(C) 2012 Azad Teimori. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of tooth-carp, Aphanius arakensis sp. n., is described from the Namak Lake basin in Iran. The new species is distinguished by the congeners distributed in Iran by the following combination of characters: 10–12 anal fin rays, 28–32 lateral line scales, 10–13 caudal peduncle scales, 8–10 gill rakers, 12–19, commonly 15–16, clearly defined flank bars in males, a more prominent pigmentation along the flank added by relatively big blotches in the middle and posterior flank segments in females, a short but high antirostrum of the otolith that has a wide excisura, and a ventral rim with some small, drop-like processes, and 19 molecular apomorphies (17 transitions, two transversions) in the cytochrome b gene. It was suggested based on the phylogenetic analysis that the new species is sister to Aphanius sophiae from the Kor River and that Aphanius farsicus from the Maharlu Lake basin is sister to Aphanius arakensis plus Aphanius sophiae. A noticeable feature of the Aphanius diversity in Iran is the conservatism of the external morphology as well as morphometric and meristic characters, while distinctive differences are present in genetic characters, otolith morphology, and male color pattern. Transformation of the latter was probably driven by sexual selection.
Male color patterm, freshwater fish, tooth-carp, biodiversity, evolution, sexual selection
Aphanius is the only representative of the Cyprinodontidae (Teleostei, Cyprinodontiformes) in Eurasia. The genus occurs in coastal (brackish) and landlocked (freshwater to saline) water bodies in the Mediterranean and Persian Gulf basins from Iberian Peninsula as far eastwards as Iran and Pakistan (
A number of isolated Aphanius populations that might deserve species status have been reported from endorheic drainages in Iran, but have not yet been investigated in detail (
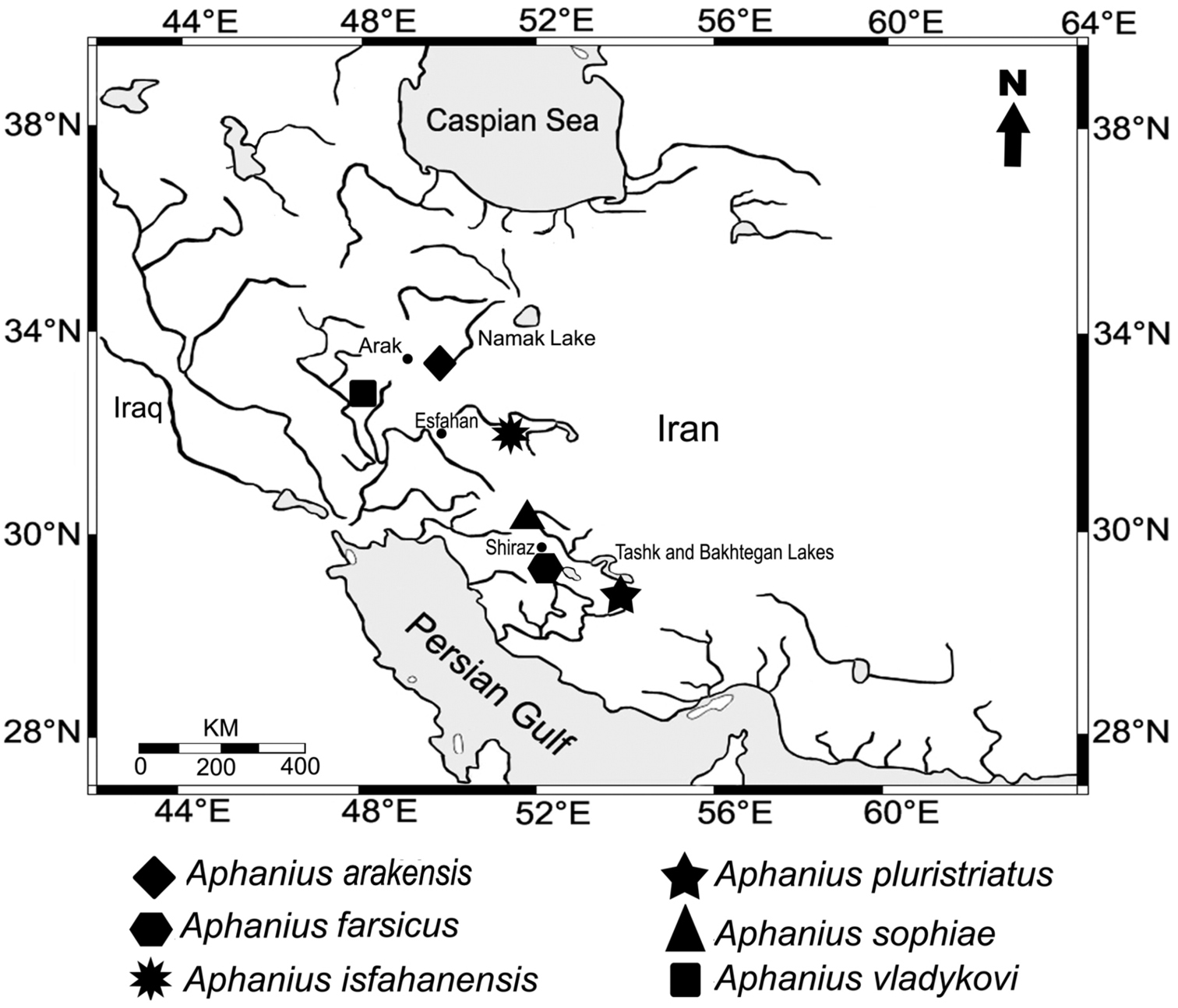
Geographic distribution of the endemic Iranian inland Aphanius species.
Institutional acronyms: ZM-CBSU, Zoological Museum of Shiraz University, Collection of Biology Department; ZSM, Zoological State Collection, Munich.
Material for morphological comparison.Aphanius sophiae: 35 males (19.3–33.3 mm SL) and 35 females (18.6–36.6 SL) from the Ghadamgah spring-stream system (close to type locality) in the Kor River Basin (Iran, Fars Province), 30°15'N, 52°25'E. Males: ZM-CBSU, 8460, 8462, 8462, 8466, 8468, 8470, 8472-73, 8475, 8477, 8479, 8481, 8483, 8485, 8487, 8479, 8489, 8491-97, 8499, 8501, 8503-09, 8511-13; females: ZM-CBSU, 8461, 8463, 8465, 8467, 8469, 8471, 8474, 8476, 8478, 8480, 8482, 8484, 8486, 8488, 8490, 8498, 8500, 8502, 8510, 8514-29.
Aphanius farsicus: 35 males (20.0–26.8 mm SL) and 35 females (20.4–35.2 mm SL) from the Barm-e-Shur spring in the Maharlu Lake Basin (type locality) (Iran, Fars Province), 29°27'N, 52°42'E. Males: ZM-CBSU, 9413, 9415, 9417, 9421, 9441, 9443, 9447, 9449, 9459, 9467, 9481, 9483, 9485, 9487, 9489, 9493, 9497, 9499, 9503, 9511, 9513, 9515, 9517, 9519, 9527, 9529, 9531, 9533, 9537, 9539, 9541, 9555, 9557, 9559, 6375; females: ZM-CBSU, 9410, 9412, 9420, 9422, 9428, 9442, 9444, 9452, 9458, 9472, 9474, 9478, 9482, 9488, 9492, 9494, 9498, 9500, 9502, 9504, 9506, 9510, 9516, 9520, 9530, 9532, 9534, 9536, 9558, 9560, 9562, 9564, 6364, 6359, 6385.
Aphanius isfahanensis: 18 males (17.6–23.8 mm SL) and 25 females (17.7–34.0 mm SL) from the Zayanderh River near Varzaneh, Esfahan Basin (type locality) (Iran, Esfahan Province), 32°25'N, 52°39'E. Males: ZM-CBSU, 6472, 6474, 6476, 6478, 6480, 6482, 6484, 6486, 6488, 6490, 6492, 6494, 6496, 6498, 6500, 8602, 8604, 8613; females: ZM-CBSU, 6471, 6473, 6475, 6477, 6479, 6481, 6483, 6485, 6487, 6489, 6491, 6493, 6495, 6497, 6499 6501, 8603, 8605-8612.
Aphanius vladykovi: 35 males (17.3–29.2 mm SL) and 35 females (16.1–41.4 mm SL) from the Chaghakhor wetland in the upper reaches of the Karoun Basin (Iran, Chahar Mahale Bakhtyari Province), 31°55'N, 50°56'E. Males: ZM-CBSU, 6408-09, 6413-14, 6416, 6418, 6420-21, 6423, 6425-27, 6430, 6433-41, 6443-44, 6446, 6448-49, 6451-57: females: ZM-CBSU, 6401-03, 6405-07, 6410-12, 6415, 6417, 6419, 6422, 6424, 6428-29, 6431-32, 6442, 6445, 6447, 6450, 6458-70.
Material for molecular comparison.Aphanius sophiae ZM-CBSU, M46, M97, M98, M174-176 (Ghadamgah spring-stream system); Aphanius farsicus ZM-CBSU, M47, M136, M177-178 (Barm-e-Shur spring); Aphanius isfahanensis ZM-CBSU, M211, M213-214 (Zayanderh River near Varzaneh); Aphanius arakensis sp. n. ZM-CBSU, M198-200 (Namak Lake Basin, 34°00'N, 49°50'E); Aphanius vladykovi ZM-CBSU, M60, M139, M209 (Chaghakhor wetland in the upper reaches of the Karoun Basin).
Materials from GenBank.Aphanius vladykovi: GenBank DQ367526; Aphanius fasciatus:GenBank AF299273; Aphanius iberus:GenBank AF299290. Poeciliopsis gracilis (GenBank AF412155) was used as outgroup.
Morphological analysisBased on the morphometric schemes introduced in
Scales removed from the left side of each fish, from the 3rd or 4th row below the dorsal fin, were mounted between microscope slides, and length and width of scales were measured to the nearest 0.1 mm by using a scale reader (Xerox 320). For each individual, scale length and scale width measurements were averaged to obtain a single length value and a single width value per individual and relative width and length of scales were calculated following
The meristic characters were counted under a stereomicroscope and consist of the numbers of (i) dorsal (ii) pectoral (iii) pelvic and (vi) anal fin rays, (v) lateral line series scales, (vi) caudal peduncle scales (the numbers of scales along the caudal peduncle, i.e. from the base of the last anal fin ray to the base of the caudal fin rays in a direct line), (vii) gill rakers and (viii) flank bars of males. Two posteriormost rays in dorsal and anal fins were calculated as one ray.
For examination of otolith morphology fish skulls were opened ventrally in order to remove the right and left otoliths. Otoliths were cleaned from tissue remains in 1% potassium hydroxide solution for 3–6 h, washed several times and finally rinsed in distilled water for 12 h. Otolith morphology was analyzed under a stereo microscope. In addition, five or six otoliths from each population were examined by a scanning electron microscope (SEM) with a LEO 1430 VP at ZSM.
Univariate analysis of variance (ANOVA, with Duncan’s post hoc test, p < 0.05) was used to test the significance of phenotypic differences among species and also between sexes. Canonical discriminant analysis (CDA) was used for multivariate analyses in order to document the classification success of the groups. The statistical analyses were carried out using PASW 19.00 (
Total genomic DNA was extracted according to phenol/chloroform procedures (
Maximum likelihood-based phylogenetic relationships were estimated by using the program SeaView version 4 (
Maximum parsimony based phylogenetic relationships were estimated using the program SeaView version 4 (
The Neighbor Joining (NJ) distance-based phylogenetic relationships were estimated by using the computer program Geneious pro v5.4 (
Morphometric characters of Aphanius arakensis sp. n. and other Iranian Aphanius species. Each cell contains mean ± standard deviation and range (minimum–maximum).
| Character |
Aphanius arakensis n=35, male |
Aphanius arakensis n=35, female |
Aphanius isfahanensis n=18, male |
Aphanius isfahanensis n=25, female |
Aphanius sophiae n=35, male |
Aphanius sophiae n=35, female |
Aphanius farsicus n=35, male |
Aphanius farsicus n=35, female |
Aphanius vladykovi n=35, male |
Aphanius vladykovi n=35, female |
Aphanius pluristriatus n=32, male |
Aphanius pluristriatus n=38, female |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Standard length | ||||||||||||
| Head length | 29.2±0.1 (26.9–30.9) |
28.5±0.9 (26.8–30.3) |
29.8±1.2 (27.7–31.6) |
29.2±1.2 (27.4–32.1) |
29.4±1.1 (27.3–31.5) |
28.0±1.3 (25.4–30.6) |
32.1±1.3 (29.8–35.2) |
31.6±1.5 (28.1–34.1) |
31.2±1.3 (28.9–33.9) |
30.9±1.4 (27.3–33.6) |
30.2±1.8 (27.1–36.8) |
28.5±1.4 (29.8–35.5) |
| Head depth | 22.1±0.8 (20.0–23.9) |
21.5±0.8 (20.0–23.5) |
20.9±1.0 (18.9–22.3) |
20.2±1.0 (17.8–22.3) |
21.9±0.8 (19.3–23.9) |
21.1±1.12 (18.8–23.9) |
23.9±1.4 (20.8–26.1) |
22.9±1.3 (20.0–25.0) |
23.7±0.9 (21.9–26) |
23.2±1.2 (20.5–25.7) |
21.6±1.1 (19.7–24.5) |
19.8±0.9 (23.3–21.2) |
| Predorsal length | 61.1±1.1 (59.0–63.8) |
61.3±1.8 (58.1–67.7) |
59.7±1.6 (55.2–62.8) |
60.1±2.3 (56.7–65.8) |
60.1±1.9 (55.2–63.5) |
61.2±1.6 (57.3–83.4) |
61.5±1.4 (58.2–64.9) |
62.5±1.1 (59.4–67.1) |
63.6±1.4 (60.5–66.4) |
63.1±1.9 (59.4–67.3) |
64.8±2.8 (59.6–75.4) |
61.0±1.7 (68.7–64.2) |
| Length of pectoral fin | 18.1±0.1 (15.5–19.8) |
17.5±1.2 (12.9–19.3) |
16.4±1.3 (12.9–19.0) |
16.2±1.2 (13.9–18.2) |
18.3±1.1 (15.8–20.1) |
17.0±1.3 (14.9–19.6) |
19.4±1.2 (17.1–21.8) |
17.2±1.1 (15.0–19.4) |
16.3±1.2(14–20.3) | 15.4±1.1 (13.5–17.6) |
18.6±1.8 (14.6–22.1) |
17.8±1.3(14–20.4) |
| Length of pelvic fin | 7.9±0.6 (6.6–9) |
7.9±0.5 (7.1–9.3) |
6.7±0.7 (5.7–8.4) |
6.6±0.8 (5.1–7.1) |
10.1±0.8 (8.7–11.7) |
8.7±0.8 (6.9–10.3) |
8.6±0.8 (6.7–10.2) |
7.6±0.9 (5.7–9.2) |
6.7±0.7 (5.4–8.1) |
6.5±0.8 (4.5–8.0) |
7.4±1.3 (4.7–11.0) |
6.7±0.6 (5.0–8.0) |
| Length of anal fin | 12.5±1.7 (9.8–14.3) |
11.7±1.2 (10.4–13.4) |
11.4±2.4 (9.6–12.7) |
10.7±2.8 (8.7–13.3) |
13.4±1.7(11–16.1) | 11.8±1.9 (14.1–11.8) |
13.4±2.6 (10.7–17.2) |
11.8±2.1 (9.7–13.3) |
12.8±1.0 (10.6–15.2) |
11.7±2.0 (9.3–16.8) |
13.7±1.0 (11.5–15.3) |
12.5±1.1 (8.9–14.5) |
| Minimum body depth | 16.5±0.6 (15.1–17.7) |
15.2±0.7 (13.8–16.5) |
14.8±0.6 (14.0–15.8) |
14.4±0.8 (12.4–15.6) |
16.6±1.1 (14.3–18.9) |
14.7±0.8 (13.3–16.8) |
15.5±1.1 (13.3–17.9) |
13.8±0.9 (11.8–15.9) |
14.8±1.0 (12.0–16.7) |
13.9±1.1 (11.6–16.1) |
17.1±1.1 (14.7–19.6) |
16.1±1.1 (12.6–17.1) |
| Pectoral - anal fins distance | 37.1±1.5 (34.3–39.3) |
38.3±1.4 (35.1–40.1) |
34.6±1.6 (31.9–37.6) |
38.2±2.8 (34.4–43.6) |
35.1±1.8 (31.5–40.6) |
39.2±2.6 (34.6–43.7) |
35.7±2.7 (32.17–42) |
37.7±2.1 (33.8–42.1) |
34.7±2.1(30–39.3) | 35.5±1.9 (31.8–39.9) |
36.8±2.4 (30.1–44.8) |
37.1±1.7 (32.0–39.5) |
| Pectoral - pelvic fins distance | 23.3±1.5 (20.5–26.4) |
23.8±1.4 (20.8–26.9) |
22.1±1.7 (19.3–25.4) |
25.2±2.8 (20.7–29.6) |
21.1±1.1 (18.4–23.2) |
23.5±2.5 (18.6–28.0) |
22.8±2.6 (18.1–30) |
24.1±2.1 (19.6–28.6) |
22.2±2.1 (17.8–25.9) |
22.7±1.6 (19.5–25.9) |
24.1±2.2 (20.1–31.5) |
23.6±1.7 (18.4–26.7) |
| Pelvic - anal fins distance | 13.1±0.8 (10.8–14.5) |
13.6±0.9 (11.9–15.2) |
11.7±1.0 (9.6–13.8) |
12.5±1.2 (10.1–14.4) |
14.1±1.1 (11.8–17.6) |
15.2±1.2 (12.9–18.0) |
13.2±1.1 (11.1–15.4) |
13.4±1.1 (11.3–16.5) |
12.1±1.2 (9.9–14.4) |
12.8±1.2 (10.5–14.9) |
13.5±0.9 (11.9–16.9) |
13±1.1 (9.5–14.9) |
| Length of caudal peduncle | 22.1±0.8 (19.9–23.9) |
21.9±0.7 (20.0–23.2) |
23.7±1.3 (21.6–27.2) |
22.8±1.1 (20.0–24.4) |
22.8±1.4 (19.7–25.7) |
23.4±1.7 (19.6–26.9) |
21.7±1.3 (18.9–23.9) |
20.8±1.4 (17.9–23.6) |
21.2±1.1 (19.1–24.1) |
21.5±1.6 (17.9–25.2) |
22.8±1.3 (19.6–25.7) |
23.1±1.4 (19.6–26.3) |
| Length of caudal fin | 20.3±1.5 (14.9–23.1) |
19.5±0.1 (16.8–21.6) |
20.2±1.9 (17.4–24.3) |
19.2±1.4 (16.4–21.3) |
20.1±1.3 (17.7–23.3) |
18.1±1.6 (14.9–22.6) |
21.7±1.0 (17.8–24.6) |
19.1±1.3 (16.7–21.6) |
20.3±1.25 (17.5–22.5) |
19.2±1.4 (16.2–22.3) |
20.4±2.3 (15.8–26.4) |
19.7±1.2 (13.3–22.7) |
| Preanal distance | 68.4±1.3 (66.3–71.2) |
69.2±0.1 (66.7–71.7) |
67.2±0.9 (62.3–71.7) |
70.5±1.0 (63.6–74.6) |
68.1±2.1 (64.2–73.3) |
69.2±1.9 (65.4–73.9) |
69.9±1.3 (66.5–76.1) |
71.2±0.9 (67.2–76.6) |
67.8±1.8 (62.9–72.4) |
67.9±1.3 (63.9–73.7) |
69.2±1.9 (65.7–73.2) |
68.1±1.4 (65.4–71.5) |
| Scale length | 3.8±0.2 (3.5–4.2) |
3.8±0.2 (3.3–4.4) |
3.1±0.2 (2.7–3.5) |
3.2±0.3 (2.6–4.0) |
3.1±0.3 (2.7–3.7) |
3.1±0.3 (2.6–3.8) |
3.5±0.4 (2.7–4.5) |
3.4±0.4 (2.5–4.6) |
2.6±0.3 (1.9–3.3) |
2.4±0.3 (1.8–3.2) |
3.9±0.2 (3.4–4.5) |
4.1±0.2 (3.8–4.5) |
| Scale width | 3.9±0.2 (3.5–3.4) |
3.8±0.2 (3.3–4.6) |
3.7±0.3 (3.3–4.3) |
3.8±0.4 (3.1–4.9) |
3.4±0.4 (2.8–4.18) |
3.3±0.4 (2.6–4.1) |
4.1±0.6 (3.2–5.4) |
3.9±0.5 (3.1–4.9) |
2.7±0.3 (1.9–3.2) |
2.5±0.4 (1.8–3.2) |
4.1±0.4 (2.8–5.4) |
4.2±0.2 (3.7–4.8) |
| % Preanal distance | ||||||||||||
| Minimum body depth | 24.1±0.1 (21.7–25.9) |
22.0±0.8 (20.2–23.4) |
22.0±1.3 (19.7–24.5) |
20.1±1.3 (18.3–23.1) |
24.4±1.4 (21.5–27.1) |
21.3±1.1 (19.3–23.3) |
22.2±1.4(19–25.1) | 19.4±1.4 (16.8–22.8) |
21.9±1.6 (17.6–24.8) |
20.5±1.7 (16.9–23.5) |
24.5±1.3 (21.2–27.1) |
23.7±1.6 (18.8–26.5) |
| Length of caudal peduncle | 32.2±1.5 (29.0–34.8) |
31.7±1.2 (28.6–34.0) |
35.3±1.7 (31.9–37.9) |
32.4±2.4 (27.4–38.4) |
33.6±2.4 (27.9–38.7) |
33.9±3.1 (27.2–40.3) |
31.1±2.5 (25.1–35.7) |
29.3±2.5 (23.9–33.8) |
31.3±1.8 (28.0–35.5) |
31.6±2.7 (26.1–38.1) |
33.1±2.2 (27.4–37.8) |
33.8±2.3 (28.2–38.1) |
| Length of caudal fin | 29.6±2.3 (21.5–33.5) |
28.2±1.7 (24.4–32.0) |
30.1±3.2 (25.7–36.6) |
27.3±2.4 (22.9–32.6) |
29.4±1.1 (25.4–33.8) |
26.2±2.7 (21.2–33.9) |
31.0±2.3 (26.6–35.4) |
26.7±2.1 (23.1–32.1) |
29.9±1.9 (27.0–33.6) |
28.2±2.2 (24.1–34.9) |
29.4±3.0 (23.2–36.6) |
28.1±2.9 (19.5–33.3) |
| Eye diameter | 12.6±0.9 (10.7–14.8) |
12.0±1.19 (10.5–13.8) |
15.5±2.6 (13.6–17.4) |
13.7±1.3 (11.1–15.9) |
13.7±0.9 (11.5–14.9) |
12.7±1.1 (10.0–15.3) |
15.1±2.6 (13.4–17) |
13.5±2.2 (10.6–16.1) |
13.7±1.1 (11.9–16.2) |
14.2±1.9 (11.3–15.3) |
14.4±0.9 (12.9–17.4) |
14.1±1.6 (12.4–18.1) |
| % Head width | ||||||||||||
| Interorbital distance | 1.1±0.06 (0.9–1.2) |
1.1±0.1 (0.9–1.2) |
0.9±0.1 (0.8–1.1) |
0.9±0.06 (0.76–1.1) |
0.9±0.06 (0.8–1.1) |
0.9±0.1 (0.8–1.1) |
1.1±0.1 (0.8–1.2) |
1.1±0.1 (0.8–1.2) |
0.8±0.05 (0.7–0.9) |
0.8±0.06 (0.7–0.9) |
0.9±0.05 (0.8–1.1) |
1.0±0.06 (0.8–1.1) |
| % Head length | ||||||||||||
| Eye diameter | 0.3±0.01 (0.2–0.3) |
0.3±0.2 (0.2–0.3) |
0.3±0.01 (0.3–0.4) |
0.3±0.02 (0.3–0.4) |
0.3±0.02 (0.3–0.35) |
0.3±0.02 (0.2–0.35) |
0.3±0.02 (0.3–0.4) |
0.3±0.03 (0.2–0.3) |
0.3±0.02 (0.2–0.3) |
0.3±0.1 (0.2–1.2) |
0.3±0.02 (0.3–0.4) |
0.3±0.02 (0.3–0.4) |
| % Eye diameter | ||||||||||||
| Preorbital distance | 0.8±0.1 (0.7–0.9) |
0.8±0.1 (0.7–1.0) |
0.7±0.05 (0.6–0.8) |
0.7±0.1 (0.6–1.0) |
0.8±0.1 (0.7–1.1) |
0.8±0.1 (0.6–1.0) |
0.8±0.1 (0.6–1.1) |
0.8±0.08 (0.7–1.1) |
0.9±0.1 (0.8–1.1) |
0.9±0.1 (0.2–1.1) |
0.8±0.05 (0.6–0.9) |
0.8±0.1 (0.6–0.9) |
| % Minimum body depth | ||||||||||||
| Length of caudal peduncle | 1.3±0.1 (1.2–1.5) |
1.4±0.1 (1.3–1.6) |
1.6±0.1 (1.5–1.9) |
1.6±0.1 (1.3–1.8) |
1.4±0.15 (1.1–1.7) |
1.6±0.15 (1.3–2.0) |
1.4±1.5 (1.1–1.8) |
1.5±0.1 (1.2–1.9) |
1.4±0.1 (1.2–1.7) |
1.5±0.2 (1.1–1.9) |
1.3±0.1 (1.2–1.6) |
1.4 ±0.2 (1.2–1.6) |
urn:lsid:zoobank.org:act:D9995F4C-AF0A-4791-9D80-D759EFEDA569
http://species-id.net/wiki/Aphanius_arakensis
Figure 2A, BMale, 38.5 mm TL, 31.5 mm SL, Iran, Arak, Namak Lake Basin, 34°00'N, 49°50'E, Altitude 1786 m, 26 September 2007, A. Teimori, M. Ebrahimi, A. Gholamifard and A. Gholmhosseini (ZM-CBSU 10999).
35 males (22.6–32.7 mm SL), 35 females (22.5–34.1 mm SL), same locality as holotype (ZM-CBSU 11000, 11051–11118).
The new species is distinguished by the congeners distributed in Iran by the following combination of characters: 10–12 anal fin rays, 28–32 lateral line scales, 10–13 caudal peduncle scales, 8–10 gill rakers, 12–19, commonly 11–13, clearly defined flank bars in males, a more prominent pigmentation along the flank added by relatively big blotches in the middle and posterior flank segments in females, a short but high antirostrum of the otolith that has a wide excisura, and a ventral rim with some small, drop-like processes and 19 molecular apomorphies (17 transitions, two transversions) in the cytochrome b gene.
The males of the new species reach approximately 32 mm SL and have 12–19 flank bars, the females are usually larger than the males and reach approximately 34 mm SL.
The morphometric characters are summarized in Table 1. Compared to the other examined Aphanius species, Aphanius arakensis sp. n. shows higher mean values of the minimum body depth, width and length of scales, distances between the pectoral and pelvic fins and the interorbital distance, but significantly lower mean values for the eye diameter and the caudal peduncle length (differences are statistically significant, p < 0.05).
The meristic characters are summarized in Table 2. The dorsal fin is characterized by a somewhat curved superior border, and has 11–14 rays; the anal fin shows a round superior border and includes 10–12 rays; the pectoral fin is rounded and consists of 14–18 rays; the pelvic fin is relatively short, positioned just anteriorly to the anal fin and comprises 6–8 rays. The caudal fin is rounded; the caudal peduncle possesses 10–13 scales. The number of lateral line series scales is 27–32. However, the ANOVA analysis reveals that only the numbers of lateral line series scales and caudal peduncle scales (in males and females), as well as the numbers of flank bars (in males), significantly differ from the values obtained for the other examined species. Moreover, there is a significant correlation between SL and numbers of flank bars (Pearson Correlation r = 0.455, p < 0.05*).
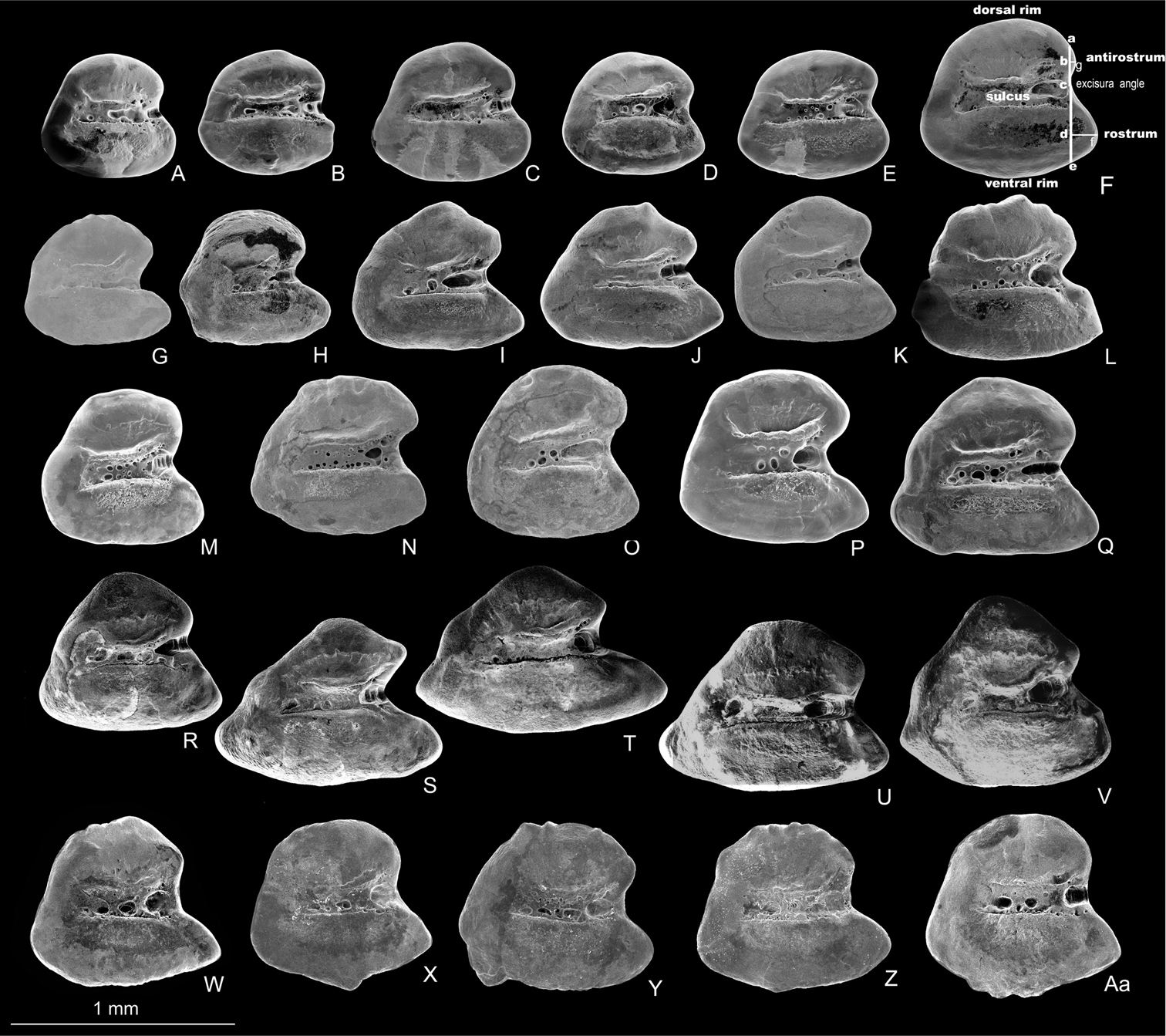
The otolith is rounded-trapezoid and characterized by a very wide excisura, a medium-sized and pointed rostrum, and a quite short antirostrum. The ventral and dorsal rims are slightly curved; the ventral rim may bear small irregular processes; the dorsal rim may show a fine crenulation; the posterior rim is steep (Fig. 3W-Aa).
The flank bars in males (Fig. 2a) are narrow and the interspaces are broader than the bars. The first bar is located above the operculum, while the posteriormost bar is located at the base of the caudal fin; the interspaces are wider at the caudal peduncle than in the anterior body part. Dorsally, the head is gray and the body is dark due to a strong melanophore pigmentation. The ventral body portion does not usually show any dark pigmentation. The dorsal, anal and caudal fins have white margins; the first rays of the dorsal fin are dark. The pectoral fins are somewhat yellowish. The pelvic fin is yellowish. Most specimens are characterized by dark blotches at the base of the dorsal and anal fins.
Females (Fig. 2b) are characterized by a grayish pigmentation of the back. The lateral flanks of the body are covered by dark pigmentations; series of blotches are present from the middle of the body to the caudal peduncle. The ventral part of the head and belly are light. The chin and sides of the head are speckled with melanophores. Below the eye there is a line of relatively dark melanophores. All fins are white.
A Aphanius arakensis, holotype, male, 31.5 mm SL (ZM-CBSU 10999) B paratype, female, 31.5 mm SL (ZM-CBSU 11054).
Left otoliths (medial view) of Aphanius isfahanensis (A–F), Aphanius farsicus (G–L), Aphanius sophiae (M–Q), Aphanius vladykovi (R–V) and Aphanius arakensis (W–Aa). Otolith terminology and taxonomic most informative morphometric distances are indicated in Fig. 3F and include height of antirostrum (a–c), height of rostrum (c–e), length of antirostrum (b–g), and length of rostrum (d– f). SEM pictures.
Meristic characters (mean ± standard deviation and range) of Iranian Aphanius species.
| Character |
Aphanius arakensis n=35, male |
Aphanius arakensis n=35, female |
Aphanius isfahanensis n=18, male |
Aphanius isfahanensis n=25, female |
Aphanius sophiae n=35, male |
Aphanius sophiae n=35, female |
Aphanius farsicus n=35, male |
Aphanius farsicus n=35, female |
Aphanius vladykovi n=35, male |
Aphanius vladykovi n=35, female |
Aphanius pluristriatus n=32, male |
Aphanius pluristriatus n=38, female |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dorsal fin rays | 12.2±0.8 (11–14) |
12.3±0.7 (11–14) |
11.7±0.6 (11–13) |
11.6±0.7 (10–13) |
13.7±0.6 (13–15) |
13.8±0.8 (13–15) |
12.1±0.8 (11–14) |
12±0.8 (10–13) |
13.2±0.8 (12–15) |
13.5±0.7 (12–15) |
13.8±0.7 (12–15) |
13.6±0.7 (12–15) |
| Pectoral fin rays | 16.7±0.9 (14–18) |
16.8±0.6 (16–18) |
15.9±0.8 (15–17) |
16.3±0.5 (15–17) |
18±0.8 (16.20) |
17.9±0.7 (17–19) |
15.4±0.7 (14–17) |
15.4±0.6 (14–17) |
16.5±0.8 (14–18) |
16.6±0.7 (15–18) |
17.1±0.7 (16–19) |
17±0.6 (16–18) |
| Pelvic fin rays | 7.3±0.5 (6–8) |
7.2±0.4 (7–8) |
7±0.6 (6–8) |
6.9±0.5 (6–8) |
7.3±0.5 (6–8) |
7.3±0.6 (6–8) |
6.7±0.4 (6–7) |
6.6±0.5 (5–7) |
7±0.6 (6–9) |
6.9±0.4 (6–8) |
7.2 ±0.5 (6–8) |
7.1± 0.4 (8–7) |
| Anal fin rays | 11.4±0.5 (10–12) |
11.5±0.5 (11–12) |
10.9±0.3 (10–11) |
11.1±0.5 (10–12) |
12.3±0.6 (11–14) |
12.7±1.1 (12–17) |
11.1±0.6 (10–12) |
11.1±0.7 (10–12) |
13.2±0.7 (12–15) |
13.2±0.8 (12–15) |
13±0.7 (12–14) |
12.5±0.6 11–14 |
| Lateral line series scales | 30.1±1.0 (29–32) |
29.6±1.1 (28–32) |
24.9±1.5 (23–27) |
26±1.4 (23–27) |
27.9±0.9 (26–29) |
27.1±1.3 (25–29) |
25.6±1.6 (22–28) |
25.4±1.5 (23–29) |
36.3±2.8 (33–43) |
37.1±2.7 (33–43) |
27.1±1.1 (24–29) |
27±1.2 (24–29) |
| Caudal peduncle scales | 11.6±0.6 (10–13) |
11.6±0.7 (10–13) |
10±0.5 (9–11) |
10.3±0.8 (9–12) |
9.9±0.8 (8–11) |
9.7±0.6 (9–11) |
9.26±0.6 (8–11) |
9.4±0.6 (8–10) |
12.6±1.1 (10–14) |
12.5±1.4 (9–15) |
9.2±0.7 (8–11) |
9.3±0.8 (8–11) |
| Gill raker | 9.2±0.5 (8–10) |
9.3±0.5 (8–10) |
10.8±0.5 (10–12) |
11.1±0.7 (10–13) |
10.7±0.7 (9–12) |
10.7±0.8 (9–12) |
10.9±0.7 (9–13) |
10.7±0.8 (9–12) |
9.7±0.1 (8–12) |
9.7±0.7 (8–11) |
9.8±0.6 (8–11) |
9.6±0.7 (8–11) |
| Flank bars | 15.9±1.4 (12–19) |
– | 10.7±0.9 (9–13) |
– | 11.9±1.5 (8–15) |
– | 12.4±1.2 (10–16) |
– | 11±1.2 (8–13) |
– | 13.8± 1.7 (11–17) |
– |
Aphanius arakensis is close to the other Iranian Aphanius species in having a similar external morphology but differs by a high number of flank bars, 12–19, commonly, 15–16 (vs. 8–13, commonly, 11–12 in Aphanius vladykovi; 10–16, commonly, 12–13, in Aphanius farsicus; 8–15, commonly, 11–13 in Aphanius sophiae; 9–13, commonly, 10–11 in Aphanius isfahanensis and 11–17, commonly, 13–14 in Aphanius pluristriatus), otolith morphology and by having 19 molecular apomorphies in the cytochrome b gene. The new species (both males and females) can be further distinguished from Aphanius vladykovi by 28–32 lateral line series scales (vs. 33–43), and by less relative width and length of scales, 3.3–4.6 and 3.3–4.5% SL, respectively (vs. 1.9–3.2 and 1.9–3.3, respectively). It differs from Aphanius sophiae in having 10–13 caudal peduncle scales (vs. 8–11), less gill rakers numbers, 8–10 (vs. 9–12), and by a greater interorbital distance, 0.9–1.2% head width (vs. 0.8–1.1). The new species differs from Aphanius farsicus in having 6–8 pelvic fin rays (vs. 6–7), and by a smaller eye diameter, 10.7–14.8% preanal distance (vs. 10.6–17.0). It can be distinguished from Aphanius isfahanensis by 8–10 gill rakers (vs. 10–13), and by a shorter caudal peduncle, 29.0–34.8% preanal distance (vs. 27.4–38.4). It differs from Aphanius pluristriatus in having 10–13 caudal peduncle scales (vs. 8–11), 28–32 lateral line series scales (vs. 24–29) and by a smaller eye diameter, 10.7–14.8% preanal distance (vs. 12.4–18.1).
The species has been collected from a small natural shallow pond (Fig. 4) in the Namak Lake basin, 5 km south east of the city of Arak (Fig. 1). This pond, which is about 6 x 4 m in size, is fed by the drainage of a nearby natural spring. During sampling, the water body was almost stagnant and water temperature was 23°C. There was no vegetation in the pond, but the surrounding area was covered with Juncus sp. and Typha sp. The bottom of the pond was generally muddy with small gravels. The habitat was in a bad condition due to anthropogenic pollution. Around collection time, the new Aphanius species was the only fish observed living in the pond. In addition, the new species can be found in several springs located in close proximity to the type locality (Fig. 5).
Natural shallow pond and type locality of Aphanius arakensis sp. n., in the Namak Lake Basin, 5 km SE of Arak city, Iran (see Fig. 1).
Male (above) and female specimens (not preserved) of Aphanius arakensis sp. n., collected from Cheshmeh Nazi (Nazi spring, 33°42'56.8"N, 50°04'21.9"E) near type locality, Namak Lake Basin.
The species name refers to the city of Arak, which is located in close proximity to the type locality. Arak is the capital of the Markazi province in north-central Iran. A proposed common name is Arak tooth-carp. Farsi name is Kapour-e-dandandar-e-Arak.
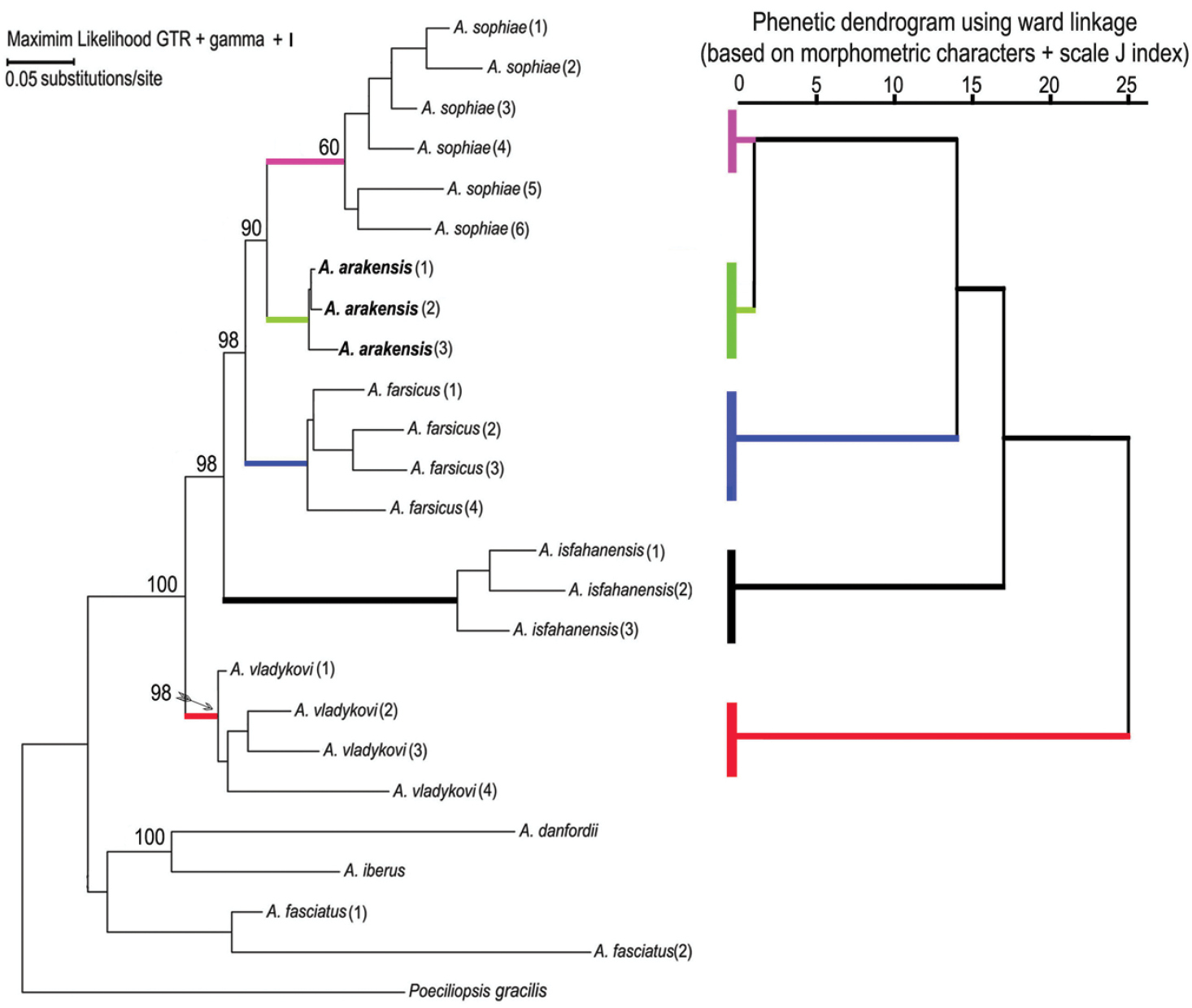
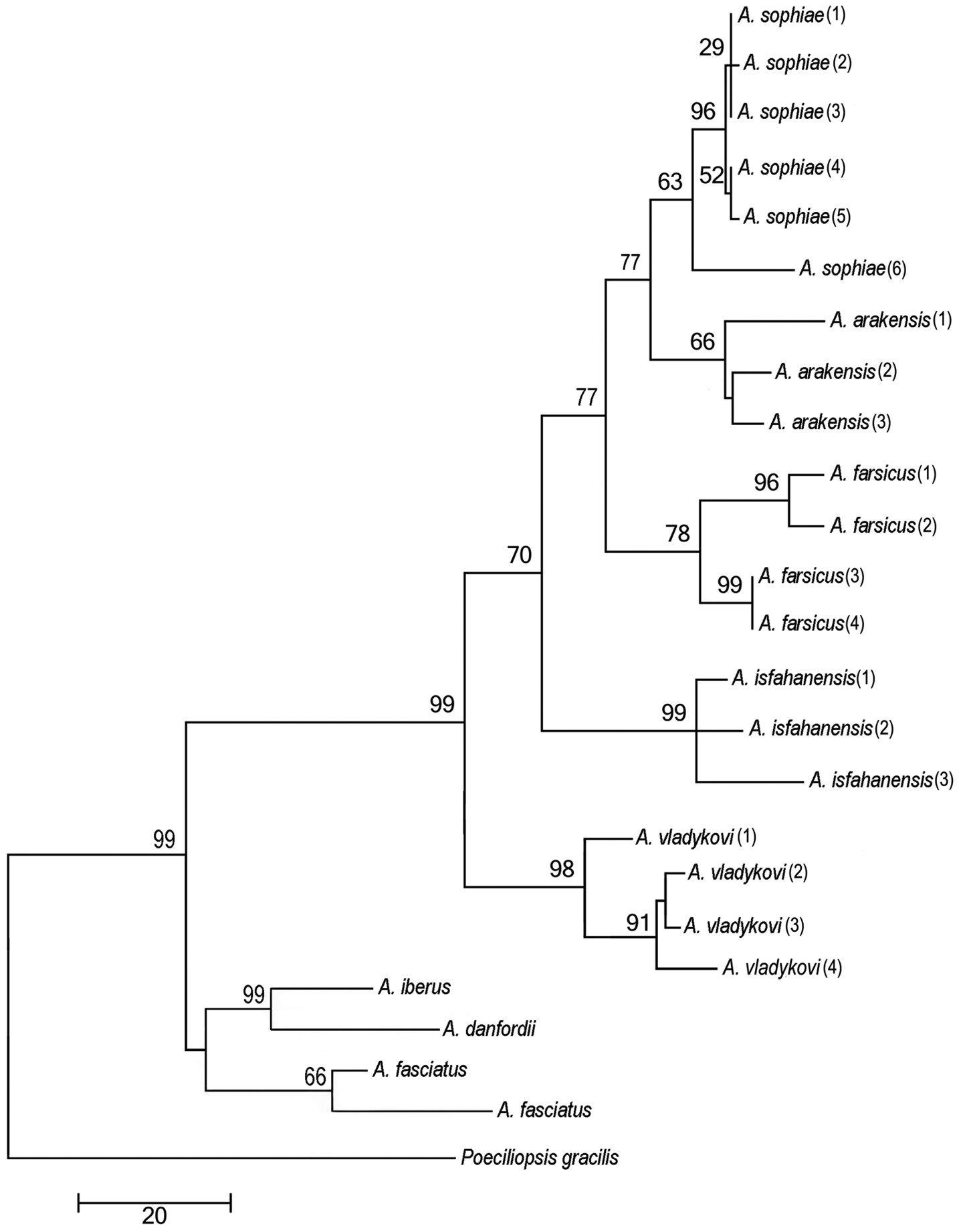
Phylogenetic relationshipsThe parameters for the maximum likelihood are ln(L) = –85.11.91237, gamma shape parameter of 1.000, proportion of invariant sites of 0.097 and parsimony = 1556. The maximum parsimony phylogeny has a CI of 0.462 and RI of 0.747. The initial tree for the maximum likelihood analysis was obtained by the BIONJ algorithm. The trees of the maximum likelihood and maximum parsimony phylogenies (Fig. 6) are not significantly different in topology (Templeton test, P > 0.05). They support the hypothesis that Aphanius arakensis diverged from the clade leading to the present-day Aphanius sophiae and is sister to this species. Moreover, Aphanius farsicus is sister to Aphanius arakensis + Aphanius sophiae; sister to these taxa is Aphanius isfahanensis, and sister to all previously mentioned species is Aphanius vladykovi. The same topology (Templeton test, P > 0.05) is observed for the tree of the Neighbor Joining (NJ) distance-based analysis. Table 4 shows the estimation of evolutionary divergence between the sequences of the new species and its relatives.
Phylogenetic relationships of Aphanius arakensis sp. n., and other endemic species of Aphanius in Iran as indicated by maximum likelihood (based on cytochrome b sequences) and phenetic (based on morphometric characters of fish specimens + J scale indices) analysis. Numbers above nodes represent maximum likelihood bootstrap values based on 2000 replicates. Species and locations correspond to those listed in the Material section.
Summary of diagnostic molecular characters that differentiate Aphanius arakensis sp. n., from other Iranian Aphanius species. Of the 19 molecular apomorphies, 17 are transitions and two are transversions. Numbers above characters indicate the character’s position in the complete molecular character matrix.
| Position | 2 | 2 | 2 | 3 | 3 | 4 | 5 | 5 | 6 | 6 | 6 | 6 | 7 | 7 | 8 | 8 | 8 | 8 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 9 | 3 | 9 | 1 | 2 | 4 | 0 | 1 | 2 | 2 | 4 | 9 | 1 | 3 | 6 | 7 | 8 | |
| 7 | 6 | 1 | 3 | 5 | 1 | 6 | 9 | 6 | 6 | 2 | 4 | 5 | 5 | 1 | 7 | 0 | 5 | 7 | |
| Aphanius arakensis | G | T | C | T | G | C | A | G | T | A | A | G | G | T | G | T | T | G | C |
| Aphanius isfahanensis | A | C | T | C | A | G | G | A | A | G | G | A | A | C | A | C | C | A | T |
| Aphanius sophiae | A | C | T | C | A | G | G | A | A | G | G | A | A | C | A | C | C | A | T |
| Aphanius farsicus | A | C | T | C | A | G | G | A | A | G | G | A | A | C | A | C | C | A | A |
| Aphanius vladykovi | A | C | T | C | A | A | G | A | A | G | G | A | A | C | A | C | C | A | A |
Several endemic Aphanius species are known that are soundly circumscribed by genetic differentiation and specific otolith morphology (see below), whereas they differ only weakly (or only in multivariate space) with regard to morphometry and meristics. Examples are Aphanius isfahanensis from central Iran, Aphanius sophiae and Aphanius farsicus from southern Iran (
Otolith morphology is known to support the distinctive taxonomic state of several Aphanius species (
Notably, the otoliths of Aphanius vladykovi are most distinctive in comparison to those of the other studied species as they are characterized by a long ventral part, angular overall shape and long rostrum (Fig. 3R–V). This uniqueness of the Aphanius vladykovi otoliths corresponds well to our and previous phylogenetic analyses, which have established Aphanius vladykovi as being sister to all other Iranian inland species that diverged approximately 10 Ma ago (
Coloration and flank bar numbers are significant characters for the identification of Aphanius species, in particular for the identification of male individuals. Among the allopatric Iranian Aphanius species, males of Aphanius arakensis have the largest number of flank bars, and flank bars are non-overlapping, whereas the number of flank bars is lowest in Aphanius sophiae. Also the flank bars of the central Anatolian Aphanius species vary in thickness and number between species (
Sexual selection has long been believed to promote species divergence among groups of animals (see
Several studies indicate that fishes can adapt to variation in underwater light environments by changing their colour, most likely as a result of a more effective intraspecific communication (
Phylogenetic relationships of Aphanius arakensis sp. n., and other endemic species of Aphanius in Iran as indicated by maximum parsimony (based on cytochrome b sequences) analysis. The maximum parsimony phylogeny has a CI of 0.462 and RI of 0.747. Numbers above nodes represent maximum parsimony bootstrap values based on 2000 replicates.
Estimation of Genetic divergence (Kimura 2-parameter model) between the sequences of the Aphanius arakensis sp. n., and other Iranian Aphanius species. Aa = Aphanius arakensis, Ai = Aphanius isfahanensis, As = Aphanius sophiae, Af = Aphanius farsicus and Av = Aphanius vladykovi.
| Aa1 | Aa2 | Aa3 | Af1 | Af2 | Af3 | Af4 | Ai1 | Ai2 | Ai3 | As1 | As2 | As3 | As4 | As5 | As6 | Av1 | Av2 | Av3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa1 | |||||||||||||||||||
| Aa2 | 0.000 | ||||||||||||||||||
| Aa3 | 0.001 | 0.001 | |||||||||||||||||
| Af1 | 0.057 | 0.057 | 0.056 | ||||||||||||||||
| Af2 | 0.054 | 0.054 | 0.053 | 0.009 | |||||||||||||||
| Af3 | 0.047 | 0.047 | 0.045 | 0.009 | 0.008 | ||||||||||||||
| Af4 | 0.047 | 0.047 | 0.045 | 0.011 | 0.007 | 0.001 | |||||||||||||
| Ai1 | 0.119 | 0.119 | 0.118 | 0.116 | 0.115 | 0.113 | 0.114 | ||||||||||||
| Ai2 | 0.098 | 0.098 | 0.096 | 0.092 | 0.093 | 0.090 | 0.092 | 0.025 | |||||||||||
| Ai3 | 0.113 | 0.113 | 0.111 | 0.111 | 0.109 | 0.105 | 0.106 | 0.012 | 0.019 | ||||||||||
| As1 | 0.041 | 0.041 | 0.040 | 0.068 | 0.066 | 0.058 | 0.060 | 0.118 | 0.097 | 0.112 | |||||||||
| As2 | 0.029 | 0.029 | 0.027 | 0.053 | 0.051 | 0.042 | 0.044 | 0.105 | 0.084 | 0.099 | 0.015 | ||||||||
| As3 | 0.036 | 0.036 | 0.034 | 0.054 | 0.051 | 0.050 | 0.051 | 0.106 | 0.085 | 0.100 | 0.018 | 0.007 | |||||||
| As4 | 0.029 | 0.029 | 0.027 | 0.053 | 0.051 | 0.042 | 0.044 | 0.105 | 0.084 | 0.099 | 0.015 | 0.000 | 0.007 | ||||||
| As5 | 0.030 | 0.030 | 0.029 | 0.051 | 0.053 | 0.044 | 0.045 | 0.107 | 0.084 | 0.100 | 0.016 | 0.001 | 0.008 | 0.001 | |||||
| As6 | 0.033 | 0.033 | 0.031 | 0.054 | 0.052 | 0.047 | 0.048 | 0.108 | 0.087 | 0.102 | 0.018 | 0.004 | 0.008 | 0.004 | 0.005 | ||||
| Av1 | 0.095 | 0.095 | 0.093 | 0.092 | 0.090 | 0.084 | 0.086 | 0.121 | 0.106 | 0.118 | 0.096 | 0.083 | 0.088 | 0.083 | 0.083 | 0.081 | |||
| Av2 | 0.082 | 0.082 | 0.081 | 0.077 | 0.073 | 0.075 | 0.076 | 0.115 | 0.098 | 0.112 | 0.086 | 0.070 | 0.073 | 0.070 | 0.072 | 0.075 | 0.027 | ||
| Av3 | 0.107 | 0.107 | 0.105 | 0.102 | 0.103 | 0.098 | 0.100 | 0.097 | 0.111 | 0.094 | 0.109 | 0.094 | 0.097 | 0.094 | 0.096 | 0.099 | 0.034 | 0.029 |
The noticeable features of the present-day diversity of the endemic Aphanius species in Iran include high genetic divergence and clear differences in otolith morphology, but only weak differences in general external morphology, morphometry and meristics. These patterns are probably caused by different rates of evolution in the mentioned characters that may be linked to the similarity of the individual environments, intra-species communication, and vicariance events. It is likely that additional Aphanius species are present in remote areas of Iran, especially in the Zagros and Alburz Mountains.
The Iranian Ministry of Sciences, Research and Technology and also DAAD (German Academic Exchange Service) are acknowledged for financial support to the first and second authors respectively. We are thankful to M. Ebrahimi, A. Gholamifard, A. Gholamhosseini for assistance in the collection of material, S. Mohsenzadeh and A. Hosseini for assistance with the DNA extraction and PCR, M. Kamran for logistic support in the field and Shiraz University for financial support (all from Shiraz University), M. Motamedi (Munich) for her constructive comments on molecular analyses, R. Melzer for providing access to the SEM at the Bavarian State Collection of Zoology (ZSM, Munich) and M. Krings (Munich) for improving the English.
The authors would like to thank four anonymous reviewers and the editor, Nina Bogutskaya, for their valuable comments and recommendations for improving earlier versions of the manuscript.