(C) 2011 Karen Ober. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Populations of the ground beetle Scaphinotus petersi are isolated in subalpine conifer forest habitats on mountain ranges or Sky Islands in southeastern Arizona. Previous work on this species has suggested these populations have been isolated since the last post-glacial maximum times as warming caused this cool adapted species to retreat to high elevations. To test this hypothesis, we inferred the phylogeny from mitochondrial DNA sequence data from several Arizona Sky Island populations of Scaphinotus petersi and estimated the divergence time of the currently isolated populations. We found two major clades of Scaphinotus petersi, an eastern clade and a western group. Our results indicated most mountain ranges form clades except the Huachucas, which are polyphyletic and the Santa Catalinas, which are paraphyletic. We estimated the Pinaleño population is much older than the last glacial maximum, but the Huachuca and Pinal populations may have been fragmented from the Santa Catalina population since the post-glacial maximum times.
carabid ground beetles, divergence dates, phylogeography
Carabidae (ground beetle family) is one of the larger families of insects with approximately 40, 000 described species (
The Sky Islands (
The goal of this study was to infer the biogeographic history of Scaphinotus petersi in southeastern Arizona and investigate how the paleoclimatic oscillations of Quaternary affected the distribution of populations in the Sky Islands. We present a preliminary genealogy of mitochondrial DNA (mtDNA) sequences and use these data to address questions about population structure of this species and examine the potential role of the Pleistocene climate changes in the differentiation some of the Sky Island populations of Scaphinotus petersi.
Methods DNA sequence dataWe collected DNA sequence data from 45 specimens of four of the six subspecies of Scaphinotus petersi in five localities in four mountain ranges (Table 1, Fig. 1). We included three outgroup species from the tribe Cychrini. One species of a related genus Sphaeroderus, and two other distantly related Scaphinotus species. Outgroup choices were limited by material available for DNA analysis. Genomic DNA was extracted following the protocol outlined in
Specimens, collection localities, and GenBank numbers included in this study.
| Specimen | Collection locality | Specimen number | COI GenBank | ND1 GenBank |
|---|---|---|---|---|
| Sphaeroderus lecontei | MA: Worcester Co. Wachusett Reservior 71.6849°W, 42.4048°N 120m elev. |
001 | JN639333 | JN641890 |
| Scaphinotus crenatus | CA: Kern Co., Silvia Rd. 37°29.789'N, 119°53.369'W |
002 | JN639334 | JN641891 |
| Scaphinotus sp. | CA: Kern Co. Hwy 49A 37°22.806'N, 119°43.879'W |
030 | JN639335 | JN641892 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Columbine Corral Camp/Ash Creek 32.7065°N, 109.9131°W elev. 2904m |
040 | JN639336 | JN641893 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Ladybug Trail 32.6589°N, 109.8540°W elev. 2716m |
041 | JN639337 | JN641894 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Columbine Corral Camp/Ash Creek 32.7065°N, 109.9131°W elev. 2904m |
075 | JN639369 | JN641926 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Columbine Corral Camp/Ash Creek 32.7065°N, 109.9131°W elev. 2904m |
076 | JN639370 | JN641927 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Columbine Corral Camp/Ash Creek 32.7065°N, 109.9131°W elev. 2904m |
077 | JN639371 | JN641928 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Columbine Corral Camp/Ash Creek 32.7065°N, 109.9131°W elev. 2904m |
078 | JN639372 | JN641929 |
| Scaphinotus petersi grahami | AZ:Graham Co., Pinaleño Mts., Columbine Corral Camp/Ash Creek 32.7065°N, 109.9131°W elev. 2904m |
079 | JN639373 | JN641930 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
044 | JN639340 | JN641897 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
045 | JN639341 | JN641898 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
046 | JN639342 | JN641899 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
047 | JN639343 | JN641900 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
048 | JN639344 | JN641901 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
049 | JN639345 | JN641902 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
050 | JN639346 | JN641903 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
051 | JN639347 | JN641904 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
052 | JN639348 | JN641947 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
073 | JN639367 | JN641924 |
| Scaphinotus petersi biedermani | AZ: Cochise Co., Huachuca Mts., Carr Canyon Trail 31.4272°N, 110.3069°W elev. 2186m |
074 | JN639368 | JN641925 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Marshall Gulch 32.4279°N, 110.7052°W elev. 2432m |
042 | JN639338 | JN641895 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Marshall Gulch 32.4279°N, 110.7052°W elev. 2432m |
043 | JN639339 | JN641896 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
053 | JN639348 | JN641905 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
054 | JN639349 | JN641906 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
055 | JN639350 | JN641907 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
056 | JN639351 | JN641908 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
058 | JN639352 | JN641909 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
059 | JN639353 | JN641910 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
060 | JN639354 | JN641911 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
061 | JN639355 | JN641912 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
062 | JN639356 | JN641913 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
063 | JN639357 | JN641914 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
064 | JN639358 | JN641915 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
065 | JN639359 | JN641916 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
066 | JN639360 | JN641917 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
067 | JN639361 | JN641918 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
068 | JN639362 | JN641919 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
069 | JN639363 | JN641920 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
070 | JN639364 | JN641921 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
071 | JN639365 | JN641922 |
| Scaphinotus petersi catalinae | AZ: Pima Co., Santa Catalina Mts., Ski Valley 32.4507°N, 110.7789°W elev. 2499m |
072 |
JN639366 | JN641923 |
| Scaphinotus petersi petersi | AZ: Gila Co., Pinal Mts., Icehouse Canyon FTrail 198 33.2925°N, 110.8311°W elev. 2302.5m |
081 | JN639375 | JN641932 |
| Scaphinotus petersi petersi | AZ: Gila Co., Pinal Mts., Icehouse Canyon FTrail 198 33.2925°N, 110.8311°W elev. 2302.5m |
082 | JN639376 | JN641933 |
| Scaphinotus petersi petersi | AZ: Gila Co., Pinal Mts., Icehouse Canyon FTrail 198 33.2925°N, 110.8311°W elev. 2302.5m |
083 | JN639377 | JN641934 |
| Scaphinotus petersi petersi | AZ: Gila Co., Pinal Mts., Icehouse Canyon FTrail 198 33.2925°N, 110.8311°W elev. 2302.5m |
084 | JN639378 | JN641935 |
| Scaphinotus petersi petersi | AZ: Gila Co., Pinal Mts., Icehouse Canyon FTrail 198 33.2925°N, 110.8311°W elev. 2302.5m |
085 | JN639379 | JN641936 |
| Scaphinotus petersi petersi | AZ: Gila Co., Pinal Mts., Icehouse Canyon FTrail 198 33.2925°N, 110.8311°W elev. 2302.5m |
086 | JN639333 | JN641890 |
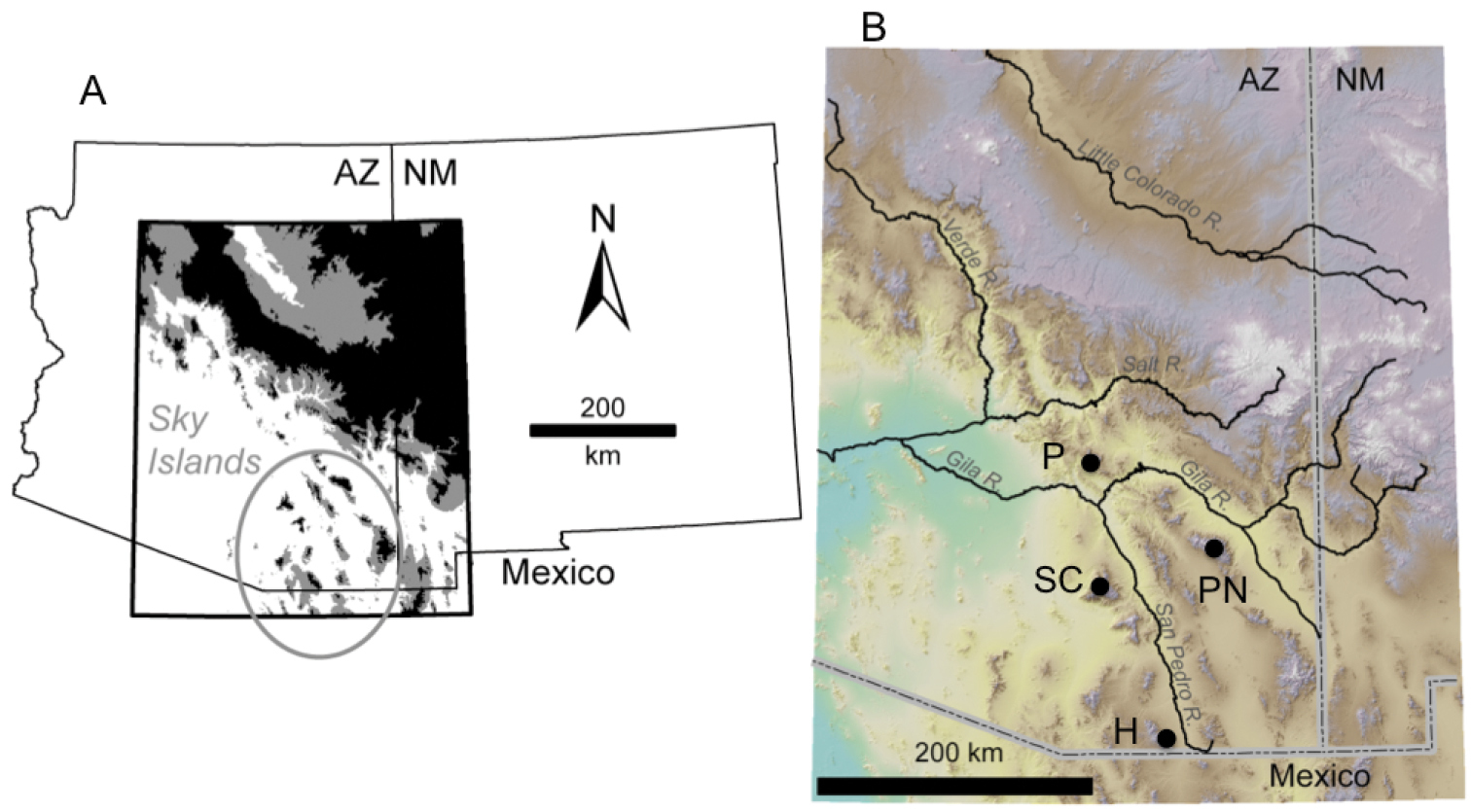
Study location A Scaphinotus petersi distribution is circled area. Habitat above 1830m is shown in black and between 1500 and 1830m is shown in grey B Shaded relief map of study area. Black dots denote sampling localities of Scaphinotus petersi used in this study (see Table 1) abbreviated as follows: P, Pinal Mountains; SC, Santa Catalina Mountains; PN, Pinaleño Mountains; and H, Huachuca Mountains. Figure courtesy of Sara Mitchell.
Phylogeographic patterns were examined by inferring phylogenetic relationships from mitochondrial sequence data from all specimens collected. The combined COI and ND1 data set (2678 characters) was partitioned in five unlinked subsets (COI pos 1 and 2, COI pos 3, ND1 pos 1 and 2, ND1 pos 3, mtRNA). Maximum likelihood models were selected using MODELTEST 3.7 (
Bayesian analyses were completed in MRBAYES 3.12 (
We inferred divergence dates of Scaphinotus petersi populations using a Bayesian relaxed clock uncorrelated lognormal method in BEAST (
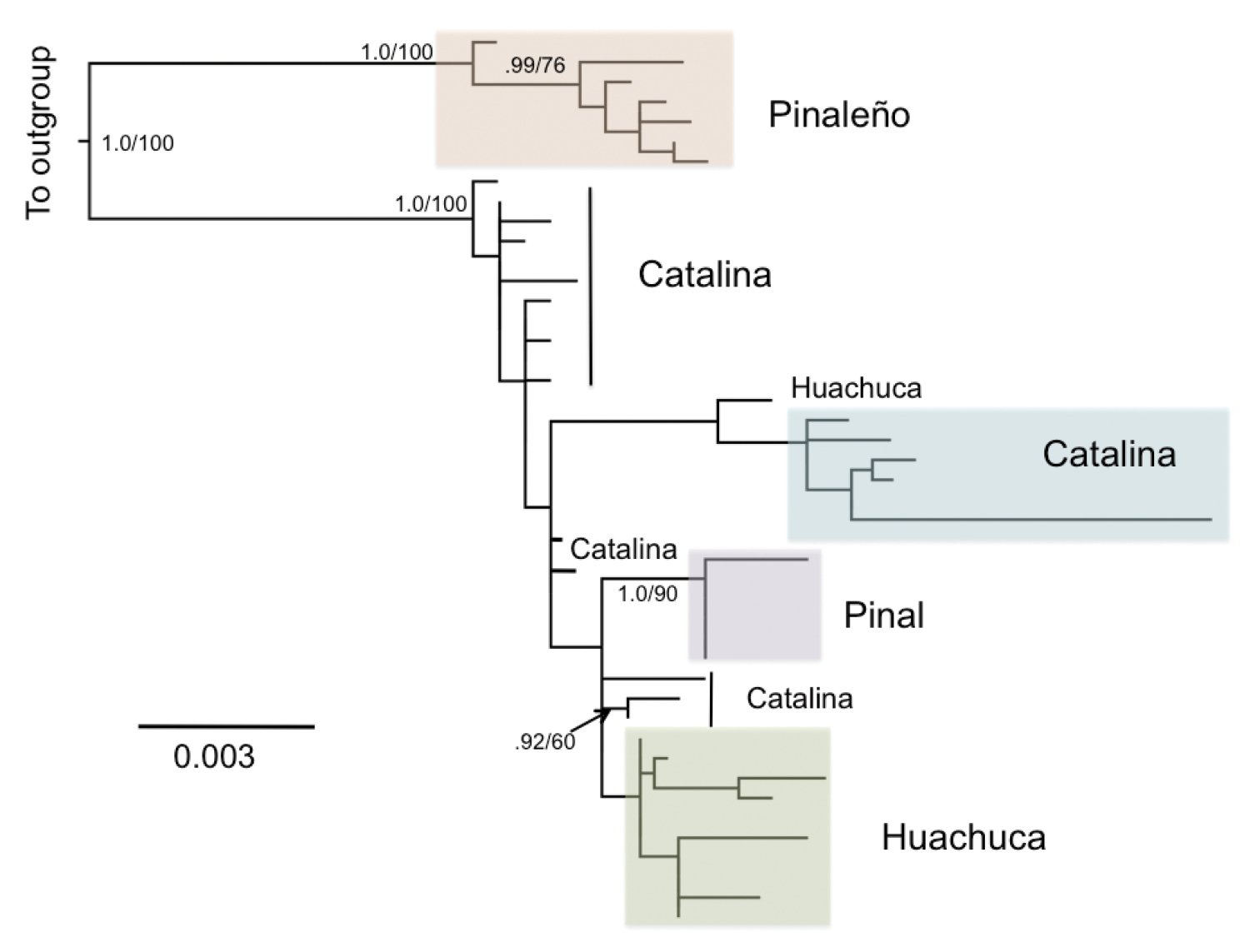
Both maximum likelihood and Bayesian analyses of mtDNA found similar topologies. The best maximum likelihood tree (Fig. 2) had a log-likelihood score of -6033.6277, and the Bayesian analysis converged on a set of trees with a mean log-likelihood score of -5797.5. Within a monophyletic Scaphinotus petersi, two well-supported major clades were identified, corresponding to geographic relationships between collection localities (Fig. 2) and spatially structured genetic variation at deep and shallow scales. A clade of Scaphinotus petersi grahami from the Pinaleño Mountains was clearly phylogenetically distinct from a western clade of Scaphinotus petersi from the Santa Catalina, Huachuca, and Pinal Mountains. The Santa Catalina population (Scaphinotus petersi catalinae) was paraphyletic with respect to a clade of Scaphinotus petersi petersi from the Pinal Mountains and Scaphinotus petersi biedermani from the Huachuca Mountains. The Scaphinotus petersi biedermani population did not appear to be monophyletic with one specimen grouping with members of Scaphinotus petersi catalinae from the Santa Catalina Mountains (Fig. 2). The overall phylogenetic tree topology estimate from GARLI and MRBAYES was similar to the BEAST analyses (Fig. 3).
Primers used for DNA amplification (PCR) and sequencing for the ND1 and COI mitochodrial genes.
| Gene | Primer | Direction | Sequence 5’ to 3’ |
|---|---|---|---|
| Cytochrome Oxidase I (COI) | SK (modification of TY-J-1460 (Simon et al. 1994)) | Forward | CGCTCTAGAACTAGTGGATCAANAAYCAYAARGAYATYG |
| Pat (L2-N-3014 (Simon et al. 1994)) | Reverse | TCCAATGCACTAATCTGCCATATTA | |
| Ron (C1-J-1751 (Simon et al. 1994)) | Forward | GGATCACCTGATATAGCATTCCC | |
| Nancy (C1-N-2191 (Simon et al. 1994)) | Reverse | CCCGGTAAAATTAAAATATAAACTTC | |
| NADH1 dehydrogenase (ND1) | ND1F | Forward | ACATGAATTGGAGCTCGACCAGT |
| 16sR (LR-N-12866 (Simon et al. 1994)) | Reverse | ACATGATCTGAGTTCAAACCGG |
Maximum likelihood tree of Scaphinotus petersi populations from combined COI and ND1 data. Outgroups are removed to show greater detail. Specimen numbers are removed, but the mountain range from which they were collected is indicated. Support for branches is indicated by Bayesian Posterior Probability/Maximum Likelihood bootstrap values. Scale bar units are substitutions per site.
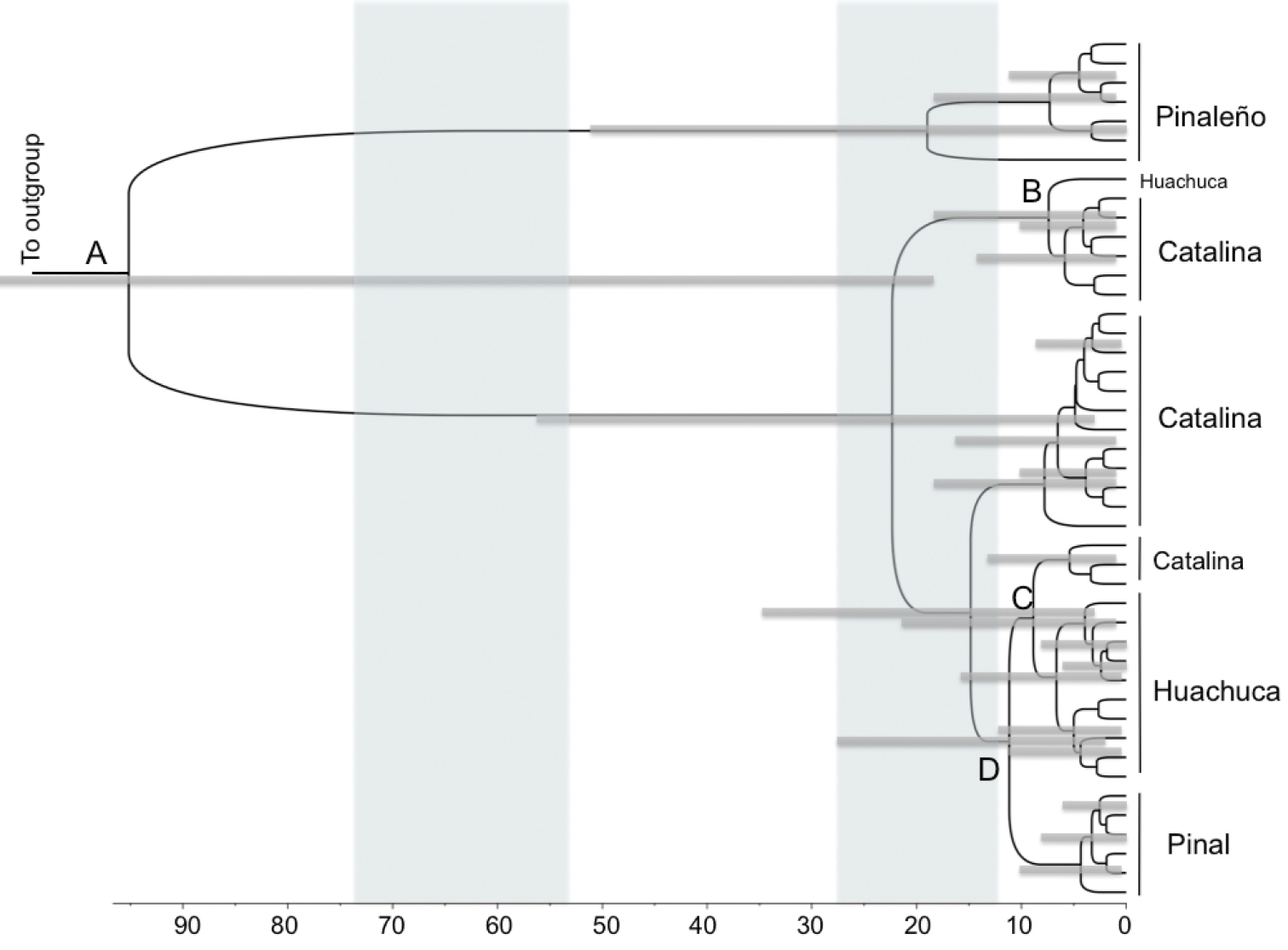
Phylogeny of Scaphinotus petersi dated using a Bayesian relaxed molecular clock in BEAST. Outgroups are removed to show greater detail. Specimen numbers are removed, but the mountain range from which they were collected is indicated. Branches are proportional to time in thousands of years. Shading indicates the two most recent glacial maxima. 95% confidence intervals for the ages of major clades in the tree are indicated with blue bars. The capital letters indicate population fragmentation between mountain ranges (see Table 3).
Divergence time estimates for mtDNA lineages from BEAST reveal a deep and complex history of diversification (Fig. 3 and Table 3). The Scaphinotus petersi grahami population in the Pinaleño Mountains diverged from the western populations in this study approximately 95, 200 years ago. The Scaphinotus petersi petersi population in the Pinal Mountains diverged from the Santa Catalina Mountain population approximately 11, 000 years ago. More than one dispersal event from the Santa Catalinas to the Huachucas may have occurred about 8, 900 years ago and also 7, 400 years ago (Fig. 3 and Table 3).
Ages of selected nodes estimated from molecular data in Scaphinotus petersi phylogeny from BEAST analysis. Letters correspond to nodes in Figure 3.
| Node | Split between populations | Age in years | 95% C.I. age in years |
|---|---|---|---|
| A | Pinaleño vs western populations | 95, 200 | 8, 000–225, 000 |
| B | Huachuca vs Catalina 1 | 7, 400 | 1, 200–18, 500 |
| C | Huachuca vs Catalina 2 | 8, 900 | 1, 500–21, 300 |
| D | Catalina vs Pinal | 11, 200 | 1, 800–28, 200 |
Our phylogenetic analyses indicated geographic and genetic structure within the Scaphinotus petersi, and most clades corresponded to isolated mountain ranges. There was strong support for two major clades in this species; an eastern clade of Scaphinotus petersi grahami from the Pinaleño Mountains and a western clade of Scaphinotus petersi petersi, Scaphinotus petersi catalinae, and Scaphinotus petersi biedermani from the Pinal Mountains, Santa Catalina Mountains, and Huachuca Mountains, respectively. While it appears the Pinaleño clade is reproductively isolated from the rest of Scaphinotus petersi, caution must be taken in interpreting genealogy patterns from mitochondrial data only, as it is a single locus and represents the maternal lineage only. The phylogenetic analyses suggested the Santa Catalina population is paraphyletic with respect to the Pinal and Huachuca populations that were derived from independent dispersal events from the Santa Catalinas. The history of the Huachuca population shows two relatively recent dispersal events from the Santa Catalinas to the Huachucas indicating there may have been suitable habitat in the past for low elevation Santa Catalina populations to migrate to the Huachucas. Based on morphological data,
In this study we sampled only four of the six subspecies of Scaphinotus petersi, and only a few of the known populations of Scaphinotus petersi petersi, Scaphinotus petersi biedermani, and Scaphinotus petersi grahami. Future work will include the additional subspecies and populations for a fuller picture of Scaphinotus petersi evolution and biogeography. We predict, with the inclusion of these samples, the phylogeography of Scaphinotus petersi subspecies will follow, in large part, Ball’s (1966) hypotheses of relationships based on morphological characteristics.
The distribution of genetic diversity in Scaphinotus petersi is structured across southeastern Arizona, indicating extrinsic barriers to gene flow are probably responsible for phylogeographic structure. It appears that a historical corridor of shared, linked habitat existed along a north – south ridge in the Western clade of Scaphinotus petersi enabling dispersal from the Santa Catalinas to the Huachuca and Pinal Mountains. This north – south ridge of connectivity pattern in biogeography has been seen in other Sky Island arthropods (
The divergence time estimates suggested the Pinaleño population (Scaphinotus petersi grahami) is considerably older than the end of the last glacial period, perhaps indicating that this population was isolated during previous interglacial events in the Pliocene and persisted during Pleistocene glaciations. The western populations of Scaphinotus petersi petersi from the Pinals and Scaphinotus petersi biedermani from the Huachucas have more recent divergence times, indicating that these areas were more recently isolated, perhaps since the end of the last glacial maximum (LGM). It is important to note that the error bars for the time estimates of nodes are large, making it difficult to pinpoint with certainty divergence dates and the impact particular changes in climate have had on population isolation. Additional loci could reduce variation in estimated time to coalescence.
Several studies have focused on the biogeography of species on the Arizona Sky Island region including plants, arthropods, birds, lizards, and mammals (
Both recent and more ancient global climate changes could be the causal mechanisms underlying the history of habitat fragmentation in Scaphinotus petersi. Our results suggest Scaphinotus petersi populations experienced a significant fragmentation into distinct eastern and western populations separated by the San Pedro River much earlier than the last glacial period. More recently, probably after the LGM, the western populations became more fragmented in the Pinal, Santa Catalina, and Huachuca Mountains. Future work will include more populations of Scaphinotus petersi and closely related species from additional mountain ranges, adding missing lineages. Additional nuclear genes will be included to provide a broader picture of genetic structure and a better estimate of divergence times. These efforts will help develop a general model for understanding the phylogeographic effects of climate change in Sky Island organisms.
The authors are deeply indebted to the work Ross and Joyce Bell have done on adephagan, rhysodine, and carabid systematics and natural history. It has formed the foundation of much of the work KAO has done on carabids, and it has truly inspired and facilitated her work in adephagan and carabid systematics. This paper is dedicated to the life and work of Ross and Joyce Bell. Some outgroup specimens were collected by Elizabeth Jockusch. The authors also thank the College of the Holy Cross, the Robert L. Ardizzone Faculty Excellence Fellowship, the Charles and Rosanna Batchelor Foundation Grant and the Richard B. Fisher Summer Research Fellowship for funding for this project. We thank Sean Devine and two anonymous reviewers for improvements to the manuscript.