(C) 2011 Christine L. Lambkin. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Bush Blitz is a three-year multimillion dollar program to document the plants and animals in hundreds of properties across Australia’s National Reserve System. The core focus is on nature discovery – identifying and describing new species of plants and animals. The Bush Blitz program has enabled the collection and description of beeflies (Diptera, Bombyliidae) from surveys in Western Australia and Queensland. Three new species of Australian beeflies belonging to the Exoprosopini are described; Palirika mackenziei Lambkin sp. n., Palirika culgoafloodplainensis Lambkin sp. n., and Larrpana bushblitz Lambkin sp. n. Phylogenetic analysis of 40 Australian exoprosopine species belonging to the Balaana generic-group Lambkin & Yeates 2003 supports the placement of the three new species into existing genera, and the erection and description of the new genus Ngalki Lambkin gen. n. for Ngalki trigonium (Lambkin & Yeates 2003) comb. n. Revised keys are provided for the genera of the Australian Balaana genus-group and the species of Palirika Lambkin & Yeates, 2003 and Larrpana Lambkin & Yeates 2003. With the description of the three new species and the transferral of Munjua trigona Lambkin & Yeates 2003 into the new genus Ngalki Lambkin gen. n., three genera are rediagnosed; Munjua Lambkin & Yeates 2003, Palirika and Larrpana.
Ngalki, Palirika, Larrpana, Munjua, Balaana genus-group , phylogenetic analysis, cybertaxonomy, Scratchpads, Morphbank
While there are more than 140, 000 published species in Australia, more than 40 per cent of continental Australia has never been comprehensively surveyed by scientists. This research was supported through funding from the Bush Blitz species discovery program, a partnership between the Australian Government, BHP Billiton and Earthwatch Australia. This innovative partnership harnesses the expertise of many of Australia’s top scientists from museums, herbaria, universities, and other institutions and organisations across the country. Bush Blitz is expected to uncover hundreds of new species and provide baseline scientific data that will help us protect our biodiversity for generations to come.
This paper describes three species of beeflies from the Exoprosopini (Diptera, Bombyliidae, Anthracinae); two captured during Bush Blitz surveys and a third species collected from south-western Queensland (Qld). All three species belong to genera recently described (Palirika Lambkin & Yeates 2003 and Larrpana Lambkin & Yeates 2003) in a large revisionary monograph (
The beeflies belong to the Family Bombyliidae, a very large, cosmopolitan family of stoutly built flies, mostly with very characteristic venation. Almost 5000 species have been described worldwide (
Australian exoprosopines are large beeflies of diverse and striking appearance (Figs 4C, 6D, 7B) with wings usually bearing distinct hyaline and black patterns. Like most bombyliids, adult Australian exoprosopines are well covered in long, dense, coloured hairs arranged in patterns, often in stripes across the dorsal surface of the abdomen, leading to their common name of beeflies. In Australian exoprosopines, like other members of the Anthracinae, many of the long hairs, especially on the dorsal surface, are modified into short, broad, flattened scales, often in contrasting stripes. The scales may be erect or upstanding, producing a “fluffy” appearance as in Larrpana bushblitz Lambkin sp. n. (Fig. 7A). Sometimes the dorsal scales are tightly adpressed, producing a smooth, often shiny appearance as in Palirika (Fig. 6D). Some beeflies have some scales or hairs that are reflective, appearing shining gold or brilliantly silver as on the terminal tergites of the male anthracine Anthrax maculatus
Adult Australian exoprosopines favour warm, sunny localities, especially in the more arid regions. Most have a strong, hovering flight, and are commonly taken from blossom, or sitting on patches of bare earth. Adults are pollen and nectar feeders, and many are important pollinators of native plants. Many species can be collected congregating on hilltops, demonstrating a landmark-based mating system (
This paper describes three new species of exoprosopine beeflies; two captured during Bush Blitz surveys and a third species collected from south-western Qld.
Palirika mackenziei sp. n. wascollected from the large grazing property, Plevna Downs, owned by the Mackenzie family, 63 km west of Eromanga, in extremely arid south-western in late December 2007. While accompanied by Noel Starick (QM volunteer) and Robyn Mackenzie, CLL hand netted a single female specimen (Fig. 6C, D) hill-toppingon the summit of Tompilly Hill (Fig. 1A, B), a jump up on Plevna Downs. This species was unlike any other Palirika collected; smaller and darker in both body and wing infuscation.
Four male specimens (Fig. 7A, B) of Larrpana bushblitz sp. n. were hand netted by CLL from Karara Pastoral Lease in Western Australia, hill-topping onForrest lookout (Fig. 1D), 24.4km SE Boiada Camp and on a nearby hilltop 23.5km ESE Boiada Camp during the Bush Blitz survey co-organised by WAM on Charles Darwin Reserve, Karara, Lochada and Kadji Kadji Pastoral Leases, 213 km ESE of Geraldton, in September 2009. This species appeared similar to the two male specimens of Larrpana zwicki Lambkin & Yeates 2003 collected only near Windorah (
Palirika culgoafloodplainensis Lambkin sp. n. was collected from Culgoa Floodplains National Park (NP) on the Queensland/New South Wales Border, 134 km WSW Dirranbandi, during the Bush Blitz survey of Culgoa Floodplains NP Qld, Culgoa NP and Ledknapper Nature Reserve (NR) NSW (NSW) organised by CLL and Noel Starick from QM between November 2009 and June 2010. A single male specimen (Fig. 4C, D) was sorted by QM volunteer John Purdie from a Malaise trap sample from 7 km NNW Toulby Gate (Fig. 1C) on Culgoa Floodplains National Park (NP). Malaise and Pitfall traps had been set at four sites on Culgoa Floodplains by CLL, Noel Starick and NP Ranger Cheryn Kelly in November 2009 as part of the Bush Blitz survey. The rangers had agreed to take monthly samples until we could return. This specimen was from a Malaise trap that had been reset on the 20th January 2010 by Ranger-In-Charge (RIC) Andy (Keith) Coward. Because of significant rain, the rangers were unable to return to take another sample until the 19th March. Subsequent flooding in March and April 2010 prevented access to the survey areas until mid-May when CLL, Noel, Rhys Smith (QM volunteer) and rangers Andy and Megan Simpson retrieved the Culgoa Floodplains NP traps. This species was similar to Palirika bouchardi Lambkin & Yeates 2003 that has been extensively collected from arid areas of central and western Australia from all states except South Australia.
Previous phylogenetic analysis of the worldwide Exoprosopini showed that the Australian bombyliids that were previously placed in Exoprosopa
Revised keys are provided for the genera of the Australian Balaana genus-group and the species of Palirika and Larrpana. The three new species are fully described; with diagnoses, distribution maps, and images of both external characters and dissected genitalia. The new genus Ngalki is described with diagnosis, and images of both external characters and dissected genitalia. With the description of three new species and the transferral of Munjua trigona Lambkin & Yeates 2003 into the new genus Ngalki, three genera are rediagnosed; Munjua Lambkin & Yeates, Palirika and Larrpana.
We attempted to use cybertaxonomic tools to produce this paper as had been used to streamline taxonomic publication of new fly species by
The following collection acronyms are used in the text: Australian Museum, Sydney, New South Wales, Australia (AM); Queensland Museum, South Brisbane, Queensland (QM); Western Australian Museum, Perth, Western Australia (WAM). Numbers quoted with individual specimens are unique identifiers (e.g. WAM 82396, T152479 (QM), K 253702 (AM)) from the respective institutions database and are attached under each specimen on a white label. A single hind leg was removed from one specimen of each species and placed into absolute ethanol for frozen tissue storage at QM for future DNA extraction. Those samples were given a tissue number (e.g. A007534) that was entered into the QM Vernon database and attached under each specimen on a yellow label.
For explanation of morphological abbreviations, see Appendix 1.
The genitalia of each species were prepared by dissecting the terminal abdominal segments and then placing in cool 10% KOH overnight. Following maceration the specimen was washed, and then dissected in distilled water. Dissected genitalia were placed in alcohol for microscopic examination and into K–Y® Jelly for photography. All dissected parts from a specimen were placed in a genitalia vial containing glycerine which was pinned beneath the identification label.
Images were taken of the whole fly, external features, and dissected genitalia. A series of multiple-focal-depth digital images were taken using a Canon EOS 500D digital camera fitted, via a Leica 10446175 1x SLR Projection Lens, to a Leica MZ6 stereo dissecting microscope, and combined into a high resolution serial montage image using Helicon Focus v.5.2 Pro (
Distribution maps were produced using ArcView GIS version 3.1 (
We intended to use cybertaxonomic methods to document these newly discovered Australian beeflies, enabling descriptions of the three new species to be generated using web resources to populate electronic documents through links to Morphbank, Life Science Identifiers, and Zoobank as had been done by
Several initiatives around the world have been developing tools to bring revisionary taxonomy to the web. Recent examples include software produced through the CATE (Creating a taxonomic e-science, http://www.cate-project.org), EDIT (European Distributed Institute of Taxonomy, http://www.e-taxonomy.eu) and the Australian TRIN (Taxonomy Research & Information Network, http://www.taxonomy.org.au/) projects. These efforts support the compilation of large distributed datasets, descriptions and identification of biota. One of the tools developed in association with the EDIT initiative are the Scratchpads (http://scratchpads.eu), a Web 2.0 Virtual Research Environment, that enable taxonomists to collaborate in the production of websites documenting the diversity of life. Using
The paper has been semantically tagged and enhanced using the Pensoft Mark Up Tool (PMT) which is based on the US National Library of Medicine’s DTD (Document Type Definitions) TaxPub extension http://sourceforge.net/projects/taxpub). We intend parallel release of the publication on paper and on-line accompanied by a) links to archived images on Morphbank, and b) with registration of authors, publications, taxon names and other nomenclatural acts in Zoobank, with assignment of Life Science Identifiers (LSIDs) for each new taxa as per the recent proposed amendment to the International Code of Zoological nomenclature for a universal register for animal names (
The nomenclatural and distributional information will be included in the Australian Faunal Directory (AFD), an open-access online catalogue of taxonomic and biological information on all animal species known to occur within Australia (ABRS, 2009), and the Australian Natural Heritage Assessment Tool (ANHAT), an open-access online map-supported database developed by the Australia Heritage Division of the Department of Sustainability, Environment, Water, Population and Communities that helps identify and prioritise areas for their natural heritage significance, focusing on biodiversity (
Phylogenetic analysis was based on 207 morphological characters from
Multistate characters used for phylogenetic analyses have been treated as unordered (non-additive
Phylogenetic analyses completed 100 random step-wise addition searches, with tree-bisection-reconnection (TBR) branch swapping, MULPARS, and branches having maximum length zero collapsed to yield polytomies in effect using PAUP*4.0b10 (
We used Bremer support (
Cladograms and character distribution were analysed in WinClada version 1.00.08 (
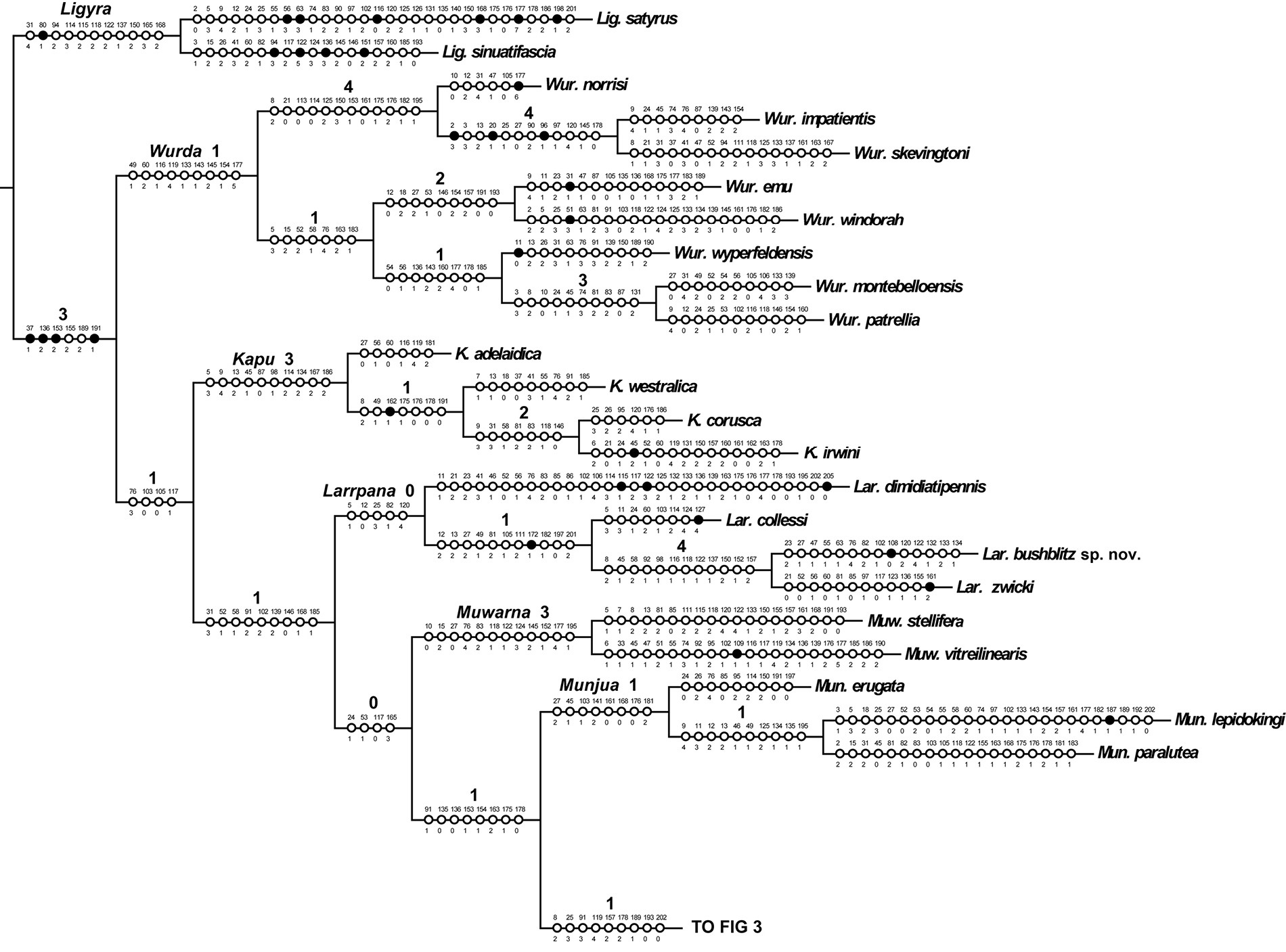
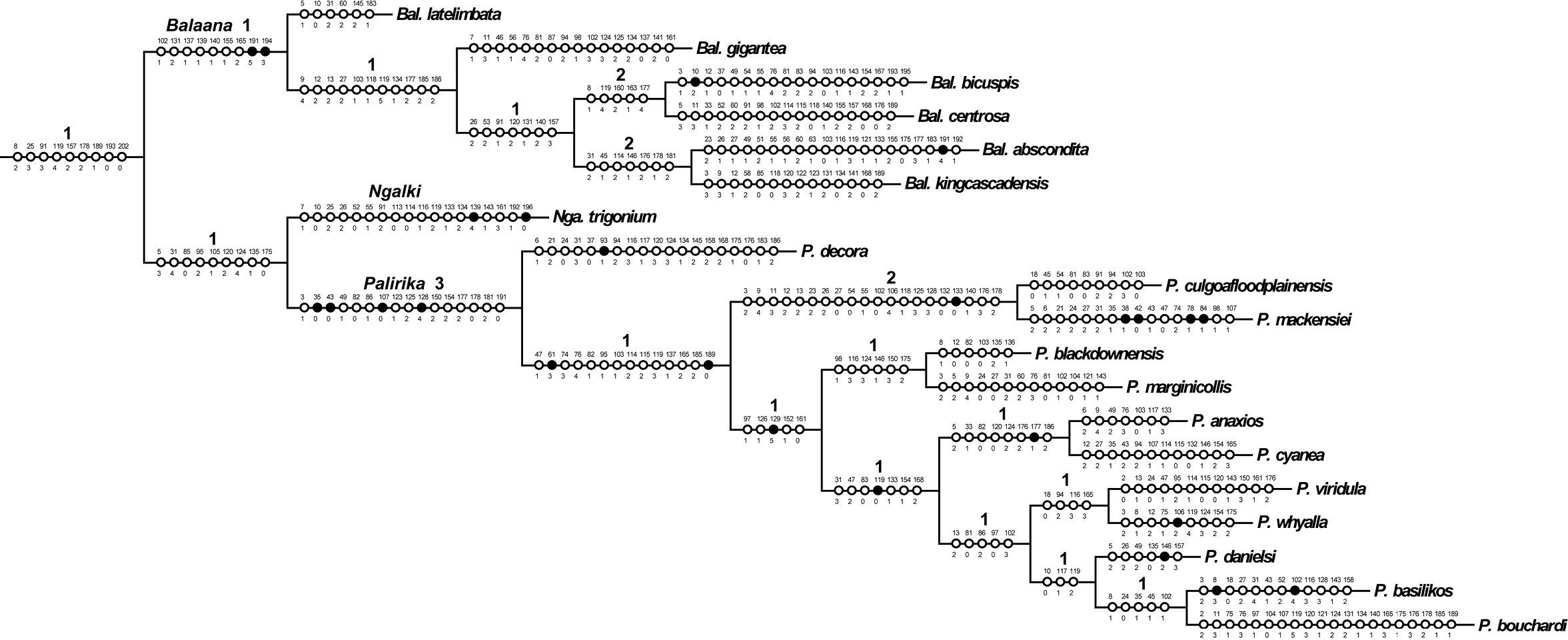
Cladistic analysis of the 42 taxa of 141 non-constant characters produced five most parsimonious trees (MPTs) of length =931, CI = 0.243, CI excluding uninformative characters = 0.231, and RI = 0.468. The five trees differ only in the placement of Larrpana dimidiatipennis (
Previous phylogenetic analysis of 207 morphological characters for the worldwide Exoprosopini showed that the Australian bombyliids that were previously placed in Exoprosopa, belonged to the monophyletic Balaana group of genera, sister to the Australian Ligyra. Phylogenetic analysis of characters of the Balaana group of genera then led to the description of seven new genera for 42 species in that genus-group (
In
In this phylogenetic analysis, Munjua trigona falls between Palirika and Balaana as sister to Palirika (Figs 2, 3). As this species clearly does not belong to Palirika, a new genus Ngalki for Ngalki trigonium is created.
With the description of the three new species and the transferral of Munjua trigona into the new genus Ngalki, the three genera Munjua, Palirika, and Larrpana require rediagnoses.
Taxonomyurn:lsid:catalogueoflife.org:taxon:d916e5f0-29c1-102b-9a4a-00304854f820:col20110201
Palirika decora, Lambkin & Yeates, 2003: 812.
Small black, rounded, dense, adpressed metallic scales dorsally on thorax and abdomen (Fig. 6D); no abdominal white scales, sternal vestiture black, not metallic. Epandrium rounded, strongly curved, red, extended smoothly basolaterally (Fig. 4E, F). Gonocoxae deeply narrowed medially, with thickened setae ventromedially, tuft of 6–8 very long, basally-directed, thick setae medially; H projecting in lateral view; EP without lateral lobes, medial projection laterally; LAEA large, convex, extending to G margin; EJA racquet-shaped, longer than the length of G (Fig. 5). Female T8 A little more than marginal thickening (Fig. 6F), spermathecal tube more than 8 × length of SP, clear thick-walled ring joining clear thick-walled BB and pigmented subquadrate SR.
Included species: Palirika anaxios Lambkin & Yeates 2003, Palirika basilikos Lambkin & Yeates 2003, Palirika blackdownensis Lambkin & Yeates 2003, Palirika bouchardi, Palirika culgoafloodplainensis sp. n., Palirika cyanea Lambkin & Yeates 2003, Palirika danielsi Lambkin & Yeates 2003, Palirika decora, Palirika mackenziei sp. n., Palirika marginicollis (Gray, 1883), Palirika viridula Lambkin & Yeates 2003, Palirika whyalla Lambkin & Yeates 2003.
urn:lsid:zoobank.org:act:85D57E72-0396-4994-8CF6-7C22B5BAC978
http://species-id.net/wiki/Palirika_culgoafloodplainensis
Figs 1C, 2–3, 4–5, 11; Morphbank Collection 692336Holotype. Queensland: ♂, 28.94°S, 146.918°E, Culgoa Floodplain NP, 7km NNW Toulby Gate, 160m, (CG4AM), Malaise, 20Jan-19Mar2010, C. Kelly, A. Coward, 19273, [dissected], PS1937, A006859, T165704 (QM). Condition: Fair (see remarks below).
Wing length 20.0 mm
Large dark flies with distinct triangular basal infuscation on the wings wings. Face and frons with transparent scales. Occiput with white scales broadly filling indentation. Collar whitish-cream. Broad laterothoracic stripe of dense white flattened scales. Scutum black with lime-green metallic scales except pink metallic scales anterolaterally to PR bristles and posterolaterally anterior to APA. Scutellum with lime-green metallic scales; very long, white, flattened-scale fringe on posterior margin. Widened base of costa with reddish-brown scales, white scales posteriorly. Wing pattern dimidiate (Fig. 4C); with distinct indentation base of first r2+3; extension along R4+5, covering basal 1/3 of first r2+3 and r5; indistinct mottling base of m1 along m-m; no infuscated band; anal and posterior cells with apically notched hyaline area, infuscation extending along CuA2; cup infuscated basal 4/5; anal infuscated basal 2/3. Squama edged with dense white scales admixed with some reddish-brown scales. T1 with Ma white dorsally, black medially and ventrally, dense very long flattened white scales posterolaterally. Abdominal tergites black with bluish-green scales. Epandrium with long setae grouped loosely apically. Epiphallus with short, medial projection. G with long black setae medially, directed basally, longest on weak ventral ridge; LAEA very large, extending well beyond G margins.
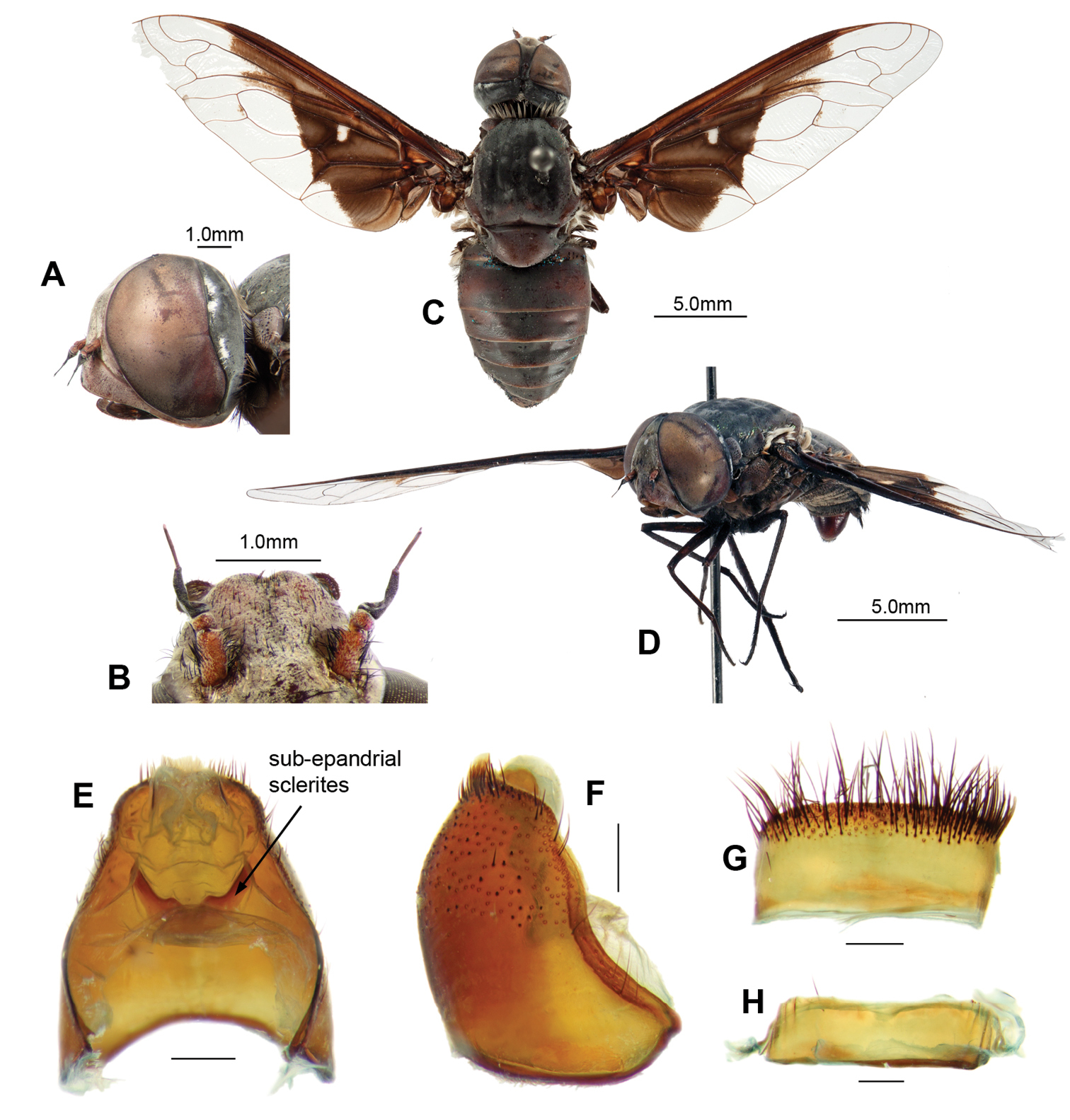
Male. Head (Figs 4A–D). Face red with transparent scales, frons brown with transparent scales; setae black, frontal depression distinct. Antennal scape red, 3 × length of pedicel, with long black setae dense laterally and ventrally; pedicel red; PP black, conical, 3 × length of pedicel, distinct apical joint; BSM rod-like, black, 3 × length of pedicel; ASM black, conical, length at least width of BSM (Fig. 4B). Occiput with white scales broadly filling indentation (Fig. 4A).
Thorax.(Figs 4C–D). Collar whitish-cream. Broad laterothoracic stripe of dense white flattened scales (Fig. 4D). Scutum black with lime-green metallic scales except pink metallic scales anterolaterally to PR bristles and posterolaterally anterior to APA; black setae. Pleural hairs black with reddish-brown iridescence. AN with black Ma; long, lightly iridescent scales at base of wing reddish-brown; long flat broad pale brown scales posteromedially. K with very long fine reddish-brown scales medially. Ma on LT black with reddish-brown iridescence. Tympanal ridge and PL with dense very long fine white flattened scales. Scutellum red, darker basally with lime-green metallic scales; very long, white, flattened-scale fringe on posterior margin. Legs. Legs reddish-brown, darkening apically, with black scales and setae, tarsi dark reddish-brown to black; fore-tarsi with straight microtrichia. Pulvilli sharp, curved, 1/3 length of mid- and hind-tarsal claws. Halter knob reddish-brown with apical margin yellow. Wing (Fig. 4C), cup narrowly open or closed only at wing margin. Patagium distinct with dense white long flat scales. Widened base of costa with reddish-brown scales, white scales posteriorly. Wing pattern dimidiate (Fig. 4C); with distinct indentation base of first r2+3; extension along R4+5, covering basal 1/3 of first r2+3 and r5; indistinct mottling base of m1 along m-m; no infuscated band; anal and posterior cells with apically notched hyaline area, infuscation extending along CuA2; cup infuscated basal 4/5; anal infuscated basal 2/3. Anal basal edge with dense black scales; alula edged with dense reddish-brown scales; squama edged with dense white scales admixed with some reddish-brown scales.
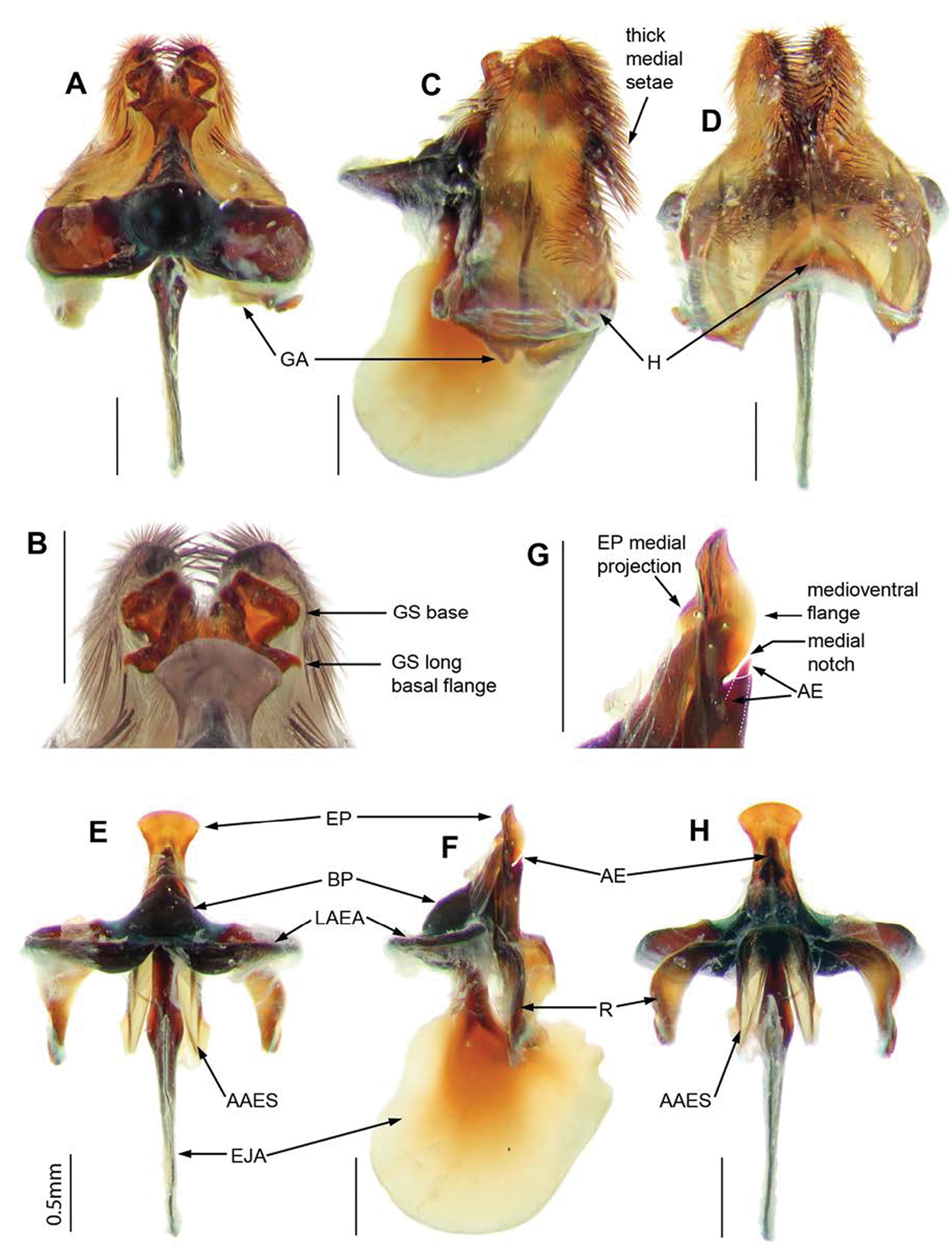
Abdomen.Black, T1–4 dark reddish-brown posterolaterally; tergites with bluish-green scales; T1 with Ma white dorsally, black medially and ventrally, dense very long flattened white scales posterolaterally; T2–7 with tufts of long, black setae laterally and posteriorly. Sternites black with dark reddish-brown scales and hairs. Genitalia (Figs 4E–H, 5A–H). Epandrium strongly convex, red with convex apical margin; tapering basal flange; long, black setae loosely grouped apically; SES large, fused medially (see Fig. 4E). Gonocoxae red, narrowed apically; GA short, triangular; thick tufts of long black setae medially, directed basally, longest on weak ventral ridge (Fig. 5C); EJA very large, extending well beyond gonocoxal margins, racquet-shaped; LAEA very large, extending well beyond G margins, deeply convex (Fig. 5A); AAES strong wedges (Fig. 5E, H); GS (Fig. 5B) cupped within G margins, large subquadrate base projecting apically; EP long, expanded slightly apically, without lateral lobes, short medial projection; medioventral flange above AE present (Fig. 5G); large recurved R (Fig. 5F, H); H triangular, projecting slightly in lateral view (Fig. 5C).
Female. Unknown.
Collection sites. A CLL showing Robyn Mackenzie the single female specimen of Palirika mackenziei sp. n. collected hill-topping on the summit of Tompilly Hill in late December 2007 B Tompilly Hill, a jump up on Plevna Downs, in extremely arid south-western Queensland C A single male specimen of Palirika culgoafloodplainensis sp. n. was collected during a Bush Blitz survey from this Malaise trap, 7 km NNW Toulby Gate on Culgoa Floodplains National Park (NP) on the Queensland/New South Wales Border, 134 km WSW Dirranbandi D Forrest lookout on Karara Pastoral Lease 213 km ESE of Geraldton in Western Australia, where two male specimens of Larrpana bushblitz sp. n. were hand netted hill-topping by CLL in September 2009 during a Bush Blitz survey. Photographs A and B by N. Starick, QM.
One of five most parsimonious cladograms (931 steps, CI = 0.24, RI =0.47). Part 1. Black circles = unique character changes, open circles = homoplasious changes. Bremer supports over branches.
One of five most parsimonious cladograms (931 steps, CI = 0.24, RI =0.47). Part 2. Black circles = unique character changes, open circles = homoplasious changes. Bremer supports over branches.
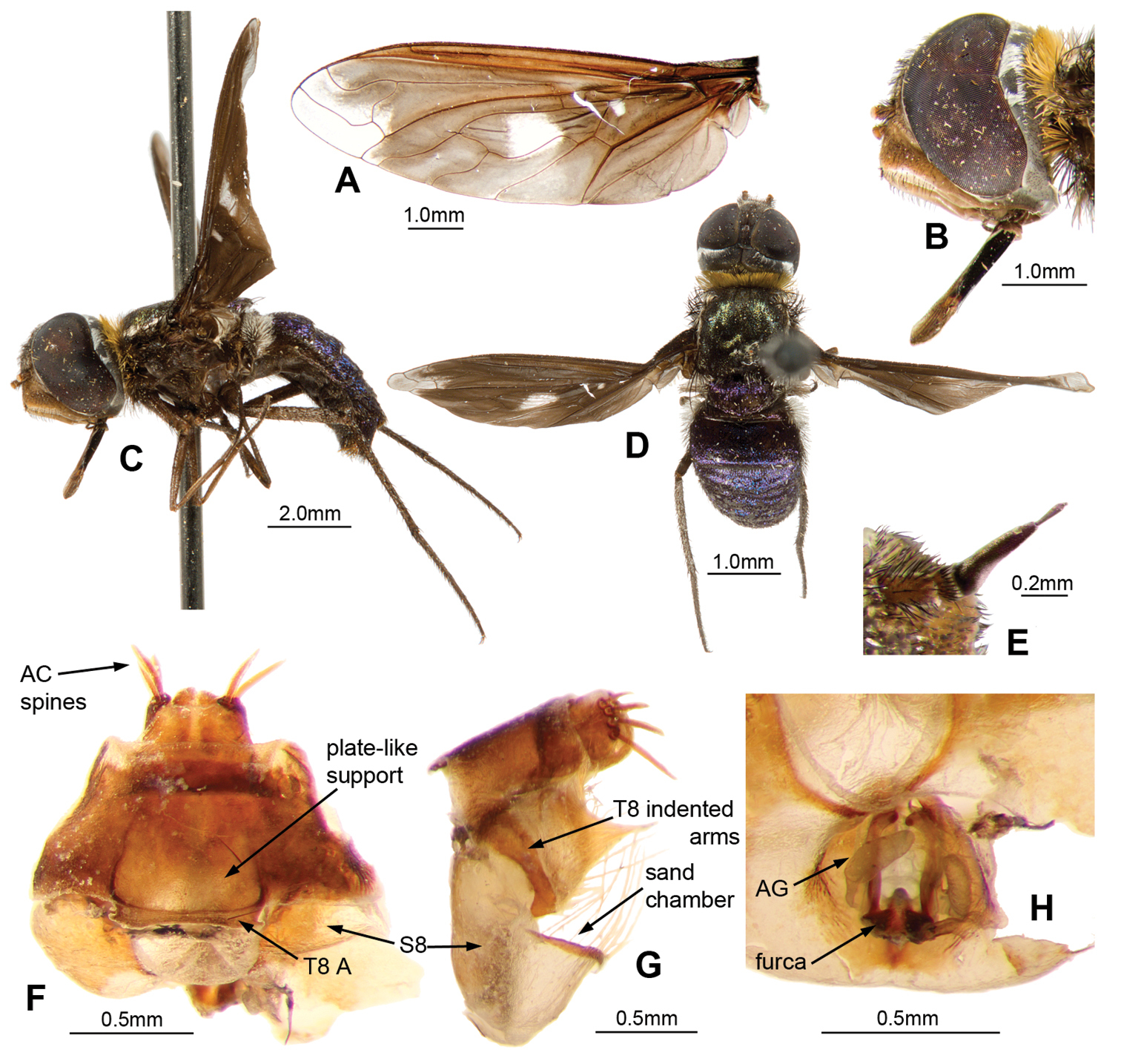
Palirika culgoafloodplainensis sp. n., Male holotype. A Head lateral B Antennae dorsal C Adult, dorsal D Adult, antero-lateral; Male genitalia: E Epandrium ventral with sub-epandrial sclerites F Epandrium lateral G T8, dorsal H S8, ventral. Scale line (E–H) = 0.5 mm.
Palirika culgoafloodplainensis sp. n., Male holotype genitalia: A Gonocoxal complex dorsal B Gonocoxal complex lateral C Gonocoxal complex ventral D Gonostyli E Adeagal complex dorsal F Adeagal complex lateral G Epiphallus lateral H Adeagal complex ventral. Scale line = 0.5 mm.
Etymology. This species is named culgoafloodplainensis after the remote Queensland Culgoa Floodplain National Park where the type specimen was collected, and where CLL and Noel Starick received so much hospitality, enthusiasm, and encouragement over the years from all the staff, but especially RIC Andy Coward.
(Fig. 11). This species has only been collected from the type locality in central south-western Queensland.
Due to extended storage in propylene glycol as retrieval of sample was prevented by extensive and prolonged flooding the specimen bears few setae, hairs or scales, therefore colour patterns referred to in the description are based on those remaining, usually at junctions of sclerites.
urn:lsid:zoobank.org:act:CD50600C-901E-46CA-840F-D4FEA863AF0E
http://species-id.net/wiki/Palirika_mackenziei
Figs 1A–B, 2–3, 6, 11; Morphbank Collection 692335Holotype. Queensland: ♀, 26°43.7'S, 142°39.1'E, Plevna Downs, Tompilly Hill summit, 13 Dec 2007, C. Lambkin, N. Starick & R. Mackenzie, 15454, sweep net, hilltopping, 220m, [dissected], PS1893, A007533, T152481 (QM). Condition: Good.
Wing length 9.0 mm.
Small dark flies with heavily infuscated wings, hyaline only apically and medial spot. Face orange with shiny reddish-brown scales, frons black with shiny black scales. Collar yellow. Narrow laterothoracic stripe of whitish scales. Scutum black with dull lime-green metallic scales except pinkish metallic scales anterolaterally and posteromedially. Scutellum dark brown, darker basally with royal-blue metallic scales, purple metallic scales laterally and posteriorly. Widened base of costa with shiny reddish-brown scales, no paler scales posteriorly. Wing pattern broadly dimidiate (Fig. 6A); black with hyaline areas, apically and medially. Apical hyaline area covering extreme apex of r1, apex of first r2+3, apical half of second r2+3, all r4, and extreme apex r5. Medial hyaline area covering middle of dc extending from M1 across m-cu and into m2. Paler prediscoidal opaque area distinct. Alula and squama edged with long broad grey scales. T1 with white Ma; long white flattened scales posterolaterally. Tergites black with royal-blue metallic scales that reflect purple (Fig. 6D). Female T8 A short, plate-like support distinct.
Female. Head.(Figs 6B–E). Face orange with shiny reddish-brown scales, frons black with shiny black scales; setae black, longest below distinct frontal depression. Antennal scape red, 3 × length of pedicel, with long black setae dense laterally and ventrally; pedicel red with black setae shorter and sparser dorsally; PP conical, 5 × length of pedicel, black with silvery pruinescence, distinct apical joint; BSM rod-like, expanded apically, reddish-brown, 2 × length of pedicel; ASM reddish, conical, length less than width of BSM (Fig. 6E). Occiput with shiny black scales broadly filling indentation.
Thorax. (Figs 6B–D). Collar yellow. Narrow laterothoracic stripe of whitish scales. Long white flattened scales posteromedially. Scutum black with dull lime-green metallic scales except pinkish metallic scales anterolaterally and posteromedially; black setae. AN with black Ma, long yellow setae anteriorly; long, lightly iridescent scales at base of wing black. Prealar bristles strong, black and long. Postalar bristles strong, black and reaching almost apex of scutellum. Pleural hairs black with reddish iridescence. Ma of LT black, reddish-brown dorsally. Tympanal ridge and PL with yellowish flattened scales. Scutellum dark brown, darker basally with royal-blue metallic scales, purple metallic scales laterally and posteriorly; strong, black apical bristles. Legs. Reddish-brown, tarsi darker; scales and setae black. Pulvilli sharp, curved, half length of mid- and hind-tarsal claws. Halter knob dark reddish-brown, posteromedial edge broadly yellow. Wing (Fig. 6A), cup open. Widened base of costa with shiny reddish-brown scales, no paler scales posteriorly. Wing pattern broadly dimidiate; black with hyaline areas, apically and medially. Apical hyaline area covering extreme apex of r1, apex of first r2+3, apical half of second r2+3, all r4, and extreme apex r5. Medial hyaline area covering middle of dc extending from M1 across m-cu and into m2. Paler prediscoidal opaque area distinct. Anal edged with long black scales basally. Alula edged with long broad grey scales. Squama edged with dense overlapping long grey scales.
Abdomen. (Figs 6C–D). T1 with white Ma; long white flattened scales posterolaterally. Tergites black with royal-blue metallic scales that reflect purple when not viewed dorsally, pleura with lateral tufts of long black setae on T2–7. Sternites black, with black scales and setae. Genitalia (Fig. 6F–H). Dorsal T8 A short, plate-like support distinct; T10 with 4 pairs of stout AC spines. Furca with 2 long broad posteriorly directed arms with small recurved hook-like dorsal extensions apically.
Male. Unknown.
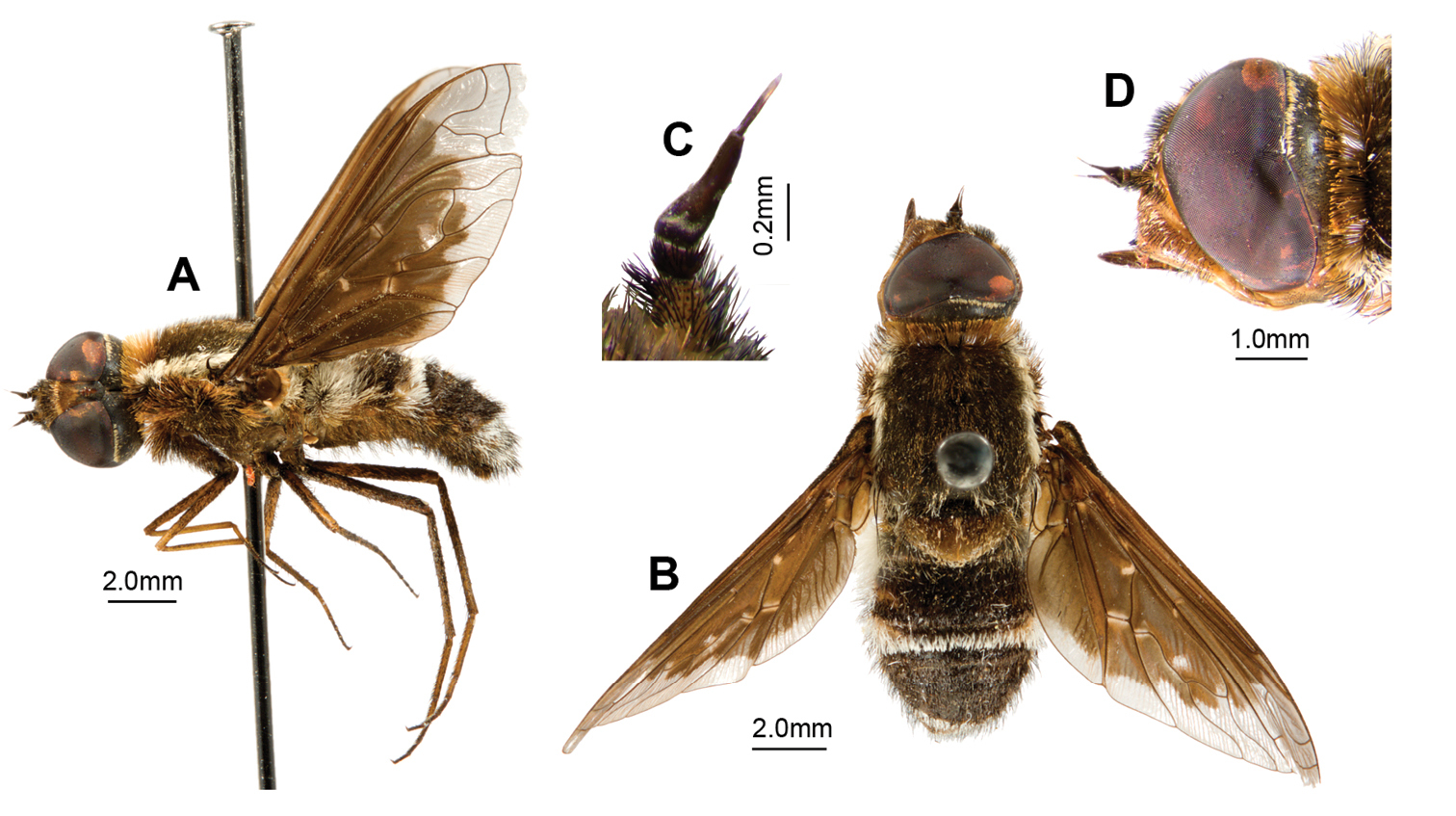
Palirika mackenziei sp. n., Female holotype. A Wing B Head lateral C Adult, lateral D Adult, dorsal; Male genitalia: E Antennae lateral; Genitalia: F Dorsal G Lateral H Dorsal, furca.
This species is named mackenziei to acknowledge the enthusiasm and interest in all kinds of natural history by the Mackenzie family of Plevna Downs Station where the type, and only, specimen was collected. Since 2007, following the discovery of dinosaurs on their property, together with a large undescribed spider, CLL and Noel Starick have been welcomed by the Mackenzie family. Robyn Mackenzie was thrilled to be helping catch hill-topping beeflies on the summit of Tompilly Hill when the only female specimen of this unusual Palirika was captured (Fig. 1A). We have happily instructed the family, the local Natural History Society, students, teachers, regional property owners and community members on the ins and outs of biodiversity of arid areas, especially the insects.
(Fig. 11). This species has only been collected from the type locality in remote far south-western Queensland.
urn:lsid:catalogueoflife.org:taxon:d9155668-29c1-102b-9a4a-00304854f820:col20110201
Exoprosopa dimidiatipennis
Dimidiate wing pattern as in Figure 7A–B, dark basally, hyaline apically; infuscation forming a distinctly separated, basal triangle leaving the apex of the posterior cubital and anal cells broadly hyaline. Cream laterothoracic stripes, white scale bands on T3, sparse white scales on T6–7. Epandrial basolateral flange longer and broader than the length of the epandrial base. Gonocoxae deeply narrowed medially, tufts of thickened setae on distinct ventral flange that projects basally; EP with rounded, projecting, lateral lobes; short, wedge-shaped AAES; EJA long. Sperm pump long with unpigmented papillae, collar or clear ring surrounding join between BB and thick-walled round SR.
Included species: Larrpana bushblitz sp. n., Larrpana dimidiatipennis, Larrpana collessi Lambkin & Yeates 2003, Larrpana zwicki.
urn:lsid:zoobank.org:act:1CFF61E9-10C6-4185-8135-CDA4DAEB69A9
http://species-id.net/wiki/Larrpana_bushblitz
Figs 1D, 2–3, 7–8, 11; Morphbank Collection 692334Holotype. Western Australia: ♂, 29.302°S, 116.725°E, Karara, 23.5km ESE Boiada Camp, 356m, 17 Sep 2009, Lambkin, sweeping, 18402, rocky hilltop, hilltopping, PS1714, WAM 82396 (WAM). Condition: Good.
Paratypes. Western Australia: 1♂, same data as holotype, T152479 (QM); 1♂, 29.309°S, 116.731°E, Karara, Forest lookout, 24.4km SE Boiada Camp, 17 Sep 2009, 18405, Lambkin, sweeping, 410m, rocky hilltop, hilltopping, [dissected], PS1894, WAM 82397 (WAM); 1♂, same data Forest lookout, A007534, T152480 (QM).
Wing length 14 mm
Medium, dark, densely setose, flies with black, dimidiate wings with five indistinct yellowish spots (Fig. 7A, B); infuscation indented in 1st r2+3; no lobe or medial band; dc infuscated except for rectangular hyaline area at junction of m-cu and m-m. Thoracic collar yellow. Dorsal surface of thorax, scutellum and abdomen covered with long upstanding setae, producing distinct fluffy appearance. T3 with uninterrupted white band of upstanding scales narrowing medially, laterally spanning entire tergite. Alula and squama edged with dense long cream scales; proximal 1/3 of anal cell edged with black scales, longest basally. Male (Fig. 8) E with basal flange very long, broad, extending basally, apically recurved. H large, subquadrate in lateral view, distinctly projecting.
Male. Head(Fig. 7A–D). Frons reddish-brown, face red, face and frons with transparent scales, black setae longest below shallow frontal depression. Antenna (Fig. 7C). Scape 2.5 × length of pedicel, red; pedicel red; PP long, 3–4 × length of pedicel, as long as scape and pedicel combined, dark reddish-brown, with reddish pruinescence; BSM dark reddish-brown, 2 × length of pedicel, not expanded apically; ASM minute blunt cone, length less than width of BSM. Narrow band of cream scales at posterior margin of eye medially.
Thorax (Fig. 7A, B). Collar yellow. Very broad distinct laterothoracic stripe of dense long white scales. Scutum black; scales long, reddish-brown, white posteriorly; long dense black setae, longest anteriorly and posteriorly. AN and PN with Ma admixed black and reddish-brown; long, slightly iridescent, reddish-brown scales at base of wing. Pleural hairs black, with reddish-brown iridescence. Scales on APA white. Laterotergite with dark reddish-brown Ma ventrally, white dorsally and red medially. Plumula with dense long white scales and TR with dense long yellow scales. Scutellum dark reddish-brown, black basally; scales black basally, transparent pale-brown medially and posteriorly, posterior scales longest; long dense, black setae. Legs reddish-yellow with black scales. Microchaetae on fore-tarsi curved apically. Pulvilli straight sharp cones, more than 1/3 length of mid- and hind-tarsal claws. Halter knob red with pale whitish apical edge. Wing (Fig. 7A, B). Widened base of C with black scales with pale band posteriorly. Spur-veins present on base of R4 extending into r4 and on apex of m-cu extending into m2 in some specimens; bump at basal bend of m-cu. Wing pattern (Fig. 7A, B) black, dimidiate, broad basal infuscation following R proximal to i-r1 to wing margin in apical 4/5 anal cell; indented in 1st r2+3; no lobe or medial band; dc infuscated except for rectangular hyaline area at junction of m-cu and m-m; apex hyaline. Dark yellowish-brown areas bordering base of CuA1, join of R1 and Rs, r-m continuing onto base of R2+3, and base m-cu; together with prediscoidal opaque area forming indistinct pentagonal pattern of spots within infuscation. Anal and cup infuscated for basal 4/5. Alula and squama edged with dense long cream scales; proximal 1/3 of anal cell edged with black scales, longest basally.
Abdomen.
Larrpana bushblitz sp. n. A Adult, lateral B Adult, dorsal C Antennae dorsal D Head lateral.
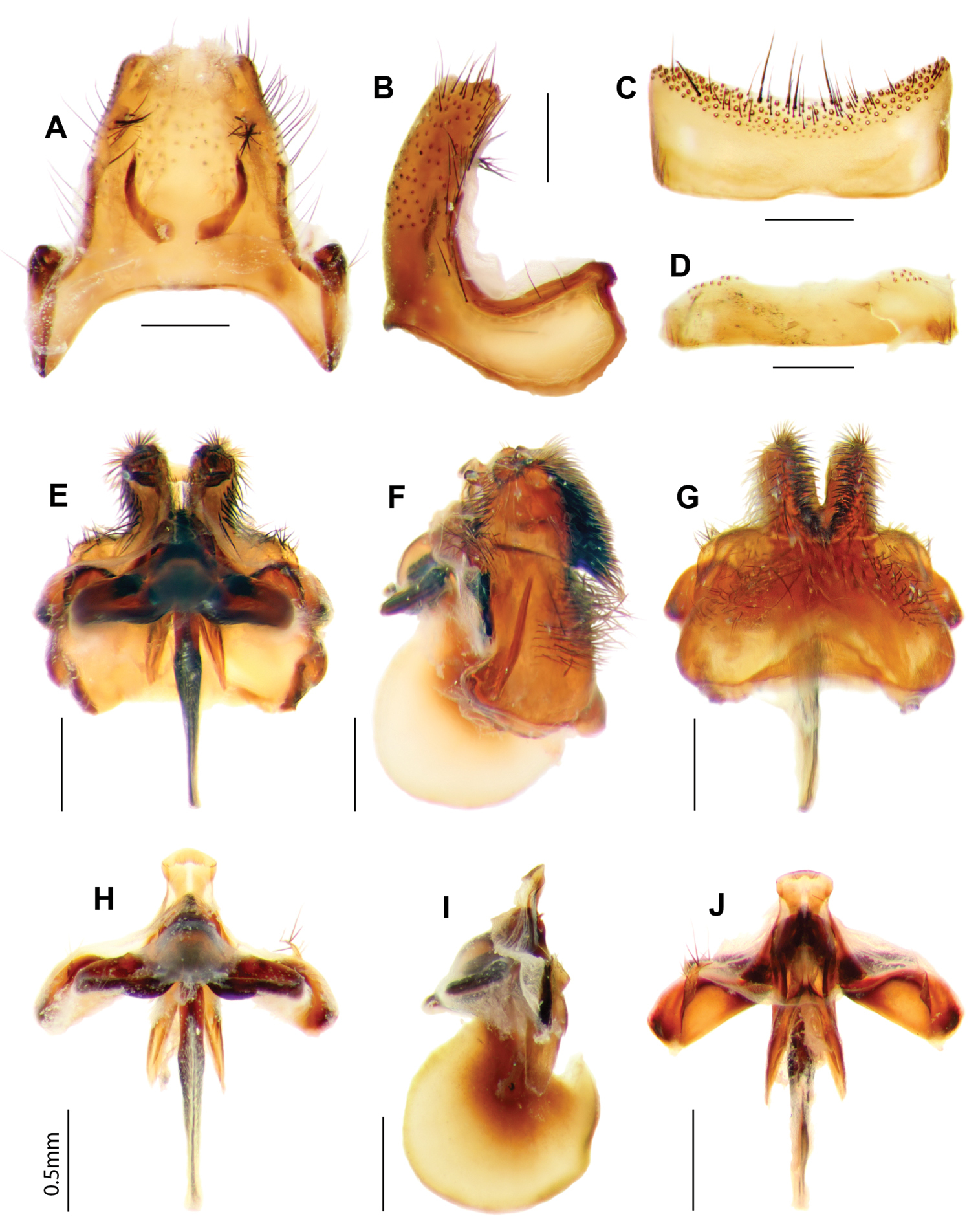
Larrpana bushblitz sp. n. Male genitalia: A) Epandrium ventral with sub-epandrial sclerites B Epandrium lateral C T8, dorsal D S8, ventral; Male genitalia: E Gonocoxal complex dorsal F Gonocoxal complex lateral G Gonocoxal complex ventral H Adeagal complex dorsal I Adeagal complex lateral J Adeagal complex ventral. Scale line = 0.5 mm.
(Fig. 7A, B). Tergites black with red anterolateral areas medially rounded on T2–3 < 1/4 width of tergite. Scales dense, black except: T3 with uninterrupted white band of upstanding scales narrowing medially, laterally spanning entire tergite; T1–3 with dense long white upstanding lateral scales; T6–7 with white scales. T1 with Ma white dorsally and laterally, yellow ventrally. T1–3 with long dense white setae laterally, pale brown anteriorly, and black posteromedially; T4–7 with long dense thick black setae. Sternites red with sparse long pale reddish scales, dense long black setae. Genitalia (Fig. 8). Epandrium red with distinct anterolateral flange bearing cluster of long black setae; basal flange very large, long, broad, extending basally and apically upcurved; setae black, loosely grouped anterolaterally; SES very long, linear, broadened basally. Gonocoxae red, strongly narrowed medially; ventral ridge projecting; LAEA deeply convex; GS cupped within G margins, subquadrate base projecting apically; very large recurved rami extending beyond G margins; setae long black, not short apically, dense tufts of long, thickened setae medially, directed basally, very long thin setae continuing laterally; H large, subquadrate in lateral view, distinctly projecting. Epiphallus 1.4 × neck width; with apical margins inturned forming projecting rounded lobes.
Female.
Unknown.
This species is named as a noun in apposition after the three-year, multimillion dollar Bush Blitz program that organised and funded the survey on Charles Darwin Reserve, Karara, Lochada and Kadji Kadji Pastoral Leases in Western Australia on which this species was collected. The core focus of the Bush Blitz program is to document the plants and animals in hundreds of properties across Australia’s National Reserve System, and on nature discovery – identifying and describing new species of plants and animals. The Bush Blitz program also funded the survey in western New South Wales and Queensland on which Palirika culgoafloodplainensis sp. n. was collected (
(Fig. 11). Larrpana bushblitz sp. n. has only been collected from Karara Pastoral Lease, 213 km ESE of Geraldton in Western Australia.
On collection, this species appeared similar to the two male specimens of Larrpana zwicki collected only near Windorah (
urn:lsid:catalogueoflife.org:taxon:d916e848-29c1-102b-9a4a-00304854f820:col20110201
Munjua erugata Lambkin & Yeates, 2003: 795.
Wing with medial hyaline band not linear and narrowing apically, apical infuscated band not meeting posterior wing margin more broadly than medial hyaline band. Gonocoxae deeply narrowed medially, broadly indented basally, with tufts of thickened setae ventromedially, H projecting but not forming a finger-like extension; AE short; EP with medioventral process above AE; very long AAES reaching G margins; EJA racquet-shaped, very long. Sperm pump short with unpigmented papillae, apical endplate simple with thin processes; thick-walled round SR with distinct basal bulb.
Included species: Munjua erugata Lambkin & Yeates 2003, Munjua lepidokingi Lambkin & Yeates 2003, Munjua paralutea Lambkin & Paramonov 2003.
See reference to the rediagnosis of the genus Munjua in the phylogenetic results.
urn:lsid:zoobank.org:act:77AACA67-1FA6-4CD9-871E-D95C34FDE977
Munjua trigona Lambkin & Yeates, 2003: 804.
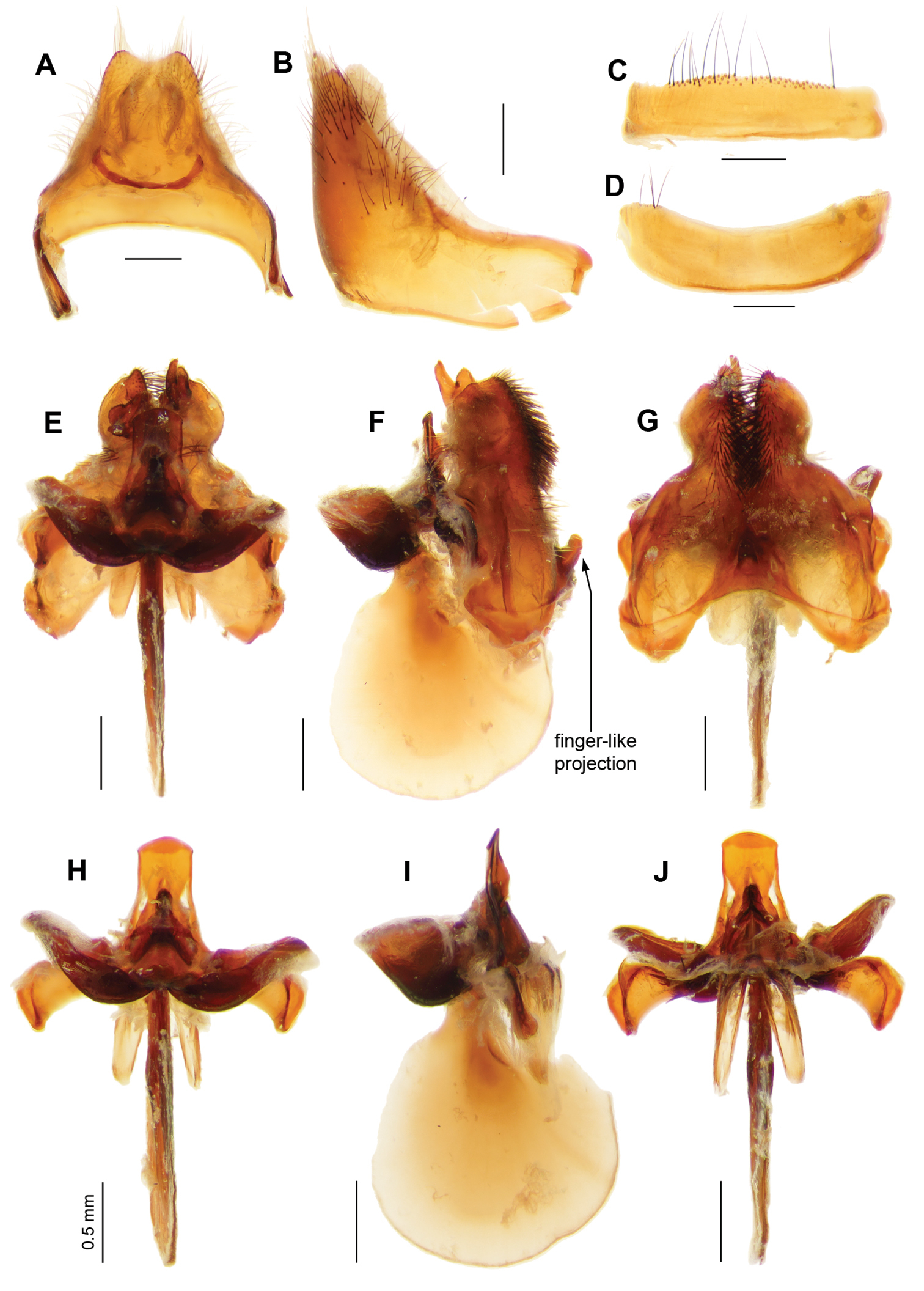
Wing with medial hyaline band linear and narrowing apically, apical infuscated band meeting posterior wing margin twice breadth of medial hyaline band (Fig. 9A, B). Gonocoxae deeply narrowed medially, broadly indented basally, with tufts of thickened setae ventromedially, H projecting forming finger-like extension (Figs 9E, 10F); AE short; EP with medioventral process above AE; very long AAES reaching G margins; EJA racquet-shaped, very long (Fig. 10). Sperm pump short with unpigmented papillae, apical endplate simple with thin processes; thick-walled round SR with no basal bulb (Fig. 9F–H).
The name for the genus Ngalki is from the aboriginal term ngalki for “little finger” from the Ngiyampaa language spoken in much of central New South Wales (
Included species: Munjua trigona Lambkin & Yeates, 2003
See reference to the erection of the genus Ngalki in the phylogenetic results.
urn:lsid:catalogueoflife.org:taxon:db706730-2dc5-11e0-98c6-2ce70255a436:col20110201
http://species-id.net/wiki/Ngalki_trigonium
Figs 23, 9–10; Morphbank Collection 692333Paratypes. New South Wales: 1♂, Round Hill Nature Reserve, 27 Dec 1976, G. Daniels, GDCB Reg # 14199, K 253709; 1♀, Round Hill Nature Reserve, same, GDCB Reg # 17925, K 253717 (AM). Victoria: 1♀, Wyperfield Nat Park, 7 Dec 1976, G. Daniels, GDCB Reg # 14163, K 253707; 3♂, Wyperfield Nat Park, 8 Dec 1976, G. Daniels, GDCB Reg # 17924, #14164, # 17923, K 253720, (PS1936) K 253702, K 253712 (AM).
New South Wales: 1♀, Round Hill area, 24–25 Nov 1991, A. Sundholm, [dissected], PS1935, K 289927 (AM). Western Australia: 1♂, Fraser Range, 8 Nov 1977, A. Atkins, [dissected], GDCB Reg # 14165, PS1934, K 253698 (AM).
Large dark flies (wing length 15-20 mm), wings as in Figure 9B, dark with narrow, linear, medial hyaline band; long, finger-like apically-directed projection on hypandrium (Figs 9E, 10F). Laterothoracic stripe creamy-white. Broad white scale band on T3, T4–5 with black scales, T6–7 with white scales. Epandrium with long golden setae; SES joined medially. Epiphallus with short medial projection. Female with no BB between pump and pale, square SR.
Male. Head (Fig. 9C). Face and frons red with reddish-yellow scales, black setae longest below deep frontal depression. Antennal scape 3 × length of pedicel, red; pedicel red; PP 3.5 × length of pedicel, black with reddish pruinescence; BSM dark reddish-brown, 3.5 × length of pedicel, expanded apically; ASM short, blunt (Fig. 9D). Narrow line of creamy-white scales on posterior margin of eye.
Thorax. Collar yellow, with tips of Ma darker, reddish. Laterothoracic stripe broad creamy-white, distinct. Scutum black with long hair-like reddish-brown scales, sparse white flattened scales posteromedially. Pleural vestiture dark-red with mauve iridescence; admixed dark-red and black Ma on PE and AN; long scales at base of wing with mauve iridescence. Anepimeral setae admixed dark-red and black. Scales on APA yellow. Plumula and TR hairs creamy yellow. LT with black Ma, red dorsally. Scutellum red with scales yellowish-red, setae black, sparse long white scale fringe on posterior margin. Legs red with dark reddish-brown scales. Pulvilli chisel-like wedges, less than a half length of mid- and hind-tarsal claws. Halter knob dark reddish-brown, with yellow apical edge. Wing (Fig. 9B). R4+5 with abrupt bend basally, cup closed at wing margin, or narrowly open. Widened base of C with dark, reddish-brown scales, paler brown scales posteriorly. M2 very sinuous, width of m1 at wing margin < 1/2 width of m2. Wing pattern (Fig. 9A, B) black, with broad infuscated band following R proximal to i-r1, obliquely through 1st r2+3, r5 and m1 to meet wing margin in m2; no infuscation in 2nd r2+3. Hyaline band very narrow, linear, from dc through m2 basally, apex of cua, cup and anal cells. Squama with reddish-yellow scales.
Abdomen (Fig. 9B). Integument with large red areas laterally, leaving black medial band; T2 with black medial band broad basally, width > 1/3 width of the tergite, tapering sharply apically; T3–4 with black medial band < 1/4 width, black apical band; T5–7 with red areas laterally < 1/4 width. T1 with Ma white, reddish-brown scales medially. T2 with dense long cream hairs anterolaterally, dark reddish-brown scales, some white scales laterally. Broad white scale band on T3, interrupted medially, dark reddish-brown scales anteromedially, black scales posteromedially. T4–5 with black scales, T6–7 with white scales. Sternites red; S2–3 and S4–5 basally with dense white scales and dense long, fine white hairs; S4–5 apically with dark reddish-brown scales, S6–7 with black scales, black setae. Genitalia (Figs 9E, 10). Epandrium red with basal flange short and broad; setae black, long golden setae apically; SES joined medially. Gonocoxae red, strongly narrowed medially; setae black, short apically, medially with thick tufts of basally directed setae, long setae laterally around apex of H; distinct ventral ridge; LAEA deeply convex, extending past G margins; GS cupped within G margins, large subquadrate base with slight projection apically; large recurved R; H crescent-shaped, laterally delimited by swollen and expanded G, laterally subrectangular, with distinct large, blunt finger-like apical projection. Epiphallus long, not expanded apically; with medial projection.
Female. Same as male. Genitalia (Fig. 9F–H). Dorsal T8 A short, entire; T10 with 4 pairs of short, thick AC spines; apical endplate with long thin processes; basal endplate with thick processes; long unpigmented papillae, no BB, narrow ring between pump and lightly pigmented, square SR.
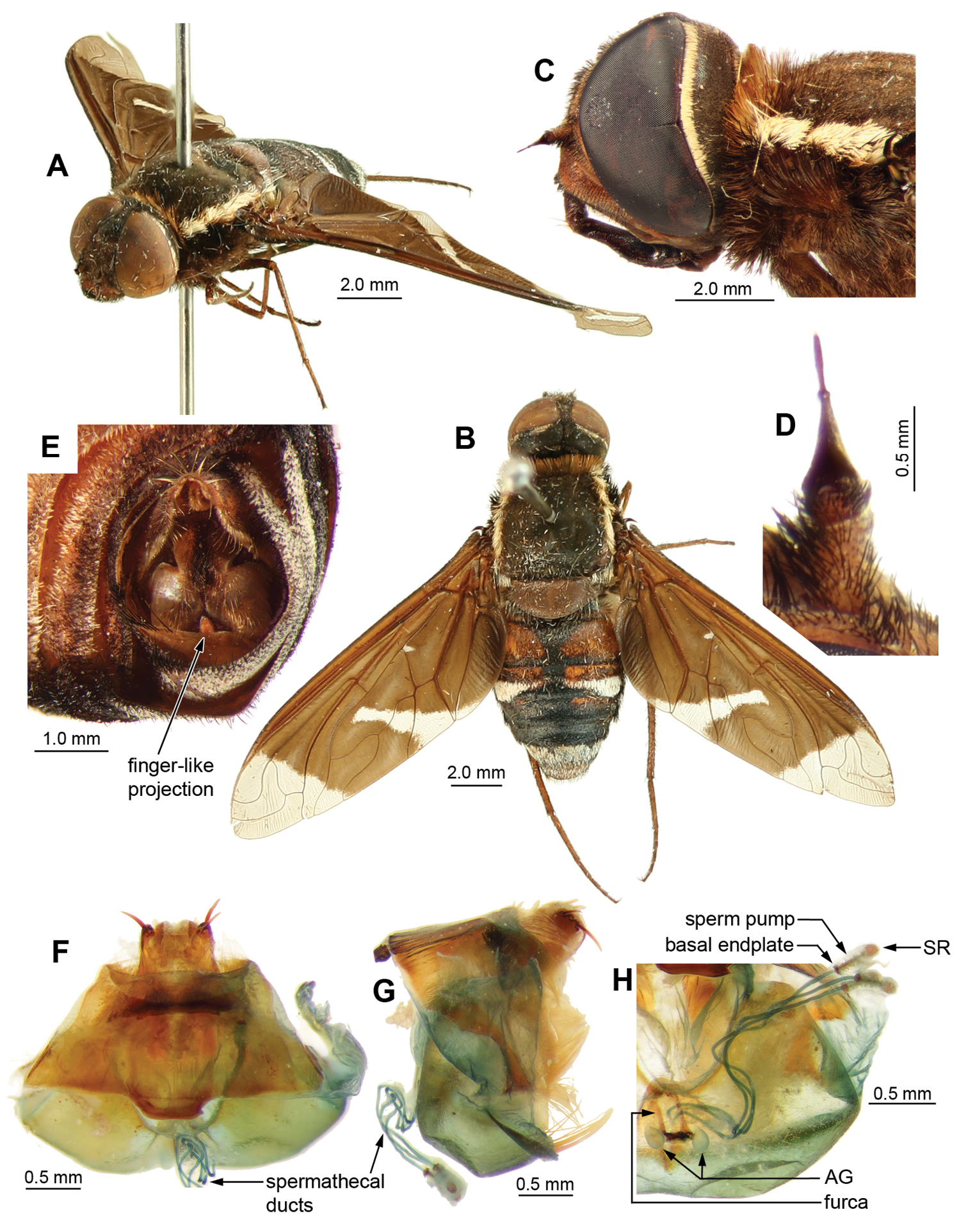
Ngalki trigonium. A Adult, antero-lateral B Adult, dorsal C Head and thorax lateral D Antennae lateral E Male genitalic complex showing diagnostic finger-like projection on hypandrium, clearly visible in situ. Female genitalia: F Dorsal G Lateral H Dorsal, furca and spermathecal complex.
Ngalki trigonium. Male genitalia: A Epandrium ventral with sub-epandrial sclerites B Epandrium lateral C T8, dorsal D S8, ventral E Gonocoxal complex dorsal F Gonocoxal complex lateral showing diagnostic finger-like projection on hypandrium G Gonocoxal complex ventral H Adeagal complex dorsal I Adeagal complex lateral J Adeagal complex ventral. Scale line = 0.5 mm.
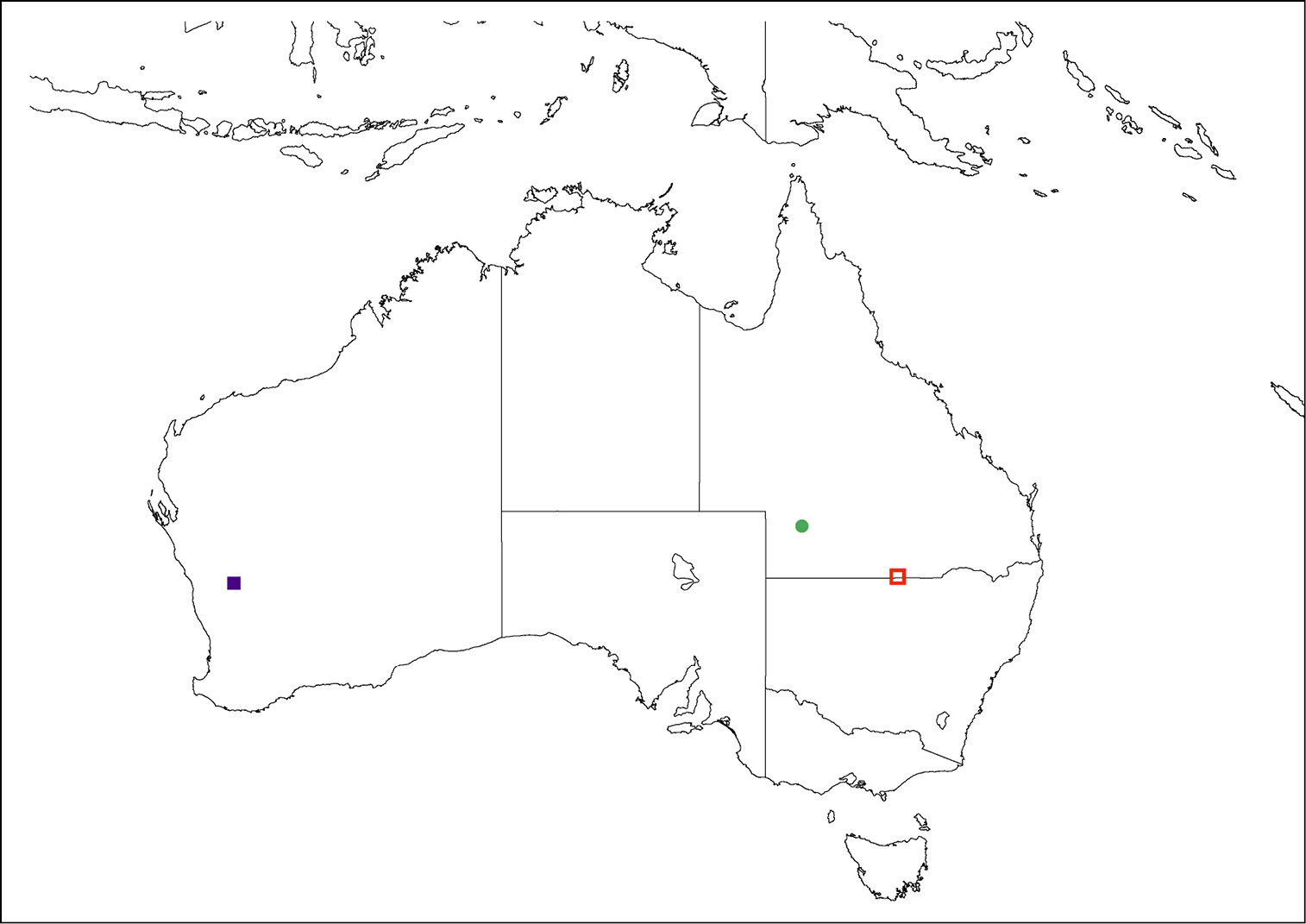
Map of distribution. Closed circle - Palirika mackenziei sp. n. Open square - Palirika culgoafloodplainensis sp. n. Closed square - Larrpana bushblitz sp. n.
In
This species has been collected in the southern Australian Bassian region, from semi-arid and arid mallee areas.
The finger-like projection on the H (Figs 9E, 10F) in the males of Ngalki trigonium is apparent without dissection and, together with the unusual wing pattern, allows easy identification.
| 1 | Metallic scales on body, black reflecting blue, bluish-black, green or maroon, no white or yellow scales on T2–7 or on S2–7 (Fig. 6D) | Palirika Lambkin & Yeates |
| – | No metallic reflecting black scales; white or yellow scales present T2–7, usually distinct bands or lateral triangles on T3 (Fig. 9B) | 2 |
| 2 (1) | Wing dimidiate and at least apical half of anal cell margin hyaline, at most short narrow lobe following m-m into m2, no medial hyaline band (Fig. 7B) | Larrpana Lambkin & Yeates |
| – | Wing not dimidiate or anal cell fully infuscated; distinct medial hyaline band usually present (Fig. 9B) | 3 |
| 3 (2) | Female spermathecal reservoir a long cylinder; T2 with black scales; T6–7 with white scales; proboscis extending beyond oral cavity, not longer than head | Balaana Lambkin & Yeates |
| – | Female spermathecal reservoir round to subquadrate never a long cylinder; T2 with some yellow scales unless T6 or T7 with black scales or proboscis longer than head .. | 4 |
| 4 (3) | Male with no medioventral process on epiphallus above aedeagus, anterior arms of aedeagal sheath long, reaching gonocoxal margins; quadrate sub-epandrial sclerites in epandrium. EITHER Deeply infuscated wings, only apex hyaline; paler yellowish spots at base of R2+3, at base of CuA1, join of R1 and Rs, r-m and base of m-cu; T6 black scales; OR medial hyaline band a narrow line; black scales forming median circle apex of T2 and base of T3, yellow scales anteriorly and laterally on T2, medially and laterally on T3 | Muwarna Lambkin & Yeates |
| – | Male with medioventral process on epiphallus above aedeagus (Figs 5G, 10I), linear or single fused (Fig. 10A) sub-epandrial sclerites in epandrium. Wings less infuscated with broad medial hyaline band broader posteriorly; IF deeply infuscated with medial hyaline band a narrow line (Fig. 9B) no median circle of black scales on T2–3 | 5 |
| 5 (4) | Male with anterior arms of aedeagal sheath long, reaching gonocoxal margins (Fig. 10E). Ventral ridge on gonocoxae small or absent, not projecting basally AND hypandrium projecting. Epiphallus without lateral lobes; ejaculatory apodeme extending beyond gonocoxae by more than length of gonostylus (Fig. 10E). EITHER yellow vestiture with wing infuscation distinctly variegated, bright yellow basally and medial band dark brown to black; OR no yellow scales on T2–7 (Fig. 9B); OR only yellow scales anteromedially on T2–3, S2–3 with dense, white scales and setae, S5–7 with dense, black scales and setae | 6 |
| – | Male with anterior arms of aedeagal sheath short, not reaching gonocoxal margins; if ventral ridge on gonocoxae very small or absent then hypandrium not projecting. Abdominal yellow scales at least anteriorly T2, S2–3 with dense, white scales; hemispherical tufts of macrochaetae laterally on T1 white or yellow, not dark reddish-brown or black; wing infuscation not distinctly variegated, IF yellow scales only anteromedially T2 then scales on S5–7 not black, at most reddish-brown | 7 |
| 6 (5) | Medial hyaline band linear, narrowing anteriorly, with apex of anal cell and cup hyaline (Fig. 9B); male gonocoxae with long, finger-like projection from hypandrium (Figs 9E, 10F) | Ngalki Lambkin gen. n. |
| – | Medial hyaline band not linear, not narrowing anteriorly; male gonocoxae with no finger-like projection from hypandrium | Munjua Lambkin & Yeates |
| 7 (5) | Male epandrium with strongly grouped setae on anterolateral flange; ventral ridge on gonocoxae large, distinctly projecting basally, hypandrium not projecting, epiphallus with rounded projecting lateral lobes, ejaculatory apodeme short, extending beyond gonocoxae by less than length of gonostylus, hind-tibial scales not protruding, dark flies, T2 and T4 mostly black scales | Kapu (Lambkin & Yeates) |
| – | Male epandrium with loose setae, without an anterolateral flange; hind-tibial scales protruding, pale yellowish flies with striped abdominal vestiture, T2 and T4 mostly yellow scales | Wurda Lambkin & Yeates |
| 1 | No pre-apical infuscated band on wing (Figs 4C, 6A) | 2 |
| – | Pre-apical infuscated band on wing present | 3 |
| 2 (1) | Infuscation of wing blade almost complete except for hyaline apical area and isolated spot over dc (Fig. 6A) | Palirika mackenziei Lambkin sp. n. |
| – | Infuscation of wing blade only extending over half wing area, indistinct extension along R4+5 and isolated mottled area along m-m (Fig. 4C) | Palirika culgoafloodplainensis Lambkin sp. n. |
| 3 (1) | Anal and posterior cells with notched hyaline area, infuscation extending along CuA2; apically-directed spur-vein on i-r1 cross vein | Palirika bouchardi |
| – | Anal and posterior cells without extension along CuA2, rarely spur-vein on i-r1 cross vein | 4 |
| 4 (3) | Hyaline medial band continues anteriorly through entire r5; dark thorax; abdomen: males dark prussian-blue, almost black; females bluish-green | Palirika cyanea |
| – | Hyaline band usually through dc anteriorly, not entirely through r5, or absent; thorax and abdomen not as above | 5 |
| 5 (4) | Collar white with contrasting tuft of black lateral Ma at base of pronotal lobe, bright green thorax, anterolaterally dark maroon; bright, dark blue abdomen; anal and cup fully infuscated | Palirika decora |
| – | Collar entirely white or yellow, at most 4 reddish Ma above postpronotal lobe; thorax, abdomen, anal and cup not as above | 6 |
| 6 (5) | Face yellow, most facial setae shiny gold; blue-green thorax and abdomen | Palirika viridula |
| – | Face orange-red to reddish-brown; if yellow, most facial setae black; thorax and abdomen not as above | 7 |
| 7 (6) | Thorax green | 8 |
| – | Thorax dark, not green | 11 |
| 8 (7) | Thorax anterolaterally with dark maroon scales; anal and cup infuscation various | 9 |
| – | Thorax entirely green; anal and cup hyaline apically | 10 |
| 9 (8) | Abdomen bluish-green; brown wing infuscation, anal and cup hyaline apically; blue face scales | Palirika whyalla |
| – | Abdomen entirely purple; black wing infuscation, anal and cup fully infuscated; purple face scales | Palirika anaxios |
| 10 (8) | Thorax bright yellowish-green; abdomen blue to bluish-green metallic scales; infuscated wing band short, much narrower than hyaline band | Palirika marginicollis |
| – | Thorax dark bluish-green; abdomen T2 blue-green, T3–7 blue, T4–6 admixed maroon at least laterally; infuscated wing band broader than hyaline band | Palirika blackdownensis |
| 11 (7) | Abdomen purple with blue scales on T4–6, Queensland | Palirika danielsi |
| – | Abdomen entirely purple, no blue scales on T4–6, Western Australia | Palirika basilikos |
| 1 | No yellow scales on T2–7 (Fig. 7A, B), Ma on T1 black, white or yellow | 2 |
| – | Abdominal yellow scales at least anteriorly T2; Ma on T1 white, not black | Larrpana collessi |
| 2 (1) | Wing without small paler yellowish spots in infuscation, m-m without infuscation | Larrpana dimidiatipennis |
| – | Wing with small paler yellowish spots in infuscation; m-m with infuscation (Fig. 7A, B) | 3 |
| 3 (2) | Wing without short narrow lobe following m-m into m2 (Fig. 7A, B) | Larrpana bushblitz Lambkin sp. n. |
| – | Wing with short narrow lobe following m-m in m1 into m2 | Larrpana zwicki |
Firstly, we thank the Bush Blitz program partners and the program managers from the Australian Biological Resources Study (ABRS), a section within the Parks Division of the Department of Sustainability, Environment, Water, Population and Communities (especially Brooke Glasser, Annabel Wheeler, and Kate Gillespie) for organising and funding the Bush Blitz field work on Charles Darwin Reserve, Karara, Lochada and Kadji Kadji Pastoral Leases in WA, funding the Bush Blitz survey of Culgoa Floodplains NP Qld, Culgoa NP and Ledknapper NR NSW (
CLL and Noel Starick thank the Mackenzie family of Plevna Downs Station for the enthusiasm and interest they always show in all forms of natural history, the welcome and hospitality they have given to us and all staff from the Queensland Museum, and the encouragement they provide to the local Natural History Society, regional property owners and community members on development of a knowledge base of biodiversity of arid areas. For providing collection permits to collect and take samples from National Reserves, we thank Jacqui Brock (Scientific Permit W1TK05498008: Queensland Parks and Wildlife Service, DERM, QLD) and Brendon Neilly (Scientific Research Licence S10016: NPWS, OEH, NSW). We thank David Britton and Jacqui Recsei (AM, Sydney) for supplying specimens of Ngalki trigonium for dissection and photography.
We thank Neal Evenhuis and an anonymous reviewer for their positive comments on the manuscript. Lastly, but not least, we gratefully acknowledge the work of Geoff Thompson (QM) for photographing specimens and guidance in the use of the imaging systems, Paul Avern (QM) for help with writing the occurrence data in Darwin Core format, Federica Turco (QM) for production of the distribution map and help with the GBIF and Dryad uploads, Vladimir Blagoderov, (Natural History Museum, London) for help with Scratchpads, Debbie Paul (School of Computational Science, Florida State University Tallahassee) for assistance with Morphbank, and Lyubo Penev and Teodor Georgiev (Pensoft Publishers, Sofia, Bulgaria) for their support and assistance with data publication through GBIF and Dryad.
For a full description of these characters see Appendix 2 in
Morphological terminology follows that of
1. Head scales: (0) absent; (1) present
2. Face scale density: (0) absent; (1) sparse; (2) overlapping; (3) carpet
3. Frons scale density: (0) absent; (1) sparse; (2) overlapping; (3) carpet; (4) distinct dense medial patch
4. Frons with vertical groove: (0) absent; (1) depression; (2) groove
5. Frons with horizontal depression: (0) absent; (1) shallow; (2) distinct; (3) deep
Dorsal6. W head/W thorax: (0) <; (1) =; (2) >
7. L antennae to compound eye/L scape: (0) <; (1) ≥; (2) ≥ 2x; (3) ≥ 4x
8. L antennal separation/L scape: (0) <; (1) ≥; (2) ≥ 2x; (3) ≥ 3x
9. Male compound eye separation /W OT: (0) meet; (1) <; (2) =; (3) ≤ 2x; (4) ≤ 3x; (5) > 3x
10. Female compound eye separation /W OT: (0) ≤ 2x; (1) ≤ 3x; (2) > 3x
11. OT to posterior margin of compound eye/ L OT: (0) ≤ OT; (1) > OT; (2) ≥ 2x; (3) ≥ 3x
12. L occiput/L OT: (0) ≤ 2x; (1) < 3x; (2) occiput long, well developed ≥ 3x
13. L vertex; i.e. L OT to occipital groove/ L OT: (0) ≤ L OT; (1) ≤ 2x; (2) wide > 2x
14. Depth occipital foveal depression: (0) no vertex; (1) not depressed; (2) shallow; (3) deep, slopes posteriorly at 45° to a short OG
15. W occipital foveal depression: (0) no vertex; (1) not depressed; (2) narrower than compound eye separation; (3) wider than compound eye separation
16. Apical occipital groove: (0) narrow; (1) wide rounded
Lateral17. Shape of head laterally: (0) round; (1) protruding but rounded, blunt; (2) conical
18. L proboscis: (0) < L oral cavity; (1) > L oral cavity; (2) > L head; (3) > 1.5x L head; (4) > 2x L head
19. L palps/ L proboscis: (0) rudimentary; (1) < 0.25x, short; (2) < 1, long
20. Genae setae surrounding oral cavity: (0) not grouped; (1) grouped laterally; (2) grouped apically, small tuft
21. L setae below antennae/L scape: (0) >; (1) ≤; (2) ≤ 0.5
22. Horizontal depression between antennae: (0) absent or shallow; (1) distinct; (2) deep
23. W of face projection/W compound eye including indentation posterior margin of eye: (0) < 1/4; (1) ≤ 1/2; (2) ≤ 1
24. W of the indentation on the posterior margin of the compound eye/L OT: (0) ≤ L OT; (1) > L OT; (2) ≥ 2x L OT
25. L of the line from the posterior margin of compound eye bisecting the compound eye facets/L OT: (0) absent; (1) <; (2) ≥; (3) ≥ 2x
Antennae26. L scape /L pedicel: (0) ≤; (1) ≤ 3x; (2) > 3x
27. L PP/ L pedicel: (0) ≤ 3x; (1) ≤ 4x; (2) > 4x
28. PP base shape: (0) broad not round base; (1) onion-like, round base abruptly narrowed; (2) medially divided, laterally; (3) conical, broad base, gradually narrowed
29. L PP rod/L base: (0) long > 2x; (1) short < 2x; (2) no thin rod
30. Distinct joint between PP and BSM: (0) absent; (1) present
31. L BSM/L pedicel: (0) absent; (1) ≤; (2) ≤ 2x; (3) ≤ 3x; (4) > 3x
32. BSM apical hairs: (0) absent; (1) present
33. L ASM/W BSM: (0) < W BSM, minute spine; (1) > W BSM; (2) > 2x; (3) conical twisted hat
Thorax34. Collar Ma: (0) pointed; (1) midstyle; (2) pectinate
35. Scm reflective vestiture: (0) bright; (1) very dull dark; (2) not reflective
36. Ma AN: (0) pointed; (1) midstyle; (2) pectinate
37. L PR bristles/ L PN: (0) > 2x; (1) >; (2) <; (3) absent
38. LT vestiture: (0) bare; (1) some hair; (2) dense hair
39. Ma LT: (0) absent; (1) pointed; (2) midstyle; (3) pectinate
40. MT vestiture: (0) bare; (1) some hair; (2) dense hair
41. L PA bristles/Scu: (0) absent; (1) < 0.5; (2) ≤; (3) >
42. L thorax/Scu: (0) ≤ 2x; (1) ≤ 3x; (2) > 3x
43. Scu vestiture reflective: (0) bright; (1) very dull dark; (2) absent
Legs44. C1 very long setae: (0) absent; (1) some; (2) dense
45. L forefemur/ L coxa: (0) ≤ 1.5x; (1) ≤ 2x; (2) ≤ 2.5x; (3) > 2.5x
46. Forefemoral spines: (0) absent; (1) short < W femur; (2) long
47. Forefemoral long hairs: (0) absent; (1) some; (2) dense
48. Foretibial spicules: (0) absent; (1) some; (2) dense
49. L foretarsus/ L foretibia: (0) ≥ 1; (1) ≥ 0.75; (2) > 0.5
50. Foretarsal microchaetae: (0) absent; (1) very few < 10; (2) present
51. Foretarsal microchaetae: (0) absent; (1) ends bulbous; (2) ends slightly bent; (3) ends distinctly bent
52. L foreclaw/ L midclaw: (0) < claw; (1) ≤ half; (2) ≤ third
53. Midfemoral spines: (0) absent; (1) short < W femur; (2) some long
54. Midfemoral long hairs: (0) absent; (1) some; (2) dense
55. Midtibial spicules: (0) absent; (1) some; (2) dense
56. L midpulvilli/ L claw: (0) ≥; (1) <; (2) ≤ half; (3) < 0.2
57. Midpulvilli: (0) large, flattened, membranous; (1) small rounded setose; (2) chisel-conical
58. Hindfemoral spines: (0) absent; (1) short < 0.5 W femur; (2) some long
59. Hindfemur long hairs: (0) absent; (1) some; (2) dense
60. Long hindtibial scales: (0) no long scales; (1) some long scales; (2) fluffy - protruding; (3) feathery; (4) very long, dense, feathered fringes
61. Hindtibial spicules: (0) absent; (1) some spicules; (2) apical patch; (3) dense spicules
62. Hindtibial spicules L: (0) absent; (1) same; (2) inner row longer
63. L hindpulvilli/L claw: (0) ≥; (1) <; (2) ≤ half; (3) ≤ 0.2
64. Hindpulvilli: (0) large, flattened, membranous; (1) small rounded setose; (2) chisel -conical
Wing65. Patagium: (0) absent hairs only; (1) present scales
66. Basicosta: (0) absent; (1) blunt; (2) sharp
Wing venation67. Crossvein forming an extra anterior apical submarginal cell: (0) absent; (1) extra apical submarginal cell
68. R2+3 join R4+5: (0) basal to r-m > L r-m; (1) at r-m
69. R2+3 rises from R4+5: (0) acutely; (1) at right angles
70. 2nd r-m crossvein: (0) absent; (1) present
71. Spurvein base R2+3: (0) absent; (1) bump or present
72. R2+3 apical loop: (0) long apical loop; (1) loop > 180°; (2) at least 90° bend; (3) absent
73. i-r1, R2+3 to R4: (0) absent; (1) present
74. i-r1 crossvein: (0) absent; (1) straight; (2) slightly sinuous; (3) distinctly sinuous
75. Spurvein i-r: (0) absent; (1) bump or present
76. L i-r1/L r-m: (0) absent; (1) ≤; (2) ≤ 2x; (3) < 3x; (4) ≥ 3x
77. Spur-vein base R4+5: (0) absent; (1) bump or present
78. Spur-vein R4: (0) absent; (1) bump or present
79. R5/R1 meet wing: (0) R5 distal to R1; (1) equal; (2) R5 basal to R1
80. i-r2, R4 to R5: (0) absent; (1) present
81. M1: (0) straight; (1) slightly sinuous; (2) sinuous
82. L m-m/r-m: (0) ≤ 2x; (1) ≤ 3x; (2) > 3x
83. m-m: (0) straight; (1) slightly sinuous; (2) sinuous
84. m-m spurvein: (0) absent; (1) into m2; (2) into discal; (3) crossvein form basal cell
85. m-m to hind wing margin: (0) oblique; (1) parallel; (2) horizontal
86. M2: (0) straight; (1) slightly sinuous; (2) sinuous
87. r5 open: (0) open; (1) narrow < r-m; (2) just closed; (3) closed and stalked - acute; (4) closed and stalked -obtuse
88. m1 open: (0) open; (1) closed and stalked
89. m2 open: (0) open; (1) closed and stalked - acute
90. L mcu/r-m: (0) > 3x; (1) < 3x; (2) ≤ 2x; (3) ≤
91. m-cu: (0) straight; (1) slightly sinuous; (2) 90° basally; (3) sinuous
92. Spur-vein m-cu: (0) absent; (1) spur into discal cell
93. Spur-vein into M2: (0) absent; (1) spur-vein into m2; (2) cross-vein to CuA1
94. W anal/ W posterior cubital: (0) ≤; (1) ≤ 1.5x; (2) ≤ 2x; (3) > 2x
95. cup open: (0) open; (1) narrow < r-m; (2) closed at wing margin (Fig. 9B); (3) closed and stalked - acute
96. Anal lobe margin: (0) hairs; (1) some scales; (2) scales dense
97. Anal cell: (0) broad rounded; (1) rounded; (2) thin linear; (3) very reduced
98. Alula reduced: (0) not reduced; (1) reduced, L < 4x W
99. Alula margin: (0) hairs; (1) some scales; (2) scales dense
100. Squamal margin: (0) hairs; (1) some scales; (2) scales dense
101. Squama reduced: (0) not reduced; (1) reduced, L < 4x W
102. wing L: (0) ≤ 10; (1) 10-15; (2) 16-20; (3) 21-25; (4) > 25
103. wing L/W: (0) ≤ 3x; (1) long > 3x
104. L wing/ L abdomen: (0) ≥ 3x; (1) ≥ 2 x; (2) > 1.5 x; (3) >
Abdomen105. Abdomen apically: (0) rounded; (1) narrowed; (2) truncate, parallel sided
106. Abdomen L/ W T2: (0) > 2.5x; (1) < 2.5x; (2) < 2x; (3) ≤ 1.5x; (4) ≤
107. Abdominal vestiture reflective: (0) bright; (1) very dull dark; (2) not reflective
108. Abdominal bristles/hair: (0) dorsally and laterally; (1) lateral and apically T7; (2) apically; (3) absent
109. Long lateral hairs > T1: (0) dense tufts; (1) some; (2) absent
110. Ma T1: (0) pointed; (1) midstyle; (2) pectinate
111. Scales: (0) absent; (1) adpressed scales; (2) upstanding scales; (3) long upstanding scale tufts
Male genitalia112. Male genitalia twisted: (0) no twisting, gonocoxae ventral; (1) 90°; (2) 180°, gonocoxae dorsal
113. E setae grouping: (0) not grouped; (1) medioapically; (2) lateroapically; (3) laterally
114. E setae group: (0) not grouped; (1) loose; (2) strong; (3) dense tufts
115. Epandrial spines: (0) absent; (1) short and broad; (2) long
116. E apically: (0) deeply indented; (1) concave-indented; (2) truncate; (3) convex rounded; (4) convex pointed
117. E apical flange: (0) absent; (1) < quarter base; (2) < third base; (3) < half base; (4) > half rest base
118. E medial flange: (0) absent; (1) slight; (2) distinct < quarter base
119. L E basal flange: (0) absent; (1) < quarter base; (2) < third base (Fig. 10B); (3) < half base; (4) ≥ half rest base; (5) > base
120. Mid W/L basal flange: (0) absent; (1) < quarter length base; (2) < half length; (3) > half length (4) > length
121. E posterolateral flange: (0) absent; (1) < quarter length base
122. E basal flange recurved: (0) absent (Fig. 10B); (1) < quarter base; (2) < third base; (3) < half base; (4) > half rest base; (5) > base
123. E basally extended: (0) absent; (1) present
124. SES: (0) absent; (1) linear; (2) triangular; (3) quadrate; (4) single (Fig. 10A)
125. L SES/G W: (0) absent; (1) < eighth; (2) < quarter; (3) > quarter
126. G setae: (0) some; (1) dense; (2) tufts
127. G setae group: (0) absent; (1) not grouped; (2) apically; (3) medially; (4) laterally; (5) basally
128. Thick G setae number: (0) absent; (1) no thick setae; (2) some; (3) many; (4) 6-8 long
129. Thick G setae position: (0) absent; (1) no thick setae; (2) apically; (3) medially; (4) laterally; (5) basally
130. G subapical indentation: (0) absent; (1) slight < third; (2) narrowed apically > third
131. W G medial indentation: (0) absent; (1) < third; (2) > third; (3) > half
132. G medial weakness: (0) absent; (1) desclerotised line; (2) lines of weakness; (3) division medially
133. G ventral division: (0) line fusion basally; (1) line fusion entire; (2) fused medially; (3) fused basally; (4) fused
134. G medioventrally: (0) deeply indented; (1) indented medially; (2) flat; (3) convex shell
135. G ventral ridge: (0) absent; (1) slight; (2) distinct
136. G basal projection of the ventral ridge: (0) absent; (1) slight; (2) distinct; (3) recurved hook
137. G basomedial margin: (0) deeply indented; (1) indented, concave; (2) smooth, linear; (3) convex
138. H: (0) absent; (1) present
139. H laterally: (0) absent; (1) indented between G, smooth; (2) projecting; (3) with spur; (4) with finger (Fig. 10F)
140. RM: (0) small; (1) > L GS; (2) large recurved
141. L G A: (0) absent; (1) < GS; (2) > GS
142. G plates dorsoapically: (0) medially parallel; (1) angled basomedial plates diverge dorsally
143. G dorsoapical plates extension apically: (0) not extended apically; (1) small apical extension; (2) long apical extension beyond the base of the gonostyli
144. GS: (0) large base, long pointed flange; (1) laterally bifid; (2) simple curved hook; (3) medial hook; (4) lateral hook
145. GS basal projection: (0) absent; (1) small; (2) < GS
146. L AE: (0) < GS; (1) = GS; (2) > GS
147. AE EP separate: (0) absent; (1) present
148. EP: (0) absent; (1) present
149. EP deep ventral notch: (0) no EP; (1) absent; (2) medial
150. EP expanded apically: (0) no EP; (1) not expanded (Fig. 10H); (2) ≤ 2x neck; (3) < 3x neck; (4) > 3 × neck
151. EP apical plate: (0) no EP; (1) absent; (2) apical plate
152. EP medioventral projection: (0) no EP; (1) absent; (2) above AE
153. EP lateroapical lobes: (0) no E; (1) absent; (2) rounded dorsally; (3) pointed dorsally
154. EP lateral projection laterally: (0) no EP; (1) absent (2) lateral
155. EP medial projection laterally: (0) no EP; (1) absent; (2) medial
156. EP pair ventral projections: (0) no EP; (1) absent; (2) medial below AE
157. L EP: (0) no EP; (1) < GS base; (2) > GS base; (3) > G margin
158. EP recurved apicomedial projection: (0) no EP; (1) absent; (2) present dorsally
159. EP recurved apically: (0) no E; (1) absent; (2) apex recurved lateral view
160. EP apical setae: (0) no EP; (1) absent; (2) present
161. BP expanded: (0) not expanded; (1) round; (2) swollen spherical; (3) bilobed
162. LAEA: (0) spoon convex up; (1) spoon concave; (2) linear; (3) absent
163. L lateral AE A: (0) < L GS; (1) < G margin; (2) = G margin; (3) absent
164. AAES: (0) spoon convex; (1) spoon concave; (2) narrow wedge; (3) linear
165. AAES: (0) < B; (1) = L GS; (2) < G margin; (3) to G margin
166. EJA: (0) racquet - round; (1) linear
167. L EJA: (0) within G; (1) = G; (2) > G < L GS; (3) > G > L GS
Female genitalia168. AC spines: (0) 3 prs; (1) 4 prs; (2) 5 prs; (3) > 5 prs; (4) > 10 prs
169. AC spines apically: (0) thin tapering; (1) broader spoon shaped
170. T9+10 sclerites: (0) 1 sclerite; (1) 3 sclerites
171. T9 dorsal medioapical unsclerotised lacuna: (0) absent; (1) present
172. T8 dorsal medioapical unsclerotised lacuna: (0) absent; (1) present
173. T8 hair: (0) apical half; (1) apical half bare medially; (2) apical edge
174. T8 laterally: (0) not indented; (1) indented arms
175. T8 A divided medially: (0) not divided; (1) slightly; (2) distinctly ≥ half width
176. T8 A lateral projections: (0) absent; (1) slight; (2) not linear; (3) linear
177. T8 A L/medial W: (0) margin thickened, sclerotised; (1) ≤ quarter; (2) ≤ half; (3) <; (4) ≥; (5) > 2x; (6) ≥ 3x
178. T8 A internal structure: (0) absent; (1) linear; (2) quadrate plate
179. S8 sclerites: (0) 1 linear 2 round; (1) 1 linear 2 round 1 medial; (2) U-shaped 2 triangular; (3) 2 round; (4) sheet
180. Furca: (0) U-shaped; (1) 3 separate sclerites; (2) 2 rods
181. ST tube: (0) short < third L pump; (1) present; (2) long > × 8 L pump
182. Basal endplate: (0) absent; (1) small; (2) present (Fig. 9H); (3) large
183. Basal endplate: (0) absent; (1) simple-thin processes; (2) thick processes; (3) funnel
184. Sperm pump: (0) short pump; (1) very long pump
185. Long pump papillae: (0) no long papillae; (1) unpigmented; (2) pigmented
186. Pump processes basally: (0) no processes; (1) short processes; (2) long papillae
187. Pump processes medially: (0) no processes; (1) short processes; (2) long papillae
188. Pump processes apically: (0) no processes; (1) short processes
189. Apical endplate: (0) large; (1) present; (2) small; (3) absent
190. Apical endplate: (0) absent; (1) simple- thin processes; (2) thick processes; (3) funnel; (4) double
191. SR: (0) L ≤ W; (1) L > W; (2) L > 2 W; (3) L > 4 W; (4) L > 6 W; (5) L > 8 W; (6) L > 30 W
192. ST basal sclerotised plate: (0) absent; (1) basal sclerotised plate
193. Tube ST to pump: (0) absent; (1) present; (2) long
194. SR shape: (0) round square; (1) oval; (2) pear, expanded apically; (3) long; (4) expanded basally
195. SR apically: (0) rounded blunt; (1) nipple; (2) narrowed; (3) knob
196. ST round basal bulb: (0) no round BB; (1) round BB
197. SR pigmented: (0) unpigmented; (1) pigmented; (2) basally unpigmented
198. ST long medial tube: (0) no medial tube; (1) tube medially
199. Long membranous base: (0) no long base; (1) long MB
200. ST long MB basally swollen: (0) no long base; (1) symmetrically; (2) asymmetrically
201. ST clear rings: (0) no rings; (1) clear ring; (2) long striated collar
202. SR to pump: (0) symmetrical; (1) asymmetrical
203. SR medially bent: (0) absent; (1) bent reservoir
204. SR apically bent: (0) absent; (1) tip only
205. Tubules: (0) absent; (1) present
206. SR walls: (0) thin unsclerotised; (1) thick sclerotised
207. SR walls: (0) no dimples; (1) with dimples thin unsclerotised
Appendix 2Matrix of 207 characters for the Australian Balaana genus-group. (http://www.pensoft.net/J_FILES/1/articles/1881/1881-SP-2-editor.htm) File format: HTML
Appendix 3
Matrix of 207 characters for the Australian Balaana genus-group. (doi: 10.3897/zookeys.150.2002.app) File format: NEXUS matrix file.
Explanation note: This NEXUS phylogenetic matrix of 207 morphological characters for 42 Australian exoprospine beeflies was created in Mesquite v2.74. The file also contains the most parsimonious tree 5 file from the PAUP* maximum parsimony phylogenetic analysis. The data is also deposited in the Dryad Repository: doi: 10.5061/dryad.5j64k and in the TREEBASE Repository at http://purl.org/phylo/treebase/phylows/study/TB2:S12050