(C) 2011 Felipe N. Soto-Adames. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Three new species of Isotomidae springtails are described from the Lake Champlain Basin (Vermont and New York, USA), Lake Willoughby and Greater Averril Pond in Vermont. Subisotoma joycei sp. n. and Scutisotoma champi sp. n. were collected in sandy beaches whereas Ballistura rossi sp. n. was found only in a constructed wetland built and managed by the University of Vermont. Scutisotoma champi sp. n. was found in Lakes Champlain and Willoughby, and Greater Averril Pond and is probably present in most lakes and large ponds in the area. Subisotoma joycei sp. n. was found only along the southern and eastern coast of South Hero, and the mainland coast facing eastern South Hero. Ballistura alpa is redescribed and transferred to the genus Pachyotoma based on the absence of tibiotarsal seta B4/B5, the presence of secondary cuticular granules, 4 prelabral setae, a full complement of guard setae on labial papilla E and in having a bifurcate outer maxillary lobe with 4 sublobal setae.
Freshwater lake sandy beach, Lake Champlain, Lake Willoughby, Great Averrill Pond, Chaetotaxy, Ballistura, Pachyotoma, Scutisotoma, Subisotoma
The springtail fauna of Vermont is poorly known. The most comprehensive list includes only 57 species (
As part of a general survey of the springtail fauna of sandy beaches on the Vermont side of Lake Champlain four species in the family Isotomidae belonging to the Proisotoma genus complex were collected. Further analysis showed that the samples included Proisotoma minuta (Tullberg), 1871 and three undescribed species. In the present contribution we describe the new species and provide additions to the description of Ballistura alpa Christiansen & Bellinger, 1980.
MethodsMost of the terminology used in the descriptions follows
The number of postlabial setae and tergal sensilla are often variable and the formula is presented in the format x (y) where x is the mode and y represents other setae number observed. If there is more than one mode the formula is expressed as x/z (y). Separation between thoracic and abdominal tagma is represented by //, segments within tagma are separated by a semicolon (;). Number of setae are given for half a tergite (i.e., formula 10//101 should be understood as 10//101 + 10//101)
Abbreviations used in the descriptions that follow are: Ant., Th., Abd., L and PAO for antenna, thorax, abdomen, leg and postantennal organ.
The types of the new species are deposited in the Insect Collection of the Natural History Survey, at the University of Illinois, Champaign, IL, USA. The slides of Pachyotoma alpa are deposited in the Zadock Thompson Natural History Collection at the University of Vermont, Burlington, Vermont, USA.
All collections were made by extracting a plug of sand or soil using a commercial bulb planter. Sand/soil plugs were placed in plastic bags in the field and transported to the laboratory. In the laboratory each plug was washed in water, the water was filtered using commercial coffee maker filters, and the content of the filters was extracted in Berlese funnels (with 15 watts light bulbs) for three days. All sand plugs were moist, as completely dry sand cannot be extracted with a bulb planter. Most samples included only sand, but others contain variable amounts of surface plant debris and the species collected could be either in the sand or on the surface plant debris. The authors collected all samples as follows (localities I and O did not contain examples of the species treated here and are not listed below):
A. Vermont, Chittenden Co., Burlington, Oakledge Park, N44.45640 W73.22513, sand, 24 September 2005.
B. Vermont, Chittenden Co., Burlington, Pine Street Canal, near water treatment plant at south end of Battery St., N 44.46859 W 73.21901, sand, 24 September 2005.
C. Vermont, Chittenden Co., Colchester, Mallets Bay, Colchester Beach, N44.54555 W73.21572, sand with sparse remains of aquatic plant debris, 1 October 2005.
D. Vermont, Chittenden, Colchester, Delta Park, mouth of Winooski River, N44.53111 W73.27396, sand, October 2005
E. Vermont, Chittenden Co., Colchester, Delta Park, beach, intersection of Widemere Way and Horizon View St., N44.53647 W73.27739, sand, October 2005.
F. Vermont, Chittenden Co., Milton, Sand Bar State Park, N44.62818 W73.23769, sand, October 2005.
G. Vermont, Grand Isle Co, South Hero, White’s Beach, N44.62189 W73.32273, sand and thick layer of aquatic plant debris, October 2005
H. Vermont, Grand Isle Co., Grand Isle, Pearl Bay, west of intersection of East Shore North Rd. and Hide Point West Rd., N44.73078 W73.26401, sand with sparse remains of aquatic plant debris, October 2005
J. Vermont, Grand Isle Co., North Hero, Knight Point State Park, eastern beach, N44.76986 W73.29399, sand with sparse remains of aquatic plant debris, October 2005.
K. Vermont, Grand Isle Co., Alburg, Alburg Dunes State Park, N44.86536 W73.30001, sand, October 2005
L. Vermont, Franklin Co., St. Albans, St. Albans Bay State Park, N44.80997 W73.14508, sand with sparse remains of aquatic plant debris, October 2005.
M. New York, Essex Co., Crown Point Historic Area, beach near ruins of Ft. Saint Frederic, N 44.03093 W 73.42768, sand and thick layer of aquatic plant debris, 9 September 2006.
N. Vermont, Addison Co., Chimney Point State Park, N44.03437 W73.42073, sand and thick layer of aquatic plant debris, 9 September 2006.
P. New York, Clinton Co., Ausable Marsh State Wildlife Management Area, N44.57269 W73.43242, sand with sparse remains of aquatic plant debris, 10 September 2006.
Q. Vermont, Orleans, Co., Westmore, Lake Willoughby northeast shore, N44.76906 W72.05338, sand with sparse remains of aquatic plant debris, 5 August 2007.
R. Vermont, Orleans, Co., Westmore, Lake Willoughby south shore, N44.71795 W72.02997, sand with sparse remains of aquatic plant debris, 5 August 2007.
S. Vermont, Orleans Co., Norton, Great Averrill Pond northwest shore, N44.99117 W71.72055, sand, 16 October 2008.
T. Vermont, Orleans, Co., Averill, Great Averill Pond, south shore, N44.97303 W71.68575, sand, 16 October 2008.
U. Vermont, Chittenden Co., South Burlington, University of Vermont Constructed Wetland, N 44.45869 W 73.18936, June 2005.
Descriptions Genus Ballistura Börner, 1906urn:lsid:zoobank.org:act:AE48D35A-87D9-4D8C-B69A-B3B8CF8207BB
Holotype– Female, locality U, slide mounted. Paratypes– locality U, 15 on slides, 3 in alcohol.
USA, Vermont, Chittenden Co., South Burlington, University of Vermont Constructed Wetland, N 44.45869 W 73.18936.
The new species is dedicated to Ross Bell in celebration of his contributions to our understanding of the entomological fauna of Vermont.
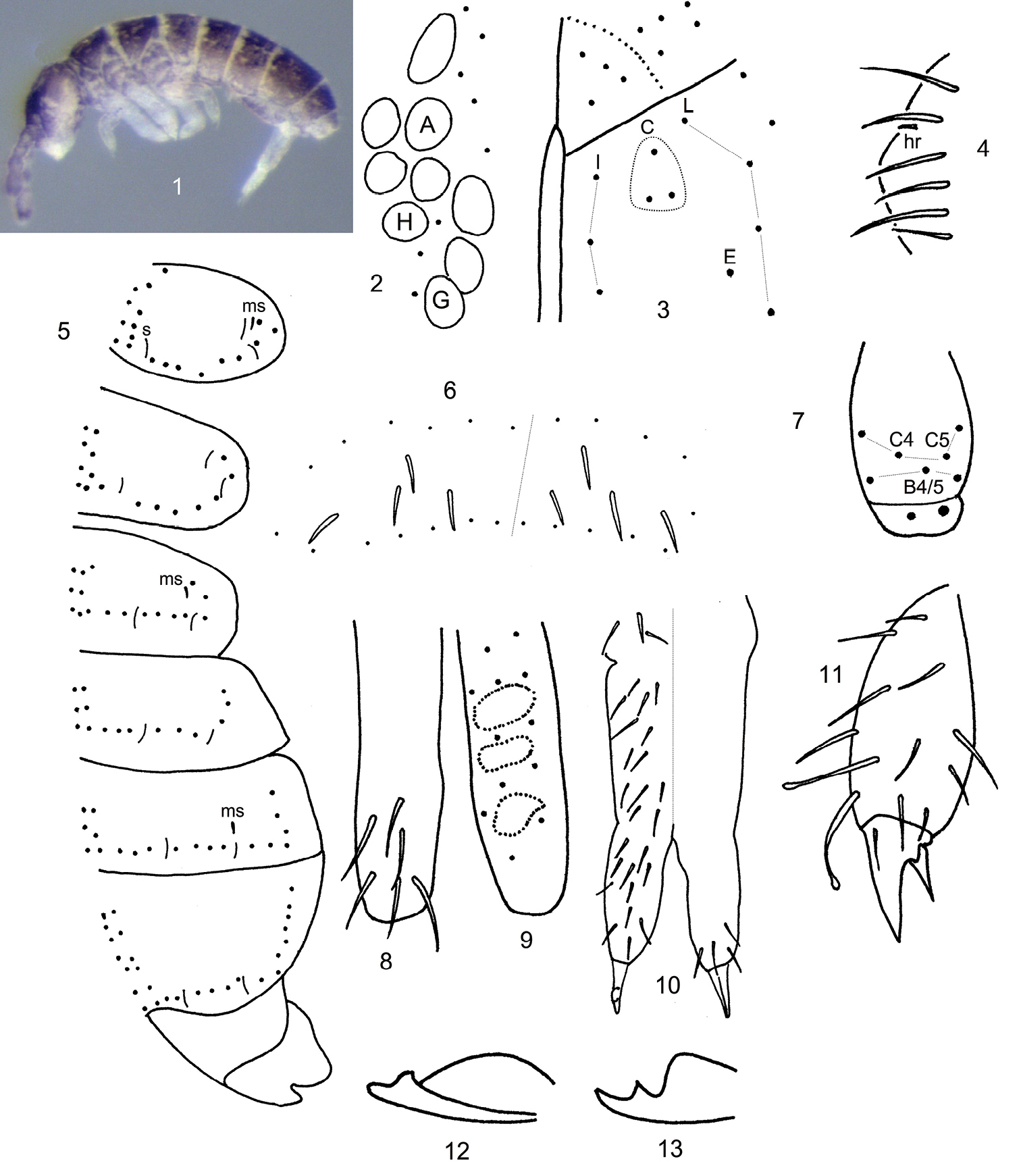
Length to 0.5 mm. Live individuals black, alcohol preserved specimens (Fig. 1) purple, with pigment more or less uniformly distributed throughout head, body and antennae, legs and manubrium purplish brown. Ant. 4 without basal microsensilla, with 8-9 well developed thin-walled sensilla, and 2-3 additional poorly developed sensilla distributed along distal 2/3 of segment; subapical sense organ with 1 differentiated microsensilla and 1 microrod in a pit. Ant. 3 with 0-1 basal microsensilla; sense organ with 2 clubbed sensilla and 2 differentiated guard sensilla; 1 lateral sensilla present. Ant. 2 with 2 basal microsensilla and 1 distal sensilla. Ant. 1 with 2 basal microsensilla, and 1 whorl of hairs comprising 11 acuminate setae and 2 sensilla. Eyes 8+8, H slightly smaller or subequal to C (Fig. 2), with 3 interocellar setae; PAO circular to elliptical, about 1-1.7X diameter of eye B, and 3 associated setae. Prelabral and labral chaetotaxy 2/554; distal labral margin smooth. Papilla of outer maxillary lobe bifurcate, sublobal plate with 2 appendages. Maxilla with lamella 1 narrow, surpassing tip of capitulum and ciliate only along external margins. Labial palps with a full complement of papillae and 3 proximal setae; papillae E with blunt lateral process and 6 guard setae, e7 absent; labial triangle with 5 anterior and 4 posterior setae; distribution of postlabial setae in columns I, C, E and L as 3, 3, 1, 3/4 (Fig. 3). Body dorsally covered by smooth hairs; some hairs on the pre-posterior row reaching base of setae on posterior row; tergal macrochaetae undifferentiated; thorax without ventral setae. Axial setae on Th. 2-Abd. 3 as 5-6, 4//3, 3, 3; Th. 3 with 14-16 setae on posterior row; microsensillar and sensillar formulae 10//101 and 33//22224, respectively (Figs. 5-6); antero-lateral sensilla on Th. 2 anterior to microsensilla, lateral sensilla on Th. 2-3 anterior to medial sensilla; medial thoracic sensilla inserted on preposterior row of setae, abdominal sensilla inserted just anterior or on posterior row of setae; lateral sensilla on Abd. 5 similar to medial sensilla (Fig. 6). Proximal and medial subcoxae on legs 1-3 with 1, 1; 1, 5; 3, 5-6 setae. Lateral valve of Abd. 6 with 1 hr seta (Fig. 4). Tibiotarsi on legs 1-3 with 20, 20, 22 setae, respectively; tibiotarsal whorl B with B4/5 (Fig. 7); only male seen apparently in reproductive quiescent instar, without modified metatibiotarsal setae; legs 1-3 with 1, 2, 2 clearly capitate tenent hairs (Fig. 11). Unguis and unguiculus toothless, unguiculus lanceolate or acuminate. Ventral tube with 4+4 apical and 1+1 posterior setae. Tenaculum with 3+3 teeth and 1 seta. Anterior and posterior furcal subcoxae with 8-11 and 4-5 setae, respectively. Proportion manubrium/dens/mucro as 3/2/1. Manubrium with 13 dorsal and 0 ventral setae (Fig. 10). Dens weakly tuberculate, with 11 (12) dorsal (Fig. 9-10) and 5 (4/6) ventral setae (Fig. 8, 10). Mucro bidentate, with a pronounced basal membrane (Figs. 12-13).
Following
Ballistura rossi sp. n. appears to be unique among Ballistura sp. in having 2 instead of 3 appendages in the sublobal plate of the outer maxillary lobe. However, this character has been reported in relatively few of the species currently placed in Ballistura and further information is needed to determine how unique the condition in Ballistura rossi sp. n. is.
Ballistura rossi sp. n. 1 Habitus 2 Eye patch and PAO 3 Labial and postlabial chaetotaxy 4 Lateral anal valve chaetotaxy 5 Chaetotaxy of Th. 2-Abd. 4 6 Chaetotaxy of Abd. 5 7 Posterior chaetotaxy of mesotibiotarsus 8 ventral chaetotaxy of dens 9 Dorsal chaetotaxy of dens, tubercles represented by dotted line 10 General anterior (left side) and posterior (right side) chaetotaxy of furcula 11 Metatibiotarsus and claw complex 12–13 Mucro from two individuals.
Comparison between Ballistura rossi sp. n., Ballistura hankoi and Ballistura tuberculata. Characters for Ballistura hankoi and Ballistura tuberculata from Europe according to
| Species Character | Ballistura rossi sp. n. | Ballistura hankoi Europe (Stach) | Ballistura tuberculata Europe (Stach) | Ballistura tuberculata Indiana/Nova Scotia (Stach) |
|---|---|---|---|---|
| Color | deep purple | dark blue | pale bluish grey | blue |
| PAO/Nearest Eye | 1-1.7 | ≈1 | 1.5 | 1.1-1.2 |
| Largest Specimen(mm) | 0.5 | 0.5 | 0.9 | 0.8 |
| Dorsal Setae Manubrium | 13 | 20 | 20 | ? |
| Mucro:Dens | 1:2 | 1:2 | 1:3 | 1:3 |
| Dorsal Setae on Dens | 11-12 | 10 | 10 | 9-10 |
| Mucronal Membrane | wider at middle | wider at middle | wider on basal half | wider at middle |
urn:lsid:zoobank.org:act:12F3C3E4-5E9E-4345-9DE6-E9D884E34410
Holotype– Locality G, Male, slide mounted. Paratypes– Locality G, 3 on slides, 3 in alcohol; H, 2 on slides, 2 in alcohol. Other Material– Locality F (9 in alcohol),
USA, Vermont, Grand Isle Co., South Hero, White’s Beach, N44.62189 W73.32273.
This species is dedicated to Joyce Bell for her contributions and support to the study of the arthropod fauna of Vermont.
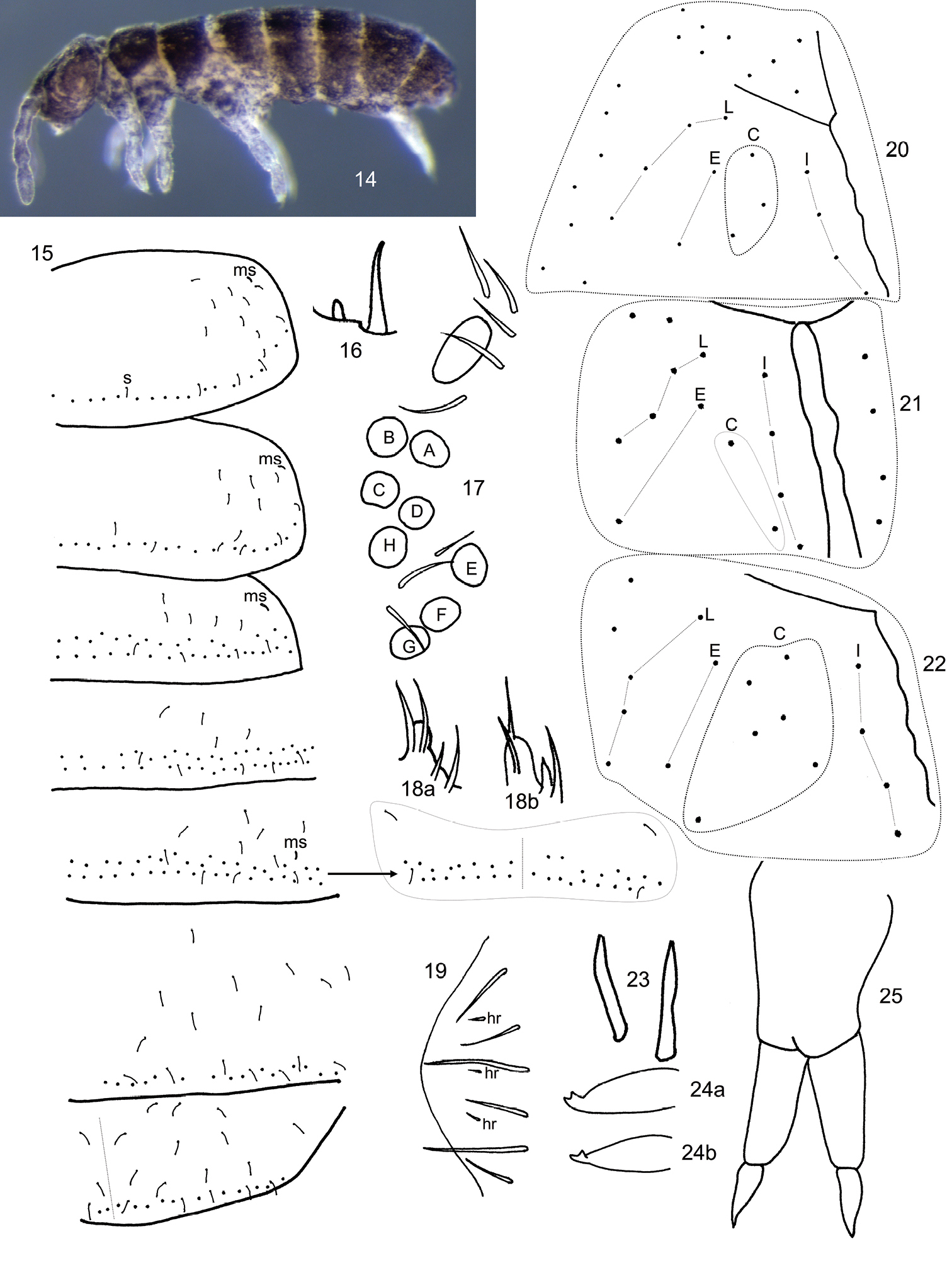
Length to 1.2 mm. Living individuals black, alcohol preserved specimens dark purplish brown to black, with pigment more or less uniformly distributed through out head, body and antennae, legs and manubrium purplish (Fig. 14); some individuals with paired black longitudinal lines extending from Th. 2-Abd. 3. General body shape short and stout, with sudden bend between Abd. 4-5 (Fig. 14) as in Folsomides. Ant. 4 without basal microsensilla (bms of
Three individuals show variation in the number and size of eyes. In the small juvenile eyes G and H are small, barely rising above the cuticle; one male is blind; and in one female all eyes in one patch are subequal, but on the other patch eye E is less than half the size of F. The axial setae are often disorganized and the number of setae in a column is open to interpretation. The number of tergal sensilla is variable. Most individuals have an asymmetric number of sensilla, and two individuals lack the microsensilla of Abd. 1 on one side. Tenent hair B7 on metathoracic legs often appears acuminate instead of capitate. Two individuals have 3+4 tenacular teeth.
The generic placement of the new species is problematic. It better fits in the genus Subisotoma (Table 2), but it is unique among species currently assigned to that genus in having more than 8+8 tergal sensilla on each segment, by the significantly larger number of dental setae (most Subisotoma species have 4 or fewer dorsal and 1-2 ventral setae, whereas Subisotoma joycei has 18-20 dorsal and 4-6 ventral setae), and by having a well developed furcula with mucro exhibiting a wide lamella and clearly separated from the dens. Subisotoma joycei is similar to species in the genus Isotopenola Potapov, Babenko, Fjellberg & Greenslade, 2009 in the presence of sensillar polychaetosis on body terga, but differs from all forms in that genus by having smooth thoracic sterna, lacking an isolated field of setae on Abd. 3 sterna and in the number of guard setae on labial papilla E. The strong polychaetotic furcula in Subisotoma joycei resembles the condition in Ballistura, but the new species clearly differs from Ballistura in maxillary palp structure, sensillar and microsensillar formulae, presence of a full complement of setae in tibiotarsal whorl B, and dens sculpturing (Table 2).
The new species is most similar to Ballistura ewingi James, 1933, sensu
The new species is also similar to Ballistura excavata Folsom, 1937, but the two species are easily separated by body color, eye number, shape of tenent hairs and unguiculus, and structure of the dens (Table 3).
Subisotoma joycei sp. n. 14 Habitus of holotype 15 Sensillar chaetotaxy of Th. 2-Abd. 5, s=sensilla, ms= microsensilla, arrow points at position of Abd. 3 medial sensilla in a different individual 16 Subapical organ of Ant. 4 17 Eye patch and PAO 18 Labial palp papilla E 18a dorsal aspect 18b ventral aspect 19 Lateral anal valve 20–22 Postlabial chaetotaxy showing variation in number of setae assigned to column C 23 Modified metatibiotarsal seta in mature male 24 Mucro 24a lateral aspect 24b oblique aspect 25 Organization of furcula.
Subisotoma joycei sp. n. 26 Posterior view of mesothoracic tibiotarsus 27 Metatibiotarsus, arrow points at abnormal seta 28 Dorsal (left side) and ventral (right side) chaetotaxy of manubrial base, manubrium and dens.
Diagnostic characters for selected genera in the Proisotoma genera complex in comparison with Subisotoma joycei sp. n. All genera, retained in the sense of
| Genus Character | Scutisotoma | Proisotoma | Folsomides | Ballistura | Isotopenola | Subisotoma | Subisotoma joycei sp. n. |
| Prelabral Seta | 4 | 3 | 2 | 2 | 2 | 2 | 2 |
| Outer Maxillary Palp/Sublobular Appendages | bifurcate/4 | simple/4 | simplebifurcate/3 | bifurcate/3 | simple/4 | simple/4 | simple/4 |
| Number Guard Setae on Labial Papilla E | 7 | 5– e4 and e7 absent | 7 | 6– e7 absent | 4-5 | 6– e7 absent | 6– e7 absent |
| Tergal Microsensillar Formula | 11//111 | 10//00000//000 | 10//000 to11//111 | 11//111 | 10//10111//111 | 10//000, 10//100, 10//001, 10//101 | 10//101 |
| Position Medial Sensilla Abd. 3 | medial row | posterior row | medial row | just anterior to posterior row | subposterior row | posterior row or just anterior to posterior row | posterior row or just anterior to posterior row |
| Sternal Thoracic Setae | presentabsent | presentabsent | absent | absent | absent | absentpresent | absent |
| Mesotibiotarsal Setae B4/5 | absent | absent | present | present | absent | absent | absent |
| Ventral Manubrial Setae | 10 | 1 | absent | 0 | 0 | 0 | 0 |
| Dorsal/Ventral Dental Setae | variable | 3-7/4-6 | 2-6/0-3 | variable | 3-8/1 | <4/1 | 18-20/4-6 |
| Tergal Sensilla Polychaetosis | absent present | absent | absent | absent | absent present | absent | present |
| Dental Sculpturing | crenulatetuberculategranulate | crenulate | smooth | tuberculate | smooth | smooth | smooth |
Comparison between Subisotoma joycei sp. n., Ballistura excavata andtwo forms of ‘Ballistura’ ewingi. The presence of smooth dens places Ballistura ewingi outside of Ballistura as currently diagnosed by
| Species Character | Subisotoma joycei sp. n. | ‘Ballistura’ ewingi James Mississippi | ‘Ballistura’ ‘ewingi’ Christiansen & Belliger Pennsylvania | Ballistura excavata Folsom |
|---|---|---|---|---|
| Eye Number | 8 | 8 | 8 | 6 |
| Color | dark purple | dark purple | dark purple | yellow |
| Tenent Hairs on L1-3 | 1, 2, 2all capitate | 2-3 on all legsall capitate | 1, 2, 2all capitate | ?acuminate |
| Unguiculus Shape | triangular, asymmetric | triangular, asymmetric | triangular, asymmetric | triangular, symmetric |
| Unguiculus Apical Filament | absent | absent | absent | present |
| Ventral Tube Distal Setae | 11 | 4 | 4 | ? |
| Teeth on Tenaculum | 3-4 | 2 | 3 | 4 |
| Dorsal Cuticle of Dens | smooth | smooth | smooth | tuberculate |
| Dorsal Seta on Dens | 18-20 | 18? ( |
? | 14-16? ( |
| Ventral Setae on Dens | 4-6 | 6 | 5 | at least 2( |
urn:lsid:zoobank.org:act:0EA01C0B-4A76-4DEE-B863-26F4952B5013
Holotype– Female, slide mounted, locality B. Paratypes– Locality B, 9 individuals on slides, 1976 in alcohol; N, 5 mounted on two slides and more than 3000 in alcohol; P, 4 individuals on slides and 59 in alcohol; Other material– Locality A, 800 individuals in alcohol; C, 7 in alcohol; D, 187 in alcohol; E, 30 in alcohol; F, 32 in alcohol; G 1186 in alcohol; H, 1290 in alcohol; J, 158 in alcohol; K, 35 in alcohol; L, 1723 in alcohol; M, 1555 in alcohol; Q, 5 in alcohol; R, 2 in alcohol; S, 1 in alcohol; T, 8 in alcohol.
USA, Vermont, Chittenden Co., Burlington, Pine Street Canal, near water treatment plant at south end of Battery St., N 44.46859 W 73.21901
The species is named after ‘Champ’ the denizen monster of Lake Champlain, were the new species seems to be most abundant.
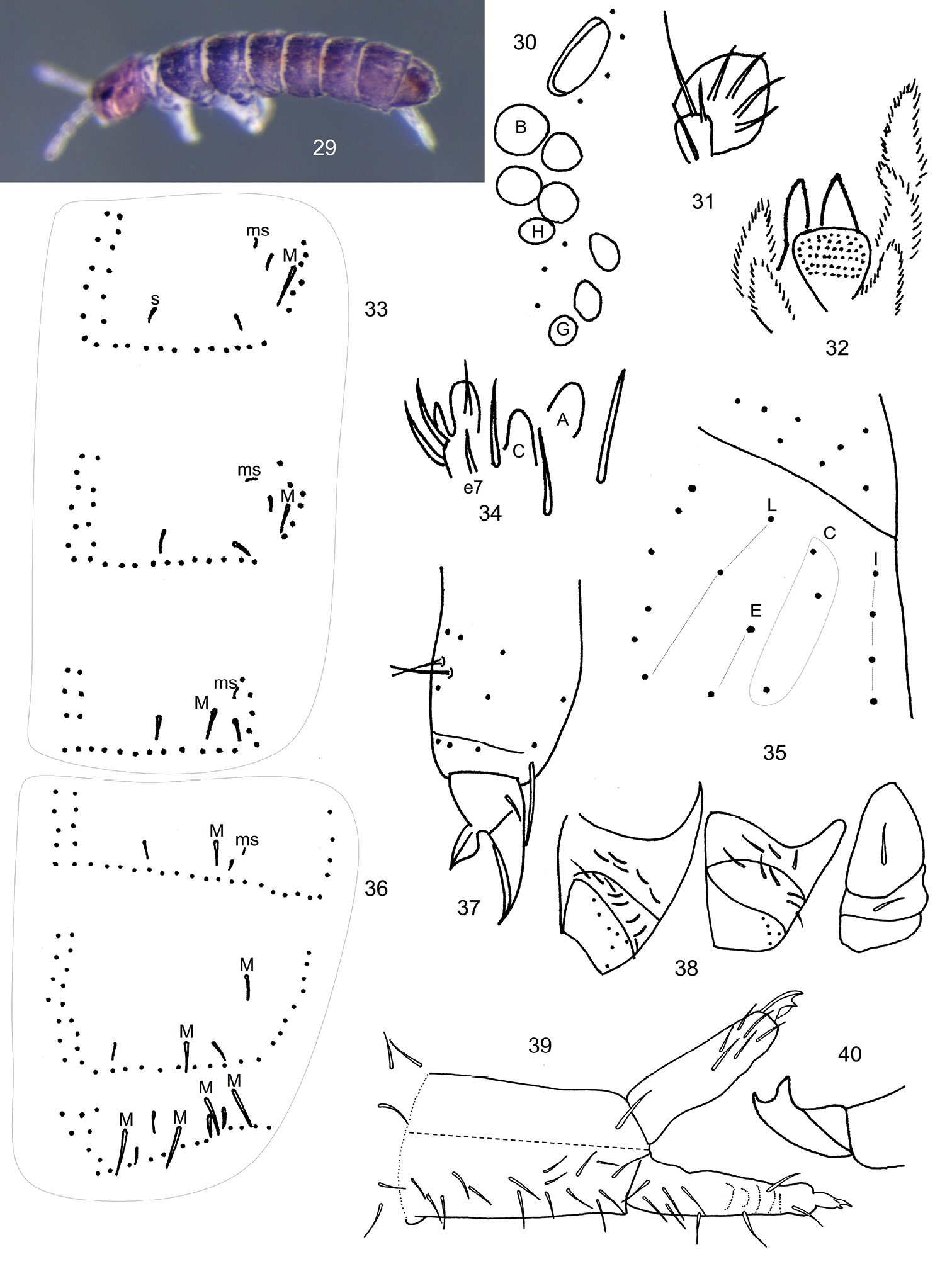
Length to 0.7 mm. Live specimens black, alcohol preserved individuals (Fig. 29) dark purple, with pigment more or less uniformly distributed throughout head, body and antennae; legs and manubrium light purplish brown. Ant. 4 with 1 basal microsensilla, and 7 poorly differentiated thin-walled sensilla distributed along apical half of segment; subapical sense organ with 1 poorly differentiated microsensilla and 1 micropeg in a pit. Ant. 3 with 1 basal microsensilla; sense organ with 2 clubbed sensilla and 2 differentiated guard sensilla; 1 lateral sensilla present; males with 2 additional dorsal sensilla. Ant. 2 with 3 basal microsensilla and 1 distal sensilla. Ant. 1 with 2 basal microsensilla and 1 whorl comprising 11 setae and 2 sensilla. Eyes 8+8, G and H slightly smaller than others (Fig. 30), with 3 interocellar setae; PAO elliptical, about 2.3X diameter of eye B, with 4-5 associated setae. Prelabral and labral chaetotaxy 4/554; distal labral margin smooth. Papilla of outer maxillary lobe bifurcate, sublobal plate with 4 appendages (Fig. 31). Maxilla with lamella 1 narrow, surpassing the tip of capitulum, with cilia confined to external margins (Fig. 32). Labial palps with a full complement of papillae and 3 proximal setae; papillae E with blunt lateral process and 7 guards, e7 detached from papilla (Fig. 34); labial triangle with 5 anterior and 4 posterior setae; distribution of postlabial setae in columns I, C, E and L as 4, 3, 2, 3 (Fig. 35). Body dorsally covered by smooth hairs; some hairs on the pre-posterior row reaching base of setae on posterior row; thorax without ventral setae; axial setae on Th. 2-Abd. 3 as 6-8, 6-7//4-5, 4-5, 4-5; Th. 3 with 20-22 setae on posterior row; microsensillar and sensillar formulae 11//111 and 33//22224, respectively (Figs. 33, 36); lateral sensilla on Abd. 5 swollen (Fig. 36), all sensilla inserted anterior to posterior row of setae, tergal macrochaetae smooth, poorly differentiated, distributed as 11//11124; Abd. 5≈ 3.2X medial macrochaeta. Proximal and medial subcoxae on legs 1-3 with 1, 1; 3(4), 6(7); 5(4, 6, 7), 7(6, 8) setae (Fig. 38). Lateral valve of Abd. 6 similar to Ballistura joycei, with 3 hr setae. Tibiotarsal whorl B complete; male metatibiotarsal setae x and B5 thin, short, bothriotrica-like, with modified sockets (Fig. 37); legs 1-3 with 1, 2, 2 tenent hairs as in Ballistura joycei, but all tenent hairs acuminate, A1 on L2 sometimes appearing weakly clubbed. Unguis and unguiculus toothless (Fig. 37). Ventral tube with 3+3 apical setae, posterior face with 1 basal and 4 distal setae. Tenaculum with 4+4 teeth and 1 seta. Anterior and posterior furcal subcoxae with 12-18 and 7-8 setae, respectively. Proportion manubrium/dens/mucro as 6/4/1. Chaetotaxy of furcula as in Figure 39: manubrium with 17-18 dorsal and 1 ventral setae; dens weakly crenulated, with 9 dorsal and 6 ventral setae. Mucro bidentate, subapical tooth longer than apical (Fig. 40).
Scutisotoma champi sp. n. is unique among Scutisotoma species in having a lamelate bidentate mucro, 4 tenacular teeth, 9 dorsal and 6 ventral setae on dens, and maxillar lamela 1 longer then the capitulum. The new species is most similar to the Central Asian Scutisotoma acorrelata Potapov, Babenko & Fjellberg, 2006 and Scutisotoma tenuidentifera Potapov, Babenko & Fjellberg, 2006, from which it can be distinguished by the characters listed in Table 4. Among North American species, Scutisotoma champi sp. n. is most similar to Scutisotoma titusi (Folsom), 1937 from which it can be easily distinguished by the number of ventral setae on dens and the number of tergal sensilla on Abd. 4 and 5 (2+2, 4+4 in champi, 7-8+7-8, 8-12+8-12 in titusi).
Scutisotoma champi sp. n. is the most common species found in sandy beaches on the northern 2/3 of Lake Champlain as well as Lake Willoughby and Greater Averill Pond, and it is likely present in most if not all lakes and large ponds in northern Vermont and southern Quebec. The species was collected in apparently healthy beaches (e.g., Pearl Bay, locality H), as well as on highly disturbed, strongly compacted beaches (e.g., Colchester Beach, locality C). The species is most abundant in beaches with aquatic plant litter, but it is also found in sand in beaches without visible surface plant remains.
http://species-id.net/wiki/Pachyotoma
USA, Vermont, Chittenden Co., Bolton, Camel’s Hump ≈1200-1230 m elevation, 7 August 1972, W. Rittenhouse, coll. Two slides, one with three individuals, the other with one individual. These are the individuals originally determined as Ballistura alpa by
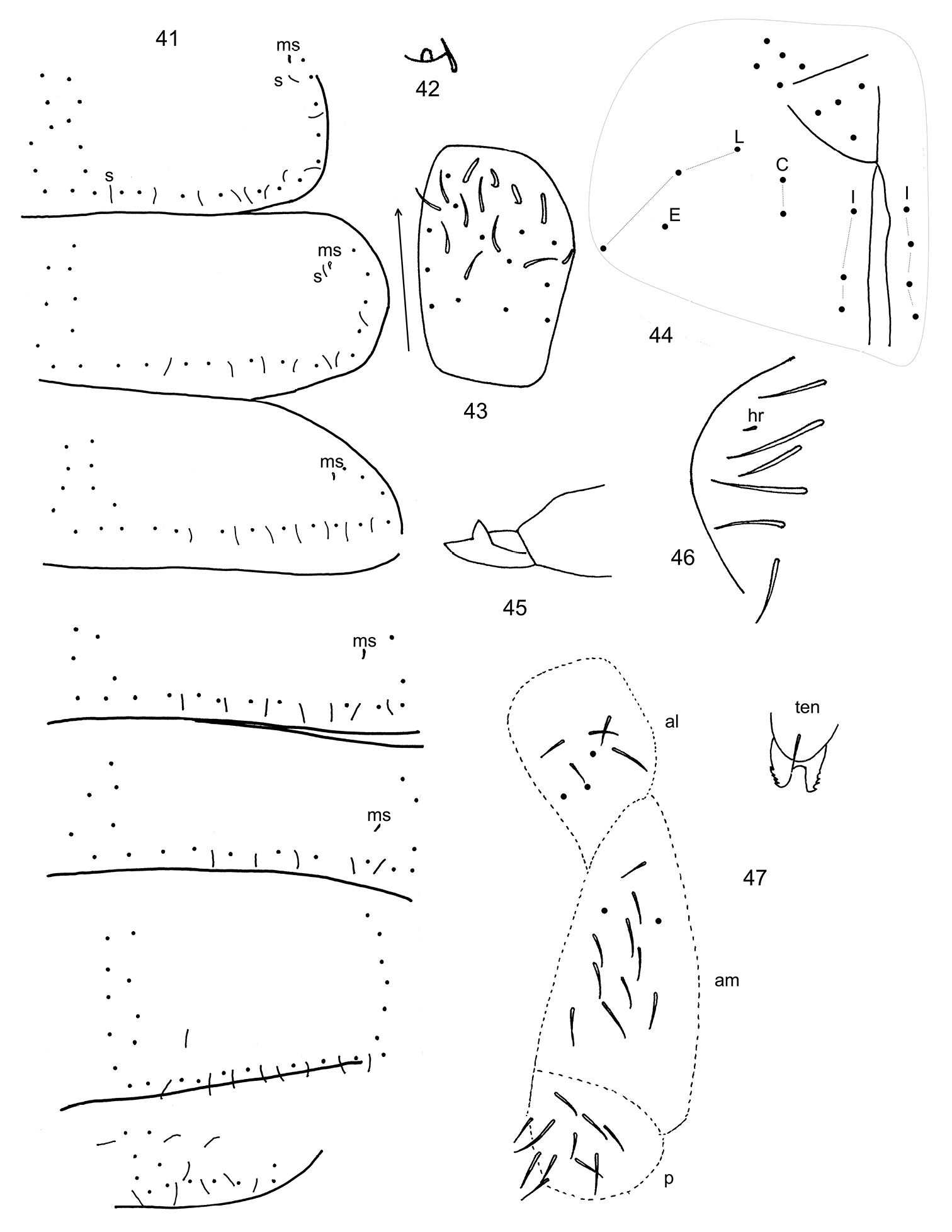
The following notes serve as a complement to the original description. Cuticle covered by many secondary granules. Organite of Ant. 4 capitate, guard sensilla not strongly modified (Fig. 42). Ant. 3 sense organ, lateral and supplementary sensilla as in Fig. 43. Ant. 2 with 1 distal sensilla and at least 1 basal microsensilla. Ant. 1 with 2 weakly modified basal microsensilla, 3-4 sensilla, and 12-14 setae. Eye patch with 3 setae, PAO with 2 guard setae. Labral formula 4/5, 5, 4. Apical seta of outer maxillary lobe bifurcate, sublobal plate with 4 appendages. Maxillary lamellae not clearly seen, but lamella 1 apparently surpassing tip of capitulum and apically acuminate (lateral margins converging well before tip of lamella), with cilia only along lateral margins. Labial papilla E with 7 guard setae, e7 detached from papilla as in Scutisotoma champi. Labial triangle with 5 anterior and 4 posterior setae. Postlabium with 3(4), 2, 1, 3 setae in columns I, C, E and L, respectively (Fig. 44). Body setae smooth and short, tip of seta on anterior rows not reaching base of setae on posterior rows. Thoracic sterna without hairs. Axial setae on Th. 2-Abd. 3 as 3-4, 5//4, 2-3, 2-4; Th. 3 with 18-20 setae on posterior row; microsensillar and sensillar formulae as 11//111 and 8(10)8//7758(9)7, respectively (Fig. 41); Th. 2 with 2 lateral sensilla inserted anterior to medial sensilla; medial sensilla on all segments inserted on p-row; Abd. 5 sensilla not swollen. Tergal macrochaetae undifferentiated. Proximal and medial subcoxae on legs 1-3 with 1, 0; 4(5), 4; 5(7), 4 setae. Lateral valves of Abd. 6 similar to Ballistura rossi, with 1 hr seta (Fig. 46). Tibiotarsal whorl B complete; legs 1-3 with 1, 2, 2 tenent hairs, all acuminate. All unguis with 1 inner tooth; unguiculus toothless, without terminal filament. Ventral tube with 7+7 apical setae, posterior face with 2 basal and 4 distal setae. Tenaculum with 4+4 teeth and 1 seta. Anterolateral, anteromedial and posterior furcal subcoxae with 7/8(6, 10), 11 (12, 13) and 7-11 setae, respectively (Fig. 47). Manubrium with 15 dorsal and 0 ventral setae. Dens granulate, with 7-8 dorsal and 6 (5) ventral setae. Mucro bidentate, subapical tooth longer than apical (Fig. 45).
This species was originally placed in the genus Ballistura, but the presence of a complete whorl B on pro- and mesothoracic legs and four prelabral setae excludes it from that genus (
Scutisotoma champi sp. n. 29 Habitus 30 Eye patch and PAO 31 Outer maxillary lobe 32 Frontal aspect of maxilla 33 Dorsal chaetotaxy of Th. 2-Abd. 1, s= sensilla, ms= microsensilla, M= macroseta 34 Ventral aspect of labial palp, terminal process of papilla A, C and E omitted 35 Labial and postlabial chaetotaxy 36 Dorsal chaetotaxy of Abd. 3-Abd. 5 37 Male metathoracic leg, lateral view 38 Chaetotaxy of pro-, meso- and meta thoracic subcoxae, from left to right, respectively 39 Dorsal (lower half) and ventral (upper half) chaetotaxy of furcula 40 mucro.
Pachyotoma alpa (Christiansen & Bellinger) 41 dorsal chaetotaxy of Th. 2-Abd. 5, s= sensilla, ms= microsensilla 42 Ant. 4 subapical sense organ 43 Ant. 3 sense organ and associated sensilla, arrow points anteriorly 45 Mucro 46 Lateral anal valve 47 Chaetotaxy of anterolateral (al), anteromedial (am) and posterior (p) furcula subcoxae and their position relative to tenaculum (ten).
Comparison between Scutisotoma champi sp. n. and similar species.
| Species Character | Scutisotoma champi sp. n. | Scutisotoma acorrelata Potapov, Babenko & Fjellberg | Scutisotoma tenuidentifera Potapov, Babenko & Fjellberg | Scutisotoma titusi (Folsom) |
|---|---|---|---|---|
| Maxillary Lamella 1 | longer than capitulum of maxilla | shorter than capitulum of maxilla | shorter than capitulum of maxilla | shorter than capitulum of maxilla |
| Abd. V Anteriormost Sensilla | s2 | s3 | s3 | s1 |
| Ventral Tube Distal Setae | 3 | 4 | 4 | 5-7 |
| Tenacular Teeth | 4 | 3 | 3 | 4 |
| Dorsal Setae on Dens | 9 | 10-11 | 6-7 | 14-15 |
| Number Mucronal Teeth | 2 | 2 | 3 | 3 |
We thank Bruce Parker, Entomology Research Laboratory, and Jim Vigoreaux, Department of Biology, for providing space at the University of Vermont during the initial phase of this study. We thank Natasha Bencivenga for assistance sorting samples. Tim Hunter and Eric Smeltzer helped locating sandy beaches on Lake Champlain. The field collection phase of this work was supported by funds from the Lake Champlain Research Consortium and the URECA! program at the University of Vermont. We also thank the two reviewers of the original manuscript, whose suggestions proved critical in the generic assignment of the species discussed here.