(C) 2012 Ivana Karanovic. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Austromesocypris bluffensis sp. n. is described and we report another species, Austromesocypris sp., both collected from subterranean aquatic habitats in Tasmania. This discovery adds a major taxonomic group to the already diverse invertebrate cave fauna of Tasmania, and is of interest because, globally, obligate subterranean aquatic species (stygobites) are poorly represented within the family Cyprididae. The genus Austromesocypris Martens, De Deckker & Rossetti, 2004 is otherwise known to comprise entirely “terrestrial or semi-terrestrial” species. The second species is not described because only juvenile specimens were collected. Both species stand apart from their congeners by the carapace shape, which is rectangular in Austromesocypris bluffensis and triangular and asymmetrical in the unnamed species. Another unique feature of the new species is the almost symmetrical uropodal rami. We also identify some broader systematic issues within the Scottiinae including the position of two New Zealand species, Scottia audax (Chapman, 1961) and Scottia insularis Chapman, 1963 in the genus, and point out their closer relationship to the Gondwana genera of Scottiinae, Austromesocypris and Mesocypris Daday, 1910, than to the Palearctic genus Scottia Brady & Norman, 1889, based on the morphology of the maxillula and mandibula. The identity of the Australian records of Scottia audax (Chapman, 1961), Austromesocypris australiensis (De Deckker, 1983) and the Boreal records of Scottia pseudobrowniana Kempf, 1971 are all considered doubtful. A key to the world species of Scottiinae is provided.
Ostracods, biodiversity, Cyprididae, Australia
Twelve freshwater podocopid ostracods belonging to the superfamily Cypridoidea and family Cyprididae are known from Tasmania (Table 1). The family Candonidae and representatives of the superfamily Cytheroidea, chiefly the family Limnocytheridae, have been recorded from Quaternary deposits (
List of ostracod species recorded from Tasmania. R - Recent, F - Fossil.
| Species | Record | Reference |
|---|---|---|
| Superfamily Cytheroidea | ||
| Australocypris robusta (De Deckker, 1974) | R |
|
| Candonocypris incosta De Deckker, 1982 | R | De Deckker (1982) |
| Diacypris spinosa De Deckker, 1981 | R | De Deckker (1981) |
| Diacypris dietzi (Herbst, 1958) | R | Herbst (1958) |
| Kennethia cristata De Deckker, 1979 | R | De Deckker, 1979 |
| Mytilocypris praenuncia (Chapman, 1966) | R |
|
| Mytilocypris splendida (Chapman, 1966) | R |
|
| Mytilocypris tasmanica McKenzie, 1966 | R |
|
| Mytilocypris mytiloides (Brady, 1886) | R |
|
| Mesocypris tasmaniensis De Deckker, 1983 | R |
|
| Newnhamia fenestrata King, 1855 | R |
|
| Sarscypridopsis aculeata (Costa, 1847) | R |
|
| Candona tecta De Deckker, 1982 | F |
|
| Candonopsis tenuis (Brady, 1886) | F |
|
| Superfamily Cytheroidea | ||
| Limnocythere mowbrayensis Chapman, 1914 | F |
|
| Gomphodella australiaca (Hussainy, 1969) | F |
|
Since their first appearance in the early Palaeozoic, podocopid ostracods have occurred in both marine and freshwater habitats, and today can be found from abyssal depths to small freshwater puddles, and even terrestrial environments (
Animals living away from free-standing or flowing water have usually very hirsute shell and soft parts, which probably allows them to retain enough moisture in semi-terrestrial surroundings (
During the recent study of Tasmanian caves, two interesting new species were collected. One is named and described here, while the other is only briefly described but left as an open nomenclature, because only a juvenile specimen was collected. They both belong to the genus Austromesocypris Martens, De Deckker and Rossetti, 2004, a very peculiar and otherwise entirely “terrestrial” genus known only from Australia (
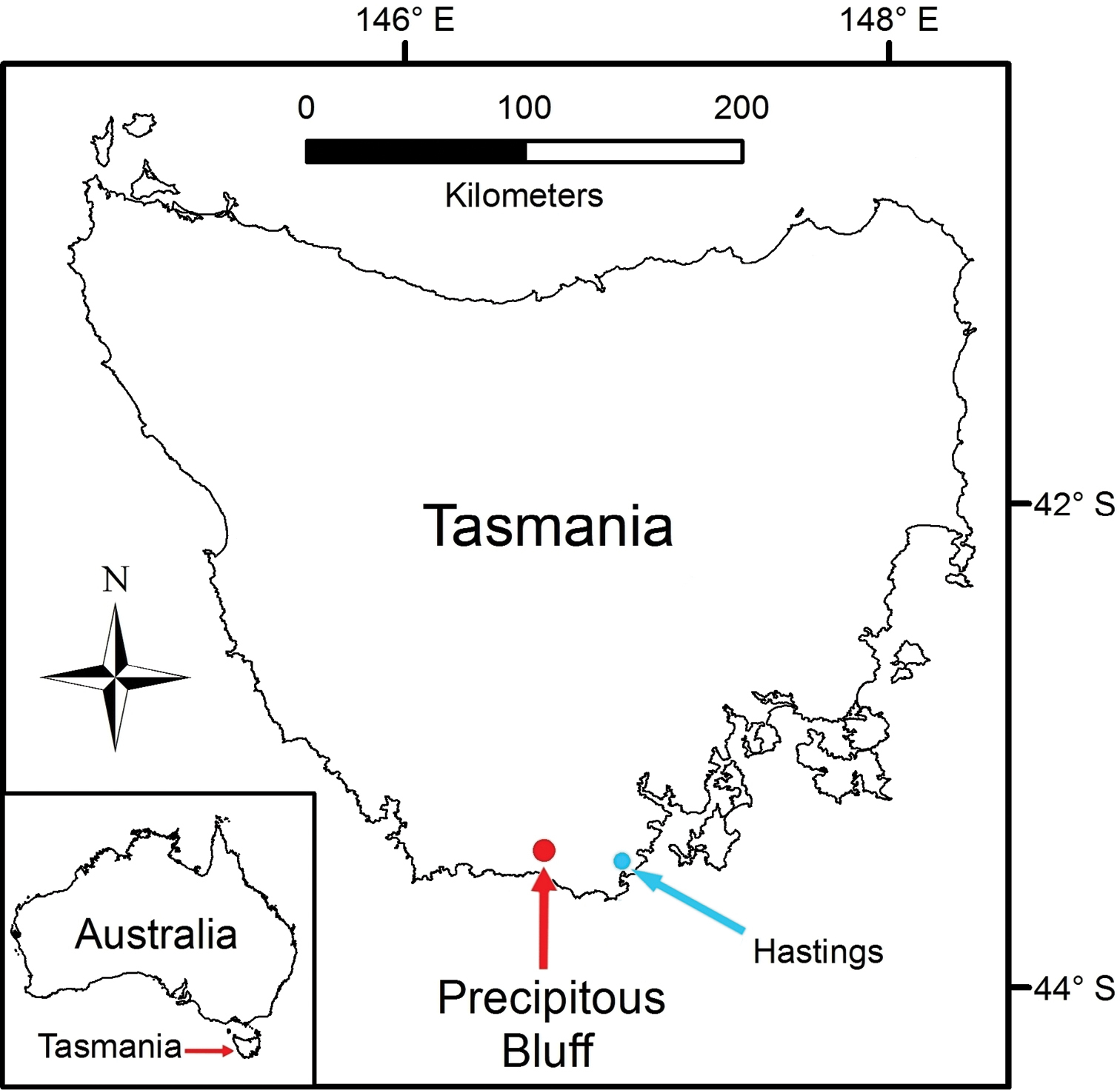
Precipitous Bluff is located near the south coast of Tasmania in a remote and inaccessible part of the Tasmanian Wilderness World Heritage Area (Figure 1). The area remains essentially undisturbed by human activities except for occasional visits by bushwalkers and cavers. An extensive deposit of highly karstic and cavernous limestone of Ordovician age outcrops over about 10 km2 between 0–300 meters above sea level (asl) on the western and southwestern flanks of Precipitous Bluff (1120 m asl) (
Tasmania, Australia, showing location of Precipitous Bluff study area.
Precipitous Bluff (1140 m asl) showing heavily forested lower slopes which contain the karst and caves.
Typical cool temperate rainforest overlying the karst and caves.
The caves have developed by allogenic recharge from streams descending from the upper slopes of the mountain which sink underground on contact with the limestone. Autogenic recharge is also received from rainfall directly on the limestone outcrop, which percolates downward via a multitude of vertical shafts and fissures in the limestone (Figure 4). The descending subsurface flow paths coalesce at lower levels to form an integrated dendritic cave drainage network which discharges from resurgence caves situated at base level on the slope-plain juncture. Damper Cave is a major resurgence cave with more than one km of surveyed underground passages (Figure 5). The cave consists of a major conduit carrying a permanent flowing stream with a bedrock-gravel-sandy bed, in addition to numerous small tributary streams and seepages.
Typical karst terrain and cool temperate rainforest vegetation in the study area.
Damper Cave entrance and stream resurgence. Collection sites were located approximately 20 to 80 m inside the cave entrance, see next figures.
The cave fauna at Precipitous Bluff was surveyed as part of a state-wide biodiversity survey of Tasmanian karsts undertaken by
Aquatic micro- and meiofauna were sampled from a variety of habitats in Damper Cave in May 2011 using 150 µm mesh plankton nets. Three types of aquatic habitats were sampled: (1) main stream (Site A); (2) small tributary stream (Site B); and (3) seepage water from dripping stalactites (Sites, C, D, E). To sample the main stream benthos, shallow interstitial and stream drift fauna, two 300 mm diameter plankton nets were installed in the main flow of the stream approximately 30 m upstream from the resurgence entrance (Figure 6). Benthic and shallow interstitial fauna was sampled by ‘kick' sampling which involved walking upstream from the nets for a distance of approximately 50 m while ‘kicking' the gravel-sandy sediments to dislodge fauna which was swept downstream into the nets. A small tributary stream approximately 20 m inside the resurgence entrance was sampled by placing a bucket and net underneath a vertical trickle (Figure 7). Seepage water from dripping stalactites was sampled in a similar manner. The nets were left in the cave for two days and then recovered, where after the net contents were elutriated and preserved in 100% ethanol.
Damper Cave main stream collection Site A with drift nets.
Damper Cave tributary stream collection Site B with net.
Specimens were dissected and mounted on microscope glass slides in Faure's medium, which was prepared following the procedure of
Terminology. In the present paper the terminology for the most posterior appendage on the body, the so-called uropodal ramus, follows
Abbreviations used in text and figures. A1-antennula; A2-antenna; LV-left valve; L5-L7-fifth-seventh limb; Md-mandibula; Mxl-maxillula; RV-right valve; UR-uropodal ramus.
Taxonomy Class Ostracoda Latreille, 1806Subclass Podocopa Müller, 1894
Order Podocopida Sars, 1866
Superfamily Cypridoidea Baird, 1845
Family Cyprididae Baird, 1845
Subfamily Scottiinae Bronstein, 1947
Austromesocypris berentsae Martens, De Deckker and Rossetti, 2004; original designation.
Austromesocypris australiensis (De Deckker, 1983); Austromesocypris bluffensis sp. n.; Austromesocypris tasmaniensis (De Deckker, 1983)
urn:lsid:zoobank.org:act:A2DDB15B-AC3D-433C-9FFC-561AFBFDEAAD
http://species-id.net/wiki/Austromesocypris_bluffensis
Figures 8A, B, 9 –11Holotype, female (TMAG 6206) Damper Cave, 43°30'S, 146°35'E, Precipitous Bluff, 90 km SW of Hobart, Tasmania, Australia, Site A, main stream, 30 m from entrance, 14 May 2011 (dissected on one slide), collected by R. and S. Eberhard, G. Perina, S. Catomore.
Ostracods with smooth, transparent shell densely covered with setulae. Dorsal margin straight, anterior and posterior margins almost equally wide. Calcified inner lamella narrow. A1 with fused third and fourth segments. A2 with only two short swimming setae and two t-setae. Md-palp with pappose γ-seta. L5 with one a-seta and one d-seta. L6 with d2-seta, short e-seta, second and third endopodal segments fused, and long terminal claw. UR with row of long, strong setulae along posterior margin; both distal claws strong with strong spines; anterior seta long. Rami almost symmetrical, only one ramus with slightly shorter setulae along posterior margin. Genital field with one clear thumb-like projection.
The species is named after its type locality.
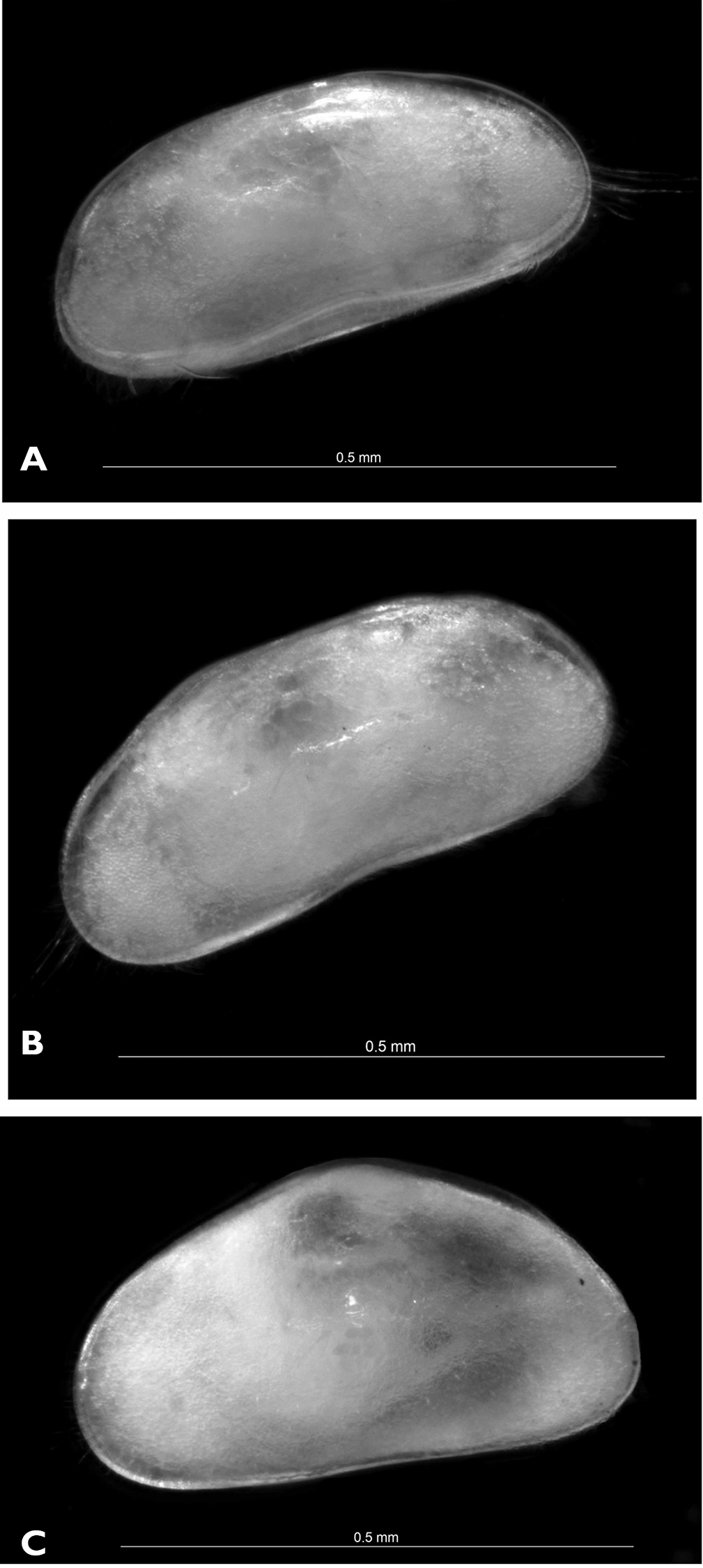
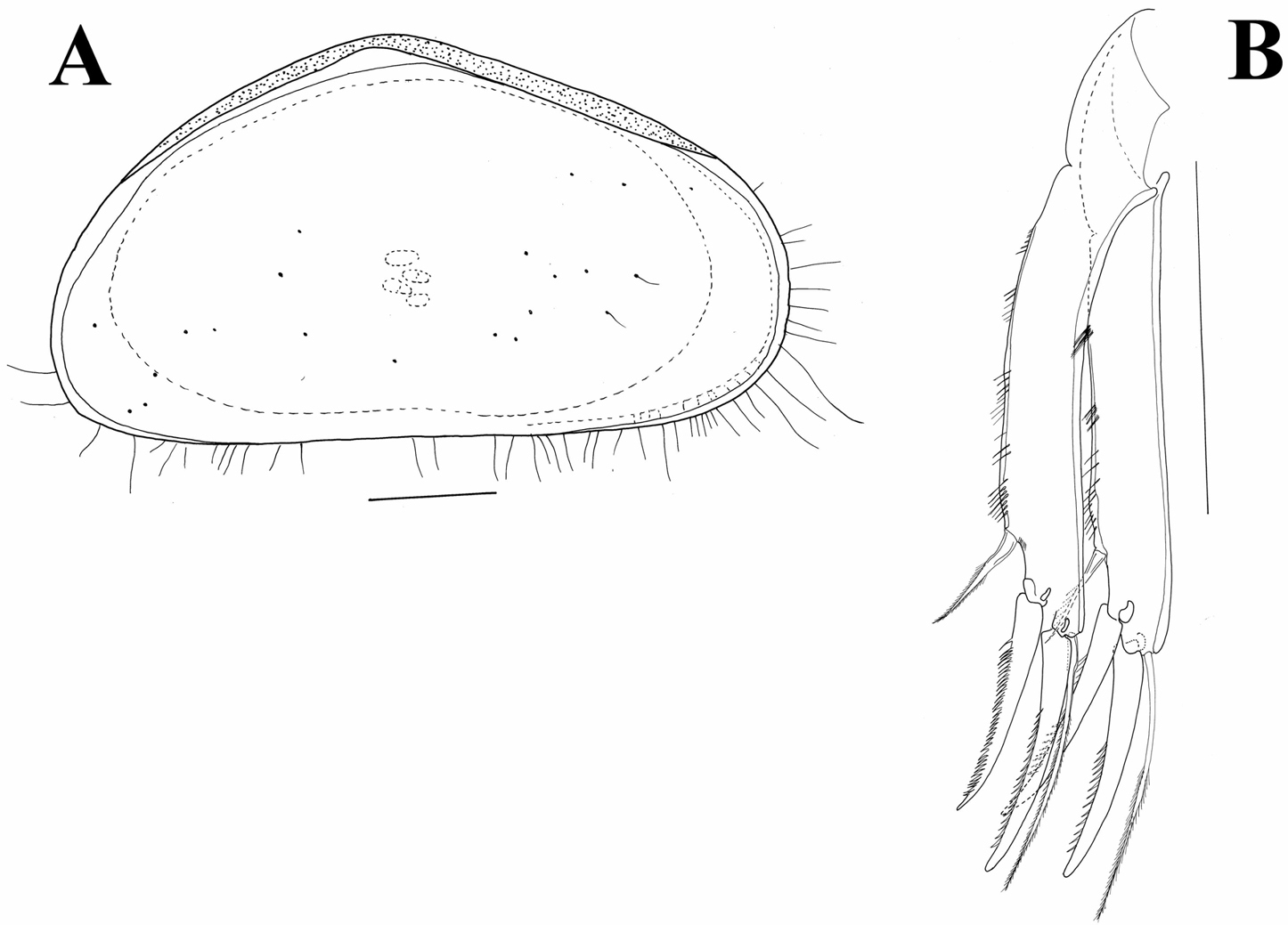
Carapace (Figs 8A, B; 9A). Rectangular in lateral view; 0.58 mm in length. Greatest height situated around middle length, equalling 0.26 mm, or 45% of length. Valves clearly asymmetrical, RV being shorter than left one. Dorsal margin straight on almost entire length, rounding towards posterior end and inclined with small recess towards anterior end. Both anterior and posterior ends rounded, anterior end more so and slightly wider than posterior one. Ventral margin straight or very slightly concave. Anterior and posterior inner calcified lamellae narrow. Marginal pore canals short, except in ventral region where enlarged. Surface densely covered with relatively short hair-like setae.
A, B Austromesocypris bluffensis (Holotype) C Austromesocypris sp. A shell, view from the right side B, C shell, view from the left side.
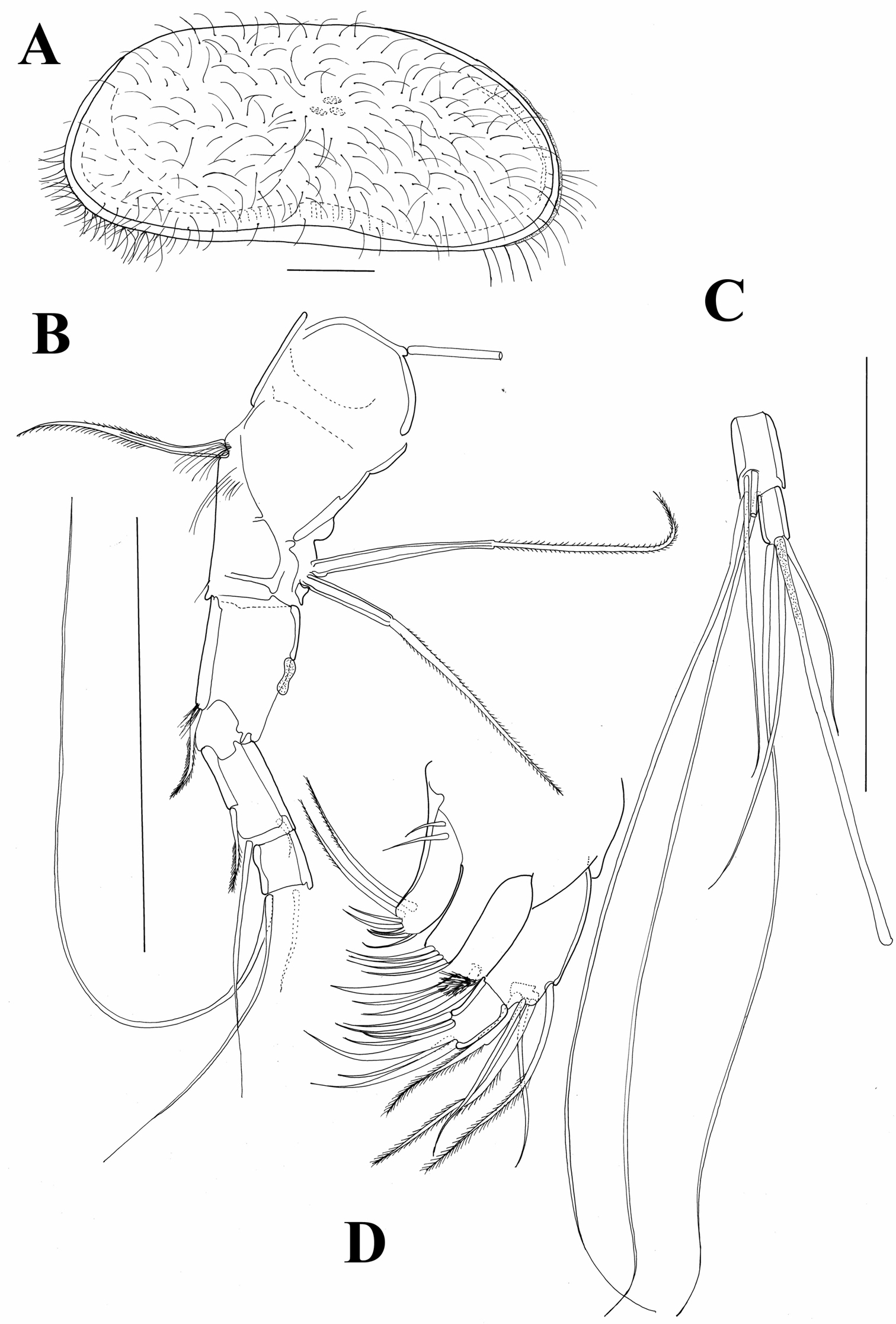
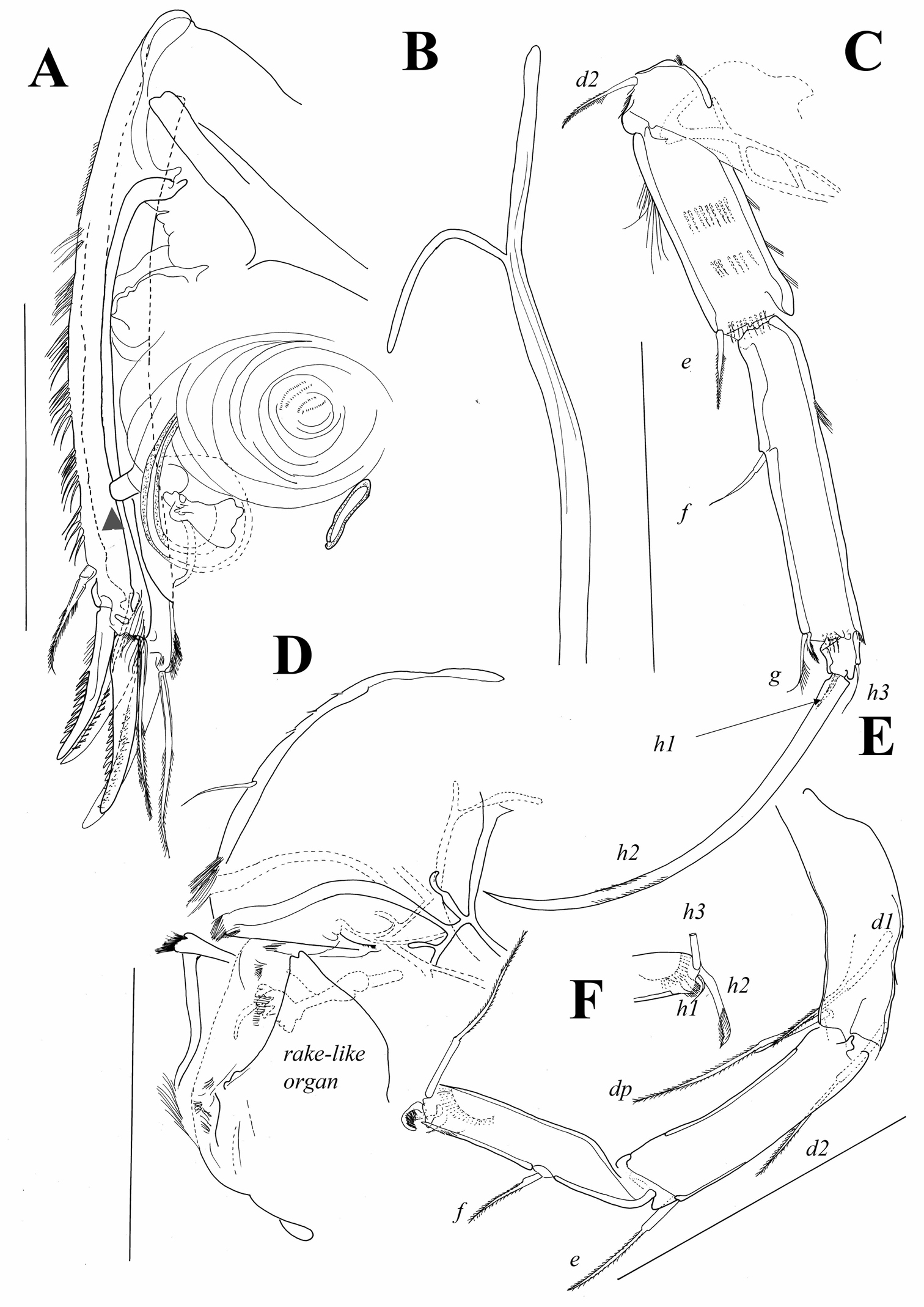
A1 (Fig. 9B, C). First segment hirsute, anteriorly with only one pappose seta, reaching middle length of following segment; posteriorly with two, almost equally long, pappose setae originating close to each other and near distal margin of segment. Wouters organ not observed. Following segment with very short Rome organ posteriorly and one pappose seta anteriorly, reaching middle of following segment. Above this seta cluster of setulae present. Third segment compound, representing fused segments three and four; point of their fusion clearly marked by one short pappose seta, which almost reaches distal margin of following free segment. Distal margin of fused segment with one posterior, short and smooth seta and one long, also smooth seta anteriorly. This seta as long as length of all segments combined. Segment following fused segments (fifth segment) very short with two long and smooth setae anteriorly and one shorter seta posteriorly. Penultimate segment with total of four anterior setae, all situated very close to each other, three being longer than length of all segments combined and one equalling 1/3 of their length. Terminal segment with one long and two shorter setae, this segment with long aesthetasc y1 being eight times longer than terminal segment. Length ratios of last four segments equalling 1.8 : 1 : 1.5 : 1.
Austromesocypris bluffensis (Holotype): A shell, lateral view from the right side B A1 C two distal segments of the A1 D Mxl. Scales = 0.1 mm.
A2 (Fig. 10A). Coxa with three pappose, equally long setae: one situated more proximally (externally) and two more distally (internally) on segment. Coxa with three rows of small setulae. Basis laterally with cluster of setulae and one anterior seta, which reaches distal end of first endopodal segment. Exopod representing small plate with three setae: most anterior one distally pappose and reaching distal margin of first endopodal segment; middle one also pappose and much shorter; most posterior one even shorter and also smooth. Endopod 3-segmented. First segment with two strong setulae along anterior margin and row of short setulae antero-distally; aesthetasc Y situated in middle of posterior margin and reaching distal end of segment; postero-distal seta pappose and extends beyond middle of second endopodal segment. Two short swimming setae situated antero-distally on segment and only one reaches 1/3 of second endopodal segment. Second endopodal segment with cluster of setulae mid-laterally; seta on middle of anterior margin distally pappose and reaches distal end of segment. Second endopodal segment posteriorly with two short aesthetascs: y1 situated more proximally and y2 at distal margin. Same segment postero-medially with three t-setae: t1 distally pappose and shorter than t2 and t3. Second endopodal segment with three smooth and equally long z-setae on distal margin, all reaching middle of terminal claws. G2 claw equalling 2/3 of G1 claw. G1 and G3 equally long and only slightly shorter than first endopodal segment. Terminal segment short with GM claw equalling 70% of first endopodal segment; Gm claw being 2/3 of GM. Same segment with one additional thin seta which is as long as Gm claw; aesthetasc y3 of same length and proximally fused with one thin seta.
Forehead and lips (Fig. 11D). Hirsute with numerous sclerotized rods and rake like organ carrying about six blunt teeth.
Austromesocypris bluffensis (Holotype): A A2 B L5 C Md-palp. Scales = 0.1 mm.
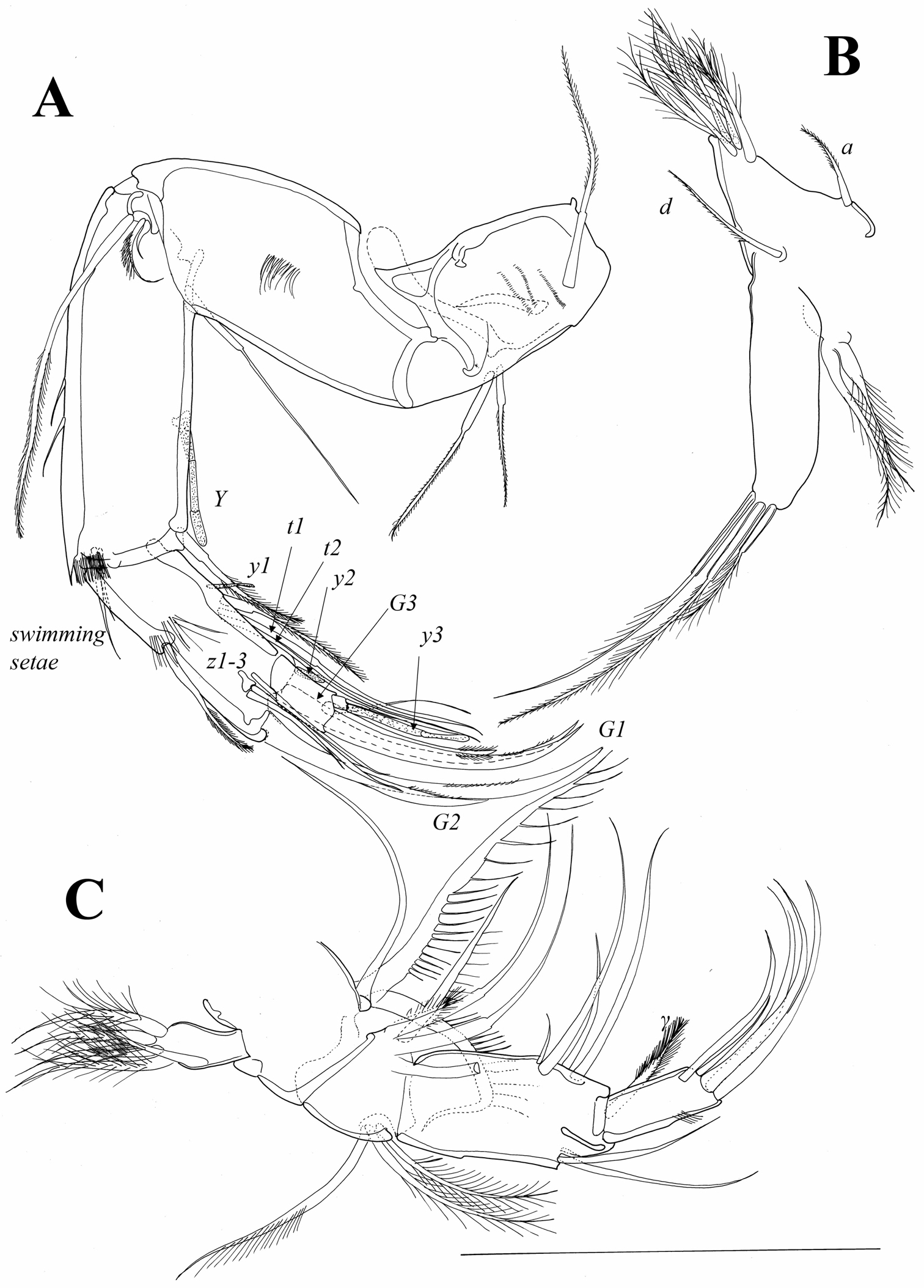
Austromesocypris bluffensis (Holotype): A UR, arrow indicating the genital process B attachment of the UR C L6 D forehead and upper lip E L7 F detail of the distal end of L7. Scales = 0.1 mm.
Md (Fig. 10C). Exopod short carrying five plumose, vibratory setae. Palp 4-segmented. First segment posteriorly with three setae, one smooth and other two with one row of long and strong setulae; one of these setae bent. Alpha seta, usually present on this segment, not observed. Second segment anteriorly with three equally long pappose setae not reaching distal margin of following segment; posteriorly with three long, smooth setae, one shorter seta with setulae along one margin, and one short and plumed seta (β-seta). Penultimate segment anteriorly with one very short seta and two long and smooth setae each exceeding distal end of terminal segment; posteriorly with total of four setae: two long and two shorter, one of which half as long as long setae, other ¼ length of these setae; γ-seta plumed, exceeding distal end of terminal segment. Terminal segment elongated (2.5 times longer than wide) with four distally curved claws and one seta half as long as claws.
Mxl (Figure 9D). Palp 2-segmented. First segment with three pappose and two smooth setae, all situated close to outer margin. Terminal segment 1.8 times longer than wide, with two claws (one fused with segment) and four setae. First endite distally with two long, pappose setae and five short claws; proximally with two short and smooth setae. Second endite with four short claws. Third endite with four claws (one fused with segment) and cluster of short setulae on anterior margin.
L5 (Fig. 10B). Exopod with two plumose vibratory setae. Endopod with three distal pappose setae, two being twice as long as third. Protopod with one pappose a-seta, one pappose d-seta and six distal pappose setae.
L6 (Fig. 11C). Basal segment (basis) with short pappose d2-seta and marginal rows of setulae. First endopodal segment with long anterior setulae, and three medial rows of shorter setulae; same segment antero-distally with short and pappose e-seta. Following segment compound, representing fused second and third segments, with f-seta near middle of anterior margin and distally with two setae (one being g-seta) and row of marginal setulae. Terminal segment with two thin setae (h1 and h3) and one strong claw (h2); latter gently serrated and 0.72 times as long as endopodal segments combined.
L7 (Fig. 11E, F). Composed of three segments plus terminal pincer. First segment with three long, pappose setae (d1, d2 and dp). Second segment with pappose e-seta reaching half length of following segment. Third segment compound, representing fused endopodal segments two and three, with f-seta near middle of anterior margin; g-seta absent. Terminal segment reduced and transformed into pincer organ. Seta h1 very thin and curved, h2-seta claw-like and distally pappose, seta h3 normal and almost as long as penultimate segment.
UR (Fig. 11A). Posterior margin with groups of long and strong setulae; posterior seta situated close to distal margin, being pappose and more than half as long as posterior claw. Posterior claw only slightly shorter than anterior one; both claws strongly serrated. Anterior seta distally pappose and as long as anterior claw. Rows of setulae on posterior margin of one ramus slightly weaker. Length ratios between anterior margin of ramus and anterior and posterior claws equalling 2.9 : 1.1 : 1.
Attachment of UR (Fig. 11B). Distally bifurcate, with no additional ventral or dorsal branches.
Genital field (Figure 11A) with clear thumb-like projection (indicated by arrow on Figure 11A).
Not known.
Austromesocypris bluffensis sp. n. stands apart from all other species of the genus in having a completely flat dorsal margin of the carapace and almost symmetrical UR. In addition, it differs from the New South Wales species, Austromesocypris berentsae Martens, De Deckker and Rossetti, 2004, in having only one segment on the A1 fused. Austromesocypris berentsae is the only species in the genus that has two segments fused (3+4, 5+6). Austromesocypris bluffensis differs from Austromesocypris australiensis (De Deckker, 1983) by having structurally similar posterior setae on both UR. Only Austromesocypris australiensis, among all species of the genus, has one seta transformed into a strong claw, the other being seta-like. The one previously described species from Tasmania, Austromesocypris tasmaniensis (De Deckker, 1983), has much stronger spines along the posterior margin of the UR. The UR of the new species is the most similar to Austromesocypris berentsae, but the former species has shorter spine-like setae along the posterior margin of the ramus. It appears that the gamma-seta on the Md-palp in Austromesocypris berentsae is not pappose (see
One juvenile female (A-7 or A-8) (TMAG 6207), Damper Cave, Precipitous Bluff, 43°30'S, 146°35'E, 90 km SW of Hobart, Tasmania, Australia, Tributary stream 20 m inside entrance, 14 May 2011 (dissected on one slide), collected by R. and S. Eberhard, G. Perina, S. Catomore
Triangular shell (Figs 8C, 12A) with left valve overlapping right one. Anterior and posterior margins rounded with long marginal hair-like setae. Surface not highly hirsute. Length 0.58 mm. UR (Fig. 12B) with anterior margin sparsely covered with strong setulae (same on both rami). Posterior seta pappose and only 1/3 length of posterior claws. Both claws strongly serrated; posterior one only slightly shorter. Anterior seta pappose and longer than anterior claw.
Austromesocypris sp.: A shell, external view from the right side B UR. Scales = 0.1 mm.
This species is assigned to the genus Austromesocypris Martens, De Deckker & Rossetti, 2004 based on the appearance of the UR. The undescribed female was probably a late juvenile (A-7 or A-8), as indicated by the well-developed appendages (including the 6-segmented A1). However, the setae on the L6 and L7 had a swollen base and the oviducts, as well as the entire genital field, were undeveloped. Until now, no Scottiinae were known with such a triangular and asymmetrical carapace shape. An unnamed fossil species, Mesocypris sp., from the Pulbeena Swamp in Tasmania (De Deckker 1982) also has a highly arched carapace, but the posterior margin in this species is broader and the LV is not higher than the RV.
Other taxa collected in the Damper Cave samples included: a) Copepoda (Diacyclops cryonastes Morton, 1985), Amphipoda (Paramelitidae), Isopoda (Styloniscidae), Oligochaeta (Phreodrilidae), Gastropoda (Hydrobiidae), Nematoda, Turbellaria (Tricladida), Diptera, Plecoptera, and Ephemeroptera from the main stream Site; and b) Isopoda (Janiridae: Heterias sp.) from the stalactite drip Site E.
No Ostracoda have previously been described from Tasmanian caves or other groundwater environments.
Discovery of cave dwelling ostracods described in this paper is of interest for two reasons. Firstly, members of Cyprididae are rarely found in subterranean waters. Secondly, the genus Austromesocypris is otherwise known to comprise entirely “terrestrial and semi-terrestrial” species. The rare finds of Cyprididae in subterranean waters are mostly records of morphologically unmodified taxa which occur as facultative or incidental inhabitants of groundwaters. The only documented case of an obligate association of cyprids with groundwaters involves the genus Pseudocypridopsis Karanovic, 1999, with two described stygobitic species from the Balkan Peninsula (
The collection of a whole specimen of Austromesocypris bluffensis sp. n. in the main stream implies that this animal could have been living in the benthos or interstitial of the main stream, but also does not preclude an origin from a tributary stream or seepage waters, of which the latter two ultimately discharge into the main stream. The collection of valves in seepage water from dripping stalactites and a complete specimen of Austromesocypris sp. in the small tributary stream confirms the occurrence of Austromesocypris in these habitats also. Notwithstanding they might still have originated from the surface which lies only a few tens of meters above Damper Cave at the point where the collections were made. Certainly the cool temperate rainforest represents a near-permanently moist habitat for hygrophilous invertebrates including, potentially, terrestrial/semi-terrestrial ostracods. The highly permeable karst at Precipitous Bluff ensures a close eco-hydrological connection between epigean and hypogean environments, confirmed by the occurrence of other normally epigean-terrestrial invertebrates such as charopid gastropods accidentally washed into the caves by percolating seepage waters (S. Eberhard personal observation).
Adaptive morphologySome adaptive morphologies, typically comprising numerous reductions, displayed in semi-terrestrial epigean ostracods are similar to those seen in aquatic subterranean ostracods as already pointed out by several authors (
The unusual shape of the two new Tasmanian species, one with trapezoidal shape, the other with triangular shape, suggests that these might also be true stygobites as such modification is very common among subterranean Candoninae (
Austromesocypris is according to
All species of Austromesocypris have only two swimming setae on the A2, while in Mesocypris the number varies from six to two. There are, however, two characters which indicate the two genera are more closely related to each other than either is to the genus Scottia. Namely, the terminal segment on the Md-palp is much more elongate and the number of setae on the first segment of the Mxl-palp is reduced in Mesocypris and Austromesocypris in comparison to Scottia. According to Chapman's (1961) illustration of the Md of Scottia audax (Chapman, 1961)and Chapman's (1963) drawings of the Mxl of Scottia insularis Chapman, 1963, the two New Zealand species appear to be more closely related to members of Mesocypridini. Scottia audax was originally described in the genus Mesocypris, but
The position of the two New Zealand species is not certain at the moment, but they definitely should be excluded from the genus Scottia. While they share many characters with Mesocypris, most of which are variable, they also have a reduced number of segments on the A1 like Austromesocypris. Fusion of A1 segments can also be partial, as noted by
In our opinion Australian records of Scottia audax (Chapman, 1961) by
In our opinion De Deckker's (1983) report of Austromesocypris australiensis also represents two species which differ most significantly in the appearance of the hemipenis, prehensile palps and the number of the rosettes on the Zenker organ. One was found in far north Queensland, and the other in New South Wales and Victoria, but both were identified as Austromesocypris australiensis by
Misidentified Scottiinae are not confined to Australian records. Reports of the “widely distributed” Scottia pseudobrowniana from Tennessee (
Scottia pseudobrowniana from Russia, as illustrated by
| 1 | First segment of the Mxl-palp with seven or eight setae, terminal segment of the Md-palp maximum 1.5 times longer than wide, “e” and “f” setae on L6 long and equal | 2 |
| – | First segment of the Mxl-palp with maximum five setae, terminal segment of the Md-palp at least two times longer than wide, “e” seta rarely long, and if so, “f” seta equals half its length | 6 |
| 2 | Swimming setae on the A2 only slightly exceed distal end of the first endopodal segment | [non] Scottia pseudobrowniana Kempf, 1971 in |
| – | Swimming setae on the A2 longer | 3 |
| 3 | Females in lateral view with dorsal margin evenly rounded towards both anterior and posterior ends, so that both ends equally wide and relatively narrow; males with inclined distal margin on the medial lobe of the lateral shield of the hemipenis towards dorsal side, and medial lobe of the lateral shield with a very short dorsal margin | [non] Scottia pseudobrowniana Kempf, 1971 in |
| – | Females in lateral view with clearly wider posterior margin and the dorsal margin more broadly rounded towards posterior than towards anterior end; males with distal margin of the medial lobe rounded, but almost parallel to the rest of the hemipenis and this lobe with a longer dorsal margin | 4 |
| 4 | Medial lobe of the lateral shield on the hemipenis very narrow, almost finger like | [non] Scottia pseudobrowniana Kempf, 1971 in |
| – | Medial lobe of the lateral shield rectangular- to square-shaped | 5 |
| 5 | Dorsal lobe of the lateral shield on the hemipenis with a prominent projection | Scottia birigida Smith et al., 2002 |
| – | No such projection present | Scottia pseudobrowniana Kempf, 1971 |
| 6 | At least one of the UR posterior seta elongated and seta-like, reaching at least half of the posterior claw | 7 |
| – | This seta very short, thick and claw-like on both UR | 14 |
| 7 | Penultimate segment on the L6 divided | 8 |
| – | Penultimate segment fused | 10 |
| 8 | Seta “h3” on the L6 reaching or exceeding half the length of the terminal claw | 9 |
| – | This seta at most reaching 1/3 of the length of the terminal claw | Scottia insularis Chapman, 1963 |
| 9 | Posterior margin of the UR covered with strong spines, and anterior end of the carapace more elongated | Scottia audax (Chapman, 1961) |
| – | Posterior margin of the UR covered with small spine-like setae and carapace in lateral view more tumid | [non] Scottia audax (Chapman, 1961) in |
| 10 | Four segments on the A1 fused, so that A1 is 5-segmented | Austromesocypris berentsae Martens, De Deckker & Rossetti, 2004 |
| – | Only two segments on the A1 fused, so that A1 is 6-segmented | 11 |
| 11 | One posterior seta on UR transformed into a thick claw, other more slender and seta-like | Austromesocypris tasmaniensis (De Deckker, 1983) |
| – | Both posterior setae slender | 12 |
| 12 | Shell with straight dorsal margin, shape almost rectangular | Austromesocypris bluffensis sp. n. |
| – | Shell with gently rounded dorsal margin, shape more reniform | 13 |
| 13 | Zenker organ with 10 rosettes of spines, hemipenis slender | [non] Austromesocypris australiensis (De Deckker, 1983) in |
| – | Zenker organ with more than 15 rosettes of spines, hemipenis robust | Austromesocypris australiensis (De Deckker, 1983) |
| 14 | Seta “h3” on the L6 claw-like | Mesocypris pauliani Danielopol & Betsch, 1980 |
| – | Seta “h3” on the L6 seta-like | 15 |
| 15 | Anterior seta on the UR short, not reaching 1/3 the length of the anterior claw | Mesocypris pubescens Daday, 1910 |
| – | This seta much longer, exceeding half the length of the anterior claw | 16 |
| 16 | Seta “e” on the L6 long, almost reaching the distal end of the penultimate segment | Mesocypris terrestris Harding, 1953 |
| – | Seta “e” on the L6 much shorter, not reaching middle of the penultimate segment | Mesocypris madagascariensis Danielopol & Betsch, 1980 |
We are very grateful to Rolan Eberhard and the Tasmanian Department of Primary Industries, Water and Environment for supporting the field study by providing permits, helicopter access and field support.
List of ostracod species considered “terrestrial or semi-terrestrial” or often associated with those habitats
Superfamily Cypridoidea Baird, 1845
Family Cyprididae Baird, 1845
Subfamily Callistocypridinae Schornikov, 1980
Genus Callistocypris Schornikov, 1980
Callistocypris mckenzie Pinto, Rocha & Martens, 2005: Saõ Paolo State, Brazil (
Callistocypris rossetti Pinto, Rocha & Martens, 2005: Saõ Paolo State, Brazil (
Callistocypris zlotini Schornikov, 1980: Solomon Islands (
Subfamily Cypridopsinae Kaufmann, 1900
Genus Bryocypris Røen, 1956
Bryocypris grandipes Røen, 1956: Cameroon (
Subfamily Scottinae Bronstein, 1947
Tribe Mesocypridini Puri, 1974
Genus Austromesocypris Martens, De Deckker & Rossetti, 2004
Austromesocypris australiensis (De Deckker, 1983): Victoria, Australia (
Austromesocypris bluffensis sp. nov.: Tasmania (present paper)
Austromesocypris berentsae Martens, De Deckker & Rossetti, 2004: New South Wales, Australia (
Austromesocypris tasmaniensis (De Deckker 1983): Tasmania, Australia (
Genus Mesocypris Daday, 1910
Mesocypris madagascariensis Danielopol & Betsch, 1980: Madagascar (
Mesocypris pauliani Danielopol & Betsch, 1980: Madagascar (
Mesocypris pubescens Daday, 1910: Kilimanjaro (
Mesocypris terrestris Harding, 1953: Malawi (
Tribe Scottini Martens, De Deckker & Rossetti, 2004
Genus Scottia Brady & Norman, 1889
Scottia audax Chapman, 1961: New Zealand (
Scottia birigida Smith, Matzke-Karasz, Kamiya & Ikeda, 2002: Japan (
Scottia insularis Chapman, 1963: New Zealand (
Scottia pseudobrowniana Kempf, 1971: Holarctic (
Family Candonidae Kaufmann, 1900
Subfamily Candoninae Kaufmann, 1900
Tribe Terrestricypridini Schornikov 1980
Genus Caaporacandona Pinto, Rocha & Martens, 2005
Caaporacandona iguassuensis Pinto, Rocha & Martens, 2005: Paraná State, Brazil (
Caaporacandona shornikovi Pinto, Martens & Rossetti, 2005: Paraná State, Brazil (
Genus Terrestricandona Danielopol & Betsch, 1980
Terrestricandona minuta Danielopol & Betsch, 1980: Madagascar (
Genus Terrestricypris Schornikov, 1980
Terrestricypris arborea Schornikov, 1980: Solomon Islands (
Terrestricypris wurdigae Pinto, Rocha & Martens, 2005: Saõ Paolo State, Brazil (
Superfamily Cytheroidea Baird, 1850
Family Limnocytheridae Klie, 1938
Subfamily Timiriaseviinae Mandelstam, 1960
Genus Intrepidocythere Pinto, Rocha & Martens, 2005
Intrepidocythere ibipora Pinto, Rocha & Martens, 2005: Saõ Paolo State, Brazil (
Superfamily Terrestricytherioidea Schornikov, 1969
Family Terrestricytheridae Schornikov, 1969
Genus Terrestricythere Schornikov, 1969
Terrestricythere elisabethae Horne, Smith, Whittaker & Murray, 2004: England (
Terrestricythere ivanovae Schornikov, 1969: Kuril Islands (
Terrestricythere pratensis Schornikov, 1980: Vladivostok, Russia (
Terrestricythere proboscidea Hiruta, Hiruta & Mawatari, 2007 (
Superfamily Darwinuloidea Brady & Norman, 1889
Family Darwinulidae Brady & Norman, 1889
Genus Penthesilenula Rossetti & Martens, 1998
Penthesilenula aotearoa (Rossetti, Eagar & Martens, 1998): Saõ Paolo State, Brazil (
Penthesilenula brasiliensis (Pinto & Kotzian, 1961): Saõ Paolo State, Brazil (
Penthesilenula reidae Pinto, Rocha & Martens, 2004: Saõ Paolo State, Brazil (
Genus Vestalenula Rossetti & Martens, 1998
Vestalenula botocuda Pinto, Rocha & Martens, 2003: Saõ Paolo State, Brazil (
Vestalenula irajai Pinto, Rocha & Martens, 2003: Saõ Paolo State, Brazil (