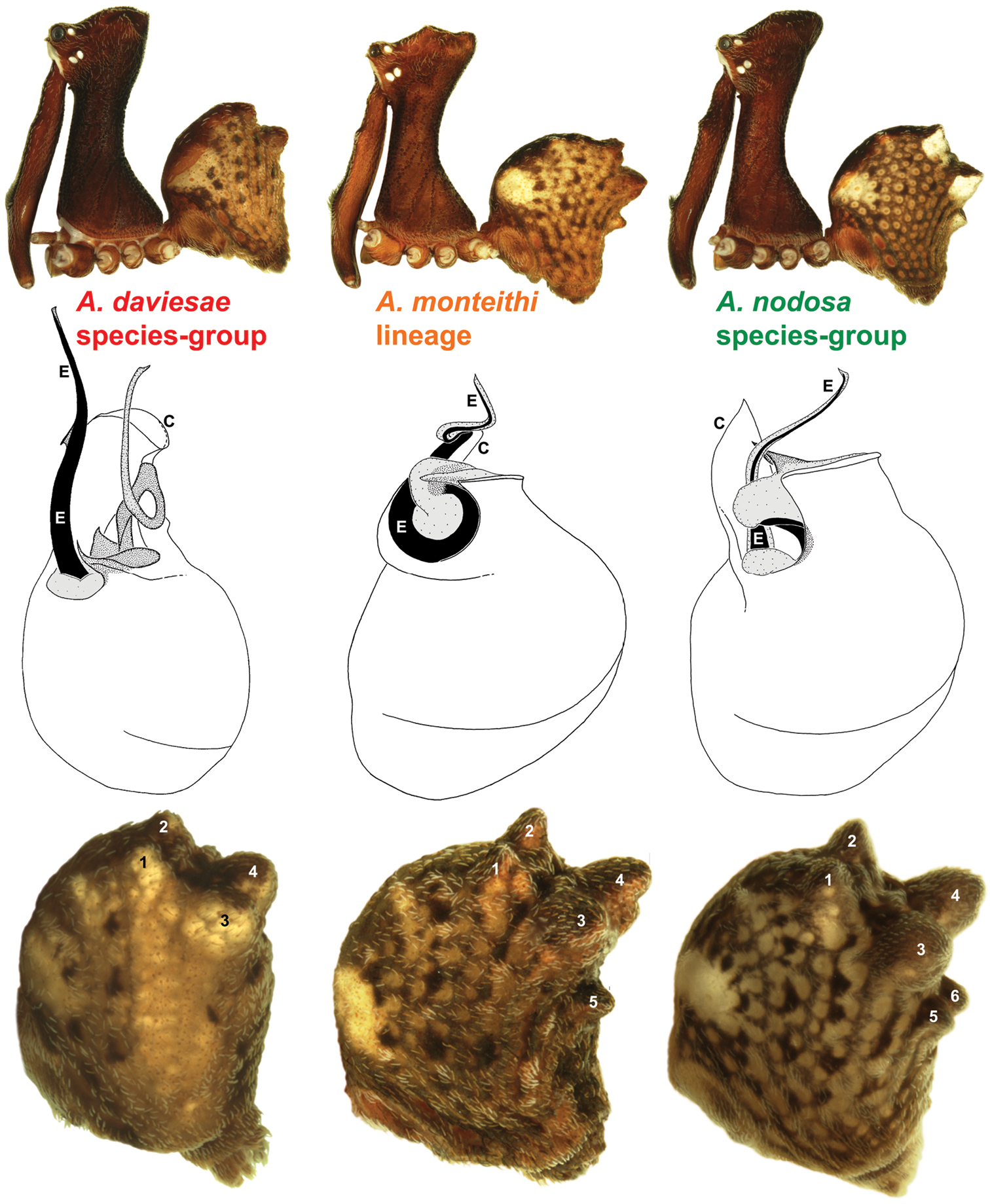
The genus Austrarchaea includes three major lineages in eastern Australia (Figs 3–4), each readily distinguished by the morphology of the abdomen and the structure of the male pedipalp (Fig. 4). The most widespread lineage (the Austrarchaea nodosa species-group) occurs south of the St Lawrence Gap, from Kroombit Tops National Park in central Queensland, south to the Badja State Forest in southern New South Wales (Fig. 3); species in this lineage possess six dorsal hump-like tubercles on the abdomen and an exposed tegular cavity with a variably scutiform conductor (Fig. 4). The second, most restricted lineage (the Austrarchaea monteithi lineage) is known only from the Gibraltar Range National Park in northern New South Wales (Fig. 3); the single known species, Austrarchaea monteithi, possesses five dorsal hump-like tubercles on the abdomen and an exposed tegular cavity with a hooked conductor (Fig. 4). The third lineage (the Austrarchaea daviesae species-group; revised in this paper) occurs north of the St Lawrence Gap, from Eungella National Park north to Cooktown (Figs 3, 25); species in this lineage possess only four dorsal hump-like tubercles on the abdomen (recumbent in Austrarchaea woodae sp. n.) and a more enclosed tegular cavity with a very large, arched conductor (Figs 4, 6–15).
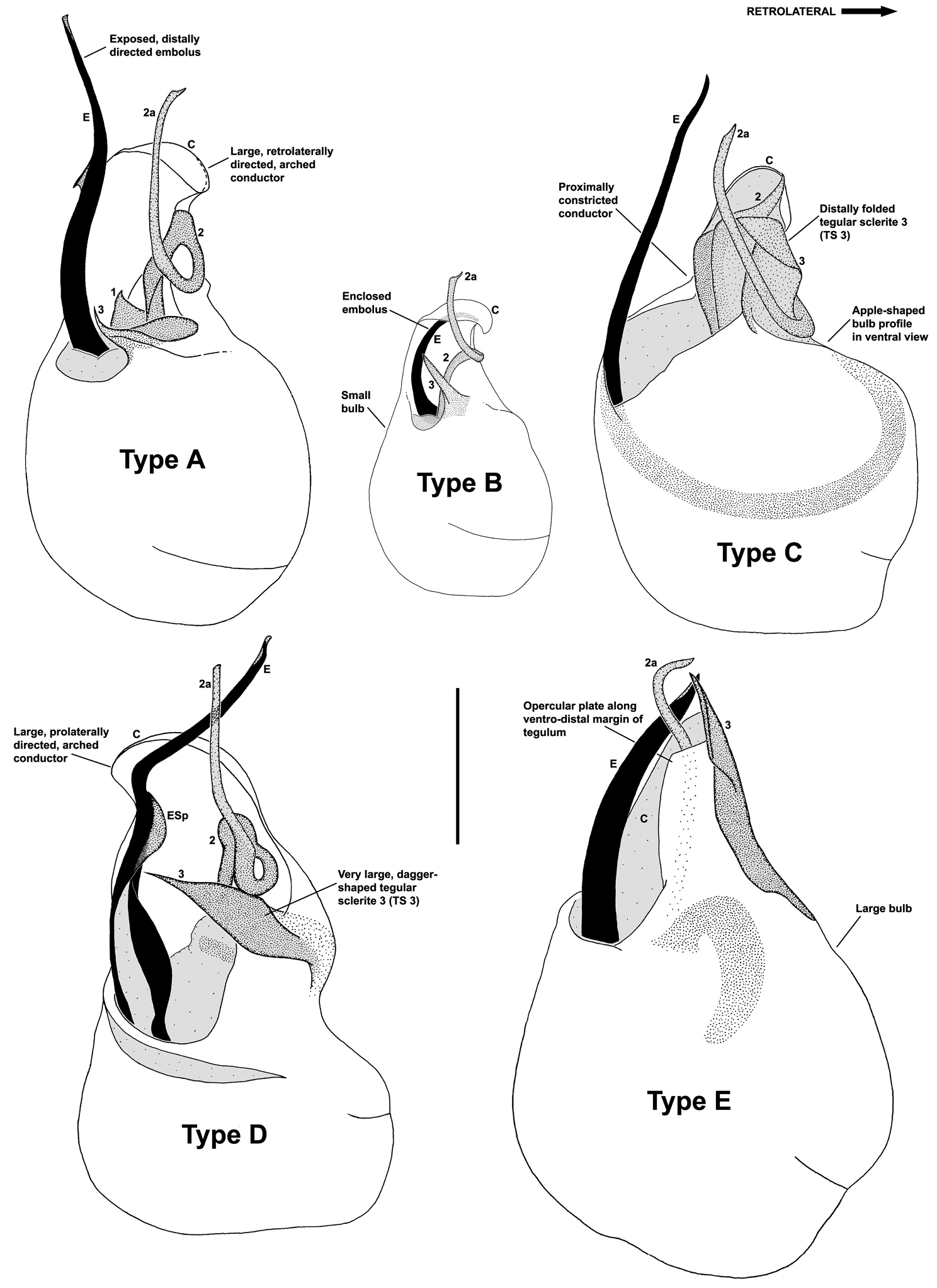
Although the derived pedipalpal morphology of Austrarchaea daviesae and its relatives is strikingly different to that of congeners further south, the distal tegular sclerites can nonetheless be broadly homologised with those of Austrarchaea nodosa and Austrarchaea monteithi on the basis of their shape and relative position in the unexpanded tegular cavity. The embolus in all nine known north-eastern Queensland species is a long, sinuous, strongly sclerotized process emerging from the distal bulb pro-ventrally, in some species bearing an additional accessory spur. Tegular sclerite 3 (TS 3) is always a prominent, pro-ventrally directed process, which is fused to the retro-ventral margin of the tegular bulb (the latter of which is usually also concomitantly modified). Tegular sclerite 2 (plus 2a, i.e. TS 2-2a) is usually inserted just behind TS 3 in the unexpanded tegular cavity, forming a distinctive, mesally-looped and distally whip-like structure common to all taxa in the Austrarchaea daviesae species-group; the extent of this very long, whip-like TS 2a is usually proximate to the distal extension of the embolus in the unexpanded state. This TS 2-2a morphology is in stark contrast to that of Austrarchaea monteithi, Austrarchaea nodosa and related species, in which TS 2a is usually covered and largely obscured by a more spur-like TS 2 process. Tegular sclerite 1 (TS 1) – generally the most prominent sclerite in species of Zephyrarchaea and other species of Austrarchaea – is reduced and often obscured in most archaeid species from north-eastern Queensland, although a few taxa possess a larger, more distinctive TS 1 posterior to the TS 2-2a complex (e.g. Fig. 9D). Inter-specific variation among taxa in the Austrarchaea daviesae species-group is pronounced, with male pedipalp morphologies usually highly autapomorphic for each species. Five broad pedipalp types (Types A-E) can be distinguished among north-eastern Queensland taxa, with Type A being the most common form, shared between five of the nine known species, and Types B-E each currently unique to single species. Figure 6 highlights differences between these different pedipalp morphologies, which are further diagnosed in the Key to species (see below).
| 1 | Distal embolus enclosed within conductor (Fig. 12D); pedipalp very small, width of bulb << 0.30 mm (Fig. 12D) (Type B pedipalp; Fig. 6) | Austrarchaea westi sp. n. |
| – | Distal embolus fully exposed, projecting distally, not enclosed within conductor (Figs 7E, 13D, 15E); pedipalp larger, width of bulb > 0.30 mm | 2 |
| 2 | Conductor arched, directed prolaterally in ventral view (Fig. 14E); tegular sclerite 3 (TS 3) very large, dagger-like, directed pro-ventrally across bulb (Figs 14E-F); embolus with prominent, rounded, fin-shaped spur (Fig. 14E) (Type D pedipalp; Fig. 6) | Austrarchaea hoskini sp. n. |
| – | Conductor directed retrolaterally in ventral view (Figs 7E, 8D, 10D); tegular sclerite 3 (TS 3) not dagger-like; embolic spur, if present, with pointed apex (Figs 9E, 11F) | 3 |
| 3 | Distal bulb and proximal conductor strongly constricted laterally, forming uniquely apple-shaped pedipalpal profile in ventral view (Figs 13C–D); tegular sclerite 3 (TS 3) large, flattened, with prominent, distally folded apex (Figs 13D-E) (Type C pedipalp; Fig. 6) | Austrarchaea woodae sp. n. |
| – | Distal bulb and proximal conductor not constricted laterally; tegular sclerite 3 (TS 3) not folded distally | 4 |
| 4 | Ventro-distal rim of tegulum distally extended to form rectangular opercular plate, covering tegular sclerite 2a (TS 2a) for most of its length (Fig. 15E); tegular sclerite 3 (TS 3) very large, flattened, extending along entire retrolateral edge of conductor (Fig. 15F) (Type E pedipalp; Fig. 6) | Austrarchaea griswoldi sp. n. |
| – | Ventro-distal rim of tegulum not forming rectangular opercular plate; tegular sclerite 3 (TS 3) shorter, spur-like (Figs 7E, 8D, 9D, 10D, 11E); conductor arched, directed retrolaterally in ventral view, not abutting TS 3 (Figs 7E, 9D, 10D, 11E) (Type A pedipalp; Fig. 6) | 5 |
| 5 | Embolus with triangular embolic spur (Figs 8D, 9E, 10D, 11F); embolus projecting beyond distal rim of conductor by > 1/3 length of exposed embolic portion (Figs 9D, 10D, 11E) | 6 |
| – | Embolus without embolic spur (Fig. 7E); embolus projecting beyond distal rim of conductor by ~1/3 length of exposed embolic portion (Figs 7D-E) | Austrarchaea daviesae Forster & Platnick, 1984 |
| 6 | Embolic spur distally positioned, situated close to pro-distal margin of conductor (slightly proximal to distal-most curve of embolus tip) (Figs 9D, 11E); tegular sclerite 1 (TS 1) relatively large, triangular, visible in ventral view posterior to TS 2-3 (Figs 8D, 9D, 11E) | 7 |
| – | Embolic spur more proximally positioned, situated near base of exposed embolic portion (Fig. 10D); tegular sclerite 1 (TS 1) small, obscured by TS 2-3, not visible in ventral view (Fig. 10D) | Austrarchaea thompsoni sp. n. |
| 7 | Tegular sclerite 3 (TS 3) with sharply pointed, claw-like apex (Figs 9D–E, 11E-F) | 8 |
| – | Tegular sclerite 3 (TS 3) with more bluntly pointed, triangular apex (Figs 8C–D) | Austrarchaea wallacei sp. n. |
| 8. | Tegular sclerite 1 (TS 1) broadly triangular in ventral view (Fig. 9D); tegular sclerite 3 (TS 3) with single, sharply pointed process distally (Fig. 9D) | Austrarchaea karenae sp. n. |
| – | Tegular sclerite 1 (TS 1) with more tapered, tooth-like triangular apex in ventral view (Fig. 11E); tegular sclerite 3 (TS 3) with second short, pointed process distally (Fig. 11E) | Austrarchaea tealei sp. n. |
http://species-id.net/wiki/Austrarchaea_daviesae
Misty Mountains Assassin SpiderFigs 7, 16, 25
Holotype male: Majors Mountain, [Tully Falls National Park], Atherton Tableland, Queensland, Australia, [17°38'25"S, 145°32'14"E], collected at night, 14–20.IV.1978, V. Davies, R. Raven (QMB S1091).
Paratypes: Allotype female, “Malaan State Forest” [= Malaan National Park], Atherton Tableland, Queensland, Australia, [17°35'S, 145°35'E], 20–24.IV.1978, V. Davies, R. Raven (QMB S1092).
AUSTRALIA: Queensland: Tully Falls National Park (Atherton Tableland): Massey Creek, 17°37'S, 145°34'E, flight intercept trap, 1000 m, 2–30.V.1996, P. Zborowski, 1♀ (ANIC). Malaan National Park (Atherton Tableland): “Malaan State Forest”, on Highway, 17°35'S, 145°35'E, pitfall trap, 850 m, 7.III.–15.V.1995, G. Monteith, J. Hasenpusch, 1 juvenile (QMB S38624); Mount Fisher, 7 km SW. of Millaa Millaa, pyrethrum knockdown, 1050–1100 m, 27–29.IV.1982, G. Monteith, D. Yeates, D. Cook, 1 juvenile (QMB S30838); next to Old Palmerston Highway, opposite Biggs Road, SSW. of Millaa Millaa, 17°35'11"S, 145°34'57"E, sifting elevated leaf litter at base of lawyer vine palms, tropical rainforest, 969 m, 18.III.2012, M. & A. Rix, 1♂, 1♀ (WAM T125183). Wooroonooran National Park: Mount Bartle Frere, inside Upper Boulder Caves, 17°23'S, 145°47'E, 1000 m, 12.V.1995, G. Monteith, D. Slaney, 1♀ (QMB S72989); same data except outside Lower Boulder Caves, 900 m, 13.V.1995, 1♀ (QMB S72987).
AUSTRALIA: Queensland: Atherton Tableland: Bally Knob, summit, 17°39'S, 145°30'E, flight intercept trap, 1100 m, 6.XII.1998–6.II.1999, G. Monteith, D. Cook, 2♀ (QMB S50332). Wooroonooran National Park: Mount Bartle Frere, on track to summit, western side, from Junction Camp carpark off Gourka Road, 17°22'42"S, 145°47'09"E, day collecting, beating high and low vegetation, rainforest, 700–1300 m, 23–26.IV.2009, H. Wood, 3♂, 1♀ (CASENT 9034523); same data, 1♂ (CASENT 9034522); same data, 1 juvenile (CASENT 9034511); same data except day collecting, sifting leaf litter and small logs, brushing logs, mini-winkler, 1♀ (CASENT 9028381); Mount Bartle Frere, 18.4 km E. of Malanda, 17°22'46"S, 145°45'46"E, rainforest, 690–800 m, 17.III.2006, C. Griswold, D. Silva, M. Ramírez, 1 juvenile (CASENT 9023672).
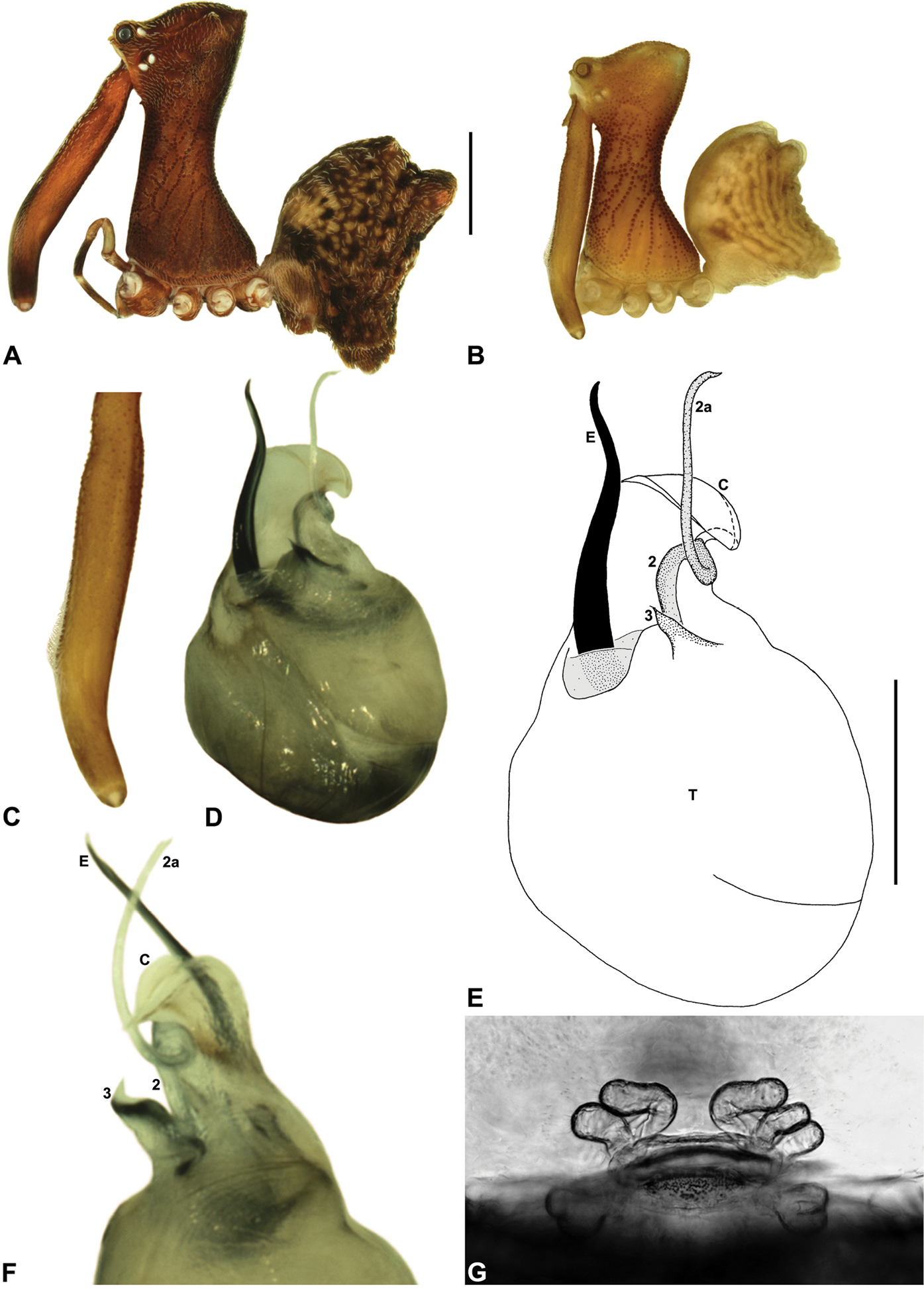
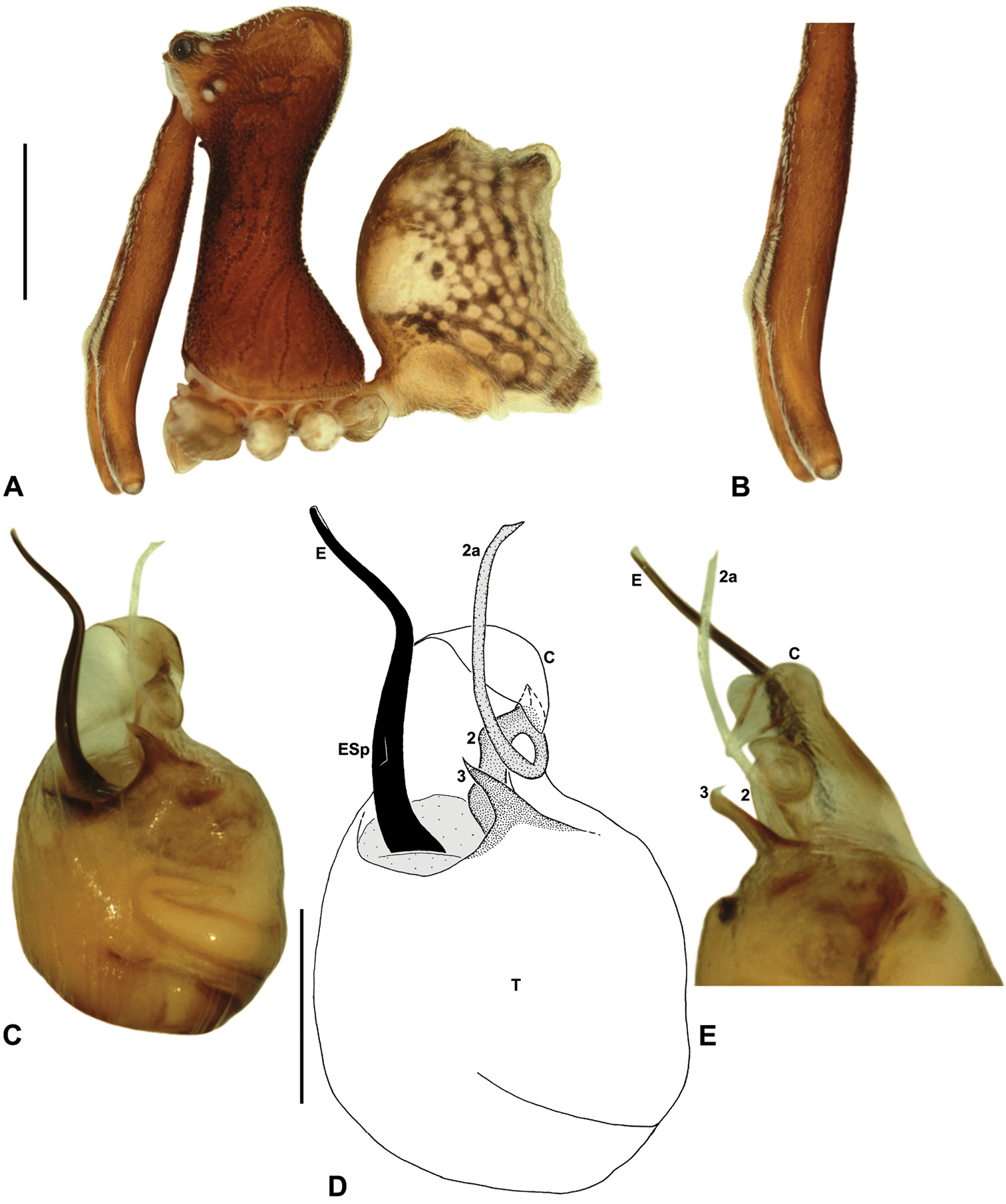
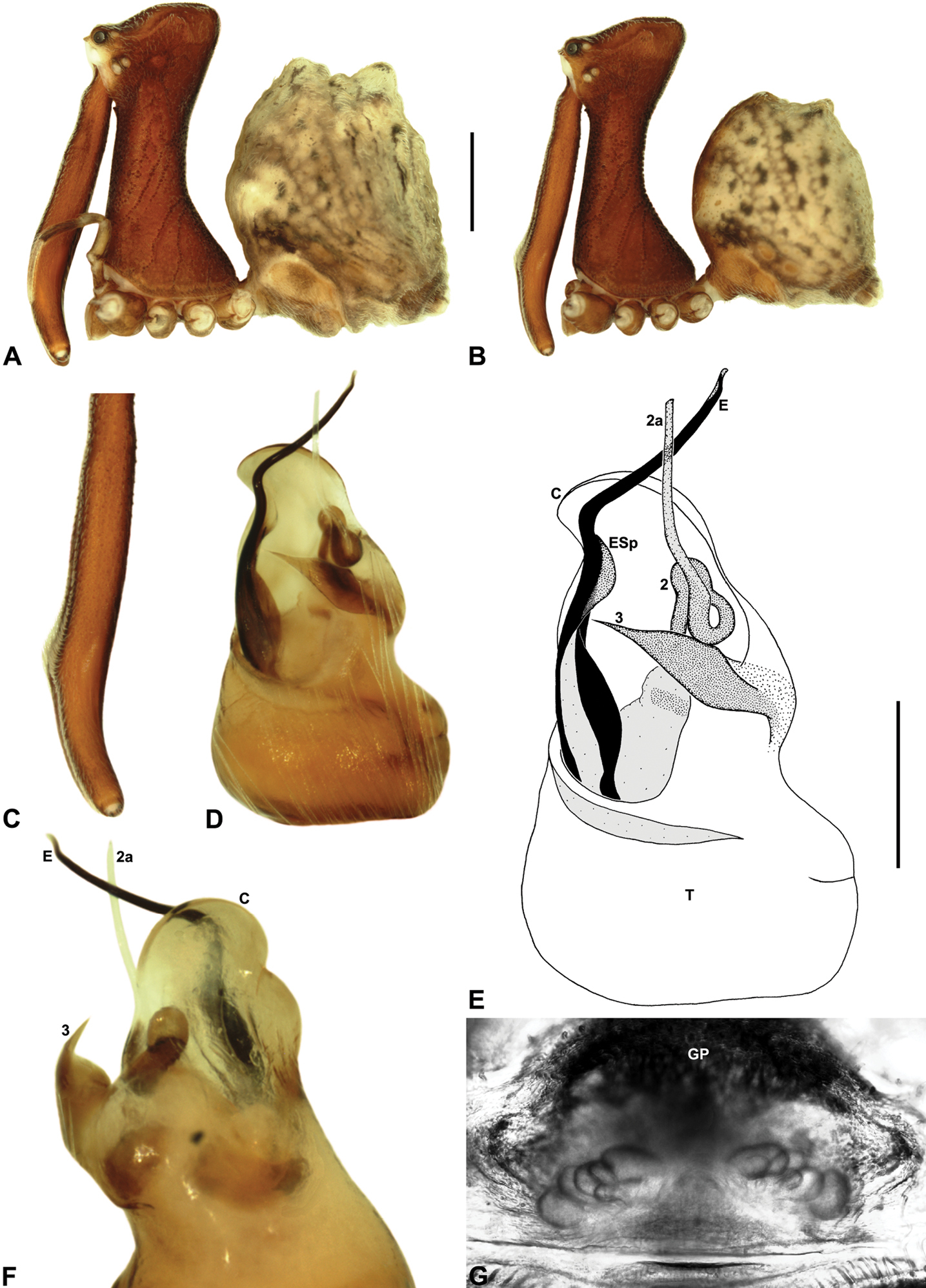
Austrarchaea daviesae can be distinguished from all other Archaeidae from north-eastern Queensland by the absence of a spur on the embolus (Fig. 7E) combined with a Type A pedipalp morphology (Fig. 6), i.e. with a large, arched, retrolaterally directed conductor (Figs 6, 7E), exposed embolus (Figs 6, 7E) and relatively short, spur-like tegular sclerite 3 (TS 3). This species can be further distinguished by the unique shape of TS 3, which has a broad tegular base and strongly hooked apex (Figs 7D-F; see also
Holotype male: Total length 2.74; leg I femur 2.73; F1/CL ratio 2.38. Cephalothorax tan-brown; legs pale tan-brown with darker annulations; abdomen mottled tan-brown and yellowish-beige (colour faded due to preservation) (Fig. 7B). Carapace tall (CH/CL ratio 2.08); 1.15 long, 2.38 high, 1.08 wide, ‘neck’ 0.62 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.67), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.27). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 7C). Abdomen 1.54 long, 1.03 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (of WAM T125183) (Figs 7D–F; see
Female (WAM T125183): Total length 3.44; leg I femur 2.97; F1/CL ratio 2.32. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled dark grey-brown and beige (Fig. 7A). Carapace tall (CH/CL ratio 2.11); 1.28 long, 2.71 high, 1.21 wide; ‘neck’ 0.71 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.63), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.26). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.54 long, 1.37 wide; with four pairs of dorsal hump-like tubercles (HT 1-4). Internal genitalia (Fig. 7G) with cluster of 4-5 variably-shaped spermathecae on either side of gonopore, clusters widely separated along midline of genital plate; innermost (anterior) spermathecae longest, sausage-shaped, bent laterally; other spermathecae variably sausage-shaped or pyriform; posterior pair of spermathecae slightly separated posteriorly.
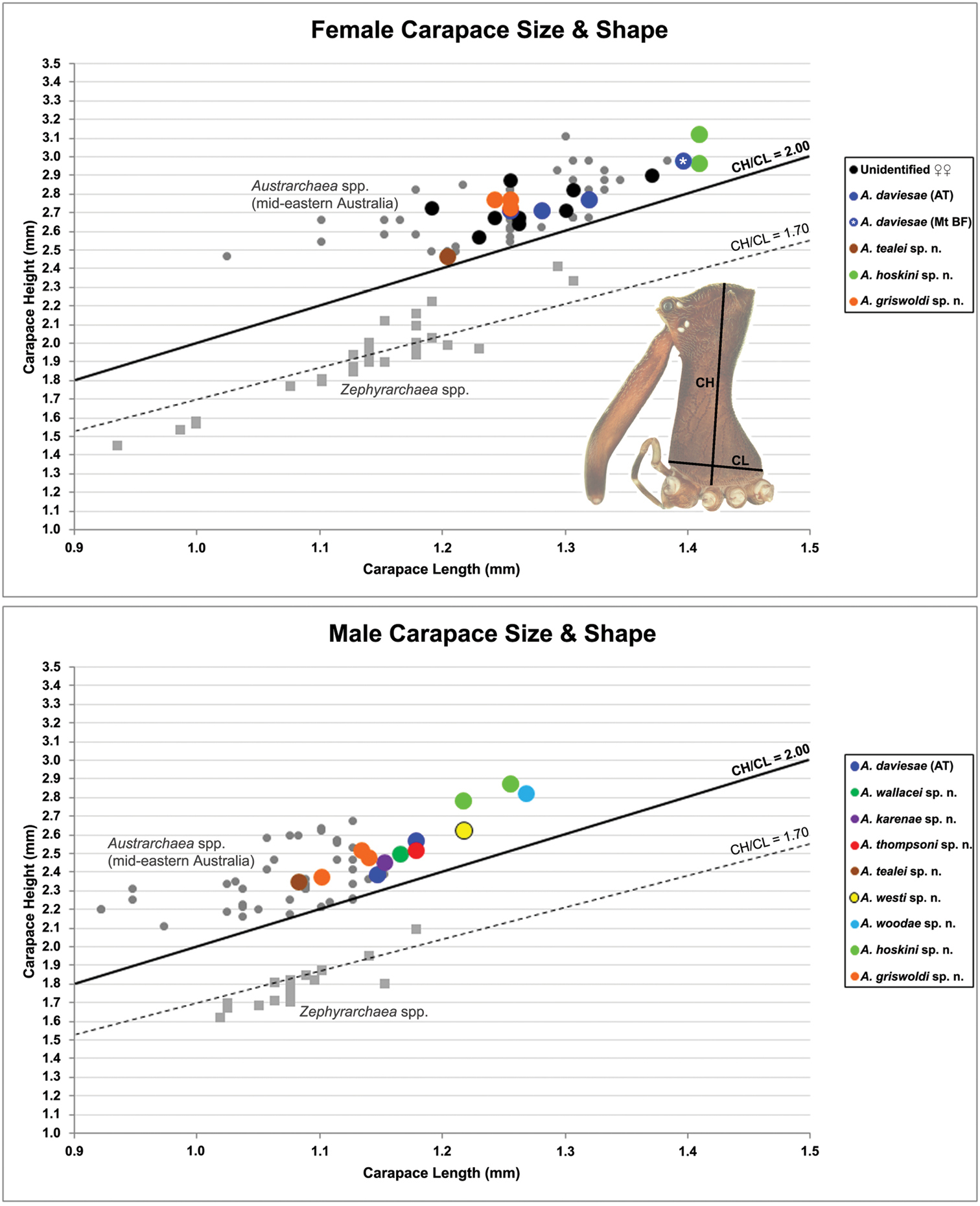
Variation: Males (Atherton Tableland; n = 2): total length 2.74–3.23; carapace length 1.15–1.18; carapace height 2.38–2.56; CH/CL ratio 2.08–2.17. Females (Atherton Tableland; n = 3): total length 3.44–3.49; carapace length 1.26–1.32; carapace height 2.7–-2.77; CH/CL ratio 2.10–2.15. Females (Mount Bartle Frere; n = 2): total length 3.64–3.79; carapace length 1.40 (invariable); carapace height 2.97 (invariable); CH/CL ratio 2.13 (invariable). Although female specimens from Mount Bartle Frere appear to be slightly larger than those from further west (Fig. 5), carapace proportions and genitalia seem otherwise very similar to specimens from the Atherton Tableland (see Remarks, below).
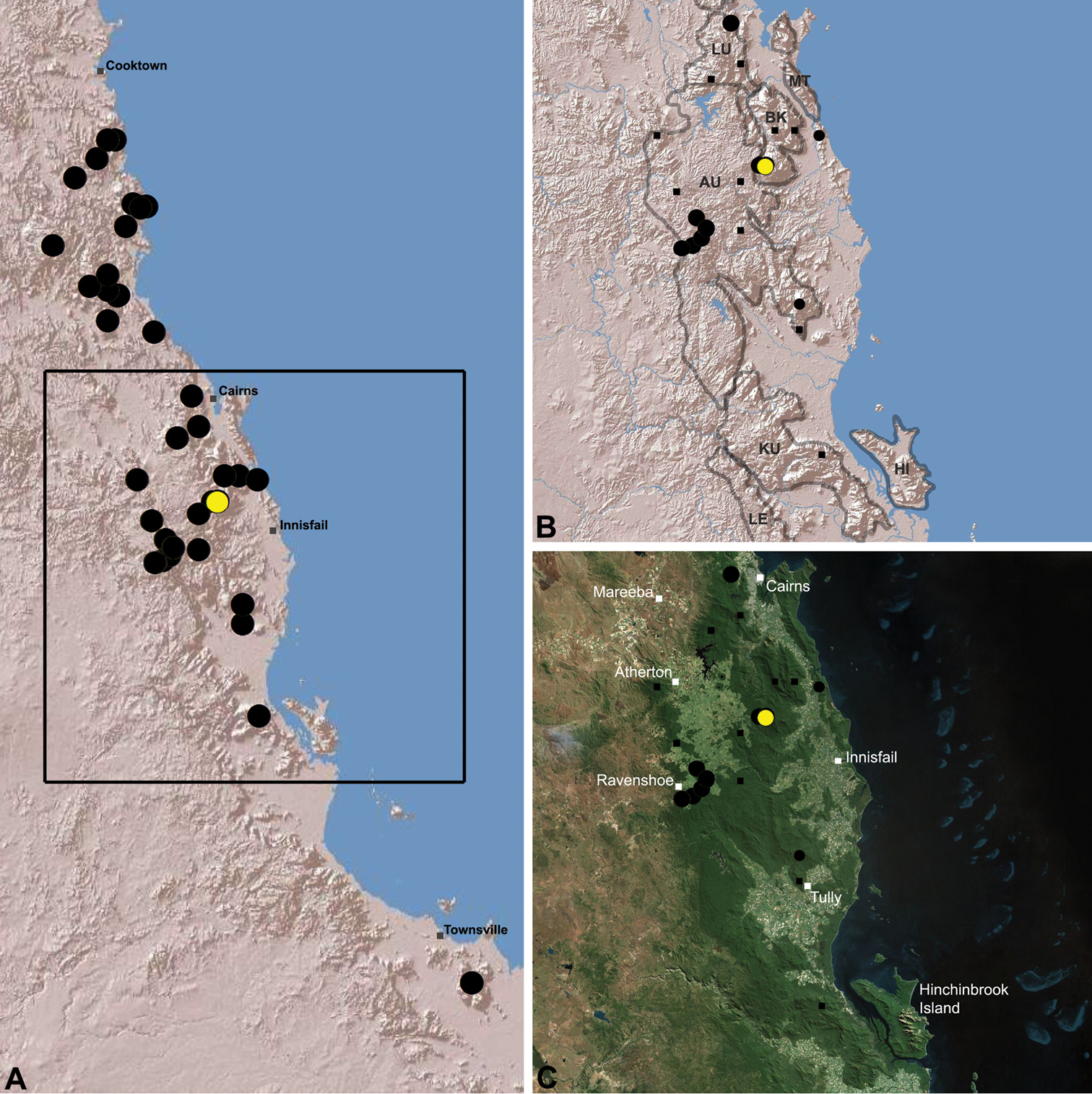
Austrarchaea daviesae is known from the ‘Misty Mountains’ region of the southern Atherton Tableland, in the vicinity of Ravenshoe and Millaa Millaa, with additional specimens also known from Mount Bartle Frere in the adjacent Wooroonooran National Park (see Remarks, below) (Figs 16, 25). Specimens have been collected in pitfall and flight intercept traps, by beating vegetation, or by beating and sifting elevated leaf litter at the bases of lawyer vine palms (Calamus spp.) in dense tropical rainforest (Fig. 1F).
This species has a relatively widespread distribution in several National Parks protected under World Heritage legislation, and is not considered to be of conservation concern.
The identification and distribution of Austrarchaea daviesae has, until recently, been difficult to ascertain, as the holotype male (QMB S1091; Fig. 7B) is without pedipalps (these presumably having been mounted on SEM stubs as per
urn:lsid:zoobank.org:act:0368CE32-E6E8-4D49-A0C0-1FD3DEFCAC23
http://species-id.net/wiki/Austrarchaea_wallacei
Mount Misery Assassin SpiderFigs 8, 17, 25
Holotype male: Mount Misery, summit, [Monkhouse Timber Reserve], Queensland, Australia, 15°52'S, 145°14'E, pitfall trap, 850 m, 6.XII.1990–17.I.1991, Queensland Museum & ANZSES (QMB S25964).
The specific epithet is a patronym in honour of the late Doug Wallace OAM (1923–2012), for his passion and enthusiasm for arachnology, for his contributions to the study of Australian (and especially Queensland) spiders, for his efforts in founding and fostering the Rockhampton Arachnological Society, and for his encouragement of MGR over many years.
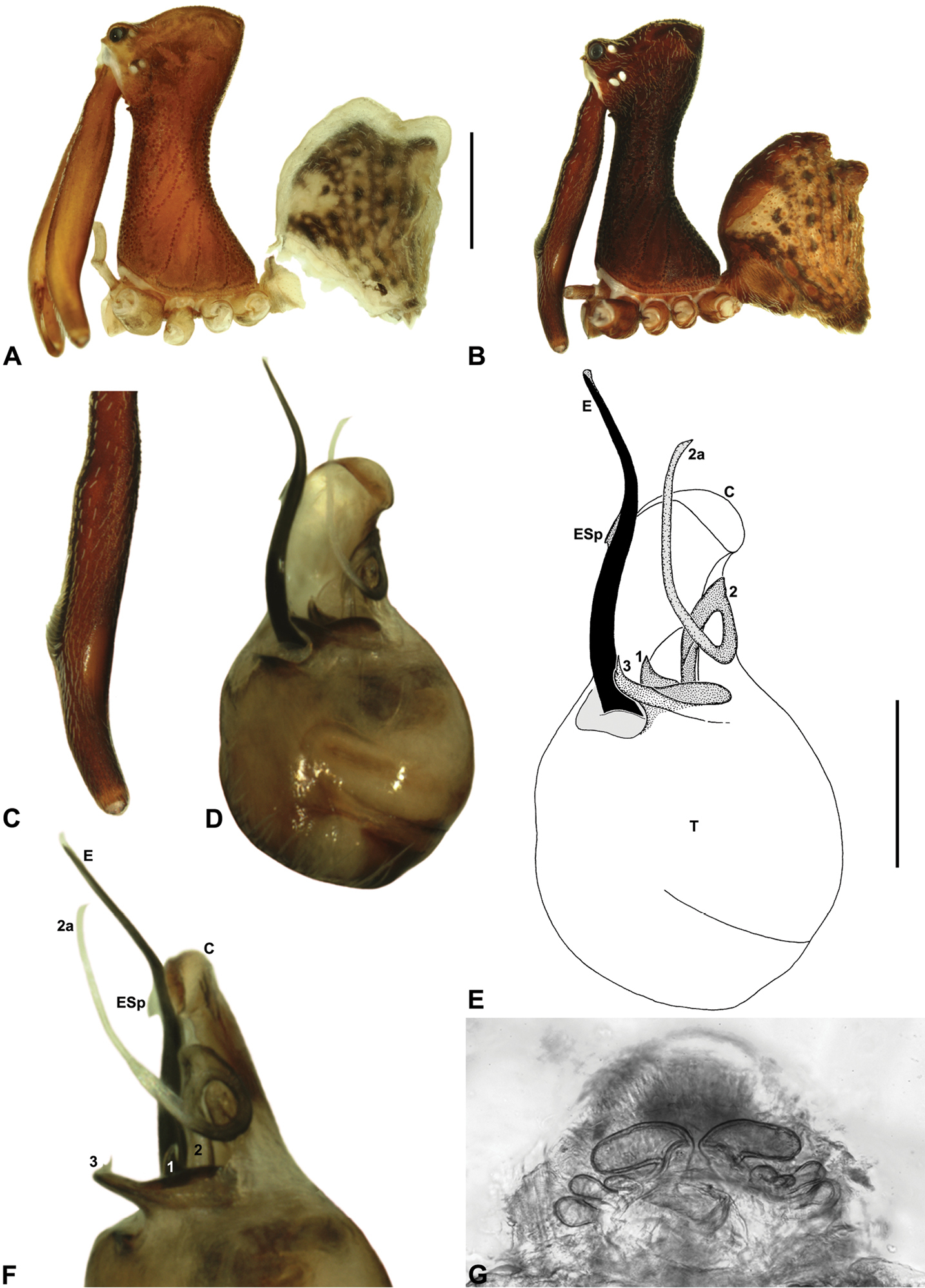
Austrarchaea wallacei can be distinguished from all other Archaeidae from north-eastern Queensland except Austrarchaea karenae sp. n., Austrarchaea tealei sp. n. and Austrarchaea thompsoni sp. n. by the presence of a triangular spur on the embolus (Fig. 8D); from Austrarchaea thompsoni sp. n. by the presence of a prominent, triangular tegular sclerite 1 (TS 1) (Fig. 8D); and from Austrarchaea karenae sp. n.and Austrarchaea tealei sp. n. by the shape of tegular sclerite 3 (TS 3), which has a bluntly pointed, triangular apex (Figs 8C–D).
Holotype male: Total length 3.28; leg I femur 3.01; F1/CL ratio 2.58. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 8A). Carapace tall (CH/CL ratio 2.14); 1.17 long, 2.49 high, 1.10 wide, ‘neck’ 0.62 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’ (ratio of HPC to post-ocular length 0.72), carapace gently sloping posterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.33). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 8B). Abdomen 1.59 long, 1.28 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Expanded pedipalp (Figs 8C–D) of Type A morphology (Fig. 6), with large, retrolaterally directed, arched conductor; embolus sinuous, with short triangular spur; tegular sclerite 3 (TS 3) short, spur-like, with flattened proximal portion and bluntly pointed, triangular apex; TS 2-2a flexed dorsally (due to haematodochal expansion), TS 2 with pointed apex; TS 1 triangular, with tapered, slightly curved tooth-like apex.
Female: Unknown.
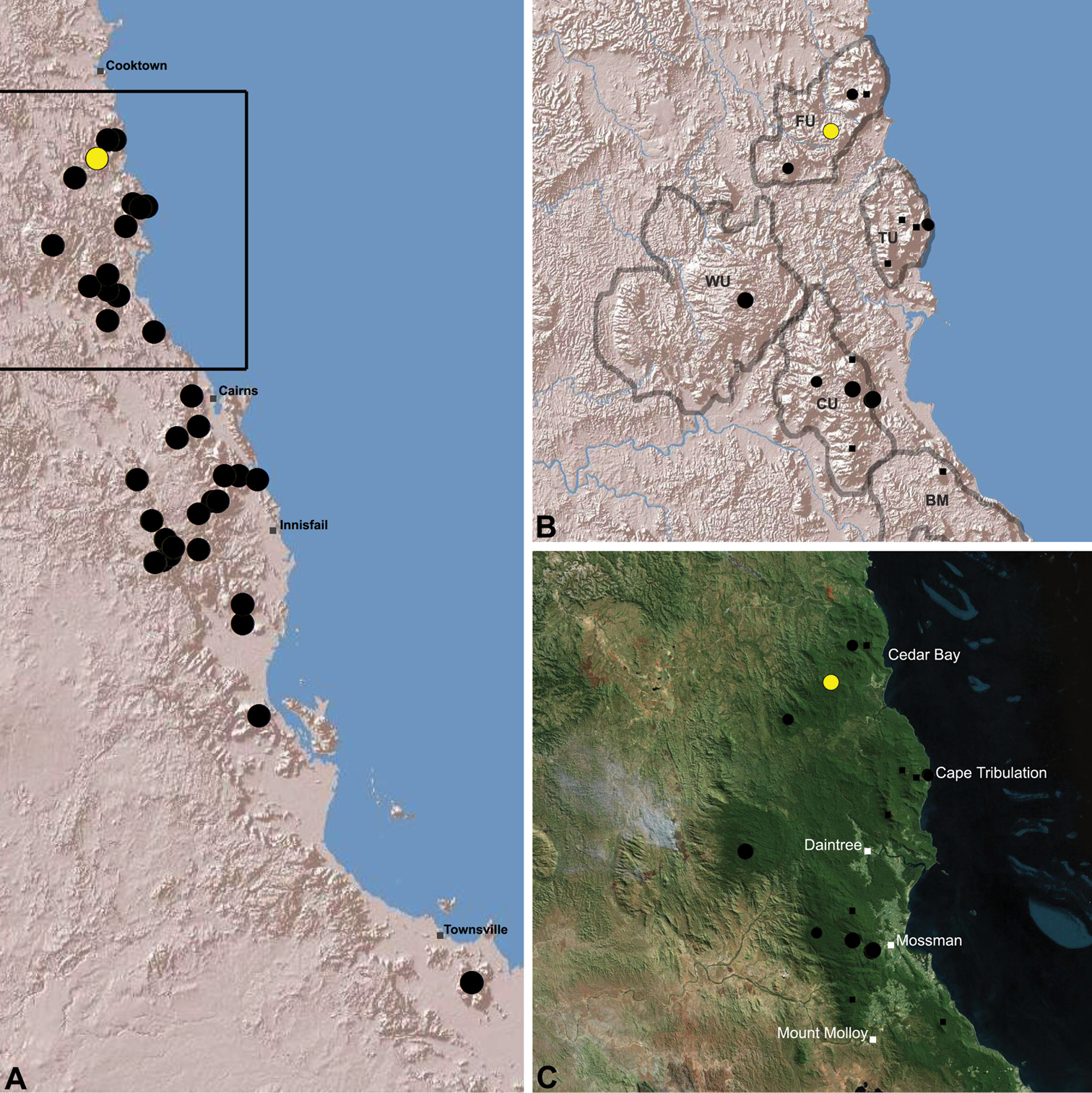
Austrarchaea wallacei is known only from the summit of Mount Misery, 34 km north-west of Cape Tribulation (Figs 17, 25). The single known specimen was collected in a pitfall trap in tropical rainforest at 850 m elevation.
Unknown (data deficient).
urn:lsid:zoobank.org:act:E010DAB8-9909-432E-BAA9-0452A1EBCCE0
http://species-id.net/wiki/Austrarchaea_karenae
Windsor Tableland Assassin SpiderFigs 9, 18, 25
Holotype male: Windsor Tableland, [Windsor Tableland National Park], 1.2 km past barracks, Queensland, Australia, 16°15'S, 145°02'E, QM berlesate, stick brushing, rainforest, 1060 m, 24.XI.1997, G. Monteith (QMB S43060).
The specific epithet is a patronym in honour of Dr Karen Edward, for her contributions to our understanding of Wet Tropics biogeography, and for her great friendship to MGR and MSH over many years.
Austrarchaea karenae can be distinguished from all other Archaeidae from north-eastern Queensland except Austrarchaea tealei sp. n., Austrarchaea thompsoni sp. n. and Austrarchaea wallacei by the presence of a triangular spur on the embolus (Figs 9D–E); from Austrarchaea thompsoni sp. n. by the presence of a prominent, triangular tegular sclerite 1 (TS 1), which is visible in ventral view (Fig. 9D); and from Austrarchaea tealei sp. n. and Austrarchaea wallacei by the shape of tegular sclerite 3 (TS 3), which has a single, sharply pointed process distally (Fig. 9D).
Holotype male: Total length 2.97; leg I femur 3.17; F1/CL ratio 2.74. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 9A). Carapace tall (CH/CL ratio 2.12); 1.15 long, 2.49 high, 1.09 wide, ‘neck’ 0.61 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.65), carapace gently sloping posterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.32). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 9B). Abdomen 1.59 long, 1.13 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 9C–E) of Type A morphology (Fig. 6), with large, retrolaterally directed, arched conductor; embolus distally directed, slightly sinuous, with short triangular spur adjacent to distal rim of conductor, embolus projecting beyond distal rim of conductor by ~1/2 length of exposed embolic portion; tegular sclerite 3 (TS 3) short, spur-like, with flattened proximal portion and sharply pointed, claw-like apex; TS 2-2a looped over retrolateral edge of conductor, TS 2 with pointed, subtriangular apex, TS 2a projecting beyond distal rim of conductor but not extending to near tip of embolus; TS 1 broadly triangular in ventral view.
Female: Unknown.
Austrarchaea karenae is known only from the Windsor Tableland, 44 km north-west of Mossman (Figs 18, 25). The single known specimen was collected in high altitude tropical rainforest.
Unknown (data deficient).
urn:lsid:zoobank.org:act:CC84B06D-AD54-41A7-8237-F2031D57F0A7
http://species-id.net/wiki/Austrarchaea_thompsoni
Carbine Tableland Assassin SpiderFigs 10, 19, 25
Holotype male: Devils Thumb area, [Daintree National Park (Mossman Gorge Section)], 10 km NW. of Mossman, Queensland, Australia, [16°27'S, 145°17'E], pyrethrum knockdown, tropical rainforest, 1000–1180 m, 10.X.1982, G. Monteith, D. Yeates, G. Thompson (QMB S30840).
. The specific epithet is a patronym in honour of Geoff Thompson, for his ongoing efforts in collecting and documenting the invertebrate rainforest fauna of the Wet Tropics, and for collecting the only known specimen of this species.
Austrarchaea thompsoni can be distinguished from all other Archaeidae from north-eastern Queensland except Austrarchaea karenae, Austrarchaea tealei sp. n. and Austrarchaea wallacei by the presence of a triangular spur on the embolus (Fig. 10D); and from Austrarchaea karenae, Austrarchaea tealei sp. n. and Austrarchaea wallacei by the very small tegular sclerite 1 (TS 1), which is not visible in ventral view (Fig. 10D), and by the more proximally positioned embolic spur, which is situated near the base of the exposed embolic portion (Fig. 10D).
Holotype male: Total length 2.97; leg I femur 3.23; F1/CL ratio 2.74. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 10A). Carapace tall (CH/CL ratio 2.13); 1.18 long, 2.51 high, 1.13 wide, ‘neck’ 0.63 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.69), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.25). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 10B). Abdomen 1.64 long, 1.23 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 10C–E) of Type A morphology (Fig. 6), with large, retrolaterally directed, arched conductor; embolus distally directed, slightly sinuous, with short triangular spur situated near base of exposed embolic portion, embolus projecting beyond distal rim of conductor by slightly less than 1/2 length of exposed embolic portion; tegular sclerite 3 (TS 3) short, spur-like, with constricted tegular base and sharply pointed, claw-like apex; TS 2-2a looped beneath overhanging retrolateral edge of conductor, TS 2 with rounded, subtriangular apex, TS 2a projecting beyond distal rim of conductor to near tip of embolus; TS 1 very small, obscured by TS 2-3, not visible in ventral view.
Female: Unknown.
Austrarchaea thompsoni is known only from Devils Thumb, on the Carbine Tableland 10 km west-north-west of Mossman (Figs 19, 25). The single known specimen was collected in high altitude tropical rainforest.
Unknown (data deficient).
urn:lsid:zoobank.org:act:60D9ADD6-1BDB-4964-B484-99587C772CAB
http://species-id.net/wiki/Austrarchaea_tealei
Mossman Gorge Assassin SpiderFigs 11, 20, 25
Holotype male: Daintree National Park (Mossman Gorge Section), Mossman Gorge, off water access road ~50 m from carpark, Queensland, Australia, 16°28'20"S, 145°19'53"E, sifting elevated leaf litter under lawyer vine palms, tropical rainforest, 78 m, 21.III.2012, M. & A. Rix (QMB S92210).
AUSTRALIA: Queensland: Daintree National Park (Mossman Gorge Section): Mossman Gorge, [16°28'20"S, 145°19'53"E], 23.IV.1967, D. Colless, 1♀ (ANIC); Mossman Gorge, Water Access Track, Site 2, 16°28'28"S, 145°19'41"E, sieved litter from around roots and rocks on shady steep section of bank, tropical rainforest, 1.IV.2009, K. Edward, J. Waldock, 1 juvenile (WAM T97462).
AUSTRALIA: Queensland: Daintree National Park (Mossman Gorge Section): Mossman Gorge, carpark, 16°28'20"S, 145°19'52"E, day collecting, turning over logs, rainforest, 45 m, 17–18.IV.2009, H. Wood, 4♂, 2♀ (CASENT 9028385).
The specific epithet is a patronym in honour of Roy Teale, for his friendship to MSH, for his efforts in facilitating systematic research at the Western Australian Museum, and for his crucial support of the Western Australian Museum’s ‘archaeid project’ since 2007.
Austrarchaea tealei can be distinguished from all other Archaeidae from north-eastern Queensland except Austrarchaea karenae, Austrarchaea thompsoni and Austrarchaea wallacei by the presence of a triangular spur on the embolus (Figs 11E–F); from Austrarchaea thompsoni by the presence of a prominent, triangular tegular sclerite 1 (TS 1), which is visible in ventral view (Fig. 11E); and from Austrarchaea karenae and Austrarchaea wallacei by the shape of tegular sclerite 3 (TS 3), which has a second short, pointed process distally (Fig. 11E).
Holotype male: Total length 2.67; leg I femur 3.09; F1/CL ratio 2.85. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled dark grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 11B). Carapace tall (CH/CL ratio 2.17); 1.08 long, 2.35 high, 1.00 wide, ‘neck’ 0.54 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’ (ratio of HPC to post-ocular length 0.71), carapace gently sloping posterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.30). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 11C). Abdomen 1.44 long, 1.05 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 11D–F) of Type A morphology (Fig. 6), with large, retrolaterally directed, arched conductor; embolus distally directed, slightly sinuous, with short triangular spur adjacent to distal rim of conductor, embolus projecting beyond distal rim of conductor by ~1/2 length of exposed embolic portion; tegular sclerite 3 (TS 3) short, spur-like, with flattened proximal portion and sharply pointed, claw-like apex bearing second short, pointed process distally; TS 2-2a looped over retrolateral edge of conductor, TS 2 with rounded, subtriangular apex, TS 2a projecting beyond distal rim of conductor but not extending to near tip of embolus; TS 1 triangular, with tapered, slightly curved tooth-like apex.
Female (ANIC): Total length 2.95; leg I femur 2.86; F1/CL ratio 2.37. Cephalothorax reddish-brown; legs pale tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 11A). Carapace tall (CH/CL ratio 2.04); 1.21 long, 2.46 high, 1.13 wide; ‘neck’ 0.69 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.65), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.23). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.64 long, 1.28 wide; with four pairs of dorsal hump-like tubercles (HT 1-4). Internal genitalia (Fig. 11G) with cluster of 4-6 variably-shaped spermathecae on either side of gonopore, clusters widely separated along midline of genital plate; innermost (anterior) spermathecae longest, sausage-shaped, bent laterally; other spermathecae variably sausage-shaped or pyriform, smallest anteriorly, becoming progressively larger posteriorly.
Austrarchaea tealei is known only from Mossman Gorge, 4.5 km west-south-west of Mossman (Figs 20, 25). Specimens have been collected under logs (as newly-hatched juveniles; H. Wood, pers. comm.), or by beating and sifting elevated leaf litter at the bases of lawyer vine palms (Calamus spp.) in lowland tropical rainforest.
Unknown (data deficient).
The female specimen described above (from the ANIC) is tentatively identified as conspecific with the holotype of Austrarchaea tealei, despite a somewhat different carapace morphology and a fairly imprecise collection locality. Austrarchaea thompsoni does occur on nearby mountains above the Mossman River, and thus it possible (albeit unlikely) that the female specimen from “Mossman Gorge” (collected in 1967) may actually belong to another species. We have described it here in the absence of evidence suggesting any sympatry in the Mossman Gorge region, given the fact that all other recently collected Mossman Gorge material appears to be conspecific with the holotype (including CAS material; H. Wood, pers. comm.), and given the similarly small body size of this female specimen and the holotype male (Fig. 5).
urn:lsid:zoobank.org:act:1D560E1E-96FB-4F5B-A826-281429ADD500
http://species-id.net/wiki/Austrarchaea_westi
Lamb Range Assassin SpiderFigs 12, 21, 25
Holotype male: Mount Williams, [Dinden National Park], 16°55'S, 145°40'E, pyrethrum, trees and logs, 1000 m, 2.XII.1993, G. Monteith, H. Janetzki (QMB S59537).
Other material examined. AUSTRALIA: Queensland: Dinden National Park: same data as holotype, 1 juvenile (QMB S59537).
The specific epithet is a patronym in honour of Paul West, for his friendship to MSH over many years, and for helping fund the Western Australian Museum’s ‘archaeid project’ from 2009–2012.
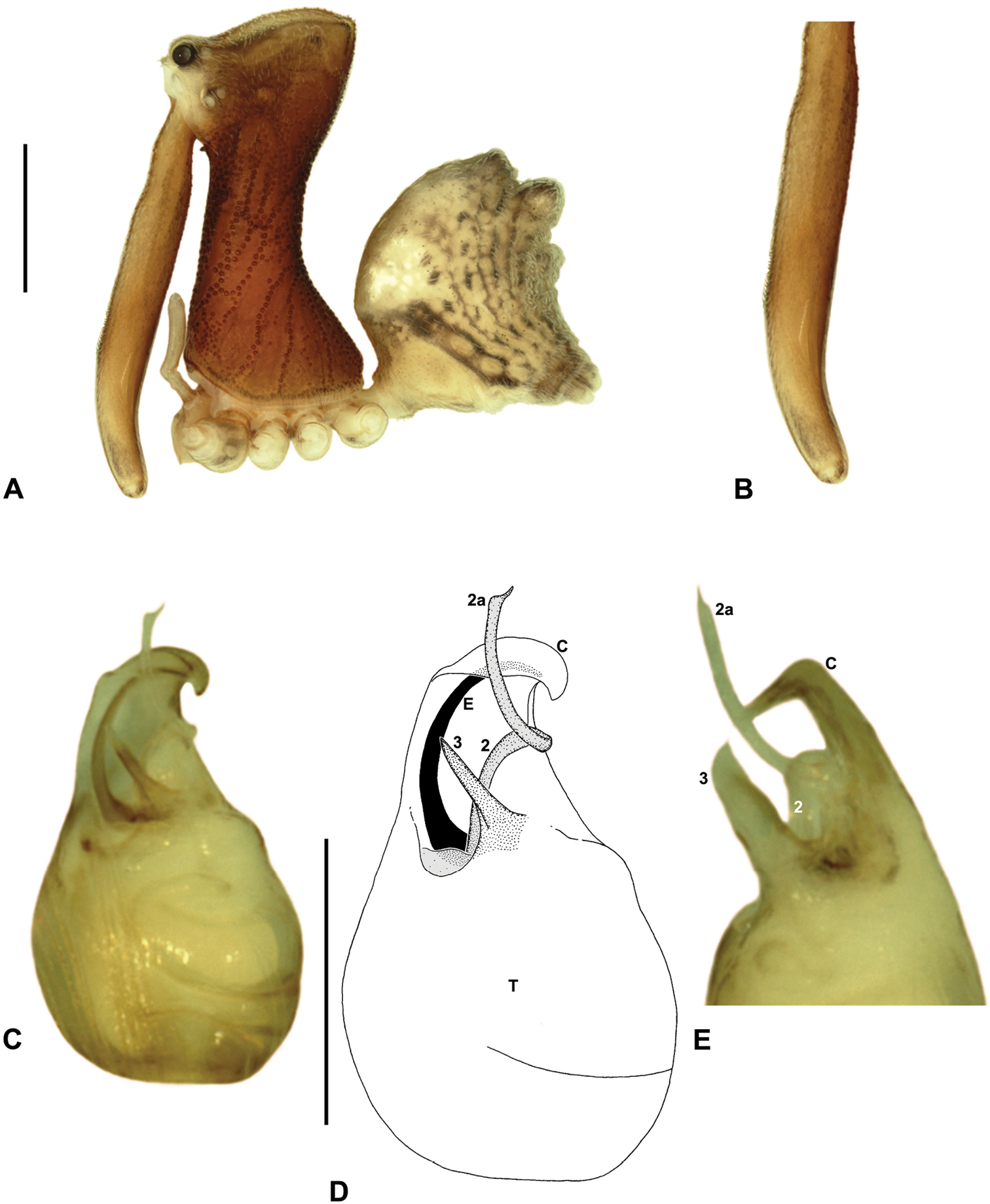
Austrarchaea westi can be distinguished from all other Archaeidae from north-eastern Queensland by the presence of a unique Type B pedipalp (Fig. 6), with very small bulb (width << 0.30 mm) (Figs 6, 12D), and by the relatively short embolus, which is distally enclosed within the conductor (Figs 6, 12D). This species can be further distinguished by the very short, barely differentiated accessory setae on the male chelicerae (Fig. 12B).
Holotype male: Total length 3.13; leg I femur 3.23; F1/CL ratio 2.65. Cephalothorax reddish-brown; legs beige with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 12A). Carapace tall (CH/CL ratio 2.15); 1.22 long, 2.62 high, 1.13 wide, ‘neck’ 0.65 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’ (ratio of HPC to post-ocular length 0.71), carapace gently sloping posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.27). Chelicerae with very short, barely differentiated accessory setae on anterior face of paturon (Fig. 12B). Abdomen 1.65 long, 1.10 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 12C-E) of Type B morphology (Fig. 6), very small in size (width of bulb << 0.30), with large, retrolaterally directed, arched conductor; embolus curved, distally enclosed within conductor, without spur; tegular sclerite 3 (TS 3) porrect, spur-like, with pointed, pro-distally directed apex; TS 2-2a looped over retrolateral edge of conductor, TS 2 not strongly developed distally, TS 2a projecting beyond distal rim of conductor; TS 1 very small, obscured by TS 2-3, not visible in ventral view.
Female: Unknown.
Austrarchaea westi is known only from Mount Williams, on the Lamb Range 11 km west of Cairns (Figs 21, 25). The two known specimens were collected in high altitude tropical rainforest.
Unknown (data deficient).
urn:lsid:zoobank.org:act:E4F1A9F7-33E3-4FCF-86CE-C14A1572F037
http://species-id.net/wiki/Austrarchaea_woodae
Mount Bartle Frere Assassin SpiderFigs 13, 22, 25
Holotype male: Mount Bartle Frere, [Wooroonooran National Park], Boulder Caves, Queensland, Australia, [17°23'S, 145°47'E], 1050 m, 8.XII.1990, G. Monteith, G. Thompson, D. Cook, R. Sheridan (QMB S72988).
The specific epithet is a patronym in honour of Dr Hannah Wood, for her pioneering research into the systematics, biology and biogeography of assassin spiders and other Palpimanoidea, and for her collaborative support of MGR and MSH during assassin spider research conducted at the Western Australian Museum.
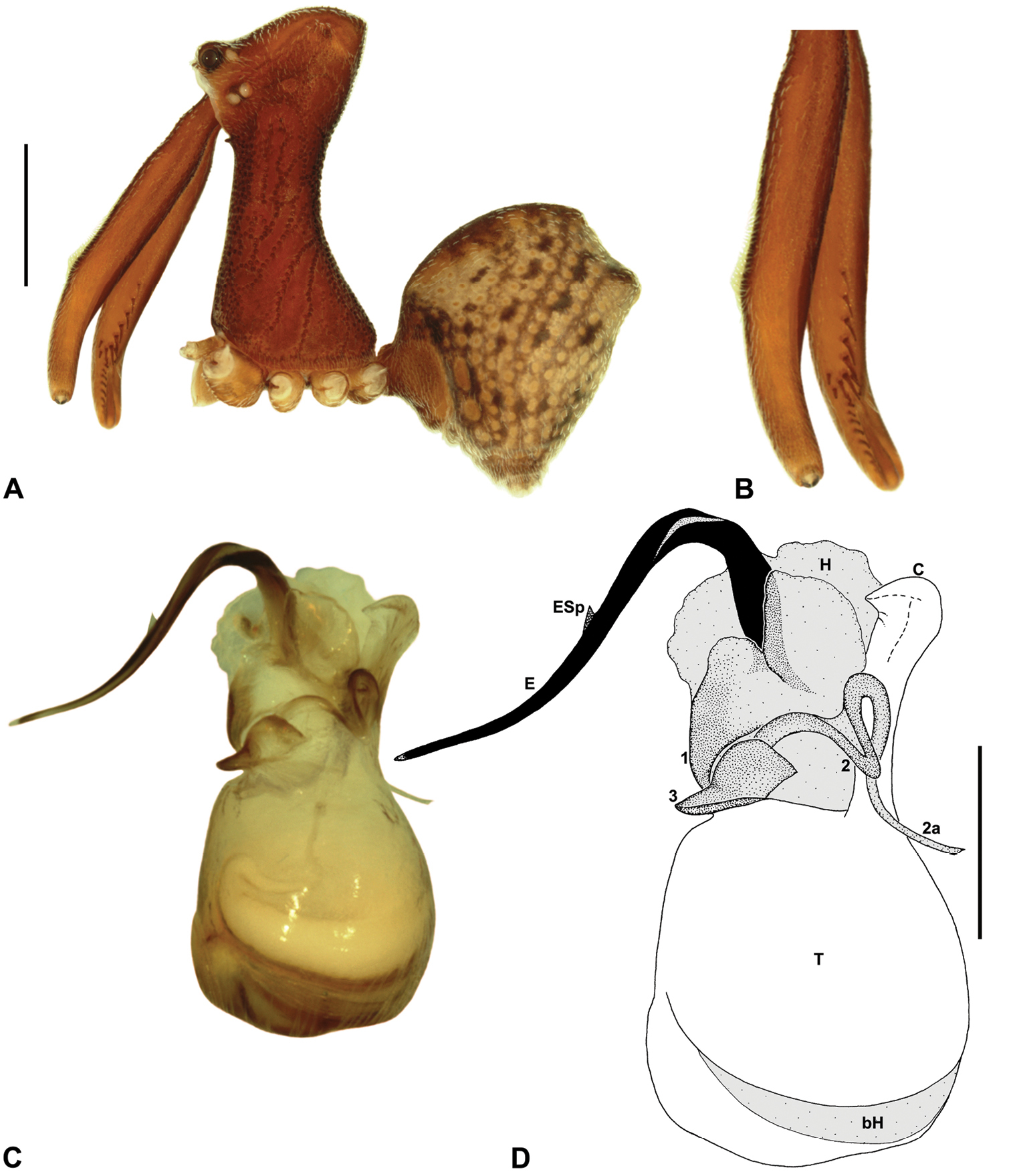
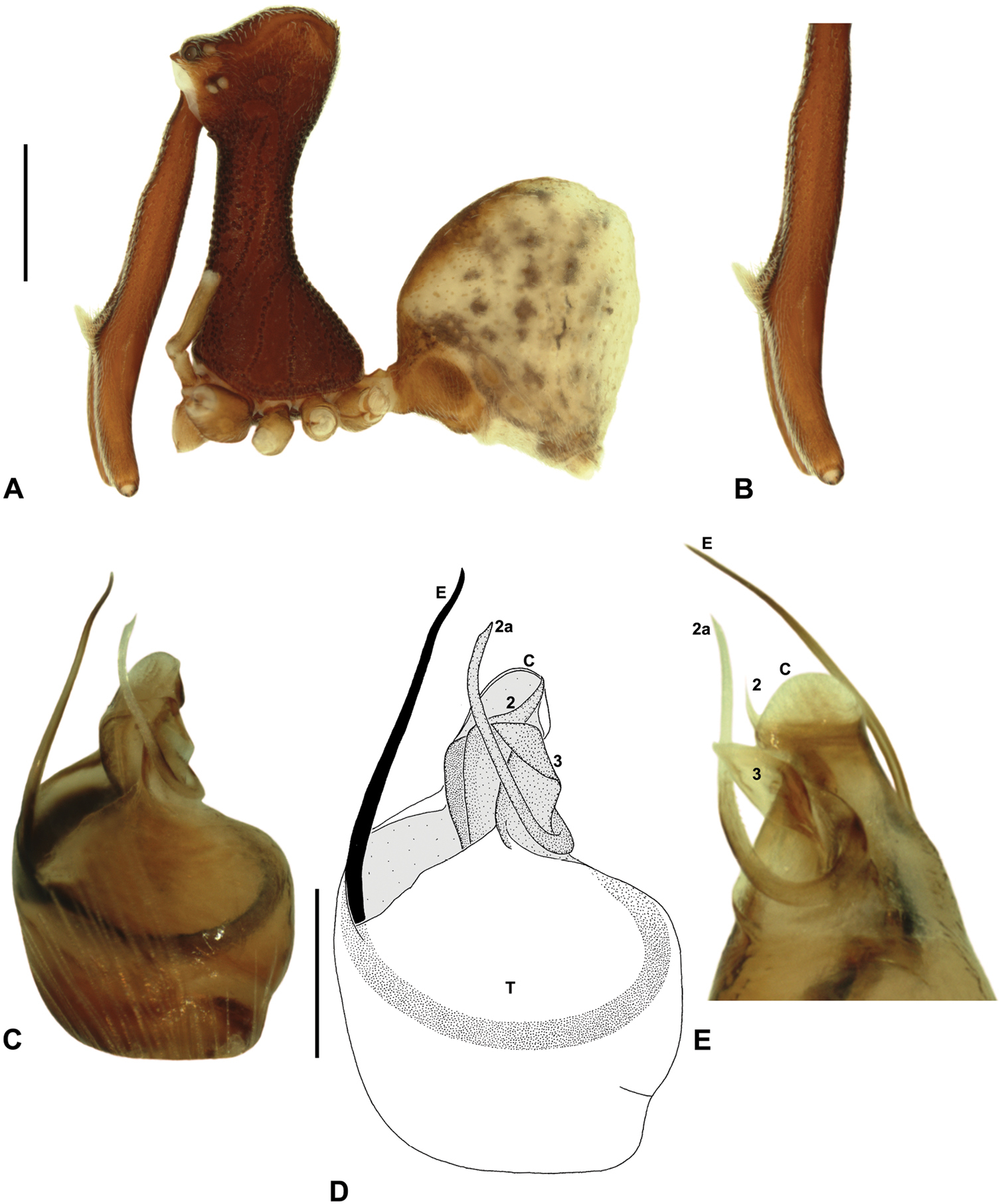
Austrarchaea woodae can be distinguished from all other Archaeidae from north-eastern Queensland by the presence of a unique Type C pedipalp (Fig. 6), with a proximally constricted conductor (Figs 6, 13D), large, flattened, distally folded tegular sclerite 3 (TS 3) (Figs 6, 13D–E), and apple-shaped bulb profile in ventral view (Figs 6, 13C–D). This species can be further distinguished by the dense, pick-like tuft of accessory setae on the male chelicerae (Fig. 13B; similar only to Austrarchaea harmsi among Australian Archaeidae), and by the almost spherical abdomen with recumbent hump-like tubercles (Fig. 13A; similar only to species of Zephyrarchaea among Australian Archaeidae).
Holotype male: Total length 3.54; leg I femur 3.74; F1/CL ratio 2.95. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 13A). Carapace very tall (CH/CL ratio 2.22); 1.27 long, 2.82 high, 1.18 wide, ‘neck’ 0.56 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.67), carapace steeply sloping and convex posterior to HPC; ‘head’ moderately elevated dorsally (post-ocular ratio 0.31). Chelicerae with dense, pick-like tuft of accessory setae on anterior face of paturon (Fig. 13B). Abdomen 1.69 long, 1.44 wide; almost spherical, with largely recumbent hump-like tubercles; dorsal scute fused anteriorly to epigastric sclerites. Unexpanded pedipalp (Figs 13C–E) of Type C morphology (Fig. 6), with retrolaterally directed, proximally constricted conductor and apple-shaped bulb profile in ventral view; embolus distally directed, slightly sinuous, without spur; tegular sclerite 3 (TS 3) large, flattened, with prominent, distally folded apex; TS 2-2a looped over retrolateral edge of conductor, TS 2 with strongly developed, spur-like apex extending to near distal rim of conductor, TS 2a looping around TS 3 proximally and projecting beyond distal rim of conductor to near tip of embolus; TS 1 indistinct, obscured by TS 2-3.
Female: Unknown.
Austrarchaea woodae is known only from near the summit of Mount Bartle Frere, 12 km south-west of Babinda (Figs 22, 25). The only known specimen was collected in high altitude tropical rainforest.
Unknown (data deficient).
See Remarks for Austrarchaea daviesae (above).
urn:lsid:zoobank.org:act:432C741C-00BA-4E9A-999C-8E0343C9F199
http://species-id.net/wiki/Austrarchaea_hoskini
Mount Elliot Assassin SpiderFigs 14, 23, 25
Holotype male: Mount Elliot, [Bowling Green Bay National Park], Upper North Creek, Queensland, Australia, [19°29'S, 146°57'E], rainforest, 1000 m, 2–5.XII.1986, G. Monteith, G. Thompson, S. Hamlet (QMB S30811).
Allotype female, Mount Elliot, [Bowling Green Bay National Park], North Creek, Queensland, Australia, 19°29'S, 146°57'E, 1000 m, 25–27.III.1991, G. Monteith, D. Cook (QMB S17937); 1 male, 1 female, same data (QMB S23045).
AUSTRALIA: Queensland: Bowling Green Bay National Park: same data as holotype, 1 juvenile (QMB S30811); same data as holotype except pitfall trap, 3–5.XII.1986, 1 juvenile (QMB S30839).
The specific epithet is a patronym in honour of Dr Conrad Hoskin, for his contributions to our understanding of Wet Tropics biogeography, and for his efforts in documenting the remarkable endemic biota of Mount Elliot.
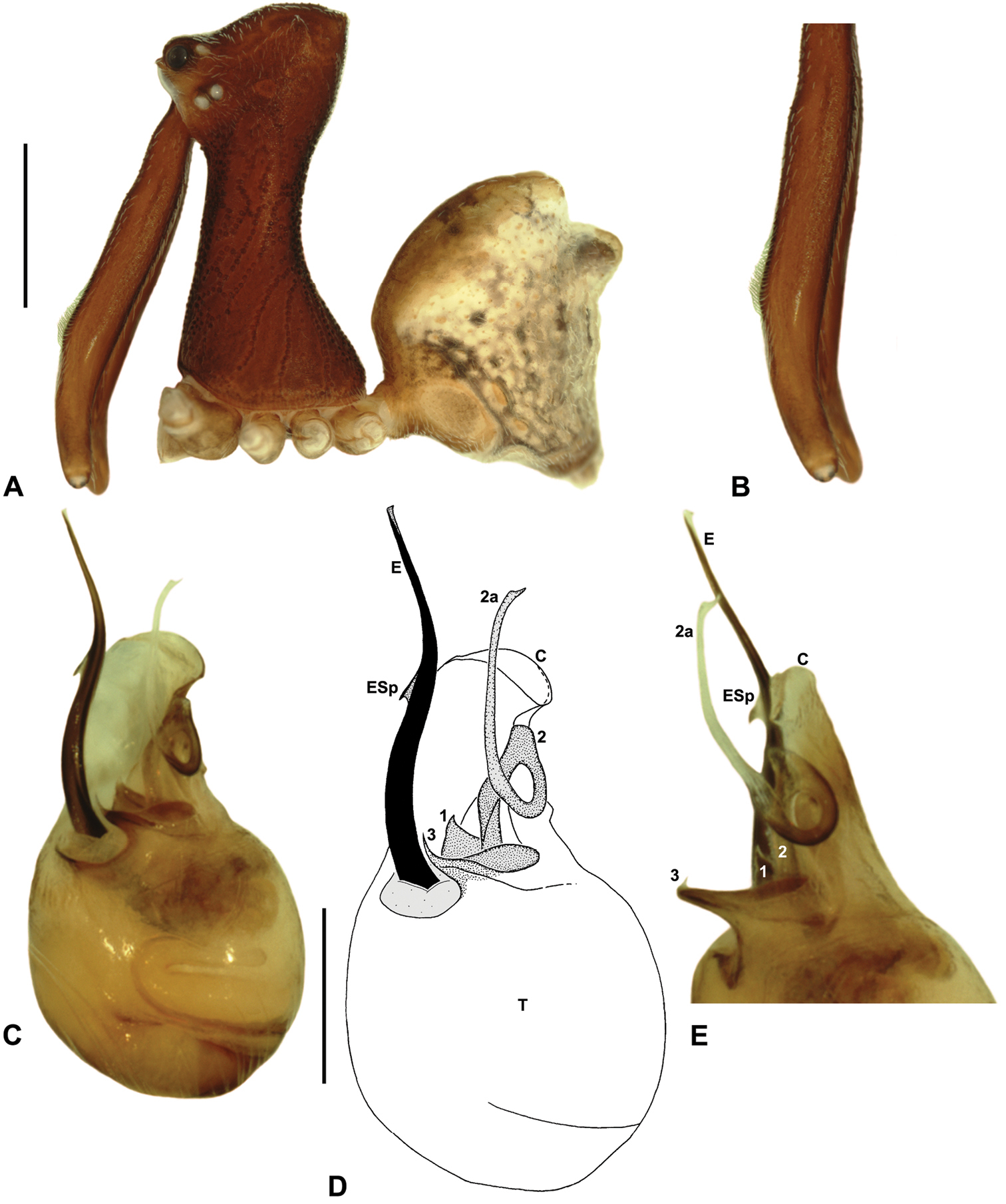
Austrarchaea hoskini can be distinguished from all other Archaeidae from north-eastern Queensland by the presence of a unique Type D pedipalp (Fig. 6), with a prolaterally directed conductor (Figs 6, 14E), very large, dagger-shaped tegular sclerite 3 (TS 3) (Figs 6, 14E–F), and prominent, rounded, fin-shaped embolic spur (Fig. 14E).
Holotype male: Total length 3.44; leg I femur 3.59; F1/CL ratio 2.86. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 14B). Carapace very tall (CH/CL ratio 2.29); 1.26 long, 2.87 high, 1.21 wide, ‘neck’ 0.59 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near posterior third of ‘head’ (ratio of HPC to post-ocular length 0.63), carapace almost horizontal posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.28). Chelicerae with short brush of accessory setae on anterior face of paturon (Fig. 14C). Abdomen 2.00 long, 1.54 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 14D–F) of Type D morphology (Fig. 6), with large, prolaterally directed, arched conductor; embolus distally directed, sinuous, with prominent, rounded, fin-shaped spur proximal to distal kink in embolus, embolus projecting beyond distal rim of conductor by ~1/3 length of exposed embolic portion; tegular sclerite 3 (TS 3) very large, dagger-like, directed pro-ventrally across bulb; TS 2-2a forming looped, figure-of-eight-shaped structure in ventral view, TS 2 rounded distally, TS 2a projecting beyond distal rim of conductor to near tip of embolus; TS 1 very small, indistinct, probably embedded within haematodochal membranes.
Allotype female: Total length 3.79; leg I femur 3.44; F1/CL ratio 2.44. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled grey-brown and beige (Fig. 14A). Carapace tall (CH/CL ratio 2.10); 1.41 long, 2.96 high, 1.31 wide; ‘neck’ 0.72 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) approaching posterior quarter of ‘head’ (ratio of HPC to post-ocular length 0.71), carapace almost horizontal anterior and slightly posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.29). Chelicerae without accessory setae on anterior face of paturon. Abdomen 2.31 long, 1.87 wide; with four pairs of dorsal hump-like tubercles (HT 1-4). Internal genitalia (Fig. 14G) with cluster of ~6 variably-shaped spermathecae on either side of gonopore, clusters widely separated along midline of genital plate; innermost (anterior) spermathecae longest, sausage-shaped, bent laterally; other spermathecae variably sausage-shaped or pyriform.
Variation: Males (n = 2): total length 3.38–3.44; carapace length 1.22–1.26; carapace height 2.78–2.87; CH/CL ratio 2.28–2.29. Females (n = 2): total length 3.59–3.79; carapace length 1.41 (invariable); carapace height 2.96–3.12; CH/CL ratio 2.10–2.21.
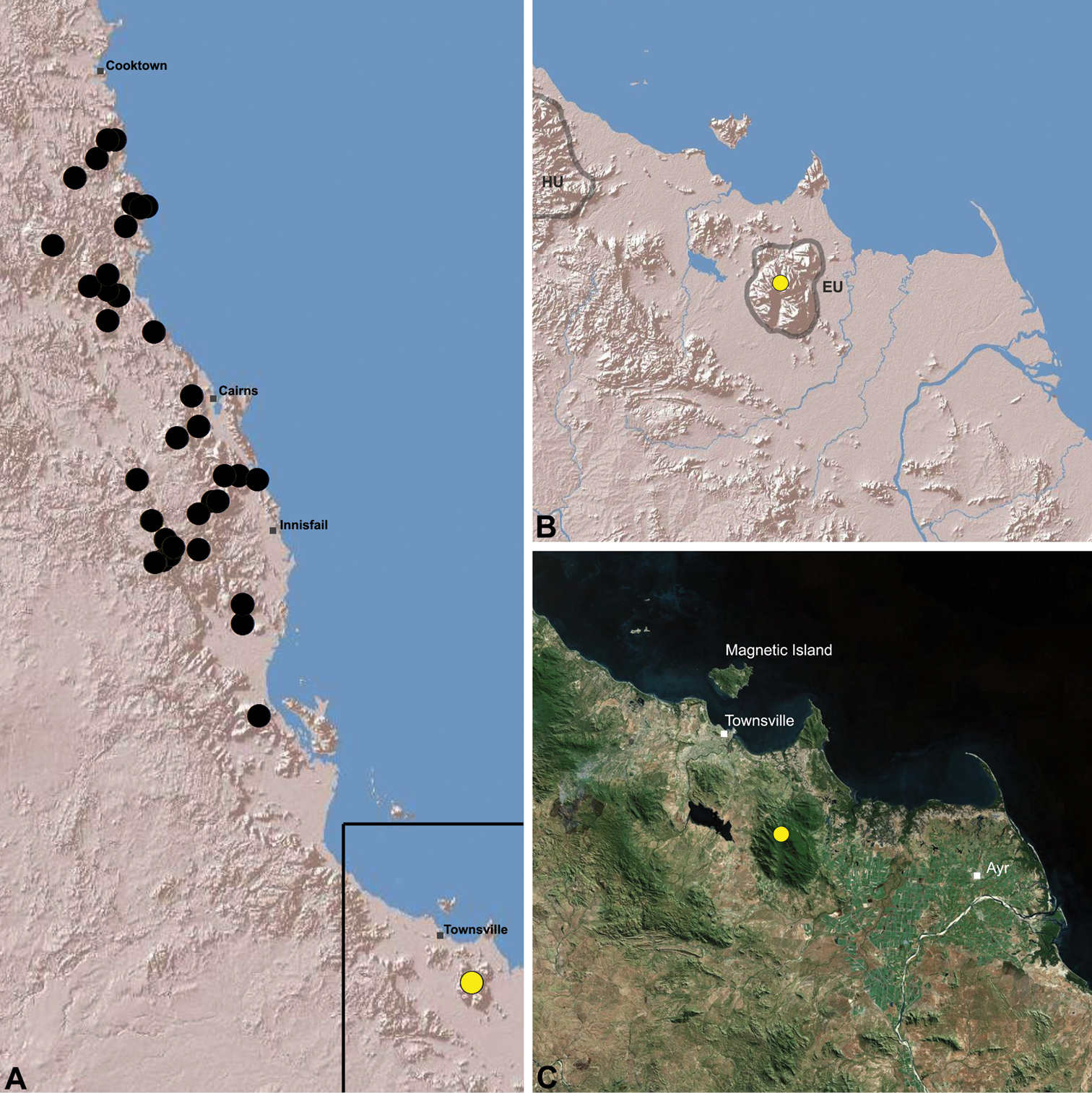
Austrarchaea hoskini is known only from Mount Elliot, 30 km south-east of Townsville (Figs 23, 25). The few known specimens were collected in high altitude rainforest along North Creek.
Unknown (data deficient).
In the absence of adult male specimens or molecular data, the following female and juvenile specimens (see Figs 16–23, 25) could not be confidently identified as known species. Species of Austrarchaea are difficult to identify (and diagnose) by females alone, and in the Wet Tropics these difficulties were compounded by the absence of representative adults from across the region. Material is thus here listed according to upland subregional zones of faunal endemism, as proposed for the Wet Tropics bioregion (see Discussion, below; Table 1; Figs 16B–23B).
List of upland subregional zones of faunal endemism identified for the Wet Tropics bioregion (by
| Wet Tropics Upland Subregion | Archaeidae | Described species |
|---|---|---|
| Mt Finnigan Uplands (FU) | YesM, F, J | Austrarchaea wallacei |
| Thornton Uplands (TU) | YesF, J | |
| Windsor Uplands (WU) | YesM | Austrarchaea karenae |
| Carbine Uplands (CU) | YesM, F, J | Austrarchaea tealei, Austrarchaea thompsoni |
| Black Mountain Corridor (BM) | YesJ | |
| Lamb Uplands (LU) | YesM, J | Austrarchaea westi |
| Malbon-Thompson Uplands (MT) | YesF, J | |
| Bellenden Ker/Bartle Frere (BK) | YesM, F, J | Austrarchaea daviesae, Austrarchaea woodae |
| Atherton Uplands (AU) | YesM, F, J | Austrarchaea daviesae |
| Kirrama Uplands (KU) | YesJ | |
| Hinchinbrook Island (HI) | N.R. | |
| Lee Uplands (LE) | N.R. | |
| Spec Uplands (SU) | N.R. | |
| Halifax Uplands (HU) | N.R. | |
| Elliot Uplands (EU) | YesM, F, J | Austrarchaea hoskini |
AUSTRALIA: Queensland: FINNIGAN UPLANDS: Monkhouse Timber Reserve: Moses Creek, 4 km NNE. of Mount Finnigan, 15°47'S, 145°17'E, berlesate, sieved rainforest litter, 14–16.X.1980, T. Weir, 1♀ (ANIC); Mount Boolbun South, 15°57'S, 145°08'E, 850–1000 m, 4–6.XI.1995, G. Monteith, D. Cook, L. Roberts, 1♀ (QMB S41070). Cedar Bay National Park: Mount Hartley, 15°47'S, 145°19'E, pyrethrum, trees & logs, 750 m, 8.XI.1995, G. Monteith, 1 juvenile (QMB). THORNTON UPLANDS: Daintree National Park (Cape Tribulation Section): Mount Sorrow Ridge Walk, centre saddle ~1.5 km from start, 16°04'35"S, 145°27'32"E, sifting elevated leaf litter under lawyer vine palms, tropical rainforest, 203 m, 20.III.2012, M. & A. Rix, 2♀, 1 juvenile (WAM T125629); same data, 1♀ (QMB S92211); on track to Mount Sorrow, 16°04'43"S, 145°27'42"E, day collecting, sifting leaf litter, mini-winklers, rainforest, 600 m, 20.IV.2009, H. Wood, 1♀ (CASENT 9028390); 4 km W. of Cape Tribulation (Site 8), 16°05'S, 145°26'E, QM berlesate, stick brushing, rainforest, 720 m, 29–30.IX.1982, G. Monteith, D. Yeates, G. Thompson, 1 juvenile (QMB S30802); 5 km W. of Cape Tribulation (Site 10), pyrethrum knockdown, rainforest, 780 m, 28.IX.1982, G. Monteith, D. Yeates, G. Thompson, 1 juvenile (QMB S30818); 4.5–5 km W. of Cape Tribulation (Top Camp), pyrethrum knockdown, rainforest, 760–780 m, 1–6.X.1982, G. Monteith, D. Yeates, G. Thompson, 1 juvenile (QMB S30825); Thornton Peak, via Daintree, 1100–1300 m, 24–27.IX.1984, G. & S. Monteith, 1 juvenile (QMB S30801). Monkhouse Timber Reserve: Mount Pieter Botte, 16°04'S, 145°24'E, pyrethrum, trees, logs, rocks, 950 m, 21.XI.1983, G. Monteith, H. Janetzki, 1 juvenile (QMB). CARBINE UPLANDS: Daintree National Park (Mossman Gorge Section): Upper Whyanbeel Creek, 16°23'S, 145°17'E, pyrethrum, mossy rocks, 1150 m, 5.IX.1992, G. Monteith, 3 juveniles (QMB S38582). Mount Lewis Forest Reserve: Mount Lewis, summit, via Julatten, QM berlesate, stick brushings, rainforest, 1200 m, 10.IX.1981, G. Monteith, D. Cook, 1 juvenile (QMB S30841). Mount Spurgeon Forest Reserve: Mount Spurgeon, summit, 16°26'S, 145°12'E, 1320 m, 21.XI.1997, G. Monteith, D. Cook, C. Burwell, 1♀ (QMB S35869). BLACK MOUNTAIN CORRIDOR: Mowbray National Park: Black Mountain, 17 km ESE. of Julatten, pyrethrum knockdown, 800–1000 m, 29–30.IV.1982, G. Monteith, D. Yeates, D. Cook, 1 juvenile (QMB S30813). LAMB UPLANDS: Danbulla National Park: Mount Haig, 17°06'S, 145°36'E, pitfall trap, 1150 m, 4–31.V.1995, P. Zborowski, 1 juvenile (ANIC). Dinden National Park: Isley Hills, 17°03'S, 145°42'E, pyrethrum, trees and rocks, 1050 m, 30.XI.1993, G. Monteith, H. Janetzki, 1 juvenile (QMB S59692). MALBON-THOMPSON UPLANDS: Russell River National Park: Graham Range, 17°17'S, 145°58'E, pyrethrum, logs, 550 m, 8–9.XII.1995, G. Monteith, G. Thompson, D. Cook, 1♀ (QMB S37969); same data except pyrethrum, trees and logs, 1.XI.1995, G. Monteith, 2 juveniles (QMB). BELLENDEN KER/BARTLE FRERE: Wooroonooran National Park: Bellenden Ker Range, 0.5 km south of Cable Tower No. 7, pyrethrum knockdown on logs, stones and tree trunks, 500 m, 17–24.X.1981, Earthwatch, QM, 1 juvenile (QMB S30828); Massey Range, 17°16'S, 145°49'E, QM berlesate, sieved litter, rainforest, 1250 m, 10.X.1991, G. Monteith, H. Janetzki, 1 juvenile (QMB S49636). ATHERTON UPLANDS: Herberton Range National Park: Longlands Gap, 17°28'S, 145°29'E, pitfall trap, 1150 m, 4.II.–6.III.1995, P. Zborowski, 1 juvenile (ANIC). Herberton Range State Forest: Baldy Mountain Road, 7 km SW. Atherton, pyrethrum, logs & trees, 1150 m, 9.XII.1988, G. Monteith, G. Thompson, 1 juvenile (QMB). Tully Gorge National Park: Mount Tyson, 2 km W. of Tully, 17°55'S, 145°54'E, QM berlesate, sieved litter, rainforest, 650 m, 7.V.1983, D. Yeates, 1 juvenile (QMB S30800); Upper Boulder Creek, via Tully, 17°50'S, 145°54'E, QM berlesate, sieved litter, rainforest, 900 m, 27.X.1983, G. Monteith, D. Yeates, G. Thompson, 1 juvenile (QMB S30805); Upper Boulder Creek, 10 km N. of Tully, 800 m, 4–5.XII.1989, G. Monteith, G. Thompson, H. Janetzki, 1♀ (QMB S73924); Upper Boulder Creek, 11 km NNW. of Tully, 850 m, 16–19.XI.1984, D. Cook, G. Monteith, G. Thompson, 1♀ (QMB S30815); same data except pyrethrum, logs & trees, 1000 m, 5.XII.1989, G. Monteith, G. Thompson, H. Janetzki, 1 juvenile (QMB). Wooroonooran National Park: Hughes Road, Topaz, 17°26'S, 145°42'E, pitfall trap, 650 m, VII.–IX.1993, G. Monteith, S. Breeden, 1 juvenile (QMB S25715); “Palmerston National Park”, 17°35'30"S, 145°42'00"E, pitfall trap, rainforest, 670 m, 25.VII.–30.XI.1992, R. Raven, P. & E. Lawless, M. Shaw, 1 juvenile (QMB S21921). KIRRAMA UPLANDS: Girringun National Park: Cardwell Range, Upper Broadwater Creek Valley, pitfall trap, rainforest, 750 m, 18.XII.1986–14.I.1987, G. Monteith, G. Thompson, S. Hamlet, 1 juvenile (QMB S30842).
urn:lsid:zoobank.org:act:5E8091DF-D073-49CB-B6AC-9D730BC126A6
http://species-id.net/wiki/Austrarchaea_griswoldi
Eungella Assassin SpiderFigs 1A–D, 15, 24–25
Holotype male: Eungella National Park (Broken River Section), Broken River Rainforest Discovery Circuit and Granite Bend Circuit, Queensland, Australia, 21°10'07"S, 148°30'22"E, sifting elevated leaf litter under palms (especially fan palms), tropical rainforest, 684 m, 23.III.2012, M. & A. Rix (QMB S92212).
Paratypes: Allotype female, same data as holotype (QMB S92213); 2 males, 1 female and 2 juveniles, same data as holotype (WAM T125630); 1 female, same data as holotype except Broken River Rainforest Discovery Circuit, hand collecting at night, 24.III.2012 (QMB S92214).
AUSTRALIA: Queensland: Eungella National Park: Broken River Rainforest Walk, 21°10'02"S, 148°30'23"E, litter, night collection, 720 m, 30.XI.2008, H. Smith, 1 juvenile (AMS KS106561); off Crediton Road Loop, 21°11'09"S, 148°31'43"E, sifting elevated leaf litter under fan palms, tropical rainforest, 673 m, 24.III.2012, M. & A. Rix, 1 juvenile (WAM T125631). Eungella: Schoolhouse rainforest general collection, 21°08'S, 148°29'E, 11–15.II.1986, R. Raven, J. Gallon, 1 juvenile (QMB S7039).
The specific epithet is a patronym in honour of Dr Charles Griswold, for his outstanding contributions to arachnology, and for his contributions to the study of Archaeidae and other basal Araneomorphae.
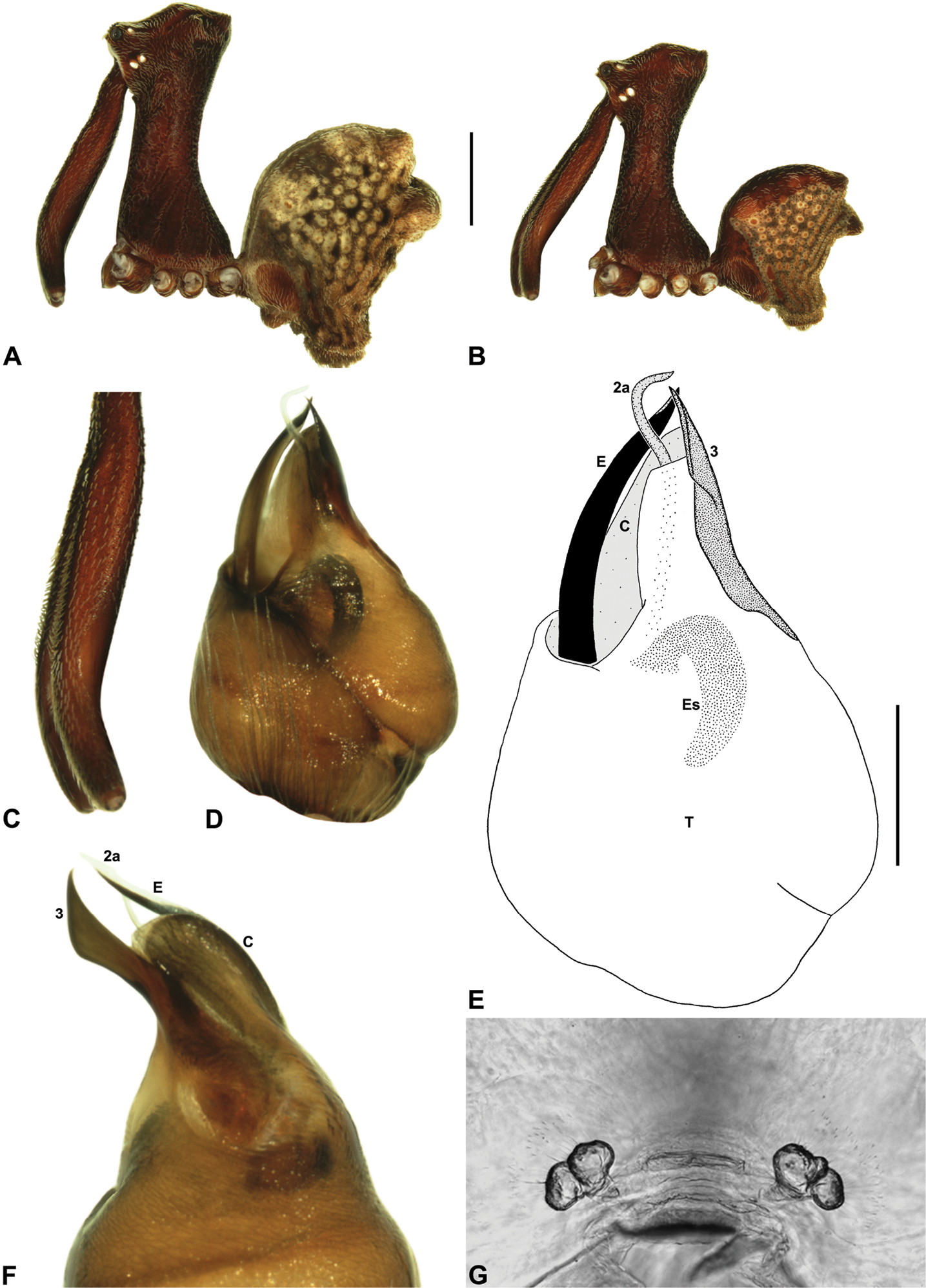
Austrarchaea griswoldi can be distinguished from all other Archaeidae from north-eastern Queensland by the presence of a unique Type E pedipalp (Fig. 6), with a very large bulb (width >> 0.30 mm) (Figs 6, 15E), modified ventro-distal rim of the tegulum forming rectangular opercular plate (Figs 6, 15E), and very large, flattened tegular sclerite 3 (TS 3), the latter extending along the entire retrolateral edge of the conductor (Fig. 15F). This species can be further distinguished by the very short, barely differentiated comb of accessory setae on the male chelicerae (Fig. 15C), and by the presence of only two pairs of female spermathecae (Fig. 15G).
Holotype male: Total length 3.08; leg I femur 2.99; F1/CL ratio 2.62. Cephalothorax dark reddish-brown; legs dark tan-brown with darker annulations; abdomen mottled dark grey-brown and beige, with darker brown dorsal scute and sclerites (Fig. 15B). Carapace tall (CH/CL ratio 2.17); 1.14 long, 2.47 high, 1.11 wide, ‘neck’ 0.58 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.56), carapace sloping in straight plane posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.27). Chelicerae with very short, barely differentiated comb of accessory setae on anterior face of paturon (Fig. 15C). Abdomen 1.47 long, 1.05 wide; with two pairs of dorsal hump-like tubercles (HT 1-4); dorsal scute fused anteriorly to epigastric sclerites, extending posteriorly to first pair of hump-like tubercles; HT 3-4 each covered by separate dorsal sclerites. Unexpanded pedipalp (Figs 15D-F) of Type E morphology (Fig. 6), very large in size (width of bulb >> 0.30), with retrolaterally directed, arched conductor; ventro-distal rim of tegulum distally extended to form rectangular opercular plate; embolus distally directed, curved, without spur, projecting only slightly beyond distal rim of conductor; tegular sclerite 3 (TS 3) very large, flattened, extending along entire retrolateral edge of conductor; TS 2-2a largely obscured by rectangular opercular plate, TS 2a projecting beyond distal rim of conductor to just past tip of embolus; TS 1 deeply embedded in bulb, obscured by opercular plate, not visible in ventral view.
Allotype female: Total length 3.68; leg I femur 2.87; F1/CL ratio 2.29. Cephalothorax dark reddish-brown; legs tan-brown with darker annulations; abdomen mottled dark grey-brown and beige (Fig. 15A). Carapace tall (CH/CL ratio 2.20); 1.26 long, 2.77 high, 1.21 wide; ‘neck’ 0.70 wide; bearing two pairs of rudimentary horns; highest point of pars cephalica (HPC) near middle of ‘head’ (ratio of HPC to post-ocular length 0.55), carapace sloping in straight plane posterior to HPC; ‘head’ not strongly elevated dorsally (post-ocular ratio 0.28). Chelicerae without accessory setae on anterior face of paturon. Abdomen 1.92 long, 1.59 wide; with four pairs of dorsal hump-like tubercles (HT 1-4). Internal genitalia (Fig. 15G) with pair of pyriform spermathecae on either side of gonopore, clusters widely separated along midline of genital plate.
Variation: Males (n = 3): total length 2.87–3.08; carapace length 1.10–1.14; carapace height 2.37–2.51; CH/CL ratio 2.15–2.22. Females (n = 3): total length 3.03–3.68; carapace length 1.24–1.26; carapace height 2.72–2.77; CH/CL ratio 2.16–2.23.
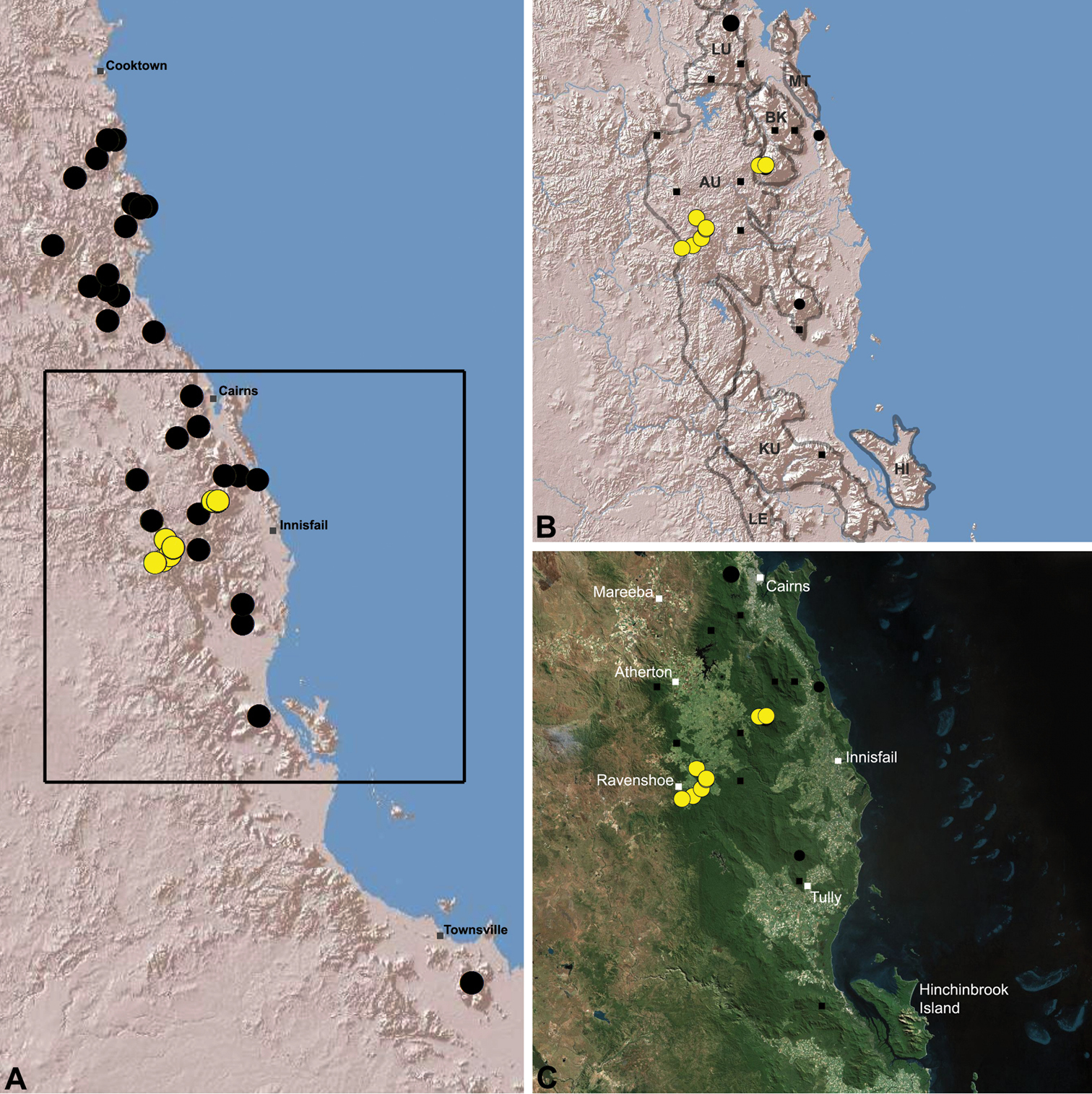
Austrarchaea griswoldi is known only from Eungella National Park, 70 km west of Mackay (Figs 24–25). Specimens have been collected by beating and sifting elevated leaf litter in tropical rainforest (Fig. 1E), especially under the dead fronds of Eungella Fan Palms (Livistona sp.).
A single female specimen was collected by MGR during night collecting in March 2012, suspended with her egg-sac in a tangled maternal web decorated with hanging debris, at the base of a large standing rainforest tree trunk. This egg-sac (Fig. 1D) was carried with both legs IV, positioned behind and against the posterior face of the abdomen, and was composed of soft brown silk. The shape of the egg-sac was irregular, with two large projections, and 18 spiderlings hatched out of the egg-sac on 3-4 April 2012.
This species appears to be a short-range endemic taxon (
In the absence of adult male specimens or molecular data, the following female specimens (see Figs 24–25) could not be confidently identified as known species.
AUSTRALIA: Queensland: Dryander National Park: Mount Dryander, [17 km WNW. of Airlie Beach], pyrethrum, 800 m, 21.XI.1992, G. Monteith, G. Thompson, H. Janetzki, 1♀ (QMB S49380). Eungella National Park: Finch Hatton [Gorge], sweeping, complex notophyll vine forest (CNVF), 7–14.IV.1975, R. Kitching, V. Davies, 1♀, 1 juvenile (QMB S1093).
The Wet Tropics World Heritage Area. The Australian Wet Tropics bioregion, situated in north-eastern Queensland between Cooktown and Townsville (Figs 16–23, 25), is a World Heritage area renowned for its rich rainforest biota and very high levels of local endemism (e.g. see
Patterns of distribution within the Wet Tropics have historically been assessed in terms of ‘regional endemism’ (i.e. those species confined to the Wet Tropics) versus ‘subregional endemism’ (i.e. those species confined to a single subregion within the Wet Tropics) (
Archaeidae in the Wet Tropics. Assassin spiders appear to be largely ubiquitous in upland rainforests throughout most of the Wet Tropics, extending from the Finnigan Uplands near Cooktown south to the Elliot Uplands near Townsville (Fig. 25). Archaeidae seem to be less common in lowland tropical rainforests (true of most species of Austrarchaea throughout their range), however populations from Mossman Gorge and near Cape Tribulation in the northern Daintree National Park suggest that they may be more widespread in lowland forest systems than current collection records suggest. Indeed, given the relatively high proportion of sites represented only by juvenile specimens or unidentified females (Fig. 25), the tendency for short-range endemism in Austrarchaea generally, and the very small number of adult male specimens available for taxonomic research, the Wet Tropics may actually be home to a significantly larger number of archaeid species than documented in this revision. For example, sites like Mount Bartle Frere support two sympatric or at least partially sympatric species (Figs 16, 22), and in the Mossman River region of the southern Daintree National Park, different taxa appear to occupy different lowland (Austrarchaea tealei) versus upland (Austrarchaea thompsoni) habitats in relatively close proximity (Figs 19–20). Apart from Austrarchaea daviesae, which is known from two adjacent subregions (Fig. 16B), all of the other seven species of Austrarchaea described from the Wet Tropics are subregional endemics, from the Finnigan Uplands (Austrarchaea wallacei), Windsor Uplands (Austrarchaea karenae), Carbine Uplands (Austrarchaea tealei, Austrarchaea thompsoni), Lamb Uplands (Austrarchaea westi) and the Bellenden Ker/Bartle Frere Uplands (Austrarchaea woodae), respectively (see Table 1).
Estimating the actual number of Archaeidae in north-eastern Queensland is a difficult task, given the surprisingly small number of collection records for the region, the very small number of adult male specimens available, and the related absence in all but two instances of anything other than single-point distributions for most known species (Fig. 25). However, available records do provide some tantalising clues, and hint at the likelihood of a possible hotspot of archaeid diversity in the Wet Tropics. Indeed, with (i) at least four other upland subregional zones with known archaeid records but for which adult male specimens are unavailable, (ii) four additional upland subregions which may harbour archaeid populations but are currently without collections (Table 1), (iii) the likelihood that at least a minority of Wet Tropical subregions may harbour multiple endemic species, either sympatrically or in upland versus lowland habitats, and (iv) the likely presence of at least one additional species in a separate upland zone of the Whitsundays region (Fig. 24), the actual number of taxa in the Austrarchaea daviesae species-group is almost certainly > 50% larger than currently recognised. Thus, at a conservative estimate, there may be 15 or more short-range endemic species in tropical Queensland, a number almost equivalent to the total archaeid diversity of mid-eastern Australia. These figures are perhaps not surprising, given the remarkable levels of diversity and endemism seen in other invertebrate groups (see
This research was made possible by an Australian Biological Resources Study (ABRS) taxonomy research grant to MSH and MGR (Grant No. 209-09), with critical initial support provided by Roy Teale (Biota Environmental Sciences) and significant additional funding provided by Cliffs Natural Resources and Verve Energy. Specimens of Austrarchaea were loaned by various curators, and the authors would like to thank Owen Seeman and Robert Raven (Queensland Museum), Bruce Halliday (Australian National Insect Collection) and Anthea Carmichael, Charles Griswold and Hannah Wood (California Academy of Sciences) for their curatorial assistance throughout this study. Specimens were collected under permit throughout north-eastern Queensland (Permit Nos. WITK10553612, WITK06874710, WITK106112112), and MGR would like to especially thank Alan Rix for his invaluable assistance during field work in March 2012. Thanks also to Karen Edward for sharing her deep knowledge of Wet Tropics biogeography and for the use of her subregional map layers utilised in Figures 16–23; and to Hannah Wood for confirming the identification of material lodged at (or on loan to) the CAS. Charles Griswold and Hannah Wood provided helpful comments on an earlier draft of the manuscript. This revision would not have been possible without the remarkable collecting efforts of staff at the Queensland Museum who have, over three decades, carefully and systematically documented the biodiversity and conservation values of the Queensland Wet Tropics World Heritage Area. We acknowledge the enormous funding and logistical investments that have been made to this end, and recognise the importance of the Queensland Museum’s collections as repositories of this remarkable natural heritage.
Habitus and habitat images of species Archaeidae from north-eastern Queensland. A–D, Habitus images of live paratype specimens of Austrarchaea griswoldi sp. n. from Eungella National Park: A newly-moulted female with recently cast cuticle; B–C, female, lateral view; D, female carrying egg-sac. E–F, Habitat images: E, tropical rainforest at Broken River, Eungella National Park – type locality of Austrarchaea griswoldi sp. n.; F, dense tropical rainforest at Malaan National Park, Atherton Tableland – locality of Austrarchaea daviesae Forster & Platnick.
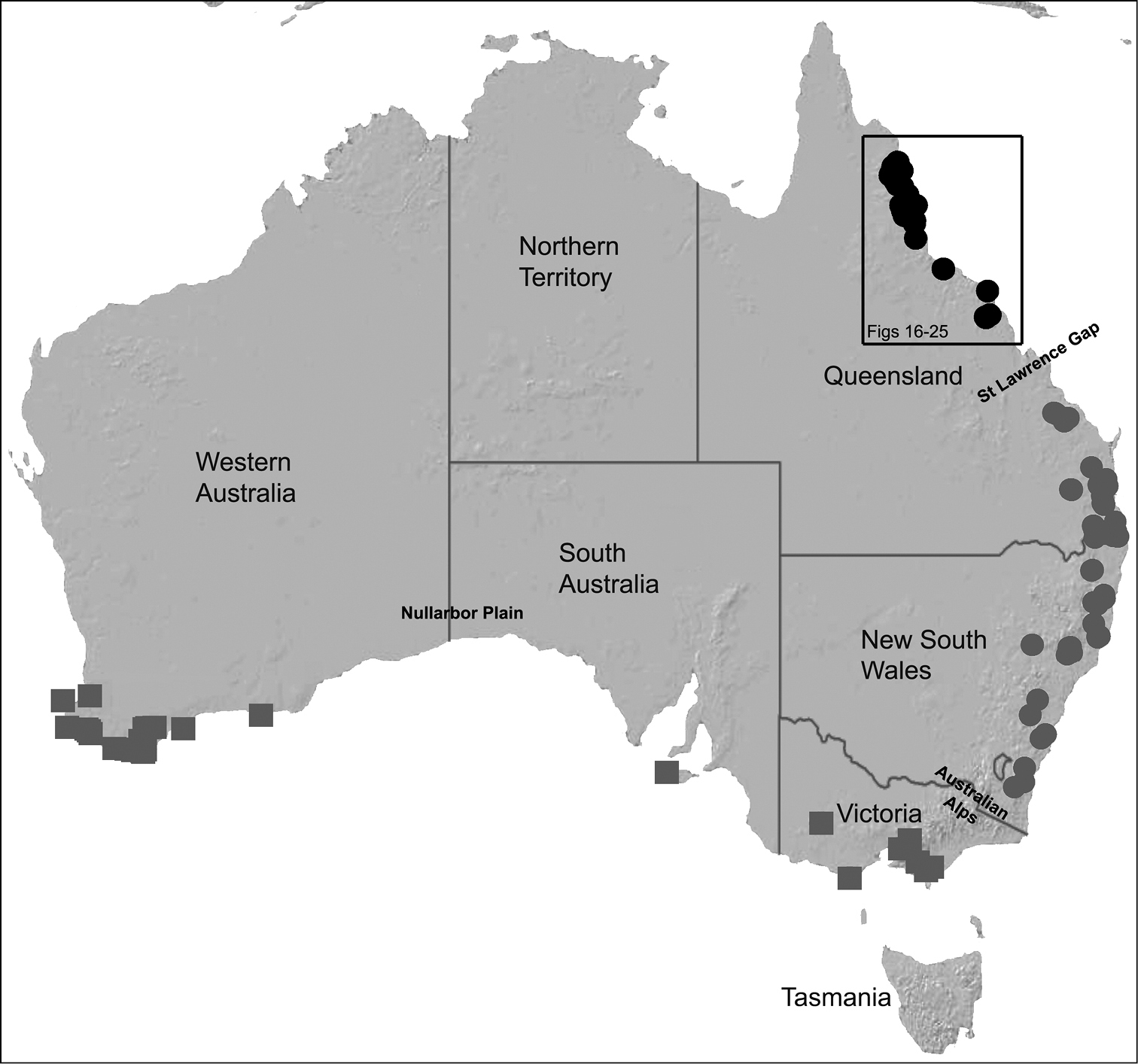
Map showing the known distribution of Archaeidae in Australia (circles for the genus Austrarchaea; squares for Zephyrarchaea), with locality records for north-eastern Queensland species of Austrarchaea in the Austrarchaea daviesae species-group highlighted in black. Note the three major biogeographic and phylogenetic disjunctions in the distribution of Australian Archaeidae (see
Distribution and phylogeny of Austrarchaea species from
Morphological differences between the three lineages of Austrarchaea (see Fig. 3). Note the variation in the shape of the male pedipalp and the marked differences in the shape and orientation of the conductor (C), embolus (E) and the distal tegular sclerites. Note also the number of abdominal hump-like tubercles (1-6): four in the Austrarchaea daviesae species-group; five in Austrarchaea monteithi; and six in the Austrarchaea nodosa species-group.
Graphs depicting the relationship between carapace length (CL) and carapace height (CH) for species of Austrarchaea from north-eastern Queensland. Smaller grey dots denote species of Austrarchaea from mid-eastern Australia (see
Morphological differences between the five pedipalp types (Types A-E) identified for species of Austrarchaea from north-eastern Queensland, with left bulbs illustrated in ventral view at scale-identical sizes. Type A pedipalps are shared among at least five species from the Wet Tropics bioregion; Types B-E are autapomorphic for single species. Note especially the variation in the size and shape of the bulb, and the shape and orientation of the conductor. C = conductor; E = embolus; ESp = embolic spur; (TS)1-3 = tegular sclerites 1-3. Scale bar = 0.2 mm.
Austrarchaea daviesae Forster & Platnick, 1984. A–B, Cephalothorax and abdomen, lateral view: A, female (WAM T125183) from Malaan National Park, Atherton Tableland, NE. Queensland; B, holotype male (QMB S1091) from Majors Mountain, Atherton Tableland, NE. Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Male (WAM T125183; from Malaan National Park, Atherton Tableland, NE. Queensland) pedipalp: D–E, bulb, ventral view; F, detail of distal tegular sclerites, retrolateral view. G, Female (WAM T125183) internal genitalia, postero-ventral view (genital plate removed). C = conductor; E = embolus; T = tegulum; (TS)2-3 = tegular sclerites 2-3. Scale bars: A-B = 1.0 mm; E = 0.2 mm.
Austrarchaea wallacei sp. n. A–D, Holotype male (QMB S25964) from Mount Misery, Monkhouse Timber Reserve, NE. Queensland: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, right pedipalpal bulb (expanded; flipped horizontal for inter-specific comparison), retrolateral view. bH = basal haematodocha; C = conductor; E = embolus; ESp = embolic spur; H = haematodocha; T = tegulum; (TS)1-3 = tegular sclerites 1-3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea karenae sp. n. A–E, Holotype male (QMB S43060) from Windsor Tableland, Windsor Tableland National Park, NE. Queensland: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, ventral view; E, detail of distal tegular sclerites, retrolateral view. C = conductor; E = embolus; ESp = embolic spur; T = tegulum; (TS)1-3 = tegular sclerites 1-3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea thompsoni sp. n. A–E, Holotype male (QMB S30840) from Devils Thumb, Daintree National Park, NE. Queensland: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, ventral view; E, detail of distal tegular sclerites, retrolateral view. C = conductor; E = embolus; ESp = embolic spur; T = tegulum; (TS)2-3 = tegular sclerites 2-3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea tealei sp. n. A–B, Cephalothorax and abdomen, lateral view: A, female (ANIC) from Mossman Gorge, Daintree National Park, NE. Queensland; B, holotype male (QMB S92210) from Mossman Gorge, Daintree National Park, NE. Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, ventral view; F, detail of distal tegular sclerites, retrolateral view. G, Female (ANIC) internal genitalia, postero-ventral view (genital plate removed). C = conductor; E = embolus; ESp = embolic spur; T = tegulum; (TS)1-3 = tegular sclerites 1-3. Scale bars: A-B = 1.0 mm; E = 0.2 mm.
Austrarchaea westi sp. n. A–E, Holotype male (QMB S59537) from Mount Williams, Dinden National Park, NE. Queensland: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing lack of defined accessory setae; C–D, pedipalpal bulb, ventral view; E, detail of distal tegular sclerites, retrolateral view. C = conductor; E = embolus; T = tegulum; (TS)2-3 = tegular sclerites 2-3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea woodae sp. n. A–E, Holotype male (QMB S72988) from Boulder Caves, Mount Bartle Frere, Wooroonooran National Park, NE. Queensland: A, cephalothorax and abdomen, lateral view; B, chelicerae, lateral view, showing accessory setae; C–D, pedipalpal bulb, ventral view; E, detail of distal tegular sclerites, retrolateral view. C = conductor; E = embolus; T = tegulum; (TS)2-3 = tegular sclerites 2-3. Scale bars: A = 1.0 mm; D = 0.2 mm.
Austrarchaea hoskini sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S17937) from Mount Elliot, Bowling Green Bay National Park, NE. Queensland; B, holotype male (QMB S30811) from Mount Elliot, Bowling Green Bay National Park, NE. Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, ventral view; F, detail of distal tegular sclerites, retrolateral view. G, Allotype female internal genitalia, postero-ventral view (as seen through posterior rim of genital plate). C = conductor; E = embolus; ESp = embolic spur; GP = genital plate; T = tegulum; (TS)2-3 = tegular sclerites 2-3. Scale bars: A-B = 1.0 mm; E = 0.2 mm.
Austrarchaea griswoldi sp. n. A–B, Cephalothorax and abdomen, lateral view: A, allotype female (QMB S92213) from Broken River, Eungella National Park, NE. Queensland; B, holotype male (QMB S92212) from Broken River, Eungella National Park, NE. Queensland. C, Holotype male chelicerae, lateral view, showing accessory setae. D–F, Holotype male pedipalp: D–E, bulb, ventral view; F, detail of distal tegular sclerites, retrolateral view. G, Allotype female internal genitalia, postero-ventral view (genital plate removed). C = conductor; E = embolus; Es = embolic sclerite; T = tegulum; (TS)1-3 = tegular sclerites 1-3. Scale bars: A-B = 1.0 mm; E = 0.2 mm.
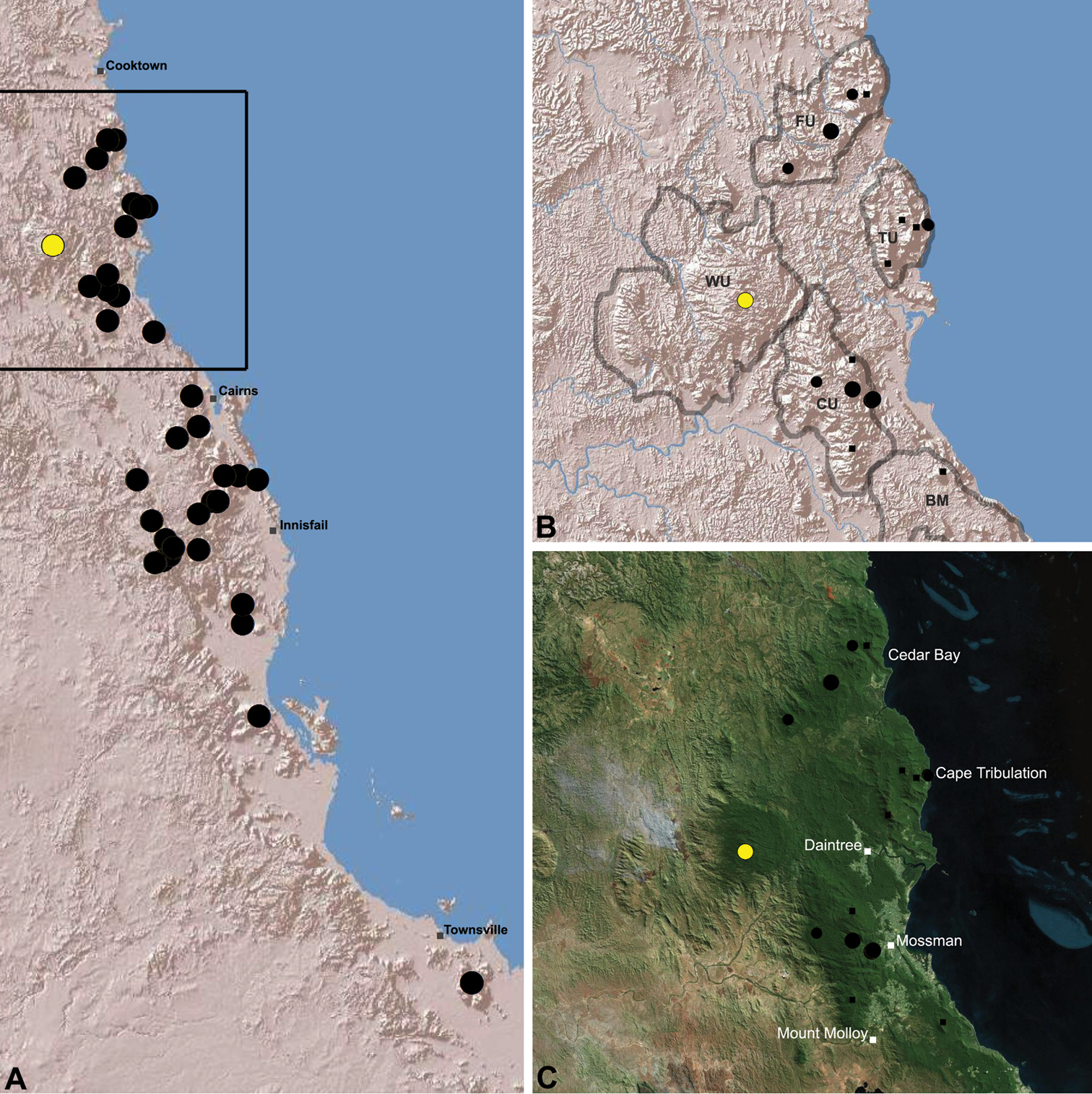
Distribution of Austrarchaea daviesae Forster & Platnick, 1984: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea daviesae highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the central Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. AU = Atherton Uplands; BK = Bellenden Ker/Bartle Frere; HI = Hinchinbrook Island; KU = Kirrama Uplands; LE = Lee Uplands; LU = Lamb Uplands; MT = Malbon-Thompson Uplands.
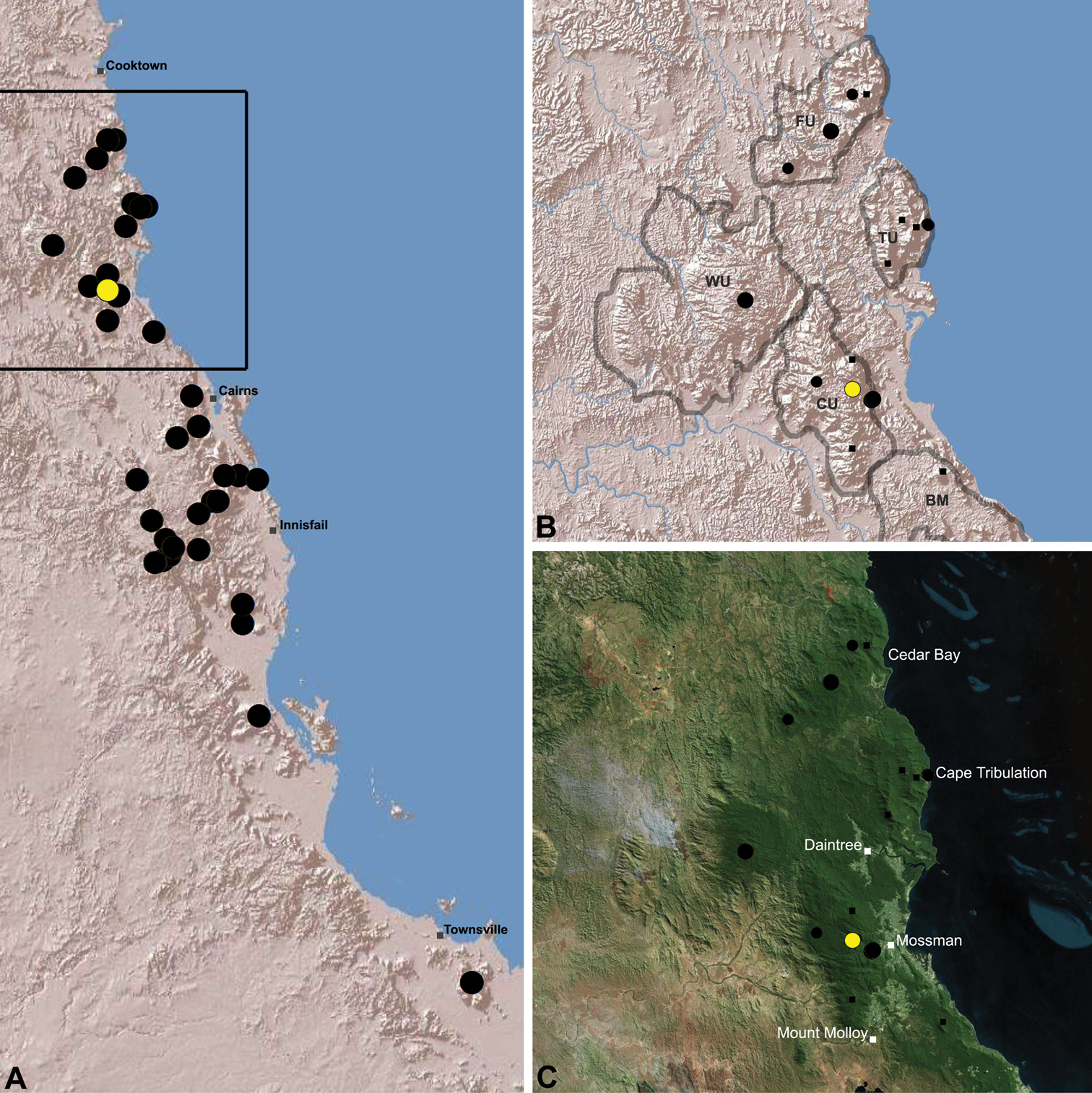
Distribution of Austrarchaea wallacei sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea wallacei highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the northern Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. BM = Black Mountain Corridor; CU = Carbine Uplands; FU = Mt Finnigan Uplands; TU = Thornton Uplands; WU = Windsor Uplands.
Distribution of Austrarchaea karenae sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea karenae highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the northern Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. BM = Black Mountain Corridor; CU = Carbine Uplands; FU = Mt Finnigan Uplands; TU = Thornton Uplands; WU = Windsor Uplands.
Distribution of Austrarchaea thompsoni sp. n.: A, topographic map showing the known distrib ution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea thompsoni highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the northern Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. BM = Black Mountain Corridor; CU = Carbine Uplands; FU = Mt Finnigan Uplands; TU = Thornton Uplands; WU = Windsor Uplands.
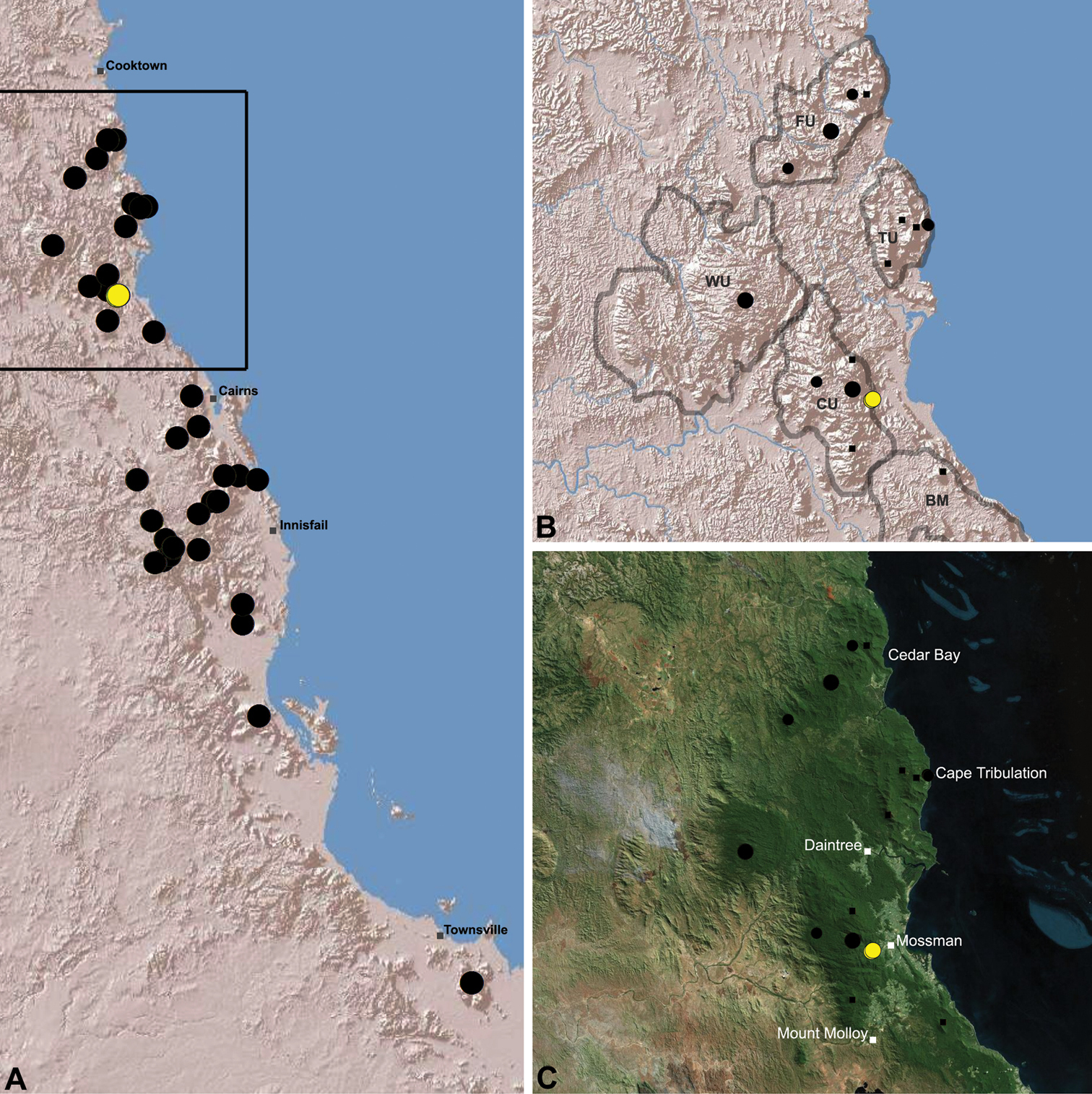
Distribution of Austrarchaea tealei sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea tealei highlighted in yellow; B-C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the northern Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. BM = Black Mountain Corridor; CU = Carbine Uplands; FU = Mt Finnigan Uplands; TU = Thornton Uplands; WU = Windsor Uplands.
Distribution of Austrarchaea westi sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea westi highlighted in yellow; B-C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the central Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. AU = Atherton Uplands; BK = Bellenden Ker/Bartle Frere; HI = Hinchinbrook Island; KU = Kirrama Uplands; LE = Lee Uplands; LU = Lamb Uplands; MT = Malbon-Thompson Uplands.
Distribution of Austrarchaea woodae sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea woodae highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the central Wet Tropics (modified from Edward 2011). Small squares in (B–C) denote unidentified juvenile specimens; small circles denote unidentified female specimens; large circles denote described species of Austrarchaea. AU = Atherton Uplands; BK = Bellenden Ker/Bartle Frere; HI = Hinchinbrook Island; KU = Kirrama Uplands; LE = Lee Uplands; LU = Lamb Uplands; MT = Malbon-Thompson Uplands.
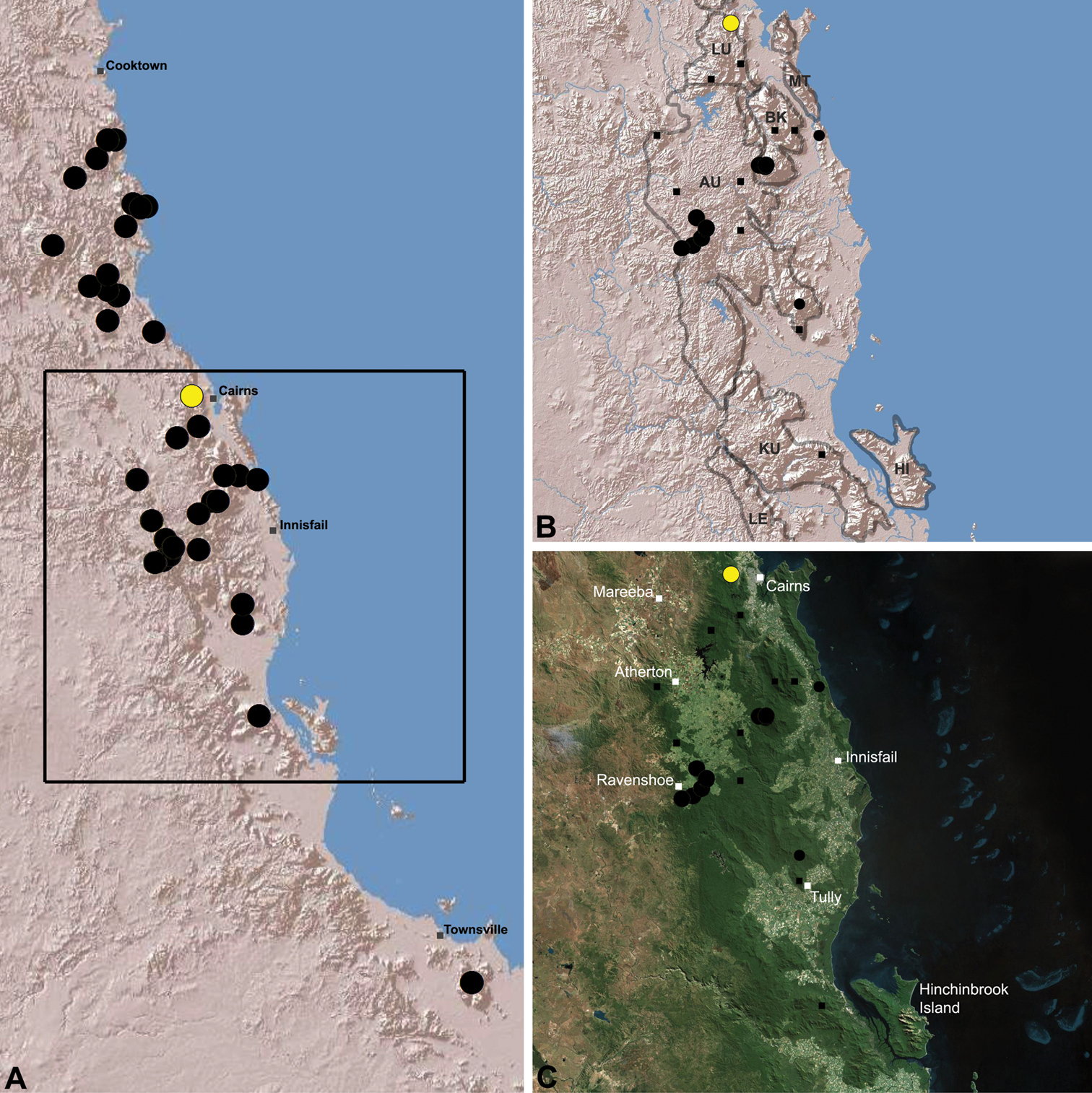
Distribution of Austrarchaea hoskini sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Wet Tropics bioregion, with collection localities for Austrarchaea hoskini highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Labelled boundaries in (B) denote upland subregional zones of faunal endemism identified by Winter et al. (1984), Williams et al. (1996) and other authors for the southern Wet Tropics (modified from Edward 2011). EU = Elliot Uplands; HU = Halifax Uplands.
Distribution of Austrarchaea griswoldi sp. n.: A, topographic map showing the known distribution of Archaeidae in the north-eastern Queensland Mackay and Whitsundays Hinterland, with collection localities for Austrarchaea griswoldi highlighted in yellow; B–C, topographic and satellite maps showing detail of inset (A). Small circles in (B–C) denote unidentified female specimens; large circles denote described species of Austrarchaea.
Figure 25. Summary distribution of the Austrarchaea daviesae species-group in tropical north-eastern Queensland, showing collections records for described species (labelled, with black circles) and unidentified juveniles or females (yellow circles) (see Table 1). Note the high proportion of unidentified specimens, especially within the Wet Tropics bioregion between Cooktown and Ingham.