(C) 2011 B. Christian Schmidt. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Phragmatobia Stephens is briefly reviewed and a diagnosis is provided. The South American species currently placed in Phragmatobia Stephens are revised to two new genera, Andesobia Schmidt and De Freina, gen. n., and Patagobia Schmidt and De Freina, gen. n. (subtribe Spilosomina). Both Andesobia and Patagobia exhibit adaptations to high altitude habitats, including micropterous females in Andesobia (Patagobia females are unknown) and diurnal flight of males. The adults, immature stages, and mating behaviour of Andesobia jelskii (Oberthür, 1881) are described. Males of Andesobia jelskii enter the female cocoon to mate, and the micropterous, flightless females remain in the cocoon following oviposition where newly hatched larvae feed initially on the female’s body.
Four species are included in Andesobia, Andesobia jelskii comb. n. (= Paracles imitatrix Rothschild, 1922, syn. n.), Andesobia flavata (Hampson, 1901), comb. n., Andesobia boliviana (Gaede, 1923), comb. n. (=Turuptiana flavescens Rothschild, 1933, syn. n.), and Andesobia sanguinea (Hampson, 1907), comb. n. Patagobia includes only Patagobia thursbyi (Rothschild, 1910), comb. n., and Patagobia thursbyi pluto Toulgoët is relegated to its synonymy. Patagobia shows affinities to Phaos Walker, 1855 of Australia, Metacrias Meyrick, 1886 of New Zealand, and Pseudophragmatobia Krüger, 2009 of South Africa, suggesting a common ancestry of circumantarctic origin. Phragmatobia karsholti Toulgoët, 1991 is transferred to Venedictoffia Toulgoët, comb. n., an unrelated genus that is removed from subtribe Arctiina and provisionally placed in the Phaegopterina. Phragmatobia oberthueri Rothschild, 1910, described from Tibet, is a synonym of Lachana alpherakii (Grum-Grzhimailo, 1891) [Erebidae: Lymantriinae], syn. n., comb. n.
Microptery, flightlessness, matrivory, biased sex ratio, Spilosomini, Spilosomina, Arctiidae, Neotropics, taxonomy, Lachana, Metacrias, Phaos, Pyrrharctia, Gondwana, circumantarctic
Although the Arctiinae are most diverse in the Neotropical realm with 55% of the approximately 11, 000 described species globally (
Here, we review the Andean species of Phragmatobia, and place most of these species in two new genera, Andesobia gen. n., and Patagobia gen. n. The life history of Andesobia jelskii (Oberthür, 1881), comb. n., is described, which shows several remarkable traits presumably in response to the high elevation environment they inhabit. Phragmatobia karsholti Toulgoët, 1991 is an unrelated species that is transferred to Venedictoffia Toulgoët, comb. n. Venedictoffia is neither in the Arctiina nor Spilosomina, and is provisionally transferred to the Phaegopterina.
Methods and materialsAdult genitalia were prepared following the methods of
Repository abbreviations are as follows:
AMNH American Museum of Natural History, New York
BMNH The Natural History Museum (formerly British Museum [Natural History]), London
CDFM Collection J. De Freina, Munich
CNC Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa
CPG Collection Pape, Grafenau, Germany
CSO Collection Speidel, Olching, Germany
CTN Collection Tannert, Nuernberg, Germany
NHMB Natural History Museum, Berlin
USNM National Museum of Natural History (formerly United States National
Museum), Washington, D.C.
ZSM Zoologische Staatssammlung, Munich
ZMUC Zoologisk Museum, Universitets Copenhagen, Copenhagen
Description of the immature stages and life history was based on four successive generations of laboratory rearings in 2009 and 2010 by JJD, obtained from live material from Peru, Junin region, Huicuash E of Tarma, 11°23'S, 75°53'W, 4100 m. All rearings were conducted indoors at ambient temperatures between 10° C and 23° C. Mortality was extremely low up to F3, but F4 larvae had higher mortality rates and females exhibited reduced fertility, both presumably symptoms of inbreeding. To obtain matings, newly emerged females were placed individually in wooden boxes screened at the top to allow air circulation. Copulation was achieved only under sunny conditions, probably because males are active only during warm, sunny periods under natural conditions. Larvae accepted both dandelion foliage (Taraxacum officinale L.) and grass (Poa sp.), with a preference for the latter.
We used the 658 bp ‘barcode’ region of the first subunit of the cytochrome oxidase (cox1) gene (
Phalaena fuliginosa Linnaeus, 1758 (by monotypy).
[Europe].
Phragmatobia includes five species distributed in the Palaearctic and Nearctic regions (including one Holarctic species, Phragmatobia fuliginosa (L.)), with the Neotropical species and one Asian species here transferred to other genera. As suggested by
Phragmatobia is a fairly homogeneous group characterized by the following combination of characters: male antennae simple; wings fully developed in both sexes, forewing transverse lines diffuse or absent; wing colours varying from pinkish red to dark vinaceous red with darkbrown to blackish markings. Microtymbal of metepisternum well developed (Phragmatobia fuliginosa) to obsolete (Phragmatobia assimilans). Male genitalia with apical process of valve finger-like and ovoid in cross section; clasper spade-like, oriented transverse to longitudinal axis of valve, originating from inner surface of valve and directed mesad (divided into a ventral and costal lobe in Phragmatobia fuliginosa); apex of aedeagus with spinose plates; paired, intersegmental coremata present between sternites 7–8. Females with ductus bursae heavily sclerotized, dorso-ventrally flattened, and nearly as wide as width of abdomen; corpus bursae globose, with two signa consisting of small flattened spicules; dorsal pheromone gland paired, each duct with 3–4 branches, the apices of which are rounded.
urn:lsid:zoobank.org:act:BA7ACA8C-9856-4B81-AE99-A344DCED0CBC
http://species-id.net/wiki/Andesobia
Figs 1–3, 5–10, 12–18Andesobia jelskii Oberthür, 1881
The name is feminine in gender, formed by combining the words Andes and –obia from the generic name Phragmatobia.
Andesobia is related to Patagobia, but is distinguished by the following combination of characters: eyes reduced and ellipsoid, 1.4–1.6 × as high as wide, gena with broader unscaled area laterally; posterior antennal rami 1.2–1.5 × and anterior rami 1.1–1.5 × longer than segment length (longest anterior and posterior rami 3 × as long as segment in Patagobia); 2nd labial segment short and stout, 1.1 × as long as wide, 2 × longer than apical segment; thoracic collar concolourous with dorsal thoracic vestiture (contrastingly paler ochre in Patagobia); thoracic vestiture sparse and shaggy, compared to dense and pilose vestiture in Patagobia;femur and tibia very stout, 3.0–3.5 × longer than wide compared to 4.5–5.6 × in Patagobia; metatibia of Andesobia with one pair of spurs, two pairs in Patagobia; medial line of forewing absent in Andesobia, present in Patagobia; postmedial line never double in Andesobia, often double in Patagobia; hindwing discal spot small and sharpor absent in Andesobia, diffuse and more elongate in Patagobia. Andesobia is endemic to the Puna grasslands of the high Andes of Peru and Bolivia.
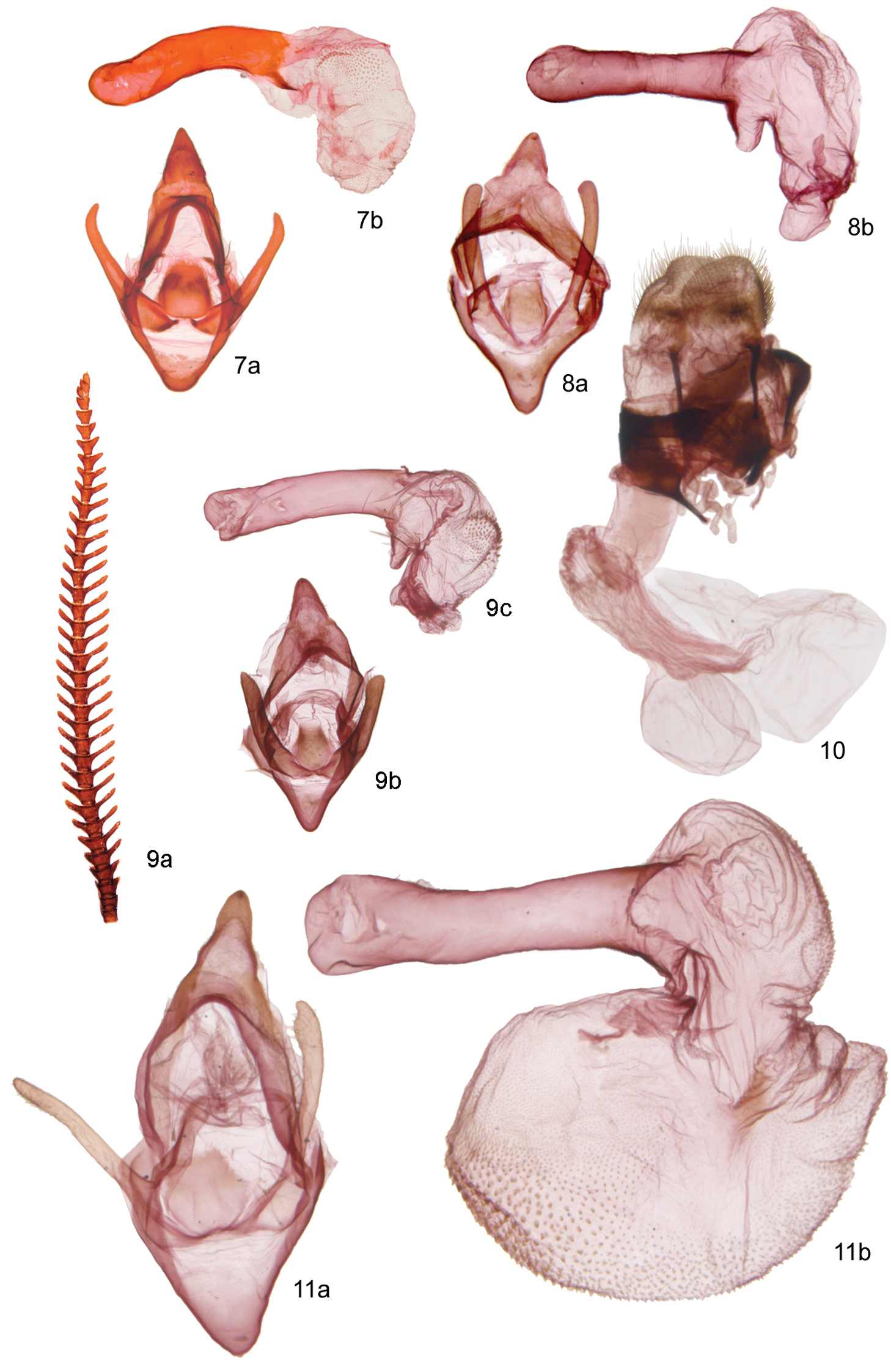
Male. Head – vestiture dark brown to black, shaggy appearance, setae long; antenna weakly bipectinate, ciliate ventrally; longest posterior rami 1.3–2.0 × segment length, longest anterior rami 1.1–1.8 × segment length; rami longest over middle third of antenna, decreasing in length toward base and apex; eye elliptical, 1.4–1.6 × as high as wide; labial palps short, not extending beyond vestiture of frons; 2nd labial segment short and stout, 1.1 × as long as wide, 2 × longer than apical segment; haustellum reduced and poorly sclerotized, presumably non-functional. Thorax – vestiture of vertex and ventrum of thorax black brown; tegulae and patagia black brown; legs black brown, dorsum of femur ochre or dull pinkish red, co-varying with hindwing and abdomen ground colour; apex of prothoracic tibia with two subequal, blunt, triangular projections; two meso- and metathoracic tibial spurs, posterior spur slightly longer than anterior, length of spurs approximately equal to tibial width at apex; metepisternum with rounded ridge along anterior margin, metepisternal microtymbals absent. Forewing – relatively small for an arctiine, forewing length 8–13 mm, elongate with apex less rounded than in Paracles and Spilosoma, length:width ratio averaging 2.2; ground colour ochre yellow, whitish to pinkish red or brownish grey; markings varying from obsoloete (Andesobia jelskii) to well defined, grey-brown transverse bands; when present, darker pattern consisting of dark-brown basal area, sub-basal band, discal spot, postmedial band and marginal band; bands occasionally confluent along anal margin; ventrally with bands obsolete except for marginal band, and with a brighter yellowish or reddish ground colour. Hindwing – ground colour slightly richer yellowish or reddish than forewing, with dark-brown to grey-brown marginal band, varying from nearly obsolete (reduced to intermittent diffuse spots extending from apex halfway to anal angle), to broad and diffuse over distal third of wing; brownish, crescentic discal spot small but usually well defined, sometimes absent; ventrally with dark markings less saturated. Abdomen – Segments A1–A3 entirely brownish black, remaining segments ochre or reddish subdorsally, with brownish-black dorsal line, widest in Andesobia flavata; ventrally, varying from entirely brownish black (Andesobia sanguinea) to black with narrow ochre border on distal margin of sternites (Andesobia flavata) or entirely ochre (Andesobia jelskii); coremata highly reduced to paired patches of sparse, deciduous setae. Genitalia – highly simplified overall with massive, triangular dorsoventrally flattened uncus characteristic of subtribe; uncus as long as width of base, broadly joined to wide, band-like tegumen; dorsal margin of tegumen caudally recurved; valve simple and digitate, lacking processes or claspers, 1–1.7 × as long as uncus-tegumen complex; vinculum semicircular, saccus v-shaped, similar in length to uncus; juxta evenly convex and hemispherical, dorsal margin slightly narrowed; aedeagus relatively large and stout, 3 × longer than wide, 1.5 × as long as width of genital capsule, curving dorsad 25–30°, proximal end approximately ⅓ narrower than apex; coecum 1/10 length of aedeagus, directed slightly ventrad; vesica directed dorso-distad, globose, finely spiculate, with small basal and poorly differentiated apical diverticulum. Female (Andesobia jelskii and Andesobia sanguinea only; female of Andesobia boliviana and Andesobia flavata unknown). Head – antennae 0.5 × as long as that of male, finely biserrate; proboscis atrophied; vestiture of closely appressed, ochre scales, lacking long, shaggy black scales present in males. Thorax – vestiture similar to that of head, notably lacking ‘shaggy’ appearance of males; legs reduced, 2/3 as long as those of male. Forewing and hindwing – micropterous and highly reduced, forewing 1.5–2.5 mm long, fully scaled and concoulours with dull tan colour of thorax, but without any discernible wing pattern. Abdomen – light ochre gray with fine, short velvety hairs, tergites well sclerotized, black, giving dorsum of abdomen appearance of a broad, black medial band; ventrally with narrower, lighter grayish-black medial band; integument broad and membranous laterally, allowing for distension caused by ova. Genitalia (based on Andesobia jelskii) – ostium and lamella antevaginalis membranous and poorly defined; lamella postvaginalis consisting of a broad, shallow sclerotized pouch; ductus bursae lightly sclerotized, dorsoventrally flattened, 2 × as long as wide; corpus bursae pear shaped, and relatively small, 2 × as longh as ductus bursae; diameter of distal, globose chamber 2 × width of ductus bursae; signum lacking; ductus seminalis wide and rugose, bulla seminalis large, diameter 1.5 × that of corpus bursae; posterior apophysis equal in length to papillae anales, anterior apophysis 0.6 × as long as papillae anales; each paired dorsal pheromone gland consisting of two tree-like subdivisions, each subdivision with 3–5 smaller diverticula.
Adult habitus of male Andesobia and Patagobia species 1 Andesobia jelskii 2 Andesobia boliviana 2b Andesobia boliviana (holotype of Estigmene boliviana Gaede) 3a, b Andesobia sanguinea 4a, b, c, d Patagobia thursbyi.
Adult habitus of female 5 Andesobia jelskii 6 Andesobia sanguinea.
Structurally, Andesobia is quite homogeneous, the main species-level differences occuring in the length and shape of the male valve and the vesica. The highly simplified, digitate male valve and massive uncus-tegumen compex is shared with several other Neotropical genera including Paracles, Patagobia, Caribarctia Ferguson and Leichosila Schmidt. The mtDNA barcode sequence (Andesobia jelskii) does not provide any additional resolution of relationships within this group, with minimum pairwise distances (uncorrected) between Andesobia, Paracles, Phragmatobia, Leichosila, Caribarctia and Phaos ranged from 6–8%. Sequences for Patagobia were not available.
Several Andean species are superficially similar to Andesobia and Patagobia, and require comment. Paracles herbuloti (Toulgoët), Paracles minuta Becker & Miller, and Paracles diminuta Becker & Miller are small species with a simple or highly reduced forewing pattern. Females of all three are unknown, but the structurally similar and probably congeneric Chilesia anguloi Ruiz, Chilesia rudis (Butler) and Chilesia watsoni Ruiz have micropterous females (
Data on the biology of Andesobia is based primarily on Andesobia jelskii and is discussed in more detail under the species account below. Andesobia is adapted to cold-temperate alpine habitats, males flying during sunny periods and the females being micropterous. Mating and oviposition occurs inside the female cocoon. The female-biased sex ratio of the broods reared during this study may indicate that females are capable of parthenogensis, as in some other cold-adapted flightless Lepidoptera (
http://species-id.net/wiki/Andesobia_jelskii
Figs 1, 5, 12–16, 18We examined over 150 specimens, obtained through four successive lab-reared generations originating from Peru, Junin region, Huicuash E of Tarma, 11°23'S, 75°53'W, 4100 m. Vouchers are deposited in CDFM, ZSM, CNC, CPG, CTN. Two specimens were included for DNA barcode analysis, voucher numbers CNC LEP 68032 (no GenBank accession number available) and CNC LEP 68033 (GenBank accession # HM416594) [CNC].
Andesobia jelskii was omitted from the catalogue of Neotropical Arctiinae (Watson and Goodger 1982), probably because
A detailed morphological description is given in the generic account of Andesobia, and the following description addresses characters specific to Andesobia jelskii. Male. Head – antenna (Fig. 9a) with posterior rami 1.6–1.9 × segment length, longest anterior rami 1.4–1.8 × segment length; eye elliptical, 1.4–1.6 × as high as wide. Thorax –vestiture and legs black brown, dorsum of femur ochre. Forewing – forewing length average 11 mm, range 8–12 mm; ground colour brownish grey with yellowish-ochre costal band varying to entirely dark brownish grey or plae ochre grey (type of luteola), indistinct black discal spot, other markings obsolete ventrally with paler yellowish ochre ground colour. Hindwing – ground colour yellowish ochre with broad, diffusely bordered grey-brown marginal band over distal third of wing, varying to entirely dark brownish grey; brownish, crescentic discal spot small but well defined; ventrally with dark markings less saturated. Abdomen – segments A1–A3 brownish black, remaining segments ochre subdorsally, with brownish-black dorsal line; ventrally entirely ochre. Genitalia (Figs 9b, c) – valve digitate, slightly flattened laterally and narrowing slightly medially; equal in length to uncus-tegumen complex; vinculum semicircular, saccus v-shaped, similar in length to uncus; aedeagus relatively large and stout, 3 × longer than wide, 1.5 × as long as width of genital capsule, curving dorsad 25–30°, proximal end approximately ⅓ narrower than apex; coecum 1/6–1/8 length of aedeagus, directed slightly ventrad; vesica directed dorso-distad, globose, finely spiculate, with poorly differentiated apical diverticulum. Female (Figs 5, 10, 13, 16). Described above in the genus account for Andesobia; differing externally from Andesobia sanguinea by the lack of yellowish-orange flush present in Andesobia sanguinea, particuarly on the ventral and lateral surfaces of the abdomen.
Egg – almost spherical, poles only very weakly flattened; micropyle very weakly sculptured, barely visible; ivory white changing to dark greyish white prior to hatching. Larva – 1st instar larva initially translucent white, becoming opaque white; setae black, yellow orange prior to molting. 2nd instar integument black, more densely setose than 1st instar. 3rd instar with jet black setae, except rusty brown on A2–A5; spiracles white. 4th instar (Figs 15a, b) with verrucae more pronounced than in previous instar; setae jet black with silver sheen apically, somewhat lighter smoky grey subventrally; colour of setae polymorphic in last instar, either with A2–A5 yellowish orange and segments A6–A8 with subdorsal and lateral silvery-white setae mixed in (Fig. 15a), or with orange setae very dark brown to black (Fig. 15b); when mature, female larva about twice as large as male larva. Pupa – cremaster short, penicillate, head compact with short, stiff setae (Fig. 12). Cocoon spherical to ovoid, reddish brown to dull brown, consisting of a single, thin and flimsy layer with incorporated larval setae (Fig. 16).
Genitalic and antennal morphology of Andesobia and Patagobia. 7 Andesobia sanguinea 8 Andesobia boliviana 9 Andesobia jelskii (male) 10 Andesobia jelskii (female) 11 Patagobia thursbyi.
Andesobia jelskii.12 female pupa 13a female, lateral aspect 13b female, dorsal aspect 14 male 15a mature larva 15b mature larva, dark form 16 male (lower arrow) and female (upper arrow) in copulo inside coccoon of female.
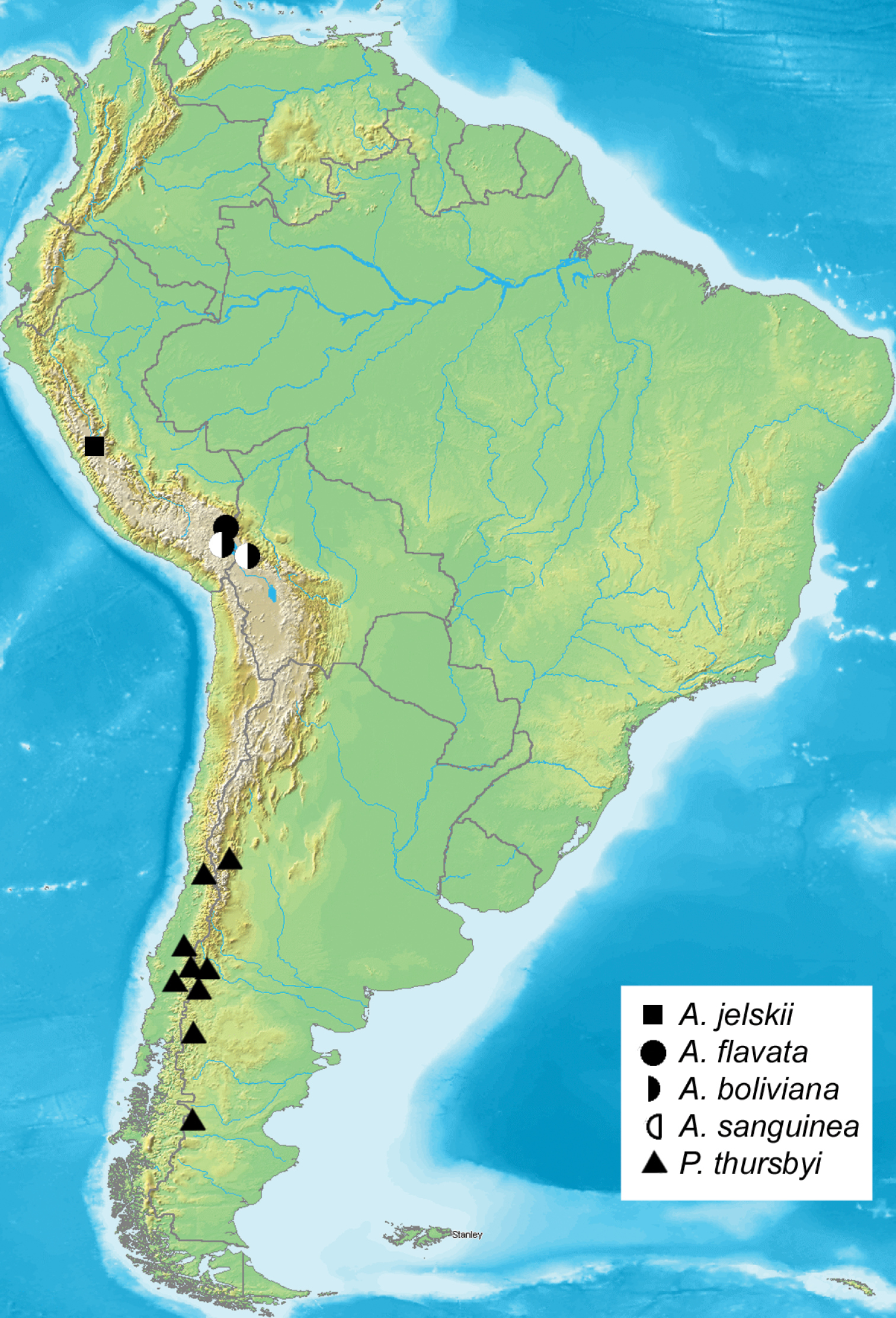
Eggs whitish, turning dark grey three days prior to hatching, hatching in about 10 days. First instar larvae initially feed on the tissue of the dead or dying female, then leave the cocoon in search of plant material. Duration of the first instar is six days, second instar five to six days. Taraxacum F.H. Wigg. and lawn grass (Poa L.) are both acceptible food plants in captivity, suggesting that larvae are polyphagous in nature. Notably, larvae emit an unpleasant odour of decay when disturbed. In late instars, female larvae are twice as large as male larvae. During the first three instars, larvae avoided sunlight, but the last two instars showed increased tolerance to sunlight, possibly to accelerate development. Cannibalism was not observed even at higher densities. Males pupated sooner than females, but the pupal stage is shorter in females lasting only a few days, so emergence of both sexes is more or less synchronous. Cocoons (Fig. 16) were spun between leaves of the food plants near the ground. The moths emerge in the morning, with relatively fast expansion of the wings. Females remain in the loosely-spun cocoon, and presumably emit mating pheromones from within the thin cocoon soon after emerging from the pupa, because males dig through the loose silk webbing to enter and mate inside the cocoon (Fig. 16). The pair remains in copula for several hours, after which the male leaves the cocoon, and the female deposits about 50 eggs inside the cocoon. Males are diurnal and fly rapidly during sunny periods. Reared cohorts of Andesobia jelskii displayed an unequal sex ratio of about 5 female: 3 male; female microptery and a female-biased sex ratio is associated with parthenogenesis in other families (Heterogynidae, Psychidae, Lymantriinae: Teia Hübner), and Andesobia may also be capable of parthenogenesis, which has not been documented in the Arctiinae. Andesobia jelskii is currently known only from the Junin region of Peru (Fig. 17), at 4100 m elevation in the Puna grassland ecoregion of the central Andes (Fig. 18). Like other members of the genus, the flight period is early in the year (January), in the middle of the four-month wet season.
Habitat of Andesobia jelskii near the type locality, Junin region, Peru (photo J. Klir).
http://species-id.net/wiki/Andesobia_flavata
Very few specimens of Andesobia flavata are known, and it is closely related to or conspecific with Andesobia boliviana. Externally, the holotype of Andesobia flavata differs from Andesobia boliviana only in having a slightly broader and more diffuse forewing marginal band, suggestive of minor intraspecific variation. However, the genitalic structure of the holotype (BMNH genitalia slide # ARCT:3421) reveals slight differences compared to Andesobia flavata, namely a slightly shorter, wider valve and a lack of the fine spicules present on the vesica of Andesobia flavata. Additional study material is needed to properly evaluate the status of these two taxa.
http://species-id.net/wiki/Andesobia_boliviana
Figs 2, 8, 18The three examined male syntypes of Turuptiana flavescens exhibit variation in the extent of the forewing markings, two specimens closely approaching the appearance of the Andesobia flavata holotype (see also Remarks under Andesobia flavata). The third syntype labeled “type” with a round, red-bordered label and a blue label reading “genitalia slide no. 3422” is here designated as lectotype; it is an almost exact match to the two specimens illustrated here (Fig. 2).
Distribution of Andesobia and Patagobia.
Subsequent to its description, Estigmene boliviana (Fig. 2b) disappeared from the literature. Although appearing in the print version of the Zoological Record for 1923, it is absent from the digital version of Zoological Record and the card index of the BMNH (Global Lepidoptera Names Index 2011). It was also omitted by
http://species-id.net/wiki/Andesobia_sanguinea
Figs 3, 6, 7, 18
Andesobia sanguinea is the only member of the genus with red colouration, prevalent on the hindwing and often the forewing, the latter varying from whitish pink (Fig. 3a) to whitish tan (Fig. 3b). Females are micropterous and are similar to Andesobia jelskii, but with a more yellowish colour (Fig. 6). The biology is unknown. It appears to be sympatric with Andesobia boliviana, and is known only from the Lake Titicaca region (Fig. 18).
urn:lsid:zoobank.org:act:AE8678AA-E7C1-4A86-8C25-7E539E5998DB
Turuptiana thursbyi Rothschild, 1910.
The name is derived from a combination of the words Patagonia and Phragmatobia.
Although Patagobia shows similarities to the Holarctic Phragmatobia in some external aspects, it differs in having longer, symmetrical rami of the male antenna, ochre thoracic collar, lack of a male clasper, pale tan forewing pattern (usually), and a restricted distribution to the Chilean Andes of South America. The wing colour and pattern is also similar to Andesobia, but structurally Patagobia has a more robust build with denser thoracic vestiture, equally long posterior and anterior male antennal rami (anterior rami shorter than posterior in Andesobia), male antennal rami up to 3 × longer than antennal segment length (up to 2 × in Andesobia); 2nd labial segment elongate, 1.8 × as long as wide, 1.5 × longer than apical segment; thoracic collar contrastingly paler ochre (conconcolourous with dorsal thoracic vestiture in Andesobia); thoracic vestiture dense and pilose (sparse and shaggy in Andesobia);femur and tibia elongate, 4.5–5.6 × longer than wide (very stout, 3.0–3.5 × longer than wide in Andesobia); metatibia with two pairs of spurs (one pair in Andesobia); forewing medial line present (absent in Andesobia); postmedial line usually double (absent in Andesobia); hindwing discal spot diffuse and elongate (sharp or absent in Andesobia). The male coremata betwen the 7th and 8th sternite are moderately developed in Patagobia, very reduced in Andesobia.
Male (female unknown). Head – vestiture dark brown to black, setae long; antenna bipectinate, ciliate ventrally; longest posterior rami 1.5–3.0 × segment length, longest anterior rami 1.1–3.0 × segment length; rami longest over middle third of antenna, decreasing in length toward base and apex; eye elliptical, 1.2–1.5 × as high as wide; labial palp short, not extending beyond vestiture of frons; haustellum reduced and poorly sclerotized, presumably nonfunctional. Thorax – vestiture of vertex and ventrum of thorax black brown; tegulae entirely black brown or black brown edged with yellowish brown; patagia yellowish brown or rarely black brown; leg vestiture brownish ochre, dorsum of femur yellow or red, co-varying with hindwing and abdomen ground colour; apex of prothoracic tibia with two subequal, blunt, triangular projections; mesotibia with two apical and two subapical spurs, length of apical spurs 1.5 × and supapical spurs 0.6 × tibial width at apex; two metatibial spurs, posterior spur slightly longer than anterior; metepisternum lacking microtymbals. Forewings – forewing length 12.9–13.2 mm (mean 13.1 mm; n = 4), length:width ratio averaging 2.1; ground colour pale ochre yellow but with broad, sometimes entirely confluent dark-brown transverse bands (Fig. 4c); pattern elements consisting of dark basal area and sinuous, diffuse dark-brown transverse lines (Fig. 4a) discal spot indistinct dorsally, but well defined ventrally; ventrally with bands obsolete except for marginal band. Hindwing – ground colour pinkish red or rarely yellow (Fig. 4d), with broad dark-brown marginal and costal band; well-defined brown, crescentic discal spot; similar ventrally but with discal spot better defined. Abdomen – Vestiture brownish black and pinkish red or yellow subdorsdally, ventrally with segmental margins yellowish ochre; abdomen entirely dark brown in melanic specimens (Fig. 4c). Coremata between sternites 7–8 in shallow pockets, scent scales approximately 0.5 × as long as sternite length. Genitalia – highly simplified, with large, triangular, dorsoventrally flattened uncus characteristic of subtribe; uncus 1.5 × longer than width of base, broadly joined to wide, band-like tegumen; dorsal margin of tegumen caudally recurved; valve simple and digitate, lacking processes or claspers, 1.5 × as long as uncus-tegumen complex; vinculum semicircular, saccus v-shaped, similar in length to uncus; aedeagus large, 5–6 × longer than wide, 2 × as long as width of genital capsule, curving dorsad slightly; coecum 1/10 length of aedeagus, directed slightly ventrad; vesica extremely large, diameter when inflated 2 × that of genital capsule; vesica directed right-laterad, globose, finely spiculate, with poorly differentiated basal chamber and large apical chamber (Fig. 11b).
http://species-id.net/wiki/Patagobia_thursbyi
Male holotype (ZMUC). Type locality: Argentina, Rio Negro, San Carlos de Bariloche, Colonia Suiza, 810 m.
The taxon pluto Toulgoët has been treated as a subspecies distinct from nominate thursbyi based on the nearly unicolourous forewing, resulting from the confluence of the transverse bands. Genitalic structure of both taxa is identical (
No detailed habitat information is available for Patagobia thursbyi, but locality information shows that it occurs from about 800 m elevation at the southern range edge (46°S) to 2700 m farther north (33°S), corresponding to temperate montane woodlands and grasslands of Patagonia. This region is well known for its high level of endemic species, and circumantarctic tree genera such as Araucaria Juss. and Nothofagus Blume (
http://species-id.net/wiki/Venedictoffia_karsholti
In his global review of Arctiina genera (as Arctiini),
BCS thanks Martin Honey, Wolfram Mey, Michel Laguerre, Thomas Simonsen, John Brown, and Marianne Horak for facilitating access to specimens in their care, and Benoit Vincent for sharing photos and DNA sequence data. Jocelyn Gill kindly provided technical support. Gary Anweiler, Don Lafontaine and Benoit Vincent provided comments and critiques that greatly improved this manuscript. Molecular analyses were carried out at the Barcode of Life Project, University of Guelph, Ontario, Canada through grants from the National Science and Engineering Research Council of Canada and Genome Canada.
JJD thanks Gerhard Pape, D-Grafenau and Rudi Tanner, D-Nuernberg, for providing specimens and livestock, Hubert Abele for his support in rearing, Ulf Buchsbaum, Zoological State Collection in Munich, for making the photo preparations, and Jiri Klir for habitat photos of Andesobia jelskii.