(C) 2011 Sarah Faulwetter. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Examination of polychaete specimens from Haifa Bay (Israel, eastern Mediterranean Sea) revealed several individuals exhibiting morphological characteristics similar to Sphaerosyllis hystrix Claparède, 1863. A detailed morphometrical analysis of the Israeli specimens in comparison to specimens of Sphaerosyllis hystrix and Sphaerosyllis boeroi Musco, Çinar & Giangrande, 2005 supported the description of the former as a new species, Sphaerosyllis levantina sp. n. Individuals of Sphaerosyllis hystrix formed a very heterogeneous group with strong character variations in the analysis and the presumed cosmopolitan distribution of the species is discussed based on literature records.
Polychaetes, Syllidae, Exogoninae, Sphaerosyllis, new species, Mediterranean, Cybertaxonomy, Scratchpads
The polychaete genus Sphaerosyllis Claparède, 1863 (Annelida) is one of the most species-rich genera of the syllid subfamily Exogoninae. At present, ca. 48 species are considered valid within Sphaerosyllis after the recent split of the group into the three genera Sphaerosyllis, Prosphaerosyllis and Erinaceusyllis (
Specimens were collected on 11 Oct. 2009 in Haifa Bay, (Israel, Eastern Mediterranean Sea) from fine to medium sands in shallow waters (10 m). Sediment samples were taken with a Van-Veen grab (KAHLSICO, model WA265/SS214) 32×35 cm, volume 20 l, penetration 20 cm. The sediment was preserved in buffered formalin 10% for 3–7 days, then sieved through a 250 µm mesh sieve and subsequently stored in 70% ethanol. Specimens were examined under an Olympus SZx12 stereomicroscope and an Olympus BX50 microscope. Illustrations in pencil were made by means of a drawing tube, subsequently scanned, imported into a graphic program (GIMP), re-drawn and saved as a vector graphic. Three specimens selected for obtaining Scanning Electron Microscope (SEM) images were dehydrated, critical point dried (Bal-Tec CPD 030), sputter-coated with gold (Bal-Tec SCD 050) and examined under a JEOL JSM-6390LV at the Department of Biology, University of Crete. Specimens are deposited in the invertebrate collection of the Smithsonian National Museum of Natural History, Washington D.C., USA (USNM) and in the Tel Aviv University Zoological Museum, Israel (TAU).
Morphometric analysesA total of 30 individuals belonging to three species (Sphaerosyllis boeroi: 3 individuals; Sphaerosyllis hystrix: 21 individuals; Sphaerosyllis levantina sp. n.: 6 individuals) were analysed. Twenty-five variables were measured: I. body length, to account for size-dependencies of other characters; II. number of chaetigers; III. length of blade of dorsalmost falciger of a) anterior, b) midbody, c) posterior chaetigers; IV. length of blade of ventralmost falciger of a) anterior, b) midbody, c) posterior chaetigers; V. ratio of length of blades of dorsalmost to ventralmost falciger in a) anterior, b) midbody, c) posterior chaetigers; VI. ratio of length of blades of falcigers in anterior to posterior chaetigers for a) dorsalmost; b) ventralmost falciger; VII. Ratio of length of dorsalmost falciger to body length in in a) anterior, b) midbody, c) posterior chaetigers; VIII. Ratio of length of ventralmost falciger to body length in in a) anterior, b) midbody, c) posterior chaetigers; IX. maximum length of serration of falcigerous blades in a) anterior, b) midbody, c) posterior chaetigers (smooth, finely serrated, strongly serrated); X. presence of a subdistal spine in dorsalmost falcigerous blades of in a) anterior, b) midbody, c) posterior chaetigers.
Body length was measured excluding antennae, anal cirri and palps. Falciger blade lengths were measured from point of insertion into shaft to distal tip. Falciger blade lengths could not always be measured on the same chaetiger in all animals if blades were broken. Instead, measurements were made in predefined body regions (anterior: first 1–5 chaetigers; posterior: last 5–7 chaetigers; midbody: in between). Three individuals of Sphaerosyllis hystrix were excluded from the multivariate statistical analysis due to missing values for some characters.
Summary statistics (mean, minimum, maximum, standard deviation, coefficient of variation and range of values) were calculated for each species (measurements and calculations available in online supplementary material:
http://polychaetes.marbigen.org/content/measured-values-sphaerosyllis-specimens
http://polychaetes.marbigen.org/content/summary-statistics-sphaerosyllis-hystrix
http://polychaetes.marbigen.org/content/summary-statistics-sphaerosyllis-boeroi
http://polychaetes.marbigen.org/content/summary-statistics-sphaerosyllis-levantina)
To take the different data types (numerical, categorical, binary) into account, Gower’s similarity coefficient (
Multivariate statistical analyses were performed with PRIMER V6, correlation of the Principal Component Scores were calculated with the R package (R package version 2.10; http://www.R-project.org).
Electronic publicationThe description of the new taxon was prepared in a Virtual Research Environment (Scratchpads) allowing for rapid and simultaneous publication of the results in print as well as electronically in a semantically enhanced form (
urn:lsid:zoobank.org:act:9CEE8F90-9596-49F6-AA22-BB79C0E816D9
http://species-id.net/wiki/Sphaerosyllis_levantina
Figures 1–4Holotype (USNM 1160540) ALA-IL-7, Haifa Bay, 10.5 m depth. Label: “Sphaerosyllis levantina, Haifa Bay, coll. B. Galil 11.10.09 [Holotype]”. Paratypes USNM 1160541–1160573: 33 individuals, TAU-AN 25006: 10 individuals; Haifa Bay, Israel, Eastern Mediterranean Sea, Station ALA-IL-7, coll. 11.10.2009, depth 10.5 m; Labels: “Sphaerosyllis levantina, Haifa Bay, coll. B. Galil 11.10.09 [Paratype X]” (where X=1–43). All material preserved in 96% Ethanol.
. Sphaerosyllis boeroi Musco, Çinar, and Giangrande, 2005 (Southern Evoikos Gulf, Aegean Sea, Greece: 3 specimens [Label: Tribe Sphaerosyllis]). Sphaerosyllis hystrix (Southern Evoikos Gulf, Aegean Sea, Greece: 1 specimen [Label: Tribe Sphaerosyllis]; Northern Evoikos Gulf, Aegean Sea, Greece: 7 specimens [Label: DI9a 7.3.91 Sphaerosyllis hystrix, checked S.Martín], all deposited the in Hellenic Centre for Marine Research, Anavyssos, Greece; Chalkida, Aegean Sea, Greece: 1 specimen [Label: 56 – Sphaerosyllis hystrix, κατώτερη µεσοπαραλιακή Χαλκίδας, Στενά Ευρίπου, Ξενοδοχείο Λούσι, St. 18, 25.9.97 0-0.5m, Άτοµα: 1, Διδακτορικού Mίλτου] (= lower intertidal zone, Chalkida, Eviros Straight, Hotel Lousi, coll. M.S. Kitsos), Chalkida, Aegean Sea, Greece: 1 specimen [Label: 26 – Sphaerosyllis hystrix, κατώτερη µεσοπαραλιακή Χαλκίδας, Στενά Ευρίπου, Ξενοδοχείο Παλίρροια, St. 1a, 24.9.97 0-0.5m, Άτοµα: 1, Διδακτορικού Mίλτου] (= lower intertidal zone, Chalkida, Eviros Straight, Hotel Palirroia, coll. M.S. Kitsos), Chalkida, Aegean Sea, Greece: 6 specimens [Label: 33 – Sphaerosyllis hystrix, κατώτερη µεσοπαραλιακή Χαλκίδας, Στενά Ευρίπου, Ξενοδοχείο Παλίρροια, St. 1α, 24.9.97 0-0.5m, Άτοµα: 6, Διδακτορικού Mίλτου] (= lower intertidal zone, Chalkida, Eviros Straight, Hotel Palirroia, coll. M.S. Kitsos), Chalkida, Aegean Sea, Greece: 4 specimens [Label: 80 – Sphaerosyllis hystrix, κατώτερη µεσοπαραλιακή Χαλκίδας, Στενά Ευρίπου, Ξενοδοχείο Παλίρροια, St. 1α, 24.9.97 0-0.5m, Άτοµα: 6, Διδακτορικού Mίλτου] (= lower intertidal zone, Chalkida, Eviros Straight, Hotel Palirroia, coll. M.S. Kitsos), Thessaloniki, Aegean Sea, Greece, 1 specimen [Label: 66 – Sphaerosyllis hystrix, κατώτερη µεσοπαραλιακή Λιµάνι Θεσσαλονίκης, 2γ, 6.10.97 0-0.5m, Άτοµα: 1, Διδακτορικού Mίλτου]) (= lower intertidal zone, Port of Thessaloniki, coll. M.S. Kitsos), all deposited the in Zoological Museum of the Aristotle University of Thessaloniki, Greece.
Eastern Mediterranean Sea, Levantine Basin, Israel, Haifa Bay (32°54.533N, 35°04.071E).
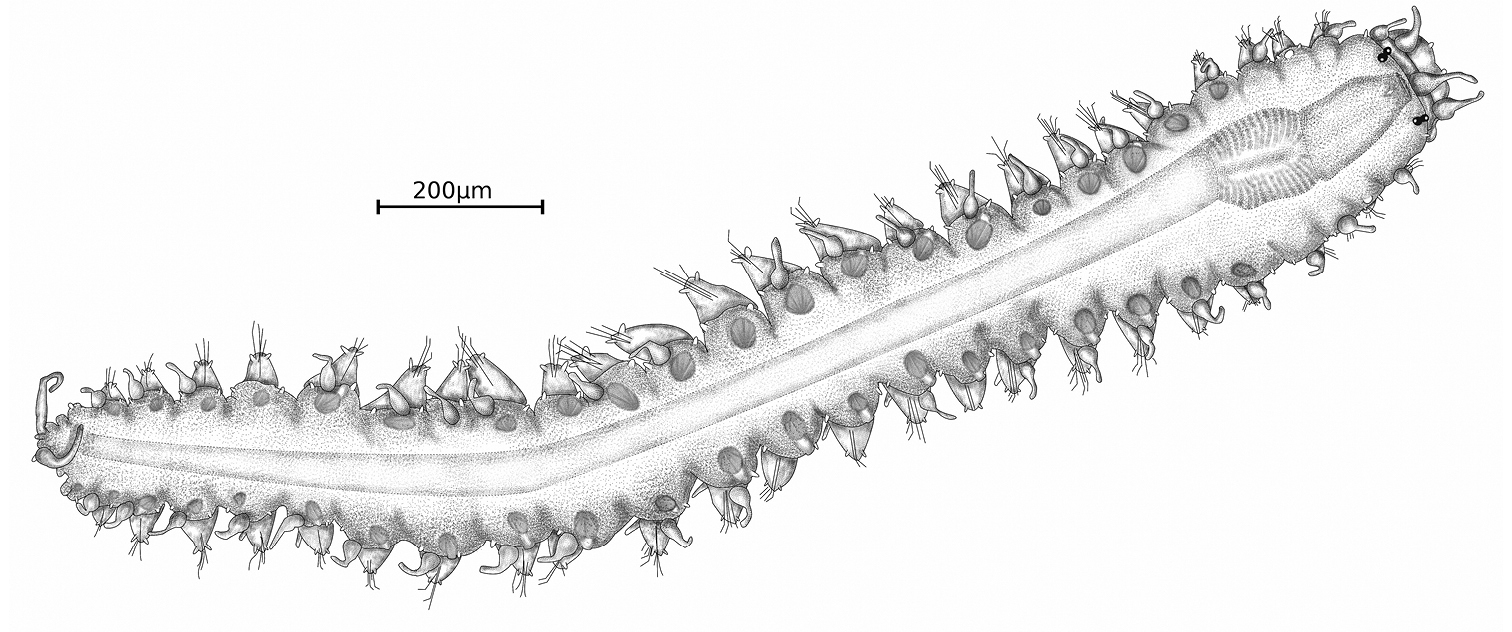
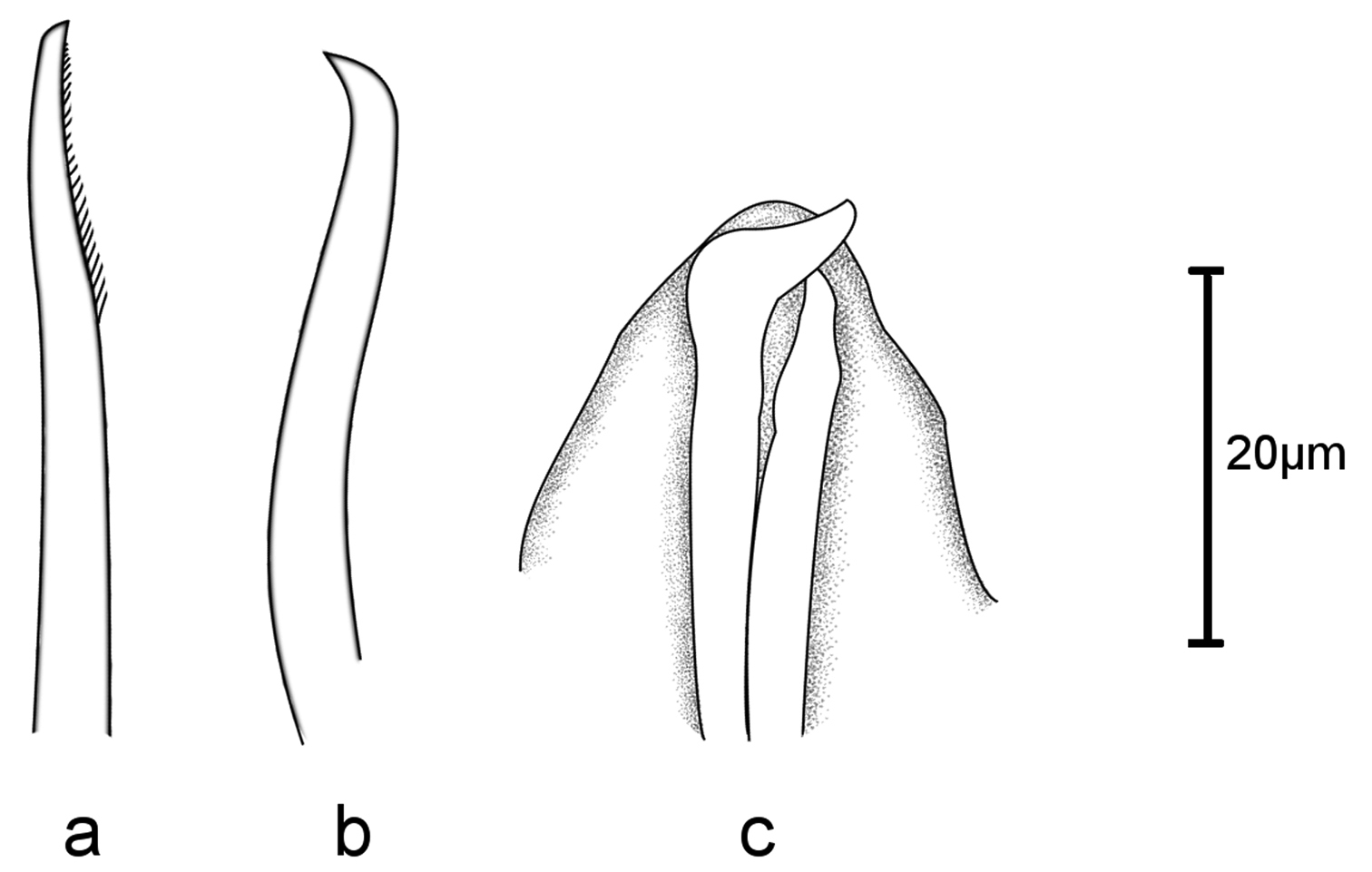
Holotype, entire animal, with 25 chaetigers, length 1.9 mm with palps but without anal cirri; width at sixth chaetiger 250 µm without parapodia, 300 µm with parapodia. Body small, slender, widest at level of proventricle (Fig. 1). Dorsal papillation on anterior chaetigers irregular, after proventricle in four longitudinal rows: two mid-dorsal rows with two papillae per segment, lateral rows with three papillae near dorsal cirri (Fig. 2a). Ventrum without visible papillation. Prostomium wider than long with 4 coalescent lensed eyes in trapezoidal arrangement. Anterior eyespots absent. Antennae pyriform with bulbous bases and elongated tips, median antenna 40 µm long, lateral ones 33 µm, longer than prostomium and palps together. Median antenna inserted between anterior pair of eyes, lateral ones attached on anterior margin of prostomium (Fig. 1). Palps directed ventrally, fused along their length, with a dorsal notch and few small papillae. Peristomium indistinct, dorsal fold partly covering prostomium. One pair of tentacular cirri, shaped like antennae but shorter (23 µm). Second chaetiger without dorsal cirri but with large papilla instead. Dorsal cirri similar in shape and length to tentacular cirri, anteriorly as long as parapodial lobes (23 µm), posteriorly slightly longer (28 µm). Ventral cirri conical, half as long as parapodial lobe, originating at bases of parapodia. Parapodial lobes triangular, with small papilla on each side of distal end. Parapodial glands with fibrillar material and with conical opening; from fourth chaetiger. Anterior parapodia with 4–5, rarely with 6 falcigers per fascicle; blades slender, unidentate with small subdistal spine and strong serration on 1–2 dorsalmost falcigers (Figs 2b–d, 3a). Dorso-ventral gradation in length of blades, dorsal ones maximally 14 µm, ventral ones 10 µm. Posteriorly, dorsal blades of similar length (13 µm), but stouter and more curved with robust subdistal spine and strong serration as long as subdistal spine (Figs 2e, f, 3b, c). Dorsalmost falciger posteriorly thicker than remaining ones in fascicle. Blades of ventral falcigers similar throughout body (Fig. 2g). All shafts with fine serration (Fig. 2c). Dorsal simple chaeta from chaetiger 1, subdistally serrated (Figs 2h, 4a). Ventral simple chaeta on posterior chaetigers, sigmoid, smooth (Fig. 4b). Anteriorly two aciculae per parapodium, one distally bent at right angle, acuminate tip curved upwards, the other straight and blunt (Fig. 4c); posteriorly only one acicula of the former type per parapodium. Pharynx occupying three chaetigers. Width more than ¾ of width of proventricle. Pharyngeal tooth located on anterior margin, surrounded by a crown of soft papillae. Proventricle in chaetigers 3–4 (120 µm long) with 15–17 muscle cell rows. Pygidium papillated, with two cirriform anal cirri twice as long as dorsal cirri (60 µm) (Fig. 1).
Sphaerosyllis levantina sp. n. holotype, dorsal view
Sphaerosyllis levantina sp. n. SEM images of a anterior end and midbody, dorsal view b–c compound chaetae, anterior chaetigers d dorsalmost compound chaetae, anterior chaetiger e compound and dorsal simple chaetae, midbody f dorsalmost compound chaeta, posterior chaetiger g ventralmost compound chaetae, posterior chaetiger h dorsal simple chaeta
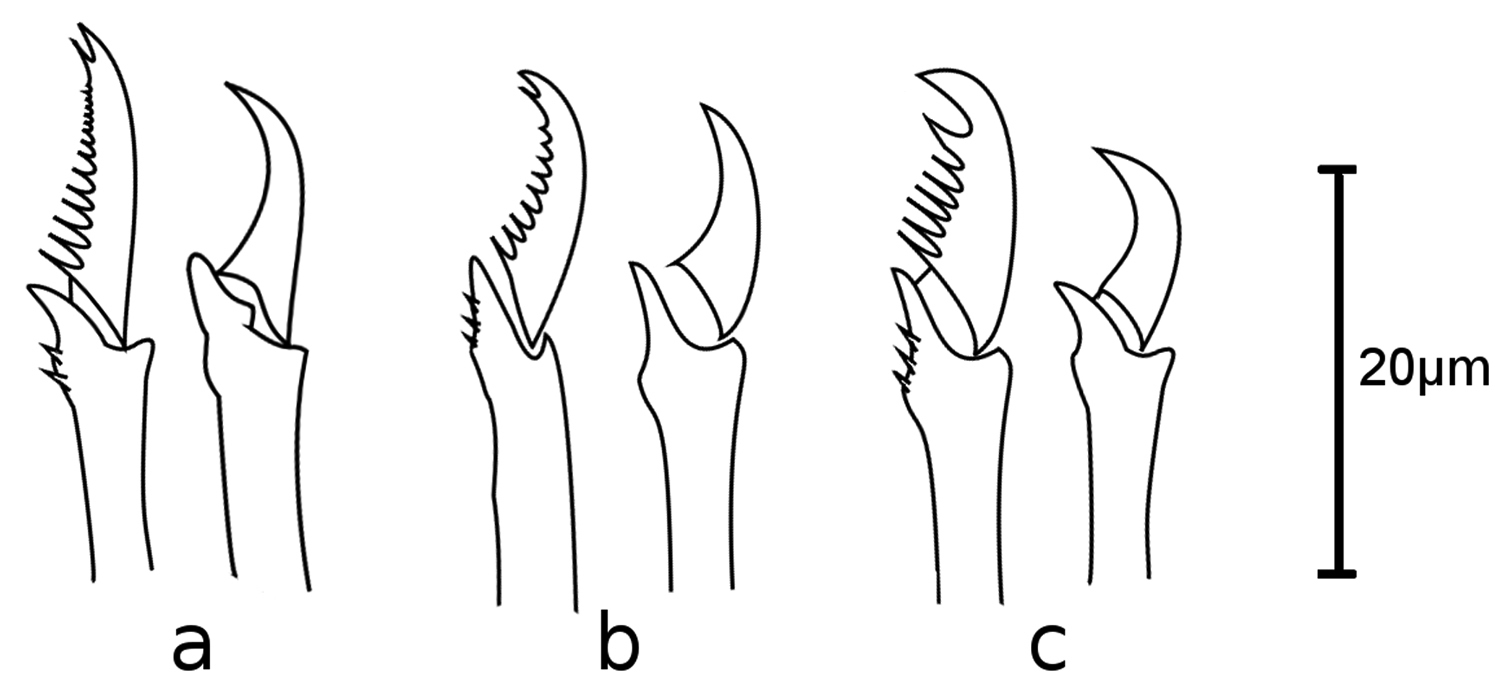
Sphaerosyllis levantina sp. n. Dorsal (left) and ventral (right) falciger of a anterior b midbody c posterior chaetiger
Sphaerosyllis levantina sp. n. a dorsal b ventral simple chaeta c aciculae, anterior chaetiger
Derived from the type locality (Levantine Basin), levantina being a neo-Latin adjective meaning “pertaining to the region where the sun raises”; feminine declination in accordance with the genus name (Syllis was a river nymph in the greek mythology and thus female).
Israeli Coast (Levantine Basin, Eastern Mediterranean Sea).
Fine to medium sands.
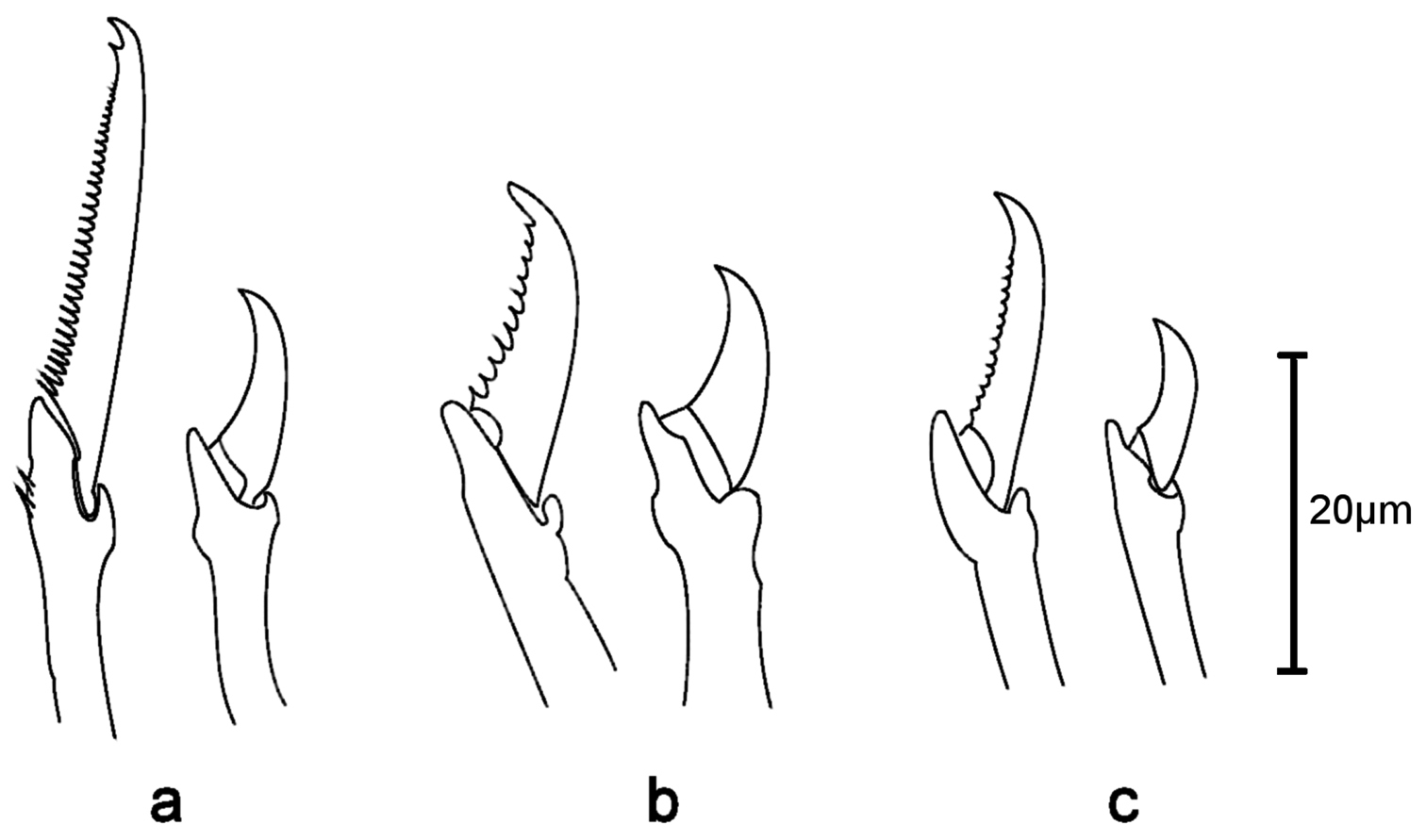
Sphaerosyllis levantina sp. n. is similar to Sphaerosyllis minima Hartmann-Schröder, 1960 in having blades of falcigers with strong serration throughout the body. However, Sphaerosyllis minima has a stronger dorso-ventral gradation of the blades of falcigers (dorsal ones twice as long as ventral ones) than Sphaerosyllis levantina sp. n. (dorsal ones 1.5 times longer than ventral ones) and the ventral cirrus is longer than the parapodial lobe in Sphaerosyllis minima, whereas is is half as long as the parapodial lobe in Sphaerosyllis levantina sp. n. Sphaerosyllis capensis Day, 1953, Sphaerosyllis taylori Perkins, 1981, and Sphaerosyllis sandrae Álvarez and San Martín, 2009 are similar to Sphaerosyllis levantina sp. n. in the shape and serration of the blades of the falcigers, but Sphaerosyllis capensis has all antennae positioned in line (median one posteriorly of lateral ones in Sphaerosyllis levantina sp. n.), Sphaerosyllis taylori shows no dorso-ventral gradation of the falciger blade length (dorsal blade 1.5 times longer than ventral one in Sphaerosyllis levantina sp. n.) and Sphaerosyllis sandrae has smooth falcigerous blades posteriorly and parapodial glands with hyaline material (strongly serrated blades throughout the body and parapodial glands with fibrillar material in Sphaerosyllis levantina sp. n.). All the above species differ from Sphaerosyllis levantina sp. n. by lacking a subdistal spine on the blades of the falcigers. The only Sphaerosyllis species known to possess this spine are Sphaerosyllis hystrix Claparède, 1863, Sphaerosyllis parabulbosa San Martín and López, 2002 and Sphaerosyllis boeroi Musco, Çinar & Giangrande, 2005. Sphaerosyllis parabulbosa clearly differs from Sphaerosyllis levantina sp. n. by having minute dorsal cirri and antennae, by the presence of a subdistal spine only on blades of the posterior falcigers and by smooth blades of posterior falcigers. Sphaerosyllis boeroi differs from Sphaerosyllis levantina sp. n. in having much longer blades of the falcigers which show a more pronounced dorso-ventral gradation (dorsal blades 2.6 times longer than ventral ones in Sphaerosyllis boeroi, 1.5 times longer in Sphaerosyllis levantina sp. n.) than those of Sphaerosyllis levantina sp. n. (Figs 3, 5, see also tables in online supplementary material), by having a subdistal spine on blades of all falcigers (only on dorsalmost ones in Sphaerosyllis levantina sp. n.) and by the dorsalmost falcigers being serrated only proximally. Sphaerosyllis hystrix, according to the literature, has a subdistal spine only on the blades of the anterior dorsalmost falcigers. However, in the examined material of Sphaerosyllis hystrix from the Aegean Sea 8 out of 21 specimens also possessed a subdistal spine in posterior falcigers. Sphaerosyllis hystrix can nevertheless be distinguished from Sphaerosyllis levantina sp. n. by having smooth or finely serrated posterior falcigers (serration less than half the length of the subdistal spine), even when the spine is present (serration almost as long as subdistal spine in Sphaerosyllis levantina sp. n.) (Figs 2f, 3, 6). Furthermore, the blades of the dorsalmost falcigers show an anteroposterior gradation in length in Sphaerosyllis hystrix (anteriorly 1.5 times longer than posteriorly), whereas they are of similar length throughout the body in Sphaerosyllis levantina sp. n. (Figs 3, 6, see also tables in online supplementary material). Finally, Sphaerosyllis hystrix has a very narrow pharynx (almost half the width of proventricle), whereas the pharynx of Sphaerosyllis levantina sp. n. is wider than ¾ of the width of the proventricle. An identification key to the Mediterranean Sphaerosyllis species is provided at the end of this manuscript.
Sphaerosyllis boeroi. Dorsal (left) and ventral (right) falciger of a anterior b midbody c posterior chaetiger
Sphaerosyllis hystrix. Dorsal (left) and ventral (right) falciger of a anterior b midbody c posterior chaetiger
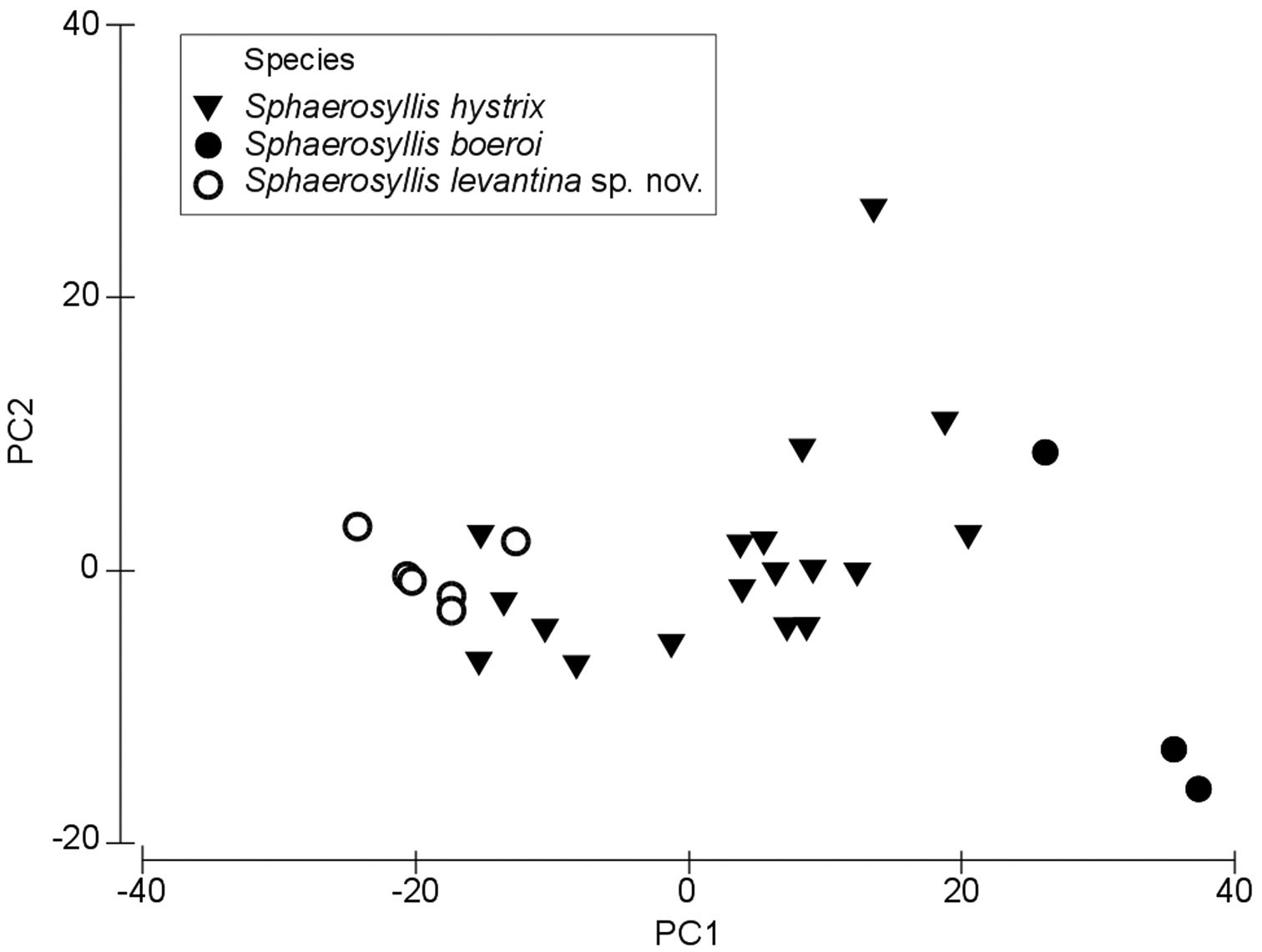
The results of the Principal Component Analysis show that the first principal component (PC1) account for 77.4% of the variability, the second (PC2) for 16.4% and the remaining 3 PCs for 5.1% (eigenvector values available at http://polychaetes.marbigen.org/content/morphometric-analysis-pca-eigenvectors). The Spearman’s correlation of the Principal Component scores with the measured character values of the individuals revealed that the length of the dorsalmost falcigerous blades in all body parts (anterior, midbody, posterior), as well as the ratio of the anterior to posterior ventralmost falcigerous blade are the most important characters discriminating between the three species (ρ-values >0.8 / <-0.8 at p < 0.005) (http://polychaetes.marbigen.org/content/spearmans-correlation-principal-component-scores-vs-measurements).
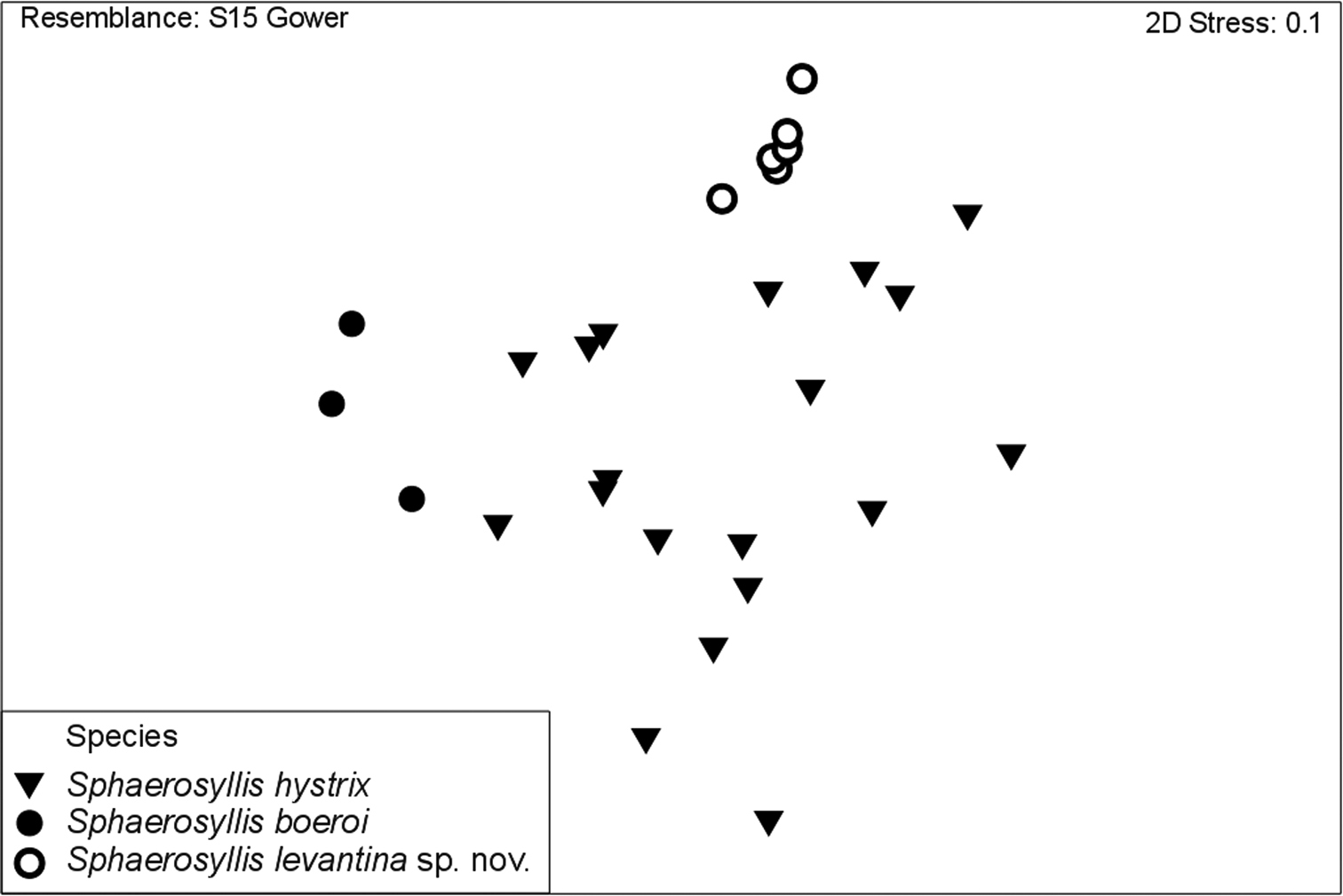
The PCA plot of the first two components show a discrimination of species into three groups, with individuals of Sphaerosyllis levantina sp. n. having the lowest PC1 scores, Sphaerosyllis boeroi the highest scores. Sphaerosyllis levantina sp. n. and Sphaerosyllis hystrix show similar PC2 scores, whereas Sphaerosyllis boeroi shows lower scores, and, except for one small-sized individual, forms a distinct group apart from the remaining species. Individuals of Sphaerosyllis levantina sp. n. likewise form a close group, however, a couple of individuals of Sphaerosyllis hystrix cannot be distinguished from this cluster (Fig. 7). The MDS diagram gives similar results, with individuals of Sphaerosyllis boeroi and Sphaerosyllis levantina sp. n. forming distinct groups, whereas individuals of Sphaerosyllis hystrix are spread as a heterogeneous group, with some of them being plotted close to individuals of either Sphaerosyllis boeroi or Sphaerosyllis levantina sp. n. (Fig. 8).
PCA plot.
MDS plot.
The PERMANOVA analysis results in a p-value of 0.001 as calculated by 999 permutations, thus the null-hypothesis (no differences between the groups) cannot be sustained. Subsequent analyses of the differences between species through pairwise tests reveals significant differences between species (Sphaerosyllis hystrix / Sphaerosyllis boeroi: p = 0.003, 713 permutations; Sphaerosyllis hystrix / Sphaerosyllis levantina sp. nov: p = 0.001, 995 permutations; Sphaerosyllis boeroi / Sphaerosyllis levantina sp. n.: p = 0.015, 84 permutations).
DiscussionThe genus Sphaerosyllis –like many of the small-sized Exogoninae genera– has a difficult and often confused taxonomy and biogeography. Among the potential causes contributing to the current confusion the following could be cited: a) lack of detail in older (before ca. 1970) species descriptions; b) difficulties of observing certain characters in fixed material (
The morphometric analysis conducted in this study support the hypothesis of several morphologically very similar species co-existing in the Mediterranean. The individuals of Sphaerosyllis levantina sp. n. and Sphaerosyllis boeroi form distinct groups in the PCA and MDS plots, however the individuals of Sphaerosyllis hystrix show a much wider spread, marginally overlapping with the other two species when only the meristic characters are taken into account. This is explained through a high character variability in the examined individuals, especially concerning the presence of a subdistal spine on the blades of the posterior falcigers and the length of the falciger blades. The presence of a subdistal spine on all dorsal falcigerous blades is invariable in Sphaerosyllis boeroi and Sphaerosyllis levantina sp. n., wheras individuals of Sphaerosyllis hystrix with otherwise very similar chaetal structures might or might not possess such spine. Another feature that seems to be highly variable in Sphaerosyllis hystrix is the length of the falciger blades in relation to body size. In fact, individuals of Sphaerosyllis levantina sp. n. with short falciger blades are located at the lower end of the size spectrum of all measured blades, Sphaerosyllis boeroi with almost spiniger-like blades at the higher end, whereas the blade lengths of the examined individuals of Sphaerosyllis hystrix form a smooth transition between the other two species.
However, when tested by strict statistical criteria, the hypothesis of different co-existing species is significantly supported, and based on their meristic characters the species show significant differences. The results of the current study suggest that Sphaerosyllis hystrix may well constitute a species complex. Given the difficult taxonomic status of the genus, similar results might be expected for other species as well, and consequently, distributions of several Sphaerosyllis species might be in fact questionable or unknown.
Key to the Mediterranean Sphaerosyllis species:The three species Sphaerosyllis claparedei Ehlers, 1864, Sphaerosyllis papillifera Naville, 1933 and Sphaerosyllis ovigera Langerhans, 1879 are poorly known. All have been described as having dorsal cirri on the second chaetiger, however, other species, such as Sphaerosyllis hystrix, were also originally described or illustrated with dorsal cirri on the second chaetiger whereas they are in fact absent. Since the three aforementioned species are exclusively known from their original description (or partly reproductions of these) and have never been re-described based on new material, they are tentatively included in the key below, but their identity remains questionable.
| 1 | Dorsal cirri on chaetiger 2 present | 2 |
| – | Dorsal cirri on chaetiger 2 absent | 4 |
| 2 | Papillae on dorsum absent | Sphaerosyllis claparedei Ehlers, 1864 |
| – | Papillae on dorsum present | 3 |
| 3 | Parapodial glands absent | Sphaerosyllis papillifera Naville, 1933 |
| – | Parapodial glands with fibrillar material | Sphaerosyllis ovigera Langerhans, 1879 |
| 4 | Parapodial glands present | 5 |
| – | Parapodial glands absent | 15 |
| 5 | Parapodial glands with fibrillar material | 6 |
| – | Parapodial glands with granular material | 12 |
| 6 | All antennae in line | Sphaerosyllis capensis Day, 1953 |
| – | Median antenna inserted more posteriorly than lateral ones | 7 |
| 7 | Dorsal cirri shorter than parapodial lobes, at least in anterior chaetigers | 8 |
| – | Dorsal cirri longer than parapodial lobes | 9 |
| 8 | Blades of falcigers strongly serrated, short (<10µm); shafts with strong spines | Sphaerosyllis thomasi San Martín, 1984 |
| – | Blades of falcigers with serration only anteriorly and dorsalmost; blades with slight dorso-ventral gradation but always longer than 10µm; shafts smooth | Sphaerosyllis parabulbosa San Martín and López, 2002 |
| 9 | Blades of falcigers without marked dorso-ventral gradation in length | Sphaerosyllis taylori San Martín, 1984 |
| – | Blades of dorsalmost falcigers at least 1.5 times the length of ventral ones | 10 |
| 10 | Blades of posterior dorsal compound falcigers smooth to finely serrated | Sphaerosyllis hystrix Claparède, 1863 |
| – | Blades of posterior dorsal compound falcigers strongly serrated (spinules of almost same length as the subdistal spine) | 11 |
| 11 | Blades of anterior dorsal compound falcigers at least twice as long as ventral ones; anteroposterior gradation of blade length; blades of both dorsal and ventral compound chaetae with a subdistal spine | Sphaerosyllis boeroi Musco, Çinar and Giangrande, 2005 |
| – | Blades of anterior dorsal compound falcigers less than twice as long as ventral ones; no anteroposterior gradation of blade length; blades of only dorsal compound chaetae with a subdistal spine | Sphaerosyllis levantina sp. n. |
| 12 | Blades of dorsalmost falcigers long (>30µm), at least twice as long as ventral ones | Sphaerosyllis magnidentata Perkins, 1981 |
| – | Blades of falcigers short (<15µm), with only slight dorso-ventral gradation | 13 |
| 13 | Dorsal cirri clearly longer than parapodial lobes | Sphaerosyllis sp. [San Martín, 2003] |
| – | Dorsal cirri as long as or shorter than parapodial lobe | 14 |
| 14 | Antennae bulbous with small tip, shorter than prostomium; dorsal simple chaetae smooth; anterior parapodia with two aciculae, one straight, one with tip bent at right angle | Sphaerosyllis sp. Del-Pilar-Ruso & San Martín, in press |
| – | Antennae pyriform, as long as prostomium, dorsal simple chaetae serrated, all parapodia with one acicula | Sphaerosyllis glandulata Perkins, 1981 |
| 15 | All aciculae straight | 16 |
| – | Tip of some aciculae bent at right angle | 17 |
| 16 | Dorsal cirri with conspicious papilla, giving cirri a bifid appearance | Sphaerosyllis gravinae Somaschini & San Martín, 1994 |
| – | Doral cirri without papilla | Sphaerosyllis bulbosa Southern, 1914 |
| 17 | All antennae in line | Sphaerosyllis austriaca Banse, 1959 |
| – | Median antenna inserted more posteriorly than lateral ones | 18 |
| 18 | Anterior parapodia with two aciculae, one straight, one with tip bent at right angle; pharyngeal glands on chaetiger 1 present | Sphaerosyllis pirifera Claparède, 1868 |
| – | All parapodia with one acicula only; pharyngeal glands on chaetiger 1 absent | Sphaerosyllis piriferopsis Perkins, 1981 |
The authors kindly acknowledge assistance from the following colleagues: Dr Nomiki Simboura (HCMR, Anavyssos) and Dr Miltiadis Kitsos (Aristotelian University of Thessaloniki) for loans of comparative material of Sphaerosyllis hystrix and Sphaerosyllis boeroi, Dr Guillermo San Martín (Universidad Autónoma de Madrid) for the provision of some of the literature resources, Mrs Alexandra Siakouli (University of Crete) for taking SEM pictures, Dr Vincent Smith, Simon Rycroft, Ben Scott (NHM, London) and Dr Lyubomir Penev (Pensoft Publishers, Bulgaria) for support with the electronic Scratchpads publication. The two reviewers (Dr Guillermo San Martín, Dr Luigi Musco) are thanked for their suggestions to improve the manuscript. Financial support was provided by the ViBRANT project (FP7, EU).
The Scratchpads version of this publication is available at:
http://polychaetes.marbigen.org/node/35
Character matrices for specimens used in the morphological analysis are available at:
http://polychaetes.marbigen.org/content/measured-values-sphaerosyllis-specimens
Summary statistics for the species are available at:
http://polychaetes.marbigen.org/content/summary-statistics-sphaerosyllis-hystrix
http://polychaetes.marbigen.org/content/summary-statistics-sphaerosyllis-boeroi
http://polychaetes.marbigen.org/content/summary-statistics-sphaerosyllis-levantina
Results of the morphometric analysis are available at:
http://polychaetes.marbigen.org/content/morphometric-analysis-pca-eigenvectors
Illustrations and graphs of the statistical analysis are available at:
http://polychaetes.marbigen.org/category/image-galleries/sphaerosyllis