(C) 2011 Michael S. Engel. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The fauna of termites (Isoptera) preserved in Early Eocene amber from the Cambay Basin (Gujarat, India) are described and figured. Three new genera and four new species are recognized, all of them Neoisoptera – Parastylotermes krishnai Engel & Grimaldi, sp. n. (Stylotermitidae); Prostylotermes kamboja Engel & Grimaldi, gen. et sp. n. (Stylotermitidae?); Zophotermes Engel, gen. n., with Zophotermes ashoki Engel & Singh, sp. n. (Rhinotermitidae: Prorhinotermitinae); and Nanotermes isaacae Engel & Grimaldi, gen. et sp. n. (Termitidae: Termitinae?). Together these species represent the earliest Tertiary records of the Neoisoptera and the oldest definitive record of Termitidae, a family that comprises >75% of the living species of Isoptera. Interestingly, the affinities of the Cambay amber termites are with largely Laurasian lineages, in this regard paralleling relationships seen between the fauna of bees and some flies. Diversity of Neoisoptera in Indian amber may reflect origin of the amber deposit in Dipterocarpaceae forests formed at or near the paleoequator.
India, Tertiary, Eocene, termites, Termitidae, Rhinotermitidae, Stylotermitidae, Neoisoptera
It is with great admiration that we dedicate this paper to our dear friend and colleague, Prof. Kumar Krishna, the authority on living and fossil termites. We have had the pleasure of working alongside Kumar for many years now and on numerous projects, none of which would have seen successful completion had it not been for his keen insight and global and encyclopedic knowledge of the Isoptera. Now 83, Kumar continues to be our guide through the wonders and subtle nuances of termite systematics and biology. We look forward to many more years of such pleasurable mentorship and amity.
IntroductionThe fossil record of termites has expanded greatly during the last 10–15 years, with numerous new taxa uncovered from deposits throughout the world. Of particular importance are the plethora of new specimens in amber which, with their exceptionally high fidelity of preservation, have permitted dramatic new insights into the history of the order and its paleobiology (e.g.,
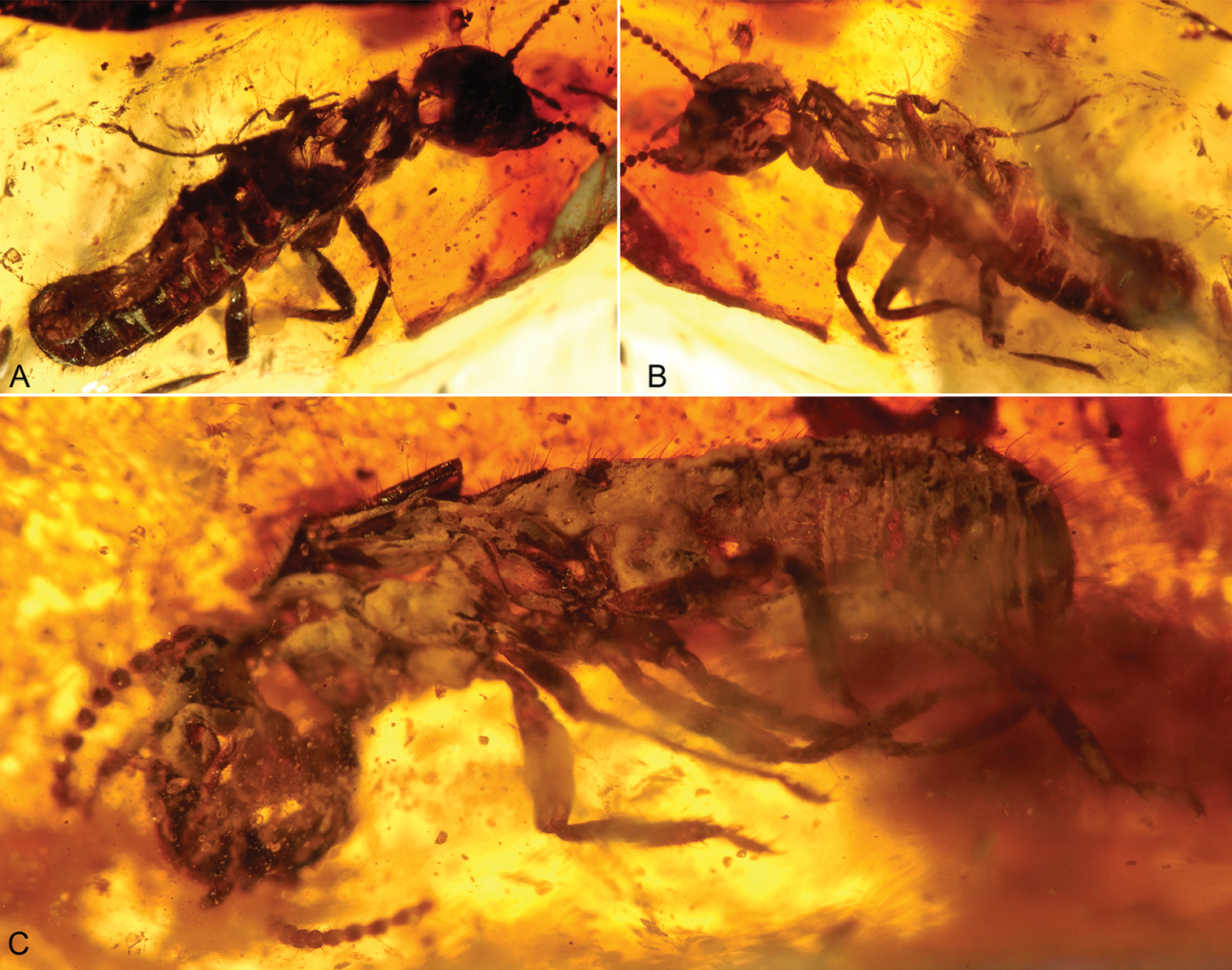
Photomicrographs of Cambay amber (Early Eocene) termites. A Nanotermes isaacae Engel & Grimaldi, gen. et sp. n., holotype (Termitidae: Tad-262) B Parastylotermes krishnai Engel & Grimaldi, sp. n., holotype (Stylotermitidae: Tad-277) C Zophotermes ashoki Engel & Singh, sp. n., holotype (Rhinotermitidae: Tad–42). Not to the same scale.
Photomicrographs of dealate male and female of Prostylotermes kamboja Engel & Grimaldi, gen. et sp. n. (Stylotermitidae: Tad-321C). A Dorsal view of female B Ventral view of female C Ventral view of male. Not to the same scale.
The Cambay amber deposits, their biotic diversity, and biogeographical significance were reviewed by
http://species-id.net/wiki/Parastylotermes
The genus Parastylotermes was erected by Snyder and Emerson (in
urn:lsid:zoobank.org:act:9B0E707D-8F26-4FCE-A806-F708CAFD518D
http://species-id.net/wiki/Parastylotermes_krishnai
Figs 1B, 3Imago (sex unknown); Tad-277 (Fig. 1B), India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 17–22 January 2010 (BSIPL).
Imago; Tad-96, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 7–12 January 2009 (AMNH). This specimen is a poorly preserved alate, with much of the specimen crushed and large portions of the upper body, wings, &c. missing. However, the front of the head is not deformed, with a good frontal view of the clypeus (Fig. 3B). The existing wing fragments show a venation very similar to that of the holotype, and the antenna has 14 antennomeres, as in the holotype. These features, along with the trimerous tarsi, strongly suggest that this is an additional individual of this species.
The new species can be distinguished from all other Parastylotermes by the apical branching of the medial vein in the forewing (branching in the apical quarter rather than being unbranched or branched only at the extreme wing apex), less reticulation, more CuA branches (10, versus 7–8 in Parastylotermes robustus) and by the smaller number of antennal articles (14 in the new species, 16–17 in Parastylotermes robustus, unknown for Parastylotermes frazieri, Parastylotermes washingtonensis, and Parastylotermes calico, which are just forewings preserved as compressions). In all other respects, Parastylotermes krishnai matches the description and lectotype (visum) for Parastylotermes robustus in Baltic amber except for in general metrics and some aspects of coloration (
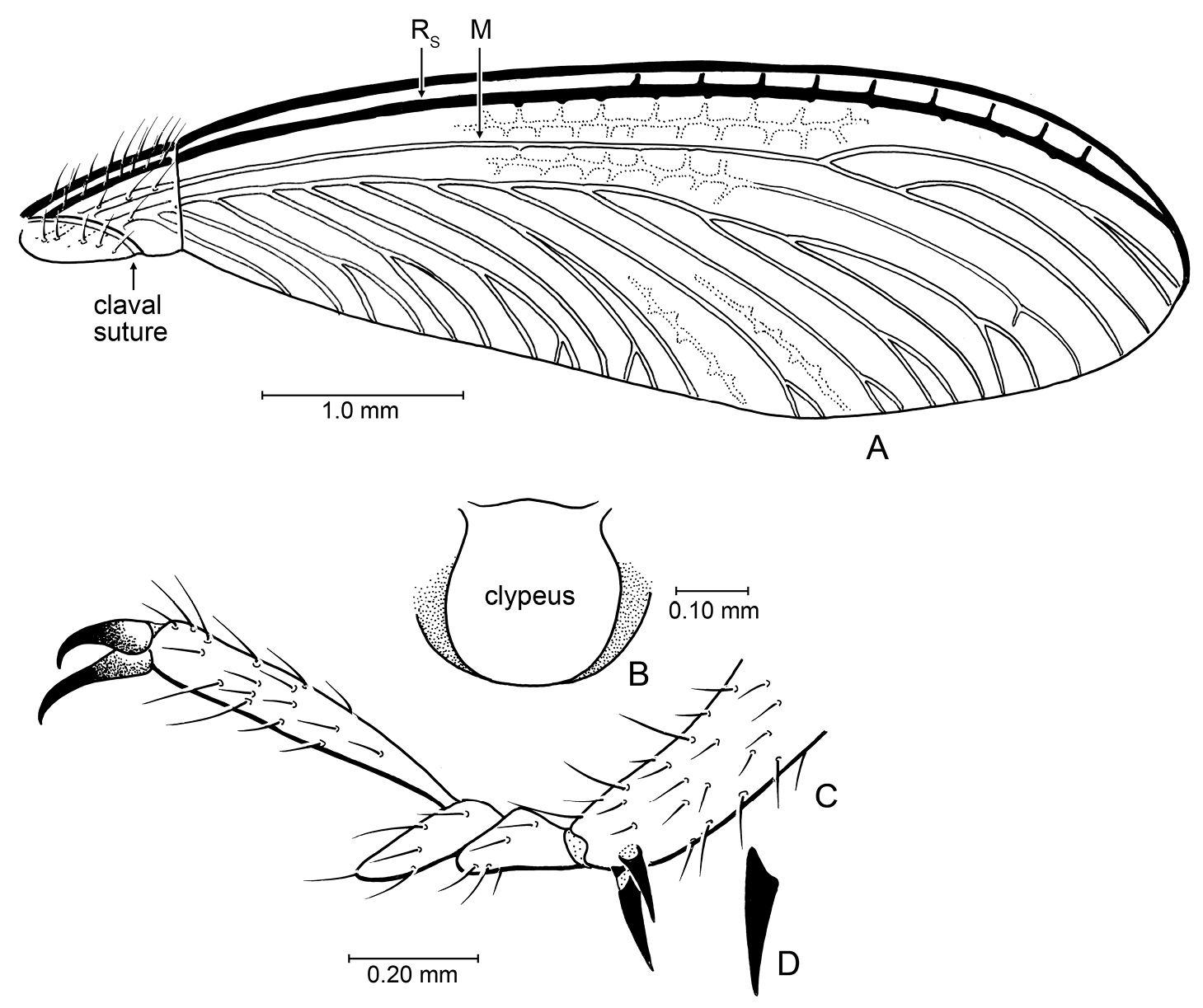
Imago: Total length without wings (as preserved) ~4.0 mm; forewing length 5.7 mm, width 1.7 mm; length of forewing scale 0.8 mm; three maxillary and labial palpomeres. Integument finely imbricate throughout; head dark brown with scattered long, erect, light brown setae, short setae exceptionally sparse; antenna brown, with 14 articles, each with scattered short setae and a few long setae apically; compound eyes round, of moderate size; ocelli not visible owing to preservation of head cuticle; Y-shaped ecdysial cleavage lines and fontanelle not evident (obscured by folding of head cuticle; however, in living Stylotermitidae the fontanelle is exceptionally small and often not visible). Pronotum brown, with scattered, long, fine, erect setae; anterolateral angle acutely rounded, posterior lateral angles broadly rounded, with small medial emargination along anterior border. Legs brown with sparse, short setae except more numerous and stout on tibiae and tarsi; tibial spur formula 2-2-2, perhaps with a single outer spine present on protibia (difficult to discern in holotype), articulating bases of spurs oblique; tarsi trimerous, apical tarsomere longer than combined lengths of basitarsus and second tarsomere, second tarsomere projecting apically beneath base of apical tarsomere; pretarsal claws simple, arolium absent. Forewing scale large, overlapping hind wing scale, humeral margin faintly convex, apical margin straight, CuP (claval fissure) gently arched, meeting posterior margin of scale well before suture, scale with numerous long, erect setae, particularly along humeral margin, without short setae; C and R more darkly pigmented than remaining veins; Sc apparently short, terminating within scale; veins more separated apically than proximally; Rs unbranched, running close and parallel to costal margin, slightly more widely separated from margin apically than proximally; M about midway between R and CuA, branching twice in apical quarter of wing, reaching to wing apex, apical branches of M strongly arched posteriad, such that apices meet wing margin posterior to wing apex; CuA with 10 primary branches reaching to posterior wing margin, apicalmost termination of CuA just posterior to wing apex; veins with sparse, minute setulae; membrane completely bare, between major veins reticulate and with strong, apically-slanting veinlets, particularly midway between R, M, and CuA. Hind wing scale with straight apical margin (suture). Abdomen brown to dark brown; largely crushed and obscured in holotype.
Details of Parastylotermes krishnai Engel & Grimaldi, sp. n., holotype (Tad-277). A Forewing venation B Clypeus (from Tad-96) C Pretarsus, tarsus, and extreme apex of tibia D Detail of spur.
The specific epithet is a patronym honoring Prof. Kumar Krishna, world authority on living and fossil termites, in recognition of his many contributions to the subject.
urn:lsid:zoobank.org:act:0B6B19C4-F041-4589-A363-8E5D605C6003
Prostylotermes kamboja Engel & Grimaldi, sp. n.
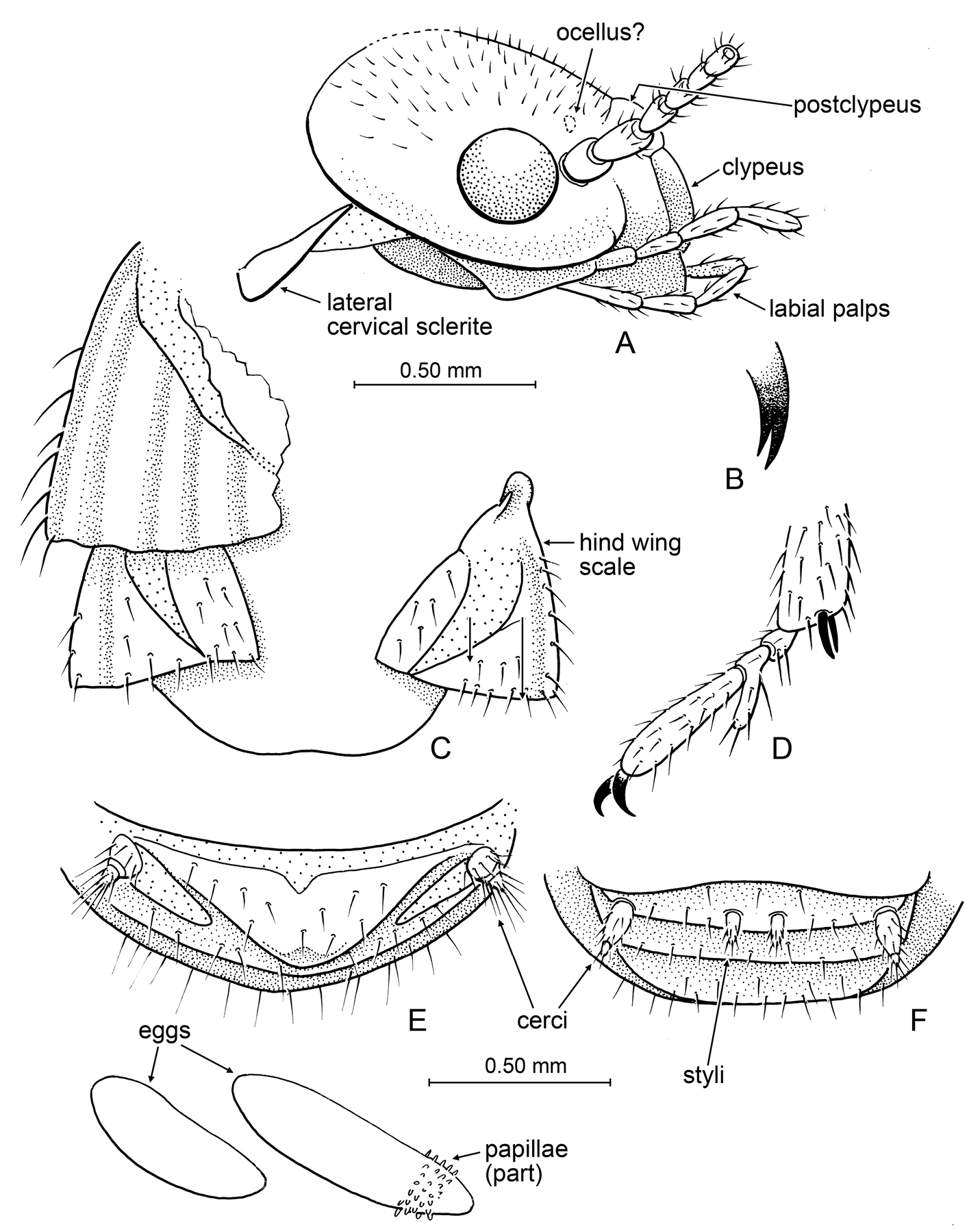
Imago: Head subcircular (Figs 2A, 2B, 4A); compound eyes small, circular; ocelli apparently present, separated from compound eye by more than ocellar diameter (Fig. 4A); postclypeus short, weakly arched (Fig. 4A); antenna with 17 articles. Pronotum flat, narrower than head; tibial spurs 2-2-2; tarsi trimerous, with second tarsomere distinctly projected ventroapically, extension longer than dorsal length of second tarsomere (Fig. 4D). Forewing with scale overlapping base of hind wing scale, slightly larger than hind wing scale (Fig. 4C), scale without numerous setae over surface, with long setae along humeral margin (Fig. 4C) (other stylotermitids have numerous and relatively long setae over the entire scale surface). Cerci short, with two cercomeres (Figs 4E, 4F); styli present in male only, not extending to abdominal apex (Fig. 4F).
Detail of Prostylotermes kamboja Engel & Grimaldi, gen. et sp. n. (Tad-321C). A Head in lateral aspect B Tip of lacinia C Dorsal view of wing scales (female specimen) D Meso-pretarsus, mesotarsus, and extreme apex of mesotibia (female specimen) E Apex of female abdomen, ventral view, with detail of eggs preserved at abdominal apex F Apex of male abdomen, ventral view. Scale bars are identical and apply to all figures except the detail enlargements of B and D.
The new genus-group name is a combination of pro (Greek, meaning “before”) and Stylotermes, type genus of the family. The name is masculine.
urn:lsid:zoobank.org:act:973DA7E6-C12C-4CB2-8FBB-A61E3CFCEFB9
http://species-id.net/wiki/Prostylotermes_kamboja
Figs 2, 4Imago ♀ (dealate) (Figs 2A, 2B); Tad-321C, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 17–22 January 2010 (BSIPL).
Imago ♂ (dealate) (Fig. 2C); Tad-321C, same piece and repository as holotype.
As for the genus (vide supra).
Imago (dealate): Total length of female 5.0 mm, of male 3.8 mm; body entirely dark brown, including wing scales and legs, pleural areas lighter. Head of female with length 1.10 mm; compound eye virtually round, diameter 0.25–0.28 mm; fine short pilosity on vertex; postclypeus weakly bulging, length ~0.20 mm, clypeal length ~0.30 mm; fontanelle and coronal ecdysial cleavage line (= Y-shaped suture) not observable as preserved; four maxillary palpomeres, three labial palpomeres; apex of lacinia bifid (Fig. 4B); antenna with 17 articles; flagellomeres slightly and gradually increasing in width distad, basal flagellomere ~0.65x width of apicalmost flagellomere. Pronotum not entirely observable, mostly lost in female and dorsal view obscured in male, portions preserved for female indicate it is narrower than head width. Only wing scales present (wings shed); forewing scale briefly overlapping hind wing scale (by nearly 0.3x length of hind wing scale); both scales with CuP fracture basally very broad, tapered to a point just before or at scale margin; fine setae on costal margin of forewing scale, none on broad surface; some fine setae on broad surface of hind wing scale. Legs with sparse, fine setae on femora and tibiae; tibial spurs 2-2-2, without preapical dorsal spines on tibiae; tarsi trimerous, basitarsomere smallest, second tarsomere with ventroapical extension; distitarsomere 2.5x length of other tarsomeres (excluding second tarsomere extension and pretarsal claws); pretarsal claws simple, arolium absent; meso- and meta- epicoxal regions bulging, slightly explanate. Abdominal tergites and sternites well developed (meeting laterally); abdomen mildly dorsoventrally flattened; apex of abdomen (terminal sternites and tergites) broad, apical margins flattnened; cerci short, with two cercomeres (apicalmost cercomere minute, sometimes separated by distinctive membrane from basal cercomeres [in female]); male with small styli; female without styli.
Eggs: Oocytes elliptical, with fine, microscopic chorionic structure; longer one with fine papillae over most of chorion (Fig. 4E). First oocyte length 0.75 mm, width 0.20 mm; second oocyte length 0.53 mm, width 0.20 mm.
The specific epithet is treated as a noun in apposition. The name Kamboja (perhaps of Scythian origin) refers to the Indo-Iranian Kshatriya tribe (Hindu warrior elites) who appear in various ancient Indian texts such as the Vamsa Brahmana and the Mahabharata. In the second century B.C. the Kambojas invaded northern India and took control of various Indo-Arayan territories such as Gujarat, eventually settling the area and lending their name to Khambat (Cambay) and the area in which the amber harboring this species was recovered.
This piece preserves together two virtually complete dealate adults – one a female, the other a male – though dorsal portions of the female have been lost at the amber surface. Interestingly, two eggs are preserved at the abdominal apex of the female (Fig. 4E).
Subfamily Prorhinotermitinae Quennedey & Deligne
urn:lsid:zoobank.org:act:07D6FDAC-35C5-4DF8-9BD0-0939B19155C7
Zophotermes ashoki Engel & Singh, sp. n.
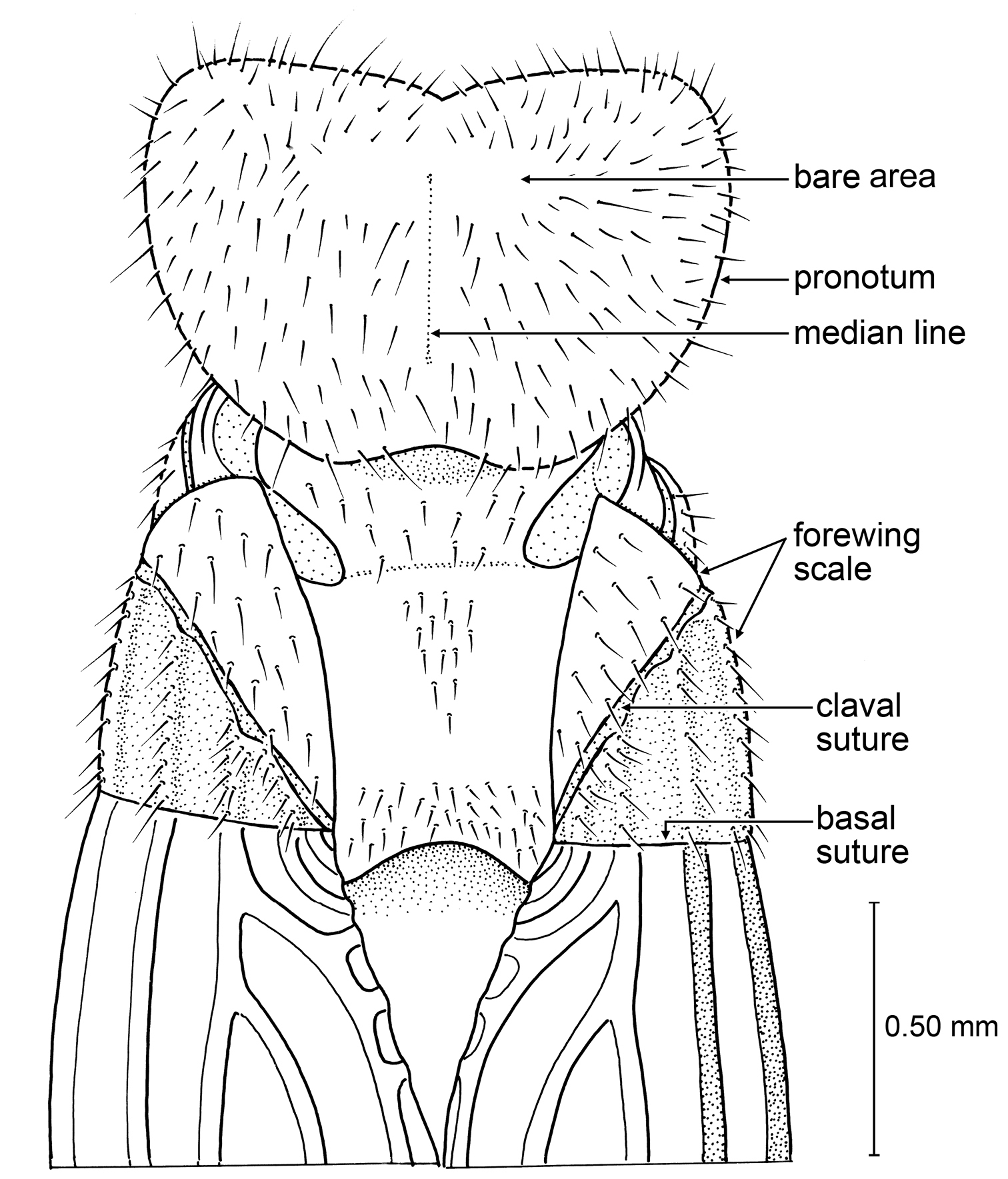
Imago: Head not flattened, narrow oval in shape, with sides somewhat parallel (appears similar to condition in Heterotermitinae but there is some compression which may be obscuring slightly roundish borders), posterior margin even; postclypeus without nose-like projection, without groove from fontanelle to apex of labrum, short relative to width, somewhat arched (as in Prorhinotermitinae); compound eyes small; ocelli present. Pronotum flat, narrower than head, anterior margin with medial emargination (Fig. 5); tibial spurs 2-2-2 (3-2-2 in Prorhinotermes Silvestri, Psammotermitinae, and Heterotermitinae); tarsi tetramerous. Forewing with scale overlapping hind wing scale; M branching from CuA outside of scale (Fig. 5) (as in Prorhinotermitinae and Psammotermitinae); wing membrane with relatively few setae (as in Prorhinotermitinae).
Detail of Zophotermes ashoki Engel and Singh, sp. n. (Tad-42), dorsal view of thorax and anterior portion of forewings (slightly reconstructed).
The new genus-group name is a combination of zophos (Greek, meaning, “nether world” or “gloom”), and Termes, type genus of the Termitidae. The name is masculine.
urn:lsid:zoobank.org:act:CA5E2EBA-B8CB-4C11-83E4-B6A5B7E5C1D9
http://species-id.net/wiki/Zophotermes_ashoki
Figs 1C, 5, 6AImago (sex unknown); Tad-42 (Fig. 1C), India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 7–12 January 2009 (BSIPL).
Imago (wings only); Tad-97, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 7–12 January 2009 (AMNH).
As for the genus (vide supra).
Imago: Total length without wings (as preserved) 4.9 mm; forewing length 6.0 mm; pronotal length (medial) 0.75 mm, width 1.20 mm; length of forewing scale 0.80 mm. Integument of head dark brown, nearly black, except antenna and mouthparts brown; pronotum and remainder of thorax dark reddish brown, legs brown; abdomen dark brown. Integument apparently finely imbricate (where evident). Head relatively large (although left side and much of vertex distorted by compression), length greater than width, lateral borders slightly convex and parallel, with scattered, erect, stout setae, such setae sparse on lateral surface behind compound eye. Compound eyes relatively small, circular, weakly exophthalmic, positioned well anterior on head, separated from posterior border of head by more than compound eye diameter. Fontanelle present, circular, located midway along tangent between middle of compound eyes. Ocelli small, semicircular, positioned anterodorsal to compound eye, separated from compound 2.5–3.0x ocellar diameter. Antenna moniliform, number of articles indeterminate owing to preservation, visible articles with moderately numerous, minute, apically-directed setae and microtrichia. Pronotum slightly wider than long, slightly broader than head, anterior margins slightly conergent mediad, apicolateral corners acutely rounded, lateral margins initially parallel in apical quarter then slightly tapering posteriorly with broadly-rounded posterior corners, medial posterior margin relatively straight; surface with numerous stout, short, suberect, posteriorly-directed setae except those along anterior margins slightly more dense and directed anteriad. Legs with numerous short to moderate-length setae; tibiae without distinct spines; tibial spur formula 2-2-2; tarsi tetramerous; pretarsal ungues simple; arolium absent. Forewing scale large, slightly overlapping hind wing scale; hind wing scale smaller than forewing scale; both fore and hind wing scales covered with numerous, stout, short, erect to suberect setae, such setae intermingled with longer setae, particularly apically along veins; hind wing with C+Sc+R and Rs thick, sclerotized, with ~5 short, perpendicular crossveins connecting them in apical third; M bifurcate in apical half, originating from CuA outside of wing scale; Cu with at least five main branches, two branches with short bifurcate branches at apex; apex of Cu reaches to 0.92x length of wing; no reticulate crossveins present; membrane very finely and densely pimplate. Abdomen apparently with scattered setae similar to those on wing scales.
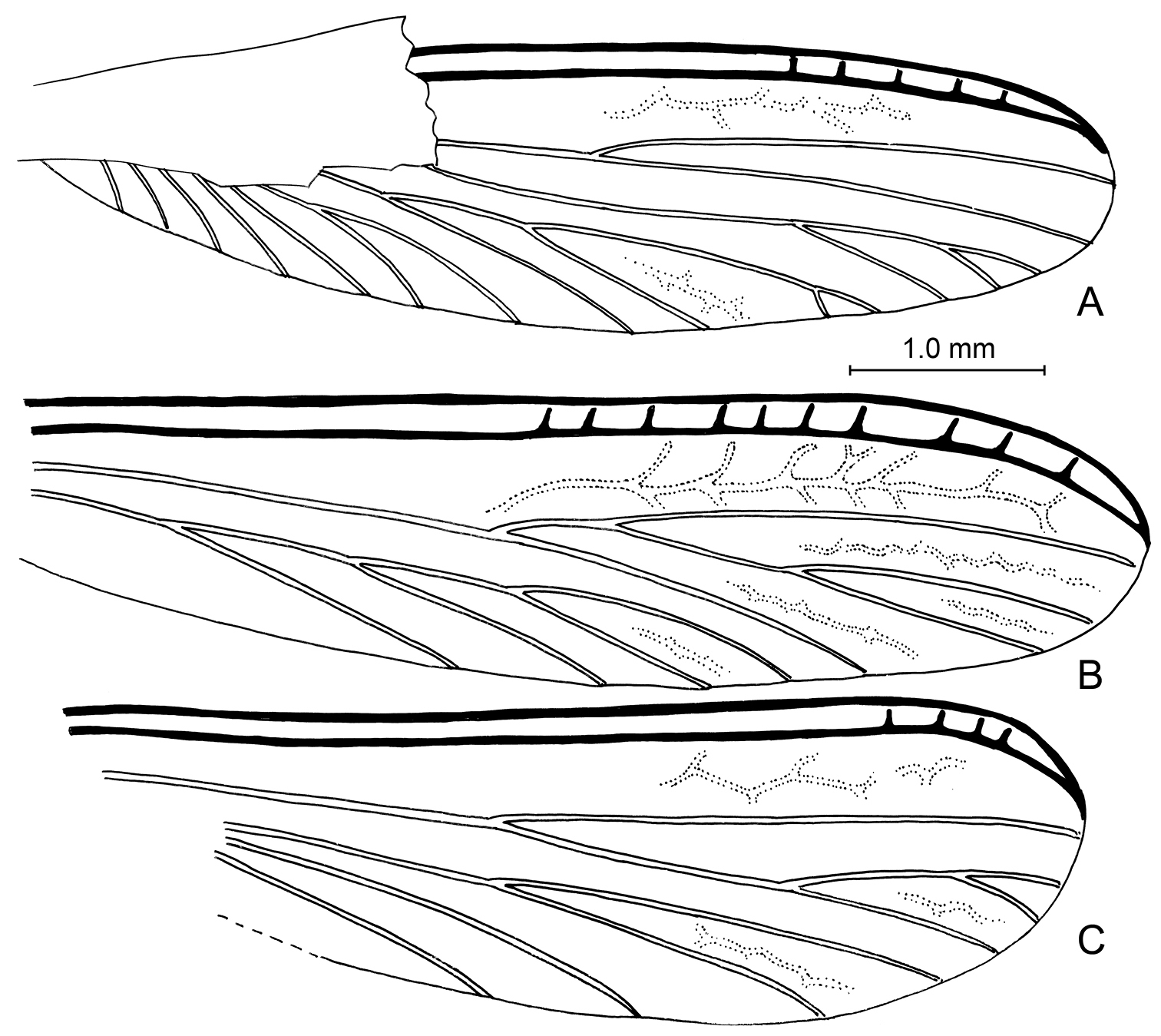
Wing venation of Cambay amber species of Zophotermes Engel, gen. n. A Hind wing of Zophotermes ashoki Engel & Singh, sp. n. (Tad-42) B Forewing of Zophotermes? sp. (Tad-95) C Hind wing of Zophotermes? sp. (Tad-278). Scale bar applies to all figures.
The specific epithet is a patronym honoring Dr. Ashok Sahni, sage of Indian paleontology and wonderful colleague.
Figs 6B, 6C
Imago (wing and fragments only); Tad-95, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 7–12 January 2009 (AMNH). Imago (wing fragment only); Tad-278, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400'N, 73°4.532'E, 17–22 January 2010 (AMNH). Imago (very badly crushed and obscured); SEMC-F000157, India: Gujarat: Tadkeshwar mine, Surat District, Cambay Basin, 21°19'26"N, 73°4'32"E, 7–12 January 2009 (SEMC). Imago (head, anterior thorax, and wing bases only); Tad-304, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 17–22 January 2010 (AMNH).
The above four specimens are too poorly preserved to permit conclusive assignment to any particular species but for each the observable details are indicative of a prorhinotermitine and they are apparently of the genus Zophotermes. Most, if not all, could be conspecific with Zophotermes ashoki described herein but we have hesitated to make a formal assignment as the condition of each is inadequate.
Imago (largely crushed dealate, sex unknown); Tad-155, India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 7–12 January 2009 (AMNH).
This specimen is badly damaged and while it certainly has the appearance of a Heterotermes, assignment as to genus, or even subfamily, cannot be made with confidence.
Subfamily Termitinae? Latreille
urn:lsid:zoobank.org:act:2D8541FC-A9DD-4593-81B3-405EAE33FBDD
Nanotermes isaacae Engel & Grimaldi, sp. n.
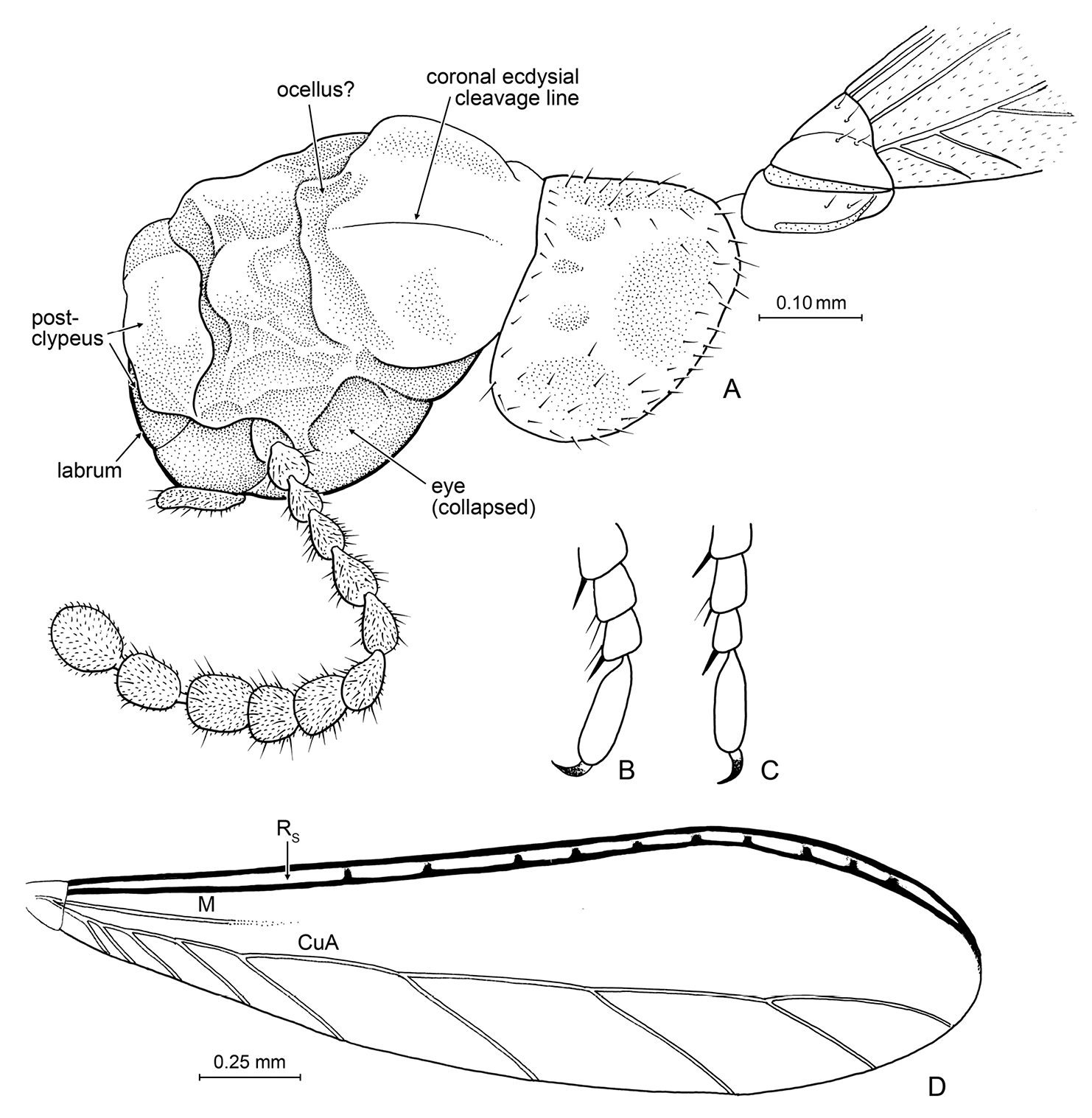
Imago: Minute termites (ca. 2.0 mm in length excluding wings, forewing ca. 2.6 mm), with head longer than wide and sparsely setose. Labrum possibly without dark, sclerotized transverse band, relatively long; postclypeus prominent and large; fontanelle apparently obscure or obscured (owing to folding of the cuticle); antenna moniliform, with 12 articles, increasing in size apicad (apical flagellomere 2x width of basal flagellomere). Forewing scale small, not overlapping hind wing scale, with basal suture relatively straight, humeral margin straight, all veins originating within scale, CuP relatively straight and terminating before basal suture; wing membrane microtrichose, not reticulate, not infuscate; Sc and R pigmented, remainder of veins faint; M and CuA become nebulous to spectral by about one-third wing length (CuA can be discerned by tilting specimen; apical two-thirds of M cannot be detected). Tibial spur formula 2-2-2; tibiae without outer spines; tarsi trimerous (similar in this respect to Indotermes Roonwal and Sen-Sarma of the Apicotermitinae: Speculitermes Group); pretarsal claws simple, arolium absent. Pronotum wider than long, slightly narrower than head; anterior margin straight, with very faint medial notch, apicolateral corners acutely rounded; lateral borders parallel-sided, with broadly-rounded posterior corners; posterior border relatively straight; setae nearly absent except a few along margins.
The new genus-group name is a combination of nanos (Gr., meaning, “small”), as this is probably the smallest known alate termite, and Termes, type genus of the Termitidae. The name is masculine.
urn:lsid:zoobank.org:act:8A3AF869-D486-4A22-94A5-C8F2DDC66D5D
http://species-id.net/wiki/Nanotermes_isaacae
Figs 1A, 7Imago; Tad-262 (Fig. 1A), India: Gujarat: Tadkeshwar lignite mine, Cambay Formation (Paleo-Eocene), 21°21.400"N, 73°4.532"E, 17–22 January 2010 (BSIPL).
As for the genus (vide supra).
As described for the genus with the following details: Imago: Total length without wings (as preserved) 2.0 mm; forewing length 2.60 mm; head length ~0.40 mm; length of head to base of clypeus 0.30 mm; clypeal medial length 0.06 mm; pronotal length (medial) 0.18 mm, width ~0.275 mm; length of forewing scale 0.15 mm. Integument of head and abdomen generally dark brown except labrum, postclypeus, and pronotum brown, antennae and legs light brown, labrum with apical margin white (as in some Nasutitermitinae). Integument (where evident) faintly imbricate to smooth. Head largely without setae except for a few laterally; forewing scale with only some sparse short setae.
Details of Nanotermes isaacae Engel & Grimaldi, gen. et sp. n. (Tad-262). A Head (as preserved, showing preservational distortion), pronotum, and base of right forewing B Protarsus, pro-pretarsus, and extreme apex of protibia C Metatarsus, meta-pretarsus, and extreme apex of metatibia D Forewing (reconstructed from both wings). Detail enlargements in B and C not to same scale.
The specific epithet is a matronym for Ms. Charlotte Isaac, who diligently processed and screened amber, and who discovered the holotype among many other interesting inclusions.
Although exploration of Cambay amber remains in its initial stages it is remarkable that such an interesting diversity of termites has already been recovered, with representatives of three different families, including the earliest evidence of the Termitidae. This relative diversity of Neoisoptera may reflect the origin of the amber deposit in dipterocarp forests formed at or near the paleoequator. The species here documented show largely Laurasian connections, particularly with the mid-Eocene fauna of Baltic amber, a pattern mirrored for many other insect lineages (e.g., bees, Pareuthychaeta [Diptera, Diastatidae]: Engel unpubl. data;
Parastylotermes krishnai can be readily distinguished from the other species of the genus owing to the more deeply branched medial vein in the forewing and the smaller number of antennal articles. A new genus could have been established for the species but this is presently unwarranted as the observed differences are relatively minor and, while putatively apomorphic, might render Parastylotermes paraphyletic. It could of course also be asked whether Parastylotermes is simply a stem group to Stylotermes and already paraphyletic with respect to the latter. While no phylogenetic analysis yet exists for the species of Stylotermitidae it would appear on the surface that Parastylotermes is monophyletic, or more precisely that at least Parastylotermes robustus and Parastylotermes krishnai are related. The fossil species from western North America are fragmentary, and their relationships are obscure. Most basal Neoisoptera have a 3-2-2 tibial spur formula and this is likely the plesiomorphic condition for this group, suggesting that the more reduced condition observed in Parastylotermes, i.e., 2-2-2 (loss of one of the protibial spurs), is synapomorphic for the genus, or perhaps between Parastylotermes and Prostylotermes, the latter discussed below.
Prostylotermes kamboja is a fascinating discovery in that it exhibits many putative plesiomorphic traits for the entire Neoisoptera clade, symplesiomorphies such as the presence of two cercomeres, styli in the male (absent in Parastylotermes and Stylotermes:
Zophotermes ashoki is particularly fascinating in that it agrees with Prorhinotermitinae in all traits except for the 2-2-2 tibial spur formula. It shares with Prorhinotermitinae and Psammotermitinae the unique branching of the forewing M from CuA outside of the scale (Fig. 5), otherwise unknown among the Rhinotermitidae. It lacks the flattened head or other distinctive traits of Psammotermitinae (vide supra). As noted above, the distribution of the 3-2-2 tibial spur formula across Rhinotermitidae, and even Neoisoptera as a whole, suggests that it is a plesiomorphic condition for these subfamilies, in which case the 2-2-2 tibial formula in Zophotermes is apomorphic. It is tantalizing to speculate that Zophotermes ashoki lacked a true worker caste as is unique to Prorhinotermes among modern Rhinotermitidae.
While it is difficult to identify phylogenetic affinities of Nanotermes given the challenge of working solely from alates, the significance of this fossil is that it is the oldest definitive representative of the Termitidae. All previous records of Termitidae are from the Late Oligocene or younger (
Partial support was provided by NSF DEB-0542909 and University of Kansas Ecology & Evolutionary Biology General Research Fund Allocation #2301465 (to MSE) and NSF DEB-0542726 (to DAG). Considerable thanks are extended to our colleagues Ashok Sahni and Rajendra S. Rana for their tireless efforts to promote research in India, and to Robert G. Goelet for his generous support of paleoentomological and exploratory research at the AMNH. Further gratitude is extended to Jennifer C. Thomas and Charlotte Isaac for their efforts in processing and screening amber at the SEMC and the AMNH, respectively, and to Enrique Peñalver and an anonymous reviewer for their suggested improvements. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum.