(C) 2012 Andrés Fabián Herrera Flórez. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the ichneumonid subfamily Labeninae, Apechoneura seminigra sp. n., is described. Specimens were collected from the Amazon Rainforest of Colombia.
Ichneumonoidea, Labenini, South America, Neotropics, nigricornis species-group , taxonomy
The Labeninae is a subfamily of Ichneumonidae containing approximately 150 described species classified in four tribes and 12 genera. Compared with other subfamilies, this group is quite well-known worldwide (
Three species of Apechoneura are found in Colombia (
During an undergraduate project focused on the subfamily Labeninae, 14 of the main entomological collections of Colombia were reviewed (view Appendix). The specimens described here are deposited in the insect collection of the Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Bogotá, Colombia. The nomenclatural treatment, morphological terminology and taxonomic characters used here follow
urn:lsid:zoobank.org:act:4CFB6077-71E1-42D0-92FB-77907767B3CC
http://species-id.net/wiki/Apechoneura_seminigra
Figures 1–13HOLOTYPE: Female, Colombia, Amazonas: Parque Nacional Natural Amacayacu Caño Mata Matá, 3°41'N, 70°15'W, Malaise trap, Martin Kelsey: 200 m, II-III.1989 (ICN 083474). PARATYPES: 1 female, same data as holotype (ICN 083472); 1 female, same locality, 300 m, 1.III.1988, bosque de tierra firme (ICN 083471).
Non-type material: 1 male, same locality, bosque de várzea (ICN 083473).
This species can be diagnosed from all other Neotropical Apechoneura by the combination of the following: head orange; mesosoma and legs mostly orange (hind leg partly black); metasoma black. Epicnemial carina absent. Metapleuron with a conspicuous sharp lateral denticle. Hind wing with first abscissa of Cu1 0.2× as long as cu-a.
Female. Fore wing length 15.0 mm.
Head. Clypeus almost flat, with a weak transverse ridge near apex; malar space 0.6× as long as basal mandibular width; lower face at narrowest point 0.9× as wide as height from clypeofacial suture to level of insertion of antenna; hypostomal carina joined to occipital carina far from base of mandible; posterior ocellus separated from eye by 1.3–1.5× its own maximum diameter. Antenna with flagellomeres 1 and 2 subequal by length; subapical flagellomere slightly elongate.
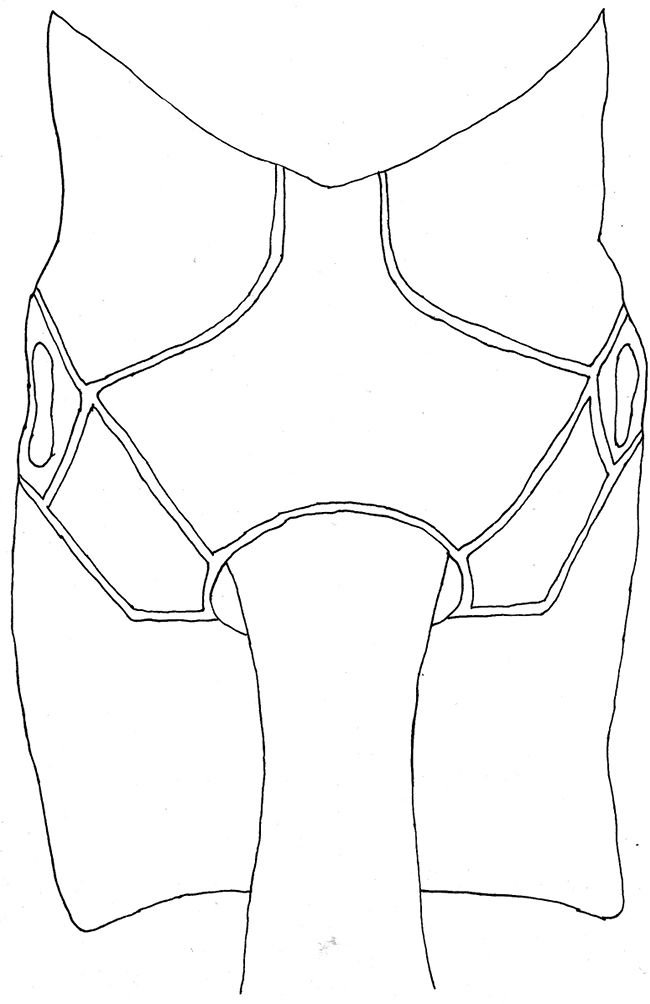
Mesosoma. Pronotum with upper hind margin swollen, forming a small conical projection; scutoscutellar groove broad and shallow; scutellum with three evident rugae posteriorly; epicnemial carina absent (Figs 1, 3, 5); sternal region of mesothorax smooth and polished; metapleuron with a rather conspicuous sharp lateral projection near posterior end; submetapleural carina narrow with a distinct low median denticle (Fig. 1). Propodeum in profile more or less flat; anterior transverse carina complete laterally, separating area spiracularis from area lateralis, mediodorsally incomplete so area basalis is not enclosed posteriorly; area basalis slightly transverse; lateromedian longitudinal carina not present behind anterior transverse carina (Figs 7, 9).
Legs. Fore leg with tibia slightly inflated, tarsus with long hairs on inner surface; mid leg with tibia bearing several stout spines.
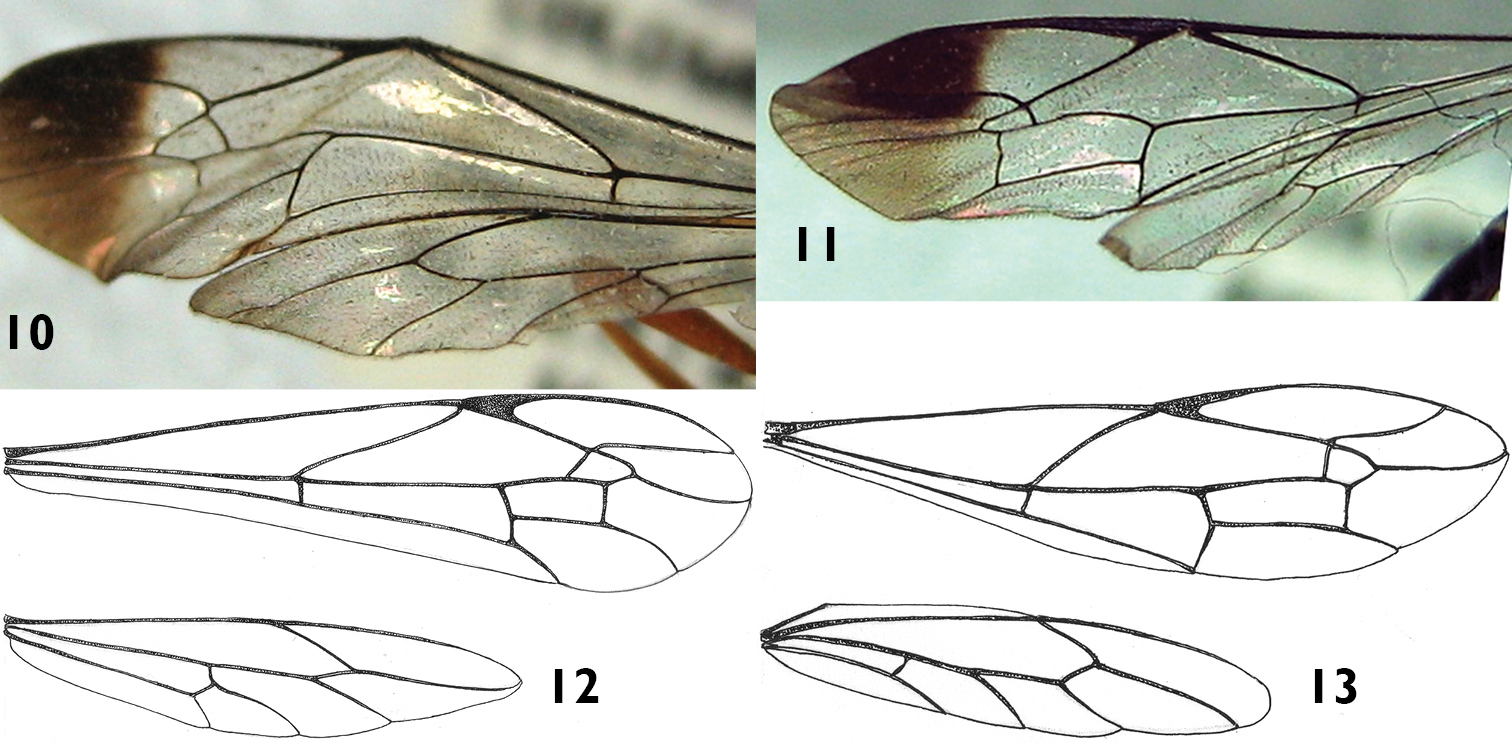
Wings. (Fig. 12) Fore wing with areolet large, anteriorly narrowly truncate, with 2m-cu joining it very slightly basal of middle; second discal cell short, with vein 1m-cu about half as long as abscissa of Cu1 between Rs&M and 1m-cu; hind wing with apical abscissa of Cu1 joining cu-a clearly closer to M than to 1A; first abscissa of Cu1 0.2× as long as cu-a.
Metasoma. Tergite 1 slender, 3.5–4.0× as long as posteriorly broad; sternite 1 short, reaching about 0.3–0.4 of length of tergite, with a median swelling centrally. Tergite 2 1.9–2.3× as long as posteriorly broad, with isolated pubescence; tergite 7 mediodorsally without an indentation posteriorly; tergite 8, in lateral view, tapered to a bluntly rounded apex, without a cornus, and with uniformly scattered pubescence; tergite 9 bearing long pubescence. Ovipositor, at rest extending beyond apex of metasoma by 3.5–3.8× the length of the metatibia.
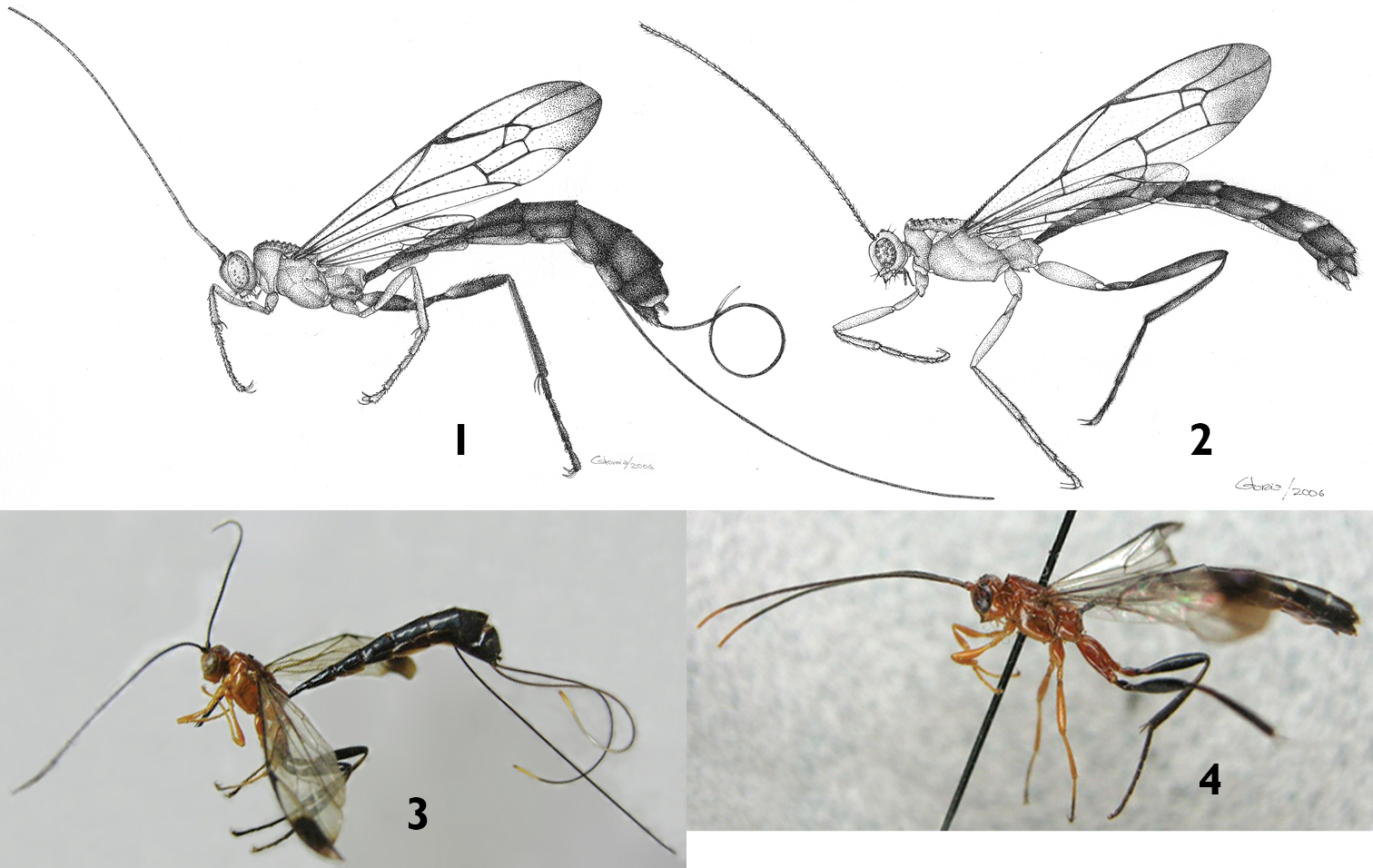
Color. (Figs 3, 5, 7, 10) Head orange; flagellum predominantly black, two basal flagellomeres ventrally reddish. Mesosoma orange. Fore and mid legs orange; hind leg with coxa orange with a ventro-lateral black spot on the apex of the outer side, trochanter and trochantellus black except for some small orange spots, femur, tibia and tarsus black. Metasoma black, hypopygium centrally orange. Ovipositor sheath black except for subapical whitish wide band. Fore wing hyaline, with a distinctive apical black band; pterostigma black.
Variation. The female identified with the code ICN 083471 has the fore wing with the areolet petiolate.
Putative Male. Similar to female in structure, but smaller (fore wing length 10.0 mm). Hind wing with apical abscissa of Cu1 arising from M apical to junction of M + Cu1 with cu-a (Figs 11, 13). Antenna black with apical flagellomeres pale (Fig. 4). Metasoma mostly black, tergites 1–6 with a yellow triangular spot at posterior margin (Figs 4, 8). Metapleuron with denticle smaller and paler than in the female.
The male exemplar was deteriorated during the drawing process. Its antenna was broken and lost.
The species name refers to its color (i.e. metasoma and most part of hind leg black).
Apechoneura seminigra sp. n., just like Apechoneura nigricornis, lacks an epicnemial carina; this characteristic separates them from the rest of the species of the genus. As Apechoneura nigricornis, Apechoneura seminigra sp. n. possesses a conical projection on the metapleuron and lacks an indentation on tergite 7. However, the metasoma is orange in Apechoneura nigricornis and black in Apechoneura seminigra sp. n. Also, in the hind wing, the first abscissa of Cu1 is 0.4× as long as cu-a in Apechoneura nigricornis and 0.2 in Apechoneura seminigra sp. n. Although these two species are rather similar morphologically, the difference in color pattern makes in this case their separation reliable.
Apechoneura seminigra sp. n. is so far only known from Colombia, Amazonian Region, northwest of Leticia. According to
Habitus of Apechoneura seminigra sp. n. 1, 3 female, holotype 2, 4 putative male 1, 2 line drawings 3, 4 photographs.
Apechoneura seminigra sp. n. 5, 7 female, holotype 6, 8 putative male 5, 6 Head, mesosoma and part of metasoma, lateral view 7, 8 head, mesosoma and part of metasoma, dorsal view.
Apechoneura seminigra sp. n., female, holotype - propodeum, dorsal view.
Wings of Apechoneura seminigra sp. n. 10, 12 female, holotype; 11, 13 putative male.
Edgard E. Palacio loaned to me the specimens that during the study belonged to his personal collection and deposited them into the ICN insect collection. Thanks to Carlos E. Sarmiento (ICN) for housing the type material. Gloria Echeverri from the Herbarium of Universidad de Antioquia (HUA) made the drawings (except Figure 9). Luciana Bueno dos Reis Fernandes edited the figures. Thanks to Ricardo Callejas, Fernando J. Muñoz-Quesada, Fernando Fernández, John Alveiro Quiroz, Diego Campos, Allan H. Smith, Víctor Hugo González and Juan Manuel Vargas who advised me during my undergraduate project. Also special thanks to the two reviewers for their helpful comments.
List of collections
- Instituto Humboldt (Acronym: IAvH-E)
- Insect Collection, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá (Acronym: ICN)
- Museo de Entomología “Francisco Luis Gallego”, Universidad Nacional, sede Medellín. (Acronym: UNCM)
- Instituto de Biología, Universidad de Antioquia, Medellín. (Acronym: CEUA)
- Facultad de Agronomía, Universidad Nacional de Colombia; Bogotá (Acronym: UNAB)
- Museo de Historia Natural, Pontificia Universidad Javeriana; Bogotá (Acronym: MUJ)
- Corporación para Investigaciones Biológicas; Medellín (Acronym: CIB)
- Universidad Pedagógica Nacional; Bogotá (Acronym: UPNC)
- Edgard Palacio Insect Collection- Colección Personal; Bogotá (Acronym: EPIC)
- Museo Universidad La Salle, Bogotá. (Acronym: U La Salle)
- Colección Entomológica “Luis María Murillo”, Instituto Colombiano Agropecuario, Tibaitabá. Bogotá (Acronym: CELM)
- Museo de Ciencias Naturales, Colegio San José. Medellín, Barrio Boston. (Acronym: CSJ)
- Colección entomológica Piedras Blancas (Comfenalco) (Acronym: CEPB)
- Colección personal del profesor Oscar E. Ortega M. (Oficina- Unalmed) (Acronym: OOCP)