(C) 2012 Charles E. Griswold. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The new spider genus and species Trogloraptor marchingtoni Griswold, Audisio & Ledford is described as the type of the new family Trogloraptoridae. The oblique membranous division of the basal segment of the anterior lateral spinnerets of Trogloraptor suggests that this haplogyne family is the sister group of the other Dysderoidea (Dysderidae, Oonopidae, Orsolobidae and Segestriidae). Trogloraptor is known only from caves and old growth forest understory in the Klamath-Siskiyou region of Oregon and California.
Haplogynae, Caves, Pacific Northwest
The spider fauna of North America is a rich one, with at least 68 families including 569 genera and comprising more than 3700 species (
Diagnostic characters for Haplogynae families. Italics represents diagnosis from Trogloraptoridae.
| Family | AME | PME distance | Cheliceral bases | Cheliceral lamina | Posterior receptaculum | 3rd Entapophyses | Posterior spiracles | Posterior spiracle(s) | Emerit’s glands | Tarsal claw number | Serrula tooth rows | Labium-sternum junction | ALS basal segment | PLs AC gland spigots | CY gland spigots | Colulus |
| Caponiidae | present | separate | fused | present | absent | separate, short | two | advanced | absent | three | one | fused | entire | dispersed | absent | small |
| Diguetidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | fused | entire | dispersed | absent | small |
| Drymusidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | fused | entire | dispersed | absent | small |
| Dysderidae | absent | contiguous | free | absent | present | separate, short | two | advanced | absent | three | one | free | divided | dispersed | absent | small |
| Filistatidae | present | contiguous | fused | present | absent | separate, long | two | posterior | absent | three | one | fused | entire | dispersed | absent | absent |
| Leptonetidae | absent | contiguous | free | absent | absent | separate, long | one | posterior | present | three | one | fused | entire | in line | present | small |
| Ochyroceratidae | absent | contiguous | free | present | absent | separate, short | one | posterior | absent | three | one | fused | entire | in line | absent | large |
| Oonopidae | absent | contiguous | free | absent | present | separate, short | two | advanced | prs; abs | two | one | free | divided | dispersed | absent | small |
| Orsolobidae | absent | contiguous | free | absent | present | separate, short | two | advanced | absent | two | one | free | divided | dispersed | absent | small |
| Periegopidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | free | entire | dispersed | absent | small |
| Pholcidae | present | separate | fused | present | absent | absent | one | posterior | absent | three | one | fused | entire | dispersed | absent | small |
| Plectreuridae | present | separate | fused | present | absent | fused | one | posterior | absent | three | one | free | entire | dispersed | absent | small |
| Scytodidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | three | one | fused | entire | dispersed | absent | large |
| Segestriidae | absent | contiguous | free | absent | present | separate, short | two | advanced | absent | three | one | free | divided | dispersed | absent | small |
| Sicariidae | absent | contiguous | fused | present | absent | fused | one | posterior | absent | two | one; abs | fused | entire | dispersed | absent | large; abs |
| Telemidae | absent | contiguous | free | absent | absent | absent | two | posterior | present | three | one | fused | entire | in line | present | large |
| Tetrablemmidae | absent | contig./sep. | free | present | absent | fused | one | posterior | absent | three | one | free | entire | dispersed | absent | small |
| Trogloraptoridae | absent | separate | free | absent | absent | separate, long | one | posterior | absent | three | multiple | fused | divided | dispersed | absent | small |
Species descriptions refer to a single adult individual for each sex, which is identified as a type or by the locality at which it was collected. All measurements are in millimeters and quantify the size of a structure at its widest or longest point. A section reporting the variation in the most conspicuous and variable features follows each description and represents multiple individuals (n), encompassing the full range in overall size.
Prior to examination with a Leo 1450VP Scanning Electron Microscope, all structures were cleaned with a fine brush and critical point dried. Spinneret preparations followed the methods of
Trogloraptoridae have simple, haplogyne genitalia, with a single opening of the female vulva for fertilization and oviposition. This family differs from most other haplogyne clades. The Palpimanoidea (Archaeidae, Huttoniidae, Mecysmaucheniidae, Palpimanidae and Stenochilidae) have a foramen around the cheliceral bases, two protrusions posterior to the labral tongue, cheliceral peg teeth and a cheliceral gland mound (
urn:lsid:zoobank.org:act:0B46EFED-0BE4-4618-B14C-0EFFAA7CA4EA
Trogloraptor marchingtoni Griswold, Audisio and Ledford, here designated.
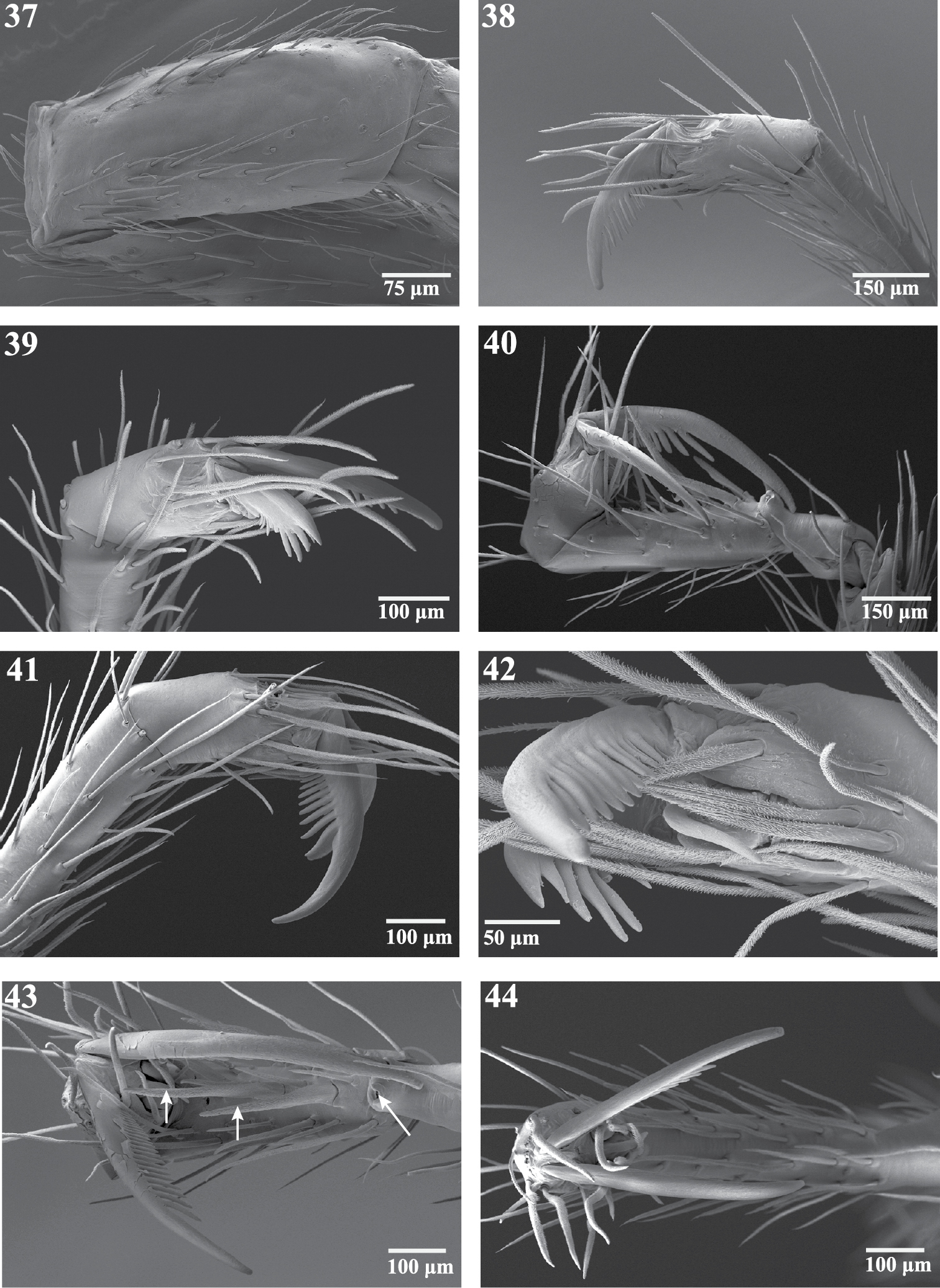
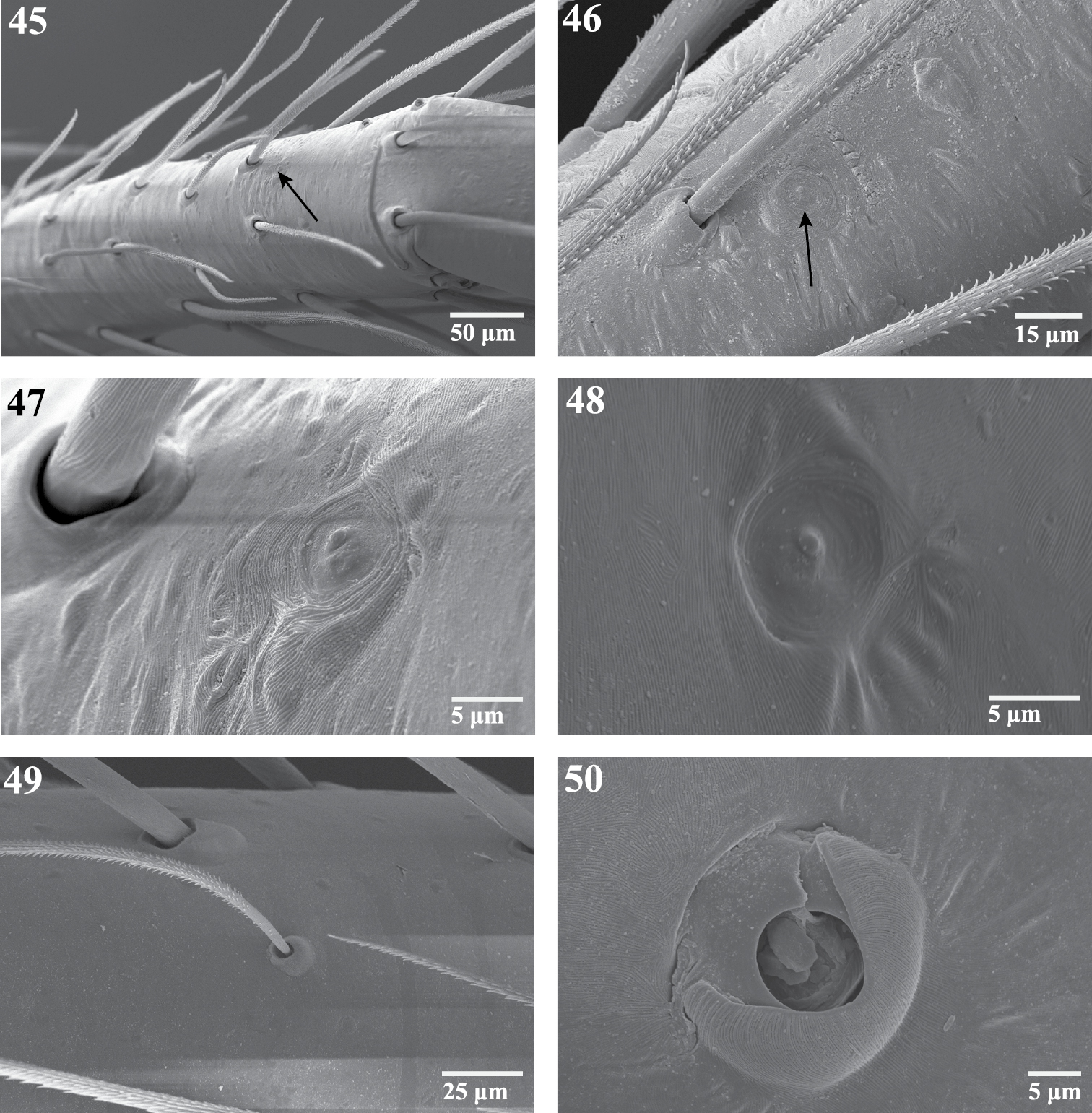
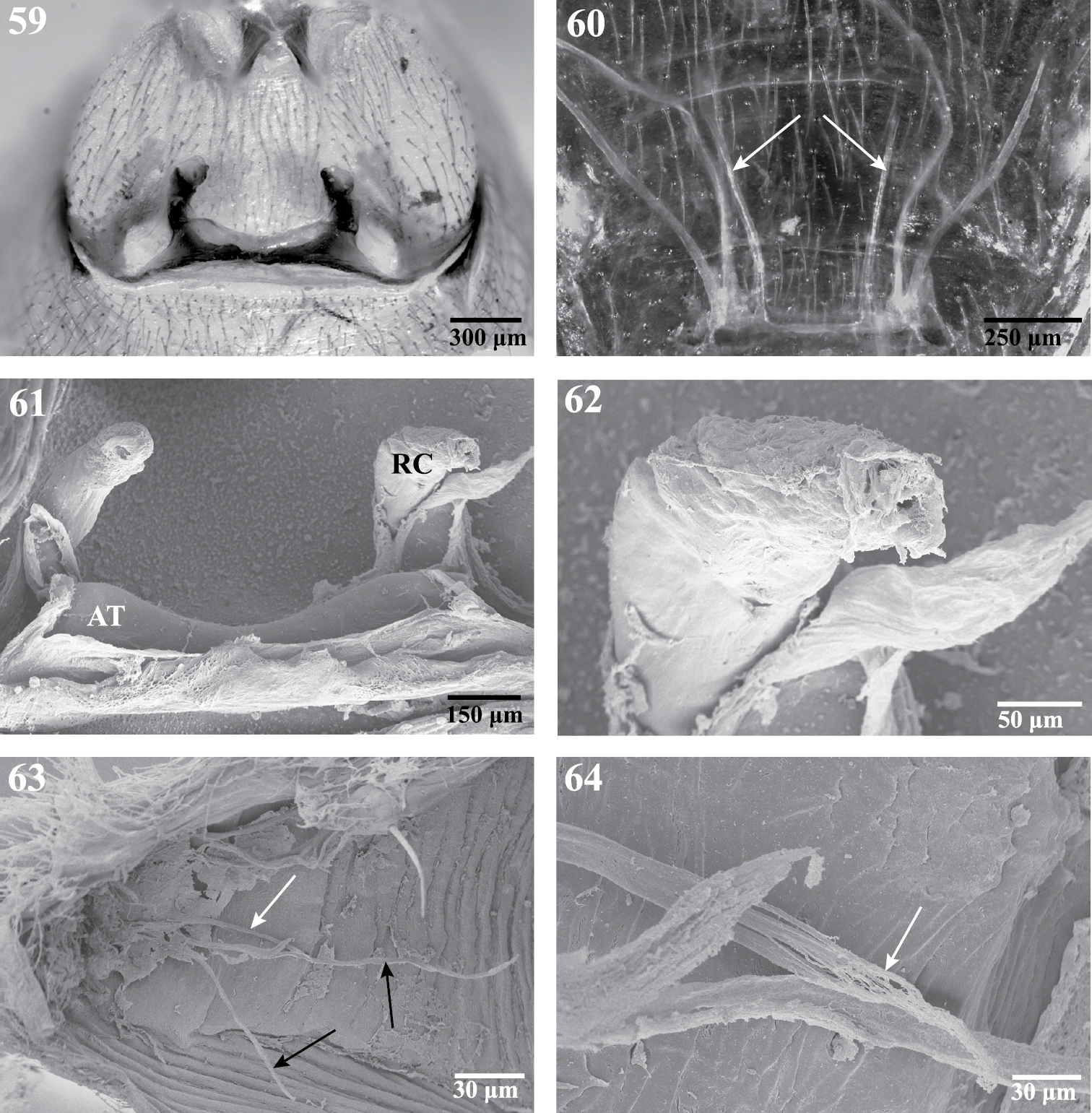
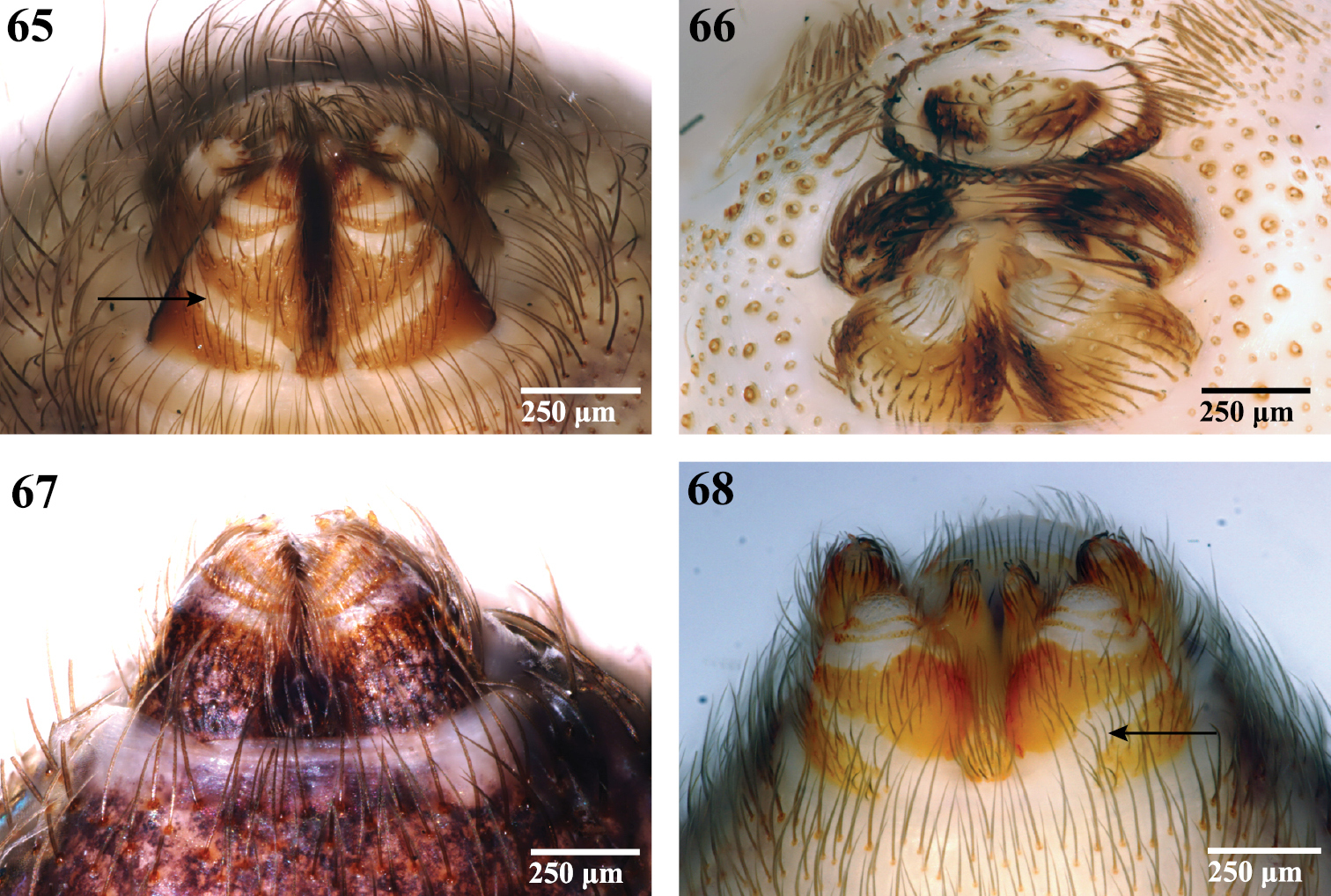
Ecribellate Haplogynae lacking AME, with ALE and PLE contiguous but PME separated (Figs 9, 11), chelicerae free at base and distally not forming a chela with fang (Figs 9, 10, 21, 24), Emerit’s glands absent from patellae and tibiae (Fig. 37), posterior respiratory system with broad spiracle closer to spinnerets than to epigastric furrow (Figs 12, 83), with paired, 2-branched lateral tracheal tubes and long, separate median entapophyses (Figs 60, 63), ALS basal article crossed by a diagonal membranous area (Fig. 68), and with all leg tarsi subsegmented and raptorial (Figs 13, 14, 29–32, 38, 44).
The extraordinary, subsegmented raptorial leg tarsi are unique among spiders and clearly autapomorphic for the family.
urn:lsid:zoobank.org:act:25F85266-612A-42BC-B2F9-72D3B5E0F7DC
Trogloraptor marchingtoninew species, here designated.
The generic name refers to the cave habitat and raptorial tarsi.
By the characters of the family.
As for the family.
Cephalothoraxwith carapace pear-shaped, narrowed anteriorly, pars cephalica faintly distinguished from pars thoracica, fovea indistinct (Figs 11, 16); six eyes, AME absent, ALE and PLE contiguous, PME separated from lateral eyes by their diameter, separated from each other by more than twice their diameter, shiny tapeta fill entire eyecup, of “primitive” type (
One species described, probably another known only from juveniles.
Known only from caves and old growth forest understory in the Klamath-Siskiyou region of Oregon and California.
urn:lsid:zoobank.org:act:DD0946CA-9479-4DC5-BCA2-384B6B82599E
http://species-id.net/wiki/Trogloraptor_marchingtoni
Figures 1–64, 68–86Holotype male from M2 Cave, 15.7 km SSW Grants Pass, Josephine Co., Oregon, USA, collected 29 July 2010 by R. S. Davis and D. S. Snyder, CASENT9040013, and paratype female from No Name Cave, Josephine Co., Oregon, 17.8 km SSW Grants Pass, collected 16 Sept. 2010 by N. Marchington, CASENT9040065, deposited in CAS.
The specific name is a patronym in honor of Neil Marchington, cave biologist, Advisory Board member of the Western Cave Conservatory, Conservation Chair, Western Region, National Speleological Society and Deschutes County Deputy Sheriff, in gratitude for his help and kindness.
By the characters of the genus.
Male (Holotype). Total length 9.70. Markings as in Figs 9–12, 15–18, cephalothorax, legs and pedipalps yellow-brown, unmarked except for dark brown v-mark posteriorly on pars cephalica, clypeus and chelicerae orange brown, abdomen purple brown with faint light chevrons posteriorly on dorsum. Carapace 4.50 long, 3.10 wide; clypeus 1.33 high; ocular area 0.45 long, 1.30 wide; ratio of eyes ALE:PME:PLE, 1.08:1.00:1.08; diameter of PME 0.18; chelicerae 2.38 long; sternum 1.75 long, 1.88 wide; labium 1.08 long, 0.70 wide; pedipalpal coxa 1.50 long, 0.45 wide; leg measurements (Femur + Patella + Tibia + Metatarsus + Tarsus = [Total]): I: 8.25 + 1.40 + 9.25 + 9.00 + 1.45 = [29.35]; II: 7.75 + 1.35 + 8.05 + 8.00 + 1.40 = [26.55]; III: 6.40 + 1.40 + 6.25 + 5.70 + 1.60 = [21.35]; IV: 7.15 + 1.40 + 6.50 + 6.35 + 1.50 = [22.90]; pedipalp: 1.55 + 0.55 + 1.70 + 2.40 = [6.20]. Pedipalp as in Figs 9, 16, 51–58.
Variation (N=2): Total length 6.90—9.70; carapace length 1.19--1.45 times width, height 0.33—0.35 times width; PER width 2.89—3.00 times OAL; clypeal height 6.43—7.00 times PME diameter; clypeal height 1.74—2.11 times cheliceral length; sternum length 0.92—0.93 times width, labium length 1.54—1.55 times width, pedipalpal coxa length 2.81—3.33 times width; femur I length 1.83—2.30 times carapace length; metatarsus I length 2.03—2.06 times carapace length.
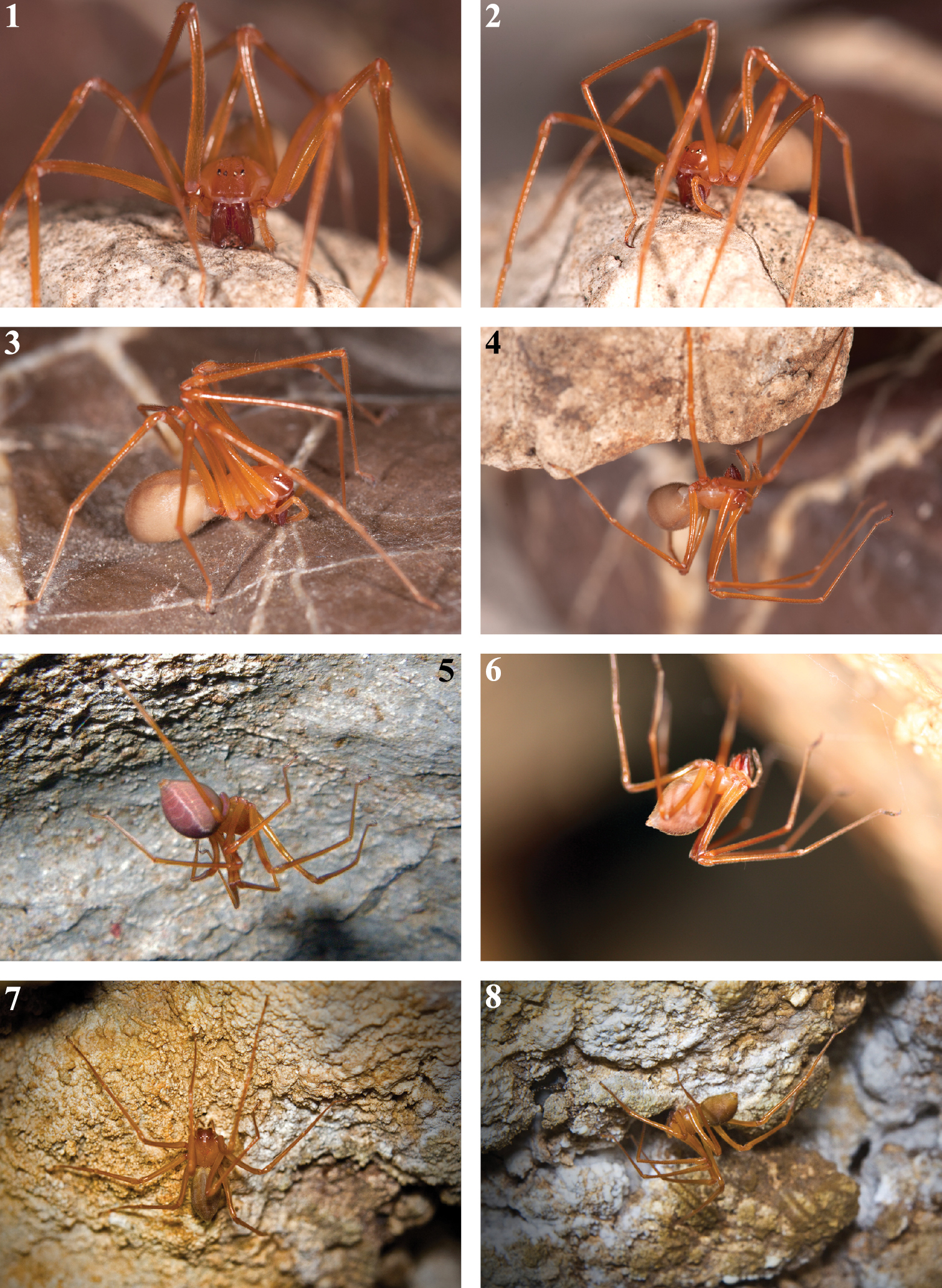
Habitus of live Trogloraptor marchingtoni. 1–4 female in captivity (JL) 5 female in Lake Cave (CG) 6 female in M2 Cave (RD) 7, 8 female in No Name Cave (BM).
Habitus and tarsi of male Trogloraptor marchingtoni (CASENT9040013) from M2 Cave. 9 front 10 mouthparts, ventral view 11 carapace, dorsal view 12 abdomen, ventral view, arrow to tracheal spiracle 13 tarsus I, prolateral; and 14 tarsus IV, prolateral.
Habitus of male Trogloraptor marchingtoni (CASENT9040013) from M2 Cave. 15, 16 dorsal views 17, 18 ventral views; note pedicel in Figure 16.
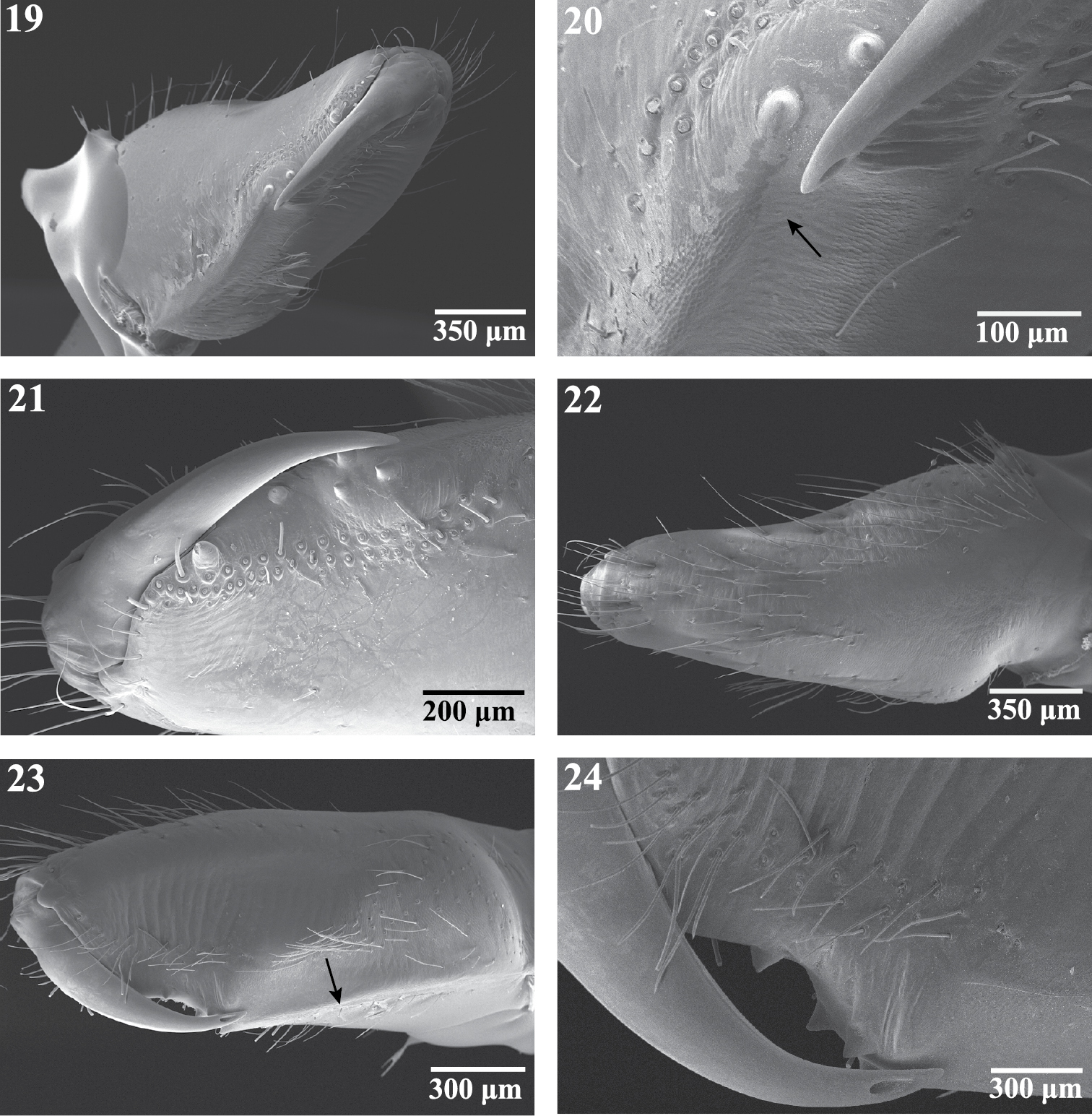
Scanning electron micrographs of the right chelicera of female Trogloraptor marchingtoni (CASENT9040051) from M2 Cave. 19 mesal view 20 mesal view, arrow to cheliceral gland openings 21 retrolateral view 22 ectal view 23 prolateral view, arrow to weak laminar ridge; and 24 prolateral view, close up of fang and opening of poison gland.
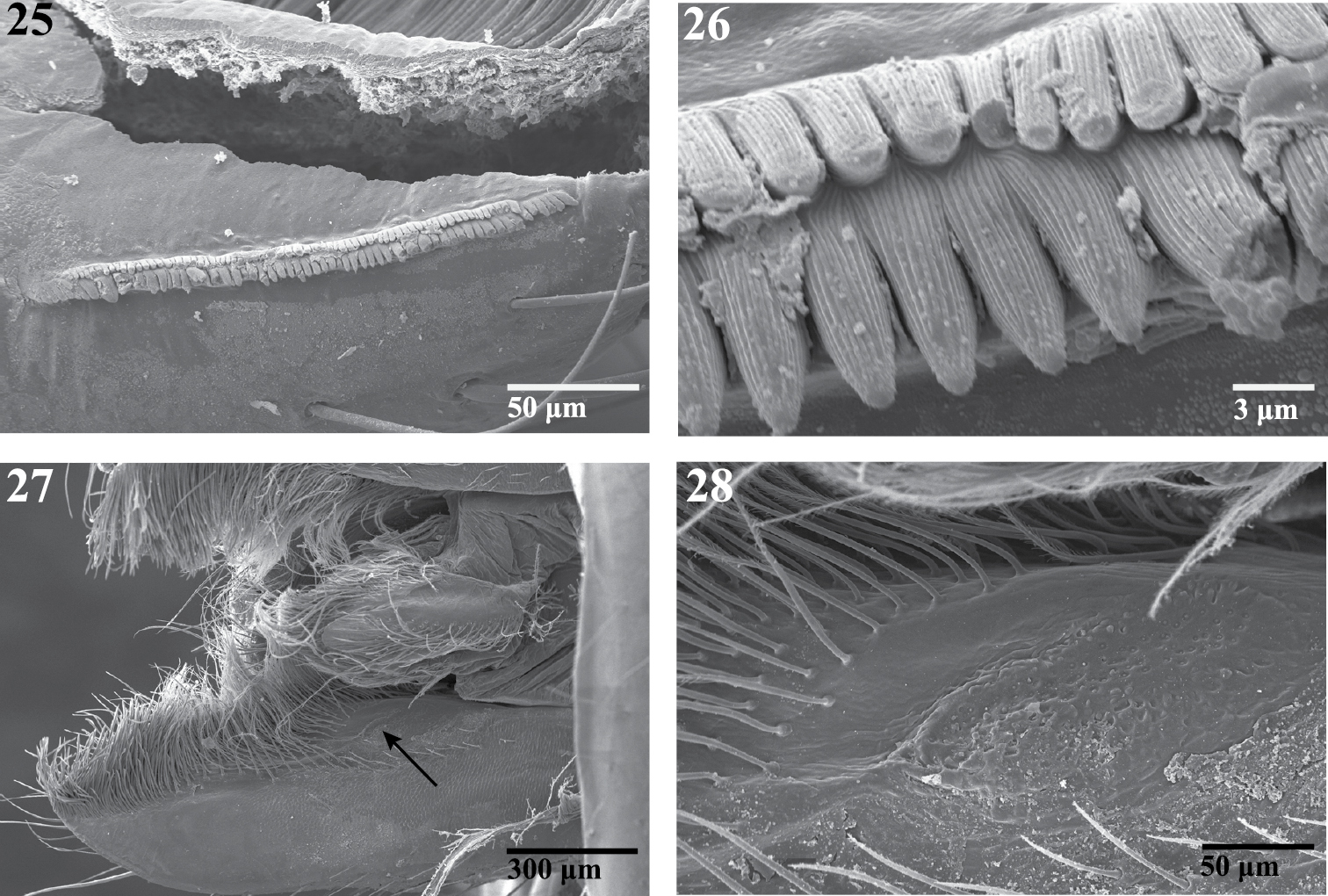
Scanning electron micrographs of the endite of a female Trogloraptor marchingtoni (CASENT9040051) from M2 Cave. 25 serrula 26 serrula, close up, note multiple tooth rows 27 labrum and left endite, dorsal view, arrow to maxillary gland opening 28 maxillary gland opening, close up.
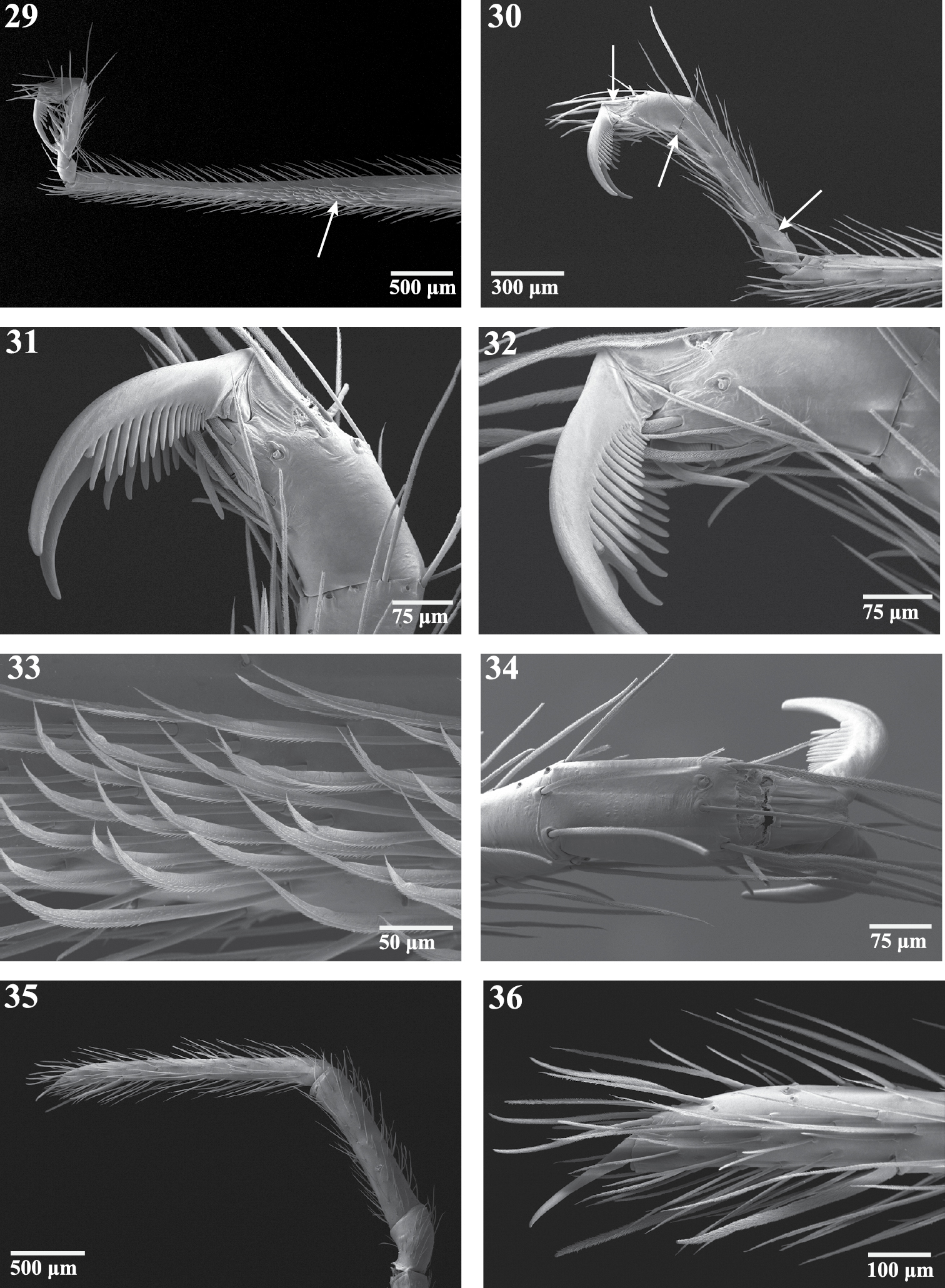
Right appendages of female Trogloraptor marchingtoni (CASENT9040051) from No Name Cave. 29 metatarsus and tarsus of leg III, prolateral view, arrow to ventrolateral patch of curved, spinose setae 30 tarsus of leg IV, prolateral view, arrows to membranous regions 31, 32 tarsus of leg IV, prolateral view 33 curved setae onmetatarsus of leg III, prolateral view 34 tarsus of leg IV, dorsal view 35 pedipalp, prolateral view; and 36 tarsal claw of pedipalp.
Scanning electron micrographs of legs of Trogloraptor marchingtoni from No Name Cave. 37 left patella IV, dorsal view 38 left tarsus IV, retrolateral view 39 left tarsus IV, prolateral view 40 right tarsus III, retrolateral view 41 right tarsus IV, retrolateral view 42 left tarsus IV, retroventral view 43 right tarsus III, ventral view, arrows to spines 44 right tarsus IV, apical view. Figures 37–39, 42 (male, CASENT9040066) 40, 41, 43, 44 (female, CASENT9040051).
Scanning electron micrographs of sensory organs of Trogloraptor marchingtoni. 45 right tarsus I, arrow to tarsal organ 46 tarsus IV, arrow to tarsal organ 47 right tarsus I, tarsal organ 48 tarsal organ on palp 49 trichobothrium on tibia of R leg III, prolateral view 50 trichobothrial base on metatarsus of right leg III. Figures 45, 47–49 (female, CASENT9040051) 46 (male, CASENT9040066) 50 (female, CASENT9040041).
Male pedipalp of Trogloraptor marchingtoni: 51–56 Scanning electron micrographs of right pedipalp 51 tarsus and bulb, apical view 52 tarsus and bulb, dorsal view 53 bulb, dorsal view 54 embolus, dorsal view 55 bulb, ventral view 56 embolus, ventral view, arrow to sperm pore 57, 58 Automontage of left pedipalp 57 prolateral view 58 retrolateral view. Figures 51–56 CASENT9040013 from M2 Cave 57, 58 CASENT9040066 from No Name Cave.
Internal anatomy of Trogloraptor marchingtoni, female (CASENT9040051) from No Name Cave. 59 vulva, dorsal view 60 female posterior respiratory system, tracheae and apodemes, with arrows to median apodemes61–64 Scanning electron micrographs of the internal anatomy 61 vulva, dorsal view, AT, atrium, RC, receptaculum 62 apex of right receptaculum 63 posterior respiratory system, dorsal view, with white arrow to median apodeme and black arrows to lateral tracheal branches; and 64 apex of apodeme (white arrow), note frayed end typical of muscle attachment. Booklungs removed from preparation in 59, 61 and 62.
Spinnerets, ventral view. 65 Segestriidae female, Ariadna sp. from Tinglewood, Western Australia (CASENT 9020550) 66 Pholcidae female, Artema atlanta from Hawaii, USA (CASENT 9047601) 67 Drymusidae female, Drymusa capensis from Table Mountain, South Africa (CASENT 9043173); and 68 Trogloraptoridae male, Trogloraptor marchingtoni (CASENT 9040065). Arrows point to membranous band on ALS base.
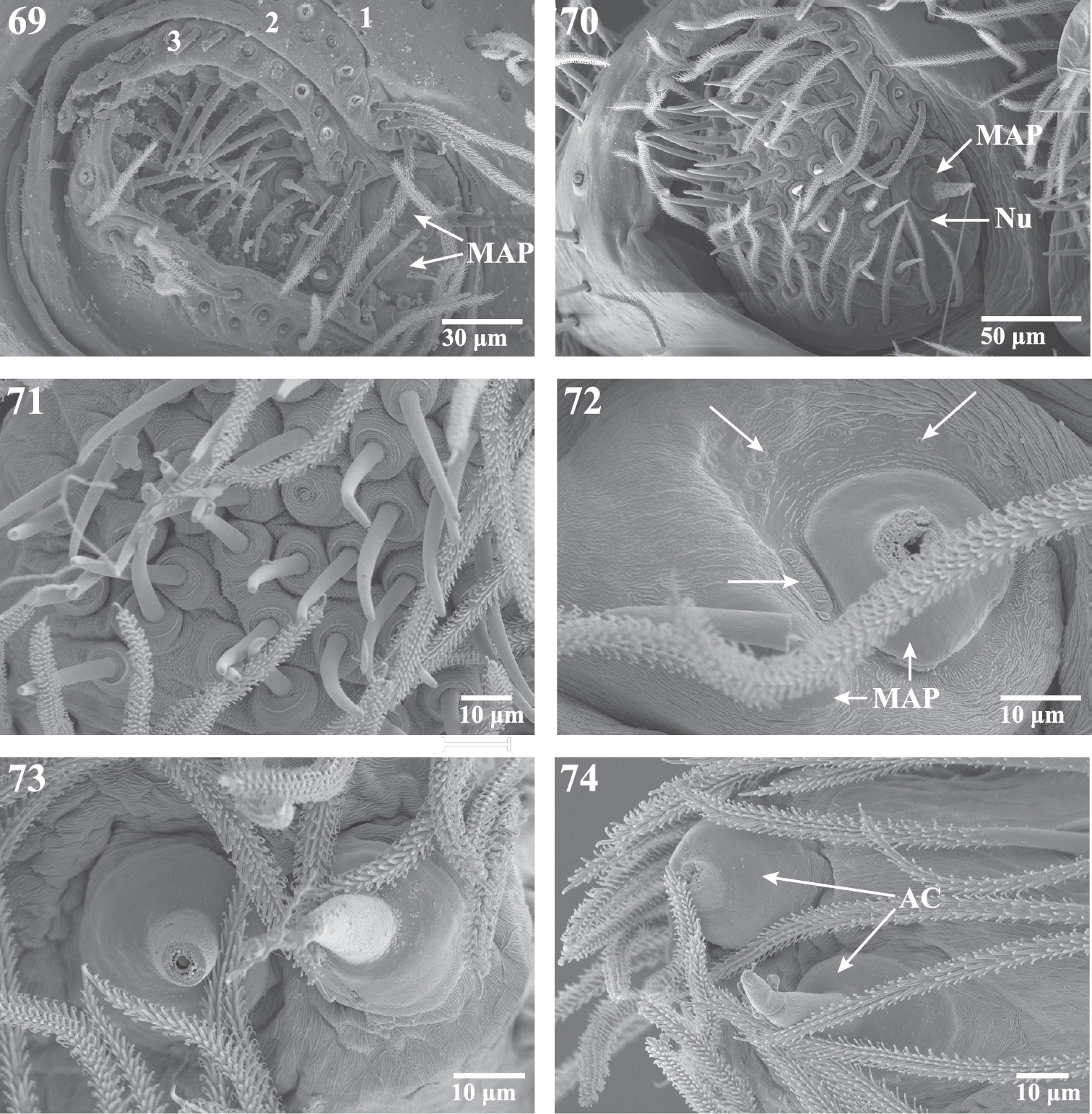
Scanning electron micrographs of the ALS and PLS of Trogloraptor marchingtoni, female (CASENT9039440) and male (CASENT9040066) from No Name Cave and penultimate female from M2 Cave (CASENT9040012). 69 penultimatefemale, right ALS, numbers refer to the three ALS segments 70 male right ALS 71 female ALS piriform gland spigots (left image flipped to appear right) 72 female major ampullate gland spigots of ALS, arrows showing individual and grouped sensillae (right image flipped to appear left) 73 female PLS apex showing aciniform gland spigots (left image flipped to appear right); and 74 female right PLS apex. AC aciniform gland spigots MAP major ampullate gland spigot(s) Nu nubbin.
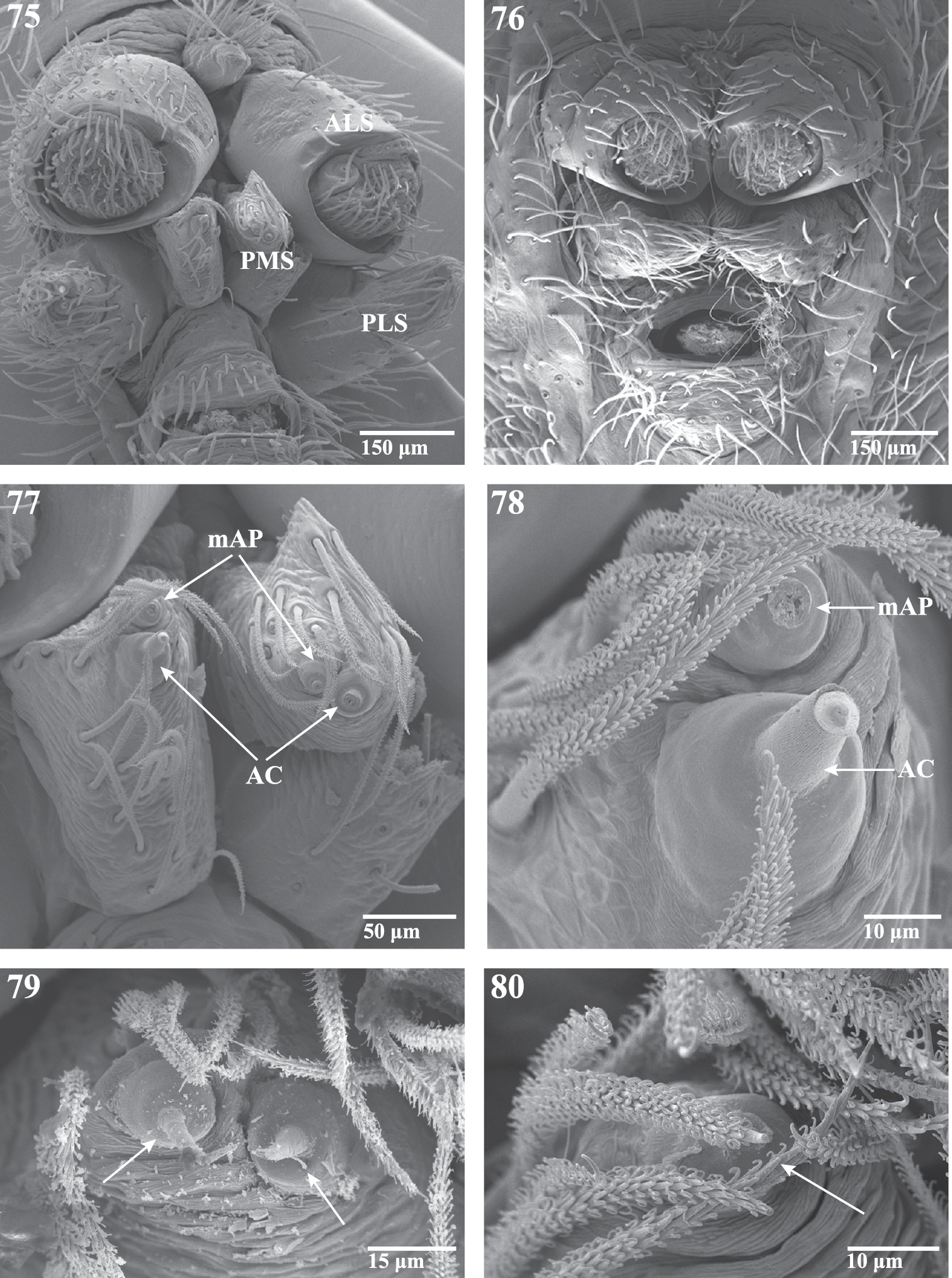
Scanning electron micrographs of spinnerets of Trogloraptor marchingtoni from No Name Cave, female (CASENT9039440), male (CASENT9040066). 75 female spinneret overview (image flipped) 76 male spinneret overview 77 female PMS overview (image flipped) 78 female left PMS (image flipped) 79 female PMS close up showing aciniform gland spigots; and 80 male left PMS apex showing single aciniform gland spigot. AC aciniform gland spigots ALS anterior lateral spinneret mAP minor ampullate gland spigot(s) PMS posterior median spinnerets PLS posterior lateral spinnerets.
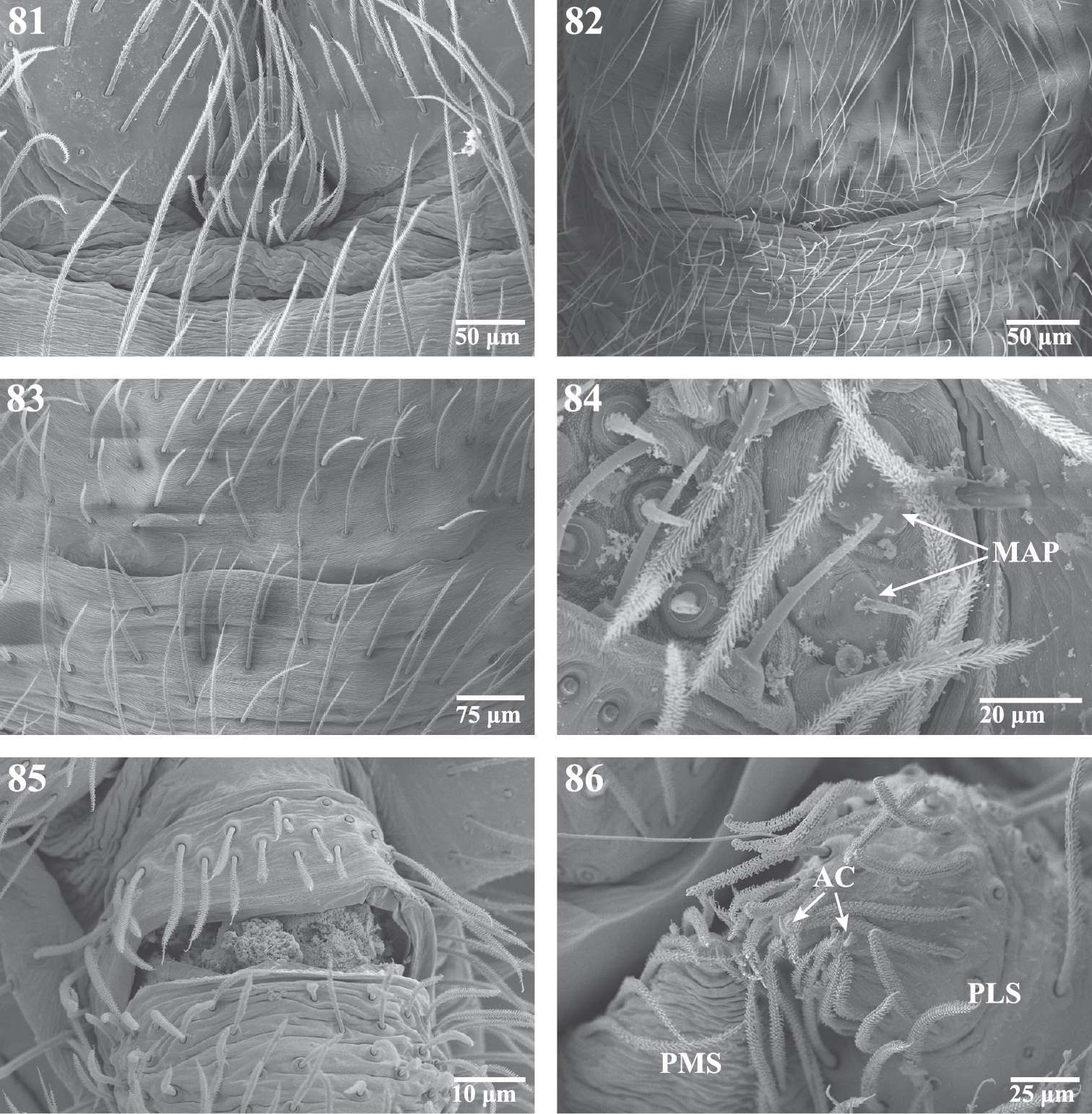
Scanning electron micrographs of the spinnerets of Trogloraptor marchingtoni female (CASENT9039440) and male (CASENT9040066) from No Name Cave, and penultimate female from M2 Cave (CASENT9040012). 81 male colulus 82 male epiandrum 83 male posterior tracheal spiracle 84 penultimatefemale right ALS 85 female anal tubercle; and 86 male left PMS and PLS spinnerets, posterior, arrows to two aciniform gland spigots on PLS. AC aciniform gland spigots MAP major ampullate gland spigot(s) PMS posterior median spinnerets PLS posterior lateral spinnerets.
(paratype): Total length 9.40. Markings as in male (Figs 1–8). Carapace 3.90 long, 2.90 wide; clypeus 0.93 high; ocular area 0.38 long, 1.28 wide; ratio of eyes ALE:PME:PLE, 1.00:1.00:1.07, diameter of PME 0.15; chelicerae 2.25 long; sternum 1.75 long, 1.88 wide; labium 0.95 long, 0.65 wide; pedipalpal coxa 1.38 long, 0.50 wide. Leg measurements (Femur + Patella + Tibia + Metatarsus + Tarsus = [Total]): I: 7.15 + 1.25 + 6.85 + 7.25 + 1.35 = [23.85]; II: 6.75 + 1.30 + 6.85 + 6.95 + 1.40 = [23.25]; III: 5.25 + 1.25 + 5.10 + 4.70 + 1.15 = [17.50]; IV: 5.85 + 1.25 + 5.60 + 5.15 + 1.20 = [19.05]; pedipalp: 1.50 + 0.50 + 1.05 + 2.05 = [5.10]. Genital region weakly sclerotized externally, vulva with median translucent atrium and sclerotized lateral receptaculae, receptacular apex membranous (Figs 59, 61, 62).
Variation (N=3): Total length 8.27—9.60; carapace length 1.30—1.40 times width, height 0.34—0.39 times width; PER width 2.71—3.40 times OAL; clypeal height 6.17—6.91 times PME diameter, 2.05—2.43 times cheliceral length; sternum length 0.93—0.96 times width; labium length 1.28—1.54 times width; pedipalpal coxa length 2.75—3.67 times width; femur I length 1.69—1.83 times carapace length; metatarsus I length 1.72—1.78 times carapace length.
This species has been collected in the dark zone of caves, hanging beneath a few strands of silk that are attached to the cave roof (Figs 5–8). Boulders and rotting logs were searched near the entrance to M2 Cave without finding any Trogloraptor. Nothing has been observed of its predatory or mating behavior. Living specimens were reared in climate controlled conditions and constructed a loose tangle of web from which they hung beneath. Multiple attempts to feed the specimens a variety of prey items failed, which may indicate a preference for specific prey.
Caves in southwestern Oregon.
(all CAS). USA: OREGON: Josephine Co., No Name Cave, 17.8 km SSW Grants Pass, 16 Sept. 2010, N. Marchington, 1 ♀, CASENT9040051, 1 ♂, CASENT9040066, same data except 13 July 2011, 1♀, CASENT9039440; M2 Cave, 15.7 km SSW Grants Pass, 31 July 2010, Geo Graening, R. S. Davis and D. S. Snyder, 1 penultimate ♀, CASENT9040012, same data except 13 July 2011, N. Marchington, T. Audisio, C. Griswold, J. Ledford, D. Ubick, H. Wood and F. Álvarez-Padilla, 3 juveniles, CASENT9047599; Lake Cave near No Name Cave, 9.05 km S Wilderville, 13 July 2011, N. Marchington, T. Audisio, C. Griswold, J. Ledford, D. Ubick, H. Wood and F. Álvarez-Padilla, 1♂ (molted to maturity in captivity) CASENT9047600; Chapman Cave, 12 July 2011, N. Marchington, 2 juveniles, CASENT9039436.
A juvenile Trogloraptor specimen has been collected under debris in old growth redwood forest in far northwest California. The markings of this juvenile differ from the cave species, Trogloraptor marchingtoni. The northwest California specimen has dusky markings laterally on leg femora, a dusky Y marking extending back from the PLE to the posterior margin of the carapace and undulate dusky markings along the lateral carapace margin. These markings suggest that there may be at least one additional Trogloraptor species currently known only from the juvenile. Records for this specimen are as follows: CALIFORNIA: Del Norte Co., NE Crescent City, Jedediah Smith Redwood State Park, Ruth Perry Hatton Grove, US199 0.2 mi E junction with Walker Rd., elev. 60m, 10.29 km NNE Crescent City, old growth redwood, under woody debris, 31 March 2011, E. Garcia, C. Richart, A. Schoenhofer, D. Sitzmann, 1 juvenile, CASENT9040069 (CAS).
Western North America, especially the Klamath-Siskiyou region of northern California and southern Oregon is rich in biodiversity, particularly with respect to its endemic plants and invertebrates (
Major funding for this project came from the Harriet Exline-Frizzell Fund with additional support coming from the Hagey Research Investment Fund of the California Academy of Sciences (CAS). Tracy and Charles acknowledge support from NSF BIR-9531307, “the CAS Summer Systematics Institute, an REU site, ” R. Mooi, PI and from NSF DEB-0613775 “PBI: Collaborative research: the megadiverse, microdistributed spider family Oonopidae” C. Griswold, PI. Joel acknowledges support from the Schlinger Foundation Postdoctoral Fellowship at CAS. We thank Geo Graening, Neil Marchington, Ron Davis, Daniel Snyder and the Western Cave Conservancy for making the first known specimens available to us, Marshal Hedin and Axel Schoenhofer (San Diego State University) for making the redwood forest specimen available, and Neil Marchington, Darrell Ubick, Hannah Wood and Fernando Álvarez-Padilla for help with fieldwork. Vic Smith helped with imaging. Photographs of the living animals are by Ron Davis, Charles Griswold, Joel Ledford and Brent McGregor. The Bureau of Land Management (BLM), Grants Pass office, facilitated cave access. Darrell Ubick and Martín Ramírez provided valuable comments on the specimens, and Fernando Álvarez-Padilla, Anthea Carmichael, Ansie Dippenaar-Schoeman, Rosie Gillespie, Mark Harvey, Gustavo Hormiga, Rudy Jocqué, Leon Lotz, Norman Platnick, Martín Ramírez, Robert Raven and Tamás Szüts commented on presentations about this new spider family. Friendly reviews of drafts of the manuscript were provided by Facundo Labarque, Martín Ramírez, Norm Platnick, Bill Shear and Darrell Ubick. Michael Rix and an anonymous reviewer provided helpful comments. We especially thank Martín Ramírez for pointing out the potential phylogenetic significance of the diagonal band across the ALS base.