(C) 2011 David A. Grimaldi. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Thirteen species of basal Brachycera (11 described as new) are reported, belonging to nine families and three infraorders. They are preserved in amber from the Early Cretaceous (Neocomian) of Lebanon, Albian of northern Spain, upper Albian to lower Cenomanian of northern Myanmar, and Late Cretaceous of New Jersey USA (Turonian) and Alberta, Canada (Campanian). Taxa are as follows, with significance as noted: In Stratiomyomorpha: Stratiomyidae (Cretaceogaster pygmaeus Teskey [2 new specimens in Canadian amber], Lysistrata emerita Grimaldi & Arillo, gen. et sp. n. [stem-group species of the family in Spanish amber]), and Xylomyidae (Cretoxyla azari Grimaldi & Cumming, gen. et sp. n. [in Lebanese amber], and an undescribed species from Spain). In Tabanomorpha: Tabanidae (Cratotabanus newjerseyensis Grimaldi, sp. n., in New Jersey amber). In Muscomorpha: Acroceridae (Schlingeromyia minuta Grimaldi & Hauser, gen. et sp. n. and Burmacyrtus rusmithi Grimaldi & Hauser gen. et sp. n., in Burmese amber, the only definitive species of the family from the Cretaceous); Mythicomyiidae (Microburmyia analvena Grimaldi & Cumming gen. et sp. n. and Microburmyia veanalvena Grimaldi & Cumming, sp. n., stem-group species of the family, both in Burmese amber); Apsilocephalidae or near (therevoid family-group) (Kumaromyia burmitica Grimaldi & Hauser, gen. et sp. n. [in Burmese amber]); Apystomyiidae (Hilarimorphites burmanica Grimaldi & Cumming, sp. n. [in Burmese amber], whose closest relatives are from the Late Jurassic of Kazachstan, the Late Cretaceous of New Jersey, and Recent of California). Lastly, two species belonging to families incertae sedis, both in Burmese amber: Tethepomyiidae (Tethepomyia zigrasi Grimaldi & Arillo sp. n., the aculeate oviscapt of which indicates this family was probably parasitoidal and related to Eremochaetidae); and unplaced to family is Myanmyia asteiformia Grimaldi, gen. et sp. n., a minute fly with highly reduced venation. These new taxa significantly expand the Mesozoic fossil record of rare and phylogenetically significant taxa of lower Brachycera.
amber, fossils, flies, Lebanon, Myanmar, New Jersey, Spain
This is the fourth paper in a series devoted to the Cretaceous record of brachyceran flies preserved in amber, the original work being a treatment of orthorrhaphans and Cyclorrhapha (
Specimens were prepared according to the protocols described in
Collection repositories of specimens are the following:
AMNH American Museum of Natural History, Entomology Section, New York.
AZ Azar Collection, presently housed in Musée national d’Histoire naturelle, Paris.
KU University of Kansas Division of Entomology, Natural History Museum, Lawrence.
MCNA Museo de Ciencias Naturales, Álava, Spain.
NHML Natural History Museum, London.
RTMP Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada.
It is a pleasure for the senior author to dedicate this paper to Kumar Krishna, world authority on the Isoptera, close colleague and friend.
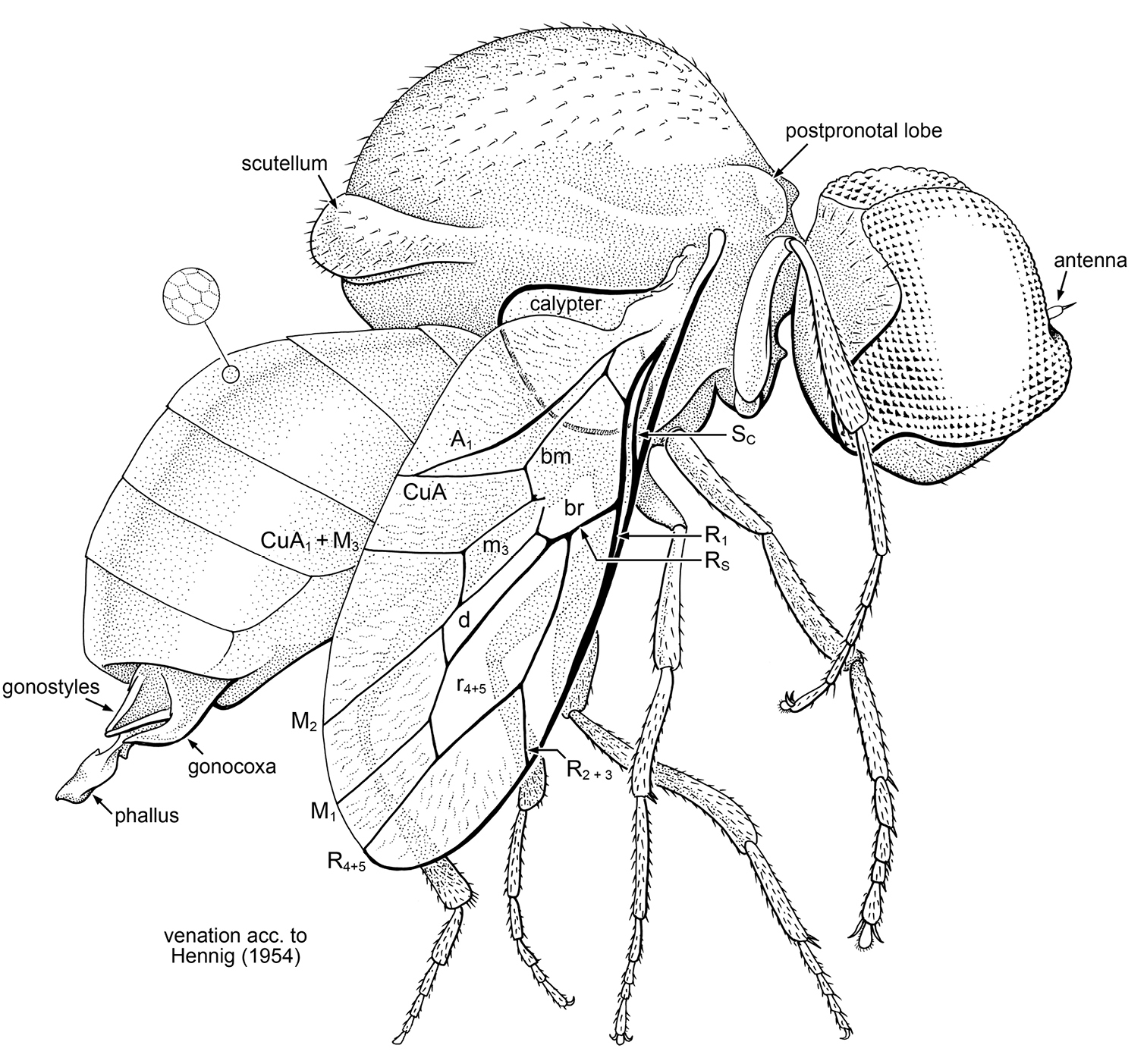
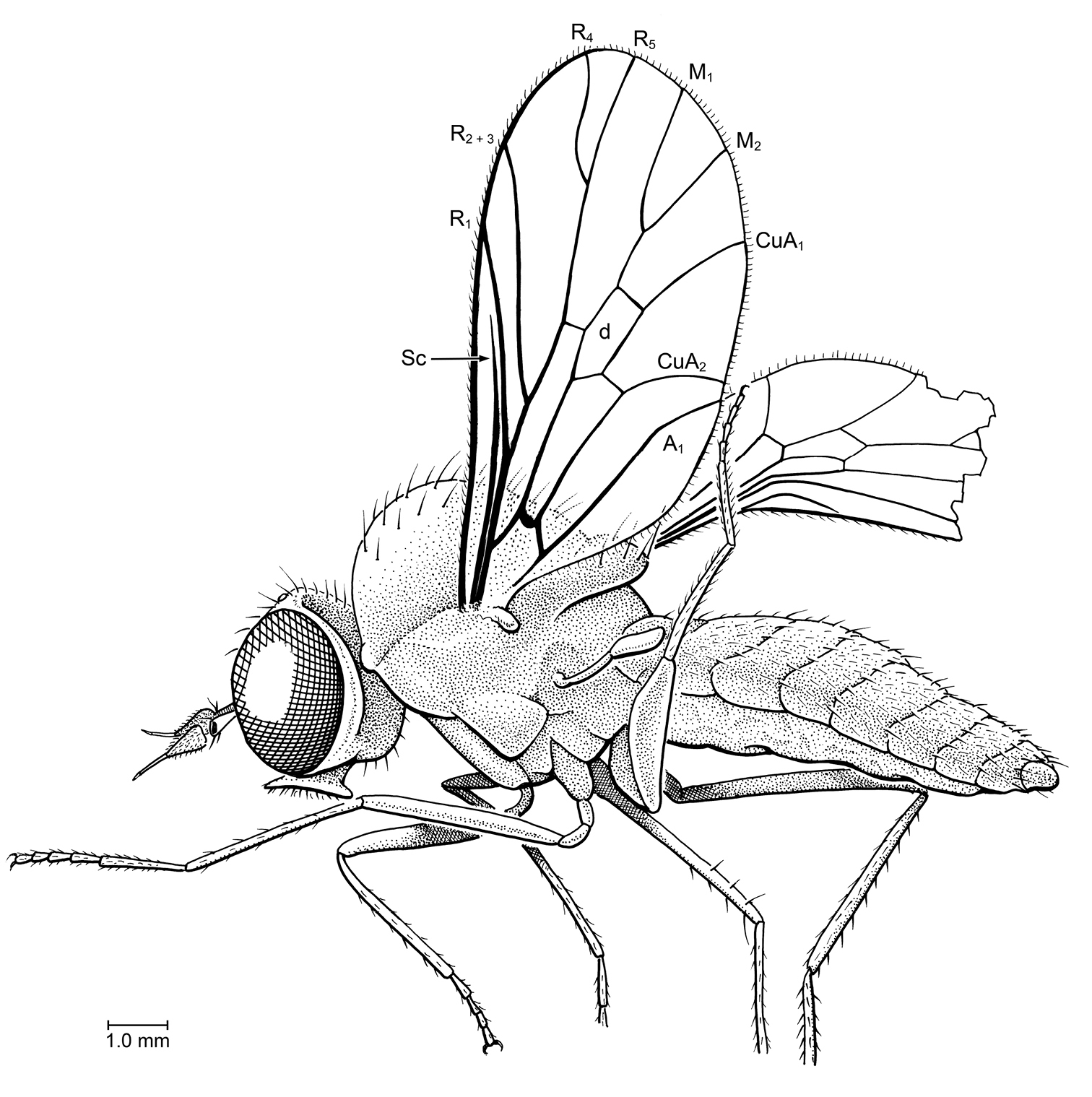
Infraorder StratiomyomorphaThis lineage comprises three living families, the Xylomyiidae (cosmopolitan; approximately 134 species in four genera), Stratiomyidae (cosmopolitan; 2651 species in 375 genera as of the year 2000 [
http://species-id.net/wiki/Cretaceogaster
We were able to study two additional specimens of this very primitive genus of stratiomyid, both in Canadian amber collected by Ted Pike from Grassy Lake, Alberta (Campanian) (
RTMP 96.9.1117: Amber is a typical clear, dark yellow with reddish flow lines; it also contains a small spider. The piece is a cylindrical runnel 12 × 4 × 2 mm, with the fly preserved near the middle, which was embedded in epoxy at the AMNH and trimmed to 9 × 13 × 4 mm (including epoxy) for better observation. The fly is laterally very flattened, especially the thorax, and is a male (though details of the genitalia are not observable). Unfortunately, the apex of the mid tibia cannot be observed in detail, so the apparent absence of tibial spurs is uncertain. Wing is slightly distended in length, but otherwise the venation is very similar to Cretaceogaster pygmaeus.
RTMP 96.9.1230: Fly is also preserved in a cylindrical runnel of amber, 7 × 3 (diam.) mm, and embedded in epoxy for careful trimming. The fly is lying at the rounded end of the runnel, with its dorsal surface against the surface of the flow. The thorax is partly decayed and wing venation is obscured. The antenna and mouthparts are visible in ventral view. Specimen is a male, but its genitalic details are also not observable. Mid tibia appears to have a small apical spur, contrary to the original description of the species but in agreement with
urn:lsid:zoobank.org:act:C038F6C9-1BFF-49D2-A756-A6235259B6A9
Antennal flagellum submoniliform, with approximately 7 short flagellomeres tapered in width apicad; articulation between basal 3 flagellomeres faint. Protibia lacking spurs; mesotibia with two short apical spurs (c. 50 µm length). Metatibia probably with one pair of short apical spurs. Vein Rs branches from R1 in the distal third of vein R. Stem of R4+5 straight, R4 curved basally, long and subparallel to R5. Cell d long and narrow, length approximately 3.5x the width; cell m3 absent.
Lysistrata emerita, sp. n., by present designation.
From the Greek, Λυσιστράτη, meaning “army disbander”, after the comedy by Aristophanes and in reference to the common name for Stratiomyidae, or “soldier flies”. Feminine.
Lysistrata is clearly within the Stratiomyomorpha, and appears closely allied with Stratiomyidae on the basis of the radial branching. The presence of two minute spurs on the mesotibia, and probably a short pair on the metatibia is indicative of either Stratiomyidae or Xylomyidae. A few Recent stratiomyids have a minute apical spur on the mesotibia, whereas xylomyids have either a 0–2–2 or 0–2–1 tibial spur formula. Pantophthalmids have one or two spurs on the mesotibia only, but are distinct from the other two families by the longer branches of R1 and Rs.
The Recent and primitive genus Parhadrestia James (consisting of two species from Chile) shares some similarities with Lysistrata, both of them possessing a long R4 vein curved only at the base and with the main branch only slightly divergent from R5. The genus Montsecia Mostovski, 1999, preserved as a compression in Early Cretaceous (Barremian) limestone of Montsec, Lérida Province, Spain (originally and incorrectly placed in the subfamily Beridinae) also has the fork of R4+R5 quite long. This long fork may be a plesiomorphic feature, seen for example in Rhagionidae and Spaniidae.
Lysistrata differs plesiomorphically from Parhadrestia by the following: antenna multiarticulate; wing longer, narrower; R2+3 slightly longer and gradually sloped to C; apex of R2+3 not close to the apex of R1; R5 and M1 slightly divergent instead of parallel; M1, M2, and CuA1 not as divergent (a condition shared with Montsecia); cell d much longer, its length approximately 3× the width (vs. 2× the width in Montsecia and 1.5× the width in Parhadrestia; in most Recent stratiomyids cell d is quite small); CuA2 more sloped toward CuP (e.g., apex of cell cup acute, instead of truncate [similar to Montsecia], although an acute cell cup is considered apomorphic by
The oldest fossil stratiomyiid is Montsecia martinezdelclosi
urn:lsid:zoobank.org:act:FD339789-7504-41CB-832A-23238FC6675A
http://species-id.net/wiki/Lysistrata emerita
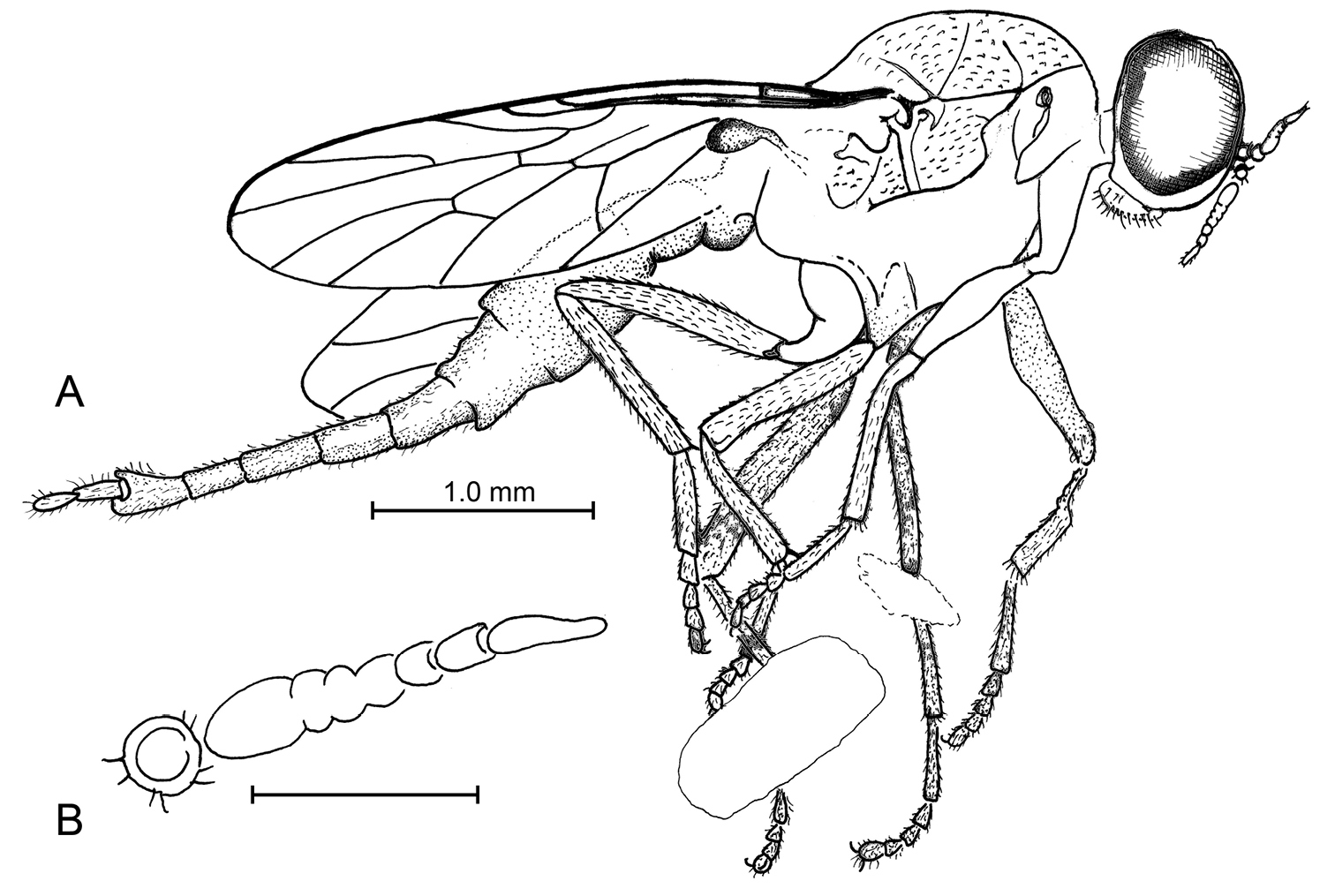
Fig. 1As for the genus.
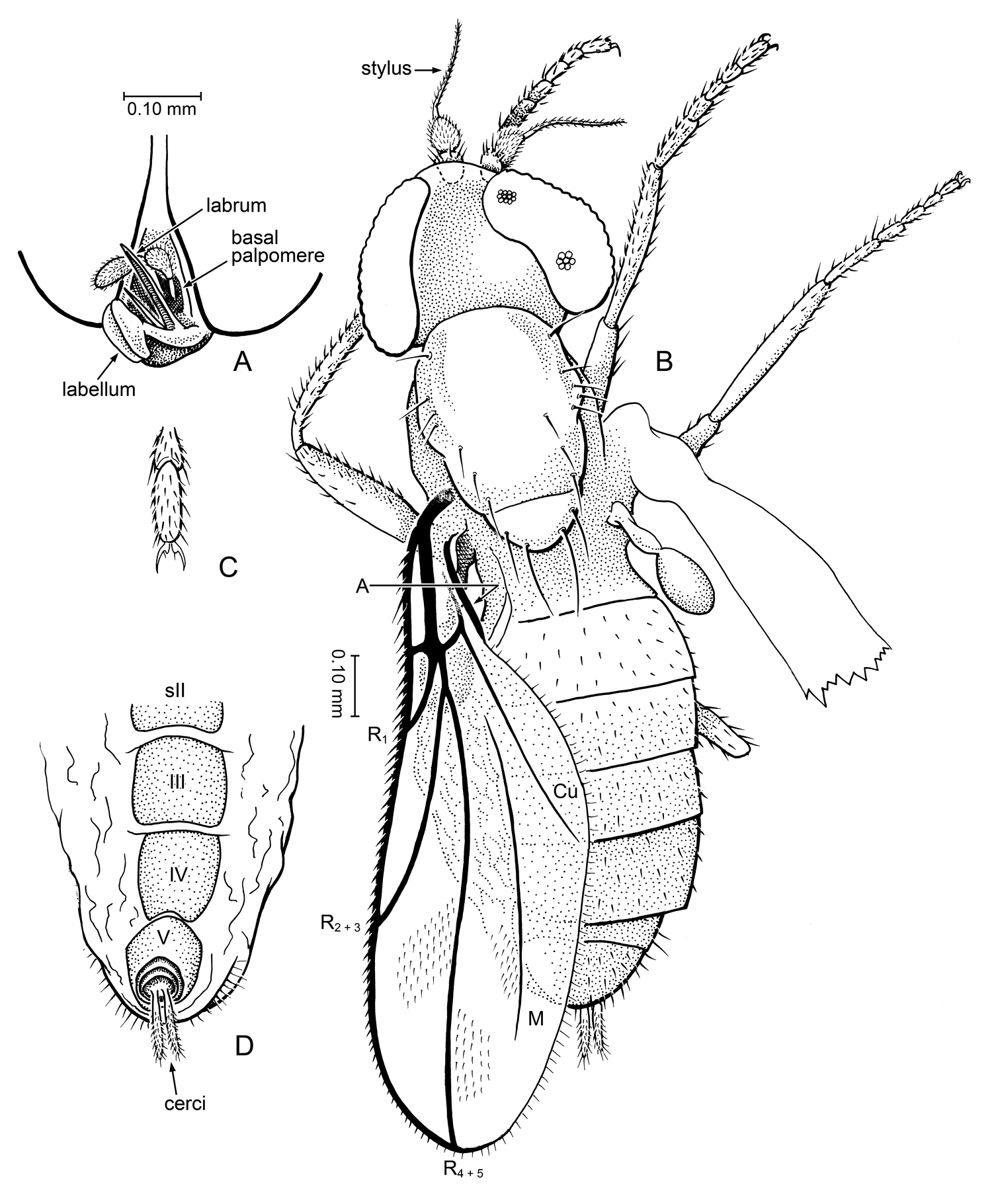
Body length 5.75 mm. Head length 0.60 mm. Specimen well preserved, but only visible in lateral view. Head slight distorted, with right antenna slightly separated from base. Eyes bare, large, covering most of head; facets not differentiated. Ocellar triangle not visible. Antenna submoniliform, with approximately 7 short flagellomeres tapered in width distad (articulations between 3 basal flagellomeres faint, number of articles difficult to discern); length of antenna approximately equal to length of head; length of flagellum 3× that of scape + pedicel combined. Distal flagellomere distinctly longer and narrower than more basal ones. Palpi reduced, segmentation not discernable; labellum well developed. Thorax: Mesonotum short and compact, finely pilose dorsally, without macrosetae. Scutellum without spines. Surface of notum slightly metallic and foveolate. All legs preserved; protibia lacking spurs; mesotibia with two apical spurs (50 µm long); probably one short apical spur on metatibiae. Length of hind basitarsomere equal to that of tarsomeres 2–5. Wing length 3.05 mm, width 0.75 mm; hyaline, vein Sc straight, length approximately 0.45× wing length, complete. Lengths of costal section of wing between apices of R2+3 and R1 equal to that between R1 and Sc. R2+3 arising distant from r-m. R4+5 straight, R4 curved at base, long and subparallel to R5. Veins M1 and M2 separated at discal cell. Cell m3 absent, vein M3 either absent or fused to CuA1. Abdomen elongate; basal 4 or 5 segments large and wide; apical 5 segments narrow and telescoping. Cercus composed of 2 segments, basal segment longer than apical one.
a, b Lysistrata emerita Grimaldi & Arillo, gen. et sp. n (Stratiomyidae) in Albian amber from Spain. Holotype, MCNA 12698 a lateral view b Antenna (scale bar 0.25 mm).
Holotype, female, MCNA 12698, SPAIN: Alava, Peñacerrada I (Moraza), Escucha Formation, Lower Cretaceous (Albian). Deposited in MCNA. Specimen is well preserved in a clear piece of amber 10 × 7 × 1.5 mm, partially missing the left side of the thorax and the left wing; the amber is embedded in epoxy 15 × 13 × 2 mm. An empidoid fly (Microphorinae) is present as a syninclusion.
From the Latin noun, Emeritus, a name given to retired Roman soldiers, used here in reference to this long-retired (i.e., extinct) species.
urn:lsid:zoobank.org:act:EB99B611-9DD4-4B20-A808-02847EC2188D
Antenna thick, greatest width (in middle) 0.25x total length, with apparently 7 flagellomeres; protibia without apical spur; most distinctive features are in venation, which distinguishes this genus from other Mesozoic xylomyids by: vein M (separating cells br and bm) weak; cell m3 very small, width and length approximately half that of cell d (these are of equivalent size in other xylomyids, or m3 is slightly smaller), and, very distinctively, vein R2+3 is uniquely lost.
Cretoxyla azari sp. n., by present designation.
From Cretaceous, and Xylomyiidae.
The closed wing cell m3 is a feature also seen in some Xylophagidae. Cretoxyla, however, apomorphically has no protibial spur (as in Stratiomyomorpha) and plesiomorphically does not have a reduced alula (a greatly reduced alula occurs in the Xylophagidae). The extent of vein C, particularly whether it extends only to the apex of M1 or M2 (
The oldest fossil record of Xylomyiidae is ?Xylomyia [sic] shcherbakovi Mostovski from the Upper Jurassic (Karabastau Formation) of Kazakhastan (
urn:lsid:zoobank.org:act:0B1DC31D-17E2-4756-BDB2-E24C5E066E05
http://species-id.net/wiki/Cretoxyla_azari
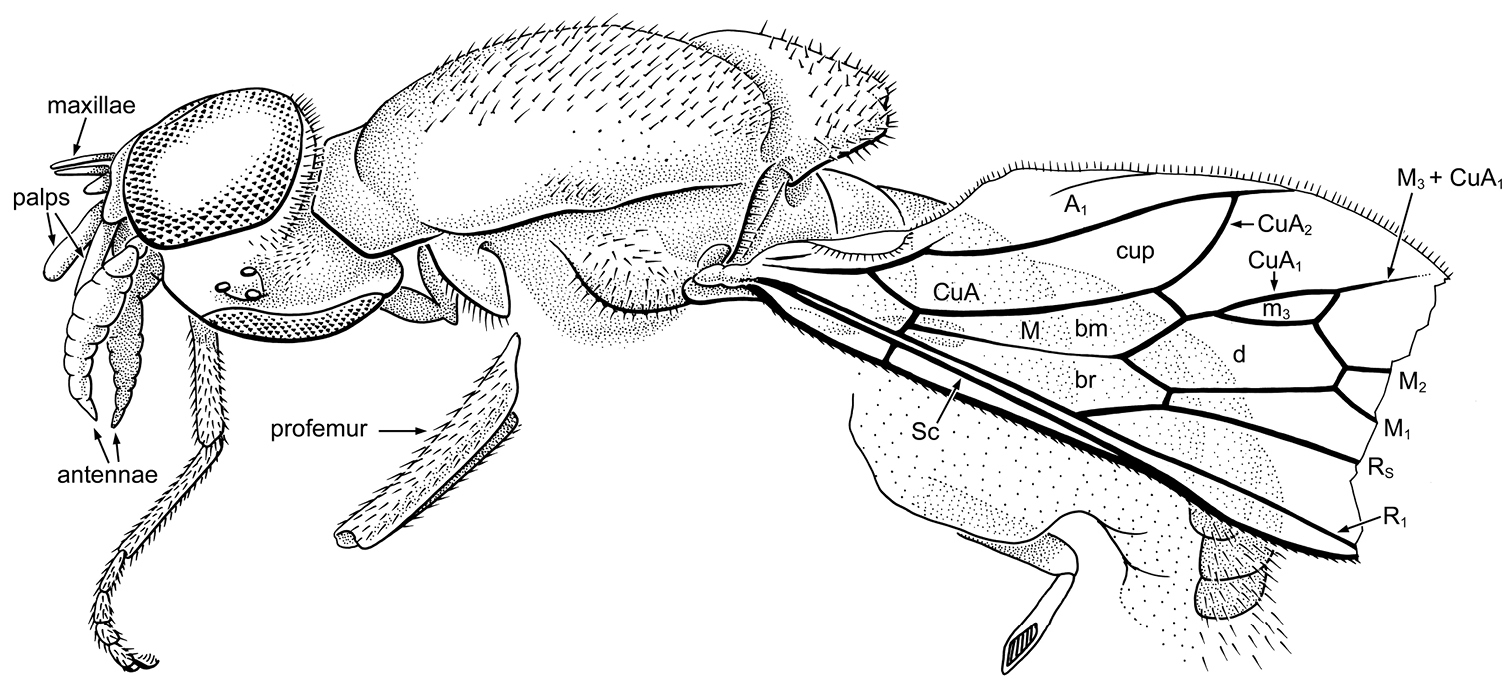
Fig. 2As for genus.
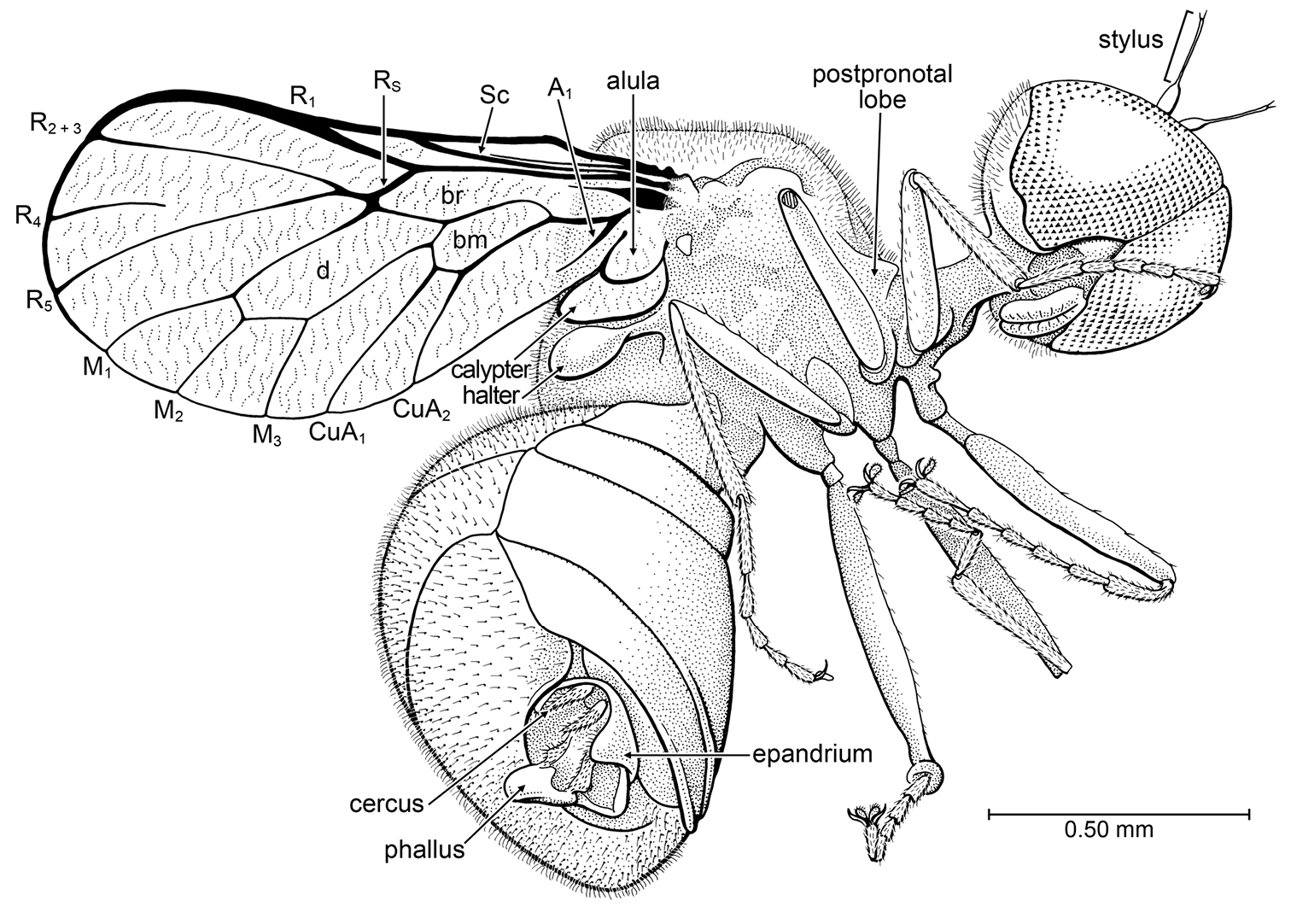
Head: Largely preserved, visible in oblique dorsal and ventral views. Head slightly flattened dorsoventrally, wider than deep, but exact proportions unclear since head seems somewhat distorted. Eyes large, bare, facets not differentiated; dorsal margins of eyes widely separated, by distance approximately 3x width of ocellar triangle. Gena/postocciput with fine pilosity; frons bare. Antenna large and thick; length equal to length of head, thickest portion of antenna near middle (width 0.25 × length of antenna). Flagellomeres difficult to discern, apparently 7, all but distal 2 are wider than long; flagellomere “2” [which may be 2 flagellomeres – if sulcus is present it is very obscure] twice the length of other flagellomeres; apical flagellomere small and conical. Mouthparts slightly prognathous, elements separated but difficult to discern; pair of stiff, stylate maxillae apparent, other elements probably include a labrum or hypopharynx, the labium and/or palps (segmentation of possible palps cannot be discerned).
Thorax: Pronotum fairly large, collar-like; mesonotum large, relatively flat; mesonotum, apical 2/3 of mesoscutellum, and anepimeron with homogeneous vestiture of fine, stiff setulae, each setula having a slightly raised, papilla-like base; row of such setulae just above wing base. Only fore leg preserved sufficiently; without spines or spurs even at apex of tibia. Empodium pulvilliform. Halter slender. Wing: Distal quarter lost at surface of amber. Sc complete, meeting C slightly beyond level of crossvein r-m. Vein h in line with short m-cu. Vein R1 straight. Vein R2+3 lost. Cells br and bm virtually equal in size, bisected by weak vein M. Cell m3 spindle-shaped, very small, approximately half the length and width of discal cell; vein M3+CuA1 incomplete (not reaching wing margin) and long, length only slightly less than length of cell m3. Cell cup very large, considerably thicker than and extended well beyond apical levels of cells br and bm. Vein A1 complete, A2 not apparent; alula present, but not particularly large.
Abdomen: Poorly preserved, genitalia lost.
Cretoxyla azari Grimaldi & Cumming, gen. et sp. n. (Xylomyidae) in Early Cretaceous amber from Lebanon. Holotype, AZ391. Body length (as preserved) 3.6 mm.
Holotype, sex unknown, Lebanon (Early Cretaceous, Neocomian): “Hammana/Mdeiru, Aptien inférieur, ” in Azar Collection no. 391, temporarily deposited in Musee National d’histoire Naturelle, Paris. The specimen is partially preserved, missing the right side of the body, most of the legs, and the right wing at the surface of the amber. It is mounted in a shallow glass well in Canada balsam on a glass slide.
Patronym, for Dany Azar, for his extensive contributions to the paleontology of Lebanese amber.
Head: Lost. Thorax: Partially preserved, relatively broad (width of mesonotum equal to length, 1.40 mm), mesoscutellum of moderate size. Notum foveolate and scutellum covered only with numerous fine setulae, no macrosetae. Left wing 3.57 mm long, it and halter entirely preserved; right wing partially preserved. Vein C ends either at apex of R4 or R5; Sc long, meets C beyond midpoint of wing length, approximately at same level as crossvein r-m. R1 parallel and very close to Sc, with slightly sclerotized, pterostigmatic membrane where they diverge slightly at apex. Base of Rs (before fork of R2+3 and R4+5) short, Rs connected to R1 quite distad, at 0.42 complete length of wing. Veins R4 and R5 forked, branches of fork relatively straight (not curved), with R5 distinctively ending at apex of wing rather than below it. Cell d small, distinctively short (length 2.5x greatest width); closed cells m3 and cup present, cell m3 triangular, short branches of M3+CuA1 and A1+CuA2 present. Alula relatively small. Halter relatively short and stout. LEG: [Presumably] hind leg without macrosetae on it; presence of an empodium difficult to discern, but pulvilli well developed. Abdomen: Relatively broad, ending short of wing apex.
?Xylomyidae sp. in Albian amber from Spain. MCNA 8833.
MCNA 8833, Spain: Álava: Peñacerrada I, Escucha Formation, Lower Cretaceous (Albian). Specimen lacks a head, and the thorax and abdomen are only partially preserved.
Because of the incomplete preservation, a precise diagnosis and family placement of the specimen is not possible, so we did not provide a name and formal description. There are genera of lower Brachycera in several families that have a venation similar to this fossil, including the closed cell m3. A distinctive feature of the fossil is vein R5 ending at the apex of the wing. This is rarely seen in the lower Brachycera, occuring, for example, in Xylomyiidae and Apsilocephala Kröber, 1914 (Apsilocephalidae). Unlike Apsilocephala, which has the branches of R4 and R5 curved, these branches in the fossil are straight. Also, Apsilocephala and most therevids usually have a longer, more slender abdomen (although see Kumaromyia, vide infra), and usually have bristle-like setae on the mesonotum. These features, plus the short branch of Rs and its distal connection to R1 indicate that the fossil is in the Stratiomyomorpha, not the Asiloidea.
http://species-id.net/wiki/Cratotabanus
Cratotabanus isdistinguished from modern tabanids by veins M1, M2, and M3 long, with lengths of M1 approximately the same as that of cell d (vs. 0.5 – 0.7× length of cell d in Recent Tabanidae); R5 only slightly deviated from the path of vein R4+5 (in most Recent tabanids, excepting Chrysops Meigen 1803, R5 curved strongly downward). Distinguished from some Cretaceous Tabanidae, as follows: Eotabanoid
urn:lsid:zoobank.org:act:B8FD8A73-669D-44EB-B923-464378B6AB94
http://species-id.net/wiki/Cratotabanus_newjerseyensis
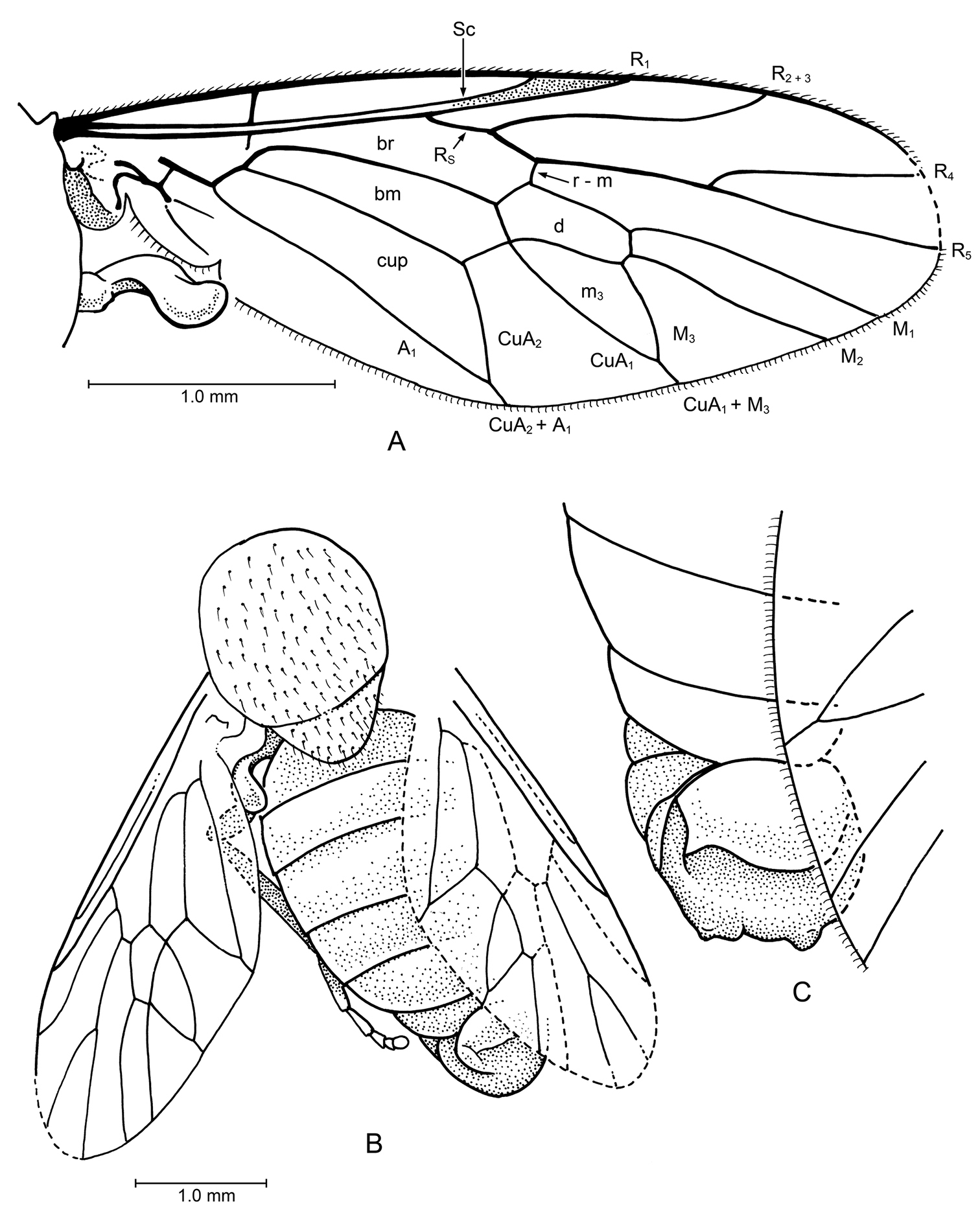
Fig. 4Venation differs from congener by Cratotabanus stenomyomorphus having vein R4 not strongly upcurved (vs. strongly upcurved) and R5 slightly downcurved (vs. nearly in line with R4+5).
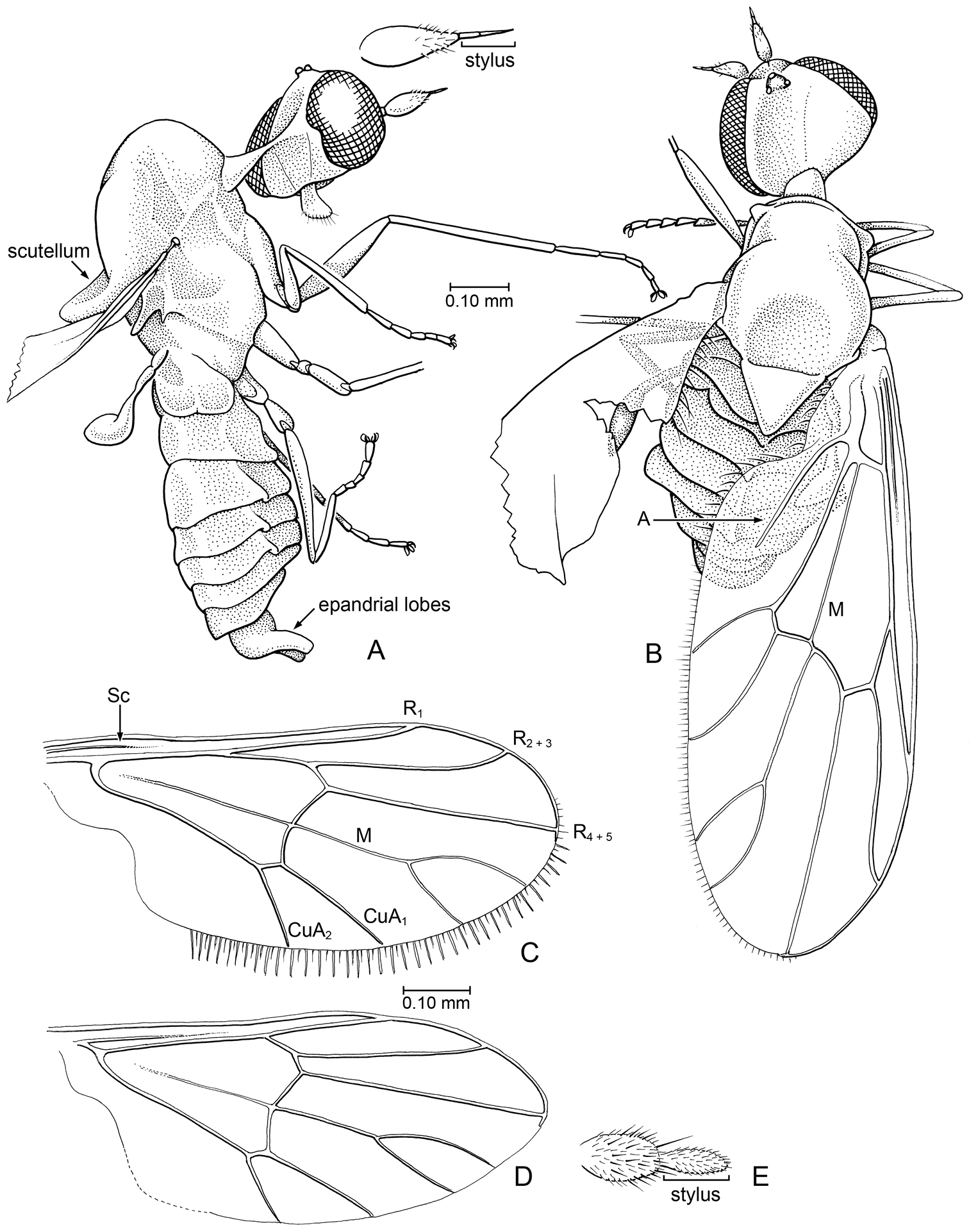
AMNH NJ-1862 (holotype): Body length 1.0 cm, wing length 8.0 mm. Most of left lateral view and some of dorsal, right lateral, and frontal view of face observable. Specimen apparently female. Head: Eyes bare, large, not dichoptic, no differentiation of facets nor apparent color patterns. Details of frons and face not entirely observable (e.g., presence of frontal callus and subcallus unlikely; development of ocelli not discernable). Antenna with scape and pedicel not observable but apparently short (not projected); flagellomere I apically narrowed to 0.5 × basal width, with 3 faint annuli; remaining 6 flagellomeres stylate, tapered apicad, articles of approximately equal lengths [best seen in frontal view]. Proboscis robust, palps barely discernable (but apparently short, length 0.4 × that of proboscis), labellum well developed; entire proboscis fairly long, length = 0.75 × depth of head. Thorax: Standard proportions for Tabanidae; legs without discernable spurs (although apices of hind tibiae not observable). Metathoracic spiracle also not observable [e.g., presence of postspiracular scale]. Wing: Completely hyaline, no patterning. Base of R2–5 nearly perpendicular to R1, not at a sharp, acute angle. Fork of R4–5 widely divergent and encompassing entire wing tip, base of R4 perpendicular to R5, then strongly and concavely curved to meet C; base of R4 without a small appendix. M1, M2, M3 nearly parallel; M3 and CuA1 convergent (not parallel); CuA1 and A1 meeting just before wing margin. A2 extended nearly to wing margin; alula very large. Abdomen: Details (e.g., segmentation of cerci) not observable.
Specimen. AMNH NJ-1081 (paratype): Thorax + abdomen length 8.2 mm, wing length 8.5 mm (from base of basicosta to wing tip). Wing: Basicosta present as a thick, scale-like lobe at base of vein C. C thickened proximally, circumambient. Short crossvein h present, where costal thickening is narrowed. Sc long, 0.6 × length of wing, straight and parallel to vein C. Veins R and base of R1 also straight, parallel, and close to Sc; apices of Sc and R1 diverging apically. Dark, heavily sclerotized pterostigma covers and surrounds R1, vein C, and extends to tip of R2+3. R2+3 straight, turned slightly upward at apex. Stem of R4 and R5 straight, base of R4 nearly perpendicular to this stem, then curved upward and meeting C anterior to tip of wing; R5 nearly in line with stem of R4+R5. Cell d large, length ca. 2.7 × the width; with veins M1, M2 and M3 each deriving directly from apical wall of cell. M veins slightly divergent, long; M1 slightly longer than cell d, M3 ca. 0.6 × length of cell d. Crossveins r-m and m-cu in line with each other. Veins CuA2 and A1 meet slightly before wing margin, forming long, complete cua cell with very short vein CuA2+A1. Vein A2 well developed, concave to A1, evanescent apically; anal lobe and anal cell well developed. Alula present but partially obscure. Abdomen: Short, broad, tergites short, typical of tabanids.
Cratotabanus newjerseyensis Grimaldi, sp. n. (Tabanidae) in Turonian amber from New Jersey, USA. Above: Lateral view of holotype, AMNH 1862. (scale 1.0 mm). Below: Wing of paratype, AMNH NJ1081.
Holotype (sex unknown), AMNH NJ-1862, New Jersey (USA): Middlesex Co., Sayreville, White Oaks [Old Crossman’s] pits (Turonian), collected by Stephen Swolensky. Observation of the fly was optimized by embedding the amber in epoxy under vacuum and trimming very close to surfaces of the fly, but the specimen is not well preserved, being occluded with a reddish, crazed layer over most of the body and by similar internal fractures in the piece, as well as by a suspension of fine particles in the amber. Piece is irregular in shape, 10 × 13 mm in largest dimensions. Study of the specimen might benefit from microtomography.
Paratype (sex unknown), AMNH NJ-1081, in Late Cretaceous (Turonian) amber from Crossman’s Pits, Sayreville, New Jersey. Fly is partially preserved: besides the entire right wing and a very small portion of left wing, only the dorsal surfaces of the abdomen and thorax remain; the head and legs are entirely lost. The amber piece is triangular and approximately 19 × 8 × 5 mm, embedded in epoxy but trimmed and polished so as to expose a dorsal view of the fly. The amber itself is light yellow and turbid, with a thick suspension of organic particles that obscures much of the fly. AMNH NJ-1081 differs from NJ-1862 by the following minor venational details: R1 slightly longer, Rs branches from R1 at a more acute angle, proximal end of cell d slightly more shallow V-shaped; A2 slightly shorter. Both specimens are also very similar in body shape and size.
“from New Jersey, ” in reference to provenance.
These are the only tabanids known to be preserved in Cretaceous amber. Other tabanids in amber are from the Miocene of the Dominican Republic and the Eocene Baltic amber (
Baissomyia redita Mostovski, Jarzembowski & Coram, 2003: Zaza Formation, Baissa, Transbaikalia, Russia.
Eotabanoid lordi Mostovski, Jarzembowski & Coram, 2003: Durlston Formation (Berriasian), Purbeck Group, Dorset UK.
“Allomyia” [sensu Ren] ruderalis Ren, 1998: Yixian Formation, China.
Eopangonius pletus Ren, 1998: Yixian Formation, China.
Palaepangonius eupterus Ren, 1998: Yixian Formation, China.
Cratotabanus stenomyomorphus Martins-Neto & Santos, 1994: Crato Formation (Aptian), Ceara, Brazil.
“Cratotabanus sp. n.”: Crato Formation (Aptian), Ceara, Brazil (in
Cratotabanus newjerseyensis sp.n.: Raritan Formation amber (Turonian), New Jersey, USA (herein).
Family Acroceridae
Acroceridae has been hypothesized to be closely related either to the family Nemestrinidae (
urn:lsid:zoobank.org:act: A659E121-9307-4454-B910-A586A82613E4
A minute, distinctive acrocerid with medial margins of male eyes contiguous above and below antennae, hind and ventral margins of eye strongly emarginate; antennae minute, in middle of head; proboscis vestigial; eyes bare, thorax with very sparse, fine setulae; postpronotal lobes of moderate size, slightly protruding; abdomen devoid of microtrichia and glabrous (possibly reflective). Mediolobus (i.e., “pulvilliform empodium”) and pulvilli pad-like. Venation distinct: All veins sclerotized, none faint; C ends at apex of R4+5; Sc short; R1 and Rs fork at ca. 0.4× length of wing; cells br and bm continuous, not bissected (vein M extremely faint or lost from this area); two closed radial cells (r4+5 and d), plus cell m3 present; R4+5 ends near apex of wing, without an apical fork of R4-R5 encompassing apex of wing.
Schlingeromyia minuta, sp. n., by present designation.
Patronym in honor of Evert Schlinger, Emeritus Professor of entomology at the University of California, Berkeley, who has devoted his career to the study of Acroceridae and who also has been a very generous patron of systematic entomology. Feminine, following the Greek myia, for fly.
This is a very distinctive, minute acrocerid – in body size quite the opposite of its generic namesake – which is unique for the venation, genitalia, and virtually bare body. Most acrocerids have long, fine pile on the thorax and abdomen, and many have it on the eyes and calypters. Vein Sc is very short in the fossil, and cells br and bm are contiguous. In addition, apparent retention of freely articulated gonostyli in the male genitalia appears to be a significant feature of the genus, since loss of articulated gonostyli through fusion with the gonocoxites is considered an apomorphy of the remainder of the family (
urn:lsid:zoobank.org:act:4881BDDC-D10C-47FE-91B1-5BA12EBE2B64
http://species-id.net/wiki/Schlingeromyia_minuta
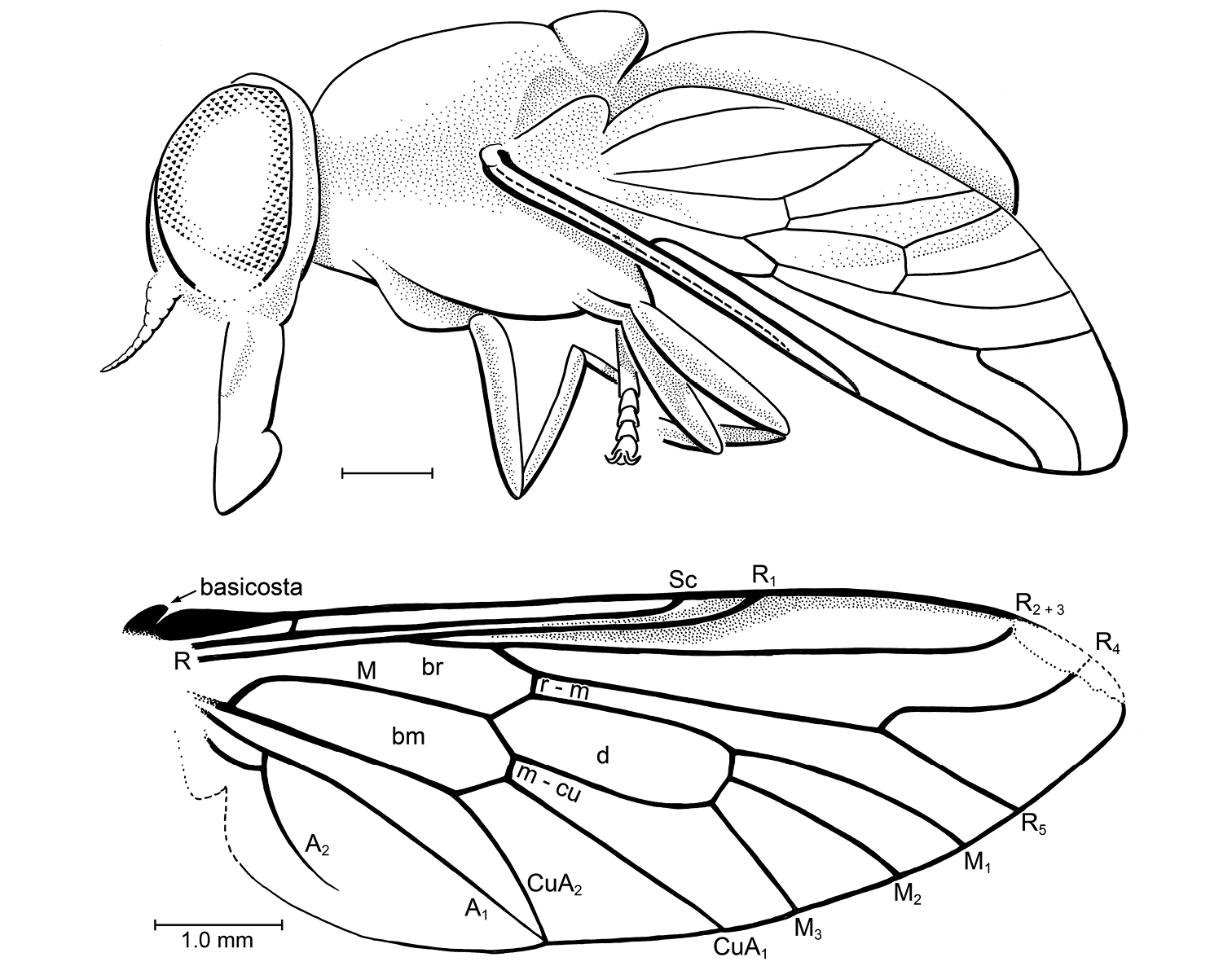
Fig. 5As for the genus.
Body length 3.0 mm, wing length 2.1 mm. Head: Large, spherical. Eyes very large, occupying most of head capsule. Eyes bare, without interfacetal setulae; no dorso-ventral or frontal differentiation of facets. Entire mesal margins of eyes above antennae are contiguous, portion of mesal eye margin below antenna also contiguous. Posterior margin of eye strongly emarginate; ventral margin of eye slightly less so. Antenna minute, length approximately equal to diameter of 2–3 eye facets; consists of small oval pedicel and minute apical style. Mouthparts vestigial. Postocciput with scattered, fine setulae. Thorax: Scutum strongly arched, very large, length of (meso)thorax 1.25 mm (nearly half the body length). Scutellum small. Position of cervical region near ventral surface of thorax. Pair of well-developed postpronotal lobes dorsal to cervical region, posterior surface of lobe slightly concave. Scutum with sparse, short setulae; scutellum with slightly thicker setulae. Legs slender, mesotibia with short pair of apical spurs; apices of tarsomeres with pair of short, thick setae. Length of basitarsomere approximately equal to that of remaining, distal tarsomeres; hind tibia expanded in width apically to approximately twice the proximal width. Pretarsus with claws large; mediolobus and pulvilli large, pad-like. Wing short and slender, length 2.10 mm, greatest width 0.75 mm; membrane with fine, faint pleating/wrinkling over apical and posterior regions, but not in closed cells [best seen in oblique views]. Calypter large, ovoid, greatest diameter 0.58 mm. All veins sclerotized, none faint. C ends at apex of R4+5; Sc short, meets C slightly distal to level of where R1 and Rs fork. R1 and Rs fork at ca. 0.4× length of wing; stem of Rs short, approximately 0.2 × total length of Rs. Rs surrounds large r4+5 cell, near middle of which R2+3 branches off to meet C. Vein R4+5 branches off of apex of cell r4+5, apex meets C slightly posterior to wing apex; M1 also short, branching subapically off of cell r4+5. Tip of wing not encompassed by an apical fork of R4-R5. Cell d bounded by M1 and M2; slender (ca. 0.3 × thickness of cell r4+5). Cell m3 slender, trapezoidal, with proximal end slightly opened. Cells br and bm continuous, not bissected (vein M extremely faint or lost from this area). A1 slender, meeting CuA shortly before wing margin. A2 not apparent; anal lobe of wing well developed. Calypter large, hemispherical, greatest diameter 0.25 × length of wing. Abdomen: Smaller than thorax, with six tergites visible (tergite I small, tI and tII virtually obscured in dorsal view under postnotum). Tergites entirely bare of microtrichia and setulae; glabrous [probably reflective], with cuticular microsculpture of minute hexagonal cells present. Spiracles not visible near lateral margins of tergites [in pleural membrane?]. Tergites VII-VIII apparently small [not discernable]. Two pairs of male genitalic appendages present: slender dorsal pair (probably gonostyli), thicker ventral pair (gonocoxites), plus terminal, central, membranous appendage, the phallus.
Schlingeromyia minuta Grimaldi & Hauser, gen. et sp. n. (Acroceridae) in latest Albian – early Cenomanian amber from Myanmar. Holotype, AMNH Bu332a. Body length 3.0 mm. Terminology for wing venation after Hennig (1954).
Holotype, Male, AMNH Bu332a, in Burmese amber. Paratype, AMNH Bu332b, in same piece of amber. Both specimens are entirely preserved, though slightly obscured by debris and a few small fractures. The specimens occur in a runnel-shaped piece of dark but transparent amber, 16 × 7 mm, which has been embedded in epoxy. The piece also contains 1 Coleoptera, 1 Hymenoptera (Serpitidae), and 6 other Diptera (Cecidomyiidae, Empidoidea), as well as twisted strands of spider webs. Interestingly, acrocerids are parasitoids of spiders.
Latin, adjective, in reference to the very small size of the species.
urn:lsid:zoobank.org:act:622EFA4E-5D9E-4244-8213-D20654E1AE15
A small, primitive acrocerid in Burmese amber easily separated from Schlingeromyia based on the well developed mouthparts; long, fine antennal stylus; dense, fine pilosity on thorax and abdominal tergites; absence of a mediolobus on the pretarsus; absence of tibial spurs; wing apex rounded; and by the venation: Vein C circumambient, cells br and bm completely separated, absence of cells r4+5 and m3, presence of a very large cell d, vein R4 present but vestigial (not connected to R5), veins CuA1 and CuA2 each present, vein A1 vestigial (cell cup not present).
Burmacyrtus rusmithi sp. n., by present designation.
Combination derived from Burma (the pre-junta name for Myanmar) and Cyrtus, nominal genus of Cyrtidae, a formerly used name of Acroceridae.
Derived acrocerid features that Burmacyrtus shares with Schlingeromyia and Recent acrocerids are the following: spherical head with large, holoptic eyes in male; apex of antennal flagellum with simple stylus; presence of a distinct cervical region; wing membrane with fine wrinkling and devoid of microtrichia; and with a large calypter. Apomorphic features in Burmacyrtus that are lacking in Schlingeromyia are fine, dense pilosity; a broadly rounded wing apex; long, fine stylus; and lack (loss) of a mediolobus. The wing shape of Burmacyrtus is similar to that of some Recent acrocerine genera such as Turbopsebius Schlinger, 1972, but the latter genus has cell r4+5 present, veins CuA1+M3 fused, and a complete vein A1, among other features. Like Schlingeromyia, Burmacyrtus is also very basal in the Acroceridae. Some of the derived features in wing venation of the two species in Burmese amber may be due to the very small body size.
urn:lsid:zoobank.org:act:17FA87A4-457F-4BB0-AC59-03ECEBF4CAA4
http://species-id.net/wiki/Burmacyrtus_rusmithi
Fig. 6As for genus.
Wing length approximately 1.4 mm, body length approximately 2.0 mm. Head: Rounded, spherical. Male eyes bare of setulae, frontally holoptic [dorsum of head not visible], occupying most of head capsule, ventromesal margins of eyes diverging around clypeus; facets in ventral portion of eye not differentiated in size; posteroventral margin of eye with shallow emargination. Basal portion of flagellum small, ovoid; stylus long and very slender, length c. 2 × that of basal portion; apex of stylus with pair of minute setulae. Labellum well developed; palps not evident. Postocciput with dense, fine pilosity. Thorax: Cervical region elongate, but not comprised of elongate postpronotal lobe (which protrudes slightly from anterior surface of scutum); cervical region connected anteroventrally to thorax. Thorax deep. Dorsal surface of mesoscutum and scutellum with dense, fine pilosity. Legs slender, metafemur longest; without spines, bristle, or tibial spurs; tibial and tarsal setulae not in regular rows. Apical portions of tibiae not distinctly broadened. Pretarsal claws large; pulvilli large, mediolobus absent [if setiform empodium present, not visible]. Wing short, with broadly rounded apex and narrow base; surface devoid of microtrichia, with fine wrinkling throughout. Vein C circumambient, though thinner past apex of M1; small hump in C midway along length of Sc. Sc complete, length ca. 0.4 × that of wing (a thin, faint, incomplete, and apparently spurious vein runs parallel and very close to Sc). Vein R1 short, length approximately 0.5 × length of stem of R; R1 and C thickened where they meet. Stem of Rs short, length approximately 0.5 × that of R1; Rs and where it meets M thickened. R4-R5 apparently a vestigial fork (R4 incomplete, not connected to R5). Cells r4+5 and m3 absent; cell d present, large; length of cell d 0.3 × that of wing. Cells br and bm present, separated by well developed basal portion of M. Veins M1, M2, M3 present, originating from apex of cell d. Veins CuA1 and CuA2 present, originating from apex of cell bm. Vein A present, but short and vestigial (cell cup absent). Alula and calypter well developed, each with fine wrinkling; calypter approximately 2 × diameter of alula. Halter apparently dark. Abdomen: larger than thorax; sternites well developed, glabrous, without setulae or punctures. Tergites large, with dense, file pilosity; each setula situated in minute puncture. Male genitalia: epandrium well developed, shallow; cerci slender and apically pointed; everted, distal portion of phallus bulbous; subapical portion flanked by pair of flat, setulose lobes. Spiracles not visible.
Burmacyrtus rusmithi Grimaldi & Hauser, gen. et sp. n. (Acroceridae) in Burmese amber. Holotype AMNH Bu-RS1.
Holotype, Male, AMNH Bu-RS1, in Burmese amber. The holotype is in excellent condition, though only the ventral and lateral portions are visible (the dorsal surface is obscured by the depth and curvature of the amber). The amber is clear yellow and the fly lies on an internal surface plane that contains bubbles and stellate trichomes. The original piece was drop-shaped, 10 × 16 mm, and contained a small spider, cecidomyiid midge, and berothid lacewing. These inclusions were separated from the fly.
Patronym, for Dr. R.D.A. (Ru) Smith, who generously donated the specimen to the AMNH from his personal collection.
Family Mythicomyiidae
urn:lsid:zoobank.org:act:7C83B2CC-BD8F-4BDA-A19D-E61110619770
R1 long, apex reaching to 2/3 length of wing; R1 branching off of the stem of R quite distad, R2+3 long, branching off of Rs in the distal half of the wing; cells br and bm large, length nearly half that of wing; M1+2 forked; vein A1 either incomplete or absent. Mesoscutum strongly arched; it and abdominal tergites devoid of bristle-like setae or long pilosity; apical tibial spurs lacking. Body size minute, ca. 1.0 mm in length.
Microburmyia analvena, sp. n. By present designation
Derived from micro- (L.), minute; -burm-, Burma; and –myia (Gr.), fly, in reference to the minute body size and provenance of this brachyceran. Feminine.
The family placement of the two new species in this genus is not entirely certain, particularly since in mythicomyiids vein R2+3 is typically short and its apex fused with R1. The genus is placed in the Mythicomyiidae since the venation bears a resemblance to the Baltic amber genus Carmenelectra Evenhuis (
Mythicomyiidae are traditionally (e.g.,
Lastly, it is interesting to note that the fossil record of Bombyliidae s.s., exclusive of mythicomyiines, is entirely Tertiary. Bombyliidae is a large, cosmopolitan family (ca. 4, 500 species) of flies that are most diverse in xeric ecosystems, where they are important pollinators of herbaceous plants. Their fossil record in sedimentary matrices and in amber (Baltic, Dominican) is quite diverse for North America and Europe (
urn:lsid:zoobank.org:act:E64C24A4-F610-4034-AC24-D65721D5BEF5
http://species-id.net/wiki/Microburmyia_analvena
Fig. 7a, bDistinguished from Microburmyia veanalvena sp. n. (below) by longer wing; presence of an anal vein; fringe of fine (vs. thick) setae on posterior wing margin; antennal style very fine, with very small article between it and basal flagellomere.
A minute fly, body length c. 1.1 mm, thorax length 0.5 mm, wing length 1.15 mm. Head: Short, somewhat flattened anteroposteriad. Cervical region long. Eyes bare, large, well separated; no dorsoventral differentiation of facets; with small, shallow emargination on posterior margin. Proboscis short [palps not visible]. Antenna with basal flagellomere drop-shaped, with sparse setulae; apical style 0.6 × length of basal flagellomere, very thin, with two articles. Three ocelli present. Postocciput expansive, concave. Thorax: Mesoscutum dorsally arched, devoid of setae or setulae; thorax deep in lateral view; mesoscutellum triangular in shape (nearly equilateral), posterior end tilted upward. Coxae of moderate size; legs slender; devoid of setae, tibiae without apical spurs. Pretarsus with large pulvilli; empodium probably setiform. Halter with slender stem, large knob. Wing long, length slightly greater than length of body, wing L/W = 2.72. Costa either without spinules or spinules minute; C reaching slightly beyond apex of R4+5. Posterior margin of wing with fringe of short, fine setae, including alula (setae longer in this area). Vein Sc with apex apparently evanescent, not reaching C. R-R1 nearly straight; R2+3 2.0 × length of R1; R4+5 straight, ends at apex of wing; proximal portion of R4+5 joined to r-m to form distal margin of cell br. Cells br and bm large, br is 0.33x length of wing, W/L cell br = 0.3; cell bm narrower and shorter. Base of M straight, with short apical fork. Crossvein bm-cu slightly shorter than r-m, not in line with each other. CuA1 and CuA2 short, curved slightly toward each other. Vein A1 present, incomplete (reaching to 0.6x distance between vein base and wing margin), apex of vein blunt, not evanescent. Anal lobe and alula small. Abdomen: Short, 1.3 × length of mesothorax, apparently devoid of setae and setulae. Tergites I – V with shallow, median keel; epandrium with pair of large ventral lobes.
Microburmyia Grimaldi & Cumming, gen. n. (Bombyliidae: Mythicomyiinae), in Burmese amber a, b Microburmyia analvena Grimaldi and Cumming sp. n. Holotype, KU-Bu079 (a lateral view, with detail of antenna b dorsal view, as preserved) c – e Microburmyia venanalvena Grimaldi and Cumming, sp. n., Holotype AMNH Bu1552 c, d left and right wings, showing variation in vein proportions. e, antenna.
Holotype, male: Myanmar: Kachin (northern Myanmar), in Burmese amber, KU Bu079 (Univ. Kansas, Division of Entomology, Natural History Museum). The amber piece containing the holotype is a very transparent, deep amber color, 14 × 7 × 5 mm, which also contains 2 scelionid wasps. The minute holotype is at the surface of a fractured corner.
in reference to the presence of an anal vein (i.e., the Latin noun vena), albeit incomplete.
urn:lsid:zoobank.org:act:440657CB-BAD8-4D35-8F8A-1D03B5752FB0
http://species-id.net/wiki/Microburmyia_veanalvena
Fig. 7c-ecf. Microburmyia analvena (above), distinguished by the absence of an anal vein; posterior fringe of setae long, thick; basal flagellomere and style setulose, style with one article, oval.
A minute fly, wing length 0.85 mm. Head: Short, somewhat flattened anteroposteriad. Cervical region with connection anteroventrally on thorax; not visible dorsally. Eyes bare, large, well separated; no dorsoventral differentiation of facets [presence of emargination on posterior margin not visible]. Proboscis short [palps not visible]. Antenna with basal flagellomere ovoid, having dense setulae (longer apicad); apical style 0.6 × length of basal flagellomere, thick (nearly 0.5 × thickness of basal flagellomere), one articled, setulose. Three ocelli present. Postocciput expansive, concave. Thorax: Mesoscutum dorsally arched, devoid of setae or setulae; thorax deep in lateral view; mesoscutellum triangular in shape (nearly equilateral). Legs slender; devoid of setae, tibiae without apical spurs. Pretarsus with large pulvilli; empodium probably setiform. Halter with slender stem, large knob. Wing W/L = 0.43. Costa either without spinules or spinules minute; C reaching slightly beyond apex of R4+5. Posterior margin of wing with fringe of long, thick setae (visible only on left wing), but margin of alula bare. Vein Sc extremely faint, evanescent. R-R1 nearly straight; R2+3 2.0 × length of R1; R4+5 straight, ends at apex of wing; proximal portion of R4+5 joined to r-m to form distal margin of cell br. Cells br and bm large, br is 0.42 × length of wing, W/L cell br = 0.27; cell bm slightly narrower and shorter. Base of M straight, with short apical fork. Length crossvein bm-cu approximately equal to that of r-m, in line with each other. CuA1 and CuA2 short, straight and diverging. Vein A1 absent. Anal lobe differentiated, alula small. Abdomen: Very broad anteriorly, devoid of setae and setulae on tergites. Tergites I – V apparently without shallow, median keel [difficult to discern with preservation]; epandrium with 5–6 long, thick setae on posterior surface, length of setae approximately equal to length of epandrium.
Holotype, Male: Myanmar: Kachin (northern Myanmar), latest Albian to earliest Cenomanian. AMNH Bu1552. Specimen is displayed with wings and legs outspread, but body is only moderately well preserved, with some details obscured beneath layer of deep reddishness. Dorsal view is better than ventral view.
ve- (Latin prefix meaning without), anal vein (L., vena), in reference to this venational character.
It could be argued that these two species might warrant separate genera, based on the differences of antennae, wing fringe, epandrial setae, and proportions of the wing. However, other than the presence/absence of the anal vein, the wing venation is very similar between the two species.
This asiloid group includes the Recent families Therevidae (cosmopolitan; 1, 063 described species), the Scenopinidae (cosmopolitan, approximately 420 described species), the monotypic family Evocoidae from Chile (
urn:lsid:zoobank.org:act:08C3574B-22B5-4654-99CE-3DE0488EDBA2
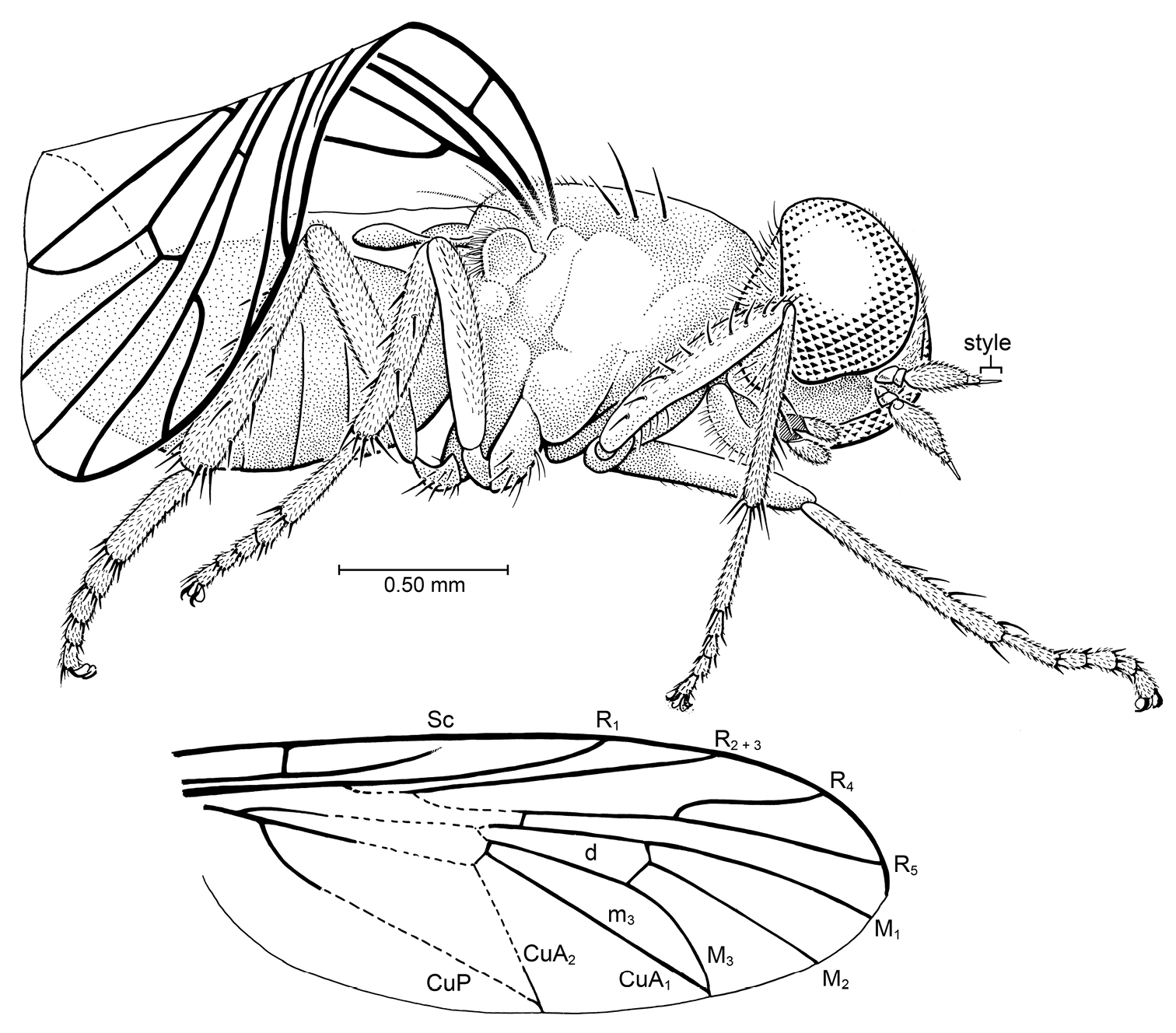
Body stout, abdomen short (length about equal to that of thorax); eyes large, bare; antenna with 3 flagellomeres, second article and third (style) minute; palp one-segmented; legs and thorax with bristle-like setae, no pilosity except for postoccipital region; hind coxa with small knob on anterior surface; thickness of metatarsi equal that of metatibial base; wing with C ending between apices of R5 and M1, apex of R5 ending slightly subapically; R4 and R5 divergent, not parallel for any part of their lengths, base of R4 not perpendicular to stem of R4+5 and R5 .
Patronym in honor of a great colleague and friend to the senior author, Prof. Kumar Krishna. Appropriately, Kumaromyia (as presently known) is preserved in amber from Burma, a place of significance in Kumar’s early years.
Kumaromyia burmitica, sp. n., by present designation.
Psilocephala electrella Cockerell, 1920, is a similar species preserved in Burmese amber, but Kumaromyia burmitica has a smaller body size (wing width 0.75 mm, vs. 1.5 mm in holotype of electrella), and differs venationally, specifically with the apex of R5 meeting C preapically (vs. slightly postapically in electrella), M1 and M2 nearly parallel (distinctly divergent in electrella), and apex of M3 distinctly curved to meet apex of CuA1 at the wing margin (vs. straight in electrella). The holotype and unique specimen of electrella (NHML In. 20148) is shown in an excellent photograph in
urn:lsid:zoobank.org:act:96FE6FDB-D380-47A8-8EDB-2FE979761B72
http://species-id.net/wiki/Kumaromyia_burmitica
Fig. 8As for the genus.
Small fly, total body length ca. 2.70 mm, thorax length 1.0 mm, wing length (estimated) 2.50 mm. Head: Large, with large eyes. Eyes bare, hemispherical in lateral view (posterior margin flat), no dorsoventral differentiation of facets; inner margins of eyes parallel, separated by distance approximately equal to width between antennal bases. Frons slightly convex, not protruding anteriad; with numerous fine setulae, without calli. Face (“subcranial cavity”) depressed, dark (sclerotized?), glabrous. Antennal scape and pedicel small, approximately equal in size, devoid of thick setae; basal flagellomere largest antennomere, drop-shaped, with dense setulae (no setae); apical two antennomeres (including apical style) small, fine, with style slightly longer than penultimate antennal article. Maxilla with bases (cardostipites) sclerotized and partially fused, palp1-segmented. Labellum slightly larger than palps. Postgena well developed, with numerous fine setae (pilosity). Thorax: Deep in lateral view, pleura apparently devoid of fine or bristle-like setae; scutum with at least 8 pairs of setae [dorsal view, including scutellum, obscured]. Scutum with 3 pairs of notopleurals and 5 pairs in supra-alar region and some setulae; no cervical/postcervical setae. Legs: With thick, stiff setae, primarily on tibiae; fore tibia slender, hind tibia thickest. Fore leg: femur with lateral row of ca. 10 fine setae, tibia with anterior row of 4–5 setae, 4 pre-apical setae. Mid leg: Femur apparently devoid of setae, tibia with 3 evenly-spaced setae on dorsal surface, 2 more ventrad, 4 apically. Hind leg: Coxa with small knob on ventral surface [best seen in left coxa]; femur devoid of setae, tibia with dorsal row of 3–4 setae, lateral row of 3 setae, ventral row of 3–4 setae. Basitarsomere on each leg equal in length to (or slightly longer than) combined length of distal tarsomeres. Each tarsomere with ca. 4 short, stiff setae on rim of distal end. Pretarsus with pair of large pulvilli, empodium setiform. Wing: Large, length nearly equal to that of body. Crossvein h long (space between Sc and C deep); Sc long, length approximately ½ that of wing and slightly shorter than length of R1; apex of Sc apparently incomplete (not meeting C). Apices of Sc and R1 without pterostigma surrounding apices. Fork of R and Rs deep, proximal to level of vein h. R2+3 straight, without apical curve. Fork of R4+5 not widely divergent; R5 in line with stem of R4+5, apex of R5 ending very near apex of wing (not posterior to it); R4 slightly curved, distinctly shorter than R5. Cell d slender, greatest width <0.25 × length. Veins M1 and M2 slightly divergent, M2 and M3 very divergent, all M veins attached to apex of cell d. Apex of M3 meeting apex of CuA1 at wing margin. ABDOMEN: Short, only slightly longer than thorax; details (e.g., sternites, genitalia) not observable.
Kumaromyia burmitica Grimaldi & Hauser, gen. et sp. n. (Therevoid family group: ?Apsilocephalidae), in Burmese amber. Right lateral habitus of holotype AMNH Bu131, as preserved. Below: wing, partially reconstructed.
Holotype, female, AMNH Bu131: Myanmar: Kachin State, near Mytikyina (mid-Cretaceous: Late Albian – Cenomanian). Specimen is complete, but the right wing (the only one observable) is folded, and most of the dorsal view is obscured, compromising a complete reconstruction of the venation (fig. 8). The fly is complete, though slightly compressed and with a slight coating of particulate matter over some areas. Its left side is lying on a rough surface of the amber, which obscures that view. The piece also contains some twisted strands of spider webbing.
In reference to the country of origin.
There is little question this fossil belongs to the therevid group, albeit unusually small (within the range in body size of some apsilocephalids and a few genera of Phycinae, such as Efflatouniella Kröber, 1927). Therevid-group features include the antennal structure, bristle-like setae on the scutum and on the legs, the small knob on the hind coxa, as well as the venation. Unlike most Therevidae, Kumaromyia lacks any pruinosity and pilosity (except for the postgena), although Xestomyzinae and Agaphotinae are also robust and have sparse pilosity. Kumaromyia lacks any thick setae that typically encircle the scape and/or pedicel subapically in Therevidae. Also, Kumaromyia has R4 and R5 above the wing tip, whereas in Therevidae these are above and below the wing tip, respectively. Unlike Apsilocephalidae, Kumaromyia has a one-segmented palp, vs. two-segmented in Apsilocephalidae, where the basal segment is distinctively thin and long (oddly, palp segmentation and structure was not described for Kaurimyia). The antennal stylus and stout body in Kumaromyia is much more similar to that of Clesthentia, as the stylus in Apsilocephala, Kaurimyia, and even Burmapsilocephala is long and thin. It is quite possible that Kumaromyia is a stem-group taxon for the therevid-family group, not necessarily belonging within Apsilocephalidae or Therevidae.
Fossil Therevidae are scarce, with only five definitive species known, all from the Tertiary.
Ambradolon grimaldii Metz and Irwin 2000: Early Miocene Dominican Republic amber
Arctogephyra agilis (Meunier 1908): mid-Eocene Baltic amber
Dasystethos hoffeinsi
Kroeberiella pinguis (Loew 1850): mid-Eocene Baltic amber
Palaeopherocera scudderi (Cockerell 1909): uppermost Eocene, Florissant, Colorado, USA
Fossil Apsilocephalidae range from the Cretaceous to early Tertiary:
Apsilocephala pusilla (Hennig 1967): mid-Eocene Baltic amber
Apsilocephala vagabunda (Cockerell 1927): uppermost Eocene, Florissant, Colorado, USA
Burmapsilocephala cockerelli
Undescribed sp.: Early Cretaceous amber, Wealden, UK (
The position of Psilocephala electrella Cockerell 1920 within the therevoid group is uncertain.
This family contains the sole Recent species Apystomyia elinguis Melander, 1950, from California, one of the world’s most relict and intriguing flies, with a dramatic history of systematic interpretation. Traditionally, Apystomyia has been placed in the Bombyliidae (e.g.,
Hilarimorphites Grimaldi & Cumming, 1999: 21. Type species: Hilarimorphites yeatesi Grimaldi and Cumming. By original designation.
urn:lsid:zoobank.org:act:BAEACD0A-8879-4761-95DB-4AF8297CADA0
http://species-id.net/wiki/Hilarimorphites_burmanica
Fig. 9Distinguished from the 4 other species in the genus (known only in New Jersey amber) by venation: vein C ending just slightly beyond apex of R4 (not at apex of R5); Sc long, distally incomplete (more so than in Hilarimorphites superba
Based on a virtually complete, well-preserved female. Body length (excluding antennae) 1.40 mm; thorax length 0.50 mm; wing length 0.95 mm. Head: Antenna with first flagellomere an elongate triangle in lateral view; apical antennal article(s) form a thin style, with possibly a minute apical article. Eyes large, glabrous. Frons with sparse, scattered setae. Proboscis with broad, flat labellum (palps not visible). Thorax: Notum dome-shaped, with sparse, fine, stiff setae; scutellum with 2 pairs of erect setae. Legs very slender, of moderate length, without distinctive spines or tibial spurs. Wing: typical of Hilarimorphites, except as given in diagnosis above [also, anal lobe may be less developed than in other species, but this area slightly folded under and obscured]. Halter of moderate length, knob slender. Abdomen: Slender, tergites unmodified, cerci and genitalia not fully visible.
Hilarimorphites burmanica Grimaldi & Cumming, sp. n. (Apystomyiiidae) in Burmese amber, as preserved. Holotype, AMNH 098.
Holotype female, AMNH Bu-098, in amber from Myanmar: Kachin, Tanai Village (on Ledo Rd. ca. 105 km Myitkyna). Amber is a deep, clear yellow, 15 × 10 × 5 mm, and was embedded in epoxy and trimmed to a wedge shape in order to maximize a full lateral view of the fly and its venation. The piece also contains a male chironomid and a thrips (Thysanoptera).
From Burma (Myanmar).
Hilarimorphites was known only from Turonian-aged amber of central New Jersey, USA, and besides the new species in Burmese amber a very similar taxon is also now known from the Upper Jurassic of Kazakhstan.
Hilarimorphites burmanica is intermediate in age between the previously known fossils, and greatly extends the geographic range. An extinct clade or grade of Apystomyiidae occurred minimally throughout Laurasia from the Upper Jurassic to the Upper Cretaceous, which is an age that is consistent with its hypothesized sister-group relationship near Eremoneura (Grimaldi and Cumming, 1999; Grimaldi and Engel, 2005; Wiegmann et al., 2011). Oddly, there are no other fossils as yet known of the family, not even from prolific and diverse Tertiary deposits like Baltic amber.
Diagnosis. Small flies 1.5 mm in total body length, with venation and other features of the wing reduced. Vein CuA1-CuA2 comprised of short fork; vein M simple; vein A either absent or reduced to vestige at base of wings. Eyes very large, extensively holoptic in males. Cervical region long, head well separated from thorax; mesonotum compact, scutellum very short.
This is a highly specialized family of Diptera known only in amber from the Cretaceous of New Jersey, USA (Grimaldi and Cumming, 1999), Spain (
http://species-id.net/wiki/Tethepomyia
(emended). Distinguished from Tethepomima by the following: Most or all of antennal flagellum lost; mesonotum bare, devoid of setae or setulae; apical tibial spurs absent; costal vein incomplete, not reaching to apex of Rs; costal spinules and fringe of fine setae on posterior margin of wing lost; alula and anal lobe lost; veins R2+3 and R4+5 lost (Rs simple, unbranched), crossvein r-m lost.
Tethepomyia thauma
urn:lsid:zoobank.org:act:1D051ABF-34FA-461F-8583-F3E6CB322FCF
http://species-id.net/wiki/Tethepomyia_zigrasi
Fig. 10Distinguished from the other two species of the genus, which are known only from males (Tethepomyia thauma Grimaldi and Cumming: New Jersey amber; and Tethepomyia buruhandi Grimaldi and Arillo: Spanish amber), by the following: thickened costal and Rs veins, bases of M and Cu complete; dorsoventral differentiation of eye facets (in female, undoubtedly more differentiated in males); U-shaped basal flagellomere large, pedicel small, indistinct. Known only from female.
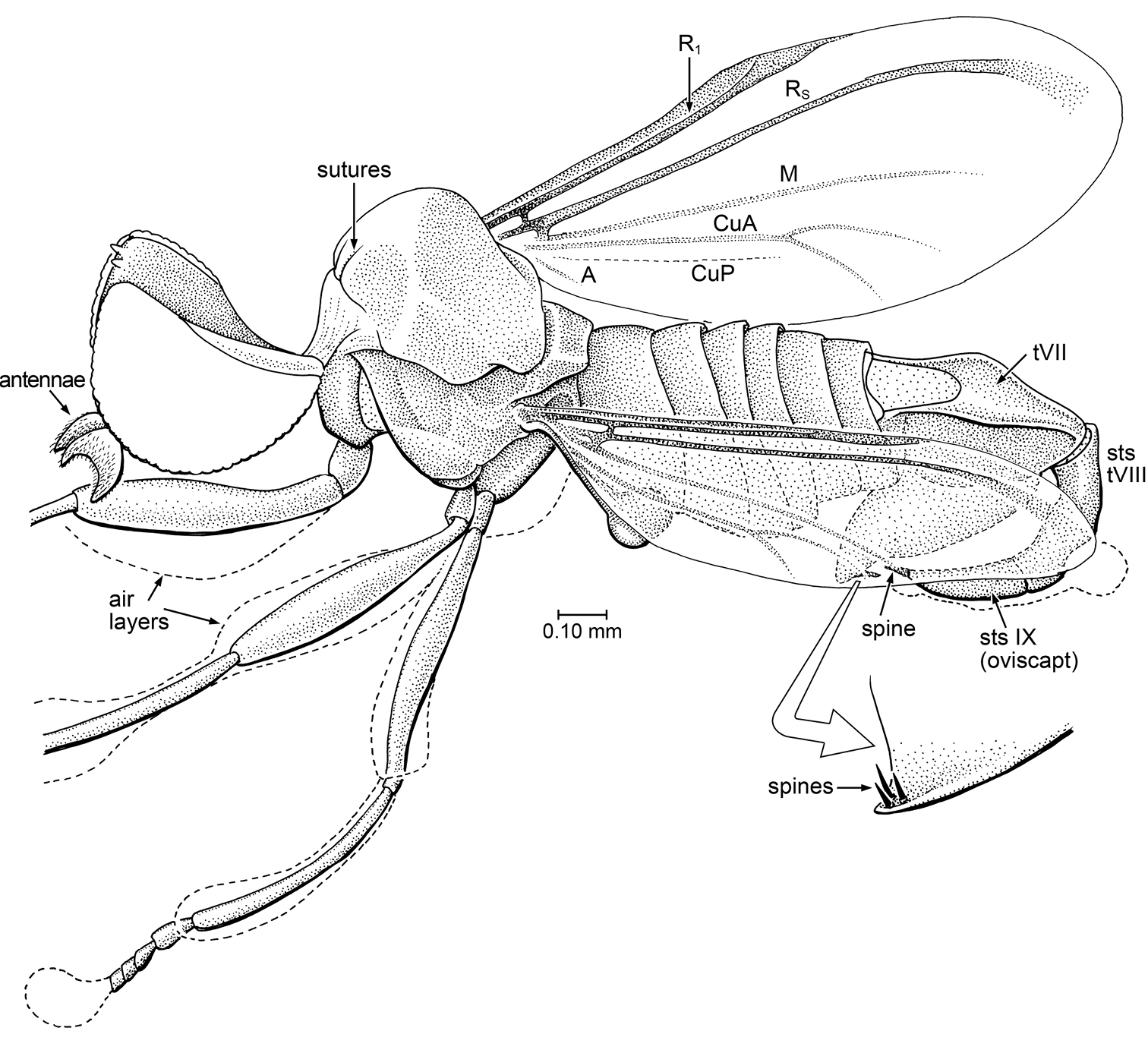
Body length (tip of basal flagellomere to posterior-most surface of tergite VIII) 2.15 mm. HEAD: Hemispherical in female; eyes very large, covering most of head, only small strip of gena exposed [view of face and frons not visible]. Dorsal eyes facets approximately 0.5× diameter of ventral facet; eye completely bare, no interfacetal setulae. No setae apparent on gena or frons. Ocelli possibly on small tubercles – small, digitate lobes in this area [but details obscure]. Antenna with large, crescent-shaped basal flagellomere; pedicel apparently small [indistinct]. Proboscis and palps not visible [ventral surface of head covered with bubble]. Posterior surface of head evenly and shallowly concave. Cervical region long; head not adpressed to pronotum.
Thorax: Small and short, L = 0.55 mm, with scutum arched, posterior half long and sloped; scutum and scutellum devoid of acrostichals or setae. Scutellum short, length ca. 0.20 × that of scutum; posterior margin flat and slightly concave, not acute. Legs bare, devoid of setae or setulae; with femora slightly swollen in middle. Fore and mid coxae adjacent, hind tibia with coxal-trochanteral articulation facing anteriad (hind legs apparently held forward). Tibiae long and slender (slightly shorter than respective femur). Metatibia slightly bowed, as if to fit tightly against ventral surface of femur. Tarsi short, with basitarsomere only slightly longer than tarsomere 2 [most tarsomeres obscured by layer of air). Halter long; knob large, length of stem approximately 2.2 × greatest diameter of knob; stem without setae. Forewing with reduced venation; veins extremely light (particularly M and Cu); microtrichia of forewing either absent or so microscopic as to not be visible; no costal spinules or fringe of fine setulae on posterior margin of wing. Vein C short, extended to only ca. 0.6 × length of wing, sclerotized, swollen towards apex; apex of R1 fused with swollen portion of C. Rs thick, width slightly increased apicad; vein incomplete, not reaching wing margin/tip. Vein M faint, complete, tip evanescent and not reaching wing margin. Vein CuA faint, with short fork CuA1–CuA2 (length of fork 0.7 × length of stem); branches of fork curved towards anal region. What appears as deep fold (CuP?) parallel and posterior to stem of CuA. Faint, short vein A at base of posterior portion of wing; anal lobe and alula not present.
Abdomen: Tergites and sternites well developed, sclerotized; segments I – VI short (I longest), tergite VII long, sclerotized, length approximately equal to that of tII through tVI, with deeply incised membranous region basally, dorsal and lateral surfaces concave. Sternite VII very large, lobe-like, suspended beneath abdomen; apex pointed, bearing three short, sharp spines. Base of tVII apparently articulating with apex of tVI; tVII+VIII formed into a curved, sclerotized, sharp ovipositor-like structure, with a small, sharp, sclerotized spine at tip. Spine at tip of abdomen/oviscapt (sts IX) apparently interdigitating between three spines of sVII.
Tethepomyia zigrasi Grimaldi & Arillo, sp. n. (Tethepomyiidae) in Burmese amber, also showing ventral detail of distal portion of abdomen. Private collection of James Zigras. sts: syntergosternite.
Holotype, Female: Myanmar, Kachin State, Early Cenomanian. Specimen is in excellent condition and is in the private collection of James Zigras.
Patronym for James Zigras, for allowing preparation and study of this remarkable specimen.
Tethepomyia zigrasi sp. n. appears to be a sister group to Tethepomyia buruhandi + thauma, from Spanish and New Jersey ambers, respectively. Tethepomyia zigrasi retains the bases of M, Rs, and Cu, which the other two species have lost. It shares with Tethepomyia buruhandi and Tethepomyia thauma many losses: of the antennal stylus, tibial spurs, crossvein r-m, veins R2+3 and R4+5, costal vestiture, fringe of marginal setulae on the wing, as well as the reduction of vein C.
With little question the oviscapt of Tethepomyia zigrasi is a hypodermic-like (“aculeus”) structure, probably used for injecting its eggs into hosts. It was probably a parasitoid. An oviscapt of similar specialization has sporadically evolved in Diptera. It occurs in a few Phoridae (e.g., Apocephalus), all Pipunculidae, and within the Schizophora in some Conopidae (e.g., Stylogaster), most Tephritoidea, all Cryptochaetidae, and probably other families. The trait appears to have evolved most often in parasitoid groups (all those listed above except tephritoids). Most tephritoids inject their eggs into fruits or stems, though a few (like Pyrgotidae) are parasitoids. Fine structure of the injecting oviscapt reveals its convergent development: what is labelled as the “ovipositor” in Pipunculidae (Hardy, 1989: 747) is probably a sclerotized, spine-like derivative of the cercus. In Cryptochaetidae the syringe-like oviscapt is sternite VIII; in Tephritoidea the oviscapt is a telescoping structure composed of segments 7–9. Interestingly, Tethepomyia has a suite of other convergent features similar to those of parasitoid families. Like Pipunculidae, Tethepomyiidae possesse large eyes; like Pipunculidae and Cryptochaetidae the family has large pulvilli; and like Cryptochaetidae the basal flagellomere is enlarged and the arista minute to lost. These are probably functionally correlated features.
urn:lsid:zoobank.org:act:7090815B-8878-487D-9980-78581DB36111
Distinctive small flies (body length less than 1.5 mm) with antennal stylus arista-like and terminal, having a single article; face without ptilinal suture; median margins of eyes very close on frons; maxillary palpus two-segmented; mesonotum with dorsocentral and scutellar setae; wing venation highly reduced, with R2+3 and R4+5 each unbranched, M unbranched and evanescent at both ends, Cu simple; female with pair of long, digitate, unsegmented cerci.
From Myanmar, country of origin, and –myia, a common suffix referring to the feminine Greek word for fly.
Myanmyia asteiformia sp. n. By present designation.
This is a perplexing little fly. Chaetotaxy of the thorax, the wing venation, and even body shape are strikingly similar to acalyptrate flies in the Asteiidae. Convergent wing features of the two groups include short R1 and R2+3 veins; a straight R4+5 that meets the tip of the wing, and even microtrichia that are arranged in rows. However, Myanmyia is not even a cyclorrhaphan, by virtue of the terminal (versus dorsal) arista-like stylus, lack of a ptilinum, and presence of two-segmented (vs. 1-segmented) palpi. With the exception of a few very basal Recent and extinct Platypezidae, almost all other Cyclorrhapha have a dorsal arista. Two-segmented palpi exclude Myanmyia from the Eremoneura (the apparent basal segment of the two segmented palpi seen in some Phoridae is probably a palpifer [
urn:lsid:zoobank.org:act:3BD4F73D-375A-4B7F-8637-5449BC695495
http://species-id.net/wiki/Myanmyia_asteiformia
Fig. 11As for genus.
Body size small, length 1.35 mm (excluding antennae and cerci), slender. Wing length 1.05 mm. Head: Slightly wider than thorax [possibly preservational, as head is slightly compressed]. Antenna with cup-like pedicel, distal edge rimmed with fine, stiff setae; basal flagellomere drop-shaped, width approximately equal to length; arista-like stylus terminal, setulose, 1-articled (no small basal articles), length approximately 3 × length of basal flagellomere. Eyes large (occupying virtually entire lateral surface of head), bare, with slight dorsoventral differentiation of facets (dorsal facets ca. 2 × diameter of ventral ones); inner margins of eyes (on frons) very close, width of separation equal to ca. 3 facet diameters. Ptilinal suture absent. Maxillary palp 2-segmented, with apical segment clavate and basal segment slender. Labrum long, very slender; hypopharynx (?) stylet-like; labellum small. Gena very shallow or barely developed (not apparent). Postocciput broad, concave. Thorax: Slender, with the following dorsal setae (per side): 1 postpronotal, 4 supra-alar/notopleurals, 3 postsutural dorsocentrals (posterior one largest), 2 pairs scutellars [pleura not visible]. Legs of moderate length, setulose, without distinctive spines/spurs. Pretarsus with claws well developed, but no pulvilli. Wing: Long, slender, W/L = 0.33; membrane microtrichia arranged in oblique rows (between R veins) and longitudinal rows (portions of space between R4+5 and M). Vein C slightly beyond apex of R4+5, no humeral or subcostal breaks; with long, sparse spinules. Sc short, very faint [best seen when tilting specimen]. Base of vein R thick, R1 short (length 0.3 × length of wing); R2+3 unbranched, meeting C at ⅔ the length of wing. R4+5 straight, extended to tip of wing, unbranched. Vein M simple, unbranched, very lightly sclerotized; both ends evanescent. Sc. Vein A thick, heavily sclerotized strip along alular edge of wing. Anal lobe and alula not developed. Halter: with large, darkened knob, stem approximately same length as knob or slightly longer. Abdomen: Tergites I—VII well developed, with sparse setulae, without macrosetae; sternites II, III, IV large, bare; segment V is tubular; VI, VII ring-like; terminal segment bearing pair of long, finger-like, one-segmented cerci. Presence/absence of abdominal muscle plaques not visible.
a-d Myanmyia asteiformia Grimaldi, gen. et sp. n. (unplaced to family), in Burmese amber (holotype, AMNH Bu1616) a Anteroventral detail of head b dorsal habitus, as preserved c pretarsus. d apex of abdomen, ventral view.
Holotype, female, AMNH Bu1616, in amber from northern Myanmar: Kachin State, Tanai Village, 105 km NW Mytikyina. The holotype is the sole inclusion in a clear amber-colored piece 9 × 6 × 2 mm. Ventral surface of the thorax and the abdomen are compressed, and a crack through the thorax obscures some details. The left wing of the unique specimen is well preserved, but venation is optimally observed by tilting and observing the piece at various oblique angles. Right wing is twisted, but in oblique view additional details of venation are visible.
L., for like, and Asteia (type genus of the Asteiidae, a family of Schizophoran flies) and Latin –formia, meaning like, in reference to the similarity of the unrelated two taxa in body shape, size, and wing venation.
We are grateful to the institutions and individuals who loaned specimens for this study and patiently awaited the long gestation of this paper, including Dany Azar, Michael Engel, Ru Smith, and James Zigras. The donation of the holotype of Burmacyrtus by Ru Smith is deeply appreciated. Field work on amber and acquisition of specimens has been generously funded by Robert G. Goelet, trustee and Chairman Emeritus of the AMNH. Paul Nascimbene (AMNH) embedded, trimmed, and prepared specimens for study, and Steve Thurston (AMNH) composed the plates of figures; the senior author is grateful to them both for their years of dedicated talent. Very helpful suggestions and editing of the ms were provided by Steve Gaimari and an anonymous reviewer. Research on amber fossils by D.G. has been funded by NSF grant DEB 0542726, and support for A.A. was provided by project CGL2008–00550/BTE from the Spanish Ministry of Science and Innovation.