(C) 2011 Rudolf H. Scheffrahn. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
An updated New World distribution of the genus Calcaritermes is given along with photographs and a key to the New World species outside Mexico. Calcaritermes recessifrons is found to be a junior synonym of Calcaritermes nigriceps. Except for Calcaritermes temnocephalus, pseudergates of the other seven studied Calcaritermes species possess a mesonotal rasp. The rasps suggest a role in propagation of microbes on gallery surfaces and microbial infusion below the wood surface. Calcaritermes temoncephalus is shown to have an unusually large physogastric queens for a kalotermitid and several species produce large eggs.
Neotropical distribution, species synonymy, field photography, taxonomic soldier key, mesonotal rasp, microbial symbiosis, queen physogastry, eggs
In his monumental revision of the family Kalotermitidae,
As with most non-pest termite genera, details of the ecology and bionomics of the Calcaritermes are completely unknown. Almost all that is published about Calcaritermes relates to identification of preserved specimens for faunal surveys (e.g.
In the current paper, the New World distribution and diversity of Calcaritermes is revised based on material in the University of Florida collection. I use field photography to show the live habitus of castes of seven Calcaritermes species and depict eight soldiers using montage photography of preserved material. I also reassess the mesonotal “rasp” of pseudergate castes of Calcaritermes and provide an example of extreme queen physogastry in the Kalotermitidae. Finally, I describe the atypical feeding galleries of this genus and hypothesize a relationship between gallery architecture and the mesonotal rasp in terms of microbial symbiosis.
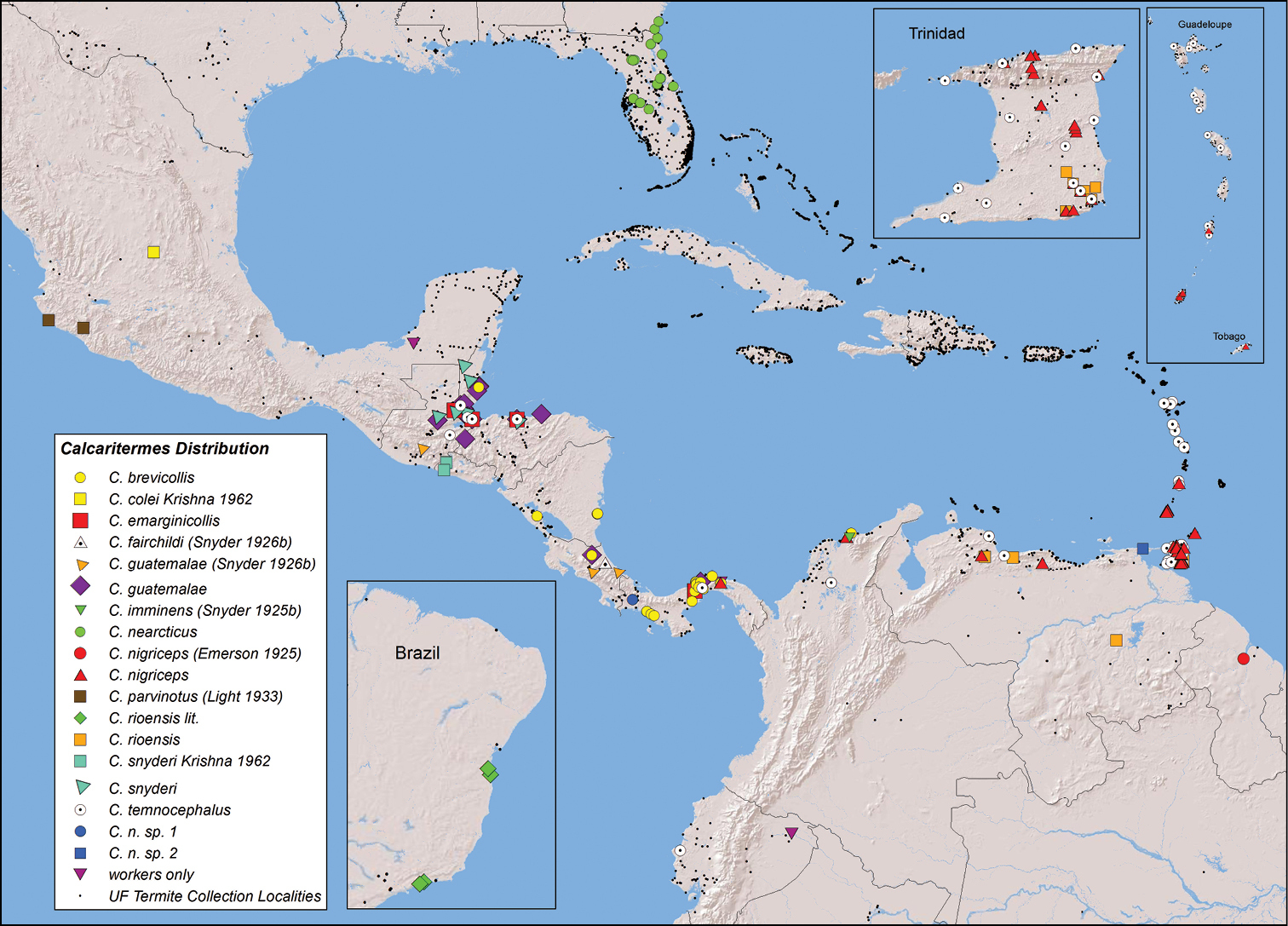
Material and methodsA total of 214 colony samples of Calcaritermes from 122 localities (Fig. 1) were collected between 1996 and 2010 and identified by the author from original descriptions and comparisons. These samples are included in the University of Florida (UF) Termite Collection, Fort Lauderdale Research and Education Center, Davie, Florida. This collection houses over 34, 000 samples, mostly from the Caribbean Basin, which the author and his colleagues have amassed since 1986. The findings herein are a direct result of field observations made while collecting Calcaritermes during various survey expeditions.
Calcaritermes distribution in the New World. Species and junior synonym names followed by citations or by “lit” are mapped from citation data only. All other species localities are mapped from records of the University of Florida Termite Collection.
Field photographs (Figs 2, 4E, 4F, and 5) were taken with a Nikon Coolpix S7c digital camera set to macro and flash mode. Specimens were usually photographed in a 5.5 cm dia. plastic Petri dish bottom lined with manila folder cardboard although natural substrate (Figs 3D and 3I) was sometimes suitable. Figures 3 and 4C were taken as multilayer montages using a Leica M205C stereomicroscope controlled by Leica Application Suite version 3 software. Montage specimens were taken from 85% ethanol and suspended in a pool of Purell® Hand Sanitizer to position the specimens in a transparent plastic Petri dish. Mesonotal rasps (Figs 4A and B) were slide-mounted with PVA mounting medium (BioQuip Products, Inc) and photographed with an Olympus BH-2 compound microscope fitted with phase contrast optics. Figure 3D was taken of a pseudergate that was freshly killed by desiccation and photographed with a Hitachi 4700 FESEM scanning electron microscope at 3-5 kV.
Photographs of live Calcaritermes specimens taken during collection. A Soldier of C. guatemalae, Honduras B Alate of Calcaritermes temnocephalus, Venezuela C Soldiers of Calcaritermes snyderi Honduras D Alate of Calcaritermes guatemalae, Belize (arrow denotes fungal hyphae growing in gallery) E Soldier of Calcaritermes temnocephalus, Venezuela F Soldier of Calcaritermes brevicollis, Colombia G Two soldiers from the same colony of Calcaritermes nigriceps, Colombia H Soldier and pseudergates of Calcaritermes rioensis, Venezuela (arrow denotes mesonotal rasp of pseudergate) I Soldier, dealate, and pseudergates of Calcaritermes nearcticus, Florida (arrow denotes fungal staining around gallery). All images to same scale.
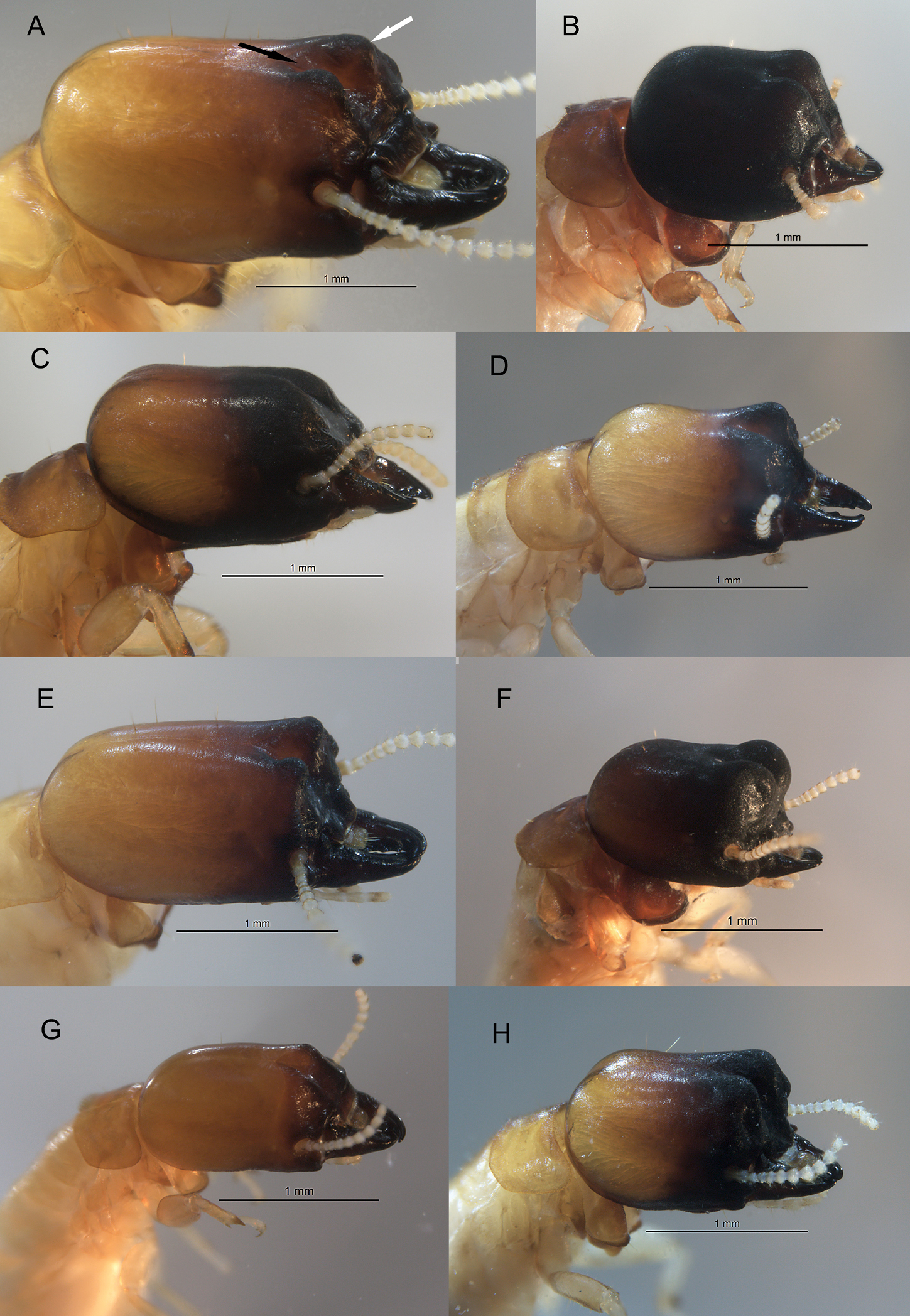
Oblique view of Calcaritermes soldiers from the University of Florida collection. A Calcaritermes guatemalae, Honduras (black arrow shows orientation of frontal furrow, white arrow points to frontal lobe) B Calcaritermes rioensis, Trinidad. C Calcaritermes nigriceps, Trinidad D Calcaritermes nearcticus, Florida E Calcaritermes emarginicollis Honduras F Calcaritermes brevicollis, Nicaragua G Calcaritermes temnocephalus, Guatemala H Calcaritermes snyderi, Guatemala. Enlarge outer tibial spines visible in B and G. Photos to same scale.
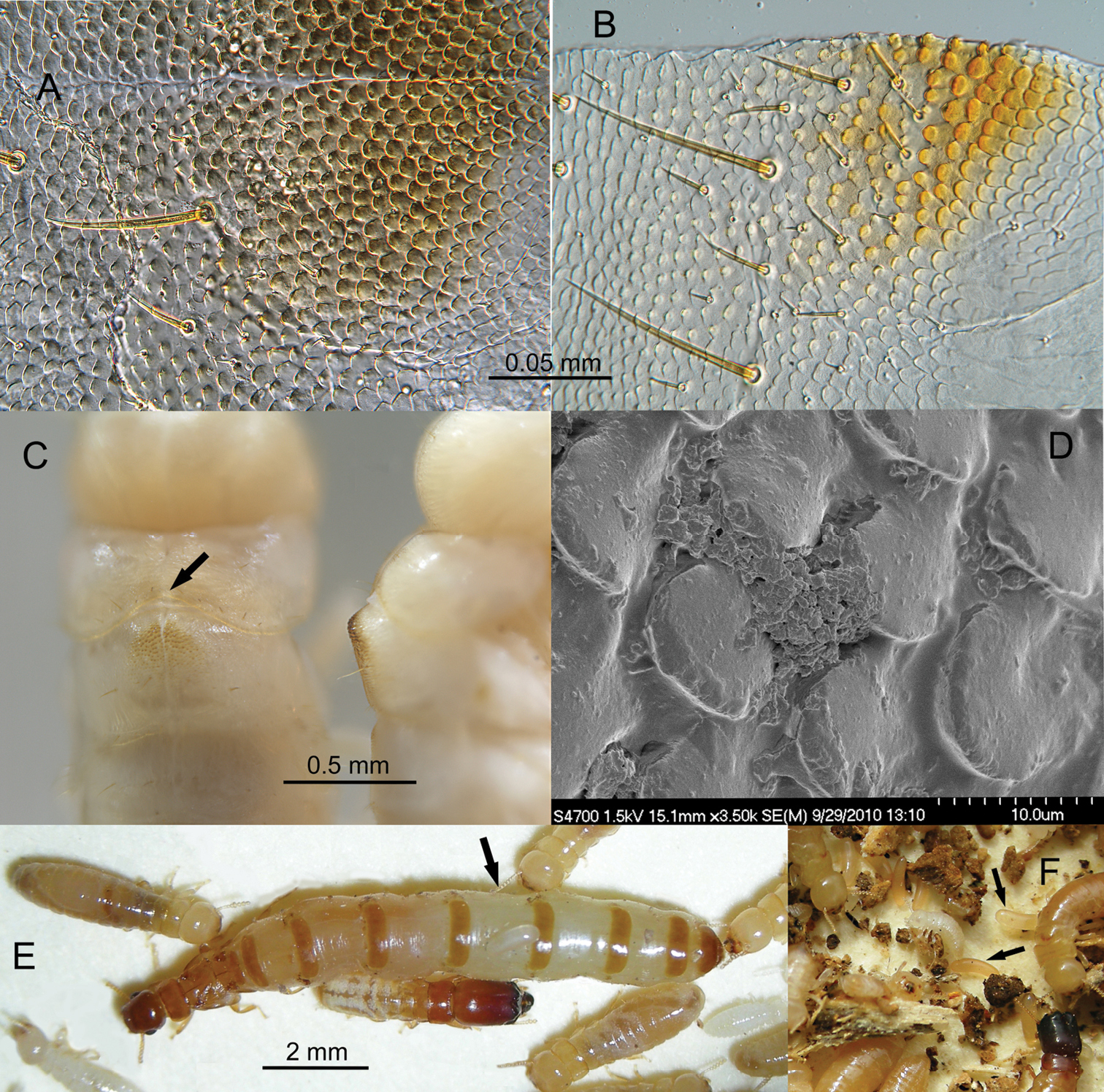
A Light micrograph of partial mesonotal rasp of Calcaritermes nearcticus pseudergate from an ethanol-preserved specimen. Mid-line is horizontal near top of figure B Micrograph of half of mesonotal rasp of Calcaritermes brevicollis pseudergate. Rasp is separated at the mid-line C Dorsal and lateral view of rasps on Calcaritermes brevicollis pseudergates (arrow denotes concavity of posterior margin of pronotum to accommodate elevation of the rasp) D SEM of rasp of Calcaritermes nearcticus from an unrinsed, freshly prepared specimen E Physogastric queen and other castes of Calcaritermes temnocephalus (arrow denotes egg on queen dorsum) F Eggs (arrows amongst nest debris) of Calcaritermes brevicollis, Colombia.
A Calcaritermes nigriceps galleries exposed in Colombia B Calcaritermes nearcticus galleries in oak wood, Florida C Calcaritermes temnocephalus galleries in Venezuela. Images not to same scale.
Calcaritermes is primarily a neotropical genus with the exceptions of a relic nearctic species, Calcaritermes nearcticus Snyder, 1933, found from central and northeastern Florida to southeastern Georgia (
Table 1. Revised New World list of Calcaritermes Snyder, 1925 and type localities.
|
Calcaritermes brevicollis ( |
Panama* |
|
Calcaritermes colei |
San Luis Potosi |
|
Calcaritermes emarginicollis ( |
Rio Chinilla, Canal Zone, Panama* |
|
Calcaritermes guatemalae ( |
Mixco, Guatemala* |
|
Calcaritermes imminens ( |
Cincinnati, Colombia |
|
Calcaritermes nearcticus ( |
Clay County, Florida* |
|
Calcaritermes nigriceps ( |
Kartabo, Guyana* |
|
Calcaritermes parvinotus ( |
Colima, Mexico |
|
Calcaritermes rioensis |
Ihla Grande, Rio de Janeiro, Brazil* |
|
Calcaritermes snyderi |
Volcan de Santa Ana, El Salvador* |
|
Calcaritermes temnocephalus ( |
Las Trincheras, Venezuela (see text)* |
| Undescribed sp. 1 | Boquette, Panama* |
| Undescribed sp. 2 | Paria Pennisula, Venezuela* |
| 1 | Pseudergates (pseudergates without large wing buds) lack mesonotal rasp or concave posterior margin of pronotum; soldier with frontal furrow rather even in depth extending length of frons at a shallow angle, frontal lobes with distinct elongate rugosity (Fig. 3G) | Calcaritermes temnocephalus |
| – | Pseudergates with mesonotal rasp and concave posterior margin of pronotum (Fig. 4C), soldier unlike above | 2 |
| 2 | Soldier maximum head width 1.4 mm or more, head capsule elongate; furrow rugose; frontal lobes clearly not overhanging frons (Fig. 3A) | Calcaritermes guatemalae |
| – | Soldier maximum head width less, or much less than 1.4 mm, head variable | 3 |
| 3 | Head capsule somewhat to clearly elongate (Figs 3C, 3D, 3E, 3H) | 4 |
| – | Head capsule truncate, mandibles short (Figs 3B, 3F) | 7 |
| 4 | Frontal furrow unsculptured; frontal lobes smooth; lobes obtuse in angle ca. 145° (Fig. 3C) | Calcaritermes nigriceps |
| – | Frontal furrow and lobes with sculpturing; lobes form angle ca. 90–100° (Figs 3D, 3E, 3H) | 5 |
| 5 | Frontal lobes rounded, ovoid depression near center of lobes (Fig. 3H) | Calcaritermes snyderi |
| – | Frontal lobes more acutely pointed, no ovoid depression near center of lobes (Figs 3D, 3E) | 6 |
| 6 | Larger species, maximum head width 1.2–1.3 mm, frontal lobes with slightly overhanging tips, neotropical distribution (Fig. 3E) | Calcaritermes emarginicollis |
| – | Smaller species, maximum head width 1.1 mm, frontal lobes evenly angled, Nearctic distribution only (Fig. 3D) | Calcaritermes nearcticus |
| 7 | Frontal lobes nearly overhanging frons; raised well above vertex; distinct ovoid depression in center of lobes (Fig. 3F) | Calcaritermes brevicollis |
| – | Frontal lobes not overhanging frons; almost even with vertex; lacking ovoid depression in center of lobes (Fig. 3B) | Calcaritermes rioensis |
Mandible dentition of pseudergate or nymphal castes has been used for generic grouping of some Kalotermitidae (
These mesonotal “rasps” were found on all apterous pseudergates, early stage brachypterous nymphs, and most soldiers of Calcaritermes for species in the UF Collection (Table 1) with the exception of Calcaritermes temnocephalus in which the rasp is absent. Under magnification, it was observed that each of these rasps actually consist of a single layer of slightly overlapping spatulate scales with basal attachments at their anterior ends (Figs 4A, 4B, 4D). The mesonotal rasps have a midline divide and form an elevated mound raised above the remainder of the dorsum (Fig. 4C, right). The posterior margin of the pronotum of all eragtoid/nymphoid castes, except again Calcaritermes temnocephalus, has a posterior marginal concavity that partially surrounds the anterior of the rasp (Fig. 4C, arrow). The pronotum is steeply angled toward the head anterior to the rasp (Fig. 4C, right). The scale patterns and lateral profile of the rasps vary somewhat among species (e.g., Figs 4A, 4B) but no species-specific morphology was investigated in this study. No rasp was found on any mature reproductive and the robustness of the rasp was inversely proportional to wing bud size disappearing when the nymphs were one molt from adulthood. The mesonotal rasp is the first external character to provide a diagnostic, generic identification of an immature kalotermitid.
Microscopic examination of the mesonotal rasps from ethanol-preserved specimens did not reveal microbial material around the scales. However, when live specimens of Calcaritermes nearcticus were prepared for SEM without cleaning or rinsing, an organic (microbial?) paste was observed between the scales (Fig 4D).
Queen physogastry. Over the years, I have observed hundreds of mature queens in kalotermitid nests but was struck by the extreme queen physogastry in Calcaritermes temnocephalus. On 26 May 2008, two colonies of Calcaritermes temnocephalus were collected by the UF survey team at Silva Seco de Capadare, Guiermo, Venezuela (11.154, -68.590, elev. 58m). Both colonies were large and occupied rather sound wood from which a mature primary queen was removed (Fig. 4E). The extent of physogastry of these queens is what is typically observed in the Rhinotermitidae or Termitidae in which the intersegmental membrane stretches well beyond the width of the tergites or sternites. Typically the extended intersegmental membrane in primary queens of the Kalotermitidae is narrower than the width of adjacent abdominal sclerites, but in the Calcaritermes temnocephalus queens, the membrane is much wider than the sclerites. Eggs from one of the Calcaritermes temnocephalus colonies (Fig. 4E, arrow) and from a Calcaritermes brevicollis colony in Panama (Fig. 4F, arrows) also appeared disproportionally large compared to other kalotermitids.
Nests. Calcaritermes colonies infest damp or wet wood, usually in the shade of forest canopy. At ground level, populations are never plentiful in a given area. However,
The gallery system of Calcaritermes differs from other kalotermitids in several distinct ways. First, the galleries are narrow and tubular, maybe allowing only two termites to pass at one time. The galleries are spaced rather far apart in the wood matrix, thus occupying a relative small volume of the colonized member (Figs 5A, 5B). Secondly, the galleries contain very few loose fecal pellets, but gallery surfaces are generously lined with what appears to be a moist fecal/microbial? paste (Figs 5A, 5B, 5C).
Given the mesonotal rasp, the low volume of wood excavated, gallery coating, and peripheral gallery staining, one can hypothesize that Calcaritermes derives some nutrition via a symbiotic relationship with microbes growing on the surface of their galleries. The rasp may be used by foragers to inoculate gallery surfaces with fungal or bacterial spores analogous to the mycangium (
I thank Kumar Krishna for setting the standard for impactful and high quality publications throughout his splendid career as a termite taxonomist. I am indebted to many contributors of material to the UF Termite Collection. I am especially grateful to the following termite survey expedition members: James A. Chase and John R. Mangold (original members, 1991-present), Julian de la Rosa Guzman (1991-1995), Jan Krecek (1994-present), Paul Ban (deceased, 1994-2009), Vinda Maharajh (deceased, 1996-2008), Yves Roisin (1997), John Warner (2001-2003), Tom Nishimura (2002-present), Bayardo Herrera and Jorge Moreno (2004), Rob Giblin-Davis and Natsumi Kanzaki (2005), Bob Setter and Tim Myles (2006-present), Brian Bahder (2006-2007), Jose Perozo (2007-2009), Solange Issa (2008) and Juan Saldarriaga (2009). Their enthusiasm, field expertise, and hard work has brought about many discoveries among the rich termite fauna of the southern Neactic and the Caribbean Basin. I thank Terminix International for funding much of our travel expenses since 2004. Many thanks to Erick James, Botany Department, University of British Columbia for Fig. 4C. The reviews by Jan Krecek and Seemanti Chakrabarti were most helpful.