(C) 2012 Jakub Straka. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The apid cuckoo bees of the Cape Verde Islands (Republic of Cape Verde) are reviewed and five species recognized, representing two genera. The ammobatine genus Chiasmognathus Engel (Nomadinae: Ammobatini), a specialized lineage of cleptoparasites of nomioidine bees is recorded for the first time. Chiasmognathus batelkai sp. n. is distinguished from mainland African and Asian species. The genus Thyreus Panzer (Apinae: Melectini) is represented by four species – Thyreus denolii sp. n., Thyreus batelkai sp. n., Thyreus schwarzi sp. n., and Thyreus aistleitneri sp. n. Previous records of Thyreus scutellaris (Fabricius) from the islands were based on misidentifications.
Apoidea, Anthophila, Apidae, cleptoparasite, taxonomy, Thyreus, Chiasmognathus, Cape Verde Islands
Although the bees of isolated archipelagos are of considerable interest and have usually attracted some degree of melittological interest, those species of the Republic of Cape Verde have been only anecdotally treated (
A list of expeditions and names of collectors of bees who visited the Cape Verde Islands and provided their material for scientific studies.
| Collector(s)/Expedition(s) | Dates | Current Repository of Material |
|---|---|---|
| HMS Challenger Expedition | 1873 | presumably NHML |
| L. Fea | 1898 | presumably MSNG, SEMC |
| H. Lindberg | 1953–1954 | MZH |
| A. van Harten | 1963–1990 | RMNH |
| E. Bauber, B. Friebe, K. Groh, H. Hölzel, W. Lobin, P. Ohm, B. Traub | 1978–1980 | FISC |
| R.T. Simon Thomas | 1988 | RMNH |
| F. La Roche | 1998–1999 | Personal coll., MICN |
| E. Aistleitner | 1999–2009 | Personal coll., NMPC, SEMC |
| J. Straka, J. Batelka | 2009–2011 | Personal coll., NMPC, SEMC |
The material considered herein is located in the following institutional and personal repositories: EAFC, Eyjolf Aistleitner, Feldkirch, Austria; NMPC, Department of Entomology, National Museum, Prague, Czech Republic; FISC, Forschungsinstitut Senckenberg, Frankfurt am Main, Germany, JSPC, Jakub Straka Collection, Charles University in Prague, Prague, Czech Republic; and SEMC, Division of Entomology, University of Kansas Natural History Museum, Lawrence, Kansas, USA. Abbreviations of additional repositories referred to in Table 1 are as follows: NHML, The Natural History Museum, London, United Kingdom; MSNG, Museo Civico di Storia Naturale “Giacomo Doria”, Genoa, Italy; MICN, Museo Insular de Ciencias Naturales, Tenerife, Canary Islands, Spain; MZH, Finnish Museum of Natural History, Helsinki, Finland; RMNH, Nationaal Natuurhistorische Museum (“Naturalis”), Leiden, The Netherlands.
Morphological terminology for the descriptions follows that of
als anterolateral mesoscutal patch (paired); frequently transverse in shape and along anterior margin and often contiguous with lpn.
deps dorsal mesepisternal patch; large patch covering upper portion of mesepisternum.
hypm hypoepimeral area patch; patch situated under wing base and posterior to pronotal lobe and often contiguous with deps.
lp lateral propodeal patch; conspicuous patch on either side of propodeum and often concealing spiracle.
lpn lateral pronotal patch (paired); transverse and placed on each side of middle on dorsal-facing surface of pronotum and toward posterior lobe.
mls mediolateral mesoscutal patch (paired); often round and situated on either side and slightly anterior to center of mesoscutum and just posterior to caudal apex of ms.
ms median mesoscutal patch (unpaired); anterior longitudinal patch from anterior of mesoscutum and extending caudad beyond als along median line; often relatively diffuse.
pls posterolateral mesoscutal patch (paired); round or hook-shaped patch in line with mls, sometimes projecting anteriorly along lateral border to meet plsa.
plsa anteriorposterolateral mesoscutal patch (paired); narrow, longitudinal patch along lateral border with tegula, often extending posteriorly to meet anteriolateral projection of pls.
ps parascutellar patch (paired), situated within the axilla.
s mesoscutellar patch (paired), typically just posterior to apicolateral angle.
t tegular patch, often posteriorly or along inner posterior angle.
veps ventral mesepisternal patch; smaller patch on ventral margin of mesepisternum.
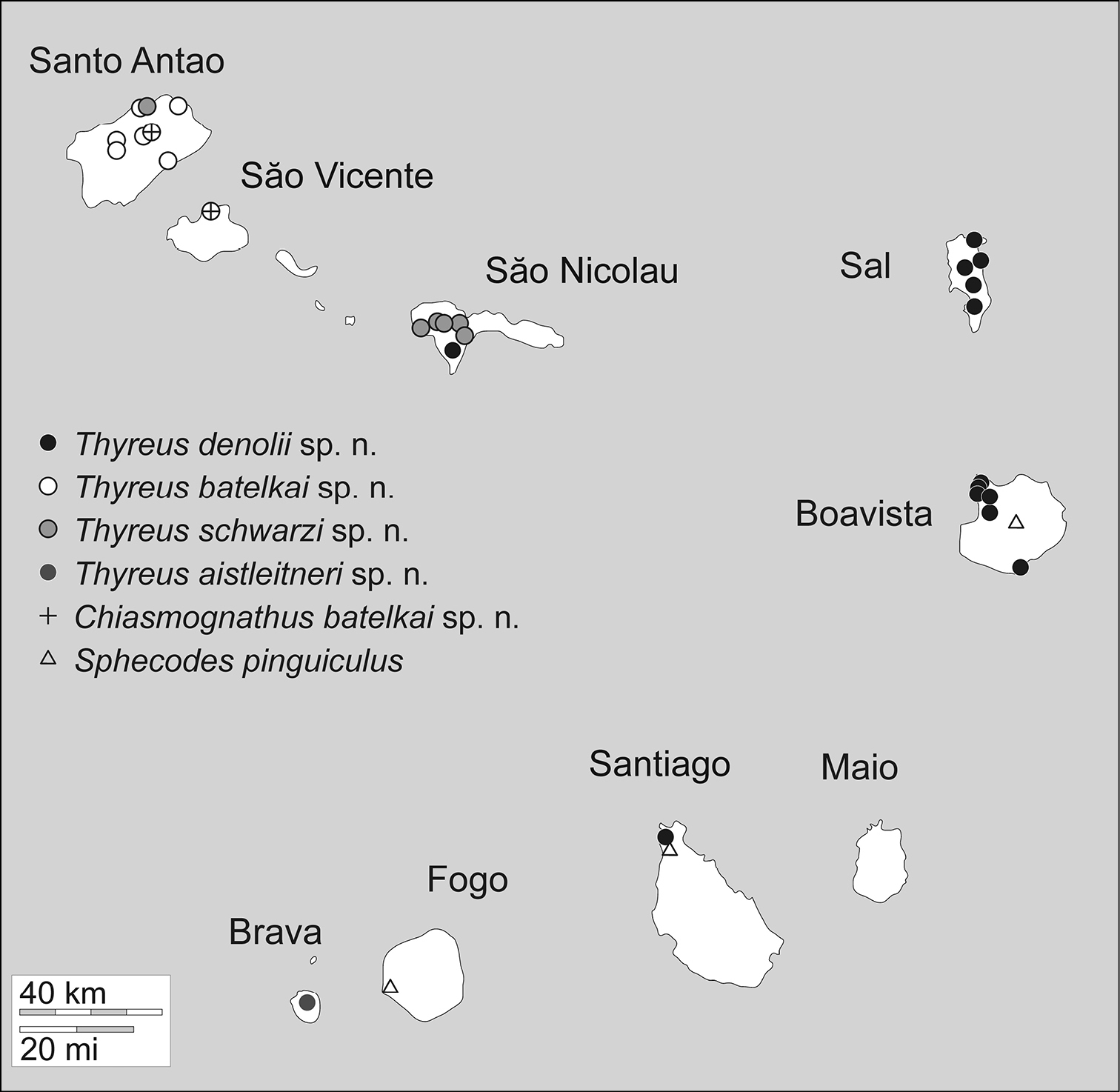
Photomicrographs were prepared using a Canon EOS 7D digital camera attached to an Infinity K-2 long-distance microscope lens. Measurements were made using an ocular micrometer attached to an Olympus SZX-12 or MBS-10 stereomicroscope. Distributions of the species across the various islands are summarized in Table 2 and Map 1 (outline map of Cape Verde came from d-maps.com).
Currently confirmed distributions of cuckoo bee species across the Cape Verde Islands (see also Map 1). All of the apid species are endemic, while Sphecodes pinguiculus Pérez is widespread (
| Taxon | Barlavento | Sotovento | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Santo Antão | São Vicente | Santa Luzia | São Nicolau | Sal | Boa Vista | Maio | Santiago | Fogo | Brava | |
| Chiasmognathus batelkai | X | X | ||||||||
| Thyreus denolii | X? | X | X | X | ||||||
| Thyreus batelkai | X | X | ||||||||
| Thyreus schwarzi | X? | X | ||||||||
| Thyreus aistleitneri | X | |||||||||
| Sphecodes pinguiculus | X | X | X | |||||||
| Totals | 3 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | ||
Cape Verde islands with distribution of all cuckoo bee species.
The genus Thyreus is the most diverse lineage of Melectini, encompassing at least 104 previously described species (e.g.,
urn:lsid:zoobank.org:act:6436F526-A122-4B3F-B173-74EF3DF4948F
http://species-id.net/wiki/Thyreus_denolii
Figs 1–11♂, Cape Verde Isl., Boavista, rock N of Sal Rei, 20.x.2009 [20 October 2009], J. Batelka & J. Straka lgt. (SEMC).
Boavista: 1♂, 2♀♀, Cabo Verde 00/41, Ilha de Boavista, Sal Rei–S, 10 m, 30.12.2000 [30 December 2000], leg. Aistleitner (EAFC); 4♂♂, Cabo Verde, Boavista, Costa de BoaEsperança, (NE Sal Rei), 50 m, 1.1.2001 [1 January 2001], leg. Aistleitner/ 46 (EAFC); 2♀♀, Cabo Verde 00/47, Ilha de Boavista, Ribeira de Rabil, 10–20 m, 2.1.2001 [2 January 2001], leg. Aistleitner (EAFC); 3♀♀, Cabo Verde 00/48, Ilha de Boavista centr., Estancia de Baixo, 60 m, 2.1.2001 [2 January 2001], leg. Aistleitner (EAFC); 1♂, 3♀♀, Cape Verde Isl., Boavista – Sal Rei, on the beach, 1.X.2009 [1 October 2009], J. Straka & J. Batelka lgt. (JSPC, FISC); 1♂, 1♀, Cape Verde Isl., Boavista – Sal Rei, dunes, sweeping, 19.X.2009 [19 October 2009], J. Batelka & J. Straka lgt. (JSPC); 3♂♂, Cape Verde Isl., Boavista – rock N of Sal Rei, 20.X.2009 [20 October 2009], J. Batelka & J. Straka lgt. (JSPC); 1♀, same data as holotype except: Sal Rei on the beach, 1.x.2009 [1 October 2009], J. Straka & J. Batelka lgt. (SEMC); Sal: 1♂, 4.11.1980 [4 Noveber 1980], SAL, Straße Flughafen – Sta. Maria, n. v. Algodoeiro, Islas do Cabo Verde – 1980, H. Hölzel, W. Lobin, P. Ohm [collectors] (FISC); 1♂, 2♀♀, Cabo Verde 00/2, Ilha do Sal, Espargos, Boa Terra, 60 m, 28.11.2000 [28 November 2000], leg. Aistleitner (EAFC); 4♂♂, 3♀♀, Cabo Verde 00/6, Ilha do Sal, Pedra Lume, Ostküste, 20–40 m, 29.11.2000 [29 November 2000], leg. Aistleitner (EAFC); 1♂, Cabo Verde 00/7, Ilha do Sal, Mte. Grande–S, 70–170 m, 30.11.2000 [30 November 2000], leg. Aistleitner (EAFC); 1♂, the same as previous except 250 m (EAFC); 2♂♂, Cabo Verde 00/54, Ilha do Sal, Mte. Grande–E, 60 m, 9.1.2001 [9 January 2001], leg. Aistleitner (EAFC); 1♀, Cabo Verde, Ilha do Sal, Pedra Lume, 19.3.2004 [19 March 2004], leg. Aistleitner (EAFC); 1♀, Cape Verde Isl., Sal – Murdeira, ribeira, 2.–3.XI.2011 [2–3 November 2011], 16°41'N, 22°55'W, J. Batelka & J. Straka lgt. (JSPC).
Sal: 1♀, Cape Verde Isl., Sal – Monte Grande, 16.XI.2011 [16 November 2011], 20–350 m, 16°49'22"N, 22°54'22"W, J. Batelka & J. Straka lgt. [remnants of meso and metasoma] (JSPC); Santiago: 4♀♀; Ilhas do Cabo Verde, S. Tiago, Tarrafal, Triebe, Goh leg., 18.–20.10.79 [18–20 October 1979] (FISC, JSPC); 1♀, the same as previous except: Goh, Lobin leg., 10.79 [October 1979] (FISC); São Nicolau: 1♀, Ilhas do Cabo Verde, S. Nicolau, Goh, Lobin leg., 10.79 [October 1979] (FISC).
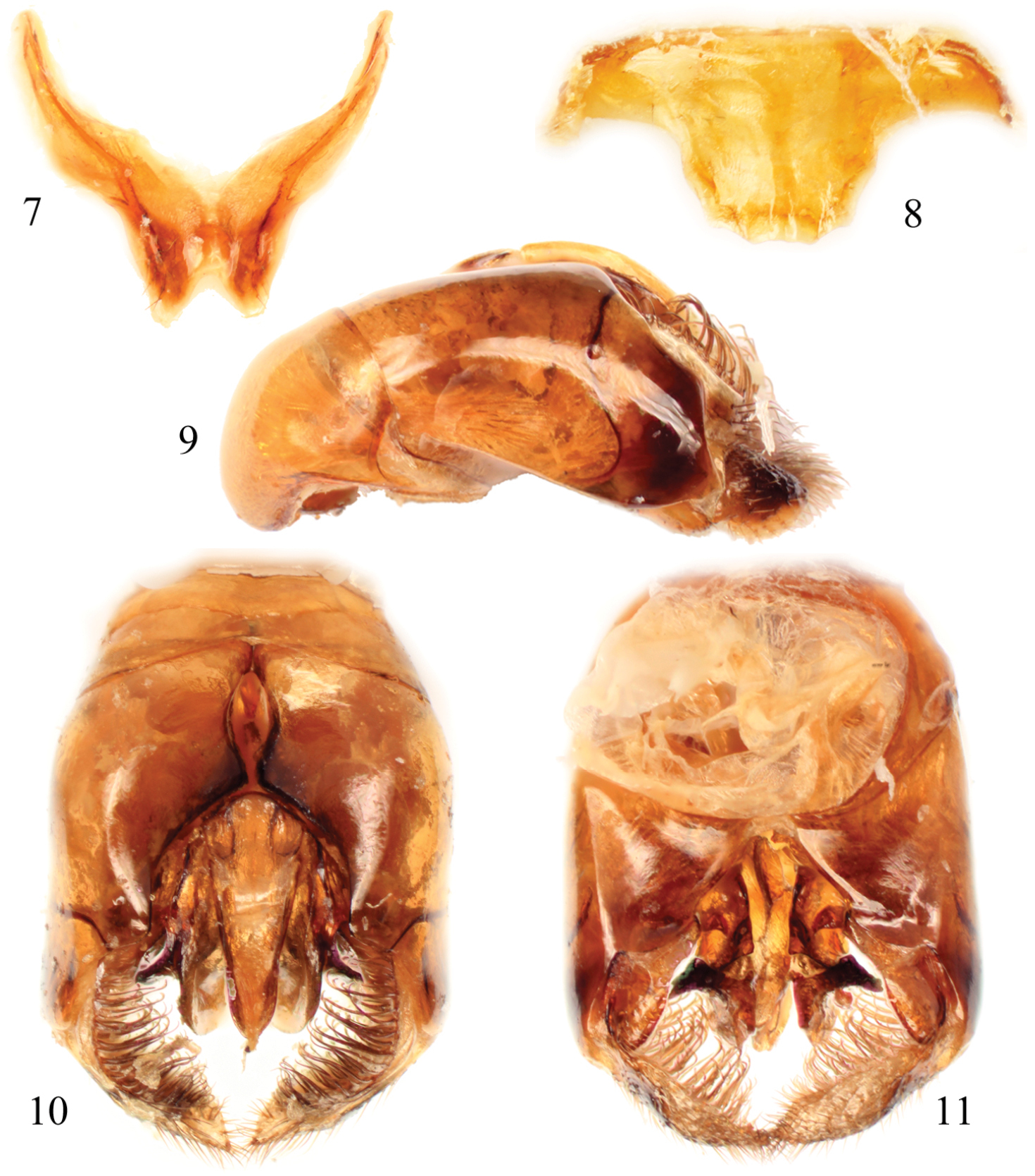
Thyreus denolii is one of the most distinctive of the Cape Verde Island species of Thyreus. The species can be readily recognized in the male by the sixth metasomal tergum with lateral white patches of the same size as on the fourth and fifth terga (rarely with the white patches of the sixth tergum reduced, but when so, then the patches are also reduced on the preceding terga) (Figs 1, 3). The female has a combination of the ventral and ventrolateral parts of the mesepisternum with distinct shiny interspaces among the punctures; the mesoscutum with plsa (anterior posterolateral mesoscutal) present and bordering the anterior portion of the tegula, but not meeting pls (posterolateral mesoscutal) posteriorly (Fig. 4); the apical depression of the fifth metasomal tergum densely punctate medially and densely setose; and the fifth tergum with lateral white patches of the same size as those on the fourth and third terga (Figs 2, 4). Both sexes have the combination of the apicolateral corners of mesoscutellum weakly pointed, forming an angle of more than 40° (although more sharply pointed in the female than the male), and the mesoscutellum finely punctate, with punctures separated, at least on the disc, by 0.5–1 times a puncture width.
♂: Total body length 9.9 mm (7.5–9.9 mm); forewing length 7.4 mm (6.0–7.9 mm). Head wider than long (length 2.4 mm, width 3.0 mm); upper interorbital distance 1.8 mm; lower interorbital distance 1.3 mm. Intertegular distance 2.4 mm (2.0–2.6 mm); mesoscutellar posterior margin often sinuate, sometimes weakly so, such that apicolateral angle projects as a prominent, broad spine and with a defined median emargination [degree of this sinuation is variable and so some males have spines and median emargination less prominent, but margins from apicolateral corners to midpoint are never straight as is usual for species such as Thyreus hohmanni Schwarz, typical Thyreus ramosus (Lepeletier de Saint Fargeau), or several Asiatic species]. Inner anterior angle of metatibia not swollen or projecting into prominence or point between metatibial spurs (e.g., in some Palearctic species this area of metatibia is prominently developed: e.g.,
Labrum with coarse, irregular, contiguous punctures except basolateral impunctate areas, such areas longer than wide and therefore ovoid in shape, basomedially with short V-shaped furrow; clypeus with small contiguous punctures, integument between (where evident) smooth; face as on clypeus except punctures slightly larger, nearly contiguous, and somewhat weaker on supraclypeal area; punctures weaker and shallower on vertex and on ocellocular area, with small impunctate area bordering lateral ocellus; punctures coarse, shallow, and nearly contiguous on gena and posterior area of postgena, anterior area of postgena smooth and impunctate. Pronotum with coarse, shallow, nearly contiguous punctures; mesoscutum with well-defined punctures separated by less than a puncture width, slightly more widely spaced around parasidal lines and medioposteriorly such that punctures are separated by about 0.5–2 times a puncture width, integument between punctures smooth and shining; mesoscutellum, including axilla, with punctures as on medioposterior section of mesoscutum except punctures separated by 0.5–1 times a puncture width; pleura with coarse, nearly contiguous punctures, integument between punctures (where evident) smooth and shining; hypoepimeral area with impunctate area bordering scrobe; propodeal lateral and posterior surfaces with coarse, shallow, ill-defined, nearly contiguous punctures. Metasoma with small punctures separated by less than a puncture width, punctures coarser, larger, and somewhat more poorly defined on more apical terga, integument between finely imbricate, apical margins narrowly impunctate and finely imbricate; sterna with similar punctation except those on discs of more basal sterna more widely spaced and becoming more poorly defined on more apical sterna.
Integument black except dark brown on tarsi, mouthparts, and apically on seventh metasomal tergum and on apical sterna. Wing membranes hyaline and slightly infumate, veins dark brown to black.
Pubescence generally fuscous to black over entire body except for presence of plumose white setae on face (Fig. 5), posterior of vertex, upper gena, outer surface of protibia, outer surface of mesotibia, outer basal surface of metatibia, and on mesosoma (using annotation system of
♀: As described for the male except in usual gender differences and as follows: Total body length 8.1–10.6 mm; forewing length 6.4–7.4 mm. Head wider than long (length 2.4 mm, width 3.0 mm); upper interorbital distance 1.9 mm; lower interorbital distance 1.3 mm (Fig. 6). Intertegular distance 2.1–2.5 mm; mesoscutellar posterior margin as in male but sometimes sinuate margin weaker and apicolateral angle not forming as prominent a spine. Apical depression of fifth tergum densely punctate and setose. Pygidial plate relatively narrow, margins converging apically, largely straight, apex narrowly rounded, surface imbricate, basal half with shallow, coarse punctures.
Clypeus with small punctures more widely spaced than in male, separated by less than a puncture width, punctures variable in size placed apically, but rather uniform medially, integument between smooth. Mesoscutum with well-defined punctures separated by less than a puncture width anteriorly and laterally, slightly more widely spaced on disc and posteriorly, there separated by 0.5–1.5 times a puncture width; mesoscutellum with punctures separated by less than a puncture width, less frequently separated by as much as a puncture width, axilla with punctures separated by less than a puncture width.
Integument and pubescence as in male except dark brown on pygidial plate; second through fifth metasomal terga with more or less transverse to rounded lateral patches (Figs 2, 4).
Lateral habitus of Thyreus denolii sp. n. from Boavista. 1 Male 2 Female.
Dorsal habitus of Thyreus denolii sp. n. from Boavista. 3 Male 4 Female.
Facial aspect of Thyreus denolii sp. n. from Boavista (facial views for the various species do not really vary and so only those for Thyreus denolii are presented). 5 Male 6 Female.
Male terminalia of Thyreus denolii sp. n. 7 Seventh metasomal sternum 8 Eighth sternum 9 Genital capsule, lateral aspect 10 Genital capsule, dorsal view 11 Genital capsule, ventral view.
The specific epithet is a patronym honoring António de Noli (ca. 1415–d. ?), a Genoese navigator who, exiled from his homeland and working on behalf of Portugal, discovered the Cape Verde Islands around 1456.
The five females collected from Santiago are not designated as part of the type series. While they most closely agree with this species the shape of the pygidial plate is slightly different and this series could possibly represent a separate species. However, given that this is the only fixed difference we can find at this time and that we lack males from Santiago, we have tentatively assigned these individuals to Thyreus denolii pending the discovery of additional material. Analogously, a single female from S. Nicolau that is clearly of Thyreus denolii is also not designated as part of the type series. This might be a mislabeled specimen, a normal part of Thyreus denolii’s distribution, or even a different species. This particular female specimen of Thyreus denolii is unique in its L-shaped white patch on the second tergum. More material and collecting are needed so as to permit a more accurate characterization of the distribution and possible variation within the species.
urn:lsid:zoobank.org:act:97D31AFF-AFE9-4234-8B7D-785D2E7B8020
http://species-id.net/wiki/Thyreus_batelkai
Figs 12–20♂, Cape Verde Isl., Santo Antao, 1382 m, Espongeiro, 13.x.2009 [13 October 2009], 17°06'17"N, 25°05'21"W, J. Straka & J. Batelka lgt. (SEMC).
Santo Antão: 1♀, 21.11.1980 [21 Noveber 1980], SANTO ANTAO, 400–1000 supra Porto Novo, Islas do Cabo Verde – 1980, H. Hölzel, W. Lobin, P. Ohm [collectors] (FISC); 1♀, Cabo Verde, Santo Antão, 5.01.99 [5 January 1999], Ribeira Grande, Orgãos, 50–200 m, leg. Aistleitner (EAFC); 1♀, Cabo Verde 00/10, Ilha d. S. Antão, Ribeira Grande, 2.12.2000 [2 December 2000], leg. Aistleitner (EAFC); 2♀♀, Cabo Verde 00/21, Ilha d, S. Antão, Cruzinha da Garça, 50 m, 7.12.2000 [7 December 2000], leg. Aistleitner (EAFC); 5♀♀, Cabo Verde 00/23, Ilha de S. Antão, Cruzinha da Garça, 10–50 m, 9.12.2000 [9 December 2000], leg. Aistleitner (EAFC); 1♂, 2♀♀, Cabo Verde 00/24, Ilha de S. Antão centr., Lagoa–E, 1150–1300 m, 10.12.2000 [10 December 2000], leg. Aistleitner (EAFC); 2♀♀, 1♂, Cape Verde Isl., Santo Antao, 1382 m, Espongeiro, 13.X.2009 [13 October 2009], 17°06'17"N, 25°05'21"W, J. Straka & J. Batelka lgt. (JSPC); 2♀♀, 1♂, same data as previous except 12.–13.X.2009 [12–13 October 2009], 17°06'N, 25°05'W (JSPC, SEMC); 8♀♀, 4♂♂, Cape Verde Isl., Santo Antao – Selada de Alto Mira – Cirio, 4.–5.XI.2011 [4–5 November 2011], 17°04'N, 25°13'W, J. Straka & J. Batelka lgt. (JSPC, FISC); 2♀, 1♂, Cape Verde Isl., Santo Antao – Curral das Vacas – Bordeira de Norte, 6.XI.2011 [6 November 2011], 17°02'N, 25°14'W, J. Batelka & J. Straka lgt. (JSPC); 1♂, Cape Verde Isl., Santo Antao – black dunes 3 km W of Porto Novo, 7.XI.2011 [7 November 2011], 17°0'47"N, 25°06'30.84"W, J. Straka & J. Batelka lgt. (JSPC); 1♀, Santo Antao, Selada de Alto Mira – Cirio, 4–5.xi.2011 [4–5 November 2011], 17°04'N, 25°13'W, J. Straka & J. Batelka lgt. (SEMC); São Vicente: 6♂♂, 10♀♀, Cabo Verde 00/25, Ilha de S. Vicente, Calhau – Baia d. Gatas, -5 m, 13.12.2000 [13 December 2000], leg. Aistleitner (EAFC).
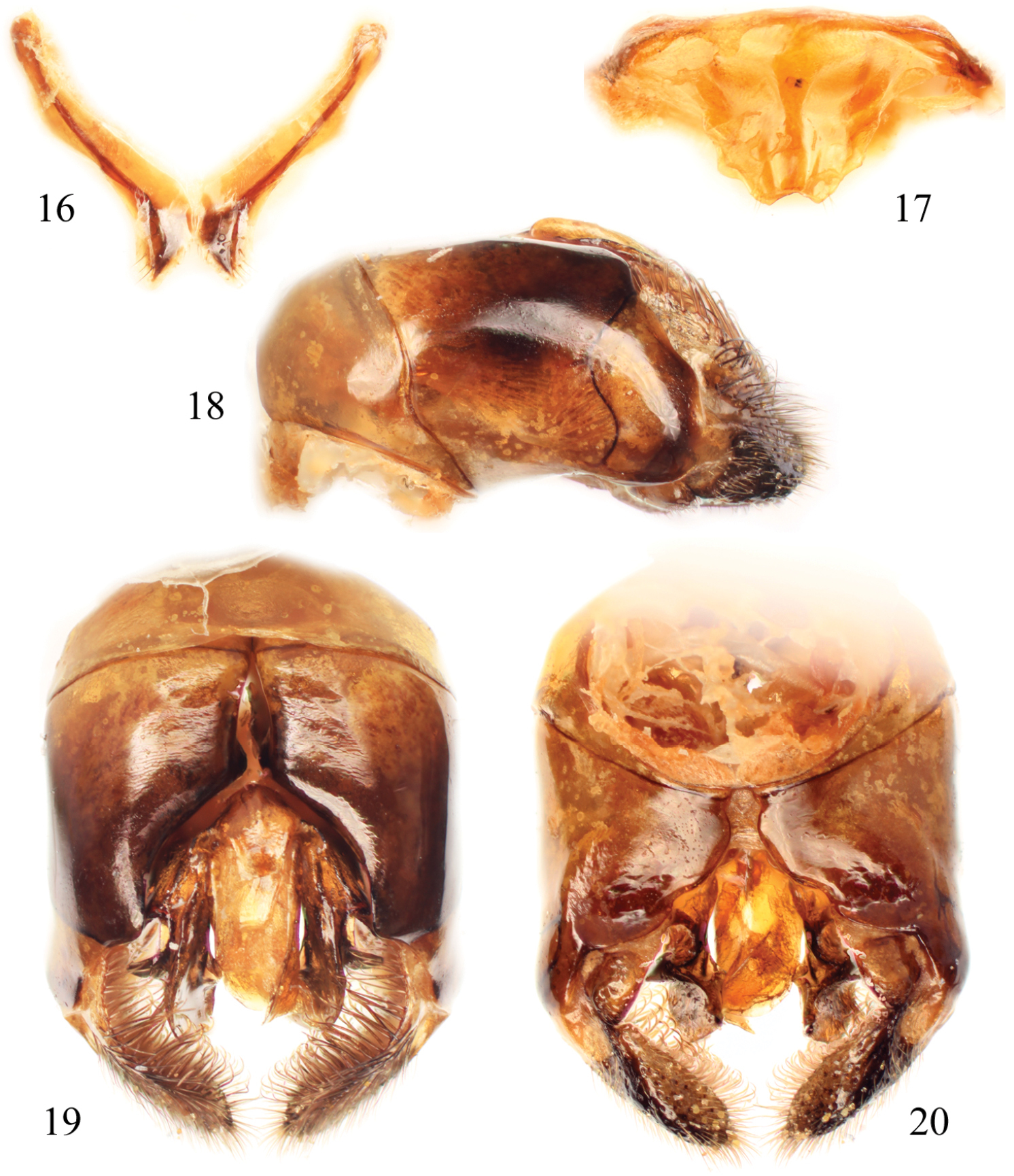
. Thyreus batelkai can be distinguished in the male by the combination of the lateral white patches of the sixth metasomal tergum greatly reduced, especially in comparison to the patches on the fourth and fifth terga, and the second tergum with the lateral white patch L-shaped (as on the first tergum) or at least with a smaller anterior patch (Fig. 14); while the female can be recognized by the mesoscutum with plsa (anterior posterolateral mesoscutal) present anterior to and along the border with the tegula, extending posteriorly and meeting, or less frequently almost meeting, pls (posterolateral mesoscutal); the second metasomal tergum with the lateral white patch L-shaped (as on the first tergum) or at least with a smaller anterior patch (Figs 12, 14); and the apical depression of the fifth tergum bare medially, rarely with a few punctures with short setae. In both sexes the mesoscutum has coarse punctures throughout; the mesoscutellum is coarsely punctate, with punctures dense and nearly contiguous; the apicolateral corners of the mesoscutellum are prominently and sharply pointed, for-ming an angle of less than 40° (Figs 14, 15); and all of the white, anterior mesoscutal patches are well developed.
As described for Thyreus denolii (vide supra) except as follows: ♂: Total body length 13.7 mm (7.9–13.7 mm); forewing length 9.6 mm (6.5–10.6 mm). Head wider than long (length 2.7 mm, width 3.5 mm); upper interorbital distance 2.2 mm; lower interorbital distance 1.6 mm. Intertegular distance 3.1 mm (2.3–3.4 mm); mesoscutellar posterior margin weakly sinuate, with apicolateral angle projecting as a prominent spine, with a defined median emargination (Fig. 15). Apex of seventh metasomal tergum with apicolateral prominences distinct, margin between frequently broadly U-shaped, weakly concave, without medial emargination or swelling; male terminalia as in Figures 16–20.
Mesoscutum with well-defined, coarse, contiguous to nearly contiguous punctures; mesoscutellum, including axilla, as on mesoscutum. Pleura with coarse, contiguous punctures; hypoepimeral area as on remainder of pleura, without impunctate area bordering scrobe.
White patches on mesosoma as follows: deps (dorsal mesepisternal), lpn (lateral pronotal), and als (anterolateral mesoscutal) present; ms (median mesoscutal) present, often extending to posterior tangent of mls (mediolateral mesoscutal); mls (mediolateral mesoscutal) present; plsa (anterior posterolateral mesoscutal) present anterior to and along border with tegula, extending posteriorly and meeting, or less frequently almost meeting, pls (posterolateral mesoscutal); t (tegular) present posteriorly on tegula; pls (posterolateral mesoscutal) present; ps (parascutellar) and s (mesoscutellar) absent; deps (dorsal mesepisternal), hypm (hypoepimeral area), and lp (lateral propodeal) present, veps (ventral mesepisternal) absent (Figs 12, 14). Metasomal terga with prominent patches of appressed, plumose white setae as follows: first metasomal tergum with large, L-shaped patches laterally; second metasomal tergum with large, L-shaped patches laterally as on first tergum, infrequently L-shape is incomplete with secondary rounded secondary anterior patch almost connecting posterior transverse patch; third through fifth metasomal terga with more or less transverse to rounded lateral patches (Figs 12, 14).
♀: As described for the male except in usual gender differences and as follows: Total body length 7.4–13.6 mm; forewing length 5.7–10.4 mm. Head wider than long (length 3.2 mm, width 3.9 mm); upper interorbital distance 2.3 mm; lower interorbital distance 1.8 mm. Intertegular distance 1.7–3.4 mm; mesoscutellar posterior margin forming a more gently, broad curve that is more straight medially between apicolateral angles, apicolateral angles forming a prominent spine, medially not to faintly emarginate. Apical depression of fifth tergum impunctate medially. Pygidial plate relatively narrow, margins converging apically, largely straight, apex narrowly rounded, surface imbricate except apicolaterally with smooth areas, basal half with shallow, coarse punctures.
Clypeus with small punctures separated by less than a puncture width, but with variable punctures in size apically and more sparsely punctate than in middle, some interspaces among punctures about as large as a puncture width, integument between smooth.
Integument and pubescence as in male except dark brown on pygidial plate; third through fifth metasomal terga with more or less transverse to rounded lateral patches (Figs 13, 15).
Lateral habitus of Thyreus batelkai sp. n. from Santo Antão. 12 Male 13 Female.
Dorsal habitus of Thyreus batelkai sp. n. from Santo Antão. 14 Male 15 Female.
Male terminalia of Thyreus batelkai sp. n. 16 Seventh metasomal sternum 17 Eighth sternum 18 Genital capsule, lateral aspect 19 Genital capsule, dorsal view 20 Genital capsule, ventral view.
The specific epithet is a patronym honoring Jan Batelka, a prominent collector of the new species, authority on the systematics of beetles, and close friend.
urn:lsid:zoobank.org:act:92D8F800-D36A-4B7E-AADE-BA3F770B96DD
http://species-id.net/wiki/Thyreus_schwarzi
Figs 21–29♂, Isl., Cabo Verde, S. Nicolau, 1.XII.80 [1 December 1980], Ribeiro Brava, Islas do Cabo Verde - 1980, H. Hölzel, W. Lobin, P. Ohm [collectors] (FISC).
São Nicolau: 1♂, same data as holotype (FISC); 1♀, Ilhas do Cabo Verde, S. Nicolau, Lobin leg. (FISC); 1♀, Cabo Verde 00/31, Ilha de S. Nicolau, Ribeira Brava–W, 100–150 m, 20.12.2000 [20 December 2000], leg. Aistleitner (EAFC); 3♂♂, Cabo Verde 00/34, Ilha de S. Nicolau, Preguiça–N, 70–100 m, 21.12.2000 [21 December 2000], leg. Aistleitner (EAFC); 4♂♂, Cabo Verde 00/36, Ilha de S. Nicolau, Monte Gordo, 12–1300 m, 22.12.2000 [22 December 2000], leg. Aistleitner (EAFC); 1♂, Cape Verde Isl., Sao Nicolau W, Barril, 11.XI.2011 [11 November 2011], 16°35'24"N, 24°23'84"W, J. Straka & J. Batelka lgt. (JSPC); 1♀, Sao Nicolau, W, south of Cachao, Cha de Caldeira, 12.xi.2011, 16°36'39"N, 24°19'58"W, J. Batelka & J. Straka lgt. (SEMC).
Santo Antão:1♀, Cabo Verde 00/21, Ilha d, S. Antão, Cruzinha da Garça, 50 m, 7.12.2000 [7 December 2000], leg. Aistleitner (EAFC).
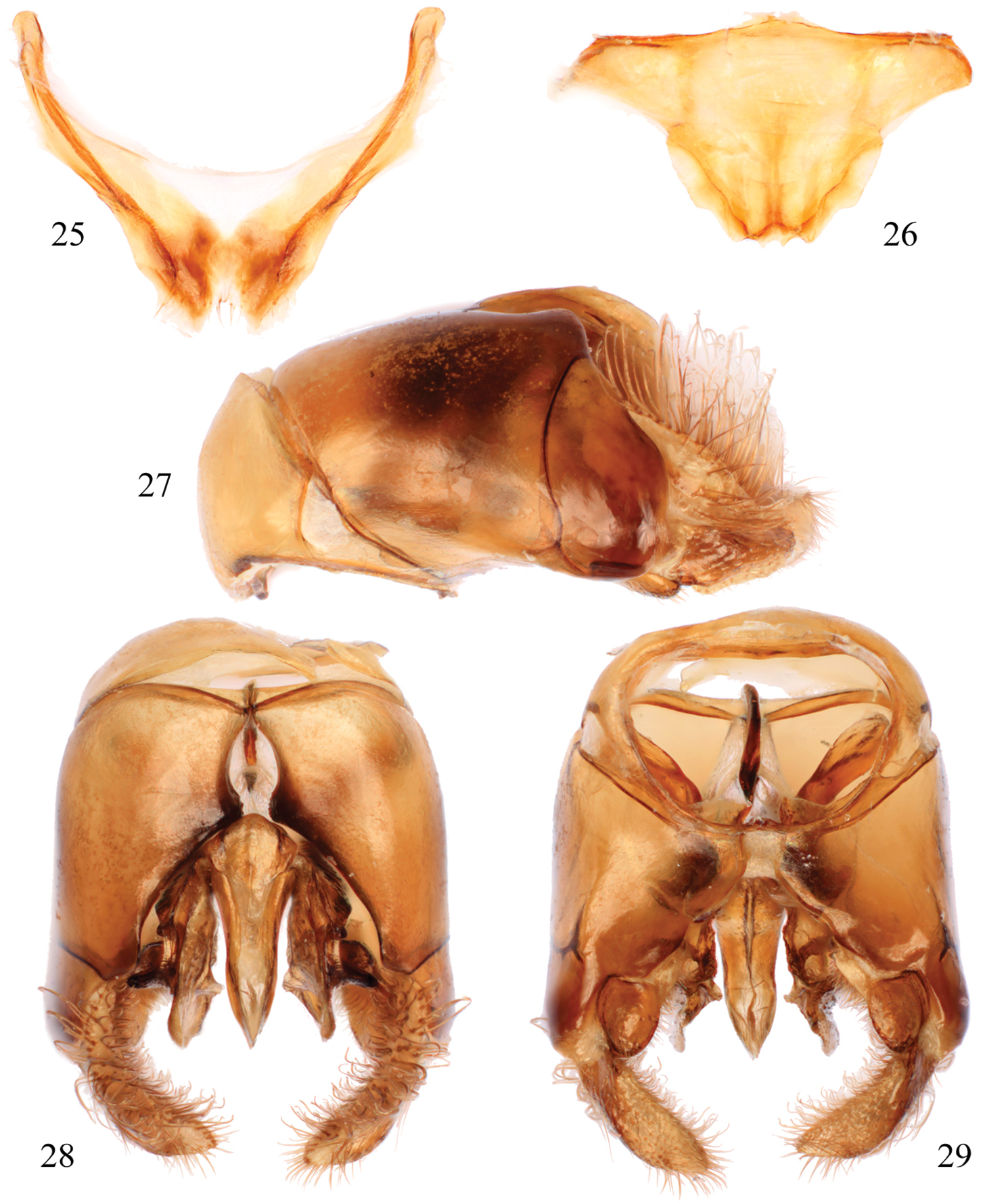
Males of Thyreus schwarzi can be recognized by the following combination of characters: white anterior mesoscutal patches, especially ms (median mesoscutal) and mls (mediolateral mesoscutal), strongly reduced to missing (Fig. 23); second metasomal tergum with lateral patch wider than long, without anterior secondary patch and never L-shaped (Fig. 23), but rarely with a few white setae in this area; and sixth tergum with lateral white patches greatly reduced, especially in comparison to patches on the fourth and fifth terga. Females can be characterized by the following combination of traits: white anterior mesoscutal patches, especially paired mls (mediolateral mesoscutal), greatly reduced to a few short setae (Fig. 24); mesoscutum with plsa (anterior posterolateral mesoscutal) present anterior to and along border with tegula, extending posteriorly and meeting, or less frequently almost meeting, pls (posterolateral mesoscutal) (Fig. 24); second metasomal tergum with lateral patch wider than long, without anterior secondary patch and never L-shaped (Fig. 24), but rarely with a few white setae in this area; and apical depression of fifth tergum with several distinct seta-bearing punctures. Both males and females have the apicolateral corners of the mesoscutellum prominently and sharply pointed, forming an angle of less than 40° (Figs 23, 24)
As described for Thyreus denolii (vide supra) except as follows: ♂: Total body length 12.8 mm (8.5–14.0 mm); forewing length 10.4 mm (6.7–10.5 mm). Head wider than long (length 3.1 mm, width 3.7 mm); upper interorbital distance 2.3 mm; lower interorbital distance 1.7 mm. Intertegular distance 3.4 mm (2.1–3.5 mm); mesoscutellar posterior margin faintly sinuate, with apicolateral angle projecting as slightly prominent spine, with median emargination. Apex of seventh metasomal tergum with apicolateral prominences distinct, truncate margin between straight, without medial emargination or swelling; male terminalia as in figures 25–29.
Mesoscutum with well-defined punctures separated by much less than a puncture width, punctures mediopically more spaced, separated by about 0.5–1 times a puncture width but more often less than a puncture width, integument between punctures smooth and shining; mesoscutellum, including axilla, with punctures separated by less than a puncture width, those laterally nearly contiguous to contiguous.
White patches on mesosoma as follows: deps (dorsal mesepisternal) and lpn (lateral pronotal) present; als (anterolateral mesoscutal) present but reduced, often faint; ms (median mesoscutal) faint to absent; mls (mediolateral mesoscutal) present to faint; plsa (anterior posterolateral mesoscutal) present anterior to and along border with tegula, extending posteriorly and meeting, or less frequently almost meeting, pls (posterolateral mesoscutal); t (tegular) present and prominent posteriorly on tegula; pls (posterolateral mesoscutal) present; ps (parascutellar) and s (mesoscutellar) absent; deps (dorsal mesepisternal), hypm (hypoepimeral area), and lp (lateral propodeal) present, veps (ventral mesepisternal) absent (Figs 21, 23). Metasomal terga with prominent patches of appressed, plumose white setae as follows: first metasomal tergum with large, L-shaped patches laterally; second metasomal tergum with lateral patch transverse, slightly wider than twice as long, never L-shaped and without rounded secondary anterior patch; third through fifth metasomal terga with more or less transverse to rounded lateral patches (Figs 21, 23).
♀: As described for the male except in usual gender differences and as follows: Total body length 11.8–13.1 mm; forewing length 9.4–10.2 mm. Head wider than long (length 3.0 mm, width 3.8 mm); upper interorbital distance 2.3 mm; lower interorbital distance 1.7 mm. Intertegular distance 2.8–3.3 mm; mesoscutellar posterior margin as in male but sinuate margin stronger. Apical depression of fifth tergum sparsely but distinctly punctate and setose. Pygidial plate relatively broad, margins converging apically, largely straight, apex narrowly rounded, surface imbricate, basal half with shallow, coarse punctures.
Mesoscutum with well-defined punctures separated by much less than a puncture width, punctures mediopically slightly more spaced but still separated by less than a puncture width; mesoscutellum, including axilla, with punctures separated by less than a puncture width.
Integument and pubescence as in male except dark brown on pygidial plate; second through fourth metasomal terga with more or less transverse to rounded lateral patches (Figs 22, 24).
Lateral habitus of Thyreus schwarzi sp. n. from São Nicolau. 21 Male 22 Female.
Dorsal habitus of Thyreus schwarzi sp. n. from São Nicolau. 23 Male 24 Female.
Male terminalia of Thyreus schwarzi sp. n. 25 Seventh metasomal sternum 26 Eighth sternum 27 Genital capsule, lateral aspect 28 Genital capsule, dorsal view 29 Genital capsule, ventral view.
The specific epithet is a patronym honoring Dr. Maximilian Schwarz, a leading authority on the systematics of cuckoo bees and a dear colleague.
A single female collected from Santo Antão is not designated as part of the type series since the specimen may be mislabeled or a rare case of introduction to a different island.
urn:lsid:zoobank.org:act:B992FB5F-C606-4CA7-BE75-5021D4AE36F7
http://species-id.net/wiki/Thyreus_aistleitneri
Figs 30–34♀, Cabo Verde, Brava, Nova Sintra, Mte. Nha Preta, 700–880 m, 25.01.01 [25 January 2001], leg. Aistleitner (SEMC).
The new species can be recognized by the following combination of features: mesoscutum with plsa (anterior posterolateral mesoscutal) present and bordering anterior portion of tegula, not meeting pls (posterolateral mesoscutal) posteriorly (Fig. 31); mesoscutellum coarsely punctate, with punctures dense, separated, at least on disc, by less than 0.5 times a puncture width; apicolateral corners of mesoscutellum weakly pointed, forming angle of more than 40° (Fig. 33); ventral and ventrolateral pleura with distinct shiny interspaces among punctures; apical depression of fifth metasomal tergum with a few isolated punctures medially; fifth tergum with lateral white patches reduced, especially in comparison to patches on fourth and third tergum (Figs 30, 31, 34).
As described for Thyreus denolii (vide supra) except as follows: ♀: Total body length 11.3 mm; forewing length 8.3 mm. Head wider than long (length 2.9 mm, width 3.5 mm) (Fig. 32); upper interorbital distance 2.1 mm; lower interorbital distance 1.55 mm. Intertegular distance 2.7 mm; mesoscutellar posterior margin faintly sinuate, apicolateral angle projects as a prominent, broad spine and with a defined median emargination (Figs 31, 33). Apical depression of fifth tergum sparsely, but distinctly, punctate and setose. Pygidial plate relatively narrow, margins converging apically, slightly sinuate, apex narrowly rounded, surface imbricate, basal half with shallow, coarse punctures.
Mesoscutum with well-defined punctures separated by much less than a puncture width, punctures mediopically more spaced, separated by about 0.5–1 times a puncture width but more often less than a puncture width, integument between punctures smooth and shining; mesoscutellum, including axilla, with punctures separated by less than a puncture width, those laterally nearly contiguous (Fig. 33).
White patches on mesosoma as follows: deps (dorsal mesepisternal) and lpn (lateral pronotal) present; als (anterolateral mesoscutal) present but reduced; ms (median mesoscutal) faint; mls (mediolateral mesoscutal) present; plsa (anterior posterolateral mesoscutal) present along anterior half of border with tegula, not meeting pls (posterolateral mesoscutal) posteriorly; t (tegular) present and prominent posteriorly on tegula; pls (posterolateral mesoscutal) present, not extending laterally to meet plsa (anterior posterolateral mesoscutal); ps (parascutellar) and s (mesoscutellar) absent; deps (dorsal mesepisternal), hypm (hypoepimeral area), and lp (lateral propodeal) present, veps (ventral mesepisternal) absent (Fig. 31). Pleural white spot reduced to two separate spots (Fig. 30). Metasomal terga with prominent patches of appressed, plumose white setae as follows: first metasomal tergum with L-shaped patch laterally interrupted in middle and thus divided into two spots, one transverse at apicolateral margin and one triangular on tergal side; second metasomal tergum with reduced lateral patch transverse, slightly wider than twice as long, without rounded secondary anterior patch; third and fourth metasomal terga with rounded lateral patches; fifth metasomal tergum with lateral spot reduced to a few setae (Figs 30, 31, 34); pygidial plate with dark brown setae.
♂: Unknown.
Female of Thyreus aistleitneri sp. n. from Brava. 30 Lateral habitus 31 Dorsal habitus.
Female of Thyreus aistleitneri sp. n. from Brava. 32 Facial aspect 33 Detail of mesoscutellum 34 Detail of metasoma in dorsal view.
The specific epithet is a patronym honoring Eyjolf Aistleitner, collector of the new species among many fine insects, and authority on the systematics of Lepidoptera.
Key to the Species of Thyreus of the Cape Verde Islands
| 1 | Males | 2 |
| – | Females | 4 |
| 2 | Sixth metasomal tergum with lateral white patches of the same size as on fourth and fifth terga; mesoscutellum finely punctate, with punctures separated, at least on disc, by 0.5–1 times a puncture width; apicolateral corners of mesoscutellum weakly pointed (angle more than 40°); metasoma densely punctate, punctures reach almost the end of metasomal terga (Boavista, Sal, Santiago*, S. Nicolau**) | Thyreus denolii sp. n. |
| – | Sixth metasomal tergum with lateral white patches greatly reduced, especially in comparison to patches on fourth and fifth tergum; mesoscutellum more coarsely punctate, with punctures dense, nearly contiguous; apicolateral corners of mesoscutellum more prominently and sharply pointed (angle less than 40°); metasoma more sparsely punctate, punctures separated from margins | 3 |
| 3 | Second metasomal tergum with lateral white patch L-shaped (as on first tergum) or at least with smaller anterior patch (Fig. 14); mesoscutum with coarse punctures throughout; all white anterior mesoscutal patches well developed (S. Antão) | Thyreus batelkai sp. n. |
| – | Second metasomal tergum with lateral patch wider than long, without anterior secondary patch and never L-shaped (Fig. 23), but rarely with few white setae in this area; mesoscutellum with finer punctures at least basomedially; white anterior mesoscutal patches, especially ms (median mesoscutal) and mls (mediolateral mesoscutal), strongly reduced to missing (Fig. 23) (S. Nicolau) | Thyreus schwarzi sp. n. |
| 4 | Apicolateral corners of mesoscutellum weakly pointed (angle more than 40°) (e.g., Fig. 33); mesoscutellum finely punctate, with punctures separated, at least on disc, by 0.5–1 times a puncture width (except Thyreus aistleitneri); ventral and ventrolateral portions of pleura with distinct shiny interspaces among punctures; mesoscutum with plsa (anterior posterolateral mesoscutal) present and bordering anterior portion of tegula, not meeting pls (posterolateral mesoscutal) posteriorly (e.g., Figs 4, 31) | 5 |
| – | Apicolateral corners of mesoscutellum sharply pointed (angle less than 40°) (Figs 15, 24); mesoscutellum more coarsely punctate, with punctures dense, nearly contiguous; pleura ventrally and ventrolaterally coarsely punctate, interspaces among most punctures indistinct (smallest specimens sometimes with sparsely punctate pleura); mesoscutum with plsa (anterior posterolateral mesoscutal) present anterior to and along border with tegula, extending posteriorly and meeting, or less frequently almost meeting, pls (posterolateral mesoscutal) (e.g., Figs 15, 24) | 6 |
| 5 | Apical depression of fifth tergum densely punctate medially and densely setose; fifth metasomal tergum with lateral white patches of same size as on fourth and third terga (Fig. 4) (rarely white patches on fifth tergum reduced, but then reduced also on preceding terga); mesoscutellum finely punctate, with punctures separated, at least on disc, by 0.5–1 times a puncture width (Boavista, Sal, Santiago*, S. Nicolau**) | Thyreus denolii sp. n. |
| – | Apical depression of fifth tergum with a few isolated punctures medially; fifth metasomal tergum with lateral white patches reduced, especially in comparison to patches on fourth and third terga (Figs 30, 31, 34); mesoscutellum more coarsely punctate, with punctures dense, separated, at least on disc, by less than 0.5 times a puncture width (Fig. 33) (Brava) | Thyreus aistleitneri sp. n. |
| 6 | Apical depression of fifth tergum bare medially, rarely with few punctures with short seta; second metasomal tergum with lateral white patch L-shaped (as on first tergum) or at least with smaller anterior patch (Fig. 15); mesoscutum with coarse punctures throughout; all white anterior mesoscutal patches well developed (Fig. 15) (Santo Antão) | Thyreus batelkai sp. n. |
| – | Apical depression of fifth tergum with several distinct seta-bearing punctures; second metasomal tergum with lateral patch wider than long, without anterior secondary patch and never L-shaped (Fig. 24), but rarely with few white setae in this area; mesoscutellum with finer punctures at least basomedially; white anterior mesoscutal patches, especially paired mls (mediolateral mesoscutal), greatly reduced to a few short setae (São Nicolau) | Thyreus schwarzi sp. n. |
* The Santiago females of Thyreus denolii are much larger, probably because of the larger host, and differ slightly in the shape of the pygidial plate. We presently interpret this as merely minor variations in the absence of more conclusive evidence suggesting a separate species status for the Santiago population.
** It seems unusual that there is a single female from S. Nicolau that is clearly of Thyreus denolii. Refer to the account for that species regarding this single individual which is perhaps mislabeled. However, this specimen of Thyreus denolii is unique for its L-shaped white patch on the second tergum. More material is needed in order to permit a more thorough understanding of their variability.
The genus Chiasmognathus comprises a series of small ammobatine bee species which are exclusively cleptoparasitic in the nests of Nomioidini (
urn:lsid:zoobank.org:act:4ED85B90-4C8B-47B7-B96D-CF4A7061A7C0
http://species-id.net/wiki/Chiasmognathus_batelkai
Figs 35–43♂, Cape Verde Isl., Santo Antao, 1382 m, Espongeiro, 13.X.2009 [13 October 2009], 17°06'17"N, 25°05'21"W, J. Straka & J. Batelka lgt. (SEMC).
São Vicente: 1♂, Cabo Verde 00/25, Ilha de S. Vicente, Calhau – Baia d. Gatas, -5 m, 13.12.2000 [13 December 2000], leg. Aistleitner (EAFC); Santo Antão: 5♂♂, same data as holotype (FISC, JSPC, SEMC); 3♂♂, 4♀♀, same data as holotype except 12–13.X.2009 [12–13 October 2009], 17°06'N, 25°05'W (JSPC, SEMC).
The new species is the largest of the known Chiasmognathus, being 3.2–4.1 mm in total length in females and 3.5–4.2 mm in males. The ocellar elevation in males is distinctly more prominent than in any of the other species, this and the combination of the size, mesoscutal sculpturing, and coloration (Figs 35, 36, 42, 43) will serve to identify Chiasmognathus batelkai from the other species of the genus, particularly those occurring in Africa (
♂: Total body length 4.0 mm (3.5–4.2 mm); forewing length 3.3 mm (3.1–3.7 mm). Head wider than long (width 1.3 mm, length 0.99 mm); inner margins of compound eyes straight, convergent below; apex of clypeus at lower tangent of compound eyes; ocelli above upper tangent of compound eyes, ocellar triangle particularly prominent, swollen above curvature of head (more so than in other species of the genus); clypeus weakly convex, nearly flat, apicolateral corners of clypeus with small patches of tightly packed, elongate, apically-sinuate setae; malar space vestigial; mandibles simple, crossing in repose but not covering labrum; frontal line carinate from just below lower tangent of antennal toruli to median ocellus. Mesoscutum with median line deeply impressed and wide, width about that of mesoscutal puncture diameter, extending to just before mesoscutal midlength. Intertegular distance (i.e., distance between inner margins of tegulae) 0.8 mm (0.7–0.9 mm). Forewing marginal cell broadly truncate; both m-cu crossveins entering second submarginal cell. Male terminalia as depicted in Figures 37–41.
Integument generally shining and smooth (Fig. 36). Labrum with punctures over entire surface, punctures nearly contiguous, integument between punctures (where evident) smooth; clypeus with shallow punctures separated by 0.5–1.5 times a puncture width centrally, punctures separated by less than a puncture width laterally; face and vertex with punctures nearly contiguous and more well defined than those centrally on clypeus, integument between (where evident) smooth, punctures on vertex posterior to ocelli somewhat weaker; punctures on gena as on sides of vertex with punctures gradually becoming more widely spaced ventrally; postgena with smaller and weaker punctures separated by 1–4 times a puncture width, integument otherwise smooth but duller than shining integument elsewhere on head. Mesoscutum punctate, anteriorly and around median line punctures separated by 0.5 times a puncture width, infrequently more widely spaced, otherwise punctures of mesoscutum separated by 1–2 times a puncture width, infrequently by less; mesoscutellum with punctures separated by 0.5 times a puncture width except in paramedial areas of disc distinctly more sparse, separated there by 1–5 times a puncture width; metanotum with punctures separated by less than a puncture width; preëpisternal area with punctures more coarse than those of meoscutum, nearly contiguous; hypoepimeral area with small punctures separated by 0.5–3 times a puncture width, ventrally with largely impunctate area bordering scrobe; mesepisternum with punctures separated by 0.5–2 times a puncture width anteriorly, posteriorly punctures more coarse and closer, often nearly contiguous, becoming more widely spaced ventrally; metepisternum with punctures separated by less than a puncture width above, punctures becoming more widely spaced ventrally; propodeum with short and narrow basal area coarsely imbricate and impunctate, otherwise integument with punctures separated by less than a puncture width. Metasomal terga and sterna finely imbricate, with scattered weak, small punctures, apical margins impunctate.
Integument of head and mesosoma black and shining (Figs 35, 36) except reddish brown on mandibular apex, brown on middle third of mandible, light brown on palpi and glossa, dark brown to black on labrum (some males with reddish brown laterally on labrum), dark brown to black on antennae, dark brown on tegula, and dark brown on legs except lighter on tarsi and at femorotibial and tibiobasitarsal joints. Wing veins brown except C and Sc+R dark brown; membranes hyaline, forewings faintly infumate. Metasoma dark brown except first tergum dark reddish brown in apical two-thirds to one-half; apical margins of terga narrowly brown to light brown.
Pubescence silvery white. Head with numerous, fine, appressed to subappressed plumose setae, such setae nearly obscuring integument of face around and below level of antennal toruli, and intermingled with a few suberect to erect finer, simple setae; such appressed plumose setae present on gena. Setae of mesosoma like those of head although more sparse centrally on mesoscutum and mesoscutellum; setae similar to those of gena on pleura (although longer and more diffuse to sparse centrally on mesepisternum), metanotum, and dorsolateral portions of propodeum. Metasoma with sparse, erect to suberect, short simple setae, without prominent apical fasciae composed of appressed, plumose, white pubescence; first metasomal tergum with small, weak apicolateral patches of appressed to subappressed plumose setae; succeeding terga with similar patches although often more diffuse or narrower than those of first tergum.
♀: As described for the male except in usual gender differences (
Metasomal terga and sterna finely imbricate, with sparse weak, minute punctures, apical margins impunctate, often broadly so.
Coloration as in male except often protibia brown as on tarsi rather than dark brown as on other more basal podites; metasoma dark brown except first tergum largely reddish brown in apical two-thirds to one-half (Figs 42, 43); apical margins of terga reddish brown to brown, most prominently so on second tergum, less so on more apical terga and sterna.
Male habitus of Chiasmognathus batelkai sp. n. 35 Lateral 36 Dorsal.
Male terminalia of Chiasmognathus batelkai sp. n. 37 Seventh metasomal sternum 38 Eighth sternum 39 Genital capsule, dorsal view 40 Genital capsule, lateral aspect 41 Genital capsule, ventral view.
Female habitus of Chiasmognathus batelkai sp. n. 42 Lateral 43 Dorsal.
The specific epithet is a patronym honoring Jan Batelka, a prominent collector of the new species, authority on the systematics of beetles, and good friend.
It appears as though the Cape Verde Islands fauna of Thyreus represents a single invasion of the archipelago with subsequent speciation across the islands. All these species resemble Thyreus hohmanni Schwarz endemic to the Canary Islands (another Macaronesian archipelago) and the more widespread Thyreus ramosus. It is possible that the Thyreus of Cape Verde came from the Canary Islands, and follow a similar pattern of relationship and introduction as is described for some plant species (
We are immensely grateful to Jan Batelka, Maximilian Schwarz, and Eyjolf Aistleitner for access to unique material; to Jan Batelka for assistance and collaboration in fieldwork; to the curators of the various institutions which permitted us to examine their holdings; to Ismael A. Hinojosa-Díaz for assistance with photography; and to Jerome G. Rozen, Jr. and Laurence Packer for valuable reviews of the manuscript. Photomicrography in the Division of Entomology was supported by the Engel Illustration Fund of the University of Kansas College of Liberal Arts & Sciences. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum. Institutional support to JS was provided by the Ministry of Education, Youth and Sports of the Czech Republic.