(C) 2012 Ekaterina Yegorenkova. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The larval instars of Pnigalio gyamiensis Myartseva and Kurashev are described in detail for the first time. This species is a larval-pupal ectoparasitoid of Chrysoesthia sexguttella (Thunberg) (Lepidoptera, Gelechiidae), which forms leaf mines in the plant Chenopodium album L. (Caryophyllales: Amaranthaceae). The female of Pnigalio gyamiensis lays a single egg on the skin of the host larva or nearby it, without any significant preference for a particular variant. The presence of long hairs on its body provides the newly-hatched first larval instar with high mobility. Some peculiarities in this parasitoid-host relationship are described.
Chalcidoidea, Eulophidae, Pnigalio gyamiensis, Chrysoesthia sexguttella, preimaginal morphology, physiological larval functions
Five species of the genus Pnigalio Schrank were reared from larva of Chrysoesthia sexguttella (
Species belonging to Pnigalio are ectoparasitoids, with solitary or gregarious larval development; most of them are polyphagous, feeding on several species of leaf miners (
Several species of Pnigalio are poorly morphologically characterized and difficult to identify. Consequently, in 2005
Our reared species is morphologically identical to Pnigalio gyamiensis. The DNA sequencing of the Pnigalio gyamiensis paratype was analyzed and revealed to be identical to the DNA sequence of Pnigalio soemius samples from Chrysoesthia sexguttella larvae on Chenopodium album and Atriplex putula. Pnigalio gyamiensis is genetically and biologically well characterized and its taxonomic validity has been confirmed (
The preimaginal stages of Pnigalio gyamiensis have never been described. The aim of this work was thus to describe morphologically the preimaginal stages, especially the larval instars; to describe any differences between the physiological functions of each of them; and to elucidate the biological strategies of solitary parasitoids developing inside leaf mines.
Materials and methodsPnigalio gyamiensis was reared without any other parasitoids from Chrysoesthia sexguttella on Chenopodium album L. (Caryophyllales: Amaranthaceae). The reared material was studied for the preimaginal stages. Samples of leaf-mines were regularly collected from five different localities in the city of Ul'yanovsk (54°16'N; 48°20'E), (separated from each other by no more than 3 km), between June and mid-September 2009. The food plant of Chrysoesthia sexguttella in the Middle Volga region is Chrysoesthia album. This plant is widespread, found in fields, gardens, and along roads and paths. Chrysoesthia sexguttella has two generations in this area: the first from May to July and the second from August to September.
In total, 500 leaf mines were collected, from which were reared 224 individuals of Chrysoesthia sexguttella and 25 specimens of Pnigalio gyamiensis. There was only one reared parasitoid of Pnigalio gyamiensis. The first generation of Pnigalio gyamiensis was reared from mid-June to early July, and the second generation from the end of July to mid-September. Mines from the leaves were stored individually in the laboratory in small glass tubes covered by several layers of wet gauze. In the present study, parasitoids were reared at 25° ± 2°С.
When mines were opened and photographed, this often prevented further development of parasitoid larvae. The total number of observations was 170. The number of observation of each larval instar is given below. In order to assess significant differences we used a non-parametric Fisher's exact test.
Video and photos of larval stages were recorded using a Canon Power Shot A-640. Light microscopy was carried out using a MC-2 ZOOM connected to a digital camera and a Mikromed microscope.
Abbreviation: F1–F4 – length of 1st, 2nd, 3rd and 4th segments of antennal funicle; SMV – submarginal, MV – marginal, PMV – postmarginal and SV – stigmal veins of forewing. Zoological Institute, Russian Academy of Science, St. Petersburg, Russia (ZISP).
Taxonomic surveyhttp://species-id.net/wiki/Pnigalio_gyamiensis
Our reared specimens were compared with type material (Zoological Institution of Russian Academy of Sciences, St. Petersburg, Russia (ZISP): “Holotype, female, Gami, 3 km W from Ashgabat, ex larva Chrysoesthia sexguttella on Atriplex sp., 13.10.1986 (Saparmamedova) Myartseva, Kurashev, 1990”, two female paratypes with the same label, and one female with label “Gami, 3 km W from Ashgabat, ex larva Chrysoesthia sexguttella on Atriplex sp., 30.10.1986 (Saparmamedova) Myartseva, Kurashev, 1990”.
Morphological diagnosis is based on a study of the type material.
Body length 1.08–1.80 mm; F1 1.1–1.3 times as long as F2; F2 1.1–1.2 times as long as F3; F3 1.0 times as short as F4; F4 1.3 times shorter than clava; callus of propodeum with 2 rows of setae: 1 row with 10–12 setae, 2 with 4 setae; sculpture of mesoscutum areolate and size of seta larger than that in scutellum. Forewing 2.3–3.5 times as long as broad; SMV 1.3–1.6 times shorter than MV; MV 1.8–2.7 times longer than PMV; PMV 2.0–3.3 longer than STV; gaster 1.5–1.8 times as long as broad. Body dark blue, the gaster brown with yellow tick at base, legs completely yellow with dark brown last segment of tarsi, hind coxae yellow with brown bracket (in the base of the coxa).
Seventeen females and eight males of Pnigalio gyamiensis reared by authors are labelled: “Ul'yanovsk, left bank of the river Volga, Verhnaya Terrasa, 56°49'N; 49°44'E, 15 June–8 August 2009 (Yegorenkova)”.
Our species belongs to Pnigalio gyamiensis and its morphological variability is less high.
Body length 1.35–1.80 mm; F1 1.2–1.3 times as long as F2; F2 1.1 times as long as F3; F3 1.0–1.1 times as short as F4; F4 1.3–1.4 times shorter than clava; forewing 2.5–2.8 times as long as broad; SMV 1.5–1.6 times shorter than MV; MV 1.7–1.8 times longer than PMV; PMV 2.7–2.8 longer than STV; gaster 1.4–1.5 times as long as broad. Body dark green with metallic tint, gaster with yellow tick or spot in the base of the gaster, legs completely yellow with dark brown last segment of tarsi, hind coxae mostly yellow without brown bracket. Male (first description): body length 1.25–1.38 mm; thorax 1.6 times as long as broad; pronotum 1.7 times as broad as long; sculpture of scutellum is the same as that of the mesoscutum; propodeum 2.3 times as broad as long; gaster 1.7–1.8 times as long as broad. Colouring is the same as in the female, sometimes hind femur and fourth tarsal segments darkened.
Turkmenistan (Myartseva, Kurashev, 1990), Italy (
Larval solitary ectoparasitoid.
Egg
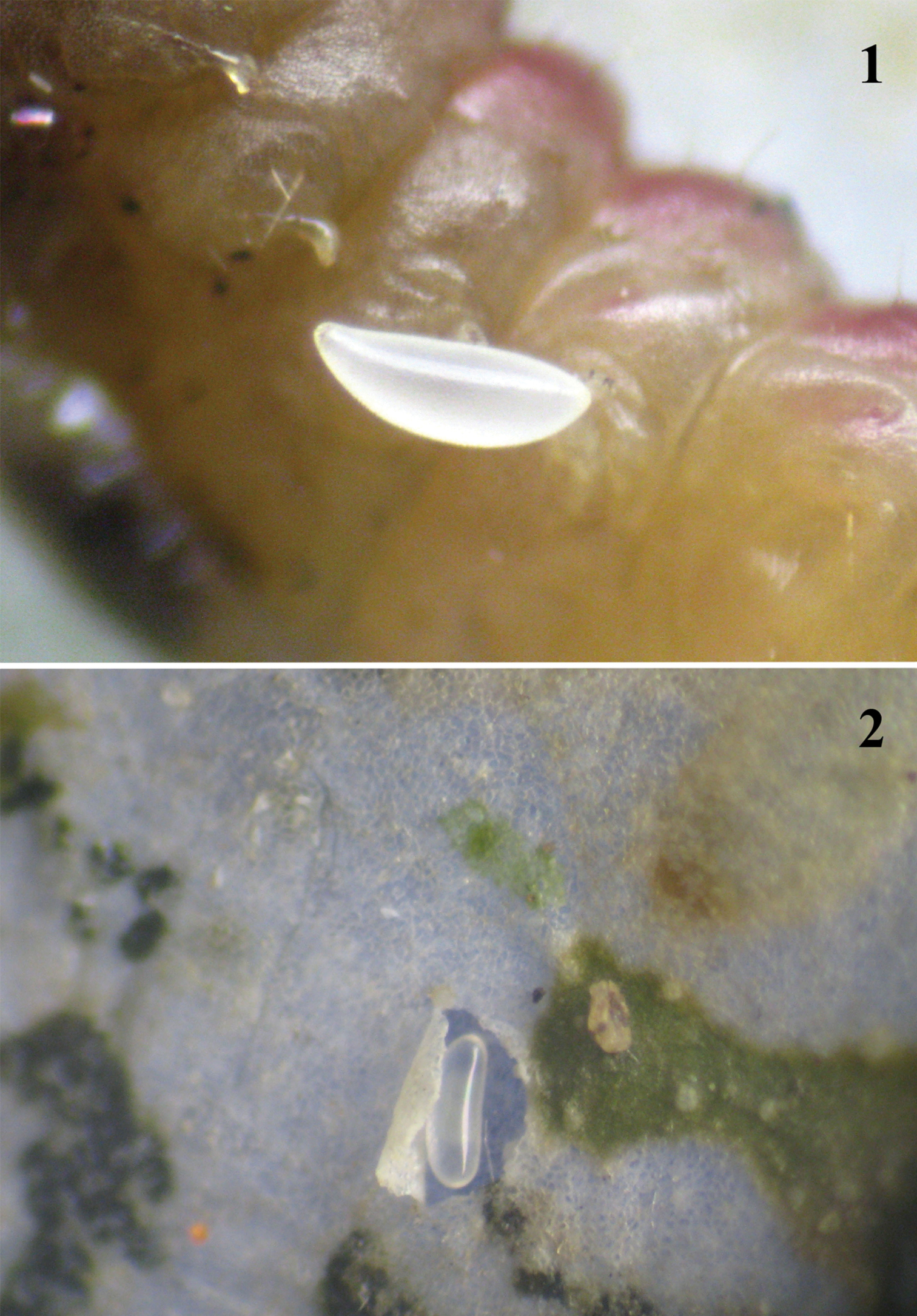
The shape of the egg changes during development of the embryo. The egg (Figure 1) just laid by a female of the parasitoid is oblong, both ends are rounded, with one a little broader than the other. The egg is white and shiny without sculpture. As the embryo develops, the egg becomes oval. Such eggs were either found beside the host (larva of dead Chrysoesthia sexguttella) (Figure 2) or lying on the surface of the host's cuticle. An egg with a fully developed embryo is elongate (Figure 3).
1 A recently laid egg of the female of Pnigalio gyamiensis, on the IV–V segments of the second larval instar of Chrysoesthia sexguttella (ventral view) 2 Pnigalio gyamiensis egg with developing embryo in an opened mine of Chrysoesthia sexguttella beside the host remains.
Eggs of species of the genus Pnigalio were previously studied by
1st instar larva
Morphology. The larva has 13 distinct segments including the extended head, which is 1.3 times as long as the second thoracic segment. The head capsule is dark yellow with one brown mandibular tooth that is used to puncture the cuticle of the host (Figure 4). The shape of the mandible is triangular. Chaetotaxy: the head capsule is covered by hairs. The body has 2 lateral rows of protuberances on segments II, III, IV, VI, VIII, X, XII and XIII, each with long hairs (total 32 hairs). Length of the hairs is equal to the diameter of the abdominal segments. Long hairs are at an angle of 45° to the body and at an obtuse angle to each other. The first dorsal hairs bend towards the head while the last ones bend back towards the anus.
. 3 Pnigalio gyamiensis egg with developing embryo on IV–V segments of Chrysoesthia sexguttella larva (ventral view) 4 1st instar Pnigalio gyamiensis larva in the mine of Chrysoesthia sexguttella
Behaviour. The larva is very active and quickly moves in the mine by means of muscle contractions, which are clearly visible. The parasitoid larva may feed on the haemolymph but does not do so solely. During observation of this instar it was noted that the larva punctured (drilled into) the cuticle of the host anticlockwise, thereby gaining access to the haemolymph. We observed such larva externally on the host larva and in a mine without a host, where it was probably searching for a host. At the end of this instar the larva becomes less active and moults to the second instar on the surface of the host's body (33 observations).This stage lasts on average 3.8 ± 0.7 days.
2nd instar larva
Morphology. The second instar larva is larger than the first, less active, and the body is segmented (Figure 5). Pulsation of the gut becomes distinct, and the food (firstly pale yellow and later on darkened) is moved to the anal part of gut. This larva moults to the 3rd instar on the surface of the host and the larva's head loses any distinctive shape as well as its hairs.
Larva of Chrysoesthia sexguttella (dorsal view) parasitized by 3rd instar larva of Pnigalio gyamiensis 6 3rd instar Pnigalio gyamiensis larva (protuberances are visible, ventral view).
Behaviour. In contrast to larva of the 1st instar that leaves the host several times, this 2nd larva stays on the host, feeding almost entirely on the haemolymph. The 2nd larval instar spends much longer periods feeding than that of the 1st instar larva, resulting in a rapid increase in size. We observed siblicide behavior between the 2nd larval and 4th larval instar of Pnigalio soemius (Figure 7). It means the 2nd larval instar begins to feed on the haemolymph of 4th instar larva (28 observations).This stage lasts on average 2.9 ± 0.6 days.
7 Siblicide behavior exhibited by 2nd and 4th larval instars of Pnigalio gyamiensis on a larva of Chrysoesthia sexguttella (dorsal view) 8 4th larval instar of Pnigalio gyamiensis on a larva of Chrysoesthia sexguttella inside its mine.
3rd instar larva
Morphology. The 3rd instar larva (Figure 6) has distinct protuberances in some segments (II–IV thoracic, VI, VIII, X and XII abdominal, and XIII anal). Length of a protuberance is equal to its width. Each protuberance has hair 1.5 times as long as length of protuberance.
Behaviour. The larva is actively feeding at this stage and its gut reveals a visible pulsation. We did not observed siblicide behavior between two larvae of 3rd instar. This stage lasts on average 2.5 ± 0.7 days (20 observations).
4th instar larva / prepupa
Morphology. The 4th larval instar (Figure 8) lacks mobility and has nine visible pairs of spiracles of the respiratory system on the II and III thoracic and I–VII abdominal segments. The fully fed larva has a dark brown gut that pulsates in one direction for 40 seconds and then in the opposite direction for 40 seconds. It is important to note that as the larva develops the frequency of pulsation decreases to a rate of 60 seconds in one direction and 60 seconds back. The prepupa loses segmentation and the body instead forms two sections between head and thorax and thorax and abdomen.
Behaviour. At the end of this stage the parasitoid larva leaves the host, stops feeding and loses mobility; its gut is full and equal to 75% of body weight. Antagonistic behavior by larvae against larvae of the same species was not observed; larvae of 4th instar may feed on the same host independently of each other (Figure 9) but might be attack by larvae of the 2nd instar (see Figure 7). This stage lasts on average 2.9 ± 0.6 days (24 observations).
9 Two 4th larval instar Pnigalio gyamiensis on a larva of Chrysoesthia sexguttella inside its mine 10 Prepupa of Pnigalio gyamiensis in an opened mine of Chrysoesthia sexguttella.
Pupa
Morphology. The pupa attaches to the leaf epidermis (Figure 10).The pupa is initially white or slightly yellow and then begins to darken to dark brown or black. The fully developed pupa of Pnigalio gyamiensis (Figure 11) has a metallic tint but the adult is never visible through the chitinized exuviae of pupa. Female pupa is recognizable by their large gaster and ovipositor visible through the light coloured cuticle of the gaster in the early stage of pupation, whereas the male pupa has a smaller gaster and darker colour.
11 Fully developed pupa of Pnigalio gyamiensis in a mine of Chrysoesthia sexguttella (dorsal view) 12 Mandibles of larval instars of Pnigalio gyamiensis: top row mandibles of 1st and 2nd larval instars, bottom row mandibles of 3rd and 4th larval instars.
The pupa is situated inside the mine ventrally to the leaf's surface. It develops on average 5.4 ± 0.8 days (28 observations).
The total duration of development is 19.8 ± 1.2 days.
Behaviour. The adult exits through the oral cavity of the pupa often in the early morning. The adult begins to clean its antennae and head and then leaves the mine.
ConclusionPnigalio gyamiensis presents four larval instars and the three moults are easy recognizable. The 1st instar larva is clearly visibly by the presence of long hairs on its body. The mandibles are very small and curved, and used to hook onto the cuticle of the host (Figure 12). Some authors have noted a difficulty in differentiating larval instars, such as in Hyssopus pallidus Askew (Tschudi-Rein and Dorn 2001), with the only discernible differences being in the shape and size of the mandibles. They did not report the long hairs on the body that the larva uses for moving across the surface of the host or inside the mine. The 2nd larval instar loses these long hairs and moves slowly; it is recognizable by its mandibles. The mandibles of 2nd, 3rd and 4th instar larvae (Figure 12) differ in size but the 4th instar has one large, well-development tooth.
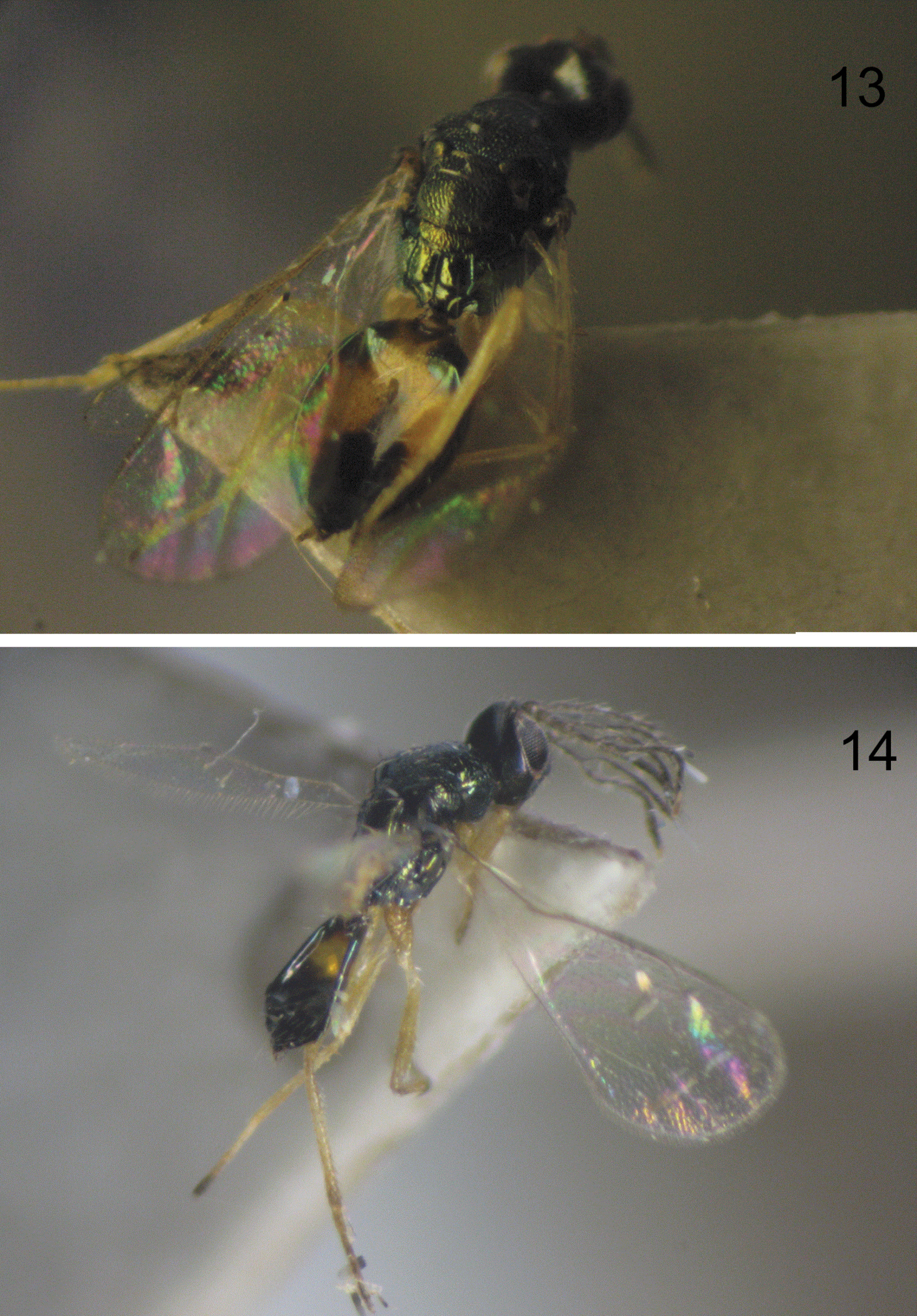
The emerged adults (both sexes) are shown in Figure 13, 14. The female parasitoid Pnigalio gyamiensis paralyses the larva Chrysoesthia sexguttella, which loses mobility, stops feeding and dies. The parasitoid larva then feeds on the killed host. Only a few cases were observed of the parasitoid female having laid an egg on the skin of a dead host larva; but in each case the parasitoid larva developed successfully. The female hid her egg on the skin of the host larva or near it without significant preference for any of the variants. The high mobility of the 1st instar allows the larva to find a host quickly and begin to feed.
13 Emerged female of Pnigalio gyamiensis 14 Emerged male of Pnigalio gyamiensis.
We thank in particular Dr Sergey Sinev (Zoological Institution of Russian Academy of Science, St. Petersburg) for identifying the parasitoid host and for his consultation on the larval development stages of Chrysoesthia sexguttella. We thank Naomi Paz for her linguistic editing of the manuscript.