(C) 2012 Martin Fikáček. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A detailed examination of specimens of Cryptopleurum sichuanicum Ryndevich, 2005 from high altitudes of Sichuan Province, China, revealed that the species belongs in the genus Nipponocercyon Satô, 1963 previously endemic to Japan. The species is here transferred in Nipponocercyon, and Nipponocercyon sichuanicus (Ryndevich, 2005), comb. n. is redescribed and compared with Nipponocercyon shibatai Satô, 1963. The male genitalia of Nipponocercyon sichuanicus is described for the first time. An adapted diagnosis of Nipponocercyon is provided, and reasons for the inclusion of Nipponocercyon sichuanicus into Nipponocercyon and the general distribution of the genus are discussed.
Hydrophilidae, Sphaeridiinae, Nipponocercyon, taxonomy, morphology, China, Oriental region, Palaearctic region
Cryptopleurum sichuanicum Ryndevich, 2005 was described from a few female specimens collected in the mountains of Sichuan Province, China (
Material examined for this study is deposited in the following collections:
CSR coll. Sergey Ryndevich, Baranovichi, Belarus;
KSEM Natural History Museum, University of Kansas, Lawrence, USA (A. Short);
NHMW Naturhistorisches Museum, Wien, Austria (M. A. Jäch, A. Komarek);
NMPC Department of Entomology, National Museum, Praha, Czech Republic (M. Fikáček);
SYSU Entomological collection of Sun Yat-sen University, Guangzhou, China (F.-L.Jia).
The current study is largely based on newly collected material of Nipponocercyon sichuanicus (18 specimens) which were compared with one paratype of Cryptopleurum sichuanicum, and on the specimens of Nipponocercyon shibatai and of other megasternine genera deposited in the collection of NMPC.
Selected specimens were dissected, with genitalia embeded in a drop of water-soluble dimethyl hydantoin resin on a piece of transparent plastic pinned below the specimen, or of alcohol-soluble Euparal resin on a small piece of glass attached below the respective specimen. The external morphology was examined using the Hitachi S-3700N environmental electron microscope at the Department of Entomology, National Museum in Prague. Habitus photographs were taken using Canon D-550 digital camera with attached Canon MP-E65mm f/2.8 1–5× macro lens, and subsequently adapted in Adobe Photoshop CS2. Figures of genitalia were prepared with the help of Photoshop CS4. The morphological terminology largely follows
The inclusion of Cryptopleurum sichuanicum into Nipponocercyon (see below) requires a modification of the differential diagnosis of the genus as follows:
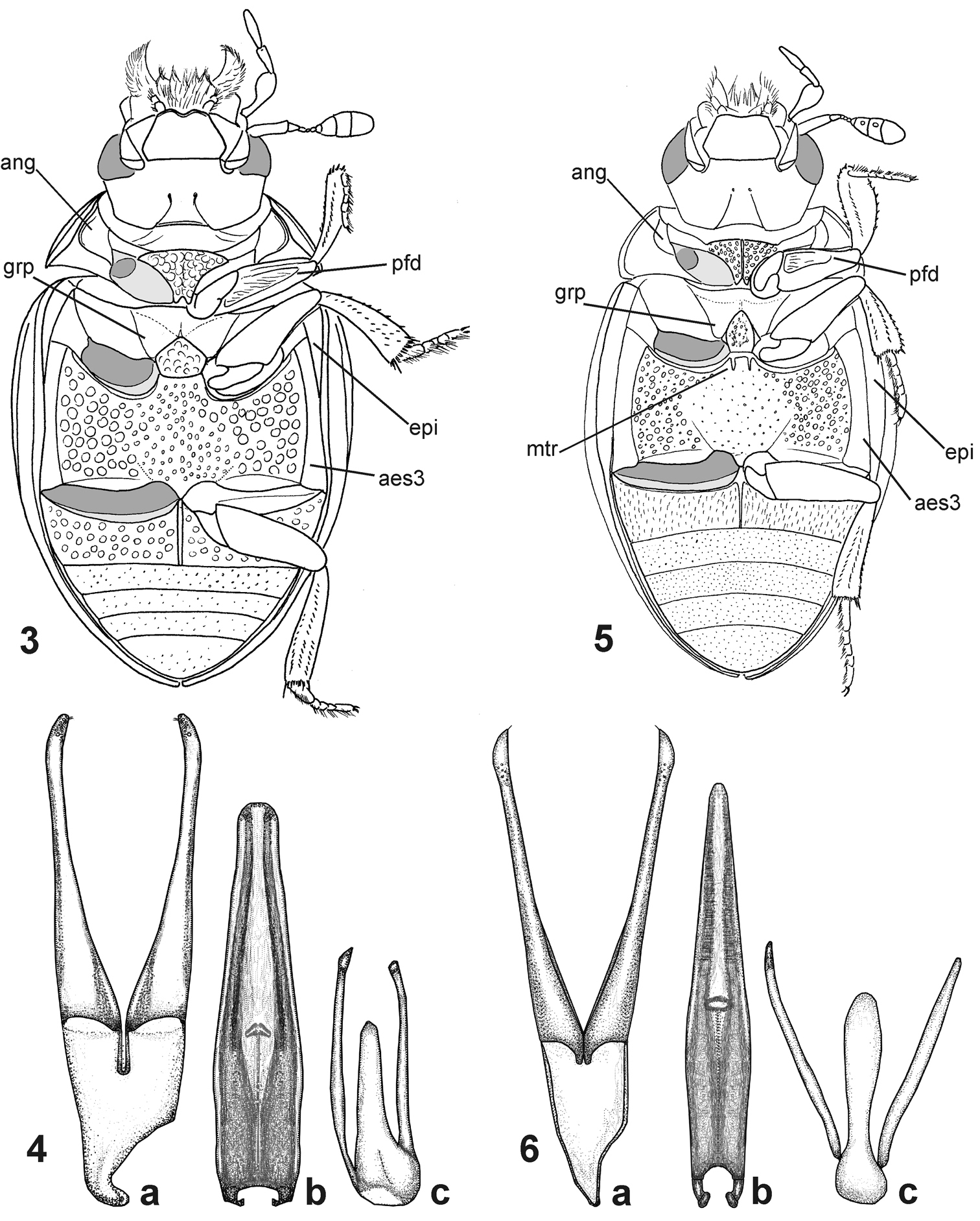
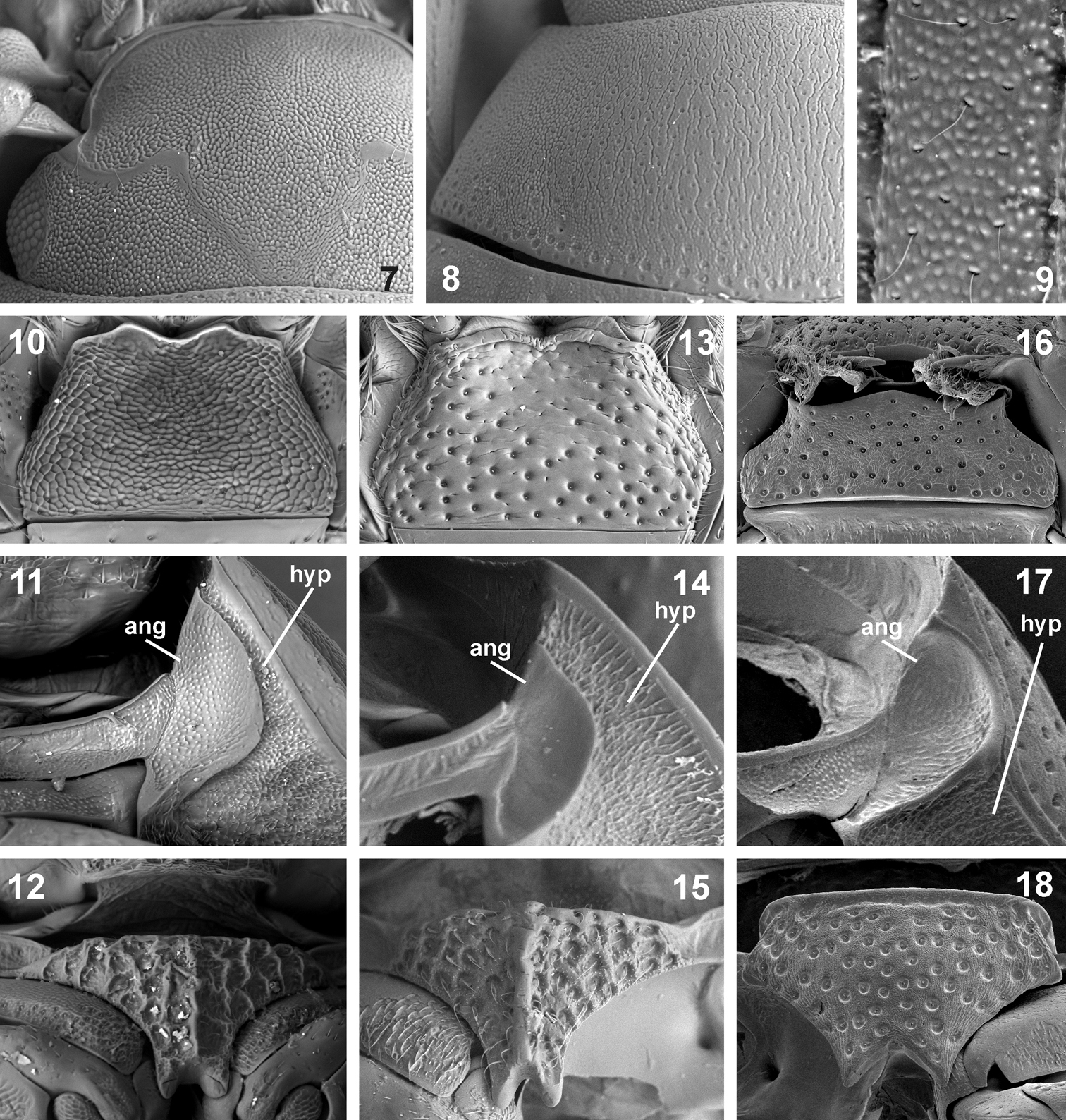
Head without transverse interantennal ridge; eyes small, separated by 9× of one eye; mentum weakly bisinuate on anterior margin; antennae with 9 antennomeres; maxilla with or without sucking disc in males; maxillary palpomere 2 strongly widened distally; posterior tentorial pits minute; pronotum evenly convex, lateral margin not deflexed or slightly deflexed; transverse row of larger punctures along posterior margin of pronotum absent (large areas without microsculpture in Nipponocercyon sichuanicus may actually resemble enlarged punctures on the first view, but the punctures are as large as those in disc when examined in detail, see Fig. 8); median portion of prosternum weakly to distinctly separated from lateral portions, bearing coarse setiferous sculpture; median portion of prosternum carinate medially (carina distinct in Nipponocercyon shibatai, partly obliterated by the sculpture but still apparent in Nipponocercyon sichuanicus, compare Figs 12 and 15); prosternal process wide, deeply excised; antennal grooves moderately large to large, not reaching lateral margin of hypomeron (Figs 11, 14); anteroventral margin of prothorax with a small denticle on the contact of prosternum and hypomeron; profemur with elongate ventral depression along anterior margin; elytron with 10 punctural series; elytral intervals flat or highly convex; lateral margins of elytra not denticulate nor serrate; mesoventral cavities for reception of procoxae large, reaching mesocoxae; preepisternal elevation subpentagonal, widely contacting metaventral process, median portion of metaventrite slightly to very distinctly elevated; postcoxal ridge lying parallel to posterior margin of mesocoxal cavity, not overlapping to lateral margin of metaventrite; lateral portions of metaventrite with coarse punctation (smaller punctures may be intermixed or absent); metanepisternum narrow, but distinct throughout; abdominal ventrite 1 carinate medially, with coarser punctation than ventrites 2–5; phallobase asymmetrical, much shorter than parameres; gonopore situated in basal half of median lobe; male sternite 9 with median tongue-like projection; male sternite 8 without median projection.
A few characters listed as diagnostic for Nipponocercyon by
By the combination of median portion of the prosternum differentiated from lateral portions, subpentagonal preepisternal elevation of the mesothorax widely contacting the metaventrite, large mesothoracic cavities for reception procoxae (reaching to anterior margin of mesoxocal cavity) and metanepisternum well developed both anteriorly and posteriorly, Nipponocercyon is most similar to the genus Australocyon Hansen, 1990. It may be easily distinguished from the Australian and Neotropical species of Australocyon by the male sternite 9 with tongue-like median portion (Fig. 4c), and male sternite 8 without median projection; from Australocyon pilocnemoides group it may be distinguished by the undifferentiated surface of the subpentagonal mesoventral plate (with a semicircular median portion defined by a wide bead in Australocyon pilocnemoides group, see Fig. 6 in
When the size of mesoventral cavities for reception of procoxae is not taken into consideration, Nipponocercyon may resemble other megasternine genera with small subpentagonal mesoventral plate, clearly defined prosternal plate and male sternite 9 tongue-like medially (characters distinguishing the respective genus from Nipponocercyon are listed in parentheses: Agna (prosternal plate without deeply excised prosternal process, antennal grooves very small and angular in shape, profemur without sculptured depression); Bolbonotum and Kahanga (elytral grooves deep and wide, reaching total base of elytra, prosternal plate projecting both anteriad and posteriad, profemur without ventral impression, mesoventral plate rhomboid when examined in detail, gonopore apical), Deltostethus (mesoventral plate with wide marginal bead, profemur without ventral depression, gonopore apical), and Pelocyon (metavetrite with complete femoral lines, prosternal plate longer than wide). Nipponocercyon sichuanicus may resemble some species of the genera Cryptopleurum, Pachysternum and Cyrtonion by its large antennal grooves, large grooves for reception of procoxae, reduced epipleura and strongly sculptured body. See below under that species for characters distinguishing it from the mentioned genera.
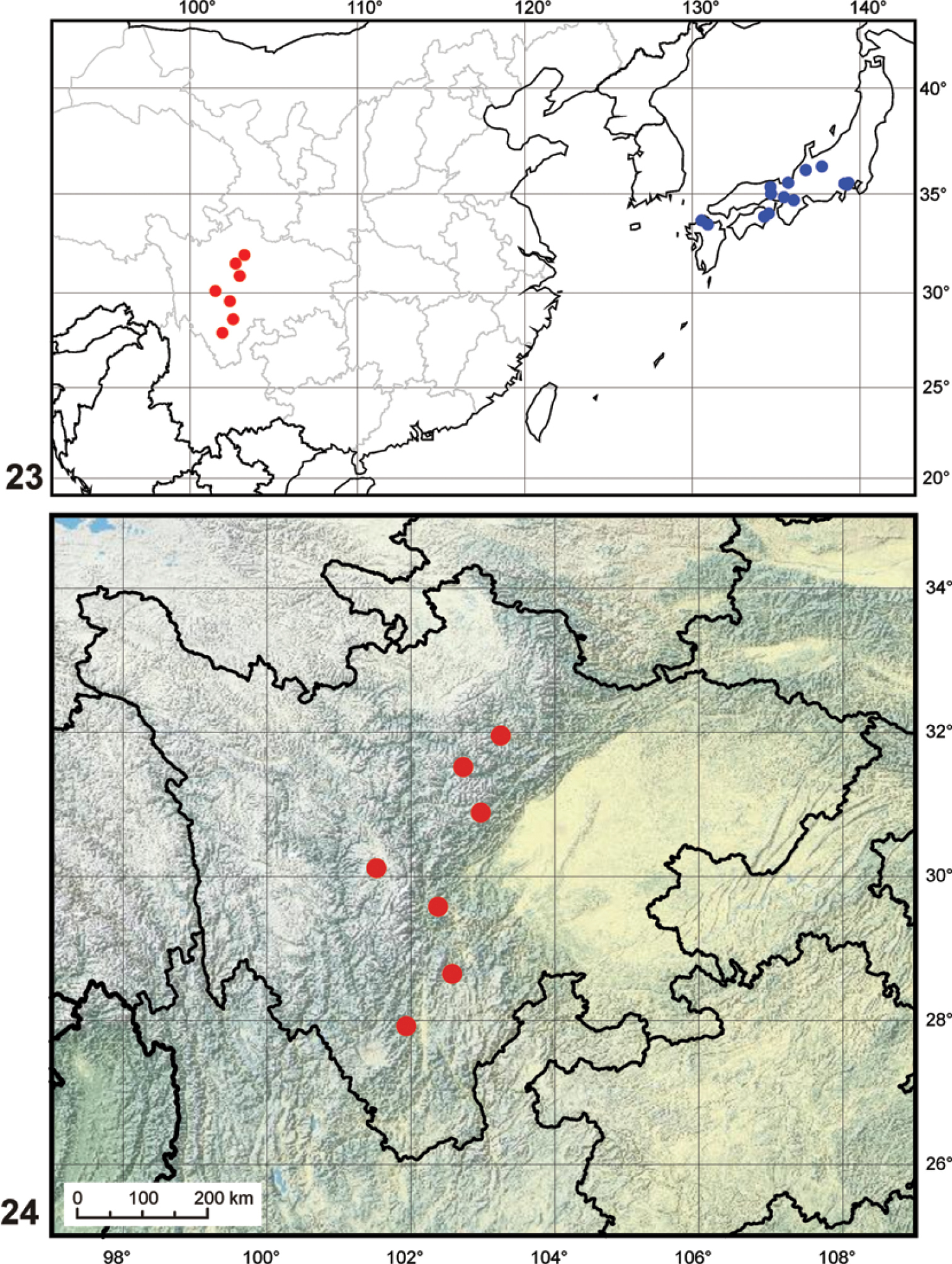
The genus now includes two species, one distributed in Kyushu, Shikoku and the southern part of the Honshu, the other occuring in high altitudes of the mountain ranges in the Chinese province of Sichuan (Fig. 23).
| 1 | Body uniformly brown (Figs 1–2). Elytral intervals strongly convex (Figs 1–2), whole dorsal surface strongly microsculptured (Figs 7–9). Male maxilla without sucking disc. Antennal club without ventral groups of peg-like sensilla (only seen at high magnifications!). Antennal grooves large, nearly reaching lateral margin of hypomeron (Fig. 11). Prosternal plate with obsolete median carina. Preepisternal elevation of mesothorax slightly wider than long (Fig. 19). Anteromedian portion of metavetrite without (or with at most very weakly developed) two short longitudinal ridges, lateral portions with very coarse punctures (Fig. 20). First abdominal ventrite with setiferous punctures many times larger than on ventrites 2–5 (Fig. 21). Protibia angulate distally. Median lobe wide apically (Fig. 4b) | Nipponocercyon sichuanicus (Ryndevich, 2005), comb. n. |
| – | Elytra pale reddish with darker spots at midlength, head and pronotum dark brown ( |
Nipponocercyon shibatai Satô, 1963 |
http://species-id.net/wiki/Nipponocercyon_sichuanicus
Figs 1 –4, 7–12, 19–21, 23–24Paratype: 1 female (CSR): 'CH, S Sichuan, near / Bijishan Village, left / tr. of Lianhegou River / 2500–3200 m, 19.6.2000 / Belousov, Kabak, Davidian // Paratype / Cryptopleurum / sichuanicum / Ryndevich S. K. // Coll. / SKR // Cryptopleurum / sp.n. / det HEBAUER'.
CHINA: Sichuan: 2 males, 1 spec. (CSR, NMPC): 2.1 km N of Dengsheng, SE of Balanguan Pass, elev. 3455 m, 30°53'3"N, 102°58'23"E, 29.viii.2004, lgt. Belousov & Kabak; 1 spec. (NHMW): 20 km N Sabdȇ, elev. 3300 m, 29°35'N, 102°23'E, 14.vii.1998, lgt. A. Smetana (C82); 1 spec. (CSR): S of Musu village, elev. 2850 m, 31°56'53"N, 103°15'11"E, 19.viii.2007, lgt. Belousov & Kabak; 1 male (NMPC): N Sichuan, SW of Baima, elev. 2980–3040 m [ca. 27°55'N, 101°56'E], 23.vi.2006, lgt. I. Kabak; 5 spec. (CSR, NMPC, SYSU, KSEM): SW of Jiabi, elev. 3240 m, 31°30'40"N, 102°43'43"E, 8–13.viii.2007, lgt. Belousov & Kabak; 1 male, 3 spec. (NHMW, NMPC): Ganzi, Daxue Shan, Mugecuo, ca. 26 km NW Kangding, elev. 3200–3400 m, 30°06'36"N, 101°31'12"E, 21.v.1997, lgt. A. Pütz.
Body widely oval, widest in anterior third of elytra. Body length 2.2–2.9 mm, body width 1.4–1.7 mm.
General coloration of dorsal surface dark brown, anterior and anterolateral margins of clypeus and lateral portions of frontoclypeal suture pale reddish, anterior margin of pronotum widely reddish, each elytron slightly paler in humeral area and at elytral apex. Ventral surface dark brown, mentum, mouthparts and posterior portions of temporae reddish brown. Maxillary palpi, antennae and legs pale reddish brown.
Head. Clypeus widely rounded, constricted above antennal bases, with very distinct marginal bead. Dorsal surface of clypeus and frons with strong scale-like microsculpture obscuring the punctation, sparsely arranged punctures not apparent among microsculpture, only evident according to long thin setae arising from punctures. Frontoclypeal suture apparent as a non-sculptured stripe directing mesad, strongly bent posteriad submesally. Mentum with sparsely arranged fine setiferous punctures medially and posteriorly, interstices with strong scale-like microsculpture. Antennal club without distinct groups of peg-like sensilla dorsally or ventrally. Maxilla of male without sucking disc ventrally.
Prothorax. Pronotum with sparsely arranged fine setiferous punctures, larger punctures along posterior margin absent. Whole dorsal surface with mesh-like microsculpture, microsculpture strong along anterior and posterior margins and on lateral portions of pronotum, obsolete on pronotal disc; pronotal disc with irregular longitudinal striae. Lateral portions of pronotum slightly deflexed (and hence seen in ventral view). Prosternum with well defined median plate 2.0× wider than long, bearing strong rugose sculpture, indistinctly carinate mesally. Anterolateral corners of prosternum (at contact with hypomeron) with small but distinct tooth. Antennal grooves large, but not quite reaching lateral margin of hypomeron. Profemur with a rather shallow sculptured depression on a large portion of ventral surface. Protibia angulate distally.
Mesothorax. Scutellar shield with sparse fine punctation, without microsculpture. Elytra with 10 punctural series, all series deeply impressed, lateral striae deeper than median ones; serial punctures minute and rather inconspicuous; elytral intervals highly convex, bearing sparsely arranged fine setiferous punctation, interstices with strong microsculpture consisting of small bumps; lateral portions of elytra deflexed laterally (hence, visible in ventral view); epipleuron present only on elytral base, reduced to extremelly narrow stripe behind level of mesocoxae. Mesoventrite with pentagonal posteromedian elevation, the elevation 1.3× wider than long, with rugose setiferous sculpture.
Metathorax. Anteromedian process with very weakly developed short longitudinal ridges, in many individuals completely obscured by microsculpture; median portion of metaventrite slightly elevated bearing densely arranged coarse setiferous punctures separated by 0.5–1.2× puncture diameter; lateral portions of metaventrite with extremelly large setiferous punctures; whole surface of metaventrite except its posteromedian portion with mesh-like microsculpture on interstices, microsculpture stronger laterally than medially. Hind wings well developed.
Abdomen. All abdominal ventrites with strong scale-like microsculpture, punctation of ventrite 1 consisting of extremelly large setiferous punctures similar to that on lateral portions of metaventrite; punctation of ventrites 2–5 sparse and very fine, nearly completely obscured by microsculpture.
Male genitalia. Parameres slender, 1.8× longer than phallobase. Median lobe robust, very wide and parallel-sided in basal 0.35, slighly and continually narrowing apicad in apical 0.65, apex widely rounded; gonopore situated in basal 0.4 of median lobe. Stenite 9 with slightly asymmetrical median projection.
General habitus of Nipponocercyon sichuanicus. 1 dorsal view 2 lateral view.
Morphological details of Nipponocercyon species. 3–4 Nipponocercyon sichuanicus:3 ventral view 4 male genitalia; 5–6 Nipponocercyon shibatai: 5 ventral view 6 male genitalia [the drawing is based on that by
Morphological details of Nipponocercyon sichuanicus and its comparison with Nipponocercyon shibatai and Cryptopleurum minutum. 7–12 Nipponocercyon sichuanicus:7 head in dorsal view 8 pronotum 9 superficial microsculpture of elytral intervals 10 mentum 11 antennal groove 12 median portion of prosternum. 13–15 Nipponocercyon shibatai:13 mentum 14 antennal groove 15 median portion of prosternum. 16–18 Cryptopleurum minutum:16 mentum 17 antennal groove 18 median portion of prosternum. Abbreviations: ang antennal groove, hyp hypomeron.
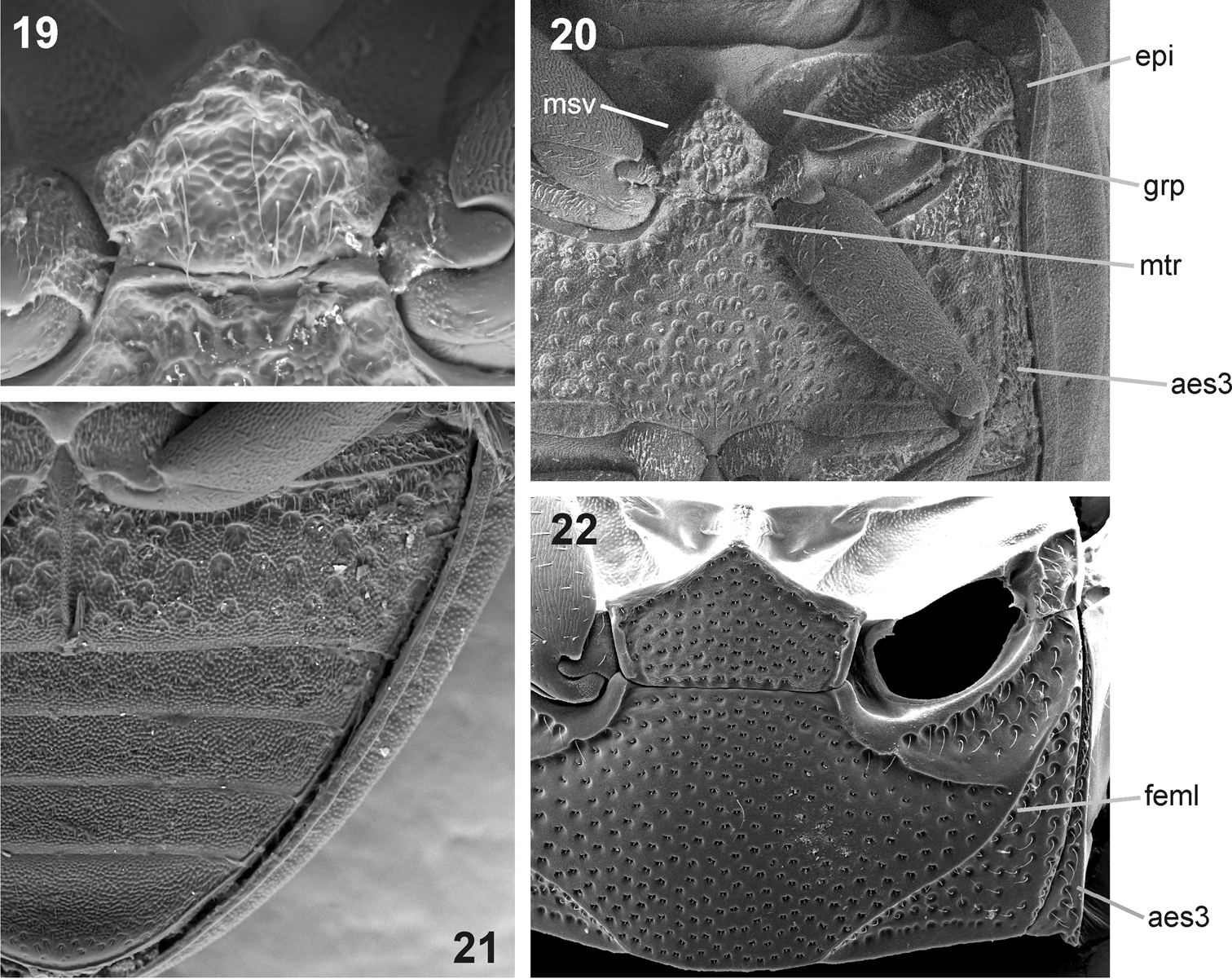
See the identification key above for characters distinguishing Nipponocercyon sichuanicus from Nipponocercyon shibatai. Nipponocercyon sichuanicus may be confused with some species of Cryptopleurum, Pachysternum or Cyrtonion (the latter not occurring in Asia, however)which are also characterized by large antennal grooves and strongly sculptured dorsal surface. Nipponocercyon shibatai may be easily distinguished from them by the combination of following characters: (1) metaventrite without femoral lines (femoral lines present in Cryptopleurum, Pachysternum and Cyrtonion, see e.g. Fig. 22, feml); (2) metanepisternum wide throughout (Fig. 20) (reduced anteriorly and widening posteriad in the above genera as well as in all other genera of the Megasternum group characterized by large antennal grooves, see e.g. Fig. 22, aes3); (3) gonopore situated in basal portion of the median lobe (Fig. 4; this character distinguishes both species of Nipponocercyon from all other Megasternini); (4) mesoventral plate only slightly wider than long, without acute angles (Fig. 19) (mesoventral plate large and distinctly transverse in Cryptopleurum, see e.g. Fig. 22); (5) anterolateral corners of mentum not sharply angulate (Fig. 10) (sharply angulate in Cryptopleurum, as in Fig. 16).
Ventral morphology of Nipponocercyon sichuanicus and its comparison with Cryptopleurum ferrugineum. 19–21 Nipponocercyon shibatai:19 mesoventral plate 20 meso- and metaventrite 21 abdominal ventrites. 22 meso- and metaventrite of Cryptopleurum ferrugineum. Abbreviations: aes3 metanepisternum, epi epipleuron, feml femoral line, grp groove for reception of procoxae, msv mesoventral plate, mtr anteromedian ridge of metaventrite.
No details on the biology are known. The terrestrial habits of Nipponocercyon shibatai (Hoshina & Fikáček 2010) as well as the vast majority of the megasternine taxa suggest that Nipponocercyon sichuanicus is a terrestrial species.
The species occurs in the mountains of the Sichuan province in South China, at altitudes between 2500–3500 m a.s.l. (Fig. 24).
Maps. 23 generaldistribution of the genus Nipponocercyon (data for Nipponocercyon shibatai adopted from
The inclusion of Cryptopleurum sichuanicum into the genus Nipponocercyon may appear contradictory, as the species differs in its external morphology from Nipponocercyon shibatai in many characters, some of which were previously considered as diagnostic at the generic level. However, a detailed comparison reveals that despite many differences, both species are very similar in terms of the morphology of the ventral side and genitalia (compare Figs 3–6) which is crucial for generic assignment of the megasternine taxa. Especially important in this respect are characters which are present in both Nipponocercyon species but absent from all other Megasternini: the basal position of the gonopore on the median lobe (Figs 4b, 6b), and the presence of a depression on the ventral surface of profemur (Figs 3, 5, pfd). Another character infrequent in the Megasternini but shared by both Nipponocercyon species is the presence of large mesothoracic grooves for the reception of the procoxae (Fig. 20, grp). Except Nipponocercyon, large grooves are only present in Australocyon and the Megasternum group of genera, of which the representatives of the latter generic group and part of Australocyon clearly seem unrelated to Nipponocercyon based on other external characters (see the differential diagnosis of Nipponocercyon for details). Moreover, general morphology of the genitalia of both speciesis very similar, with principal differences found only in the shape of the apical portion of the median lobe. Eyes with extremelly small dorsal portion are also unusual within the Megasternini but shared by both species. All these characters indicate that both species are more closely related to each other than they are to other megasternine taxa, which justifies the tranfer of Cryptopleurum sichuanicum into Nipponocercyon.
Characters in which Nipponocercyon sichuanicus is contradicting the diagnosis of the genus Nipponocercyon used by previous authors (
The autapomorphies of Nipponocercyon sichuanicus make the species similar in habitus to Cryptopleurum and related genera, which is the reason why the species was originally described within Cryptopleurum. However, a detailed examination shows that this similarity is due to the parallelism, as the body parts which are responsible for the Cryptopleurum-like appearance of Nipponocercyon sichuanicus differ between Cryptopleurum and Nipponocercyon sichuanicus in detailed morphology:
(1) The antennal grooves of Nipponocercyon sichuanicus are large, but still do not reach the lateral margins of hypomeron, as they do in Cryptopleurum and related taxa (compare Figs 11 and 17).
(2) The meso- and metathorax of Nipponocercyon sichuanicus resemble Cryptopleurum and related genera by their extremely coarse punctation and large grooves for reception of procoxae, but the overall thoracic morphology is totally different from these genera due to its well developed metepisternum (not reduced anteriorly, Fig. 20: aes3) and the ventral side of mesothorax not steeply declined, possessing unmodified mesepimera.
(3) The prosternal plate of Nipponocercyon sichuanicus is feebly carinate even though the carina is partly obscured by the strong microsculpture (ecarinate in Crypopleurum and related genera, see Fig. 18).
The above differences indicate that despite its superficial resemblance, Nipponocercyon sichuanicus is not related to the Cryptopleurum group of genera, but probably represents another example of highly sculptured sphaeridiine taxon derived from a non-sculptured ancestor. The shift from non-sculptured to sculptured phenotype was shown to lead to similar ‚sculptured‘ morphology in distantly related taxa in the Megasternini (Oosternum and Emmidolium, see Fikáček 2007), and similar examples also exist in some other groups of the Sphaeridiinae (e.g., the omicrine genera Noteropagus and Peratogonus resemble the megasternine genus Cryptopleurum by the same characters which are responsible for the Cryptopleurum-like appearance of Nipponocercyon sichuanicus).
The inclusion of Cryptopleurum sichuanicum into Nipponocercyon extends the range of the genus (previously endemic to Japan) to the mainland Asia. The isolated occurrence of the genus at high altitudes of the mountain ranges in Sichuan and in mountain areas of southern Japan (Fig. 23) suggests that the current distribution may represent relictual remnants of the former wider distribution of the genus. The situation hence resembles that of the myxophagan genus Satonius Endrödy-Younga, 1997 which was originally considered as Japanese endemic (
Hoshina and Fikáček (2008) examined 35 specimens of Japanese Nipponocercyon from the entire Japanese range of the genus, including the types of all three taxa described from Japan by
We are indebted to M. A. Jäch for the opportunity to examine the specimens deposited in NHMW, to H. Hoshina (Fukui University, Fukui, Japan) for reexamining several morphological details of Nipponocercyon shibatai and taking their photographs for us, to J. Růžička (University of Life Sciences, Prague, Czech Republic) for his help with generating the maps, and to the collectors of the additional specimens presented above for the kind donation of these specimens to the second author. The study was financially supported by the Ministry of Culture of the Czech Republic (DKRVO000023272) and the institutional resources of Ministry of Education, Youth and Sports of the Czech Republic for the support of science and research. Examination of the specimens using scanning electron microscope Hitashi-3700N was possible due to Barrande I Project partially supported by the European Union.